Abstract
Aging is associated with reduced slow wave (SW) density (number SW/min in nonrapid-eye movement sleep) and amplitude. It has been proposed that an age-related decrease in SW density may be due to a reduction in electroencephalogram (EEG) amplitude instead of a decline in the capacity to generate SW. Here, we propose a data-driven approach to adapt SW amplitude criteria to age and sex. We predicted that the adapted criteria would reduce age and sex differences in SW density and SW characteristics but would not abolish them. A total of 284 healthy younger and older adults participated in one night of sleep EEG recording. We defined age- and sex-adapted SW criteria in a first cohort of younger (n = 97) and older (n = 110) individuals using a signal-to-noise ratio approach. We then used these age- and sex-specific criteria in an independent second cohort (n = 77, 38 younger and 39 older adults) to evaluate age and sex differences on SW density and SW characteristics. After adapting SW amplitude criteria, we showed maintenance of an age-related difference for SW density whereas the sex-related difference vanished. Indeed, older adults produced less SW compared with younger adults. Specifically, the adapted SW amplitude criteria increased the probability of occurrence of low amplitude SW (<80 µV) for older men especially. Our results thereby confirm an age-related decline in SW generation rather than an artifact in the detection amplitude criteria. As for the SW characteristics, the age- and sex-adapted criteria display reproducible effects across the two independent cohorts suggesting a more reliable inventory of the SW.
Keywords: slow oscillations, aging, sex differences, slow waves, EEG, sleep, nonrapid eye movement sleep
Statement of Significance.
Aging and sex-related differences in regard to slow oscillations, in particular slow waves (SW), have already been reported in the literature. It has been proposed that an age-related decrease in SW density (number SW/min in nonrapid eye movement sleep [NREM]) may be due to a reduction in electroencephalogram amplitude instead of a decline in the capacity to generate SW. Here, we propose a data-driven approach to adapt the automatic detection of SW based on age and sex. Indeed, we offer a more reliable way to detect SW, which in addition, gives a more exhaustive portrayal of NREM slow-wave sleep in aging. This study highlights the importance of methodological considerations in aging and sex-related changes in sleep research.
Introduction
Several studies suggest that the sleeping brain is a proxy of cognitive integrity in older individuals and may be used as a biomarker of neurodegeneration [1–6]. Reductions in slow-wave sleep (SWS), slow waves (SW; <4 Hz and >75uV) and slow-wave activity (SWA; spectral power 0.5–4 Hz) constitute one of the most robust age-related modifications in sleep [5, 7, 8]. Studies have shown that cortical thinning and lower grey matter volume in frontal and prefrontal areas explain statistically SWA and SW reductions in older individuals [1, 3, 4]. Higher SWA and SW density (number of SW per minute of NREM sleep) are linked to better cerebral and cognitive health in older adults [9, 10], whereas age-related changes in SWA and SW density explain reduced sleep-dependent memory consolidation in aging [4, 11–13]. Growing evidence also suggests that SWS, and SW in particular, could play a role in the clearance of cerebrospinal fluid beta-amyloid [3, 14, 15]. In light of these reports, current studies are developing ways to enhance SWS in the hopes of reducing age-related depletion of sleep and to enhance memory consolidation [13, 16].
Findings on the functional impact of age-related differences on SW characteristics depend on the algorithm used to automatically detect SW [17]. Standard detection of SW relies on amplitude criteria including absolute peak-to-peak (PtP) amplitude greater or equal to 75 µV [1, 18–20]. Although electroencephalogram (EEG) amplitude mainly reflects synchronization of post-synaptic synaptic potentials [21, 22], it is also modulated by tissue conductivity of the scalp, skull, cerebrospinal fluid, grey and white matter [23]. Furthermore, distance between the electrode and the cortex will directly impact EEG amplitude. These factors may very well vary with aging and between men and women.
Indeed, an early hypothesis proposed that the observed age-related changes in SW density reflect a general decrease in sleep EEG amplitude that may not be specific to SW generation mechanisms but linked to confounding factors associated with age and sex [24]. In other words, it is possible that an age-related decrease in SW density could be merely due to a reduction in SW amplitude, impacting the number of SW detected with present standard detection criteria. This hypothesis supports the notion that amplitude criteria to detect SW should be lowered in older adults [24]. Given that some studies indirectly corroborate that age-related changes in SWA and SW density are not only due to a general decrease in EEG amplitude [2, 6, 25, 26], the need for age-adjusted SW criteria appears crucial in order to ensure the validity of age-related effects [17].
Although less robust than age-related decreases in SWA and SW, several studies reported that women show more SWS, as well as higher SWA and SW density than men [7, 25–32]. In order to explain these sex differences in SWS and SWA, it was hypothesized that women would generally have higher EEG amplitude than men. Some studies have tried to exclude sex-related influences by using relative spectral measures because their variations are less sensitive to various external factors such as skull thickness [33]. In doing so, sex differences were indeed attenuated when spectral power was expressed as relative power [25, 32].This last finding supports the notion that a mechanism unrelated to SW generation (e.g. skull thickness) could explain the observation of more SWS, higher SWA and SW amplitude in women [26, 27, 29]. The fact that older women showed more SWS than older men, also led to the proposition that objective sleep in men might “age” more rapidly [34] than in women. Indeed, a few studies indicated stronger age-related decreases in the SWS of men compared with women [25, 34–36]. However, other studies do not report significant sex differences in age-related changes in sleep polysomnographic (PSG) and spectral EEG variables [7, 32].
This study is a response to the notion that age- and sex-related differences in SW could be mainly due to differences unrelated to sleep physiological processes (e.g. distance between the cortex and the skull, skull thickness leading to general differences in EEG amplitude). Solving the EEG detection hypothesis of the potential bias-effect of amplitude is thus crucial to evaluate whether age- and sex-related differences are associated with changes in mechanisms of SW generation. Here, we address the automatic detection of SW amplitude criteria and we propose a data-driven approach to adapt these criteria to age and sex. The first objective of the present work was to use a data-driven approach to find SW amplitude criteria detection thresholds from a first cohort (the learning cohort). The second objective aimed to apply the age- and sex-adapted criteria to an independent database (the testing cohort) to assess age and sex differences. We predicted that the data-driven criteria would reduce age and sex differences in SW characteristics but would not abolish them.
Methods
Participants
Two independent cohorts called learning and testing cohorts comprised of 284 healthy adults were used in this work. The learning cohort included 207 younger and older adults (younger: N = 97, 52 women, 23.8 ± 2.8 years, [20–30 years] and older: N = 110, 61 women, 57.5 ± 5.1 years, [50–70 years]). Data from participants in the learning cohort were obtained from PSG adaptation and screening nights conducted in a chronobiology laboratory from 1998 to 2012. A phone interview was conducted to exclude potential subjects who smoked, used sleep-affecting medication, and reported sleep complaints or unusual sleep duration (i.e. < 7 or > 9 h). Participants who engaged in night work or transmeridian travel 3 months prior to the study were also excluded. All PSG evaluations included nasal/oral thermistor and electromyogram (EMG) leg electrodes to screen for sleep disturbances.
