Abstract
Background:
Although aspects of brain morphology have been associated with chronic posttraumatic stress disorder (PTSD), limited work has investigated multimodal patterns in brain morphology that are linked to acute posttraumatic stress severity. In the present study, we utilized multimodal magnetic resonance imaging (MRI) to investigate if structural covariance networks (SCNs) assessed acutely following trauma were linked to acute posttraumatic stress severity.
Methods:
Structural MRI data were collected around 1-month after civilian trauma exposure in 78 participants. Multimodal MRI data fusion was completed to identify combinations of SCNs, termed structural covariance profiles (SCPs), related to acute posttraumatic stress severity collected at 1 month. Analyses assessed the relationship between SCP participant loadings, acute posttraumatic stress severity, the change in posttraumatic stress severity between 1 and 12 months, and depressive symptoms.
Results:
We identified an SCP that reflected greater gray matter properties of the anterior temporal lobe, fusiform face area, and visual cortex (i.e., the ventral visual stream) that varied curvilinearly with acute posttraumatic stress severity and the change in PTSD symptom severity between one and twelve months. The SCP was not associated with depressive symptoms.
Discussion:
We identified combinations of multimodal SCNs that are related to variability in PTSD symptoms in the early aftermath of trauma. The identified SCNs may reflect patterns of neuroanatomical organization that provide unique insight into acute posttraumatic stress. Further, these multimodal SCNs may be potential candidates for neural markers of susceptibility to both acute posttraumatic stress and the future development of PTSD.
Keywords: Stress, Trauma, Multimodal MRI, PTSD, Neuroimaging, Data fusion
1. Introduction
Traumatic events can have acute, adverse effects on cognitive and affective function. Acute stress in the early aftermath of trauma is linked to later development of chronic, debilitating dysfunction in the form of posttraumatic stress disorder (PTSD) (1,2). Acute posttraumatic stress experienced in the peritraumatic period (i.e., < 1 to ~2 months following trauma exposure) often naturally subsides over time in contrast to the enduring dysfunction that characterizes chronic PTSD (i.e., symptoms that last for ~3 months or greater). However, there is considerable individual variability in susceptibility to acute posttraumatic stress and the development of chronic PTSD, and not everyone who experiences a traumatic event will go on to develop acute or chronic dysfunction (3). Identifying the underlying neural basis for variability in stress susceptibility may provide targets for early intervention and treatment of chronic stress-related disorders. Prior research demonstrates chronic PTSD is related to alterations in brain gray and white matter morphology (4–8). However, limited work has investigated the relationship between brain morphology and posttraumatic stress soon after a traumatic event. Given that chronic stress (e.g., stress experienced as part of PTSD) may in itself impact brain morphology (9,10), posttraumatic stress during the acute phase of trauma may show different relationships with brain structure compared to those observed in chronic PTSD. Characterization of the relationship between brain morphology and posttraumatic stress in the early aftermath of trauma is thus critical for the development of accurate neural markers of susceptibility to both acute and chronic PTSD.
Chronic PTSD has been tied to reduced volume of the hippocampus and amygdala in addition to reduced volume and thickness of the dorsomedial and ventromedial prefrontal cortex (PFC) (7,8,11–15). Further, prior work has observed alterations in white matter microstructure of tracts such as the uncinate fasciculus, dorsal cingulum, and corona radiata in individuals with PTSD (4,16–18). These brain regions and white matter tracts are part of a neural circuit that supports threat learning and memory processes which are dysfunctional in PTSD (for review, see (19,20)). Together, these findings demonstrate that chronic PTSD is linked to differences in gray and white matter brain morphology particularly within threat learning and memory circuitry.
Compared to research on brain morphology in chronic PTSD, relatively little is known about the relationship between brain morphology and acute posttraumatic stress severity. Further, the limited findings to date are at times inconsistent with the literature from chronic PTSD. For example, although hippocampal volume is reduced in PTSD (12), and some studies have suggested hippocampal volume reductions are a vulnerability factor for PTSD (21–23), these volumes are not necessarily reduced during the acute phase of trauma exposure (24). Recent findings in individuals with acute stress disorder (i.e., PTSD symptoms lasting less than one month) initially show reduced gray matter volume (GMV) within the visual cortex, and only later show reductions in ventromedial PFC volume, compared to non-ASD controls (25). Other research has observed that individuals who develop PTSD within six months following trauma show reduced gray matter within the ventromedial PFC during the acute phase (26). Further, although acutely assessed white matter microstructure of the uncinate fasciculus, dorsal cingulum, and fornix vary with expression of posttraumatic stress symptoms, the microstructure of other tracts such as the corticospinal tract, inferior longitudinal fasciculus, and inferior-frontal occipital fasciculus are also tied to the development of PTSD symptoms (27–29). Thus, data on assessments of brain gray and white matter as informative of future PTSD symptoms have been somewhat mixed and may be reflective of stress sensitization processes in traumatized individuals (30). Specifically, although the initial stressor of the trauma may not necessarily lead to structural changes, it may facilitate continual dysfunction of stress-regulation systems that ultimately leads to changes in brain morphology. Stress sensitization is also thought to partially explain why individuals with prior experiences of trauma are more likely to develop PTSD after a recent trauma (31). Although the mechanisms are currently unclear and the findings are somewhat mixed, these data at least provide initial support for the idea that early assessments of brain morphology may be linked to acute posttraumatic stress.
An important consideration of prior published reports is that the findings have been largely based on unimodal outcomes (i.e., gray or white matter investigated separately). Gray and white matter aspects of the brain likely do not vary completely independently of one another, such that structural variability may be linked across regions and across modalities (32–34). Further, combining data across modalities may increase the power and predictive utility of brain imaging for identifying traumatic stress susceptibility by accounting for relationships between the modalities. Thus, identifying accurate and robust brain structure markers of acute – and potentially future – posttraumatic stress may be improved by multimodal brain structure investigations. Importantly, multimodal or ‘data fusion’ approaches have been developed to analyze multimodal imaging data that can identify joint spatial patterns across multiple modalities. Further multimodal data fusion patterns may better disentangle neural substrates supporting processes of interest from comorbid conditions such as postconcussive syndrome (35,36). Particularly relevant to understanding the neuroetiology of PTSD is the separation of neural substrates of the disorder from depression, which is highly comorbid with PTSD. Limited investigations however have sought to determine if multimodal patterns related to PTSD are separate from depression.
