Abstract
Background
Premature ovarian insufficiency (POI) is one of the major causes of infertility. We previously demonstrated that transplantation of menstrual blood-derived stromal cells (MenSCs) effectively improved ovarian function in a murine model of POI. Recent studies indicated that mesenchymal stem cell-derived exosomes were important components in tissue repair. In this study, we investigated the therapeutic effects of MenSCs-derived exosomes (MenSCs-Exos) in a rat model of POI and its mechanism in restoring ovulation.
Methods
Ovaries of 4.5-day-old Sprague Dawley rats (SD rats) were cultured in vitro to evaluate the effects of MenSCs-Exos exposure on early follicle development. Furthermore, POI in rats was induced by intraperitoneal administration of 4-vinylcyclohexene diepoxide (VCD). Forty-eight POI rats were randomly assigned to four groups, each receiving a different treatment: PBS, MenSCs, MenSCs-Exos, and Exo-free culture supernatant of MenSCs. Estrous cyclicity, ovarian morphology, follicle dynamics, serum hormones, pregnancy outcomes, and molecular changes were investigated.
Results
Exposure to MenSCs-Exos promoted the proliferation of granulosa cells in primordial and primary follicles in vitro and increased the expression of early follicle markers Deleted In Azoospermia Like (DAZL) and Forkhead Box L2 (FOXL2) while inhibiting follicle apoptosis. In vivo, MenSCs-Exos transplantation effectively promoted follicle development in the rat model of POI and restored the estrous cyclicity and serum sex hormone levels, followed by improving the live birth outcome. In addition, transplantation of MenSCs-Exos regulated the composition of the ovarian extracellular matrix and accelerated the recruitment of dormant follicles in the ovarian cortex and increased proliferation of granulosa cells in these follicles.
Conclusion
MenSCs-Exos markedly promoted follicle development in vitro and in vivo and restored fertility in POI rats, suggesting a restorative effect on ovarian functions. The therapeutic effect of MenSCs-Exos transplantation was sustainable, consistent with that of MenSCs transplantation. Our results suggested that MenSCs-Exos transplantation may be a promising cell-free bioresource in the treatment of POI.
Keywords: Premature ovarian insufficiency, Menstrual blood-derived stromal cells, MenSCs, Exosomes, Rat model
Background
Premature ovarian insufficiency (POI) is defined as the loss of ovarian activity before the age of 40 and is characterized by hormone imbalances, menstrual disorders (amenorrhea or oligomenorrhea), anovulation, and infertility [1]. According to global epidemiological data, POI affects approximately 1% of women younger than age 40, 0.1% of women younger than age 30, and 0.01% of women younger than age 20 [2, 3]. Iatrogenic factors, autoimmune diseases, and genetic abnormalities are the main causes of POI [4, 5].
The clinical features of POI are follicle dysfunction and follicle depletion [6]. The majority of POI patients are diagnosed with diminished ovarian reserve (DOR), which is manifested by the rapid depletion of primordial follicle pool [7]. Between 25 and 50% of POI patients retain menstrual cycles with detectable follicles; however, most of these remnant follicles are dormant. It is difficult for POI patients to restore fertility, and only about 5% of POI patients achieved a clinical pregnancy as result of spontaneous remission [8, 9]. In severe cases, conventional assisted reproductive technology provided a low prognosis unless follicle donation was accepted [10]. In addition, low ovarian response is common in POI patients. Only about 24% of POI patients are able to get ovulatory resumption after hormone supplementation, with a live birth rate of 4%, suggesting that fertile follicles may still be present in some ovaries [11, 12]. Therefore, restoring ovarian reserve and activating dormant follicles are potential strategies for POI treatment.
Recently, transplantation of mesenchymal stem cells (MSCs) was found to be a promising therapy for POI patients. MSCs derived from bone marrow (BMMSCs), umbilical cord (UCMSCs), adipose (ADMSCs), uterine endometrium (EnSCs), and amniotic membrane (AMSCs) have been reported to improve ovarian functions in murine models [13–17]. Our studies using POI mouse models also showed ovulatory improvement and fertility restoration after transplantation of menstrual blood-derived stromal cells (MenSCs) [18]. Recent studies suggested that in situ proliferation and trans-differentiation may not be the main mechanisms through which MSCs improve ovarian function [19–21].
Exosomes (Exos) are extracellular vesicles with a size range between 40 and 150 nm in diameter and could play paracrine roles. By delivering cytoplasmic components of proteins, non-coding RNA, and growth factors, Exos participate in biological processes such as cell proliferation, apoptosis, cytokine production, and immune and metabolic regulation [22]. It has been shown that Exos derived from MSCs have the same efficacy in tissue regeneration and injury restoration as their source cells [23].
Here, we aimed to evaluate whether Exos derived from MenSCs could promote follicular recruitment and development in vitro and investigated the possibility of MenSCs-Exos transplantation in ovulatory restoration in vivo as a cell-free substitute for MenSCs as a strategy for POI treatment.
Materials and methods
Ethical approval
This study was approved by the Ethics Committee of the Shengjing Hospital of China Medical University (2020PS461K) and has been performed in accordance with the principles of the Declaration of Helsinki.
Culture and identification of MenSCs
The culture of MenSCs was consistent with our previous study [24]. Briefly, MenSCs were separated from menstrual blood samples and were cultured in DMEM/F12 medium (1:1, HyClone, Logan, UT, USA) with 10% fetal bovine serum (Gibco, Waltham, MA, USA) under 37 °C with atmosphere containing 5% CO2. The culture medium was changed every 3 days. Cells of passage 3 (P3) were collected to identify MSC surface markers of CD44, CD73, CD90, and CD105 using flow cytometry. In this study, P3-P6 cells were selected and labeled with green fluorescent protein (GFP) by lentiviral transfection (MOI 20).
Extraction and identification of MenSCs-Exos
Briefly, when MenSCs reached 80% confluence, P3-P6 MenSCs were washed twice with PBS and cultured with serum-free DMEM/F12 for 24 h. The supernatant was collected and centrifuged at 2000×g for 10 min to remove cell debris and filtered through a 0.22-μm filter. Then, the supernatant was ultracentrifuged at 10,000×g for 1 h and 100,000×g for 4 h at 4 °C to concentrate exosomes (HITACHI CS120FNX, Japan). Exosomes were labeled with DiI (5 μl/ml, Invitrogen, USA, Cat#C70001) by incubation at 37 °C for 5 min and 4 °C for 15 min before ultracentrifugation at 100,000×g for 30 min to remove the un-conjugated dye. The enriched exosomes were diluted in PBS and filtered through a 0.22-μm filter before stored at − 80 °C (named as MenSCs-Exos). The supernatant with exosomes removed was filtered through a 0.22-μm filter and stored at − 80 °C (named as Exo-clear). Transmission electron microscopy (TEM, HITACHI H-7650, Japan) and nanoparticle tracking analysis (NTA) were used to detect the morphology and size of exosomes. Bicinchoninic acid (BCA) protein quantification kit (Beyotime, China, Cat#P0010S) was used to evaluate the protein concentration. Western blot was used to evaluated protein expressions of CD81 and TSG101 (1:1000, Proteintech, China) to identify exosomes.
Ovarian culture in vitro and analysis
Ovaries from 4.5-day-old female SD rats were harvested and cultured in 8-μm transwell inserts under 37 °C with atmosphere containing 5% CO2. The culture medium was 10% FBS-DMEM medium containing 0.05 IU/ml follicle-stimulating hormone (FSH) (LIVZON, China). MenSCs-Exos (2 μg/ml or 20 μg/ml) were added. Ovaries were randomly assigned to receive different treatment (n = 5): control, Low-Exos (2 μg/ml), and High-Exos (20 μg/ml). Ovaries were incubated in 300 μl culture medium for 2, 4, and 6 days. The culture medium was changed every 48 h. 1:300 5-ethynyl-2′-deoxyuridine (EdU) solution was added to the medium to detect the proliferation of ovarian cells, before the end of culturing. After EdU incubation for 4 h, the ovaries were collected and fixed in 4% paraformaldehyde. Paraffin sections of 5 μm were prepared. EdU immunofluorescence staining was prepared following the instructions (Riobio, China, C10310). Expressions of DAZL and FOXL2 were evaluated using immunofluorescence using specific antibodies (1:100, Proteintech). Cell apoptosis was detected using TUNEL staining (TransDetect® In Situ Fluorescein TUNEL Cell Apoptosis Detection Kit, FA201-01) and evaluated by using Nikon Eclipse Ni (Nikon, Tokyo, Japan) and Image Pro Plus 6.
