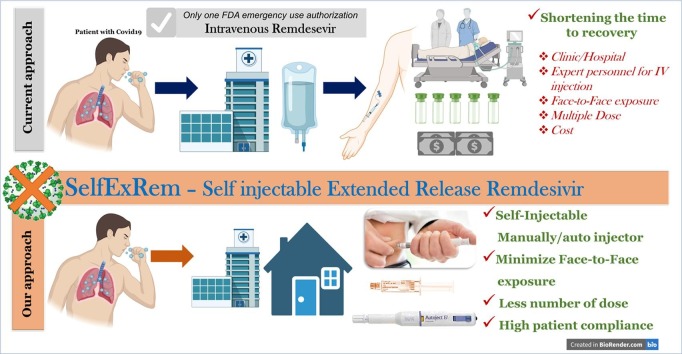
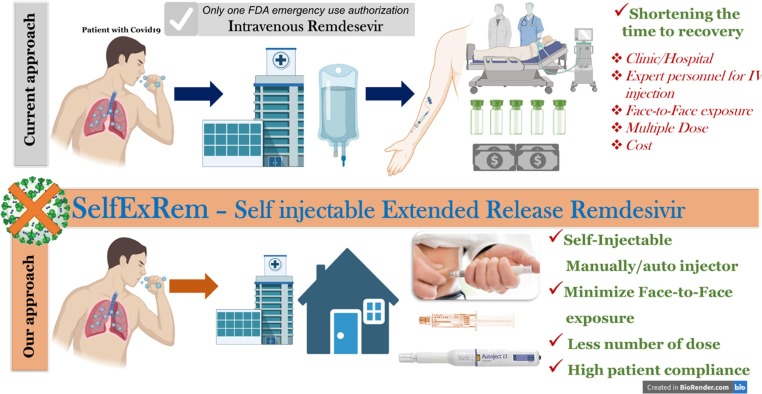
Graphical abstract
Keywords: Covid19, SARS-CoV2, Remdesivir, Long-acting injectables, In situ depot formulations
Abstract
There has been a growing and evolving research to find a treatment or a prevention against coronavirus 2019 (COVID-19). Though mass vaccination will certainly help in reducing number of COVID-19 patients, an effective therapeutic measure must be available too. Intravenous remdesivir (RDV) was the first drug receiving Food and Drug Administration (FDA) approval for the treatment of COVID-19. However, in a pandemic like COVID-19, it is essential that drug formulations are readily available, affordable and convenient to administer to every patient around the globe. In this study, we have developed a Self-injectable extended release subcutaneous injection of Remdesivir (SelfExRem) for the treatment of COVID-19. As opposed to intravenous injection, extended release subcutaneous injection has the benefits of reducing face-to-face contact, minimizing hospitalization, reducing dosing frequency and reducing overall health care cost. SelfExRem was developed using a biodegradable polymer, poly(lactic-co-glycolic acid) (PLGA), dissolved in a biocompatible vehicle. Six different batches were formulated using 2 different grades of low molecular weight PLGA and 3 different PLGA concentration. The force of injection of various polymeric solutions through 23–30-gauge needles were analyzed using a TA.XTplus texture analyzer. The time required for injection was evaluated both manually and by using an autoinjector. In vitro release of all the batches were carried out in 1% v/v tween 80 in phosphate buffer saline. The study indicated that SelfExRem developed with 15% w/v PLGA (75:25) provided a steady release of drug for 48 h and may be a breakthrough approach for the treatment of COVID-19.
1. Introduction
The 2019 novel coronavirus (2019-nCoV; severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)) has spread rapidly since its recent identification in patients with severe pneumonia in Wuhan, China (WHO, 2020, Sharma et al., 2020). The 2019-nCoV belongs to the Betacoronavirus genus, which also contains SARS-CoV and Middle East respiratory syndrome CoV (MERS-CoV) (Petrosillo et al., 2020). An efficient approach to drug discovery is to test whether existing antiviral drugs are effective in treating related viral infections. Several drugs, such as ribavirin, interferon, lopinavir-ritonavir and corticosteroids, have been used in patients with SARS or MERS, although the efficacy of some of these drugs remains controversial. In a study by Wang et al., five FDA-approved drugs, including ribavirin, penciclovir, nitazoxanide, nafamostat, chloroquine and two well-known broad-spectrum antiviral drugs, remdesivir (GS-5734) and favipiravir (T-705), were tested in vitro against a clinical isolate of 2019-nCoV (Wang et al., 2020). Amongst the various drugs tested, Remdesivir has been recently recognized as a promising antiviral drug with efficacy against a wide array of RNA viruses (including SARS/MERS-CoV) in cell culture, murine and nonhuman primate (NHP) models (Amirian and Levy, 2020, Wang et al., 2020).
On 1st May 2020, the U.S. Food and Drug Administration (FDA) authorized Emergency use of Remdesivir (RDV) for COVID-19 infection (USFDA, 2020). Later, it was approved for COVID-19 treatment. RDV is an adenosine analogue, which is incorporated into nascent viral RNA chains, resulting in pre-mature termination. Currently, RDV is available as a sterile, preservative-free lyophilized powder (100 mg), which is reconstituted with 19 mL of sterile water for injection and diluted into 0.9% saline prior to intravenous (IV) administration. Post reconstitution, each single-dose vial contains a 5 mg/mL RDV concentrated solution (USFDA, 2020). However, there are certain problems and restrictions associated with an IV injection, especially in the case of COVID-19 infections. It needs to be administered by a medical practitioner and requires a hospital setting. This increases the chance of person-person contact and risk of further spreading the infection (Fig. 1 ). Intravenous injection or infusion is usually painful and less agreeable for the patient and can produce patient anxiety, especially in patients with trypanophobia. Conventional injection can also present risks and challenges to the health care provider, such as the potential for human error and contamination when drawing up the dose in the syringe as well as the risk of needlestick injury (McMurtry et al., 2015, Taddio et al., 2012).
Fig. 1.
Schematic representation of SelfExRem. Current COVID-19 treatment, intravenous remdesivir has some limitations restricting its patient compliance. Our proposed formulation approach, SelfExRem, a novel self injectable depot formulation would help in improving the minimizing the cost associated with drug administration, face-to-face interaction, requirement of expert personnel etc.
