Abstract
Background
Peptic ulcers recur, suggesting that ulcer healing may leave tissue predisposed to subsequent damage. In mice, we have identified that the regenerated epithelium found after ulcer healing will remain abnormal for months after healing.
Aim
To determine whether healed gastric mucosa has altered epithelial function, as measured by electrophysiologic parameters.
Method
Ulcers were induced in mouse gastric corpus by serosal local application of acetic acid. Thirty days or 8 months after ulcer induction, tissue was mounted in an Ussing chamber. Transepithelial electrophysiologic parameters (short-circuit current, Isc. resistance, R) were compared between the regenerated healed ulcer region and the non-ulcerated contralateral region, in response to luminal hyperosmolar NaCl challenge (0.5 M).
Results
In unperturbed stomach, luminal application of hyperosmolar NaCl transiently dropped Isc followed by gradual recovery over 2 h. Compared to the starting baseline Isc, percent Isc recovery was reduced in 30-day healing mucosa, but not at 8 months. Prior to NaCl challenge, a lower baseline Isc was observed in trefoil factor 2 (TFF2) knockout (KO) versus wild type (WT), with no Isc recovery in either non-ulcerated or healing mucosa of KO. Inhibiting Na/H exchanger (NHE) transport in WT mucosa inhibited Isc recovery in response to luminal challenge. NHE2-KO baseline Isc was reduced versus NHE2-WT. In murine gastric organoids, NHE inhibition slowed recovery of intracellular pH and delayed the repair of photic induced damage.
Conclusion
Healing gastric mucosa has deficient electrophysiological recovery in response to hypertonic NaCl. TFF2 and NHE2 contribute to Isc regulation, and the recovery and healing of transepithelial function.
Keywords: Ulcer, Gastric, Epithelial cell, Repair, TFF2, NHE2, Ussing chamber, Confocal microscopy, Photodamage, Actin
Introduction
Peptic ulcers are a major burden in health care and despite a decreasing incidence are still diagnosed in about 5–10% in the general population [1, 2]. Although H. pylori eradication or antacid medication facilitates ulcer healing and reduces ulcer recurrence, past clinical observations have reported a cumulative 20% probability of ulcer recurrence at 6 months [3] and 5% at 5 years [4] among patients previously diagnosed and treated for H. pylori infection. Seo et al. [4] also reported the 5-year cumulative probability of ulcer recurrence in the absence of H. pylori infection was 36.4%. Our previous work in animal models suggested that even after visual confirmation of ulcer healing, the restored mucosa is physiologically dysfunctional [5].
Previous studies in mice suggest that after ulcer healing, residual abnormalities in the regenerated epithelium could increase the risk of further damage or continued peptic ulcer disease [5–9]. These abnormalities included poorly differentiated cells, degenerative changes in the glandular cells, increased connective tissue, and a disorganized microvasculature [6–9]. Indeed, the presence of nonsteroidal anti-inflammatory drugs (NSAIDs) or H. pylori infection contributes to ulcer relapse in experimental animal models [5, 10, 11] and in patients [3, 4].
Trefoil factor 2 (TFF2), a peptide secreted into mucus, protects the gastric mucosa and promotes cell migration in response to injury in vivo [12]. TFF2 gene and protein expression are upregulated in the healing epithelium following ulcer induction [5, 13, 14]. Interestingly, TFF2-KO mice are susceptible to NSAID-induced gastric mucosal injury and display delayed ulcer healing [12, 14], suggesting that in TFF2 replete tissue, TFF2 strengthens the epithelial barrier in order to prevent further disease progression during ulcer healing. In addition, solute carrier family (SLC) 9 (sodium/hydrogen exchanger [NHE]) isoforms facilitate and regulate epithelial sodium absorption, intracellular pH (pHi), cell volume, migration, and wound repair [15–19]. Our laboratory has shown that TFF2 requires NHE2 activity to facilitate the repair of gastric epithelial micro-injury induced by two-photon laser irradiation [19]. Interestingly, following induced injury TFF2 is upregulated in the regenerating gatric epithelium while NHE2 expression is downregulated [5]. Although the impact of these observations on epithelial barrier function is unknown, we and others observed that following repair from an initial ulcer, ulcer relapse was enhanced by further administration of H. pylori or NSAIDs [5, 10, 11].
Hagen et al. [20] reported that gastric restitution is Na+-dependent in bullfrog, and EGF-induced restitution is mediated in part by NHE in guinea pigs [17]. On the basis of these prior observations, we hypothesized that the healing mucosa would have abnormal ion transport activity possibly due to the downregulation of NHE2 following induced ulceration, which could make the healing mucosa more susceptible to ulcer relapse. We sought to test our hypothesis using electrophysiology to study active transport in the healing mucosa.
Gastric transepithelial resistance (R) and short-circuit current (Isc), a measure of active ion transport, are classically measured in Ussing chambers [21, 22] using isolated bullfrog or guinea pig gastric mucosa [23–25]. In the present study, we investigated events, detected by Isc and R, during repair of mild murine gastric mucosal damage in response to hyperosmolar NaCl. We compared tissues that had healed from experimentally induced ulcers for short (30 day) and long (8 months) periods and also investigated pHi in gastric organoids exposed to photic injury.
Methods
Animals and Surgery
C57BL/6 J mice (Jackson Lab, Bar Harbor, ME), trefoil factor 2 knockout (TFF2-KO) mice backcrossed onto a C57BL/6J background, solute carrier family 9 (sodium/hydrogen exchanger), and member 2 knockout (NHE2-KO) mice on a FVB/N background were used in Ussing chamber experiments. Two-photon laser-induced microlesion experiments were performed in HuGE heterozygous mice [26]. HuGE mice carry one green fluorescent protein (GFP)—human actin knock-in allele in the profilin 1 locus [26], confirmed by PCR genotyping. Male and female mice were used for experimentation at 2–4 months of age, except one group of gastric ulcer mice were not analyzed until 8 months after ulcer induction. All mice were bred in-house. Animals were fed a standard rodent chow diet and had free access to water. Mice were maintained in an Association for Assessment, Accreditation of Laboratory Animal Care (AAALAC)-approved facility, and all animal studies were approved by the Institutional Animal Care and Use Committee of the University of Cincinnati.
Gastric ulcers were produced by acetic acid, as previously described [5, 7]. Under isoflurane-inhaled anesthesia, the abdomen was incised and the intact stomach was exposed. A microcapillary tube (0.7 mm in diameter; Drummond Scientific, Co, Broomall, PA) filled with acetic acid (99%) was placed in contact with the exterior surface of the stomach corpus region and left in place for 25 s. After the acetic acid was removed, the exterior of the stomach was wiped with gauze and the stomach was reinserted into the abdomen and closed with absorbable suture obviating the need for gastric incision. Buprenorphine hydrochloride (0.1 mg/kg intraperitoneal (Buprenex; Reckitt Benckiser Pharmaceuticals, Inc., Richmond, VA) was given as analgesia. The animals were maintained with regular food and water.
Tissue Preparation
The mouse stomach was isolated 30 days or 8 months after ulcer induction, or from non-ulcerated controls, and immediately placed in warmed 37 °C HEPES-buffered Krebs–Ringer solution (hereafter called nutrient solution). The stomach was opened along the greater curvature, and the muscle was removed. The ulcer-induced area was visually located by serosal observation of white discolored muscle tissue; as previously described with a photograph [5]. Then the region of gastric corpus was mounted into an Ussing chamber (0.1 cm2 area slider, P2303A, Physiological Instruments, San Diego, CA). Intact gastric mucosa was obtained, from the same mouse, from mucosa opposite to the ulcerated tissue. The mucosal and serosal tissue surfaces were bathed in the same nutrient solution (5 mL in the reservoirs) containing 100 mM NaCl, 5.4 mM KCl, 1.2 mM CaCl2, 1.2 mM MgCl2, 25 mM NaHCO3, 20 mM HEPES, 10 mM glucose. Mucosal and serosal reservoir solutions were gassed with 95% O2–5% CO2 and maintained at 37 °C by a circulating water bath behind the reservoir chambers.