Establishment and treatment of the POI rat model
SD rats were purchased from HFK Bioscience Co. (Beijing, China) and housed in an SPF (specific pathogen-free) lab (SYXK 2017-0004, China) in an environment with a temperature of 22 ± 1 °C, relative humidity of 50 ± 1%, and a light/dark cycle of 12/12 h. Sterilized food and water were available ad libitum. All animal studies (including the euthanasia procedure) were performed in compliance with the regulations and guidelines of China Medical University institutional animal care and conducted according to the AAALAC and the IACUC guidelines.
The chemical compound VCD (4-vinylcyclohexene diepoxide, Merk Millipore) was intraperitoneally injected into female SD rats with regular estrous cycles (7 weeks old, body weight 180–200 g) for 15 consecutive days (80 mg/kg per day). Vaginal smears for 7 consecutive days were taken from the 8th day of injection to confirm the modeling effect. After that, forty-eight modeled rats were randomly signed into four groups: PBS injection group (control group), 5 × 105 MenSCs per ovary suspended in PBS injection group (MenSCs group,), 25 μg MenSCs-Exos per ovary suspended in PBS injection group (MenSCs-Exos group), and exosome-removed MenSC culture supernatant injection group (Exo-clear group). The received fluid amount was 50 μl per ovary, and intra-ovary injection was performed with a 32G syringe. Every 5 days, all rats received caudal intravenous injection of 100 μl: PBS for control and MenSCs group, 50 μg exosomes for MenSCs-Exos group, and exosome-removed MenSC culture supernatant for Exo-clear group. Daily vaginal smears were performed from the 7th day of treatment for 21 days. After 28 days of treatments, eight rats of each group were sacrificed at the first diestrous phase and then the ovaries and serum samples were harvested. The main organs (brain, heart, lung, liver, spleen, kidney, uterus, and ovary) of MenSCs and MenSCs-Exos group were used for bioluminescence imaging (BLI) using the in vivo MS FX Pro system (Carestream, USA) and analyzed by the Carestream MI SE software. After removing the surrounding fat pads and fallopian tubes, ovary weights and ovary/body weight ratios were evaluated (ovary ratio = ovary weight/body weight × 100%). Then, the left ovary was fixed for histology and the right ovary and serum samples were stored at − 80 °C. Eight healthy female SD rats were assigned as the normal group.
ELISA
Serum anti-Mullerian hormone (AMH), follicle-stimulating hormone (FSH), and estradiol (E2) levels were monitored according to the standard method of ELISA kit (CUSABIO, China). Ninety-six-well plates with antibodies were incubated with 1:10 diluting serum, and the reaction was conducted at room temperature for 2 h (n = 8). Absorbance was recorded by using a microplate reader, to determine serum hormone concentration.
Evaluation of reproductive potential
After 28 days of treatment, 4 rats in each group were randomly selected to mate with healthy male SD rats (1:2). The second morning, sperm plugs represented successful mating. Each female rat was mated again after delivery and lactation (3 weeks postpartum). The number of cubs of the two births was recorded. Female rats that were sperm positive for three consecutive times but did not give birth were judged to be infertile.
Histological analysis
Rat ovaries were fixed with 4% paraformaldehyde, dehydrated, and embedded in paraffin. A series of 5-μm sections were prepared and one of every five sections was selected for hematoxylin and eosin staining (H&E). Ovarian morphology was observed and photographed with an optical microscope (Nikon 80i). The total number of primordial follicles, primary follicles, secondary follicles, antral follicles, and preovulatory follicles were counted.
Real-time PCR
The extraction of ovarian RNA and RT-PCR analysis were described by us previously [24]. Briefly, total RNA was extracted from tissues by using the RNAiso Plus kit (Takara, China, Cat#9108) before reverse transcribed to generate cDNA (Takara, China, Cat#RR047A). Quantitative real-time PCR using 96-well optical plates was performed in a SYBR Green (Takara, China, Cat#RR820A) format with 7500 Fast Real-Time PCR Detection Systems (Life Technology); each sample was done in triplicates. We detected the genes related to apoptosis of Bcl2 (Bcl2, apoptosis regulator), Bad (Bcl2 associated agonist of cell death), Bax (Bcl2 associated X), and Casp8 (Caspase 8) between the control group and low-Exos group after 2 days of in vitro culturing (n = 6). We also evaluated the gene expression of follicles development: Zp3 (zona pellucida glycoprotein 3), Th (tyrosine hydroxylase), Amh (anti-Mullerian hormone), and Fshr (follicle stimulating hormone receptor) in POI ovaries after treatments (n ≥ 6). Primer sequences used for each target gene are summarized in Additional file 1: Table S1. Analyses of relative gene expressions were performed using 2−ΔΔCT methods. Ratios of mRNA expression were given as fold-changes relative to untreated controls after normalizing to allogeneic Gapdh housekeeping gene.
Immunohistochemistry and immunofluorescence
Ovarian paraffin sections were dewaxed with xylene and rehydrated with alcohol gradient before immunohistochemistry and immunofluorescence. Following the instructions of the immunohistochemical kit (SP-9000, ZSGB-BIO, China), after the sodium citrate solution heating and repairing (ZLI-9064 pH 6.0, ZSGB-BIO, China), all slices were sealed with H2O2 and 10% goat serum. The sections were then incubated with the first antibody at 4 °C overnight (Ki-67, Collagen 1 and FOXL2,1:100, Proteintech, China; Collagen IV, FN1 and Laminin,1:100, Bioss, China). On the second day, all sections were washed with PBS and incubated with the secondary antibody for 2 h at room temperature. The primary antibody was replaced by PBS as a negative control. The detection of bound antibody was performed using avidin-biotin complex. DAB was performed at room temperature for 1 min as chromogen. The nuclei were counterstained with Mayer’s hematoxylin for 2 min. Staining intensity was recorded as 0 (negative), 1 (weak), 2 (medium), and 3 (strong). The positive cells were observed and evaluated using an optical microscope (Nikon 80i).
Statistical analysis
One-way analysis of variance (ANOVA) was used to analyze variables among groups, and Bonferroni post hoc tests were performed for multiple comparisons when statistical significance was recognized. All numerical values are presented as the mean ± standard deviation (SD), and *P < 0.05, **P < 0.01, and ***P < 0.001 were considered to indicate a statistically significant difference. All statistical analyses were performed using GraphPad Prism 8 software (San Diego, CA, USA).
Results
Characterization of MenSCs and MenSCs-Exos
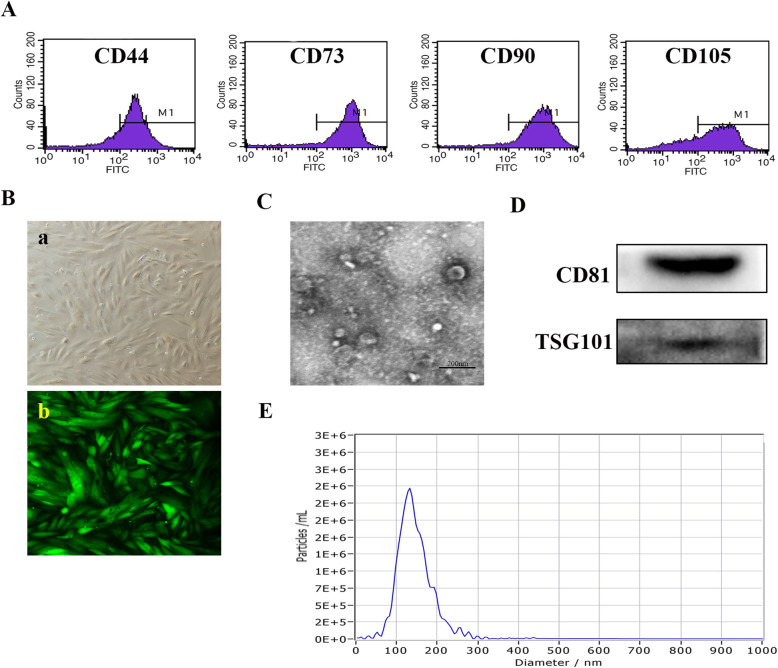
As our previous studies described [24–26], MenSCs had a spindle shape, fibroblast-like morphology, and tested positive for CD44, CD73, CD90, and CD105 (Fig. 1A, B). Exosomes isolated from serum-free culture supernatant of MenSCs were characterized by TEM, NTA, and western blotting. MenSCs-Exos exhibited a sphere-shaped morphology with a concentration of 1.1 × 1010 particles/ml and a peak diameter of 128 nm. BCA analysis showed that the protein concentration of MenSCs-Exos was 1.75 mg/ml. MenSCs-Exos were confirmed to be positive for CD81 and TSG101 by Western blot analysis (Fig. 1C–E).
Fig. 1.
