Abstract
Mesenchymal stem cells (MSCs) represent an ideal source of autologous cell‐based therapy for chronic wounds. Functional characteristics of MSCs may benefit wound healing by exerting their multi‐regenerative potential. However, cell ageing resulting from chronic degenerative diseases or donor age could cause inevitable effects on the regenerative abilities of MSCs. A variety of studies have shown the relationship between MSC ageing and age‐related dysfunction, but few associate these age‐related impacts on MSCs with their ability of repairing chronic wounds, which are common in the elderly population. Here, we discuss the age‐associated changes of MSCs and describe the potential impacts on MSC‐based therapy for chronic wounds. Furthermore, critical evaluation of the current literatures is necessary for understanding the underlying mechanisms of MSC ageing and raising the corresponding concerns on considering their possible use for chronic wound repair.
Keywords: Ageing, Chronic wound, Mesenchymal stem cells (MSCs), Regenerative capabilities
Introduction
Chronic wounds or non‐healing wounds remain a clinical challenge in medicine and represent a significant health burden in modern society 1. While standard therapies, including debridement, pressure offloading, dressing regimens, hyperbaric oxygen, antibiotics and topical growth factors, have improved management of wounds and relatively shortened the healing time, there are few therapeutic approaches that effectively reverse the consequence of fibrosis or scar. As the general population continues to age, the number of patients with diabetes and other chronic ageing‐related diseases is growing dramatically 2; hence the need for more effective approaches to treat many chronic diseases, including non‐healing wounds becomes more important. One promising solution, cell therapy, involving the transplantation of progenitor/stem cells to patients through local or systemic delivery, has heralded a new area of research for the treatment of wounds with delayed healing 3.
Mesenchymal stem cells (MSCs) are of great interest because of their unique regenerative potential. The beneficial effect of exogenous MSCs on chronic wound healing had been shown in a variety of animal models and in reported clinical cases 4. However, there appears to be an inevitable link with ageing‐associated impact on MSCs and their regenerative capabilities on chronic wound healing. Accumulated data indicate that MSCs from elderly donors or long‐term ex vivo cultivation show great difference compared with that of young donors in cell morphology, proliferation potential, differentiation potential, telomerase length and activity, as well as special molecular markers 5, 6. Moreover, several researchers found that passages of in vitro culture share equal importance with donor age when considering the proliferative and differential properties of MSCs 7, 8. The decline of regenerative capacity of MSCs is predicted to be caused by cellular ageing 9. Thus, regenerative potential of MSCs might change with age or cultured passages, which suggests a possible limitation in their use for chronic wound repair.
In this review, the ageing‐associated changes in MSCs and the related molecular mechanism is summarized. In particular, the impact of these changes on the regenerative capabilities of MSCs and current improvements is described, specifically for chronic wounds.
Age‐related changes of MSC features
Age‐related changes in MSCs not only mediate cell morphology, proliferation and differentiation, but they also impact on the telomere length, telomerase activity and cellular senescence markers (Figure 1).
Figure 1.

Ageing‐induced alternations in mesenchymal stem cells in terms of morphology, proliferation potential, differential potential, telomere and senescence markers.
Cell morphology
After 20–30 population divisions, MSC morphology shows obviously larger 10 and wider alterations, and the proliferative ability becomes more slow 11 than that of young counterparts. The alternations in cell morphology are typically associated with the Hayflick limitation of cellular senescence 12. Bonab 13 observed that the cytoplasm became granular with many cell inclusions, and debris was formed in the medium after 3 months of in vitro culture. Aged MSCs show reduced numbers of spindle‐shaped (young) cells in culture, which is exhibited in very early stage of cultivation in MSCs from young donors and is gradually lost with increased cultivation time 10. Notably, transfecting simian vacuolating virus 40 (sv40) 14 or telomerase 15 into MSCs considerably reduces the cell sizes compared with their initial sizes.
Proliferation
The decrease in proliferation capability of ageing cells is manifested in MSCs. Hayflick 16 observed that somatic cells division happens for a limited number of times. The maximal in vitro population doubling of MSCs is 30–40, while embryonic stem cells (ESCs) show no such loss 17, 18. MSCs from aged donors show a significant decrease in the growth rate 19 and decline in replicative lifespan of ageing somatic cells 20. These results suggest that proliferative capacity is gradually lost with increasing cultured time and donor age.
Differentiation
The capability of MSC differentiation appears to change with age in some cases 17. In chondrocyte induction test, the specific genes SOX9, COL2A and AGG sharply decreased with donor age 21. However, the age of the MSC donors had little effect on the ALPase activity or the calcium content for osteogenesis induction and glyceraldehyde‐3‐phosphate dehydrogenase (GPDH) activity on oil red O staining for adipogenesis induction 22, while another study reported the age‐dependent changes of MSCs in relation to the osteogenic potential 23. In addition, with the increased culture time in vitro, the homing ability of transplanted MSCs was severely reduced 24.
Telomere length and telomerase activity
Telomere shortening was found in almost all the ageing cells and was thought to be the first possible mechanism of cellular senescence 25. Cells generally lose proliferation capacity and enter senescence once the telomeres reach a certain length 26. It is reported that telomere shortening happens in MSCs at the rate of 100 bp per every two passages, and in early to late passage cells, the telomere lengths decrease from an average of 10·4 to 7·1 kbp 8. On this basis, the correlation between the dividing capacity and telomere length was studied, in both in vitro and in vivo stem cell ageing 27.