Recording Electrophysiology Parameters
The instrument gain (VCC MC8; Physiological Instruments, San Diego, CA) was set to 10; the fluid resistance control (FRC) master was set to 100, making a capacity of 1000 Ω, and fast (every 1 s) pulsing. Sets of voltage and current electrodes connected the Ussing chambers to a digital recorder via 3 M KCl-agar bridges.
After compensating for fluid resistance, tissues were mounted, the remote electronic monitoring (REM) was engaged, and a reference was taken. Isc was measured by clamping the voltage to zero and was reported as μA/cm2 to account for the area of the exposed mucosa. R was calculated by Ohm’s law with a rectangular pulse of 3 mV and the change in R, was measured in ohms (Ω) and reported as Ω cm2 to account for mucosal surface area. Transmural voltage difference was calculated by Ohm’s law. With the voltage clamped, the tissue was allowed to equilibrate to a basal steady state for a minimum of 30 min.
Induction of Surface Epithelial Damage
Nutrient solution was applied to the mucosal side with variable additions of NaCl (0–1 M) in order to determine a NaCl concentration sufficient to induce damage to the gastric epithelium. The tissue was challenged with a hypertonic NaCl solution for 5 min and then removed with one rinse of normal mucosal nutrient solution. After the hypertonic NaCl challenge, the Isc and R were monitored for a minimum of 135 min. In some cases, the luminal and serosal chambers also included HOE 694 (3-methylsulphonyl-4-piperidinobenzoyl, guanidine hydrochloride, 20 μM to assure inhibition of apical NHE2 and basolateral NHE1) [27, 28], or omeprazole [29] (OMZ, 100 μM applied a minimum of 30 min prior to the NaCl challenge, Sigma-Aldrich, St. Louis, MO, to inhibit gastric acid secretion by blocking H,K-ATPase activity [29]). Final dimethyl sulfoxide (DMSO) concentration was < 0.2%; an equal amount of DMSO was added to nutrient solution in the control group. In a preliminary study, either ion-permeable plastic wrap (Kroger Home Sense, Cincinnati, OH) or a non-permeable plastic coverslip (Electron Microscopy Sciences, Hatfield, PA) was mounted into the Ussing chamber followed by a hypertonic NaCl (0.5 M) challenge. Relative Isc recovery was calculated by the following formula [30]: percent Isc recovery = (C − B) × 100%/(A − B), where A is the basal-state Isc before mucosal damage, B is the Isc nadir after hypertonic NaCl exposure, and C is the Isc at time 135 min (or 2 h after hypertonic NaCl exposure).
Hematoxylin and Eosin (H&E) Staining
Muscle-stripped non-ulcerated gastric tissue was mounted in an Ussing chamber with nutrient solution. Either 0 M or 0.5 M NaCl was then added to the mucosal side for 5 min. After 5 min, the solution was changed back to nutrient solution, after which the tissue was immediately removed from the chamber and fixed in 4% paraformaldehyde for 2 h. Next, the tissue was rinsed with phosphate-buffered saline, sucrose saturated to 30% at 4 °C, and then embedded into Optimal Cutting Temperature solution (Sakura Finetek, Inc., Torrance, CA). Tissue sections (10 μm) were prepared by cryostat and were stained with H&E (Sigma-Aldrich, St. Louis, MO).
Gastroid Generation
To generate gastric organoids (gastroids), mouse gastric mucosa from the corpus was isolated and incubated, with gentle rocking, at 4 °C for 2 h in 10 mM EDTA in Dulbecco’s phosphate-buffered saline (DPBS) pH 7.4 without Ca2+ and Mg2+. To dissociate the individual glands, the tissue was incubated and shaken in ice-cold buffer (43.3 mM sucrose (Thermo Fisher Scientific, Waltham, MA), and 54.9 mM D-sorbitol, (Sigma-Aldrich, St. Louis, MO), in DPBS). Glands were centrifuged at 150×g for 5 min at 4 °C, and the pellet was re-suspended in Matrigel® (Corning, Corning, NY) and added to an eight-well Lab-Tek chamber with coverglass (Thermo Fisher Scientific, Waltham, MA). The Matrigel® was allowed to polymerize at 37 °C. Then medium was added to the wells and replaced every 4 days and cultured in a 5% CO2 incubator at 37 °C. Gastroid media consisted of advanced DMEM/F12 supplemented with 2 mM GlutaMax® (Thermo Fisher Scientific, Waltham, MA), 10 mM HEPES (Research Organics, Cleveland, OH), 100 U/mL penicillin/100 μg/mL streptomycin, 1 × N2 and 1 × B27 supplements (Thermo Fisher Scientific, Waltham, MA), Wnt3a-conditioned medium (50%), R-spondin-conditioned medium (10%), Noggin-conditioned medium (10%), [Leu15]-Gastrin I (10 nM, Sigma-Aldrich, St. Louis, MO), and epithelial growth factor (50 ng/mL, PeproTech, Rocky Hill, NJ) [31–33].
In Vitro Gastroid Induction of Two‑Photon Laser‑Induced Microlesion
Experiments were performed as previously described [33] in gastroid culture medium under 5% CO2 at 37 °C (incubation chamber, PeCon, Erbach, Germany) on an inverted confocal microscope (Zeiss LSM 510 NLO) and imaged with a C-Achrophan NIR 40 × objective. Gastroids were pre-incubated with Hoechst 33342 (10 μg/mL, Thermo Fisher Scientific, Waltham, MA) for 30 min to visualize nuclei. Images of Hoechst 33342 (Ti-Sa laser, excitation of 730 nm, emission of 435–485 nm) and GFP–actin (excitation 488 nm, emission 500–550 nm) in the gastroid were collected simultaneously with a transmitted light. To measure pHi [33, 34], SNARF-5F-AM (5 μM, excitation 543 nm, emission 550–600 and 620–680 nm, Thermo Fisher Scientific, Waltham, MA) was added to the medium of the HuGE gastroids. To induce a single-cell microlesion within a gastroid, located ∼ 150–300 μm from the cover glass in the Matrigel®, a rectangular region (≈ 5 μm2) of a single cell was repetitively scanned at high Ti-Sa laser power (730 nm; 650 mW average) for 500 iterations (duration, 2–3 s). In some cases 5-(N-ethyl-N-isopropyl) amiloride (EIPA, 100 μM), potent inhibitor of several NHE isoforms including NHE2, was added to the medium. Final DMSO concentration was < 0.1% and an equal amount of DMSO was added to medium in the control group. The damage–repair cycle was measured once per gastroid, and outcomes from at least six different gastroids were compiled for each experimental protocol.
Image Analysis
Images were analyzed usingImage J [35] and/or Meta-Morph software (Molecular Devices, Downingtown, PA) as previously described [33, 34]. To measure pHi, back-ground-corrected SNARF-5F fluorescence was measured directly in the migrating cells adjacent to the damaged site. Then ratio values of SNARF-5F (F620–680/F550–600) were converted to pH using a standard curve of the relevant pure dye in different pH of solution. To measure actin dynamics in the HuGE mice expressing GFP-actin as performed previously [33], background-corrected GFP intensity was measured in the basal site of the damaged area. Then the value was normalized to a value of 1 set for the averaged pre-damage baseline condition. Furthermore, the damaged area was measured as the region with cellular loss of GFP or SNARF-5F fluorescence.
Data Analysis
All data are presented as the mean ± SEM. Statistical significance was determined using either paired or unpaired Student’s t test, one-way ANOVA with Dunnett’s Multiple Comparison Test, or column statistics to test whether the mean of a column was different from zero. A P value of < 0.05 was considered significant. Data statistics were performed using GraphPad Prism 5 (GraphPad Software, San Diego, CA).
