Abstract
Advanced brain aging is commonly regarded as a risk factor for neurodegenerative diseases, for example, Alzheimer’s dementia, and it was suggested that sleep disorders such as obstructive sleep apnea (OSA) are significantly contributing factors to these neurodegenerative processes. To determine the association between OSA and advanced brain aging, we investigated the specific effect of two indices quantifying OSA, namely the apnea–hypopnea index (AHI) and the oxygen desaturation index (ODI), on brain age, a score quantifying age-related brain patterns in 169 brain regions, using magnetic resonance imaging and overnight polysomnography data from 690 participants (48.8% women, mean age 52.5 ± 13.4 years) of the Study of Health in Pomerania. We additionally investigated the mediating effect of subclinical inflammation parameters on these associations via a causal mediation analysis. AHI and ODI were both positively associated with brain age (AHI std. effect [95% CI]: 0.07 [0.03; 0.12], p-value: 0.002; ODI std. effect [95% CI]: 0.09 [0.04; 0.13], p-value: < 0.0003). The effects remained stable in the presence of various confounders such as diabetes and were partially mediated by the white blood cell count, indicating a subclinical inflammation process. Our results reveal an association between OSA and brain age, indicating subtle but widespread age-related changes in regional brain structures, in one of the largest general population studies to date, warranting further examination of OSA in the prevention of neurodegenerative diseases.
Keywords: obstructive sleep apnea, brain age, SHIP-Trend, brain atrophy, dementia
Statement of Significance.
Accelerated brain aging is commonly regarded as a risk factor for neurodegenerative diseases such as dementia and it has been suggested that sleep disorders are significantly contributing to these processes. Using polysomnography and magnetic resonance imaging data from 690 individuals of the SHIP-Trend large-scale population study, Weihs et al. show an association between obstructive sleep apnea and brain age, a score quantifying age-related brain patterns, which is partially mediated by subclinical inflammation parameters. There is a high demand to identify treatable conditions that facilitate the development of neurodegenerative diseases and OSA is a valuable candidate within this research field.
Introduction
Advanced brain aging is commonly considered to be a risk factor for neurodegenerative diseases like Alzheimer’s dementia [1, 2], whose prevalence in Europe is 0.6% for subjects aged between 60 and 64 years, increasing to 46.8% in subjects aged above 90 [3]. It is commonly accepted that neurodegeneration starts decades before the clinical manifestation of the disease, with possible contributing factors including age, hypertension, diabetes, obesity, or traumatic brain injury [4]. Previous studies have suggested that lack of sleep and sleep disorders, especially hypoxia and obstructive sleep apnea (OSA), might contribute to neurodegenerative diseases [5–7].
OSA is a common form of chronic sleep-disordered breathing affecting around 1/7th of the world’s adult population [8] and is characterized by recurrent complete or partial upper airway obstruction during sleep [9]. The disorder often lacks symptoms and can lead to systemic hypertension, coronary heart diseases, obesity, or cognitive impairment [5, 6, 10]. The gold standard for diagnosing OSA is an overnight polysomnography (PSG) that provides objective characteristics of sleeping behavior including the two commonly used measures to quantify the severity of OSA: (1) the apnea–hypopnea index (AHI), the standard parameter used to diagnose OSA, which combines multiple characteristics such as absence of airflow, reduction of airflow accompanied by oxygen desaturations or arousals and (2) the oxygen desaturation index (ODI), which, unlike AHI, focuses on the drop in oxygen levels and can therefore be interpreted as the clean oxygen desaturation component of OSA [9, 11, 12].
In clinical samples, OSA is associated with structural brain alterations, such as extensive alterations in white matter regions [13, 14] or reductions in gray matter areas [15–17], both being linked with the aging brain [18–20]. Limitations in these studies are the relatively high load of comorbid disorders like metabolic disorders or hypertension and obesity in patients with moderate to severe OSA, all associated with structural brain changes themselves [1, 2, 21, 22], as well as the small sample sizes ranging only from 16 [17] to 60 [16] subjects.
Gaining a deeper insight into the impact of OSA on the brain as a whole is of the utmost importance to understand age-related brain changes and hence enable us to combat age-related disorders such as dementia [23]. Using subjects from the Study of Health in Pomerania (SHIP-Trend baseline) general population study [24], we, therefore, investigated the association between OSA and three MRI-based indices assessing brain aging, namely total brain volume, total gray matter volume, and brain age [25], a global brain score similar to the one used in Habes et al.[1] and Janowitz et al.[26]. While the first two are univariate measures quantifying age-related changes in the brain as a whole [19] and the latter is calculated using typical age-related brain atrophy patterns and increases in ventricle volumes and is therefore more sensitive to subtle but widespread deviations in regional brain structures [27]. We further looked at local changes caused by OSA using a voxel-based morphometry analysis and addressed the putative mediating role of inflammatory markers between OSA and brain aging.
Methods
Participants in SHIP-Trend
We included all subjects from the baseline examination of the SHIP-Trend study [24], who had complete data sets (N = 690). The sample is a subset of 8,016 subjects randomly drawn from the general adult population living in Western Pomerania in Germany in September 2008, with the data collection of 4,420 subjects concluding in September 2012. After excluding subjects who refused participation or who fulfilled exclusion criteria (e.g. presence of a pacemaker), N = 2,154 participants volunteered for an MRI scanning [28] and, after inviting all subjects participating in the SHIP-Trend study, N = 1,264 individuals underwent PSG assessment, with the main reason for nonparticipation being the requirement of staying overnight [9]. Of those, 703 participants passed both the PSG and MRI quality control and a further 13 subjects had to be excluded due to missing covariable data, leaving 690 subjects in the analysis sample (Supplementary Figure S1). For the causal mediation analysis only, another 23 samples were excluded from the high-sensitive C-reactive protein (hsCRP) analysis, one from the white blood cell (WBC) count analysis and five from the fibrinogen analysis due to missing data. An overview of the included and excluded samples can be seen in Supplementary Figure S1 and Table 1. All participants gave written informed consent and the Ethics Committee of the University Medicine Greifswald approved the SHIP-Trend study.
