Abstract
Empathy is an essential component of social communication that involves experiencing others sensory and emotional states. We observed that a brief social interaction with a mouse experiencing pain or morphine analgesia resulted in the transfer of these experiences to its social partner. Optogenetic manipulations demonstrated that the anterior cingulate cortex (ACC) and its projections to the nucleus accumbens (NAc) were selectively involved in the social transfer of both pain and analgesia. In contrast, the ACC→NAc circuit was not necessary for the social transfer of fear, which instead depended on ACC projections to the basolateral amygdala. These findings reveal that the ACC, a brain area strongly implicated in human empathic responses, mediates distinct forms of empathy in mice by influencing different downstream targets.
One Sentence Summary:
Mice exhibit different empathy-like behaviors that are dependent on anterior cingulate cortex projections to distinct downstream targets.
Empathy plays an essential role in social communication and involves integrated behavioral, cognitive, and affective processes that facilitate the adoption of a sensory or affective state that is more appropriate to another’s situation than one’s own (1–3). Evolutionarily conserved behavioral antecedents of human empathy have been identified in a range of species (2–5) including rodents, which display emotional contagion (6, 7), socially transferred pain (8–11), observational fear (3, 5, 12), and prosocial behaviors such as consolation (13) and “helping” (14, 15).
The ACC is a principle node in the neural circuitry thought to mediate empathy (16–18). In both humans and rodents, the ACC is particularly critical for affective and motivational responses to direct and observed pain as well as the social transfer of pain (10, 13, 19, 20). The ACC is thought to communicate with a broad range of brain regions that regulate emotional and motivational states including the thalamus, insula, amygdala and NAc (16, 21–23). However, the roles of these specific ACC circuit elements in empathy-related behaviors are unknown.
Rapid transfer of pain behavior to bystander mice
The presence of a conspecific in pain can modulate the expression of pain behavior in a test animal already experiencing pain (6) and cause hyperalgesia in “bystander” (BY) mice that have not been subjected to any pain-inducing stimuli (9, 10), a phenomenon known as the “social transfer of pain”. We examined whether a brief (1 hour), direct social interaction between a male BY mouse and a male cage mate experiencing inflammatory hyperalgesia [due to intraplantar injection of complete Freund’s adjuvant (CFA), which induces long lasting arthritis-like pain, (24,25)] would lead to the social transfer of pain (Fig. 1A). Following this 1 hour social interaction, BY mice exhibited mechanical hypersensitivity (as measured by stimulation with von Frey hairs using the “up-down” technique (26)) equivalent to that of CFA mice, whereas control mice subjected to the same procedures (in the absence of a nociceptive stimulus in either mouse) exhibited no change in mechanical thresholds (Fig. 1, B and C). Following von Frey testing, all mice were separated and housed with treatment matched cagemates (fig. S1). Repeated mechanical testing revealed that the hyperalgesia in BY mice lasted 4, but not 24 hours (Fig. 1B); was not influenced by prolonging the social interaction to 2 hours (fig. S2A) or by delaying the start of the social interaction to 24 hours after CFA injection (fig. S2B); and still occurred when capsaicin was used to induce pain and the social interaction was limited to 30 minutes (fig. S2C). Females demonstrated similar social transfer of pain (fig. S3A), although they expressed lower basal mechanical thresholds and enhanced CFA-induced hypersensitivity (fig. S3, A–C).
Fig. 1. Rapid social transfer of pain to bystander mice.
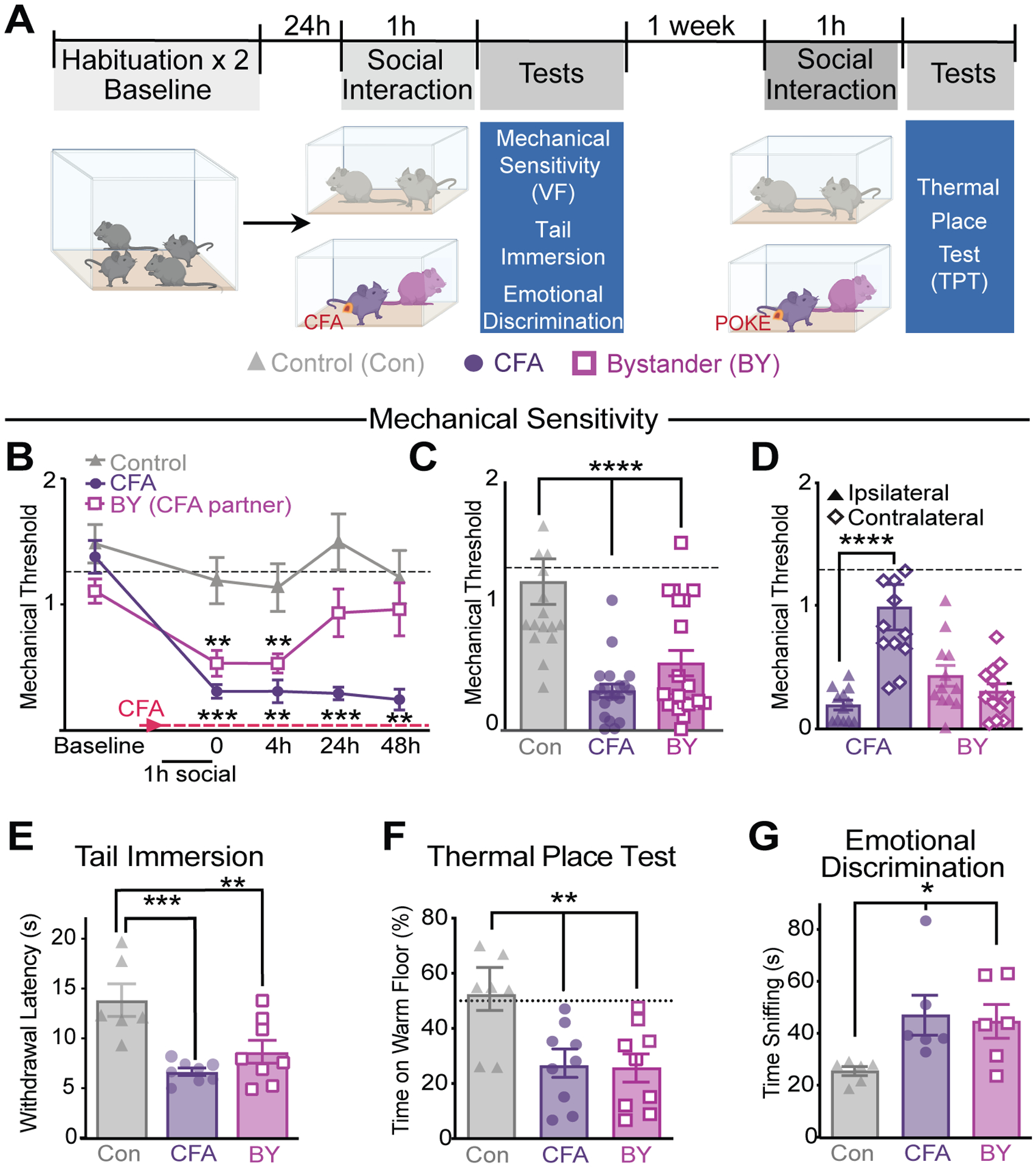
(A) Timeline of social transfer of pain protocol. (B) Timecourse of mechanical von Frey (VF) sensitivity at 0, 4, 24 and 48 h post 1h social interaction in Con/Con and CFA/BY pairs. (C) Mechanical thresholds immediately after the 1 h social interaction. (D) Mechanical thresholds of the ipsilateral vs. contralateral hindpaws. (E) Tail withdrawal latencies in the tail immersion test. (F) Time (%) spent on the warm floor (40° C) in the thermal place test (TPT). (G) Time (s) a stranger conspecific spent sniffing each group. Data are means ± s.e.m. Dotted lines (---) represents mean baseline thresholds for all groups (B, C, D); Statistical tests included two-way repeated measures (A), one-way (B, E, F, G) and two-way (D) ANOVA with Holm-Sidak post hoc tests; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 representative of post hoc comparisons. All statistical measure details are presented in Table S1A.