Results
Damage Recovery in Control Gastric Mucosa
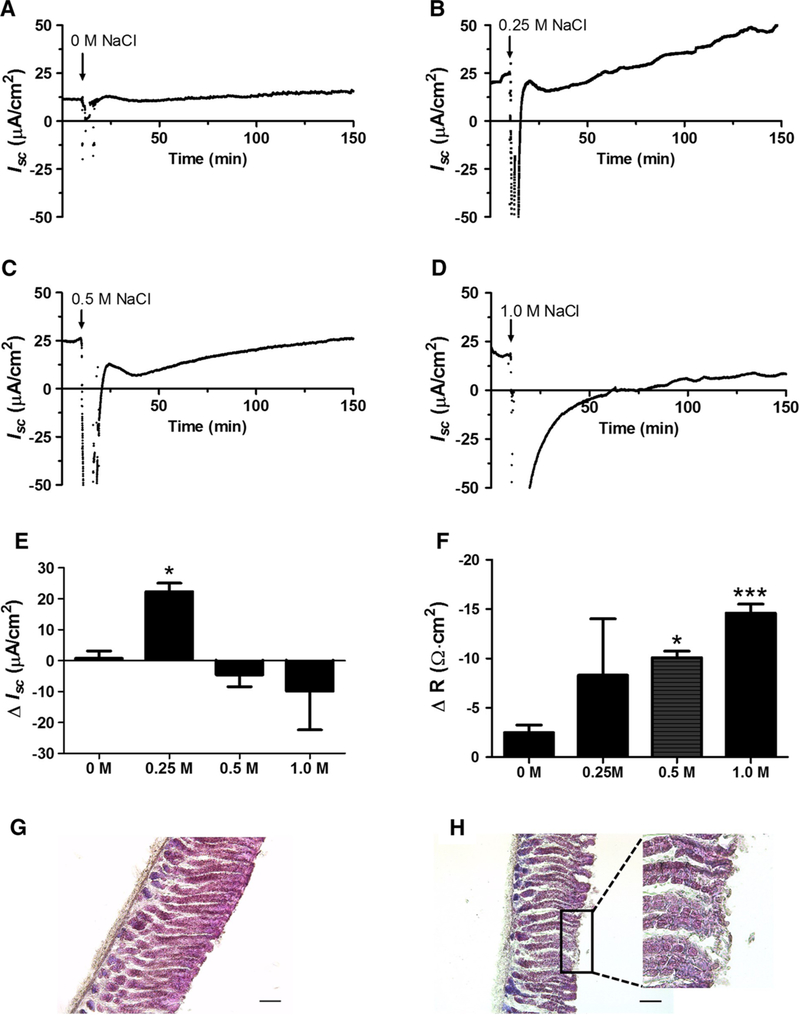
We first determined the concentration of NaCl that mildly damaged the mouse gastric epithelium in order to monitor events after damage. Figure 1a–d depicts representative time course plots of Isc in response to each NaCl concentration. Figure 1e compiles the observed change in (Δ) Isc (μA/cm2) from baseline at time 135 min (2 h after hypertonic NaCl exposure, n = 4–5). For simplicity, after reaching a steady state, only the 10 min of basal Isc, immediately preceding hypertonic NaCl addition are shown and used in the calculations (Fig. 1e). The control (0 M additional NaCl) showed no change in Isc after a mock challenge of removing and adding fresh nutrient solution (Fig. 1a, e). After 0.25 M NaCl, Isc showed a mean recovery of 22 ± 3 μA/cm2 increase over baseline (Fig. 1b, e). After exposure to 0.5 M NaCl, a rapid recovery in Isc was followed by a decline termed the nadir [30], and then an increase back to the basal Isc over 2–3 h (Fig. 1c). In contrast, 1.0 M NaCl produced a varying range of functional loss that, in most cases, lacked a nadir or Isc recovery, outcomes considered indicative of more extensive damage (Fig. 1d, e). The extent of damage in these conditions was compared by measuring the change in (Δ) R from basal to 15 min after the NaCl challenge (Fig. 1F and Supplementary Fig. 1). At 15 min after 0 or 0.25 M NaCl challenge, R was not significantly different from basal levels. At 15 min after 0.5 M or 1 M NaCl challenge, a significant decrease in R was observed (ΔR of − 10 ± 0.64 Ω cm2 or − 15 ± 0.90 Ω cm2, respectively, n = 4). Results show that the 0.5 M NaCl challenge was the lowest salt concentration that produced a significant ΔR in the short term (demonstrating damage) and that allowed a recovery of Isc over the measurement period of 2 h.
Fig. 1.
Hypertonic NaCl effects on transepithelial parameters. Wild-type mouse muscle-stripped gastric corpus was mounted into an Ussing chamber (area = 0.1 cm2). Short-circuit current (Isc) and resistance (R) were measured as described in Methods. a–d Time course of Isc before and after addition of 0 M, 0.25 M, 0.5 M, or 1.0 M NaCl to luminal nutrient solution. Time zero starts 10 min baseline measurement prior to 5 min (arrow) exposure to NaCl challenge (n = 4–5). e Change in (Δ) Isc from baseline, measured 2 h after NaCl challenge (t = 135 min) (n = 4–5). *P < 0.05 versus zero, mean ± SEM. f Change in (Δ) R between baseline and 15 min after NaCl chalenge (t = 30 min), *P < 0.05, ***P < 0.001 mean ± SEM (One-way ANOVA P = 0.0023; Dunnett’s Multiple Comparison Test to 0 M NaCl). H&E of tissue challenged 5 min with 0 M (g) or 0.5 M NaCl (h) while mounted in the Ussing chamber, then tissue removed and fixed immediately. Scale bar = 100 μm. Inset in h shows magnified representative region of mild epithelial damage
We also evaluated morphology of tissue mounted into the Ussing chamber by H&E staining (Methods), to confirm that the 0.5 M NaCl challenge mildly damaged the gastric surface epithelium. Figure 1g shows control gastric epithelium, after a 0 M NaCl mock challenge, with no surface epithelial damage, whereas Fig. 1h shows gastric epithelium with mild surface damage immediately after a 0.5 M NaCl challenge (5 min) with patchy loss of surface epithelial cells. In all subsequent experiments, application of 0.5 M NaCl for 5 min was used to induce mild tissue damage.
The immediate drop in Isc during hypertonic NaCl was tested to determine whether the drop was an artifact or was generated by ions flowing across the mucosa. A plastic cover slip (with no ion permeability) was inserted into the Ussing chamber with resultant steady state Isc = 0. No Isc was generated during or after hypertonic NaCl addition to the reservoir that we designated and used as our standard luminal side when inserting tissue (Supplementary Fig. 2A). To demonstrate that the drop in Isc was due to asymmetric salt concentrations during hypertonic challenge (diffusion potentials), we mounted ion-permeable plastic wrap into the Ussing chamber and observed an immediate drop in Isc during hypertonic NaCl with an immediate return to the initial steady state Isc = 0 after one chamber rinse and addition of normal nutrient solution (Supplementary Fig. 2B). Ions spontaneously move down a concentration gradient by passive diffusion; therefore, we hypothesize, based on work by Silen’s group [23, 24], that the drop in Isc observed after the addition of hypertonic NaCl was due to an electrical gradient generated by the combination of ion diffusion and possibly active sodium transport activity across the gastric mucosa. Results clarify that the drop is an artifact of the challenge, but that it recovers such that tissue data after the nadir can be used to reliably report on tissue activity.