Table 1.
Baseline characteristics
| Study sample (N = 690) | Excluded SHIP-trend sample (N = 3,730) | P-value (test-statistic) | |
|---|---|---|---|
| Age, mean (SD), years | 52.5 (13.4) | 51.5 (14.3) | 0.1 (1.64)a |
| Gender, N (%), female | 337 (48.8) | 1,938 (52.0) | 0.14 (2.14)b |
| Body size, mean (SD), cm | 170.45 (9.01) | 169.71 (9.44) | 0.049 (1.97)a |
| Waist-height ratio, mean (SD) | 0.53 (0.07) | 0.54 (0.09) | 0.03 (−2.13)a |
| Intracranial volume, mean (SD), mm3 | 1,585,832 (162,128.6) | 1,593,723 (157,411.3) | 0.29 (−1.06)a |
| Disorders | |||
| Diabetes, N (%), yes | 66 (9.57) | 483 (13.0) | 0.02 (5.93)b |
| Hypertension, N (%), yes | 308 (44.6) | 1,815 (48.9) | 0.045 (4.03)b |
| Lifetime depression, N (%), yes | 215 (31.2) | 1,008 (27.8) | 0.08 (3.06)b |
| HbA1c, mean (SD), % | 5.35 (0.75) | 5.38 (0.86) | 0.47 (−0.72)a |
| Systolic blood pressure, mean (SD), mmHg | 126.54 (16.5) | 128.26 (19.1) | 0.01 (−2.45)a |
| Diastolic blood pressure, mean (SD), mmHg | 77.37 (9.5) | 77.27 (10.4) | 0.8 (0.25)a |
| Smoking | <0.001 (33.03)b | ||
| Never smoked, N (%) | 292 (42.3) | 1,313 (35.4) | |
| Ex-smoker, N (%) | 273 (39.6) | 1,337 (36.1) | |
| Current smoker, N (%) | 125 (18.1) | 1,058 (28.5) | |
| Avg. alcohol consumption per day, mean (SD), g/day | 8.88 (11.9) | 8.37 (13.7) | <0.001 (1,390,222)c |
| Triglyceride, mean (SD), mmol/L | 1.57 (1.08) | 1.69 (1.25) | 0.004 (1,196,620)c |
| HDL-C, mean (SD), mmol/L | 1.44 (0.36) | 1.43 (0.39) | 0.9 (0.12)a |
| LDL-C, mean (SD), mmol/L | 3.44 (0.93) | 3.33 (0.97) | 0.005 (2.82)a |
| Educational attainment | <0.001 (65.88)b | ||
| <10 years, N (%) | 93 (13.5) | 936 (25.2) | |
| 10 years, N (%) | 355 (51.5) | 1,913 (51.5) | |
| >10 years, N (%) | 242 (35.1) | 868 (23.4) | |
| Physical activity, mean (SD) | 3.57 (2.12) | 3.14 (2.19) | <0.001 (1,422,620)c |
This table shows the baseline characteristics of the study sample and the remaining data in the excluded sample. The p-value shows the results of the comparison of the two sets. HbA1c, glycated hemoglobin A1c; HDL-C, high-density cholesterol; LDL-C, low-density cholesterol.
a t-test.
bChi-squared test.
cWilcoxon-test.
Data assessment in SHIP-Trend
The clinical data were collected using a computer-assisted face-to-face interview, recording, among others, smoking status, educational attainment, alcohol consumption [29], physical activity, and the participants’ medication. All subjects underwent medical examinations in order to assess their waist-height ratio, blood pressure [24], glycated hemoglobin A1c (HbA1c), blood sugar levels, high-density cholesterol (HDL-C), low-density cholesterol (LDL-C), triglyceride and hsCRP, WBC, and fibrinogen concentration. Details can be found in Supplementary Method S1, as can the definitions of hypertension [30], diabetes [31, 32], and lifetime depression [33].
OSA parameter assessment
The PSG examination was carried out as described by Fietze et al. [9] and the evaluation of respiratory events, arousals, and sleep stages was performed according to AASM 2007 criteria by trained PSG scorers. AHI was defined as the average number of apnea (peak signal excursion drop by ≥90% for at least 10 s with at least 90% of the duration showing such amplitude reduction) and hypopnea events (either a flow drop of ≥30% for at least 10 s with an oxygen desaturation of ≥4% or a flow drop of ≥50% with an oxygen desaturation of ≥3% or an arousal) per hour of sleep [9]. ODI was defined as the average number of drops in arterial oxygen saturations ≥3% per hour of sleep [34].