In this study, we have systematically developed and characterized a self-injectable extended release RDV (SelfExRem) for the treatment of COVID-19. SelfExRem is designed to be administered manually or via autoinjector to an appropriate subcutaneous site. The ability to self-administer the drug will help reduce person-person contact, minimize exposure to a clinic or hospital, reduce dosing frequency and reduce health care costs (Fig. 1). A novel RDV formulation – SelfExRem - is an organic solvent based in-situ depot injection. It consists of a syringeable and injectable solution of a polymer, poly(lactic-co-glycolic acid) (PLGA), that is dissolved with the drug in a water-miscible solvent like N-methyl-2-pyrrolidone (NMP). NMP is a biocompatible, non-toxic solvent. It is listed in the FDA inactive ingredient database (IID) list for the SC and periodontal routes (Ahmed et al., 2014). An in-situ depot injection involves a less complex manufacturing process compared to solid implants and microparticles (Rahnfeld and Luciani, 2020) and can be developed relatively quickly.
Injectability force, penetration force, needle-syringe selection, viscosity and comfort on injection are very important parameters for self-injectable formulations. Like any other injectable formulation, syringeability and injectability are important for desired product performance, the former being the ability of the formulation to be drawn from the vial to the syringe and the latter being the performance of the formulation. Over the past decade, self-injectable formulations have proven to be highly beneficial. The needle geometry, viz. the needle’s inner diameter, length, shape and surface finish, all play an important role, particularly in the self-injectable formulations. In addition to affecting the pain associated with the injection, these parameters also impact the injectability of the formulations. Hence, a comprehensive understanding of all these parameters for a self-injectable formulation is imperative.
An auto-injector is a device that completely or partially replaces the activities involved in parenteral drug administration with a conventional syringe and needle (Travanty et al., 2018). Such devices are increasingly being developed for use in the clinical setting or home environment for treatment of acute and chronic conditions. A major advantage that may be expected with an auto-injector is that it will give the flexibility to patients to take the medication in a home setting and avoid going to a hospital, thereby decreasing the risk of spreading the coronavirus. It will help to improve patient compliance and most importantly may reduce health care costs. Another potential advantage includes reduction in patient anxiety related to trypanophobia since the patient does not see the needle. It will also lead to reduction of errors in drawing up the dose consistently and will prevent accidental drug contamination.
After a thoughtful consideration of various commercially viable self-injectable Long Acting Injectable (LAI) technologies, scale-up feasibility, dose of RDV and pharmacokinetic/physicochemical properties of RDV, we decided to develop a PLGA-NMP based in-situ depot forming system. There is no previous report describing preclinical or clinical investigation of subcutaneous RDV. The main objective of this paper is to optimize the RDV/NMP/PLGA ratio to achieve desired sustained release and injectability using a commonly used autoinjector and needle.
2. Materials
Remdesivir was purchased from Medchemexpress (Monmouth Junction, NJ). Poly(D,L-lactide-co-glycolide) (PLGA) Resomer 752H and Resomer 502H polymers were purchased from Sigma-Aldrich (St. Louis, MO). 1 methyl-2-pyrrolidinone (NMP), high performance liquid chromatography (HPLC) grade water, acetonitrile, needles of gauge sizes ranging from 23G to 30G were purchased from Fisher Scientific (Waltham, MA). Kolliphor TPGS and Kolliphor HS15 were received as a gift sample from BASF (Florham Park, NJ). Tween 80 was received as a gift sample from Croda (Edison, NJ). Luer Lock glass syringes were purchased from Becton Dickinson (BD Hypak, USA).
3. Methods
3.1. Analytical method
A quantitative reverse-phase HPLC method was developed and validated to determine RDV release in vitro from the various formulations. The HPLC analysis was carried out using a Waters Alliance system equipped with a 2998 PDA (Photo diode array) detector and hypersil ODS column (250 mm × 4.6 mm, 5 µm). Chromatographic separation was carried out using acetonitrile with 0.1% (v/v) trifluoroacetic acid (TFA) and phosphate buffer (pH 3.5) in a ratio of (65:35). The flow rate was maintained at 1 mL/min keeping an injection volume of 10 µL. The output signal was monitored using Empower 3 software. The column was maintained at room temperature (25 °C). The retention time of RDV was 5.1 ± 0.3 min detected at 244 nm.
3.2. Saturation solubility of RDV
The saturation solubility of RDV was carried out in NMP, PLGA solution (Resomer 502H, Resomer 752H) in NMP and various surfactant solutions in water (1% v/v). An excess amount of RDV was added to individual vials containing different surfactant solutions in water (TPGS, Tween 80, Kolliphor HS15), NMP, PLGA502H 20% in NMP, PLGA752H 20% in NMP. The tubes were kept on a mechanical shaker at 37 °C for 24 h. After 24 h, the samples were centrifuged at 12000 rpm for 5 min. The supernatant was collected and diluted with acetonitrile. RDV concentration in the supernatant was determined by HPLC.
3.3. Development of SelfExRem
Initially, Resomer 502H [50:50 Poly (DL-lactide-co-glycolide)] and Resomer 752H [75:25 Poly (DL-lactide-co-glycolide] were solubilized in a water miscible organic solvent, NMP, to prepare 15, 20, and 30% w/v solutions of PLGA in NMP as shown in Table 1 . The tubes containing the various ratios were vortexed and kept on a shaker at 37 °C to form a homogenous mixture. SelfExRem was prepared by adding 5 mg of RDV to each of 6 different tubes. The PLGA-NMP solution was added to the RDV containing tubes until a clear solution was obtained. Percent w/w RDV loading was calculated using the following formula;
Table 1.
Composition of various RDV batches.
| Batch | Amount of RDV (mg) | PLGA |
%RDV loading (w/w) | ||
|---|---|---|---|---|---|
| Grade | Concentration (% w/v of PLGA in NMP) | Volume (µL) | |||
| 1 | 5 | 502H | 15 | 15 | 25 |
| 2 | 752H | 15 | 15 | 25 | |
| 3 | 502H | 20 | 20 | 20 | |
| 4 | 752H | 20 | 20 | 20 | |
| 5 | 502H | 30 | 25 | 16 | |
| 6 | 752H | 30 | 25 | 16 | |
#RDV loading was reduced with increase in PLGA concentration.
The concentration and volume of PLGA-NMP solution required to solubilize 5 mg RDV is given in Table 1.
3.4. Determination of injectability: panel test
The injectability of the different batches prepared was assessed by 5 volunteers who received different needle-syringe systems (Table 2 ) each filled with an aliquot of 1 mL of the different batches. The volunteers participated in a blind study. Each volunteer was asked to evaluate the injectability in terms of the ease of injection and the formulation flow through the needle, using an arbitrary score from 1 to 4. In particular, the arbitrary scores for both parameters were as follows
score 1 = injection: very difficult; flow: no flow or drop wise
score 2 = injection: difficult; flow: initially drop wise, then continuous
score 3 = injection: moderate; flow: continuous
score 4 = injection: easy; flow: continuous.