Effect of Hypertonic NaCl on the Regenerating Epithelium
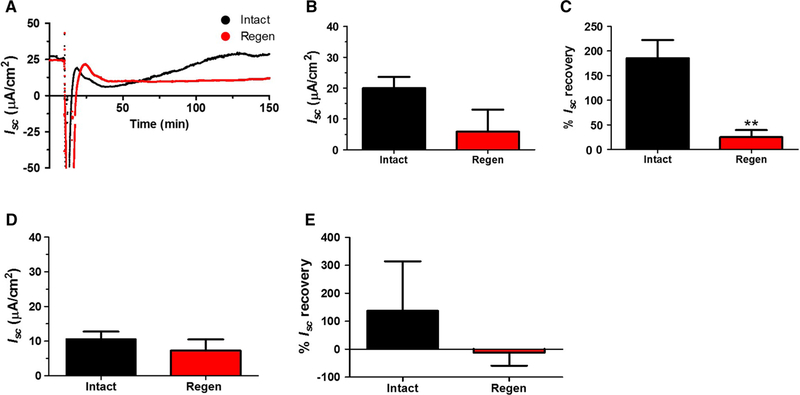
Serosal application of acetic acid to one side of corpus induces necrosis locally and is a well-established mouse model used to mimic human ulcers, where the other side of the corpus remains intact and undamaged [7]. We previously demonstrated that the ulcerated area was visually covered by newly generated healing epithelium 30 days after ulcer induction, although the regenerated gastric tissue was significantly thinner than the normal epithelium [5]. Mouse corpus stomach tissue, 30 days after ulceration (labeled as Regen) and non-ulcerated tissue (labeled as Intact) from the same stomach were mounted in an Ussing chamber. Figure 2a depict 0.5 M NaCl challenge representative plot of Isc measurements for both 30-day regenerated (Regen, red) and non-ulcerated (Intact, black) tissue. Basal Isc values trended lower, but not significantly, in the regenerated epithelium (Fig. 2b). The percent (%) Isc recovery of the regenerated mucosa (Methods), measured 2 h after hypertonic saline exposure, was significantly lower (25 ± 14%) than in non-ulcerated intact mucosa (185 ± 36%) (Fig. 2c).
Fig. 2.
Effect of hypertonic NaCl on the healing corpus Isc. Experimental ulcers were induced to one side of the gastric corpus in wild-type mice by acetic acid (Methods). 30 days or 8 months later, muscle-stripped tissue was mounted in the Ussing chamber from the regenerated healed ulcer region (Regen, red) and the non-ulcerated contralateral region (Intact, black). Tissues were exposed to 0.5 M NaCl challenge as in Fig. 1. a Representative time course of Isc for tissue 30 days after ulceration. b Baseline Isc of 30-day tissue after ulcer induction. c Percent (%) Isc recovery (Methods) of 30-day mucosa 2 h after 0.5 M NaCl. d Baseline Isc of tissue 8 months after ulceration. e Percent (%) Isc recovery of 8-mo mucosa 2 h after hypertonic NaCl. Mean ± SEM (n = 4 30-day or 8 months) for each tissue condition **P < 0.01 Intact versus Regen (two-tailed t test)
To determine the effect of acid secretion on the events, we monitor after mild damage, we added the H,K-ATPase inhibitor omeprazole (OMZ, 100 μM) [29] to the serosal and luminal chambers a minimum of 30 min prior to the challenge. In WT mice, addition of OMZ increased Isc significantly from basal (Supplementary Fig. 3A) with no change in R (data not shown). The % Isc recovery was similar between WT mice treated with OMZ versus untreated WT intact tissue previously reported in Fig. 2c (Supplementary Fig. 3B). Results suggest acid secretion is not a component of the Isc response during damage recovery.
We asked if a longer healing time would normalize the electrophysiological response of the recovering epithelium to hypertonic saline challenge. Previously, we observed that the epithelium 8 months after ulcer induction contained normally differentiated cells [5]. In Ussing chamber measurements, we noted that tissues in these older animals tended toward reduced nadir and Isc recovery phases after NaCl challenge (Fig. 2d, e and data not shown), compared to 30-day recovery tissues (Fig. 2b, c). However, the 8-month regenerated epithelium showed no difference in basal or % Isc recovery versus the non-ulcerated mucosa from the same animals (Fig. 2d, e).
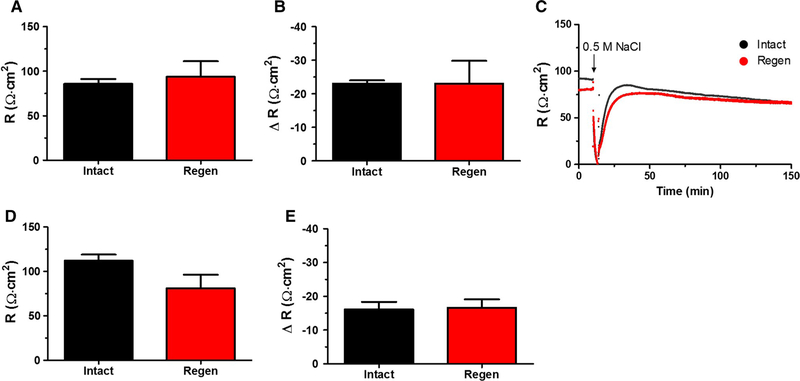
The basal R, 30 days and 8 months after ulcer induction, was the same between the intact and regenerated epithelium (Fig. 3a, d, respectively). Figure 3c depicts a representative time course plot of R measurements taken every second for both regenerated (Regen, red) and non-ulcerated (Intact, black) tissue exposed to 0.5 M NaCl challenge. R dropped to zero during a 5 min hypertonic saline exposure followed by rapid R recovery, after which R slowly decreased. We compared R at 120 min after hypertonic challenge as a surrogate measure of repair reported at the same time that Isc recovery was evaluated. The ΔR at 2 h after hypertonic saline exposure was the same comparing between post-ulcer regenerated mucosa and intact mucosa for both for 30 day and 8 months recovered tissues (Fig. 3b, e, respectively).
Fig. 3.
Effect of hypertonic NaCl on the healing corpus transepithelial resistance. Using tissues studied as in Fig. 2, Ussing chamber measurements of transepithelial R were made for regenerated healed ulcer region (Regen, red) and the non-ulcerated contralateral region (Intact, black). a Baseline transepithelial R of tissue 30 days after ulcer induction. b Change in (Δ) R from baseline for tissue 30-day post-ulceration, 2 h after 0.5 M NaCl challenge. c Representative time course of R of 30-day mucosa. d Basal R of tissue 8 months after ulcer induction. e Change in (Δ) R from baseline for tissue 8 months post-ulceration, 2 h after 0.5 M NaCl challenge. Mean ± SEM (n = 4 30 day or 8 months)
Involvement of TFF2 and NHE in Regulation of Active Ion Transport
TFF2 has been implicated in gastric injury repair through its ability to promote restitution of a damaged epithelium [5, 14]. In addition, we previously observed TFF2 expression was upregulated at 30 days and decreased at 8 months post-ulceration, with this transient expression most pronounced in the bottom of the glands in the regenerating mucosa [5].
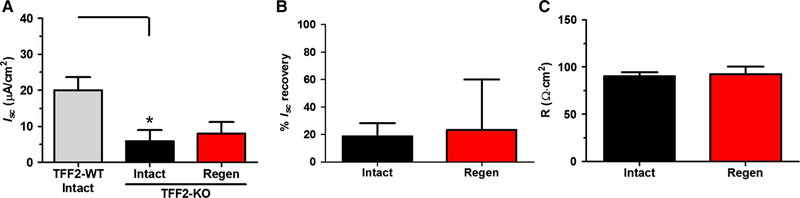
TFF2-KO intact mucosa had a significantly lower basal Isc (5.9 ± 3.1 μA/cm2) than TFF2-WT intact mucosa (20 ± 4 μA/ cm2) (Fig. 4a), yet with no difference in basal R (data not shown). Examining TFF2-KO mucosa 30 days after ulceration, and subjecting both intact and healing mucosa to 0.5 M NaCl challenge, we observed Isc recovered only an average of 18.8 ± 9.6% and 23.4 ± 37%, respectively (Fig. 4b). Interestingly, TFF2-KO intact % Isc recovery (18.8 ± 9.6%) was significantly (P = 0.0017) lower than WT intact (186 ± 36%) Isc recovery (Figs. 4b, 2c, respectively). The TFF2-KO had no difference between basal Isc, % Isc recovery, and basal R between TFF2-KO intact and 30-day regenerated epithelium (Fig. 4a–c, respectively).
