Abstract
Aims
Delay of progression from paroxysmal to persistent atrial fibrillation (AF) is an important measure of long-term success of AF treatment. However, published data on the impact of catheter ablation on AF progression are limited. This study evaluates whether radiofrequency (RF) catheter ablation delays the progression of AF compared with antiarrhythmic drug (AAD) treatment using current AF management guidelines.
Methods
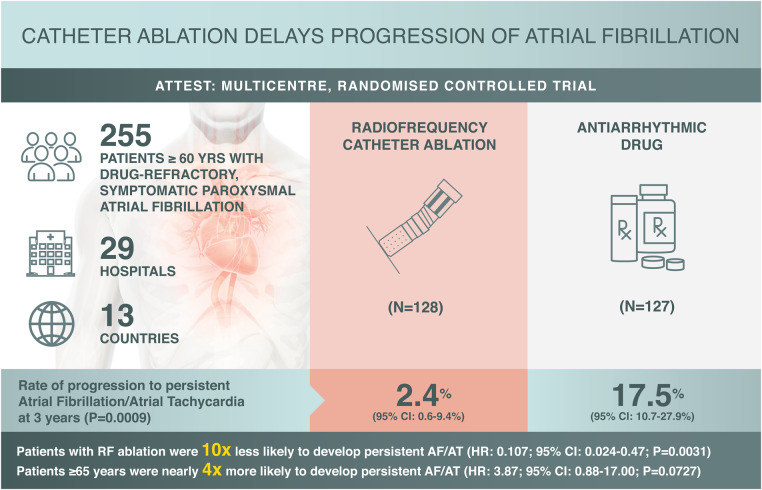
This prospective, randomized, controlled, two-arm, open-label trial was conducted at 29 hospitals and medical centres across 13 countries. Patients were randomized 1 : 1 to RF ablation or AAD treatment. The primary endpoint was the rate of persistent AF/atrial tachycardia (AT) at 3 years.
Results
After early study termination following slow enrolment, 255 (79%) of the planned 322 patients were enrolled (RF ablation, n = 128, AAD, n = 127); 36% of patients in the RF ablation group and 41% in the AAD group completed 3 years of follow-up. For the primary endpoint, the Kaplan–Meier estimate of the rate of persistent AF/AT at 3 years was significantly lower with RF ablation [2.4% (95% confidence interval (CI), 0.6–9.4%)] than with AAD therapy [17.5% (95% CI, 10.7–27.9%); one-sided P = 0.0009]. Patients ≥65 years were ∼4 times more likely to progress to persistent AF/AT than patients <65 years, suggesting RF ablation can delay disease progression [hazard ratio: 3.87 (95% CI, 0.88–17.00); P = 0.0727]. Primary adverse events were reported for eight patients in the RF ablation group.
Conclusions
Radiofrequency ablation is superior to guideline-directed AAD therapy in delaying the progression from paroxysmal to persistent AF.
Keywords: Persistent atrial fibrillation, Atrial tachycardia, Antiarrhythmic drugs, Radiofrequency ablation, Progression
Graphical Abstract
Graphical Abstract.
What’s new?
The ATTEST trial was a multicentre, randomized, prospective study in patients with paroxysmal atrial fibrillation (AF) designed to assess whether radiofrequency (RF) ablation is more effective in delaying the progression to persistent AF than antiarrhythmic drugs (AADs).
Although study termination occurred due to slow enrolment, the results demonstrated that patients treated with RF ablation were significantly less likely to develop persistent AF or persistent atrial tachycardia (AT) at 3 years post-study initiation than patients treated with AADs.
Patients ≥65 years were significantly more likely to progress to persistent AF/AT than patients <65 years, suggesting that early RF ablation may be an effective treatment strategy for delaying AF progression.
This study demonstrates that catheter ablation may be a more effective treatment option for patients with paroxysmal AF than AAD therapy, thereby potentially offering additional clinical value beyond the second-line, symptomatic treatment.
Introduction
Progression from paroxysmal to persistent atrial fibrillation (AF) results in an increased risk of myocardial infarction, thromboembolism, acute decompensation of heart failure, and stroke1,2; delay of progression is therefore an important measure of cardiovascular treatment outcome. However, published data on the long-term clinical impact of treatment in delaying AF progression are limited.
Some studies have shown that, over follow-up periods of 1–5 years, there is a significant benefit of catheter ablation compared with antiarrhythmic drug (AAD) treatment for reducing the recurrence of AF episodes in patients with paroxysmal AF.3,4 However, none of those studies has assessed the prevention of progression to persistent AF. Therefore, the objective of the current study was to determine, in patients with paroxysmal AF, whether ablation treatment using an irrigated catheter in conjunction with a three-dimensional electroanatomic mapping system delays progression of AF compared with drug therapy (either rate or rhythm control) using current AF management guidelines.