While CFA mice exhibited hyperalgesia only in the ipsilateral, CFA injected hindpaw, BY mice exhibited hyperalgesia in both hindpaws (Fig. 1D), suggesting the involvement of higher brain regions in mediating the pain transfer. Both CFA and BY mice also displayed thermal hypersensitivity to tail immersion in hot water (Fig. 1E) and thermal place aversion when given the choice between a warm (40° C) or room temperature (30° C) floor in a thermal place test (TPT, Fig. 1F). To determine whether the socially transferred pain experienced by BY mice led to affective changes that could be detected by a conspecific, we conducted an emotional discrimination task (27), which demonstrated that a stranger mouse spent more time exploring both CFA and BY mice compared to controls (Fig. 1G).
Activation of an ACC to NAc core circuit by the social transfer of pain
To elucidate the brain regions potentially contributing to the social transfer of pain, we identified neurons activated during the social interaction using a reporter line generated by crossing FosCreERT2 (TRAP2) mice with the Ai14-TdTomato reporter line (28, 29). Administering 4-hydroxytamoxifen (4-OHT) immediately prior to a 4 hour social interaction between BY and CFA mice (Fig. 2A) generated activated neurons in BY mice in brain regions previously associated with empathy and social motivation, such as the ACC and NAc, as well as regions associated with pain transmission, such as the thalamus, central amygdala and periaqueductal grey (Fig. 2, B and C). Because the numbers of activated neurons in the ACC and NAc were greater in BY mice than in both control and CFA mice (Fig. 2C) and the fact that the ACC and NAc are important for social behaviors (30–33), we hypothesized that ACC neurons synapse onto NAc cells that are activated during the social transfer of pain. Thus, we first injected AAV-CaMKIIα-YFP into the ACC and verified that ACC pyramidal neurons send projections to the NAc, preferentially in its core region (Fig 2D). To determine if there are direct synaptic connections between ACC neurons and activated NAc neurons during social transfer, we applied monosynaptic rabies virus tracing (34, 35) in TRAP2-BY and -CFA mice. Injection of AAVs expressing Cre-dependent RG (rabies glycoprotein) and TVA (avian tumor virus receptor A) into the NAc core followed by injection of EnvA-pseudotyped RG-deleted rabies virus expressing GFP (Fig 2E), resulted in similar levels of GFP expression throughout the ACC in both CFA and BY mice (Fig 2F, fig. S4).
Fig. 2. Activation of an ACC→NAc core circuit by the social transfer of pain.
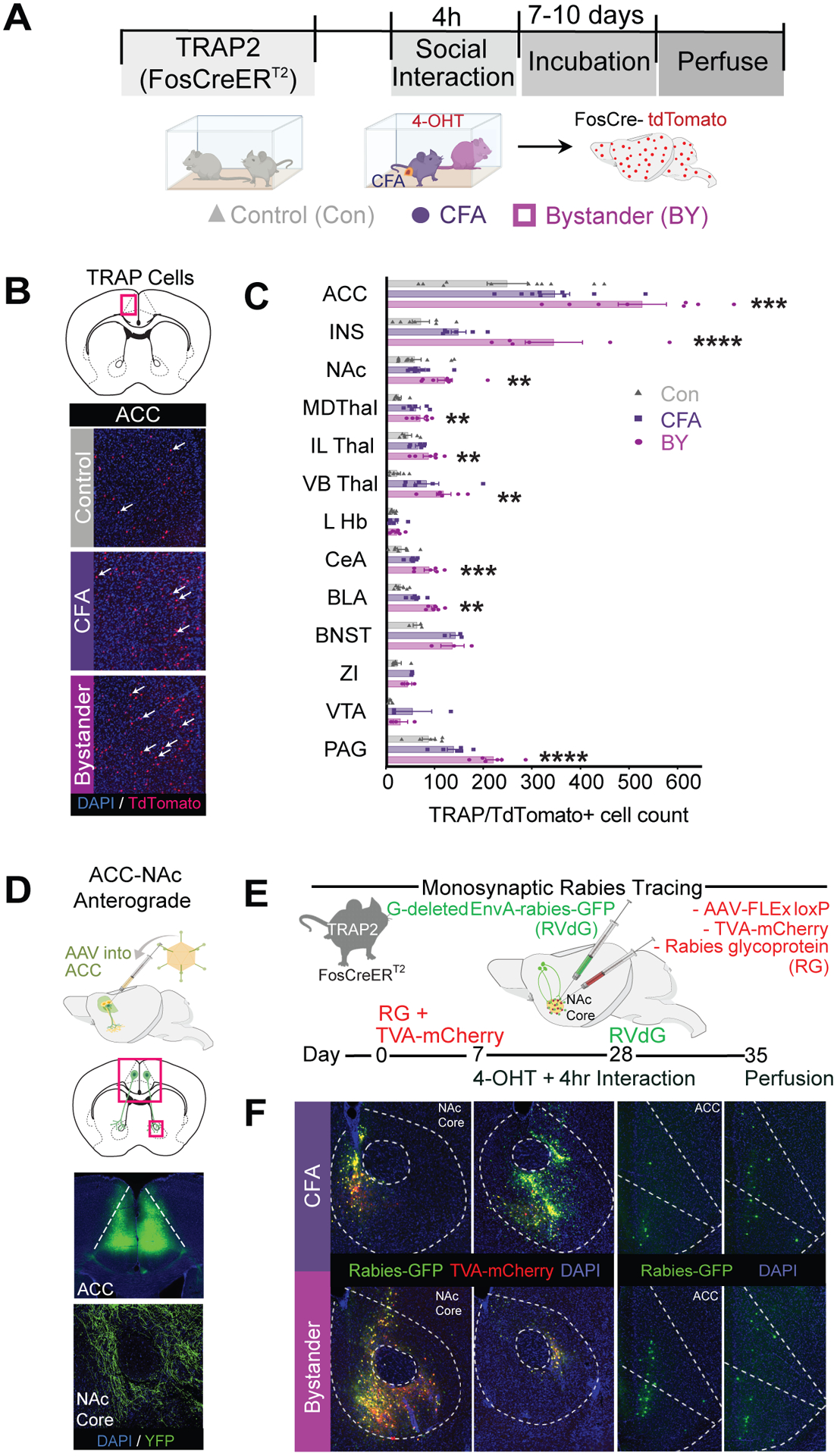
(A) Timeline to “TRAP” activated neurons during social transfer of pain. (B) Representative photomicrographs of TdTomato positive cells (white arrows) in the ACC of Con, CFA and BY mice after social transfer of pain (C) Quantification of Ai14-positive/TRAPed cells across 13 brain regions (n = 6–9/group). (D) Schematic of AAV injection and representative photomicrographs of ACC injection site and fibers in the NAc core. (E) Schematic and timeline of monosynaptic rabies tracing. (F) Representative photomicrographs of NAc core RVdG/RG injection site and ACC GFP expression in CFA and BY mice. Data are means ± s.e.m. One-way ANOVA with Holm-Sidak post hoc tests comparing Con vs. BY, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. All statistical measure details are presented in Table S1B. Anterior cingulate (ACC), insula (INS), nucleus accumbens core (NAc), mediodorsal thalamus (MD Thal), interlaminar thalamus (IL Thal), ventrobasal thalamus (VB Thal), lateral habenula (L Hb), central amygdala (CeA), basolateral amygdala complex (BLAc), bed nucleus of the stria terminalis (BNST), zona inserta (ZI), ventral tegmental area (VTA), periaqueductal gray (PAG).