Fig. 4.
TFF2 effect on baseline and recovery of Isc after hypertonic challenge. Experimental ulcers were induced to one side of the gastric corpus in TFF2 knockout (TFF2-KO) mice by acetic acid (Methods), and animals allowed to heal for 30 days. Tissue was then analyzed as in Fig. 2, examining regenerated healed ulcer region (Regen, red) and the non-ulcerated contralateral region (Intact, black). a Baseline Isc of TFF2-WT intact taken from Fig. 2b (Intact, gray), and TFF2-KO Intact and Regen mucosa, as defined above. *P < 0.05 TFF2-WT intact versus TFF2-KO intact (two-tailed t test). b Percent (%) Isc recovery of TFF2-KO tissues. c Baseline R of TFF2-KO tissues. Mean ± SEM (n = 5 TFF2-KO, n = 4 TFF2-WT)
The % Isc recovery of TFF2-KO mice pre-treated with OMZ was similar to the TFF2-KO intact tissue previously reported in Fig. 4b (Supplementary Fig. 3C). Results suggest that Isc recovery in response to NaCl does not involve gastric acid secretion in C57BL/6 mice (with or without functional TFF2).
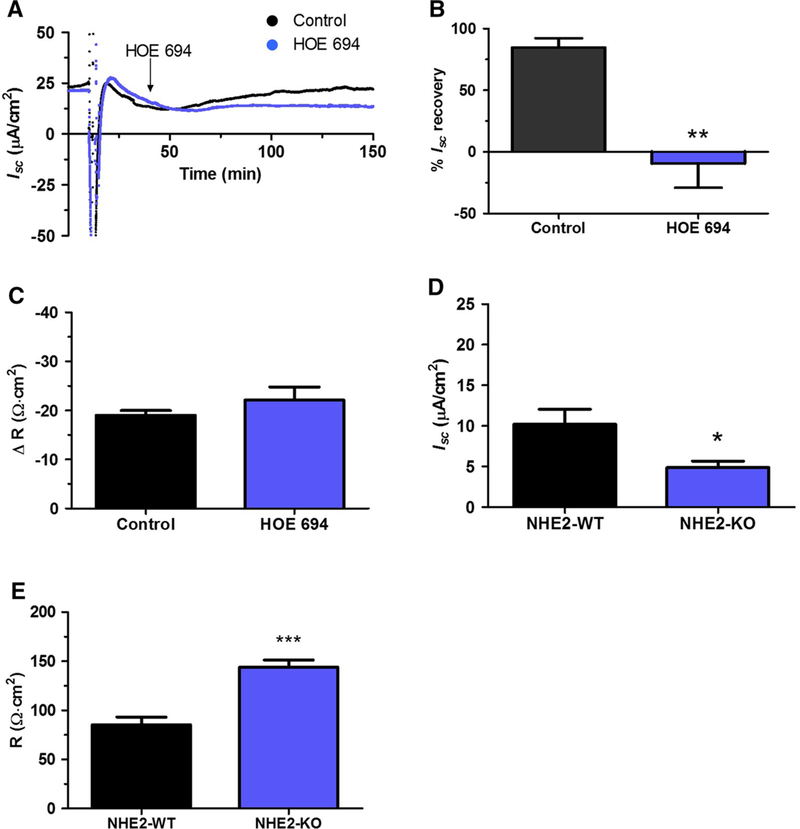
We previously reported downregulated NHE2 protein and gene expression in the regenerated epithelium 30 days after ulceration [5], and we also demonstrated that NHE2, but not NHE1, is involved in gastric repair [19, 36]. Therefore, we hypothesized that downregulation of NHE2 may be the reason why the healing mucosa does not recover after NaCl challenge. We tested Isc recovery in the presence of the NHE1–2 inhibitor HOE 694 (20 μM), using non-ulcerated C57BL/6J mice. HOE 694 was applied 25 min after luminal hypertonic saline exposure for 5 min (Fig. 5a), as shown by an arrow in the representative plot. The addition of DMSO or HOE 694 did not alter basal Isc in control experiments (data not shown). HOE 694 significantly inhibited the % Isc recovery (− 9.6 ± 7.6%) versus control (DMSO only) mice 84.7 ± 7.57%) (Fig. 5b); we also observed that NHE inhibition did not alter the change in R 2 h after hypertonic NaCl exposure (Fig. 5c).
Fig. 5.
NHE inhibition impairs Isc recovery in response to hypertonic NaCl and NHE2-KO lowers basal Isc. Wild-type (Control, C57BL6/J), or NHE2-WT and NHE2-KO (FVB/N) tissues were mounted into an Ussing chamber and WT challenged with 0.5 M NaCl at t = 10 min. Results compared Control tissue in the absence (black) versus presence (blue) of 20 μM HOE 694 added at t = 40 min (arrow) to both luminal and serosal bath, measuring a representative time course of Isc, b percent (%) Isc recovery 2 h after 0.5 M NaCl, or c change in (Δ) transepithelial R 2 h after 0.5 M NaCl. Mean ± SEM. (n = 4 Control, n = 5 HOE 694) **P < 0.01 Control versus HOE 694 (two-tailed t test). d Baseline Isc or e transepithelial R of FVB/N NHE2-WT (black, n = 7) versus NHE2-KO (blue, n = 8). Mean ± SEM. *P < 0.01 and ***P < 0.001 NHE2-WT versus NHE2-KO (two-tailed t test)
Our NHE2-KO mice were bred on a FVB/N and not a C57BL/6J background. We observed that the FVB/N NHE2 wild-type (NHE2-WT) mucosa did not recover after 0.5 M NaCl exposure; therefore, we only compared basal Isc of NHE2-WT to NHE2-KO using non-ulcerated mucosa. The NHE2-KO basal Isc (5.3 ± 1.8 μA/cm2) was significantly lower (P = 0.0228) than the NHE2-WT basal Isc (13.7 ± 1.8 μA/cm2) (Fig. 5d). The basal R was also significantly increased (P < 0.0001) in NHE2-KO (FVBN-WT 85.4 ± 7.6 and NHE2-KO 144 ± 7.1 Ω cm2) (Fig. 5e).
NHE Contributes to Intracellular pH
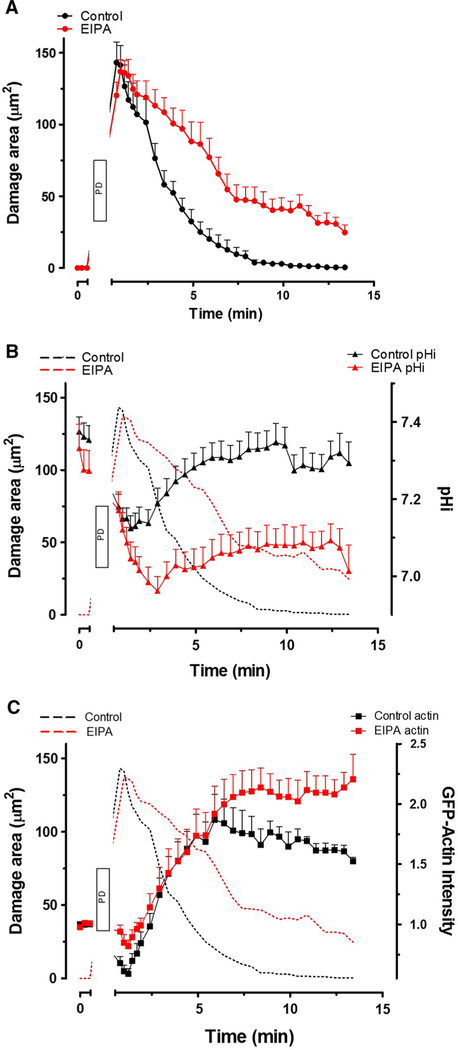
To clarify the contribution of NHE activity to epithelial restitution, we used a two-photon-induced restitution model using gastric organoids [33]. Damage area, identified by reflectance and lack of NAD(P)H, showed epithelial damage was repaired within ~ 10 min in control organoids (black), but repair was inhibited by the NHE inhibitor EIPA (red) (Fig. 6a). In response to single cell damage, pHi in the migrating cells adjacent to the damage area decreased followed by recovery during repair after photon-induced damage (Fig. 6b). Therefore, EIPA inhibited both pHi recovery (Fig. 6b) and damage repair (Fig. 6a, b). Utilizing gastric organoids from mice expressing the human GFP-actin (HuGE) transgene [26], actin dynamics, after photon-induced damage, at the damage site was followed by GFP intensity. Yet, EIPA did not alter the initial rate of actin closure at the basal pole of the damaged area (< 6 min), as GFP-actin intensity increased similar to control (Fig. 6c). Results suggest that NHE activity regulates pHi and restitution in the organoid model.
