Abstract
Considering the high incidence of postoperative complications of open fracture, management of this injury is an intractable challenge for orthopaedist, and surgical site infection (SSI) is the devastate one. Screening for high‐risk patients and target them with appropriate interventions is important in clinical practice. The aim of this study was to identify modifiable factors that were associated with SSI following operative treatment of open fractures. This retrospective, multicentre study was conducted at three hospitals. A total of 2692 patients with complete data were recruited between June 2015 and July 2018. Demographic characteristics, operation relative variables, additional comorbidities, and biochemical indexes were extracted and analysed. Receiver operating characteristic analysis was performed to detect the optimum cut‐off value for some variables. Univariate and multivariate logistic analysis models were performed, respectively, to identify the independent risk factors of SSI. The overall incidence of SSI was 18.6%, with 17.0% and 1.6% for superficial and deep infection, respectively. Results of univariate and multivariate analyses showed the following: fracture type, surgical duration > 122 minutes, anaesthesia time > 130 minutes, intraoperative body temperature < 36.4°C, blood glucose (GLU) > 100 mg/dL, blood platelet (PLT) < 288 × 109, and white blood cells (WBC) > 9.4 × 109 were independent risk factors of postoperative wound infection following operative treatment of open fractures. Six modifiable factors such as surgical duration > 122 minutes, anaesthesia time > 130 minutes, intraoperative body temperature < 36.4°C, GLU > 100 mg/dL, PLT < 288 × 109, and WBC > 9.4 × 109 play an important role in the prevention of SSI, and these factors should be optimized perioperatively.
Keywords: open fractures, hyperglycaemia, hypothermia, haematoma, infection
1. INTRODUCTION
A majority of open fractures are caused by high‐energy trauma, and management of open fracture is an intractable challenge for orthopaedist since the soft tissue envelope in consecutive wound exposes the fracture haematoma to contamination.1 Surgical site infection (SSI) is a feared complication and most often requiring surgical revision, and infection is a common cause of nonunion and loss of function following open fractures.2 About 0% to 70% of open fractures are contaminated with bacteria at the time of trauma.3 Regarding economic costs, previous studies reported that mean hospital inpatient costs in the 3‐ to 6‐month time period were higher in infectious cases following open fracture of lower extremity, and SSI account for 17% of nosocomial infections and cost between US $1 and 10 billion in direct and indirect medical expenses annually in the United States.4 At present, studies on the epidemiological characteristics of open fracture are still rare, and most of them have focused on the specific sites of the body (such as tibial plateau, upper limb, and lower limb).2, 5, 6, 7 However, deep understanding of the feature of SSI and identification of the associated risk factors could be of significant importance for the estimation of patients at risk and in optimisation of the perioperative treatment strategy. Moreover, screening for patients with high‐risk infections and targeting them with appropriate interventions are more cost‐effective.
Given that, we designed this study to retrospectively analyse the incidence of SSI in adult patients following operative treatment of open fracture and to identify prognostic risk factors, especially the modifiable variables, which were associated with postoperative wound infection.
2. MATERIAL AND METHODS
2.1. Study design
This study was designed as retrospective and multicentre, and it was conducted at three level I hospitals from June 2015 to July 2018. After being approved by the Ethics Committee of all the participant hospitals, data of patients 18 years or older who underwent operative treatment for open fracture were extracted and collected from the electronic medical records (EMRs) by three well‐trained investigators. The exclusion criteria were (a) soft tissue injury without fracture; (b) open fracture with amputation; (c) open fracture with conservative treatment (debridement combined with skin or skeletal traction; plaster immobilisation or thermoplastic plate brace); (d) patients younger than 18 years. All the enrolled patients were followed up for any evidence of SSI occurrence via telephone assessment, interview, and collection of medical records when they discharged from hospital.
2.2. SSI definition
Definition of SSI was based on the criteria of the United States Centers for Disease Control and Prevention.8 According to this classification system, SSI was categorised into three types: (a) superficial incisional; (b) deep soft tissue of the incision; (c) organ/space infection. Superficial SSI, defined as infection occurring within 30 days postoperative, involves the skin and subcutaneous tissue of the surgical site only; one or more symptoms are observed: redness, swelling, and pain of the incision; purulent discharge; spontaneous wound dehiscence; and positive results of bacterial culture. Deep wound infection is defined as infection that occurs within 90 days postoperative and involves the fascial and muscular layer.
