Abstract
Context
Fructose compared to glucose has adverse effects on metabolic function, but endocrine responses to oral sucrose vs glucose is not well understood.
Objective
We investigated how oral sucrose vs glucose affected appetite-regulating hormones, and how biological factors (body mass index [BMI], insulin sensitivity, sex) influence endocrine responses to these 2 types of sugar.
Design
Sixty-nine adults (29 men; 23.22 ± 3.74 years; BMI 27.03 ± 4.96 kg/m2) completed the study. On 2 occasions, participants consumed 300-mL drinks containing 75 g of glucose or sucrose. Blood was sampled at baseline, 10, 35, and 120 minutes post drink for plasma glucose, insulin, glucagon-like peptide (GLP-1)(7–36), peptide YY (PYY)total, and acyl-ghrelin measures. Hormone levels were compared between conditions using a linear mixed model. Interaction models were performed, and results were stratified to assess how biological factors influence endocrine responses.
Results
Sucrose vs glucose ingestion provoked a less robust rise in glucose (P < .001), insulin (P < .001), GLP-1 (P < .001), and PYY (P = .02), whereas acyl-ghrelin suppression was similar between the sugars. We found BMI status by sugar interactions for glucose (P = .01) and PYY (P = .03); obese individuals had smaller increases in glucose and PYY levels after consuming sucrose vs glucose. There were interactions between insulin sensitivity and sugar for glucose (P = .003) and insulin (P = .04), and a sex by sugar interaction for GLP-1 (P = .01); men demonstrated smaller increases in GLP-1 in response to oral sucrose vs glucose.
Conclusion
Sucrose is less efficient at signaling postprandial satiation than glucose, and biological factors influence differential hormone responses to sucrose vs glucose consumption.
Keywords: sucrose, glucose, periphery, obesity, insulin resistance, sex
Over the past several decades, global obesity rates have risen dramatically, and a growing body of evidence suggests that parallel increases in sugar consumption may be contributing to this public health concern (1-3). The common dietary sugars glucose and fructose are absorbed and metabolized differently by the body, and prior studies have shown that these monosaccharides have differential effects on hormones involved in energy homeostasis, which may significantly affect appetitive behavior and obesity risk (4-6).
One of the limitations acknowledged by prior studies is that fructose is rarely consumed in isolation, and additional studies are necessary to examine how real-world sugars, such as sucrose, affect endocrine responses (6, 7). Sucrose is a disaccharide composed of equal parts glucose and fructose and is commonly referred to as table sugar (8). Few studies have attempted to compare peripheral glucose and hormone responses to acute sucrose vs glucose consumption in humans, and findings from the limited number of studies to date have been difficult to interpret because of several factors, including comparisons of unequal doses of glucose and sucrose (9), testing the effects of sucrose and glucose beverages consumed in combination with meals (10), and very small sample sizes (10-13). Only one study to date has investigated the effects of oral sucrose vs glucose on incretin hormone responses, and they observed no differences in circulating levels of glucagon-like peptide-1 (GLP-1) following sucrose relative to glucose ingestion (13). However, Yau et al tested a low dosage of each sugar, and the small sample size precluded examination of possible associations between GLP-1 responses and participant characteristics (13). Thus, there is a lack of knowledge regarding the effects of acute sucrose ingestion, relative to glucose, on acute gut hormone responses. Furthermore, the existing studies that compared endocrine responses to glucose vs sucrose ingestion have been limited to all male (10, 11, 13) and/or lean (9, 11, 12) cohorts, and no study to date has examined whether peripheral responses to sucrose compared to glucose are affected by sex or obesity. Given that prior evidence demonstrated significant effects of obesity on GLP-1 and ghrelin responses to fructose compared to glucose (6, 7), investigating how adiposity is associated with peripheral hormone responses to sucrose compared to glucose is critically important. There is also evidence to suggest that insulin resistance may propagate the potential deleterious metabolic effects of fructose consumption; oral fructose does not suppress orexigenic hormones, a phenomenon that is even more pronounced in individuals with obesity/insulin resistance, compared to their lean/insulin sensitive counterparts (6). However, whether insulin sensitivity, independent of body mass index (BMI), affects GLP-1 and ghrelin responses to sucrose compared to glucose ingestion is unknown and worthy of investigation. Given that personalized nutrition must take into account differential responses to nutrients that arise because of the influence of biological processes (14), understanding how individual characteristics (ie, sex, adiposity, and insulin sensitivity) affect peripheral hormone responses to glucose and sucrose ingestion will provide new insights that might be important considerations for personalized nutrition.
This study aimed to address gaps in the literature by investigating the following questions: (1) What are the effects of acute sucrose compared to glucose ingestion on plasma glucose, insulin, and gut hormones (GLP-1, peptide YY [PYY], and ghrelin) involved in the regulation of energy homeostasis; and (2) do differential hormone responses to sucrose vs glucose vary based on level of adiposity, insulin sensitivity, and/or by sex? We hypothesized that sucrose and glucose would provoke differential hormone responses after oral ingestion, and that these differential peripheral responses would differ by obesity, and insulin resistance, and sex.
Materials and Methods
Participants
Young adults age 18 to 35 years were recruited for this study. Participants were right-handed, nonsmokers, weight-stable for at least 3 months prior to the study visits, nondieters, not on any medication (except oral contraceptives), and with no history of diabetes, eating disorders, illicit drug use, or other medical diagnoses. Participants provided written informed consent compliant with the University of Southern California Institutional Review Board (IRB No. H-09-00395).
Study procedures
The study reported here is part of a larger study examining neuroendocrine responses to high-reward foods (NCT02945475). This substudy consisted of an initial screening visit, an oral glucose tolerance test (OGTT), and an oral sucrose tolerance test (OSTT). All the visits were conducted after a 12-hour overnight fast. During the screening visit, we assessed eligibility for participation in the study and demographic information and anthropometric measurements were obtained. Height was measured to the nearest 0.1 cm using a stadiometer and weight to the nearest 0.1 kg using a bioelectrical impedance analysis scale (Model No. SC-331S, TANITA Corporation of America Inc), and BMI was calculated as weight in kilograms divided by height in meters squared.