Fig. 6.
Effect of NHE inhibition on intracellular pH and actin dynamics during restitution in mouse gastroids in vitro. A single gastric organoid epithelial cell was photodamaged (PD) in the presence of EIPA (red, n = 7) or DMSO only (Control, black, n = 7). Compiled results showing time course of damaged area (a), with these results replicated in subsequent panels to provide a reference time frame. b Time course of pHi measured in migrating cells repairing damage. C Time course of GFP-actin intensity measured in the lateral spaces of migrating cells as previously [33]. Mean ± SEM
Discussion
Several studies have reported that the site of ulcer healing is also the site of ulcer recurrence [3, 5, 10, 11, 37] and that the regenerating mucosa is metaplastic for at least 4 months after ulcer induction in animal models [5]. For this study, we chose the time points of 30 days and 8 months after ulceration based on our previous work which used these same time points [5]. By 30 days acetic acid-induced gastric ulcers were visually healed, yet with mucosal imperfections (e.g., lack of parietal cells) that were sustained until at least 4 months. We selected 8 months as the long-term healing period in the current study because this was the earliest time point at which we had observed the return of parietal cells in the regenerating mucosa [5]. Many ion transporters have upregulated gene expression in the metaplastic gastric epithelium such as the cystic fibrosis transmembrane regulator (CFTR), and SLCs, in addition to mucus secretion [5, 38–41]. This upregulation led us to hypothesize that the 30 days regenerating epithelium would have increased electrogenic transport activity [42] and strengthened barrier function. In contrast, we found that the post-ulcer healing epithelium had impaired Isc recovery after hypertonic NaCl-induced epithelial injury compared with undamaged epithelium. This was similar to the observation that baseline Isc was lower in H. pylori-infected Mongolian gerbils [43]. These data suggest that metaplastic gastric epithelium lacks differentiated functions such as active ion transport that may contribute to longstanding increased susceptibility to mucosal injury.
We assume our measurements of Isc made in previously ulcerated tissue are largely representative of the condition of the regenerated epithelium versus surrounding normal tissue. As we reported, the ulcers we make are ~ 6.6 mm2 at day 1 post-ulcer [13]. Assuming that the healing/regenerating area is the entire area of the original ulcer, this area can be readily identified by a white color compared to the pinkish red of normal tissue. This healing area is reliably centered into the Ussing chamber slider by eye under a dissecting scope. However, there may be some normal (intact) area exposed in the rectangular measurement orifice, as the healing area is more rounded instead of rectangular, so the calculations for healing mucosa (regen) Isc and R basal and % Isc recovery may reflect this limitation.
High concentrations of luminal NaCl disrupt the mucosal barrier presumably through the creation of a steep transmucosal osmotic gradient. [44]. Although hypertonic saline has been used mostly in experimental injury models, the gastric osmolarity can approach 0.5 M shortly after ingestion of sweetened or salty foods, providing clinical relevance to this experimental intervention [45]. Because restitution rapidly occurs in vitro, without a requirement for cell proliferation or blood flow, hypertonic NaCl is often used to damage the gastric epithelium, which can be monitored in the Ussing chamber. Rutten et al. [46] studied restitution in guinea pig gastric mucosa mounted in Ussing chambers using a more severe damage model (1.25 M NaCl) where the mucosa showed extensive damage and exfoliation of surface cells [46]. In contrast, our 0.5 M NaCl mildly damaged the epithelium with only some sporadic loss of surface cells (Fig. 1h), yet in both damage models R was restored by 2 h [46]. Mucosal restoration is also confirmed by our ΔR at 2 h post-NaCl confirming the mucosa is similarly restored in both intact and regen tissue, yet the activity measured by Isc is disrupted in regenerated epithelium.
We used a lower NaCl concentration compared to prior reports in frog [25, 47] or guinea pig [23, 46], and the mouse response to NaCl may not be as sensitive or may be very quick to repair. We previously reported that in response to epithelial damage, the basal pole of migrating cells meets quickly in the murine gastroid restitution model during cell exfoliation and sustains epithelial barrier function, unless actin dynamics is inhibited [33]. It is not known if mouse epithelial cells perform this function more efficiently (or in a different way) versus other animal stomachs. R recovery was also more rapid than we expected, and we observed Isc recovery to be slower than R recovery suggesting that Isc recovery is still occurring even if epithelial repair has completed.
Most reports, under severe damage conditions with frog or guinea pig gastric mucosa, use potential difference under open circuit measurements and the change in R to measure restitution [23–25]. Epithelial repair is an energy-requiring process [48]; therefore in our current study, we wanted to see whether other energy-requiring processes, such as active ion transport, were also affected during healing; hence, the voltage was clamped and Isc measured. Our observations of rapid R recovery were identical in all our current gastric study conditions with mice. We also found that the Isc response was a better model for mice, suggesting that an electrogenic ion transport mechanism might be more sensitive to a mild injury model; therefore, we report Isc observing active ion transport, rather than potential difference and restitution as past studies have reported.
After removal of luminal hypertonic NaCl, we observed in control mice an immediate increase in Isc followed by a decline (the nadir) and then an increase back to basal Isc or higher. Silen’s group [23, 24] hypothesized that during hyperosmolar NaCl exposure, a rapid flow of isotonic serosal fluid crosses the mucosa, decreasing the damaging effect of NaCl and reducing the extent of injury. This postulated serosal to luminal fluid flow appears to contain ions that initially increase Isc after hypertonic NaCl removal, suggestive of active ion transport. As the rapid serosal flow dissipates, the Isc returns to expected levels after injury causing the nadir and the eventual Isc increase back to basal-state levels.