MRI acquisition and image processing
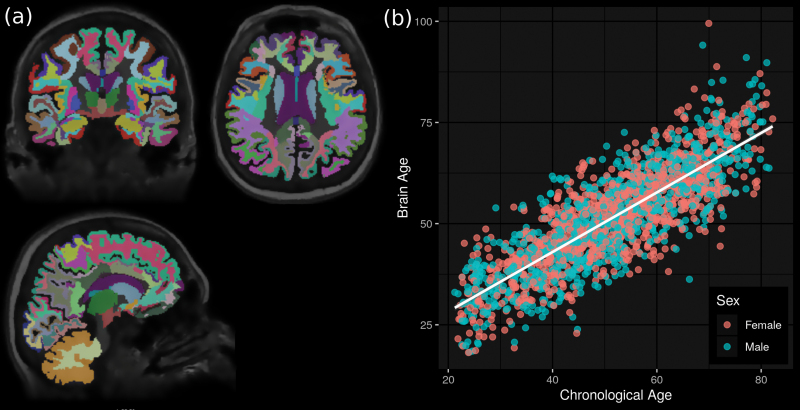
We considered head T1-weighted MRI (1.5T Magnetom Avanto scanner, Siemens, Erlangen, Germany) [35] using the following set of parameters: orientation = axial plane; repetition time = 1,900 ms; echo time = 3.37 ms; flip angle = 15°; slice thickness = 1 mm; resolution = 1 mm × 1 mm. After removing 103 subjects, 3 due to strong frontal darkening in their scans and 100 due to the presence of structural abnormalities (e.g. tumors, cysts) or cases of cerebral strokes, we ran the images through an image segmentation pipeline using the FreeSurfer image analysis suite (version 5.3, http://surfer.nmr.mgh.havard.edu), which resulted in the removal of an additional 81 subjects (4 failed the pipeline and 77 failed QC). The pipeline included the removal of non-brain tissue using a hybrid watershed/surface deformation procedure [36], automated Talairach transformation, segmentation of subcortical white matter and deep gray matter volumetric structures (including hippocampus, amygdala, caudate, putamen, and ventricles) [37–39], intensity normalization [40], tessellation of gray matter–white matter boundary, automated topology correction [41, 42], and surface deformation following intensity gradients to optimally place gray/white and gray/cerebrospinal fluid borders at the location where the greatest shift in intensity defines the transition to the other tissue class [43–45]. The individual images were then registered onto a spherical atlas based on individual cortical folding patterns, in order to match cortical geometry across subjects [46]. Next, the cerebral cortex was parceled into 68 units with respect to gyral and sulcal structures [47, 48] and the cortical white matters, that is, white matter up to 5 mm below the gray matter boundary, into 68 units by assigning each white matter voxel the label of the closest cortical voxel [20]. Although being part of the standard FreeSurfer output, we excluded the 5th ventricle because it was not detected in all scans (zero volume), the brain stem and the optic chiasm, leading to a total 169 out of 172 regions of gray matter, white matter and the ventricular system being used to estimate brain age (Figure 1, A and Supplementary Method S2). At this stage, FreeSurfer also measured the total intracranial volume.
Figure 1.
Overview of the brain age estimation. (A) Each individual’s brain was segmented into distinct cortical and subcortical regions using the automated image processing pipeline FreeSurfer. (B) Brain age was then estimated based on the volumes of these regions.
Statistical quality control was performed by first regressing out age, gender, and intracranial volume for each brain region separately, and then rejecting those cases where the residual was more than five standard deviations away from the whole-sample mean.
Additionally to brain age, we considered two global brain measurements, namely the total brain volume (the volume of all brain voxels neither belonging to the brain stem, ventricles, cerebrospinal fluid nor choroid plexus) and total gray matter volume (the volume of voxels belonging to the cortex, gray matter of the cerebellum and subcortical gray matter, including thalamus, caudate, putamen, pallidum, hippocampus, amygdala, nucleus accumbens, ventral diencephalon, and substantia nigra, if there).
Brain age estimation
Aging is accompanied by spatially heterogeneous atrophy of gray matter [18, 19], white matter [20], and increases in ventricle volumes [19] with age-related changes in regional brain volumes being stronger in males than females [49]. Brain age was therefore calculated using gender-stratified ordinary least squares regression, with chronological age as the dependent variable and the volumes of the 169 brain regions as the independent variables. More specifically, the brain age of an individual was defined as his/her predicted age using a model based on all 169 regional brain volumes from the remaining individuals of the same gender (=gender-stratified leave-one-out cross-validation). Similar approaches have been successfully applied to MRI [2, 50–53] and metabolic data [54], among others. Instead of looking at the differences between brain age and chronological age as done by, for example, Cole et al. [55], which would require additional statistical assumptions, for example, brain age being an unbiased estimator of chronological age across the whole age-range and has been shown to potentially result in false-positive associations [56], we considered the residuals after regressing brain age onto chronological age [25]. Rather than explicitly calculating these residuals, we, following common epidemiological practice, added chronological age as a covariable in all statistical analyses.
Voxel-based morphometry
To identify local areas influenced by OSA, we searched for changes in regional brain volumes by means of a voxel-based morphometry analysis using SPM12 (https://www.fil.ion.ucl.ac.uk/spm/) and the CAT12 toolbox (developed by Christian Gaser, University of Jena, Germany, http://www.neuro.uni-jena.de). The images were bias-corrected, spatially normalized using high-dimensional DARTEL normalization, segmented into the different tissue classes, modulated for nonlinear warping, and affine transformations and smoothed using a Gaussian kernel of 8 mm FWHM. The homogeneity of gray matter images was checked using the covariance structure of each image with all other images (outliers ≥3 standard deviations from the mean), as implemented in the check data quality function in the CAT12 toolbox. To identify regional changes, we conducted an ordinary least squares linear regression adjusted for age, gender, age × gender, and intracranial volume for both AHI and ODI as the independent variables and the individual gray matter within each voxels as the dependent variable.
Statistical analysis
The data were analyzed using R version 3.6 [57] unless stated otherwise. We used ordinary least squares multivariable regression models to test if AHI and ODI are associated with total brain volume, total gray matter volume, and brain age. All models were adjusted for age, gender, age × gender, and intracranial volume, referred to as base covariables (AHI/ODI models: Brain Parameters ~ (AHI or ODI) + Base Covariables), and model outliers were removed after visual inspection of the diagnostic plots of the regression analyses (one sample in the brain age analysis and two samples in the total gray matter volume analysis).
To assess the robustness of the significant associations, we analyzed various confounders suspected to have an impact on the brain parameters (Supplementary Table S1) by comparing the base model (Brain Parameter ~ Base Covariable) to an expanded model (Brain Parameter ~ Confounder + Base Covariables). Confounders for whom the base model’s explained variance changed significantly, were then added one by one to the AHI/ODI models (extended AHI/ODI models: Brain Parameters ~ (AHI or ODI) + Confounder + Base Covariables) to assess the possible confounding effect of these covariables on the effect of AHI and ODI. Hypertension and blood pressure, as well as diabetes and HbA1c were always added in tandem as the blood pressure and HbA1c levels are influenced by the corresponding disorder’s presence.