Participants arrived at the Dornsife Social and Behavioral Phlebotomy Lab at University of Southern California at 0800 after a 12-hour overnight fast for their OGTT and OSTT visits. The order of the drink sessions was randomized, and the time interval between the 2 sessions was between 2 and 30 days. Participants consumed either a standard 75-g glucose or 75-g sucrose load, mixed with 0.45 g of nonsweetened zero-calorie cherry flavoring (Kool-Aid Unsweetened Cherry Drink Mix; Kraft Foods) for palatability, dissolved in 300 mL of water. The drinks were equicaloric (300 kcal each). Participants were asked to finish consuming each drink within 2 minutes. During the study visits, blood samples were collected in accordance with a standard OGTT: at baseline (0), 10, 35, and 120 minutes post drink to measure plasma glucose, insulin, acyl-ghrelin, GLP-1, and PYY. Whole-body insulin sensitivity index (ISI) was computed on the 2-hour OGTT (15, 16). Participants were classified as whole-body insulin resistant if ISI was equal or lower than 2.5 and insulin sensitive if ISI was above 2.5 (17). Female participants underwent study visits during the follicular phase of their menstrual cycles (18, 19).
Hormone analysis
Plasma glucose was measured enzymatically using glucose oxidase (YSI 2300 STAT PLUS Enzymatic Electrode-YSI analyzer, Yellow Springs Instruments). Plasma insulin, acyl-ghrelin (active ghrelin), and GLP-1(7–36) (active) were measured via Luminex multiplex technology (Millipore, Billerica), and PYY (total) was measured using a human PYY enzyme-linked immunosorbent assay kit (Millipore, Billerica). To represent overall response to ingestion of each drink, we calculated 120 minutes’ total area under the curve (AUC) for plasma hormones using the trapezoid method (also known as the trapezoidal rule) (20-23). Trapezoidal rule AUC is calculated by adding the areas under the graph between each pair of consecutive observations, as previously described (20, 23).
Statistical analysis
Comparisons were conducted to measure the endocrine responses to sucrose compared to glucose ingestion. The primary outcome was the total AUC difference in endocrine responses to sucrose vs glucose over the 120-minute testing period. We found AUC values to be right-skewed; for statistical analyses, AUC values were cubic root–transformed to better meet the assumptions of normality. Baseline levels of hormones between glucose and sucrose visits were compared using paired t tests. Baseline measures of hormones between BMI, ISI, and sex groups were compared using unpaired t tests and analysis of variance. We used a linear mixed-effects regression model to examine the differential endocrine responses to sucrose compared to glucose, adjusting for age, sex, BMI (continuous variable), with a random intercept for drink randomization order. For longitudinal models that included repeated measurements over time, a random intercept for participant was included with an unstructured covariance matrix. Given known effects of obesity, insulin sensitivity, and sex on endocrine responses to sugar consumption (6, 7, 10, 24) we tested for interactions between (1) BMI status (lean, overweight, or obese) and sugar; (2) sex and sugar; and (3) insulin sensitivity and sugar on the differential response to sucrose vs glucose, where BMI status, sex, and insulin sensitivity were treated as categorical variables. P values less than .05 were interpreted as statistically significant. SAS 9.4 statistical software (SAS Institute) was used for all data analyses, with regression models performed in PROC MIXED.
Results
Participants
Sixty-nine adults (29 men, 40 women) with a mean (±SD) age of 23.22 ± 3.74 years completed the study (Table 1). Participants had a mean BMI of 27.03 ± 4.96 kg/m2; 25 lean, 24 overweight, 20 obese based on Centers for Disease Control and Prevention criteria (25). Participants had a mean ISI of 4.12 ± 3.40; 42 insulin sensitive, 26 insulin resistant (17). One man (lean) completed only the sucrose visit but not the glucose visit.
Table 1.
Participant characteristics (N = 69)
| Characteristic | N or mean ± SD | Range |
|---|---|---|
| Age, y | 23.22 ± 3.74 | 18-35 |
| Sex | Male: N = 29 | |
| Female: N = 40 | ||
| BMI, kg/m2 | 19.18-40.27 | |
| Lean: BMI ≥ 18 < 25 | N = 25 | |
| Overweight: BMI ≥ 25 < 30 | N = 24 | |
| Obese: BMI ≥ 30 | N = 20 | |
| Insulin sensitivity category | 0.69-15.13 | |
| Insulin sensitive (ISI > 2.5) | N = 42 | |
| Insulin resistant (ISI ≤ 2.5) | N = 26 |
Abbreviations: BMI, body mass index; ISI, insulin sensitivity index.
Differential endocrine responses to sucrose vs glucose ingestion
Baseline levels of plasma glucose, insulin, acyl-ghrelin, GLP-1, and PYY were not different between the glucose and sucrose conditions (Supplementary Table 1, which can be found in the digital repository) (26). Expectedly, individuals with overweight and obesity had higher fasting insulin levels compared to lean participants (P = .03), and lean individuals had higher fasting acyl-ghrelin than individuals with overweight and obesity (P = .02). There were no significant differences in baseline levels of glucose, GLP-1, or PYY between BMI groups, although there was a trend toward higher fasting glucose levels in overweight and obese compared to lean individuals (SI Table 2) (26). Correspondingly, insulin-resistant individuals had higher fasting insulin levels than insulin-sensitive participants (P < .001) and tended to have higher fasting glucose levels (P = .05), but there were no differences in fasting acyl-ghrelin, GLP-1, or PYY between insulin sensitivity groups (SI Table 3) (26). Baseline levels of plasma glucose, insulin, acyl-ghrelin, GLP-1, and PYY were not different between men and women (SI Table 4) (26).