Table 2.
Score of Manual Injectability of various formulations.
| Formulation | Concentration (%w/v) | Needle gauge | Injectability (Y/N) | Score |
|---|---|---|---|---|
| 502H | 15 | 23G | Y | 4 |
| 25G | Y | 4 | ||
| 28G | Y | 4 | ||
| 30G | N | 2 | ||
| 20 | 23G | Y | 4 | |
| 25G | Y | 4 | ||
| 28G | N | 2 | ||
| 30G | N | 2 | ||
| 30 | 23G | Y | 4 | |
| 25G | N | 2 | ||
| 28G | N | 1 | ||
| 30G | N | 1 | ||
| 752H | 15 | 23G | Y | 4 |
| 25G | Y | 4 | ||
| 28G | Y | 4 | ||
| 30G | N | 2 | ||
| 20 | 23G | Y | 4 | |
| 25G | Y | 4 | ||
| 28G | N | 1 | ||
| 30G | N | 1 | ||
| 30 | 23G | Y | 3 | |
| 25G | N | 1 | ||
| 28G | N | 1 | ||
| 30G | N | 1 |
# 23G needle is suitable for all batches of SelfExRem, while 25G was not suitable for batches prepared with polymer concentrations >20% w/v. 28G and higher are not suitable for SC injection of such formulations.
3.5. Evaluating the injectability/expulsion force using texture analyzer
Six different formulations were tested for their injectability. The samples were drawn into the syringe. Each sample was tested by a texture analyzer equipped with a 50 kg load cell (TA. XT Plus, Stable Microsystems, South Hamilton, MA). A pushing force was required to evaluate these properties. Compression mode was used for analysis, where a 1 mL syringe with a 23, 25, 28 or 30 gauge (G) needle was used for each test, respectively. The test was performed at a speed of 1 mm/s with a hold time of 60 s with a maximum distance of 90 mm (equivalent to 1 mL). The force required to push out the formulation was recorded. All measurements were carried out in triplicate.
For further analysis, the work done for each injection was calculated by using the following equation (1):
| (1) |
where WT is the total work done per injection, Dmax is the maximum distance the plunger reaches to extrude 1 mL of the formulation, F and d are force and distance recorded during the course of injection. By applying this equation, a traditional AUC was calculated which is a more descriptive representation of the injectability (Zhang et al., 2018).
3.6. In vitro release study
To determine the rate of drug release from SelfExRem, an in vitro release study was performed by injecting different formulations into 15 mL of 0.01 M phosphate buffer saline (PBS) pH 7.4 containing 1% (v/v) Tween 80. At time points of 30 min, 2 h, 4 h, 24 h, and 48 h, the sample was withdrawn and the amount of RDV released was estimated using HPLC. For mass balance, the amount of RDV remaining within the depot was also evaluated by dissolving the depot in acetonitrile. All experiments were performed in triplicate.
3.7. Time required for injection by an auto injector
The autoinjector (Owen Mumford, Autoinject 2) was filled with the optimized formulation (Batch 2) and attached to 3 different gauge needles (25, 28, 30G). The time required to inject 1 mL of the optimized formulation using an autoinjector was measured. The time required to inject SelfExRem was compared with the time required to inject water using the same method. Each test was performed in triplicate.
3.8. Accelerated stability study
The optimized batch of RDV (Batch 2) was stored in an individual scintillation vial at 4 °C, 25 °C and 40 °C. After a period of 2 weeks (14 days) and a month (31 days) of incubation, samples were collected and analyzed using HPLC for RDV content. The formulations were also visually inspected for any change in their physical state, i.e. color, turbidity, and consistency.
3.9. Statistical analysis
The data are shown as the mean ± standard deviation (SD). Student's t-test or one way ANOVA was used to determine the significance of the differences among treatment groups using GraphPad Prism version 5.0 (San Diego, CA), where p < 0.05 was considered statistically significant.
4. Results
4.1. Saturation solubility
Since the proposed SelfExRem in-situ depot formulation is based on Atrigel® technology, it was essential to evaluate the solubility of RDV in NMP. RDV was found to be freely soluble in NMP (>1 g/mL), however its solubility was substantially decreased in PLGA-NMP solution. Furthermore, the addition of RDV led to the precipitation of PLGA from the NMP solution. Thus, it was apparent that in order to maintain the sink condition during the release study, a surfactant had to be added. Since the release study was performed for 48 h, it was important to determine whether the surfactant used could maintain the solubility of RDV for 48 h. The saturation solubility of RDV was found to be very similar in all three surfactants used, i.e. Kolliphor RH 40, TPGS and Tween 80 (>1000 µg/ml).
4.2. Development of SelfExRem
Various batches of SelfExRem were prepared using two different grades of low molecular weight PLGA. Resomer 502H is PLGA with molecular weight of 7000–17,000 and L:G ratio of 50:50. Resomer 752H is PLGA with molecular weight of 4000–15,000 and L:G ratio of 75:25 (Babos et al., 2018). PLGA solutions of 15, 20 and 30% w/v were prepared by dissolving appropriate amounts of PLGA in 1 mL of NMP. PLGA-NMP solution was added to an accurately weighed 5 mg amount of RDV in increments of 2.5 µL until a clear system was obtained. Batches were intermittently vortexed to facilitate the solubilization of RDV. After the addition of 15 µL to batches 3–6, a white solid mass was still observed at the bottom of the tube. Hence more PLGA-NMP solution was added in increments of 2.5 µL to dissolve the solid mass in these tubes, until a clear system was obtained. Thus, as shown in Table 1, the volume of PLGA solution required to solubilize 5 mg of RDV was higher for batches with higher PLGA concentrations (20%, 30%). Batches were inspected for 24 h for any signs of precipitation before any further studies.
4.3. Determination of injectability: panel test
It is crucial to determine how the viscosity of a formulation affects its ejection from the syringe via a needle to the injection site. Hence the injectability of the various formulations were manually assessed by the panel test. As the needle size might influence patients’ comfort and compliance, in this study needles consistent with intramuscularly and subcutaneously injections were tested. It was found that a 23G needle was suitable for all batches of SelfExRem, while 25G was not suitable for batches prepared with polymer concentrations >20% w/v (Table 2). There was not much difference observed in manual injectability of 502H and 752H at a concentration of 15%. However, 752H was slightly better when tested by texture analyzer. Batches with 30% PLGA concentration were very difficult to inject through needles above 25G.
