Abstract
Context
Although the development of immune checkpoint inhibitors has transformed treatment strategies of several human malignancies, research models to study immunotherapy in adrenocortical carcinoma (ACC) are lacking.
Objective
To explore the effect of anti-PD1 immunotherapy on the alteration of the immune milieu in ACC in a newly generated preclinical model and correlate with the response of the matched patient.
Design, Setting, and Intervention
To characterize the CU-ACC2-M2B patient-derived xenograft in a humanized mouse model, evaluate the effect of a PD-1 inhibitor therapy, and compare it with the CU-ACC2 patient with metastatic disease.
Results
Characterization of the CU-ACC2-humanized cord blood-BALB/c-Rag2nullIl2rγnullSirpaNOD model confirmed ACC origin and match with the original human tumor. Treatment of the mice with pembrolizumab demonstrated significant tumor growth inhibition (60%) compared with controls, which correlated with increased tumor infiltrating lymphocyte activity, with an increase of human CD8+ T cells (P < 0.05), HLA-DR+ T cells (P < 0.05) as well as Granzyme B+ CD8+ T cells (<0.001). In parallel, treatment of the CU-ACC2 patient, who had progressive disease, demonstrated a partial response with 79% to 100% reduction in the size of target lesions, and no new sites of metastasis. Pretreatment analysis of the patient's metastatic liver lesion demonstrated abundant intratumoral CD8+ T cells by immunohistochemistry.
Conclusions
Our study reports the first humanized ACC patient-derived xenograft mouse model, which may be useful to define mechanisms and biomarkers of response and resistance to immune-based therapies, to ultimately provide more personalized care for patients with ACC.
Keywords: Adrenocortical carcinoma, anti-PD-1, humanized mouse PDX model, immunotherapy
Introduction
Adrenocortical carcinoma (ACC) is an aggressive, orphan endocrine malignancy with limited treatment options and poor survival rates (<35% at 5 years) (1). Progress in the field has been hampered by the scarcity of preclinical research models; thus, development of ACC models to test novel druggable targets, before their use in human studies, is urgently needed (2–4).
With paucity of research models, efforts in the field toward developing new targeted therapies has been mostly focused on small cohort patient studies. ACC transcriptome landscape analyses have led to the identification of a robust overexpression of insulin growth factor 2 in ACC tumors (5, 6), but the effect of insulin growth factor 2/IGF1R pathway inhibitor had a limited effect in patients with advanced ACC (7, 8). Other efforts toward targeted therapy in patients with ACC have included phase 2 studies that, at best, had modest effect on tumor progression (9–12).
Although most ACC tumors are sporadic, several hereditary syndromes have an increased risk of developing ACC, including ~3% of patients with ACC tumors who are found to have mutations in DNA mismatch repair genes, mutS homolog 2, mutS homolog 6, PMS1 homolog 2 (mismatch repair system component), mutL homolog 1, and epithelial cell adhesion molecule, consistent with Lynch syndrome. Specific treatment strategies for these patients have not yet been determined.
Recent introduction of immune checkpoint inhibitors into the clinic has transformed cancer treatment regimens and improved survival in patients with multiple human malignancies (13–16). The PD-1 checkpoint inhibitor, pembrolizumab (Keytruda, Merck), was recently US Food and Drug Administration approved for mismatch repair-deficient or high microsatellite instability (MSI-H) solid tumors (17). The broad efficacy of immunotherapy in adrenocortical carcinoma has not been reported to date, although recent case reports in patients with Lynch syndrome and associated ACC treated with anti-PD-1 yielded variable results (18, 19). Although several clinical studies (NCT02720484, NCT03333616) are recruiting patients with advanced ACC for immunotherapy trials with PD-1 checkpoint inhibitors, relevant preclinical models to examine the efficacy, cell-specific mechanisms of action, and potential pathways of resistance to immunotherapy are lacking.
In this study, we describe the first CU-ACC2-M2B patient-derived xenograft (PDX) model to examine the in vivo effects of the PD-1 inhibitor, pembrolizumab. In parallel, the CU-ACC2 patient was treated with pembrolizumab in an attempt to halt progressive, metastatic disease. The patient showed a remarkable response, with changes in immune markers similar to that observed in the animal model, suggesting that checkpoint blockade should be considered for subsets of patients with metastatic ACC and that humanized mouse models may be relevant in elucidating mechanism of action and detection of response-associated biomarkers in studies of combination therapies.
Materials and methods
Mice
BALB/c-Rag2nullIl2rγnullSirpaNOD (BRGS) recipient mice were bred, engrafted, and maintained on a diet including Septra (Uniprim diet, Harlan) every 2 weeks to prevent opportunistic infections (20, 21). Mice were kept in a biosafety level 2 room at the University of Colorado Denver Anschutz Medical Center vivarium. As previously decribed, 5- to 6-week-old female athymic nude (nu/nu) mice were purchased from Envigo (formally Harlan Sprague Dawley). At the time of surgery, a sample of human adrenal tumor tissue was obtained and immediately implanted subcutaneously into both flanks of female athymic nude mice (4, 22). These studies were conducted following approval from the University of Colorado Animal Care and Use Committee and in a facility accredited by the American Association for Accreditation of Laboratory Animal Care.
Establishment of the ACC-002-humanized mouse model
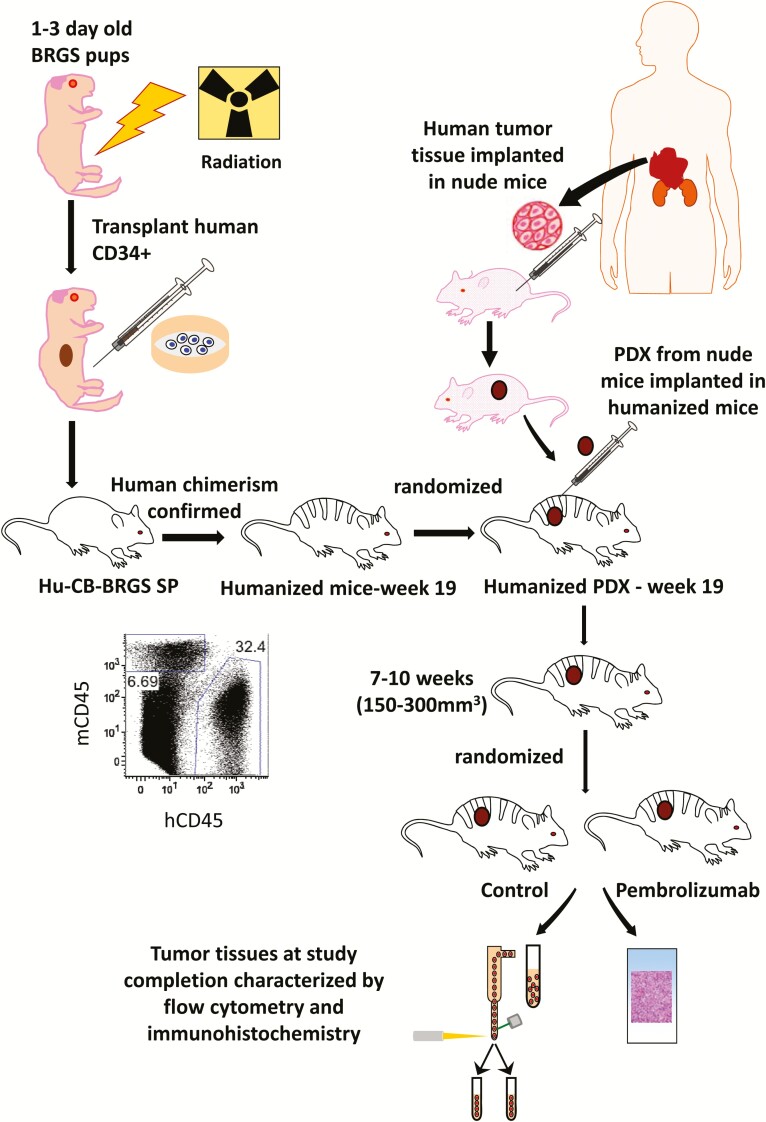
As previously described (21, 23–25), the generation of humanized cord blood BRGS (hu-CB-BRGS) mice was accomplished using human umbilical CB obtained from deidentified samples from University of Colorado Cord Blood Bank at ClinImmune Labs (Aurora, CO), and in compliance with the University of Colorado institutional review board (23). In brief, CB mononuclear cells were isolated and CD34+ cells selected using AutoMACS (Miltenyi Biotech) and cultured in complete medium (IMDM supplemented with 10% fetal bovine serum, 50 μM 2-ME, 2 nM Glutamax) with the addition of interleukin-6 (IL-6; 10 ng/mL), stem cell factor (20 ng/mL), and FLT3 ligand (10 ng/mL) for 3 to 6 days. Humanized mice were generated by intravenous or intrahepatic injection of CD34+ cells (~100,000 to 700,000 per mouse) in phosphate-buffered saline into sublethally irradiated (300 rad) newborn BRGS pups. Previously established CU-ACC2-M2B PDX, from a liver metastasis in a nude mouse model, was used to establish the ACC humanized mouse PDX. Institutional review board protocol was approved, and informed consent was obtained in compliance with National Institutes of Health policies for establishing human tumor-derived xenografts in mice. Specifically, CU-ACC2-M2B PDX was passaged in nude mice three times and tissue samples were used for generation of humanized mouse model, which we refer to as CU-ACC2-hu-CB-BRGS mice.
Animal studies
For in vivo studies, a group of 12 BRGS mice was generated from the same CB. At 19 weeks post-CD34+ cell transplantation, CU-ACC2-M2B PDX tissue obtained from nude mice was implanted into both flanks of humanized hu-CB-BRGS mice to generate CU-ACC2-hu-CB-BRGS mice. Once tumors reached 150 to 300 mm3 (7 to 10 weeks posttumor injection), pembrolizumab treatment was initiated at a dose of 30 mg/kg intraperitoneally twice weekly in mice, randomized according to human chimerism. Both control and treated mice were monitored twice weekly for signs of toxicity. To better visualize flank tumors, mice were shaved and tumor size was evaluated twice weekly by caliper measurements using the following equation: tumor volume = (length × width2) × 0.52 and recorded in the Study Director software package (Studylog Systems, South San Francisco, CA). The animals were euthanized at end of the study or when total tumor burden reached 3000 mm3.