2.3. Data collection and definition of variables
About 70 interesting variables were included in this study to describe a clear picture for the epidemiological characteristic and prognostic risk factors of SSI after open fracture. These variables were divided into five aspects. Demographic variables included age, gender, occupation, residential status (rural, urban), cigarette consumption, and body mass index (BMI); BMI was divided into six groups according to the Chinese reference criteria: <18.5, underweight; 18.5 to 23.9, normal; 24 to 27.9, overweight; 28 to 31.9, obesity; ≥32, morbid obesity. Characteristics of fracture included injured site (such as upper extremity, spine, pelvis and acetabulum, lower extremity), fracture type (Gustilo‐Anderson Classification; I, II, IIIA, IIIB, and IIIC), and injury causes (high‐ or low‐energy trauma). Operation‐related variables included time to surgery (from initial injury to the start of surgery), fixation method (internal or external fixation), surgeons' experience (chief, associate chief, attending), interoperative blood loss, interoperative blood transfusion (autotransfusion or allogenic blood transfusion), surgical duration, anaesthetic type (intrathecal, general, and combination), anaesthesia time, interoperative body temperature (the lowest temperature during operation), fluoroscopy times, pre‐ and intro‐ and postoperative intravenous antibiotics (protocol of administration of antibiotics from the beginning and postoperative treatment is the same in the three hospitals), drainage usage and type (tube, strip, or negative pressure suction device), and length of hospital stay. Cleanliness of operating room was classified into four levels (class 100, 1000, 10 000, and over 100 000), and based on the bacterial quantity per square meter (m2) and the American Society of Anesthesiologists (ASA, I‐IV) classification system,9 evaluation of the patients' physical status and tolerance to surgery were performed. Additional comorbidities of patients included: diabetes mellitus, hypertension, cardio or cerebrovascular disease, rheumatoid disease, liver and kidney disease, chronic respiratory diseases, anaemia, tumour (benign or malignant), and immune system disease. Preoperative laboratory indexes (24 hours within hospitalisation) included white blood cell (WBC), neutrophil granulocyte (NEUT), lymphocyte (LYM), monocyte (MON), eosinophil granulocyte (EOS), basophilic granulocyte (BAS), red blood cell (RBC), haemoglobin (HGB), blood platelet (PLT), serum total protein (TP), albumin (ALB), globulin (GLOB), blood glucose (GLU), and electrolyte (K+, Na+, Cl+, Ca+, P+, and Mg+).
All enrolled patients were divided into two groups according to the occurrence of SSI. The case group was defined as patients with SSI, and the control group included patients who were not suffered from infection. All the data mentioned earlier were extracted and collected by three well‐trained investigators.
2.4. Statistical analysis
Whitney U test was used for non‐normally distributed continuous variables and t test for normally distributed variables. Receiver operating characteristic (ROC) analysis was performed to detect the optimum cut‐off value for continuous variables (such as age, surgical duration, anaesthesia time, intraoperative blood loss, and body temperature). Factors demonstrated to associate with the occurrence of SSI in univariate analysis were entered into the multivariable logistic analysis to identify independent predictors of SSI. A stepwise backward elimination approach was used to exclude confounding covariates from the final multivariate model, and a P value less than .05 was considered to be statistically significant. The Hosmer‐Lemeshow test was used to evaluate goodness of fit of the final model, and an acceptable fitness was enacted as P > .05. All statistical procedures were performed by using the SPSS 19.0 software package (SPSS Inc., Chicago, Illinois).