The testing cohort consisted of 38 healthy younger (19 women, mean = 22.7 ± 2.4 years, [20–28 years]) and 39 older participants (20 women, mean = 59.6 ± 5.4 years, [50–71 years]). Data were drawn retrospectively from various studies conducted with control participants under baseline conditions. All participants included in this study were free from active pharmacological manipulation. Symptoms of depression (score higher than 13 at the Beck depression inventory) [37] and anxiety (score higher than 7 at the Beck Anxiety Inventory [38]) were ruled out for each participant. All participants had an apnea–hypopnea index of <10 and Periodic Leg Movement Index (PLMI) of < 10. Pre-menopausal women reported a regular menstrual cycle (25–32 days) during the previous year. Menopausal women of the cohorts showed no menstrual cycle for at least a year and reported no vasomotor complaints (i.e. night sweat and hot flashes). Peri-menopausal women were excluded.
The study was approved by the Ethics Board of the “Centre Intégré Universitaire de Santé et Services Sociaux du Nord-de-l’Ile-de-Montréal.” All subjects signed an informed consent form and received monetary compensation for their participation.
Procedures
PSG recording
PSG recordings included EEG electrodes (10–20 system, referential montage with linked ears), chin EMG and left and right electrooculography (EOG). For the learning cohort, the montage included only C3, C4, O1, and O2, whereas 20 EEG derivations were recorded in the testing cohort. PSG was recorded using a Grass Model 15 amplifier system (gain 10,000; bandpass 0.3–100 Hz). Signals were digitalized at a sampling rate of 256 Hz using commercial software (Harmonie, Stellate System). Sleep stages were visually scored on 30-s epochs on a computer screen according to standard criteria of the American Academy of Sleep Medicine (AASM) [39]. EEG artifacts were detected automatically [40]: muscle artifacts were removed by filtering out bursts of myogenic activity within high frequency signals (power density in the 26.25 and 32 Hz). More specifically, the median of the 45 values in the surrounding symmetric 3-min window of each 4-s epoch (“the moving median procedure”) was chosen as an estimate for the local level of the background level of high frequency activity across the sleep episode. Four second values that exceeded the median value of the local 3-min window by a certain factor (4X) was considered artifactual and were flagged as artifacts. We then visually inspected the EEG to ensure appropriate rejection from analysis and to remove other artifacts (e.g. eye movements). PSG sleep variables for younger and older men and women in the learning and testing cohorts are shown in the supplemental materials.
SW characteristics
The following SW characteristics were obtained for both cohorts (learning and testing): (1) SW density is defined as the number of SW per minute of NREM sleep (N2–N3 combined), (2) SW PtP amplitude expresses the difference in voltage (i.e. microvolt) between negative and positive peak of filtered signal, and (3) SW slope is the ratio between the PtP amplitude and the delay between the two peaks (i.e. microvolt per second). All these SW characteristics are then averaged over all-night NREM sleep (N2–N3 combined).
Standard SW automatic detection
For both cohorts, SW were automatically detected on artifact-free-NREM epochs for left and right central derivations (i.e. C3 and C4) using published criteria [19]. Briefly, EEG was initially bandpass filtered between 0.3 and 4.0 Hz using a linear phase Finite Impulse Response filter (−3 dB at 0.3 and 4.0 Hz; −23 dB at 0.1 and 4.2 Hz). Then, SW were individually detected on the filtered signal using these criteria: (1) PtP ≥ 75 µV, (2) negative peak amplitude (NegA) ≥ 40 µV, (3) duration of negative deflection ≥ 125 and ≤ 1,500 ms, and (4) duration of positive deflection ≤ 1,000 ms [1, 18–20, 41].
Development of age- and sex-adapted amplitude SW criteria
A data-driven approach to derive age- and sex-adapted SW amplitude criteria was developed using the learning cohort. The proposed approach is a compromise between the sensitivity of the detector and some specific characteristics of the sleep SW.
A sensitivity criterion of the detector
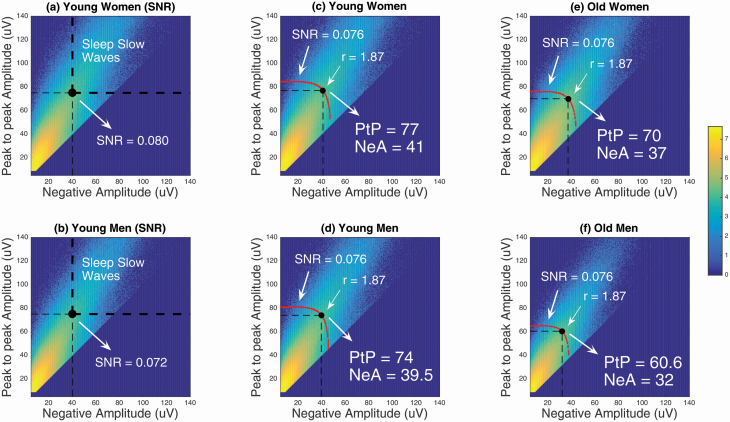
To take into account a documented sex difference in SW and SWA in younger adults [7, 25–27, 32], SW detection was performed on the filtered signal separately in younger women and younger men of the learning cohort, using very low PtP amplitude and NegA thresholds, that is, 9 and 5 µV, respectively. Duration of negative deflection (≥ 125 and ≤ 1,500 ms) and positive deflection (≤ 1,000 ms) was similar to standard criteria. This permissive detection is very sensitive but not specific to sleep SW. Figure 1, A and B shows the density of occurrence of all detected slow oscillations with respect to their PtP amplitude and NegA, for younger women and younger men, respectively. The bold dashed lines are the usual thresholds of the standard detection criteria. Among all events, the standard-detected SW, defining the “signal” part of the detection (PtP ≥ 75 µV and NegA ≥ 40 µV), are events located in the upper right quadrant of each graph. The detected events in the other three quadrants were considered as “noise” (PtP < 75 µV or NegA < 40 µV) and the ratio of the number of “signal” events over the number of “noise” events defined a signal-to-noise ratio (SNR) that characterizes the detector: SNR = 0.08 for younger women; SNR = 0.072 for younger men. However, we assume that as for any filtering algorithm, the detection is characterized with a unique SNR. We estimate this value from the standard detection algorithm applied to the younger cohort. We must, however, take into account the putative difference between men and women in the learning cohort. To do so, the best estimate of this characteristic is provided with the average of the two SNR estimated from the younger women and men, respectively. The averaged SNR = 0.076 thus obtained reflects the sensitivity of the detector found in the younger adults. We further assume that the SNR of the algorithm should not depend on aging. With this later assumption, we favor the hypothesis that SW density would mirror a global sleep EEG amplitude reduction rather than be an impairment of SW generation mechanisms.