Characterization of MenSCs and MenSCs-Exos. a Surface markers of MenSCs detected by Flow cytometry. b Morphology of P3 MenSCs and GFP-labeled MenSCs (200×). c Morphology of MenSCs-Exos observed by TEM (scale bar = 200 nm). d Western blots of CD81 and TSG101 of MenSCs-Exos. e Size distribution of MenSCs-Exos examined by NTA
MenSCs-Exos treatment promoted early follicular development and inhibited apoptosis in vitro
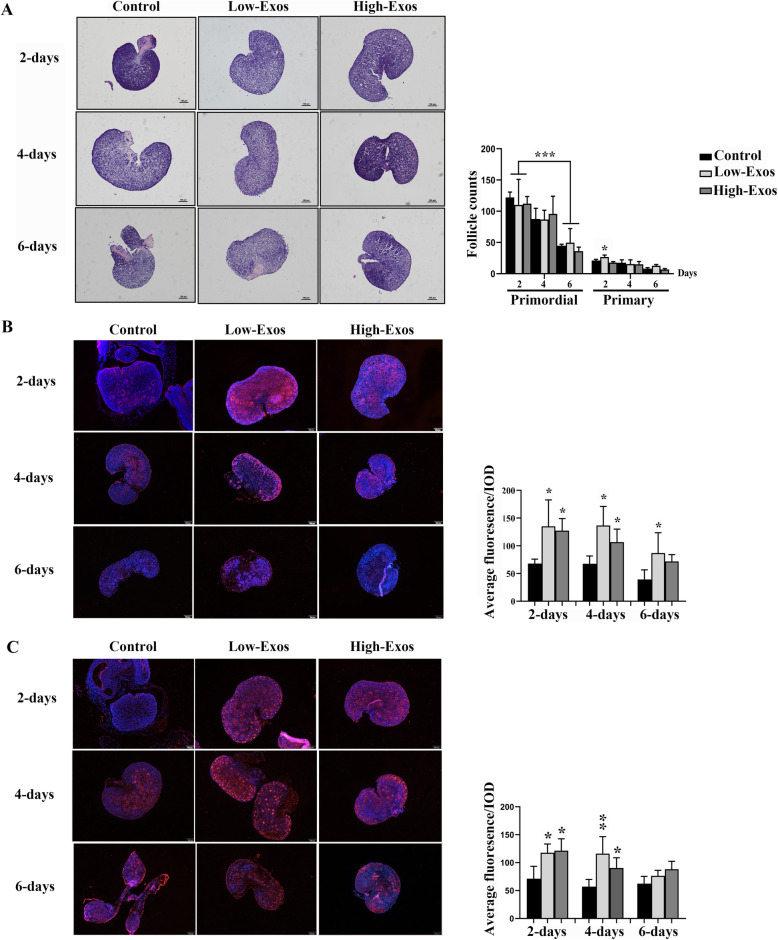
The ovaries of 4.5-day-old rats were cultured in culture medium containing 0, 2 μg/ml (low-Exos), or 20 μg/ml (high-Exos) of MenSCs-Exos for 2, 4, and 6 days. With the extension of culture time, there was significant decrease in the number of primordial follicles with nuclei in the ovaries of all groups. At the same time, atretic follicles were observed. Low-Exos treatment for 2 days significantly increased the number of primary follicles in ovaries cultured in vitro (Fig. 2a). Next, we evaluated follicle markers in the 3 groups. Compared to the control group, Low-Exo treatment for 2–6 days, and High-Exo treatment for 2 and 4 days, significantly increased the DAZL immunofluorescence intensity in the ovaries cultured in vitro (Fig. 2b). In addition, after 2 and 4 days of treatment, a significant increase in the expression of FOXL2, a marker for granulosa cells, was detected in the ovaries of the Low-Exos and High-Exos groups (Fig. 2c).
Fig. 2.
Ovarian morphology and immunofluorescence analysis of rat ovaries cultured in vitro. a H&E staining of ovaries sections (× 100) and follicles counts. b Immunofluorescence of DAZL in ovaries (red) and statistics of fluorescence intensity. Nuclei were stained by using DAPI (blue, 100×, n ≥ 4). c Immunofluorescence of FOXL2 of ovaries and statistics of fluorescence intensity. Nuclei were stained by using DAPI (blue, × 100, n ≥ 4). Data were represented as mean ± SD, *p < 0.05, **p < 0.01, ***p < 0.001
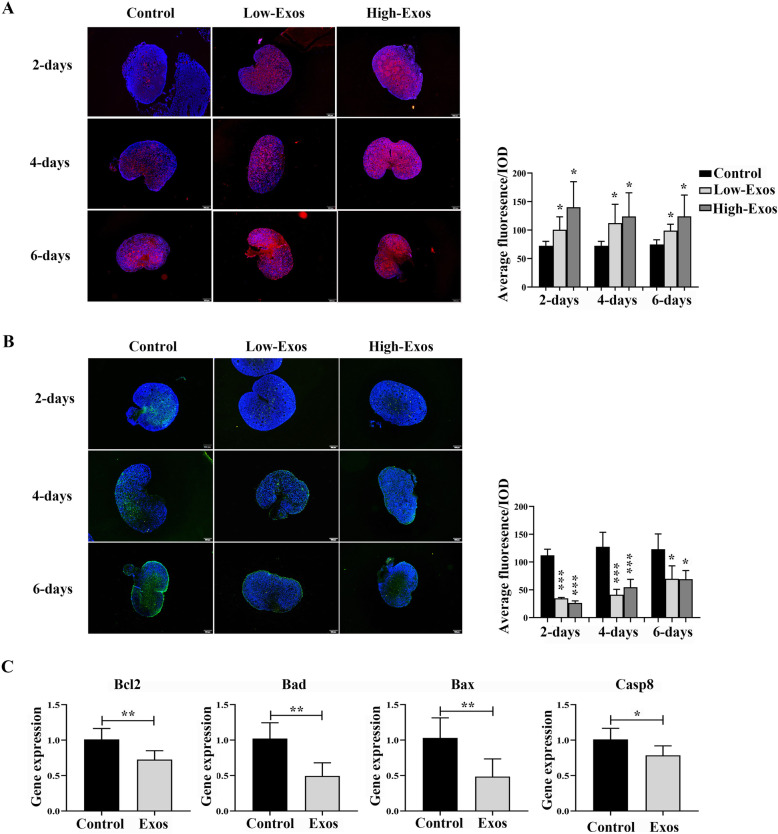
EdU-positive cells, as indicated by the high fluorescence intensity, were detected in both the Low-Exos and High-Exos groups after 2, 4, and 6 days of culture (Fig. 3a). With the extension of culture time, we observed that in the ovaries of all groups, TUNEL-positive cells increased from the center of the ovary with some follicles being TUNEL-positive. A significant decrease in TUNEL fluorescence intensity levels was detected in both the Low-Exos and High-Exos groups during the entire ovarian culture experiment (Fig. 3b). There was no difference between the Low-Exos and High-Exos groups with respect to either EdU- or TUNEL-positive fluorescence intensity. In addition, we examined the expression of apoptosis-related genes in the control and Low-Exos groups after 2 days of in vitro incubation (n = 6). The results indicated that MenSCs-Exos treatment for 2 days significantly downregulated the expression of Bcl2 (p = 0.0059), Bad (p = 0.0013), Bax (p = 0.0054), and Casp8 (p = 0.0302) (Fig. 3c). The above results were consistent with the TUNEL staining. These results indicated that MenSCs-Exos could act on the early stage of oocytes, promote follicular development and granulosa cell proliferation, and inhibited tissue apoptosis.
Fig. 3.
