Abstract
The purpose of this study was to examine the clinical outcomes of negative pressure wound therapy (NPWT) using reticulated open‐cell foam (ROCF) in the adjunctive management of abdominal wounds with exposed and known infected synthetic mesh. A non randomised, retrospective review of medical records for 21 consecutive patients with infected abdominal wounds treated with NPWT was conducted. All abdominal wounds contained exposed synthetic mesh [composite, polypropylene (PP), or knitted polyglactin 910 (PG) mesh]. Demographic and bacteriological data, wound history, pre‐NPWT and comparative post‐NPWT, operative procedures and complications, hospital length of stay (LOS) and wound healing outcomes were all analysed. Primary endpoints measured were (1) hospital LOS prior to initiation of NPWT, (2) total time on NPWT, (3) hospital LOS from NPWT initiation to discharge and (4) wound closure status at discharge. A total of 21 patients with abdominal wounds with exposed, infected mesh were treated with NPWT. Aetiology of the wounds was ventral hernia repair (n = 11) and acute abdominal wall defect (n = 10). Prior to NPWT initiation, the mean hospital LOS for the composite, PP and PG meshes were 76 days (range: 21–171 days), 51 days (range: 32–62 days) and 19 days (range: 12–39 days), respectively. The mean hospital LOS following initiation of NPWT for wounds with exposed composite, PP and PG mesh were 28, 31 and 32 days, respectively. Eighteen of the 21 wounds (86%) reached full closure after a mean time of 26 days of NPWT and a mean hospital LOS of 30 days postinitiation of NPWT. Three wounds, all with composite mesh left in situ, did not reach full closure, although all exhibited decreased wound dimensions, granulating beds and decreased surface area exposure of mesh. During NPWT/ROCF, one hypoalbuminemic patient with exposed PP mesh developed an enterocutaneous fistula over a prior enterotomy site. This patient subsequently underwent total mesh extraction, takedown of the fistula and PP mesh replacement followed by reinstitution of NPWT and flap closure. In addition to appropriate systemic antibiotics and nutritional optimisation, the adjunctive use of NPWT resulted in successful closure of 86% of infected abdominal wounds with exposed prosthetic mesh. Patient hospital LOS (except those with PG mesh), operative procedures and readmissions were decreased during NPWT compared with treatment prior to NPWT. Future multi‐site prospective, controlled studies would provide a strong evidence base from which treatment decisions could be made in the management of these challenging and costly cases.
Keywords: Infected abdominal wound, Negative pressure wound therapy, NPWT, Synthetic mesh, Vacuum assisted closure
INTRODUCTION
The incidence of acute abdominal wall defects is on the rise because of new approaches in surgical management (1). Common aetiologies for acute abdominal wall defects are trauma, prior surgery and necrotising fascial infections (1). Newer strategies in patient management require repeated fascial opening and suturing, which can lead to fascial necrosis and necrotising infection. Attempting to close the abdominal wall under excess tension can result in potential failure, respiratory compromise and possibly intestinal ischaemia (2). In the absence of fascia, or when re‐approximation without tension is not possible, surgeons are increasingly using a variety of prostheses to maintain abdominal wall integrity and allow for re‐exploration through the prosthesis.
In response to this increased demand for abdominal prosthetic materials, a broad range of meshes has been introduced into the market. The use of synthetic mesh to strengthen the abdominal wall was popularised following an animal study by Usher and Gannon in 1959 and a subsequent clinical study by Usher (3). Synthetic meshes are designed with goals of maintaining their strength, incorporating with surrounding tissues and not stimulating adhesions (4). Overall, synthetic mesh can be credited with allowing tension‐free repairs and doubtlessly reducing hernia recurrence rates (5).