Proliferation was greatly related to telomerase activity but telomerase activity is markedly decreased in somatic cells and other non‐embryonic stem cells such as progenitors after 30–40 population doubling 28. Although many studies have detected telomerase activity in MSCs 29, whether MSCs possess telomerase activity is controversial. The divergence in various results obtained in MSCs is due to varying donor age and species 18. Other researchers state that forced telomerase up‐regulation could increase the division times and enhance differentiation potential in human MSCs 30, suggesting that telomerase activity may play a vital role in maintaining the proliferative and differentiation potential of MSCs, and its dysfunction leads to the MSC ageing process.
Cellular senescence markers
The first identified senescence marker is senescence‐associated β‐galactosidase (SA‐βgal); its activity reflects the growing lysosomal compartment that usually occurs in aged cells 12. Recently, more senescence markers were identified: p16 INK4A, DEC1, p15 INK4B and DCR2 31 as well as cytological markers: senescence‐associated heterochromatin foci (SAHFs) and senescence‐associated DNA damage foci (SDFs) 32, 33. SDFs are abundant in proteins that are related to DNA damage and exist in senescent cells from both mice and humans. Expression of genes that promote cell cycle progression (i.e. c‐FOS, cyclin A, cyclin Band PCNA) 34 is suppressed in ageing cells. In the aged MSCs, apoptosis‐associated proteins such as p53, caspase 3, caspase 8, caspase 9 down‐regulated and Bcl‐2, which enhance survival of many cell types, are expressed at a high level 35.
Mechanism of MSC ageing
Although the beneficial effect of exogenous MSCs on chronic wound healing had been evidenced in pre‐clinical and clinical studies, autologous treatment with MSCs from older donors appeared to be less effective than application of their younger counterparts 36. These age‐related differences remain incompletely understood, as do their functional consequences. Thus, exploring the mechanism of ageing MSCs is vital to indicating age‐related MSC impacts on chronic wound healing (Figure 2).
Figure 2.
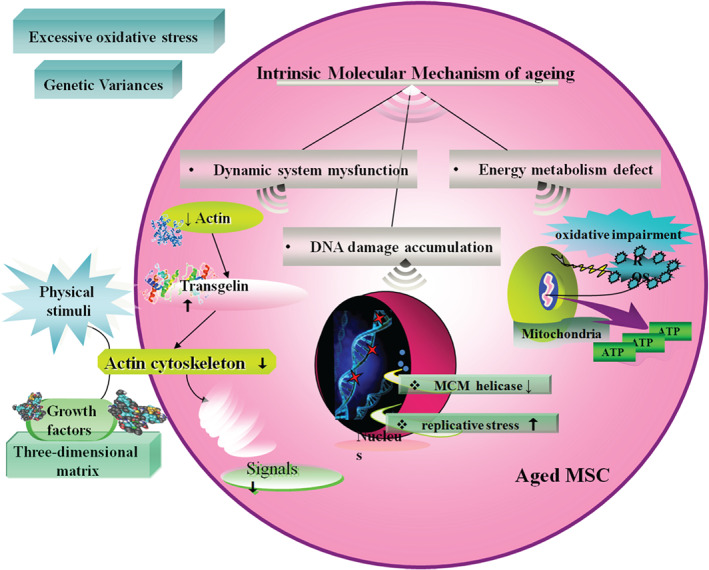
Intrinsic and extrinsic molecular triggers for mesenchymal stem cells ageing.
Intrinsic molecular mechanism
Dynamic system mysfunction
Actin turnover significantly decreases in ageing MSCs, leading to the up‐regulation of one actin cross‐linking protein, transgelin, also the biomarker of ageing, which reduces the actin cytoskeleton dynamics. Actin cytoskeleton translates and processes the external physical stimuli or biochemical molecules into intracellular signals through various growth factors and three‐dimensional extracellular matrix structure. Considering this, the actin cytoskeleton could not respond adequately to these factors and was less dynamic. For instance, the mechanical signals regulating bone homeostasis and regeneration are transduced via actin cytoskeleton 37, which may be responsible for the frequent bone‐related diseases in elderly patients. By contrast, it is intriguing that mechanical requirements appear to change with age in vivo 38. Thus, lower actin dynamics result in regenerative potential decrease of senescent MSCs due to slower and less response to the environmental signals, both biological and mechanical 39.
Energy metabolism defect
Almost all aged cells suffer mitochondrial damage and oxidative impairment 40. Mitochondria are the central organelle supplying energy in the form of ATP. Energy generation, which is accompanied by unstable reactive oxygen species (ROS), may damage both the mitochondrion itself and other components of the cell, which finally causes ageing as a result of damage accumulation 41. Mitochondria are essential for all cells in life and death, and energy support is fundamental for cell differentiation 42. In adult MSCs, early passage cells contain more undifferentiated stem cells that form a significant cluster of mitochondria around the nucleus 43. Frequent self‐renewal of stem cells requires more mitochondria resistant to the cellular senescence.
In aged MSCs, expression of several proteins involved in antioxidant defence up‐regulate along with decreased antioxidant power, which appears to occur in other cells, such as the increased expression of peroxiredoxins in mouse embryonic fibroblasts with age 23. This might expose cells to higher ROS levels, as well as age‐dependent reduced metabolic activities, resulting in insufficient capacity to defend effectively. Thus, despite decreased antioxidative proteins not being the cause for MSC ageing, the molecular damage results from reduced ROS elimination capability which may induce cell dysfunction over time 44.