4.4. Injectability/expulsion force
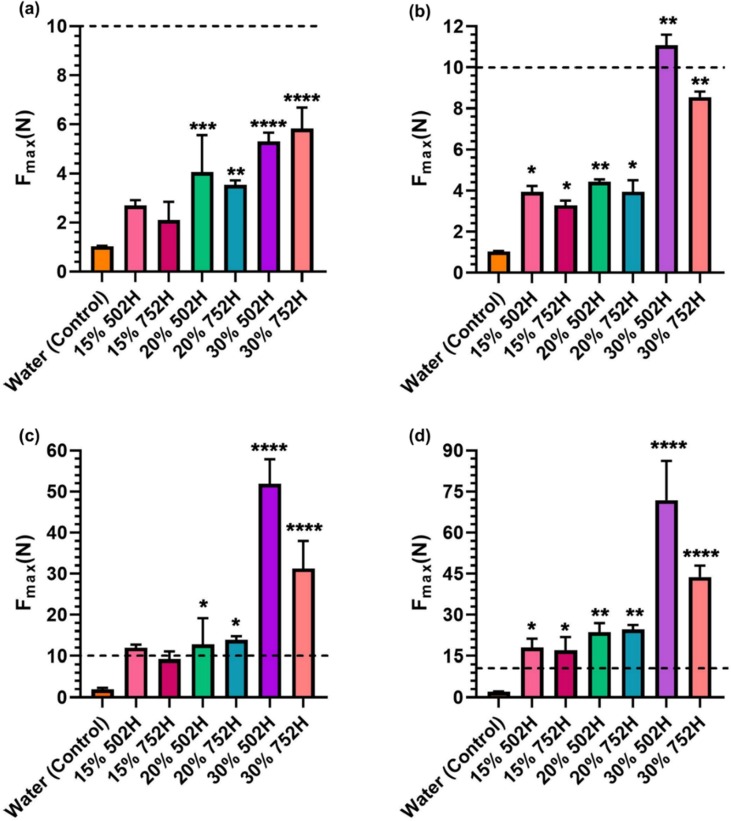
The injectability of the formulations was tested quantitively by texture analyzer. It is evident from Fig. 2 that the injection force was increased with the needle gauge and polymer concentration. As shown in Fig. 2a, formulations containing 15, 20 and 30% of 502H and 752H had an average maximum force requirement in the range of 2–5.5 N when injected using a 23G needle. The dotted line in Fig. 2 represents the average force required for an easy injection based on the panel test. Estimation of the injection force through a 25G needle revealed that formulations of 502H and 752H had a maximum force requirement in the range of 3–11 N (Fig. 2b). It was observed that around twice the amount of force was required for injection with a needle with a decrease in inner diameter from 23G to 25G. For 28G, formulations of 502H had an injection force requirement range of 11–52 N. However, a decrease in the range of force requirement was observed for 752H formulations, which had a maximum force requirement of 11–32 N (Fig. 2c). A similar trend is shown in Fig. 2d. The injectability profile of the formulation with a 30G needle suggested that the formulations of 502H required a force in the range of 18–72 N. 752H had a maximum force requirement in the range of 17–44 N. A statistically significant difference in Fmax requirement was observed for all the batches, apart from 15% 502H and 752H, when injected by 23 and 28G when compared to water (control). All these results complement the panel test results. As described earlier, it was observed that as the inner diameter of the needle decreased, the difficulty to inject the formulation increased. A linear relationship between Fmax and inner diameter was observed. It was hence confirmed that there is a direct relationship between the force required to inject the formulation and the inner diameter of the needle, independent of the polymeric concentration. From these results it can be inferred that the 23G and 25G needles are ideal as far as the injectability of SelfExRem. If we compare the results obtained from the panel test and the injectability using the texture analyzer (Fig. 2), batches with scores of 4 (easy to inject manually) required less than 10 N of force. Hence, we can infer that batches with Fmax of 10 N or less are very easy to inject, while batches with Fmax higher than 10 N have poor injectability.
Fig. 2.
Evaluation of injectability/expulsion force of 502H and 752H (15, 20 and 30%w/v). Comparison of injectability/expulsion force of the formulation was performed using different needle gauges, (a) 23G, (b) 25G, (c) 28G and (d) 30G. (----) represents the force corresponding to 10 N, i.e. the minimum force required for an effortless injection. Injection force was found to be increased with the needle gauge and polymer concentration. Fine needles (28G and 30G) are not suitable to inject such formulations.
4.5. Injectability of SelfExRem: work done, plunger-breaking force (PBF) and dynamic glide force (DGF)
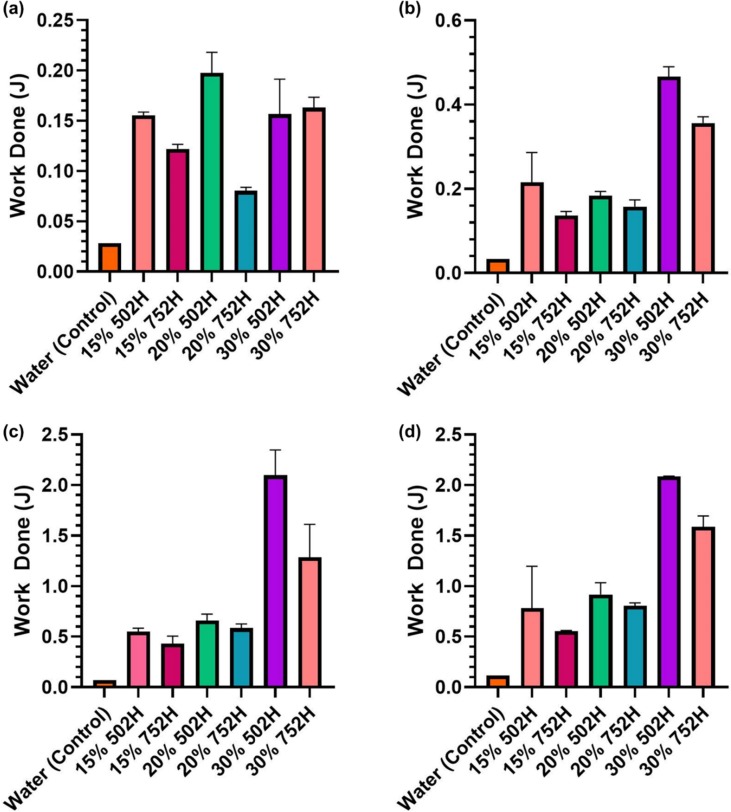
To understand the complete energy requirement during the course of injection, total work done was calculated. As represented in Fig. 3 a, the formulations required total energy (J) between 0.1 and 2.5 J. A direct relationship between the inner diameter and energy requirement was observed, with the total work done (J) increasing as the inner diameter was decreased. The batch with 20% 752H required less energy in comparison to the other formulations. However, as the inner diameter decreased, the total energy required for injection of 15% 752H was significantly less than any other formulation. The recorded values for 15% 752H injected from 25, 28 and 30G needles were 0.13, 0.42 and 0.55 J, respectively (Fig. 3b–d).