Although the use of synthetic mesh is ever increasing, no single mesh material has gained universal acceptance (4). Polypropylene (PP) mesh has a long reported history of successful adjunctive use in hernia and abdominal wall repair. It is a stiff, non absorbable, screen‐like knitted mono‐filament that is commonly used in these repairs because of its long‐term tensile strength. Complications associated with PP mesh include severe adhesions, infection and seroma and fistula development 1, 6. Polyglactin 910 (PG) mesh, by comparison, is an absorbable, soft, pliable mesh made from 90% glycolide and 10% l‐lactide and is known to be inert, non antigenic and non pyrogenic. The mesh is a knitted, microporous tightly woven broadcloth designed to encourage tissue in‐growth and evoke minimal inflammation and adhesion. Dissolving by hydrolysis, PG serves as a lattice for the formation of granulation tissue and is used for temporary wound support. It is easy to remove because of suppuration between the mesh and the granulating wound after a few weeks (1). The composite mesh is a bi‐product prosthetic mesh compromised of PP on one side and expanded polytetraflouroethylene (ePTFE) on the other. The PP allows tissue in‐growth and helps secure the mesh while the ePTFE is meant to act as a barrier to prevent the PP side from contacting adjacent organs and reducing risk of adhesion.
There is no doubt that introducing synthetic mesh in abdominal wall defect management has profoundly enhanced long‐term abdominal wall closure rates. As an example, of the over 2 million abdominal procedures performed annually in the USA, an estimated 2–11% of patients will develop an incisional hernia (7). Creating a tension‐free repair with a prosthetic material has lowered the previously reported hernia recurrence rate 30–50% of cases following primary repair to a recurrence rate between 0% and 10% 8, 9, 10.
It is also well understood that introducing foreign material, such as synthetic mesh, into a wound increases the risk of infection. Delayed infections can be seen with the presence of prosthetic meshes months or years after the procedure (11). Dramatically different mesh infection rates are reported in the literature, varying from 0·6% (12) to 22·0% (13). Nevertheless, infection is known to be the leading cause of failure in hernia repair, regardless of biomaterial used (14), with higher infection rates found in hernia repairs with mesh compared with those without mesh (5). Infection is also one of the most common complications following abdominal repair (5) and is associated with prolonged hospital stay and costs. Aggressive management of these infected wounds with exposed mesh is important in reducing the burden to the patient and the entire healthcare system.
Negative pressure wound therapy (NPWT) using reticulated open‐cell foam (ROCF) has been used as an adjunctive treatment for dehisced abdominal wounds since its introduction in 1995 (15). NPWT is a wound management technique that applies negative pressure to a proprietary dressing placed within the wound cavity. The negative pressure induces micro‐deformations of the tissue, which has been shown to increase cell proliferation and migration 16, 17. Wound exudate is also removed, which enhances the removal of inhibitory mediators and matrix metalloproteinases and improves wound healing (18). Studies have shown that NPWT can enhance local blood perfusion, reduces oedema, draws wound edges together 19, 20 and significantly increase growth of reparative granulation tissue 21, 22, which is of prime importance in these types of wounds.
Infected abdominal wounds containing exposed prosthetic mesh are commonly managed in the USA with conventional normal saline moistened gauze applied twice daily 23, 24. These wounds often become recalcitrant, resulting in protracted hospital stays, increased bacterial burden, patient pain and frustration, multiple readmissions and operative procedures for drainage of abscesses and serial debridements. Proactive management of these wound infections is needed to decrease cost and burden to the patient and healthcare system. In this series, we document our outcomes of using NPWT as delivered by V.A.C.® Therapy (KCI Licensing, Inc., San Antonio, TX) as an adjunctive treatment for abdominal wound infections with exposed mesh. For each patient, total hospital length of stay (LOS), complications and procedures performed were documented prior to and post‐NPWT initiation to determine efficacy of NPWT in the treatment of this type of wound.
METHODS
A non randomised, retrospective review of medical records for 21 consecutive patients with infected abdominal wounds treated with NPWT was conducted. Inclusion criteria into the study were patients with a primary diagnosis of infected abdominal wound with exposed mesh following a failed acute abdominal wall closure or ventral hernia repair from 1999 to 2004.