Methods
Trial design and study participants
ATTEST was a prospective, multicentre, randomized, controlled, two-arm open-label trial performed at 29 sites worldwide (Supplementary material online, Appendix) between 13 February 2012 and 29 May 2018 (ClinicalTrials.gov Identifier: NCT01570361, https://clinicaltrials.gov/ct2/show/NCT01570361). Full details of the inclusion and exclusion criteria and definitions are presented in Supplementary material online, Table S1. Briefly, adults ≥60 years of age with paroxysmal AF for ≥2 years and with ≥2 episodes over the 6 months preceding enrolment were included. Eligible patients had failed treatment with 1–2 AADs, had a HATCH score (hypertension = 1, age >75 = 1, transient ischaemic attack or stroke = 2, chronic obstructive pulmonary disease = 1, heart failure = 2) between 1 and 4, and were randomized (1 : 1; stratified by gender and study site) to pulmonary vein isolation via radiofrequency (RF) ablation or AAD therapy. Exclusion criteria included reversible AF, a previous diagnosis of persistent/permanent AF/atrial tachycardia (AT), cardioversion >48 h after onset of AF/AT, and recent cardiovascular events.
Consent
The protocol, amendments, and informed consent forms were reviewed and approved by ethics committees at each individual centre and local authorities (as needed). The trial was conducted in accordance with Good Clinical Practice Guidelines and the principles of the Declaration of Helsinki. Written informed consent was obtained from all patients at the time of their screening visit.
Interventions
In the RF ablation group, pulmonary vein isolation was achieved using an irrigated catheter (6-hole irrigated THERMOCOOL® catheter family with or without contact force [CF] sensing or porous-tip THERMOCOOL® SF catheter, Biosense Webster, Inc., Irvine, CA, USA) in conjunction with a 3-dimensional electroanatomic mapping system (CARTO® 3, CARTO® XP, or CARTO® RMT systems, Biosense Webster, Inc., Irvine, CA, USA). Procedures were performed as per standard of care and processes required by the participating sites. In the AAD group, medication was managed according to current guidelines5,6 at the investigators’ discretion. At the time of trial enrolment, if patients randomized to the AAD group had already been switched from a failed medication to a new AAD, they continued with this agent after enrolment; otherwise, a newly prescribed AAD regimen was initiated. Patients randomized to AAD could cross over to the RF ablation group after optimization of AAD and management of noncompliance. For management of arrhythmia recurrence, investigators’ usual clinical practice was followed as closely as possible. Changes in AAD treatment during the trial were permitted in accordance with current AF management guidelines.5,6 Follow-up procedures are described in detail in Supplementary material online, Appendix.
Outcomes measured
The primary endpoint of the trial was the (first documented) occurrence of persistent AF/AT, following a 90-day treatment initiation phase (‘blanking period’) starting with randomization. Persistent AF/AT was defined as AF/AT episodes monitored through TTM (>30 s, weekly TTM from Days 104–300, followed by monthly monitoring from Day 300 until the last follow-up visit up to 3 years; additional daily TTM was conducted if AF symptoms were present) lasting for >7 consecutive days or requiring termination by cardioversion after 48 h. Primary endpoint events were assessed out to 3 years. Secondary endpoints included rates of persistent AF/AT at 1 and 2 years, time to recurrent AF/AT, as well as the number of repeat ablations and new AADs. Factors potentially associated with AF progression, including gender, age, cardiac parameters, HATCH score, and certain comorbid conditions, were also explored. Safety endpoints are described in Supplementary material online, Appendix.
Statistical analysis
The trial hypothesis was that RF ablation for symptomatic paroxysmal AF would demonstrate a longer time to persistent AF/AT compared with AAD treatment. The primary endpoint of first documented occurrence of persistent AT/AF was assessed by the Kaplan–Meier method [event rate estimates at 3 years with 95% confidence intervals (CIs) derived from the Kaplan–Meier curve] in the intention-to-treat (ITT) population over 3 years of follow-up. The time to progression from paroxysmal to persistent AF/AT was compared between treatment groups using a one-sided superiority log-rank test with an overall α = 0.025; the one-sided α was chosen since the primary objective of the study was to assess whether RF ablation was superior to AAD in delaying AF progression to persistent AF/AT. Additional statistical analysis methodology is provided in Supplementary material online, Appendix.
