Abstract
Aims
Clinical trials have demonstrated that a reduction in low-density lipoprotein cholesterol (LDL-C) reduces cardiovascular (CV) events. This has, however, not yet been shown in a real-world setting. We aimed to investigate the association between LDL-C changes and statin intensity with prognosis after a myocardial infarction (MI).
Methods and results
Patients admitted with MI were followed for mortality and major CV events. Changes in LDL-C between the MI and a 6- to 10-week follow-up visit were analysed. The associations between quartiles of LDL-C change and statin intensity with outcomes were assessed using adjusted Cox regression analyses. A total of 40 607 patients were followed for a median of 3.78 years. The median change in LDL-C was a 1.20 mmol/L reduction. Patients with larger LDL-C reduction (1.85 mmol/L, 75th percentile) compared with a smaller reduction (0.36 mmol/L, 25th percentile) had lower hazard ratios (HR) for all outcomes (95% confidence interval): composite of CV mortality, MI, and ischaemic stroke 0.77 (0.70–0.84); all-cause mortality 0.71 (0.63–0.80); CV mortality 0.68 (0.57–0.81); MI 0.81 (0.73–0.91); ischaemic stroke 0.76 (0.62–0.93); heart failure hospitalization 0.73 (0.63–0.85), and coronary artery revascularization 0.86 (0.79–0.94). Patients with ≥50% LDL-C reduction using high-intensity statins at discharge had a lower incidence of all outcomes compared with those using a lower intensity statin.
Conclusions
Larger early LDL-C reduction and more intensive statin therapy after MI were associated with a reduced hazard of all CV outcomes and all-cause mortality. This supports clinical trial data suggesting that earlier lowering of LDL-C after an MI confers the greatest benefit.
Keywords: Myocardial infarction, Secondary prevention, LDL-C, Cardiovascular outcomes, Cardiovascular mortality, Statin
Graphical Abstract
 Listen to the audio abstract of this contribution.
Listen to the audio abstract of this contribution.
See page 253 for the editorial comment on this article (doi: 10.1093/eurheartj/ehaa1008)
Introduction
Epidemiological, genetic, and interventional studies indicate that higher low-density lipoprotein cholesterol (LDL-C) levels cause atherosclerotic cardiovascular (CV) events.1 Indeed, multiple clinical trials have shown a decreased risk of CV-related morbidity associated with lowering of LDL-C levels.2–5 Hence, a cornerstone for secondary prevention of CV disease is treatment with LDL-C-lowering therapies.6
Data suggest that regardless of pre-treatment LDL-C concentrations, the relative CV risk reduction acquired per unit reduction of LDL-C is consistent.7 Clinical trials have shown that patients with higher baseline LDL-C levels appear to benefit the most from LDL-C-lowering therapy, with a larger absolute LDL-C lowering associated with the greatest reduction in mortality.8 However, studies assessing this relationship in trials of patients with predefined characteristics are often not representative of patients seen in real-world clinical practice.9 There is a paucity of information assessing the association between early changes in LDL-C level and intensity of statin therapy after a myocardial infarction (MI) with long-term prognosis from real-life patient populations. The aim of this study was to investigate the association between early LDL-C changes and statin intensity with mortality and major adverse CV outcomes after an MI.
Methods
Study population
This was an observational research study using data obtained from the SWEDEHEART registry. SWEDEHEART is a Swedish nationwide MI quality registry that records patient characteristics, medication, and outcomes as well as data on acute coronary care, coronary interventions, and secondary prevention. All patients admitted with an MI to any of the 74 coronary care units in Sweden are included in the registry.10 This study included patients between 30 and <75 years of age, admitted for first or recurrent MI, between January 2006 and December 2016 and who were alive at a follow-up visit within cardiac rehabilitation (CR), 6–10 weeks after discharge. Patients who had no measurements of plasma LDL-C levels were excluded (Supplementary material online, Figure S1). In the registry, data on LDL-C were only available for patients <75 years of age and at these two time points. If patients were hospitalized more than once during the study time period, only the first hospitalization was included. The register was cross-referenced with three mandatory national registers held by the National Board of Health and Welfare: the patient register including all ICD codes for all hospital admissions, the cause of death register, and the prescribed drug register containing data on all dispensed prescription drugs.
Exposure variables
Blood samples were drawn in a fasting state within 24 h after hospital admission for the index event, and within 2 weeks of the CR visit. In most cases, LDL-C was assessed by the Friedewald equation [LDL-C = total cholesterol − HDL-C − (0.45 × triglycerides)].
Statin usage and intensity were identified based on data collected from the prescribed drug register and the SWEDEHEART registry (details and definitions in Supplementary material online, Table S1). The usage was assessed at the time of admission—referred to as ongoing therapy, at discharge, at the CR visit, and at 8–12 months after the index event.
Outcomes
Included outcomes were: all-cause mortality; CV mortality; MI; ischaemic stroke; hospitalization for heart failure; coronary artery revascularization (coronary artery bypass grafting or percutaneous coronary intervention); a composite of CV mortality, MI, and ischaemic stroke (i.e. major adverse CV event [MACE]); and a composite of CV mortality, MI, ischaemic stroke, and coronary artery revascularization (major vascular event). Outcomes were censored until the CR visit (Supplementary material online, Figure S2 and Table S2).