Figure 1.
Joint-probability densities of occurrence of SW for the learning cohort. PtP Amplitude, peak-to-peak amplitude; NegA, negative amplitude; SNR, signal-to-noise ratio. Each graph represents a heat map of the density of SW detected given the NegA (> 5 μV) and PtP amplitude (> 9 μV) on the x- and y-axis, respectively, over all-night NREM sleep (N2–N3 combined). The range displayed is in logarithmic scale (power of 10), from 0 to 107 SW/(μV)2. (A) Distribution of detected SW in younger women. (B) Distribution of detected SW in younger men. The upper right quadrant defined by the bold dashed lines in (a) and (b) represents the “signal” of conventional SW detections. From the distributions of the younger cohorts (A and B), the mean of the SNR obtained for the women (SNR = 0.08) and the men (SNR = 0.072) defines the general SNR = 0.076 of the detector. (C–F) The intersection between the SNR curve and the “ratio-r line” defines the adaptive SW amplitude detection criteria for the younger and older women and men, respectively.
A criterion of specificity of the SW detection
In each group of the learning cohort, the red curves in Figure 1, C–F represents the points with equal SNR. All possible pairs of adapted PtP amplitude (on y-axis) and NegA (on x-axis) for which the SNR equals the estimated value (SNR = 0.076) follow those curves. Since minimal PtP amplitude (75 µV) and minimal NegA (40 µV) are the accepted thresholds for the usual SW detection [1, 18–20, 41], we used this ratio-r (75 µV/40 µV = 1.87) between these amplitude thresholds for our detector. This ratio corresponds as a criterion of “specificity” of the SW detection algorithm, and only concerns the SW at the detectability threshold. This specificity threshold allows minimal negativity threshold to detect SW, but most SW will not demonstrate this specific ratio. In other words, we assume that the lowest amplitudes (PtP, NegA) satisfy the necessary relation PtP ≈1.875 NegA at the detectability threshold for any group. This relation is not verified for all the detected SW.
Consequently, the “optimal” sensitive and specific detection criteria of SW is obtained at the intersection of the SNR curve and the “ratio-r line” (red lines of slope equal to 1.87 in Figure 1, C–F) for younger women, younger men, older women and older men, respectively. These new thresholds define the data-driven SW detection adapted to age and sex (see Table 1) and will be further evaluated in a testing cohort. Note that the SW detection for both the learning and the testing cohort was also performed on artifact-free and filtered NREM epochs for left and right central derivations (i.e. C3 and C4) using published criteria [19].
Table 1.
Age and sex-adapted SW amplitude thresholds.
| Age- and sex-adapted criteria | |||||
|---|---|---|---|---|---|
| Young | Middle-aged | ||||
| Standard criteria | Men | Women | Men | Women | |
| Peak-to-peak SW Amplitude | 75 µV | 74 µV | 77 µV | 60 µV | 70 µV |
| Negative SW Amplitude | 40 µV | 39.5 µV | 41 µV | 32 µV | 37 µV |
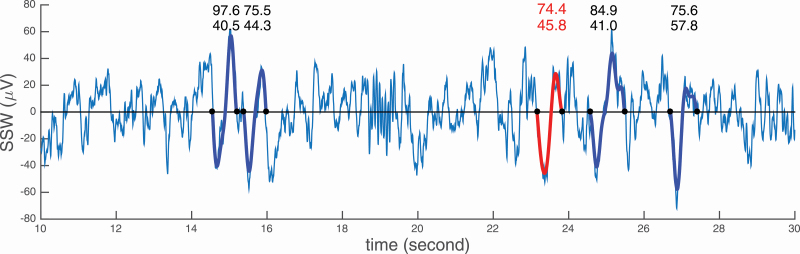
We show a concrete depiction of individual SW detected using the two SW criteria in Figure 2.
Figure 2.
Twenty seconds of sleep EEG (C3) in stage N2 of an older male healthy subject. In blue, the sleep slow waves as detected with the standard SW criteria. In red, the extra SW obtained with the data-driven thresholds adapted for age (older here) and sex (male). The numbers above each detection indicate the PtP amplitude and NegA, respectively.
Statistical analyses
To evaluate age and sex effects on all-night SW characteristics detected on average central derivation, two-way ANOVAs with two independent factors (two age groups and two sex groups) were performed on the two cohorts separately (first in the learning cohort and then in the testing cohort). The separate analysis performed in the testing cohort ensures that the observed effects are independent of the SW amplitude criteria emerging from the learning cohort. These analyses were performed on the standard SW detection and on age- and sex-adapted SW detection, respectively, for each cohort. Although SW slope and SW amplitude are highly dependent on each other, we decided to not include these SW characteristics in the same statistical model because of their high collinearity. SW slope and SW amplitude were highly correlated regardless of the SW detection and the cohort (Learning cohort: Standard SW detection: r = 0.78, p < 0.0001, R2 = 0.61, Adaptative SW detection: r = 0.84, p < 0.0001, R2 = 0.71; Testing cohort: Standard SW detection: r = 0.81, p < 0.0001, R2 = 0.66; Adaptative SW detection: r = 0.85, p < 0.0001, R2 = 0.72).
Differences in main effects were assessed with post hoc multiple mean comparisons and interactions were decomposed using simple effect analyses. p values for post hoc comparisons were adjusted with Bonferroni corrections. Effect sizes (ES) were measured using the partial eta squared. Results were considered significant when p < 0.05.
Results
General descriptive measures of SW detection characteristics, with either the standard SW criteria or the adapted SW criteria, are displayed in Table 2 for the learning cohort and in Table 3 for the testing cohort.
Table 2.
General descriptive measures of SW detection characteristics for the learning cohort.
| Standard SW criteria | Adapted SW criteria | |||||||
|---|---|---|---|---|---|---|---|---|
| Younger | Older | Younger | Older | |||||
| Women | Men | Women | Men | Women | Men | Women | Men | |
| SW density (number of SW/ min of NREM sleep) | 8.69 | 7.74 | 5.59 | 3.14 | 8.31 | 7.85 | 6.67 | 5.73 |
| (3.45) | (3.55) | (3.27) | (2.78) | (3.39) | (3.57) | (3.67) | (4.12) | |
| SW amplitude(µV) | 127.64 | 123.75 | 108.28 | 101.46 | 129.79 | 122.20 | 103.11 | 86.38 |
| (12.72) | (9.62) | (7.57) | (9.29) | (12.73) | (9.41) | (7.38) | (8.55) | |
| SW slope(µV/s) | 356.72 | 330.74 | 286.50 | 260.91 | 361.22 | 329.46 | 277.68 | 236.13 |
| (42.24) | (45.48) | (52.83) | (44.01) | (42.61) | (45.25) | (50.81) | (38.94) | |
Untransformed mean (standard deviation).