Chimerism evaluation
Human chimerism of hu-CB-BRGS mice was determined as previously described (21, 23). The hu-CB-BRGS mice were bled three times between weeks 10 and 19 post-CD34+ cell transplantation, peripheral blood mononuclear cells were isolated and evaluated for mouse and human hematopoietic (mCD45 or hCD45), human T (hCD3, hCD8), human B (hCD20) chimerism, and human PD-1 expression. Mice were randomized into equivalent experimental and control groups based on their human hematopoietic and T-cell chimerism before tumor implantation at 19 weeks of age (23–26).
Tissue harvesting and flow cytometry
At the completion of the study, 26 days after start of treatment or when total tumor burden reached 3000 mm3 in volume, the animals were euthanized and tissues were collected. Blood was collected from the heart soon after euthanasia and sera were frozen for future analysis. Lymph nodes (LN), spleen (SP), bone marrow (BM), and tumor-infiltrating leukocytes (TILs) were collected and digested into single-cell suspensions as previously described (25, 27). Each tumor was evaulated and a small portion was formalin-fixed for further histological analyses.
Single-cell suspensions were incubated with fluorescently-labeled antibodies to evaluate: mouse leukocytes (mouse CD45), human lymphocytes (human CD45, CD3, CD4, CD8, CD19, CD20), T regulatory cells (FoxP3), activated T cells [HLA-antigen D related (DR)], effector T cells [GrB, interferon-γ (IFN-γ), Tbet, CD28), inhibitory receptors (PD-1, Tim3, CTLA-4), chemokine receptor CXCR3, myeloid cell subsets (CD33, CD11b, CD11c, HLA-DR), and the immune status of the tumor (HLA class I and class II, PD-L1), and run on a Cyan analyzer (Beckman Coulter) at the University of Colorado Denver Cancer Center Flow cytometry core (26). We excluded mice with <5% human chimerism in the SP from the study. To analyze IFN-γ production by human T cells, approximately 2e6 LN, SP, or TIL cells were stimulated overnight in RPMI complete media with Cell Stimulation Cocktail (Invitrogen). Unstimulated (negative control) and stimulated (positive control) human peripheral blood mononuclear cells were included in each assay. GolgiPlug (BD) was added to each well after an overnight incubation. Four hours later, the cells were spun, washed one time with staining buffer, and extracellular staining performed to identify human T and CD8 cells. Subsequently, an intracellular staining for detection of IFN- γ and Tbet was performed. All cells were gated on a single-cell gate based on forward and side scatter followed by doublet discrimination and FlowJo software (Tree Star) was used for data analysis.
Multispectral fluorescence immunohistochemistry
Tumor tissue was fixed in formalin and paraffin-embedded for multispectral imaging on the Vectra 3.0 Automated Quantitative Pathology Imaging System (Perkin Elmer) (28–30). Four-micron sections mounted on glass slides were sequentially stained for human Granzyme B (GrB), CD3, PD-1, TIM-3, CD8, and inhibin-α (panel 1), or CD3, CD33, IL-10, CD11b, HLA-DR, and inhibin- α (panel 2) on a Bond RX autostainer (Leica). Slides were dewaxed (Leica), heat treated in ER2 (epitope retrieval solution 2) antigen retrieval buffer for 20 minutes at 93oC (Leica), blocked in antibody (Ab) Diluent (Perkin Elmer), incubated for 30 min with the primary antibody, 10 minutes with horseradish peroxidase-conjugated secondary polymer (anti-mouse/anti-rabbit, Perkin Elmer), and 10 minutes with horseradish peroxidase-reactive OPAL fluorescent reagents (Perkin Elmer). Slides were washed between staining steps with Bond Wash (Leica) and stripped between each round of staining with heat treatment in antigen retrieval buffer. After the final staining round, the slides were heat-treated in ER1 antigen retrieval buffer, stained with spectral 4′,6-diamidino-2-phenylindole (Perkin Elmer), and coverslipped with Prolong Diamond mounting media (Thermo Fisher). Whole slide scans were collected using the 10× objective at a resolution of 1.0 μm. Approximately 10 regions of interest were chosen in tumors from the hu-CB-BRGS mice spanning the tumor tissue. Approximately 30 regions of interest were chosen from the human ACC tumor in areas either near the tumor border or in the center of the tumor. Regions of interest were scanned for multispectral imaging with the 20× objective at a resolution of 0.5 μm. The multispectral images were analyzed with inForm software (Perkin Elmer) to unmix adjacent fluorochromes; subtract autofluorescence; segment the tissue into tumor regions and stroma; segment the cells into nuclear, cytoplasmic, and membrane compartments; and to phenotype the cells according to cell marker expression. Minimal thresholds for phenotyping human immune markers were set using staining intensities in ACC tumors harvested from nude mice.
Radiographic studies
Computed topography (CT) images were analyzed in Philips IntelliSpace PACS Radiology (version 4.4.516.42, Koninklijke Philips N.V., Amsterdam, Netherlands) by a board-certified abdominal imaging radiologist with 5 years of subspecialty experience. Two target lesions were established per RECIST 1.1 criteria on a baseline contrast-enhanced CT acquired per standard clinical protocol. These lesions were assessed with contrast-enhanced CT images at 12, 10, 7, 6, 4, and 2 months before the start of treatment and on subsequent imaging on therapy at 3, 5, 7, 9, 12, 14, and 16 months' follow-up by the same radiologist, and two-dimensional sum of diameters relative to baseline and diameter-sum percent decrease relative to baseline were computed.
Statistical analysis
Average tumor volumes from treated (Vt) and control (Vvc) groups were used to calculate tumor growth inhibition according to the following equation: TGI = 100 × (Vt final – Vt initial)/(Vvc final – Vvc initial). Two-tailed Student t test of equal variance or Welch's correction were used to calculate statistical significance of immuno measurements using Prism software (GraphPad software).
Results
Clinical features of the CU-ACC2 patient
A 24-year-old woman with a history of Lynch syndrome was diagnosed with a left-sided adrenocortical carcinoma after spontaneous rupture of an adrenal mass and underwent laparoscopic resection at an outside institution. Pathology revealed a 4-cm fragmented specimen consistent with ACC with MSI-H. Ki67 and mitotic count were not commented on without stage assigned. Subsequently with largely positive margins and tumor seeding in the peritoneal cavity, the patient underwent reexploration with left nephrectomy 1 month after primary surgery. With the high risk of recurrence, the patient elected to undergo adjuvant mitotane and radiation therapy to the resected bed. Five months later, a CT scan demonstrated metastatic disease and, subsequently, there were several recurrences, including a metastatic liver lesion 1.5 years postoperatively (treated with radiation), a metastatic peritoneal implant 2.5 years postoperatively, and new liver masses and anterior abdominal wall nodules 3 years postoperatively. The patient was consented for an institutional review board–approved study at the University of Colorado and underwent partial hepatectomy, peritoneal nodule resection, and perfusion with chemotherapy (HIPEC with 50.1 mg of mitomycin C). The CU-ACC2-M2B PDX model in nude mice was derived from the metastatic liver lesion (4). Four years after primary resection, a positron emission tomography/CT scan showed multiple FDG-avid bilateral pulmonary nodules, the left nephrectomy bed mass, and an FDG-avid liver lesion, all consistent with rapid disease progression.
Humanization and PDX tumor implantation in hu-CB-BRGS mice
In an attempt to characterize the effect of PD-1 blockade in a unique setting of MSI-H adrenocortical cancer, we used our recently developed u-CB-BRGS model (25) to examine the effects of immunotherapy on this tumor in the preclinical setting. As illustrated in Fig. 1, hu-CB-BRGS mice, generated as pups by injection of hematopoietic stem cells from a single CB, were confirmed of human chimerism in the blood at 15 and 19 weeks postengraftment, and randomized into two groups based on human hematopoietic and T-cell chimerism (31). CU-ACC2-M2B PDX tumor tissue was then injected into bilateral flanks of the humanized mice to generate CU-ACC2-hu-CB-BRGS mice, and treatment begun when tumors approximated 150 to 300 mm3. Because the PDX grows relatively slowly, treatment start dates were variable (between 7 and 10 weeks). At harvest, a portion of the tumor was collected and short tandem repeat analysis confirmed a correspondence between CU-ACC2-M2B PDX and CU-ACC2-hu-CB-BRGS and the respective human CU-ACC2 tumor tissue (31), as well as steroidogenic factor 1 immunohistochemical positivity (31), as previously described (4). A portion of the tumor was fixed in formalin for subsequent immunohistochemistry (IHC) analysis.
Figure 1.
Experimental timeline for evaluation of pembrolizumab for CU-ACC2-M2B PDX tissue in hu-CB-BRGS mice. Newborn BRGS mice were irradiated with 300 rad before injection of CB CD34+ human hematopoietic stem cells. Following confirmation of human chimerism in the blood at 10, 15, and 19 weeks, mice were randomized into equivalent groups based on chimerism and implanted with CU-ACC2 PDX tissue that had been developed and passaged in a nude mouse. Once tumors reached 150 to 300 mm3, pembrolizumab treatments were begun. Mice were euthanized when tumor volumes approximated 2000 mm3, tumors and lymph organs were excised, and immune parameters were evaluated by flow cytometry and immunohistochemistry.