ACC→NAc projections bidirectionally control social transfer of pain
To investigate if the ACC→NAc pathway is required for the social transfer of pain, we first tested the necessity of the ACC itself by injecting AAVs expressing the inhibitory opsin halorhodopsin (NpHR; AAV-DJ-CaMKIIα-NpHR) or eYFP as a control (YFP; AAV-DJ-CaMKIIα-eYFP) and placing an optical fiber directly above the ACC (Fig. 3A). Activating NpHR during the social interaction between BY and CFA mice (Fig. 3A) attenuated the hyperalgesia in BY mice but not CFA mice, whereas YFP-expressing BY and CFA mice exposed to the same light stimulation displayed the expected mechanical hypersensitivity (Fig. 3B). Acute light exposure in the same mice during the mechanical testing had no consistent effect on mechanical thresholds (Fig. 3C), suggesting that acute inhibition of ACC neurons does not directly alter mechanical sensation.
Fig. 3. ACC→NAc projections bidirectionally control social transfer of pain.
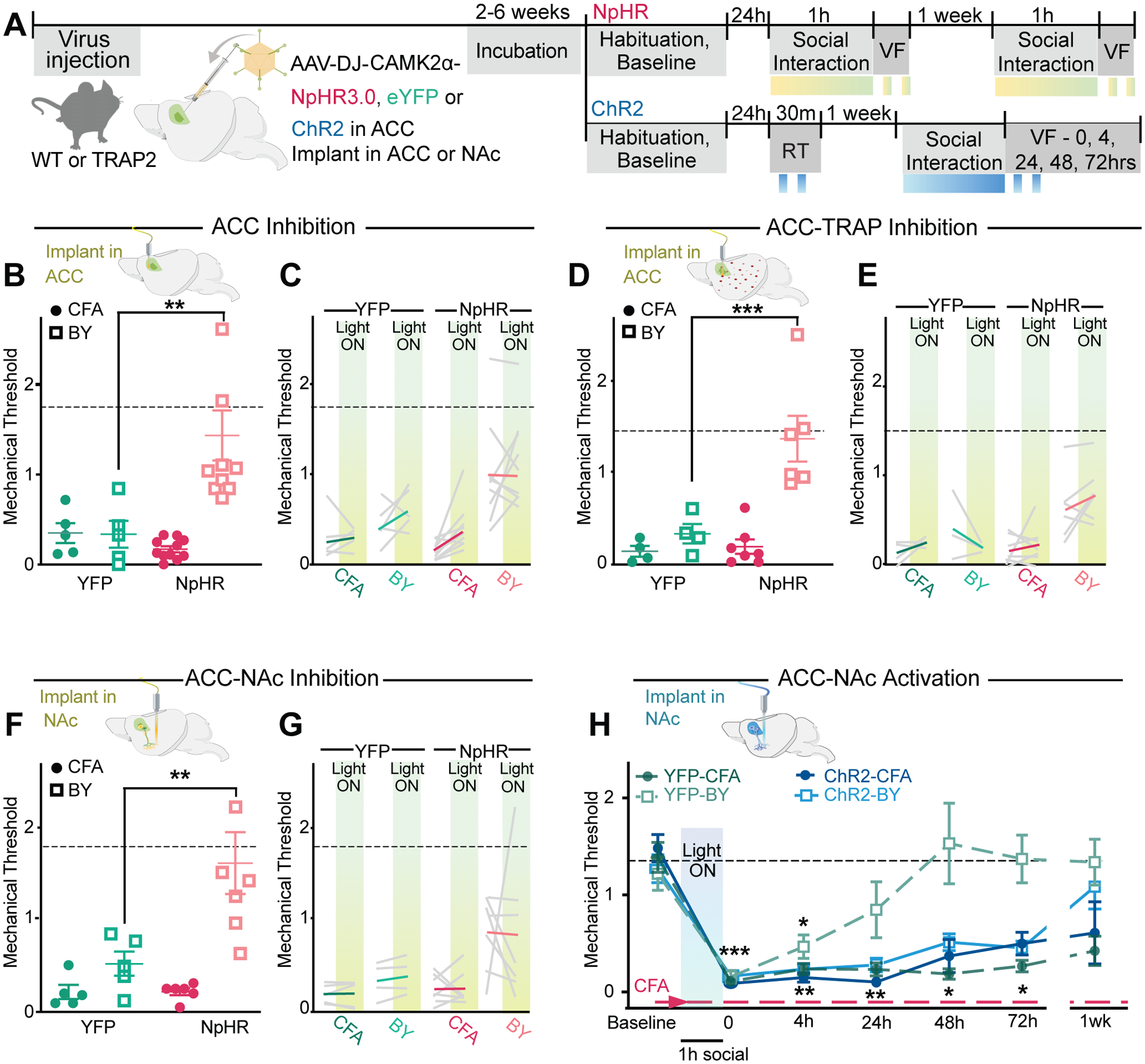
(A) Schematic of viral injection and experimental timeline. Light stimulation (~5–15 mW, 8 s on/2 s off) periods represented by yellow-green boxes. (B) Schematic shows fiber optic implant above the ACC. First light off test of mechanical sensitivity (VF) of YFP and NpHR injected mice immediately after 1 h social transfer with ACC inhibition. (C) Mechanical sensitivity during averaged (n=2) light off and light on sessions of ACC inhibition. (D) Schematic shows fiber optic implant above the ACC. First light off test of mechanical sensitivity of YFP and NpHR injected TRAP2 mice immediately after 1 h social transfer with ACC-TRAP inhibition. (E) Mechanical sensitivity during averaged (n=2) light off and light on sessions of ACC-TRAP inhibition. (F) Schematic of bilateral fiber optic implants above the NAc core. First light off test of mechanical sensitivity of YFP and NpHR injected mice immediately after 1 h social transfer with ACC→NAc input inhibition. (G) Mechanical sensitivity during averaged (n=2) light off and light on sessions of ACC→NAc input inhibition. (H) First light off test of mechanical sensitivity at 0, 4, 24, 48, 74 h and 1 wk post 1 h social interaction with ACC→NAc input inhibition. Data are means ± s.e.m. Dotted line (---) represents mean baseline thresholds for all groups. *P < 0.05, **P < 0.01, ***P < 0.001, **** P < 0.0001; One-way ANOVA with Holm-Sidak post hoc tests comparing YFP to matched NpHR groups (B–G) and two-way repeated measures ANOVA with Holm-Sidak post hoc tests comparing treatment groups to baseline at each timepoint, notation is the least significant p value of all comparisons (H). All statistical measure details are presented in Table S1C.