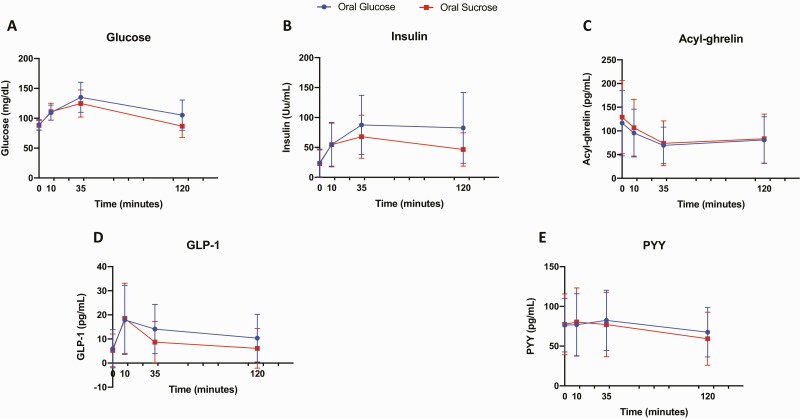
Trajectories for plasma glucose, insulin, ghrelin, GLP-1, and PYY responses at each time point (baseline/0 minutes and 10, 35, and 120 minutes post drink) to oral glucose and sucrose are shown in Fig. 1. Maximum acyl-ghrelin suppression occurred at 35 minutes, reaching approximately 40% change after oral glucose, and 43% change after oral sucrose. Maximum PYY stimulation occurred at 35 minutes after oral glucose and 10 minutes after oral sucrose, reaching approximately 8% change post glucose ingestion, and 4% change after sucrose consumption.
Figure 1.
Trajectories for plasma A, glucose; B, insulin; C, acyl-ghrelin; D, glucagon-like peptide-1 (GLP-1); and E, peptide YY (PYY) values after glucose (blue) and sucrose (red) drinks. Time point values are expressed as mean ± SD for all participants.
Sucrose relative to glucose ingestion led to reduced AUC for plasma glucose (P < .001), insulin (P < .001), GLP-1 (P < .001), and PYY (P = .02). AUC for acyl-ghrelin was not significantly different following sucrose relative to glucose ingestion (Table 2).
Table 2.
Area under the curve for plasma glucose, insulin, ghrelin, glucagon-like peptide-1, and peptide YY after glucose and sucrose ingestion
| Glucose drink | Sucrose drink | P | |
|---|---|---|---|
| Glucose, mg/dL·min | 13 700.87 (12 596.88-15 386.36) | 12 824.53 (11 421.48-13 972.95) | < .001a |
| Insulin, µU/mL·min | 8034.30 (5772.20-13 098.26) | 5734.98 (4508.60-8774.42) | < .001a |
| Acyl-ghrelin, pg/mL·min | 8544.63 (5241.98-12 121.78) | 8835.50 (5739.83-12 600.18) | .54 |
| GLP-1(7–36), pg/mL·min | 1392.70 (880.48-2108.74) | 931.88 (500.05-1478.63) | < .001a |
| PYY(total) , pg/mL·min | 8548.16 (6125.24-11 184.06) | 7757.02 (5489.22-10 416.23) | .02a |
Data are median (25th percentile-75th percentile).
Abbreviations: GLP-1, glucagon-like peptide-1; PYY, peptide YY.
a P values are statistically significant at P less than .05. Adjusted for age, sex, and body mass index.
Influence of body mass index status on endocrine responses to sucrose vs glucose ingestion
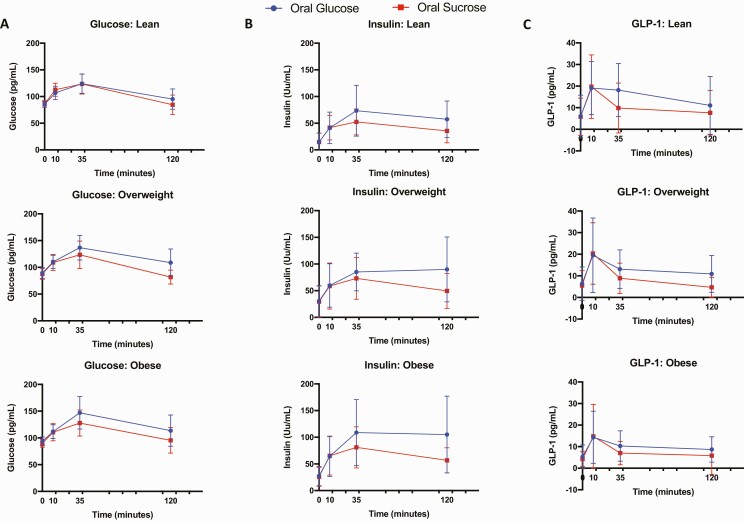
There was a significant interaction between BMI status and sugar on increases in plasma glucose (P = .01) and PYY (P = .03) levels. In results stratified by BMI status (Table 3, Fig. 2, SI Figure 1) (26), among lean individuals there were no significant differences in plasma AUC glucose levels after sucrose vs glucose ingestion (P = .10), whereas among individuals with overweight (P < .001) and obesity (P = .002), plasma AUC glucose levels were lower after sucrose vs glucose ingestion (Fig. 2A, Supplementary Figure 1) (26). Plasma AUC insulin levels were lower in lean (P = .001), overweight (P < .001), and obese (P = .001) groups after consuming sucrose vs glucose (Fig. 2B, Supplementary Figure 1) (26). Acyl-ghrelin AUC levels were not different between the sucrose vs glucose conditions in lean, overweight, or obese individuals (Table 3). Given the known influence of obesity on fasting glucose, insulin, and ghrelin levels, we also calculated incremental area under the curve (iAUC) in a post hoc analysis to account for baseline variations. Glucose, insulin, and acyl-ghrelin iAUC after sucrose vs glucose in lean, overweight, and obese groups were similar to total AUC results (Supplementary Table 5) (26). Lean (P = .01) and overweight (P = .001) individuals had a smaller GLP-1 AUC increase after sucrose relative to glucose ingestion, whereas individuals with obesity did not have significant differences in GLP-1 AUC between the glucose and sucrose conditions (P = .11) (Fig. 2C, Supplementary Figure 1) (26). Individuals with obesity had reduced AUC for PYY after consuming sucrose relative to glucose (P = .002), whereas lean (P = .34) and overweight (P = .96) individuals had no differences in AUC for PYY between the drink conditions (Table 3).
Figure 2.