Table 3.
General descriptive measures of SW detection characteristics for the testing cohort.
| Standard SW criteria | Adapted SW criteria | |||||||
|---|---|---|---|---|---|---|---|---|
| Younger | Older | Younger | Older | |||||
| Women | Men | Women | Men | Women | Men | Women | Men | |
| SW density (number of SW/ min of NREM sleep) | 8.21 | 8.48 | 5.95 | 3.22 | 7.83 | 8.60 | 7.10 | 5.82 |
| (3.97) | (2.73) | (3.43) | (2.42) | (3.86) | (2.76) | (3.87) | (3.69) | |
| SW amplitude (µV) | 125.37 | 124.14 | 108.32 | 101.91 | 127.56 | 123.41 | 103.09 | 86.88 |
| (13.13) | (7.28) | (6.82) | (6.87) | (13.30) | (7.26) | (6.61) | (6.62) | |
| SW slope (µV/s) | 362.43 | 334.07 | 281.92 | 264.32 | 367.31 | 332.63 | 273.83 | 239.23 |
| (62.30) | (47.01) | (52.83) | (51.84) | (63.41) | (46.78) | (50.75) | (46.17) | |
Untransformed mean (standard deviation).
Density of the SW
Learning cohort
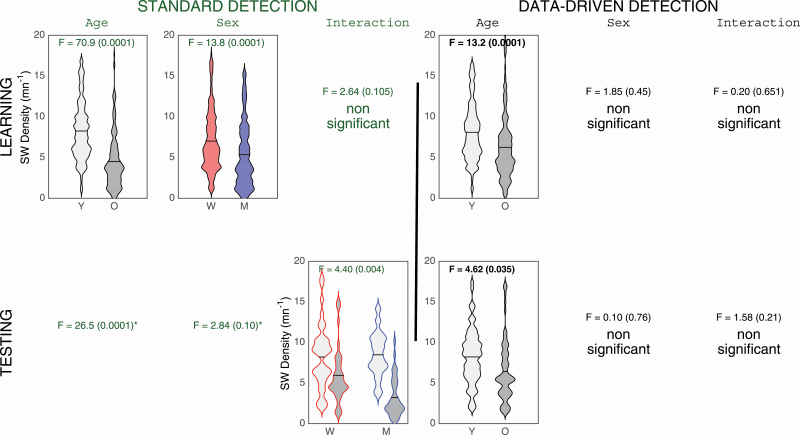
With standard SW criteria, older adults showed lower SW density compared with younger adults (age effect: F(1,203) = 70.9, p < 0.0001, ES = 0.26) and women had a higher SW density than men regardless of age (sex effect: F(1,203) = 13.8, p < 0.0001, ES = 0.064; see first row of Figure 3). With the adapted SW criteria, only a significant age effect was found. Older subjects showed lower SW density compared with younger subjects (age effect: F(1,203) = 13.2, p < 0.0001, ES = 0.06; see first row of Figure 3).
Figure 3.
SW density for NREM sleep with standard (left side) and data-driven criteria (right side). Only the significant interactions or main effects are presented. Younger subjects (Y) are represented in light grey and older participants (O) are represented in dark grey. Women (W) are in red and Men (M) are in blue. SW density (number of SW/min) is represented on the y-axis. When a similar effect is observed in both the learning (upper row) and testing cohort (lower row), the ANOVA results are shown in bold.
Testing cohort
With standard criteria, we observed a significant main effect of age (F(1,73) = 26.5, p < 0.0001, ES = 0.27), and a significant interaction between age and sex groups (F(1,73) = 8.9, p = 0.0004, ES = 0.11; see second row of Figure 3). Older adults had lower density compared with younger adults, although this effect was stronger for men (F(1,73) = 25.7, p < 0.0001, ES = 0.26) than women (F(1,73) = 4.8, p = 0.03, ES = 0.06). With the adapted criteria, older adults showed lower SW density as compared with the younger participants, regardless of sex (effect of age: F(1,73) = 4.6, p = 0.035, ES = 0.06; see second row of Figure 3).
Characteristics of the SW
SW amplitude
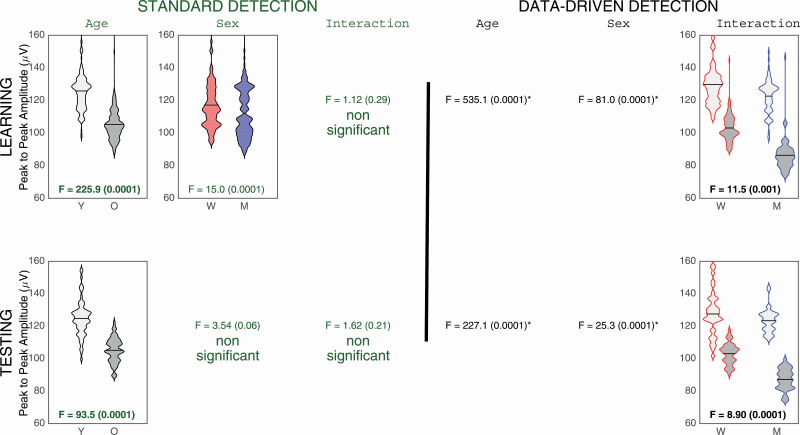
Learning cohort
With standard criteria, older adults showed a significantly lower SW amplitude than younger adults (main age effect: F(1,203) = 225.9, p < 0.0001, ES = 0.26), and women had a higher SW amplitude than men regardless of age (main sex effect: F(1,203) = 15.0, p < 0.0001, ES = 0.07; see first row of Figure 4). With the adapted criteria, an age and sex interaction was found (F(1,203) = 11.5, p = 0.001, ES = 0.05; see first row of Figure 4), as well as main age (F(1,203) = 535.1, p < 0.0001, ES = 0.73) and sex effects (F(1,203) = 81.0, p < 0.0001, ES = 0.29). Older adults had lower SW amplitude compared with younger adults but the effect was stronger in men (F(1,203) = 322.7, p < 0.0001, ES = 0.61) than in women (F(1,203) = 214.2, p < 0.0001, ES = 0.51).
Figure 4.