Results
Patients
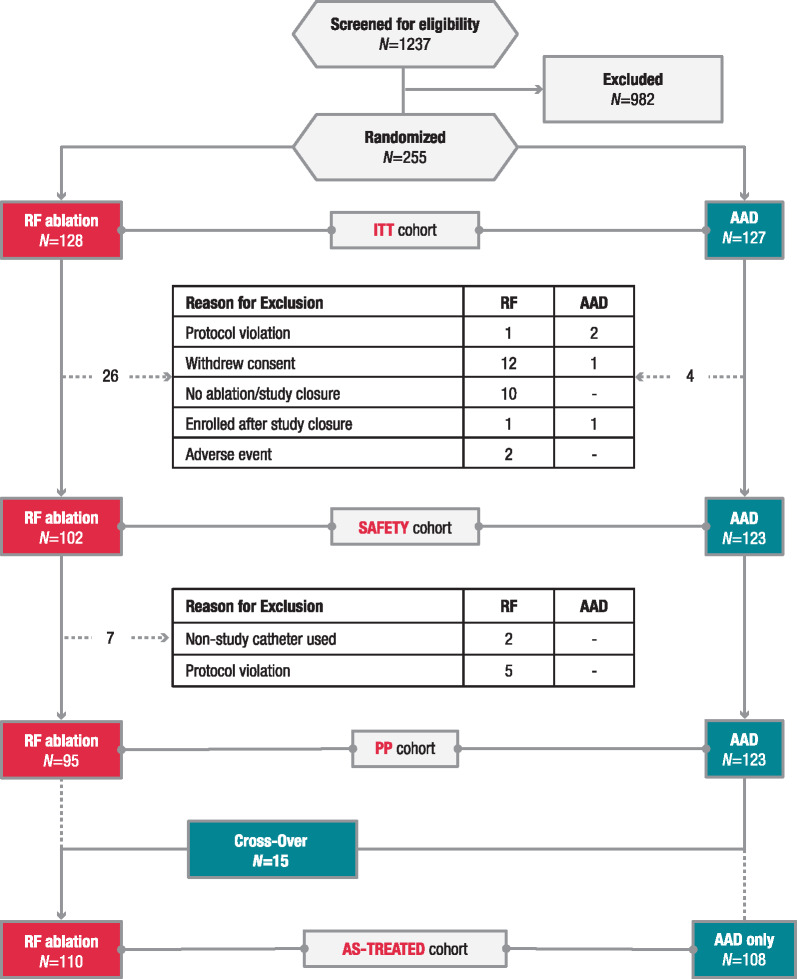
By the early termination date following slow enrolment, ∼1 year after the second interim analysis, 79% (255 of 322) of the targeted sample size was enrolled and randomized (ITT population). The ITT population included 128 RF and 127 AAD patients, whereas the safety population (all patients who received initial treatment as assigned by randomization) included 225 patients (102 RF and 123 AAD); the PP population comprised 218 patients (95 RF and 123 AAD), and the as-treated population was the same cohort grouped differently [110 RF (including 15 crossovers from AAD)] and 108 AAD only; Figure 1]. Demographic and baseline characteristics were generally similar between the two treatment groups [ITT: mean ± standard deviation (SD) age, 67.7 ± 4.7 years; male, 42%; Table 1]. Baseline AAD daily doses were similar in both groups (Supplementary material online, Table S2). The overall TTM compliance was 98.8%, 96.9%, and 86.2% at 1, 2, and 3 years, respectively.
Figure 1.
Patient disposition. AAD, antiarrhythmic drug; ITT, intention-to-treat; PP, per-protocol; RF, radiofrequency.
Table 1.
Baseline patient characteristics (ITT population)
| Characteristic a | RF ablation (n = 128) | AAD (n = 127) | Total (N = 255) |
|---|---|---|---|
| Age, mean ± SD (years) | 67.8 ± 4.8 | 67.6 ± 4.6 | 67.7 ± 4.7 |
| Male sex | 54 (42.2) | 53 (41.7) | 107 (42.0) |
| Months since first experience of AF, median (range) | 51.2 (19–625) | 49.8 (25–366) | 50.3 (19–625) |
| Number of AF/AT episodes during prior 6 months, median (range) | 6.5 (2–180) | 6.0 (0–180) | 6.0 (0–180) |
| Lone AF | 38 (29.7) | 39 (30.7) | 77 (30.2) |
| HATCH score,b mean ± SD | 1.5 ± 0.9 | 1.7 ± 0.9 | 1.6 ± 0.9 |
| Congestive heart failure | 24 (18.8) | 27 (21.3) | 51 (20.0) |
| Hypertension | 120 (93.8) | 123 (96.9) | 243 (95.3) |
| Cardiomyopathyc | 6 (4.7) | 2 (1.6) | 8 (3.1) |
| Left ventricular hypertrophy | 26 (20.3) | 23 (18.1) | 49 (19.2) |
| Atrial flutter | 15 (11.7) | 10 (7.9) | 25 (9.8) |
| Diabetes | 13 (10.2) | 14 (11.0) | 27 (10.6) |
| Hyperlipidaemia/dyslipidaemia | 67 (52.3) | 67 (52.8) | 134 (52.5) |
| Renal insufficiency | 3 (2.3) | 4 (3.1) | 7 (2.7) |
| Transient ischaemic attack/stroke | 12 (9.4) | 8 (6.3) | 20 (7.8) |
| AAD class I/III at baseline | 61 (47.7) | 69 (54.3) | 130 (51.0) |
| Left ventricular ejection fraction, mean ± SD (%) | 61.8 ± 5.8 | 62.3 ± 5.2 | 62.0 ± 5.5 |
| Left atrial diameter, mean ± SD (mm) | 42.1 ± 6.1 | 43.4 ± 5.6 | 42.7 ± 5.9 |
AAD, antiarrhythmic drug; AF, atrial fibrillation; AT, atrial tachycardia; ITT, intent-to-treat; RF, radiofrequency; SD, standard deviation.