Plasma A, glucose; B, insulin; and C, glucagon-like peptide-1 (GLP-1) responses to glucose (blue) and sucrose (red) drinks, stratified by body mass index groups. Values are expressed as mean ± SD.
Table 3.
Area under the curve for plasma glucose, insulin, ghrelin, glucagon-like peptide-1, and peptide YY after glucose and sucrose ingestion in lean, overweight, and obese groups
| Lean | Overweight | Obese | P S × BMI | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Glucose drink | Sucrose drink | P | Glucose drink | Sucrose drink | P | Glucose drink | Sucrose drink | P | ||
| Glucose, mg/dL·min | 12 748.82 (11 947.21-14 797.15) | 12 482.29 (11 667.45-13 764.18) | .10 | 14 572.29 (13 567.81-15 204.15) | 12 820.34 (11 023.39-13 697.80) | < .001a | 15 436.02 (13 136.42-16 931.52) | 13 032.50 (12 071.29-14 987.96) | .002a | .01a |
| Insulin, µU/mL·min | 6579.12 (3862.73-8919.79) | 4619.56 (3309.80-5783.88) | .001a | 8480.10 (6198.94-13 347.20) | 5587.19 (4652.37-9151.62) | < .001a | 9513.27 (7036.50-15 265.49) | 7698.57 (5935.15-10 225.52) | .001a | .69 |
| Acyl-ghrelin, pg/mL·min | 10 034.11 (7380.00-14 811.13) | 10 629.30 (7959.30-16 524.70) | .70 | 8188.05 (5445.88-10 748.05) | 8760.66 (6057.68-13 389.05) | .70 | 6147.23 (4153.58-11 395.90) | 7185.10 (4857.48-10 719.95) | .81 | .99 |
| GLP-1(7–36), pg/mL·min | 1862.18 (1039.51-2269.69) | 973.63 (744.63-1514.05) | .01a | 1579.65 (857.58-2033.08) | 1075.30 (421.74-1577.58) | .001a | 1123.38 (890.65-1557.03) | 675.11 (536.09-1270.79) | .11 | .38 |
| PYY(total), pg/mL·min | 8965.25 (6521.88-11 678.61) | 7970.68 (6513.69-10 236.38) | .34 | 8548.16 (5954.87-9764.59) | 8191.97 (5169.75-12 870.40) | .96 | 8371.34 (6206.44-10 767.04) | 7092.04 (4665.72-10 169.37) | .002a | .03a |
Data are median (25th percentile-75th percentile).
Abbreviations: BMI, body mass index; GLP-1, glucagon-like peptide-1; PYY, peptide YY.
a P values are statistically significant between glucose and sucrose drinks. Adjusted for age, sex, and BMI; PS × BMI, P value for sugar × BMI group interaction, statistical significance if P less than .05.
Influence of insulin sensitivity on endocrine responses to sucrose vs glucose ingestion
There were interactions between ISI and sugar on AUC for plasma glucose (P = .003) and insulin (P = .04). In models stratified by insulin sensitivity (Table 4), the diminished increase in plasma glucose after sucrose vs glucose consumption was more pronounced in insulin-resistant (P < .001) compared to insulin-sensitive (P = .01) individuals. In contrast, we observed a smaller increase in plasma insulin levels after oral sucrose vs glucose both among insulin-sensitive (P < .001) and insulin-resistant (P < .001) participants. Given the known influence of insulin resistance on fasting glucose and insulin levels, we also calculated iAUC in a post hoc analysis to account for baseline variations, and glucose and insulin iAUC results were similar to total AUC results (Supplementary Table 6) (26). The smaller increase in GLP-1 levels in response to sucrose vs glucose ingestion was more pronounced in insulin sensitive (P < .001) than insulin resistant (P = .04) individuals. Acyl-ghrelin and PYY responses to the sucrose vs glucose conditions were not different between insulin sensitivity groups (Table 4).
Table 4.
Area under the curve for plasma glucose, insulin, ghrelin, glucagon-like peptide-1, and peptide YY after glucose and sucrose ingestion in insulin-resistant and insulin-sensitive groups
| Insulin sensitive | Insulin resistant | P S × ISI | |||||
|---|---|---|---|---|---|---|---|
| Glucose drink | Sucrose drink | P | Glucose drink | Sucrose drink | P | ||
| Glucose, mg/dL·min | 13 264.88 (12 413.55-14 825.31) | 12 530.69 (11 363.28-13 205.90) | .01a | 15 397.35 (13 569.86-16 931.52) | 13 266.63 (11 748.96-14 608.50) | < .001a | .003a |
| Insulin, µU/mL·min | 6307.66 (4703.55-7976.26) | 4862.89 (3586.12-5759.43) | < .001a | 13 648.60 (10 793.31-15 997.07) | 9604.56 (8286.41-11 555.07) | < .001a | .04a |
| Acyl-ghrelin, pg/mL·min | 8951.28 (4969.70-14 162.35) | 9902.84 (5634.84-13 389.05) | .26 | 8207.06 (5445.88-10 748.05) | 7896.60 (5779.95-9810.10) | .48 | .21 |
| GLP-1(7–36), pg/mL·min | 1200.21 (836.30-2111.15) | 824.08 (286.95-1427.50) | < .001a | 1546.71 (1123.38-2033.08) | 1162.45 (691.80-1570.08) | .04a | .23 |
| PYY(total), pg/mL·min | 8322.75 (6040.05-11 008.48) | 7525.82 (5541.10-10 462.49) | .09 | 9752.61 (6206.44-11 338.45) | 8870.51 (5187.50-10 416.23) | .11 | .83 |
Data are median (25th percentile-75th percentile).
Abbreviations: BMI, body mass index; GLP-1, glucagon-like peptide-1; ISI, insulin sensitivity index; PYY, peptide YY.
a P values are statistically significant between glucose and sucrose drinks. Adjusted for age, sex, and BMI. PS × ISI, P value for sugar × ISI group interaction, statistical significance if P less than .05.