For multimodal structural MRI, linked independent components analysis (LICA) has previously been used to identify structural covariance networks (SCNs) that combine both gray and white matter metrics (37–39). SCNs describe spatial patterns of shared variability and have previously focused on single modalities (e.g., brain gray matter). Prior unimodal reports of brain gray matter SCNs have observed altered covariance patterns in youth with PTSD compared to controls (40). In contrast, multimodal SCNs describe shared spatial patterns of variability across multiple brain structure modalities (e.g., brain gray and white matter), however no work has investigated multimodal SCNs in PTSD. Other psychiatric investigations have tied multimodal SCNs to aging and aging-related pathologies (37,38) and suggested these SCNs may discriminate between typical controls and individuals with psychiatric conditions such as autism spectrum disorders (41). SCNs may therefore also be related to other psychiatric disorders such as PTSD. To the best of our knowledge, no prior work has investigated multimodal SCNs in an acute trauma sample to determine if these are tied to posttraumatic stress in the early aftermath of trauma. Identifying linked spatial patterns of covariation among modalities in acutely traumatized individuals may better elucidate the neural substrates associated with acute posttraumatic stress and provide insight into the neurobiology of traumatic responses.
In the present study, we investigated SCNs in acutely trauma-exposed individuals to identify relationships between brain structure and acute posttraumatic stress. We further investigated whether these SCNs may also be related to changes in posttraumatic stress between the acute (i.e., at 1 month) and chronic (i.e., 12 month) trauma phases. We collected magnetic resonance imaging (MRI) data from recently trauma-exposed civilians (i.e., around 1 month post-trauma) to generate SCNs of gray and white matter metrics using LICA. We anticipated that acute posttraumatic stress assessed one-month post-trauma would be associated with SCNs that overlap with previously identified PTSD-associated brain regions (e.g., PFC, hippocampus, amygdala, visual cortex) and white matter tracts (e.g., uncinate fasciculus, cingulum bundle, inferior longitudinal fasciculus). Further, we hypothesized the identified SCNs would also be related to changes in posttraumatic stress over time. Importantly, the SCN modeling approach may allow for a better understanding of the broader interacting networks related to variability in stress responses early after trauma, beyond traditional univariate associations. The present study provides a data-driven characterization of the brain structural correlates of acute traumatic stress reactivity, and it elucidates linked neural profiles that may aide identification of individuals most susceptible to the acute effects of trauma exposure.
2. Methods and Materials
2.1. Participants
A total of 83 participants were recruited from the emergency department (ED) at Grady Memorial Hospital as part of an ongoing Grady Trauma Project ED longitudinal study to understand the impact and aftermath of trauma exposure (NIH R01MH094757). Participants were recruited within 24 hours after presenting to the ED, dependent on inclusion and exclusion criteria. Inclusion criteria included endorsement of a traumatic event that met Criterion A in the DSM-IV, English-speaking, and between 18–65 years of age. Exclusion criteria included typical magnetic resonance imaging (MRI) contraindications (e.g., metal or implanted device), prior hospitalization for mental health, suicide attempt within the past three months or suicidal ideation within the past month, and current intoxication or otherwise altered mental status. For the present analyses, participants were further excluded if they did not have complete MRI data or artifacts prevented successful data processing. Three participants did not have complete MRI data (i.e., lacking either a T1-weighted or diffusion weighted dataset) and were excluded from analyses. MRI data for two participants were excluded due to motion/scanner artifacts identified during quality inspection that prevented successful data processing (see below and supplemental material). Thus, 78 participants (Age: M = 35.5 years, SD = 12.45 years; 45 males) were included in the present analyses. In total, 62 participants (79%) had experienced an automobile-related accident (motor vehicle, motorcycle crash, or pedestrian in an auto crash), 7 participants (9%) had experienced an assault (sexual or nonsexual, stabbing, or gunshot wound), and another 9 participants (12%) had experienced other traumatic events (e.g., animal attacks, industrial or home accidents, or bicycle accidents). All participants provided informed consent as approved by the Emory University Institutional Review Boards and Grady Memorial Hospital Research Oversight Committee.
2.2. Psychological and demographic assessment
At time of enrollment in the ED, participants completed a battery of questionnaires to assess demographics, prior trauma history, and current trauma characteristics as described in prior reports (28,42,43). For the present analyses, the Posttraumatic Diagnostic Scale (PDS) was completed within the ED to assess baseline trauma history (i.e., the number of traumatic events the participants endorsed) and PTSD symptoms (44,45). A modified PTSD Symptom Scale (mPSS) (46) and Beck Depression Inventory (BDI-II) were completed by participants around 1-month after trauma exposure and served as a measure of acute posttraumatic stress severity. The mPSS assessed DSM-IV criterion for PTSD including avoidance symptoms (e.g., “have you persistently been making efforts to avoid thoughts or feelings associated with the traumatic event?”), re-experiencing symptoms (e.g., “have you had the experience of suddenly reliving the traumatic event, flashbacks of it, acting or feeling as if it were re-occurring?”), and arousal symptoms (e.g., “have you been jumpier, more easily startled, since the traumatic event?”). The mPSS was administered again at 12 months post-trauma to assess chronic PTSD symptoms. At the 1-month assessment, 34 participants met all three PTSD criteria while 14 participants met these criteria at 12-months. Participant demographics are detailed in Table 1. In addition, participants also completed the Mini International Neuropsychiatric Schedule (47) to assess comorbid psychiatric conditions during the ED visit. Comorbid diagnoses and prescription medication use assessed in the ED are presented in Table S1.
Table 1.
Demographic and clinical characteristics
| Mean (SD) or Count (%) | |
|---|---|
| Age | 35.5 (12.45) |
|
| |
| Gender | 45 (58%) M / 33 (42%) F |
|
| |
| Race | |
| Black | 58 (74%) |
| White | 13 (17%) |
| Mixed-race | 4 (5%) |
| Other | 3 (4%) |
|
| |
| Education | |
| Some high school | 7 (9%) |
| High school degree | 22 (28%) |
| Associates degree/Some college | 38 (49%) |
| Bachelor’s degree | 6 (8%) |
| Some graduate school | 1 (1%) |
| Master’s degree or higher | 4 (5%) |
|
| |
| Clinical Characteristics | |
| BDI scores (n =78) | 14.73 (11.11) |
| Number of prior traumas | 2.44 (1.76) |
| Baseline PDS scores (n = 75) | 6.63 (8.67) |
| mPSS total scores (1mo) (n = 78) | 16.36 (11.46) |
| Re-experiencing scores | 4.46 (3.71) |
| Avoidance scores | 5.96 (5.19) |
| Arousal scores | 5.94 (3.95) |
| mPSS total scores (12mo) (n = 65) | 9.02 (10.01) |
| Re-experiencing scores | 1.85 (2.93) |
| Avoidance scores | 3.34 (4.36) |
| Arousal scores | 3.83 (3.59) |
Note: BDI = Beck Depression Inventory-II, PDS = Posttraumatic Diagnostic Scale, mPSS = modified Posttraumatic Symptom Scale, M = Male, F = Female.