Data are number of patients (%) unless otherwise specified.
HATCH score was calculated as: hypertension = 1 point, age >75 years = 1 point, transient ischaemic attack or stroke = 2 points, chronic obstructive pulmonary disease = 1 point, and heart failure = 2 points.
Includes ischaemic, non-ischaemic dilated, and hypertrophic obstructive cardiomyopathy.
Intervention and treatment
The most commonly used catheters during the index ablation procedure were 6-hole irrigated non-CF catheters (58.8%), followed by CF catheters (31.4%; Supplementary material online, Table S3). The majority of patients initiated a new AAD regimen at the start of the trial in the RF ablation group [94 of 127 patients (74.0%)], AAD group [82 of 108 patients (75.9%)], and crossover group [12 of 15 patients (80.0%)]. About half of the patients in the RF ablation group [59 of 128 (46.1%)] and AAD group [68 of 127 (53.5%)] started a new AAD regimen during the trial. The mean ± SD number of new AADs initiated during the trial period was similar in both groups (RF ablation, 0.8 ± 1.1; AAD, 1.0 ± 1.2). Excluding beta-blockers and calcium channel blockers, 53 of 127 patients in the AAD arm (41.7%) and 30 of 128 patients in the RF ablation arm (23.4%) were initiated with new Class I/III AADs (Supplementary material online, Table S4). A summary of medication and dose after randomization throughout the study follow-up period can be found in Supplementary material online, Table S5. Complete pulmonary vein isolation was achieved in all 102 patients (100%) who underwent the treatment. Additional details on ablation outcomes are available in Supplementary material online, Table S6.
Efficacy outcomes
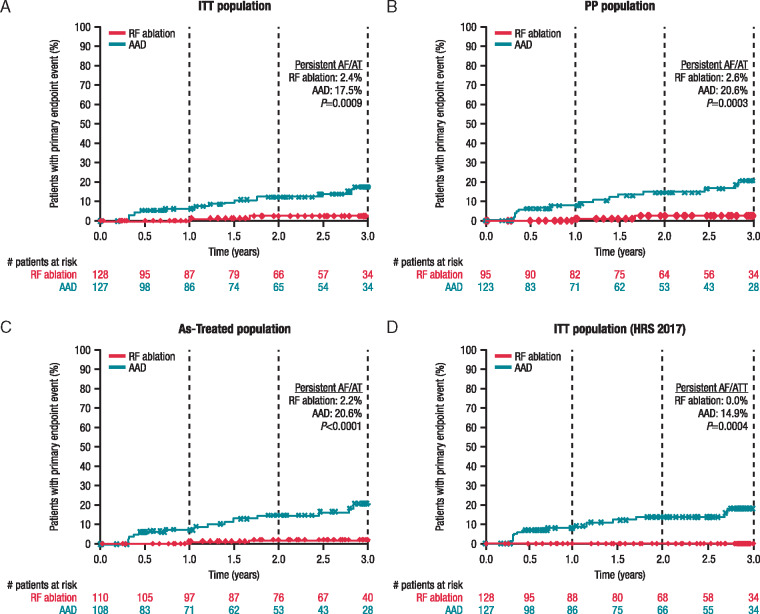
In the ITT population, 2 patients in the RF ablation group and 15 patients in the AAD group had developed persistent AF/AT at 3 years (primary endpoint), corresponding to a significantly lower 3-year Kaplan–Meier event rate estimate in the RF ablation group than the AAD group [2.4% (95% CI, 0.6–9.4%) vs. 17.5% (95% CI, 10.7–27.8%); one-sided P = 0.0009; Figure 2A]. Lower progression rates in the RF ablation group than in the AAD treatment group were already apparent at 1 and 2 years (secondary endpoints; point estimates, 1.3% vs. 6.5%, P = 0.0237 and 2.4% vs. 12.4%, P = 0.0082, respectively).
Figure 2.