3. RESULTS
During the 37‐month investigation period, 105 patients were lost (3.8%); therefore, 2692 patients with complete data were included in the final analysis, with a mean follow‐up time of 26.2 ± 14.7 months (ranging 13‐48 months). There were 2027 males and 665 females of the study sample and their mean age was 42.0 ± 13.2 years (18‐96 years). There were 1903 injury cases located in the upper limb, 1066 cases in the lower limb, and 87 in pelvis and acetabulum. Among the 2692 patients, 678 (25.2%) were of type I: 1254 (46.6%) were of type II; 509 (18.9%) were of type IIIA, 138 (5.1%) were of type IIIB, and 113 (4.2%) were of type IIIC, according to the Gustilo‐Anderson classification system of open fracture. A total of 1870 patients undergone open reduction and internal fixation and 822 patients were treated with an external fixator.
Totally, 501 patients were diagnosed as surgical site infection, indicating a cumulative incidence of 18.6%. Of them, 459 were with superficial SSI and 42 patients suffered from deep infection, with an incidence rate of 17.0% and 1.6% for superficial and deep SSI, respectively. Infection occurred in 45 (6.6%) of the 678 type I, 164 (13.1%) of the 1254 type II, and 292 (38.4%) of the 760 type III open fracture wounds. Mean length values of hospital stay for SSIs and no‐infection patients were 36.2 and 16.2 days, respectively, and the difference was statistically significant (P = .000). The earliest and latest occurrence of SSI was, respectively, 3 and 71 days postoperatively for the infectious patients after operative treatment of open fracture. Microorganism examination results showed that Enterococcus faecalis was the most common pathogenic bacteria, followed by Pseudomonas aeruginosa, Staphylococcus aureus, Acinetobacter baumannii, and Staphylococcus epidermidis.
ROC analysis was performed to identify the optimum cut‐off value for age, BMI, surgical duration, interoperative blood loss, body temperature, HGB, and other biochemical indexes (Figure 1). The area under the curve (AUC), their corresponding 95% confidence interval, and the optimum cut‐off value for these variables are presented in Table 1. Demographic data, perioperative characteristics, and preoperative biochemical indexes of the two groups are presented in Table 2. Of the 27 risk variables listed, 12 factors have been demonstrated to be associated with SSI, these variables included age > 40 years (OR = 1.30; 95% CI, 1.06‐1.59), fracture type (OR = 1.86; 95% CI, 1.58‐2.18), experience of surgeon (OR = 1.15; 95% CI, 1.05‐1.27), cleanliness of operating room (OR = 1.34; 95% CI, 1.09‐1.66), surgical duration >122 minutes (OR = 1.79; 95% CI, 1.46‐2.28), anaesthesia time > 130 minutes (OR = 1.31; 95% CI, 1.07‐1.61), interoperative body temperature < 36.4°C (OR = 1.40; 95%, 1.13‐1.73), length of hospital stay >32 days (OR = 1.51; 95% CI, 1.09‐2.10), WBC > 9.4 × 109 (OR = 1.34; 95% CI, 1.08‐1.66), PLT < 288 × 109 (OR = 1.45; 95% CI, 1.10‐1.91), TP < 68.9 g/L (OR = 1.37; 95% CI, 1.11‐1.67), GLU > 100 mg/dL (OR = 1.36; 95% CI, 1.11‐1.67).
Figure 1.
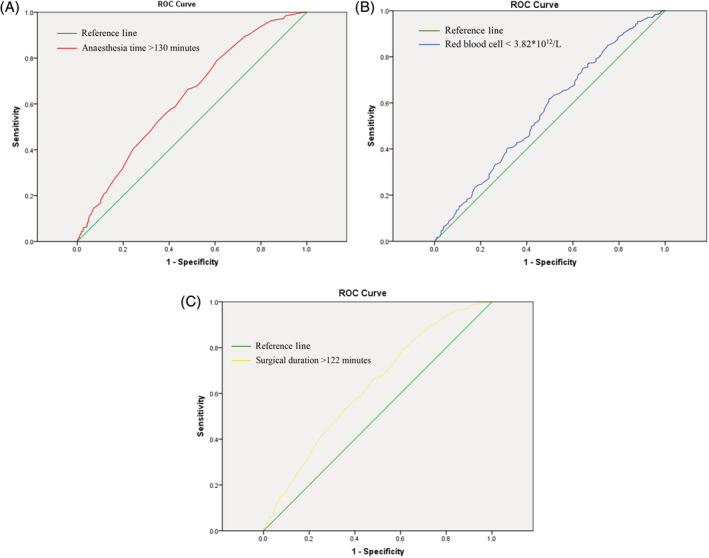
Cut‐off value of anaesthesia time, RBC, and surgical duration identified by the ROC analysis. RBC, red blood cell; ROC, receiver operating characteristic
Table 1.