2.3.1. Magnetic Resonance Imaging
Brain imaging data were acquired on three separate Siemens 3 Tesla Magnetom TIM Trio MRI scanners (Siemens, Malvern, PA) each using a 12-channel head coil. Multiple scanners were used as the scanning facility upgraded systems over the course of the study. MRI was completed within a three-week window of the 1-month assessment (M = 17.77, SD = 12.85, range = 2 – 63 days). Detailed information on acquisition and pre-processing for T1-weighted and diffusion weighted images (DWI) across all three scanners are provided in the supplement (Table S2). Scan parameters for two scanners were harmonized while the third scanner used different T1 and diffusion weighted sequences. The same preprocessing pipelines were used regardless of scanner type to facilitate analysis standardization. Visual inspection of MRI data was completed to evaluate inclusion for multimodal analyses based on acquisition or preprocessing artifacts, and we performed linked independent components analysis (LICA) at a high dimensionality to further account for both scanner and participant-noise related artifacts (see below for further details). Briefly, DWI data were preprocessed using the FMRIB Software Library’s (FSL) “eddy” routine and “dtifit” while following previously suggested methods for susceptibility distortion correction using T1-weighted images (48,49) Tract-based based spatial statistics and the recommended ENIGMA-DTI working group processing steps were followed to generate white matter skeletal maps of fractional anisotropy (FA), mean diffusivity (MD), and mode of the diffusion tensor (MO) for each participant (50,51). T1-weighted anatomical data were processed using standard processing procedures implemented in FSLVBM (48,52,53) to obtain maps of gray matter volume (GMV). T1-weighted data were also processed through freesurfer using the FMRIPREP pipeline to obtain maps of cortical thickness (CT) and pial surface area (PSA) (54–56).
2.3.2. Linked Independent Component Analysis
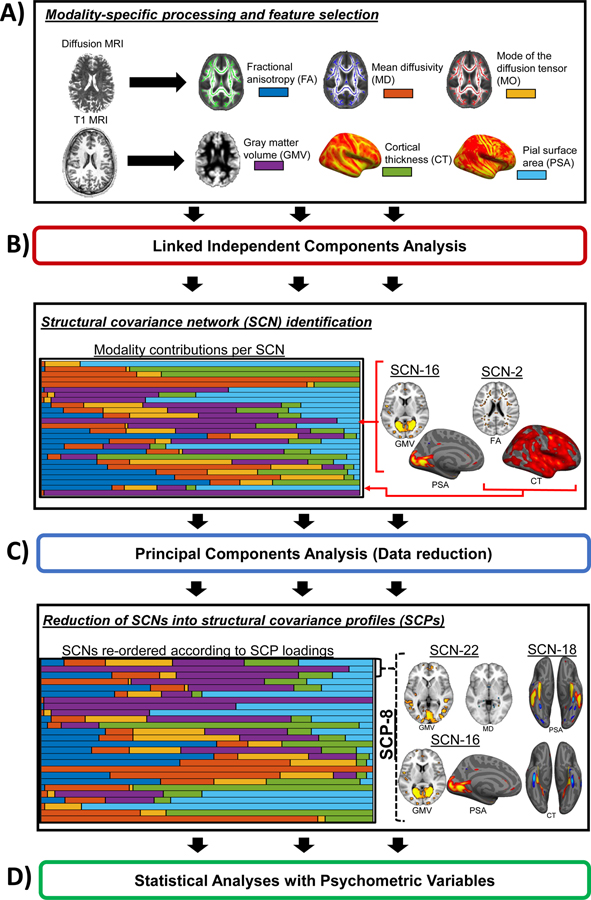
Linked independent component analysis (LICA) was completed to perform multimodal data fusion (37,39). The FA, MD, MO, GMV, CT, and PSA maps were used as spatial features. LICA identifies multimodal spatial covariance patterns that reflect variability in the sample and derives a set of participant loadings for each pattern that reflect the strength of the pattern in each participant. LICA was completed at a high dimensionality (L = 34, the maximum estimated within LICA) to better separate signal and noise (i.e., participant-dominated and motion) components as has been completed in prior LICA analyses (37,41,57). Of the 34 identified components, 8 were determined to be driven predominantly by a single subject (i.e., noise-related) and were excluded from analyses resulting in 26 SCNs. Given high ICA dimensionalities can result in fractionalization of brain networks (58), and given the limited information as to what canonical multimodal SCNs should look like, we performed a data-reduction step (principle components analysis; PCA) to the participant loadings for all SCNs to reduce the SCNs into structural covariance profiles (SCPs) that may be related to acute posttraumatic stress reactivity (Figure 1). The goal was to retain a separation of signal and noise components through LICA while reducing the number of multimodal components to assess. One alternative to the present approach would have been to visually group the SCNs into spatially similar groups, however such groupings may then be biased by an assumption of the spatial patterns. Importantly, the PCA approach to grouping SCNs is data-driven and therefore not limited by assumptions as to which SCNs should covary together. Visual inspection of scree plots and assessment of variance explained for each eigenvalue was completed to select a reduced set of SCPs that also allowed for a general clustering of the original SCNs. We sought to minimize the number of derived SCPs while also selecting components that together explained a fair amount of the variability in the SCNs. Thus, we selected an 8 eigenvalue solution that accounted for 49.4% of the variance in the present data and applied a varimax rotation to the component matrix with a Kaiser normalization to derive the SCPs (SCN loadings are detailed in Table S3 and the SCPs are detailed in Figure S1). The distributions of the SCN and SCP loadings are visualized in Figure S2.
Figure 1. Data fusion and analytic strategy.
A) Diffusion magnetic resonance imaging (MRI) and T1-weighted anatomical MRI data were processed through modality specific pipelines to derive measures of white matter microstructure (i.e., fractional anisotropy, FA; mean diffusivity, MD; mode of the diffusion tensor, MO) and gray matter morphology (i.e., gray matter volume, GMV; cortical thickness, CT; pial surface area, PSA) which were then used as features in a linked independent components analysis (LICA). B) LICA was completed and 34 components were calculated, with 8 being identified as predominantly driven by a single participant and excluded from subsequent analyses. The resulting components represented multimodal structural covariance networks (SCNs) composed of differing weights per modality. C) We further reduced the set of SCNs into structural covariance profiles (SCPs) in a data-driven fashion (principle components analysis) to identify sets of SCNs that shared variability. D) The final set of SCP loadings were then used in statistical analyses with psychometric variables assessing levels of posttraumatic stress and depression severity.