Cell proliferation and apoptosis of rat ovaries cultured in vitro. a EdU staining of ovaries (red) and statistics of fluorescence intensity. Nuclei were stained by using DAPI (blue, × 100, n ≥ 4). b TUNEL staining of apoptosis (green) and statistics of fluorescence intensity. Nuclei were stained by using DAPI (blue, × 100, n ≥ 4). c RT-PCR analysis of Bcl2, Bad, Bax, and Casp8 in ovaries (n = 6). Data were represented as mean ± SD, *p < 0.05, **p < 0.01, ***p < 0.001
MenSCs-Exos showed tropism toward the ovaries of POI rats
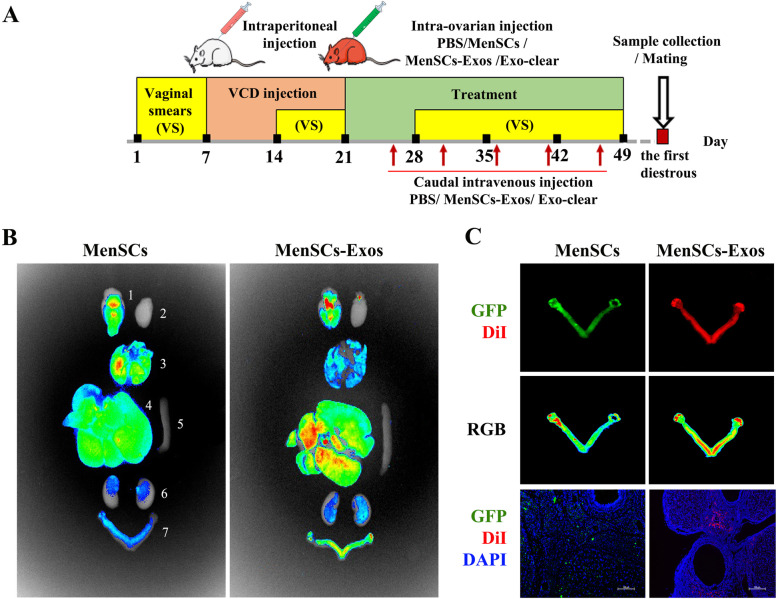
Scheme of in vivo experiments was shown in Fig. 4a. Briefly, we administered VCD intraperitoneally to SD rats (with regular motility cycles) for 15 consecutive days to establish the POI phenotype. After confirming the irregular cycle, 48 POI rats were randomly grouped for a single intra-ovarian injection treatment. Subsequently for every 5 days, rats in the MenSCs-Exos group received exosomes, the Exo-clear group received exosome-removed MenSC culture supernatant and other rats received PBS via caudal intravenous injection. Vaginal smear was observed continuously for 28 days during treatment. At the first diestrous post-treatment, 8 rats in each group were sacrificed and 4 rats were caged with males to observe two-times fertility outcomes.
Fig. 4.
Experimental procedure chart and locations of transplants in MenSCs and MenSCs-Exos group. a Scheme of this experiment. b Fluorescence intensity (RGB) in main organs ((1) brain, (2) heart, (3) lung, (4) liver, (5) spleen, (6) kidney, (7) genital system; blue, low fluorescence intensity, green, moderate fluorescence intensity, red, high fluorescence intensity). c Fluorescence localization in genital system and in frozen sections (green, GFP, red, DiI, blue, DAPI, × 100)
To trace the location of the transplanted cells and exosomes, MenSCs were transfected with GFP and MenSCs-Exos were labeled with DiI. According to the results of BLI, GFP intensity was strong in the basal ganglia and lungs; moderate in the liver; weak in the ovaries, uterus, and kidneys; and non-existent in the heart and spleen after 28 days of treatment. As for the fluorescence of DiI, the intensity was strong in the liver and basal ganglia, moderate in ovaries and uteri, weak in the lungs and kidneys, and non-existent in the heart and spleen (Fig. 4b). A strong GFP signal was detected in the bilateral ovarian cortex. The DiI signal was detected in the bilateral ovarian cortex and the lower uterine segment. It was observed that MenSCs and MenSCs-Exos were integrated in the somatic cells of the ovarian cortex using immunofluorescence (Fig. 4c). These results indicated that both MenSCs and MenSCs-Exos showed tropism toward the ovarian cortex in POI rats.
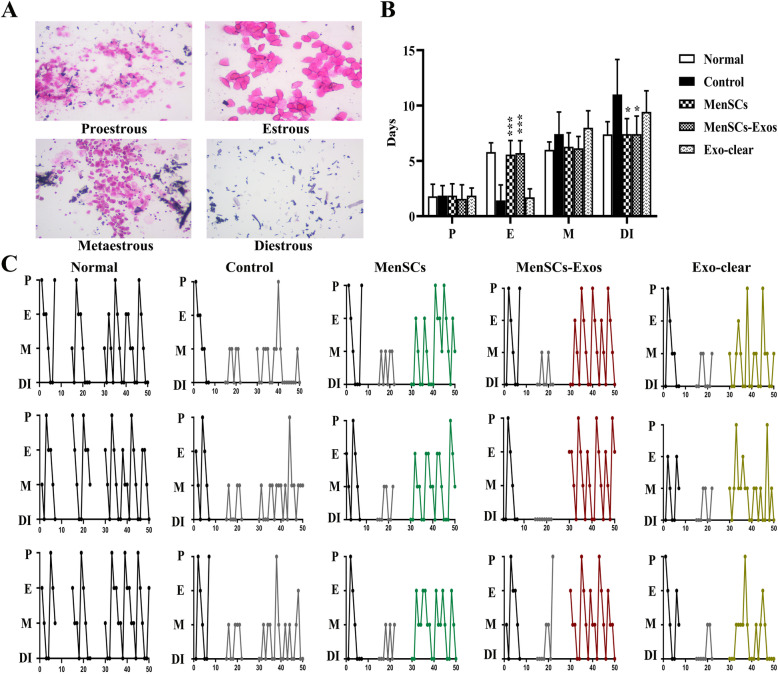
MenSCs-Exo treatment restored the estrous cyclicity in POI rats in vivo
We compared the stages of the estrous cycle in 4 treatment groups and the control group. Regular estrous cycles were founded in control female rats, showing a stable pattern. In the control group, the diestrus stage (DI stage) was predominant, followed by metestrus stage (M stage), almost without proestrus stage (P stage), and estrus stage (E stage). Compared with the control group, the frequency of E stage was significantly increased in the MenSCs and MenSCs-Exos groups (PMenSCs < 0.0001, PMenSCs-Exos < 0.0001), and the DI stage was significantly decreased (PMenSCs = 0.0250, PMenSCs-Exos = 0.0262). There was no statistical difference between the Exo-clear group (Fig. 5b) and the control. After VCD injection, it was observed that the estrous cycle of SD rats mainly changed into the M stage and the DI stage. The rats in the control group and the Exo-clear group had irregular estrus cycles after treatment. After MenSC transplantation, regular estrous cycles were restored in 11 rats, and a total of 40 cycles were observed with an average of 4.85 days. All rats in the MenSCs-Exos group showed restored regular estrous cycles, and 45 cycles were observed with an average of 4.44 days. Four stages were observed in the Exo-clear group but without a regular pattern (Fig. 5c).
Fig. 5.
Statistics of estrous cycle in different group of rats. a H&E staining of vaginal smears (× 100×). b Compositions of estrous cycle in different groups (n = 8; P, proestrous; E, estrous; M, metaestrous; DI, diestrous). c Typical estrous cycle patterns in normal rats and in four treatment groups. Data were represented as mean ± SD, *p < 0.05, **p < 0.01, ***p < 0.001
MenSCs-Exos improved ovarian morphology, increased follicle numbers, regulated serum hormones, and restored fertility in POI rats in vivo
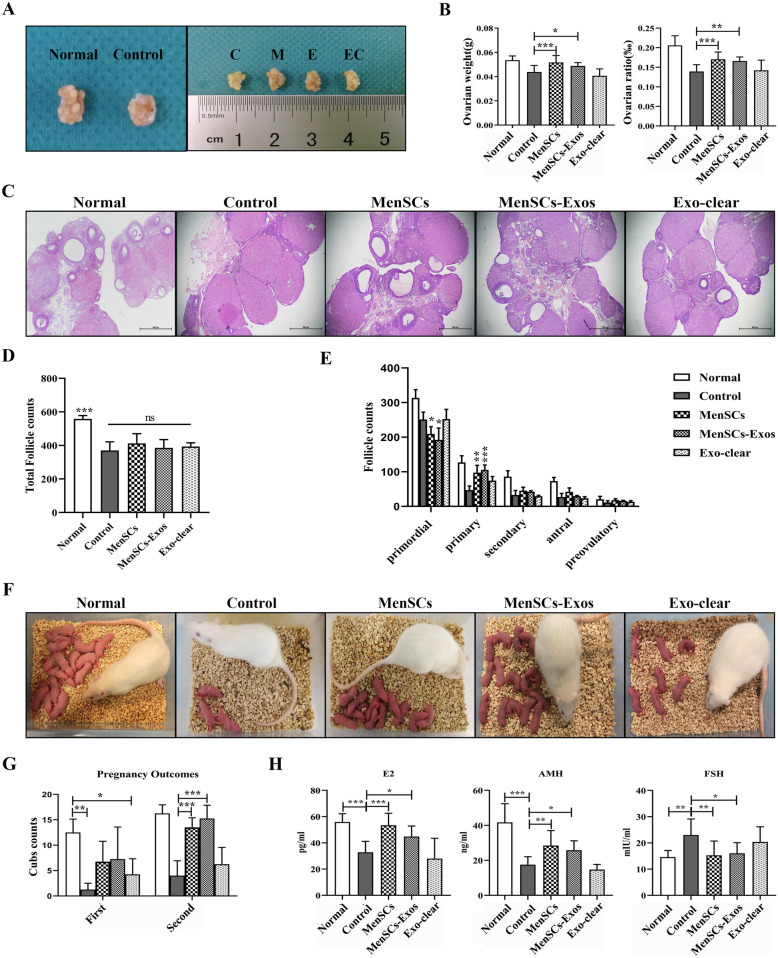
VCD treatment decreased ovarian sizes whereas ovarian sizes of POI model rats increased after receiving MenSCs or MenSCs-Exos transplantation (Fig. 6a). Compared to the control group, the ovarian weight (PMenSCs = 0.0008, PMenSCs-Exos = 0.0054) and ovarian ratios (PMenSCs = 0.0001, PMenSCs-Exos < 0.0001) were significantly increased in both the MenSCs and MenSCs-Exos groups, but not in the Exo-clear group (Fig. 6b).