DNA damage accumulation
Haematopoietic stem cells (HSCs), another kind of multi‐potent stem cell, show a dramatic decline in regenerative function activity with age, resulting in degraded blood production and impaired engraftment following transplantation. Flach 45 demonstrated that cycling old HSCs in mice has heightened levels of replication stress associated with cell cycle defects and chromosome gaps or breaks, which are due to decreased expression of mini‐chromosome maintenance (MCM) helicase components and altered dynamics of DNA replication forks. As HSCs share several features with MSCs, such as various differential capability and self‐renewing ability, we assume that the DNA damage accumulates because the high replicative stress is a potential driver of functional decline in MSCs from aged donors.
Extrinsic molecular mechanism
Excessive oxidative stress
Cells cultured in vitro enter senescence after a certain number of cell divisions. Telomeres can shorten during expansion and excessive oxidative stress can cut down the rate of telomere loss 46. Ageing is considered as a complicated stress response triggered by activation of three main mechanisms: telomere erosion, DNA damage and INK4/ARF locus repression. All of these pathways relate to the tumour suppressors p53 and RB, and are highly consistent with the production of oxidative stress during cell culture, named stress‐induced premature senescence (SIPS) 47, 48.
Genetic variances
Complete characterization of expanded MSCs is essential in serial passages as ageing imposes restriction on MSC application; especially MSC‐based therapies have achieved some success in many disease treatments 49, 50. Cai 51 performed a whole‐genome sequencing of MSCs in ex vivo culture to locate the genetic variances. There are no obvious changes in copy‐number variation and low levels of single‐nucleotide changes (SNCs) in the initial phase but a significant number of SNCs is found in passage 13. In primary culture and early passage, MSCs had low possibility of SNC mutations but reached a high frequency in passage 13, which clarifies that genomic composition of ex vivo MSC cultures tends to be unstable with extended expansion and may be responsible for the ageing process.
Environmental influence from chronic wounds on MSCs
Apart from the systemic ageing, chronic wounds and their pathological microenvironment may negatively affect MSCs itself and their positive properties, and impaired repairing properties may diminish the effectiveness of autologous cell therapy in patients with chronic wounds 52. For example, in the case of chronic wounds in diabetic patients, the high level of glucose induces up‐regulation of BAX in MSCs which encourages apoptosis of the stem cells before differentiation or proliferation 53. On the other hand, the advanced glycation end products in the wounds of diabetic patients could inhibit the level of Bcl‐2 and increase the caspases, FAS and BAX to promote cell apoptosis by giving rise to higher oxidative stress. Besides, cells near the wound could secrete cytokines such as IL‐2, IL‐4, IL‐7 and so on inducing MSC apoptosis by suppressing Bcl‐2 expression. The dysfunction of TGF‐β1 up‐regulation after impaired and over‐expression of TGF‐β3 in diabetic ulcers also causes limitation in MSCs to prevent their repair capability 54, 55. While ample evidence exists that microenvironment of chronic diseases severely affect MSCs in the regenerative capability, further investigation on their interaction and mechanisms is important for effective clinical application.
Possible impacts of ageing in MSCs on chronic wound healing
The application of MSCs in cell therapy is being studied in several areas of medicine, including chronic wound healing. Badiavas et al. directly injected autologous bone marrow derived MSCs (BM‐MSCs) to the edge of a wound, and achieved complete closure of the wound 56. Dash and Lu treated 24 patients having chronic ulcers in the lower limb with autologous BM‐MSCs intramuscularly and significantly reduced the ulcer size 57, 58. The clinical trial carried out by Hernández and coworkers on 22 patients with pressure ulcers due to spinal cord injury showed that injected autologous BM‐MSCs topically had a better healing effect compared with traditional surgical treatment 59. Although MSCs show dramatic therapeutic effects on chronic wound healing, more attention should be paid to non‐healing wounds that occur in ageing patients. As mentioned above, because donor age or culture senescence impacts the regenerative capability and differential potential of MSCs, it seriously limits the autologous application of MSCs in the ageing population. Therefore, it is imperative to highlight the impact of this relationship between MSC ageing and curative effects on chronic wound healing.
In recent studies, researchers demonstrated that aged adipose tissue derived MSCs are unable to rescue age‐associated impairments in cutaneous wound healing because of their significantly compromised ability to support vascular network formation. Through single‐cell transcriptional profile analysis, they found a sub‐population of MSCs with depleted pro‐vascular characteristics 60. Also, there is increasing evidence that aged cells lacked the anti‐inflammatory, protective effect due to changes in the expression levels of inflammatory response genes, which indicate that MSCs undergo an age‐related decline in their immunomodulatory activity 61. Because angiogenesis dysfunction and inflammation deletion mainly account for chronic wounds, there is no doubt that the therapeutic potential of MSCs in chronic wound healing will be hampered heavily by decreasing these capabilities.
Potential solutions for age‐related impacts
Reduced oxygen concentration
A series of molecular and cellular changes occur in MSCs during in vitro culture 62. Treatment of MSC lines from donors of various ages with 5% oxygen environment permits the cells to grow more robustly and with less oxidative stress than the traditional 21% oxygen concentration 63. Although the MSCs are obtained from donors of different ages, they share similar cellular fitness, suggesting that low‐oxygen concentration may neutralise the negative effects of age, effectively.