TFF2, secreted by gastric neck cells, is important for the maintenance of gastric epithelial barrier function, including restitution and ulcer healing, and may prevent further progression to gastric cancer [5, 12, 14, 19, 49]. We observed that basal Isc was significantly lower in TFF2-KO mouse stomach compared with WT and lacks Isc recovery in response to 0.5 M NaCl-induced epithelial damage, suggesting that TFF2 is involved in the regulation of Isc recovery during restitution. TFF2 is upregulated in metaplastic gastric epithelium in a specialized epithelium termed spasmolytic polypeptide-expressing metaplasia (SPEM) [5, 50], supporting the contribution of TFF2 to the regulation of Isc. We previously reported that NHE2 activity, but not NHE1, is necessary for restitution and is a TFF2 effector in gastric healing [14, 19, 36]. The NHE family of transporters are electroneutral and therefore cannot themselves directly contribute to the electrogenic current reported as Isc. However, since NHE2 is essential for restitution and is downregulated in healing epithelium, we speculated that the lack of Isc recovery is due to a lack of NHE2 activity. Here, we demonstrated that NHE inhibition impaired Isc recovery in response to 0.5 M NaCl-induced gastric epithelial damage and that NHE2-KO mice had a lower basal Isc. The NHE2-KO stomach lacks parietal cells and has upregulated TFF2 protein expression, similar to regenerating epithelium [5, 51]. Schultheis et al. [51] showed that NHE2 is not required for parietal cell acid secretion, whereas it is also well known that NHE is a major pHi regulator [52–54]. In this study, we directly measured pHi during restitution and observed that pHi decreased in migrating cells in response to damage and that pHi recovered in a NHE-dependent manner during restitution. Interestingly, we found that initiation of actin dynamics in response to damage was normal, suggesting that any role of NHE occurred downstream of those activating actin mobilization, although it is possible that pHi changes may affect actin dynamics in the later phases of restitution. Thus, NHE2 may be important for restitution by helping regulate pHi. The driving force of NHE activity is a sodium concentration gradient, which is maintained by basolateral Na+:K+-ATPase (NKA). Since NHE2 electroneutrally exchanges Na+ and H+ in a 1:1 ratio, it cannot directly affect the Isc. However, NHE2 affects the cellular gradients of Na+ and H+ during restitution, suggesting that an electrogenic transporter for one or both of these ions may be implicated in the generation of the Isc that we observe during restitution. For example, enhanced NHE activity may stimulate a transmural electrogenic Na+ absorption with excess intracellular sodium exported by the electrogenic NKA, generating a potential difference and possibly activating other Na+-dependent transporters, such as sodium-bicarbonate cotransporters and sodium-dependent anion exchangers [55, 56], involved in electrogenic anion secretion, thereby increasing Isc. Dissection of these mechanisms will require extensive work that can build upon the observations and findings reported here.
In conclusion, post-ulcer healing mucosa has impaired electrophysiological recovery following hypertonic saline exposure which may be tied to the downregulation of NHE2 activity in the regenerating mucosa. The inhibition of NHE in epithelial restitution also impairs pHi regulation. Epithelial downregulation of NHE2 may contribute to increased mucosal susceptibility leading to further ulcer relapse.
Supplementary Material
Acknowledgments
We thank H.J. Lang, PhD (Aventis Pharma Deutschland) for the generous gift of HOE 694, John Cuppoletti, PhD (University of Cincinnati) for supplying the Ussing chamber, and Chet Closson (University of Cincinnati) for technical assistance with the microscopes. We are very grateful to Timothy C. Wang, MD (Columbia University) for supplying the TFF2-KO, Gary E. Shull, PhD and Roger T. Worrell, PhD (University of Cincinnati) for supplying the NHE2-KO, and Walter Witke, PhD and Jerrold R. Turner, MD, PhD for supplying the HuGE mice.
Funding This work was supported by the National Institutes of Health (NIH) grant R01DK102551 (M.H.M., E.A.), the University of Cincinnati Research Council Faculty Research Grant (E.A.), Ryuji Ueno Award co-sponsored by the S&R Foundation and American Physiological Society (E.A.), and a VA Merit Award to JDK. This project was also supported in part by the NIH P30 DK078392; Live Microscopy Core and DNA Sequencing and Genotyping Core of the Digestive Disease Research Core Center in Cincinnati.
Footnotes
Compliance with Ethical Standards
Conflict of interest The authors declare that they have no conflict of interest.
Human and animal rights All procedures performed in studies involving animals were in accordance with the ethical standards of the Institutional Animal Care and Use Committee of the University of Cincinnati. This article does not contain any studies with human participants performed by any of the authors.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s10620-019-05825-x) contains supplementary material, which is available to authorized users.
References
- 1.Lanas A, Chan FKL. Peptic ulcer disease. Lancet. 2017;390:613–624. [DOI] [PubMed] [Google Scholar]
- 2.Everhart JE. The burden of digestive diseases in the United States Washington, DC: US Government Printing Office. NIH Publication No. 09–6443. 2008; p. 97– 106. [Google Scholar]
- 3.Laine L, Hopkins RJ, Girardi LS. Has the impact of Helicobacter pylori therapy on ulcer recurrence in the United States been overstated? A meta-analysis of rigorously designed trials. Am J Gastroenterol. 1998;93:1409–1415. [DOI] [PubMed] [Google Scholar]
- 4.Seo JH, Hong SJ, Kim JH, et al. Long-term recurrence rates of peptic ulcers without Helicobacter pylori. Gut Liver.. 2016;10:719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aihara E, Matthis AL, Karns RA, et al. Epithelial regeneration after gastric ulceration causes prolonged cell-type alterations. Cell Mol Gastroenterol Hepatol.. 2016;2:625–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blom H. The structure of normal and regenerating rat oxyntic mucosa. Scand J Gastroenterol Suppl. 1985;110:73–80. [DOI] [PubMed] [Google Scholar]
- 7.Okabe S, Amagase K. An overview of acetic acid ulcer models— the history and state of the art of peptic ulcer research. Biol Pharm Bull. 2005;28:1321–1341. [DOI] [PubMed] [Google Scholar]
- 8.Tarnawski A, Stachura J, Krause WJ, Douglass TG, Gergely H. Quality of gastric ulcer healing: a new, emerging concept. J Clin Gastroenterol. 1991;13:S42–S47. [DOI] [PubMed] [Google Scholar]
- 9.Young Oh T, Ok Ahn B, Jung Jang E, et al. Accelerated ulcer healing and resistance to ulcer recurrence with gastroprotectants in rat model of acetic acid-induced gastric ulcer. J Clin Biochem Nutr. 2008;42:204–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keto Y, Ebata M, Tomita K, Okabe S. Influence of Helicobacter pylori infection on healing and relapse of acetic acid ulcers in Mongolian gerbils. Dig Dis Sci. 2002;47:837–849. 10.1023/A:1014760504955. [DOI] [PubMed] [Google Scholar]
- 11.Wang GZ, Huang GP, Yin GL, et al. Aspirin can elicit the recurrence of gastric ulcer induced with acetic acid in rats. Cell Physiol Biochem. 2007;20:205–212. [DOI] [PubMed] [Google Scholar]
- 12.Farrell JJ, Taupin D, Koh TJ, et al. TFF2/SP-deficient mice show decreased gastric proliferation, increased acid secretion, and increased susceptibility to NSAID injury. J Clin Invest. 2002;109:193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aihara E, Closson C, Matthis AL, et al. Motility and chemotaxis mediate the preferential colonization of gastric injury sites by Helicobacter pylori. PLoS Pathog. 2014;10:e1004275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aihara E, Engevik KA, Montrose MH. Trefoil factor peptides and gastrointestinal function. Annu Rev Physiol. 2017;79:357–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boivin GP, Schultheis PJ, Shull GE, Stemmermann GN. Variant form of diffuse corporal gastritis in NHE2 knockout mice. Comp Med. 2000;50:511–515. [PubMed] [Google Scholar]