Statistical analyses
Continuous variables are presented as medians, quartiles 1 and 3 (Q1, Q3), and groups compared using Kruskal–Wallis tests. Categorical variables are presented as counts and percentages, and groups compared using χ2-tests. The difference in LDL-C between the MI hospitalization and the CR visit was calculated. The patients were stratified according to quartile change in LDL-C level from index event to the CR visit. Demographics and other baseline characteristics were compared across these quartiles. In addition, the patients were stratified according to increase/no reduction, <50%, and ≥50% reduction in LDL-C. The relation of change in LDL-C and statin intensity to each clinical outcome is presented as cumulative Kaplan–Meier curves and analysed with Cox proportional hazards models with LDL-C values both as a continuous and as a categorical variable (see Supplementary material online for method validity).
Secondary analyses were performed to take the potential LDL-C decline after ischaemic onset as well as the size of myocardial damage into account. These included time from onset of MI symptoms to LDL-C sampling and left ventricular ejection fraction into the models. The Friedewald equation may be biased in patients with high triglycerides and very low LDL-C levels, and alternative methods have been proposed.11,12 To account for this, all analyses were also made by an additional LDL-C calculation (see Supplementary material online). Lastly, a stratification was made by ongoing statin therapy at admission for index event.
Cardiovascular and mortality event rates were calculated 12 months after the index event and at the end of available follow-up period. Hazard ratios (HR) were calculated comparing the LDL-C change at the 75th percentile with the LDL-C change at the 25th percentile as reference, as well as for 1 mmol/L change in LDL-C. Analyses were adjusted for clinical characteristics and established cardiovascular risk factors (Supplementary material online, Table S3). The relationships between change in LDL-C, as well as statin intensity and outcomes, were explored using restricted cubic splines to allow for non-linearity, as well as with a linear model. Furthermore, the association between degree of LDL-C change and event rate was assessed.
The recently suggested E-value13 for both the observed association estimate (adjusted HR) and the limit of the confidence interval closest to the null was calculated. The E-value is defined as the minimum strength of association that one or more unmeasured confounders would need to have with both the treatment and the outcome to fully explain away a specific treatment-outcome association, conditional on the measured covariates.
Missing data on covariates were imputed with multiple imputations by chained equations. For most variables, there were no or very scarce missing data. All analyses were performed at the Uppsala Clinical Research Center, Uppsala University, Uppsala, Sweden using R Core Team (2019) R Foundation for Statistical Computing, Vienna, Austria.
The Regional Ethics Committee in Stockholm approved the study in accordance with the Helsinki Declaration (approval numbers 2012/6013/2 and 2018/1957-32).
Results
Baseline patient characteristics
A total of 40 607 MI patients were included, yielding 168 769 patient-years of observation with a median follow-up of 3.8 years (Q1, Q3: 1.9, 6.5, range from time of CR visit to 11 years). The median LDL-C at the time of the index event was 3.1 (2.4, 3.9) mmol/L for the whole population, 3.4 (2.8, 4.1) mmol/L for statin naïve patients, and 2.2 (1.8, 2.8) mmol/L for patients with ongoing statin therapy. Patients in the quartile with the largest LDL-C reduction were less likely to have comorbidities such as hypertension, diabetes mellitus, prior MI, and prior coronary revascularization compared with those with the smallest LDL-C reduction (Table 1).
Table 1.
Patient characteristics at admission for index event
| LDL-C reduction from index event to cardiac rehabilitation visit (mmol/L) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Overall | <0.36 | 0.36–1.17 | 1.17–1.85 | >1.85 | ||||||
| Number of patients | 40 607 | 10 262 | 10 152 | 10 131 | 10 062 | ||||||
| Demographics | |||||||||||
| Age (years) | 64 | (57–69) | [0] | 66 | (59–71) | 64 | (57–69) | 63 | (56–69) | 62 | (55–68) |
| Female | 10 321 | (25%) | [0] | 2741 | (27%) | 2427 | (24%) | 2427 | (24%) | 2726 | (27%) |
| BMI (kg/m2) | 27 | (25–30) | [2761] | 27 | (25–30) | 27 | (25 – 30) | 27 | (25–30) | 27 | (25–30) |
| Medical history | |||||||||||
| Current smoker | 11 902 | (30%) | [882] | 2753 | (28%) | 3193 | (32%) | 3079 | (31%) | 2877 | (29%) |
| Hypertension | 16 927 | (42%) | [3] | 5498 | (54%) | 4342 | (43%) | 3721 | (37%) | 3366 | (34%) |
| Diabetes mellitus | 7768 | (19%) | [55] | 3234 | (32%) | 2021 | (20%) | 1351 | (13%) | 1162 | (12%) |
| Prior myocardial infarction | 4935 | (12%) | [3] | 2793 | (27%) | 1182 | (12%) | 554 | (6%) | 406 | (4%) |
| Prior revascularizationa | 5400 | (13%) | [3] | 3230 | (32%) | 1248 | (12%) | 538 | (5%) | 384 | (4%) |
| Prior heart failure | 989 | (2%) | [0] | 604 | (6%) | 232 | (2%) | 90 | (1%) | 63 | (1%) |
| Prior ischaemic stroke | 1512 | (4%) | [28] | 751 | (7%) | 381 | (4%) | 207 | (2%) | 173 | (2%) |
| Laboratory variables | |||||||||||
| LDL-C (mmol/L) | 3.1 | (2.4–3.9) | [0] | 2.1 | (1.7–2.7) | 2.8 | (2.3–3.2) | 3.4 | (3.0–3.8) | 4.3 | (3.8–4.8) |
| eGFR | 87 | (73–96) | [589] | 84 | (68–94) | 88 | (74–96) | 88 | (75–96) | 88 | (76–96) |
| Systolic blood pressure (mmHg) | 150 | (130–170) | [511] | 145 | (130–165) | 150 | (130–167) | 150 | (133–170) | 155 | (137–173) |
| Diastolic blood pressure (mmHg) | 88 | (77–100) | [1613] | 84 | (74–95) | 86 | (75–98) | 90 | (79–100) | 90 | (80–100) |
| Lipid-lowering therapy | |||||||||||
| Ongoing statin therapy | |||||||||||
| No therapy | 31 263 | (77%) | [0] | 4335 | (42%) | 7737 | (76%) | 9521 | (94%) | 9670 | (96%) |
| Low intensity | 719 | (2%) | 370 | (4%) | 257 | (2%) | 63 | (1%) | 29 | (<1%) | |
| Medium intensity | 7289 | (18%) | 4592 | (45%) | 1895 | (19%) | 486 | (5%) | 316 | (3%) | |
| High intensity | 1336 | (3%) | 965 | (9%) | 263 | (3%) | 61 | (1%) | 47 | (1%) | |
Patient characteristics at admission, overall, and by LDL-C reduction quartile. Values are medians (interquartile ranges) and n (%) for categorical variables. [n] is numbers of missing values.
