Abstract
Chronic wounds are a significant problem in Australia. The health care‐related costs of chronic wounds in Australia are considerable, equivalent to more than AUD $3.5 billion, approximately 2% of national health care expenditure. Chronic wounds can also have a significant negative impact on the health‐related quality of life of affected individuals. Studies have demonstrated that evidence‐based care for chronic wounds improves clinical outcomes. Decision analytical modelling is important in confirming and applying these findings in the Australian context. Epidemiological and clinical data on chronic wounds are required to populate decision analytical models. Although epidemiological and clinical data on chronic wounds in Australia are available, these data have yet to be systematically summarised. To address these omissions and clarify the state of existing evidence, we conducted a systematic review of the literature on key epidemiological and clinical parameters of chronic wounds in Australia. A total of 90 studies were selected for inclusion. This paper presents a synthesis of the evidence on the prevalence and incidence of chronic wounds in Australia, as well as rates of infection, hospitalisation, amputation, healing, and recurrence.
Keywords: Australia, chronic wounds, systematic review, incidence, prevalence
1. INTRODUCTION
Chronic wounds are defined as wounds that have failed to heal or to reach anatomic and functional integrity.1, 2 There are four categories of chronic wounds, each with differing aetiologies: arterial ulcers (AUs), diabetic foot ulcers (DFUs), venous leg ulcers (VLUs), and pressure injuries (PIs). All categories are a significant problem in Australia. The costs of chronic wounds in Australia are considerable, equivalent to more than AUD $3.5 billion, approximately 2% of national health care expenditure.3 Chronic wounds can also have a major negative impact on the health‐related quality of life (HRQoL) of affected individuals.4, 5, 6, 7, 8
Studies have demonstrated that evidence‐based care for chronic wounds improves clinical outcomes9, 10 and is cost‐effective.11, 12, 13, 14 However, economic models have been complicated by problems with input data. Decision analytical modelling is an approach for economic evaluation that ideally uses evidence from randomised controlled trials and other high‐quality sources.15 The findings should provide evidence to support or reject a practice change against the criterion of value for money.16 Epidemiological and clinical data on chronic wounds are required to populate decision analytical models about the cost‐effectiveness of alternate models of care for chronic wounds.17 The identification and synthesis of evidence to populate decision analytical models should emerge from a systematic review of the literature.18
Although epidemiological and clinical data on chronic wounds in Australia—including on prevalence and incidence, as well as rates of infection, hospitalisation, amputation, healing, and recurrence—are available, these data have yet to be summarised in a reproducible review. In current economic evaluations of evidence‐based care for chronic wounds in Australia, values for these parameters originate from sources of varying quality, from small quasi‐experimental studies to expert opinions. In many cases, key values are derived from studies published in other countries and from older studies that lack relevance to the current health context.
To address this and clarify the state of the existing evidence, we conducted a systematic review of the literature on key epidemiological and clinical parameters of chronic wounds in Australia. Our aims were: to identify sources of primary data on the key epidemiological and clinical parameters for chronic wounds in Australia and to identify the knowledge gaps in the evidence that need to be addressed. Apart from informing economic modelling, such an integrated summary will have both clinical and public health applications. To the best of our knowledge, this review is the first to summarise the evidence on key clinical and epidemiological parameters relating to chronic wounds in Australia.
2. METHODS
The review was conducted according to the guidelines recommended by the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement19 (Supporting Information Appendix S1).
2.1. Search strategy
Searches were conducted on the electronic databases CINAHL, Cochrane Library, EMBASE, PubMed, and Scopus up to May 2, 2017. Information on the search strings used is available in the review protocol (Appendix S2). In addition to the database searches, other sources were identified by searching official websites (such as the Australian Bureau of Statistics [ABS] and Australian Institute of Health and Welfare [AIHW]) and contacting various experts in the field. The reference lists of selected studies were screened for other relevant studies. Additional studies and doctoral theses were also identified through direct contact with authors.
2.2. Inclusion and exclusion criteria
Criteria for inclusion and exclusion were defined prior to conducting the searches (Appendix S2). Sources were only included in the review if they were published, and if they reported primary data, and if they related to any chronic wound type(s) (AU, DFU, PI, VLU), and if they measured any of the outcome(s) of interest (prevalence, incidence, rates of infection, hospitalisation, amputation, healing, and/or recurrence), and if they were conducted in Australia. Studies reporting wound types discretely and in combination were considered for inclusion. Studies conducted using routinely collected health data as well as epidemiological studies on chronic wounds were considered for inclusion. Sources were limited by language (English). For relevancy in reporting, sources were also limited by date (January 1, 1990 to May 2, 2017 inclusive).
2.3. Screening
The sources retrieved were screened by title and abstract; those that appeared to meet the inclusion criteria were then retrieved and read in full. Two researchers (L.M. and S.R.) independently assessed the sources for eligibility. Where disagreements occurred, reviewers discussed these with the study's primary investigator (R.P.) to reach consensus.
2.4. Quality assessment
The quality of the selected sources was assessed using a tool designed to assess the risk of bias in population‐based prevalence studies,20 which was modified for our study (Appendix S2).
2.5. Data extraction
A data extraction tool was developed by the research team to extract 10 data items about key features of the studies, including publication details, setting, design, sample, instrument, and parameters of interest (Appendix S3). Data were extracted collaboratively by two researchers (L.M. and S.R.).
Two researchers (L.M. and S.R.) independently evaluated each of the sources for quality (Appendix S4). Again, disagreements were resolved through discussions with a senior team member (R.P.) until consensus was reached. The total quality score for each study was the sum of the scores for each individual assessment item. This was converted to a proportional quality score (the total quality score divided by the maximum score possible expressed as a percentage). A source received an unfavourable rating on any quality evaluation question where there was insufficient information reported within it to answer the evaluation question with confidence.20
3. RESULTS
Of 1274 records screened, 90 studies met the criteria for inclusion (Figure 1).
Figure 1.
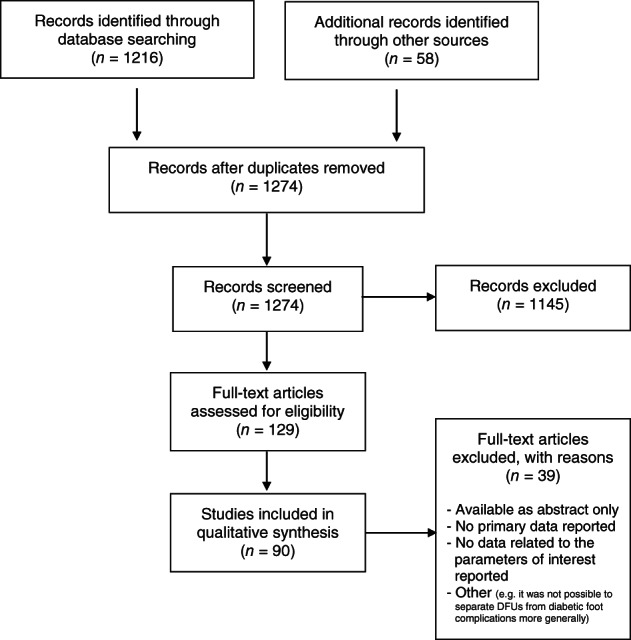
The PRISMA flowchart illustrating study selection
A summary of study characteristics for each of the 90 studies selected for inclusion is presented in Appendix S3. The studies were published from 199121 to 201622, 23, 24, 25, 26, 27 inclusive. Cohorts from all six states and two territories in Australia were included in at least one study. The studies were a mix of retrospective and prospective designs, undertaken in acute health care facilities (eg, hospitals), non‐acute health care facilities (eg, residential aged care settings), and/or community settings. Most studies were published in peer‐reviewed journals, although a number of government reports and two Doctor of Philosophy theses were also included. The studies each reported on one or more parameters of interest in relation to one or more chronic wound types.
Other key features of the studies are presented in Table 1.
Table 1.