Effects of sex on endocrine responses to sucrose vs. glucose ingestion
We found a sex-by-sugar interaction for GLP-1 AUC (P = .01). In models stratified by sex (Table 5), men had a less pronounced increase in plasma GLP-1 after sucrose vs glucose consumption (P < .001) than women (P = .02). Men did not have differential PYY responses to sucrose vs glucose (P = .33), whereas women had decreased PYY AUC after sucrose relative to glucose ingestion (P = .03). Plasma glucose, insulin, and acyl-ghrelin responses to sucrose vs glucose ingestion were not different between male and female participants (see Table 5).
Table 5.
Area under the curve for plasma glucose, insulin, ghrelin, glucagon-like peptide-1, and peptide YY after glucose and sucrose ingestion in males and females
| Male | Female | P S × sex | |||||
|---|---|---|---|---|---|---|---|
| Glucose drink | Sucrose drink | P | Glucose drink | Sucrose drink | P | ||
| Glucose, mg/dL·min | 14 170.76 (12 803.66-15 204.15) | 12 416.06 (11 293.88-13 810.34) | < .001a | 13 601.18 (12 465.00-15 538.34) | 12 928.98 (11 856.36-14 290.73) | < .001a | .82 |
| Insulin, µU/mL·min | 7797.36 (5481.36-9513.27) | 5068.72 (4508.60-7949.36) | < .001a | 8726.71 (6416.37-13 635.52) | 6304.22 (4402.47-9860.87) | < .001a | .41 |
| Acyl-ghrelin, pg/mL·min | 7151.43 (5253.65-11 127.90) | 7846.65 (5739.83-12 603.78) | .69 | 9501.78 (5230.30-13 306.05) | 9691.73 (6042.45-12 483.66) | .72 | .90 |
| GLP-1(7–36), pg/mL·min | 1524.10 (904.00-2111.15) | 876.30 (309.65-1570.08) | < .001a | 1361.90 (830.56-2071.20) | 952.75 (642.51-1436.01) | .02a | .01a |
| PYY(total), pg/mL·min | 8548.16 (6826.03-12 653.67) | 8555.00 (6097.26-12 464.78) | .33 | 8603.44 (5994.90-10 799.97) | 7600.76 (5187.50-9483.62) | .03a | .46 |
Data are median (25th percentile-75th percentile).
Abbreviations: GLP-1, glucagon-like peptide-1; PYY, peptide YY.
a P values are statistically significant between glucose and sucrose drinks. Adjusted for age, sex, and body mass index. PS X sex, P value for sugar × sex interaction, statistical significance if P less than .05.
Discussion
This study describes the effects of acute sucrose compared to glucose ingestion on peripheral glucose, insulin, and gut hormones involved in appetite regulation, and how individual characteristics (ie, sex, adiposity, and insulin sensitivity) contribute to differential endocrine responses to sucrose vs glucose consumption. We found that sucrose compared to glucose ingestion provokes reduced circulating glucose, insulin, GLP-1, and PYY levels. We found no differences in acyl-ghrelin suppression between the sucrose vs glucose conditions, which is consistent with prior reports in rodents and humans (10, 13, 27). We found BMI status-by-sugar interactions for glucose and PYY indicating that individuals with obesity showed lower circulating glucose and PYY levels after consuming sucrose relative to glucose, whereas lean individuals had no significant differences in glucose or PYY levels after sucrose vs glucose. Independent of BMI, there were interactions between insulin sensitivity and sugar on plasma glucose and insulin levels, and stratified models additionally showed that the smaller increase in GLP-1 levels in response to sucrose vs glucose ingestion was more pronounced in insulin-sensitive than insulin-resistant individuals. We also found a sex-by-sugar interaction for GLP-1 indicating that men had lower increases in plasma GLP-1 after sucrose vs glucose consumption than women. Our findings suggest that oral sucrose may be less efficient at postprandial peripheral satiety signaling compared to oral glucose, and that individual biological factors influence differential peripheral hormone responses to sucrose vs glucose consumption.
This is the first study to demonstrate that sucrose relative to glucose ingestion leads to a less robust increase in circulating GLP-1, an incretin hormone known to trigger postprandial satiation. Only one additional study has examined the effects of oral sucrose vs glucose on postprandial GLP-1 in humans; Yau et al did not detect differential GLP-1 responses to sucrose and glucose, which may be because of the low dose of sugar ingested, their measurement of total GLP-1 (as opposed to active GLP-1), and/or the small and selected study cohort of 7 healthy men (13). We additionally demonstrated that lean and overweight individuals had lower GLP-1 levels after sucrose relative to glucose ingestion, whereas individuals with obesity had an attenuated increase in GLP-1 levels in response to ingestion both of sucrose and glucose, suggesting that adults with obesity had overall impairments in GLP-1 secretion that were not differentiated by the 2 sugar conditions (see Fig. 2, Supplementary Figure 1) (26). These findings build on previous reports regarding GLP-1 responses to fructose vs glucose consumption; whereas fructose relative to glucose resulted in smaller increases in GLP-1 among lean adults (5, 28, 29), adults with obesity had attenuated GLP-1 responses both to oral fructose and glucose (30).
Our results also revealed sex differences in the GLP-1 responses to ingestion of sucrose relative to glucose whereby differences in circulating GLP-1 levels after sucrose vs glucose were more pronounced in men than women. Prior studies have reported equivocal findings regarding sex differences in postprandial GLP-1 secretion: either no difference between men and women (31), higher in women (32), or higher in men (33). To the best of our knowledge, this is the first study to explore how sex influences endocrine response to oral sucrose vs glucose, and additional studies are warranted to confirm potential sex differences in incretin secretion in response to different types of sugars.
