ABSTRACT
Background
Lutein and zeaxanthin are carotenoids associated with better cognition at older age. To our knowledge, no previous study has evaluated their cognitive implications in the prenatal period, when the brain undergoes its most rapid development.
Objective
The objective of this study was to examine associations of maternal lutein and zeaxanthin (L/Z) intake during pregnancy with child cognition.
Design
Among 1580 mother-child pairs in Project Viva, a prospective cohort, we assessed maternal intake of L/Z during pregnancy using food frequency questionnaires and offspring cognition by the Visual Recognition Memory paradigm in infancy, the Peabody Picture Vocabulary Test and the Wide Range Assessment of Visual Motor Abilities (WRAVMA) in early childhood, and the Kaufman Brief Intelligence Test (KBIT-II), the WRAVMA drawing subtest, and the Wide Range Assessment of Memory and Learning in mid-childhood. Parents completed the Behavior Rating Inventory of Executive Function (BRIEF) and Strengths and Difficulties Questionnaire.
Results
Mothers consumed a daily mean (SD) of 2.6 (2.0) mg L/Z in the first and second trimesters of pregnancy. Mean mid-childhood KBIT-II verbal scores were higher with greater maternal L/Z intake [difference of Q4–Q1 means for first trimester: 2.67 (95% CI: 0.13, 5.20) and for second trimester: 3.55 (95% CI: 0.81, 6.28)], indicating better verbal intelligence. Secondary analyses on cognitive subtests showed that mean mid-childhood BRIEF Behavioral Regulation Index scores were lower with greater maternal L/Z intake [difference of Q4–Q1 means for first trimester: –1.63 (95% CI: –3.22, –0.04) and for second trimester: –1.89 (95% CI: –3.58, –0.21)], indicating better behavior regulation ability.
Conclusions
Higher maternal L/Z intake during pregnancy was associated with better offspring verbal intelligence and behavior regulation ability in mid-childhood, suggesting a potential benefit during prenatal development. We did not find a benefit of higher maternal L/Z intake on other child cognitive or behavioral outcomes. Project Viva is registered at clinicaltrials.gov as NCT02820402.
Keywords: birth cohort, lutein and zeaxanthin, maternal diet during pregnancy, early life nutrition, prenatal nutrition, childhood cognition, cognitive development, early development, programming
Introduction
Current evidence supports a role for lutein and its isomer zeaxanthin in cognitive function in older adults (1, 2), and emerging evidence also suggests a role in early neurodevelopment (3, 4). Although not the major dietary contributors to carotenoid intake, lutein and zeaxanthin are the dominant carotenoids in adult central nervous system tissues; they are the only 2 carotenoids that cross the blood-retina barrier to form the macular pigment in the eye (5) and preferentially accumulate in the human brain (6). A possible role in brain development is especially compelling because lutein was found to also preferentially accumulate in infant brain in comparison to other carotenoids that are predominant in the diet, and the relative contribution of lutein to total carotenoids in infant brains is twice that in older adults, accounting for more than half the concentration of total carotenoids (4, 6).
During pregnancy, lutein is transferred to the fetus via the placenta, and cord blood concentrations are correlated with maternal plasma concentrations, which in turn vary with maternal dietary intake (7–9). In a US study of 82 mother-infant pairs, lutein and zeaxanthin were the most prevalent carotenoids in the placenta and umbilical cord blood, despite not being the major carotenoids in maternal serum or diet (10). In that study, lutein and zeaxanthin had the highest maternal to fetal transfer rate among carotenoids (16%), underlining the distinctive roles they may play during gestation (10). In addition, lutein and zeaxanthin are actively transported into breast milk, and lutein is the predominant carotenoid in mature breast milk (11).
Despite the apparent importance of lutein and zeaxanthin in neurodevelopment, we are not aware of any previous study that directly evaluated the association between maternal dietary intake and cognitive function in the offspring. Recent studies demonstrated that child macular pigment optical density (MPOD), a biomarker of lutein and zeaxanthin concentrations in the brain (12), was associated with child cognitive function (13–16), and maternal serum zeaxanthin concentrations were correlated with infant MPOD (17). A potential association between maternal lutein and zeaxanthin intake and offspring cognitive function is also supported by evidence from adults showing that higher lutein and zeaxanthin status is related to better cognitive function (6, 18–20) and lutein and/or zeaxanthin supplementation improve cognitive performance (21–23).
Given the gaps in our current understanding of the role of maternal lutein and zeaxanthin intake in neurodevelopment in early life, the primary aim of this study was to examine the hypothesis that higher maternal dietary intake of these carotenoids during pregnancy is associated with better measures of child cognition in infancy, early childhood, and mid-childhood, and with better measures of behavior and social-emotional development in mid-childhood. The secondary aim was to examine associations of maternal intake of the main food sources of lutein and zeaxanthin during pregnancy with the same child cognitive and behavioral outcomes.
Subjects and Methods
Subjects
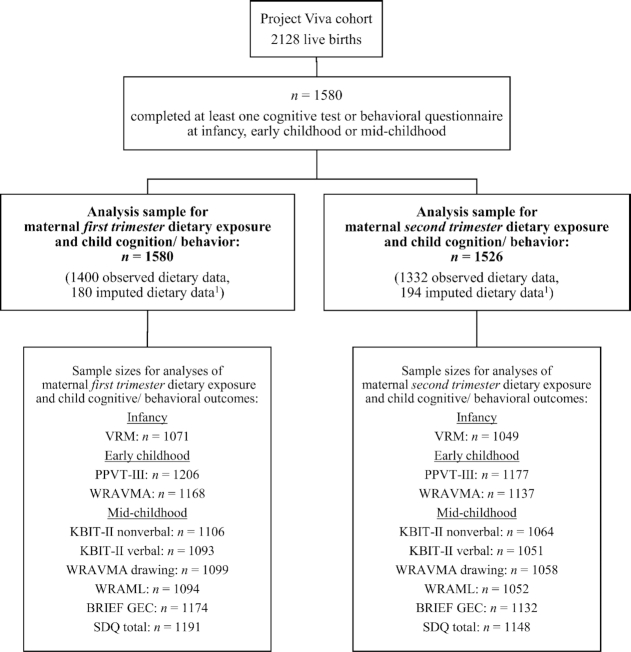
We studied mother-child pairs from Project Viva, a prospective cohort study investigating pre- and perinatal factors in relation to child health outcomes (24). Pregnant women were recruited in 1999–2002 at their first prenatal care visit from 8 obstetric offices of a multispecialty group practice in eastern Massachusetts. Exclusion criteria included multiple gestation, inability to answer questions in English, gestational age ≥22 wk at the time of the initial prenatal visit, and plans to move out of the area before delivery. Women who agreed to participate in the study (65% of those eligible) completed the first study visit after their obstetric appointment. The first visit was completed in the first trimester, and the second visit was completed in the second trimester. The institutional review boards of participating institutions authorized the study protocols, and pregnant women provided written informed consent. The Project Viva cohort consists of 2128 liveborn singleton infants and their mothers. For this analysis, we included only mother-child pairs in which children completed at least 1 cognitive or behavioral assessment at infancy, early or mid-childhood, and mothers completed a study visit (i.e., were eligible for a diet assessment) in the first (n = 1580) and/or second trimester (n = 1526) of pregnancy.
Measurements
Primary exposure: Maternal intake of L/Z during pregnancy
We derived maternal dietary intake from self-administered semiquantitative food frequency questionnaires (FFQ) that mothers completed during the early and mid-pregnancy visits. Current nutrient databases report lutein and zeaxanthin content of foods together; therefore, we assessed dietary intake of lutein and zeaxanthin combined as 1 exposure, and refer to them as L/Z for the remainder of the article.
The 166-item FFQ used in Project Viva was derived from one previously validated against multiple diet records and biomarkers in other cohorts of nonpregnant women and men, and was modified for use in pregnancy (25, 26). This questionnaire was calibrated in pregnant women by comparing dietary intake values obtained using the FFQ against blood concentrations of several nutrients and food components, including individual carotenoids (27). The first FFQ was administered at enrollment (median 9.9 wk of gestation), and the reference period was time since the last menstrual period to reflect intakes during the first trimester of pregnancy. The second FFQ was administered at 26 to 28 wk of gestation (median 27.9 wk of gestation), and the reference period was the previous 3 mo, roughly reflecting intake during the second trimester.
L/Z content of each food was derived from the Harvard nutrient composition database, which is based on USDA publications and is supplemented by additional published sources and communications with laboratories and manufacturers (28). We adjusted dietary estimates for total energy intake using the nutrient residual method to provide an estimate of the associations independent of energy intake and to reduce the impact of measurement error (29).
Secondary exposure: Maternal intake of L/Z-rich foods during pregnancy
We defined the L/Z-rich foods exposure as the sum of servings per day of the top 10 L/Z food contributors in the Project Viva cohort (cooked spinach, raw spinach, romaine or leaf lettuce, kale, broccoli, peas or lima beans, orange juice, corn, eggplant or zucchini, mixed vegetables), plus any food with L/Z content ≥1 mg per 100 g (Brussels sprouts, dark squash, popcorn, whole eggs).
Different parts and functions of the brain develop at different times during pregnancy (30, 31), and the mechanisms and timing of action of lutein and zeaxanthin in the brain are still not understood. Therefore, we examined all associations with maternal dietary intake in the first and second trimesters separately.
Outcomes: Child cognition
Child cognition was assessed using different standardized tests at each of the infancy, early childhood, and mid-childhood visits.
At the infancy study visit (median age 6.4 mo, range 5.2–10.0), cognitive testing was performed using the visual recognition memory (VRM) paradigm. Tests were performed at the child's home or in a research clinic. The infant was placed on the mother's lap, and trained test administrators presented the infant with 2 identical pictures of a face or a geometric design at a distance of 18 in. in front of the infant (32). The familiarization trial lasts until the infant has accumulated either 10 or 20 s of visual fixation time, depending on the type of picture. In the test trial, the infant is again simultaneously presented with 2 photos, the previously seen photo and a novel photo. Test trials last a total of 5 s beginning with the first fixation. Using a laptop computer, administrators tracked the amount of time that the infant looked at each stimulus. Then, a novelty preference score is calculated as the percentage of the total test time that the infant spent looking at the novel stimulus rather than the familiar stimulus. The VRM paradigm consists of 9 sets of trials. Each set includes 1 familiarization trial and 2 test trials, and the final score is determined as the mean of the 2 trials. This test reflects the infant's ability to encode a stimulus into memory, to recognize that stimulus, and to look preferentially at a novel stimulus; higher percent novelty preference scores indicate better visual recognition memory. Infant recognition memory is predictive of later measures of cognitive abilities (including IQ and language) as well as recognition memory in later childhood (33–36).