Demographic and bacteriological data, wound history, pre‐NPWT and comparative post‐NPWT operative procedures and complications, hospital LOS and wound healing outcomes were entered into an electronic database and analysed. All patient data were de‐identified for Health Insurance Portability and Accountability Act (HIPAA) compliance. Primary endpoints measured were (1) hospital LOS prior to initiation of NPWT, (2) total time on NPWT, (3) hospital LOS from initiation of NPWT to discharge and (4) wound closure status at discharge.
All abdominal wounds were dressed with a ROCF (V.A.C.® GranuFoam™, KCI Licensing, Inc., San Antonio, TX) dressing in accordance with manufacturer recommendations at a continuous negative pressure of 125 mmHg. The ROCF dressing was placed within the entire wound cavity and covered with a semi‐occlusive transparent adhesive drape. Tubing was applied over a hole cut into the drape. The distal end of the tubing was connected to the fluid collection canister, and pressure was initiated. Daily wound assessments were performed until granulation tissue was present and clinical signs of infection were obviated, and then patients were converted to every 48 hour dressing changes. Nutritional supplementation was administered as needed. All patients were treated in‐patient during the entire course of therapy.
Intra‐operative tissue and abscess aspirate cultures were taken within 24 hours prior to NPWT initiation, and results were recorded in the database. Infectious disease (ID) consultation and follow‐up were provided for all 21 patients. Systemic antibiotics were administered in all cases consistent with ID recommendations and intra‐operative bacteriological results. The type, dosage and duration administered were patient‐dependent and not recorded in the database.
Analysis was performed using the SAS statistical analysis system V9·3 (SAS Institute Inc., Cary, NC). Wounds were classified into three different groups according to type of mesh used: PP mesh (Marlex, Bard Vascular, Billerica, MA), knitted PG mesh (Vicryl, Ethicon, Inc, Somerville, NJ) and a PP/expanded polytetrafluoroethylene composite mesh (Bard Composix, Davol Inc, Cranston, RI). Outcomes were analysed for the entire patient set and by type of mesh used. Additional evaluation of procedures performed prior to and during NPWT as well as complications experienced during NPWT was undertaken to help determine best practices of NPWT in treating these types of wounds. The total LOS prior to NPWT was the sum of all of the days spent in‐hospital for treatment of the wound, including recurrent admissions. The post‐NPWT LOS included all hospital days from day 1 of NPWT initiation to complete wound closure and/or hospital discharge. All categorical variables are expressed as frequency while continuous variables are expressed as the median and range unless otherwise stated. Approval to perform this study was granted by the institutional review board.
RESULTS
Twenty‐one patients with abdominal wounds with exposed, infected mesh were treated with NPWT. Six patients were male and 15 were female. The median age was 71 years (range: 36–87 years). Wound aetiologies, baseline patient comorbidities and mesh type used are summarised in Table 1. Pre‐NPWT intra‐operative cultures identified Methicillin‐resistant Staphylococcus aureus (MRSA) as the single bacteria in 15 wounds; MRSA and Pseudomonas aerunginosa in 4 wounds and Pseudomonas in 2 wounds.
Table 1.
Demographic data
| Comorbidities | n | % |
|---|---|---|
| Hypoalbuminemia | 18 | 86 |
| Smoker | 7 | 33 |
| Diabetes mellitus | 5 | 24 |
| Morbid obesity | 5 | 24 |
| Chronic Obstructive Pulmonary Disease (COPD) | 2 | 10 |
| Radiation Therapy (RT) | 1 | 5 |
| Wound aetiology | ||
| Ventral hernia repair | 11 | 52 |
| Acute abdominal wall closure | 10 | 48 |
| Mesh type | ||
| Composite | 8 | 38 |
| Marlex (PP) | 8 | 38 |
| Vicryl (PG) | 5 | 24 |
COPD, ; RT, ; PP, polypropylene; PG, polyglactin 910.