Time to primary endpoint (occurrence of persistent atrial fibrillation/tachycardia) by treatment group: (A) ITT patient cohorts, (B) PP patient cohorts, (C) as-treated patient cohorts, and (D) ITT patient cohorts using the 2017 expert consensus Heart Rhythm Society definition of persistent atrial fibrillation. Note adherence to 90-day (0.25 year) blanking period in all panels. AAD, antiarrhythmic drug therapy; AF, atrial fibrillation; AT, atrial tachycardia; ITT, intention-to-treat; PP, per-protocol; RF, radiofrequency.
Sensitivity analyses
Sensitivity analyses of the primary endpoint in the PP and as-treated populations as well as in the ITT population using the Heart Rhythm Society 2017 expert consensus definition of persistent AF confirmed the above findings. In all three analyses, the Kaplan–Meier estimate of the rate of persistent AF/AT at 3 years was significantly lower in the RF ablation than the AAD group [PP population: 2.6% (95% CI, 0.6–9.9%) vs. 20.6% (95% CI, 12.7–32.5%); one-sided P = 0.0003 (Figure 2B); as-treated population: 2.2% (95% CI, 0.5–8.5%) vs. 20.6% (95% CI, 12.7–32.5%); one-sided P < 0.0001 (Figure 2C); ITT population using the Heart Rhythm Society 2017 expert consensus definition of persistent AF: 0.0% vs. 14.9% (95% CI, 8.7–24.8%); two-sided P = 0.0004 (Figure 2D)].
Factors associated with atrial fibrillation progression
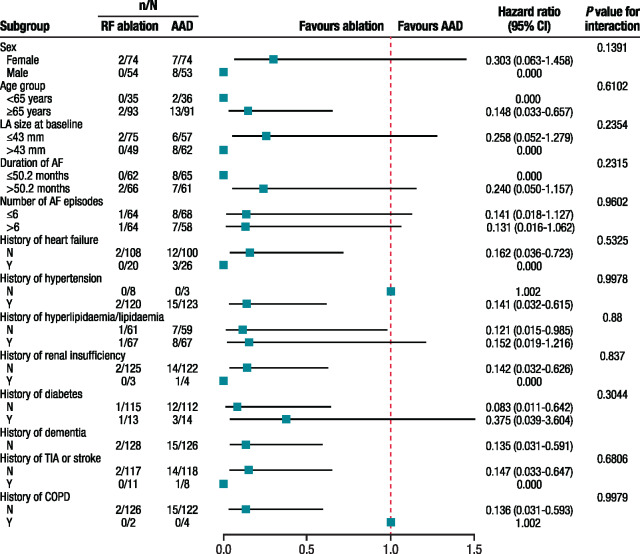
Using a Cox model with multiple baseline covariates and treatment as the time-dependent covariate in the PP population, treatment modality was significantly associated with AF progression. Patients treated with RF ablation, as opposed to AAD, were almost 10 times less likely to develop persistent AF/AT [hazard ratio: 0.107 (95% CI, 0.024–0.47]; P = 0.0031]. Patients ≥65 years were ∼4 times more likely [hazard ratio: 3.87 (95% CI, 0.88–17.00); P = 0.0727] to progress to persistent AF/AT than patients <65 years, suggesting early RF ablation can delay disease progression.
In the ITT population, there were no baseline conditions that significantly impacted the occurrence of AF/AT progression (Figure 3).
Figure 3.
Association of baseline conditions with the occurrence of persistent AF/AT (ITT population). AAD, antiarrhythmic drug treatment; AF, atrial fibrillation; AT, atrial tachycardia; CI, confidence interval; COPD, chronic obstructive pulmonary disease; ITT, intention-to-treat; LA, left atrial; RF, radiofrequency; TIA, transient ischaemic attack.
Secondary endpoints
The incidence of recurrent AF/AT and recurrent AF was consistently lower with RF ablation than with AAD treatment from 6 months through the 3-year follow-up period (Supplementary material online, Figures S1A and B). At 3 years, the Kaplan–Meier estimate of the rate of recurrent AF/AT was significantly lower in the RF ablation group than in the AAD group [49.2% (95% CI, 39.0–60.6%) vs. 84.8% (95% CI, 76.0–91.7%); two-sided P < 0.0001]. The rates of AT recurrence were similar between both groups. Excluding AT recurrence, the Kaplan–Meier estimated rates of persistent AF at 3 years were 0.0% in the RF ablation group and 10.2% in the AAD group (P = 0.0002; Supplementary material online, Figure S2).
In the ITT population, there was a mean of 0.9 and 1.1 ablations in the catheter ablation and crossover groups, respectively. The Kaplan–Meier estimate of the rate of repeat ablations at 3 years was 17.1% (95% CI, 10.8–27.6%; Supplementary material online, Figure S3). Kaplan–Meier estimates of the rate of new AADs at 3 years in the ITT population were similar between the RF ablation group [47.3% (95% CI, 37.3–58.6%)] and the AAD group [50.4% (95% CI, 40.2–61.4%); two-sided P = 0.411; Supplementary material online, Figure S4], implying that, after the blanking period, about half the patients in either treatment group underwent a change in their antiarrhythmic medication over the course of the study.