Cut‐off values of variables identified by the ROC analysis
| Variables | Cut‐off value | AUC | 95% CI |
|---|---|---|---|
| Age (year) | 40 | 0.532 | 0.503‐0.560 |
| Length of hospital stay (day) | 32 | 0.786 | 0.756‐0.816 |
| Surgical duration (minute) | 122 | 0.629 | 0.594‐0.664 |
| Intraoperative body temperature (°C) | 36.4 | 0.537 | 0.508‐0.566 |
| WBC (109/L) | 9.4 | 0.545 | 0.505‐0.585 |
| BMI (kg/m2) | 23 | 0.532 | 0.504‐0.560 |
| GLU (mg/dL) | 100 | 0.544 | 0.505‐0.583 |
| TP (g/L) | 68.9 | 0.541 | 0.500‐0.581 |
| Intraoperative blood loss (ml) | 320 | 0.510 | 0.501‐0.539 |
| PLT (109/L) | 288 | 0.533 | 0.504‐0.562 |
| RBC (1012/L) | 3.82 | 0.538 | 0.509‐0.567 |
| HGB (g/L) | 146 | 0.546 | 0.506‐0.585 |
| Serum ALB (g/L) | 40 | 0.558 | 0.519‐0.598 |
Abbreviations: ALB, albumin; AUC, area under the curve; BMI, body mass index; GLU, blood glucose; HGB, haemoglobin; PLT, blood platelet; RBC, red blood cell; ROC, receiver operating characteristic; TP, total protein; WBC, white blood cell.
Table 2.
Relationship between interesting factors and SSI after operative treatment of open fracture
| Variables | SSI (n = 501, 18.6%) | No SSI (n = 2191, 81.4%) | P value |
|---|---|---|---|
| Age > 40 (years) | 268 (53.5) | 1052 (48.0) | .011a |
| Fracture type (Gustilo‐Anderson III) | 126 (25.1) | 499 (22.8) | .000a |
| Cigarette consumption | 123 (24.6) | 400 (18.3) | .109 |
| Injured site (lower extremity) | 148 (29.5) | 663 (30.3) | .378 |
| Gender (male) | 392 (78.2) | 1635 (74.6) | .332 |
| Surgeon experience (attending) | 271 (54.1) | 997 (45.5) | .005a |
| BMI < 23 kg/m2 | 189 (37.7) | 1041 (47.5) | .200 |
| Diabetes mellitus | 28 (5.6) | 148 (6.8) | .375 |
| Hypertension | 70 (14.0) | 266 (12.1) | .306 |
| Anaemia | 3 (0.6) | 4 (0.2) | .299 |
| Length of hospital stay (>32d) | 50 (10.0) | 286 (13.1) | .013a |
| Surgical duration (>122 minutes) | 118 (23.6) | 579 (26.4) | .037 |
| Anaesthesia time (>130 minutes) | 193 (38.5) | 975 (44.5) | .010 |
| Intraoperative blood loss (320 mL) | 31 (6.2) | 171 (7.8) | .170 |
| ASA score (III‐IV) | 32 (6.4) | 151 (6.9) | .063 |
| Intraoperative body temperature (<36.4°C) | 319 (63.7) | 1570 (71.7) | .002a |
| Cleanliness of operating room | .973 | ||
| Drainage usage | 131 (26.1) | 540 (24.6) | .182 |
| Erythrocyte count (RBC < 3.82 × 1012/L) | 78 (15.6) | 254 (11.6) | .055 |
| TP (<68.9 g/L) | 265 (52.9) | 1324 (60.4) | .003a |
| ALB (<40 g/L) | 88 (17.6) | 464 (21.2) | .852 |
| GLOB (<24.1) | 176 (35.1) | 968 (44.2) | .006a |
| Leukocyte count (WBC < 9.4 × 109/L) | 168 (33.5) | 594 (27.1) | .009a |
| HGB (<146 g/L) | 319 (63.7) | 1512 (69.0) | .721 |
| GLU (>100 mg/dL) | 224 (44.7) | 814 (37.2) | .003a |
| PLT (<288 × 109/L) | 425 (84.8) | 1728 (78.9) | .008a |
Abbreviations: ALB, albumin; ASA, American Society of Anaesthesiologists; BMI, body mass index; GLOB, globulin; GLU, blood glucose; HGB, haemoglobin; PLT, blood platelet count; RBC, red blood cell; TP, total protein; SSI, surgical site infection; WBC, white blood cell.