Prior to the initiation of NPWT, the mean wound duration was 86 days (range: 5–295 days). The mean wound duration prior to NPWT by mesh type was as follows: composite mesh 149 days (range: 30–295 days), PP mesh 70 days (range: 37–171 days) and PG mesh 10 days (range: 5–17 days). Mean hospital LOS for patients associated with an admitting diagnosis of infected abdominal wound prior to initiation of NPWT for the composite, PP and PG meshes was 76 (range: 21–171 days), 51 (range: 32–62 days) and 19 days (range: 12–39 days), respectively. The mean hospital LOS following initiation of NPWT for wounds with exposed composite, PP and PG mesh was 28 (24 NPWT days), 31 (25 NPWT days) and 32 days (28 NPWT days), respectively.
Prior to initiation of NPWT, 14 patients were readmitted for operative drainage of abscesses for a total of 20 such admissions. Fifteen patients underwent operative partial mesh debridements pre‐NPWT for a total of 25 admissions. Total mesh extractions were performed in five patients pre‐NPWT/ROCF and four more patients during NPWT, with one patient undergoing three operative extractions and three replacements of PP mesh. Comparative data is presented for the same population post‐NPWT initiation in Table 2. The incidence of complications pre‐ and post‐NPWT are described in Table 3.
Table 2.
Procedures pre‐ and post‐NPWT/ROCF
| Pre‐NPWT/ROCF | Post‐NPWT/ROCF | |||
|---|---|---|---|---|
| n | % | n | % | |
| Incision and Drainage (I&D) | 14 | 67 | 2 | 10 |
| Mesh debridement | 15 | 71 | 3 | 14 |
| Total mesh extraction | 5 | 24 | 4 | 19 |
| Mesh replacement | 2 | 10 | 3 | 14 |
I&D, ; NPWT, negative pressure wound therapy; ROCF, reticulated open‐cell foam.
Table 3.
Complications pre‐ and post‐NPWT
| Pre‐NPWT | Post‐NPWT | |||
|---|---|---|---|---|
| n (patient) | % | n (patient) | % | |
| Fistula(e) | 5 | 24 | 1 | 5 |
| Small bowel obstruction | 3 | 14 | 0 | 0 |
| Abscess | 12 | 57 | 1 | 5 |
| Sinus | 4 | 19 | 3 | 14 |
NPWT, negative pressure wound therapy.
Eighteen of the 21 wounds (86%) reached full closure after a mean time of 26 days of NPWT and a mean hospital LOS of 30 days postinitiation of NPWT. Closure methods are outlined in Figure 1. Three wounds, all with composite mesh left in situ, did not reach full closure, although all exhibited decreased wound dimensions, granulating beds and decreased surface area exposure of mesh. Conversely, five patients with composite mesh underwent total mesh extraction and all subsequently healed (Table 4).
Figure 1.
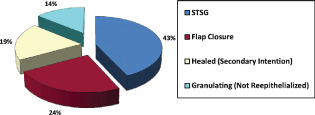
V.A.C.® healing outcomes.
Table 4.
Mesh disposition by percentage healed
| n | % | % Healed | |
|---|---|---|---|
| Total mesh extraction | 9 (PP 4; Composite 5) | 43 | 100 |
| Partial PP mesh extraction | 4 | 19 | 100 |
| PG mesh left in situ | 5 | 24 | 100 |
| Composite mesh left in situ | 3 | 14 | 0 |
PP, polypropylene; PG, polyglactin 910.
During NPWT, one hypoalbuminemic patient with exposed PP mesh developed an enterocutaneous fistula over a prior enterotomy site. This patient subsequently underwent total mesh extraction, takedown of the fistula and PG mesh replacement followed by reinstitution of NPWT and flap closure with no recurrence. Among those with retained composite mesh sinus tracts developed in three wounds during the course of NPWT (Table 3).
DISCUSSION
In this series, we documented our outcomes of using NPWT as an adjunctive treatment for abdominal wound infections with exposed PP, PG or composite (PP/ePTFE) mesh. Overall, use of NPWT resulted in an 86% wound closure rate with decreased operative procedures and readmissions. Hospital LOS was also decreased but only for those patients with PP or composite mesh. Among those patients with PG mesh all were hypoalbuminemic with wounds secondary to acute abdominal closure and intra‐operative polymicrobial cultures (MRSA and P. aerunginosa).