SW characteristics for NREM sleep SW amplitude with standard (left side) and data-driven criteria (right side). Only the significant interactions or main effects interactions are presented. Younger subjects (Y) are represented in light grey and older participants (O) are represented in dark grey. Women (W) are in red and Men (M) are in blue. SW PtP amplitude (μV) is represented on the y-axis. When a similar effect is observed in both the learning (upper row) and testing cohort (lower row), the ANOVA results are shown in bold.
Testing cohort
SW detection with standard criteria showed only a main age effect, where SW amplitude was lower for older adults than for younger adults (F(1,73) = 93.5, p < 0.0001, ES = 0.56; see second row of Figure 4). The use of the adapted SW criteria showed a significant interaction between age and sex (F(1,73) = 8.9, p = 0.0004, ES = 0.11; see second row of Figure 4), as well as main age (F(1,73) = 227.1, p < 0.0001, ES = 0.76) and sex effects (F(1,73) = 25.3, p < 0.0001, ES = 0.26). Older individuals showed lower SW amplitude than younger adults but this effect was, once again, stronger in men (F(1,73) = 160.8, p < 0.0001, ES = 0.69) than in women (F(1,73) = 74.0, p < 0.0001, ES = 0.50).
Probability of occurrence of SW PtP amplitude when using the standard and adapted criteria in the testing cohort
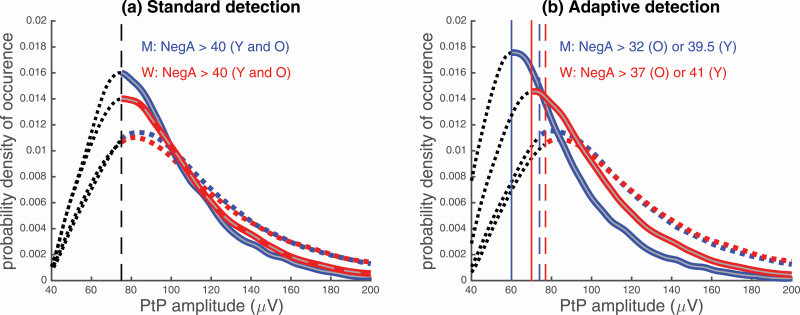
We sought to understand the difference in SW amplitude results between the standard and the age- and sex-adapted criteria in the testing cohort (refer to Figure 4 for SW amplitude results). To do so, we considered the probability distribution of the SW PtP amplitude provided by the two detectors using their respective NegA amplitude criterion. Figure 4 illustrates the probability of occurrence of sleep SW PtP amplitude when using standard and adapted SW detection in (A) younger and (B) older adults.
In the younger adults, we observed that the change in the SW PtP amplitude with the age- and sex-adapted criteria compared with the standard criteria does not affect the probability of occurrence of the SW (Figure 5, A). In both the standard and the adapted SW detection criteria, the highest probability of occurrence, as shown by the distributions’ peaks, is located between 80 and 90 μV.
Figure 5.
Probability of occurrence of sleep SW PtP amplitude in (A) younger and (B) older adults in the testing cohort. The probability distributions were calculated so that the probability of occurrence (on the y-axis) is equal to one. Hence, the area under the curve of the histograms conveniently normalized must be equal to one. Each graph contains the standard SW detection (dashed lines), and the adaptive SW detection that refers to the data-driven SW detection (full lines). The color blue refers to the men, and the color red is for women. Vertical lines indicate each group’s respective PtP amplitude detection criterion. Dashed black lines before the vertical lines represent the “noise” detections. NegA represents negative amplitude of the detected SW.
For older adults, we observed that the highest probability of occurrence with the standard criteria is around the threshold PtP amplitude value of 75 μV. We also observed a distinctly higher probability of occurrence of lower amplitude SW (< 80 μV) for older adults (Figure 5, B), as compared with younger adults (Figure 5, A). When reducing the amplitude threshold with age- and sex-adapted criteria, there was a shift in the highest probability of occurrence of PtP amplitude for older adults (Figure 5, B), shifting to about 60 μV for older men and 70 μV for older women. With this age- and sex-adapted criteria, older men consequently showed a highest probability of occurrence for lower amplitude SW (<80 μV) as compared with older women. In summary, this qualitative assessment of the adapted SW criteria reveals that older adults, and especially men, produce a higher probability of lower amplitude SW. This finding explains age and sex interaction of SW amplitude that was statistically highlighted when using the adapted criteria (see second row of Figure 4).
SW slope
Learning cohort
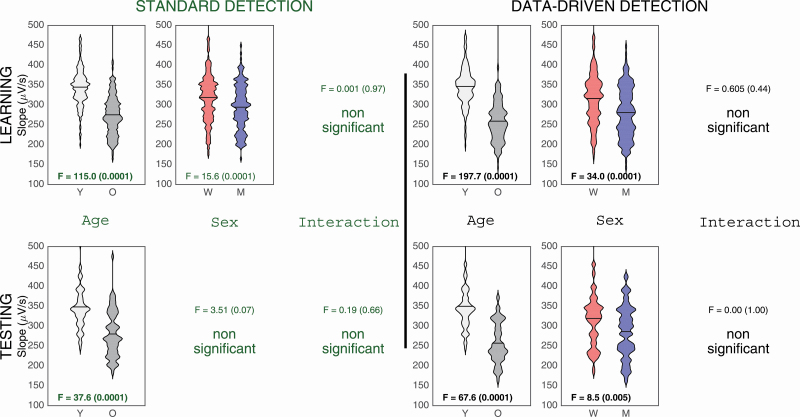
With the standard criteria, older adults had a lower SW slope compared with younger adults (main age effect: F(1,203) = 115.0, p < 0.0001, ES = 0.36) and women had a higher SW slope than men regardless of age (main sex effect: F(1,203) = 15.6, p < 0.0001, ES = 0.07; see first row of Figure 6). Similar results were obtained when using adapted criteria (main age effect: F(1,203) = 197.7, p < 0.0001, ES = 0.49; main sex effect: F(1,203) = 34.0, p < 0.0001, ES = 0.14; see first row of Figure 6).
Figure 6.
SW characteristics for all-night NREM sleep SW slope. Only the significant interaction or main effects are presented. Younger subjects (Y) are represented in light grey and older participants (O) are represented in dark grey. Women (W) are in red and Men (M) are in blue. SW slope (μV/s) is represented on the y-axis. When a similar effect is observed in both the learning (upper row) and testing cohort (lower row), the ANOVA F results are shown in bold.
Testing cohort
The use of the standard SW detector revealed an age effect for SW slope (F(1,73) = 37.6, p < 0.0001, ES = 0.34; see second row of Figure 6) with the younger adults having a steeper slope compared with older adults. This age effect was still preserved when using the adapted SW criteria (F(1,73) = 61.6, p < 0.0001, ES = 0.46; see second row of Figure 6). However, with the adaptive SW detection only, women had a steeper SW slope compared with men regardless of age (main sex effect: F(1,73) = 8.5, p = 0.005, ES = 0.10; see second row of Figure 6).