- 16.Muthusamy S, Cheng M, Jeong JJ, Kumar A, Dudeja PK, Malakooti J. Extracellular acidosis stimulates NHE2 expression through activation of transcription factor Egr-1 in the intestinal epithelial cells. PLoS One. 2013;8:e82023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yanaka A, Suzuki H, Shibahara T, Matsui H, Nakahara A, Tanaka N. EGF promotes gastric mucosal restitution by activating Na+/H+ exchange of epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2002;282:G866–G876. [DOI] [PubMed] [Google Scholar]
- 18.Furukawa O, Matsui H, Suzuki N, Okabe S. Epidermal growth factor protects rat epithelial cells against acid-induced damage through the activation of Na+/H+ exchangers. J Pharmacol Exp Ther. 1999;288:620–626. [PubMed] [Google Scholar]
- 19.Xue L, Aihara E, Wang TC, Montrose MH. Trefoil factor 2 requires Na/H exchanger 2 activity to enhance mouse gastric epithelial repair. J Biol Chem. 2011;286:38375–38382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hagen SJ, Morrison SW, Law CS, Yang DX. Restitution of the bullfrog gastric mucosa is dependent on a DIDS-inhibitable pathway not related to HCO3− ion transport. Am J Physiol Gastrointest Liver Physiol.. 2004;286:G596–G605. [DOI] [PubMed] [Google Scholar]
- 21.Clarke LL. A guide to Ussing chamber studies of mouse intestine. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1151–G1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li H, Sheppard DN, Hug MJ. Transepithelial electrical measurements with the Ussing chamber. J Cyst Fibros. 2004;3:123–126. [DOI] [PubMed] [Google Scholar]
- 23.Ito S, Lacy ER, Rutten MJ, Critchlow J, Silen W. Rapid repair of injured gastric mucosa. Scand J Gastroenterol Suppl. 1984;101:87–95. [PubMed] [Google Scholar]
- 24.Silen W, Ito S. Mechanisms for rapid re-epithelialization of the gastric mucosal surface. Annu Rev Physiol. 1985;47:217–229. [DOI] [PubMed] [Google Scholar]
- 25.Svanes K, Ito S, Takeuchi K, Silen W. Restitution of the surface epithelium of the in vitro frog gastric mucosa after damage with hyperosmolar sodium chloride. Morphologic and physiologic characteristics. Gastroenterology. 1982;82:1409–1426. [PubMed] [Google Scholar]
- 26.Gurniak CB, Witke W. HuGE, a novel GFP-actin-expressing mouse line for studying cytoskeletal dynamics. Eur J Cell Biol. 2007;86:3–12. [DOI] [PubMed] [Google Scholar]
- 27.Loh SH, Sun B, Vaughan-Jones RD. Effect of Hoe 694, a novel Na+-H+ exchange inhibitor, on intracellular pH regulation in the guinea-pig ventricular myocyte. Br J Pharmacol. 1996;118:1905–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scholz W, Albus U, Lang HJ, et al. Hoe 694, a new Na+/H+ exchange inhibitor and its effects in cardiac ischaemia. Br J Pharmacol. 1993;109:562–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fellenius E, Berglindh T, Sachs G, et al. Substituted benzimidazoles inhibit gastric acid secretion by blocking (H+ + K+) ATPase. Nature.. 1981;290:159–161. [DOI] [PubMed] [Google Scholar]
- 30.Miller MA, Bunnett NW, Debas HT. Laminin mediates the restitution of rat gastric mucosa in vitro. Exp Physiol. 1994;79:647–659. [DOI] [PubMed] [Google Scholar]
- 31.Mahe MM, Aihara E, Schumacher MA, et al. Establishment of gastrointestinal epithelial organoids. Curr Protoc Mouse Biol. 2013;3:217–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schumacher MA, Aihara E, Feng R, et al. The use of murine-derived fundic organoids in studies of gastric physiology. J Physiol. 2015;593:1809–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aihara E, Medina-Candelaria NM, Hanyu H, et al. Cell injury triggers actin polymerization to initiate epithelial restitution. J Cell Sci. 2018;131:jcs216317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Demitrack ES, Soleimani M, Montrose MH. Damage to the gastric epithelium activates cellular bicarbonate secretion via SLC26A9 Cl−/HCO3−. Am J Physiol Gastrointest Liver Physiol. 2010;299:G255–G264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Engevik KA, Hanyu H, Matthis AL, et al. Trefoil factor 2 activation of CXCR35 requires calcium mobilization to drive epithelial repair in gastric organoids. J Physiol. 2019;597:2673–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Take S, Mizuno M, Ishiki K, et al. Seventeen-year effects of eradicating Helicobacter pylori on the prevention of gastric cancer in patients with peptic ulcer; a prospective cohort study. J Gastroenterol. 2015;50:638–644. [DOI] [PubMed] [Google Scholar]
- 38.Meyer AR, Goldenring JR. Injury, repair, inflammation and metaplasia in the stomach. J Physiol. 2018;596:3861–3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mills JC, Sansom OJ. Reserve stem cells: differentiated cells reprogram to fuel repair, metaplasia, and neoplasia in the adult gastrointestinal tract. Sci Signal. 2015;8:re8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weis VG, Sousa JF, LaFleur BJ, et al. Heterogeneity in mouse spasmolytic polypeptide-expressing metaplasia lineages identifies markers of metaplastic progression. Gut. 2013;62:1270–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Companioni O, Sanz-Anquela JM, Pardo ML, et al. Gene expression study and pathway analysis of histological subtypes of intestinal metaplasia that progress to gastric cancer. PLoS One. 2017;12:e0176043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng AM, Morrison SW, Yang DX, Hagen SJ. Energy dependence of restitution in the gastric mucosa. Am J Physiol Cell Physiol. 2001;281:C430–C438. [DOI] [PubMed] [Google Scholar]
- 43.Sun YQ, Soderholm JD, Petersson F, Borch K. Long-standing gastric mucosal barrier dysfunction in Helicobacter pylori-induced gastritis in mongolian gerbils. Helicobacter. 2004;9:217–227. [DOI] [PubMed] [Google Scholar]
- 44.Tamura M, Matsui H, Nagano YN, et al. Salt is an oxidative stressor for gastric epithelial cells. J Physiol Pharmacol. 2013;64:89–94. [PubMed] [Google Scholar]
- 45.Fordtran JS, Locklear TW. Ionic constituents and osmolality of gastric and small-intestinal fluids after eating. Am J Dig Dis. 1966;11:503–521. [DOI] [PubMed] [Google Scholar]
- 46.Rutten MJ, Ito S. Morphology and electrophysiology of guinea pig gastric mucosal repair in vitro. Am J Physiol. 1983;244:G171–G182. [DOI] [PubMed] [Google Scholar]
- 47.Critchlow J, Magee D, Ito S, Takeuchi K, Silen W. Requirements for restitution of the surface epithelium of frog stomach after mucosal injury. Gastroenterology. 1985;88:237–249. [DOI] [PubMed] [Google Scholar]
- 48.Kuipers D, Mehonic A, Kajita M, et al. Epithelial repair is a two-stage process driven first by dying cells and then by their neighbours. J Cell Sci. 2014;127:1229–1241. [DOI] [PubMed] [Google Scholar]
- 49.Shi SQ, Cai JT, Yang JM. Expression of trefoil factors 1 and 2 in precancerous condition and gastric cancer. World J Gastroenterol. 2006;12:3119–3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmidt PH, Lee JR, Joshi V, et al. Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma. Lab Invest. 1999;79:639–646. [PubMed] [Google Scholar]
- 51.Schultheis PJ, Clarke LL, Meneton P, et al. Targeted disruption of the murine Na+/H+ exchanger isoform 2 gene causes reduced viability of gastric parietal cells and loss of net acid secretion. J Clin Invest. 1998;101:1243–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jang IS, Brodwick MS, Wang ZM, Jeong HJ, Choi BJ, Akaike N. The Na+/H+ exchanger is a major pH regulator in GABAergic presynaptic nerve terminals synapsing onto rat CA3 pyramidal neurons. J Neurochem. 2006;99:1224–1236. [DOI] [PubMed] [Google Scholar]
- 53.Praetorius J, Andreasen D, Jensen BL, Ainsworth MA, Friis UG, Johansen T. NHE1, NHE2, and NHE3 contribute to regulation of intracellular pH in murine duodenal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2000;278:G197–G206. [DOI] [PubMed] [Google Scholar]
- 54.Valles PG, Bocanegra V, Gil Lorenzo A, Costantino VV. Physiological functions and regulation of the Na+/H+ exchanger [NHE1] in renal tubule epithelial cells. Kidney Blood Press Res. 2015;40:452–466. [DOI] [PubMed] [Google Scholar]
- 55.Damkier HH, Nielsen S, Praetorius J. Molecular expression of SLC4-derived Na+-dependent anion transporters in selected human tissues. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2136–R2146. [DOI] [PubMed] [Google Scholar]
- 56.Rossmann H, Bachmann O, Vieillard-Baron D, Gregor M, Seidler U. Na+/HCO3− cotransport and expression of NBC1 and NBC2 in rabbit gastric parietal and mucous cells. Gastroenterology. 1999;116:1389–1398. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.