In this study, hospital LOS was used as a benchmark to determine effectiveness of the wound care therapy to treat an infected abdominal wound. The resulting pre‐NPWT LOS was 1·7 times greater than the LOS from NPWT initiation to wound closure/discharge (53 versus 30 days, respectively). In most cases, standard care for these open abdominal wounds includes saline moistened gauze and if necessary, removal of the infected mesh (25). However, this can lead to a more complex wound, such as an underlying abdominal wall weakness or other complications 26, 27, 28, and most certainly will result in subsequent hernia recurrences and longer hospital stays 27, 29. The loss of intra‐abdominal domain can be detrimental in these patients and can lead to more complex surgical procedures resulting in longer hospital stays. Historically, our options were limited to mesh removal; hence, salvage procedures were rarely attempted. With the advent of newer technologies for management of complicated infected wounds, our goal is to always attempt salvage if possible (30). As always, sound surgical techniques should be used and all wounds should be surgically debrided prior to initiation of any innovative technology. The aim of our treatment is to minimise donor site morbidities while maximising function. Rapid healing allows for earlier hospital discharge and return to normal daily activity. Our data suggests that NPWT transitioned long recalcitrant hospital stays into relatively short stays with positive outcomes among patients with PP and composite mesh.
For those patients with PP mesh, there was only one mesh extracted and one mesh replaced during NPWT compared with five extractions and four replacements prior to NPWT. This decrease in procedures may be because of NPWT's effectiveness in removing excess wound fluid (18), increasing local tissue perfusion(19) and promoting granulation tissue formation through the mesh 26, 28.
For 18 patients, 13 of whom underwent total or partial mesh extraction, wound closure was achieved in an average of 26 days with NPWT. The average duration of these non healing abdominal wounds prior to initiation of NWPT was 86 days with an average of 2·4 readmissions per patient (51 total readmissions) for operative procedures. Specifically, for those patients with composite mesh, the five wounds with mesh removed or replaced closed completely, whereas all three wounds with mesh left in situ showed improvement but did not close. The removal and/or replacement of the mesh, in conjunction with NPWT use, may have allowed for complete closure of the wounds. These results support previous findings that describe NPWT as an effective adjunct in managing wounds with infected mesh 26, 28, 29.
These data are also in agreement with other recent studies using NPWT for the treatment of abdominal wounds. One case series documented the use of NPWT on 21 patients with dehisced abdominal wounds (31). Thirteen of the patients had fascial dehiscence and nine of these had frank bowel exposure. Following definitive fascial closure in nine patients, stable cutaneous coverage was ultimately achieved in all patients. The authors concluded that integration of NPWT in the management of postlaparotomy wound dehiscence proved to be a successful and viable therapy for these compromised patients (31). Another study retrospectively reviewed 100 charts of patients who underwent abdominal wall reconstruction following NPWT. Their findings showed that NPWT served as a temporary dressing to control for wound dehiscence and to maintain abdominal wall integrity when oedema prevented closure. It also shortened the time to abdominal wall reconstruction (32).
One other recent publication documented outcomes of 29 cases where NPWT had been used in conjunction with laparostomy in the management of open abdominal wounds (33). Six of 29 patients developed leakage of small bowel contents into the abdominal wound cavity because of intestinal fistulisation during NPWT. Reasons for this were multi‐factorial; however, based on their experience and the results, the authors recommended caution when using NPWT on patients with bowel anastomoses or enterotomy repairs.