Probability of occurrence of SW slope when using the standard and adapted criteria in the testing cohort
We sought to understand the difference in SW slope results between the SW criteria, mainly the additional sex effect found with the age and sex-adapted criteria in the testing cohort. To do so, we considered the probability distribution of the SW slope as shown in Figure 6 for the two detection criteria and the sex and age groups.
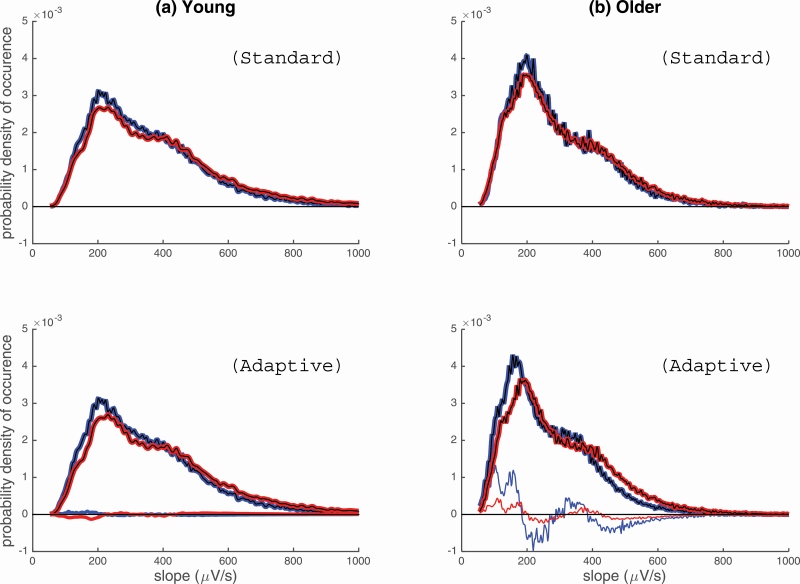
With the standard criteria, sex does not affect the distribution of the slope as observed with similar pattern distribution for both men and women in each age group. We observed a change in probability distribution with age. Older participants showed a higher probability distribution around 200 μV/s (see first row of Figure 7). Younger adults, on the other hand, showed a similar mode but with a broader distribution of the probability distribution for the SW slope. This implies that younger adults produce SW of more varying slopes compared with older adults which tend to form SW that are less steep.
Figure 7.
Probability density of SW slope in (A) younger and (B) older adults, for women (red) and men (blue). The probability distributions were calculated so that the probability of occurrence (on the y-axis) is equal to one. Hence, the area under the curve of the histograms conveniently normalized must be equal to one. The standard SW detection is displayed in the first row, while the adaptive SW detection is displayed in the second row. For the graphs in the second row, thin lines exhibit the difference between the standard and adaptive distributions, for men and women.
When adapting the amplitude threshold to age and sex, a similar aging effect to the standard detection was observed. However, an additional independent sex difference, which emerged statistically (results in Figure 6), was perceivable in the probability distribution of flatter SW slopes for men in both age groups (< 200 μV/s; see second row of Figure 7). To better illustrate this sex effect, we computed the contrast between the standard and adaptive detection on Figure 7 (second row). An additional value of using the adapted detection is that it allows the observation of a vaster inventory of SW, which reveals that younger men produce SW of a flatter slope (peak around 200 μV/s) compared with younger women. Compared with older women, we also observed for older men, a higher production of a flatter SW slope (peak of probability around 150 μV/s) in addition to a marked reduction of SW of steeper slopes (peak of probability above 200 μV/s). In summary, men have a higher probability to produce the dominant low SW slope (< 200 μV/s). Women’s highest probability density tends to shift toward a steeper SW slope (>200 μV/s for younger and older women). This shift in “slope production” could explain an additional sex effect that is absent while using standard criteria.
Discussion
This work proposes a data-driven approach to adapt the automatic detection of SW to age and sex. The age and sex-adapted criteria on the amplitude thresholds have been obtained from learning cohort, as the optimal tradeoff between two notions. First, the sensibility of the algorithm has been quantified with the SNR, that is, the proportion of detected SW in the exhaustive inventory of oscillations with equivalent properties (durations) but the amplitudes. As an intrinsic property of the detection algorithm, this proportion (i.e. the SNR) is to be taken as in all the groups and thus makes the detection independent from age and sex. Second, a specific constraint was defined on the smallest PtP amplitude at the minimal off-state amplitude (NegA) of the SW. As an intrinsic property of the SW, this amplitude ratio of the smallest SW is assumed to be a general requirement, independent from age and sex, in order to obtain, at the detectability threshold, a legitimate off-on transition in a SW. All in all, this adaptive approach puts male, female, younger and older individuals on an equal footing. Using these adapted criteria, we confirm a robust age-related decrease in SW density. However, sex-related differences for SW density faded away when using this adapted detection. These results suggest that aging reduces SW generation, whereas the usual sex difference in SW density with standard detection is driven by EEG amplitude criteria.
Age impacts SW density, but sex does not
The reduction in SW density is one of the most robust age-related changes reported, and a great deal of research showed their implications in cerebral and cognitive health in older individuals [1–6, 20]. Although the origin and the neurophysiological significance of age-dependent changes in SW density is not fully understood, structural brain atrophy has consistently been identified as one factor governing an age-related decrease in SW density [1, 3, 4]. However, there is still to date an ongoing controversy regarding the hypothesis that an age-related decrease in SW density could be a mere reflection factor associated with EEG amplitude reduction, which would impact the number of SW detected. In order to make robust inferences about group differences, SW must be reliably detected while controlling a priori confounding factors associated with age and sex that may modulate EEG amplitude. In this respect, our algorithm allows us to define group-specific thresholds that are age- and sex-adapted. With the use of our adaptive algorithm, we observed persistent age-related changes in SW density, suggesting an impact of age on SW generation mechanisms.
Sex-related differences in SW density were abolished when using the age- and sex-adapted approach. This finding supports the hypothesis that sex may not impact sleep regulatory mechanisms associated with SW generation but rather be caused by other factors, such as the difference between men and women in scalp thickness, modulating the amplitude of SW [26, 27, 29]. Indeed, studies have shown that a thinner skull thickness of women may explain greater EEG amplitude detected on the scalp [42–44].