At the early childhood study visit (median age 3.2 y, range 2.8–6.2 y), researchers administered the Peabody Picture Vocabulary Test, Third Edition (PPVT-III), a test of receptive language correlated with intelligence tests (37), and the Wide Range Assessment of Visual Motor Abilities (WRAVMA), including the pegboard, matching, and drawing subtests, to assess fine-motor, visual-spatial, and visual-motor abilities, respectively (38). WRAVMA subtest scores were combined to generate a visual motor total composite score. The PPVT-III and WRAVMA are each scaled to a mean (SD) score of 100 (15).
At the mid-childhood study visit (median age 7.7 y, range 6.6–10.9 y), researchers administered the Kaufman Brief Intelligence Test, Second Edition (KBIT-II) to assess verbal and nonverbal global intelligence and the WRAVMA drawing subtest, a measure of visual-motor integration (39). The KBIT-II and WRAVMA are each scaled to a mean (SD) score of 100 (15). In addition, memory and learning were assessed with the Wide Range Assessment of Memory and Learning (WRAML) design memory and picture memory subtests (40). The 2 WRAML subtests are scaled to a mean (SD) of 10 (3) and were summed to yield a total visual memory score. For all cognitive tests, the administrators noted their confidence on the extent to which the test was conducted without distractions or other concerns that might influence the results, and we excluded results for which the administrators did not have confidence in the test performance (<1%).
At the mid-childhood study visit, parents also completed 2 behavioral questionnaires regarding their children, the Behavioral Rating Inventory of Executive Function (BRIEF) and the Strengths and Difficulties Questionnaire (SDQ). The BRIEF is a validated 86-item questionnaire designed to assess executive function behaviors in home environments (41, 42) and includes the following subscales: inhibit, shift, emotional control, initiate, working memory, plan/organize, organization of materials, and monitor. The subscales form 2 indices: the Behavioral Regulation Index (BRIEF BRI), which reflects the ability of the child to shift cognitive set and modulate emotions and behavior via appropriate inhibitory control, and the Metacognition Index, which indicates the child's ability to initiate, plan, organize, and sustain future-oriented problem-solving in working memory. The BRIEF indices are each scaled to a mean (SD) of 50 (10). The Global Executive Composite (BRIEF GEC) combines the 2 indices and represents a summary measure of executive function. Higher BRIEF scores indicate worse executive function.
The SDQ is a validated 23-item questionnaire designed to assess social, emotional, and behavioral functioning (43). The SDQ is used extensively in clinical and research settings (44) and includes 5 subscales (prosocial behavior, hyperactivity/inattention, emotional symptoms, conduct problems, and peer relationship problems). Possible scores range from 0 to 40 points. Higher scores represent greater difficulties on all except the prosocial subscale, on which a higher score is more favorable. Normative data for the SDQ derive from a representative sample of US children (45).
Covariates
Using a combination of questionnaires and interviews, Project Viva collected information on maternal age, race/ethnicity, education, household income, marital status, prepregnancy weight and height, parity, smoking history, and breastfeeding duration (24). Data on maternal dietary intake were obtained from the FFQs administered at the early and mid-pregnancy visits. The child's sex, birthweight, and date of birth were obtained from hospital medical records. We calculated gestational age from the date of the last menstrual period or from the second trimester ultrasound. We calculated sex-specific birthweight-for-gestational-age z score using a US national reference (46). Maternal intelligence was evaluated using the PPVT-III at the early childhood visit and the KBIT-II at the mid-childhood visit. We used the Home Observation Measurement of the Environment–Short Form (HOME-SF), completed by mothers at the mid-childhood visit, to assess the child's home environment for cognitive stimulation and emotional support (47).
Statistical analysis
We used SAS software, version 9.4 (SAS Institute) for all analyses. All cognitive and behavioral outcomes were age and sex standardized except for the SDQ, and all were analyzed as continuous variables. We saw evidence of a nonlinear relation for some exposure-outcome associations; therefore, we modeled maternal intake of L/Z and L/Z-rich foods categorized into quartiles to allow a common analytical approach for all associations. We analyzed data using 3 sequential multivariable linear regression models for each cognitive and behavioral outcome: model 0 adjusted for child age and sex, model 1 additionally adjusted for selected maternal sociodemographic characteristics, and model 2 additionally adjusted for trimester-specific maternal dietary factors. We selected covariates that we considered a priori to be confounders and/or that were associated with maternal dietary intake of L/Z and at least 1 child cognitive or behavioral outcome in binary analyses. The final multivariable model (model 2) adjusted for child sex and age, maternal age, race/ethnicity, marital status, parity, education, income, prepregnancy BMI, smoking history, and trimester-specific intake of total energy, DHA, folate, choline, vitamin B-12, and alcohol. We present results from the 3 models to illustrate the extent to which addition of covariates changes effect estimates.
Secondary analyses
Because of the number of cognitive tests, many of which were based on subtests, our primary analyses included only the composite test scores. We performed secondary analyses on the subtests and present results in the supplemental material. As part of our secondary analyses, we also adjusted for maternal cognition. Although maternal cognition was assessed temporally after the maternal exposure, at the early and mid-childhood visits, it reflects a stable construct and likely represents maternal cognition before/during pregnancy and can act as a confounder. But much of the variance in maternal cognition may be captured by maternal education and household income, which we already adjusted for in model 2. In addition, since previous evidence suggests that dietary intake of L/Z is associated with cognitive health (3, 22, 48), we were concerned that maternal intake would simultaneously affect maternal and child cognition.
As part of our secondary analyses, for the mid-childhood cognitive outcomes, we also adjusted for the child's home environment assessed by the HOME-SF score. The home environment is a possible confounder in the maternal diet and child cognition associations, but it was only assessed at the mid-childhood visit (median age 7.7 y), which may not accurately reflect the home environment several years earlier during pregnancy.
We also considered adjustment for breastfeeding status but ultimately decided not to include this variable in our main models because it may be in the causal pathway between maternal diet and child cognition and might act as a mediator. We mention findings of these additional secondary models in the Results section if their effect estimates were meaningfully different from those from model 2.
Imputation of missing data
We used multiple imputation (MI) methods to impute missing data. We generated 50 imputed data sets using chained imputation and combined estimates using Rubin's rules (49, 50). All 2128 participants were used in generating the imputed dataset. Once the MI data set was created, we determined eligibility for analysis, as shown in Figure 1. We did not use imputed values for missing child outcome data; therefore, our first eligibility criterion was that the child had completed at least 1 cognitive or behavioral assessment at any of the infancy, early childhood, or mid-childhood visits (n = 1580) (Figure 1). Missing data resulted from loss to follow-up or refusal or inability to complete all visits or the cognitive component of a particular visit. We used imputed values for missing maternal dietary data if the mother was eligible to take the dietary assessment (i.e., attended the study visit). For the first-trimester analyses, we did not further limit the 1580 sample based on the availability of maternal dietary data, as all mothers attended the early pregnancy visit (i.e., we used imputed values for all missing first-trimester dietary exposures). For the second-trimester analyses, we used imputed values for missing second-trimester dietary exposures only for mothers who attended the mid-pregnancy visit; therefore, the 1580 sample was further limited to 1526 for the second-trimester analyses. We used imputed values for all missing covariate data in the analysis sample. Because we did not use imputed values for missing child outcome data, sample sizes for each association varied depending on the child cognitive/behavioral assessment and the trimester of maternal exposure. All analyses were performed using both original and imputed data, and results were similar. Therefore, we present results only from the imputed analyses throughout the article.
FIGURE 1.
Flow diagram for inclusion in study population. 1Exposure data were imputed for participants who had missing data but were eligible to complete the measure (i.e., completed the study visit but did not complete an FFQ).
Results
Participant characteristics
Characteristics of the included 1580 mother-child pairs are presented in Table 1. Women in our eligible sample consumed a mean (SD) 2.6 (2.0) mg/d of L/Z in both the first and second trimesters of pregnancy. Mean (SD) L/Z-rich foods intake was 1.8 (1.2) and 1.9 (1.2) servings per day in the first and second trimesters, respectively. Women in our sample were predominantly white (69%), were married or living with a partner (92%), were college-educated (68%), had a normal prepregnancy BMI (60%), and never smoked (69%).
TABLE 1.