All wounds in this series were infected. Infection was defined as exhibiting signs and symptoms of infection (e.g. advancing cellulitis, frank pus, pyrexia, warmth, oedema and tenderness) and positive intra‐operative bacteriological tissue and abscess aspirate cultures of 105 or greater. According to Fry (5), infection can be caused by braiding effects from the sutures or from the ‘crinkling’ effects of redundant mesh, which creates pockets of dead space at the site of placement. Necrotic tissue and inadequate haemostasis can also enhance the risk of infection. When infection is present, the wound should be opened completely so that local wound care can be administered. Avoidance of fully opening a wound may lead to missed areas of contamination, delay in recovery and more difficult subsequent wound care (2). The most important factor in the successful management of infected wounds with biologic or synthetic mesh is adequate multiple debridements with initiation of early NPWT. It is critical to start decreasing bioburden early in this period while minimising the oedema with the use of NPWT. This can also aid in making an early but critical decision regarding whether removal of mesh is indicated if no progression in a short period of time is noted.
Various studies have shown that systemic antibiotics can be effective in addressing cellulitis, but otherwise are of little value in reducing infection in these cases (2). The use of systemic antibiotics should be minimised in these cases since it will have little effect on the local bioburden. In this case series, systemic antibiotics were administered in accordance with intra‐operative bacteriological culture results and ID recommendations.
Controversy remains with regard to completely removing the infected mesh because of the risk of recurrent herniation and fistulisation, time and cost involved in its removal as well as long‐term compromise in abdominal wall integrity 5, 26, 27, 34. When infection occurs with the mesh, the removal of knots and redundant mesh is recommended (5). The decision to partially debride or completely remove the mesh can be difficult in these critical situations. Authors recommend that if the wound does not improve with partial debridement, complete removal of all unincorporated mesh and associated sutures is required (5). According to Bendavid et al. (11), removal of PP mesh is implied when the mesh is sequestered and bathing in purulent exudates. Although the sample size is too small to determine significance, outcome data in this study suggest that incomplete removal of the composite mesh may have affected wound closure outcomes in three cases.
In light of these findings, some case studies have used NPWT in the treatment and salvage of infected meshes after hernia repair 26, 28, 29, 35. In a recent study by Tamhankar et al., four patients with mesh infection following abdominal wall hernia repair were treated with NPWT. Three patients had complete mesh preservation, whereas one patient had partial mesh excision. In all cases, NPWT promoted granulation tissue formation through the mesh, which occurred 1 to 7 weeks after its use. Thus, our data and those of others support the use of NPWT as adjunctive therapy for abdominal wounds with infected mesh and should be considered as alternative to mesh removal. As noted in these studies, NPWT improves salvage rates of infected abdominal wound meshes.
Complications recorded in this study are documented in Table 3 and include fistulae, sinus tracts, abscesses and small bowel obstruction. Prior to initiation of NPWT and associated mesh extraction and debridements, 5 patients were treated for fistulae, 14 were admitted for drainage of abscesses for a total of 20 such admission, 4 for sinus tracts and 3 for small bowel obstruction. Following initiation of NPWT, three patients with retained composite mesh were treated for sinus tracts, two abscesses developed requiring incision and drainage and one patient with retained PP mesh was treated for an enterocutaneous fistula. A total of 32 complications were recorded prior to NPWT versus 6 during NPWT. These results suggest a reduction in the number of complications with the use of NPWT versus standard care.
Generalising results from this retrospective review presents obvious limitations, given the small sample size and lack of randomisation and control. Future multi‐site, prospective, controlled studies would provide a strong evidence base from which treatment decisions could be made in the management of these challenging and costly cases. Additionally, cost‐benefit studies examining use of newer adjunctive technologies, such as V.A.C. Instill® Wound Therapy and GranuFoam Silver (KCI Licensing, Inc., San Antonio, TX) dressing, earlier in the treatment course are needed in addition to the use of alternative biologic meshes of both human and porcine matrices.
DISCLAIMER
This study was not performed by the Veterans Administration, but rather at a large teaching hospital in New York where the primary author was previously employed as the Director of Wound Healing.