Overall summary of characteristics of included studies
| Chronic wound type | Number of papers | Scope of papers | States/territories included | Range of quality scores |
|---|---|---|---|---|
| Arterial ulcers (AUs) | 119, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37 | All studies included small local (single‐site) or regional (multi‐site) populations, in single states | NSW31; QLD9, 34; TAS33; VIC37; WA28, 29, 30, 32, 35, 36 | 40%34, 36 to 80%35 |
| Diabetic foot ulcers (DFUs) | 239, 22, 28, 32, 34, 35, 37, 38, 39, 40, 41, 42, 86, 87, 88, 89, 92, 95, 96, 97, 98, 99, 100 | Most studies included small local (single‐site) or regional (multi‐site) populations in single states/territories; there were two studies that included state‐wide cohorts40, 42 and one that included a multi‐state cohort89 | NSW88, 92; NT95, 96, 98; QLD,9, 22, 34, 42 VIC37, 41, 86, 87, 100; WA28, 32, 35, 38, 39, 40, 97, 99 | 30%87 to 80%35, 38, 39 |
| There was one study that included a multi‐state population89 | ||||
| Venous leg ulcers (VLUs) | 249, 21, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 43, 93, 94, 101, 102, 103, 104, 105, 106, 107, 108, 109 | Most studies included small local (single‐site) or regional (multi‐site) populations in single states; there were also studies that included state‐wide cohorts94 and two studies that included a multi‐state cohort43, 103 | NSW31; QLD9, 34, 93, 101, 102, 104, 105, 106, 109; TAS33; VIC37, 94, 108; WA21, 28, 29, 30, 32, 35, 36, 107 | 40%34, 36 to 80%35, 106 |
| There were two studies that included a multi‐state cohort43, 103 | ||||
| Pressure injuries (PIs) | 5223, 24, 25, 26, 30, 33, 35, 36, 37, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 90, 91 | Most studies included small local (single‐site) or regional (multi‐site) populations in single states; there were a number that included state‐wide or multi‐state cohorts24, 26, 43, 49, 61, 66, 68, 74 | ACT79; NSW26, 43, 51, 53, 55, 68, 69, 70; QLD23, 24, 25, 44, 45, 46, 48, 50, 52, 57, 73, 75, 76, 77, 78, 91; SA59; TAS33, 62; VIC37, 47, 54, 56, 58, 63, 66, 83; WA30, 35, 36, 60, 61, 64, 67, 74, 80, 81, 82, 90 | 40%36, 48, 52, 56, 76, 91 to 90%51, 66 |
| There was one study where the location was unclear71 | ||||
| There were a number of studies that included multi‐state cohorts43, 49, 65, 72 | ||||
| Leg ulcers (LUs) (generally, without dividing these into wounds of arterial, diabetic, venous, or other aetiology) | 1129, 31, 32, 34, 35, 42, 43, 61, 74, 84, 85 | Most studies included small local (single‐site) or regional (multi‐site) populations in single states; there were three studies that included state‐wide or multi‐state cohorts43, 61, 74 | NSW31, 43, 85; QLD34, 42; WA29, 32, 35, 61, 74, 84 | 40%34 to 90%42, 61 |
3.1. Prevalence
3.1.1. Arterial ulcers
All of the studies on AUs reported prevalence. Most measured prevalence in people with lower‐extremity ulcers specifically.9, 28, 29, 30, 31, 32, 33, 34, 35 Prevalence of AUs as a primary cause of ulceration in this population ranged from 3.0%32 to 19.0%.33 Other studies measured prevalence in people with all types of wounds (including chronic, surgical, and traumatic wounds).36, 37 Prevalence of AUs as a primary cause of ulceration in this population ranged from 1.0%37 to 10.9%.36 One study found that 74.5% of people with foot ulcers specifically had associated arterial disease.28
3.1.2. Diabetic foot ulcers
Many of the papers on DFUs reported prevalence. Some measured prevalence in people with lower‐extremity ulcers specifically.9, 28, 32, 34, 35 Prevalence of DFUs as a primary cause of ulceration in this population ranged from 2.5%28 to 12.0%.35 One paper measured the prevalence of DFUs in people with all types of wounds (including chronic, surgical, and traumatic wounds) and reported this to be 2.6%.37
Several studies reported on the prevalence of DFUs in all people with diabetes38, 39, 40; this ranged from 1.2%38 to 2.5%.40 Prevalence of DFUs was reported to be 1.0% in the first year of diabetes diagnosis.40 One study found that, of people with diabetes‐related foot complications, 32.6% had a DFU specifically.41 Other studies reported that diabetes mellitus was found in 48.5%28 to 85.0%42 of people with foot ulcers.
3.1.3. Venous leg ulcers
Most of the studies on VLUs reported prevalence. Some measured prevalence in people with lower‐extremity ulcers specifically.9, 28, 29, 30, 31, 32, 33, 34, 35, 43 Prevalence of VLUs as a primary cause of ulceration in this population ranged from 1.0%35 to 70.5%.34 Two studies measured prevalence in people with all types of wounds (including chronic, surgical, and traumatic wounds); the prevalence of VLUs as a primary cause of ulceration in this population was reported to be between 3.1%37 and 53.1%.36 In a large, population based study in Perth, prevalence in people ≥60 years was 3.3 per 1000.21
3.1.4. Pressure injuries
Most of the studies on PIs reported prevalence. Some measured the prevalence of PIs in acute health care facilities (eg, hospitals).33, 35, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68 Prevalence ranged from 0.2%49 to 29.6%59 in hospital settings. Other papers reported prevalence of PIs in specific populations in acute health care settings, including in medical patients: 3.8%,68 in surgical patients: 4.1%,68 in people undergoing coronary artery bypass graft: 2.9%,69 in people undergoing orthopaedic hip replacement: 3.3%,69 in people with dementia: 4.0%,68 in people receiving intensive care: 11.5%24 to 50.0%,70 and in long‐stay patients (≥91 days): 25.0%.67
Some papers measured the prevalence of PIs in non‐acute health care facilities (eg, residential aged care settings).26, 71, 72, 73 Prevalence ranged from 0.03%73 to 25.9%.72
Some larger studies involved a mix of acute and non‐acute health care facilities26, 74; these measured the prevalence of PIs to be between 9.1%26 and 12.5%.74 In people in acute and non‐acute health care facilities who were classified as malnourished, the prevalence of PIs was measured at 31.5%.75
Many of the studies that measured PI prevalence in health care facilities reported on rates of health care‐, versus community‐, acquired PIs.24, 26, 47, 50, 53, 54, 60, 64, 66, 76 One study found the prevalence of PIs on admission to hospital to be 4.9%, versus prevalence at discharge of 5.7%.77 Another study measured the prevalence of medical device‐related PIs in acute health care settings specifically to be 6.1%.76
Most of the studies that measured PI prevalence in acute and non‐acute health care facilities also reported on PI staging.24, 26, 37, 44, 45, 47, 51, 53, 55, 58, 59, 60, 61, 63, 64, 66, 67, 69, 72, 73, 74, 78, 79, 80, 81, 82 The majority of PIs in these studies were at Stage I (non‐blanchable erythema only). In a state‐wide sample of acute and non‐acute health care settings, the proportion of PIs in Stage I was estimated at 44.0%.26
Some of the studies measured the prevalence of PIs in the community. In studies involving general practitioners or community nursing services,26, 80, 81 the prevalence of PIs—as a percentage of total presentations—ranged from 7.7%26 to 42.3%.80 One study measured prevalence in people with lower‐extremity ulcers in the community specifically, 5.0%.43 Other papers reported on prevalence in people with wounds generally (including chronic, surgical, and traumatic wounds)30, 36, 37; prevalence of PIs as a primary cause of ulceration in this population ranged from 6.0%30 to 11.0%.37
It is important to acknowledge that some of the health care facilities involved in the above studies had PI improvement initiatives in place, whereas others did not. Several studies reported on declines, often significant, in PI prevalence as a result of such interventions.53, 56, 57, 59, 60, 65, 70, 71, 75, 79, 83 For these studies, baseline (pre‐intervention) PI prevalence is reported above.
