Abstract
The potential use of stem cell‐based therapies for the repair and regeneration of various tissues and organs offers a paradigm shift in plastic and reconstructive surgery. The use of either embryonic stem cells (ESC) or induced pluripotent stem cells (iPSC) in clinical situations is limited because of regulations and ethical considerations even though these cells are theoretically highly beneficial. Adult mesenchymal stem cells appear to be an ideal stem cell population for practical regenerative medicine. Among these cells, adipose‐derived stem cells (ADSC) have the potential to differentiate the mesenchymal, ectodermal and endodermal lineages and are easy to harvest. Additionally, adipose tissue yields a high number of ADSC per volume of tissue. Based on this background knowledge, the purpose of this review is to summarise and describe the proliferation and differentiation capacities of ADSC together with current preclinical data regarding the use of ADSC as regenerative tools in plastic and reconstructive surgery.
Keywords: ADSC, Reconstructive surgery, Stem cells, Tissue engineering
Introduction
In recent years, worthwhile advances in the field of plastic and reconstructive surgery have been achieved in both basic science and clinical research with subsequent translation into patient care. Plastic surgery aims to restore form and function following a wide range of congenital or acquired defects, with procedures often transcending the anatomical boundaries that limit other specialties. With the recent advances in medical imaging, microsurgery, composite tissue allotransplantation, nanotechnology, cell biology and biomaterials, treatment options for patients are wider than ever. For centuries, the ‘reconstructive ladder’ was restricted to local flaps and skin grafts, and with the advent of microsurgery, the reconstructive armamentarium was vastly expanded. These autologous options are reliable and are generally functionally and aesthetically acceptable. However, many plastic surgeons have become increasingly cognizant that there is the real potential for a paradigm shift in reconstructive surgery. The worldwide surgical community is becoming increasingly aware of the research landscape. Tissue engineering, also known by the term regenerative medicine, is a modern, interdisciplinary field combining principles of both engineering and the life sciences. It shares a common objective with plastic and reconstructive surgery, namely to maintain or restore tissue function.
Tissue engineering offers like‐for‐like reconstruction without donor site morbidity or immunosuppression by combining stem cell therapy with 3D scaffolds made from biological or synthetic biomaterials and growth factors for custom‐designed regenerative solutions. Significant progress has recently been made in the application of mesenchymal stem cells in soft tissue reconstruction and cutaneous wound healing based on the inherent capacity of these cells to enhance angiogenesis, minimise inflammation 1, self‐renew and differentiate to specialised cell types under specific physiological conditions 2, 3, 4. Autologous adult stem cells have been the predominant cells used as they are immunocompatible, and their use has few ethical concerns as long as informed consent is obtained prior to harvest. This is in contrast to embryonic (ESC), umbilical cord mesenchymal (UCMSC) and induced pluripotent stem cells (iPSC), which have limited clinical use because of problems with cellular regulation and teratoma formation, ethical considerations, immunogenicity (ESC), genetic manipulation (iPSC) and difficulties with long‐term storage (UCMSC) 5, 6.
Adult mesenchymal stem cells (MSC) are non‐haematopoietic cells of mesodermal derivation that are present in a number of organs and connective tissues, including adipose tissue 7, 8, 9, 10, 11, 12, 13, 14. Adipose tissue is the predominant source of these cells used clinically and experimentally because significant quantities of adipose tissue can be removed with minimal morbidity. While other tissues can be used, their biopsy volume is more limited without causing morbidity, and therefore, the lower yield of cells requires in vitro expansion before clinical use 2. Since their first description in 2002 15, there has been significant interest in the potential offered by autologous adipose‐derived stem cell (ADSC) (Figure 1) to bridge the translational gap. A number of preclinical and clinical trials have established the safety and efficacy of ADSC 16, 17 and range from breast reconstruction and correction of defects 18 to neural regeneration in spinal cord injuries 19. In light of this recent progress in translational ADSC research, it is time to review recent progress in the application of (ADSC) both to reconstruct soft tissue defects and in the enhancement of cutaneous wound healing and determine the remaining challenges to the widespread clinical application of this approach. The Celution system (Cytori Therapeutics, San Diego, CA), currently approved for clinical use, utilises adipose tissue to yield an ADSC‐enriched cell mixture. Among other applications, this mixture has been shown to be a valuable method for the treatment of chronic ulcers in lower limbs of arteriopathic patients 20.
Figure 1.
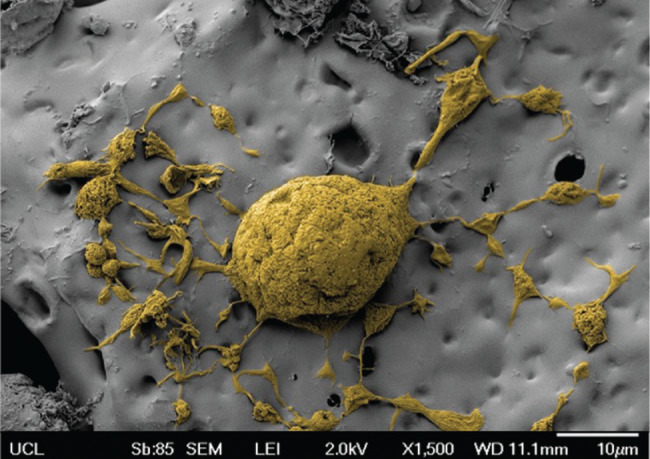
Scanning electron microscopy image of adipose‐derived stem cells (ADSCs) on a polyhedral oligomeric silsesquioxane (POSS)‐modified poly(caprolactone urea‐urethane) (POSS‐PCL) nanocomposite polymer scaffold.
Adipose‐derived stem cells: definition, preparation and application
Whilst ADSCs share many of the characteristics of bone marrow‐derived mesenchymal stem cells (BMSC) 21, 22, 23, 24, they can be obtained more easily with a 100–1000 times greater cellular yield 25, and in practice, many patients are eager for the harvest of unwanted fat. ADSCs are isolated from the stromal vascular fraction (SVF), which is obtained from adipose tissue by enzymatic digestion (Figure 2). The SVF shows a heterogeneous immunophenotype based on flow cytometry with additional cell types including lymphocytes, endothelial cells, fibroblasts, macrophages, pericytes and preadipocytes 26, 27, 28. Subsequent cultures of SVF cells on a tissue culture surface yield an adherent population; these ADSCs are more homogeneous based on their surface immunophenotype 2, 26, 27, 29. Although ADSCs are of mesodermal origin, they can also be differentiated under the correct conditions into cells of ectodermal or endodermal origin. Thus, ADSCs have the potential to differentiate several lineages and into adipocytes, chondrocytes, osteoblasts, neurons, Schwann cells, vascular endothelial cells, tenogenic cells and others, both in vitro and in vivo 2, 30, 31, 32, 33, 34, 35 (Figure 3).
Figure 2.

Schematic representation of adipose‐derived stem cell (ADSC) isolation from adipose tissue and/or lipoaspirate. After enzymatic digestion, the effect of the enzyme is reversed by foetal bovine serum (FBS), and the mixture is filtered through a cell strainer. The cell pellet remains after centrifuging the mixture and discarding the supernatant.
Figure 3.

Adipose‐derived stem cells (ADSCs) have the potential to differentiate along several lineages and into adipocytes, chondrocytes, osteoblasts, neurons, Schwann cells, vascular endothelial cells and tenogenic cells among other cell and tissue types.
Both ADSCs and SVF cells display regenerative capacity when applied to a range of animal models of human disease 36, and multiple mechanisms have been proposed to explain their properties. Initial studies suggested that ADSCs act by differentiating along a mesenchymal lineage, thereby helping to replace defective or ablated cells in vivo 37, 38, 39. ADSCs may also act through the paracrine release of growth factors required to accelerate and direct tissue repair by host‐derived cells 40, 41. The introduction of ADSCs into an ischaemic area may, for example, result in their secretion of vascular endothelial growth factor (VEGF), leading to the increased recruitment of local endothelial cells and angiogenesis. The secretion of immunomodulatory factors, such as prostaglandin E2, by ADSCs may suppress host inflammatory responses following an ischaemic event and thereby enhance recovery 26, 42, 43. Secretion of VEGF, hepatocyte growth factor, FGF‐2, and insulin‐like growth factor 1 (IGF‐1) may enhance angiogenesis and tissue regeneration to the benefit of wound healing. This is of particular interest in challenging wounds such as burn or radiation injuries 44, 45. The use of platelet‐rich plasma (PRP) in combination with ADSCs has seen great popularity recently. PRP in association with insulin greatly potentiates adipogenesis in human ADSCs through a FGFR‐1‐ and ErbB2‐regulated Akt mechanism and ameliorates clinical fat graft maintenance, showing promising results for the translational applications of combined PRP–insulin treatment in regenerative medicine 46.
Phenotypical and functional heterogeneity of adipose‐derived stem cells
Heterogeneity, both donor‐to‐donor and within ADSC populations, can make assessments of their utility, challenging and predicting how culture expansion and external factors such as donor age, method and site of harvest influence the properties of the harvested ADSCs 47, 48, 49, 50, 51, 52, 53, 54. This may be because of variability in the rate of proliferation as illustrated by Kalbermatten et al. (47) who isolated cells from the superficial and deep layers of abdominal adipose tissue and found that cells isolated superficially proliferated more quickly. In animal models, a study focusing on the use of ADSCs in nerve regeneration found that after culture with specific growth factors, tissue harvested from the neck and flank region showed the highest levels of neural‐specific proteins 49. Oedayrajsingh‐Varma et al. (48) proposed that while adipose tissue harvested from the hips and abdomen* was suitable for tissue engineering, the presence of glandular cells in tissue harvested from the breasts could cause potentially unacceptable variability in cell counts. The cellular function may be adversely affected by surgical technique, with a number of studies suggesting that an atraumatic harvest† versus traditional liposuction may result in a healthier cell population that is more capable of surviving transplantation 50, 51, 52. The angiogenic potential of cells has also been shown to decline with advancing donor age, caused by a reduction in the quantities of pro‐angiogenic factors being produced 55.