Selected characteristics of included Project Viva mother-child pairs according to maternal intake of lutein and zeaxanthin (L/Z) during pregnancy (N = 1580)1
| Quartiles of first-trimester L/Z intake | Quartiles of second-trimester L/Z intake | ||||
|---|---|---|---|---|---|
| Characteristic | Overall | Quartile 1 | Quartile 4 | Quartile 1 | Quartile 4 |
| Maternal characteristics | |||||
| Age at enrollment (y) | 32.1 (5.2) | 30.7 (6.0) | 32.9 (5.2) | 30.9 (5.7) | 33.1 (4.9) |
| Race/ethnicity | |||||
| White | 1087 [68.8] | 255 [64.5] | 263 [66.9] | 259 [67.8] | 256 [67.2] |
| Black | 238 [15.1] | 61 [15.5] | 60 [15.2] | 53 [13.9] | 55 [14.5] |
| Asian | 83 [5.3] | 12 [3.0] | 33 [8.4] | 14 [3.7] | 41 [10.8] |
| Hispanic | 106 [6.7] | 47 [11.9] | 21 [5.2] | 39 [10.3] | 14 [3.6] |
| Other | 66 [4.2] | 20 [5.1] | 17 [4.3] | 16 [4.3] | 15 [3.8] |
| Education | |||||
| Less than college degree | 506 [32.0] | 186 [47.1] | 85 [21.5] | 175 [46.0] | 71 [18.6] |
| 4-y college or more | 1074 [68.0] | 209 [52.9] | 309 [78.5] | 206 [54.0] | 310 [81.4] |
| Annual household income | |||||
| $70,000 or less | 641 [40.6] | 208 [52.6] | 138 [35.1] | 202 [53.1] | 133 [35.0] |
| >$70,000 | 939 [59.4] | 187 [47.4] | 256 [64.9] | 179 [46.9] | 247 [65.0] |
| Married or cohabitating | |||||
| Yes | 1455 [92.1] | 352 [89.2] | 370 [93.9] | 340 [89.0] | 363 [95.3] |
| No | 125 [7.9] | 43 [10.8] | 24 [6.1] | 42 [11.0] | 18 [4.7] |
| Prepregnancy BMI (kg/m2) | |||||
| <18.5 (underweight) | 49 [3.1] | 13 [3.4] | 10 [2.5] | 10 [2.7] | 16 [4.3] |
| 18.5–24.9 (normal) | 952 [60.2] | 211 [53.5] | 261 [66.1] | 200 [52.5] | 251 [66.1] |
| 25.0–29.9 (overweight) | 345 [21.8] | 98 [24.7] | 73 [18.5] | 104 [27.1] | 69 [18.1] |
| ≥30 (obese) | 234 [14.8] | 73 [18.4] | 51 [12.9] | 67 [17.7] | 44 [11.5] |
| Parity | |||||
| Nulliparous | 755 [47.8] | 181 [45.8] | 208 [52.8] | 168 [44.0] | 209 [55.0] |
| Parity 1 or more | 825 [52.2] | 214 [54.2] | 186 [47.2] | 214 [56.0] | 171 [45.0] |
| Smoking status | |||||
| Never | 1089 [68.9] | 260 [65.9] | 277 [70.2] | 248 [65.0] | 265 [69.7] |
| Former | 313 [19.8] | 67 [17.0] | 93 [23.6] | 67 [17.6] | 86 [22.6] |
| During pregnancy | 178 [11.3] | 68 [17.1] | 25 [6.2] | 66 [17.4] | 30 [7.8] |
| First-trimester dietary intake | |||||
| L/Z (mg/d) | 2.6 (2.0) | 1.0 (0.4) | 5.0 (2.0) | ||
| L/Z-rich foods (servings/d) | 1.8 (1.2) | 1.2 (0.8) | 2.7 (1.4) | ||
| Total fruit and vegetables (servings/d) | 5.9 (3.2) | 4.2 (2.4) | 7.8 (3.8) | ||
| Total energy (kcal/d) | 2080 (684) | 2100 (741) | 2090 (776) | ||
| DHA (mg/d) | 116 (128) | 90.0 (148) | 148(137) | ||
| Folate (μg/d) | 928 (429) | 784 (387) | 1040 (456) | ||
| Choline (mg/d) | 331 (66.8) | 309 (69.2) | 355 (68.5) | ||
| Vitamin B-12 (μg/d) | 10.7 (17.5) | 9.9 (7.9) | 10.9 (11.9) | ||
| Alcohol (servings/d) | 0.2 (0.4) | 0.2 (0.2) | 0.2 (0.2) | ||
| Second-trimester dietary intake | |||||
| L/Z (mg/d) | 2.6 (2.0) | 1.0 (0.4) | 5.1 (2.1) | ||
| L/Z-rich foods (servings/d) | 1.9 (1.2) | 1.2 (0.8) | 2.7 (1.2) | ||
| Total fruit and vegetables (servings/d) | 5.9 (3.1) | 4.2 (2.3) | 7.8 (3.3) | ||
| Total energy (kcal/d) | 2160 (683) | 2190 (733) | 2170 (703) | ||
| DHA (mg/d) | 104 (90.2) | 81.9 (81.9) | 128 (107) | ||
| Folate (μg/d) | 1260 (406) | 1120 (453) | 1370 (400) | ||
| Choline (mg/d) | 324 (69.5) | 300 (69.0) | 352 (74.0) | ||
| Vitamin B-12 (μg/d) | 10.6 (6.6) | 10.0 (4.5) | 11.2 (7.8) | ||
| Alcohol (servings/d) | 0.02 (0.0) | 0.02 (0.0) | 0.02(0.0 | ||
| Child characteristics | |||||
| Child sex | |||||
| Male | 810 [51] | 216 [54.6] | 192 [48.8] | 214 [56.0] | 179 [46.9] |
| Female | 770 [49] | 179 [45.4] | 202 [51.2] | 168 [44.0] | 202 [53.1] |
| Gestational age at birth (wk) | 39.5 (2.0) | 39.4 (2.0) | 39.6(1.8) | 39.5 (1.8) | 39.5 (1.8) |
| Birthweight-for-gestational-age z score | 0.2 (0.8) | 0.2 (1.0) | 0.07 (1.0) | 0.1 (1.0) | 0.1 (1.0) |
| Breastfeeding status at 6 mo | |||||
| Formula only, never breastfed | 172 [10.9] | 72 [18.3] | 21 [5.3] | 67 [17.5] | 16 [4.2] |
| Weaned | 592 [37.4] | 167 [42.3] | 126 [31.9] | 160 [41.8] | 131 [34.5] |
| Mixed | 400 [25.3] | 79 [19.9] | 113 [28.6] | 82 [21.6] | 109 [28.6] |
| Breast milk only, no formula | 417 [26.4] | 77 [19.5] | 135 [34.2] | 73 [19.0] | 125 [32.7] |
| Cognitive outcomes in infancy2 | |||||
| VRM (% novelty preference) | 63.8 (16.3) | 65.3 (16.8) | 62.2 (16.7) | 64.6 (17.5) | 64.1 (16.0) |
| Cognitive outcomes in early childhood3 | |||||
| PPVT-III | 104 (14.4) | 101 (15.1) | 105 (13.9) | 101 (14.1) | 105 (14.2) |
| WRAVMA total | 102 (11.3) | 102 (11.1) | 102 (11.8) | 102 (11.0) | 103 (11.4) |
| Cognitive/behavioral outcomes in mid-childhood4 | |||||
| KBIT-II verbal | 112 (15.0) | 108 (15.2) | 114 (14.8) | 108 (15.8) | 115 (14.1) |
| KBIT-II nonverbal | 106 (16.8) | 105 (15.8) | 109 (17.0) | 104 (16.4) | 108 (17.1) |
| WRAVMA drawing | 92.1 (16.7) | 92.6 (17.5) | 91.8 (16.0) | 90.2 (18.0) | 93.3 (16.3) |
| WRAML summary score | 16.9 (4.4) | 16.5 (4.3) | 16.9 (4.5) | 16.6 (4.6) | 16.8 (4.3) |
| BRIEF GEC | 48.7 (9.2) | 49.8 (10.1) | 48.2 (8.7) | 49.5 (9.9) | 47.7 (8.7) |
| SDQ, total difficulties | 6.6 (4.8) | 7.5 (5.4) | 6.1 (4.6) | 7.5 (5.2) | 6.1 (4.5) |
Values are mean (SD) or frequency [%]. Median maternal L/Z intake (range), in mg/d for each quartile: first trimester (n = 1580): Q1 = 0.97 (0.09–1.43), Q4 = 4.50 (3.25–16.3); second trimester (n = 1526): Q1 = 0.98 (0.05–1.43), Q4 = 4.48 (3.19–15.2). Nutrient values were adjusted for total energy intake using the residual model. BRIEF GEC, Behavioral Rating Inventory of Executive Function–Global Executive Composite; KBIT-II, Kaufman Brief Intelligence Test, Second Edition; PPVT-III, Peabody Picture Vocabulary Test, Third Edition; Q, quartile; SDQ, Strengths and Difficulties Questionnaire; VRM, visual recognition memory (percent novelty preference); WRAML, Wide Range Assessment of Memory and Learning, Second Edition; WRAVMA, Wide Range Assessment of Visual Motor Abilities.
Infancy cognitive tests were administered at median 6.4 mo of age.
Early childhood cognitive tests were administered at median 3.2 y of age.
Mid-childhood cognitive tests were administered at median 7.7 y of age.
Mean maternal intake of L/Z and their main food sources during the first and second trimesters were similar between the participants included and excluded from analysis. Compared with the 548 participants not included in our analysis, included mothers were slightly older in age (32 y compared with 31 y, P < 0.001), more likely to be college-educated (68% compared with 55%, P < 0.001), to be white (69% compared with 60%, P < 0.001), to have an annual household income of >$70,000 (59% compared with 54%, P < 0.05), and to have breastfed a longer duration (6 mo compared with 5 mo, P < 0.001). We did not observe differences between included and excluded participants in marital status; prepregnancy BMI; parity; smoking history; maternal intake of total energy, alcohol, DHA, folate, choline, and vitamin B-12; child sex; gestational age at birth; and birthweight-for-gestational-age z score.
Primary exposure: Maternal intake of L/Z during pregnancy
Infancy
Infants with mothers in the highest quartile category of first-trimester L/Z intake had a lower mean VRM score (percent novelty preference) than those with mothers in the lowest quartile category [difference of Q4–Q1 means: –3.22 (95% CI: –6.39, –0.05)] (Table 2), indicating worse visual recognition memory. We observed a similar difference in means between the first and third quartile categories of maternal second-trimester L/Z intake [Q3–Q1 means: –3.35 (95% CI: –6.44, –0.26)].
TABLE 2.