2.4. Statistical Analysis
We completed statistical analyses using IBM SPSS Version 24 (Armonk, NY). Participant loadings from the eight identified SCPs were used as dependent variables in a multivariate analysis of covariance (MANCOVA) to identify linear and quadratic associations with mPSS total scores at 1-month post-trauma (i.e., acute posttraumatic stress severity) while controlling for scanner, age, sex/gender, trauma history (from the PDS), sex, and baseline PDS scores (i.e., within the ED). Participant age, trauma history, baselines PDS scores, and mPSS total scores were mean-centered prior to analysis. We tested for quadratic effects given that SCNs revealed through LICA have previously revealed non-linear relationships and given that prior work has demonstrated neural activation may vary non-linearly with cognitive-affective processes (37,59). Significant omnibus multivariate effects between mPSS total scores at 1 month and SCPs were followed up with multiple linear regressions. Follow-up comparisons were considered significant at a Bonferroni corrected threshold of p < 0.003 (p = 0.05/16; eight SCPs and two contrasts for each). Exploratory analyses were completed to assess relationships with mPSS subscale scores (i.e., avoidance, re-experiencing, and arousal scores) for significant SCPs. Additional multiple regressions with BDI-II scores were completed to determine if the identified SCPs varied specifically with posttraumatic stress or reflected cognitive-affective dysfunction more broadly. Of note, BDI-II scores were significantly correlated with mPSS scores (r = 0.78, p < 0.001). To further assess if the identified SCPs were also informative of progression to PTSD, similar multiple regressions were completed using the change in mPSS scores from 1 to 12 months (i.e., 12-month mPSS scores minus 1-month mPSS scores).
3. Results
3.1. Multivariate relationships with acute posttraumatic stress
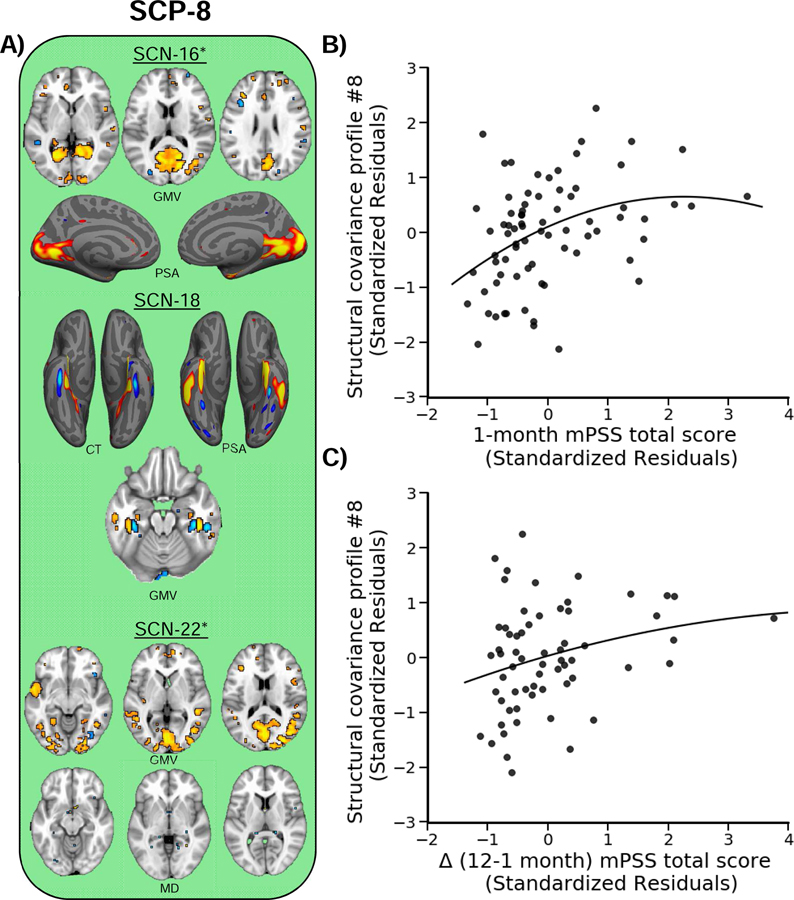
We first analyzed structural covariance profiles (SCPs) associated with 1-month mPSS scores (i.e., acute posttraumatic stress symptoms). The MANCOVA revealed a significant curvilinear relationship between SCPs and 1-month mPSS scores [F(8,59) = 2.11, p = 0.049, Wilks’ λ = 0.79]. Follow-up regressions revealed this effect was driven by a curvilinear relationship between 1 month mPSS scores and SCP-8 [t(66) = 3.19, p = 0.002, β = 0.43] (Figure 2). Although it did not survive multiple comparison correction, a second relationship was observed between SCP-3 and 1 month mPSS scores [t(66) = 2.33, p = 0.023, β = 0.31] (Figure S3).
Figure 2. Structural covariance profile eight (SCP-8) varies with posttraumatic stress severity.
A) SCP-8 reflected GMV, PSA, and MD of the visual cortex, anterior temporal lobe, and fusiform gyrus. The loading for this profile varied curvilinearly with both B) mPSS scores at 1 month and C) the change in mPSS scores between 1 and 12 months. Warm colors (red/yellow/orange) reflect positive, and cool colors (blue/light blue) reflect relative negative, relationships of each modality with each SCN loading. Plots represent the standardized residuals for the SCP and mPSS scores to show the unique curvilinear relationship between the variables, accounting for covariates. Dots represent individual participant points and the solid line represents the line of best fit. Asterisks indicate other modalities contributed significant (i.e., greater than or equal to 15%) to the SCN, however no voxels survived the |2| threshold.
3.2. Characteristics of structural covariance profile eight and acute posttraumatic stress
SCP-8 reflected a positive association with SCN-16, SCN-18, and SCN-22. Together, these components reflected increased GMV, cortical thickness (CT), and PSA within the visual cortex, anterior temporal lobe, and dorsomedial PFC, as well as reduced MD within the inferior longitudinal fasciculus (Figures 2A and S1). Follow-up regression analyses revealed SCP-8 varied curvilinearly with re-experiencing [t(66) = 3.46, p = 0.001, β = 0.47], avoidance [t(66) = 2.41, p = 0.019, β = 0.45], and arousal [t(66) = 2.18, p = 0.033, β = 0.38] scores. Further, SCP-8 did not vary curvilinearly with BDI-II [t(66) = 0.22, p = 0.824, β = 0.03] scores.