Regenerative applications of ADSCs
Conventional vascularised tissue transfer is the mainstay of current tissue reconstruction, but it produces donor‐site morbidity, and not all defects can be reconstructed adequately with this approach. While composite vascularised tissue allotransplantation (CTA) is an emerging field used for the reconstruction of complex tissues such as hands and the face, the replacement of the whole structure as a unit is usually necessary, and life‐long immune suppression is still mandatory for these patients. The use of adult stem cells for cell‐based tissue engineering and regeneration strategies represents a promising approach for the repair of many critical tissues, including vasculature, muscle, nerves, cartilage and skin. Tissues that are not currently reconstructible, such as cardiac muscle or central nervous tissue, which transforms into non‐functional fibrous tissue following infarction, would be ideal for this approach and reduce the need for organ or tissue transplantation as a result. For this approach to be successful, the aetiology of the tissue deficit must be addressed. For example, a patient with a systemic myopathy may be unsuitable for this approach, but studies of the regenerative capacities of stem cells in this setting may provide new insights into the treatment of these types of conditions.
Regeneration and angiogenesis
Several studies suggest that ADSCs have the potential to differentiate into endothelial cells, secrete paracrine factors that stimulate endothelial repair and prevent neointimal formation. As a result of their angiogenic properties, ADSCs have novel therapeutic potential in a wide range of ischaemic conditions, such as myocardial infarctions, reno‐vascular, peripheral vascular and cerebrovascular diseases. Studies examining the effects of ADSCs in renal artery stenosis (RAS) found the suppression of inflammatory cytokines and contribution to neovascularisation 56 through the initial paracrine delivery of factors such as VEGF 57. Similarly, during in vivo testing of ischaemic animal tissue, ADSCs were found to promote new vessel formation and angiogenesis along with vascular remodelling 58. Functional improvements associated with these vascular changes were also demonstrated, with ADSCs having a positive effect on cardiac remodelling with a decrease in fibrotic change and cardiac hypertrophy in the months following myocardial infarction 59, 60.
Neural regeneration
Peripheral
Neural tissue exemplifies the paradox between the use of the most suitable cell for neural regeneration (e.g., the Schwann cell) and the donor deficit in harvesting autologous Schwann cells for this approach. Schwann cell (SC) transplantation has been shown to enhance peripheral nerve repair, but the clinical application of this technique is limited by donor‐site morbidity and the inability to generate sufficient numbers of SC quickly 61. Instead, incorporation of ADSCs into nerve conduits has been shown to enhance neural regeneration similar to autograft transplantation by offering a more nerve‐like environment and releasing several neurotrophic factors 62, 63, 64 (Table 1). It has been well reported that better functional and histological results can be obtained by differentiating the ADSCs into an SC‐like phenotype prior to transplantation 62, 65. However, further investigation of the long‐term ADSC survival and functionality combined with nerve conduits in peripheral nerve repair is required.
Table 1.
Nerve tissue regeneration with ADSCs
| Year (reference) | ADSC | In vivo/in vitro | Scaffold | Growth factors | Differentiation | Tissue regeneration |
|---|---|---|---|---|---|---|
| 2013 130 | Dog | Dog | PTFE conduit | – | – | Facial nerve regeneration |
| 2013 131 | Rat | In vitro | TCP, laminin/ fibronectin coated TCP | Coculture with various neural cells | – | Enhanced NTF release by SC‐like cells |
| 2013 132 | Human | Rat | NGF‐hydrogel | NGF within hydrogel | – | Erectile function improvement |
| 2012 133 | Human | In vitro | – | BHA, RA, EGF, and bFGF | Neuron‐like cells | PNR in vivo |
| 2012 134 | Rat | In vitro | – | DM | – | PNR in vitro |
| 2012 135 | Rat | Rat | GGT* | DM | Neuro‐like cells | PNR in vivo |
| 2012 136 | Rat | Rat | Silicon | DM | SC | PNR in vivo |
| 2012 62 | Rat | Rat | DNA | DM | SC‐like cells | PNR in vivo |
| 2012 137 | Rat | In vitro | – | N2, ABAM, B27, EGF, and bFGF | Glial‐like cells | PNR in vitro |
| 2012 138 | Rat | Rat | GGT | – | – | PNR in vivo |
| 2011 139 | Rat | Rat | PCL | DM | SC | Prevention of DRG neuronal loss |
| 2011 140 | Rat | Rat | Allogeneic artery | DM | SC | PNR and functional recovery in vivo |
| 2011 141 | Rat | Rat | ANAs | – | – | PNR in vivo |
| 2011 142 | Rat | In vitro | – | DM | SC‐like cells | – |
| 2011 143 | Rat | Rat | Chitosan/ silk fibroin | – | – | PNR in vivo |
| 2011 144 | Human | In vitro | – | BHA, RA, EGF, bFGF | Neuron‐like cells | Neuronal differentiation |
| 2011 145 | Rat | Rat | ADMT | – | – | Cavernous nerve in vivo |
| 2011 146 | Rat | Rat | Fibrin | DM | SC‐like cells | PNR in vivo |
| 2011 147 | Rat | In vitro | – | DM | SC‐like cells | ADSC from SC and PN fat were more effective† |
| 2011 148 | Mice | Mice | Matrigel | – | – | NTF release |
| 2011 149 | Rat | Rat | Vein graft | – | – | PNR in vivo |
| 2010 150 | Rat | Rat | PHB sheet | – | – | PNR in vivo |
| 2010 151 | Rat | In vitro | PCL and PLLA | DM | SC | PNR in vitro |
| 2010 152 | Rat | Rat | Fibrin conduit | DM | SC‐like cells | PNR in vivo |
| 2010 153 | Rat | Rat | XANM | DM | SC | PNR in vivo |
| 2010 154 | Rat | Rat | – | DM | SC | CNR in vivo |
| 2009 155 | Rat | In vitro | – | DM | SC‐like cells | – |
| 2009 156 | Rat | In vitro | – | DM | Glial cell | NTF release |
| 2009 157 | Human | Nude rat | PCL | – | – | PNR in vivo |
| 2008 158 | Rat | In vitro | – | DM | SC‐like cells | Myelin formation |
| 2007 159 | Rat | In vitro | – | DM and forskolin | SC‐like morphology | PNR in vivo |
ADMT, adipose tissue‐derived acellular matrix thread; ANA, acellular nerve allografts; bFGF, basic fibroblast growth factor; BHA, butylated hydroxyanisole; CNR, Central nerve tissue regeneration; DM, differentiation medium containing PDGF, bFGF and GGF‐2; DNA, decellularized nerve allografts; EGF, epidermal growth factor; NGF, nerve growth factor; NTF, neurotrophic factor; PCL, poly‐ϵ caprolactone; PHB, poly‐3‐hydroxybutyrate; PLLA, poly‐d‐l‐lactic acid; PN, perinephric; PNR, peripheral nerve tissue regeneration; PTFE,, polytetrafluoroethylene; RA, retinoic acid; SC: subcutaneous. TCP, tissue culture plastic; XANM: Xenogenic acellular nerve matrix.
GGT nerve conduit containing genipin crosslinked gelatin annexed with tricalcium phosphate.
Compared to epididymal fat in Wistar rats.
Central
ADSCs have a significant role in tissue repair by virtue of their ability to home to the injured central nervous system coupled with their ability to suppress inflammation. This property may have therapeutic benefit in autoimmune diseases such as multiple sclerosis and encephalomyelitis 66, 67, 68, 69, and research into these areas is on‐going.
Adipose regeneration
Several studies suggest that ADSCs are highly effective in forming de novo adipose tissue 49, 70, 71, 72, 73, 74 (Table 2, Figure 4) with evidence supporting the use of collagen scaffolds to provide support during the process of neo‐adipogenesis 56, 59. The paracrine mechanism of ADSCs promotes the secretion of several growth factors, potentially enhancing their ability to regenerate adipose tissue 75. Adipose tissue regeneration has been shown to benefit breast reconstruction by enhancing volume and improving cosmesis and breast symmetry 15, 31, 76. Lipofilling is a minimally invasive technique transplanting autologous cells, which, in addition to creating volume, also provides a complementary effect within the receptor zone 77. The small population of stem cells within the fat graft may contribute to the improved remodelling through the production of signalling molecules and growth factors 66. These techniques are particularly useful and increasingly employed following breast‐conserving surgery for breast cancer. In this context, however, we must consider the possibility that autologously grafted MSCs might contribute to carcinogenesis and invasive potential by autocrine and paracrine effects 78, 79. A number of studies are currently examining the local and systemic effects of ADSCs on breast cancer cells 80, 81, 82, 83.
Table 2.
Adipose tissue regeneration with ADSC
| Year (reference) | ADSC | In vivo/in vitro | Scaffold | Growth factors | Differentiation | Tissue regeneration |
|---|---|---|---|---|---|---|
| 2013 127 | Human | Both – nude mice | Collagen | – | Adipocytes endothelial cells | Adipose and loose connective tissues in vivo |
| 2013 160, * | Human and rat | Both – rats | DAT | – | Adipocytes | Adipose tissue and angiogenesis in vivo |
| 2013 71 | – | Rabbit | PPP mesh, collagen | – | – | Adipose tissue in vivo |
| 2012 161 | Human | In vitro | – | – | Adipocytes | – |
| 2012 162 | Human | Both – rats | Cross–linked DAT | – | Adipocytes | Adipose tissue and angiogenesis in vivo |
| 2012 163 | Porcine | In vivo pigs | Collagen | – | – | Increased thickness connective tissue |
| 2012 164 | Human | In vivo nude mice | Collagen/gelatin | bFGF | Adipocytes | Increased adipogenesis and angiogenesis with 1 mg/cm2 bFGF |
| 2011 165 | Human | In vitro | Silk | VEGF/laminin | Adipocytes | – |
| 2010 31 | Mice | In vitro | – | – | Adipocytes | – |
| 2010 72 | Mice |
In vivo athymic mice |
Collagen type I, PGA, HA | – | Adipocytes | Collagen regenerated more adipose tissue than two other scaffolds |
| 2008 166 | Human | In vitro | – | – | Adipocytes | Adipogenesis with collagen fibres and vessels at 4 months |
| 2008 167 | GFP Mice | Both – athymic mice | Fibrin glue | – | Adipocytes | Adipogenesis |
| 2008 168 | Human | Both – athymic mice | Gelatin, PGA in PPP mesh | – | Adipocytes | Adipose tissue in vivo, preservation of scaffold shape at 6 months |
ADSC, adipose‐derived stem cell; DAT, decellularised adipose tissue; HA, hyaluronic acid; PGA, polyglycolic acid; PPP: polypropylene.