3.1.5. Leg ulcers
Some studies reported prevalence in people presenting to community health care services31, 43, 84, 85; prevalence was reported at 1.184 to 7.043 per 1000 patient encounters and at 0.1%31 and 0.3%85 of all patient encounters. Prevalence was estimated at 5.9 per 1000 in people aged ≥60 years,21 at 0.6% in people aged ≥65 years,31 and at 24 per 1000 in people aged ≥75 years.43 Among people presenting to a community health care service with a wound (including chronic, surgical, and traumatic wounds), 48.2% had an LU.29 Two studies measured the prevalence of LUs in hospitalised patients; prevalence ranged from 2.3%61 to 2.8%.74 One study reported the prevalence of all‐cause foot ulcers among hospitalised patients; 9.8% of people reported having a previous foot ulcer, and 6.3% were found to have a current foot ulcer.27
3.2. Incidence
3.2.1. Arterial ulcers
None of the studies on AUs reported incidence.
3.2.2. Diabetic foot ulcers
Some of the papers on DFUs reported incidence. One study reported that 6.3% of people with diabetes mellitus developed a new DFU in a 3‐month study period.86 Another study found that 34.2% of people developed a new DFU in the study period, but this was a short report, and the study period was not specified.87 Another paper found that 6.3% of people with diabetes mellitus and neuropathy developed a DFU, compared with 0.5% of people with diabetes mellitus but without neuropathy.88 An Australia‐wide retrospective cross‐sectional population survey found that 19.6% of people with diabetes mellitus had clinical features, which placed them “at risk” of developing a DFU89; however, this paper did not measure or estimate how many of these people actually developed a DFU.
3.2.3. Venous leg ulcers
None of the studies on VLUs reported on incidence.
3.2.4. Pressure injuries
Several papers on PIs reported incidence.23, 45, 46, 55, 59, 64, 67, 78, 79, 82, 83, 90, 91 These papers measured incidence over a variety of time periods, from 7 days55 to 12 months.78 Some papers reported the incidence of PIs in general medical patients in acute health care settings (eg, hospitals)46, 55, 59, 67, 79, 83, 90; incidence ranged from 6.5% in 7 days (shortest time period)55 to 16.6% in 6 months (longest time period).83 Other papers reported incidence of PIs in people undergoing various surgical procedures23, 45, 82, 91; incidence ranged from 11.1% in 6 weeks (shortest time period)82 to 11.8% in 7 months (longest time period).23 One study reported on the incidence of PIs in people in intensive care settings, 30.4% in 12 months.78 One study estimated the risk of developing a healthcare‐associated PI in a hospital to be between 9.8% and 12.0%, equating to 7.2 to 7.6 per 1000 bed days.64
3.2.5. Leg ulcers
None of the studies on LUs reported incidence.
3.3. Infection
3.3.1. Arterial ulcers
One paper found that 16.7% of the people with AUs showed signs of infection; however, this equated to just 1 of 6 people with AUs included in the study.9
3.3.2. Diabetic foot ulcers
Three of the papers on DFUs reported rates of infection9, 41, 92; between 14.6%41 and 49.7%92 of DFUs showed clinical sign(s) of infection.
3.3.3. Venous leg ulcers
Three of the papers on VLUs reported rates of infection.9, 93, 94 In groups receiving standard care or baseline cohorts, infection ranged from 5.9%93 to 58.1%.94
3.3.4. Pressure injuries and leg ulcers
None of the included studies reported rates of infection for PIs and LUs.
3.4. Hospitalisation
3.4.1. Arterial ulcers
None of the studies on AUs reported rates of hospitalisation.
3.4.2. Diabetic foot ulcers
One paper found that an infected DFU was the primary cause of hospitalisation in 79 admissions per 100 000 person‐years.95 This study also reported that the median duration of hospital stay once admitted with DFU‐related complication(s), and particularly infection, was 29.0 days.95 Another study measured the incidence of first‐ever hospital admission for DFU to be 5.21 per 1000 patient‐years.38 Another study found that 1.8% of people with diabetes mellitus had been hospitalised for complications related to a DFU.39
3.4.3. Venous leg ulcers
One study reported that 6.0% of people with VLUs were admitted to hospital because of failure of the wound to heal and/or wound deterioration.32
3.4.4. Pressure injuries
None of the studies on PIs reported rates of hospitalisation; rather, reporting focused on mean length of hospital stay. One study found that the mean length of hospital stay for general medical and surgical patients who developed a PI was 61.1 days.65 Another reported that the mean length of hospital stay for general medical and surgical patients who developed a PI was 34.0 days, versus 25.0 days for people who did not develop a PI.67 Another study measured the hospital stay for people undergoing coronary artery bypass graft who developed a PI at 22.4 days, versus 12.7 days for patients who did not develop a PI, and for people undergoing an orthopaedic hip replacement who developed a PI at 31.2 days, versus 19.7 days for patients who did not develop a PI.69
3.4.5. Leg ulcers
Two papers on LUs reported rates of hospitalisation; these studies found that between 4.5%34 and 13.8%32 of people with LUs were admitted to hospital because of complications with their wound.
3.5. Amputation
3.5.1. Arterial ulcers
None of the studies on AUs reported on rates of amputation.
3.5.2. Diabetic foot ulcers
Several studies on DFUs reported on rates of DFU‐related amputation.22, 92, 95, 96, 97, 98 The studies measured rates of ≥1 minor amputation (below the ankle), ranging from 2.1%92 to 36.5%,96 and rates of ≥1 major amputation (above the ankle), ranging from 0.5%92 to 23.0%.96 One study found that, in people who had one minor amputation for a DFU‐related complication, 26.0% also had at least one subsequent minor amputation, and 18.5% had at least one subsequent major amputation.95
One study reported that DFU was a significant independent predictor of first‐ever lower‐extremity amputation in people with diabetes mellitus (hazard ratio [95% CI]: 5.56 [1.24‐25.01]).97 Another found that DFU was the major cause of amputation in 17.2% of all amputations performed in a major metropolitan hospital in a 2‐year period.99 Another study concluded that, of the 7.0% of people with diabetes mellitus who experienced an amputation (minor or major), 34.0% were the direct result of a DFU.98
3.5.3. Venous leg ulcers
None of the studies on VLUs reported on rates of amputation.
3.5.4. Pressure injuries
None of the included studies on PIs reported on rates of amputation.
3.5.5. Leg ulcers
One study found that, among people with LUs receiving standard care, 13.9%35 had an amputation.