3.3. Structural covariance profile eight and changes in posttraumatic stress over time
We next sought to investigate if the SCP identified to vary with mPSS scores at 1 month also predicted changes in mPSS scores between 1 and 12 months. In total, 13 participants did not provide 12 month mPSS data (n = 65). In the present sample, mPSS total scores showed a general decline between the 1-month (M = 16.36, SD = 11.46) and 12 months (M = 9.02, SD = 10.01) assessments. Further, mPSS scores at 1 month were significantly associated with mPSS scores at 12 months (r = 0.67, p < 0.001) post-trauma. Thus, in this sample, acute traumatic stress reactivity was linked to future expression of PTSD symptoms. We observed a significant curvilinear relationship between SCP-8 and the change in mPSS scores between 1 and 12 months [Figure 2C; t(54) = 2.03, p = 0.048, β = 0.32]. However, SCP-8 loadings did not uniquely vary with the change in re-experiencing [t(54) = 0.61, p = 0.542, β = 0.08], avoidance [t(54) = 1.25, p = 0.217, β = 0.23], or arousal [t(54) = 1.40, p = 0.168, β = 0.22] scores.
4. Discussion
Although chronic posttraumatic stress disorder (PTSD) is associated with alterations in gray and white matter brain morphology, the relationship between brain morphology and acute posttraumatic stress has received little attention. Further, limited work to date has utilized multimodal data fusion to link patterns across different structural magnetic resonance imaging (MRI) metrics (i.e., structural covariance networks; SCNs) that may be informative of susceptibility to acute posttraumatic stress. Characterization of brain morphology that underlies acute posttraumatic stress is necessary for a full understanding of the neurobiology that mediates the development of chronic PTSD, and for developing predictive markers of PTSD susceptibility. Therefore, the present study utilized multimodal MRI to investigate SCNs in acutely traumatized individuals that may be associated with posttraumatic stress. We identified a group of SCNs (i.e., structural covariance profile; SCP) that varied with acute posttraumatic stress severity (i.e., mPSS total scores at 1 month post-trauma). The SCP was related to acute re-experiencing, avoidance, and arousal symptoms. Further, the SCP was also related to changes in posttraumatic stress severity between 1 and 12 months post-trauma. The present findings suggest brain morphology is linked to acute traumatic stress responses and may be critical for identifying individuals likely to transition to future PTSD.
In the present study we observed that a multimodal structure covariance profile (SCP-8) reflected SCNs that overlapped with the ventral visual processing stream (60). The SCP was curvilinearly related to acute posttraumatic symptom severity and predicted the chronic maintenance of symptoms out to 12 months post-trauma. These data suggest morphological variability in the structure of the ventral visual stream may play a role in the development and maintenance of PTSD symptoms. Suggestion that the ventral visual stream may be related to the development of PTSD symptoms is consistent with prior functional MRI research. Specifically, differences in visual processing, particularly of affective stimuli, and neural activity within the ventral visual stream have been observed in PTSD (61–65). Previous work in individuals with chronic PTSD observed elevated activity of the amygdala and visual cortex compared to those without PTSD and that these individuals also show reduced functional connectivity between the amygdala and ventromedial prefrontal cortex (PFC) (61). Further, individuals with PTSD show a positive relationship between symptom severity and neural activity within the dorsolateral PFC during an attentional bias to threat task (63). Individuals with PTSD also show a positive relationship between activation in lingual gyrus (i.e., visual cortex) during this task and behavioral bias to threat. Interestingly, recent resting-state investigations have suggested that PTSD is associated with reduced functional connectivity between the hippocampus and visual cortex (66) and attentional control training can modulate visual processing pathways in individuals with PTSD (67). Together, prior functional MRI research provides support for the present results suggesting ventral visual processing stream dysfunction may be associated with PTSD.
Threat and affect related processes supported by the amygdala, hippocampus, and PFC that are disrupted in PTSD likely rely on interactions with regions that support processing of visual stimuli. However, a significant portion of neurocircuitry investigations of PTSD have focused on amygdala, hippocampus, and PFC contributions to the disorder such that it is unclear why these differences in visual processing areas may occur (20). Both human and animal model research has demonstrated that amygdala activation modulates visual cortex activity, and connectivity between these regions is thought to be important for attending to novel or threatening events (68–70). Some have postulated that dysfunction of affective visual processing circuitry, particularly between the amygdala and ventral visual stream, may drive hyperarousal symptoms that partially underlie the development of re-experiencing (i.e., intrusion) symptoms in PTSD (71). Partially consistent with this view, SCP-8 was associated with re-experiencing, avoidance, and arousal symptoms in the present study. Given the importance of visual processing for attentional processes, the consistency of the findings related to PTSD, and the lack of research in this area, future studies are needed to build a more comprehensive circuit related to the etiology and pathophysiology of PTSD.
In contrast to typically employed univariate analyses, our approach suggests that some structural variability related to acute posttraumatic stress and PTSD may be uniquely multivariate. Thus multimodal and data-driven approaches may reveal novel aspects of neurobiology important for our understanding of PTSD. Several of the observed SCNs in the present study (e.g., SCN-16 and SCN-22) have been reported the other linked independent components analyses (LICA) (37). These SCNs may reflect intrinsic patterns of neuroanatomical organization within the general population similar to canonical resting-state functional networks (72). However, limited work to date has investigated the replicability of these SCNs across different samples. Thus, the generalizability of these multimodal SCNs within the wider population remains unclear. If the observed SCNs are generalizable to the greater population, these SCNs may be future candidates for neuroimaging markers of acute and future posttraumatic stress development.