BMI, body mass index; eGFR, estimated glomerular filtration rate calculated by the Chronic Kidney Disease Epidemiology Collaboration equation; LDL-C, low-density lipoprotein cholesterol.
Prior revascularization is defined as percutaneous coronary intervention or coronary artery bypass grafting.
Statin therapy and low-density lipoprotein cholesterol change
The median change in LDL-C was a 1.2 mmol/L reduction. The largest mean LDL-C reduction was achieved in statin naïve patients discharged with a high-intensity statin (mean reduction 1.7 mmol/L), whereas low- and medium-intensity statin therapy resulted in a mean LDL-C reduction of 1.2 mmol/L in these patients. In patients with low- or medium-intensity statin therapy at admission, the average LDL-C reduction was 0.6 mmol/L when discharged with a high-intensity statin, whereas no change in statin therapy resulted in a reduction of 0.1 mmol/L. Ezetimibe was used by 459 patients (1%) at the time of the index event and 2767 (7%) after the CR visit. See Supplementary material online, Table S4 and Figure S3 for absolute LDL-C values and statin intensity during the study period.
Study outcomes
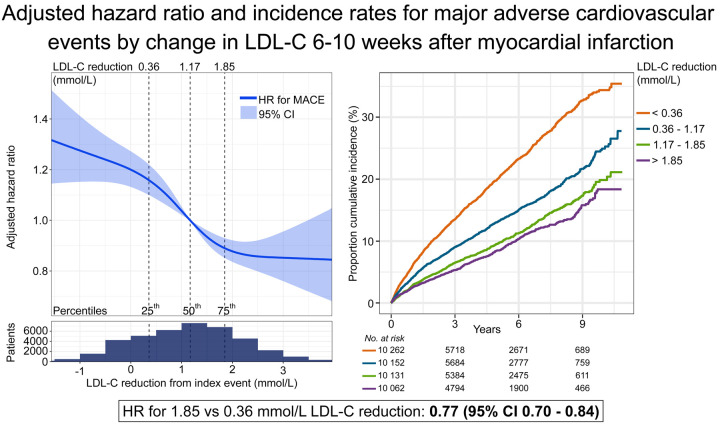
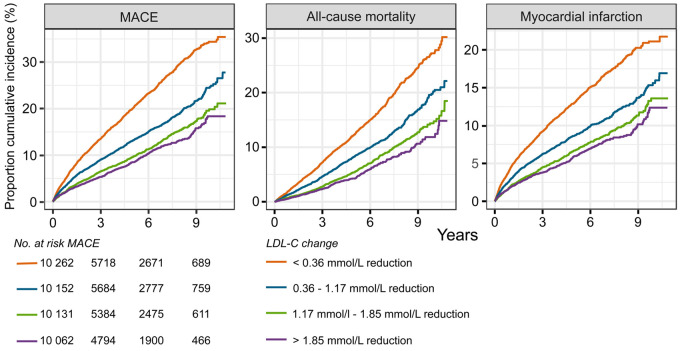
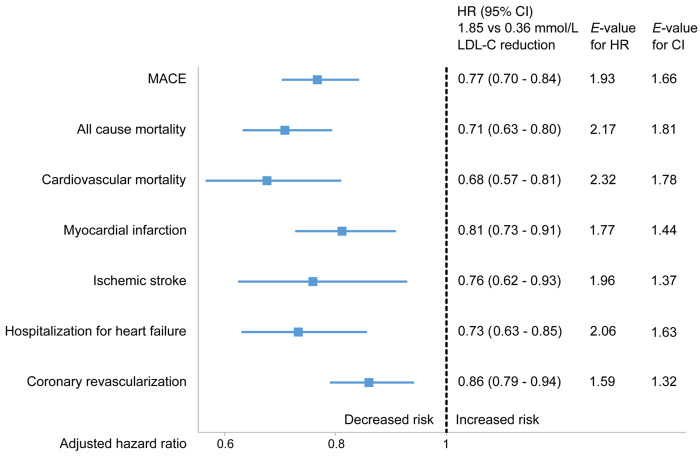
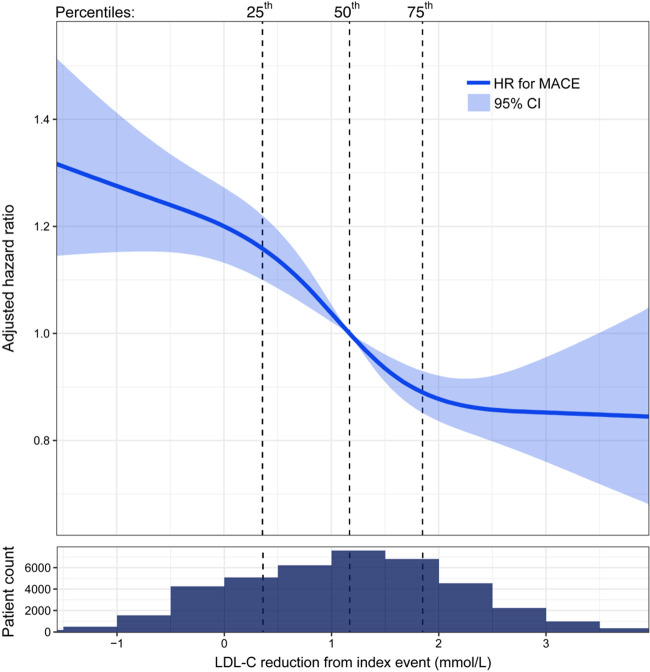
During follow-up, 2991 (7%) patients died from any cause, 3000 (7%) had a recurrent MI, and 4609 (11%) patients had a MACE. The unadjusted frequency of all outcomes was higher in the quartiles with less LDL-C reduction (Supplementary material online, Figure S4). The distribution of event curves through all 11 years of follow-up across quartiles of LDL-C change showed a stepwise lower risk of all outcomes with larger LDL-C reduction (Figure 1, Supplementary material online, Figure S5). Patients in the 75th percentile of LDL-C reduction (1.85 mmol/L) had a lower hazard of all outcomes compared with patients in the 25th percentile (0.36 mmol/L) (Figure 2). The reduction in HR appeared linear across the interquartile range of LDL-C reduction for MACE (Figure 3), all-cause mortality, and MI (Supplementary material online, Figure S6).