Fig. 6.
Ovarian morphological, ELISA assays, and live birth outcomes after treatments. a Representative ovarian morphology ((C) control group, (M) MenSCs group, (E) MenSCs-Exos group, (EC) Exo-clear group). b Ovarian weight (left) and ovarian/body weight ratio (right). c H&E staining of ovarian sections (× 40). d Total follicle counts. e Counting of follicles at different stages. f Live births photos of normal rats and four treatment groups. g Live births counts. h Serum levels for E2 (left), AMH (middle), and FSH (right) (n = 8). Data were represented as mean ± SD, *p < 0.05, **p < 0.01, ***p < 0.001
Histological analyses indicated that VCD treatment led to ovarian atrophy, mainly consisting of corpora lutea. In the MenSCs and MenSCs-Exos groups, there were a number of primordial, primary, secondary, and antral follicles together with corpora lutea. In contrast, only a few follicles were observed in the Exo-clear group (Fig. 6c). The total number of follicles in all POI ovaries decreased significantly when compared with the normal group, and there was no difference in the total number of follicles between the four treatment groups (Fig. 6d). Compared to the control group, after MenSCs and MenSCs-Exos transplantation, the number of primordial follicles significantly decreased while the number of primary follicles increased markedly. There was no difference in the number of secondary follicles, antral follicles, and preovulatory follicles in each group (Fig. 6e).
The fertility of POI rats was assessed with consecutive mating experiments. All females were mated again at the end of the lactation period. After the first mating, an increasing trend in the number of live births was observed in the MenSCs and MenSCs-Exos groups, but without statistical significance. The second litter size was greater than the first, for all groups. After the second mating, the number of births in the MenSCs and MenSCs-Exos groups increased significantly (PMenSCs = 0.02, PMenSCs-Exos = 0.005), when compared to the control group, but not in the Exo-clear group (Fig. 6f, g).
Next, we evaluated the serum concentrations of E2, AMH, and FSH at the DI stage. Compared to non-treated rats, serum E2 and AMH levels in POI rats were significantly decreased (p < 0.001) and the FSH level was increased (p = 0.002). After the MenSC and MenSCs-Exo treatments, serum E2 and AMH levels in POI rats significantly increased (PMenSCs < 0.001 and PMenSCs-Exos = 0.03 for E2, PMenSCs = 0.001 and PMenSCs-Exos = 0.03 for AMH) while the FSH level significantly decreased (PMenSCs = 0.004 and PMenSCs-Exos = 0.02). There was no difference in the levels of these hormones between the MenSCs group and the MenSCs-Exos group (Fig. 6h).
These results indicated that MenSCs-Exos treatment improved the ovarian atrophy caused by VCD, effectively restoring estrous cyclicity and promoting follicle development. Multiple transplantation of MenSCs-Exos had a restorative effect on the female sex hormones, thus improving fertility in POI rats, and achieving an equivalent therapeutic effect similar to single intra-ovarian transplantation of MenSCs.
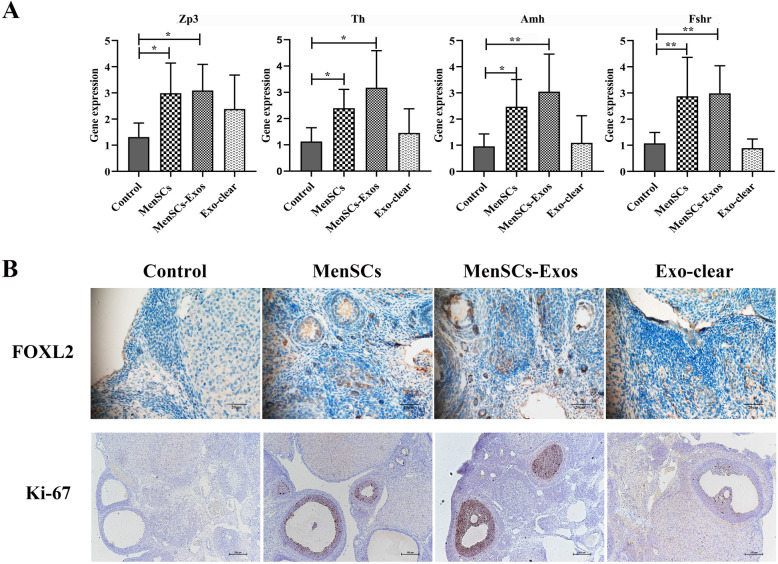
MenSCs-Exos promoted follicle development and increased the proliferation of granulosa cells in vivo
Ovarian gene expression was evaluated by RT-PCR, immunofluorescence, and immunochemistry. As evaluated by RT-PCR, Zp3 (PMenSCs = 0.04, PMenSCs-Exos = 0.02), Th (PMenSCs = 0.03, PMenSCs-Exos = 0.04), Amh (PMenSCs = 0.03, PMenSCs-Exos = 0.004), and Fshr (PMenSCs = 0.01, PMenSCs-Exos = 0.005) were significantly upregulated after MenSCs and MenSCs-Exos transplantation (Fig. 7a). Compared to the control and Exo-clear groups, there was a significant expression of FOXL2 detected in the single-layer granulosa cells of primordial follicles after the MenSCs and MenSCs-Exos treatments. The expression of Ki-67 was mainly detected in the multiple layers of granulosa cells of follicles. An increase in the levels of Ki-67-positive cells was found in the MenSCs and MenSCs-Exos groups when compared with the control group. Moreover, Ki-67-positive cells were also observed in the ovarian stroma (Fig. 7b).
Fig. 7.
Real-time PCR and immunohistochemistry analysis of follicle development in four groups. a RT-PCR analysis of Zp3, Th, Amh, and Fshr in ovaries (n ≥ 6). b Typical IHC staining of FOXL2 (× 400) and Ki-67 (× 100), n ≥ 4
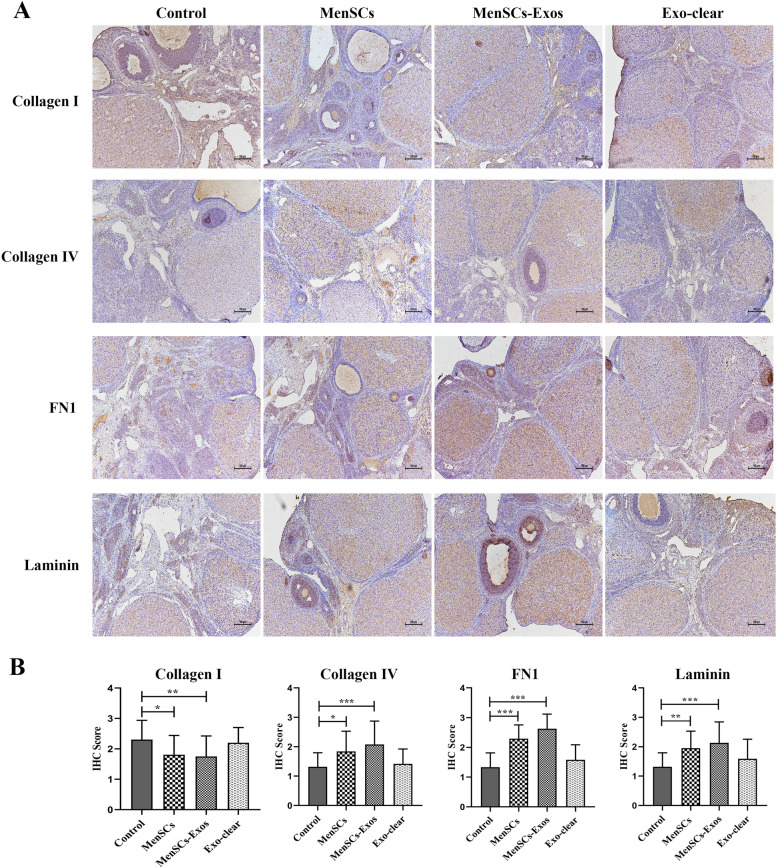
MenSCs-Exos regulated the ovarian extracellular matrix (ECM) in POI
ECM components were analyzed by immunohistochemistry. Compared to the control group, there was a significant decrease in the expression of Collagen I in the POI ovaries after MenSCs and MenSCs-Exos transplantation. In the MenSCs and MenSCs-Exos groups, the expression of Collagen IV, FN1, and Laminin significantly increased in the follicular basement membrane and ovarian stroma of the cortex. The increased expression of FN1 and Laminin was also observed in the granulosa cells. There was no statistical difference between the Exo-clear group and the control group. The above results revealed that MenSCs-Exos regulated the ECM components of POI ovaries and had the same effect as MenSCs (Fig. 8a, b).