Ours is also the first study in humans to examine the effects of acute sucrose compared to glucose ingestion on circulating PYY, a gut-derived hormone implicated in the regulation of hunger. We observed that PYY levels were lower after sucrose relative to glucose consumption. There was also a BMI status-by-sugar interaction for PYY response to sucrose vs glucose, indicating that oral sucrose induces a less favorable satiety response than oral glucose among individuals with obesity. This finding implies that obesity itself may enhance the obesogenic metabolic effects of sucrose, which is in line with previous reports suggesting that excessive sucrose consumption may play a role in the etiology and perpetuation of obesity (1-3). Little is known about the acute PYY responses to different types of sugars. Prior reports in lean adults have observed either greater PYY levels after fructose compared to glucose ingestion (5) or no differential PYY response between the fructose and glucose conditions (29), whereas a study in rodents found higher PYY levels after 24 hours of glucose and sucrose, but not fructose, feeding (27). However, it has been well documented that obese individuals have blunted PYY responses to mixed-meal caloric intake compared to their lean counterparts (34-37). Therefore, inconsistencies between the present findings and previous reports regarding PYY responses to different types of sugars in lean individuals (5, 29) may be due to obesity-driven differences in postprandial PYY secretion. Differences in the biochemical measurements (total PYY vs active PYY(3–36)) and/or differences in the assay used to determine PYY levels may also lead to discrepancies in results.
Previous studies in lean adults have demonstrated that sucrose compared to glucose ingestion elicits disparate insulin secretion than would be expected from the glycemic response (9, 12), and we also observed a disparity between insulinemic and glycemic responses to oral sucrose in the present study (see Fig. 1). Notably, we found a BMI status–by-sugar interaction for plasma glucose, and stratified models demonstrated that lean participants had similar plasma glucose responses between the 2 drink conditions, whereas individuals with overweight/obesity had significantly greater plasma glucose levels in response to glucose compared to sucrose ingestion (see Fig. 2). Correspondingly, there was also an insulin sensitivity–by-sugar interaction for plasma glucose, with stratified results revealing that the higher AUC for plasma glucose following the ingestion of glucose relative to sucrose was more pronounced in insulin-resistant compared to insulin-sensitive individuals. Prior tracer studies have shown that approximately 30% to 50% of ingested fructose is converted into glucose (38), and the conversion percentage from fructose into glucose was lower in individuals with obesity and diabetes (39). In keeping with earlier findings, our data suggest obesity and insulin resistance may have resulted in a reduction in the conversion of the fructose subunit of sucrose into glucose. In contrast, participants across all BMI groups (lean, overweight, and obese) and insulin sensitivity groups (insulin sensitive and insulin resistant) had increased insulin responses to glucose compared to sucrose ingestion. Importantly, these disparate patterns between plasma glucose and insulin responses to the drink conditions in lean and insulin-sensitive individuals paralleled a more robust increase in GLP-1 after oral glucose compared to sucrose within both the lean and insulin-sensitive groups (see Fig. 2, Table 4). Given the insulinotropic effects of GLP-1 action in healthy individuals (40), and the fact that above-basal insulin secretion depends linearly on GLP-1 concentration and its rate of change (41, 42), we postulate that the disproportionate insulinemic, compared to glycemic, responses to glucose relative to sucrose among lean, insulin-sensitive individuals might be partly explained by incretin potentiation.
Our cohort and study design had several strengths. Unlike previous studies that investigated endocrine responses to oral sucrose and glucose, we had a larger cohort that included men and women and individuals who were classified as lean, overweight, and obese as well as insulin sensitive or insulin resistant; therefore, we were able to test for sex, BMI status, and insulin sensitivity as potential moderators of the relationship between sugar consumption and hormone levels. However, our study also had some limitations. Blood samples were collected in accordance with a standard OGTT (15, 16) as part of our parent study design. Although previous reports have demonstrated that among adults with normal glucose tolerance (including individuals with obesity and insulin resistance), plasma glucose, insulin, and incretin hormones peak at 30 minutes during a standard OGTT (43, 44), additional blood sampling time points could have provided useful information regarding glucose and hormone trajectories during the OGTT and OSTT. In addition, although a previous report suggested that obese-insulin resistant and obese-insulin sensitive individuals have differential acyl-ghrelin responses to oral fructose vs glucose (6), we did not perform subgroup analyses to examine the role of insulin sensitivity within the obese group because it would have required a larger sample size. Furthermore, our cohort was limited to young adults (ages 18-35 years), and studies examining endocrine responses to sucrose vs glucose in children and older adults are warranted. Additional work is needed to investigate both longer-term consequences and dose-dependent effects of sucrose relative to glucose consumption on endocrine responses. Future studies should also incorporate additional factors that may play a role in the differential peripheral responses to sugars, such as race/ethnicity (45), genetic variants (46), the gut microbiome (47), and food form (ie, sugar-sweetened beverages vs sugar-sweetened solids) (48-50).
In conclusion, oral sucrose, a typically consumed, dietary-added sugar widely known as “table sugar,” produced lower increases in peripheral hormones involved in satiety signaling compared to oral glucose. Furthermore, individual biological factors (adiposity, insulin sensitivity, sex) influenced differential peripheral hormone responses to sucrose relative to glucose consumption. Individuals with obesity had smaller increases in plasma glucose and PYY levels after consuming sucrose compared to glucose. Independent of BMI, there were interactions between insulin sensitivity and sugar type on the increase in plasma glucose and insulin levels. In addition, when compared to women, men had smaller increases in GLP-1 in response to oral sucrose vs glucose. These findings highlight the importance of considering individual characteristics that may affect the metabolic consequences of consumption of specific nutrients, such as different types of sugar.
Acknowledgments
The authors would like to thank the volunteers who participated in this study. The authors would also like to thank Ana Romero, Enrique Trigo, Reina Maniego, Esther Jahng, Brandon Ge, Lloyd Nate Overholtzer, Jada Hislop, Martina Erdstein, and Priyanka Dave for assisting with study visits and recruiting volunteers, and the staff at the Dornsife Cognitive Neuroimaging Center and Diabetes and Obesity Research Institute of the University of Southern California.
Financial Support: This work was supported by the National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases (grant No. R01DK102794 (principal investigator: K.A.P). A Research Electronic Data Capture, REDCap, database was used for this study, which is supported by the Southern California Clinical and Translational Science Institute through NIH grant number UL1TR001855.
Clinical Trial Information: Clinical trial registration number NCT02945475 (registered October 26, 2016).