As OSA and brain aging have been previously associated with inflammation [26, 58, 59], we conducted a causal mediation analysis to study potential mediation effects of hsCRP, WBC, and fibrinogen, as well as WBC subtypes as a secondary explorative sub-analyses, on the significant associations using the popular R package mediation [60]. The mediation and direct effects, as well as their 95% confidence intervals, were estimated via bootstrapping with 100,000 simulations each. Furthermore, a log transformation was performed on all inflammation markers to normalize the distributions [26] and all models were adjusted for the base covariables as well as the intake of anti-inflammatory medication.
We reported all effect sizes other than the ones in the mediation analyses as standardized effect sizes, which were calculated by standardizing all numerical variables (subtracting the mean and dividing by the standard deviation) and refitting the models with the new values. To correct for multiple testing according to Bonferroni, in the initial and main mediation analyses, effects were considered significant if the p-value was <0.017 (0.05/3) and borderline significant if <0.033 (0.1/3), with AHI and ODI being considered as two different test series.
Data availability
The data used in this study can be applied for at the University Medicine Greifswald (https://fvcm.med.uni-greifswald.de/dd_service/data_use_intro.php) or by contacting the corresponding author.
Results
Baseline characteristics of the sample population
We included 690 individuals (Supplementary Figure S1) with 48.8% women, a mean age of 52.5 years (95% CI: [51.5; 53.5]) and an average waist-height ratio of 0.53 (95% CI: [0.53; 0.54]). About 9.57% suffered from diabetes, 44.6% from hypertension, and 31.12% from lifetime depression (Table 1). The mean [95% CI] time difference between the MRI and PSG measurements was 6.43 [−1.04; 13.91] days and 33.68 [25.46; 41.90] days between PSG and clinical data assessment. Regarding the brain outcomes, as expected, total gray matter volume (mean [95% CI]: 612,415 mm3 [607,712; 617,117]) and total brain volume (mean [95% CI]: 1,116,948 mm3 [1,108,214; 1,125,683]) were strongly positively correlated (Pearson’s r: 0.94), while brain age (mean [95% CI]: 52.16 [51.27; 53.05]) was negatively correlated with the two volumetric scores (Pearson’s r total gray matter volume: −0.6, total brain volume: −0.45; Supplementary Figure S2).
Prevalence of oxygen saturation impairment
The mean AHI was 8.2 (95% CI: [7.3; 9.1]) events per hour with, using AHI as a diagnostic criterion, 41.2% of the study population exhibiting mild to severe (AHI ≥ 5), and 6.5% exhibiting severe OSA (AHI ≥ 15) [9]. The mean ODI was 5.8 (95% CI: [5.0; 6.5]) events per hour with 31.6% exhibiting mild to severe (ODI ≥ 5) and 3.9% exhibiting severe OSA (ODI ≥ 15) when using ODI as a diagnostic criterion [61, 62]. Comparing the two parameters, AHI and ODI were strongly positively correlated (Pearson’s r: 0.96; Supplementary Figure S2). These results approximately reflect the OSA prevalence of the whole SHIP-Trend PSG sample [9] (Supplementary Table S2).
OSA parameters are associated with brain age
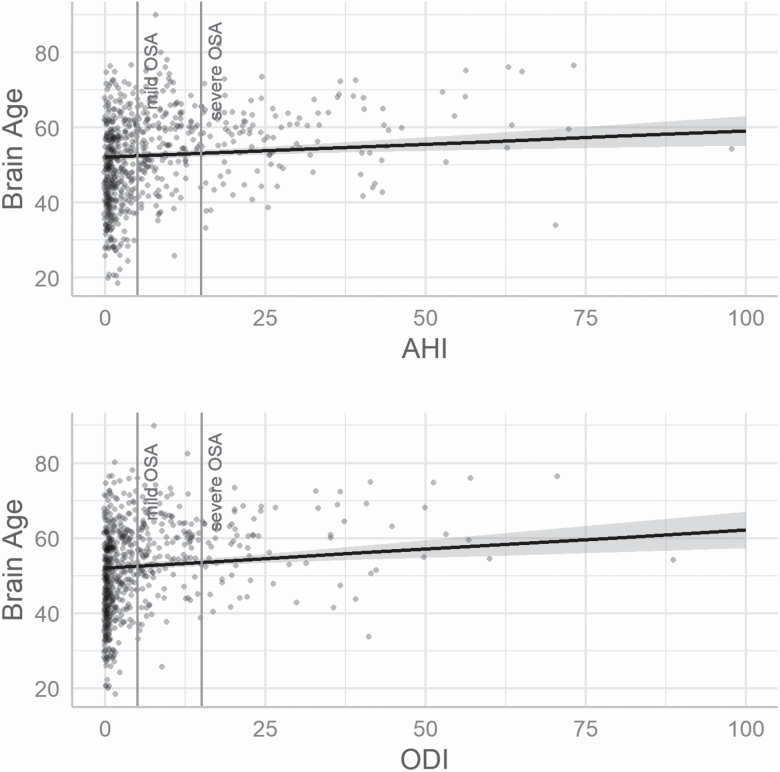
Multivariable regression models adjusted for age, gender, age × gender, and intracranial volume revealed significant positive associations (p-value < 0.017) between brain age and AHI (std. estimate [95% CI]: 0.07 [0.03; 0.12], p-value: 0.002) and brain age and ODI (std. estimate [95% CI]: 0.09 [0.04; 0.13], p-value: < 0.0003). No significant association was found between AHI (std. estimate [95% CI]: −0.02 [−0.04; 0.01], p-value: 0.27) or ODI (std. estimate [95% CI]: −0.02 [−0.05; 0.005], p-value: 0.11) and total brain volume nor between AHI (std. estimate [95% CI]: −0.02 [−0.05; 0.01], p-value: 0.18), or ODI and total gray matter volume (std. estimate [95% CI]: −0.03 [−0.07; 0.0009], p-value: 0.04; Tables 2 and 3 and Figure 2). To assess the robustness of the significant brain age effects, we analyzed the effect of various potential confounders on the base models (Supplementary Table S1). We found that diabetes, HbA1c, waist-height ratio, triglyceride concentration, diastolic blood pressure, educational attainment, alcohol consumption, and % of total sleep time spent in stage 1 nonrapid eye movement sleep had a significant impact on the brain age models (Supplementary Figure S3). After adding these variables one by one to both brain age models, the effect of AHI and ODI remained significant, irrespective of the added extra confounder (Supplementary Table S3).