To determine if the subset of ACC neurons activated during an initial social interaction is necessary for subsequent socially transferred pain, TRAP2:Ai14 mice that had received ACC injections of AAV-DIO-NpHR or AAV-DIO-eYFP were given 4-OHT prior to the social transfer of pain. One week later, optogenetic inhibition of the TRAPed ACC neurons during a second social interaction prevented BY mice from developing mechanical hypersensitivity, compared to control YFP-BY mice, which expressed robust hyperalgesia (Fig. 3D). Immediately following the initial light off test, the same mice were given light stimulation of the TRAPed neurons during the mechanical testing, and this manipulation had no consistent effect (Fig. 3E).
We next tested the necessity of ACC→NAc projections specifically by bilaterally injecting NpHR or YFP-expressing AAVs in the ACC and placing optical fibers immediately above the NAc core. Similar to the effects of inhibiting the ACC, inhibition of ACC→NAc projections during the 1 hour social interaction strongly impaired the social transfer of mechanical hypersensitivity to BY mice, while having no effects on CFA mice or YFP-expressing BY and CFA mice (Fig. 3F). Furthermore, repeated inhibition of the ACC→NAc projections during mechanical testing in the same mice had no consistent effect on mechanical thresholds (Fig. 3G).
To further evaluate the role of the ACC→NAc pathway in the social transfer of pain, we expressed ChR2 in the ACC and activated ACC→NAc projections during the 1 hour social transfer. This caused a robust prolongation of the duration of hyperalgesia in the BY mice, which lasted >72 hours as opposed to the expected 4–24 hours, as seen in YFP-expressing BY mice (Fig. 3H). Prior to any nociceptive stimulation, acute activation of ACC→NAc projections during mechanical testing had no consistent effect on mechanical sensitivity (fig. S5A) and also did not have acute aversive or reinforcing effects as assayed by a real time place preference test (fig. S5B).
Distinct ACC projections control the social transfer of pain and fear
To examine the generalizability of ACC→NAc control over socially transferred behaviors, we examined the role of ACC→NAc projections in the well-established phenomenon of the social transfer of fear (5, 12). BY mice were exposed to shock 24 hours prior to placement in a distinct context, which allowed observation of a demonstrator (Shock) mouse being repeatedly shocked (Fig 4A). Shock pre-exposure enhances the magnitude of freezing behavior in BY mice and is thought to more closely model human empathy (12, 20, 36). During the short observation period, BY mice exhibited significant increases in freezing (Fig. 4B) and this was maintained 24 hours later during re-exposure to the shock observation context (“retrieval”, Fig. 4C). Inhibition of ACC→NAc projections in BY mice during the conditioning phase (Fig. 4D) had no effect on their acquisition of freezing behavior (Fig. 4E) and also did not affect freezing during the context-induced retrieval (Fig. 4F), where light was applied every other minute (fig. S6A). However, when the same mice were subjected to the social transfer of pain, inhibition of the ACC→NAc projections during a 1 hour social interaction between CFA and BY mice impaired the acquisition of hyperalgesia in the BY mice (Fig. 4G), thereby providing evidence that the optogenetic inhibition of ACC→NAc input activity was effective in these mice.
Fig. 4. Distinct ACC projections control the social transfer of pain and fear.