In vitro human ADSC differentiation and in vivo rat ADSC implantation.
Figure 4.
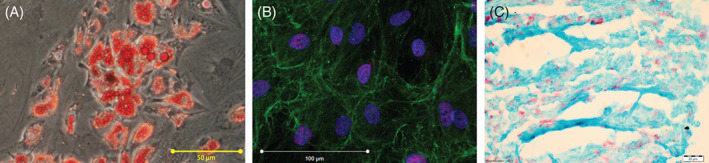
Adipose‐derived stem cells (ADSCs) differentiated into adipocytes – Oil Red O (red) stained (A), osteoblasts – RunX2 (red), collagen I (green) and Dapi (blue) stained (B) and chondrocytes – alcian blue (blue) and neutral red (red) stained (C).
Tendon regeneration
The goal of primary tendon repair is to restore tensile strength at the time of mobilisation. Current repair strategies using primary repair, suitable autografts and freeze‐dried allografts lead to a slow repair process that is sub‐optimal and often fails to restore function completely 84. A repaired tendon never completely regains the biological and mechanical properties of an undamaged tendon; the collagen fibrils remain thinner, and the tendon's mechanical strength is reduced 85. Tendon regeneration using MSCs has been described in several studies; however, the use of ADSCs for tendon tissue engineering has only recently been considered (Table 3). Local administration of ADSCs to the site of injury appears to accelerate tendon repair 86, as exhibited by a significant increase in tensile strength, direct differentiation of ADSCs toward tenocytes and endothelial cells and increases in angiogenic growth factors 87.
Table 3.
Tendon tissue regeneration with ADSC
| Year (reference) | ADSC | In vivo/in vitro | Scaffold | Growth factors | Differentiation | Tissue regeneration |
|---|---|---|---|---|---|---|
| 2012 86 | Rabbit | In vivo rabbit Achilles tendon injury | – | PRP with or without ADSC | Tenocytes | ADSC improved primary tendon healing, increased collagen type I, VEGF and FGF production and decreased TGF‐β1, 2, 3 levels |
| 2011 84 | Rat | In vitro | PLAGA 3D fibre scaffold compared to 2D sheet | GDF‐5 | Tenocytes | Increased collagen type I gene expression with GDF‐5 stimulation of ADSC on 3D scaffolds |
| 2011 87 | Rabbit | In vivo rabbit Achilles tendon injury | – | PRP with or without ADSC | Tenocytes and endothelial cells | ADSC increase tensile strength, differentiate toward tenocytes and endothelial cells, and increases angiogenic growth factors. |
| 2010 88 | Rat | In vitro | – | GDF‐5 | Tenocytes | GDF‐5 induces tenogenic differentiation of ADSC. |
FGF, fibroblast growth fator; GDF‐5, growth differentiation factor‐5; PLAGA, poly(dl‐lactide‐co‐glycolide); PRP, platelet‐rich plasma; VEGF, vascular endotelial growth factor; TGF‐β, transforming growth factor beta.
Three‐dimensional scaffolds have been shown to support the proliferation and adhesion of ADSCs, which, under the influence of growth differentiation factor‐5 (GDF‐5), have successfully produced type 1 collagen 84. However, while a number of studies have shown the potential for the use of ADSCs in tendon repair and identified that GDF‐5 may have a significant role to play in tendonogenic differentiation, further studies are needed to determine the ideal concentration of growth factors and conditions required for effective tendon repair 2, 24, 84, 87, 88.
Cartilage regeneration
In our aging populations, the high rates of arthritis impact joint mobility and quality of life (Table 4). As a result of this massive market, there has been intense interest in the use of stem cell‐based therapies for articular cartilage regeneration. The current treatments for articular cartilage injury revolve primarily around symptom control, having little effect on disease progression 89. There is a lack of inherent mechanisms for the regeneration of mature articular cartilage, and surgical options for repair or replacement generally result in the formation of fibrocartilage rather than hyaline 90, 91, 92, 93. After initial experiments demonstrating the potential for regeneration, MSCs under the right conditions have since been shown to differentiate into chondrocytes 77, 94, 95, 96, 97 (Figure 4). There are a number of growth factors that affect the repair of cartilage and the differentiation of MSCs to chondrocytes; in vitro studies examining growth factors have shown that TGFβ and FGF‐2 induces the proliferation of cells 92, 96, 98, 99, 100 and promotes the chondrogenic differentiation of MSCs 101, 102. While studies examining ADSCs have demonstrated the successful production of cartilage in vivo 95, the focus of many studies remains on the use of stem cells derived from bone marrow (BM), which has been shown to produce more architecturally stable cartilage 95, 101, 103. In addition, this has implications for nasal and auricular cartilage engineering, potentially negating the need for donor‐site morbidity and multiple stage operations to achieve a cosmetically desirable result.
Table 4.
ADSC in cartilage tissue regeneration
| Year (reference) | ADSC | In vivo/in vitro | Scaffold | Growth factors | Differentiation | Tissue regeneration |
|---|---|---|---|---|---|---|
| 2010 92 | Rabbit | In vivo Rabit articular cartilage | Gellan Gum Hydrogels |
TGF‐β1 BMP‐2 |
Chondrocytes |
ADSC combined with TGF‐β1 & BMP‐2 showed up‐regulation of type II collagen and aggrecan |
| 2010 97 | Human | Both – nude mice | Fibrin glue | – | Chondrocytes | Concentration of GAGs increased over time, ADSC differentiated and formed new cartilage |
| 2008 96 | Human | In vitro | – |
TGF‐β2 BMP‐2,6,7 |
Chondrocytes | Combination of TGF‐β2 and BMP‐7 enhance chondrogenesis |
| 2008 100 | Human | Both – nude mice | PLGA | TGF‐β1 | Chondrocytes | ADSC‐seeded PLGA scaffolds expressed a stable chondrogenic phenotype |
ADSC, adipose‐derived stem cell; BMP, bone morphogenetic protein; GAGs, glycosaminoglycans; GF, growth factor; PLGA, poly(lactic‐co‐glycolic acid); TGF‐β, transforming growth factor beta.
Muscle regeneration
Skeletal muscle
Satellite cells are generally regarded as the myocyte precursor within adult tissues. Regeneration of adult skeletal muscle occurs when this normally dormant and non‐proliferative population of mononucleated myocyte precursors are activated following injury 104. However, the number of these satellite cells within mature muscle represents only 1–5% of the total cell number 105, and their potential to self‐renew decreases with age and diseases that cause a gradual and continuous degeneration of the muscle fibres, such as Duchenne muscular dystrophy (DMD) 106. ADSCs have been shown to participate in myofibre formation when exposed to a regenerating muscle environment 2, 107 and may additionally contribute to muscle regeneration by modifying gene expression post fusion with the host cells 108.
Smooth muscle
Smooth muscle is a major component of, and essential for, the normal function of cardiovascular, gastrointestinal, reproductive and urinary systems. In the field of cellular therapeutics, one major limitation is a reliable source of smooth muscle cells (SMC) as biopsies of organs containing these cells can be impractical and have associated morbidity. The ease of harvest and low donor‐site morbidity of ADSCs make them ideal for use, and their ability to differentiate along multiple cell lineages has been well established 1, 2, 15. Studies have shown that these cells are capable of inducing the expression of a smooth muscle phenotype and respond as SMCs to common pharmacological agents 109.
Wound healing and skin regeneration
Wound repair is a complex process of inflammation, angiogenesis, formation of new tissue and finally remodelling 110, 111. Stem cells are thought to integrate themselves with the local environment, contributing to wound healing by secreting signalling molecules, accelerating repair and differentiating into the required cells, encouraging similar differentiation within adjacent cells 112. The paracrine effect of ADSCs results from the secretion of cytokines such as TGF‐β, VEGF, granulocyte/macrophage colony‐stimulating factor (GM‐CSF), hepatocyte growth factor (HGF) and stromal derived factor 1 (SDF‐1) 40, 113, 114. In addition to facilitating wound healing, they can also recruit and stimulate endogenous stem cells to participate in the process 105.
The use of progenitor cells in wound healing has been extensively investigated (Table 5) and the use of ADSCs, as previously discussed, has a number of advantages regarding ease of harvest and low rate of donor‐site complications 115, 116, 117. The autologous transplantation of ADSCs has been shown to increase the survival of full‐thickness skin grafts and promote wound healing 118. In recent human studies, there was an improvement reported in the skin quality overlying fat injection sites 119, scar reduction in patients with severe burns 120 and marked improvement in severe radiodermatitis sustained post cancer treatment 121. Delivery systems have also been explored with dermal polymer scaffolds in development to act as a skin substitute to allow optimal stem cell interface with the wound to encourage healing 122.
Table 5.