Fig. 3.
Evaluating of total work done (WT) during injection of 502H and 752H (15, 20 and 30% w/v). WT was assessed using different needle gauges, (a) 23G, (b) 25G, (c) 28G, and (d) 30G. Except for 23G, 30% w/v PLGA batches showed significantly higher work done compared to 15 and 20 %w/v batches.
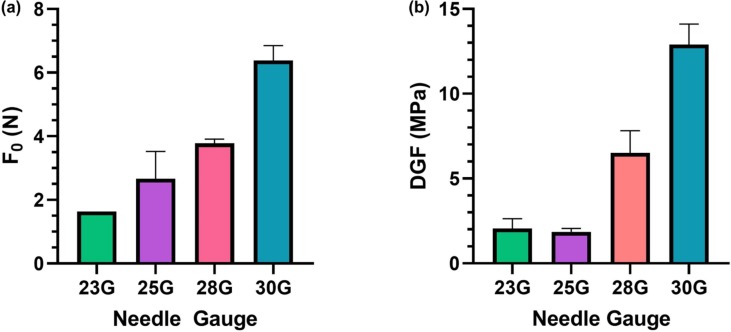
Due to a significantly lower Fmax and WT for 15% 752H, batch 2 was investigated further for its PBF and DGF characteristics. On further evaluation of 15% 752H, it was observed that there was a relative increase in the initial force (F0) with a decrease in the inner diameter of the needle. As the diameter was reduced from 0.34 to 0.16 mm, a statistically significant increase in F0 was observed (Fig. 4 a). A similar trend was observed in the DGF, which also varied with changes in the diameter of the needle. The DGF was observed to be 2.05, 1.54, 6.8 and 12.89 MPa for needles with inner diameters of 0.34, 0.26, 0.18 and 0.16 mm, respectively (Fig. 4b).
Fig. 4.
Injectability parameters of the optimized formulation 15% 752H (a) Plunger-breaker loose force and (b) dynamic glide force profiles of injection of SelfExRem batches by different needle gauges. A sharp increase in the initial force (F0) with a decrease in the inner diameter of the needle was observed.
4.6. In vitro release study
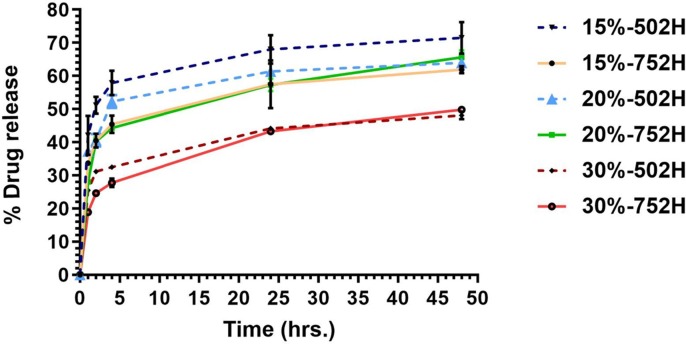
In vitro release of various RDV formulations (batches 1–6) was carried out in phosphate buffer with 1% w/v Tween 80. As shown in Fig. 5 , it was confirmed that the drug release was significantly dependent on the type of PLGA used and its concentration. In the case of batches prepared with Resomer 502H (batche 1, 3, 5), higher burst release was observed in batch 1. After 48 h, the release of RDV from batches 1, 3, 5 was around 70, 58, 40%, respectively. Whereas for batches prepared using Resomer 752H (batches 2, 4, 6), release of RDV was around 51, 58 and 41%, respectively after 48 hr. Although release of batch 6 was found to be slowest at 30%, there was no significant difference observed between batches 2 and 4 (PLGA752H 15 and 20%). At the 15% concentration, comparatively less burst release was observed with batches prepared using 752H than batches prepared with 502H. Based on release profile and percentage drug loading, batch 2 was selected for further studies.
Fig. 5.
In vitro release study of SelfExRem prepared (six different batches) using 2 different grades of PLGA. Comparatively less burst release was observed with batches prepared using 752H than batches prepared with 502H. Increasing the polymer concentration from 15% to 30%, decreased the RDV release.
4.7. Time required for injection using an autoinjector
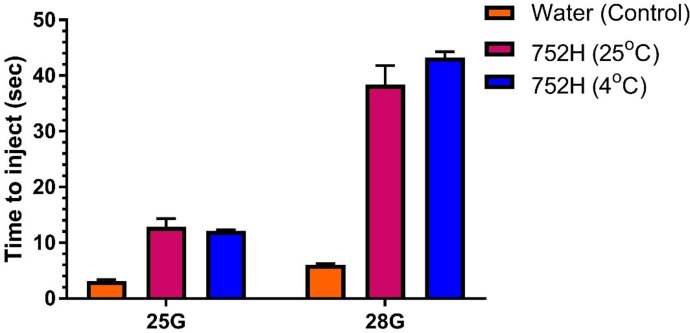
It is imperative to determine the time required to inject the formulation using an autoinjector. A patient must be able to inject the formulation with ease. As shown in Fig. 6 , the optimized formulation took a longer time compared to water owing to the higher viscosity of the formulation. However, for 25G and 28G, the optimized formulation was injected in 12 and 38 s, respectively. When a 30G needle was attached to the autoinjector, it was observed that the formulation was very difficult to inject and hence the time required to inject could not be recorded. As shown in Fig. 6, there was no significant difference in the time required for injection when the formulation temperature was 25 °C or 4 °C.
Fig. 6.
Time required to inject optimized formulation using an autoinjector. The optimized formulation was easily injected using 25G needle. It required significantly lesser time (1/4th) using a 25G needle compared with a 28G needle.