Significant variables.
All of the 12 prognostic risk factors were entered into the multivariable analysis model for adjustment. The results showed that fracture type (OR = 3.18; 95% CI, 2.67‐5.39; P = .000), operative duration >122 minutes (OR = 2.52; 95% CI, 2.03‐4.23; P = .006), anaesthesia time > 130 minutes (OR = 1.43; 95% CI , 1.13‐1.81; P = .003), interoperative body temperature < 36.4°C (OR = 1.98; 95% CI, 1.42‐2.76; P = .000), WBC >9.4 × 109 (OR = 1.36; 95% CI, 1.08‐1.72; P = .010), PLT < 288×109 (OR = 1.51; 95% CI, 1.13‐1.67; P = .005), GLU > 100 mg/dL (OR = 2.06; 95% CI, 1.20‐3.21; P = .002) were independent risk factors of postoperative SSI (Table 3). The result of Hosmer‐Lemeshow test demonstrated a preferable fitness (X 2 = 14.746; P = .064).
Table 3.
Multivariate analysis of prognostic risk factors of SSI following open fracture
| Variable | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Fracture type | 3.18 | 2.67‐5.39 | .000 |
| Surgical duration > 122 minutes | 2.52 | 2.03‐4.23 | .006 |
| Anaesthesia time > 130 minutes | 1.43 | 1.13‐1.81 | .003 |
| Body temperature < 36.4°C | 1.98 | 1.42‐2.76 | .000 |
| GLU > 100 mg/dL | 2.06 | 1.20‐3.21 | .002 |
| PLT < 288 × 109 | 1.51 | 1.13‐1.67 | .005 |
| WBC > .4 × 109 | 1.36 | 1.08‐1.72 | .010 |
Abbreviations: GLU, blood glucose; PLT, blood platelet count; WBC, white blood cell.
4. DISCUSSION
In the present study, we have characterised surgical site infection following operative treatment in open fracture. The incidence rate of SSI after the surgery of open fractures was 18.6%, with 17.0% and 1.56% for superficial SSI and deep infection, respectively. More importantly, seven risk factors were identified to be associated with SSI, namely, fracture type, surgical duration, anaesthesia time, intraoperative body temperature, the level of blood glucose, and platelet and leukocyte count. Among the seven mentioned predictors, six risk factors were approximately modifiable, and we have observed their significant role in protecting patients against postoperative wound infection.
Guistilo and Anderson's original classification of open fracture wounds based on the extent of soft‐tissue injury was used to categorise the fracture.10 According to the definition of Guistilo and Anderson's classification system, types I and II included an open fracture with a wound less or more than 1 cm long and without extensive soft‐tissue damage or flaps, or avulsions. Type III open fracture included open segmental fractures and fractures with extensive soft‐tissue damage, flaps or avulsions, and fractures with associated vascular or neurologic injuries requiring repair. The incidence rate of postoperative SSI has been reported to be ranging from 0% to 2% for type I fractures, from 2% to 10% for type II fractures, and from 10% to 50% for type III fractures.10, 11 In this study, incidence rates of SSI for grades I and II were 1.7% and 6.1%, respectively. Among the 760 type III open fracture, 10.8% (292 patients) were suffered from superficial or deep wound infection. For major type I and type II open fractures, immediate fixation of the fracture and primary closure of the wound could be performed after resuscitation, irrigation, and debridement. However, soft tissue procedure (skin graft or flap) was often needed in the management of type III fracture, which usually accompanied by contaminated even infected wounds.12 Generally, the increase in infection rate is associated with the transition from grade I to grade III of open fracture in patients following operative treatment, owing to the extensive damage in bone and soft tissue, more surgical procedure, and prolonged operative time.