Pembrolizumab (anti-PD-1) treatment inhibited tumor growth in CU-ACC2-hu-CB-BRGS mice
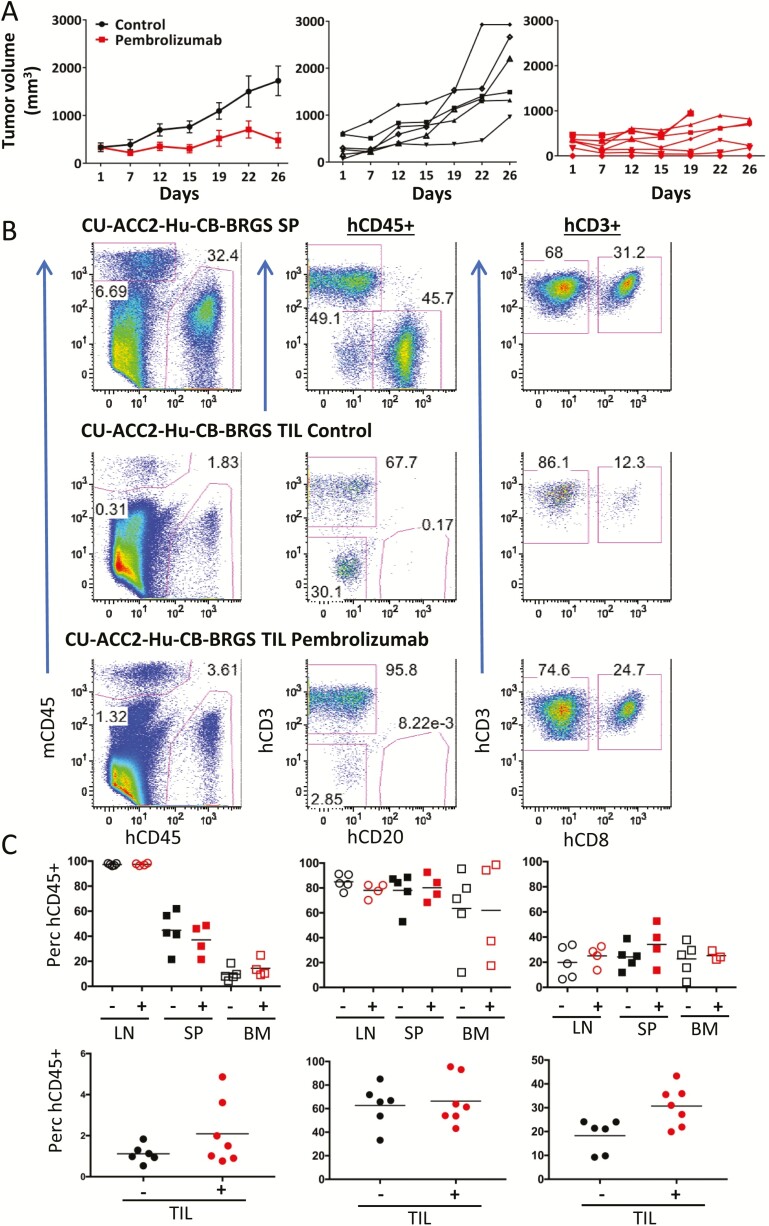
A total of 13 CU-ACC2-M2B PDX tumors grew within the 2-month experimental window in 12 hu-CB-BRGS mice with an overall tumor take rate of 54%. Measurements of palpable tumors in CU-ACC2-hu-CB-BRGS mice demonstrated significantly reduced tumor growth (60%) in the pembrolizumab-treated mice (P = 0.002) (Fig. 2A). Although 6 mice were initially injected per treatment group with CU-ACC2-M2B PDX tissues in both flanks, 5 mice in the vehicle control group (n = 6 tumors) and 4 mice in the pembrolizumab-treated group (n = 7 tumors) were available for analysis because of delayed tumor growth at the completion of the study.
Figure 2.
Reduced tumor growth correlates with increased CD8+ T-cell infiltration into tumor of CU-ACC2-hu-CB-BRGS mice treated with pembrolizumab. A, Tumor growth curves of untreated (black) or pembrolizumab-treated (red) CU-ACC2-hu-CB-BRGS mice. Top graph shows mean ± standard error of the mean and individual data points are shown in middle (control) and bottom (pembrolizumab) graphs. B, Human hematopoietic and T-cell chimerism of peripheral lymph organs and TILs of CU-ACC2-hu-CB-BRGS mice. Representative flow cytometry plots distinguishing mouse (mCD45, myeloid lineage) and human (hCD45) hematopoietic cells (left), human T (CD3) and B (CD20) lymphocytes among the hCD45+ cells (middle), and CD8 T cells (right) in indicated tissues (SP or TIL) of ACC2-hu-CB-BRGS mice, either untreated (SP, TIL; top) or treated with pembrolizumab (TIL; bottom). C, The graphs show percentages of human hematopoietic cells (top), human T cells (middle) within the hCD45+ gate, and CD8+ cells of the T cells (bottom) from all mice in the indicated tissues with (+) or without (-) anti-PD-1 treatments. Lines are arithmetic means. P values: *< 0.05, **< 0.01.
Pembrolizumab-treated CU-ACC2-hu-CB-BRGS mice exhibit increased frequency of CD8+ T-cell tumor-infiltrating lymphocytes
An advantage to the humanized mouse model is the access to abundant lymph and tumor tissue to study alterations in the human immune response upon checkpoint therapy (32). We have previously shown that triple negative breast cancer cell lines or colorectal cancer PDXs grow similarly in hu-CB-BRGS and nonhumanized BRGS unirradiated hosts; however, anti-PD-1 therapy alters tumor growth only in the humanized mice (25). To understand the nature of the response of the human immune system (HIS) responsible for the CU-ACC2 growth suppression, single-cell suspensions of the tumor, lymph tissues (LN, SP, and BM were extracted from CU-ACC2-hu-CB-BRGS mice for human chimerism and human immune subsets using flow cytometry.
Flow cytometry analysis of lymph organs (LN, SP, and BM) from CU-ACC2-hu-CB-BRGS mice confirmed the human hematopoietic chimerism (% hCD45+ of total mouse + human CD45+), with no differences observed among treated or control groups (SP control: 22% to 62%, pembrolizumab: 22% to 49%, Figs. 2B and C) or absolute hCD45+ cell numbers (SP range for all mice: 10 to 26 × 106 human cells (31)). Human T cells were also detected among the TILs of both control and pembrolizumab-treated CU-ACC2-hu-CB-BRGS mice at similar frequencies (Fig. 2B (31)). Although interrogation into hCD45+ subsets revealed no significant differences in the frequencies of T cells in lymph organs or TILs or the numbers of T cells in lymph organs, we did observe an increased number of T cells in four of the seven tumors from pembrolizumab-treated mice (Fig. 2D (31)). Notably, we observed an increase in the frequency of CD8+ T cells in the TILs, but not the peripheral lymph organs, of pembrolizumab-treated CU-ACC2-hu-CB-BRGS mice relative to controls (P < 0.05) (Fig. 2E).).
CD8+ T cells in tumors of pembrolizumab-treated CU-ACC2-hu-CB-BRGS mice exhibit increased activation and GrB production
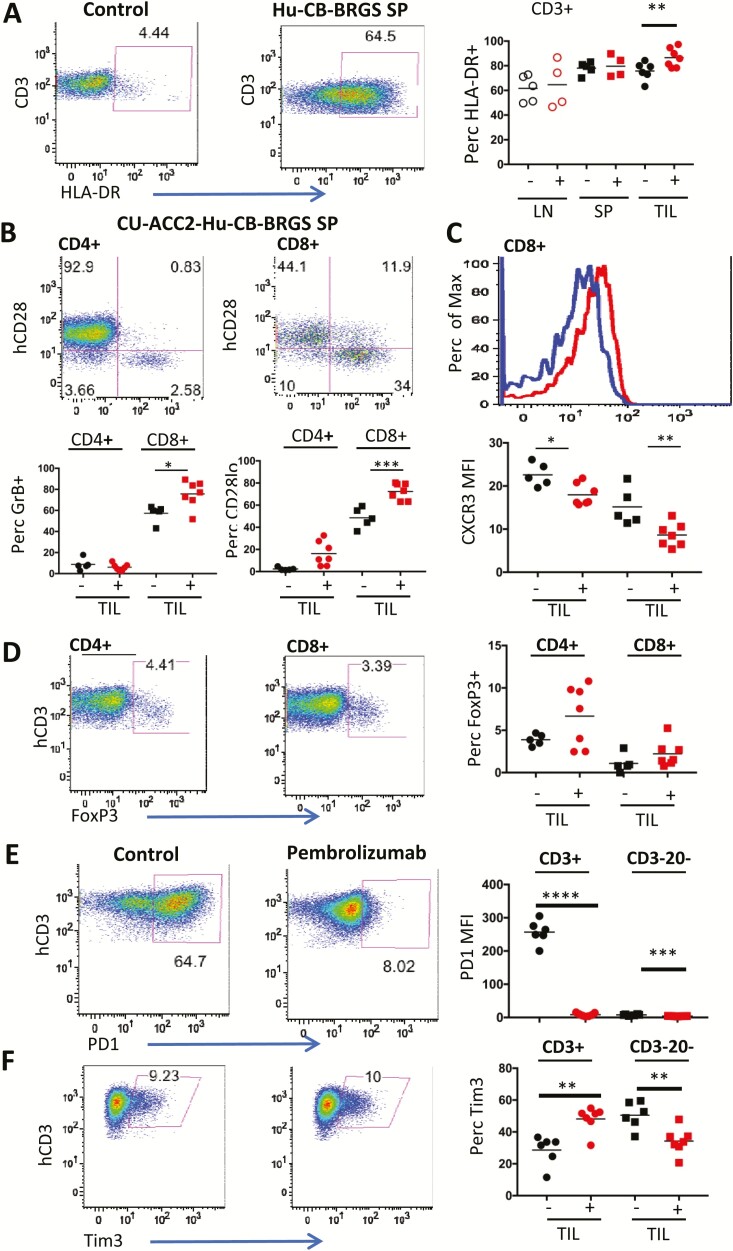
The immune system is remarkably complex, with immune cells possessing the capability to mount either a destructive or suppressive response (33). To determine the functional capacity of the immune response in our model, we next investigated whether the T cells in the lymph and tumors of treated vs control CU-ACC2-hu-CB-BRGS demonstrated differences in signs of activation, cytotoxic effector function, or regulatory subsets. T cells were stained with HLA-DR activation markers (Fig. 3A), and no differences were observed in the frequencies of T cells in the lymph organs for either marker. However, there was an increase in HLA-DR+ activated T cells in the TILs of the anti-PD-1–treated mice (P < 0.05) (Fig. 3A), which correlated with an increase in cytotoxic GrB production by CD8+ T cells (P < 0.05), but not IFN-α, a Th1-associated cytokine that can contribute to tumor regression (33) (Fig. 3B and (31)). No differences in GrB production were observed in the lymph organs in the pembrolizumab-treated mice (31). In all tissues, the expression of GrB was associated with decreased expression of both costimulatory molecule CD28, an important target of PD1 (34), and CXCR3, a chemokine involved in trafficking of T cells into tumors, which translated into significant differences among both the CD4+ (P < 0.05) and CD8+ T (P < 0.01) cells in the TILs of the anti-PD-1–treated mice (Fig. 3B and 3C (31)). Additionally, we examined whether pembrolizumab altered the frequency of suppressive FoxP3+ T-regulatory cells by intracellular staining for the FoxP3 transcription factor (Fig. 3D). Although we could detect FoxP3+ among T-cell subsets in all tissues, we did not observe a difference in their frequencies between experimental groups (Fig. 3D (31)).