Safety outcomes
In the RF ablation group, adverse events (AEs) occurring within 7 days of the intervention (‘primary’ AEs) were reported for eight patients (7.8%; Table 2). The primary AEs of vascular pseudoaneurysm and haematoma were reported in two patients (2%) each, while atrial flutter, bradycardia, cardiac tamponade, and pericardial effusion were reported in one patient (1%) each. All primary AEs were resolved without sequelae. Catheter-, procedure-, and drug-related serious AEs (SAEs) are summarized in Table 2. The number of patients who experienced catheter/device-related SAEs was similar to the number of patients who experienced serious adverse drug reactions.
Table 2.
SAEs related to catheter, procedure, or AAD (safety population)
| AE, n (%) | RF ablation (n = 102) | AAD only (n = 108) | Crossover (n = 15) |
|---|---|---|---|
| Procedure-related SAEs | 12 (11.8) | — | 0 |
| Pericardial effusion | 1 | — | 0 |
| Tamponade | 1 | — | 0 |
| Pericarditis | 2 | — | 0 |
| Intracardiac thrombus | 1 | — | 0 |
| Atrial fibrillation | 1 | — | 0 |
| Atrial flutter | 3 | — | 0 |
| Congestive heart failure | 1 | — | 0 |
| Vascular access complication | 2 | — | 0 |
| Serious ADRsa | 5 (4.9) | 6 (5.6) | 2 (13.3) |
| Haemorrhage | 1 | 2 | 0 |
| CVA/stroke | 0 | 1 | 0 |
| Atrial fibrillation | 0 | 0 | 1 |
| Tachycardia | 0 | 1 | 0 |
| Bradycardia | 2 | 1 | 0 |
| Epistaxis | 1 | 0 | 0 |
| Lung disorder | 1 | 0 | 0 |
| Back pain | 0 | 1 | 0 |
| Cardiac ablation | — | — | 1 |
| Primary AEsb | 8 (7.8) | — | 0 |
| Vascular pseudoaneurysm | 2 | — | 0 |
| Haematoma | 2 | — | 0 |
| Atrial flutter | 1 | — | 0 |
| Bradycardia | 1 | — | 0 |
| Cardiac tamponade | 1 | — | 0 |
| Pericardial effusion | 1 | — | 0 |
AAD, antiarrhythmic drug; ADR, adverse drug reaction; AE, adverse event; CVA, cerebrovascular accident; RF, radiofrequency; SAE, serious adverse event.
Patients in the RF ablation group also received AAD therapy.
Occurring within 7 days of the procedure.
Discussion
This study demonstrated that RF catheter ablation—as part of standard-of-care AF management including AADs—is superior to guideline-directed AAD therapy alone in delaying the progression to persistent AF in patients with drug-refractory, recurrent paroxysmal AF. To date, pulmonary vein isolation by catheter ablation is primarily indicated for second-line, symptomatic treatment of paroxysmal AF after failure of ≥1 AAD.7 Nonetheless, cumulative evidence has demonstrated clinically meaningful outcomes or superiority of RF ablation over AAD for different outcomes. First, consistent with our findings, RF ablation has been shown to be superior to AAD in reducing long-term AF/AT recurrence in patients with drug-refractory paroxysmal AF.8 Secondly, catheter ablation has been shown to be superior to AAD in reducing death from any cause and hospitalization related to heart failure or any cardiovascular cause in AF patients with heart failure.9 Thirdly, first-line catheter ablation has been associated with a significant reduction in AF burden at 2–5 years.3,4 Our study results showing superiority of RF catheter ablation over AAD in delaying AF progression in paroxysmal AF patients add to the wealth of data on the clinical benefits of catheter ablation. Of note, the superior treatment effect of RF catheter ablation was evidenced as early as 1 year post-treatment and sustained through 3 years of follow-up.