Figure 1.
Kaplan–Meier curves of the cumulative incidence rates by quartile low-density lipoprotein cholesterol (LDL-C) change from index event to the cardiac rehabilitation visit. Outcomes are assessed after the cardiac rehabilitation visit. Numbers at risk shown for MACE. MACE, major adverse cardiovascular event is the composite outcome of cardiovascular mortality, myocardial infarction, and ischaemic stroke.
Figure 2.
Association between low-density lipoprotein cholesterol (LDL-C) change and outcomes. Cox proportional hazards analysis adjusted for clinical characteristics and established cardiovascular risk factors. Comparing the 75th percentile of low-density lipoprotein cholesterol reduction (1.85 mmol/L) with the 25th percentile (0.36 mmol/L) between index event and cardiac rehabilitation visit. Hazard ratio (HR) with 95% confidence interval (CI), E-value for hazard ratio, and confidence interval. MACE, major adverse cardiovascular event is the composite outcome of cardiovascular mortality, myocardial infarction, and ischaemic stroke. Coronary revascularization is defined as coronary artery bypass grafting or percutaneous coronary artery intervention.
Figure 3.
Hazard ratio (HR) for the composite outcome MACE by change in low-density lipoprotein cholesterol (LDL-C,mmol/L) from index event to cardiac rehabilitation visit, adjusted for clinical characteristics, and established cardiovascular risk factors. Solid line: hazard ratio with 95% confidence interval (CI), shadowed area, in relation to low-density lipoprotein cholesterol change using restricted cubic splines with four knots. Vertical dotted lines: percentiles. X-axis presented on a linear scale. Population distribution in relation to change in low-density lipoprotein cholesterol below spline. MACE, major adverse cardiovascular event is the composite outcome of cardiovascular mortality, myocardial infarction, and ischaemic stroke.