In this study, surgical duration > 122 minutes and anaesthesia time > 130 minutes were confirmed to increase the risk of SSI by 2.52 and 1.43 times, respectively. Generally, surgical duration and anaesthesia time were significant markers of adverse physiological condition, severity of open fracture, technical difficulty, more extensive soft stripping, and exposure of the wounds. It has been reported that the risk of SSI increased approximately 78% with every extra hour of surgical duration in tibial plateau fractures.13 With the increase of surgical duration, exposure time of surgical incision and deep tissues to the airborne pathogenic bacteria will increase correspondingly, and immunity of human body to microorganism will be compromised. Therefore, well‐experienced and highly skilled orthopaedists along with a tacitly cooperative surgical team play a definitive role in shortening operative time and subsequently reducing the incidence rate of SSI. In a large‐scale observational study, 2.5% patients in the 3909 hip or knee arthroplasties were diagnosed as periprosthetic joint infection, and the authors found several aesthetic agents that were commonly used in general anaesthesia and may significantly inhibit leukocyte chemotactic migration, phagocytosis, lymphocyte function, inflammation, or even directly support bacterial growth in the case of contamination14; moreover, the tissue oxygenation might reduce with the prolongation of anaesthesia time. This result suggested that nerve block and spinal anaesthesia should be the first choice in the procedure of fracture fixation and soft tissue reconstruction in open fracture.
Body temperature is vital in maintaining normal human activities, and it has been reported that hypothermia occurs when the core temperature of an organism is <36.0°C, commonly affecting up to 70% of surgical patients perioperatively.15 Kurz et al16 observed that the incidence of SSI increased 3‐fold when a patient's body temperature is 2°C lower than the normal temperature. According to the results of our study, intraoperative body temperature lower than 36.4°C would increase the risk of SSI by 1.98 times in open fracture. Interoperative use of anaesthetic agents and intravenous solutions, especially multiple application of cold irrigation in debridement of open fractures, was due to low body temperature in anaesthetic‐surgical procedures. Hypothermia can lead to dysfunction of coagulation and contraction of vasculature around surgical site, this circumstance will compromise the immunity of tissue to pathogenic microorganism, and the underlying mechanism of low temperature affecting immune response has been reported.17 However, inadvertent intraoperative hypothermia is common but preventable adverse event. Various reliable body heat‐regulating systems have been designed and developed with an aim to maintain an adequate body temperature. A pilot randomised controlled clinical study18 prospectively recruited 62 patients who underwent thoracic surgery or hip replacement surgery, and the authors demonstrated that the volume of blood loss was more in the passive warming group than in the active warming group (682 ± 426 mL vs 464 ± 324 mL). Our findings highlight the significance of intraoperative body temperature maintained in the prevention of severe complications in open fracture patients.