The present results should be interpreted in light of several limitations. First, a non-trauma-exposed comparison group was not included in the present study. While the present results suggest multimodal imaging is associated with PTSD symptom severity, it is unclear if the observed components are specific to trauma-exposed individuals or may reflect more general neurobiological patterns across the population. Although several of the identified components have been identified in prior work (37), it remains unclear if these may differ as a function of trauma exposure itself. Although the present data demonstrate that peritraumatic assessments of multimodal networks are related to posttraumatic stress processes, it is important to determine if the identified SCPs and SCNs could be identified in non-trauma-exposed individuals as a potential pretraumatic marker for posttraumatic stress susceptibility. Second, it is difficult to determine the specific function of the identified SCPs and SCNs. It will be necessary to better characterize the present SCNs to determine the specific cognitive-affective functions they support. Assessing the generalizability of these SCNs coupled with the assessment of neuropsychological assessments may help to better identify the cognitive and behavioral correlates of these SCNs. Third, although the present results demonstrate multimodal analyses provide novel information on the neurobiology of posttraumatic stress, some findings may appear to be incongruent with unimodal analyses. For example, in a prior unimodal report, we found that generalized fractional anisotropy (FA) of the fornix/stria terminalis was positively related to acute and future PTSD severity (27). However, we did not observe a multimodal component reflecting fornix/stria terminalis FA in the present study (see supplementary discussion). One potential reason for the discrepancy is that the present results reflect spatial patterns account for data across all modalities while unimodal data often only considers one region in one modality without accounting for shared spatial variability. Further research into the utility of unimodal and multimodal approaches to studying PTSD will be necessary. Finally, data were only available for a total of 78 participants. Although these numbers are likely adequately powered and larger than many existing neuroimaging investigations of trauma and PTSD, particularly in the acute aftermath of trauma, additional samples will be needed to complete replication cohorts and optimize SCN identification.
In conclusion, acute posttraumatic stress severity appears to be tied to multimodal variability in brain structure. Specifically, we observed SCNs that reflected variation in FA, MD, MO, GMV, CT, and PSA and which were associated with acute posttraumatic stress. A multimodal spatial pattern representing greater structural integrity of the ventral visual stream was related to the severity of posttraumatic stress symptoms acutely following trauma. This pattern also positively related to changes in posttraumatic stress severity over time. Our findings highlight the unique associations between multimodal imaging data and PTSD symptoms. In particular, these findings suggest multivariate and multimodal approaches elucidate important neural substrates of PTSD symptomatology that may not be readily identifiable through univariate and unimodal analyses. Further research into the robustness of multimodal spatial patterns, particularly assessed in the early aftermath of trauma, will be needed to develop actionable neural signatures of PTSD and contribute to effective screening and early intervention tools for susceptibility to trauma and stress-related disorders.
Supplementary Material
Acknowledgements:
The authors would like to thank Lauren Lebois and Huanjie Li for helpful comments and analytic advice in drafting this manuscript.
Funding and Support: This research was supported by the National Institute of Mental Health K00 MH119603, R01 MH094757, R21 MH106902, F32 MH101976, K01 MH102415, and U01 MH110925
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Ressler has received consulting income from Alkermes, and is on scientific advisory boards for Janssen, Verily, and Nobilis. He has also received sponsored research support from Takeda and Brainsway. None of these sources of industry support are related to the present work. The other authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Kleim B, Ehlers A, Glucksman E (2007): Early predictors of chronic post-traumatic stress disorder in assault survivors. Psychol Med 37: 1457–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McLean SA, Ressler K, Koenen KC, Neylan T, Germine L, Jovanovic T, et al. (2019): The AURORA Study: a longitudinal, multimodal library of brain biology and function after traumatic stress exposure. Mol Psychiatry 1–14. [DOI] [PMC free article] [PubMed]
- 3.Bonanno GA, Mancini AD (2012): Beyond resilience and PTSD: Mapping the heterogeneity of responses to potential trauma. Psychol Trauma Theory, Res Pract Policy 4: 74–83. [Google Scholar]
- 4.Fani N, King TZ, Shin J, Srivastava A, Brewster RC, Jovanovic T, et al. (2016): Structural and functional connectivity in posttraumatic stress disorder: Associations with FKBP5. Depress Anxiety 33: 300–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bremner JD, Randall P, Scott TM, Bronen RA, Seibyl JP, Southwick SM, et al. (1995): MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry 152: 973–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villarreal G, Hamilton DA, Petropoulos H, Driscoll I, Rowland LM, Griego JA, et al. (2002): Reduced hippocampal volume and total white matter volume in posttraumatic stress disorder. Biol Psychiatry 52: 119–125. [DOI] [PubMed] [Google Scholar]
- 7.Wrocklage KM, Averill LA, Scott JC, Averill CL, Schweinsburg B, Trejo M, et al. (2017): Cortical thickness reduction in combat exposed U. S. veterans with and without PTSD. Eur Neuropsychopharmacol 27: 515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rauch SL, Shin LM, Segal E, Pitman RK, Carson MA, McMullin K, et al. (2003): Selectively reduced regional cortical volumes in post-traumatic stress disorder. Neuroreport 14: 913–916. [DOI] [PubMed] [Google Scholar]
- 9.Chao LL, Yaffe K, Samuelson K, Neylan TC (2014): Hippocampal volume is inversely related to PTSD duration. Psychiatry Res - Neuroimaging 222: 119–123. [DOI] [PubMed] [Google Scholar]
- 10.Felmingham K, Williams LM, Whitford TJ, Falconer E, Kemp AH, Peduto A, Bryant RA (2009): Duration of posttraumatic stress disorder predicts hippocampal grey matter loss. Neuroreport 20: 1402–1406. [DOI] [PubMed] [Google Scholar]