Associations of maternal intake of lutein and zeaxanthin (L/Z) during pregnancy with child cognitive and behavioral outcomes1
| Child outcome Trimester Model2 | Quartiles of maternal L/Z intake | |||
|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |
| (reference) | β (95% CI)3 | β (95% CI) | β (95% CI) | |
| Infancy | ||||
| VRM | ||||
| T1 (n = 1071) | ||||
| Model 0 | 0 | −1.30 (−4.20, 1.59) | −2.78 (−5.64, 0.09) | −3.34 (−6.20, −0.47)4 |
| Model 1 | 0 | −1.40 (−4.32, 1.52) | −2.33 (−5.27, 0.61) | −2.92 (−5.92, 0.08) |
| Model 2 | 0 | −1.49 (−4.44, 1.46) | −2.36 (−5.34, 0.63) | −3.22 (−6.39, −0.05)4 |
| T2 (n = 1049) | ||||
| Model 0 | 0 | −0.95 (−3.99, 2.09) | −2.87 (−5.80, 0.06) | −1.06 (−3.95, 1.83) |
| Model 1 | 0 | −0.41 (−3.49, 2.67) | −2.41 (−5.42, 0.61) | −0.30 (−3.33, 2.74) |
| Model 2 | 0 | −0.87 (−3.96, 2.22) | −3.35 (−6.44, −0.26)4 | −1.34 (−4.54, 1.85) |
| Early childhood | ||||
| PPVT-III | ||||
| T1 (n = 1206) | ||||
| Model 0 | 0 | 3.29 (0.90, 5.68)4 | 3.13 (0.74, 5.52)4 | 3.55 (1.18, 5.92)4 |
| Model 1 | 0 | 1.97 (−0.17, 4.11) | 1.12 (−1.04, 3.28) | 1.13 (−1.03, 3.30) |
| Model 2 | 0 | 1.69 (−0.46, 3.84) | 1.00 (−1.20, 3.19) | 0.91 (−1.38, 3.20) |
| T2 (n = 1177) | ||||
| Model 0 | 0 | 2.71 (0.31, 5.12)4 | 3.88 (1.45, 6.31)4 | 4.40 (2.00, 6.80)4 |
| Model 1 | 0 | 0.90 (−1.28, 3.09) | 1.34 (−0.90, 3.58) | 2.07 (−0.17, 4.31) |
| Model 2 | 0 | 0.90 (−1.31, 3.11) | 1.39 (−0.90, 3.68) | 2.17 (−0.23, 4.57) |
| WRAVMA total | ||||
| T1 (n = 1168) | ||||
| Model 0 | 0 | 0.71 (−1.15, 2.57) | 0.25 (−1.60, 2.10) | 0.10 (−1.73, 1.92) |
| Model 1 | 0 | 0.20 (−1.62, 2.03) | −0.77 (−2.60, 1.07) | −0.94 (−2.78, 0.89) |
| Model 2 | 0 | 0.06 (−1.78, 1.90) | −0.89 (−2.76, 0.99) | −1.14 (−3.07, 0.79) |
| T2 (n = 1137) | ||||
| Model 0 | 0 | 0.07 (−1.85, 1.99) | 0.72 (−1.19, 2.64) | 1.01 (−0.88, 2.89) |
| Model 1 | 0 | −0.42 (−2.32, 1.47) | −0.27 (−2.17, 1.63) | 0.02 (−1.90, 1.94) |
| Model 2 | 0 | −0.35 (−2.26, 1.57) | −0.12 (−2.07, 1.84) | 0.31 (−1.75, 2.37) |
| Mid-childhood | ||||
| KBIT-II nonverbal | ||||
| T1 (n = 1106) | ||||
| Model 0 | 0 | 1.96 (−1.05, 4.97) | 1.95 (−1.09, 4.99) | 4.79 (1.82, 7.76)4 |
| Model 1 | 0 | 1.01 (−1.99, 4.01) | 0.10 (−2.96, 3.16) | 3.09 (0.07, 6.12)4 |
| Model 2 | 0 | 0.86 (−2.16, 3.89) | −0.04 (−3.11, 3.04) | 2.87 (−0.31, 6.05) |
| T2 (n = 1064) | ||||
| Model 0 | 0 | 1.05 (−2.09, 4.19) | 3.75 (0.62, 6.87)4 | 3.38 (0.35, 6.42)4 |
| Model 1 | 0 | −0.20 (−3.32, 2.91) | 1.42 (−1.76, 4.60) | 1.00 (−2.17, 4.17) |
| Model 2 | 0 | −0.26 (−3.44, 2.91) | 1.28 (−1.96, 4.52) | 0.60 (−2.73, 3.94) |
| KBIT-II verbal | ||||
| T1 (n = 1093) | ||||
| Model 0 | 0 | 5.19 (2.47, 7.91)4 | 5.02 (2.33, 7.72)4 | 7.13 (4.50, 9.75)4 |
| Model 1 | 0 | 2.48 (0.08, 4.88)4 | 1.33 (−1.11, 3.76) | 3.23 (0.82, 5.65)4 |
| Model 2 | 0 | 2.21 (−0.21, 4.64) | 1.05 (−1.44, 3.53) | 2.67 (0.13, 5.20)4 |
| T2 (n = 1051) | ||||
| Model 0 | 0 | 3.49 (0.66, 6.32)4 | 5.78 (2.96, 8.61)4 | 7.56 (4.84, 10.27)4 |
| Model 1 | 0 | 1.34 (−1.22, 3.89) | 2.10 (−0.50, 4.71) | 3.70 (1.13, 6.28)4 |
| Model 2 | 0 | 1.30 (−1.30, 3.90) | 1.99 (−0.69, 4.66) | 3.55 (0.81, 6.28)4 |
| WRAVMA drawing | ||||
| T1 (n = 1099) | ||||
| Model 0 | 0 | −0.94 (−3.94, 2.05) | −0.40 (−3.41, 2.61) | −0.76 (−3.66, 2.13) |
| Model 1 | 0 | −1.46 (−4.54, 1.61) | −1.55 (−4.67, 1.58) | −1.87 (−4.93, 1.18) |
| Model 2 | 0 | −1.77 (−4.88, 1.33) | −1.87 (−5.03, 1.29) | −2.49 (−5.71, 0.73) |
| T2 (n = 1058) | ||||
| Model 0 | 0 | 0.45 (−2.63, 3.53) | 3.25 (0.21, 6.28) | 2.37 (−0.59, 5.32) |
| Model 1 | 0 | −0.17 (−3.30, 2.96) | 2.20 (−0.95, 5.35) | 1.31 (−1.86, 4.48) |
| Model 2 | 0 | 0.03 (−3.15, 3.21) | 2.38 (−0.85, 5.61) | 1.44 (−1.91, 4.79) |
| WRAML | ||||
| T1 (n = 1094) | ||||
| Model 0 | 0 | 0.83 (0.06, 1.61)4 | 0.72 (−0.07, 1.50) | 0.59 (−0.18, 1.36) |
| Model 1 | 0 | 0.75 (−0.04, 1.54) | 0.53 (−0.28, 1.33) | 0.40 (−0.41, 1.21) |
| Model 2 | 0 | 0.61 (−0.19, 1.41) | 0.40 (−0.42, 1.23) | 0.13 (−0.73, 0.98) |
| T2 (n = 1052) | ||||
| Model 0 | 0 | 0.33 (−0.49, 1.15) | 0.67 (−0.14, 1.49) | 0.08 (−0.72, 0.87) |
| Model 1 | 0 | 0.10 (−0.73, 0.93) | 0.39 (−0.45, 1.24) | −0.29 (−1.14, 0.56) |
| Model 2 | 0 | 0.14 (−0.71, 0.99) | 0.44 (−0.43, 1.31) | −0.27 (−1.18, 0.63) |
| BRIEF GEC | ||||
| T1 (n = 1174) | ||||
| Model 0 | 0 | −0.68 (−2.23, 0.86) | −1.99 (−3.55, −0.44)4 | −1.82 (−3.35, −0.29)4 |
| Model 1 | 0 | −0.46 (−2.01, 1.09) | −1.54 (−3.12, 0.03) | −1.52 (−3.09, 0.06) |
| Model 2 | 0 | −0.45 (−1.99, 1.10) | −1.42 (−3.01, 0.16) | −1.47 (−3.11, 0.17) |
| T2 (n = 1132) | ||||
| Model 0 | 0 | −0.59 (−2.23, 1.05) | −1.17 (−2.80, 0.46) | −1.92 (−3.50, −0.34)4 |
| Model 1 | 0 | −0.30 (−1.96, 1.36) | −0.69 (−2.36, 0.99) | −1.81 (−3.46, −0.15)4 |
| Model 2 | 0 | −0.07 (−1.72, 1.58) | −0.30 (−2.00, 1.40) | −1.35 (−3.08, 0.38) |
| SDQ total | ||||
| T1 (n = 1191) | ||||
| Model 0 | 0 | −0.71 (−1.54, 0.12) | −1.41 (−2.22, −0,59)4 | −1.33 (−2.13, −0.53)4 |
| Model 1 | 0 | −0.36 (−1.17, 0.45) | −0.84 (−1.65, −0.03) | −0.81 (−1.61, −0.01)4 |
| Model 2 | 0 | −0.31 (−1.12, 0.50) | −0.81 (−1.63, 0.00) | −0.75 (−1.59, 0.09) |
| T2 (n = 1148) | ||||
| Model 0 | 0 | −0.92 (−1.76, −0.08)4 | −1.33 (−2.17, −0.49)4 | −1.32 (−2.13, −0.50)4 |
| Model 1 | 0 | −0.51 (−1.34, 0.32) | −0.69 (−1.54, 0.16) | −0.78 (−1.61, 0.05) |
| Model 2 | 0 | −0.37 (−1.21, 0.46) | −0.50 (−1.37, 0.37) | −0.54 (−1.42, 0.34) |
Median maternal L/Z intake (range), in mg/d, for each quartile. First trimester: Q1 = 0.97 (0.09–1.433); Q2 = 1.78 (1.434–2.200); Q3 = 2.64 (2.201–3.251); Q4 = 4.51 (3.253–16.307). Second trimester: Q1 = 0.98 (0.05–1.426); Q2 = 1.82 (1.427–2.209); Q3 = 2.59 (2.210–3.187); Q4 = 4.48 (3.194–15.205). Nutrient values were adjusted for total energy intake using the residual model. BRIEF GEC, Behavioral Rating Inventory of Executive Function–Global Executive Composite; KBIT-II, Kaufman Brief Intelligence Test, Second Edition; PPVT-III, Peabody Picture Vocabulary Test, Third Edition; Q, quartile; SDQ, Strengths and Difficulties Questionnaire; VRM, visual recognition memory (percent novelty preference); WRAML, Wide Range Assessment of Memory and Learning, Second Edition; WRAVMA, Wide Range Assessment of Visual Motor Abilities.
Model 0 (crude): adjusted for child age and sex. Model 1 (multivariable): model 0 adjusted for maternal sociodemographic characteristics (age, race/ethnicity, marital status, parity, education, income, smoking history, prepregnancy BMI). Model 2 (multivariable): model 1 also adjusted for trimester-specific intake of total energy, DHA, folate, choline, vitamin B-12, and alcohol.