Lower temperature of culture
Cell culture in low temperature requires less oxygen consumption, such as a 10% reduction in hybridoma and baby hamster kidney (BHK) cells 64 which directly reduce the radical level produced by aerobic respiration 65 and decreased culture temperature can also alleviate stress‐induced senescence and apoptosis levels 66. Similarly, in MSCs, culturing at a low temperature 32°C shows a significant decline in oxidative damage, radical production and induction of glutathione peroxidase activity by reducing oxidative stress. Culturing temperature also regulates stem cell capacity of self‐renewal and maintains the multi‐potency of MSCs by raising p53 and p21 levels to suppress differentiation 67, 68.
Growth factor addition
MSCs gradually lose differentiation potential in culture as the result of culture stress or in vitro senescence. Some batches of foetal bovine serum markedly enhance culture stress or in vitro senescence of MSCs. In contrast, MSCs expanded with fibroblast growth factor‐2 (FGF‐2) maintain their trilineage differentiation potential at high levels throughout many mitotic divisions. Furthermore, FGF‐2 markedly enhances MSC proliferation 69. FGF‐2 may affect the innate properties of MSCs, but MSCs readily lose multi‐potency in culture without FGF‐2. Some other studies also used FGF‐2 in MSC cultures 70.
Natural environment cultures are beneficial to maintain the properties of MSCs compared with traditional medium. Wharton's jelly extract (WJE) from UC‐MSC niche, a commonly used supplement, which is abundant in collagen, fibronectin and insulin‐like growth factor I (IGF‐I) and basic fibroblast growth factor‐b (bFGF) 71, is reported to preserve MSC properties effectively 72. The bottom of culture vessel coated with WJE facilitates in suppressing the MSC senescence through up‐regulation of p53 and p16INK4a/pRb expression. Analysis at the molecular level shows decreased intracellular ROS in MSCs 73.
hTERT over‐expression
Transfecting with human telomerase reverse transcriptase (hTERT) vector, the telomerase catalytic subunit, in vitro culture MSCs enhance genome stability by maintaining mitochondrial physiology to keep the oxygen consumption rate (OCR) and oxidative stress at a low level and normal antioxidant defences such as SOD2, which is significantly over‐expressed in ageing cells. hTERT has also been demonstrated to be a transcriptional modulator that promotes metabolism and decreases ROS production 74. In addition, it markedly reduces aneuploidy level and prevents the dysregulation of ploidy‐controlling genes through up‐regulation of hTERT 75.
Nanog transfection
Pluripotency maintained transcription factor, Nanog 76, reverses the decline of proliferation and differentiation potential in BM‐MSCs from adult donors through activation of the TGF‐β pathway. The young MSCs express a high level of Nanog and microarray analysis results showed that adult BM‐MSCs transfected Nanog close to the neonatal MSCs. It also made genes involved in the cell cycle, DNA replication and DNA damage repair up‐regulated, which is definitely facilitating the proliferation rate and clonogenic capacity. Notably, Nanog will be beneficial in treating cardiovascular diseases that are more likely to happen in elderly patients for its restoration of the myogenic differentiation potential and contractile function of BM‐MSC 77. Besides, considering the safety factor, up‐regulation of oncogenes may lead to cancer and man‐made viral vectors may have potential to infect human cells. Therefore, it is essential to develop safer solutions for these obstacles.
Other approaches for compensation of MSC ageing
Although MSCs have demonstrated a reduced ability to improving chronic wound healing in case of ageing, there is still some compensation to ageing‐induced impacts on MSCs. According to previous work, one of the underlying molecular mechanisms of mesenchymal stem cells facilitating chronic wound healing is through the paracrine factors to promote angiogenesis and improve impaired metabolism 78, 79, 80. Hence, we assume that up‐regulation of the angiogenic factors and IGF‐1 in the MSCs could neutralise the negative effects of ageing to some extent. On the other hand, beneficial niche for MSC regulation produced by tissue engineering approaches would be an effective strategy to remedy the potential limitation of MSC ageing. For instance, constructing microparticles with growth factors not only could enhance their interaction with MSCs by increasing its local concentration, but might also ameliorate the therapeutic effects on chronic wounds directly 81.
Summary and future prospective
Despite numerous advances in wound repair, some wounds never heal and become chronic problems that result in significant morbidity and mortality to the patient. Stem cell therapy for these chronic cutaneous wounds has recently emerged on investigation as a potential solution. Especially, MSCs are a promising source of adult progenitor cells as they are easy to isolate and expand and have been shown to differentiate into various cell lineages. However, along with ageing, MSCs are likely to suffer significant loss in number and functionality, resulting in progressive decline in tissue maintenance and regenerative capacity in the long‐term 82. On the other hand, the prevalence of chronic wounds usually occurs in the ageing population. Thus, the ageing population has profound impact on MSC regenerative capacity and subsequently on its curative effects.
Age‐associated changes in MSCs should be taken into account when they are intended for application in research or for cytotherapy for chronic wound healing. It is required to investigate the entire organism for the internal drivers and extracellular interactions at the molecular, cellular and organ levels, based on the substantial individual variation, with multiple experimental methods. It is also crucial for better approaches to isolate, expand and characterise MSC populations. In addition, resolutions of ageing‐related stem cell changes are required to identify specific pathways involved in the activation of MSCs, which is important in the regeneration of a complete and functional tissue.