Our results support the notion of increased benefit with catheter ablation. This is the first randomized study to introduce progression from paroxysmal to persistent AF as a clinical endpoint in an AF study. Progression of paroxysmal to persistent AF involves electrical and structural abnormalities or remodelling. Previous AF registries have shown that progression from paroxysmal to persistent AF occurred at rates between 8% at 1 year and 24% at 5 years.10,11 At 10 years after the first presentation of paroxysmal AF, >50% of patients have progressed to persistent AF.12 Similarly, data from a single centre with 564 patients showed that 11% of paroxysmal AF patients awaiting ablation progressed to persistent AF within 6–14 months.13 Our results further demonstrated that patients <65 years of age were ∼4 times less likely to progress to persistent AF than patients ≥65 years. Persistent AF patients have a higher risk of death and stroke than paroxysmal AF patients,14 and continued AAD therapy in patients who have failed prior, albeit different, AAD treatment yields low overall efficacy.15 Furthermore, the results of catheter ablation are worse than in paroxysmal AF if patients have progressed to persistent AF.16 In addition, there are currently no standardized ablation protocols beyond pulmonary vein isolation for persistent AF, and the results of additional ablation modalities are still controversial.7 Taken together, these data necessitate prudent consideration of catheter ablation to prevent progression to persistent AF in paroxysmal AF patients who have failed ≥1 AAD. Thus, this study suggests that catheter ablation may represent a valuable treatment option in patients with drug-resistant paroxysmal AF apart from symptomatic improvement, namely, the delay of progression to persistent AF. These results are in line with findings of a previous systematic review that showed a substantially lower rate of progression to persistent AF with catheter ablation (1.5%) compared with general population patients (30.9%).17
The rate of primary AEs associated with catheter ablation in the current study was within the range reported in other AF ablation studies8,17 and was similar to the rate for the AAD group. The current study protocol included atrial flutter (one case) and bradycardia (one case) as primary AEs. More recent catheter ablation studies18,19 excluded these events from the list of primary AEs, which would result in a lower catheter ablation complication rate. Since both treatment modalities are associated with different types of AEs, it is not possible to compare the safety of RF catheter ablation and AAD in a meaningful way. Nonetheless, no unanticipated AEs occurred during the study.
Limitations
Only RF catheters were used in the current study. Applicability of these catheter ablation results to other ablation technologies is unknown and requires further evaluation. In addition, approximately half of the patients in the RF ablation arm started a new AAD regimen during the study after the blanking period, which could have presented a confounding factor; however, a comparable proportion of patients in the AAD group also started a new AAD regimen during the study following the blanking period.
Conclusions
This multicentre, randomized, controlled study demonstrated that, in patients with drug-refractory paroxysmal AF, standard-of-care RF ablation was superior to rhythm or rate control drug therapy in delaying progression to persistent AF. This study suggests that catheter ablation may offer clinical value beyond symptom improvement in patients with paroxysmal AF.
Supplementary material
Supplementary material is available at Europace online.
Supplementary Material
Acknowledgements
We thank the following individuals for their contributions to study conduct, statistical analysis, and editorial assistance: Nathalie Macours, Liesbeth Vanderlinden, Karola Köhler, Lee Ming Boo, and Christina Kaneko. We thank Megan Knagge, PhD, of MedErgy for providing editorial assistance, which was paid for by the sponsor.
Funding
This work was supported by Biosense Webster, Inc.
Conflict of interest: K.-H.K reports research grants (moderate) from Biosense Webster, Impulse Dynamics, and Biotronik. D.S.L. reports honoraria (modest) from Biosense Webster, Medtronic, Biotronik, and Boehringer Ingelheim during the conduct of the study. E.N.M. reports research grants (modest) from Biosense Webster during the conduct of the study and reports honoraria (modest) from Boehringer Ingelheim. K.D. reports research grants (modest) from Biosense Webster during the conduct of the study, and from Abbott and Medtronic outside of the submitted work. S.W. reports research grants (modest) and honoraria (modest) from Biosense Webster, Boston Scientific, and Abbott during the conduct of the study. A.R., L.G., O.K., T.N., Y.K.O., S.P., M.G.B., M.S., and F.O. have nothing to disclose. The trial was designed by the investigators in conjunction with the sponsor, Biosense Webster, Inc. Data analysis was conducted independently by Premier Research, Durham, NC, USA. A full list of the trial investigators is included in Supplementary material online. All authors had full access to the data and vouch for the completeness and accuracy of the presented data and analyses and for the adherence of the trial to the protocol. All authors reviewed and approved the manuscript before it was submitted for publication.
Data availability
Johnson & Johnson Medical Devices Companies have an agreement with the Yale Open Data Access (YODA) Project to serve as the independent review panel for evaluation of requests for clinical study reports and participant level data from investigators and physicians for scientific research that will advance medical knowledge and public health. Requests for access to the study data can be submitted through the YODA Project site at http://yoda.yale.edu.