Figure 3.
Functional analysis of T cells in untreated or pembrolizumab-treated CU-ACC2-hu-CB-BRGS mice. A, Representative flow plot illustrating expression and gating of HLA-DR on human T (hCD45+CD3+) cells in control (top, CB T cells) or spleen from untreated humanized mouse (bottom) and graph on bottom displays the percentage of HLA-DR+ for each mouse in indicated tissue. B, Representative flow plots illustrating gating strategy for expression of CD28 and GrB among CD4 (top) or CD8 (bottom) T cells (hCD45+CD3+) in the spleen of an untreated CU-ACC2-hu-CB-BRGS mouse. The graphs on the right show the percentages of GrB+ (top) or CD28lo (bottom) for CD4+ (left) or CD8+ (middle) T cells in the TILs. C, Histogram illustrating the expression of chemokine receptor CXCR3 on CD8+ T cells from the spleen in panel B that were either secreting GrB or not. The graph on the right shows the MFI of CXCR3 for CD4+ (left) or CD8+ (right) T cells in TILs. D, T regulatory cells in CU-ACC2-hu-CB-BRGS mice. Flow plots show the gating strategy for expression of FoxP3 among CD4+ and CD8+ human T cells (hCD45+, hCD3+) in a spleen from a CU-ACC2-hu-CB-BRGS mouse and data from each tumor from untreated (-) or pembrolizumab-treated (+) are shown in the graph on the right. Expression of inhibitory receptors (E) PD-1 and (F) Tim3. Flow cytometry panels (left) from a spleen of a humanized mouse depicting the expression of (E) PD-1 and (F) Tim3 and data for expression in T cells (hCD45+,CD3+, left) and myeloid cells (hCD45+,CD3–,CD20–, right) for each tumor is shown. For all graphs, each dot represents data from the indicated organ from an individual humanized mouse that was either untreated (-) or treated with pembrolizumab (+). Lines are arithmetic means. P values: *< 0.05, **< 0.01, ***< 0.001, ****< 0.0001. MFI, mean fluorescence intensity.
PD-1 blockade altered the expression of inhibitory receptors on human immune cells in CU-ACC2-hu-CB-BRGS mice
Upon activation, immune cells upregulate several inhibitory receptors to control the response (33). To examine whether blocking PD-1 resulted in an altered inhibitory receptor expression, T cells and myeloid (CD3–CD20–hCD45+) cells were stained for expression of PD-1, Tim3, and CTLA-4. Consistent with previous experiments, using the anti–PD-1, nivolumab (25), a loss of PD-1 expression was observed in all cell types examined in all tissues, likely resulting from epitope blocking by detecting antibody, but suggesting saturation of PD1 receptor by pembrolizumab (P < 0.001) (Fig. 3E (31)). Although there were no significant differences in CTLA-4 expression on CD4+ T cells, there was an increased frequency of Tim3+ T cells accompanied by a decreased percentage of Tim3+ myeloid cells, detected only in the TILS (P < 0.01) ((31) Fig. 3F). Thus, pembrolizumab treatment specifically altered expression of immune inhibitory receptors on multiple immune cell types, with a predominant action within the tumors.
Human B-cell and myeloid populations in CU-ACC2-hu-CB-BRGS mice
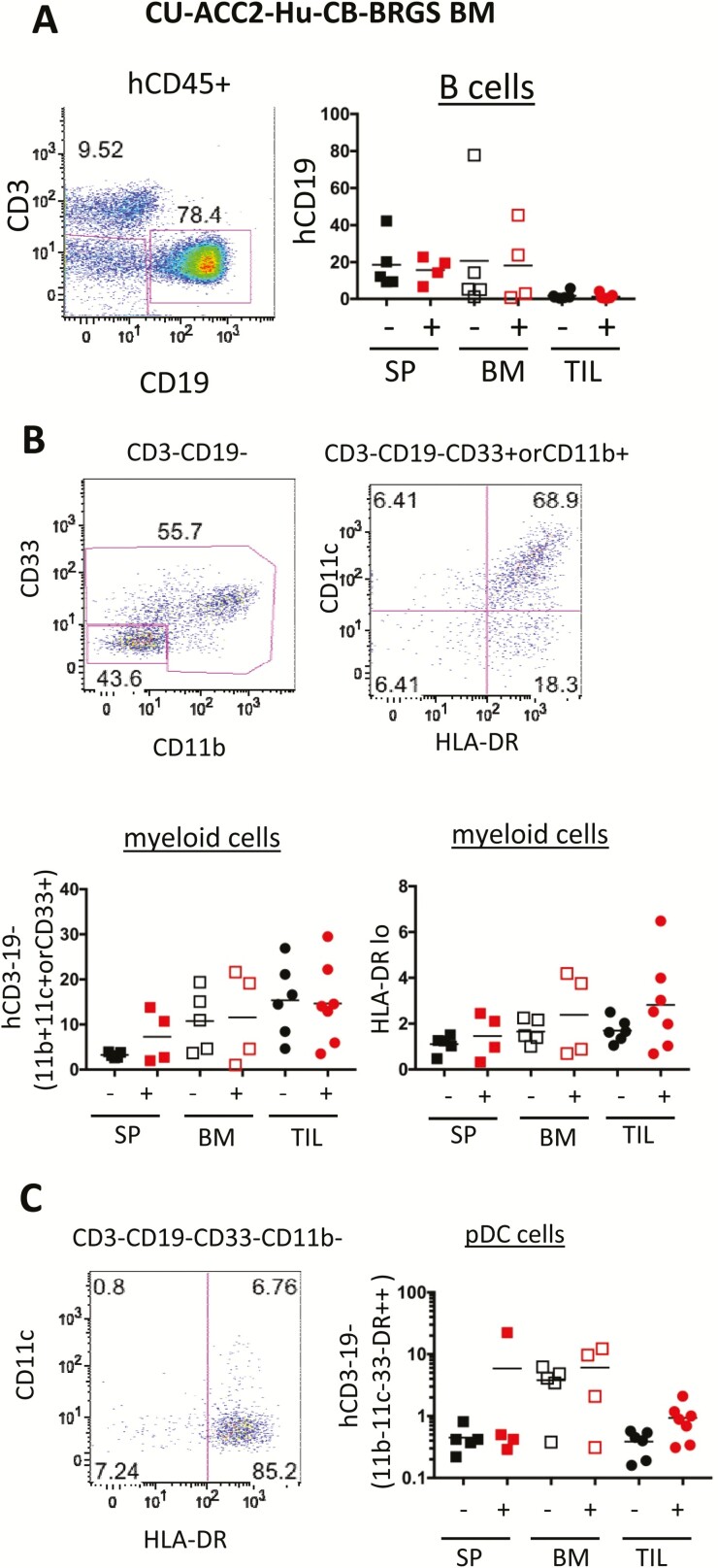
Although T cells are critical for tumor immune responses, the immune system is a highly integrated organ consisting of multiple cell types, each of which can also have immune activating or suppressive functions (33). Therefore, we examined the expression of human B cells, which were abundant in the peripheral lymph tissues, but nearly absent in the tumor in the CU-ACC2-hu-CB-BRGS model, consistent with other tumor-bearing CB-derived humanized mouse models (Fig. 4A) (25, 35).
Figure 4.
Analysis of human B-cell and myeloid populations in the peripheral lymph organs and TILs of CU-ACC2-hu-CB-BRGS mice. A, Representative flow cytometry plot from the BM of a CU-ACC2-hu-CB-BRGS mouse indicating the gating strategy for quantification of B cells (hCD45+CD19+) with data from each mouse indicated in the lower graph. B, Representative flow cytometry plots (upper), and graphs below showing individual analysis for each CU-ACC2-hu-CB-BRGS mouse, from the bone marrow of the same CU-ACC2-hu-CB-BRGS mouse as in panel A indicating the gating strategy for myeloid cells (left, hCD45+, CD3–CD19–, CD33+, CD11b+, or CD11c+); or HLA-DRlo/– among these myeloid cells. C, Representative flow cytometry plot (upper), and graph below showing individual analysis for each CU-ACC2-hu-CB-BRGS mouse, from the BM of the same CU-ACC2-hu-CB-BRGS mouse as in panel A indicating further gating strategy for pDCs (hCD45+, CD3–, CD19–, CD33–, CD11b–, CD11c–, HLA-DRhi). For all graphs, each dot represents data from the indicated organ from an individual humanized mouse that was either untreated (-) or treated with pembrolizumab (+). Lines are arithmetic means. pDC, plasmacytoid dendritic cells.
In addition, the diverse myeloid lineage, which we identified as CD3–CD19–(CD33+, CD11b+, or CD11c+), was more abundant in the BM and TILs of CU-ACC2-hu-CB-BRGS mice; however, with similar frequencies in untreated and pembrolizumab-treated mice (Fig. 4B). Myeloid-derived suppressor cells express lower levels of human antigen-presenting molecule HLA class II. Although clear populations of the HLA-DRlo/– myeloid cells were detected in the humanized mice, there were no differences between the experimental groups (Fig. 4B). In addition, human plasmacytoid dendritic cells (pDC), defined as hCD45+, CD3–CD19–CD11b–CD11c–CD33–HLA-DR+, which we confirmed with coexpression of CD123 (data not shown), were measured, and although definite populations were detected, especially in the BM, there were no significant differences in lymph organs between treated and untreated mice. There was, however, a trend of increased pDCs in the TILs of the pembrolizumab group (P = 0.056) (Fig. 4C). In summary, differences in B-cell and myeloid populations did not contribute significantly to the increased tumor response in the anti-PD-1–treated CU-ACC2-hu-CB-BRGS mice.