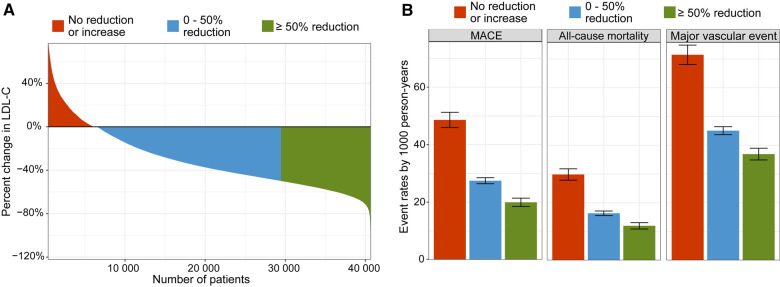
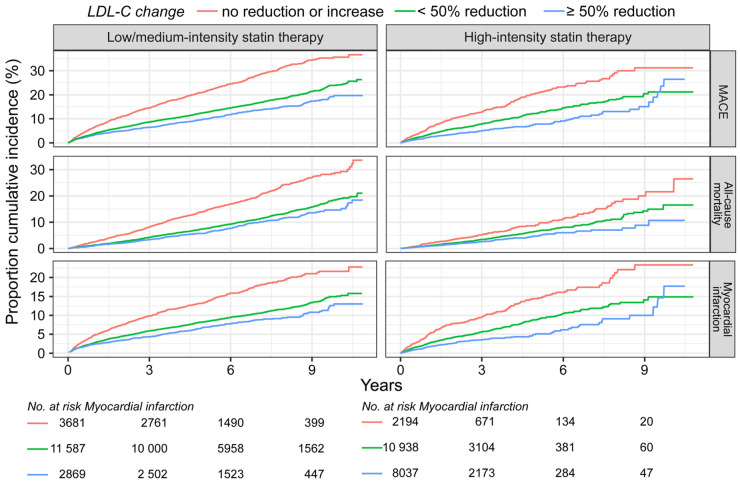
Between the index event and the CR visit, 10 995 (27%) had an LDL-C reduction of 50% or more, and 23 055 patients (57%) had an LDL-C reduction of <50% (Figure 4A). Of the remaining 6557 (16%) who had no reduction or an increase in LDL-C, 56% were treated with low- or medium-intensity statin, 33% with high-intensity, and 10% were not treated with statins. The magnitude of reduction in LDL-C was directly related to the event rates of MACE, all-cause mortality, and major vascular event (Figure 4B). Patients with ≥50% reduction in LDL-C and a high-intensity statin after the CR visit had a lower incidence of all outcomes compared with patients achieving ≥50% reduction in LDL-C with a low- or medium-intensity statin (Figure 5, Supplementary material online, Table S5 and Figures S7 and S8). Among patients receiving high-intensity statin therapy, comparing those achieving <50% to ≥50% reduction in LDL-C, the event curves started to separate for MACE, MI, and coronary revascularization after ∼4 months (Supplementary material online, Figures S7 and S8). For all-cause and CV mortality, the event curve separation was observed after 1 year. The curve separation was not as apparent in patients receiving medium-intensity statins. Patients with LDL-C increase or no reduction had higher event rates and an increased risk of all outcomes (Figures 3, 4B, and 5, Supplementary material online, Figures S7 and S8) compared with patients with any LDL-C reduction.
Figure 4.
Change in low-density lipoprotein cholesterol (LDL-C) and incidence rates. Data are shown for no reduction or an increase in low-density lipoprotein cholesterol (red), >0 but <50% reduction (blue), and ≥50% reduction (green) between index event and cardiac rehabilitation visit. Waterfall plot for change in low-density lipoprotein cholesterol (A) and concordant incidence rates per 1000 person-years with confidence intervals (B). MACE, major adverse cardiovascular event is the composite outcome of cardiovascular mortality, myocardial infarction, and ischaemic stroke. Major vascular event is the composite outcome of cardiovascular mortality, myocardial infarction, ischaemic stroke, and coronary revascularization (coronary artery bypass grafting or percutaneous coronary artery intervention).
Figure 5.
Kaplan–Meier curves of the cumulative incidence rates by statin therapy intensity after cardiac rehabilitation visit and change in low-density lipoprotein cholesterol (LDL-C) from index event to cardiac rehabilitation visit . Data are shown for no reduction or an increase in low-density lipoprotein cholesterol, >0 but <50% reduction, and ≥50% reduction between index event and cardiac rehabilitation visit. Numbers at risk shown for myocardial infarction. MACE, major adverse cardiovascular event is the composite outcome of cardiovascular mortality, myocardial infarction, and ischaemic stroke.
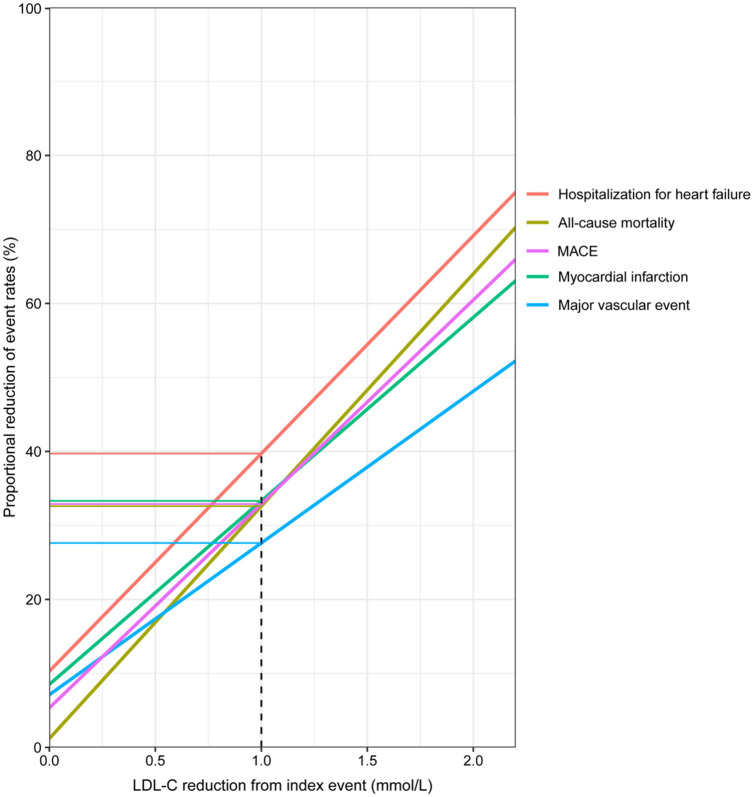
The association between degree of LDL-C reduction and event rate reduction was assessed: for every 1 mmol/L reduction in LDL-C, there was an ∼25% relative event rate reduction for major vascular events (Figure 6). Similar associations were observed for the separate outcomes of MACE, all-cause mortality, MI, and with an even larger reduction in event rate for hospitalization for heart failure.