Table 2.
Associations between brain age, total gray matter volume, or total brain volume and AHI
| Brain age ~ AHI + base covariates | GM volume ~ AHI + base covariates | Brain volume ~ AHI + base covariates | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Std. estimate [95% CI] | P-value | Partial R2 | Std. estimate [95% CI] | P-value | Partial R2 | Std. estimate [95% CI] | P-value | Partial R2 | |
| (Intercept) | 0.05 [−0.02, 0.12] | <0.0003*** | 0.03 [−0.02, 0.07] | <0.0003*** | 0.03 [−0.02, 0.07] | <0.0003*** | |||
| AHI | 0.07 [0.03, 0.12] | 0.002** | 0.02 | −0.02 [−0.05, 0.01] | 0.18 | 0.003 | −0.02 [−0.04, 0.01] | 0.27 | 0.002 |
| Age, years | 0.83 [0.77, 0.89] | <0.0003*** | 0.64 | −0.45 [−0.49, −0.40] | <0.0003*** | 0.46 | −0.29 [−0.33, −0.25] | <0.0003*** | 0.30 |
| Gender, female | −0.09 [−0.20, 0.02] | 0.09 | 0.004 | −0.06 [−0.14, 0.02] | <0.0003*** | 0.02 | −0.07 [−0.13, 0.004] | <0.0003*** | 0.03 |
| ICV, mm3 | −0.09 [−0.14, −0.04] | 0.001** | 0.02 | 0.73 [0.69, 0.77] | <0.0003*** | 0.67 | 0.84 [0.80, 0.87] | <0.0003*** | 0.78 |
| Age × gender | −0.10 [−0.19, −0.016] | 0.02+ | 0.008 | 0.12 [0.06, 0.18] | <0.0003*** | 0.02 | 0.11 [0.06, 0.16] | <0.0003*** | 0.02 |
| R 2 = 0.69 (Adjusted R2 = 0.68) | R 2 = 0.84 (Adjusted R2 = 0.84) | R 2 = 0.88 (Adjusted R2 = 0.88) | |||||||
This table shows the results of the multiple regression models between AHI and the different brain parameters. The effect sizes have been standardized. The base covariables, for which the models have been adjusted are age, gender, age × gender, and intracranial volume. AHI, apnea–hypopnea index; ICV, intracranial volume.
+ p < 0.03.
*p < 0.017.
**p < 0.003.
***p < 0.0003.
Table 3.
Associations between brain age, total gray matter volume or total brain volume, and ODI
| Brain age ~ ODI + base covariates | GM volume ~ ODI + base covariates | Brain volume ~ ODI + base covariates | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Std. estimate [95% CI] | P-value | Partial R2 | Std. estimate [95% CI] | P-value | Partial R2 | Std. estimate [95% CI] | P-value | Partial R2 | |
| (Intercept) | 0.05 [−0.02, 0.11] | <0.0003*** | 0.03 [−0.02, 0.08] | <0.0003*** | 0.03 [−0.01, 0.07] | <0.0003*** | |||
| ODI | 0.09 [0.04, 0.13] | <0.0003 *** | 0.02 | −0.03 [−0.07, −0.0009] | 0.04 | 0.006 | −0.02 [−0.05, 0.005] | 0.11 | 0.004 |
| Age, years | 0.83 [0.77, 0.89] | <0.0003 *** | 0.64 | −0.44 [−0.49, −0.40] | <0.0003*** | 0.47 | −0.29 [−0.33, −0.25] | <0.0003*** | 0.3 |
| Gender, female | −0.09 [−0.20, 0.02] | 0.09 | 0.004 | −0.07 [−0.14, 0.01] | <0.0003*** | 0.02 | −0.07 [−0.14, 0.0003] | <0.0003*** | 0.03 |
| ICV, mm3 | −0.09 [−0.14, −0.03] | 0.002 ** | 0.02 | 0.73 [0.69, 0.77] | <0.0003*** | 0.67 | 0.83 [0.80, 0.87] | <0.0003*** | 0.78 |
| Age × Gender | −0.10 [−0.18, −0.02] | 0.021+ | 0.008 | 0.11 [0.05, 0.18] | <0.0003*** | 0.02 | 0.11 [0.06, 0.16] | <0.0003*** | 0.02 |
| R 2 = 0.69 (Adjusted R2 = 0.69) | R 2 = 0.84 (Adjusted R2 = 0.84) | R 2 = 0.88 (Adjusted R2 = 0.88) | |||||||
The table shows the results of the multiple regression models between ODI and the different brain parameters. The effect sizes have been standardized. The base covariables, for which the models have been adjusted are age, gender, age × gender, and intracranial volume. ODI, oxygen desaturation index; ICV, intracranial volume.
+ p < 0.03.
*p < 0.017.
**p < 0.003.
***p < 0.0003.
Figure 2.
Predicted brain age and gray matter volume values. The values were predicted after adjusting for the base covariates. For the prediction, the base covariables were fixed at age = 52.49 years, gender = male and intracranial volume = 1,585,949.67 mm3. These values correspond to the mean values in the data set. Mild OSA corresponds to an AHI or ODI value below 5 and severe OSA corresponds to an AHI or ODI value below 15. AHI, apnea–hypopnea index; ODI, oxygen desaturation index; OSA, obstructive sleep apnea.
Descriptive voxel-based morphological maps
No family-wise error corrected significant voxels could be identified in the voxel-based morphometry analysis. More details can be found in Supplementary Tables S4 and S5.