The older adults show globally less SW but they have a higher probability to produce lower amplitude SW compared with younger adults
Using our adapted amplitude criteria, older adults showed lower SW amplitude compared with younger adults, but the age effect was stronger in men. Moreover, looking at the probability of SW occurrence with the adapted amplitude criteria, older men produced more low amplitude SW (< 80 μV) compared with older women and younger adults. It is important to notice that the SW under 75 μV of PtP amplitude are currently not detected with the standard criteria used in the literature. In this study, we show significant effects emerging when considering these undetected SW. Our study highlights the relevance of using adapted criteria to get a more complete inventory of SW production in aging, especially in older men. Given that SW amplitude reflects the degree of synchronization in neuronal firing [45, 46], it is possible that older men still generate SW but would show impairments in synchronizing extended cortical areas during SW.
SW slope: distinct age and sex effects
For SW slope, we showed independent age and sex effects when using our adaptive metrics as compared with standard SW amplitude criteria. Compared with older subjects, we found that younger individuals have sharper and steeper SW. Women also have generally sharper and steeper SW compared with men. We also observed a pattern of reduction in the production rate of abrupt SW slope (200 μV/sec) in favor of flatter slopes in older men. This shift in “slope production” is less pronounced for aging women. SW slope has been modeled to represent the speed of recruitment of neurons alternating between an active and silent phase during SW [47]. A steeper slope would imply faster synchronization between neurons [48]. Our results therefore suggest distinct effects of age and sex on the speed of recruitment during sleep SW.
The use of our adapted criteria solves conflicting results in the literature regarding sex differences and interaction between age and sex
Sex effects and interactions between age and sex differed in the learning and testing cohorts when using standard SW criteria. For instance, a principal effect of sex was found for SW amplitude in the learning cohort whereas no significant effect of sex was observed in the testing cohort. Our conflicting sex effects and age and sex interaction between the two cohorts with the standard detection SW criteria, mirror the inconsistency of results found between studies [49, 50]. Using adapted SW criteria, discrepancies between cohorts for SW density, SW amplitude, and SW slope vanish. Regardless of the cohort used, similar and consistent effects were found for each SW characteristics (e.g. an age and sex interaction for SW amplitude in both the learning and testing cohort).
Limitations to the study and avenues for future research
We assume that the putative difference between younger men and women by averaging their respective SNR, does not discriminate younger adults on the basis of sexual differences. This average estimator was done in order to obtain a unique characteristic of the detection algorithm (the SNR) for the younger adults. Moreover, this study mainly focused on the amplitude criteria of the SW and did not explore the SW duration criteria, because targeting duration criteria that are already circumscribed to large interval of durations will only decrease and reduce the options of shape for the sleep SW.
Due to recent computational and mathematical advances, the consideration of interindividual variability within a group is starting to become more common, and promising work is already underway (e.g. [17]) Nonetheless, to make valid interindividual analyses using our method, a large amount of data (several nights) from each individual would be required to compute a robust individual SNR. Future studies could consider interindividual differences in each age group and sex category. Also, to ensure comparability with other studies and future studies, particular attention needs to be paid to the age range of the older adults in our study as they were between 50 and 70 years old. Moreover, there is an undeniable topographical effect in SW with SW being more prominent in frontal derivations [19, 20]. The choice of derivations for this study was imputable to electrodes restriction from our learning cohort. However, most studies which evaluated topography used the same amplitude criteria for all derivations [18, 19]. Importantly, contrary to age and sex effects which we evaluated between group differences (the objective of this manuscript), topography effects consider intraindividual differences (between two electrodes) making interindividual differences in skull thickness, distance between the cortex and the skull, and skin resistance less prominent. Nonetheless, whether amplitude criteria should be adapted to topography (e.g. using stricter amplitude detection criteria for more anterior derivations) remains an interesting avenue to explore. Future studies should evaluate whether our conclusions also apply for other derivations.
Conclusion
Our adapted approach confirms the validity of age-related changes found in previous reports in the literature [1, 5, 26, 35, 49, 51]. Sex-related differences in SW density are circumscribed to EEG amplitude, and these differences may explain inconsistencies in previous literature when detection criteria are not adapted. As for the SW characteristics, while we still observe robust age and inconsistent sex effects with the standard criteria, the age- and sex-adapted criteria clearly show a more consistent and exhaustive inventory of the SW. We found age- and sex-related effects on SW amplitude and SW slope that were consistent regardless of the population of study (i.e. learning and testing cohorts). Finally, our study shows the importance of methodological considerations in the measurement of SW.
Supplementary Material
Acknowledgments
The authors would like to thank Sonia Frenette, Tyna Paquette, and all sleep technologists from our lab for their help with data collection and analysis. This work was supported by the Canadian Institutes of Health Research (CIHR), grant number PJT-153259 and the Natural Sciences and Engineering Research Council of Canada (NSERC), grant number RGPIN-2016–05149 (J.C.) and by a CIHR doctorate scholarship (T.R.).
Disclosure statement
Financial disclosure: none.
Non-financial disclosure: none.