Fig. 8.
Immunohistochemistry of ovarian ECM components in four groups. a Typical IHC staining of Collagen I, Collagen IV, FN 1, and Laminin (× 100). b IHC scores. Data were represented as mean ± SD, *p < 0.05, **p < 0.01, ***p < 0.001
Discussion
This study demonstrated that MenSCs-Exos treatment is effective in restoring ovarian functions and fertility in a rodent model of POI. Short-term incubation with MenSCs-Exos enhanced the growth and development of early stage follicles during ovarian culture in vitro, while inhibiting the apoptosis of ovarian cells. In the POI rat model, repeated applications of MenSCs-Exos reversed ovarian insufficiency induced by a chemo-toxic drug, markedly promoted follicular development, and restored hormonal profiles, ovarian ECM remodeling, and fertility in vivo. The therapeutic effect of repeated applications of MenSCs-Exos was comparable to that of MenSC transplantation, suggesting a promising biological alternative to the use of cell-free bioresource for the restoration of ovarian function.
Over the last decade, MSC transplantation has been shown to be effective in the repair of systemic injuries such as in the motor system, nervous system, digestive system, and more specific reproductive system [27]. MenSCs are endometrial stem cells isolated from menstrual blood shedding with typical MSC characteristics. In addition to having similar functions and mechanisms to common sources of MSCs (BDMSCs, ADMSCs, and UCMSCs), MenSCs’ periodic non-invasive acquisition and ability to be used for autologous transplantation highlights their great potential for clinical applications [28]. In our rodent study, we demonstrated that MenSCs effectively restored ovulation and fertility in a POI murine model [18]. Although MSC application is widely considered to be safe and almost nontumorigenic, the risk of contamination and embolization should not be overlooked [29, 30]. It is worth noting that the therapeutic effects of stem cell preparations or derivatives have gradually been confirmed. Several studies suggested that the culture medium of MSCs (MSC-CM) could have therapeutic effects in POI murine models. Promotions of follicular number, ovarian angiogenesis, and decrease of granulosa cell apoptosis were detected after MSC-CM application, suggesting a paracrine effect of MSCs in ovarian treatment [31, 32]. Compared to MSC transplantation, Exos are biocompatible and non-immunogenic, thus allowing allo-transplantation. Therefore, Exos treatment may be a promising cell-free therapy in regenerative medicine. In addition, the application of Exos can be personalized which makes MSC-Exo-based therapy a safer and more effective treatment than MSC transplantation [33]. Exos derived from ADMSCs and AMSCs that were demonstrated to restore ovulatory function in a POI mouse model, mainly acted on granulosa cells [34, 35]. Recently, UCMSCs-Exos were found to improve oocyte production and quality in aged female mice in vivo and accumulated primordial follicles in vitro, suggesting a new approach of mitigating fertility retardation [36]. Similarly, we demonstrated therapeutic effects of MenSCs-Exos in restoring ovarian function in rodent POI model.
In this study, we established a rat model of POI by intraperitoneal injection of VCD. VCD is a chemical compound causing small preantral (primordial and primary) follicle degeneration in rats [37]. Therapeutically, gonadotropin treatments mainly act on growing follicles, while the remaining dormant primordial follicles are not affected in the POI ovaries. For POI patients, in vitro activation (IVA) was effective by following in vitro treatment of ovarian cortical pieces with Akt-stimulating drugs to activate dormant primordial follicles and then re-transplanted under the serous membrane of the fallopian tube [38]. Recently, IVA application without the use of PTEN/PI3K/Akt signaling modulators has been demonstrated effective in follicular activation [39]. However, the operational procedures and equipment requirements remain as challenges for the clinical application of IVA. Our recent results indicated the benefits of MenSCs-Exos in ovarian culture in vitro. In this study, we found the promotion of primordial follicular activation and development by using cell-free MenSCs-Exos, as well as inhibition of cell apoptosis during ovarian culture in vitro.
The successful maturation of oocytes is associated with hormone regulation, granulosa cell function, corpus luteum development, and ovarian microenvironment [40]. Li et al. suggested that the synchronous development of oocytes and ovarian cells plays a key role in follicular formation [41]. Increased FOXL2 expression was also detected in the MenSCs and MenSCs-Exos groups. As a marker of pre-granulosa cells, FOXL2 is essential for the functional maintenance of follicle development. An absent expression of FOXL2 is suggested to be related with abnormal structure and activity of granulosa cells, resulting in follicular formation failure [42, 43]. The ovarian stroma has been shown to play a key role in follicular development. In the cortex of the adult ovary, there is a continuous basal layer between the ovarian stroma, corpus luteum, follicle, and the ovarian epithelium. These basal layers generally consisted of laminin, collagen, and fibronectin, which constitute the follicular ECM. In addition, follicular ECM is believed to be involved in maintaining follicular growth, which is considered a part of the follicular microenvironment [44]. In this study, we found that MenSCs-Exos enhanced the expression of Laminin, Collagen IV, and FN1 in POI ovaries, suggesting an effect on ovarian microenvironment regulation. In 2019, Macdonald et al. indicated that the addition of Collagen IV promoted meiosis and follicular formation in vitro. Laminin is suggested as an important ECM component for human follicular development. Secreted by oocytes, Laminin has been proved to promote ovarian stem cell differentiation in vitro [45].
There are some limitations in the present study that need to be pointed out: (1) In this study, it was preliminarily confirmed that MenSCs-Exos promoted both oocytes and granulosa cells. Yet follicular maturation is dependent on the interaction between oocyte and granulosa cells. Further explorations of MenSCs-Exos effects on oocyte-granulosa cross-talking or gap-junction are necessary. (2) The molecular mechanism involved in MenSCs-Exos needs to be further explored. Identifying the active components of MenSCs-Exos for improving ovarian function (such as protein or micro-RNA) is conducive to improving the efficiency of MenSCs-Exos in the treatment of POI.
Conclusions
In summary, we confirmed that MenSCs-Exos treatments were beneficial in vitro and had a significant therapeutic effect in restoring ovarian functions in a rat POI model. Ovarian reserve, serum hormones, and fertility were all improved after MenSCs-Exo transplantation. However, the molecular mechanisms by which MenSCs-Exos restore ovarian function remain to be further explored. Overall, our results suggested that MenSC-Exos transplantation may be a promising cell-free bioresource in the treatment of POI.
Supplementary Information
Additional file 1: Table S1. Quantitative polymerase chain reaction primer sequences.
Acknowledgements
We are grateful to Aaron J.W. Hsueh for insightful comments and writing guidance. We wish to express our gratitude to Penghui Feng, Xudong Zhang, and Chao Han for figure guidance and writing guidance.
Consent
All authors agreed to publish this article.
Abbreviations
- POI
Premature ovarian insufficiency
- MenSCs
Menstrual blood-derived stromal cells
- MenSCs-Exos
MenSCs-derived exosomes
- SD rats
Sprague Dawley rats
- VCD
4-Vinylcyclohexene diepoxide
- DAZL
Deleted In Azoospermia Like
- FOXL2
Forkhead Box L2
- DOR
Diminished ovarian reserve
- MSCs
Mesenchymal stem cells
- BMMSCs
Bone marrow-derived stem cells
- UCMSCs
Umbilical-derived stem cells
- ADMSCs
Adipose-derived stem cells
- EnSCs
Endometrium-derived stem cells
- AMSCs
Amniotic membrane-derived stem cells
- Exos
Exosomes
- P3
Passage 3
- P6
Passage 6
- GFP
Green fluorescent protein
- PBS
Phosphate-buffered saline
- TEM
Transmission electron microscopy
- NTA
Nanoparticle tracking analysis
- BCA
Bicinchoninic acid
- FSH
Follicle-stimulating hormone
- EdU
5-Ethynyl-2′-deoxyuridine
- SPF
Specific pathogen-free
- BLI
Bioluminescence imaging
- AMH
Anti-Mullerian hormone
- E2
Estradiol
- H&E
Hematoxylin and eosin
- ELISA
Enzyme-linked immunosorbent assay
- Bcl2
Bcl2, apoptosis regulator
- Bad
Bcl2-associated agonist of cell death
- Bax
Bcl2-associated X
- Casp8
Caspase 8
- Zp3
Zona pellucida glycoprotein 3
- Th
Tyrosine hydroxylase
- Fshr
Follicle-stimulating hormone receptor
- ECM
Extracellular matrix
- ANOVA
One-way analysis of variance
- SD
Standard deviation
Authors’ contributions
SZ conducted the experiments, collected and analyzed the data, and prepared the manuscript; PS established the animal models; QC prepared the exosomes; PL and ZY provided the technical guidance; AS and XZ collected the data; BH provided technical and writing guidance; JT designed the experiments, supervised the whole work, and approved the final version of the manuscript. All authors read and approved the final manuscript.