3.6. Healing
3.6.1. Arterial ulcers
Three studies reported on median time to healing for AUs. One study reported that 33.3% of AUs healed in ≤12 months.36 In another study, median time to healing of AUs was measured at 107.0 days.37 In a third study, data on median time to healing were presented graphically and could not be quantified.9
3.6.2. Diabetic foot ulcers
The studies on DFUs reported healing in a variety of ways. Some measured healing in a given period. One study reported that 74.8% of DFUs in people receiving standard care healed in ≤28 days.100 Another study found that 47.0% of DFUs healed in 12 weeks, and 72.0% healed in 20 weeks.41 Three studies reported median time to healing for DFUs in people receiving standard care,37, 41, 42 ranging from 6.042 to 15.7 weeks.41 In one study, time to healing for DFUs was presented graphically and could not be quantified.9
3.6.3. Venous leg ulcers
The studies on VLUs also reported healing in a variety of ways. Some reported healing in groups receiving standard care—at ≤12 weeks,93, 101, 102, 103 ranging from 23.5%93 to 45.1%103; at 24 weeks: 38.5%104; at 6 months: 73.6%32; and at 12 months: 67.7%.36 Some reported healing in groups receiving specialist care—at ≤12 weeks,9, 93, 101, 102, 103 ranging from 43.6%93 to 73.0%103; and at 24 weeks: 57.6%.104 In a group receiving specialist care, 96.8% of low‐risk patients and 25.0% of high‐risk patients healed in 24 weeks.105
Other studies reported healing of VLUs in comparison groups receiving different specialist interventions—for example, three‐layer versus four‐layer compression bandaging (72.0% vs 84.0% healing in 24 weeks),106 and with different types of dressings, ranging from 58.7% to 86.0% in 9 months.107
One study found the median time to healing for VLUs to be 63.9 days.37 In one study, time to healing for VLUs was presented graphically and could not be quantified.9
3.6.4. Pressure injuries
The papers on PIs reported healing in a variety of ways. One study found that the average time to healing of a PI was 57.9 days; the average time to healing for Stage I PIs was 45.6 days, Stage II PIs was 56.5 days, Stage III PIs was 58.9 days, and Stage IV PIs was 58.3 days.37 Another study reported that, among people presenting to a community wound clinic with a PI, 100.0% had healed in ≤12 months.36 A third study found that, with an intensive nutrition intervention, 58.1% of malnourished people with a PI healed within the period of their hospital admission, with length of admission averaging 14.0 days.25
3.6.5. Leg ulcers
Studies on LUs reported outcomes related to healing in a variety of ways. Studies reported that, with standard care, between 20.3%31 and 38.8%32 of LUs healed in 3 months, 67.0%32 healed in 6 months, and 92.6%32 healed in 12 months. Another study reported that, in uncomplicated LUs, mean time to healing was 4.6 weeks, and in LUs with one or more complications, mean time to healing was 23.9 weeks.34 In a control group, mean rate of healing by ulcer area was reported to be 6.3% per week.35 The mean duration of LUs prior to healing among the people participating in one study was reported to be 9.0 years.31
3.7. Recurrence
3.7.1. Arterial ulcers
None of the studies on AUs reported rates of recurrence.
3.7.2. Diabetic foot ulcers
One study found that 3.6% of people who presented to a health care service with a DFU had had at least one previous DFU.92 Another study reported a 37.0% rate of recurrence.42
3.7.3. Venous leg ulcers
The studies on VLUs defined and measured rates of recurrence in multiple ways. In most studies, recurrence was defined as a new ulcer developing after the patient healed and could be on the other leg or in another location. Some measured the number of people with a current VLU who reported a previous VLU21, 93, 94, 108; this ranged from “half”, assumed to be 50.0%,108 to 81.7%.94 Other studies measured recurrence after healing within 5 weeks: 23.1%103; at 3 months, ranging from 5.6%9 to 36.0%109; at 6 months: 73.5%32; and at 12 months,9, 109 ranging from 16.7%9 to 20.0%.109 Other studies reported a median time to recurrence ranging from 11.194 to 63.0 weeks.9
3.7.4. Pressure injuries
None of the studies on PIs reported rates of recurrence.
3.7.5. Leg ulcers
One study found that 65.0% of people who presented to a community health care service with an LU had at least one previous LU.28
3.8. Study quality and risk of bias
Appendix S4 describes the quality assessment and scoring results for each of the studies selected for inclusion. Overall quality scores ranged from 30% to 90%. Of the 90 included studies, 4 scored 90%27, 51, 61, 66; we concluded that these studies had relatively high internal and external validity, and risk of bias was considered minimal in these studies. Twenty‐two of the studies scored ≤50% in terms of quality; we concluded that risk of bias was relatively high for these studies, particularly regarding representativeness of the study population, selection bias, non‐response bias, and lack of use of an acceptable case definition. The quality of the included studies was moderate, with an average quality score of 64%.
4. DISCUSSION
To the best of our knowledge, this is the first review of published studies reporting on the prevalence, incidence and rates of infection, hospitalisation, amputation, healing, and recurrence of chronic wounds in Australia. A total of 90 studies were included.
A key finding to emerge from this review is that all types of chronic wounds—AUs, DFUs, VLUs, and PIs—are highly prevalent in Australia. There was a considerable amount of data on the prevalence of all wound types in a variety of cohorts. However, of the studies selected for inclusion, most were published prior to 2010 and were not representative of the Australian population. Given population ageing and the obesity epidemic, the prevalence of chronic wounds has probably increased in recent years. Prevalence was reported in specific populations—for example, in people with lower‐extremity ulcers, people presenting to community wound services, people admitted to hospital, and people with comorbidities such as diabetes mellitus. None of the studies selected for inclusion gave an estimate of the prevalence of chronic wounds in the general Australian population. As a result, it remains difficult to estimate the number or people currently affected with chronic wounds in Australia.
It is interesting to compare our findings about the prevalence of chronic wounds in Australia—a key parameter for economic modelling—with the international literature. A recent literature review involving 69 international studies110 returned the following findings:
4.1. Arterial ulcers
Internationally, the prevalence of AUs in the community was 0.02% to 0.35%110 (compared with our finding of 3.0% to 19.0% in people with lower‐extremity ulcers and 0.7% to 10.9% in people with wounds generally). This review supported our finding of a paucity of evidence on the prevalence (and incidence) of AUs.110
4.2. Diabetic foot ulcers
Internationally, the prevalence of DFUs in acute health care facilities (eg, hospitals) ranged from 1.2% to 20.4%, and in non‐acute health care facilities (eg, residential aged care settings), it ranged from 0.02% to 9.0%110 (compared with our finding of 2.5% to 12.0% in people with lower‐extremity ulcers and 2.6% in people with wounds generally).
4.3. Venous leg ulcers
Internationally, the prevalence of VLUs in acute health care facilities (eg hospitals) was 0.05%; in non‐acute health care facilities (eg, residential aged care settings), it was 2.5%; and in the community, it ranged from 0.05% to 1.0%110 (compared with our finding of 1.0% to 70.5% in people with lower‐extremity ulcers and 2.3% to 53.1% in people with all types of wounds).
4.4. Pressure injuries
The prevalence of PIs in acute health care facilities (eg hospitals) ranged from 1.1% to 26.7%110 (compared with our finding of 0.2%‐29.6%); in people receiving intensive care, it ranged from 13.1% to 28.7%110 (compared with our finding of 11.5%‐50.0%); and in non‐acute health care facilities (eg residential aged care settings), it ranged from 7.6% to 53.2%110 (compared with our finding of 0.03%‐25.9%).
The same problem we encountered with reporting prevalence—as noted above, that this was population‐specific—was also found regarding incidence. Again, there was a considerable amount of data on the incidence of all wound types, in a variety of cohorts; however, incidence was typically reported in specific populations (such as those listed above). Aside from one study that provided an estimated risk of developing a health care‐associated PI during a hospital admission,64 none of the studies reported incidence rates of PIs in the Australian general population. In addition, incidence was measured over a variety of time frames, making comparison with the international literature review described above110 difficult. There were some difficulties with determining the difference between incidence and recurrence; in all instances, we used the same terminology as the study authors.
This review also returned important findings in relation to the clinical outcomes of interest—rates of infection, hospitalisation, amputation, healing, and recurrence. The literature selected for inclusion reported highly variable rates of infection for most chronic wound types; this was possibly because of problems with the definition and diagnosis of “infection,” discussed later. For most chronic wound types, rates of hospitalisation were relatively low; however, once a person was admitted to hospital for complications associated with a chronic wound, or if they developed a chronic wound whilst hospitalised (eg, a PI), their length of stay was likely to be considerable.
Rates of amputation were relevant mainly to DFUs, and the rates of both minor and major amputation for people with this type of chronic wound were high. There was a considerable amount of data on rates of healing for all wound types, and again, this was highly variable; this was possibly because of problems with treatment and confounding factors affecting the rates of healing, again discussed later. Finally, there were limited data on recurrence, but available data suggest that the risk of recurrence is high for DFUs and VLUs in particular.
Although some data were available on a few parameters for all chronic wound types—AUs, DFUs, VLUs, and PIs—in the studies selected for inclusion, there was a particularly large amount of data on PIs. Indeed, 60% of the studies identified for inclusion (n = 52) reported on PIs. There were a moderate number of studies on VLUs (n = 24) and DFUs (n = 23) but a relative paucity of data on AUs (n = 11). This is an important finding considering this review suggests that AUs are not significantly less prevalent than DFUs, and perhaps VLUs, by some measures. The apparent paucity of literature on AUs may also be related to the inconsistencies, and lack of clarity, in defining different ulcer types, particularly distinguishing between AUs and VLUs.