REFERENCES
- 1. Fabian TC, Croce MA, Pritchard FE, Minard G, Hickerson WL, Howell RL, Schuur MJ, Kudsk KA. Planned ventral hernia. Staged management for acute abdominal wall defects. Ann Surg 1994;219:643–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cliby WA. Abdominal incision wound breakdown. Clin Obstet Gynaecol 2002;45:507–17. [DOI] [PubMed] [Google Scholar]
- 3. Morris‐Stiff GJ, Hughes LE. The outcomes of nonabsorbable mesh placed within the abdominal cavity: literature review and clinical experience. J Am Coll Surg 1998;186:352–67. [DOI] [PubMed] [Google Scholar]
- 4. Jenkins SD, Klamer TW, Parteka JJ, Condon RE. A comparison of prosthetic materials used to repair abdominal wall defects. Surgery 1983;94:392–8. [PubMed] [Google Scholar]
- 5. Fry DE. Wound infection in hernia repair. In: Fitzgibbons RJ Jr, Greenburg AG, editors. Nyhus and Condon's hernia. 5th edn. Philadelphia: Lippincott Williams and Wilkins; 2002:279–90. [Google Scholar]
- 6. LeBlanc KA, Bellanger D, Rhynes KV, Baker DG, Stout RW. Tissue attachment strength of prosthetic meshes used in ventral and incisional hernia repair. A study in the New Zealand White rabbit adhesion model. Surg Endosc 2002;16: 1542–6. [DOI] [PubMed] [Google Scholar]
- 7. Leber GE, Garb JL, Alexander AI, Reed WP. Long‐term complications associated with prosthetic repair of incisional hernias. Arch Surg 1998;133: 378–82. [DOI] [PubMed] [Google Scholar]
- 8. Langer S, Christiansen J. Long‐term results after incisional hernia repair. Acta Chir Scand 1985; 151:217–9. [PubMed] [Google Scholar]
- 9. George CD, Ellis H. The results of incisional hernia repair: a twelve year review. Ann R Coll Surg Engl 1986;68:185–7. [PMC free article] [PubMed] [Google Scholar]
- 10. Usher FC. The repair of incisional and inguinal hernias. Surg Gynecol Obstet 1970;131:525–30. [PubMed] [Google Scholar]
- 11. Bendavid R. Complications of groin hernia surgery. Surg Clin North Am 1998;78:1089–103. [DOI] [PubMed] [Google Scholar]
- 12. Martin RE, Sureih S, Clasen JN. Polypropylene mesh in 450 hernia repairs: evaluation of wound infections. Contemp Surg 1982;20:46–8. [Google Scholar]
- 13. Drainer IK, Reid DK. Recurrence‐free ventral herniorrhaphy using a polypropylene mesh prosthesis. J R Coll Surg Edinb 1972;17:253–60. [PubMed] [Google Scholar]
- 14. Lamb JP, Vitale T, Kaminski DL. Comparative evaluation of synthetic meshes used for abdominal wall replacement. Surgery 1983;93:643–8. [PubMed] [Google Scholar]
- 15. Argenta LC, Morykwas MJ. Vacuum‐assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 1997;38:563–76. [PubMed] [Google Scholar]
- 16. Saxena V, Hwang CW, Huang S, Eichbaum Q, Ingber D, Orgill DP. Vacuum‐assisted closure: microdeformations of wounds and cell proliferation. Plast Reconstr Surg 2004;114:1086–96. [DOI] [PubMed] [Google Scholar]
- 17. Buttenschoen K, Fleischmann W, Haupt U, Kinzl L, Buttenschoen DC. The influence of vacuum‐assisted closure on inflammatory tissue reactions in the postoperative course of ankle fractures. Foot Ankle Surg 2001;7:165–73. [Google Scholar]
- 18. Banwell PE, Musgrave M. Topical negative pressure therapy: mechanisms and indications. Int Wound J 2004;1:95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wackenfors A, Sjogren J, Gustafsson R, Algotsson L, Ingemansson R, Malmsjo M. Effects of vacuum‐assisted closure therapy on inguinal wound edge microvascular blood flow. Wound Repair Regen 2004;12:600–6. [DOI] [PubMed] [Google Scholar]