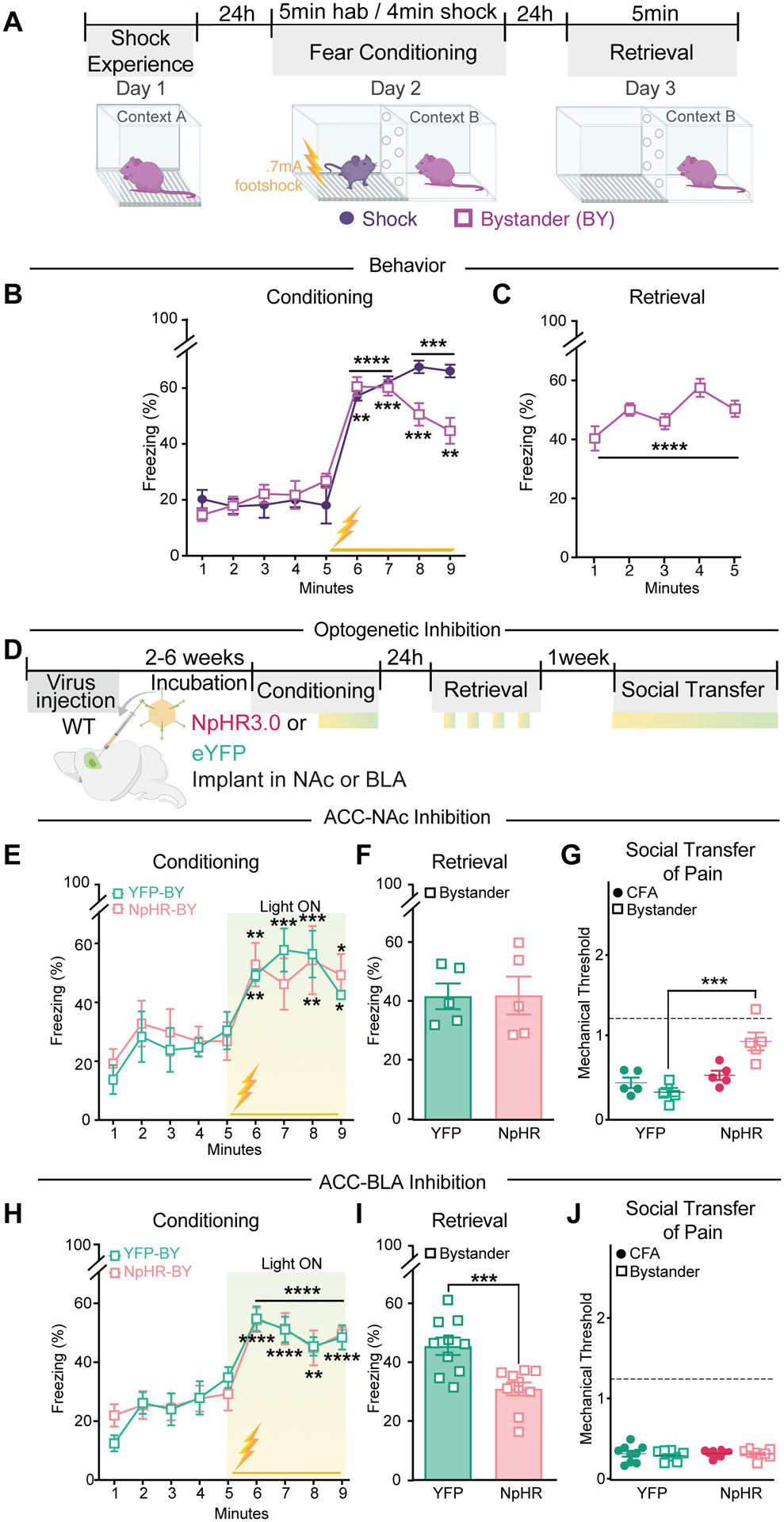
(A) Schematic and timeline of social transfer of fear (Shock Experience: 2 × 0.7 mA, 2 s duration, 1 min intervals; Shock Conditioning: 24 × 0.7 mA for 2 s duration, 10 s intervals, total of 4 min). (B) Freezing behavior of Shock and Bystander (BY) during conditioning phase of socially transferred fear. (C) Freezing behavior of BY mice during retrieval phase of socially transferred fear. (D) Schematic of YFP and NpHR injection and experimental timeline of optogenetic stimulation in NAc or BLA. Light stimulation (~10–15 mW, 8 s on/2 s off) periods represented by green boxes. (E) Conditioning session with inhibition of ACC→NAc projections in YFP- and NpHR-BY mice during shock observation. (F) Freezing behavior of YFP- and NpHR-BY mice during retrieval phase. (G) First light off session measuring mechanical sensitivity following NpHR inhibition of ACC→NAc projections during social transfer of pain in the same mice from E, F. Dotted line (---) represents mean baseline of all groups. (H) Conditioning session with inhibition of ACC→BLA projections in BY mice during shock observation. (I) Freezing behavior of YFP- and NpHR-BY mice during retrieval phase. (J) First light off session measuring mechanical sensitivity following NpHR inhibition of ACC→BLA projections during social transfer of pain in the same mice from H, I. Dotted line (---) represents mean baseline of all groups. Data are means ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Two-way repeated measures ANOVA with Holm Sidak post hoc tests comparing freezing to the first minute, notation is the least significant p value of all comparisons (B, E, H), paired t-test (C) or unpaired t-test (F,I) comparing average baseline to average freezing during retrieval, or one-way ANOVA with Holm-Sidak post hoc tests (G,J). All statistical measure details are presented in Table S1D.
ACC projections to the basolateral amygdala (BLA) are necessary for cue-induced retrieval of socially transferred fear (36). To evaluate if ACC→BLA projections also are necessary for context-induced retrieval of socially transferred fear behavior, we inhibited this pathway during acquisition of socially transferred fear and intermittently during retrieval (Fig. 4D). Inhibition of ACC→BLA projections in BY mice had no effect on their acquisition of freezing behavior (Fig. 4H), but did attenuate freezing behavior during retrieval (Fig 4I), regardless of whether the light was on or off (fig. S6B). In contrast, when the same mice were tested for the social transfer of pain, there was no effect of ACC→BLA input inhibition during the social interaction on their mechanical thresholds (Fig. 4J).
ACC→NAc projections regulate the social transfer of analgesia
Although the social transfer of pain and fear in rodents are well established, it is unknown if the experience of pain relief (i.e. analgesia) can be transferred socially. To examine this possibility, all mice were administered CFA to induce pain and then one quarter of mice were also given an analgesic dose of morphine (10 mg/kg, CFA-Mor) at which time mice were paired for a 1 hour social interaction (Fig. 5A). Despite prior administration of CFA, CFA-Analg-BY mice paired with morphine treated mice (CFA-Mor) exhibited diminished reductions in mechanical threshold (i.e. lessened pain responses) compared to CFA-CFA control pairs (CFA-Con; Fig. 5B). Following separation from CFA-Mor partners, the social transfer of analgesia in CFA-Analg-BY mice lasted 4 hours but not 24 hours (Fig. 5B). Because morphine causes hyperlocomotion, which prevents measurement of mechanical thresholds in CFA-Mor mice, we used the TPT to directly compare the magnitude of the analgesia in CFA-Analg-BY and CFA-Mor mice. In contrast to mechanical hyperalgesia, thermal place aversion was not present 1 hour after CFA injection, but was robust one week later (Fig. 5C), at which point one quarter of CFA mice were again administered morphine immediately prior to a 1 hour social interaction with CFA-Analg-BY partners (Fig. 5A). CFA-induced thermal aversion (CFA-Con) was reduced in the CFA-Analg-BY mice to the same extent as morphine administration (CFA-Mor; Fig. 5D).