Future directions for research in this field might focus on optimization of MSC efficiency in the chronic wound context, both by improving cell function via independent technologies or in combination with tissue engineering designs as well as niche regulation. Moreover, ongoing promising strategies to extend MSC survival and optimise cell delivery continue to emerge, which will improve the regenerative potential of these ageing MSCs in the future.
Acknowledgements
This article was supported in part by the National Nature Science Foundation of China (81121004, 81230041 and 81372066) and the National Basic Science and Development Program (973 Program, 2012CB518105).
The authors declare that they have no competing financial interests.
References
- 1. Nunan R, Harding KG, Martin P. Clinical challenges of chronic wounds: searching for an optimal animal model to recapitulate their complexity. Dis Model Mech 2014;7:1205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vogeli C, Shields AE, Lee TA, Gibson TB, Marder WD, Weiss KB, Blumenthal D. Multiple chronic conditions: prevalence, health consequences, and implications for quality, care management, and costs. J Gen Intern Med 2007;22:391–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nuschke A. Activity of mesenchymal stem cells in therapies for chronic skin wound healing. Organogenesis 2014;10:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maxson S, Lopez EA, Yoo D, Danilkovitch‐Miagkova A, Leroux MA. Concise review: role of mesenchymal stem cells in wound repair. Stem Cells Transl Med 2012;1:142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stolzinga A, Jonesb E, McGonagleb D, Scutta A. Age‐related changes in human bone marrow‐derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev 2008;129:163–73. [DOI] [PubMed] [Google Scholar]
- 6. Asumda FZ, Chase PB. Age‐related changes in rat bone‐marrow mesenchymal stem cell plasticity. BMC Cell Biol 2011;12:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Justesen J, Stenderup K, Eriksen EF, Kassem M. Maintenance of osteoblastic and adipocytic differentiation potential with age and osteoporosis in human marrow stromal cell cultures. Calcif Tissue Int 2002;71:36–44. [DOI] [PubMed] [Google Scholar]
- 8. Stenderup K, Justesen J, Clausen C, Kassem M. Ageing is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone 2003;33:919–26. [DOI] [PubMed] [Google Scholar]
- 9. Raggi C, Berardi AC. Mesenchymal stem cells, ageing and regenerative medicine. Muscles Ligaments Tendons J 2012;2:239–42. [PMC free article] [PubMed] [Google Scholar]
- 10. Baxter MA, Wynn RF, Jowitt SN, Wraith JE, Fairbairn LJ, Bellantuono I. Study of telomere length reveals rapid ageing of human marrow stromal cells following in vitro expansion. Stem Cells 2004;22:675–82. [DOI] [PubMed] [Google Scholar]
- 11. Colter DC, Sekiya I, Prockop DJ. Identification of a subpopulation of rapidly self‐renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proc Natl Acad Sci U S A 2001;98:7841–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes Dev 2010;24:2463–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bonab MM, Alimoghaddam K, Talebian F, Ghaffari SH, Ghavamzadeh A, Nikbin B. Aging of mesenchymal stem cell in vitro . BMC Cell Biol 2006;7:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Negishi Y, Kudo A, Obinata A, Kawashima K, Hirano H, Yanai N, Obinata M, Endo H. Multipotency of a bone marrow stromal cell line, TBR31‐2, established from ts‐SV40 T antigen gene transgenic mice. Biochem Biophys Res Commun 2000;268:450–5. [DOI] [PubMed] [Google Scholar]
- 15. Kobune M, Kawano Y, Ito Y, Chiba H, Nakamura K, Tsuda H, Sasaki K, Dehari H, Uchida H, Honmou O, Takahashi S, Bizen A, Takimoto R, Matsunaga T, Kato J, Kato K, Houkin K, Niitsu Y, Hamada H. Telomerized human multipotent mesenchymal cells can differentiate into hematopoietic and cobblestone area‐supporting cells. Exp Hematol 2003;31:715–22. [DOI] [PubMed] [Google Scholar]
- 16. Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res 1965;37:614–36. [DOI] [PubMed] [Google Scholar]
- 17. Banfi A, Muraglia A, Dozin B, Mastrogiacomo M, Cancedda R, Quarto R. Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: implications for their use in cell therapy. Exp Hematol 2000;28:707–15. [DOI] [PubMed] [Google Scholar]
- 18. Sethe S, Scutt A, Stolzing A. Aging of mesenchymal stem cells. Ageing Res Rev 2006;5:91–116. [DOI] [PubMed] [Google Scholar]
- 19. Mendes SC, Tibbe JM, Veenhof M, Bakker K, Both S, Platenburg PP, Oner FC, de Bruijn JD, van Blitterswijk CA. Bone tissue‐engineered implants using human bone marrow stromal cells: effect of culture conditions and donor age. Tissue Eng 2002;8:911–20. [DOI] [PubMed] [Google Scholar]