No changes in tumor HLA or PDL1 expression with pembrolizumab treatment in CU-ACC2-hu-CB-BRGS mice
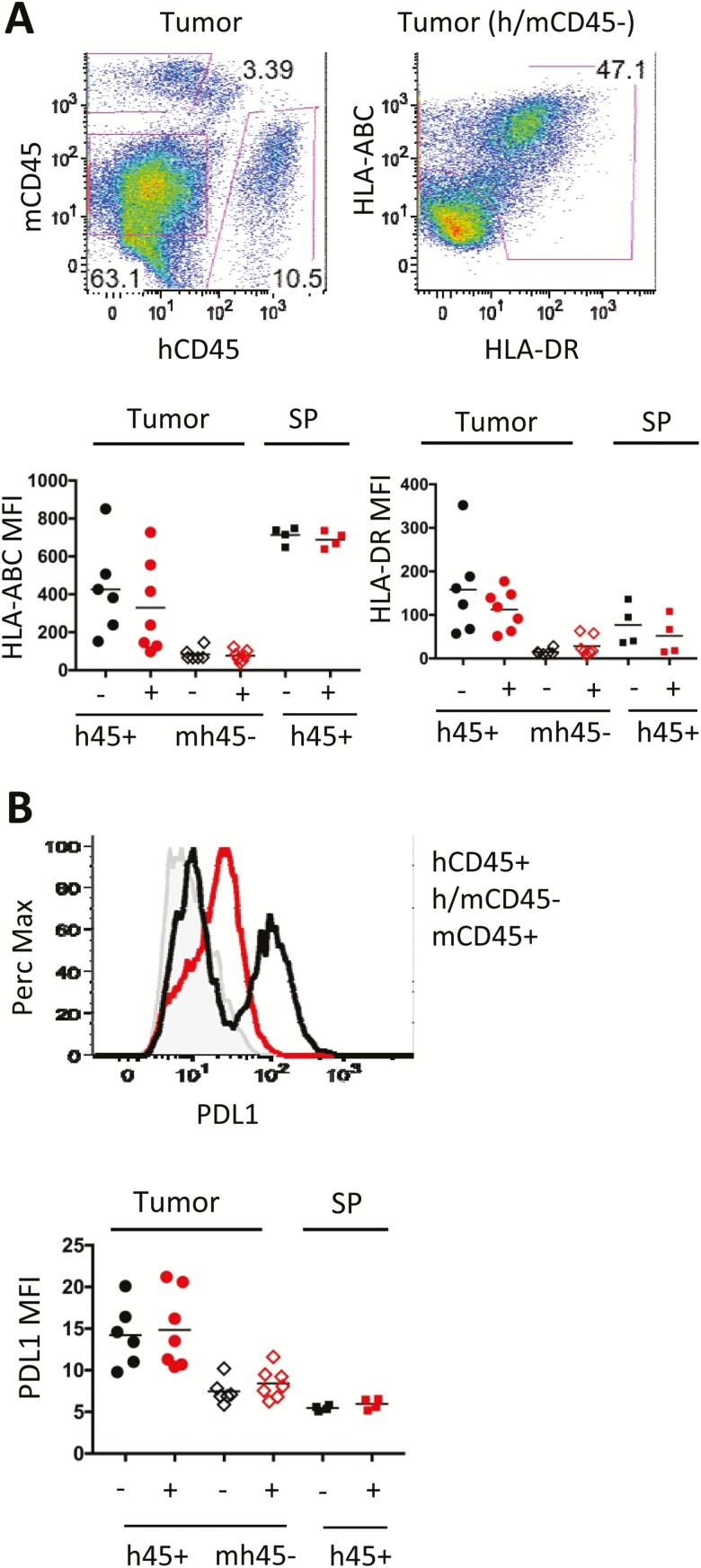
Next, the expression of human HLA class I (ABC), class II (DR), or PDL1 in the tumors was assessed. The tumor and SP cell suspensions were stained to identify both the hematopoietic populations of mouse (mCD45), as negative control, and human (hCD45), as positive control (Fig. 5A). Because the ACC lacks robust tumor-specific markers (e.g., EpCam), the mouse/human CD45- population were gated to identify tumor cells by expression of human HLA molecules (Fig 5A). As expected, high levels of hHLA-ABC class I were observed on human hematopoietic cells in the SP with lower expression on the hCD45+ TILs and even lower levels on tumor cells (Fig. 5A, lower left). hCD45+ cells in the TILS expressed higher HLA-DR class II as well as the inhibitory receptor ligand PDL1 than in the SP (Figs. 5A and B). In contrast, the expression of HLA-class I and II or PDL1 were low on all tumor cells, indistinguishable from expression on negative control mouse cells (Fig. 5A and B and data not shown). Thus, treatment with pembrolizumab did not alter expression of any of these molecules on any subset within the TIL or SP.
Figure 5.
Expression of human antigen-presentation molecules and PDL1 in the tumor in CU-ACC2-hu-CB-BRGS mice. A, Flow cytometry plots of a digested tumor showing gating strategy to identify the tumor cells (mCD45–, hCD45–, left plot and HLA-ABC+, HLA-DR+, right plot). B, Flow histogram from a digested tumor showing relative expression of PDL1 on tumor cells (red line) and compared with expression on human hematopoietic cells (black line) or negative control mouse hematopoietic (mCD45+) cells (gray). A-B, The graphs show the expression of HLA-ABC (A, left) or HLA-DR (A, right) and PDL1 (B) for each tumor for the indicated populations (human hematopoietic: h45+, tumor: mh45–). Human (h45+) hematopoietic cells from the spleen serve as positive controls for each stain. For all graphs, each dot represents data from the indicated organ from an individual humanized mouse that was either untreated (-) or treated with pembrolizumab (+). Lines are arithmetic means.
PD-1 blockade increased an immune infiltrate in the tumor microenvironment
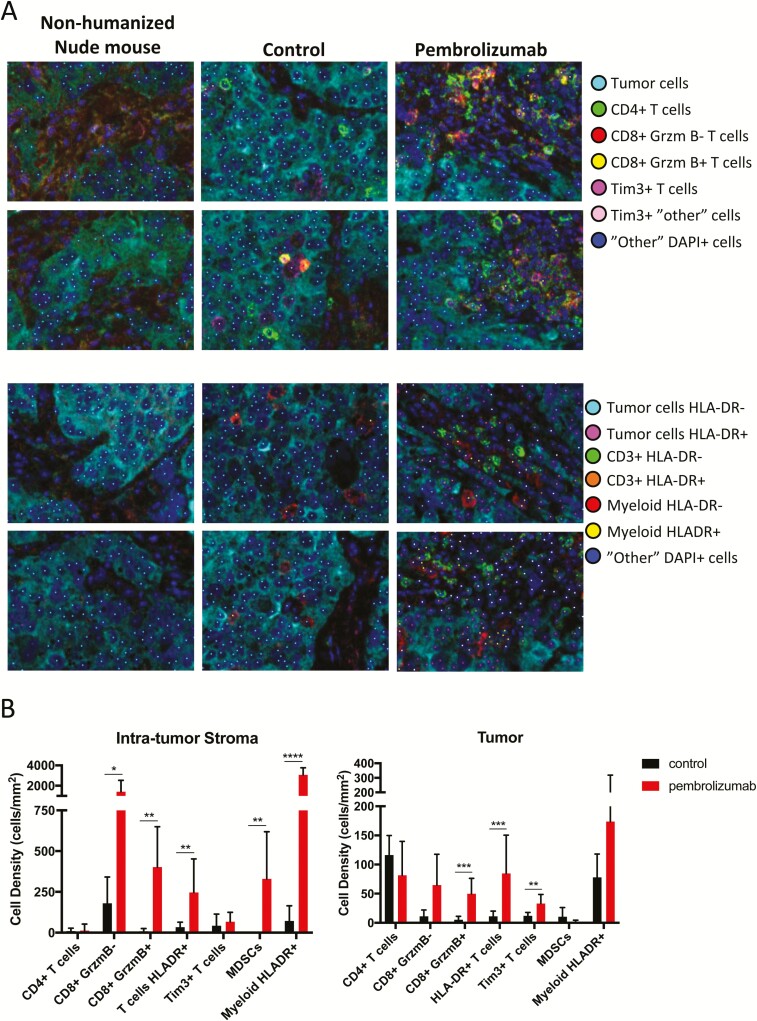
We next investigated the location of immune cells within the tumor microenvironment and whether the results obtained in freshly harvested tissue by flow cytometry would be verified in fixed tissue using quantitative multispectral immunohistochemistry. After confirming the correspondence between CU-ACC2-hu-CB-BRGS tumor tissue and matching human ACC tissue (31), we distinguished tumor from intratumor stromal regions using the expression of inhibin alpha (36). Most immune cells were located in the intratumor stromal regions of the tumor microenvironment, with substantial increases in CD8+ T cells, and GrB and HLA-DR expression after treatment with pembrolizumab (Fig. 6A and B (31)). Similar to the results of flow cytometry, GrB+ CD8+ T cells, HLA-DR+ T cells, and Tim3+ T cells were also significantly increased in tumor regions after treatment. In addition, increases in myeloid cells in the intratumor stroma were observed after pembrolizumab treatment in these representative tumors.
Figure 6.