Preoperative biochemical indexes are of great significance in the evaluation of physical condition for traumatic patients. Many studies have demonstrated that serum albumin,19 fast blood glucose,20 and other specific laboratory markers21, 22 as independent risk factors of SSI. Diabetes mellitus has an adverse effect on the process of wound healing, and this variable will increase the risk of SSI in surgical patients significantly. Unlike previous research studies, we found GLU > 100 mg/dL rather than diabetes mellitus was a predictor of infection, and patients with GLU > 100 mg/dL were at twice the risk of SSI when compared with those with a normal level of blood glucose in this study. In clinical practice, emergency surgeries were needed for patients with open fracture, and GLU was routinely measured within preoperative 24 hours; therefore, GLU could better reflect the status of glycaemic level than the history of diabetes mellitus. Haematoma formation was also associated with an increased risk of superficial and deep infection in orthopaedic surgery.23 Cheung et al24 reported a high incidence rate of infection in shoulder arthroplasty patient who underwent reoperation for haematoma formation. Blood platelet (PLT) count plays a crucial role in haemostasis, in the present study, PLT < 288 × 109/L was an independent risk factor for wound infection. Patients with a low level of blood platelet were prone to forming haematoma, and the collection of fluids in surgical incision provides pabulum for bacterial growth and impairs wound healing by increasing wound tension and reducing tissue perfusion.25
White blood cell is a sensitive indicator in the human body; therefore, the increase of leukocyte count in blood is often regarded as the one of the diagnostic criteria of SSI. An interesting finding was that preoperative WBC >9.4 × 109 was identified as the risk factor for SSI in our study; however, the outcome was weak (P = 1.36). We believe this finding was not difficult to explain: patients that were critically injured and accompanied by contaminated wounds were prone to having severe stress reactions, and this condition would result in a higher level of preoperative leukocyte. Although the bacteria may be in a stage of rapid reproduction, the white blood cells were only slightly elevated due to the decline of the body's immune response, and the risk of SSI for these patients would increase substantially. Therefore, the high level of leukocyte may be a sign of severe injury and suspicious infection for open fracture patients.
The present study had several strengths: it evaluated the association between both clinical variables, laboratory indexes and open fracture in a large population. Among these continuous variables, ROC analysis was performed to detect a highly sensitive cut‐off value. A figure of modifiable factors such as operating time > 107 minutes, serum albumin < 41.6 g/L, and BMI > 26.6 kg/m2 were clearly demonstrated by this investigation. However, some particular limitations of our study should be recognised. Firstly, retrospective study inevitably inherits the selective bias; secondly, some infected patients were identified via telephone review after being discharged from hospital and the incidence rate of SSI may be underestimated.
5. CONCLUSION
In summary, the overall incidence of SSI for open fractures following operative treatment was 18.6%. Multiple operation relative variables and patients' factors were identified to have association with increased risk of SSI, and these factors included fracture type, surgical duration > 122 minutes, anaesthesia time > 130 minutes, and intraoperative body temperature < 36.4°C. Three of the biochemical indexes, namely GLU > 100 mg/dL, PLT < 288 × 109, and WBC > 9.4 × 109, were demonstrated to be independent risk factor of infection. These variables would play a significant role in reducing the incidence of SSI in open fractures, and orthopaedist could consider choosing prophylactic procedures once the patients were under risk.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Hu Q, Zhao Y, Sun B, Qi W, Shi P. Surgical site infection following operative treatment of open fracture: Incidence and prognostic risk factors. Int Wound J. 2020;17:708–715. 10.1111/iwj.13330
REFERENCES
- 1. Diwan A, Eberlin KR, Smith RM. The principles and practice of open fracture care, 2018. Chin J Traumatol. 2018;21(4):187‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dellinger EP, Miller SD, Wertz MJ, Grypma M, Droppert B, Anderson PA. Risk of infection after open fracture of the arm or leg. Arch Surg. 1988;123(11):1320‐1327. [DOI] [PubMed] [Google Scholar]
- 3. Ojo OD, Oluwadiya KS, Ikem IC, Oginni LM, Ako‐Nai AK, Daniel FV. Superficial swab cultures in open fracture management: insights from a resource‐poor setting. J Wound Care. 2010;19(10):432‐438. [DOI] [PubMed] [Google Scholar]