This review also found an absence of data for a number of key clinical outcomes. There were no data reported in the studies selected for inclusion on the rates of infection in PIs; rates of amputation in AUs, VLUs or PIs; and rates of recurrence in AUs and PIs. Of note was the limited data available on rates of hospitalisation because of complications for specific types of chronic wounds. This represents an important gap in the existing knowledge and a possible focus for future Australian research.
As noted, the majority of studies were small local (single‐site) or slightly larger regional (multi‐site) studies. There were only a few state‐wide studies, fewer multi‐state studies, and two nation‐wide studies43, 89 identified. Most studies included small cohorts from specific locations—often, a single or small group of health care facilities—limiting generalisability. This is particularly problematic as the quality assessment indicated that the likelihood of non‐response bias and selection bias in many of the studies was high. The few larger studies also had limitations. The state‐wide and multi‐state studies focused on New South Wales,26, 43, 65, 68, 71, 72 Queensland,24, 42, 49, 65 South Australia,71, 72 Victoria,49, 65, 66, 71, 72, 94 and Western Australia,40, 61, 65, 71, 72, 74 with the less‐populated states and territories of Tasmania, the Northern Territory, and the Australian Capital Territory nearly entirely overlooked. In addition, two nation‐wide studies that were included had significant limitations. The first did not directly measure any of the outcomes of interest for this review but instead reported on the concept of people with diabetes mellitus “at risk” of developing a DFU.89 The second was reported as a conference abstract only.42
5. STUDY LIMITATIONS
The findings of this systematic review should be interpreted in light of a number of limitations of our review. There were significant problems with how the different chronic wound types (AU, DFU, VLU, and PI) were defined in the studies. Some used clear definitions of wound types—based on an international consensus definition (eg, those contained in a reliable and valid assessment tool) or clear diagnostic criteria—but many did not. This made it difficult to determine the accuracy of outcomes reported about a particular wound type. This was especially problematic in the retrospective studies, where it was typically difficult to determine how chronic wounds were assessed, their aetiology diagnosed, and if this was a standardised process for all participants included in the study. These studies frequently received low‐quality scores for this reason. There were also problems with the definitions used by the small number of studies that considered “leg ulcers” a group; some of these studies included in their definition of “leg ulcers” other types of wounds such as skin tears, burns, and malignancies. Again, for this reason, these studies typically received relatively low‐quality scores.
Many of the studies used non‐standardised definitions for the other key outcomes—in particular, for wound infection and also for hospitalisation, healing, and recurrence. This led to outcomes being measured in different ways—for example, hospitalisation may have been measured as rate of hospital admission or length of stay. Similarly, recurrence may have been measured as recurrence of a known wound or history of previous chronic wound(s) of the same aetiology as the current wound. Non‐standardised definitions also resulted in variability in outcomes between studies—for example, the two studies reporting on rates of infection in VLUs, which included comparable cohorts and involved similar research methods, reported highly discrepant rates of infection: 5.9%93 and 58.1%.94 Different definitions precluded a meta‐analysis and resulted in difficulties reporting results in meaningful ways.
There were also problems with the way in which healing was measured and reported in many of the studies. Some studies compared rates of healing in standard care (control) against specialist care (intervention) groups, but many did not. A large number of studies reported “healing” without specifying the treatment(s), if any, used on the wound. This outcome was therefore highly exposed to confounding and difficult to report with accuracy.
A number of studies on DFUs originally identified for inclusion in the review111, 112, 113 were subsequently excluded because they grouped DFUs with other diabetes‐related foot complications—for example, peripheral neuropathy, peripheral vascular insufficiency, cellulitis, Charcot arthropathy, or osteomyelitis. When reporting on outcomes such as amputation, it was not possible to determine in these studies if amputation was because of a DFU specifically (as per our inclusion criteria) or other diabetes‐related foot complications more generally or even a combination of both. For this reason, these studies were excluded.
There were also some limitations with the review process that must be acknowledged. A limited number of databases were searched, and it is possible that sources, including grey literature, published elsewhere were missed. The data extraction tool was not validated. Although three researchers were involved in the assessment of the study quality process (LM, SR, RP), only one (LM) conducted the final synthesis of the data, and no rigorous inter‐rater checks were conducted.
6. CONCLUSION AND RECOMMENDATIONS
In this paper, we have presented the method and findings of a reproducible literature review regarding evidence on important epidemiological parameters of prevalence and incidence and key clinical parameters of rates of infection, hospitalisation, amputation, healing, and recurrence of chronic wounds in Australia. We demonstrate that there are large gaps and limitations in the existing evidence. The knowledge gaps in some key parameters need to be addressed as a matter of urgency. The effective implementation and evaluation of evidence‐based wound care depends on the availability of reliable and comparable information, and as better‐quality evidence becomes available, future economic modelling will be more accurate and reliable.
We recommend targeted primary research to establish the epidemiological profile of chronic wounds in Australia. A nationally representative prevalence survey should be conducted at regular intervals and in line with international best practice to identify the baseline prevalence and size of the problem in Australia. In addition, a national wound registry should be established to provide real patient data on clinical wound outcomes and facilitate comparative effectiveness research to identify patients needing advanced treatment. For this to be achieved, a number of barriers to collaboration between sectors must be overcome—including establishment costs and jurisdictional funding issues, sensitivities around data sharing, and the challenge of the sustainability of chronic wound services.
ACKNOWLEDGEMENTS AND FUNDING STATEMENT
The authors acknowledge the support of the Australian Government's Cooperative Research Centres Program. The Wound Management Innovation Cooperative Research Centre (WMI CRC) received funding from the Australian Government, Curtin University of Technology, Queensland University of Technology, Smith & Nephew Pty Limited, Southern Cross University, University of South Australia, Wounds Australia, Blue Care, the Department of Health South Australia, the Department of Health Victoria, Ego Pharmaceuticals Pty Ltd, Metropolitan Health Service/Wounds West, Queensland Health, Royal District Nursing Service Limited, Royal Melbourne Institute of Technology, and Silver Chain Group. The funders had no role in the design of the study and collection, analysis and interpretation of data, and writing and submitting the manuscript for publication.
Supporting information
Appendix S1. PRISMA Checklist
Appendix S2. Review protocol
Appendix S3. Summary of Study Characteristics
Appendix S4. Quality assessment of the studies selected for inclusion.
McCosker L, Tulleners R, Cheng Q, et al. Chronic wounds in Australia: A systematic review of key epidemiological and clinical parameters. Int Wound J. 2019;16:84–95. 10.1111/iwj.12996
REFERENCES
- 1. Nunan R, Martin P. Clinical challenges of chronic wounds: searching for an optimal animal model to recapitulate their complexity. Dis Models Mech. 2014;7(11):1205‐1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wedin F, Schaller H, Rennekampff H. Evidence‐based management strategies for treatment of chronic wounds. J Plast Surg. 2009;9(19):169‐179. [PMC free article] [PubMed] [Google Scholar]
- 3. Graves N, Zheng H. Modelling the direct health care costs of chronic wounds in Australia. Wound Pract Res. 2014;22(1):20‐33. [Google Scholar]
- 4. Gonzalez‐Consuegra R, Verdu J. Quality of life in people with venous leg ulcers: an integrative review. J Adv Nurs. 2011;67(5):926‐944. [DOI] [PubMed] [Google Scholar]
- 5. Hopman W, van der Kerkhof EG, Carley ME, Kuhnke JL, Harrison MB. Factors associated with health‐related quality of life in chronic leg ulceration. Qual Life Res. 2014;23(6):1833‐1840. [DOI] [PubMed] [Google Scholar]
- 6. Palfreyman S. Assessing the impact of venous ulceration on quality of life. Nurs Times. 2008;104(41):34‐37. [PubMed] [Google Scholar]
- 7. Hopman W, van der Kerkhof EG, Carley ME, Harrison MB. Health‐related quality of life at healing in individuals with chronic venous or mixed‐venous leg ulceration: a longitudinal assessment. J Adv Nurs. 2016;72(11):2869‐2878. [DOI] [PubMed] [Google Scholar]
- 8. Brown A. Chronic leg ulcers, part 2: do they affect a patient's social life? Br J Nurs. 2005;14(18):986‐989. [DOI] [PubMed] [Google Scholar]
- 9. Edwards H, Finlayson K, Courtney M, Graves N, Gibb M, Parker C. Health service pathways for patients with chronic leg ulcers: identifying effective pathways for facilitation of evidence based wound care. BMC Health Serv Res. 2013;13:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harrison MN, Graham ID, Lorimer K, Friedberg E, Pierscianowski T, Brandys T. Leg ulcer care in the community, before and after implementation of an evidence‐based service. Can Med Assoc J. 2005;172(11):1447‐1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bayoumi A, John‐Baptiste A, Chen M, Chen W, Farahati F, Krahn M, et al. The cost‐effectiveness of prevention strategies for pressure ulcers in long‐term care homes in Ontario: Projections of the Ontario pressure ulcer model. Toronto, Ontario, Canada; 2008.