Activated immune cells are increased in the tumor and the intra-tumor stroma following anti-PD-1 treatment. A, Tumors from CU-ACC2-hu-CB-BRGS mice stained with two seven-color IHC panels and analyzed by multispectral imaging. Cellular phenotypes were analyzed using inForm software and are indicated by the colored dots. B, The density of cells for each indicated cellular phenotype was determined for intratumor stroma regions (top) or tumor regions (bottom) in control tumors (black) or pembrolizumab treated tumors (red). Lines indicate standard deviations. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
CU-ACC2 patient demonstrated a remarkable response to pembrolizumab
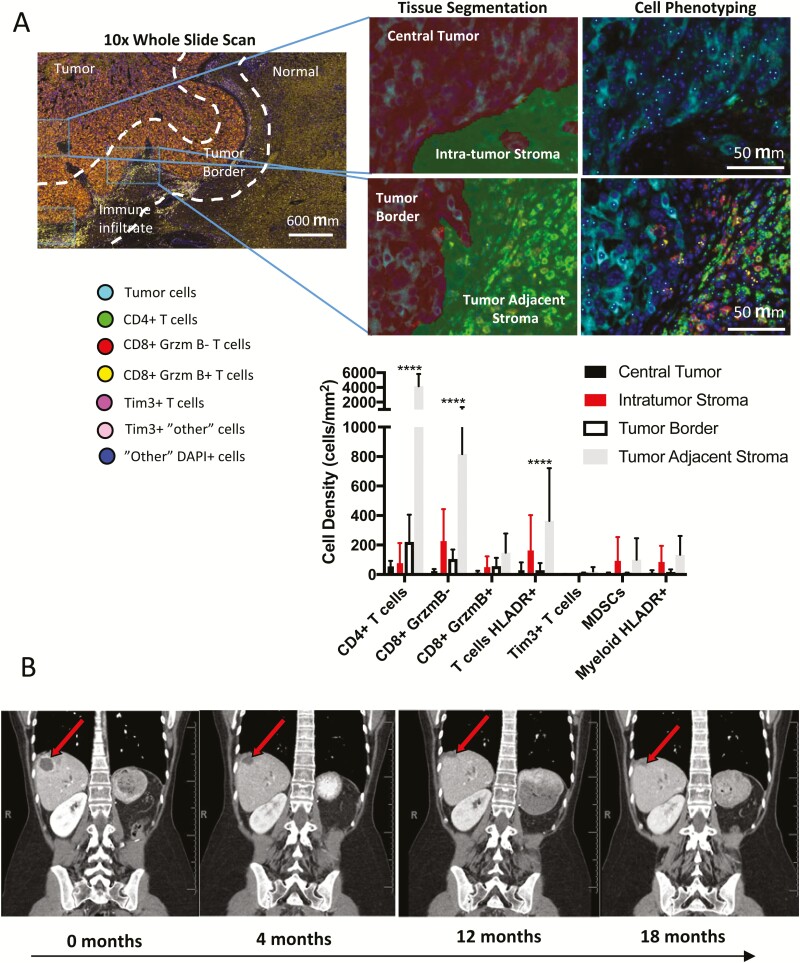
Four years after her initial diagnosis, our patient exhibited evidence of progressive intraabdominal and pulmonary metastatic disease. Important for predicting the potential efficacy of pembrolizumab in treating this patient, we determined whether her metastatic liver lesion exhibited evidence of immune infiltrate similar to the preclinical animal model. Using multispectral immunohistochemistry panels as previously, a robust presence of T cells that were primarily concentrated along the tumor border and adjacent stromal region was observed (Fig. 7A (31)). A subset of these T cells also expressed HLA-DR, indicating immune activation.
Figure 7.
CU-ACC2 patients shows a partial response to treatment with anti-PD-1 pembrolizumab. A, Pre-pembrolizumab multispectral fluorescence immunohistochemistry of ACC-M2B lesion shows evidence of CD4+ and CD8+ immune cell infiltration in the tumor adjacent stroma. Regions on the tumor border or those away from the tumor border were chosen for quantification and segmented into tumor or stroma areas using inForm software. The density of cells for each indicated phenotype was determined for central tumor regions (black), intratumor stroma (red), tumor regions near the tumor border (white), or tumor adjacent stroma (gray). B, Changes in representative target lesion 2 over 16-month period using serial contrast-enhance CT.
Based on the microsatellite instability status and pretreatment tumor immunogenicity, treatment with anti-PD-1 therapy was recommended on a compassionate basis (before US Food and Drug Administration drug approval for solid tumors) and the patient was started on pembrolizumab 200 mg every 3 weeks. To accurately assess the disease progression before the start of treatment, we analyzed CT imaging studies covering slightly over 1 year before initiation of anti-PD1, with lesions measured by the radiologist per RECIST 1.1 criteria. This demonstrated that the two target lesions were not measurable on contrast-enhanced CTs performed 12, 10, and 7 months before baseline. The target liver metastasis was visible as a new lesion on a study 6 months before baseline, and the target lesion by the lung was visible as a new lesion on the study 4 months before baseline. The liver metastasis additionally grew by 178% between its appearance and the subsequent 2 months. After initiation of anti-PD1 therapy, two metastatic target lesions (liver and lung) were followed on successive contrast-enhanced CTs per RECIST 1.1 criteria (31). Comparing the last CT, performed after 16 months on anti-PD-1 therapy, to the baseline, target lesion 1 underwent a 79% decrease in sum of two diameters (Fig. 7B). Target lesion 2 was not measurable on all scans from 3 to 16 months following baseline, effectively a 100% decrease in sum of two diameters. Nontarget lung nodules remained on the subsequent imaging, yielding an overall partial response. No new metastases have been detected since the initiation of the anti-PD-1 treatment for 18 months.
Discussion
Efforts to modulate the immune system for a therapeutic benefit in human cancers span over a century (37), but it was not until the recent success of immune checkpoint inhibitors that immunotherapy has emerged as a transformative clinical strategy in multiple solid cancer types (16, 38, 39). In this study, we developed the first humanized mouse ACC PDX model to test the hypothesis that the effects of an immune checkpoint inhibitor, anti-PD1, on tumor microenvironment would recapitulate effects of the immune milieu of the tumor in the matching patient. Effective tumor growth inhibition was demonstrated in the humanized mice similar to the response observed in the matched patient.
Activating the immune system using dendritic cell vaccines in two patients with metastatic ACC was one of the initial efforts to use immunotherapy in human patients with ACC (40). With the emergence of immune checkpoint inhibitors, investigators explored the expression of PD-L1 on ACC tumor membranes and tumor infiltrating mononuclear cells, and concluded that, although 10% of ACC tissues were PD-L1 positive, there was no correlation with tumor grade or overall survival (41).
In the CU-ACC2-hu-CB-BRGS mice, we were able to interrogate the human immune system (HIS) in depth, in multiple mice and tumors. With adequate tissue samples, the HIS composition could be measured, not only by subset, but also by function through extended analyses of activation and inhibitory proteins, secretion of cytotoxic proteins and expression of inflammatory and regulatory transcription factors. The response of TILs and immune cells in the peripheral lymph organs (i.e., LN, SP, and BM) were compared, providing a more thorough investigation of the immune response than human peripheral blood alone would allow. Flow analyses indicated that increased HLA-DR+, GrB expressing CD8+ T cells, specifically in the TILs of anti-PD-1–treated mice, correlated with reduced tumor growth. Flow cytometry analysis, with higher quantitative power, was also verified by quantitative immunohistochemistry. In this humanized PDX, there was no difference of suppressor T or myeloid cells with anti-PD-1 therapy, although increased myeloid cells were observed among a subset of treated tumors by IHC. This may be due to a difference in markers used to identify the populations, that the IHC was performed on fewer samples, or differences in sensitivity of these techniques. An increase in Tim3 expression on T cells suggests that the upregulation of this inhibitory receptor could have compensated for the loss of PD-1, and may therefore represent an additional, future target in ACC patients following anti-PD-1 therapy. Furthermore, the loss of Tim3 on the human myeloid populations could favor increased phagocytosis, IL-12 production, and T-cell activation in the pembrolizumab-treated humanized mice (42).
Because of differences in tumor growth rates in individual mice, the samples in this study were not all processed at the same time. It is important to take this into consideration because we have recently demonstrated differences in immune responses within TILs collected at different time points (25). As in any tumor analysis, whether using IHC, flow cytometry, RNA, or protein, the data we collected represent a snapshot in time of a dynamic tumor-immune system interaction.
There are several advantages and also some limitations of this humanized mouse model. The hu-CB-BRGS model develops a HIS with high frequencies of T and B lymphocytes as well as less frequent populations of human myeloid, monocyte, and dendritic cells, similar to the recently published NSG [nondiabetic obese (NOD) SCID gamma] humanized mouse model (35). Unlike the NSG model, the BALB/c strain is resistant to radiation and lacks known defects in mouse myeloid cells and complement systems that have been reported in the NOD mice (43–46). The use of either of these models for oncoimmune studies relies on careful examination of chimerism and understanding of the HIS development to ensure adequate T-cell development, which can take months, before tumor implantation. The humanized mice are also more costly than syngeneic mouse models because of the increased time required for HIS development, reagents associated with purification of stem cells, and the “trained” labor needed to generate the mice. We also consider the variability of chimerism among individual mice, and allocate our treatment groups to reduce these effects. In both the NSG and BRGS models developed from hematopoietic stem cells, the HIS is tolerant to the mouse host and offers the continued production of human immune cells from human stem cells resident in the bone marrow. This prevents a graft-versus-host response that is overwhelming in other oncoimmune models in which human immune cells (e.g., from a patient) are injected into an immunodeficient host harboring a human tumor. However, the lack of HLA-matching of the CB donor and tumor implies the response in the hematopoietic stem cell–derived models is not solely tumor-antigen specific, but also contains alloreactivity, as we have previously discussed (25). To date, there is no human preclinical in vivo model that ensures a T-cell syngeneic human thymic selection fully matched to the human immune system and the patient's tumor. Given the enormity of HLA polymorphisms, this will be a very costly and heroic endeavor, although current efforts are under way to develop single HLA-transgenic mice for these humanized mouse studies, which will facilitate a partial HLA match between the humanized immune system and the tumor.
This system is further complicated by selection of human T cells on a mouse thymus. The T cells in these CB-derived humanized mice become exhausted, as indicated by high expression of PD-1, much like T cells in a tumor microenvironment (TME). This immunosuppressed environment allows the growth of allo- and xenogeneic tumor grafts in the mouse, which would be rejected in an immunocompetent setting. We view this quality as an advantage of our model and reason that the tumor-specific responses we observe with checkpoint blockade are actually from release of this exhaustion. We and others have shown that the TME of various PDXs in this model largely recapitulate that seen in the patient, with “cold” tumors showing low lymphocyte infiltration and “hot” tumors having more lymphocyte infiltration (25, 47, 48). However, in the absence of immunotherapy, the T cells in the mice are unable to respond, and, in fact, upon immunotherapy treatments, respond well to “hot” tumors and less well to “cold” tumors. Thus, the immune response is essentially a readout of the TME and how that can be altered by immunotherapy drugs, alone or in combination.
We can also use the model to test the induction of autoimmunity, a common side effect of immunotherapy treatments, although we acknowledge that this is likely related to the genetics of the human immune system. In this study, no autoimmunity was observed, as noted by health assessments, or more conclusively by no increases in autoantibody concentrations in the sera, as measured using a standardized HEP-2 assay (data not shown).