Fig. 5. ACC→NAc projections regulate social transfer of analgesia.
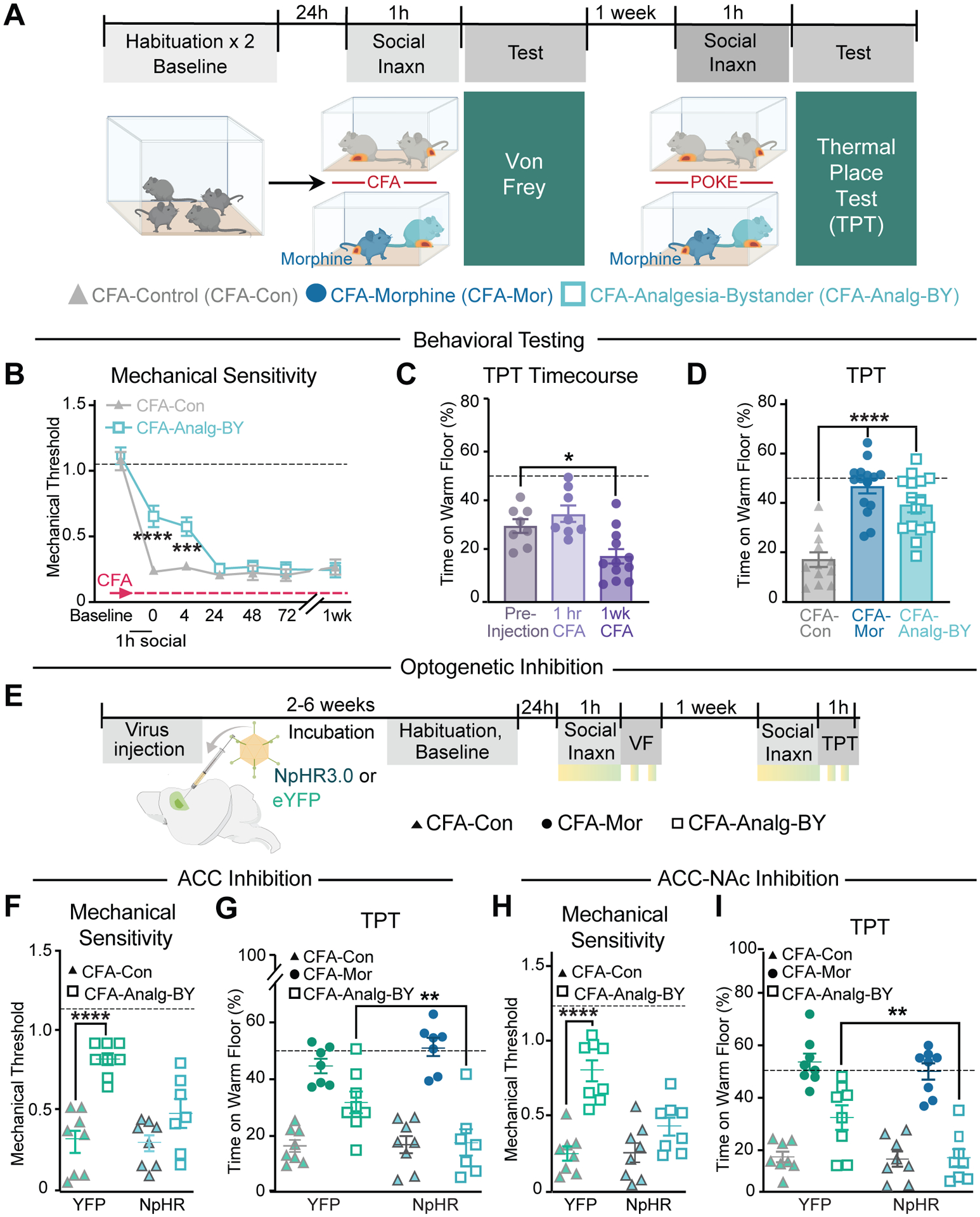
(A) Schematic of social transfer of analgesia protocol and timeline. (B) Mechanical sensitivity at 0, 4, 24, 48, 72 h and 1 week post 1 h social interaction in CFA-Con/CFA-CON pairs and CFA-Mor/CFA-Analg-BY pairs. (C) Time (%) on the warm (40° C) floor in the thermal place test (TPT) prior to and 1 h and 1 week (wk) post CFA injection. (D) Time (%) on the warm (40° C) floor in the TPT immediately after the second 1 h social transfer of analgesia, 1 week post CFA injection. (E) Schematic of YFP and NpHR injection and optogenetic stimulation in ACC or NAc. Light stimulation (~10–15 mW, 8 s on/2 s off) periods represented by yellow-green boxes. (F) First light off session measuring mechanical sensitivity following light stimulation of ACC in YFP- and NpHR-expressing CFA-Con and CFA-Analg-BY mice during social transfer of analgesia. (G) Time (%) on the warm (40° C) floor in the TPT following light stimulation of ACC in YFP- and NpHR-expressing CFA-Con, CFA-Mor, and CFA-Analg-BY mice during the second social transfer of analgesia test. (H) First light off session measuring mechanical sensitivity following light stimulation of ACC→NAc projections in YFP- and NpHR-expressing CFA-Con and CFA-Analg-By mice during social transfer of analgesia. (I) Time (%) on the warm (40°C) floor in the TPT following ACC→NAc light stimulation in YFP- and NpHR-expressing CFA-Con, CFA-Mor, and CFA-Analg-BY mice during the second social transfer of analgesia test. Data are means ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001, **** P < 0.0001. Two-way repeated measures (B) or one-way (C,D,F–I) ANOVA with Holm-Sidak post hoc tests comparing between groups. Not all significant post hoc analyses are displayed. All statistical measure details are presented in Table S1E.