- 12. Cheng Q, Lazzarini P, Gibb M, et al. A cost‐effectiveness analysis of optimal care for diabetic foot ulcers in Australia. Int Wound J. 2017;14(4):616‐628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ragnarson‐Tennvall G, Apelqvist J. Prevention of diabetes‐related foot ulcers and amputations: a cost‐utility analysis based on Markov model simulations. Diabetologia. 2001;44(11):2077‐2087. [DOI] [PubMed] [Google Scholar]
- 14. Korn PPS, Heller JA, Deitch JS, Krishnasastry KV, Bush HL, Kent KC. Why insurers should reimburse for compression stockings in patients with chronic venous stasis. J Vasc Surg. 2002;35(5):1‐8. [DOI] [PubMed] [Google Scholar]
- 15. Cooper N, Coyle D, Abrams K, Mugford M, Sutton A. Use of evidence in decision models: an appraisal of helth technology assessments in the UKsince 1997. J Health Serv Res Policy. 2005;10(4):245‐250. [DOI] [PubMed] [Google Scholar]
- 16. Briggs A, Claxton K, Sculpher M. Decision Modelling for Health Economic Evaluation. Oxford, UK: Oxford University Press; 2006. [Google Scholar]
- 17. Petrou S, Gray A. Economic evaluation using decision analytical modelling: design, conduct, analysis, and reporting. BMJ. 2011;342:d1766. 10.1136/bmj.d1766 [DOI] [PubMed] [Google Scholar]
- 18. Weinstein M, O'Brien B, Hornberger J, et al. Principles of good practice for decision analytic modelling in health‐care evaluation: report of the ISPOR task force on good research practices—modeling studies. Value Health. 2006;6(1):9‐17. [DOI] [PubMed] [Google Scholar]
- 19. Moher D, Liberati A, Tetzlaff D, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. PLoS Med. 2009;6(7): e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hoy DBP, Woolf A, Blyth F, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65(9):934‐939. [DOI] [PubMed] [Google Scholar]
- 21. Baker S, Stacey M, Jopp‐McKay A, Hoskin S, Thompson P. Epidemiology of chronic venous ulcers. Br J Surg. 1991;78(7):864‐867. [DOI] [PubMed] [Google Scholar]
- 22. Rodrigues B, Vangaveti VN, Malabu UH. Prevalence and risk factors for diabetic lower limb amputation: a clinic‐based case control study. J Diab Res. 2016;2016:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McRae P, Walker PJ, Peel NM, et al. Frailty and geriatric syndromes in vascular surgical ward patients. Ann Vasc Surg. 2016;35:9‐18. [DOI] [PubMed] [Google Scholar]
- 24. Coyer F, Miles S, Gosley S, et al. Pressure injury prevalence in intensive care versus non‐intensive care patients: A state‐wide comparison. Aus Crit Care. 2017; 30(5): 244‐250. [DOI] [PubMed] [Google Scholar]
- 25. Banks M, Ross L, Webster J, et al. Pressure ulcer healing with an intensive nutrition intervention in an acute setting: a pilot randomised controlled trial. J Wound Care. 2016;25(7):384‐392. [DOI] [PubMed] [Google Scholar]
- 26. Clinical Excellence Commission . 2016 NSW Pressure Injury Point Prevalence Survey Report. Sydney: Clinical Excellence Commission, 2017.
- 27. Lazzarini P, Hurn S, Kuys S, et al. Direct inpatient burden caused by foot‐related conditions: a multisite point‐prevalence study. BMJ Open. 2016;6:e010811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Baker S, Stacey M, Singh G, Hoskin S, Thompson P. Aetiology of chronic leg ulcers. Eur J Vasc Surg. 1992;6:245‐251. [DOI] [PubMed] [Google Scholar]
- 29. Carville K, Lewin G. Caring in the community: a wound prevalence survey. Prim Intent. 1998;6(2):54‐62. [Google Scholar]
- 30. Carville K, Smith J. A report on the effectiveness of comprehensive wound assessment and documentation in the community. Prim Intent. 2004;12(1):41‐49. [Google Scholar]
- 31. Hoskins A, Ramstadius B, Sibbald J. The Illawarra leg ulcer study. Prim Intent. 1997;5(3):24‐30. [Google Scholar]
- 32. Jopp‐McKay A, Stagey MC, Rohr JB, Baker SR, Thompson PJ, Hoskin SE. Outpatient treatment of chronic venous ulcers in a specialised clinic. Australas J Dermatol. 1991;32(3):143‐149. [DOI] [PubMed] [Google Scholar]
- 33. Liew I, Sinha S. A leg ulcer clinic: audit of the first three years. J Wound Care. 1998;7(8):405‐407. [DOI] [PubMed] [Google Scholar]
- 34. Muller M, Morris K, Coleman K. Venous leg ulcer management: the Royal Brisbane Hospital leg ulcer clinic experience. Prim Intent. 1999:162‐166. http://www.woundsaustralia.com.au/journal/0704_04.pdf [Google Scholar]
- 35. Santamaria N, Carville K, Ellis I, Prentice J. The effectiveness of digital imaging and remote expert wound consultation on healing rates in chronic lower leg ulcers in the Kimberley region of Western Australia. Prim Intent. 2004;12(2):62‐70. [Google Scholar]
- 36. Rayner R. A review of the effectiveness of a nurse‐led rural community wound clinic. Prim Intent. 2007;15(3):130‐137. [Google Scholar]
- 37. Walker J, Cullen M, Chambers H, Mitchell E, Steers N, Khalil H. Identifying wound prevalence using the Mobile wound care program. Int Wound J. 2014;11(3):319‐325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Baba M, Davis W, Norman PE, Bruce DG, Davis TM, Davis T. A longitudinal study of foot ulceration and its risk factors in community‐based patients with type 2 diabetes: the Fremantle diabetes study. Diab Res Clin Pract. 2014;106:42‐49. [DOI] [PubMed] [Google Scholar]
- 39. Baba M, Davis W, Norman P, Davis T. Temporal changes in the prevalence and associates of foot ulceration in type 2 diabates: the Freemantle diabetes study. J Diabetes Complications. 2015;29:356‐361. [DOI] [PubMed] [Google Scholar]
- 40. Clarke P, Leal J, Kelman C, Smith M, Colagiuri S. Estimating the cost of complications of diabetes in Australia using administrative health‐care data. Value Health. 2008;11(2):199‐206. [DOI] [PubMed] [Google Scholar]
- 41. Perrin B. A retrospective audit of a diabetic foot clinic. Australas J Podiatr Med. 2006;40(2):23‐29. [Google Scholar]
- 42. Lazzarini P, O'Rourke SR, Russell AW, et al. Queensland's high risk foot database: tracking the length and width of Queensland's foot ulcers. J Foot Ankle Res. 2013;6(S1):201. [Google Scholar]
- 43. Charles J, Harrison C, Britt H. Chronic skin ulcers. Aust Fam Physician. 2014;43(9):587. [PubMed] [Google Scholar]
- 44. Webster J, Coleman K, Mudge A, et al. Pressure ulcers: effectiveness of risk‐assessment tools: a randomised controlled trial (the ULCER trial). BMJ Qual Saf. 2011;20:297‐306. [DOI] [PubMed] [Google Scholar]
- 45. Webster J, Lister C, Corry J, Holland M, Coleman K, Marquart L. Incidence and risk factors for surgically acquired pressure ulcers. J Wound Ostomy Continence Nurs. 2015;42(2):138‐144. [DOI] [PubMed] [Google Scholar]
- 46. Webster J, Gavin N, Nicholas C, Coleman K, Gardner G. Validity of the Waterlow scale and risk of pressure injury in acute care. Br J Nurs. 2010;19(6):S14, S16, S18. [DOI] [PubMed] [Google Scholar]
- 47. Wright R, Tiziani A. Pressure ulcer point prevalence study. Prim Intent. 1996;4:18‐23. [Google Scholar]