Funding
This study was granted from the 2020 Central Government Special Fund for Local Science and Technology Development (2020JH6/10500006), the National Natural Science Foundation of China (61873257), National Key Research and Development Program (2018YFC1002105), the Key Research and Development Program of Liaoning Province (2018020222), and the Major Special Construction Plan for Discipline Construction Project of China Medical University (3110118033), the Shengjing freelance researcher plan of Shengjing Hospital of China Medical University.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
All protocols were approved by the Ethics Committee of the Shengjing Hospital affiliated to China Medical University (2020PS461K) and were consistent with the principles of the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Siwen Zhang, Email: zhangsiwenivy@foxmail.com.
Boxian Huang, Email: huangboxiannj@163.com.
Peng Su, Email: animal_sugongzuo@163.com.
Qiyuan Chang, Email: shelleychang1994@163.com.
Pingping Li, Email: lipingping0227@163.com.
Aixin Song, Email: ssci0905@163.com.
Xinyang Zhao, Email: 1207312386@qq.com.
Zhengwei Yuan, Email: yuanzw@hotmail.com.
Jichun Tan, Email: tjczjh@163.com.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13287-021-02255-3.
References
- 1.Webber L, Davies M, Anderson R, Bartlett J, Braat D, Cartwright B, Cifkova R, de Muinck K-SS, Hogervorst E, Janse F, Liao L, Vlaisavljevic V, Zillikens C, Vermeulen N. ESHRE guideline: management of women with premature ovarian insufficiency. Hum Reprod. 2016;31(5):926–937. doi: 10.1093/humrep/dew027. [DOI] [PubMed] [Google Scholar]
- 2.Michel DV, Paul D, Fauser Bart CJM. Primary ovarian insufficiency. Lancet. 2010;376(9744):911–921. doi: 10.1016/S0140-6736(10)60355-8. [DOI] [PubMed] [Google Scholar]
- 3.Cooper Amber R, Baker Valerie L, Sterling Evelina W, Ryan Mary E, Teresa W, Nelson Lawrence M. The time is now for a new approach to primary ovarian insufficiency. Fertil. Steril. 2011;95(6):1890–1897. doi: 10.1016/j.fertnstert.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barchi CE, Maria CD, Amaro DSA, Bianca B, Parente BC. Genetic aspects of premature ovarian failure: a literature review. Arch. Gynecol. Obstet. 2011;283(3):635–643. doi: 10.1007/s00404-010-1815-4. [DOI] [PubMed] [Google Scholar]
- 5.Haller-Kikkatalo K, Uibo R, Kurg A, Salumets A. The prevalence and phenotypic characteristics of spontaneous premature ovarian failure: a general population registry-based study. Hum Reprod. 2015;30:1229–1238. doi: 10.1093/humrep/dev021. [DOI] [PubMed] [Google Scholar]
- 6.Nelson LM. Clinical practice. Primary ovarian insufficiency. N. Engl. J Med. 2009;360(6):606–614. doi: 10.1056/NEJMcp0808697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ophelia Y, Kamaria C, Segars James H. In vitro activation: s dip into the primordial follicle pool? J. Clin. Endocrinol. Metab. 2016;101(10):3568–3570. doi: 10.1210/jc.2016-2837. [DOI] [PubMed] [Google Scholar]
- 8.Ben-Nagi J, Panay N. Premature ovarian insufficiency: how to improve reproductive outcome? Climacteric. 2014;17(3):242–246. doi: 10.3109/13697137.2013.860115. [DOI] [PubMed] [Google Scholar]
- 9.Ikko K, Kazuhiro K. Disorganization of the germ cell pool leads to primary ovarian insufficiency. Reproduction. 2017;153(6):R205–R213. doi: 10.1530/REP-17-0015. [DOI] [PubMed] [Google Scholar]
- 10.Torrealday Saioa., Kodaman Pinar., Pal Lubna. Premature ovarian insufficiency - an update on recent advances in understanding and management. F1000Res. 2017; 6(undefined), 2069. doi:10.12688/f1000research.11948.1 [DOI] [PMC free article] [PubMed]
- 11.Bachelot Anne, Nicolas Carole, Bidet Maud., Dulon Jérôme, Leban Monique, Golmard Jean Louis, Polak Michel, Touraine Philippe. Long-term outcome of ovarian function in women with intermittent premature ovarian insufficiency. Clin. Endocrinol. (Oxf). 2017; 86(2): 223–228. doi:10.1111/cen.13105 [DOI] [PubMed]
- 12.Chen Xin, Chen Shi-Ling, Ye De-Sheng, Liu Yu-Dong, He Yu-Xia, Tian Xiao-Long, Xu Li-Juan., Tao Ting. Retrospective analysis of reproductive outcomes in women with primary ovarian insufficiency showing intermittent follicular development. Reprod. Biomed. Online. 2016;32(4): 427–33. doi:10.1016/j.rbmo.2015.12.011 [DOI] [PubMed]
- 13.Wang Shufang, Yu Ling, Sun Min, Mu Sha, Wang Changyong, Wang Deqing, Yao Yuanqing. The therapeutic potential of umbilical cord mesenchymal stem cells in mice premature ovarian failure. Biomed Res Int. 2013(undefined), 690491. doi:10.1155/2013/690491 [DOI] [PMC free article] [PubMed]
- 14.Abd-Allah Somia H, Shalaby Sally M, Pasha Heba F, El-Shal Amal S, Nermin R, Shabrawy Sheren M, Awad Hanan A, Amer Mona G, Gharib Mahmoud A, El Gendy Eman A, Raslan Amal A, El-Kelawy Hassan M. Mechanistic action of mesenchymal stem cell injection in the treatment of chemically induced ovarian failure in rabbits. Cytotherapy. 2013;15(1):64–75. doi: 10.1016/j.jcyt.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Min S, Wang S, Yi L, Yu L, Fang G, Wang C, Yuanqing Y. Adipose-derived stem cells improved mouse ovary function after chemotherapy-induced ovary failure. Stem Cell Res Ther. 2013;4(4):80. doi: 10.1186/scrt231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Te L, Yongyi H, Jian Z, Wenxing Q, Huiying C, Jiulin C, Yu Z, Chuan C. Transplantation of human menstrual blood stem cells to treat premature ovarian failure in mouse model. Stem Cells Dev. 2014;23(13):1548–1557. doi: 10.1089/scd.2013.0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rongxia L, Xiaoyu Z, Zhenhai F, Wang Y, Guanping Y, Wan X, Zulin L, Yang B, Yu L. Human amniotic mesenchymal stem cells improve the follicular microenvironment to recover ovarian function in premature ovarian failure mice. Stem Cell Res Ther. 2019;10(1):299. doi: 10.1186/s13287-019-1315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Penghui F, Pingping L, Tan J. Human menstrual blood-derived stromal cells promote recovery of premature ovarian insufficiency via regulating the ECM-dependent FAK/AKT signaling. Stem Cell Rev Rep. 2019;15(2):241–255. doi: 10.1007/s12015-018-9867-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He Y, Dongmei C, Lingling Y, Qiaoni H, Huiming M, Xian X. The therapeutic potential of bone marrow mesenchymal stem cells in premature ovarian failure. Stem Cell Res Ther. 2018;9(1):263. doi: 10.1186/s13287-018-1008-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Z, Wang Y, Yang T, Jing L, Yang X. Study of the reparative effects of menstrual-derived stem cells on premature ovarian failure in mice. Stem Cell Res Ther. 2017;8(1):11. doi: 10.1186/s13287-016-0458-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai Dongmei, Wang Fangyuan, Yao Xiaofen, Zhang Qiuwan, Wu Xiaoxing, Xiang Charlie. Human endometrial mesenchymal stem cells restore ovarian function through improving the renewal of germline stem cells in a mouse model of premature ovarian failure. J Transl Med. 2015;13(undefined): 155. doi:10.1186/s12967-015-0516-y [DOI] [PMC free article] [PubMed]
- 22.Bidarimath Mallikarjun, Khalaj Kasra, Kridli Rami T, Kan Frederick W K, Koti Madhuri., Tayade Chandrakant. Extracellular vesicle mediated intercellular communication at the porcine maternal-fetal interface: a new paradigm for conceptus-endometrial cross-talk. Sci Rep. 2017;7(undefined):40476. doi:10.1038/srep40476 [DOI] [PMC free article] [PubMed]