Wound healing and skin regeneration with the aid of ADSCs
| Year (reference) | ADSC | In vivo/in vitro | Scaffold | Growth factors | Differentiation | Tissue regeneration |
|---|---|---|---|---|---|---|
| 2013 111 | Rabbit | Both – Rabbit | – | – | – | ADSC increase endothelial cell recruitment and enhance wound repair |
| 2012 128 | Human | In vitro | – | BGFG, EGF | Keratinocytes | Formation of epidermal layer and expression of Keratin 10. Detection of Desmosomes and hemidesmosomes |
| 2012 129 | Human | In vitro | – | – | Keratinocytes | ADSC protein extracts improved human keratinocyte proliferation and migration |
| 2011 126 | Mice | Both – GFP transgenic mice | Co‐CS‐HA and ADM | – | – | ADSC‐seeded scaffolds enhanced angiogenesis and wound healing |
| 2011 118 | Sprague– Dawley rats | Both – rat | – | – | – | ADSC under skin grafts increased angiogenesis and improved wound healing |
| 2009 124 | Human | Both – athymic mice | Silk fibroin‐chitosan | – | Epithelial, endothelial & fibrovascular | ADSC seeded scaffolds enhance wound healing |
| 2009 119 | Human | In vivo – nude mice | – | – | – | Grafting of adipose tissue stimulates neosynthesis of collagen increasing dermal thickness |
| 2008 120 | Human | In vivo – humans | – | – | – | New collagen deposition, local hypervascularity and dermal hyperplasia |
| 2007 125 | Human | Both – nude mice | Collagen | – | Fibroblast proliferation | ADSC enhance collagen synthesis by HDFs and activate HDF proliferation & migration |
| 2007 121 | Human | In vivo – humans | – | – | – | Improvement of ultrastructure, neovessel formation and systematic improvement |
ADM, acellular dermal matrix; BFGF, basic fibroblast growth factor; Co‐CS‐HA, collagen‐chondroitin sulfate‐hyaluronic acid; EGF, epidermal growth factor; GFP, 6‐green fluorescent protein; HDF, human dermal fibroblast; HGF, hepatocyte growth factor.
Multiple groups worldwide are investigating tissue‐engineering approaches that combine ADSCs with fibroblasts or other progenitor cells and seeding onto scaffolds to ‘manufacture’ implantable constructs 123. Various materials have been evaluated, both natural and synthetic, including silk fibroin‐chitosan 124, collagen 125 collagen‐chondroitin sulfate‐hyaluronic acid 126 and acellular dermal matrix 126. While there is no consensus on the most appropriate material to construct a scaffold, key features can be identified as desirable between each of the studies. In addition to conforming to the wound shape, they should provide sufficient stability to allow cells to proliferate and mature while resorbing in sufficient time so as to not interfere with wound maturation 127. Scaffolds can create a wound–stem cell interface to control the interaction and the rate at which the cells are introduced to the wound 112. Scaffolds can affect cell adhesion, with some studies suggesting that a type 1 collagen sponge is ideally suited because of its porous structure 114. It is also important that the collagen degrades to allow for the migration, proliferation and differentiation of cells 71. A mixture of fibroblasts and ADSCs can improve the epidermal morphogenesis of tissue‐engineered skin 128. ADSCs have the ability to grow on porous biomaterials and differentiate into fibrovascular, endothelial and epithelial tissues 124. ADSCs also enhance skin regeneration through their secretory effects on dermal fibroblasts 125 and keratinocytes 129.
Conclusion
The clinical applications of ADSCs are broadly ranged; the ease of cell harvest and high yield with minimal donor‐site morbidity make them an ideal cell source. Additionally, the multi‐lineage potential of these cells demonstrates the significant opportunities they present within the field of tissue engineering, with studies successfully demonstrating the ability to produce a range of tissue types. Future challenges lie within the application of these technologies and the steps required to bridge the transitional gap that currently exists. In particular, the mechanism of action of ADSCs, their interactions with extracellular matrix microenvironments and long‐term fate require further clarification. Additionally, randomised trials demonstrating the continued safety of transplanted ADSCs as well as the efficacy of cellular therapies in comparison to commonly applied conventional techniques are fundamental.
Acknowledgements
We would like to thank Belinda Colton MSc for colouring of ADSCs in Figure 1, Douglas Neil for designing Figure 2 and Steve Atherton MA RMIP MIMI for designing Figure 3.
This work is attributed to: Reconstructive Surgery Regenerative Medicine Group, Institute of Life Sciences (ILS), Swansea University Medical School, Swansea, UK.
Footnotes
Harvested by either tumescent liposuction or resection.
Coleman technique or cannula and syringe.
References
- 1. Mizuno H. Adipose‐derived stem cells for tissue repair and regeneration: ten years of research and a literature review. J Nippon Med 2009;76:56–66. [DOI] [PubMed] [Google Scholar]
- 2. Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim PH, Lorenz P, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell‐based therapies. Tissue Eng 2001;7:211–28. [DOI] [PubMed] [Google Scholar]
- 3. Zuk PA. The adipose‐derived stem cell: looking back and looking ahead. Mol Biol Cell 2010;21:1783–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Watson D, Keller GS, Lacombe V, Fodor PB, Rawnsley J, Lask GP. Autologous fibroblasts for treatment of facial rhytids and dermal depressions. A pilot study. Arch Facial Plast Surg 1999;1:165–70. [DOI] [PubMed] [Google Scholar]
- 5. Lenoir N. Europe confronts the embryonic stem cell research challenge. Science 2000;287:1425–7. [DOI] [PubMed] [Google Scholar]
- 6. Ben‐David U, Benvenisty N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat Rev Cancer 2011;11:268–77. [DOI] [PubMed] [Google Scholar]
- 7. Dodson MV, Hausman GJ, Guan L, Du M, Rasmussen TP, Poulos SP, Mir P, Bergen WG, Fernyhough ME, McFarland DC, Rhoads RP, Soret B, Reecy JM, Velleman SG, Jiang Z. Skeletal muscle stem cells from animals I. Basic cell biology. Int J Biol Sci 2010;6:465–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Belicchi M, Pisati F, Lopa R, Porretti L, Fortunato F, Sironi M, Scalamonga M, Parati EA, Bresolin N, Torrente Y. Human skin‐derived stem cells migrate throughout forebrain and differentiate into astrocytes after injection into adult mouse brain. J Neurosci Res 2004;77:475–86. [DOI] [PubMed] [Google Scholar]
- 9. Feng J, Mantesso A, Sharpe PT. Perivascular cells as mesenchymal stem cells. Expert Opin Biol Ther 2010;10:1441–51. [DOI] [PubMed] [Google Scholar]
- 10. Shi M, Ishikawa M, Kamei N, Nakasa T, Adachi N, Deie M, Asahara T, Ochi M. Acceleration of skeletal muscle regeneration in a rat skeletal muscle injury model by local injection of human peripheral blood‐derived CD133‐positive cells. Stem Cells 2009;27:949–60. [DOI] [PubMed] [Google Scholar]
- 11. Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A 2003;100:5807–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Seo B‐M, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004;364:149–55. [DOI] [PubMed] [Google Scholar]
- 13. Baksh D, Yao R, Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells 2007;25:1384–92. [DOI] [PubMed] [Google Scholar]
- 14. Musina RA, Bekchanova ES, Sukhikh GT. Comparison of mesenchymal stem cells obtained from different human tissues. Bull Exp Biol Med 2005;139:504–9. [DOI] [PubMed] [Google Scholar]
- 15. Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 2002;13:4279–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gimble JM, Katz AJ, Bunnell BA. Adipose‐derived stem cells for regenerative medicine. Circ Res 2007;100:1249–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tobita M, Orbay H, Mizuno H. Adipose‐derived stem cells: current findings and future perspectives. Discov Med 2011;11:160–70. [PubMed] [Google Scholar]
- 18. Yoshimura K, Sato K, Aoi N, Kurita M, Hirohi T, Harii K. Cell‐assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose‐derived stem/stromal cells. Aesthetic Plast Surg 2008;32:48–55; discussion 56–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Khalifian S, Sarhane KA, Tammia M, Ibrahim Z, Mao H‐Q, Cooney DS, Shores JT, Lee WPA, Brandacher G. Stem cell‐based approaches to improve nerve regeneration: potential implications for reconstructive transplantation? Arch Immunol Ther Exp (Warsz) 2015;63:15–30. [DOI] [PubMed] [Google Scholar]
- 20. Marino G, Moraci M, Armenia E, Orabona C, Sergio R, De Sena G, Capuozzo V, Barbarisi M, Rosso F, Giordano G, Iovino F, Bargarisi A. Therapy with autologous adipose‐derived regenerative cells for the care of chronic ulcer of lower limbs in patients with peripheral arterial disease. J Surg Res 2013;185:36–44. [DOI] [PubMed] [Google Scholar]