Inflammation parameters mediate the OSA parameters’ effects on brain age
We tested for possible mediation effects of the subclinical inflammation parameters hsCRP, WBC, and fibrinogen, on the significant associations between OSA and brain age, while adjusting for the base covariables. WBC significantly mediated the effect of AHI (average causal mediation effect [95% CI]: 0.01 [0.005; 0.03], p-value: 0.0007, proportion mediated: 0.19) and ODI (average causal mediation effect [95% CI]: 0.02 [0.006; 0.03], p-value: 0.007, proportion mediated: 0.17). No mediation effect was found for fibrinogen or hsCRP (Supplementary Tables S6–S8). In the exploratory analyses of WBC subtypes, we found that monocytes and lymphocyte concentration mediated the effects in both the AHI and ODI brain age models (Supplementary Tables S9–S13).
Discussion
OSA is a disorder affecting roughly 1/7th of the world’s adult population [8] and was shown to be implicated in neurodegenerative diseases like Alzheimer’s dementia [5, 6]. While there are several small-scale studies analyzing associations between OSA and brain subsections [13–16, 63, 64], none have, to our knowledge, analyzed the relationship between OSA and the brain as a whole.
In the current study, we aimed to fill this gap by investigating, next to total brain volume and total gray matter volume, possible associations between two parameters quantifying OSA, namely the classically used AHI and ODI, which solely describes oxygen desaturation, and brain age, a score specifically quantifying age-related brain changes, in the large-scale population-based SHIP-Trend study. We have found significant positive associations between both AHI and ODI and brain age, but found no effects of AHI or ODI on total gray matter volume nor on total brain volume. Considering, that total gray matter volume and total brain volume provide univariate volumetric measures that quantify global age-related changes and that brain age is more sensitive in detecting and quantifying subtle but widespread deviations in regional brain structures [27], this result indicates that OSA may not affect the brain in a global unspecific manner, but rather follows age-related regional pathways of brain atrophy.
Furthermore, we found that the significant effects of the two OSA parameters on brain age were not altered by the presence of additional factors that independently influence brain age. Diabetes and waist-height ratio had the biggest impact on the AHI and ODI effects and, interestingly, have been related to mechanisms potentially causing these associations: oxidative stress, chronic brain inflammation, or brain damage caused by repeated oxygen deficiency [65–67].
Oxidative stress and chronic inflammation have been observed in diabetes patients [65] and both have been associated with OSA [59]. Oxidative stress, an imbalance in the redox status between the production and removal of reactive oxygen species, is thought to play a role in the development of syndromes like Parkinson’s and Alzheimer’s disease [68] and was suggested to be responsible for the age-related gradual decline of brain function [68]. In animal models, oxidative stress was linked to chronic inflammation [59], which is involved in neural cell death in neurological diseases [68]. We therefore performed a causal mediation analysis to test if hsCRP, WBC, or fibrinogen concentration mediate the effect of AHI/ODI on brain age. While hsCRP and fibrinogen showed no significant mediation effects after correcting for multiple testing, WBC was found to significantly mediate both effects.
Another potential mechanism is the energy imbalance in the brain cells due to the repeated lack of oxygen resulting in cell swelling and, in extreme cases, a cytotoxic edema [69]. Animal models exposed to OSA-like breathing events exhibited cellular injury in the hippocampus, anterior fornix, regions throughout the thalamus and forebrain, the brainstem, frontal cortex, cerebral Purkinje cells, and deep nuclei. While some damage can be recovered during waking periods, repeated nocturnal apneic events would likely lead to progressive pathological changes [13, 69].
This study has some limitations: (1) Only approximately one third of the SHIP-Trend population underwent a PSG and half an MRI examination, possibly leading to a selection bias. Comparing the study sample with the remaining SHIP-Trend sample revealed that the study sample was slightly healthier and more educated. This was also observed by Fietze et al. [9] in the whole PSG data set, who concluded that selection bias may not be that critical. (2) The PSG measurements have only been performed over one night. Multiple studies have shown night-to-night variations in PSG-based assessments of OSA caused by alternating episodes of stable breathing and episodes of obstructive apnea and hypopneas during the night, suggesting that an OSA assessment should always be based on multi-night measurements [62, 70]. This could not be performed in our setting and it should be noted that one-night PSG measurements are common practice in clinical and research settings [15, 71, 72]. (3) Only cross-sectional and not longitudinal data were used in this study, limiting the interpretation of the results. While longitudinal PSG or MRI data were unfortunately not yet available, further analyses in this regard are planned in future. (4) OSA diagnoses and treatments prior to the study participation were not recorded, possibly affecting the estimated brain age. However, due to a small diagnosis rate of OSA of only 20%, a putative treatment for OSA is unlikely to have confounded the results [9]. Moreover, an unknown OSA treatment, especially surgical interventions, in some participants would have led to an underestimation of the true effect sizes rather than to false-positive associations.
In conclusion, our study shows a positive association between OSA and brain age, in one of the largest population-based samples with both MRT and PSG data. The relationship remained stable even with the introduction of covariables suspected of having an effect on the brain. We have additionally found that these effects are mediated by the systemic inflammation parameter WBC. These results indicate that OSA might be a worthwhile study target combatting neurodegenerative diseases.
Supplementary Material
Disclosure statement
Financial disclosure: None
Funding
The Study of Health in Pomerania (SHIP) is part of the Community Medicine Research net (CMR) (http://www.medizin.uni-greifswald.de/icm) of the University Medicine Greifswald, which is supported by the German Federal State of Mecklenburg-West Pomerania. MRI scans in SHIP and SHIP-TREND have been supported by a joint grant from Siemens Healthineers, Erlangen, Germany and the Federal State of Mecklenburg-West Pomerania. PSG assessment was in part supported by the German RLS organization (Deutsche Restless Legs Vereinigung). This study was further supported by EU Joint Programme-Neurodegenerative Disease Research funding for BRIDGET (grant number: 01ED1615) and by National Institutes of Health (NIH, grant number: AG059421). This work specifically was supported by the Deutsche Forschungsgemeinschaft (DFG, grant number: GR 1912/13-1). HJG has received travel grants and speakers honoraria from Fresenius Medical Care, Neuraxpharm, Servier, and Janssen Cilag as well as research funding from Fresenius Medical Care.