Author Contributions: Dr Page had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis. Dr Page was responsible for study conceptualization and design and obtained funding for this study. Ms Yunker, Dr Dorton, Mr Angelo, and Ms DeFendis were responsible for management and coordination of the study execution. Dr Pickering performed the statistical analysis. Ms Yunker and Dr Page drafted the manuscript. Ms Yunker, Dr Luo, Dr Jones, Dr Alves, Dr Pickering, Dr Monterosso, and Dr Page provided critical review, commentary, and revisions to the manuscript. All authors approved the final manuscript as submitted.
Glossary
Abbreviations
- BMI
body mass index
- iAUC
incremental area under the curve
- ISI
insulin sensitivity index
- AUC
area under the curve
- GLP-1
glucagon-like peptide-1
- OGTT
, oral glucose tolerance test
- OSTT
, oral sucrose tolerance test
- PYY
peptide YY.
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability
The data sets generated and analyzed during the present study are available from the corresponding author on reasonable request.
References
- 1. Bray GA, Popkin BM. Dietary sugar and body weight: have we reached a crisis in the epidemic of obesity and diabetes? Health be damned! Pour on the sugar. Diabetes Care. 2014;37(4):950-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Malik VS, Willett WC, Hu FB. Global obesity: trends, risk factors and policy implications. Nat Rev Endocrinol. 2013;9(1):13-27. [DOI] [PubMed] [Google Scholar]
- 3. Hu FB, Malik VS. Sugar-sweetened beverages and risk of obesity and type 2 diabetes: epidemiologic evidence. Physiol Behav. 2010;100(1):47-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stanhope KL, Schwarz JM, Keim NL, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009;119(5):1322-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Page KA, Chan O, Arora J, et al. Effects of fructose vs glucose on regional cerebral blood flow in brain regions involved with appetite and reward pathways. JAMA. 2013;309(1):63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Van Name M, Giannini C, Santoro N, et al. Blunted suppression of acyl-ghrelin in response to fructose ingestion in obese adolescents: the role of insulin resistance. Obesity (Silver Spring). 2015;23(3):653-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Galderisi A, Giannini C, Van Name M, Caprio S. Fructose consumption contributes to hyperinsulinemia in adolescents with obesity through a GLP-1-mediated mechanism. J Clin Endocrinol Metab. 2019;104(8):3481-3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vos MB, Kimmons JE, Gillespie C, Welsh J, Blanck HM. Dietary fructose consumption among US children and adults: the Third National Health and Nutrition Examination Survey. Medscape J Med. 2008;10(7):160. [PMC free article] [PubMed] [Google Scholar]
- 9. Crapo PA, Reaven G, Olefsky J. Plasma glucose and insulin responses to orally administered simple and complex carbohydrates. Diabetes. 1976;25(9):741-747. [PubMed] [Google Scholar]
- 10. Stanhope KL, Griffen SC, Bair BR, Swarbrick MM, Keim NL, Havel PJ. Twenty-four-hour endocrine and metabolic profiles following consumption of high-fructose corn syrup-, sucrose-, fructose-, and glucose-sweetened beverages with meals. Am J Clin Nutr. 2008;87(5):1194-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Macdonald I, Keyser A, Pacy D. Some effects, in man, of varying the load of glucose, sucrose, fructose, or sorbitol on various metabolites in blood. Am J Clin Nutr. 1978;31(8):1305-1311. [DOI] [PubMed] [Google Scholar]
- 12. Lee BM, Wolever TM. Effect of glucose, sucrose and fructose on plasma glucose and insulin responses in normal humans: comparison with white bread. Eur J Clin Nutr. 1998;52(12):924-928. [DOI] [PubMed] [Google Scholar]
- 13. Yau AMW, McLaughlin J, Gilmore W, Maughan RJ, Evans GH. The acute effects of simple sugar ingestion on appetite, gut-derived hormone response, and metabolic markers in men. Nutrients. 2017;9(2):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Verma M, Hontecillas R, Tubau-Juni N, Abedi V, Bassaganya-Riera J. Challenges in personalized nutrition and health. Front Nutr. 2018;5:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462-1470. [DOI] [PubMed] [Google Scholar]
- 16. DeFronzo RA, Matsuda M. Reduced time points to calculate the composite index. Diabetes Care. 2010;33(7):e93. [DOI] [PubMed] [Google Scholar]
- 17. Kernan WN, Inzucchi SE, Viscoli CM, et al. Pioglitazone improves insulin sensitivity among nondiabetic patients with a recent transient ischemic attack or ischemic stroke. Stroke. 2003;34(6):1431-1436. [DOI] [PubMed] [Google Scholar]
- 18. Dye L, Blundell JE. Menstrual cycle and appetite control: implications for weight regulation. Hum Reprod. 1997;12(6):1142-1151. [DOI] [PubMed] [Google Scholar]
- 19. Krishnan S, Tryon RR, Horn WF, Welch L, Keim NL. Estradiol, SHBG and leptin interplay with food craving and intake across the menstrual cycle. Physiol Behav. 2016;165:304-312. [DOI] [PubMed] [Google Scholar]
- 20. Tai MM. A mathematical model for the determination of total area under glucose tolerance and other metabolic curves. Diabetes Care. 1994;17(2):152-154. [DOI] [PubMed] [Google Scholar]
- 21. Allison DB, Paultre F, Maggio C, Mezzitis N, Pi-Sunyer FX. The use of areas under curves in diabetes research. Diabetes Care. 1995;18(2):245-250. [DOI] [PubMed] [Google Scholar]
- 22. Le Floch JP, Escuyer P, Baudin E, Baudon D, Perlemuter L. Blood glucose area under the curve. Methodological aspects. Diabetes Care. 1990;13(2):172-175. [DOI] [PubMed] [Google Scholar]
- 23. Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. Bmj. 1990;300(6719):230-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Teff KL, Grudziak J, Townsend RR, et al. Endocrine and metabolic effects of consuming fructose- and glucose-sweetened beverages with meals in obese men and women: influence of insulin resistance on plasma triglyceride responses. J Clin Endocrinol Metab. 2009;94(5):1562-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. CDC. Overweight & Obesity. Defining Adult Overweight and Obesity. https://www.cdc.gov/obesity/adult/defining.html. Updated September 17, 2020. Accessed January 10, 2018.