We next tested the necessity of ACC activity for the social transfer of analgesia. Optogenetic inhibition of ACC neurons using NpHR during the 1 hour social interaction (Fig. 5E) prevented the social transfer of analgesia in CFA-Analg-BY mice as assayed by both mechanical sensitivity (Fig. 5F) and the TPT (Fig. 5G) but had no effect on acute mechanical thresholds, thermal place aversion, or the analgesic action of morphine during the testing of CFA-Con and CFA-Mor mice. Inhibition of ACC→NAc input activity specifically had essentially identical effects, also preventing the social transfer of analgesia without affecting mechanical or thermal sensitivity directly (Fig. 5, H and I).
Discussion
We investigated the neural mechanisms of simple forms of empathy in mice by establishing a protocol that results in the rapid social transfer of two types of pain behavior: hyperalgesia and analgesia. Although the definition of “empathy” is subject to debate (37), the BY mice in this bidirectional behavioral model appear to fulfill one critical feature of the expression of empathy, the adoption of another’s sensory and affective state (1–3, 37). The social transfer of hyperalgesia to BY mice required only 1 hour of social interaction, lasted 4–24 hours, and generalized to several different pain modalities. Surprisingly, analgesia could also be transferred to a mouse in pain and lasted at least 4 hours. These results provide further evidence for the critical importance of the social environment to the experience of pain, including an innovative model for socially-induced pain relief, which can be tested in human subjects.
The social interactions in mice resulted in increased activity in ACC and several of its downstream targets including the NAc, a key node of the circuitry involved in a range of affective and motivated behaviors, including those triggered by pain (38–41). A critical role for ACC to NAc communication was established by demonstrating that bidirectional manipulation of activity in ACC→NAc inputs influences the acquisition of socially transferred pain, but not the expression of mechanical sensitivity itself. Specifically, inhibiting this activity during the one hour social interaction reduced hyperalgesia in BY mice, whereas increasing activity in ACC→NAc inputs prolonged the duration of the hyperalgesia evoked by the brief social interaction. ACC→NAc input activity was also necessary for the social transfer of analgesia, but not for the social transfer of fear, which requires activity in ACC projections to the BLA (36).
These results suggest that the ACC, which has been proposed to be a key brain area for mediating the emotional aspects of pain as well as encoding information about the affective state of others (23, 30, 42), generates a specific and appropriate empathic behavioral response through accessing distinct downstream targets. The specificity of the neural circuit and behavioral response generated during socially transferred pain and fear may, at least in part, be due to the sensory modalities required for these two forms of social transfer. The social transfer of pain does not require visual or auditory stimuli but can be generated by exposure to used bedding from mice experiencing pain, suggesting that olfactory cues are sufficient for this form of social transfer (9). In contrast, the social transfer of fear requires visual and/or auditory cues (3,12). Further elucidation of the mechanisms by which this specificity in empathic neural and behavioral responses occurs will be important for developing interventions that promote social-context appropriate empathic responses. Furthermore, a better understanding of the neural circuits mediating specific empathic responses will greatly facilitate the development of therapies that target pathological forms of empathy, or its absence, in a variety of neuropsychiatric disorders.
Historically, empathy, as defined by the ability to experience and share the emotions of others, was often considered a high level, affective-cognitive process experienced almost exclusively by humans (1, 2, 43). However, most investigators now accept that empathy can be deconstructed into specifiable, evolutionarily conserved components, many of which can be studied in rodents in order to elucidate their underling neural mechanisms (3–5, 12, 44). Our results provide additional evidence that mice can rapidly and reliably adopt the sensory-affective state of a social partner, regardless of the valence of the information (pain, fear, or pain relief). Although it is conceivable that the behavioral responses of our BY mice reflect “imitative” or “mimicry” behavior (45) rather than “empathy,” several findings suggest that at least for the social transfer of pain and analgesia, the BY mice manifest changes in their behavior because they are experiencing an altered sensory-affective state. Importantly, the BY mice were tested using several different behavioral assays and did not always have direct visual access to their social partners during testing. In addition, control mice spend more time interacting with a BY mouse that had recently been exposed to a CFA mouse than with another control mouse, indicating that the BY mouse is in an altered affective state that is sufficient to attract control mice. Finally, as mentioned above, the social transfer of pain can be generated by bedding (9), which provides no opportunity for imitative or mimicry-like behavior.
The behavioral protocols we established are relatively easy to implement and generate multifaceted empathy-like behavior in mice, a species that offers many advantages for rapid, direct, and highly-specific manipulation of neural circuit activity. Mechanistic findings from rodents and other experimentally accessible species provide new hypotheses that researchers studying human empathy can explore using tools such as neuromodulation methods and brain imaging. Advancing our understanding of the evolutionarily conserved brain mechanisms of empathy will, hopefully, also expedite the development of novel interventions that promote the empathic social interactions that the world, apparently, desperately needs.
Supplementary Material
Acknowledgments:
We thank the Luo lab for generously providing the TRAP2 breeding pairs; Max Lenail for contributing to the cell counting in the TRAP2 analysis; the Sudhof lab for access to fear conditioning equipment; Brandon Bentzley for aid in modifying the behavioral chambers used in the social transfer of fear experiments.
Funding: M.L.S. was supported by the National Institute on Drug Abuse (T32DA035165-06) and a Stanford University School of Medicine Deans Fellowship.
Footnotes
Competing interests: R.C.M. is a co-founder and scientific advisor of MapLight Therapeutics. He is also on the scientific advisory board of Cerevance, Inc., The Brave Neuroscience Co., AZ Therapies, and Cognition Therapeutics.
Data and materials availability: All data are available in the main text or the supplementary materials. Additional data are stored on google drive as well as on external hard drives (property of the Malenka lab at Stanford University) and may be requested from the authors.