- 4. Perencevich EN, Sands KE, Cosgrove SE, Guadagnoli E, Meara E, Platt R. Health and economic impact of surgical site infections diagnosed after hospital discharge. Emerg Infect Dis. 2003;9(2):196‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lack WD, Karunakar MA, Angerame MR, et al. Type III open tibia fractures: immediate antibiotic prophylaxis minimizes infection. J Orthop Trauma. 2015;29(1):1‐6. [DOI] [PubMed] [Google Scholar]
- 6. Spencer J, Smith A, Woods D. The effect of time delay on infection in open long‐bone fractures: a 5‐year prospective audit from a district general hospital. Ann R Coll Surg Engl. 2004;86(2):108‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yokoyama K, Itoman M, M, Kai H, Ueta S, Kobayashi A. Deep infection and fracture healing in immediate and delayed locked intramedullary nailing for open femoral fractures. Orthopedics. 1999;22(5):485‐490. [PubMed] [Google Scholar]
- 8. Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Am J Infect Control. 1992;13(10):606‐608. [PubMed] [Google Scholar]
- 9. Dripps R. New classification of physical status. Anesthesiology. 1963;24:111. [Google Scholar]
- 10. Gustilo RB, Anderson JT. Prevention of infection in the treatment of one thousand and twenty‐five open fractures of long bones: retrospective and prospective analyses. J Bone Joint Surg Am. 1976;58(4):453‐458. [PubMed] [Google Scholar]
- 11. Gustilo RB, Gruninger RP, Davis T. Classification of type III (severe) open fractures relative to treatment and results. Orthopedics. 1987;10(12):1781‐1788. [PubMed] [Google Scholar]
- 12. Mahfood GH. Ways to prevent infection after open fracture of the lower limb. Clujul Med. 2014;86(3):240‐244. [PMC free article] [PubMed] [Google Scholar]
- 13. Colman M, Wright A, Gruen G, Siska P, Pape HC, Tarkin I. Prolonged operative time increases infection rate in tibial plateau fractures. Injury. 2013;44(2):249‐252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Scholten R, Leijtens B, Hannink G, Kamphuis ET, Somford MP, van Susante JLC. General anesthesia might be associated with early periprosthetic joint infection: an observational study of 3,909 arthroplasties. Acta Orthop. 2019;24:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Burger L, Fitzpatrick J. Prevention of inadvertent perioperative hypothermia. Br J Nurs. 2009;18(18):1114‐1116–9. [DOI] [PubMed] [Google Scholar]
- 16. Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical‐wound infection and shorten hospitalization. Study of wound infection and temperature group. N Engl J Med. 1996;334(19):1209‐1215. [DOI] [PubMed] [Google Scholar]
- 17. Shao L, Pang N, Yan P, et al. Control of body temperature and immune function in patients undergoing open surgery for gastric cancer. Bosn J Basic Med Sci. 2018;18(3):289‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yi J, Liang H, Song R, Xia H, Huang Y. Maintaining intraoperative normothermia reduces blood loss in patients undergoing major operations: a pilot randomized controlled clinical trial. BMC Anesthesiol. 2018;18(1):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhu Y, Liu S, Zhang X, Chen W, Zhang Y. Incidence and risks for surgical site infection after adult tibial plateau fractures treated by ORIF: a prospective multicentre study. Int Wound J. 2017;14(6):982‐988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ma T, Lu K, Song L, et al. Modifiable factors as current smoking, Hypoalbumin, and elevated fasting blood glucose level increased the SSI risk following elderly hip fracture surgery. Journal of investigative surgery. J Invest Surg. 2019; Mar;19:1‐9. [DOI] [PubMed] [Google Scholar]
- 21. Richards JE, Kauffmann RM, Zuckerman SL, Obremskey WT, May AK. Relationship of hyperglycemia and surgical‐site infection in orthopaedic surgery. J Bone Joint Surg Am. 2012;94(13):1181‐1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sørensen LT. Wound healing and infection in surgery: the clinical impact of smoking and smoking cessation: a systematic review and meta‐analysis. Arch Surg. 2012;147(4):373‐383. [DOI] [PubMed] [Google Scholar]
- 23. Saleh K, Olson M, Resig S, et al. Predictors of wound infection in hip and knee joint replacement: results from a 20‐year surveillance program. J Orthop Res. 2010;20(3):506‐515. [DOI] [PubMed] [Google Scholar]
- 24. Cheung EV, Sperling JW, Cofield RH. Infection associated with hematoma formation after shoulder arthroplasty. Clin Orthop Relat Res. 2008;466(6):1363‐1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alexander JW, Korelitz J, Alexander NS. Prevention of wound infections. A case for closed suction drainage to remove wound fluids deficient in opsonic proteins. Am J Surg. 1976;132(1):59‐63. [DOI] [PubMed] [Google Scholar]