4.8. Accelerated stability study
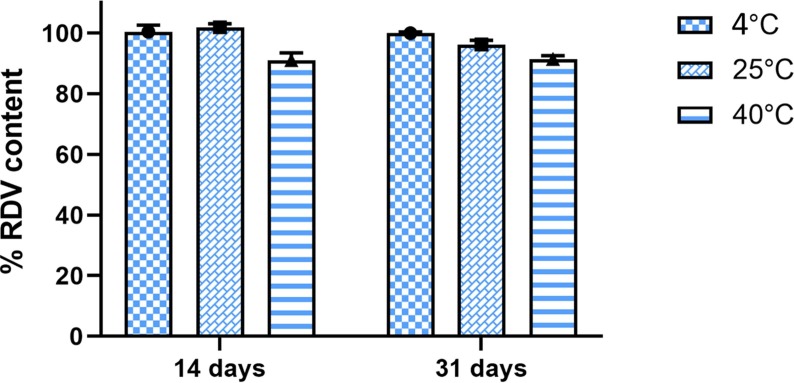
As shown in Fig. 7 , after 14 days, the RDV content of SelfExRem was found to be similar i.e around 99 ± 2% at 4 °C and 25 °C. However, at 40 °C, RDV content decreased by 10% within 14 days. After a month, at 4 °C RDV content remained the same. RDV content reduced by 5%-10% in the batches stored 25 °C and 40 °C. The substantial decrease in drug content at higher temperature indicated that formulation is susceptible to thermal degradation. Samples stored at 4 °C showed no signs of drug or polymer precipitation, color change or turbidity. Thus, we suggest that refrigeration would be suitable for long term storage and transportation of SelfExRem.
Fig.7.
Stability study of SelfExRem. SelfExRem was chemically and physically stable at 4 °C but RDV content was reduced in the batches stored at 25 °C and 40 °C.
5. Discussion
Currently, RDV is available for emergency use as an IV injection/infusion. Since it is an IV injection, skilled medical personnel are required to administer it (USFDA, 2020). During the COVID-19 pandemic, it is highly recommended to avoid social contact of any sort. However, a patient who is COVID positive is left with no choice but to be admitted to the hospital to receive the injection. This could further increase the risk of spread because of the high transmission rate of SARS-CoV-2. Hence, a kind of dosage form of RDV which a COVID positive patient can self-administer and also a high-risk population like medical professionals can take prophylactically would be invaluable. After thoughtful consideration of various technologies, physicochemical and metabolic properties of RDV, we designed an in-situ depot which can be self-injectable via the subcutaneous route.
There are three different types of in-situ depot injection systems, i.e. oil based, aqueous based and organic solvent based. A saturation solubility study revealed that RDV is freely soluble in NMP (>1 g/mL), while poorly soluble in oils. Due to the high solubility of RDV in NMP, we used NMP as a water miscible organic solvent in the in-situ depot formulation. Comparatively sustained release is not so readily attainable by non-PLGA systems, which do not have commercial priority and lack the so-called “real world biomaterial” status (Schwendeman et al., 2014). When a formulation's benefits offset the risks, it is usually approved by the FDA. This might be the reason why the latest two formulations approved by the FDA in 2017 and 2018 (Sublocade, Perseris) are in-situ gel formulations (Park et al., 2019). Thus, considering the translational feasibility, we chose two low molecular weight PLGAs to formulate the in-situ depot formulation.
Many reports suggest that low molecular weight PLGA helps increase drug loading (Brauner et al., 2020, Patel et al., 2010). Accordingly, we anticipated that SelfExRem batches with as high as 50% drug loading could be prepared. However, we observed an anomalous characteristic of our formulation. RDV loading was inversely proportional to PLGA concentration. Addition of polymer solution to RDV resulted in precipitation of PLGA at very low volume. Therefore, PLGA solution was added until a clear homogenous system was observed. Thus, maximum drug loading of 25% was achieved with a PLGA concentration of 15% w/v. Although solubilization of RDV was quicker and easier in 752H batches than in 502H batches, the volume of a given concentration of PLGA solution required to solubilize 5 mg of RDV was unchanged. However, batches prepared using 20% w/v PLGA solution (batches 3 and 4) required 5 µL more compared to batches prepared using 15% w/v PLGA solution. A further increase in PLGA concentration to 30% w/v resulted in reduction in RDV loading to 16%. We speculate that RDV and PLGA compete for solubilization in NMP. An increase in the PLGA concentration resulted in a smaller amount of free NMP. The concentration of NMP in SelfExRem is 63.7% w/v (batch 2) which is similar to some of the commercial products - Eligard® and Sublocade® which contain 50–64% NMP.
After determining the ratio of NMP and PLGA in various batches (1–6), another aim was to evaluate the effect of different batches of SelfExRem on the ease of injectability. The perplexing issue with a PLGA-NMP based depot system is the problem of injectability due to the high viscosity. Thus, we carried out a panel test to determine which injection-needle system would facilitate patient compliance because of ease of injectability. Usually 25G and above needles are used for SC injection, however it was difficult to inject SelfExRem with a 25G needle for polymer concentrations above 20% w/v. Next we performed a force of ejection study. Injectability can be defined as the amount of force required to push the formulation out. However, there are limitations to quantifying these results, and more reproducible models should be developed and used (Rahman et al., 2017). In our case it was observed that there was a linear relationship between the force required to inject the formulation and the inner diameter of the needle. As the inner diameter decreased from 0.34 to 0.16 mm (23G–30G), it was observed that the force required to inject both the 502H and 752H formulations increased by 8–9-fold. Along with this, due to the increase in the kinematic viscosity of the formulation, there was a 2–4-fold increment in the force required for injection. This is in accordance with a previous report by Rungseevijitprapa et al. where the authors report a marked increase in the force of injection when there is an increase in concentration (Rungseevijitprapa and Bodmeier, 2009, Voigt et al., 2012). Apart from these two factors the polymer chemistry may also affect the injectability of the formulation. Due to the difference in the lactic and glycolic acid concentration the internal meshwork of the PLGA particle can vary and hence exert a different pressure during injection. In the case of 502H, where the lactide:glycolide ratio is 50:50, there can be a higher injectability force requirement than with 752H (lactide:glycolide ratio of 75:25) (Gentile et al., 2014, Makadia and Siegel, 2011). Our results echo previous reports that higher lactide content results in lower force of injection.