Figure 6.
Proportional reduction of event rates by degree of mean absolute low-density lipoprotein cholesterol (LDL-C, mmol/L) reduction . MACE, major adverse cardiovascular event is the composite outcome of cardiovascular mortality, myocardial infarction, and ischaemic stroke. Major vascular event is the composite outcome of cardiovascular mortality, myocardial infarction, ischaemic stroke, and coronary revascularization.
Secondary analyses
The early effect of myocardial ischaemia on LDL-C levels, as well as the influence of the degree of myocardial damage on LDL-C levels, was explored in secondary analyses. Neither of these adjustments altered the overall results (see details in Supplementary material online). The overall results were also confirmed in fitting linear LDL-C reduction into Cox regression models (Supplementary material online, Figure S9). For statin naïve patients, the results were almost identical to those of the total study population (Supplementary material online, Figure 10A). There were no associations between LDL-C change and outcomes in patients with ongoing statin therapy at the time of the index MI (Supplementary material online, Figures S10B and S11). Furthermore, the overall results for the total population were almost identical when using alternative LDL-C equation (Supplementary material online, Figures S12 and S13).
Discussion
In this nationwide study of MI patients, early LDL-C reduction after MI was associated with lower incidence and reduced adjusted hazard of MACE, all-cause mortality, CV mortality, MI, ischaemic stroke, hospitalization for heart failure, and coronary revascularization. These findings were most evident in patients with the largest LDL-C reduction, whereas patients that did not have a reduction, or had an increase in LDL-C, had the highest risk. The relationship between LDL-C reduction and event rate decline was linear and comparable to the Cholesterol Treatment Trialists’ Collaboration meta-analysis of statin treatment trials.7
Previous large-scale, observational studies examining the long-term effects related to LDL-C reduction immediately following MI are limited. In a Taiwanese register-based study on patients with stable CV disease, failure to achieve an LDL-C target level of <2.6 mmol/L, irrespective of statin use, was associated with increased risk of MACE.14 In patients with ongoing statins and established CV disease, no additional risk reduction could be shown in those reaching LDL-C < 1.8 mmol/L.15 However, a lower event rate for MACE was seen after 6 months in patients with established CV disease reaching LDL-Cof 1.8–2.6 mmol/L vs. those reaching 2.6–3.3 mmol/L.15 In our study, the results robustly showed that the larger the LDL-C decline, the lower the risk. This was consistent for all outcomes assessed and quantified by assessing proportional reduction of event rates by degree of mean absolute LDL-C reduction.
Interestingly, we observed a large reduction in hospitalization for heart failure, on par with mortality, with larger LDL-C reduction. This finding may be due to a true effect of intensive LDL-C lowering on reducing the final stages of ischaemic heart disease, or of an unbalanced baseline population.
Comparing patients achieving ≥50% LDL-C reduction, we found lower incidence of all CV events and all-cause mortality in patients with high-intensity statin therapy, compared with those treated with low- or medium-intensity statins. Our results are analogous with data from a previous observational study in patients with established CV disease showing reduced hazard in patients receiving more intensive compared with less intensive therapy after discharge and revealing differences in event rates for mortality already at 6 months.16 Reduced mortality rates with intensive statin therapy were also seen in a smaller study in patients hospitalized for a coronary heart disease.17 However, in a study where the majority of patients had ongoing statin therapy, no significant differences in the 1-year rates of death, MI, or target-vessel revascularization were seen when treated with high-intensity vs. lower intensity statins, although there was a trend towards reduced mortality.18 The risk reduction in our study was also similarly less apparent in patients with ongoing statin therapy. In the ODYSSEY OUTCOMES trial with a large proportion of patients achieving LDL-C reduction of ≥50%, the risk of death declined with lower achieved LDL-C, similarly as in our study.19
Our findings on timing of events in relation to the index MI are similar to the PROVE IT20 and the A–Z2 trials comparing high- and low-intensity statin therapy, where the event curves for the combined endpoints diverged early, within a few months. In contrast, the intensified LDL-C lowering therapy with ezetimibe in addition to high-dose statin in the IMPROVE-IT3 trial did not show differences in events until after 1 year. Similarly, in the two outcomes trials studying effects of proprotein convertase subtilisin–kexin type 9 (PCSK9) inhibitors4,5 in addition to high-intensity standard of care, the divergence for the combined primary endpoints was apparent later, at 6–12 months. The discrepancies in the timing of the results between our study and these three trials with lipid-lowering therapy in addition to statin therapy might be expected. An explanation could be that the present study identifies early LDL-C lowering, whereas in the PCSK9 inhibitor trials, the additional LDL-C reduction occurred later. Furthermore, in these three trials, the proportion of subjects with ongoing, high-intensity statin therapy was high, possibly delaying a beneficial effect of additional treatments. Indeed, in a subgroup analysis of FOURIER in patients with a recent MI, the effect of LDL-C lowering was larger and appeared earlier when compared with patients with a more remote MI.21 Overall, both in our study and in those clinical trials cited above, the benefit of reducing LDL-C on CV outcomes was consistent. Furthermore, given the observational nature of the study, the suggested pleiotropic effects of statins cannot be addressed.22,23
Strengths and limitations
Due to Sweden having universal and publicly funded health care with 100% of the coronary care units reporting to SWEDEHEART, we were able to follow a large proportion of the total Swedish MI population. The high-quality data in the Swedish registers have been previously validated.24 Thus, this study yields important information on long-term trends with limited selection bias, loss to follow-up, and with very few missing data. Still, there are limitations in our study. Due to the observational nature of the study design, it is vulnerable to systematic errors such as classification bias or residual confounding as well as the inability to establish causality. However, the E-values13 estimated from the Cox regression analyses, range from 1.3 to 2.3. This suggests that in order to believe that there is no causal relationship between e.g., CV mortality and a larger reduction in LDL-C, there would need to be one or more unmeasured confounders that increase the risk of CV mortality 2.3 times (E = 2.3) and are at the same time 2.3 times more common in the group with the larger LDL-C reduction. This seems unlikely given the vast amount of knowledge available regarding CV risk factors.