- 11.Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, et al. (1997): Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse - A preliminary report. Biol Psychiatry 41: 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Logue MW, van Rooij SJH, Dennis EL, Davis SL, Hayes JP, Stevens JS, et al. (2018): Smaller Hippocampal Volume in Posttraumatic Stress Disorder: A Multisite ENIGMA-PGC Study: Subcortical Volumetry Results From Posttraumatic Stress Disorder Consortia. Biol Psychiatry 83: 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamasue H, Kasai K, Iwanami A, Ohtani T, Yamada H, Abe O, et al. (2003): Voxel-based analysis of MRI reveals anterior cingulate gray-matter volume reduction in posttraumatic stress disorder due to terrorism. Proc Natl Acad Sci 100: 9039–9043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bing X, Qiu MG, Ye Z, Zhang JN, Min L, Han C, et al. (2013): Alterations in the cortical thickness and the amplitude of low-frequency fluctuation in patients with post-traumatic stress disorder. Brain Res 1490: 225–232. [DOI] [PubMed] [Google Scholar]
- 15.Rogers MA, Yamasue H, Abe O, Yamada H, Ohtani T, Iwanami A, et al. (2009): Smaller amygdala volume and reduced anterior cingulate gray matter density associated with history of post-traumatic stress disorder. Psychiatry Res - Neuroimaging 174: 210–216. [DOI] [PubMed] [Google Scholar]
- 16.Sanjuan PM, Thoma R, Claus ED, Mays N, Caprihan A (2013): Reduced white matter integrity in the cingulum and anterior corona radiata in posttraumatic stress disorder in male combat veterans: A diffusion tensor imaging study. Psychiatry Res - Neuroimaging 214: 260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koch SBJ, Van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, Olff M (2017): Decreased uncinate fasciculus tract integrity in male and female patients with PTSD: A diffusion tensor imaging study. J Psychiatry Neurosci 42: 331–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olson EA, Cui J, Fukunaga R, Nickerson LD, Rauch SL, Rosso IM (2017): Disruption of white matter structural integrity and connectivity in posttraumatic stress disorder: A TBSS and tractography study. Depress Anxiety 34: 437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.VanElzakker MB, Kathryn Dahlgren M, Caroline Davis F, Dubois S, Shin LM (2014): From Pavlov to PTSD: The extinction of conditioned fear in rodents, humans, and anxiety disorders. Neurobiol Learn Mem 113: 3–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harnett NG, Goodman AM, Knight DC (2020): PTSD-related neuroimaging abnormalities in brain function, structure, and biochemistry. Experimental Neurology, vol. 330. 10.1016/j.expneurol.2020.113331 [DOI] [PubMed] [Google Scholar]
- 21.Van Rooij SJH, Kennis M, Sjouwerman R, Van Den Heuvel MP, Kahn RS, Geuze E (2015): Smaller hippocampal volume as a vulnerability factor for the persistence of post-traumatic stress disorder. Psychol Med 45: 2737–2746. [DOI] [PubMed] [Google Scholar]
- 22.Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK (2002): Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci 5: 1242–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubin M, Shvil E, Papini S, Chhetry BT, Helpman L, Markowitz JC, et al. (2016): Greater hippocampal volume is associated with PTSD treatment response. Psychiatry Res - Neuroimaging 252: 36–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonne O, Brandes D, Gilboa A, Gomori JM, Shenton ME, Pitman RK, Shalev AY (2001): Longitudinal MRI study of hippocampal volume in trauma survivors with PTSD. Am J Psychiatry 158: 1248–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cwik JC, Vahle N, Woud ML, Potthoff D, Kessler H, Sartory G, Seitz RJ (2019): Reduced gray matter volume in the left prefrontal, occipital, and temporal regions as predictors for posttraumatic stress disorder: a voxel-based morphometric study. Eur Arch Psychiatry Clin Neurosci. 10.1007/s00406-019-01011-2 [DOI] [PubMed]
- 26.Hu H, Sun Y, Su S, Wang Y, Qiu Y, Yang X, et al. (2018): Cortical surface area reduction in identification of subjects at high risk for post-traumatic stress disorder: A pilot study. Aust N Z J Psychiatry 52: 1084–1091. [DOI] [PubMed] [Google Scholar]
- 27.Harnett NG, Ference EW, Knight AJ, Knight DC (2018): White matter microstructure varies with post-traumatic stress severity following medical trauma. Brain Imaging Behav 1–13. [DOI] [PMC free article] [PubMed]
- 28.Fani N, Michopoulos V, van Rooij SJH, Clendinen C, Hardy RA, Jovanovic T, et al. (2019): Structural connectivity and risk for anhedonia after trauma: A prospective study and replication. J Psychiatr Res 116: 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu H, Zhou Y, Wang Q, Su S, Qiu Y, Ge J, et al. (2016): Association of abnormal white matter integrity in the acute phase of motor vehicle accidents with post-traumatic stress disorder. J Affect Disord 190: 714–722. [DOI] [PubMed] [Google Scholar]
- 30.Smid GE, Van Der Velden PG, Lensvelt-Mulders GJLM, Knipscheer JW, Gersons BPR, Kleber RJ (2012): Stress sensitization following a disaster: A prospective study. Psychol Med 42: 1675–1686. [DOI] [PubMed] [Google Scholar]
- 31.Breslau N, Chilcoat HD, Kessler RC, Davis GC (1999): Previous exposure to trauma and PTSD effects of subsequent trauma: Results from the detroit area survey of trauma. Am J Psychiatry 156: 902–907. [DOI] [PubMed] [Google Scholar]
- 32.Pareek V, Rallabandi VS, Roy PK (2018): A Correlational Study between Microstructural White Matter Properties and Macrostructural Gray Matter Volume Across Normal Ageing: Conjoint DTI and VBM Analysis. Magn Reson Insights 11: 1178623X1879992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mechelli A, Friston KJ, Frackowiak RS, Price CJ (2005): Structural covariance in the human cortex. J Neurosci 25: 8303–8310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alexander-Bloch A, Giedd JN, Bullmore E (2013): Imaging structural co-variance between human brain regions. Nature Reviews Neuroscience, vol. 14. pp 322–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stout DM, Buchsbaum MS, Spadoni AD, Risbrough VB, Strigo IA, Matthews SC, Simmons AN (2018): Multimodal canonical correlation reveals converging neural circuitry across trauma-related disorders of affect and cognition. Neurobiol Stress 9: 241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rangaprakash D, Deshpande G, Daniel TA, Goodman AM, Robinson JL, Salibi N, et al. (2017): Compromised hippocampus-striatum pathway as a potential imaging biomarker of mild-traumatic brain injury and posttraumatic stress disorder. Hum Brain Mapp 38: 2843–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Groves AR, Smith SM, Fjell AM, Tamnes CK, Walhovd KB, Douaud G, et al. (2012): Benefits of multi-modal fusion analysis on a large-scale dataset: Life-span patterns of inter-subject variability in cortical morphometry and white matter microstructure. Neuroimage 63: 365–380. [DOI] [PubMed] [Google Scholar]
- 38.Douaud G, Groves AR, Tamnes CK, Westlye LT, Duff EP, Engvig A, et al. (2014): A common brain network links development, aging, and vulnerability to disease. Proc Natl Acad Sci U S A 111: 17648–17653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Groves AR, Beckmann CF, Smith SM, Woolrich MW (2011): Linked independent component analysis for multimodal data fusion. Neuroimage 54: 2198–2217. [DOI] [PubMed] [Google Scholar]