- 48. Graves N, Birrell F, Whitby M. Effect of pressure ulcers on length of hospital stay. Infect Control Hosp Epidemiol. 2005;26(3):293‐297. [DOI] [PubMed] [Google Scholar]
- 49. Jackson T, Nghiem HS, Rowell D, Jorm C, Wakefield J. Marginal costs of hospital‐acquired conditions: information for priority‐setting for patient safety programmes and research. J Health Serv Res Policy. 2011;16(3):141‐146. [DOI] [PubMed] [Google Scholar]
- 50. Miles S, Fulbrook P, Nowicki T, Franks C. Decreasing pressure injury prevalence in an Australian general hospital: a 10‐year review. Wound Pract Res. 2013;21(4):148. [Google Scholar]
- 51. Pearson A, Francis K, Hodgkinson B, Curry G. Prevalence and treatment of pressure ulcers in northern New South Wales. Aust J Rural Health. 2000;8(2):103‐110. [DOI] [PubMed] [Google Scholar]
- 52. Roosen K, Fulbrook P, Nowicki T. Pressure injury prevention: continence, skin hygiene and nutrition management. Aust J Nurs. 2010;18(2):31‐34. [PubMed] [Google Scholar]
- 53. Asimus M, Maclellan L, Li P. Pressure ulcer prevention in Australia: the role of the nurse practitioner in changing practice and saving lives. Int Wound J. 2011;8(5):508‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Barker A, Kamar J, Tyndall T, et al. Implementation of pressure ulcer prevention best practice recommendations in acute care: an observational study. Int Wound J. 2013;10(3):313‐320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Charlier C. Prevalence, incidence and risk: a study of pressure ulcers at a rural base hospital. Prim Intent. 2001;9(1):12. [Google Scholar]
- 56. Davenport J. Let's take the pressure off. J Stomal Ther Aust. 1999;17(2):5‐9. [Google Scholar]
- 57. Hunter M, Kelly J, Stanley N, et al. Pressure injury success in a regional hospital. Contemp Nurse. 2014;49:75‐82. [DOI] [PubMed] [Google Scholar]
- 58. Martin RK. AM the incidence and management of pressure ulcers in a metropolitan teaching hospital. Prim Intent. 1994;2(2):31‐34. [Google Scholar]
- 59. McErlean B, Prendergast J, Sandison S, et al. Implementation of a preventative pressure management framework. Prim Intent. 2002;10(2):61‐66. [Google Scholar]
- 60. McGowan S, Hensley L, Madocks J. Monitoring the occurrence of pressure ulcers in a teaching hospital: a quality improvement project. Prim Intent. 1996;4(1):9‐17. [Google Scholar]
- 61. Mulligan S, Prentice J, Scott L. WoundsWest Wound Prevalence Survey. Ambulatory Care Services, WA Government Department of Health; 2011.
- 62. Young C, Stoker F. A four‐year review of pressure ulcer prevalence. Prim Intent. 2000;8(1):6‐12. [Google Scholar]
- 63. Gardner A, Millar L, Legg S, Gomez Y, McGillion T, Mulcahy A. Pressure injury prevalence in a private health service: risks and recommendations. Wound Pract Res. 2009;17(3):134‐145. [Google Scholar]
- 64. Morey P, Porock D. A quality improvement survey of presure ulcers at a tertiary teaching hospital. Prim Intent. 1997;5(2):18‐25. [Google Scholar]
- 65. Prentice J. An Evaluation of Clinical Practice Guidelines for the Prediction and Prevention of Pressure Ulcers. Perth, Western Australia: University of Western Australia; 2007. [Google Scholar]
- 66. Services QaSB‐VGDoH . PUPPS 3—Pressure ulcer point prevalence survey statewide report 2006; 2006.
- 67. Young J, Nikoletti S, McCaul K, Twigg D, Porey P. Risk factors associated with pressure ulcer development at a major Western Australian teaching hospital from 1998‐2000: secondary data analysis. J Wound Ostomy Continence Nurs. 2002;29(5):234‐241. [DOI] [PubMed] [Google Scholar]
- 68. Bail K, Berry H, Grealish L, et al. Potentially preventable complications of urinary tract infections, pressure areas, pneumonia, and delirium in hospitalised dementia patients: retrospective cohort study. BMJ Open. 2013;3(6). https://bmjopen.bmj.com/content/3/6/e002770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lapsley HV, Vogels R. Cost and prevention of pressure ulcers in an acute teaching hospital. Int J Qual Health Care. 1996;8(1):61‐66. [DOI] [PubMed] [Google Scholar]
- 70. Elliott R, McKinley S, Fox V. Quality improvement program to reduce the prevalence of pressure ulcers in an intensive care unit. Am J Crit Care. 2008;17(4):328‐334. [PubMed] [Google Scholar]
- 71. Ellis I, Santamaria N, Carville K, et al. Improving pressure ulcer management in Australian nursing homes: results of the PRIME trial organisational study. Prim Intent. 2006;14(3):106‐111. [Google Scholar]
- 72. Santamaria N, Carville K, Prentice J, et al. Pressure ulcer prevalence and its relationship to comorbidity in nursing home residents: results from phase 1 of the PRIME trial. Prim Intent. 2005;13(3):107‐112. [Google Scholar]
- 73. Madsen W, Leonard M. Monitoring pressure ulcers in nursing homes. J Qual Clin Pract. 1997;17(1):209‐213. [PubMed] [Google Scholar]
- 74. Santamaria N. WoundsWest: identifying the prevalence of wounds within Western Australia's public health system. EWMA J. 2009;9(3):13‐18. [Google Scholar]
- 75. Banks M, Bauer J, Graves N, Ash S. Malnutrition and pressure ulcer risk in adults in Australian health care facilities. Nutrition. 2010;26:896‐901. [DOI] [PubMed] [Google Scholar]
- 76. Coyer F, Stotts N, Blackman V. A prospective window into medical device‐related pressure ulcers in intensive care. Int Wound J. 2014;11(6):656‐664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lakhan P, Jones M, Wilson A, Courtney M, Hirdes J, Gray LC. A prospective cohort study of geriatric syndromes among older medical patients admitted to acute care hospitals. J Am Geriatr Soc. 2011;59(11):2001‐2008. [DOI] [PubMed] [Google Scholar]
- 78. Coyer F, Gardner A, Doubrovsky A, et al. Reducing pressure injuries in critically ill patients by using a patient skin integrity care bundle (InSPiRE). Am J Crit Care. 2015;24(3):199‐210. [DOI] [PubMed] [Google Scholar]
- 79. Cubit K, McNally B, Lopez V. Taking the pressure off in the emergency department: evaluation of the prophylactic application of a low shear, soft silicon sacral dressing on high risk medical patients. Int Wound J. 2013;10(5):579‐584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lewin G, Carville K, Newall N, Phillipson M, Smith J, Prentice J. Determining the effectiveness of implementing the AWMA guidelines for the prediction and prevention of pressure ulcers in silver chain, a large home care agency. Prim Intent. 2003;11(2):57‐72. [Google Scholar]
- 81. Lewin G, Carville K, Newall N, Phillipson M, Smith J, Prentice J. Skin safe: implementing clinical guidelines to prevent pressure ulcers in home care clients. Prim Intent. 2007;15(1):4‐12. [Google Scholar]
- 82. Young J, Morey P, Browne R, Nikoletti S. A study on the incidence of presuer ulcers in the acute orthopaedic setting. Prim Intent. 2000;8(4):142‐147. [Google Scholar]