- 20. Morykwas MJ, Argenta LC, Shelton‐Brown EI, McGuirt W. Vacuum‐assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg 1997;38:553–62. [DOI] [PubMed] [Google Scholar]
- 21. Armstrong DG, Lavery LA, Diabetic Foot Study Consortium. Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet 2005;366:1704–10. [DOI] [PubMed] [Google Scholar]
- 22. Joseph E, Hamori CA, Bergman S, Roaf E, Swann NF, Anastasi GW. A prospective, randomized trial of vacuum‐assisted closure versus standard therapy of chronic nonhealing wounds. Wounds 2000;12:60–7. [Google Scholar]
- 23. Kaplan M, Banwell P, Orgill DP, Ivatury RR, Demetriades D, Moore FA, Miller P, Nicholas J, Henry S. Guidelines for the management of the open abdomen. Wounds 2005;17(Suppl 1):S1–24. [Google Scholar]
- 24. Gaddnas F, Saarnio J, Ala‐Kokko T, Laurila J, Koivukangas V. Continuous retention suture for the management of open abdomen: a high rate of delayed fascial closure. Scand J Surg 2007;96: 301–7. [DOI] [PubMed] [Google Scholar]
- 25. Paton BL, Novitsky YW, Zerey M, Sing RF, Kercher KW, Heniford BT. Management of infections of polytetrafluoroethylene‐based mesh. Surg Infect 2007;8:337–41. [DOI] [PubMed] [Google Scholar]
- 26. Kercher KW, Sing RF, Lohr C, Matthews BD, Heniford BT. Successful salvage of infected PTFE mesh after ventral hernia repair. Ostomy Wound Manage 2002;48:40–5. [PubMed] [Google Scholar]
- 27. Delikoukos S, Tzovaras G, Liakou P, Mantzos F, Hatzitheofilou C. Late‐onset deep mesh infection after inguinal hernia repair. Hernia 2007;11:15–7. [DOI] [PubMed] [Google Scholar]
- 28. Tamhankar AP, Ravi K, Everitt NJ. Vacuum Assisted Closure therapy in the treatment of mesh infection after hernia repair. Surgeon 2009;7:316–8. [DOI] [PubMed] [Google Scholar]
- 29. Steenvoorde P, de Roo RA, Oskam J, Neijenhuis P. Negative pressure wound therapy to treat peri‐prosthetic methicillin‐resistant Staphylococcus aureus infection after incisional herniorrhaphy. A case study and literature review. Ostomy Wound Manage 2006;52:52–4. [PubMed] [Google Scholar]
- 30. Gabriel A, Shores J, Bernstein B, De Leon J, Kamepalli R, Wolvos T, Baharestani MM, Gupta S. A clinical review of infected wound treatment with Vacuum Assisted Closure (V.A.C.) therapy: experience and case series. Int Wound J 2009;6(Suppl 2):1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Heller L, Levin SL, Butler CE. Management of abdominal wound dehiscence using vacuum assisted closure in patients with compromised healing. Am J Surg 2006;191:165–72. [DOI] [PubMed] [Google Scholar]
- 32. DeFranzo AJ, Pitzer K, Molnar JA, Marks MW, Chang MC, Miller PR, Letton RW, Argenta LC. Vacuum‐assisted closure for defects of the abdominal wall. Plast Reconstr Surg 2008;121:832–9. [DOI] [PubMed] [Google Scholar]
- 33. Rao M, Burke D, Finan PJ, Sagar PM. The use of vacuum‐assisted closure of abdominal wounds: a word of caution. Colorectal Dis 2007;9:266–8. [DOI] [PubMed] [Google Scholar]
- 34. Szczerba SR, Dumanian GA. Definitive surgical treatment of infected or exposed ventral hernia mesh. Ann Surg 2003;237:437–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Agrawal S, Hayhurst C, Joseph T, Prinsloo D, Morgan RH, Pherwani AD. Successful salvage of infected and exposed non‐absorbable mesh following decompressing laparostomy after emergency repair of ruptured abdominal aortic aneurysm using vacuum‐assisted closure system. Eur J Vasc Endovasc Surg 2008;15:1–2. [Google Scholar]