- 21. Gronthos S, Franklin DM, Leddy HA, Robey PG, Storms RW, Gimble JM. Surface protein characterization of human adipose tissue‐derived stromal cells. J Cell Physiol 2001;189:54–63. [DOI] [PubMed] [Google Scholar]
- 22. De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, Dragoo JL, Ashjian P, Thomas B, Benhaim P, Chen I, Fraser J, Hedrick MH. Comparison of multi‐lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs 2003;174:101–9. [DOI] [PubMed] [Google Scholar]
- 23. Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, Blake J, Schwager C, Eckstein V, Ansorge W, Ho AD. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol 2005;33:1402–16. [DOI] [PubMed] [Google Scholar]
- 24. Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006;24:1294–301. [DOI] [PubMed] [Google Scholar]
- 25. Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol 2006;24:150–4. [DOI] [PubMed] [Google Scholar]
- 26. McIntosh K, Zvonic S, Garrett S, Mitchell JB, Floyd ZE, Hammill L, Kloster A, Di Halvorsen Y, Ting JP, Storms RW, Goh B, Kilroy G, Wu X, Gimble JM. The immunogenicity of human adipose‐derived cells: temporal changes in vitro. Stem Cells 2006;24:1246–53. [DOI] [PubMed] [Google Scholar]
- 27. Mitchell JB, McIntosh K, Zvonic S, Garrett S, Floyd ZE, Kloster A, Di Halvorsen Y, Storms RW, Goh RW, Kilroy G, Wu X, Gimble JM. Immunophenotype of human adipose‐derived cells: temporal changes in stromal‐associated and stem cell‐associated markers. Stem Cells 2006;24:376–85. [DOI] [PubMed] [Google Scholar]
- 28. Zimmerlin L, Donnenberg VS, Pfeifer ME, Meyer EM, Péault B, Rubin JP, Donnenberg AD. Stromal vascular progenitors in adult human adipose tissue. Cytometry A 2010;77:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pachón‐Peña G, Yu G, Tucker A, Wu X, Vendrell J, Bunnell BA, Gimble JM. Stromal stem cells from adipose tissue and bone marrow of age‐matched female donors display distinct immunophenotypic profiles. J Cell Physiol 2011;226:843–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Planat‐Benard V, Silvestre J‐S, Cousin B, André M, Nibbelink M, Tamarat R, Clergue M, Manneville C, Saillan‐Barreau C, Duriez M, Tedgui A, Levy B, Penicaud L, Casteilla L. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation 2004;109:656–63. [DOI] [PubMed] [Google Scholar]
- 31. Konno M, Hamazaki TS, Fukuda S, Tokuhara M, Uchiyama H, Okazawa H, Okochi H, Asashima M. Efficiently differentiating vascular endothelial cells from adipose tissue‐derived mesenchymal stem cells in serum‐free culture. Biochem Biophys Res Commun 2010;400:461–5. [DOI] [PubMed] [Google Scholar]
- 32. Rangappa S, Fen C, Lee EH, Bongso A, Sim EKW, Wei EKS. Transformation of adult mesenchymal stem cells isolated from the fatty tissue into cardiomyocytes. Ann Thorac Surg 2003;75:775–9. [DOI] [PubMed] [Google Scholar]
- 33. Halvorsen YC, Wilkison WO, Gimble JM. Adipose‐derived stromal cells – their utility and potential in bone formation. Int J Obes Relat Metab Disord 2000;24(Suppl 4):S41–4. [DOI] [PubMed] [Google Scholar]
- 34. Uysal AC, Mizuno H. Tendon regeneration and repair with adipose derived stem cells. Curr Stem Cell Res Ther 2010;5:161–7. [DOI] [PubMed] [Google Scholar]
- 35. Tobita M, Mizuno H. Periodontal disease and periodontal tissue regeneration. Curr Stem Cell Res Ther 2010;5:168–74. [DOI] [PubMed] [Google Scholar]
- 36. Gimble JM, Guilak F, Bunnell BA. Clinical and preclinical translation of cell‐based therapies using adipose tissue‐derived cells. Stem Cell Res Ther 2010;1:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Noël D, Caton D, Roche S, Bony C, Lehmann S, Casteilla L, Jorgensen C, Cousin B. Cell specific differences between human adipose‐derived and mesenchymal‐stromal cells despite similar differentiation potentials. Exp Cell Res 2008;314:1575–84. [DOI] [PubMed] [Google Scholar]
- 38. Lin G, Garcia M, Ning H, Banie L, Guo Y‐L, Lue TF, Lin CS. Defining stem and progenitor cells within adipose tissue. New Rochelle, NY: Mary Ann Liebert, Inc., 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hong L, Peptan IA, Colpan A, Daw JL. Adipose tissue engineering by human adipose‐derived stromal cells. Cells Tissues Organs 2006;183:133–40. [DOI] [PubMed] [Google Scholar]
- 40. Rehman J, Traktuev D, Li J, Merfeld‐Clauss S, Temm‐Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 2004;109:1292–8. [DOI] [PubMed] [Google Scholar]
- 41. Miranville A, Heeschen C, Sengenès C, Curat CA, Busse R, Bouloumié A. Improvement of postnatal neovascularization by human adipose tissue‐derived stem cells. Circulation 2004;110:349–55. [DOI] [PubMed] [Google Scholar]
- 42. Puissant B, Barreau C, Bourin P, Clavel C, Corre J, Bousquet C, Taureau C, Cousin B, Abbal M, Laharrague P, Penicaud L, Casteilla L, Blancher A. Immunomodulatory effect of human adipose tissue‐derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol 2005;129:118–29. [DOI] [PubMed] [Google Scholar]
- 43. Yañez R, Lamana ML, García‐Castro J, Colmenero I, Ramírez M, Bueren JA. Adipose tissue‐derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft‐versus‐host disease. Stem Cells 2006;24:2582–91. [DOI] [PubMed] [Google Scholar]
- 44. Markeson D, Pleat JM, Sharpe JR, Harris AL, Seifalian AM, Watt SM. Scarring, stem cells, scaffolds and skin repair. J Tissue Eng Regen Med 2013; 9(6):649–68. [DOI] [PubMed] [Google Scholar]
- 45. Zahorec P, Koller J, Danisovic L, Bohac M. Mesenchymal stem cells for chronic wounds therapy. Cell Tissue Bank 2014;16:19–26. [DOI] [PubMed] [Google Scholar]
- 46. Cervelli V, Scioli MG, Gentile P, Doldo E, Bonanno E, Spagnoli LG, Orlandi A. Platelet‐rich plasma greatly potentiates insulin‐induced adipogenic differentiation of human adipose‐derived stem cells through a serine/threonine kinase Akt‐dependent mechanism and promotes clinical fat graft maintenance. Stem Cells Transl Med 2012;1:206–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kalbermatten DF, Rieger UM, Uike K, Erba P, Laifer G, Hintermann B, Pierer G. Infection with Aeromonas hydrophila after use of leeches (Hirudo medicinalis) in a free microvascular osteo‐(myo‐)cutaneous flap – suggestions for successful management. Handchir Mikrochir Plast Chir 2007;39:108–11. [DOI] [PubMed] [Google Scholar]
- 48. Oedayrajsingh‐Varma MJ, van Ham SM, Knippenberg M, Helder MN, Klein‐Nulend J, Schouten TE, Ritt M, van Milligen FJ. Adipose tissue‐derived mesenchymal stem cell yield and growth characteristics are affected by the tissue‐harvesting procedure. Cytotherapy 2006;8:166–77. [DOI] [PubMed] [Google Scholar]
- 49. Engels PE, Tremp M, Kingham PJ, di Summa PG, Largo RD, Schaefer DJ, Kalbermatten DF. Harvest site influences the growth properties of adipose derived stem cells. Cytotechnology 2013;65:437–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pu LLQ, Cui X, Fink BF, Cibull ML, Gao D. The viability of fatty tissues within adipose aspirates after conventional liposuction: a comprehensive study. Ann Plast Surg 2005;54:288–92; discussion 292. [PubMed] [Google Scholar]
- 51. Pu LLQ. Towards more rationalized approach to autologous fat grafting. J Plast Reconstr Aesthet Surg 2012;65:413–9. [DOI] [PubMed] [Google Scholar]
- 52. Boschert MT, Beckert BW, Puckett CL, Concannon MJ. Analysis of lipocyte viability after liposuction. Plast Reconstr Surg 2002;109:761–5; discussion 766–7. [DOI] [PubMed] [Google Scholar]
- 53. Phinney DG. Functional heterogeneity of mesenchymal stem cells: implications for cell therapy. J Cell Biochem 2012;113:2806–12. [DOI] [PubMed] [Google Scholar]
- 54. Ross RJ, Shayan R, Mutimer KL, Ashton MW. Autologous fat grafting: current state of the art and critical review. Ann Plast Surg 2014;73:352–7. [DOI] [PubMed] [Google Scholar]
- 55. Efimenko A, Dzhoyashvili N, Kalinina N, Kochegura T, Akchurin R, Tkachuk V, Parfyonova Y. Adipose‐derived mesenchymal stromal cells from aged patients with coronary artery disease keep mesenchymal stromal cell properties but exhibit characteristics of aging and have impaired angiogenic potential. Stem Cells Transl Med 2014;3:32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Eirin A, Zhu X‐Y, Krier JD, Tang H, Jordan KL, Grande JP, Lerman A, Textor SC, Lerman LO. Adipose tissue‐derived mesenchymal stem cells improve revascularization outcomes to restore renal function in swine atherosclerotic renal artery stenosis. Stem Cells 2012;30:1030–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhu X‐Y, Urbieta Caceres V, Krier JD, Textor SC, Lerman A, Lerman LO. Mesenchymal stem cells and endothelial progenitor cells decrease renal injury in experimental swine renal artery stenosis through different mechanisms. Stem Cells 2013;31:117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fan W, Sun D, Liu J, Liang D, Wang Y, Narsinh KH, Li Y, Qin X, Liang J, Tian J, Cao F. Adipose stromal cells amplify angiogenic signaling via the VEGF/mTOR/Akt pathway in a murine hindlimb ischemia model: a 3D multimodality imaging study. PLoS One 2012;7:e45621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mazo M, Hernández S, Gavira JJ, Abizanda G, Araña M, López‐Martínez T, Moreno C, Juana M, Martino‐Rodriguez A, Uixeira A, Garcia de Jalon JA, Pastrana J, Martinez‐Caro D, Prosper F. Treatment of reperfused ischemia with adipose‐derived stem cells in a preclinical swine model of myocardial infarction. Cell Transplant 2012;21:2723–33. [DOI] [PubMed] [Google Scholar]