As a self-injectable formulation, it is important that SelfExRem be easy and fast to inject to minimize patient discomfort and increase compliance. Reports suggests that while formulations requiring forces above 50 N are injectable, they are administered with greater difficulty (Cilurzo et al., 2011). Injection forces less than 25 N allow easy injection for most depot formulations (Rahnfeld and Luciani, 2020). Injectability profiles have been previously reported to be only a representation of Fmax with some correlation to the dynamic glide force (Cilurzo et al., 2011). This report suggest that this is not an entirely accurate parameter to describe the injection completely. Hence, to understand the complete course of injection, total work done per injection should be determined. We found that the higher the concentration of the polymeric solution, the greater the increase in the total work done per injection. The total work done is also directly impacted by the decrease in the inner diameter of the needle. Hence, this suggests that the work done per injection would increase with an increase in the polymeric concentration and also as the inner diameter decreases. These results are in accordance with a previous report by Zhang et al. (2018). However, an injectability profile for an injectable formulation can ideally be divided into two phases, Phase-1: plunger-break loose force (N) and Phase-2: dynamic glide force (MPa). The plunger-break loose force (PBF), is defined as the initial force (F0) required to start the course of the injection at the initial distance (d0). In other words, it is the first resilience the formulation exerts on the plunger when the extrusion starts. The dynamic glide force (DGF) is defined as the force required to continue and complete the course of injection. It is the continuous force exerted on the plunger during the injection. Both these phases are directly proportional to the function of the formulation’s kinematic viscosity and concentration. For a formulation like SelfExRem, it is essential that both these characteristics be in an ideal range. Especially when a patient is suffering from COVID19, it very important that the course of the injection be easy both physically and psychologically. Higher Fmax may result in the wear and tear of an autoinjector over a period of time thereby affecting its longevity. Concomitantly, the DGF should be minimal and consistent over the course of injection. A higher DGF would impede the process of injection and increase the pain during injection. If there is a spike or decrease in the DGF, the in-situ depot formulation may also be affected thus rendering unpredictable release of the drug. In our case, it was observed that as the inner diameter of the syringe decreased, the glide force increased.
SelfExRem is in a liquid state at 4 °C and above. Upon injection into an aqueous environment, SelfExRem showed a fast sol–gel transition and formed a soft gel depot. This gel depot constantly released RDV at a steady release rate for 48 h with an initial burst release of ~36%. The relatively high initial burst release of RDV from the gel depot can be directly linked to the fast sol–gel transition of the formulation. The quick gel formation process was driven by the rapid transfer of the water miscible organic solvent NMP from the solution formulation into the aqueous environment with a simultaneous rapid phase separation of PLGA polymer (Ahmed et al., 2016, Lee and Pokorski, 2018). This is followed by formation of large pores leading to the formation of water accessible channels on the surface and inner core. Therefore, we hypothesize that RDV present on the surface and in the water accessible channels quickly diffused out from the in-situ depot system. Since in situ gel formulations provide no physical barrier for drug release until the solvent (NMP) is removed from the gel, they are not really designed to prevent initial burst release. However, a higher burst release is favorable in this scenario as the dose of RDV (IV) is 200 mg at day 1 followed by 100 mg on subsequent days. We speculate that the initial burst release might help to achieve the immediate plasma concentration of RDV, which is then followed by a sustained release that maintains a constant plasma RDV level. Collectively, it may help in reducing the dosing frequency from 5 to 8 doses (intravenous remdesivir) to 2–3 doses (extended release subcutaneous injection). Finally, another advantage of the in-situ depot is that the manufacturing process is less complex relative to solid implants and microparticles.
The formulations with a lower L/G ratio (Resomer 502H) exhibited faster drug release than those with a higher L/G ratio (Resomer 752H). This can be attributed to a slower hydrolytic degradation rate of PLGA 752H due to a higher content of lactide units. Siegel et al. mentioned that the glycolic acid units were shown to have hydrolyzed ~1.3 times faster than lactic acid units, regardless of the temperature, morphology, or composition. It is well established that increasing the glycolic acid content of PLGA polymers results in a higher hydrolysis rate, mainly because the higher hydrophilicity of glycolic repeat units results in a greater degree of water uptake during hydrolysis (Keles et al., 2015, Vey et al., 2011). Hence, as seen in the in vitro release study, after 4 h, the release from 502H was around 10% higher compared to that from 752H at a concentration of 15%. Along with the above-mentioned reasons, the PLGA ester bonds are less stable than the bonds with LA, and thus a higher content of glycolic acid facilitates water uptake and increases the rate of degradation of the polymer. Thus, the higher glycolic acid content might have been the reason why drug release from batches prepared using 502H showed faster release. Thus, considering the higher drug loading achieved with 15% 752H and comparatively smaller burst release, batch 2 was chosen as the optimized formulation. The in vivo release profile may differ slightly/moderately from the in vitro release profile due to differences in microenvironment, volume of release medium, agitation speed and, most importantly, degradation rate of the PLGA matrix. The amount of time required to inject such a formulation is also very important for patient compliance. Many reports have suggested that the ideal time required for injection should be approximately 30 s in a simulated use setting. Noteworthily, 1 mL of the optimized SelfExRem (batch 2) was injected in less than 12 s using a 25G needle. We would not recommend higher gauge needles for injection of SelfExRem.
Reduction in RDV content in the batches stored at 25 °C and 40 °C after 31 days, clearly indicated that RDV in the PLGA-NMP system undergoes thermal degradation. Also, PLGA matrix itself is not stable at such high temperatures. Keles et al. have reported higher degradation rates for both lactic and glycolic units at higher temperature (70 °C) (Keles et al., 2015). Thus, based on our accelerated stability data, we recommend storing SelfExRem at 4–8 °C. Also, since RDV is not found to be photosensitive (data not shown), the final formulation can be used with any glass/plastic syringe. Currently, we are evaluating the long term stability of SelfExRem and planning to perform a pharmacokinetic study in an in vivo model.
6. Conclusion
RDV loaded in-situ depot forming injection was successfully developed using FDA approved ingredients – a biodegradable low molecular weight PLGA (752H) and a biocompatible solvent (NMP). Optimized SelfExRem (15% PLGA) showed controlled release of RDV for 48 h, was easy to inject manually or by auto-injector using a 25G needle and showed no sign of chemical or physical degradation when stored in a refrigerator. SelfExRem with self-injectability and extended release properties could greatly enhance our therapeutic arsenal against COVID-19. It could significantly improve the availability of RDV for patients at an early stage of infection and for patients who have limited access to a hospital or clinical facility.
CRediT authorship contribution statement
Manali Patki: Conceptualization, Methodology, Software, Formal analysis, Investigation, Data curation, Writing - original draft, Writing - review & editing. Siddhant Palekar: Conceptualization, Methodology, Software, Formal analysis, Investigation, Data curation, Writing - original draft, Writing - review & editing. Sandra Reznik: Resources, Conceptualization, Writing - review & editing. Ketan Patel: Conceptualization, Methodology, Validation, Resources, Writing - review & editing, Visualization, Supervision, Project administration.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Patel, Reznik, Patki and Palekar acknowledge department of Pharmaceutical Sciences, St. John’s University, NY for funding the project. Authors are co-inventors on related intellectual property (US Patent pending).