β (95% CI) represent the difference in mean cognitive/behavioral scores compared with the lowest quartile of maternal L/Z intake (reference).
95% CI for the difference in mean cognitive/behavioral scores excludes zero.
Early childhood
In child age- and sex-adjusted models, higher maternal intake of L/Z in the first and second trimesters of pregnancy was associated with better child PPVT-III scores (Table 2). However, the associations were attenuated and no longer statistically significant after additional adjustment for maternal sociodemographic and dietary factors. We did not find any associations of maternal L/Z intake during pregnancy with child WRAVMA total scores in early childhood. Secondary analyses of WRAVMA subtests showed that higher maternal second-trimester intake of L/Z was associated with a higher mean child WRAVMA drawing score after adjustment for maternal sociodemographic characteristics [Q4–Q1 means: 1.99 (95% CI: 0.11, 3.87)], indicating better visual-motor integration; and the association remained similar with slight attenuation after additional adjustment for maternal dietary factors (Supplemental Table 1).
Mid-childhood
In models adjusting for child age and sex and maternal sociodemographic characteristics, children whose mothers were in the highest quartile category of first-trimester L/Z intake had a mean KBIT-II nonverbal score 3.09 (95% CI: 0.07, 6.12) points higher than those with mothers in the lowest quartile category. After additional adjustment for maternal dietary factors, the difference in means remained similar with a slight attenuation and somewhat wider confidence intervals [Q4–Q1 means: 2.87 (95% CI: –0.31, 6.05)]; this loss of precision may account for much of the change in statistical significance (Table 2).
In child age- and sex-adjusted models, higher maternal intake of L/Z in the first and second trimesters was associated with significantly higher child KBIT-II verbal scores. After additional adjustment for maternal characteristics and dietary factors, these associations were attenuated but remained statistically significant such that children with mothers in the highest quartile category of first- and second-trimester L/Z intake had mean KBIT-II verbal scores 2.67 (95% CI: 0.13, 5.20) and 3.55 (95% CI: 0.81, 6.28) points higher, respectively, than those with mothers in the lowest quartile category, indicating better verbal intelligence (Table 2). In secondary analyses, the associations between maternal first-trimester L/Z and child KBIT-II verbal were attenuated after additional adjustment for breastfeeding status [Q4–Q1 means: 1.82 (95% CI: −0.70, 4.34)], maternal KBIT-II score [Q4–Q1 means: 2.03 (95% CI: –0.44, 4.50)], or HOME-SF score [Q4–Q1 means: 2.39 (95% CI: –0.14, 4.92)]. We found no associations between maternal L/Z intake and WRAVMA drawing or WRAML scores in mid-childhood. Secondary analyses of cognitive subtests showed that children with mothers in the second and third quartile categories of first-trimester L/Z intake had significantly higher WRAML design memory scores [Q3–Q1 means: 0.62 (95% CI: 0.11, 1.13)] than those with mothers in the lowest quartile category, indicating better short-term ability to remember new visual information (Supplemental Table 1).
In child age- and sex-adjusted models, higher maternal intake of L/Z in the first and second trimesters of pregnancy was associated with a lower mean child BRIEF GEC score, indicating better executive function. The associations were slightly attenuated after additional adjustment for maternal sociodemographic characteristics and remained statistically significant for the second-trimester exposure only [Q4–Q1 means: –1.81 (95% CI: –3.46, –0.15)] (Table 2). After additional adjustment for maternal dietary factors, the difference in means remained similar with a slight attenuation and somewhat wider confidence intervals; this loss of precision may account for much of the change in statistical significance.
In secondary analyses, higher maternal first- and second-trimester L/Z intake was associated with lower mean child BRIEF BRI scores [Q4–Q1 means for first trimester: –1.63 (95% CI: –3.22, –0.04); second trimester: –1.89 (95% CI: –3.58, –0.21)], after adjustment for maternal sociodemographic and dietary factors, indicating fewer behavioral regulation problems (Supplemental Table 1). Additional adjustment for HOME-SF score slightly attenuated these associations [Q4–Q1 means for first trimester: –1.31 (95% CI: –2.87, 0.26); second trimester: –1.43 (95% CI: –3.10, 0.24)].
In child age- and sex-adjusted models, higher maternal L/Z intake in the first and second trimesters was associated with lower child SDQ total scores, indicating fewer behavioral and social-emotional difficulties. These associations were slightly attenuated after additional adjustment for maternal characteristics and remained statistically significant only for the first-trimester exposure [Q4–Q1 means: –0.81 (95% CI: –1.61, –0.01)]. After additional adjustment for maternal dietary factors, the difference in means remained similar with a slight attenuation and somewhat wider confidence intervals [Q4–Q1 means: –0.75 (95% CI: –1.59, 0.09)] (Table 2). Additional adjustment of model 2 for HOME-SF score attenuated the association [Q4–Q1 means: –0.57 (95% CI: –1.39, 0.25)].
Secondary exposure: Maternal intake of L/Z-rich foods during pregnancy
We did not observe statistically significant associations of maternal L/Z-rich foods intake with VRM scores in infancy, PPVT-III and WRAVMA scores in early childhood, or KBIT-II nonverbal, KBIT-II verbal, WRAVMA drawing, WRAML total, and BRIEF GEC scores in mid-childhood (Table 3). Secondary analyses showed that higher first-trimester intake of L/Z-rich foods was associated with a lower mean child BRIEF BRI score [Q4–Q1 means: –1.76 (95% CI: –3.45, –0.08)], after adjusting for maternal sociodemographic and dietary factors, indicating better behavioral regulation ability (Supplemental Table 2). The mean SDQ total score was 1.02 (95% CI: –1.91, –0.12) points lower among children whose mothers consumed the highest number of servings of L/Z-rich foods in the first trimester compared to those with mothers who consumed the lowest number of servings, indicating fewer behavioral difficulties (Table 3).
TABLE 3.
Associations of maternal intake of lutein and zeaxanthin-rich (L/Z-rich) foods during pregnancy with child cognitive and behavioral outcomes1
| Child outcome Trimester Model3 | Quartiles of maternal L/Z-rich foods intake2 | |||
|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartiles 4 | |
| (reference) | β (95% CI)4 | β (95% CI) | β (95% CI) | |
| Infancy | ||||
| VRM | ||||
| T1 (n = 1071) | ||||
| Model 0 | 0 | −1.01 (−3.92, 1.91) | −0.50 (−3.36, 2.36) | −1.48 (−4.33, 1.37) |
| Model 1 | 0 | −0.95 (−3.88, 1.97) | −0.06 (−2.93, 2.81) | −1.06 (−3.93, 1.81) |
| Model 2 | 0 | −0.91 (−3.89, 2.07) | −0.47 (−3.55, 2.60) | −1.72 (−5.11, 1.67) |
| T2 (n = 1049) | ||||
| Model 0 | 0 | −1.36 (−4.28, 1.57) | −0.89 (−3.81, 2.04) | −1.26 (−4.11, 1.59) |
| Model 1 | 0 | −0.63 (−3.57, 2.31) | −0.65 (−3.52, 2.22) | 0.66 (−1.34, 2.66) |
| Model 2 | 0 | −1.86 (−4.88, 1.17) | −1.55 (−4.63, 1.54) | −2.08 (−5.48, 1.33) |
| Early childhood | ||||
| PPVT-III | ||||
| T1 (n = 1206) | ||||
| Model 0 | 0 | 0.50 (−1.88, 2.87) | −0.47 (−2.85, 1.92) | 1.26 (−1.11, 3.62) |
| Model 1 | 0 | −0.26 (−2.34, 1.82) | −1.01 (−3.10, 1.09) | 0.58 (−1.50, 2.67) |
| Model 2 | 0 | −0.05 (−2.17, 2.07) | −0.75 (−2.98, 1.48) | 1.15 (−1.31, 3.61) |
| T2 (n = 1177) | ||||
| Model 0 | 0 | −1.07 (−3.50, 1.36) | −0.11 (−2.52, 2.30) | 0.49 (−1.90, 2.87) |
| Model 1 | 0 | −0.97 (−3.13, 1.18) | −1.13 (−3.27, 1.01) | −0.03 (−2.16, 2.11) |
| Model 2 | 0 | −1.09 (−3.28, 1.11) | −1.03 (−3.29, 1.23) | −0.23 (−2.74, 2.28) |
| WRAVMA total | ||||
| T1 (n = 1168) | ||||
| Model 0 | 0 | −0.43 (−2.27, 1.42) | −1.40 (−3.25, 0.45) | −0.67 (−2.48, 1.15) |
| Model 1 | 0 | −0.93 (−2.72, 0.87) | −1.89 (−3.70, −0.09)5 | −1.00 (−2.77, 0.76) |
| Model 2 | 0 | −0.76 (−2.59, 1.06) | −1.62 (−3.54, 0.31) | −0.40 (−2.46, 1.66) |
| T2 (n = 1137) | ||||
| Model 0 | 0 | −0.44 (−2.33, 1.46) | 0.56 (−1.31, 2.43) | −0.31 (−2.18, 1.56) |
| Model 1 | 0 | −0.65 (−2.50, 1.21) | −0.01 (−1.85, 1.83) | −0.66 (−2.50, 1.18) |
| Model 2 | 0 | −0.22 (−2.12, 1.68) | 0.69 (−1.25, 2.63) | 0.64 (−1.53, 2.81) |
| Mid-childhood | ||||
| KBIT-II nonverbal | ||||
| T1 (n = 1106) | ||||
| Model 0 | 0 | 1.50 (−1.54, 4.54) | 0.57 (−2.44, 3.59) | 2.48 (−0.46, 5.41) |
| Model 1 | 0 | 1.56 (−1.37, 4.48) | 0.29 (−2.61, 3.20) | 2.02 (−0.86, 4.89) |