- 20. Rubin H. Promise and problems in relating cellular senescence in vitro to ageing in vivo . Arch Gerontol Geriatr 2002;34:275–86. [DOI] [PubMed] [Google Scholar]
- 21. Kanawa M, Igarashi A, Ronald VS, Higashi Y, Kurihara H, Sugiyama M, Saskianti T, Pan H, Kato Y. Age‐dependent decrease in the chondrogenic potential of human bone marrow mesenchymal stromal cells expanded with fibroblast growth factor‐2. Cytotherapy 2013;15:1062–72. [DOI] [PubMed] [Google Scholar]
- 22. Dexheimer V, Mueller S, Braatz F, Richter W. Reduced reactivation from dormancy but maintained lineage choice of human mesenchymal stem cells with donor age. PLoS One 2011;6:e22980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kretlow JD, Jin YQ, Liu W, Zhang WJ, Hong TH, Zhou G, Baggett LS, Mikos AG, Cao Y. Donor age and cell passage affects differentiation potential of murine bone marrow‐derived stem cells. BMC Cell Biol 2008;9:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rombouts WJ, Ploemacher RE. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia 2003;17:160–70. [DOI] [PubMed] [Google Scholar]
- 25. Flores I, Blasco MA. The role of telomeres and telomerase in stem cell aging. FEBS Lett 2010;584:3826–30. [DOI] [PubMed] [Google Scholar]
- 26. Parsch D, Fellenberg J, Brummendorf TH, Eschlbeck AM, Richter W. Telomere length and telomerase activity during expansion and differentiation of human mesenchymal stem cells and chondrocytes. J Mol Med 2004;82:49–55. [DOI] [PubMed] [Google Scholar]
- 27. Sharpless NE, DePinho RA. Telomeres, stem cells, senescence, and cancer. J Clin Invest 2004;113:160–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Armstrong L, Lako M, Lincoln J, Cairns PM, Hole N. mTert expression correlates with telomerase activity during the differentiation of murine embryonic stem cells. Mech Dev 2000;97:109–16. [DOI] [PubMed] [Google Scholar]
- 29. Filioli Uranio M, Valentini L, Lange‐Consiglio A, Caira M, Guaricci AC, L'Abbate A, Catacchio CR, Ventura M, Cremonesi F, Dell'Aquila ME. Isolation, proliferation, cytogenetic, and molecular characterization and in vitro differentiation potency of canine stem cells from foetal adnexa: a comparative study of amniotic fluid, amnion, and umbilical cord matrix. Mol Reprod Dev 2011;78:361–73. [DOI] [PubMed] [Google Scholar]
- 30. Shi S, Gronthos S, Chen S, Reddi A, Counter CM, Robey PG, Wang CY. Bone formation by human postnatal bone marrow stromal stem cells is enhanced by telomerase expression. Nat Biotechnol 2002;20:587–91. [DOI] [PubMed] [Google Scholar]
- 31. Collado M, Gil J, Efeyan A, Guerra C, Schuhmacher AJ, Barradas M, Benguría A, Zaballos A, Flores JM, Barbacid M, Beach D, Serrano M. Tumour biology: senescence in premalignant tumours. Nature 2005;436:642. [DOI] [PubMed] [Google Scholar]
- 32. Narita M, Nũnez S, Heard E, Narita M, Lin AW, Hearn SA, Spector DL, Hannon GJ, Lowe SW. Rb mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell 2003;113:703–16. [DOI] [PubMed] [Google Scholar]
- 33. Di Micco R, Cicalese A, Fumagalli M, Dobreva M, Verrecchia A, Pelicci PG, di Fagagna F. DNA damage response activation in mouse embryonic fibroblasts undergoing replicative senescence and following spontaneous immortalization. Cell Cycle 2008;7:3601–6. [DOI] [PubMed] [Google Scholar]
- 34. Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol 2007;8:729–40. [DOI] [PubMed] [Google Scholar]
- 35. Alt EU, Senst C, Murthy SN, Slakey DP, Dupin CL, Chaffin AE, Kadowitz PJ, Izadpanah R. Ageing alters tissue resident mesenchymal stem cell properties. Stem Cell Res 2012;8:215–25. [DOI] [PubMed] [Google Scholar]
- 36. Rauscher FM, Goldschmidt‐Clermont PJ, Davis BH, Wang T, Gregg D, Ramaswami P, Pippen AM, Annex BH, Dong C, Taylor DA. Ageing, progenitor cell exhaustion, and atherosclerosis. Circulation 2003;108:457–63. [DOI] [PubMed] [Google Scholar]
- 37. Banes A, Lee G, Graff R, Otey C, Archambault J, Tsuzaki M, Elfervig M, Qi J. Mechanical forces and signaling in connective tissue cells: cellular mechanisms of detection, transduction, and responses to mechanical deformation. Curr Opin Orthop 2001;12:389–96. [Google Scholar]
- 38. Strube P, Sentuerk U, Riha T, Kaspar K, Mueller M, Kasper G, Matziolis G, Duda GN, Perka C. Influence of age and mechanical stability on bone defect healing: age reverses mechanical effects. Bone 2008;42:758–64. [DOI] [PubMed] [Google Scholar]
- 39. Kasper G, Mao L, Geissler S, Draycheva A, Trippens J, Kühnisch J, Tschirschmann M, Kaspar K, Perka C, Duda GN, Klose J. Insights into mesenchymal stem cell ageing: involvement of antioxidant defense and actin cytoskeleton. Stem Cells 2009;27:1288–97. [DOI] [PubMed] [Google Scholar]
- 40. Li G, Luna C, Liton PB, Navarro I, Epstein DL, Gonzalez P. Sustained stress response after oxidative stress in trabecular meshwork cells. Mol Vis 2007;13:2282–8. [PMC free article] [PubMed] [Google Scholar]