- 40.Sun D, Haswell CC, Morey RA, De Bellis MD (2019): Brain structural covariance network centrality in maltreated youth with PTSD and in maltreated youth resilient to PTSD. Dev Psychopathol 31: 557–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Itahashi T, Yamada T, Nakamura M, Watanabe H, Yamagata B, Jimbo D, et al. (2015): Linked alterations in gray and white matter morphology in adults with high-functioning autism spectrum disorder: A multimodal brain imaging study. NeuroImage Clin 7: 155–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Rooij SJH, Stevens JS, Ely TD, Hinrichs R, Michopoulos V, Winters SJ, et al. (2018): The Role of the Hippocampus in Predicting Future Posttraumatic Stress Disorder Symptoms in Recently Traumatized Civilians. Biol Psychiatry 84: 106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stevens JS, Kim YJ, Galatzer-Levy IR, Reddy R, Ely TD, Nemeroff CB, et al. (2017): Amygdala Reactivity and Anterior Cingulate Habituation Predict Posttraumatic Stress Disorder Symptom Maintenance After Acute Civilian Trauma. Biol Psychiatry 81: 1023–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Foa EB (1995): The posttraumatic diagnostic scale (PDS) manual. Minneapolis, MN Natl Comput Syst 1–5. [Google Scholar]
- 45.Foa EB, Cashman L, Jaycox L, Perry K (1997): The validation of a self-report measure of posttraumatic stress disorder: The posttraumatic diagnostic scale. Psychol Assess 9: 445–451. [Google Scholar]
- 46.Foa EB, Tolin DF (2000): Comparison of the PTSD Symptom Scale-Interview Version and the Clinician-Administered PTSD Scale. J Trauma Stress 13: 181–191. [DOI] [PubMed] [Google Scholar]
- 47.Pinninti NR, Madison H, Musser E, Rissmiller D (2003): MINI International Neuropsychiatric Schedule: Clinical utility and patient acceptance. Eur Psychiatry 18: 361–364. [DOI] [PubMed] [Google Scholar]
- 48.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, et al. (2004): Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, vol. 23 23. 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- 49.Wang S, Peterson DJ, Gatenby JC, Li W, Grabowski TJ, Madhyastha TM (2017): Evaluation of field map and nonlinear registration methods for correction of susceptibility artifacts in diffusion MRI. Front Neuroinform 11. 10.3389/fninf.2017.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. (2006): Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage 31: 1487–1505. [DOI] [PubMed] [Google Scholar]
- 51.Jahanshad N, Kochunov PV, Sprooten E, Mandl RC, Nichols TE, Almasy L, et al. (2013): Multi-site genetic analysis of diffusion images and voxelwise heritability analysis: A pilot project of the ENIGMA-DTI working group. Neuroimage 81: 455–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Good CD, Johnsrude IS, Ashburner J, Henson RNA, Friston KJ, Frackowiak RSJ (2001): A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage 14: 21–36. [DOI] [PubMed] [Google Scholar]
- 53.Douaud G, Smith S, Jenkinson M, Behrens T, Johansen-Berg H, Vickers J, et al. (2007): Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain 130: 2375–2386. [DOI] [PubMed] [Google Scholar]
- 54.Esteban O, Markiewicz CJ, Blair RW, Moodie CA, Isik AI, Erramuzpe A, et al. (2019): fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat Methods 16: 111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gorgolewski K, Burns CD, Madison C, Clark D, Halchenko YO, Waskom ML, Ghosh SS (2011): Nipype: A flexible, lightweight and extensible neuroimaging data processing framework in Python. Front Neuroinform 5. 10.3389/fninf.2011.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fischl B (2012): FreeSurfer. Neuroimage 62: 774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li H, Smith SM, Gruber S, Lukas SE, Silveri MM, Hill KP, et al. (2020): Denoising scanner effects from multimodal MRI data using linked independent component analysis. Neuroimage 208. 10.1016/j.neuroimage.2019.116388 [DOI] [PubMed] [Google Scholar]
- 58.Abou-Elseoud A, Starck T, Remes J, Nikkinen J, Tervonen O, Kiviniemi V (2010): The effect of model order selection in group PICA. Hum Brain Mapp 31: 1207–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harnett NG, Wheelock MD, Wood KH, Ladnier JC, Mrug S, Knight DC (2015): Affective state and locus of control modulate the neural response to threat. Neuroimage 121: 217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kravitz DJ, Saleem KS, Baker CI, Ungerleider LG, Mishkin M (2013): The ventral visual pathway: An expanded neural framework for the processing of object quality. Trends in Cognitive Sciences, vol. 17. pp 26–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stevens JS, Jovanovic T, Fani N, Ely TD, Glover EM, Bradley B, Ressler KJ (2013): Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. J Psychiatr Res 47: 1469–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mueller-Pfeiffer C, Schick M, Schulte-Vels T, O’Gorman R, Michels L, Martin-Soelch C, et al. (2013): Atypical visual processing in posttraumatic stress disorder. NeuroImage Clin 3: 531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fani N, Jovanovic T, Ely TD, Bradley B, Gutman D, Tone EB, Ressler KJ (2012): Neural correlates of attention bias to threat in post-traumatic stress disorder. Biol Psychol 90: 134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fani N, Tone EB, Phifer J, Norrholm SD, Bradley B, Ressler KJ, et al. (2012): Attention bias toward threat is associated with exaggerated fear expression and impaired extinction in PTSD. Psychol Med 42: 533–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kleim B, Ehring T, Ehlers A (2012): Perceptual processing advantages for trauma-related visual cues in post-traumatic stress disorder. Psychol Med 42: 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Misaki M, Phillips R, Zotev V, Wong CK, Wurfel BE, Krueger F, et al. (2018): Connectome-wide investigation of altered resting-state functional connectivity in war veterans with and without posttraumatic stress disorder. NeuroImage Clin 17: 285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Badura-Brack A, McDermott TJ, Becker KM, Ryan TJ, Khanna MM, Pine DS, et al. (2018): Attention training modulates resting-state neurophysiological abnormalities in posttraumatic stress disorder. Psychiatry Res - Neuroimaging 271: 135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Silverstein DN, Ingvar M (2015): A multi-pathway hypothesis for human visual fear signaling. Front Syst Neurosci 9. 10.3389/fnsys.2015.00101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ousdal OT, Andreassen OA, Server A, Jensen J (2014): Increased amygdala and visual cortex activity and functional connectivity towards stimulus novelty is associated with state anxiety. PLoS One 9. 10.1371/journal.pone.0096146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Amaral DG, Behniea H, Kelly JL (2003): Topographic organization of projections from the amygdala to the visual cortex in the macaque monkey. Neuroscience 118: 1099–1120. [DOI] [PubMed] [Google Scholar]
- 71.Weston CSE (2014): Posttraumatic stress disorder: A theoretical model of the hyperarousal subtype. Front Psychiatry 5. 10.3389/fpsyt.2014.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Beckmann CF, DeLuca M, Devlin JT, Smith SM (2005): Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc B Biol Sci 360: 1001–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.