- 26. Yunker AG. Appetite regulating hormones are reduced after sucrose compared to glucose ingestion and influenced by obesity, insulin resistance and sex. Published online August 30, 2020. https://osf.io/p5mqn/?view_only=7904eb7f3a304abe9cf2f80819a909cf. doi: 10.17605/OSF.IO/P5MQN. Accessed October 26, 2020. [DOI]
- 27. Lindqvist A, Baelemans A, Erlanson-Albertsson C. Effects of sucrose, glucose and fructose on peripheral and central appetite signals. Regul Pept. 2008;150(1-3):26-32. [DOI] [PubMed] [Google Scholar]
- 28. Kong MF, Chapman I, Goble E, et al. Effects of oral fructose and glucose on plasma GLP-1 and appetite in normal subjects. Peptides. 1999;20(5):545-551. [DOI] [PubMed] [Google Scholar]
- 29. Kuhre RE, Gribble FM, Hartmann B, et al. Fructose stimulates GLP-1 but not GIP secretion in mice, rats, and humans. Am J Physiol Gastrointest Liver Physiol. 2014;306(7):G622-G630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bowen J, Noakes M, Clifton PM. Appetite hormones and energy intake in obese men after consumption of fructose, glucose and whey protein beverages. Int J Obes (Lond). 2007;31(11):1696-1703. [DOI] [PubMed] [Google Scholar]
- 31. Kim BJ, Carlson OD, Jang HJ, Elahi D, Berry C, Egan JM. Peptide YY is secreted after oral glucose administration in a gender-specific manner. J Clin Endocrinol Metab. 2005;90(12):6665-6671. [DOI] [PubMed] [Google Scholar]
- 32. Færch K, Torekov SS, Vistisen D, et al. GLP-1 response to oral glucose is reduced in prediabetes, screen-detected type 2 diabetes, and obesity and influenced by sex: the ADDITION-PRO Study. Diabetes. 2015;64(7):2513-2525. [DOI] [PubMed] [Google Scholar]
- 33. Carroll JF, Kaiser KA, Franks SF, Deere C, Caffrey JL. Influence of BMI and gender on postprandial hormone responses. Obesity (Silver Spring). 2007;15(12):2974-2983. [DOI] [PubMed] [Google Scholar]
- 34. Batterham RL, Cohen MA, Ellis SM, et al. Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med. 2003;349(10):941-948. [DOI] [PubMed] [Google Scholar]
- 35. Stock S, Leichner P, Wong AC, et al. Ghrelin, peptide YY, glucose-dependent insulinotropic polypeptide, and hunger responses to a mixed meal in anorexic, obese, and control female adolescents. J Clin Endocrinol Metab. 2005;90(4):2161-2168. [DOI] [PubMed] [Google Scholar]
- 36. le Roux CW, Batterham RL, Aylwin SJ, et al. Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology. 2006;147(1):3-8. [DOI] [PubMed] [Google Scholar]
- 37. Mittelman SD, Klier K, Braun S, Azen C, Geffner ME, Buchanan TA. Obese adolescents show impaired meal responses of the appetite-regulating hormones ghrelin and PYY. Obesity (Silver Spring). 2010;18(5):918-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sun SZ, Empie MW. Fructose metabolism in humans—what isotopic tracer studies tell us. Nutr Metab. 2012;9(1):1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Paquot N, Schneiter P, Jéquier E, et al. Effects of ingested fructose and infused glucagon on endogenous glucose production in obese NIDDM patients, obese non-diabetic subjects, and healthy subjects. Diabetologia. 1996;39(5):580-586. [DOI] [PubMed] [Google Scholar]
- 40. Aulinger BA, Vahl TP, Wilson-Pérez HE, Prigeon RL, D’Alessio DA. β-Cell sensitivity to GLP-1 in healthy humans is variable and proportional to insulin sensitivity. J Clin Endocrinol Metab. 2015;100(6):2489-2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dalla Man C, Micheletto F, Sathananthan A, Rizza RA, Vella A, Cobelli C. A model of GLP-1 action on insulin secretion in nondiabetic subjects. Am J Physiol-Endocrinol Metab. 2010;298(6):E1115-E1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dalla Man C, Micheletto F, Sathananthan M, Vella A, Cobelli C. Model-based quantification of glucagon-like peptide-1–induced potentiation of insulin secretion in response to a mixed meal challenge. Diabetes Technol Ther. 2016;18(1):39-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Muscelli E, Mari A, Casolaro A, et al. Separate impact of obesity and glucose tolerance on the incretin effect in normal subjects and type 2 diabetic patients. Diabetes. 2008;57(5):1340-1348. [DOI] [PubMed] [Google Scholar]
- 44. Wang Q, Jokelainen J, Auvinen J, et al. Insulin resistance and systemic metabolic changes in oral glucose tolerance test in 5340 individuals: an interventional study. BMC Med. 2019;17(1):217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Michaliszyn SF, Lee S, Bacha F, et al. Differences in β-cell function and insulin secretion in Black versus White obese adolescents: do incretin hormones play a role? Pediatr Diabetes. 2017;18(2):143-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. van der Klaauw AA, Keogh JM, Henning E, et al. Divergent effects of central melanocortin signalling on fat and sucrose preference in humans. Nat Commun. 2016;7(1):13055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Utzschneider KM, Kratz M, Damman CJ, Hullar M. Mechanisms linking the gut microbiome and glucose metabolism. J Clin Endocrinol Metab. 2016;101(4):1445-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Apolzan JW, Leidy HJ, Mattes RD, Campbell WW. Effects of food form on food intake and postprandial appetite sensations, glucose and endocrine responses, and energy expenditure in resistance trained v. sedentary older adults. Br J Nutr. 2011;106(7):1107-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Leidy HJ, Apolzan JW, Mattes RD, Campbell WW. Food form and portion size affect postprandial appetite sensations and hormonal responses in healthy, nonobese, older adults. Obesity (Silver Spring). 2010;18(2):293-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mourao DM, Bressan J, Campbell WW, Mattes RD. Effects of food form on appetite and energy intake in lean and obese young adults. Int J Obes (Lond). 2007;31(11):1688-1695. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated and analyzed during the present study are available from the corresponding author on reasonable request.