| Model 2 | 0 | 1.67 (−1.32, 4.65) | 0.64 (−2.46, 3.75) | 2.64 (−0.69, 5.96) |
| T2 (n = 1064) | ||||
| Model 0 | 0 | −1.25 (−4.37, 1.86) | 1.31 (−1.84, 4.45) | 0.53 (−2.56, 3.62) |
| Model 1 | 0 | −1.84 (−4.85, 1.16) | 0.29 (−2.72, 3.30) | −0.52 (−3.53, 2.48) |
| Model 2 | 0 | −1.78 (−4.86, 1.30) | 0.27 (−2.96, 3.51) | −0.54 (−4.16, 3.09) |
| KBIT-II verbal | ||||
| T1 (n = 1093) | ||||
| Model 0 | 0 | −0.03 (−2.72, 2.67) | −0.53 (−3.22, 2.15) | 1.45 (−1.20, 4.10) |
| Model 1 | 0 | −0.62 (−2.93, 1.69) | −1.47 (−3.78, 0.83) | 0.29 (−1.99, 2.57) |
| Model 2 | 0 | −0.61 (−2.96, 1.74) | −1.51 (−3.96, 0.93) | 0.21 (−2.43, 2.85) |
| T2 (n = 1051) | ||||
| Model 0 | 0 | 1.15 (−1.64, 3.93) | 2.18 (−0.72, 5.08) | 2.58 (−0.16, 5.33) |
| Model 1 | 0 | 0.25 (−2.14, 2.65) | 0.28 (−2.17, 2.74) | 0.88 (−1.51, 3.26) |
| Model 2 | 0 | 0.04 (−2.43, 2.50) | 0.05 (−2.58, 2.67) | 0.35 (−2.51, 3.20) |
| WRAVMA drawing | ||||
| T1 (n = 1099) | ||||
| Model 0 | 0 | −0.47 (−3.45, 2.51) | −1.33 (−4.30, 1.64) | −0.89 (−3.77, 2.00) |
| Model 1 | 0 | −0.41 (−3.40, 2.57) | −1.53 (−4.52, 1.46) | −1.17 (−4.07, 1.74) |
| Model 2 | 0 | −0.75 (−3.81, 2.31) | −2.27 (−5.46, 0.92) | −2.32 (−5.71, 1.07) |
| T1 (n = 1058) | ||||
| Model 0 | 0 | −0.68 (−3.68, 2.33) | −0.01 (−3.06, 3.05) | 0.14 (−2.85, 3.14) |
| Model 1 | 0 | −1.26 (−4.27, 1.75) | −0.61 (−3.67, 2.44) | −0.53 (−3.55, 2.49) |
| Model 2 | 0 | −0.89 (−3.98, 2.20) | −0.16 (−3.41, 3.08) | 0.27 (−3.36, 3.90) |
| WRAML total | ||||
| T1 (n = 1094) | ||||
| Model 0 | 0 | −0.21 (−1.00, 0.57) | 0.09 (−0.68, 0.86) | 0.33 (−0.43, 1.09) |
| Model 1 | 0 | −0.18 (−0.96, 0.61) | 0.02 (−0.75, 0.79) | 0.25 (−0.51, 1.01) |
| Model 2 | 0 | −0.29 (−1.09, 0.51) | −0.18 (−1.01, 0.65) | −0.03 (−0.92, 0.86) |
| T2 (n = 1052) | ||||
| Model 0 | 0 | −0.19 (−1.01, 0.64) | −0.55 (−1.36, 0.27) | 0.17 (−0.64, 0.98) |
| Model 1 | 0 | −0.27 (−1.09, 0.55) | −0.72 (−1.53, 0.09) | 0.01 (−0.80, 0.83) |
| Model 2 | 0 | −0.20 (−1.04, 0.64) | −0.70 (−1.57, 0.16) | 0.07 (−0.90, 1.05) |
| BRIEF GEC | ||||
| T1 (n = 1174) | ||||
| Model 0 | 0 | 0.00 (−1.54, 1.54) | 0.16 (−1.41, 1.72) | −0.18 (−1.70, 1.33) |
| Model 1 | 0 | 0.27 (−1.26, 1.80) | 0.40 (−1.15, 1.94) | 0.10 (−1.41, 1.60) |
| Model 2 | 0 | −0.23 (−1.77, 1.32) | −0.28 (−1.90, 1.33) | −1.43 (−3.17, 0.30) |
| T2 (n = 1132) | ||||
| Model 0 | 0 | 1.32 (−0.33, 2.96) | −0.12 (−1.72, 1.48) | 0.45 (−1.19, 2.08) |
| Model 1 | 0 | 1.65 (0.02, 3.27)5 | 0.16 (−1.43, 1.75) | 0.79 (−0.83, 2.41) |
| Model 2 | 0 | 1.50 (−0.13, 3.14) | −0.40 (−2.07, 1.27) | −0.07 (−1.95, 1.81) |
| SDQ total | ||||
| T1 (n = 1191) | ||||
| Model 0 | 0 | −0.48 (−1.29, 0.32) | −0.14 (−0.95, 0.67) | −0.81 (−1.60, −0.02)5 |
| Model 1 | 0 | −0.25 (−1.02, 0.53) | 0.10 (−0.67, 0.87) | −0.54 (−1.30, 0.23) |
| Model 2 | 0 | −0.39 (−1.17, 0.39) | −0.09 (−0.90, 0.72) | −1.02 (−1.91, −0.12)5 |
| T2 (n = 1148) | ||||
| Model 0 | 0 | 0.28 (−0.56, 1.13) | 0.02 (−0.80, 0.85) | −0.14 (−0.96, 0.69) |
| Model 1 | 0 | 0.55 (−0.27, 1.38) | 0.35 (−0.45, 1.16) | 0.22 (−0.58, 1.02) |
| Model 2 | 0 | 0.57 (−0.25, 1.40) | 0.24 (−0.61, 1.09) | 0.07 (−0.86, 1.01) |
L/Z-rich foods: top 10 contributors to total maternal lutein and zeaxanthin intake in eligible sample (cooked spinach, raw spinach, romaine or leaf lettuce, kale, broccoli, peas or lima beans, orange juice with calcium, corn, eggplant or zucchini, mixed vegetables) and any food with lutein and zeaxanthin ≥1 mg per 100 g (Brussels sprouts, dark squash, popcorn, eggs). BRIEF GEC, Behavioral Rating Inventory of Executive Function–Global Executive Composite; KBIT-II, Kaufman Brief Intelligence Test, Second Edition; PPVT-III, Peabody Picture Vocabulary Test, Third Edition; Q, quartile; SDQ, Strengths and Difficulties Questionnaire; VRM, visual recognition memory (percent novelty preference); WRAML, Wide Range Assessment of Memory and Learning, Second Edition; WRAVMA, Wide Range Assessment of Visual Motor Abilities.
Median maternal L/Z-rich foods intake (range), in servings/d, for each quartile. First trimester: Q1 = 0.77 (0.07≤ and ≤1.06), Q2 = 1.35 (1.06< and ≤1.63), Q3 = 1.93 (1.63< and <2.35), Q4 = 3.06 (2.35≤ and ≤9.58). Second trimester: Q1 = 0.84 (0.00≤ and ≤1.13), Q2 = 1.42 (1.13< to ≤1.70), Q3 = 1.99 (1.70< to ≤2.42), Q4 = 3.21 (2.42< to ≤10.44).
Model 0 (crude): Adjusted for child age and sex. Model 1 (multivariable): model 0 adjusted for maternal sociodemographic characteristics (age, race/ethnicity, marital status, parity, education, income, smoking history, prepregnancy BMI). Model 2 (multivariable): model 1 also adjusted for trimester-specific intake of total energy, DHA, folate, choline, vitamin B-12, and alcohol.
β (95% CI) represent the difference in mean cognitive/behavioral scores compared with the lowest quartile of maternal L/Z-rich foods intake (reference).
95% CI for the difference in mean cognitive/behavioral scores excludes zero.
Discussion
To our knowledge, this is the first study to evaluate maternal intake of L/Z during pregnancy in relation to child cognition, behavior, and social-emotional development. In this prospective cohort study, higher maternal intake of L/Z during the first and second trimesters of pregnancy was associated with better verbal intelligence (main analyses) and behavioral regulation ability (secondary analyses) in mid-childhood, independently of child sex and age at testing and various maternal sociodemographic and dietary factors. We also observed that higher maternal first-trimester intake of L/Z-rich foods was associated with better social-emotional development and behavioral regulation ability in mid-childhood.
We found little evidence of any association between higher maternal intake of L/Z during pregnancy and early childhood cognitive outcomes. This suggests that the benefits of higher L/Z exposure in utero may be manifested later in childhood perhaps as a consequence of long-term programming of cognition by prenatal carotenoid exposure. It is also possible that the 2 cognitive tests administered in early childhood (PPVT-III and WRAVMA) were not sufficiently sensitive to detect subtle differences in cognitive performance or that the cognitive domains assessed by these tests were simply not associated with maternal dietary L/Z. Recent studies in children suggest that MPOD is associated with measures of academic performance, memory, global intelligence, verbal ability, and executive processes (13–16). Moreover, a study of the distribution of carotenoids in infant brains found that lutein was the predominant carotenoid in the occipital cortex, auditory cortex, hippocampus, and frontal lobe, which are associated with vision, audition, memory, and executive function, respectively (4). This supports a role for lutein in early neurodevelopment and is consistent with our finding of an association between maternal intake of L/Z with child verbal intelligence and executive function.
However, we also observed that higher maternal intake of L/Z during pregnancy was associated with worse VRM scores in infants, contrary to our hypothesis. This finding also disagrees with the only study relating lutein to infant VRM, which demonstrated synergism between lutein and choline in breast milk, with higher concentrations of both related to better recognition memory in 6-mo-old infants using an objective assessment of VRM based on electroencephalogram-measured event-related potentials (51). The relation of lutein exposure in early life with measures of cognition in infancy requires further investigation.
The underlying protective mechanisms of lutein and zeaxanthin on neural tissues are not yet understood, but it is theorized that they exert their effects through their antioxidant, anti-inflammatory, and structural properties (3, 52, 53). Given that oxidative stress and inflammation are believed to be implicated in the pathogenesis of cognitive decline, these mechanisms may be more relevant in older ages. Other suggested mechanisms include modulation of membrane stability, neurotrophic support, enhancement of gap junction communications between neurons, modulation of synaptic membranes, and epigenetic modifications (3, 54). Indeed, these proposed mechanisms by which lutein and zeaxanthin may support brain function would also apply in early life. Postmortem metabolomic analyses on human infant brain tissues showed that lutein concentrations correlated with many compounds in the brain, including lipid and energy pathway metabolites, and amino acid neurotransmitters that are known to influence cognition (55).