- 83. Jolley D, Wright R, McGowan S, et al. Preventing pressure ulcers with the Australian medical sheepskin: an open‐label randomised controlled trial. Med J Aust. 2004;180:324‐327. [DOI] [PubMed] [Google Scholar]
- 84. Baker R, Stacey M. Epidemiology of chronic leg ulcers in Australia. ANZ J Surg. 1994;64:258‐261. [DOI] [PubMed] [Google Scholar]
- 85. Johnson M. The prevalence of leg ulcers in older people: implications for community nursing. Public Health Nurs. 1995;12(4):269‐275. [DOI] [PubMed] [Google Scholar]
- 86. Perrin B, Gardner MJ, Kennett SR. The foot‐health of people with diabetes in a regional Australian population: a prospective clinical audit. J Foot Ankle Res. 2012;5(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Perrin B, Swerissen H, Payne C. The relationship between cognitive and emotional representations of peripheral neuropathy and incident diabetes‐related foot ulceration. J Foot Ankle Res. 2011;4(S1):37. [Google Scholar]
- 88. McGill M, Molyneaux L, Yue DK. Which diabetic patients should receive podiatry care? An objective analysis. Int Med J. 2005;35(8):451‐456. [DOI] [PubMed] [Google Scholar]
- 89. Tapp R, Shaw J, de Courten M, Dunstan D, Welborn T, Zimmet P. Foot complications in type 2 diabetes: an Australian population‐based study. Diab Med. 2003;20(2):105‐113. [DOI] [PubMed] [Google Scholar]
- 90. McGowan S, Montgomery K, Jolley D, Wright R. The role of sheepskins in preventing pressure ulcers in elderly orthopaedic patients. Prim Intent. 2000;8:127‐134. [Google Scholar]
- 91. McRae P, Peel NM, Walker PJ, de Looze JWM, Mudge AM. Geriatric syndromes in individuals admitted to vascular and urology surgical units. J Am Geriatr Soc. 2014;62(6):1105‐1109. [DOI] [PubMed] [Google Scholar]
- 92. Haji Zaine N, Burns J, Vicaretti M, Fletcher JP, Begg L, Hitos K. Characteristics of diabetic foot ulcers in Western Sydney, Australia. J Foot Ankle Res. 2014;7(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Edwards H, Courtney M, Finlayson K, Lewis C, Lindsay E, Dumble J. Improved healing rates for chronic venous leg ulcers: pilot study results from a randomized controlled trial of a community nursing intervention. Int J Nurs Pract. 2005;11(4):169‐176. [DOI] [PubMed] [Google Scholar]
- 94. Kapp S, Miller C, Donohue L. The clinical effectiveness of two compression stocking treatments on venous leg ulcer recurrence: A randomised controlled trial. Int J Low Extrem Wounds. 2013;12(3):189‐198. [DOI] [PubMed] [Google Scholar]
- 95. Commons R, Robinson C, Gawler D, Davis J, Price R. High burden of diabetic foot infections in the top end of Australia: an emerging health crisis (DEFINE study). Diab Res Clin Pract. 2015;110(2):147‐157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. O'Rourke I, Heard S, Treacy J, Gruen R, Whitbread C. Risks to feet in the top end: outcomes of diabetic foot complications. Aust NZ J Surg. 2002;72(4):282‐286. [DOI] [PubMed] [Google Scholar]
- 97. Davis W, Norman P, Bruce D, Davis T. Predictors, consequences and costs of diabetes‐related lower extremity amputation complicating type 2 diabetes: the Fremantle diabetes study. Diabetologia. 2006;49:2634‐2641. [DOI] [PubMed] [Google Scholar]
- 98. Ewald D, Patel M, Hall G. Hospital separations indicate increased need for prevention of diabetic foot complications in Central Australia. Aust J Rural Health. 2001;9:275‐279. [DOI] [PubMed] [Google Scholar]
- 99. Lim T, Finlayson A, Thorpe JM, et al. Outcomes of a contemporary amputation series. ANZ J Surg. 2006;76(5):300‐305. [DOI] [PubMed] [Google Scholar]
- 100. Santamaria N, Ogce F, Gorelik A. Healing rate calculation in the diabetic foot ulcer: comparing different methods. Wound Repair Regen. 2012;20(5):786‐789. [DOI] [PubMed] [Google Scholar]
- 101. Edwards H, Courtney M, Finlayson K, et al. Chronic venous leg ulcers: effect of a community nursing intervention on pain and healing. Nurs Stand. 2005;19(52):47‐54. [DOI] [PubMed] [Google Scholar]
- 102. O'Brien J, Edwards H, Stewart I, Gibbs H. A home‐based progressive resistance exercise programme for patients with venous leg ulcers: a feasibility study. Int Wound J. 2013;10(4):389‐396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Weller C, Evans S, Staples M, Aldons P, McNeil J. Randomised clinical trial of three‐layer tubular bandaging system for venous leg ulcers. Wound Repair Regen. 2012;20(6):822‐829. [DOI] [PubMed] [Google Scholar]
- 104. Edwards H, Courtney M, Finlayson K, Shuter P, Lindsay E. A randomised controlled trial of a community nursing intervention: improved quality of life and healing for clients with chronic leg ulcers. J Clin Nurs. 2009;18(11):1541‐1549. [DOI] [PubMed] [Google Scholar]
- 105. Parker C. Predicting the likelihood of non‐healing: A venous leg ulcer risk assessment tool. Queensland University of Technology; 2014.
- 106. Finlayson K, Courtney MD, Gibb MA, O'Brien JA, Parker CN, Edwards HE. The effectiveness of a four‐layer compression bandage system in comparison with class 3 compression hosiery on healing and quality of life in patients with venous leg ulcers: a randomised controlled trial. Int Wound J. 2014;11(1):21‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Stacey M, Jopp‐McKay A, Rashic P, Hoskin S, Thompson P. The influence of dressings on venous leg ulcer healing: a randomised trial. J Geophys Res Biogeosci. 1997;13:174‐179. [DOI] [PubMed] [Google Scholar]
- 108. Smith E, McGuinness W. Managing venous leg ulcers in the community: personal financial costs to sufferers. Wound Pract Res. 2010;18(3):134‐139. [Google Scholar]
- 109. Finlayson K, Edwards H, Courtney M. Factors associated with recurrence of venous leg ulcers: a survey and retrospective chart review. Int J Nurs Stud. 2009;46:1071‐1078. [DOI] [PubMed] [Google Scholar]
- 110. Graves N, Zheng H. The prevalence and incidence of chronic wounds: a literature review. Wound Pract Res. 2014;22(1):4‐19. [Google Scholar]
- 111. Lazzarini PA, O'Rourke SR, Russell AW, Derhy PH, Kamp MC. Reduced incidence of foot‐related hospitalisation and amputation amongst persons with diabetes in Queensland, Australia. PLoS One. 2015;10(6):e0130609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Malone M, Lau NS, White J, et al. The effect of diabetes mellitus on costs and length of stay in patients with peripheral arterial disease undergoing vascular surgery. J Geophys Res Biogeosci. 2014;48(4):447‐451. [DOI] [PubMed] [Google Scholar]
- 113. Wu J, Chan T, Bowring G. Functional outcomes of major lower limb amputation 1994–2006: a modern series. Intern Med J. 2010;40:8‐9. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. PRISMA Checklist
Appendix S2. Review protocol
Appendix S3. Summary of Study Characteristics
Appendix S4. Quality assessment of the studies selected for inclusion.