References
- Ahmed T.A., Alharby Y.A., El-Helw A.R., Hosny K.M., El-Say K.M. Depot injectable atorvastatin biodegradable in situ gel: development, optimization, in vitro, and in vivo evaluation. Drug Des. Devel. Ther. 2016;10:405–415. doi: 10.2147/DDDT.S98078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed T.A., Ibrahim H.M., Samy A.M., Kaseem A., Nutan M.T., Hussain M.D. Biodegradable injectable in situ implants and microparticles for sustained release of montelukast: in vitro release, pharmacokinetics, and stability. AAPS PharmSciTech. 2014;15:772–780. doi: 10.1208/s12249-014-0101-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amirian E.S., Levy J.K. Current knowledge about the antivirals remdesivir (GS-5734) and GS-441524 as therapeutic options for coronaviruses. One Health. 2020;9 doi: 10.1016/j.onehlt.2020.100128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babos G., Biro E., Meiczinger M., Feczko T. Dual drug delivery of sorafenib and doxorubicin from PLGA and PEG-PLGA polymeric nanoparticles. Polymers (Basel) 2018;10 doi: 10.3390/polym10080895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauner B., Schuster C., Wirth M., Gabor F. Trimethoprim-loaded microspheres prepared from low-molecular-weight PLGA as a potential drug delivery system for the treatment of urinary tract infections. ACS Omega. 2020;5:9013–9022. doi: 10.1021/acsomega.0c00981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cilurzo F., Selmin F., Minghetti P., Adami M., Bertoni E., Lauria S., Montanari L. Injectability evaluation: an open issue. AAPS PharmSciTech. 2011;12:604–609. doi: 10.1208/s12249-011-9625-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile P., Chiono V., Carmagnola I., Hatton P.V. An overview of poly (lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014;15:3640–3659. doi: 10.3390/ijms15033640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keles H., Naylor A., Clegg F., Sammon C. Investigation of factors influencing the hydrolytic degradation of single PLGA microparticles. Polym. Degrad. Stab. 2015;119:228–241. [Google Scholar]
- Lee P.W., Pokorski J.K. Poly(lactic-co-glycolic acid) devices: production and applications for sustained protein delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018 doi: 10.1002/wnan.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makadia H.K., Siegel S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers. 2011;3:1377–1397. doi: 10.3390/polym3031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurtry, C.M., Noel, M., Taddio, A., Antony, M.M., Asmundson, G.J., Riddell, R.P., Chambers, C.T., Shah, V., HelpinKids, Adults, T., 2015. Interventions for individuals with high levels of needle fear: systematic review of randomized controlled trials and quasi-randomized controlled trials. Clin. J. Pain 31, S109–123. [DOI] [PMC free article] [PubMed]
- Park K., Skidmore S., Hadar J., Garner J., Park H., Otte A., Soh B.K., Yoon G., Yu D., Yun Y., Lee B.K., Jiang X., Wang Y. Injectable, long-acting PLGA formulations: analyzing PLGA and understanding microparticle formation. J. Control. Release. 2019;304:125–134. doi: 10.1016/j.jconrel.2019.05.003. [DOI] [PubMed] [Google Scholar]
- Patel R.B., Carlson A.N., Solorio L., Exner A.A. Characterization of formulation parameters affecting low molecular weight drug release from in situ forming drug delivery systems. J. Biomed. Mater. Res. A. 2010;94:476–484. doi: 10.1002/jbm.a.32724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrosillo N., Viceconte G., Ergonul O., Ippolito G., Petersen E. COVID-19, SARS and MERS: are they closely related? Clin. Microbiol. Infect. 2020;26:729–734. doi: 10.1016/j.cmi.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman Z., Zidan A.S., Korang-Yeboah M., Yang Y., Siddiqui A., Shakleya D., Khan M.A., Cruz C., Ashraf M. Effects of excipients and curing process on the abuse deterrent properties of directly compressed tablets. Int. J. Pharm. 2017;517:303–311. doi: 10.1016/j.ijpharm.2016.12.015. [DOI] [PubMed] [Google Scholar]
- Rahnfeld L., Luciani P. Injectable lipid-based depot formulations: where do we stand? Pharmaceutics. 2020;12 doi: 10.3390/pharmaceutics12060567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rungseevijitprapa W., Bodmeier R. Injectability of biodegradable in situ forming microparticle systems (ISM) Eur. J. Pharm. Sci. 2009;36:524–531. doi: 10.1016/j.ejps.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Schwendeman S.P., Shah R.B., Bailey B.A., Schwendeman A.S. Injectable controlled release depots for large molecules. J. Control. Release. 2014;190:240–253. doi: 10.1016/j.jconrel.2014.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A., Tiwari S., Deb M.K., Marty J.L. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): a global pandemic and treatment strategies. Int. J. Antimicrob. Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.106054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddio A., Ipp M., Thivakaran S., Jamal A., Parikh C., Smart S., Sovran J., Stephens D., Katz J. Survey of the prevalence of immunization non-compliance due to needle fears in children and adults. Vaccine. 2012;30:4807–4812. doi: 10.1016/j.vaccine.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Travanty M.N., Calawa B., Shalaby W.S., Jozwiakowski M.J., Haraldsen K.B. Development and usability of a new subcutaneous auto-injector device to administer hydroxyprogesterone caproate to reduce the risk of recurrent preterm birth. Med. Devices (Auckl) 2018;11:241–252. doi: 10.2147/MDER.S157114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USFDA, 2020. Fact sheet for health care providers emergency use authorization (EUA) of Veklury® (remdesivir), FDA fact sheet.
- Vey E., Rodger C., Booth J., Claybourn M., Miller A.F., Saiani A. Degradation kinetics of poly (lactic-co-glycolic) acid block copolymer cast films in phosphate buffer solution as revealed by infrared and Raman spectroscopies. Polym. Degrad. Stab. 2011;96:1882–1889. [Google Scholar]
- Voigt M., Koerber M., Bodmeier R. Improved physical stability and injectability of non-aqueous in situ PLGA microparticle forming emulsions. Int. J. Pharm. 2012;434:251–256. doi: 10.1016/j.ijpharm.2012.05.029. [DOI] [PubMed] [Google Scholar]
- Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (WHO), W.H.O., 2020. Coronavirus disease 2019 (COVID-19) Situation Report – 97.
- Zhang Q., Fassihi M.A., Fassihi R. Delivery Considerations of highly viscous polymeric fluids mimicking concentrated biopharmaceuticals: assessment of injectability via measurement of total work done “W T”. AAPS PharmSciTech. 2018;19:1520–1528. doi: 10.1208/s12249-018-0963-x. [DOI] [PubMed] [Google Scholar]