Notably, the difference in means that we found in KBIT-II verbal scores between the highest and lowest quartile category of maternal L/Z intake was similar to a previously published difference in mean scores between children who were ever breastfed and those who were never breastfed in the same cohort (56). Given the compelling evidence that breast milk benefits child neurodevelopment (57, 58), the trends we found in child KBIT-II verbal scores by maternal L/Z intake appear clinically meaningful. Moreover, KBIT-II scores are correlated with the Wechsler Intelligence Scale for Children III (59) and associated with academic achievement in young children (60). Therefore, the difference we found in child KBIT-II verbal scores by maternal intake of L/Z may be practically relevant in terms of overall intelligence and academic achievement. In our analyses, additional adjustment of the main models for breastfeeding status did not meaningfully change associations of maternal L/Z with child BRIEF BRI but slightly attenuated the association with child KBIT-II verbal scores, suggesting that breastfeeding could be confounding and/or mediating at least part of the associations.
Our study has several strengths. Project Viva is a prospective cohort, and maternal dietary intake was assessed during early and mid-pregnancy, when the major structures of the brain and the central nervous system are established (30, 31). We assessed neurocognitive outcomes using validated tests at each of infancy, early childhood, and mid-childhood. Given the novelty of our investigation, we could not hypothesize which child cognitive domains are likely to be associated with maternal L/Z and at what age in childhood the associations or lack thereof are likely to manifest. Therefore, we chose to use a battery of pertinent tests and questionnaires, administered at 3 different stages of child development, to give an overall picture of cognitive function in childhood.
Nevertheless, our study had several limitations. First, measurement errors in dietary assessment are always a concern. However, the FFQs used were previously validated in nonpregnant women and men, as well as calibrated for use in pregnancy to assess carotenoid intake, and FFQs should accurately rank mothers with regard to their L/Z intake after adjustment for total energy intake. Random error in the exposure measurement would likely have attenuated our effect estimates. Second, there is possibility for measurement error in the cognitive test scores. However, the tests were administered by trained research assistants, we excluded results for which the administrator did not have confidence in the test performance, and any error in the dependent variable would have reduced the precision of our effect estimates, rendering our results conservative. Third, as in any observational study, there is possibility that unmeasured confounding may explain at least part of the observed findings. However, we controlled for potential important confounders, including maternal socioeconomic status and intake of other nutrients that were shown to be related to neurodevelopment. Last, the generalizability of our results may be limited given that all participants in Project Viva resided in eastern MA and received health care, most were college-educated, and consumed higher L/Z compared with women of childbearing age in the US (61). Like many food components, the benefits of increased L/Z intake may be most apparent among those with the lowest intakes. Nevertheless, our findings showed that, even in women who are apparently well-nourished and socioeconomically advantaged, small differences in maternal diet at the critical period of pregnancy may have implications for child cognition. The lack of detected associations with many of the child cognitive/behavioral outcomes and modest effect sizes could be due in part to our sample being at low risk for nutritional deficiencies or in whom low intake may not lead to measurable changes in child outcomes due to compensation over time by the many factors (other than nutrition) that affect neurodevelopment.
In a prospective cohort study of mother-child pairs, higher maternal intake of L/Z during pregnancy was associated with better verbal intelligence and behavior regulation ability in the offspring at mid-childhood. The role of L/Z in early neurodevelopment deserves further investigation.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Gail Rogers, senior statistician in the Nutritional Epidemiology Program at the Jean Mayer–USDA Human Nutrition Research Center on Aging, for statistical support.
The authors’ contributions were as follows—HAM, KMS, TMS, EJJ, and PFJ: designed the research; KMS, SLR-S, and EO: oversaw data collection and provided access to the Project Viva data set; HAM: analyzed the data and drafted the paper; HAM and PFJ: had primary responsibility for final content; and all authors: read and approved the final manuscript.
Data availability
Data described in the manuscript and the associated code book and analytic code are available from Project Viva upon request, pending application and approval.
Notes
Sources of support: Kemin Foods, L.C. (HAM, EJJ, and PFJ); Project Viva grants NIH UH3 OD023286 and NIH/NICHD R01 HD034568 (KMS, SLR-S, and EO); and USDA Agricultural Research Service, agreement no. 58-1950-4-003 (PFJ). Neither Project Viva nor any Project Viva investigators received any funding from Kemin Foods. The funder (Kemin Foods) was not involved in the study design, study implementation, interpretation of the results, or manuscript preparation.
Author disclosures: The authors report no conflicts of interest.
Supplemental Tables 1 and 2 are available from the “Supplementary Data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn.
Abbreviations used: BRIEF, Behavioral Rating Inventory of Executive Function; BRIEF BRI, Behavioral Rating Inventory of Executive Function Behavioral Regulation Index; BRIEF GEC, Behavioral Rating Inventory of Executive Function Global Executive Composite; HOME-SF, Home Observation Measurement of the Environment–Short Form; KBIT-II, Kaufman Brief Intelligence Test, Second Edition; L/Z, lutein and zeaxanthin; MI, multiple imputation; MPOD, macular pigment optical density; PPVT-III, Peabody Picture Vocabulary Test, Third Edition; SDQ, Strengths and Difficulties Questionnaire; VRM, visual recognition memory; WRAML, Wide Range Assessment of Memory and Learning, Second Edition; WRAVMA, Wide Range Assessment of Visual Motor Abilities.
Contributor Information
Hiya A Mahmassani, Dorothy J and Gerald R Friedman School of Nutrition and Science Policy at Tufts University, Boston, MA, USA.
Karen M Switkowski, Department of Population Medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston, MA, USA.
Tammy M Scott, Dorothy J and Gerald R Friedman School of Nutrition and Science Policy at Tufts University, Boston, MA, USA.
Elizabeth J Johnson, Dorothy J and Gerald R Friedman School of Nutrition and Science Policy at Tufts University, Boston, MA, USA.
Sheryl L Rifas-Shiman, Department of Population Medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston, MA, USA.
Emily Oken, Department of Population Medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston, MA, USA; Department of Nutrition, Harvard T. H. Chan School of Public Health, Boston, MA, USA.
Paul F Jacques, Dorothy J and Gerald R Friedman School of Nutrition and Science Policy at Tufts University, Boston, MA, USA; Jean Mayer–USDA Human Nutrition Research Center on Aging at Tufts University, Boston, MA, USA.
References
- 1. Johnson EJ. A possible role for lutein and zeaxanthin in cognitive function in the elderly. Am J Clin Nutr. 2012;96:1161S. [DOI] [PubMed] [Google Scholar]
- 2. Erdman JW, Smith JW, Kuchan MJ, Mohn ES, Johnson EJ, Rubakhin SS, Wang L, Sweedler JV, Neuringer M. Lutein and brain function. Foods. 2015;4:547–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johnson EJ. Role of lutein and zeaxanthin in visual and cognitive function throughout the lifespan. Nutr Rev. 2014;72:605–12. [DOI] [PubMed] [Google Scholar]
- 4. Vishwanathan R, Kuchan MJ, Sen S, Johnson EJ. Lutein and preterm infants with decreased concentrations of brain carotenoids. J Pediatr Gastroenterol Nutr. 2014;59:659–65. [DOI] [PubMed] [Google Scholar]
- 5. Renzi LM, Johnson EJ. Lutein and age-related ocular disorders in the older adult. J Nutr Elder. 2008;26:139–57. [DOI] [PubMed] [Google Scholar]
- 6. Johnson EJ, Vishwanathan R, Johnson MA, Hausman DB, Davey A, Scott TM, Green RC, Miller LS, Gearing M, Woodard J et al. Relationship between serum and brain carotenoids, -tocopherol, and retinol concentrations and cognitive performance in the oldest old from the Georgia Centenarian Study. J Aging Res. 2013;20:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yeum KJ, Ferland G, Patry J, Russell RM. Relationship of plasma carotenoids, retinol and tocopherols in mothers and newborn infants. J Am Coll Nutr. 1998;17:442–7. [DOI] [PubMed] [Google Scholar]
- 8. Woollett LA. Review: transport of maternal cholesterol to the fetal circulation. Placenta. 2011;32:S218–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Curran-Celentano J, Hammond BR, Ciulla TA, Cooper DA, Pratt LM, Danis RB. Relation between dietary intake, serum concentrations, and retinal concentrations of lutein and zeaxanthin in adults in a Midwest population. Am J Clin Nutr. 2001;74:796–802. [DOI] [PubMed] [Google Scholar]
- 10. Thoene M, Anderson-Berry A, Van Ormer M, Furtado J, Soliman GA, Goldner W, Hanson C. Quantification of lutein + zeaxanthin presence in human placenta and correlations with blood levels and maternal dietary intake. Nutrients. 2019;11;134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lietz G, Mulokozi G, Henry JCK, Tomkins AM. Xanthophyll and hydrocarbon carotenoid patterns differ in plasma and breast milk of women supplemented with red palm oil during pregnancy and lactation. J Nutr. 2006;136:1821–7. [DOI] [PubMed] [Google Scholar]