Formalin-fixed paraffin-embedded tissue blocks that can be analyzed by IHC are a more readily available source of human tissue than fresh tumor samples that are required for flow cytometry analysis. However, the assessment of immune infiltrate by IHC is quantitatively limited by the analysis of individual tissue sections containing thousands of cells, compared with flow cytometry in which entire tumors with hundreds of thousands of cells can be analyzed. Despite this limitation, we found similar increases in CD8+ T cells, GrB+ T cells, and HLA-DR+ T cells by multispectral IHC and flow cytometry following pembrolizumab treatment in humanized mice.
Recent case reports using anti-PD-1 in patients with mismatch repair mutated metastatic ACC found that PD-1 checkpoint inhibitor was variably effective (18, 19), despite the reported sensitivity of Lynch syndrome associated solid tumors to anti-PD-1 therapy (17). Unlike our case report, the ACC tumors in these studies were associated with excess cortisol production, which may contribute to poor efficacy of anti-PD-1 therapy resulting from the general immune suppressive effects of cortisol. In addition, a recent phase 1B clinical trial using anti-PD-L1 therapy in 50 patients with ACC showed moderate clinical activity potentially related to tumor PD-L1 positivity (49). Further clinical and preclinical studies are necessary to determine whether hormonal excess, PD-L1 expression, mutation load (MSIhi), or other biomarkers are potential predictors of response in patients with ACC.
In conclusion, this study demonstrates the establishment of a relevant and novel humanized mouse ACC PDX model to elucidate the effects of immunotherapy and its mechanism of action. We demonstrate that the tumor response to anti-PD-1 therapy was observed in both the preclinical model and the patient. These data are similar to the previous reports of PDX mice as “avatars” for personalized cancer treatment with therapy corresponding between PDX and matched human tumor (50–52). Our single-case sentinel study presents a humanized mouse PDX as an avatar for our patient with ACC. Single patients' avatars are, however, time-consuming and cost-prohibiting and therefore unlikely to be used as feasible or time-efficient approach in clinical care for a single patient. However, the availability of multiple preclinical models, which are under development, with a variety of molecular signatures that reflect the genomic heterogeneity seen in ACC, will be an approach to guide more personalized clinical treatment decisions. With this proposed approach, the clinical decisions would be guided based on results in the preclinical model tumor similar molecular signature. Future studies across different preclinical ACC subsets may further elucidate mechanism of resistance to checkpoint inhibitors in “cold” ACC tumors and identify novel combination approaches for the treatment of ACC and define the subset of ACC tumors that might best respond to immunotherapy modalities.
Acknowledgments
We thank the patient for her contribution to this research. We appreciate the technical support from the University of Colorado Cancer Center Flow cytometry staff.
Financial Support: This work was supported by NIH K12CA086913-12 (to K.K.-V), NIH K08CA222620 (to K.K.-V.), Cancer League of Colorado Award (to K.K.-V. and S.L.), Doris Duke CU-FSRC (to K.K.V.), Veterans Affairs Merit Review Award 001 (to M.E.W.), CPRIT Scholar REI RR160093 (to S.G.E.), University of Colorado Cancer Center Support Grant P30-CA046934, and University of Colorado School of Medicine Human Immunology and Immunotherapy Initiative (J.L., K.R.J). The funding bodies had no role in the design of the study and collection, analysis, and interpretation of data or in writing the manuscript.
Author contributions: J.L, A.C., S.G.E., and K.K.-V conceived and designed the paper; J.L., A.C., K.R.J., and K.K.-V. developed the methodology; J.L., A.C., B.M.F., S.M.B., J.B., A.K., B.W.Y., K.D.T., L.S.H., T.J.C., T.M.P., H.S., J.D.F., S.L., M.E.W., and K.K.-V. acquired data (provided animals, acquired and managed patients, provided facilities, etc.); J.L. A.C., K.R.J., M.E.W., and K.K.-V. analyzed and interpreted the data; J.L., A.C., K.R.J., L.S.H., T.M.P., J.D.F., S.L., W.A.M., S.G.E., S.L., M.E.W., and K.K.-V. wrote, reviewed, and/or revised the manuscript; all authors provided administrative, technical, or material support (i.e., reporting or organizing data, constructing databases); and J.L., A.C., and K.K.-V. supervised the study.
Glossary
Abbreviations
- ACC
adrenocortical carcinoma
- BM
bone marrow
- BRGS
BALB/c-Rag2nullIl2rγnullSirpaNOD
- CB
cord blood
- CT
computed tomography
- GrB
Granzyme B
- HIS
human immune system
- IFN
interferon
- IHC
immunohistochemistry
- IL
interleukin
- LN
lymph node
- MSI-H
high microsatellite instability
- NOD
nondiabetic obese
- pDC
plasmacytoid dendritic cells
- PDX
patient-derived xenograft
- SP
spleen
- TIL
tumor-infiltrating leukocyte
- TME
tumor microenvironment
- Vt
treated group
- Vvc
control group
Additional Information
Disclosure Summary: The authors declare no potential conflicts of interest.
Data Availability
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Else T, Kim AC, Sabolch A, Raymond VM, Kandathil A, Caoili EM, Jolly S, Miller BS, Giordano TJ, Hammer GD. Adrenocortical carcinoma. Endocr Rev. 2014;35(2):282–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang T, Rainey WE. Human adrenocortical carcinoma cell lines. Mol Cell Endocrinol. 2012;351(1):58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hantel C, Shapiro I, Poli G, Chiapponi C, Bidlingmaier M, Reincke M, Luconi M, Jung S, Beuschlein F. Targeting heterogeneity of adrenocortical carcinoma: evaluation and extension of preclinical tumor models to improve clinical translation. Oncotarget. 2016;7(48):79292–79304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kiseljak-Vassiliades K, Zhang Y, Bagby SM, Kar A, Pozdeyev N, Xu M, Gowan K, Sharma V, Raeburn CD, Albuja-Cruz M, Jones KL, Fishbein L, Schweppe RE, Somerset H, Pitts TM, Leong S, Wierman ME. Development of new preclinical models to advance adrenocortical carcinoma research. Endocr Relat Cancer. 2018;25(4):437–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Fraipont F, El Atifi M, Cherradi N, Le Moigne G, Defaye G, Houlgatte R, Bertherat J, Bertagna X, Plouin PF, Baudin E, Berger F, Gicquel C, Chabre O, Feige JJ. Gene expression profiling of human adrenocortical tumors using complementary deoxyribonucleic acid microarrays identifies several candidate genes as markers of malignancy. J Clin Endocrinol Metab. 2005;90(3):1819–1829. [DOI] [PubMed] [Google Scholar]
- 6. Giordano TJ, Thomas DG, Kuick R, Lizyness M, Misek DE, Smith AL, Sanders D, Aljundi RT, Gauger PG, Thompson NW, Taylor JM, Hanash SM. Distinct transcriptional profiles of adrenocortical tumors uncovered by DNA microarray analysis. Am J Pathol. 2003;162(2):521–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barlaskar FM, Spalding AC, Heaton JH, Kuick R, Kim AC, Thomas DG, Giordano TJ, Ben-Josef E, Hammer GD. Preclinical targeting of the type I insulin-like growth factor receptor in adrenocortical carcinoma. J Clin Endocrinol Metab. 2009;94(1):204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fassnacht M, Berruti A, Baudin E, Demeure MJ, Gilbert J, Haak H, Kroiss M, Quinn DI, Hesseltine E, Ronchi CL, Terzolo M, Choueiri TK, Poondru S, Fleege T, Rorig R, Chen J, Stephens AW, Worden F, Hammer GD. Linsitinib (OSI-906) versus placebo for patients with locally advanced or metastatic adrenocortical carcinoma: a double-blind, randomised, phase 3 study. Lancet Oncol. 2015;16(4):426–435. [DOI] [PubMed] [Google Scholar]
- 9. Kroiss M, Quinkler M, Johanssen S, van Erp NP, Lankheet N, Pollinger A, Laubner K, Strasburger CJ, Hahner S, Muller HH, Allolio B, Fassnacht M. Sunitinib in refractory adrenocortical carcinoma: a phase II, single-arm, open-label trial. J Clin Endocrinol Metab. 2012;97(10):3495–3503. [DOI] [PubMed] [Google Scholar]
- 10. O'Sullivan C, Edgerly M, Velarde M, Wilkerson J, Venkatesan AM, Pittaluga S, Yang SX, Nguyen D, Balasubramaniam S, Fojo T. The VEGF inhibitor axitinib has limited effectiveness as a therapy for adrenocortical cancer. J Clin Endocrinol Metab. 2014;99(4):1291–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gross DJ, Munter G, Bitan M, Siegal T, Gabizon A, Weitzen R, Merimsky O, Ackerstein A, Salmon A, Sella A, Slavin S, Israel Glivec in Solid Tumors Study G . The role of imatinib mesylate (Glivec) for treatment of patients with malignant endocrine tumors positive for c-kit or PDGF-R. Endocr Relat Cancer. 2006;13(2):535–540. [DOI] [PubMed] [Google Scholar]
- 12. Jesús García-Donas SHP GM, Duran MAC, Méndez-Vidal MJ, Jiménez-Fonseca P, Laínez N, Mateos LL, Moreno F, Gonzalez ERS, Duran I, Perez FJ, Rodriguez-Moreno JF, Maciá S. Phase II study of dovitinib in first line metastatic or (non resectable primary) adrenocortical carcinoma (ACC): SOGUG study 2011-03. 2014.