Nonfinancial disclosures: none.
References
- 1. Habes M, et al. White matter hyperintensities and imaging patterns of brain ageing in the general population. Brain. 2016;139(Pt 4):1164–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Habes M, et al. Advanced brain aging: relationship with epidemiologic and genetic risk factors, and overlap with Alzheimer disease atrophy patterns. Transl Psychiatry. 2016;6:e775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alzheimer Europe. Dementia in Europe Yearbook 2019 Estimating the prevalence of dementia in Europe. In: Georges J, Miller O, Bintener C, eds. Dementia in Europe yearbooks. Luxembourg: Alzheimer Europe; 2020: 108. [Google Scholar]
- 4. Daulatzai MA. Cerebral hypoperfusion and glucose hypometabolism: key pathophysiological modulators promote neurodegeneration, cognitive impairment, and Alzheimer’s disease. J Neurosci Res. 2017;95(4):943–972. [DOI] [PubMed] [Google Scholar]
- 5. Gosselin N, et al. Obstructive sleep apnea and the risk of cognitive decline in older adults. Am J Respir Crit Care Med. 2019;199(2):142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yaffe K, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306(6):613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shokri-Kojori E, et al. β-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc Natl Acad Sci USA. 2018;115(17):4483–4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Benjafield AV, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7(8):687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fietze I, et al. Prevalence and association analysis of obstructive sleep apnea with gender and age differences—results of SHIP-trend. J Sleep Res. 2019;28(5):e12770. [DOI] [PubMed] [Google Scholar]
- 10. May AM, et al. Obstructive sleep apnea: role of intermittent hypoxia and inflammation. Semin Respir Crit Care Med. 2014;35(5):531–544. [DOI] [PubMed] [Google Scholar]
- 11. Netzer N, et al. Overnight pulse oximetry for sleep-disordered breathing in adults: a review. Chest. 2001;120(2):625–633. [DOI] [PubMed] [Google Scholar]
- 12. Oeverland B, et al. Pulseoximetry: sufficient to diagnose severe sleep apnea. Sleep Med. 2002;3(2):133–138. [DOI] [PubMed] [Google Scholar]
- 13. Macey PM, et al. Brain structural changes in obstructive sleep apnea. Sleep. 2008;31(7):967–977. [PMC free article] [PubMed] [Google Scholar]
- 14. Tummala S, et al. Associations between brain white matter integrity and disease severity in obstructive sleep apnea. J Neurosci Res. 2016;94(10):915–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Joo EY, et al. Reduced brain gray matter concentration in patients with obstructive sleep apnea syndrome. Sleep. 2010;33(2):235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morrell MJ, et al. Changes in brain morphology in patients with obstructive sleep apnoea. Thorax. 2010;65(10):908–914. [DOI] [PubMed] [Google Scholar]
- 17. Torelli F, et al. Cognitive profile and brain morphological changes in obstructive sleep apnea. Neuroimage. 2011;54(2):787–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fox NC, et al. Imaging cerebral atrophy: normal ageing to Alzheimer’s disease. Lancet. 2004;363(9406):392–394. [DOI] [PubMed] [Google Scholar]
- 19. Resnick SM, et al. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci. 2003;23(8):3295–3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Salat DH, et al. Regional white matter volume differences in nondemented aging and Alzheimer’s disease. Neuroimage. 2009;44(4):1247–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Meissner A. Hypertension and the brain: a risk factor for more than heart disease. Cerebrovasc Dis. 2016;42(3–4):255–262. [DOI] [PubMed] [Google Scholar]
- 22. Yoon S, et al. Brain changes in overweight/obese and normal-weight adults with type 2 diabetes mellitus. Diabetologia. 2017;60(7):1207–1217. [DOI] [PubMed] [Google Scholar]
- 23. Peters R. Ageing and the brain. Postgrad Med J. 2006;82(964):84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Völzke H, et al. Cohort profile: the study of health in Pomerania. Int J Epidemiol. 2011;40(2):294–307. [DOI] [PubMed] [Google Scholar]
- 25. Hertel J, et al. The informative error: a framework for the construction of individualized phenotypes. Stat Methods Med Res. 2019;28(5):1427–1438. [DOI] [PubMed] [Google Scholar]
- 26. Janowitz D, et al. Inflammatory markers and imaging patterns of advanced brain aging in the general population. Brain Imaging Behav. 2020;14(4):1108–1117. doi: 10.1007/s11682-019-00058-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Franke K, et al. Ten years of BrainAGE as a neuroimaging biomarker of brain aging: what insights have we gained? Front Neurol. 2019;10:789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Grabe HJ, et al. Alexithymia and brain gray matter volumes in a general population sample. Hum Brain Mapp. 2014;35(12):5932–5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baumeister SE, et al. Riskanter Alkoholkonsum und alkoholbezogene Störungen in Vorpommern: die Studie “Leben und Gesundheit in Vorpommern” (SHIP) und der Bundesgesundheitssurvey 1998 im Vergleich. Gesundheitswesen. 2005;67(01):39–47. [DOI] [PubMed] [Google Scholar]
- 30. Guidelines Subcommittee. World Health Organization-International Society of Hypertension Guidelines for the management of hypertension. Guidelines Subcommittee. J Hypertens. 1999;17(2):151–183. [PubMed] [Google Scholar]
- 31. World Health Organization, International Diabetes Federation. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia. Geneva, Switzerland: World Health Organization; 2006: 50. [Google Scholar]
- 32. International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32(7):1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Grabe HJ, et al. Association of mental distress with health care utilization and costs: a 5-year observation in a general population. Soc Psychiatry Psychiatr Epidemiol. 2009;44(10):835–844. [DOI] [PubMed] [Google Scholar]
- 34. Berry RB, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. J Clin Sleep Med. 2012;8(5):597–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hegenscheid K, et al. Whole-body magnetic resonance imaging of healthy volunteers: pilot study results from the population-based SHIP study. Rofo. 2009;181(8):748–759. [DOI] [PubMed] [Google Scholar]