- 60. Cai L, Johnstone BH, Cook TG, Tan J, Fishbein MC, Chen P‐S, March KL. IFATS collection: human adipose tissue‐derived stem cells induce angiogenesis and nerve sprouting following myocardial infarction, in conjunction with potent preservation of cardiac function. Stem Cells 2009;27:230–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kitada M. Mesenchymal cell populations: development of the induction systems for Schwann cells and neuronal cells and finding the unique stem cell population. Anat Sci Int 2012;87:24–44. [DOI] [PubMed] [Google Scholar]
- 62. Wang Y, Zhao Z, Ren Z, Zhao B, Zhang L, Chen J, Xu WJ, Lu S, Zhao Q, Peng J. Recellularized nerve allografts with differentiated mesenchymal stem cells promote peripheral nerve regeneration. Neurosci Lett 2012;514:96–101. [DOI] [PubMed] [Google Scholar]
- 63. Abdanipour A, Tiraihi T, Delshad A. Trans‐differentiation of the adipose tissue‐derived stem cells into neuron‐like cells expressing neurotrophins by selegiline. Iran Biomed J 2011;15:113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sowa Y, Imura T, Numajiri T, Nishino K, Fushiki S. Adipose‐derived stem cells produce factors enhancing peripheral nerve regeneration: influence of age and anatomic site of origin. Stem Cells Dev 2012;21:1852–62. [DOI] [PubMed] [Google Scholar]
- 65. Liu Y, Zhang Z, Qin Y, Wu H, Lv Q, Chen X, Deng W. A new method for Schwann‐like cell differentiation of adipose derived stem cells. Neurosci Lett 2013;551:79–83. [DOI] [PubMed] [Google Scholar]
- 66. Payne NL, Sun G, McDonald C, Layton D, Moussa L, Emerson‐Webber A, Veron N, Siatskas C, Herszfeld D, Price J, Bernard CCA. Distinct immunomodulatory and migratory mechanisms underpin the therapeutic potential of human mesenchymal stem cells in autoimmune demyelination. Cell Transplant 2013;22:1409–25. [DOI] [PubMed] [Google Scholar]
- 67. Hedayatpour A, Ragerdi I, Pasbakhsh P, Kafami L, Atlasi N, Pirhajati Mahabadi V, Ghasemi S, Reza M. Promotion of remyelination by adipose mesenchymal stem cell transplantation in a cuprizone model of multiple sclerosis. Cell J 2013;15:142–51. [PMC free article] [PubMed] [Google Scholar]
- 68. Constantin G, Marconi S, Rossi B, Angiari S, Calderan L, Anghileri E, Gini B, Bach SD, Martinello M, Bifari F, Galiè M, Turano E, Budui S, Sbarbati A, Krampera M, Bonetti B. Adipose‐derived mesenchymal stem cells ameliorate chronic experimental autoimmune encephalomyelitis. Stem Cells 2009;27:2624–35. [DOI] [PubMed] [Google Scholar]
- 69. Semon JA, Maness C, Zhang X, Sharkey SA, Beuttler MM, Shah FS, Pandey AC, Gimble JM, Zhang S, Scruggs BA, Strong AL, Strong TA, Bunnell BA. Comparison of human adult stem cells from adipose tissue and bone marrow in the treatment of experimental autoimmune encephalomyelitis. Stem Cell Res Ther 2014;5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Choi J, Minn KW, Chang H. The efficacy and safety of platelet‐rich plasma and adipose‐derived stem cells: an update. Arch Plast Surg 2012;39:585–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tsuji W, Inamoto T, Ito R, Morimoto N, Tabata Y, Toi M. Simple and longstanding adipose tissue engineering in rabbits. J Artif Organs 2013;16:110–4. [DOI] [PubMed] [Google Scholar]
- 72. Itoi Y, Takatori M, Hyakusoku H, Mizuno H. Comparison of readily available scaffolds for adipose tissue engineering using adipose‐derived stem cells. J Plast Reconstr Aesthet Surg 2010;63:858–64. [DOI] [PubMed] [Google Scholar]
- 73. Charles W, Patrick J. Breast tissue engineering. Ann Rev Biomed Eng 2004;6:109–30. [DOI] [PubMed] [Google Scholar]
- 74. Gomillion CT, Burg KJL. Stem cells and adipose tissue engineering. Biomaterials 2006;27:6052–63. [DOI] [PubMed] [Google Scholar]
- 75. Suga H, Glotzbach JP, Sorkin M, Longaker MT, Gurtner GC. Paracrine mechanism of angiogenesis in adipose‐derived stem cell transplantation. Ann Plast Surg 2014;72:234–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rodriguez A‐M, Elabd C, Amri E‐Z, Ailhaud G, Dani C. The human adipose tissue is a source of multipotent stem cells. Biochimie 2005;87:125–8. [DOI] [PubMed] [Google Scholar]
- 77. Vallejo A, Urban C, Zucca‐Matthes G, Rietjens M. Is there enough evidence to use lipofilling in breast cancer reconstruction? Plast Reconstr Surg 2013;132:689e–91. [DOI] [PubMed] [Google Scholar]
- 78. Pearl RA, Leedham SJ, Pacifico MD. The safety of autologous fat transfer in breast cancer: lessons from stem cell biology. J Plast Reconstr Aesthet Surg 2012;65:283–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zhao Y, Gao J, Lu F. Human adipose‐derived stem cell adipogenesis induces paracrine regulation of the invasive ability of MCF‐7 human breast cancer cells in vitro. Exp Ther Med 2013;6:937–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bertolini F, Lohsiriwat V, Petit J‐Y, Kolonin MG. Adipose tissue cells, lipotransfer and cancer: a challenge for scientists, oncologists and surgeons. Biochim Biophys Acta 2012;1826:209–14. [DOI] [PubMed] [Google Scholar]
- 81. Bielli A, Scioli MG, Gentile P, Agostinelli S, Tarquini C, Cervelli V, Orlandi A. Adult adipose‐derived stem cells and breast cancer: a controversial relationship. SpringerPlus 2014;3:345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B, Wang YY, Meulle A, Salles B, Le Gonidec S, Garrido I, Escourrou G, Valet P, Muller C. Cancer‐associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res 2011;71:2455–65. [DOI] [PubMed] [Google Scholar]
- 83. Chandler EM, Seo BR, Califano JP, Eguiluz RCA, Lee JS, Yoon CJ, Tims DT, Wang JX, Cheng L, Mohanan S, Buckley MR, Cohen I, Nikitin AY, Williams RM, Gourdon D, Reinhart‐King CA, Fischbach C. Implanted adipose progenitor cells as physicochemical regulators of breast cancer. Proc Natl Acad Sci 2012;109:9786–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. James R, Kumbar SG, Laurencin CT, Balian G, Chhabra AB. Tendon tissue engineering: adipose‐derived stem cell and GDF‐5 mediated regeneration using electrospun matrix systems. Biomed Mater 2011;6:025011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Frank C, McDonald D, Shrive N. Collagen fibril diameters in the rabbit medial collateral ligament scar: a longer term assessment. Connect Tissue Res 1997;36:261–9. [DOI] [PubMed] [Google Scholar]
- 86. Uysal CA, Tobita M, Hyakusoku H, Mizuno H. Adipose‐derived stem cells enhance primary tendon repair: biomechanical and immunohistochemical evaluation. J Plast Reconstr Aesthet Surg 2012;65:1712–9. [DOI] [PubMed] [Google Scholar]
- 87. Uysal AC, Mizuno H. Differentiation of adipose‐derived stem cells for tendon repair. Methods Mol Biol 2011;702:443–51. [DOI] [PubMed] [Google Scholar]
- 88. Park A, Hogan MV, Kesturu GS, James R, Balian G, Chhabra AB. Adipose‐derived mesenchymal stem cells treated with growth differentiation factor‐5 express tendon‐specific markers. Tissue Eng Part A 2010;16:2941–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Simon LS. Osteoarthritis: a review. Clin Cornerstone 1999; 2(2):26–37. [DOI] [PubMed] [Google Scholar]
- 90. Khan IM, Francis L, Theobald PS, Perni S, Young RD, Prokopovich P, Conlan S, Archer CW. In vitro growth factor‐induced bio engineering of mature articular cartilage. Biomaterials 2013;34:1478–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Khan WS, Johnson DS, Hardingham TE. The potential of stem cells in the treatment of knee cartilage defects. Knee 2010;17:369–74. [DOI] [PubMed] [Google Scholar]
- 92. Oliveira JT, Gardel LS, Rada T, Martins L, Gomes ME, Reis RL. Injectable gellan gum hydrogels with autologous cells for the treatment of rabbit articular cartilage defects. J Orthop Res 2010;28:1193–9. [DOI] [PubMed] [Google Scholar]
- 93. Khan IM, Gilbert SJ, Singhrao SK, Duance VC, Archer CW. Cartilage integration: evaluation of the reasons for failure of integration during cartilage repair. A review. Eur Cell Mater 2008;16:26–39. [DOI] [PubMed] [Google Scholar]
- 94. Caplan AI, Elyaderani M, Mochizuki Y, Wakitani S, Goldberg VM. Overview: principles of cartilage repair and regeneration. Clin Orthop Relat Res 1997;342:254–69. [PubMed] [Google Scholar]