- 41. Navarro A, Torrejon R. Role of nitric oxide on mitochondrial biogenesis during the ovarian cycle. Front Biosci 2007;12:1164–73. [DOI] [PubMed] [Google Scholar]
- 42. Rehman J. Empowering self‐renewal and differentiation: the role of mitochondria in stem cells. J Mol Med (Berl) 2010;88:981–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lonergan T, Brenner C, Bavister B. Differentiation‐related changes in mitochondrial properties as indicators of stem cell competence. J Cell Physiol 2006;208:149–53. [DOI] [PubMed] [Google Scholar]
- 44. Baron W, Metz B, Bansal R, Hoekstra D, de Vries H. PDGF and FGF‐2 signaling in oligodendrocyte progenitor cells: regulation of proliferation and differentiation by multiple intracellular signaling pathways. Mol Cell Neurosci 2000;15:314–29. [DOI] [PubMed] [Google Scholar]
- 45. Flach J, Bakker ST, Mohrin M, Conroy PC, Pietras EM, Reynaud D, Alvarez S, Diolaiti ME, Ugarte F, Forsberg EC, Le Beau MM, Stohr BA, Méndez J, Morrison CG, Passegué E. Replication stress is a potent driver of functional decline in ageing haematopoietic stem cells. Nature 2014;512:198–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Richter T, von Zglinicki T. A continuous correlation between oxidative stress and telomere shortening in fibroblasts. Exp Gerontol 2007;42:1039–42. [DOI] [PubMed] [Google Scholar]
- 47. Toussaint O, Weemaels G, Debacq‐Chainiaux F, Scharffetter‐Kochanek K, Wlaschek M. Artefactual effects of oxygen on cell culture models of cellular senescence and stem cell biology. J Cell Physiol 2011;226:315–21. [DOI] [PubMed] [Google Scholar]
- 48. Shay JW, Wright WE. Tissue culture as a hostile environment: identifying conditions for breast cancer progression studies. Cancer Cell 2007;12:100–1. [DOI] [PubMed] [Google Scholar]
- 49. Salem HK, Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells 2010;28:585–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang S, Qu X, Zhao RC. Clinical applications of mesenchymal stem cells. J Hematol Oncol 2012;5:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cai J, Miao X, Li Y, Smith C, Tsang K, Cheng L, Wang QF. Whole‐genome sequencing identifies genetic variances in culture‐expanded human mesenchymal stem cells. Stem Cell Reports 2014;3:227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kuhn NZ, Tuan RS. Regulation of stemness and stem cell niche of mesenchymal stem cells: implications in tumorigenesis and metastasis. J Cell Physiol 2010;222:268–77. [DOI] [PubMed] [Google Scholar]
- 53. Bhan S, Mitra R, Arya AK, Pandey HP, Tripathi K. A study on evaluation of apoptosis and expression of bcl‐2‐related marker in wound healing of streptozotocin‐induced diabetic rats. ISRN Dermatol 2013;2013:739054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Berlanga‐Acosta J, Schultz GS, López‐Mola E, Guillen‐Nieto G, García‐Siverio M, Herrera‐Martínez L. Glucose toxic effects on granulation tissue productive cells: the diabetics' impaired healing. BioMed Res Int 2012;2013:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Arya AK, Tripathi R, Kumar S, Tripathi K. Recent advances on the association of apoptosis in chronic non healing diabetic wound. World J Diabetes 2014;15:756–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Badiavas EV, Abedi M, Butmarc J, Falanga V, Quesenberry P. Participation of bone marrow derived cells in cutaneous wound healing. J Cell Physiol 2003;196:245–50. [DOI] [PubMed] [Google Scholar]
- 57. Dash NR, Dash SN, Routray P, Mohapatra S, Mohapatra PC. Targeting nonhealing ulcers of lower extremity in human through autologous bone marrow‐derived mesenchymal stem cells. Rejuvenation Res 2009;12:359–66. [DOI] [PubMed] [Google Scholar]
- 58. Lu D, Chen B, Liang Z, Deng W, Jiang Y, Li S, Xu J, Wu Q, Zhang Z, Xie B, Chen S. Comparison of bone marrow mesenchymal stem cells with bone marrow‐derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: a double‐blind, randomized, controlled trial. Diabetes Res Clin Pract 2011;92:26–36. [DOI] [PubMed] [Google Scholar]
- 59. Sarasúa JG, López SP, Viejo MA, Basterrechea MP, Rodríguez AF, Gutiérrez AF, Gala JG, Menéndez YM, Augusto DE, Arias AP, Hernández JO. Treatment of pressure ulcers with autologous bone marrow nuclear cells in patients with spinal cord injury. J Spinal Cord Med 2011;34:301–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Duscher D, Rennert RC, Januszyk M, Anghel E, Maan ZN, Whittam AJ, Perez MG, Kosaraju R, Hu MS, Walmsley GG, Atashroo D, Khong S, Butte AJ, Gurtner GC. Aging disrupts cell subpopulation dynamics and diminishes the function of mesenchymal stem cells. Sci Rep 2014;4:7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Go AS et al. Heart disease and stroke statistics – 2013 update: a report from the American Heart Association. Circulation 2013;127:e6–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Stolzing A, Scutt A. Age–related impairment of mesenchymal progenitor cell function. Aging Cell 2006;5:213–24. [DOI] [PubMed] [Google Scholar]