- 13. Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ferris RL, Blumenschein G, Jr., Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, Worden F, Saba NF, Iglesias Docampo LC, Haddad R, Rordorf T, Kiyota N, Tahara M, Monga M, Lynch M, Geese WJ, Kopit J, Shaw JW, Gillison ML. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, Larkin J, Lorigan P, Neyns B, Blank CU, Hamid O, Mateus C, Shapira-Frommer R, Kosh M, Zhou H, Ibrahim N, Ebbinghaus S, Ribas A, investigators K . Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–2532. [DOI] [PubMed] [Google Scholar]
- 16. Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, Burke MM, Caldwell A, Kronenberg SA, Agunwamba BU, Zhang X, Lowy I, Inzunza HD, Feely W, Horak CE, Hong Q, Korman AJ, Wigginton JM, Gupta A, Sznol M. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, Wong F, Azad NS, Rucki AA, Laheru D, Donehower R, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Greten TF, Duffy AG, Ciombor KK, Eyring AD, Lam BH, Joe A, Kang SP, Holdhoff M, Danilova L, Cope L, Meyer C, Zhou S, Goldberg RM, Armstrong DK, Bever KM, Fader AN, Taube J, Housseau F, Spetzler D, Xiao N, Pardoll DM, Papadopoulos N, Kinzler KW, Eshleman JR, Vogelstein B, Anders RA, Diaz LA, Jr. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Casey RT, Giger O, Seetho I, Marker A, Pitfield D, Boyle LH, Gurnell M, Shaw A, Tischowitz M, Maher ER, Chatterjee VK, Janowitz T, Mells G, Corrie P, Challis BG. Rapid disease progression in a patient with mismatch repair-deficient and cortisol secreting adrenocortical carcinoma treated with pembrolizumab. Semin Oncol. 2018;45(3):151–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mota JM, Sousa LG, Braghiroli MI, Siqueira LT, Neto JEB, Chapchap P, Hoff AAO, Hoff PM. Pembrolizumab for metastatic adrenocortical carcinoma with high mutational burden: two case reports. Medicine (Baltimore). 2018;97(52):13517–13524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Legrand N, Huntington ND, Nagasawa M, Bakker AQ, Schotte R, Strick-Marchand H, de Geus SJ, Pouw SM, Bohne M, Voordouw A, Weijer K, Di Santo JP, Spits H. Functional CD47/signal regulatory protein alpha (SIRP(alpha)) interaction is required for optimal human T- and natural killer- (NK) cell homeostasis in vivo. Proc Natl Acad Sci U S A. 2011;108(32):13224–13229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lang J, Zhang B, Kelly M, Peterson JN, Barbee J, Freed BM, Di Santo JP, Matsuda JL, Torres RM, Pelanda R. Replacing mouse BAFF with human BAFF does not improve B-cell maturation in hematopoietic humanized mice. Blood Adv. 2017;1(27):2729–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bagby S, Messersmith WA, Pitts TM, Capasso A, Varella-Garcia M, Klauck PJ, Kim J, Tan AC, Eckhardt SG, Tentler JJ, Arcaroli J. Development and maintenance of a preclinical patient derived tumor xenograft model for the investigation of novel anti-cancer therapies. J Vis Exp. 2016(115). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lang J, Kelly M, Freed BM, McCarter MD, Kedl RM, Torres RM, Pelanda R. Studies of lymphocyte reconstitution in a humanized mouse model reveal a requirement of T cells for human B cell maturation. J Immunol. 2013;190(5):2090–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Coleman CB, Lang J, Sweet LA, Smith NA, Freed BM, Pan Z, Haverkos B, Pelanda R, Rochford R. Epstein-Barr virus type-2 infects T-cells and induces B-cell lymphomagenesis in humanized mice. J Virol. 2018;92(21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Capasso A, Lang J, Pitts TM, Smith NA, Freed BM, Pan Z, Haverkos B, Pelanda R, Rochford R. Characterization of immune responses to anti-PD-1 mono and combination immunotherapy in hematopoietic humanized mice implanted with tumor xenografts. J Immunother Cancer. 2018;7(1):37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lang J, Ota T, Kelly M, Strauch P, Freed BM, Torres RM, Nemazee D, Pelanda R. Receptor editing and genetic variability in human autoreactive B cells. J Exp Med. 2016;213(1):93–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lang J, Weiss N, Freed BM, Torres RM, Pelanda R. Generation of hematopoietic humanized mice in the newborn BALB/c-Rag2null Il2rgammanull mouse model: a multivariable optimization approach. Clin Immunol. 2011;140(1):102–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huang W, Hennrick K, Drew S. A colorful future of quantitative pathology: validation of Vectra technology using chromogenic multiplexed immunohistochemistry and prostate tissue microarrays. Hum Pathol. 2013;44(1):29–38. [DOI] [PubMed] [Google Scholar]
- 29. Stack EC, Wang C, Roman KA, Hoyt CC. Multiplexed immunohistochemistry, imaging, and quantitation: a review, with an assessment of tyramide signal amplification, multispectral imaging and multiplex analysis. Methods. 2014;70(1):46–58. [DOI] [PubMed] [Google Scholar]
- 30. Gartrell RD, Marks DK, Hart TD, et al. Quantitative analysis of immune infiltrates in primary melanoma. Cancer Immunol Res. 2018;6(4):481–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lang J, Capasso A, Jordan KR, Jena D French, Kar A, Bagby SM, Barbee J, Yacob BW, Head LS, Tompkins KD, Freed BM, Somerset H, Clark TJ, Pitts TM, Messersmith WA, Eckhardt SG, Wierman ME, Leong S, Kiseljak-Vassiliades K. Development of an adrenocortical cancer humanized mouse model to characterize anti-PD1 effects on tumor microenvironment in. Dryad Digital Repository 2019. 10.5061/dryad.f8q4350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Morton JJ, Bird G, Refaeli Y, Jimeno A. Humanized mouse xenograft models: narrowing the tumor-microenvironment gap. Cancer Res. 2016;76(21):6153–6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12(4):269–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Slaney CY, Kershaw MH, Darcy PK. Trafficking of T cells into tumors. Cancer Res. 2014;74(24):7168–7174. [DOI] [PubMed] [Google Scholar]
- 35. Wang M, Yao LC, Cheng M, Cai D, Martinek J, Pan CX, Shi W, Ma AH, De Vere White RW, Airhart S, Liu ET, Banchereau J, Brehm MA, Greiner DL, Shultz LD, Palucka K, Keck JG. Humanized mice in studying efficacy and mechanisms of PD-1-targeted cancer immunotherapy. FASEB J. 2018;32(3):1537–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Arola J, Liu J, Heikkila P, Ilvesmaki V, Salmenkivi K, Voutilainen R, Kahri AI. Expression of inhibin alpha in adrenocortical tumours reflects the hormonal status of the neoplasm. J Endocrinol. 2000;165(2):223–229. [DOI] [PubMed] [Google Scholar]
- 37. Hall SS. A Commotion in the Blood: Life, Death, and the Immune System. New York: Henry Holt; 1997. [Google Scholar]
- 38. Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Aren Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Haddad H, Rini BI. Current treatment considerations in metastatic renal cell carcinoma. Curr Treat Options Oncol. 2012;13(2):212–229. [DOI] [PubMed] [Google Scholar]
- 40. Papewalis C, Fassnacht M, Willenberg HS, Domberg J, Fenk R, Rohr UP, Schinner S, Bornstein SR, Scherbaum WA, Schott M. Dendritic cells as potential adjuvant for immunotherapy in adrenocortical carcinoma. Clin Endocrinol (Oxf). 2006;65(2):215–222. [DOI] [PubMed] [Google Scholar]
- 41. Fay AP, Signoretti S, Callea M, Telomicron GH, McKay RR, Song J, Carvo I, Lampron ME, Kaymakcalan MD, Poli-de-Figueiredo CE, Bellmunt J, Hodi FS, Freeman GJ, Elfiky A, Choueiri TK. Programmed death ligand-1 expression in adrenocortical carcinoma: an exploratory biomarker study. J Immunother Cancer. 2015;3:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ocana-Guzman R, Torre-Bouscoulet L, Sada-Ovalle I. TIM-3 regulates distinct functions in macrophages. Front Immunol. 2016;7:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Verma MK, Clemens J, Burzenski L, Sampson SB, Brehm MA, Greiner DL, Shultz LD. A novel hemolytic complement-sufficient NSG mouse model supports studies of complement-mediated antitumor activity in vivo. J Immunol Methods. 2017;446:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Baxter AG, Cooke A. Complement lytic activity has no role in the pathogenesis of autoimmune diabetes in NOD mice. Diabetes. 1993;42(11):1574–1578. [DOI] [PubMed] [Google Scholar]
- 45. Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7(2):118–130. [DOI] [PubMed] [Google Scholar]
- 46. O'Brien BA, Huang Y, Geng X, Dutz JP, Finegood DT. Phagocytosis of apoptotic cells by macrophages from NOD mice is reduced. Diabetes. 2002;51(8):2481–2488. [DOI] [PubMed] [Google Scholar]
- 47. Gammelgaard OL, Terp MG, Preiss B, Ditzel HJ. Human cancer evolution in the context of a human immune system in mice. Mol Oncol. 2018;12(10):1797–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Morton JJ, Bird G, Keysar SB, Astling DP, Lyons TR, Anderson RT, Glogowska MJ, Estes P, Eagles JR, Le PN, Gan G, McGettigan B, Fernandez P, Padilla-Just N, Varella-Garcia M, Song JI, Bowles DW, Schedin P, Tan AC, Roop DR, Wang XJ, Refaeli Y, Jimeno A. XactMice: humanizing mouse bone marrow enables microenvironment reconstitution in a patient-derived xenograft model of head and neck cancer. Oncogene. 2016;35(3):290–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Le Tourneau C, Hoimes C, Zarwan C, Wong DJ, Bauer S, Claus R, Wermke M, Hariharan S, von Heydebreck A, Kasturi V, Chand V, Gulley JL. Avelumab in patients with previously treated metastatic adrenocortical carcinoma: phase 1b results from the JAVELIN solid tumor trial. J Immunother Cancer. 2018;6(1):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bousquet G, Feugeas JP, Ferreira I, Vercellino L, Jourdan N, Bertheau P, de Bazelaire C, Barranger E, Janin A. Individual xenograft as a personalized therapeutic resort for women with metastatic triple-negative breast carcinoma. Breast Cancer Res. 2014;16(1):401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hidalgo M, Amant F, Biankin AV, Budinska E, Byrne AT, Caldas C, Clarke RB, de Jong S, Jonkers J, Maelandsmo GM, Roman-Roman S, Seoane J, Trusolino L, Villanueva A. Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov. 2014;4(9):998–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Weroha SJ, Becker MA, Enderica-Gonzalez S, Harrington SC, Oberg AL, Maurer MJ, Perkins SE, AlHilli M, Butler KA, McKinstry S, Fink S, Jenkins RB, Hou X, Kalli KR, Goodman KM, Sarkaria JN, Karlan BY, Kumar A, Kaufmann SH, Hartmann LC, Haluska P. Tumorgrafts as in vivo surrogates for women with ovarian cancer. Clin Cancer Res. 2014;20(5):1288–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.