- 36. Ségonne F, et al. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22(3):1060–1075. [DOI] [PubMed] [Google Scholar]
- 37. Fischl B, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. [DOI] [PubMed] [Google Scholar]
- 38. Fischl B, et al. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23(suppl. 1):69–84. [DOI] [PubMed] [Google Scholar]
- 39. Han X, et al. Atlas renormalization for improved brain MR image segmentation across scanner platforms. IEEE Trans Med Imaging. 2007;26(4):479–486. [DOI] [PubMed] [Google Scholar]
- 40. Sled JG, et al. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17(1):87–97. [DOI] [PubMed] [Google Scholar]
- 41. Fischl B, et al. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20(1):70–80. [DOI] [PubMed] [Google Scholar]
- 42. Ségonne F, et al. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans Med Imaging. 2007;26(4):518–529. [DOI] [PubMed] [Google Scholar]
- 43. Dale AM, et al. Improved localizadon of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: a linear approach. J Cogn Neurosci. 1993;5(2):162–176. [DOI] [PubMed] [Google Scholar]
- 44. Dale AM, et al. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. [DOI] [PubMed] [Google Scholar]
- 45. Fischl B, et al. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 2000;97(20):11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fischl B, et al. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999;8(4):272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tzourio-Mazoyer N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. [DOI] [PubMed] [Google Scholar]
- 48. Fischl B, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14(1):11–22. [DOI] [PubMed] [Google Scholar]
- 49. Good CD, et al. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14(1 Pt 1):21–36. [DOI] [PubMed] [Google Scholar]
- 50. Franke K, et al. Estimating the age of healthy subjects from T1-weighted MRI scans using kernel methods: exploring the influence of various parameters. Neuroimage. 2010;50(3):883–892. [DOI] [PubMed] [Google Scholar]
- 51. Gaser C, et al. BrainAGE in mild cognitive impaired patients: predicting the conversion to Alzheimer’s disease. PLoS One. 2013;8(6):e67346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cole JH, et al. Brain age predicts mortality. Mol Psychiatry. 2018;23(5):1385–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Frenzel S, et al. A biomarker for Alzheimer’s disease based on patterns of regional brain atrophy. Front Psychiatry. 2019;10:953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hertel J, et al. Measuring biological age via metabonomics: the metabolic age score. J Proteome Res. 2016;15(2):400–410. [DOI] [PubMed] [Google Scholar]
- 55. Cole JH, et al. Brain age and other bodily ‘ages’: implications for neuropsychiatry. Mol Psychiatry. 2019;24(2):266–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Smith SM, et al. Estimation of brain age delta from brain imaging. Neuroimage. 2019;200:528–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. R Core Team. R: A Language and Environment for Statistical Computing. 2018. https://www.r-project.org/. Accessed 14 September 2020.
- 58. Spicuzza L, et al. Obstructive sleep apnoea syndrome and its management. Ther Adv Chronic Dis. 2015;6(5):273–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Snyder B, et al. Chronic intermittent hypoxia induces oxidative stress and inflammation in brain regions associated with early-stage neurodegeneration. Physiol Rep. 2017;5(9):e13258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tingley D, et al. Mediation : R package for causal mediation analysis. J Stat Softw. 2014;59(5):0–38. [Google Scholar]
- 61. Senaratna CV, et al. Comparison of apnoea-hypopnoea index and oxygen desaturation index when identifying obstructive sleep apnoea using type-4 sleep studies. J Sleep Res. 2019;28(5):e12804. [DOI] [PubMed] [Google Scholar]
- 62. Stöberl AS, et al. Night-to-night variability of obstructive sleep apnea. J Sleep Res. 2017;26(6):782–788. [DOI] [PubMed] [Google Scholar]
- 63. Owen JE, et al. Neuropathological investigation of cell layer thickness and myelination in the hippocampus of people with obstructive sleep apnea. Sleep. 2019;42(1). doi: 10.1093/sleep/zsy199 [DOI] [PubMed] [Google Scholar]
- 64. Chen HL, et al. White matter damage and systemic inflammation in obstructive sleep apnea. Sleep. 2015;38(3):361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hamed SA. Brain injury with diabetes mellitus: evidence, mechanisms and treatment implications. Expert Rev Clin Pharmacol. 2017;10(4):409–428. [DOI] [PubMed] [Google Scholar]
- 66. Tucsek Z, et al. Obesity in aging exacerbates blood-brain barrier disruption, neuroinflammation, and oxidative stress in the mouse hippocampus: effects on expression of genes involved in beta-amyloid generation and Alzheimer’s disease. J Gerontol A Biol Sci Med Sci. 2014;69(10):1212–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Farr SA, et al. Obesity and hypertriglyceridemia produce cognitive impairment. Endocrinology. 2008;149(5):2628–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Vila J, et al. Oxidative stress and the aging brain. In: Riddle DR, ed. Brain Aging: Models, Methods, and Mechanisms. 1st ed. Boca Raton, FL: Taylor & Francis; 2007:353–374. [Google Scholar]
- 69. Kumar R, et al. Altered global and regional brain mean diffusivity in patients with obstructive sleep apnea. J Neurosci Res. 2012;90(10):2043–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ahmadi N, et al. Clinical diagnosis of sleep apnea based on single night of polysomnography vs. two nights of polysomnography. Sleep Breath. 2009;13(3):221–226. [DOI] [PubMed] [Google Scholar]
- 71. Mesas AE, et al. The bidirectional association between physical activity and sleep in middle-aged and older adults: a prospective study based on polysomnography. Sleep. 2018;41(9). doi: 10.1093/sleep/zsy114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yamauchi M, et al. Oxidative stress in obstructive sleep apnea. Chest. 2005;127(5):1674–1679. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this study can be applied for at the University Medicine Greifswald (https://fvcm.med.uni-greifswald.de/dd_service/data_use_intro.php) or by contacting the corresponding author.