- 95. Beane OS, Darling EM. Isolation, characterization, and differentiation of stem cells for cartilage regeneration. Ann Biomed Eng 2012;40:2079–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kim H‐J, Im G‐I. Combination of transforming growth factor‐beta2 and bone morphogenetic protein 7 enhances chondrogenesis from adipose tissue‐derived mesenchymal stem cells. Tissue Eng Part A 2009;15:1543–51. [DOI] [PubMed] [Google Scholar]
- 97. Jung S‐N, Rhie JW, Kwon H, Jun YJ, Seo J‐W, Yoo G, Oh DY, Ahn ST, Woo J, Oh J. In vivo cartilage formation using chondrogenic‐differentiated human adipose‐derived mesenchymal stem cells mixed with fibrin glue. J Craniofac Surg 2010;21:468–72. [DOI] [PubMed] [Google Scholar]
- 98. Khan IM, Evans SL, Young RD, Blain EJ, Quantock AJ, Avery N, Archer CW. Fibroblast growth factor 2 and transforming growth factor β1 induce precocious maturation of articular cartilage. Arthritis Rheum 2011;63:3417–27. [DOI] [PubMed] [Google Scholar]
- 99. Rosier RN, O'Keefe RJ, Crabb ID, Puzas JE. Transforming growth factor beta: an autocrine regulator of chondrocytes. Connect Tissue Res 1989;20:295–301. [DOI] [PubMed] [Google Scholar]
- 100. Mehlhorn AT, Zwingmann J, Finkenzeller G, Niemeyer P, Dauner M, Stark B, Südkamp NP, Schmal H. Chondrogenesis of adipose‐derived adult stem cells in a poly‐lactide‐co‐glycolide scaffold. Tissue Eng Part A 2009;15:1159–67. [DOI] [PubMed] [Google Scholar]
- 101. Indrawattana N, Chen G, Tadokoro M, Shann LH, Ohgushi H, Tateishi T, Tanaka J, Bunyaratvej A. Growth factor combination for chondrogenic induction from human mesenchymal stem cell. Biochem Biophys Res Commun 2004;320:914–9. [DOI] [PubMed] [Google Scholar]
- 102. Furumatsu T, Tsuda M, Taniguchi N, Tajima Y, Asahara H. Smad3 induces chondrogenesis through the activation of SOX9 via CREB‐binding protein/p300 recruitment. J Biol Chem 2005;280:8343–50. [DOI] [PubMed] [Google Scholar]
- 103. Li Q, Tang J, Wang R, Bei C, Xin L, Zeng Y, Tang X. Comparing the chondrogenic potential in vivo of autogeneic mesenchymal stem cells derived from different tissues. Artif Cells Blood Substit Immobil Biotechnol 2011;39:31–8. [DOI] [PubMed] [Google Scholar]
- 104. Schultz E, McCormick KM. Skeletal muscle satellite cells. In: Reviews of physiology, biochemistry and pharmacology. Vol. 123. Berlin/Heidelberg: Springer Berlin Heidelberg, 1994:213–57. [DOI] [PubMed] [Google Scholar]
- 105. Schultz E, Lipton BH. Skeletal muscle satellite cells: changes in proliferation potential as a function of age. Mech Ageing Dev 1982;20:377–83. [DOI] [PubMed] [Google Scholar]
- 106. Heslop L, Morgan JE, Partridge TA. Evidence for a myogenic stem cell that is exhausted in dystrophic muscle. J Cell Sci 2000;113(Pt 12):2299–308. [DOI] [PubMed] [Google Scholar]
- 107. Andersen DC, Schrøder HD, Jensen CH. Non‐cultured adipose‐derived CD45‐ side population cells are enriched for progenitors that give rise to myofibres in vivo. Exp Cell Res 2008;314:2951–64. [DOI] [PubMed] [Google Scholar]
- 108. Vieira NM, Brandalise V, Zucconi E, Jazedje T, Secco M, Nunes VA, Strauss BE, Vainzof M, Zatz M. Human multipotent adipose‐derived stem cells restore dystrophin expression of Duchenne skeletal‐muscle cells in vitro. Biol Cell 2008;100:231–41. [DOI] [PubMed] [Google Scholar]
- 109. Rodríguez LV, Alfonso Z, Zhang R, Leung J, Wu B, Ignarro LJ. Clonogenic multipotent stem cells in human adipose tissue differentiate into functional smooth muscle cells. Proc Natl Acad Sci U S A 2006;103:12167–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev 2003;83:835–70. [DOI] [PubMed] [Google Scholar]
- 111. Hong SJ, Jia S‐X, Xie P, Xu W, Leung KP, Mustoe TA, Galiano RD. Topically delivered adipose derived stem cells show an activated‐fibroblast phenotype and enhance granulation tissue formation in skin wounds. PLoS One 2013;8:e55640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair—current views. Stem Cells 2007; 25(11):2896–902. [DOI] [PubMed] [Google Scholar]
- 113. Schäffler A, Büchler C. Concise review: adipose tissue‐derived stromal cells‐‐basic and clinical implications for novel cell‐based therapies. Stem Cells 2007;25:818–27. [DOI] [PubMed] [Google Scholar]
- 114. Murohara T, Shintani S, Kondo K. Autologous adipose‐derived regenerative cells for therapeutic angiogenesis. Curr Pharm Des 2009;15:2784–90. [DOI] [PubMed] [Google Scholar]
- 115. Meliga E, Strem BM, Duckers HJ. Adipose‐derived cells. Cell 2007; 16(9):963–70. [DOI] [PubMed] [Google Scholar]
- 116. Klinger FM, Vinci V, Forcellini D, Caviggioli F. Basic science review on adipose tissue for clinicians. Plast Reconstr Surg 2011;128:829–30. [DOI] [PubMed] [Google Scholar]
- 117. Locke M, Feisst V, Dunbar PR. Concise review: human adipose‐derived stem cells: separating promise from clinical need. Stem Cells 2011;29:404–11. [DOI] [PubMed] [Google Scholar]
- 118. Zografou A, Tsigris C, Papadopoulos O, Kavantzas N, Patsouris E, Donta I, Perrea D. Improvement of skin‐graft survival after autologous transplantation of adipose‐derived stem cells in rats. J Plast Reconstr Aesthet Surg 2011;64:1647–56. [DOI] [PubMed] [Google Scholar]
- 119. Mojallal A, Lequeux C, Shipkov C, Breton P, Foyatier J‐L, Braye F, Damour O. Improvement of skin quality after fat grafting: clinical observation and an animal study. Plast Reconstr Surg 2009;124:765–74. [DOI] [PubMed] [Google Scholar]
- 120. Klinger M, Marazzi M, Vigo D, Torre M. Fat injection for cases of severe burn outcomes: a new perspective of scar remodeling and reduction. Aesthetic Plast Surg 2008;32:465–9. [DOI] [PubMed] [Google Scholar]
- 121. Rigotti G, Marchi A, Galiè M, Baroni G, Benati D, Krampera M, Pasini A, Sbarbati A. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose‐derived adult stem cells. Plast Reconstr Surg 2007;119:1409–22; discussion 1423–4. [DOI] [PubMed] [Google Scholar]
- 122. Chawla R, Tan A, Ahmed M, Crowley C, Moiemen NS, Cui Z, Butler PE, Seifalian AM. A polyhedral oligomeric silsesquioxane‐based bilayered dermal scaffold seeded with adipose tissue‐derived stem cells: in vitro assessment of biomechanical properties. J Surg Res 2014;188:361–72. [DOI] [PubMed] [Google Scholar]
- 123. Hemmrich K, Heimburg von D. Biomaterials for adipose tissue engineering. Expert Rev Med Devices 2006;3:635–45. [DOI] [PubMed] [Google Scholar]
- 124. Altman AM, Yan Y, Matthias N, Bai X, Rios C, Mathur AB, Song YH, Alt EU. IFATS collection: human adipose‐derived stem cells seeded on a silk fibroin‐chitosan scaffold enhance wound repair in a murine soft tissue injury model. Stem Cells 2009;27:250–8. [DOI] [PubMed] [Google Scholar]
- 125. Kim W‐S, Park B‐S, Sung J‐H, Yang J‐M, Park S‐B, Kwak S‐J, Park JS. Wound healing effect of adipose‐derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci 2007;48:15–24. [DOI] [PubMed] [Google Scholar]
- 126. Liu S, Zhang H, Zhang X, Lu W, Huang X, Xie H, Zhou J, Wang W, Zhang Y, Liu Y, Deng Z, Jin Y. Synergistic angiogenesis promoting effects of extracellular matrix scaffolds and adipose‐derived stem cells during wound repair. Tissue Eng Part A 2010;17:725–39. [DOI] [PubMed] [Google Scholar]
- 127. Ferraro GA, De Francesco F, Nicoletti G, Paino F, Desiderio V, Tirino V, D'Andrea F. Human adipose CD34+ CD90+ stem cells and collagen scaffold constructs grafted in vivo fabricate loose connective and adipose tissues. J Cell Biochem 2013;114:1039–49. [DOI] [PubMed] [Google Scholar]
- 128. Lu W, Yu J, Zhang Y, Ji K, Zhou Y, Li Y, Deng Z, Jin Y. Mixture of fibroblasts and adipose tissue‐derived stem cells can improve epidermal morphogenesis of tissue‐engineered skin. Cells Tissues Organs 2012;195:197–206. [DOI] [PubMed] [Google Scholar]
- 129. Moon KM, Park Y‐H, Lee JS, Chae Y‐B, Kim M‐M, Kim D‐S, Kim BW, Nam SW, Lee JH. The effect of secretory factors of adipose‐derived stem cells on human keratinocytes. Int J Mol Sci 2012;13:1239–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Ghoreishian M, Rezaei M, Beni BH, Javanmard SH, Attar BM, Zalzali H. Facial nerve repair with Gore‐Tex tube and adipose‐derived stem cells: an animal study in dogs. J Oral Maxillofac Surg 2013;71:577–87. [DOI] [PubMed] [Google Scholar]
- 131. di Summa PG, Kalbermatten DF, Raffoul W, Terenghi G, Kingham PJ. Extracellular matrix molecules enhance the neurotrophic effect of Schwann cell‐like differentiated adipose‐derived stem cells and increase cell survival under stress conditions. Tissue Eng Part A 2013;19:368–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Kim IG, Piao S, Lee JY, Hong SH, Hwang T‐K, Kim SW, Kim CS, Ra JC, Noh I, Lee JY. Effect of an adipose‐derived stem cell and nerve growth factor‐incorporated hydrogel on recovery of erectile function in a rat model of cavernous nerve injury. Tissue Eng Part A 2013;19:14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Cardozo AJ, Gómez DE, Argibay PF. Neurogenic differentiation of human adipose‐derived stem cells: relevance of different signaling molecules, transcription factors, and key marker genes. Gene 2012;511:427–36. [DOI] [PubMed] [Google Scholar]