References
- 1. Chiang CE, Naditch-Brule L, Murin J, Goethals M, Inoue H, O'Neill J. et al. Distribution and risk profile of paroxysmal, persistent, and permanent atrial fibrillation in routine clinical practice: insight from the real-life global survey evaluating patients with atrial fibrillation international registry. Circ Arrhythm Electrophysiol 2012;5:632–9. [DOI] [PubMed] [Google Scholar]
- 2. Ganesan AN, Chew DP, Hartshorne T, Selvanayagam JB, Aylward PE, Sanders P. et al. The impact of atrial fibrillation type on the risk of thromboembolism, mortality, and bleeding: a systematic review and meta-analysis. Eur Heart J 2016;37:1591–602. [DOI] [PubMed] [Google Scholar]
- 3. Cosedis Nielsen J, Johannessen A, Raatikainen P, Hindricks G, Walfridsson H, Kongstad O. et al. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N Engl J Med 2012;367:1587–95. [DOI] [PubMed] [Google Scholar]
- 4. Nielsen JC, Johannessen A, Raatikainen P, Hindricks G, Walfridsson H, Pehrson SM. et al. Long-term efficacy of catheter ablation as first-line therapy for paroxysmal atrial fibrillation: 5-year outcome in a randomised clinical trial. Heart 2017;103:368–76. [DOI] [PubMed] [Google Scholar]
- 5. Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH. et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Europace 2012;14:1385–413. [DOI] [PubMed] [Google Scholar]
- 6. Wann LS, Curtis AB, January CT, Ellenbogen KA, Lowe JE, Estes NA III et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (updating the 2006 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2011;123:1144–23. [DOI] [PubMed] [Google Scholar]
- 7. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L. et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation Europace 2018;20:e1–e160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wilber DJ, Pappone C, Neuzil P, De Paola A, Marchlinski F, Natale A. et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. Jama 2010;303:333–40. [DOI] [PubMed] [Google Scholar]
- 9. Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Poole JE, for the CABANA Investigators et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA 2019;321:1261–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pappone C, Radinovic A, Manguso F, Vicedomini G, Ciconte G, Sacchi S. et al. Atrial fibrillation progression and management: a 5-year prospective follow-up study. Heart Rhythm 2008;5:1501–7. [DOI] [PubMed] [Google Scholar]
- 11. Kerr CR, Humphries KH, Talajic M, Klein GJ, Connolly SJ, Green M. et al. Progression to chronic atrial fibrillation after the initial diagnosis of paroxysmal atrial fibrillation: results from the Canadian Registry of Atrial Fibrillation. Am Heart J 2005;149:489–96. [DOI] [PubMed] [Google Scholar]
- 12. Padfield GJ, Steinberg C, Swampillai J, Qian H, Connolly SJ, Dorian P. et al. Progression of paroxysmal to persistent atrial fibrillation: 10-year follow-up in the Canadian Registry of Atrial Fibrillation. Heart Rhythm 2017;14:801–7. [DOI] [PubMed] [Google Scholar]
- 13. Kochhauser S, Dechering DG, Trought K, Hache P, Haig-Carter T, Khaykin Y. et al. Predictors for progression of atrial fibrillation in patients awaiting atrial fibrillation ablation. Can J Cardiol 2016;32:1348–54. [DOI] [PubMed] [Google Scholar]
- 14. Steinberg BA, Hellkamp AS, Lokhnygina Y, Patel MR, Breithardt G, Hankey GJ, on behalf of the ROCKET-AF Steering Committee and Investigators et al. Higher risk of death and stroke in patients with persistent vs. paroxysmal atrial fibrillation: results from the ROCKET-AF Trial. Eur Heart J 2015;36:288–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Curtis AB, Seals AA, Safford RE, Slater W, Tullo NG, Vidaillet H. et al. Clinical factors associated with abandonment of a rate-control or a rhythm-control strategy for the management of atrial fibrillation in the AFFIRM study. Am Heart J 2005;149:304–8. [DOI] [PubMed] [Google Scholar]
- 16. Verma A, Jiang CY, Betts TR, Chen J, Deisenhofer I, Mantovan R. et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 2015;372:1812–22. [DOI] [PubMed] [Google Scholar]
- 17. Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J. et al. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation 2005;111:1100–5. [DOI] [PubMed] [Google Scholar]
- 18. Chinitz LA, Melby DP, Marchlinski FE, Delaughter C, Fishel RS, Monir G. et al. Safety and efficiency of porous-tip contact-force catheter for drug-refractory symptomatic paroxysmal atrial fibrillation ablation: results from the SMART SF trial. Europace 2018;20:f392–400. [DOI] [PubMed] [Google Scholar]
- 19. Natale A, Reddy VY, Monir G, Wilber DJ, Lindsay BD, McElderry HT. et al. Paroxysmal AF catheter ablation with a contact force sensing catheter: results of the prospective, multicenter SMART-AF trial. J Am Coll Cardiol 2014;64:647–56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Johnson & Johnson Medical Devices Companies have an agreement with the Yale Open Data Access (YODA) Project to serve as the independent review panel for evaluation of requests for clinical study reports and participant level data from investigators and physicians for scientific research that will advance medical knowledge and public health. Requests for access to the study data can be submitted through the YODA Project site at http://yoda.yale.edu.