To take early LDL-C decline caused by to the MI25 into account, secondary analyses were performed including time span between ischaemic symptom onset and laboratory sampling, and the extent of myocardial damage estimated by left ventricular ejection fraction. None of these factors affected the results. Using the commonly used Friedewald equation to calculate LDL-C may introduce some imprecision. In patients with high triglyceride levels, above 4.5 mmol/L, the equation is inaccurate, and these patients were excluded. Furthermore, the equation does not take atherogenic particles such as lipoprotein (a) into account causing underestimation of the atherogenic risk by solely using LDL-C,6 and may also show negative bias at low LDL-C levels.11,12 To account for some of these limitations, the analyses were recalculated with an alternative LDL-C calculation. Additionally, as evaluation of adherence to the drug therapies was not available, data on prescription filling were used as a proxy for drug adherence. Approximately 13% of patients had stopped filling their statin prescriptions 8–12 months after the index event. Discontinuation of statin treatment could be due to adverse events or low adherence.
Conclusion
Early and aggressive LDL-C lowering after MI appears to reduce the risk of major CV outcomes and mortality. For the first time, to our knowledge, large-scale observational data now substantiate the risk reductions seen in clinical trials. The results suggest that the benefit of LDL-C-lowering may be extended to the general MI population with effects as early as after 6 months.
Supplementary material
Supplementary material is available at European Heart Journal online.
Data availability
SWEDEHEART does not allow individual data sharing to third party. Access to aggregated data might be granted following review by the SWEDEHEART steering committee.
Funding
This work was partially funded by the Swedish Heart and Lung foundation and by a grant from Amgen Inc. The funding supported the costs of data extraction, data management, and analyses. For the detailed contributions of each author, please refer to Supplementary material online.
Conflict of interest: J.S.: grants from Amgen during the conduct of the study. B.L., H.M., H.R., M.L., and P.U.: no conflict of interest to disclose. A.Y.: institutional grants from MSD outside the submitted work. T.J.: grants from MSD and Novartis outside the submitted work, and honoraria from Sanofi, MSD, Astra Zeneca, and Bayer for lecturing and consulting. S.J.: grants from Amgen and Astra Zeneca outside the submitted work. S.R.R., P.J.D., and A.W.H. are employees and stockholders of Amgen, Inc., CA, USA. E.H.: grants from Amgen during the conduct of the study, grants, and honoraria from Amgen, Sanofi, Bayer, and NovoNordisk outside the submitted work.
Supplementary Material
References
- 1.Borén J, Chapman MJ, Krauss RM, Packard CJ, Bentzon JF, Binder CJ, Daemen MJ, Demer LL, Hegele RA, Nicholls SJ, Nordestgaard BG, Watts GF, Bruckert E, Fazio S, Ference BA, Graham I, Horton JD, Landmesser U, Laufs U, Masana L, Pasterkamp G, Raal FJ, Ray KK, Schunkert H, Taskinen M-R, Sluis BVD, Wiklund O, Tokgozoglu L, Catapano AL, Ginsberg HN.. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J Oxford Academic 2020;41:2313–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Lemos JA, Blazing MA, Wiviott SD, Lewis EF, Fox KAA, White HD, Rouleau J-L, Pedersen TR, Gardner LH, Mukherjee R, Ramsey KE, Palmisano J, Bilheimer DW, Pfeffer MA, Califf RM, Braunwald E, Investigators. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA 2004;292:1307–1316. [DOI] [PubMed] [Google Scholar]
- 3.Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, De Ferrari GM, Ruzyllo W, De Lucca P, Im K, Bohula EA, Reist C, Wiviott SD, Tershakovec AM, Musliner TA, Braunwald E, Califf RM.. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372:2387–2397. [DOI] [PubMed] [Google Scholar]
- 4.Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR.. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, Jukema JW, Lecorps G, Mahaffey KW, Moryusef A, Pordy R, Quintero K, Roe MT, Sasiela WJ, Tamby J-F, Tricoci P, White HD, Zeiher AM.. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med 2018;379:2097–2107. [DOI] [PubMed] [Google Scholar]
- 6.Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen M-R, Tokgozoglu L, Wiklund O, ESC Scientific Document Group. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur Heart J 2020;41:111–188. [DOI] [PubMed] [Google Scholar]
- 7.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R, Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet Lond Engl 2005;366:1267–1278. [DOI] [PubMed] [Google Scholar]