- 12. Vishwanathan R, Neuringer M, Snodderly DM, Schalch W, Johnson EJ. Macular lutein and zeaxanthin are related to brain lutein and zeaxanthin in primates. Nutr Neurosci. 2013;16:21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barnett SM, Khan NA, Walk AM, Raine LB, Moulton C, Cohen NJ, Kramer AF Jr, Renzi-Hammond L, Hillman CH. Macular pigment optical density is positively associated with academic performance among preadolescent children. Nutr Neurosci. 2018;21:632–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hassevoort KM, Khazoum SE, Walker JA, Barnett SM, Raine LB, Hammond BR, Renzi-Hammond LM, Kramer AF, Khan NA, Hillman CH et al. Macular carotenoids, aerobic fitness, and central adiposity are associated differentially with hippocampal-dependent relational memory in preadolescent children. J Pediatr. 2017;183:108–114.e1. [DOI] [PubMed] [Google Scholar]
- 15. Walk AM, Khan NA, Barnett SM, Raine LB, Kramer AF, Cohen NJ, Moulton CJ, Renzi-Hammond LM, Hammond BR, Hillman CH. From neuro-pigments to neural efficiency: the relationship between retinal carotenoids and behavioral and neuroelectric indices of cognitive control in childhood. Int J Psychophysiol. 2017;118:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Saint SE, Renzi-Hammond LM, Khan NA, Hillman CH, Frick JE, Hammond BR. The macular carotenoids are associated with cognitive function in preadolescent children. Nutrients. 2018;10:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Henriksen BS, Chan G, Hoffman RO, Sharifzadeh M, Ermakov IV, Gellermann W, Bernstein PS. Interrelationships between maternal carotenoid status and newborn infant macular pigment optical density and carotenoid status. Invest Ophthalmol Vis Sci. 2013;54:5568–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vishwanathan R, Iannaccone A, Scott TM, Kritchevsky SB, Jennings BJ, Carboni G, Forma G, Satterfield S, Harris T, Johnson KC et al. Macular pigment optical density is related to cognitive function in older people. Age Ageing. 2014;43:271–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Feeney J, Finucane C, Savva GM, Cronin H, Beatty S, Nolan JM, Kenny RA. Low macular pigment optical density is associated with lower cognitive performance in a large, population-based sample of older adults. Neurobiol Aging. 2013;34:2449–56. [DOI] [PubMed] [Google Scholar]
- 20. Kelly D, Coen RF, Akuffo KO, Beatty S, Dennison J, Moran R, Stack J, Howard AN, Mulcahy R, Nolan JM. Cognitive function and its relationship with macular pigment optical density and serum concentrations of its constituent carotenoids. J Alzheimers Dis. 2015;48:261–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Johnson EJ, McDonald K, Caldarella SM, Chung H-Y, Troen AM, Snodderly DM. Cognitive findings of an exploratory trial of docosahexaenoic acid and lutein supplementation in older women. Nutr Neurosci. 2008;11:75–83. [DOI] [PubMed] [Google Scholar]
- 22. Bovier ER, Renzi LM, Hammond BR. A double-blind, placebo-controlled study on the effects of lutein and zeaxanthin on neural processing speed and efficiency. PLoS One. 2014;9:e108178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hammond BR, Miller LS, Bello MO, Lindbergh CA, Mewborn C, Renzi-Hammond LM. Effects of lutein/zeaxanthin supplementation on the cognitive function of community dwelling older adults: a randomized, double-masked, placebo-controlled trial. Front Aging Neurosci. 2017;9:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oken E, Baccarelli AA, Gold DR, Kleinman KP, Litonjua AA, De Meo D, Rich-Edwards JW, Rifas-Shiman SL, Sagiv S, Taveras EM et al. Cohort profile: Project Viva. Int J Epidemiol. 2015;44:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. [DOI] [PubMed] [Google Scholar]
- 26. Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–26.; discussion 1127–36. [DOI] [PubMed] [Google Scholar]
- 27. Fawzi WW, Rifas-Shiman SL, Rich-Edwards JW, Willett WC, Gillman MW. Calibration of a semi-quantitative food frequency questionnaire in early pregnancy. Ann Epidemiol. 2004;14:754–62. [DOI] [PubMed] [Google Scholar]
- 28. US Department of Agriculture, Agricultural Research Service . USDA National Nutrient Database for Standard Reference, Release 14. [Internet]. 2001. [Accessed 2019 Nov 21]. Available from: https://www.ars.usda.gov/ARSUserFiles/80400525/Data/SR14/sr14_doc.pdf [Google Scholar]
- 29. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65:1220S–8S.; discussion 1229S–31S. [DOI] [PubMed] [Google Scholar]
- 30. Stiles J, Jernigan TL. The basics of brain development. Neuropsychol Rev. 2010;20:327–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cusick SE, Georgieff MK. The role of nutrition in brain development: the golden opportunity of the “first 1000 days.”. J Pediatr. 2016;175:16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Oken E, Wright RO, Kleinman KP, Bellinger D, Amarasiriwardena CJ, Hu H, Rich-Edwards JW, Gillman MW. Maternal fish consumption, hair mercury, and infant cognition in a U.S. cohort. Environ Health Perspect. 2005;113:1376–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rose SA, Feldman JF, Jankowski JJ. Infant visual recognition memory. Dev Rev. 2004;24:74–100. [DOI] [PubMed] [Google Scholar]
- 34. Rose SA, Feldman JF, Wallace IF. Infant information processing in relation to six-year cognitive outcomes. Child Dev. 1992;63:1126–41. [PubMed] [Google Scholar]
- 35. McCall RB, Carriger MS. A meta-analysis of infant habituation and recognition memory performance as predictors of later IQ. Child Dev. 1993;64:57–79. [PubMed] [Google Scholar]
- 36. Rose SA, Feldman JF. Prediction of IQ and specific cognitive abilities at 11 years from infancy measures. Dev Psychol. 1995;31:685–96. [Google Scholar]
- 37. Dunn LM, Dunn LM. Examiner's manual for the PPVT-III, Peabody Picture Vocabulary Test, Third Edition. Circle Pines (MN): American Guidance Service; 1997. [Google Scholar]
- 38. Adams W, Sheslow D. Wide Range Assessment of Visual Motor Abilities. Wilmington (DE): Wide Range, Inc.; 1995. [Google Scholar]
- 39. Kaufman A, Kaufman N. Kaufman Brief Intelligence Test, Second Edition (KBIT-2). Bloomington (MN): Pearson, Inc; 2004. [Google Scholar]
- 40. Sheslow D, Adams W. Wide Range Assessment of Memory and Learning, Second Edition (WRAML2). Lutz (FL): Psychological Assessment Resources, Inc; 2003. [Google Scholar]
- 41. Gioia GA, Isquith PK, Guy SC, Kenworthy L. TEST REVIEW Behavior Rating Inventory of Executive Function. Child Neuropsychol. 2000;6:235–8. [DOI] [PubMed] [Google Scholar]
- 42. Gioia GA, Isquith PK, Retzlaff PD, Espy KA. Confirmatory factor analysis of the Behavior Rating Inventory of Executive Function (BRIEF) in a clinical sample. Child Neuropsychol. 2002;8:249–57. [DOI] [PubMed] [Google Scholar]
- 43. Goodman R. Psychometric properties of the Strengths and Difficulties Questionnaire. J Am Acad Child Adolesc Psychiatry. 2001;40:1337–45. [DOI] [PubMed] [Google Scholar]
- 44. Vostanis P. Strengths and Difficulties Questionnaire: research and clinical applications. Curr Opin Psychiatry. 2006;19:367–72. [DOI] [PubMed] [Google Scholar]
- 45. Bourdon KH, Goodman R, Rae DS, Simpson G, Koretz DS. The Strengths and Difficulties Questionnaire: U.S. normative data and psychometric properties. J Am Acad Child Adolesc Psychiatry. 2005;44:557–64. [DOI] [PubMed] [Google Scholar]
- 46. Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Frankenburg WK, Coons CE. Home screening questionnaire: its validity in assessing home environment. J Pediatr. 1986;108:624–6. [DOI] [PubMed] [Google Scholar]
- 48. Renzi-Hammond L, Bovier E, Fletcher L, Miller L, Mewborn C, Lindbergh C, Baxter J, Hammond B. Effects of a lutein and zeaxanthin intervention on cognitive function: a randomized, double-masked, placebo-controlled trial of younger healthy adults. Nutrients. 2017;9:1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Statist Med. 2011;30:377–99. [DOI] [PubMed] [Google Scholar]
- 50. Rubin DB. Multiple imputation for nonresponse in surveys. Hoboken (NJ): John Wiley; 2004. [Google Scholar]
- 51. Cheatham CL, Sheppard KW. Synergistic effects of human milk nutrients in the support of infant recognition memory: an observational study. Nutrients. 2015;7:9079–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rubin LP, Chan GM, Barrett-Reis BM, Fulton AB, Hansen RM, Ashmeade TL, Oliver JS, Mackey AD, Dimmit RA, Hartmann EE et al. Effect of carotenoid supplementation on plasma carotenoids, inflammation and visual development in preterm infants. J Perinatol. 2012;32:418–24. [DOI] [PubMed] [Google Scholar]
- 53. Wang M-X, Jiao J-H, Li Z-Y, Liu R-R, Shi Q, Ma L. Lutein supplementation reduces plasma lipid peroxidation and C-reactive protein in healthy nonsmokers. Atherosclerosis. 2013;227:380–5. [DOI] [PubMed] [Google Scholar]
- 54. Picone S, Ritieni A, Fabiano A, Graziani G, Paolillo P, Livolti G, Galvano F, Gazzolo D. Lutein levels in arterial cord blood correlate with neuroprotein activin A in healthy preterm and term newborns: a trophic role for lutein?. Clin Biochem. 2018;52:80–4. [DOI] [PubMed] [Google Scholar]
- 55. Lieblein-Boff JC, Johnson EJ, Kennedy AD, Lai C-S, Kuchan MJ. Exploratory metabolomic analyses reveal compounds correlated with lutein concentration in frontal cortex, hippocampus, and occipital cortex of human infant brain. PLoS One. 2015;10:e0136904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Belfort MB, Rifas-Shiman SL, Kleinman KP, Guthrie LB, Bellinger DC, Taveras EM, Gillman MW, Oken E. Infant feeding and childhood cognition at ages 3 and 7 years: effects of breastfeeding duration and exclusivity. JAMA Pediatr. 2013;167:836–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Horta BL, Victora CG. Long-term effects of breastfeeding: a systematic review. Geneva (Switzerland): World Health Organization; 2013. [Google Scholar]
- 58. Belfort MB. The science of breastfeeding and brain development. Breastfeed Med. 2017;12:459–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Grados JJ, Russo-Garcia KA. Comparison of the Kaufman Brief Intelligence Test and the Wechsler Intelligence Scale for Children–Third Edition in economically disadvantaged African American youth. J Clin Psychol. 1999;55:1063–71. [DOI] [PubMed] [Google Scholar]
- 60. Lassiter KS, Bardos AN. The relationship between young children's academic achievement and measures of intelligence. Psychol Schs. 1995;32:170–7. [Google Scholar]
- 61. US Department of Agriculture, Agricultural Research Service . What we eat in America, NHANES 2015–2016. [Internet]. [Accessed 2019 Dec 1]. Available from: www.ars.usda.gov/nea/bhnrc/fsrg. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.