Supplementary Materials:
Materials and Methods
Figures S1-S6
Table S1
References (46, 47)
References and Notes
- 1.Hoffman ML, Developmental synthesis of affect and cognition and its implications for altruistic motivation. Develop. Psychol 11, 607–622 (1975). [Google Scholar]
- 2.de Waal FBM, Putting the altruism back into altruism: the evolution of empathy. Annu. Rev. Psychol 59, 279–300 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Panksepp JB, Lahvis GP, Rodent empathy and affective neuroscience. Neurosci. Biobehav. Rev 35, 1864–1875 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sivaselvachandran S, Acland EL, Abdallah S, Martin LJ, Behavioral and mechanistic insight into rodent empathy. Neurosci. Biobehav. Rev 91, 130–137 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Keum S et al. , Variability in empathic fear response among 11 inbred strains of mice. Genes, Brain, Behav 15, 231–242 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Langford DJ et al. , Social modulation of pain as evidence for empathy in mice. Science 312, 1967–1970 (2006). [DOI] [PubMed] [Google Scholar]
- 7.Baptista-de-Souza D et al. , Mice undergoing neuropathic pain induce anxiogenic-like effects and hypernociception in cagemates. Behav. Pharmacol 26, 664–672 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Li Z et al. , Social interaction with a cagemate in pain facilitates subsequent spinal nociception via activation of the medial prefrontal cortex in rats. Pain 7, 1–9 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Smith ML, Hostetler CM, Heinricher MM, Ryabinin AE, Social transfer of pain in mice. Sci. Adv 2, e1600855 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith ML, Walcott AT, Heinricher MM, Ryabinin AE, Anterior cingulate cortex contributes to alcohol withdrawal- Induced and Socially Transferred Hyperalgesia. eNeuro. 4, ENEURO.0087–17.2017 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu Y-F et al. , Social interaction with a cagemate in pain increases allogrooming and induces pain hypersensitivity in the observer rats. Neurosci. Lett 662, 385–388 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Kim A, Keum S, Shin H-S, Observational fear behavior in rodents as a model for empathy. Genes, Brain, Behav 18, e12521 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Burkett JP et al. , Oxytocin-dependent consolation behavior in rodents. Science 351, 375–378 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ueno H et al. , Helping-Like Behaviour in Mice towards conspecifics constrained inside tubes. Science Rep. 9, 1–11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartal IB-A, Decety J, Mason P, Empathy and pro-social behavior in rats. Science 334, 1427–1430 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamm C, Decety J, Singer T, Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage 54, 2492–2502 (2011). [DOI] [PubMed] [Google Scholar]
- 17.Timmers I et al. , Is empathy for pain unique in its neural correlates? A meta-analysis of neuroimaging studies of empathy. Front. Behav. Neurosci 12, 289 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson PL, Brunet E, Meltzoff AN, Decety J, Empathy examined through the neural mechanisms involved in imagining how I feel versus how you feel pain. Neuropsychologia 44, 752–761 (2006). [DOI] [PubMed] [Google Scholar]
- 19.Jeon D et al. , Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nature Neurosci. 13, 482–488 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carrillo M et al. , Emotional mirror neurons in the rat’s anterior cingulate cortex. Curr Biol. 29, 1301–1312.e6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fillinger C, Yalcin I, Barrot M, Veinante P, Efferents of anterior cingulate areas 24a and 24b and midcingulate areas 24aʹ and 24bʹ in the mouse. Brain Struct. Funct 223, 1747–1778 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Jackson PL, Meltzoff AN, Decety J, How do we perceive the pain of others? A window into the neural processes involved in empathy. NeuroImage. 24, 771–779 (2005). [DOI] [PubMed] [Google Scholar]
- 23.Stevens FL, Hurley RA, Taber KH, Anterior cingulate cortex: unique role in cognition and emotion. J. Neuropsych. Clin. Neurosci 23, 121–125 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Larson AA, Brown DR, el-Atrash S, Walser MM, Pain threshold changes in adjuvant-induced inflammation: a possible model of chronic pain in the mouse. Pharmacol. Biochem. Behav 24, 49–53 (1986). [DOI] [PubMed] [Google Scholar]
- 25.Ren K, Dubner R, Inflammatory models of pain and hyperalgesia. ILAR J. 40, 111–118 (1999). [DOI] [PubMed] [Google Scholar]
- 26.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL, Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Meth 53, 55–63 (1994). [DOI] [PubMed] [Google Scholar]
- 27.Ferretti V et al. , Oxytocin signaling in the central amygdala modulates emotion discrimination in mice. Curr. Biol 29, 1938–1953.e6 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Guenthner CJ, Miyamichi K, Yang HH, Heller HC, Luo L, Permanent genetic access to transiently active neurons via TRAP: targeted recombination in active populations. Neuron 78, 773–784 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allen WE et al. , Thirst-associated preoptic neurons encode an aversive motivational drive. Science 357, 1149–1155 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Apps MAJ, Rushworth MFS, Chang SWC, The anterior cingulate gyrus and social cognition: Tracking the motivation of others. Neuron 90, 692–707 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walsh JJ et al. , 5-HT release in nucleus accumbens rescues social deficits in mouse autism model. Nature 560, 589–594 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hung LW et al. , Gating of social reward by oxytocin in the ventral tegmental area. Science 357, 1406–1411 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Apps MAJ, Lockwood PL, Balsters JH, The role of the midcingulate cortex in monitoring others’ decisions. Front. Neurosci 7, 251 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Callaway EM, Luo L, Monosynaptic circuit tracing with glycoprotein-deleted rabies viruses. J. Neurosci 35, 8979–8985 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim EJ, Jacobs MW, Ito-Cole T, Callaway EM, Improved monosynaptic neural circuit tracing using engineered rabies virus glycoproteins. Cell Rep 15, 692–699 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allsop SA et al. , Corticoamygdala transfer of socially derived information gates observational learning. Cell. 173, 1329–1342.e18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Waal FBM, Preston SD, Mammalian empathy: behavioural manifestations and neural basis. Nat. Rev. Neurosci 18, 498–509 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Watanabe M, Narita M, Brain reward circuit and pain. Adv. Exp. Med. Biol 1099, 201–210 (2018). [DOI] [PubMed] [Google Scholar]
- 39.Harris HN, Peng YB, Evidence and explanation for the involvement of the nucleus accumbens in pain processing. Neural Regen. Res 15, 597–605 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klawonn AM, Malenka RC, Nucleus accumbens modulation in reward and aversion. Cold Spring Harb. Symp. Quant. Biol 83, 119–129 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwartz N et al. , Decreased motivation during chronic pain requires long-term depression in the nucleus accumbens. Science. 345, 535–542 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singer T et al. , Empathy for pain involves the affective but not sensory components of pain. Science. 303, 1157–1162 (2004). [DOI] [PubMed] [Google Scholar]
- 43.Preston SD, de Waal FBM, Empathy: Its ultimate and proximate bases. Behav. Brain Sci 25, 1–20 (2002). [DOI] [PubMed] [Google Scholar]
- 44.Meyza KZ, Bartal IB-A, Monfils MH, Panksepp JB, Knapska E, The roots of empathy: Through the lens of rodent models. Neurosci. Biobehav. Rev 76, 216–234 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ueno H, et al. , Conformity-like behaviour in mice observing the freezing of other mice: a model of empathy. BMC Neurosci. 21:19 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beier KT et al. , Rabies screen reveals GPe control of cocaine-triggered plasticity. Nature. 549, 345–350 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paxinos G, Franklin KBJ, Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates (Academic Press, 2012). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