- 63. Rosová I, Dao M, Capoccia B, Link D, Nolta JA. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells 2008;26:2173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chuppa S, Tsai YS, Yoon S, Shackleford S, Rozales C, Bhat R, Tsay G, Matanguihan C, Konstantinov K, Naveh D. Fermentor temperature as a tool for control of high‐density perfusion cultures of mammalian cells. Biotechnol Bioeng 1997;55:338–441. [DOI] [PubMed] [Google Scholar]
- 65. Jorjani P, Ozturk SS. Effects of cell density and temperature on oxygen consumption rat for different mammalian cell lines. Biotechnol Bioeng 1999;64:349–56. [DOI] [PubMed] [Google Scholar]
- 66. Cheng T, Rodrigues N, Shen H, Yang Y, Dombkowski D, Sykes M, Scadden DT. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science 2000;287:1804–8. [DOI] [PubMed] [Google Scholar]
- 67. Marcotte R, Wang E. Replicative senescence revisited. J Gerontol A Biol Sci Med Sci 2002;57:257–69. [DOI] [PubMed] [Google Scholar]
- 68. Stolzing A, Scutt A. Effect of reduced culture temperature on antioxidant defences of mesenchymal stem cells. Free Radic Biol Med 2006;41:326–38. [DOI] [PubMed] [Google Scholar]
- 69. Tsutsumi S, Shimazu A, Miyazaki K, Pan H, Koike C, Yoshida E, Takagishi K, Kato Y. Retention of multilineage differentiation potential of mesenchymal cells during proliferation in response to FGF. Biochem Biophys Res Commun 2001;288:413–9. [DOI] [PubMed] [Google Scholar]
- 70. Bianchi G, Banfi A, Mastrogiacomo M, Notaro R, Luzzatto L, Cancedda R, Quarto R. Ex vivo enrichment of mesenchymal cell progenitors by fibroblast growth factor 2. Exp Cell Res 2003;287:98–105. [DOI] [PubMed] [Google Scholar]
- 71. Sobolewski K, Malkowski A, Bankowski E, Jaworski S. Wharton's jelly as a reservoir of peptide growth factors. Placenta 2005;26:747–52. [DOI] [PubMed] [Google Scholar]
- 72. Guillot PV, Gotherstrom C, Chan J, Kurata H, Fisk NM. Human first trimester fetal MSC express pluripotency markers and grow faster and have longer telomeres than adult MSC. Stem Cells 2007;25:646–54. [DOI] [PubMed] [Google Scholar]
- 73. Hao H, Chen G, Liu J, Ti D, Zhao Y, Xu S, Fu X, Han W. Culturing on Wharton's Jelly extract delays mesenchymal stem cell senescence through p53 and p16INK4a/pRb pathways. PLoS One 2013;8:e58314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sharma NK, Reyes A, Green P, Caron MJ, Bonini MG, Gordon DM, Holt IJ, Santos JH. Human telomerase acts as a hTR‐independent reverse transcriptase in mitochondria. Nucleic Acids Res 2011;40:712–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Estrada JC, Torres Y, Benguría A, Dopazo A, Roche E, Carrera‐Quintanar L, Pérez RA, Enríquez JA, Torres R, Ramírez JC, Samper E, Bernad A. Human mesenchymal stem cell‐replicative senescence and oxidative stress are closely linked to aneuploidy. Cell Death Dis 2013;4:e691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Do JT, Scholer HR. Regulatory circuits underlying pluripotency and reprogramming. Trends Pharmacol Sci 2009;30:296–302. [DOI] [PubMed] [Google Scholar]
- 77. Han J, Mistriotis P, Lei P, Wang D, Liu S, Andreadis ST. Nanog reverses the effects of organismal aging on mesenchymal stem cell proliferation and myogenic differentiation potential. Stem Cells 2012;30:2746–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gao D, Xie J, Zhang J, Feng C, Yao B, Ma K, Li J, Wu X, Huang S, Fu X. MSC attenuate diabetes‐induced functional impairment in adipocytes via secretion of insulin‐like growth factor‐1. Biochem Biophys Res Commun 2014;452:99–105. [DOI] [PubMed] [Google Scholar]
- 79. Gao D, Gu C, Wu Y, Xie J, Yao B, Li J, Feng C, Wang J, Wu X, Huang S, Fu X. Mesenchymal stromal cells enhance wound healing by ameliorating impaired metabolism in diabetic mice. Cytotherapy 2014;16:1467–75. [DOI] [PubMed] [Google Scholar]
- 80. Gu C, Huang S, Gao D, Wu Y, Li J, Ma K, Wu X, Fu X. Angiogenic effect of mesenchymal stem cells as a therapeutic target for enhancing diabetic wound healing. Int J Low Extrem Wounds 2014;13:88–93. [DOI] [PubMed] [Google Scholar]
- 81. Huang S, Lu G, Wu Y, Jirigala E, Xu Y, Ma K, Fu X. Mesenchymal stem cells delivered in a microsphere‐based engineered skin contribute to cutaneous wound healing and sweat gland repair. J Dermatol Sci 2012;66:29–36. [DOI] [PubMed] [Google Scholar]
- 82. Bellantuono I, Aldahmash A, Kassem M. Ageing of marrow stromal (skeletal) stem cells and their contribution to age‐related bone loss. Biochim Biophys Acta 2009;1792:364–70. [DOI] [PubMed] [Google Scholar]