- 8.Navarese EP, Robinson JG, Kowalewski M, Kolodziejczak M, Andreotti F, Bliden K, Tantry U, Kubica J, Raggi P, Gurbel PA.. Association between baseline LDL-C level and total and cardiovascular mortality after LDL-C lowering: a systematic review and meta-analysis. JAMA 2018;319:1566–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kennedy-Martin T, Curtis S, Faries D, Robinson S, Johnston J.. A literature review on the representativeness of randomized controlled trial samples and implications for the external validity of trial results. Trials 2015;16:495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jernberg T, Attebring MF, Hambraeus K, Ivert T, James S, Jeppsson A, Lagerqvist B, Lindahl B, Stenestrand U, Wallentin L.. The Swedish Web-system for Enhancement and Development of Evidence-based care in Heart disease Evaluated According to Recommended Therapies (SWEDEHEART). Heart 2010;96:1617–1621. [DOI] [PubMed] [Google Scholar]
- 11.Martin SS, Blaha MJ, Elshazly MB, Toth PP, Kwiterovich PO, Blumenthal RS, Jones SR.. Comparison of a novel method vs the Friedewald equation for estimating low-density lipoprotein cholesterol levels from the standard lipid profile. JAMA 2013;310:2061–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sampson M, Ling C, Sun Q, Harb R, Ashmaig M, Warnick R, Sethi A, Fleming JK, Otvos JD, Meeusen JW, Delaney SR, Jaffe AS, Shamburek R, Amar M, Remaley AT.. A new equation for calculation of low-density lipoprotein cholesterol in patients with normolipidemia and/or hypertriglyceridemia. JAMA Cardiol 2020;5:540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.VanderWeele TJ, Ding P.. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med 2017;167:268–274. [DOI] [PubMed] [Google Scholar]
- 14.Yeh Y-T, Yin W-H, Tseng W-K, Lin F-J, Yeh H-I, Chen J-W, Wu Y-W, Wu C-C, on behalf of the Taiwanese Secondary Prevention for Patients with AtheRosCLErotic Disease (T-SPARCLE) Registry Investigators. Lipid lowering therapy in patients with atherosclerotic cardiovascular diseases: which matters in the real world? Statin intensity or low-density lipoprotein cholesterol level? ‒ Data from a multicenter registry cohort study in Taiwan. PLoS One 2017;12:e0186861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leibowitz M, Karpati T, Cohen-Stavi CJ, Feldman BS, Hoshen M, Bitterman H, Suissa S, Balicer RD.. Association between achieved low-density lipoprotein levels and major adverse cardiac events in patients with stable ischemic heart disease taking statin treatment. JAMA Intern Med 2016;176:1105–1113. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez F, Maron DJ, Knowles JW, Virani SS, Lin S, Heidenreich PA.. Association between intensity of statin therapy and mortality in patients with atherosclerotic cardiovascular disease. JAMA Cardiol 2017;2:47–54. [DOI] [PubMed] [Google Scholar]
- 17.Fintel D, Joyce A, Mackell J, Graff J, Kuntze E, Ollendorf DA.. Reduced mortality rates after intensive statin therapy in managed-care patients. Value Health 2007;10:161–169. [DOI] [PubMed] [Google Scholar]
- 18.Guedeney P, Baber U, Claessen B, Aquino M, Camaj A, Sorrentino S, Vogel B, Farhan S, Faggioni M, Chandrasekhar J, Kalkman DN, Kovacic JC, Sweeny J, Barman N, Moreno P, Vijay P, Shah S, Dangas G, Kini A, Sharma S, Mehran R.. Temporal trends, determinants, and impact of high-intensity statin prescriptions after percutaneous coronary intervention: results from a large single-center prospective registry. Am Heart J 2019;207:10–18. [DOI] [PubMed] [Google Scholar]
- 19.Steg PG, Szarek M, Bhatt DL, Bittner VA, Brégeault M-F, Dalby AJ, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, Jukema JW, Lecorps G, Mahaffey KW, Moryusef A, Ostadal P, Parkhomenko A, Pordy R, Roe MT, Tricoci P, Vogel R, White HD, Zeiher AM, Schwartz GG, For the ODYSSEY OUTCOMES Committees and Investigators. Effect of alirocumab on mortality after acute coronary syndromes. Circulation 2019;140:103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA, Skene AM.. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004;350:1495–1504. [DOI] [PubMed] [Google Scholar]
- 21.Gencer B, Mach F, Murphy SA, Ferrari GMD, Huber K, Lewis BS, Ferreira J, Kurtz CE, Wang H, Honarpour N, Keech AC, Sever PS, Pedersen TR, Sabatine MS, Giugliano RP.. Efficacy of evolocumab on cardiovascular outcomes in patients with recent myocardial infarction: a prespecified secondary analysis from the FOURIER trial. JAMA Cardiol 2020;5:952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marzilli M. Pleiotropic effects of statins: evidence for benefits beyond LDL-cholesterol lowering. Am J Cardiovasc Drugs 2010;10:3–9. [DOI] [PubMed] [Google Scholar]
- 23.Wiviott SD, de Lemos JA, Cannon CP, Blazing M, Murphy SA, McCabe CH, Califf R, Braunwald E.. A tale of two trials. Circulation 2006;113:1406–1414. [DOI] [PubMed] [Google Scholar]
- 24.Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, Heurgren M, Olausson PO.. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenson RS. Myocardial injury: the acute phase response and lipoprotein metabolism. J Am Coll Cardiol 1993;22:933–940. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
SWEDEHEART does not allow individual data sharing to third party. Access to aggregated data might be granted following review by the SWEDEHEART steering committee.