- 23.Isabel H-D, Chen-Yu Z, Antonio V-P. Extracellular vesicles: novel mediators of cell communication in metabolic disease. Trends Endocrinol Metab. 2017;28(1):3–18. doi: 10.1016/j.tem.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Siwen Z, Pingping L, Zhengwei Y, Tan J. Platelet-rich plasma improves therapeutic effects of menstrual blood-derived stromal cells in rat model of intrauterine adhesion. Stem Cell Res Ther. 2019;10(1):61. doi: 10.1186/s13287-019-1155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siwen Z, Pingping L, Zhengwei Y, Tan J. Effects of platelet-rich plasma on the activity of human menstrual blood-derived stromal cells in vitro. Stem Cell Res Ther. 2018;9(1):48. doi: 10.1186/s13287-018-0795-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan J, Pingping L, Wang Q, Yaxuan L, Xiaoni L, Dongni Z, Xu X, Kong L. Autologous menstrual blood-derived stromal cells transplantation for severe Asherman’s syndrome. Hum Reprod. 2016;31(12):2723–2729. doi: 10.1093/humrep/dew235. [DOI] [PubMed] [Google Scholar]
- 27.Gomez-Salazar Mario, Gonzalez-Galofre Zaniah N, Casamitjana Joan, Crisan Mihaela., James Aaron W, Péault Bruno. Five decades later, are mesenchymal stem cells still relevant? Front Bioeng Biotechnol. 2020; 8(undefined): 148. doi:10.3389/fbioe.2020.00148 [DOI] [PMC free article] [PubMed]
- 28.Lijun C, Jingjing Q, Cheng T, Xin C, Charlie X. Menstrual blood-derived stem cells: toward therapeutic mechanisms, novel strategies, and future perspectives in the treatment of diseases. Stem Cell Res Ther. 2019;10(1):406. doi: 10.1186/s13287-019-1503-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Makhlough A, Shekarchian S, Moghadasali R, Einollahi B, Dastgheib M, Janbabaee G. et al. Bone marrow-mesenchymal stromal cell infusion in patients with chronic kidney disease: a safety study with 18 months of follow-up. Cytotherapy. 2–18;20, 660–669 10.1016/j.jcyt.2018.02.368. [DOI] [PubMed]
- 30.Bartolucci J, Verdugo FJ, Gonzalez PL, Larrea RE, Abarzua E, Goset C, et al. Safety and efficacy of the intravenous infusion of umbilical cord mesenchymal stem cells in patients with heart failure: a phase 1/2 randomized controlled trial (RIMECARD trial [randomized clinical trial of intravenous infusion umbilical cord mesenchymal stem cells on cardiopathy]) Circ. Res. 2017;121:1192–1204. doi: 10.1161/CIRCRESAHA.117.310712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qiuwan Z, Shixia B, Junyan S, Xu M, Xiaofen Y, He K, Dongmei L. Paracrine effects of human amniotic epithelial cells protect against chemotherapy-induced ovarian damage. Stem Cell Res Ther. 2017;8(1):270. doi: 10.1186/s13287-017-0721-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li L, Xiushan F, Tianqin W, Wang Y, Wang Y, Wang Z, Dongyuan T, Luo Y, Zhengai X. Human amnion-derived mesenchymal stem cell (hAD-MSC) transplantation improves ovarian function in rats with premature ovarian insufficiency (POI) at least partly through a paracrine mechanism. Stem Cell Res Ther. 2019;10(1):46. doi: 10.1186/s13287-019-1136-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seong TW, Chai LR, Bin Z, Kiang LS. MSC exosome works through a protein-based mechanism of action. Biochem Soc Trans. 2018;46(4):843–853. doi: 10.1042/BST20180079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liping S, Dong L, Kun S, Jianlu W, Shu Y, Zhao L, Xuantao S, Xiuli J, Lan C, Deng X, Kong B, Li L. Exosomes derived from human umbilical cord mesenchymal stem cells protect against cisplatin-induced ovarian granulosa cell stress and apoptosis in vitro. Sci Rep. 2017;7(1):2552. doi: 10.1038/s41598-017-02786-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boxian H, Lu J, Chenyue D, Qinyan Z, Wang W, Hong L. Exosomes derived from human adipose mesenchymal stem cells improve ovary function of premature ovarian insufficiency by targeting SMAD. Stem Cell Res Ther. 2018;9(1):216. doi: 10.1186/s13287-018-0953-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang Weijie, Zhang Jing, Xu Boqun, He Yuanlin, Liu Wei, Li Jiazhao, Zhang Songying, Lin Xiaona, Su Dongming, Wu Tinghe, Li Jing. HucMSC-derived exosomes mitigate the age-related retardation of fertility in female mice. Mol. Ther, 2020; undefined (undefined), undefined. doi:10.1016/j. ymthe.2020.02.003. [DOI] [PMC free article] [PubMed]
- 37.Rémi B, Pascale H, Sophie C-M, Vincent B. Occupational exposures to chemicals as a possible etiology in premature ovarian failure: a critical analysis of the literature. Reprod Toxicol. 2012;33(3):269–279. doi: 10.1016/j.reprotox.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Nao S, Nobuhito Y, Seido T, Yodo S, Midori T, Shu H, Yoshiharu M, Kazuhiro K. Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency. Hum Reprod. 2015;30(3):608–615. doi: 10.1093/humrep/deu353. [DOI] [PubMed] [Google Scholar]
- 39.Fabregues F, Ferreri J, Calafell JM, Moreno V, Borrás A, Manau D, Carmona F. Pregnancy after drug-free in vitro activation of follicles and fresh tissue autotransplantation in primary ovarian insufficiency patient: a case report and literature review. J Ovarian Res. 2018;11(1):76. doi: 10.1186/s13048-018-0447-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGuire Megan M, Bowden Wayne, Engel Natalie J, Ahn Hyo Won, Kovanci Ertug, Rajkovic Aleksandar. Genomic analysis using high-resolution single-nucleotide polymorphism arrays reveals novel microdeletions associated with premature ovarian failure. Fertil Steril 2011;95(5), 1595–1600. doi:10.1016/j.fertnstert.2010.12.052. [DOI] [PMC free article] [PubMed]
- 41.Lei L, Zhang H, Jin S, Wang F, Fu M, Wang H, Xia G. Stagespecific germ-somatic cell interaction directs the primordial folliculogenesis in mouse fetal ovaries. J Cell Physiol. 2006;208:640–647. doi: 10.1002/jcp.20702. [DOI] [PubMed] [Google Scholar]
- 42.Uhlenhaut N Henriette, Jakob Susanne, Anlag Katrin, Eisenberger Tobias, Sekido Ryohei, Kress Jana, Treier Anna-Corina, Klugmann Claudia., Klasen Christian, Holter Nadine I, Riethmacher Dieter, Schütz Günther, Cooney Austin J, Lovell-Badge Robin, Treier Mathias. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell. 2009;139(6), 1130–1142. doi:10.1016/j.cell.2009.11.021. [DOI] [PubMed]
- 43.Ernst EH, Franks S, Hardy K, Villesen P, Lykke-Hartmann K. Granulosa cells from human primordial and primary follicles show differential global gene expression profiles. Hum Reprod. 2018;33(4):666–679. doi: 10.1093/humrep/dey011. [DOI] [PubMed] [Google Scholar]
- 44.Oktay K, Karlikaya G, Akman O, Ojakian GK, Oktay M. Interaction of extracellular matrix and activin-A in the initiation of follicle growth in the mouse ovary. Biol Reprod. 2000;63(2):457–461. doi: 10.1095/biolreprod63.2.457. [DOI] [PubMed] [Google Scholar]
- 45.MacDonald Julie A, Takai Yasushi, Ishihara Osamu, Seki Hiroyuki, Woods Dori C, Tilly Jonathan L. Extracellular matrix signaling activates differentiation of adult ovary-derived oogonial stem cells in a species-specific manner. Fertil Steril 2019; 111(4): 794–805. doi:10.1016/j.fertnstert.2018.12.015. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Quantitative polymerase chain reaction primer sequences.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.