- 134. Faroni A, Terenghi G, Magnaghi V. Expression of functional γ‐aminobutyric acid type A receptors in Schwann‐like adult stem cells. J Mol Neurosci 2012;47:619–30. [DOI] [PubMed] [Google Scholar]
- 135. Liu B‐S, Yang Y‐C, Shen C‐C. Regenerative effect of adipose tissue‐derived stem cells transplantation using nerve conduit therapy on sciatic nerve injury in rats. J Tissue Eng Regen Med 2014;8:337–50. [DOI] [PubMed] [Google Scholar]
- 136. Orbay H, Uysal AC, Hyakusoku H, Mizuno H. Differentiated and undifferentiated adipose‐derived stem cells improve function in rats with peripheral nerve gaps. J Plast Reconstr Aesthet Surg 2012;65:657–64. [DOI] [PubMed] [Google Scholar]
- 137. Adams AM, Arruda EM, Larkin LM. Use of adipose‐derived stem cells to fabricate scaffoldless tissue‐engineered neural conduits in vitro. Neuroscience 2012;201:349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Shen C‐C, Yang Y‐C, Liu B‐S. Peripheral nerve repair of transplanted undifferentiated adipose tissue‐derived stem cells in a biodegradable reinforced nerve conduit. J Biomed Mater Res A 2012;100:48–63. [DOI] [PubMed] [Google Scholar]
- 139. Reid AJ, Sun M, Wiberg M, Downes S, Terenghi G. Nerve repair with adipose‐derived stem cells protects dorsal root ganglia neurons from apoptosis. Neuroscience 2011; 199:151–22. [DOI] [PubMed] [Google Scholar]
- 140. Sun F, Zhou K, Mi W‐J, Qiu J‐H. Combined use of decellularized allogeneic artery conduits with autologous transdifferentiated adipose‐derived stem cells for facial nerve regeneration in rats. Biomaterials 2011;32:8118–28. [DOI] [PubMed] [Google Scholar]
- 141. Liu Y‐P, Li S‐Z, Yuan F, Xia J, Yu X, Liu X, Yu GR. Infrapatellar fat pad may be with tendon repairing ability and closely related with the developing process of patella Baja. Med Hypotheses 2011;77:620–3. [DOI] [PubMed] [Google Scholar]
- 142. Faroni A, Mantovani C, Shawcross SG, Motta M, Terenghi G, Magnaghi V. Schwann‐like adult stem cells derived from bone marrow and adipose tissue express γ‐aminobutyric acid type B receptors. J Neurosci Res 2011;89:1351–62. [DOI] [PubMed] [Google Scholar]
- 143. Wei Y, Gong K, Zheng Z, Wang A, Ao Q, Gong Y, Zhang X. Chitosan/silk fibroin‐based tissue‐engineered graft seeded with adipose‐derived stem cells enhances nerve regeneration in a rat model. J Mater Sci Mater Med 2011;22:1947–64. [DOI] [PubMed] [Google Scholar]
- 144. Cardozo AJ, Gómez DE, Argibay PF. Transcriptional characterization of Wnt and Notch signaling pathways in neuronal differentiation of human adipose tissue‐derived stem cells. J Mol Neurosci 2011;44:186–94. [DOI] [PubMed] [Google Scholar]
- 145. Lin G, Albersen M, Harraz AM, Fandel TM, Garcia M, McGrath MH, Konety BR, Lue TF, Lin CS. Cavernous nerve repair with allogenic adipose matrix and autologous adipose‐derived stem cells. Urology 2011;77:1509.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. di Summa PG, Kalbermatten DF, Pralong E, Raffoul W, Kingham PJ, Terenghi G. Long‐term in vivo regeneration of peripheral nerves through bioengineered nerve grafts. Neuroscience 2011;181:278–91. [DOI] [PubMed] [Google Scholar]
- 147. Kaewkhaw R, Scutt AM, Haycock JW. Anatomical site influences the differentiation of adipose‐derived stem cells for Schwann‐cell phenotype and function. Glia 2011;59:734–49. [DOI] [PubMed] [Google Scholar]
- 148. Lopatina T, Kalinina N, Karagyaur M, Stambolsky D, Rubina K, Revischin A, Pavlova G, Parfyonova Y, Tkachuk V. Adipose‐derived stem cells stimulate regeneration of peripheral nerves: BDNF secreted by these cells promotes nerve healing and axon growth de novo. PLoS One 2011;6:e17899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Mohammadi R, Azizi S, Delirezh N, Hobbenaghi R, Amini K. Comparison of beneficial effects of undifferentiated cultured bone marrow stromal cells and omental adipose‐derived nucleated cell fractions on sciatic nerve regeneration. Muscle Nerve 2011;43:157–63. [DOI] [PubMed] [Google Scholar]
- 150. Erba P, Mantovani C, Kalbermatten DF, Pierer G, Terenghi G, Kingham PJ. Regeneration potential and survival of transplanted undifferentiated adipose tissue‐derived stem cells in peripheral nerve conduits. J Plast Reconstr Aesthet Surg 2010;63:e811–7. [DOI] [PubMed] [Google Scholar]
- 151. Tse K‐H, Sun M, Mantovani C, Terenghi G, Downes S, Kingham PJ. In vitro evaluation of polyester‐based scaffolds seeded with adipose derived stem cells for peripheral nerve regeneration. J Biomed Mater Res A 2010;95:701–8. [DOI] [PubMed] [Google Scholar]
- 152. di Summa PG, Kingham PJ, Raffoul W, Wiberg M, Terenghi G, Kalbermatten DF. Adipose‐derived stem cells enhance peripheral nerve regeneration. J Plast Reconstr Aesthet Surg 2010;63:1544–52. [DOI] [PubMed] [Google Scholar]
- 153. Zhang Y, Luo H, Zhang Z, Lu Y, Huang X, Yang L, Xu J, Yang W, Fan X, Du B, Gao P, Hu G, Jin Y. A nerve graft constructed with xenogeneic acellular nerve matrix and autologous adipose‐derived mesenchymal stem cells. Biomaterials 2010;31:5312–24. [DOI] [PubMed] [Google Scholar]
- 154. Chi GF, Kim M‐R, Kim D‐W, Jiang MH, Son Y. Schwann cells differentiated from spheroid‐forming cells of rat subcutaneous fat tissue myelinate axons in the spinal cord injury. Exp Neurol 2010;222:304–17. [DOI] [PubMed] [Google Scholar]
- 155. Kingham PJ, Mantovani C, Terenghi G. Notch independent signalling mediates Schwann cell‐like differentiation of adipose derived stem cells. Neurosci Lett 2009;467:164–8. [DOI] [PubMed] [Google Scholar]
- 156. Radtke C, Schmitz B, Spies M, Kocsis JD, Vogt PM. Peripheral glial cell differentiation from neurospheres derived from adipose mesenchymal stem cells. Int J Dev Neurosci 2009;27:817–23. [DOI] [PubMed] [Google Scholar]
- 157. Santiago LY, Clavijo‐Alvarez J, Brayfield C, Rubin JP, Marra KG. Delivery of adipose‐derived precursor cells for peripheral nerve repair. Cell Transplant 2009;18:145–58. [DOI] [PubMed] [Google Scholar]
- 158. Xu Y, Liu L, Li Y, Zhou C, Xiong F, Liu Z, Gu R, Hou X, Zhang C. Myelin‐forming ability of Schwann cell‐like cells induced from rat adipose‐derived stem cells in vitro. Brain Res 2008;1239:49–55. [DOI] [PubMed] [Google Scholar]
- 159. Kingham PJ, Kalbermatten DF, Mahay D, Armstrong SJ, Wiberg M, Terenghi G. Adipose‐derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp Neurol 2007;207:267–74. [DOI] [PubMed] [Google Scholar]
- 160. Yu C, Bianco J, Brown C, Fuetterer L, Watkins JF, Samani A, Flynn LE. Porous decellularized adipose tissue foams for soft tissue regeneration. Biomaterials 2013;34:3290–302. [DOI] [PubMed] [Google Scholar]
- 161. Khan WS, Adesida AB, Tew SR, Longo UG, Hardingham TE. Fat pad‐derived mesenchymal stem cells as a potential source for cell‐based adipose tissue repair strategies. Cell Prolif 2012;45:111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Wu I, Nahas Z, Kimmerling KA, Rosson GD, Elisseeff JH. An injectable adipose matrix for soft‐tissue reconstruction. Plast Reconstr Surg 2012;129:1247–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Lequeux C, Oni G, Wong C, Damour O, Rohrich R, Mojallal A, Brown SA. Subcutaneous fat tissue engineering using autologous adipose‐derived stem cells seeded onto a collagen scaffold. Plast Reconstr Surg 2012;130:1208–17. [DOI] [PubMed] [Google Scholar]
- 164. Ito R, Morimoto N, Liem PH, Nakamura Y, Kawai K, Taira T, Tsuji W, Toi M, Suzuki S. Adipogenesis using human adipose tissue‐derived stromal cells combined with a collagen/gelatin sponge sustaining release of basic fibroblast growth factor. J Tissue Eng Regen Med 2014;8:1000–8. [DOI] [PubMed] [Google Scholar]
- 165. Choi JH, Bellas E, Vunjak‐Novakovic G, Kaplan DL. Adipogenic differentiation of human adipose‐derived stem cells on 3D silk scaffolds. Methods Mol Biol 2011;702:319–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. D'Andrea F, De Francesco F, Ferraro GA, Desiderio V, Tirino V, De Rosa A, Papaccio G. Large‐scale production of human adipose tissue from stem cells: a new tool for regenerative medicine and tissue banking. Tissue Eng Part C Methods 2008;14:233–42. [DOI] [PubMed] [Google Scholar]
- 167. Mizuno H, Itoi Y, Kawahara S, Ogawa R, Akaishi S, Hyakusoku H. In vivo adipose tissue regeneration by adipose‐derived stromal cells isolated from GFP transgenic mice. Cells Tissues Organs 2008;187:177–85. [DOI] [PubMed] [Google Scholar]
- 168. Lin S‐D, Wang K‐H, Kao A‐P. Engineered adipose tissue of predefined shape and dimensions from human adipose‐derived mesenchymal stem cells. Tissue Eng Part A 2008;14:571–81. [DOI] [PubMed] [Google Scholar]