References
- 1. Dubé J, et al. Cortical thinning explains changes in sleep slow waves during adulthood. J Neurosci. 2015;35(20): 7795–7807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Latreille V, et al. Age-related cortical signatures of human sleep electroencephalography. Neurobiol Aging. 2019;76:106–114. [DOI] [PubMed] [Google Scholar]
- 3. Mander BA, et al. β-amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation. Nat Neurosci. 2015;18(7):1051–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mander BA, et al. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nat Neurosci. 2013;16(3):357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mander BA, et al. Sleep and human aging. Neuron. 2017;94(1):19–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sun H, et al. Brain age from the electroencephalogram of sleep. Neurobiol Aging. 2019;74:112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carrier J, et al. The effects of age and gender on sleep EEG power spectral density in the middle years of life (ages 20–60 years old). Psychophysiology. 2001;38(2):232–242. [PubMed] [Google Scholar]
- 8. Landolt HP, et al. Effect of age on the sleep EEG: slow-wave activity and spindle frequency activity in young and middle-aged men. Brain Res. 1996;738(2):205–212. [DOI] [PubMed] [Google Scholar]
- 9. Pótári A, et al. Age-related changes in sleep EEG are attenuated in highly intelligent individuals. Neuroimage. 2017;146:554–560. [DOI] [PubMed] [Google Scholar]
- 10. Yaffe K, et al. Connections between sleep and cognition in older adults. Lancet Neurol. 2014;13(10):1017–1028. [DOI] [PubMed] [Google Scholar]
- 11. Papalambros NA, et al. Acoustic enhancement of sleep slow oscillations and concomitant memory improvement in older adults. Front Hum Neurosci. 2017;11:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Westerberg CE, et al. Memory improvement via slow-oscillatory stimulation during sleep in older adults. Neurobiol Aging. 2015;36(9):2577–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wilckens, KA, et al. Slow-wave activity enhancement to improve cognition. Trends Neurosci, 2018;41(7):470–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ju YE, et al. Sleep and Alzheimer disease pathology–a bidirectional relationship. Nat Rev Neurol. 2014;10(2):115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ju YS, et al. Slow wave sleep disruption increases cerebrospinal fluid amyloid-β levels. Brain. 2017;140(8):2104–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang Y, et al. Can slow-wave sleep enhancement improve memory? A review of current approaches and cognitive outcomes. Yale J Biol Med. 2019;92(1):63–80. [PMC free article] [PubMed] [Google Scholar]
- 17. Muehlroth BE, et al. Understanding the interplay of sleep and aging: Methodological challenges. Psychophysiology. 2020;57(3):e13523. [DOI] [PubMed] [Google Scholar]
- 18. Dang-Vu, TT, et al. Spontaneous neural activity during human slow wave sleep. Proc Natl Acad Sci U S A, 2008;105(39):15160–15165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Massimini M, et al. The sleep slow oscillation as a traveling wave. J Neurosci. 2004;24(31):6862–6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carrier J, et al. Sleep slow wave changes during the middle years of life. Eur J Neurosci. 2011;33(4):758–766. [DOI] [PubMed] [Google Scholar]
- 21. Bondar, AT, Fedotchev, AI. [Dynamic changes in EEG spectral structure during voluntary movements in man]. Fiziol Cheloveka, 1999;25(5):64–73. [PubMed] [Google Scholar]
- 22. Novak P, et al. Periodic amplitude modulation of EEG. Neurosci Lett. 1992;136(2):213–215. [DOI] [PubMed] [Google Scholar]
- 23. Laarne P, et al. Effects of tissue resistivities on electroencephalogram sensitivity distribution. Med Biol Eng Comput. 1999;37(5):555–559. [DOI] [PubMed] [Google Scholar]
- 24. Webb WB, et al. A modified method for scoring slow wave sleep of older subjects. Sleep. 1982;5(2):195–199. [DOI] [PubMed] [Google Scholar]
- 25. Luca G, et al. Age and gender variations of sleep in subjects without sleep disorders. Ann Med. 2015;47(6):482–491. [DOI] [PubMed] [Google Scholar]
- 26. Mourtazaev MS, et al. Age and gender affect different characteristics of slow waves in the sleep EEG. Sleep. 1995;18(7):557–564. [DOI] [PubMed] [Google Scholar]
- 27. Dijk DJ, et al. Sex differences in the sleep EEG of young adults: visual scoring and spectral analysis. Sleep. 1989;12(6):500–507. [DOI] [PubMed] [Google Scholar]
- 28. Fukuda, N, et al. Gender difference of slow wave sleep in middle aged and elderly subjects. Psychiatry Clin Neurosci, 1999. 53(2): p. 151–153. [DOI] [PubMed] [Google Scholar]
- 29. Ma J, et al. EEG power spectra response to a 4-h phase advance and gaboxadol treatment in 822 men and women. J Clin Sleep Med. 2011;7(5):493–501A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mongrain V, et al. Difference in sleep regulation between morning and evening circadian types as indexed by antero-posterior analyses of the sleep EEG. Eur J Neurosci. 2006;23(2):497–504. [DOI] [PubMed] [Google Scholar]
- 31. Rediehs MH, et al. Sleep in old age: focus on gender differences. Sleep. 1990;13(5):410–424. [PubMed] [Google Scholar]
- 32. Svetnik V, et al. EEG spectral analysis of NREM sleep in a large sample of patients with insomnia and good sleepers: effects of age, sex and part of the night. J Sleep Res. 2017;26(1):92–104. [DOI] [PubMed] [Google Scholar]
- 33. Duffy FH, et al. The pattern of age-related differences in electrophysiological activity of healthy males and females. Neurobiol Aging. 1993;14(1):73–84. [DOI] [PubMed] [Google Scholar]
- 34. Ehlers CL, et al. Slow-wave sleep: do young adult men and women age differently? J Sleep Res. 1997;6(3):211–215. [DOI] [PubMed] [Google Scholar]
- 35. Della Monica C, et al. Rapid eye movement sleep, sleep continuity and slow wave sleep as predictors of cognition, mood, and subjective sleep quality in healthy men and women, aged 20–84 years. Front Psychiatry. 2018;9:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hume KI, et al. A field study of age and gender differences in habitual adult sleep. J Sleep Res. 1998;7(2):85–94. [DOI] [PubMed] [Google Scholar]
- 37. Beck, AT, et al. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev. 1988;8(1):77–100. [Google Scholar]
- 38. Beck AT, et al. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56(6):893–897. [DOI] [PubMed] [Google Scholar]
- 39. Iber, C, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester, IL: American Association of Sleep Medicine; 2007. [Google Scholar]
- 40. Brunner DP, et al. Muscle artifacts in the sleep EEG: automated detection and effect on all-night EEG power spectra. J Sleep Res. 1996;5(3):155–164. [DOI] [PubMed] [Google Scholar]
- 41. Lafortune M, et al. Reduced slow-wave rebound during daytime recovery sleep in middle-aged subjects. PLoS One. 2012;7(8):e43224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dannhauer M, et al. Modeling of the human skull in EEG source analysis. Hum Brain Mapp. 2011;32(9):1383–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Frodl T, et al. The effect of the skull on event-related P300. Clin Neurophysiol. 2001;112(9):1773–1776. [DOI] [PubMed] [Google Scholar]
- 44. Pfefferbaum, A, Rosenbloom, M. Skull thickness influences P3 amplitude. Psychopharmacol Bull. 1987;23:493–496. [Google Scholar]
- 45. Nir Y, et al. Regional slow waves and spindles in human sleep. Neuron. 2011;70(1):153–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vyazovskiy VV, et al. Sleep homeostasis and cortical synchronization: II. A local field potential study of sleep slow waves in the rat. Sleep. 2007;30(12):1631–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Riedner BA, et al. Sleep homeostasis and cortical synchronization: III. A high-density EEG study of sleep slow waves in humans. Sleep. 2007;30(12):1643–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Esser SK, et al. Sleep homeostasis and cortical synchronization: I. Modeling the effects of synaptic strength on sleep slow waves. Sleep. 2007;30(12):1617–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Carrier J, et al. Sex differences in age-related changes in the sleep-wake cycle. Front Neuroendocrinol. 2017;47:66–85. [DOI] [PubMed] [Google Scholar]
- 50. Mong JA, et al. Sex differences in sleep: impact of biological sex and sex steroids. Philos Trans R Soc Lond B Biol Sci. 2016;371(1688):20150110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ujma PP, et al. Individual slow-wave morphology is a marker of aging. Neurobiol Aging. 2019;80:71–82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.