Abstract
Background
ECG abnormalities are associated with adverse outcomes in the general population, but their prognostic significance in severe mental illness (SMI) remains unexplored. We investigated associations between no, minor, and major ECG abnormalities and fatal cardiovascular disease (CVD) among patients with SMI compared with controls without mental illness.
Methods and Results
We cross‐linked data from Danish nationwide registries and included primary care patients with digital ECGs from 2001 to 2015. Patients had SMI if they were diagnosed with schizophrenia, bipolar disorder, or severe depression before ECG recording. Controls were required to be without any prior mental illness or psychotropic medication use. Fatal CVD was assessed using hazard ratios (HRs) with 95% CIs and standardized 10‐year absolute risks. Of 346 552 patients, 10 028 had SMI (3%; median age, 54 years; male, 45%), and 336 524 were controls (97%; median age, 56 years; male, 48%). We observed an interaction between SMI and ECG abnormalities on fatal CVD (P<0.001). Severe mental illness was associated with fatal CVD across no (HR, 2.17; 95% CI, 1.95–2.43), minor (HR, 1.90; 95% CI, 1.49–2.42), and major (HR, 1.40; 95% CI, 1.26–1.55) ECG abnormalities compared with controls. Across age‐ and sex‐specific subgroups, SMI patients with ECG abnormalities but no CVD at baseline had highest standardized 10‐year absolute risks of fatal CVD.
Conclusions
ECG abnormalities conferred a poorer prognosis among patients with SMI compared with controls without mental illness. SMI patients with ECG abnormalities but no CVD represent a high‐risk population that may benefit from greater surveillance and risk management.
Keywords: ECG, primary care, risk prediction, severe mental illness
Subject Categories: Electrophysiology, Mental Health
Nonstandard Abbreviations and Acronyms
- SMI
severe mental illness
Clinical Perspective
What Is New?
Both minor (eg, QT prolongation) and major (eg, Q waves) ECG abnormalities conferred a poorer prognosis among patients with severe mental illness compared with controls without any prior mental illness or psychotropic medication use.
Patients with severe mental illness and ECG abnormalities but no cardiovascular disease at baseline had a higher 10‐year absolute risk of fatal cardiovascular disease relative to if the patient did not have either ECG abnormalities or severe mental illness.
What Are the Clinical Implications?
Patients with severe mental illness have excess cardiovascular morbidity and mortality.
Early detection of ECG abnormalities may offer a simple way to identify patients with severe mental illness at greater cardiovascular risk.
Patients with severe mental illness (SMI) comprising schizophrenia, bipolar disorder, and severe depression have excess cardiovascular risk and a reduced life expectancy of ≈20 years compared with the general population. 1 , 2 , 3 The mortality risk of cardiovascular disease (CVD), such as myocardial infarction, is 30% to 40% among these patients compared with 10% to 15% in the general population. 4 Although several reasons may explain differences in outcomes, patients with SMI are overall less likely to receive timely and proper medical care, including invasive coronary management, and tend to neglect cardiovascular symptoms such as chest pain, palpitations, or presyncope. 5 , 6 Therefore, there is a significant interest in early detection of subclinical CVD and its outcomes among patients with SMI.
The ECG remains a readily available and inexpensive tool to assess cardiovascular risk and preexisting CVD. Although prior studies have demonstrated that commonly encountered ECG abnormalities predict adverse outcomes in the general population, 7 , 8 , 9 , 10 , 11 , 12 there are no published data on the prognostic significance of ECG abnormalities among patients with SMI. Recent work from our group suggests that these patients more often demonstrate elevated heart rate, corrected QT prolongation, and Q waves as a sign of prior myocardial infarction, and less left ventricular hypertrophy and atrial fibrillation/flutter on ECGs compared with background population controls. 13
Therefore, using a large contemporary clinical laboratory database including nearly 1 million digital ECGs cross‐linked with Danish nationwide administrative registries, we investigated the association between ECG abnormalities and fatal CVD among patients with SMI compared with controls without any prior mental illness or psychotropic medication use. Considering the higher prevalence of CVD and overrepresentation of certain ECG abnormalities among patients with SMI, we hypothesized that risk prediction incorporating the ECG may be useful in this vulnerable population.
Methods
Data were obtained from Danish nationwide administrative registries, which were available on a Statistics Denmark server through remote access. The authors declare that all supporting data are available within the article and its online supplementary files.
Study Design and Population
This was a registry‐based retrospective cohort study including patients with their latest available digital ECG recorded between January 1, 2001, and December 31, 2015, at the Copenhagen General Practitioners' Laboratory in Denmark. The index date was the day of ECG recording. The central core facility serviced primary care and specialty outpatient clinics including psychiatry, with various clinical examinations such as ECG recordings, as described previously. 14
Patients were excluded in case of missing data on age or sex or if they were <16 years of age at the index date based on data obtained from the Danish Civil Registration System. 15 Data on vital status were obtained from the Danish Registry of Causes of Death. 16
SMI Definition
We used the Danish Psychiatric Central Research Registry 17 to identify inpatient or outpatient encounters for schizophrenia (International Classification of Diseases, Tenth Revision [ICD‐10] code: F20), bipolar disorder (ICD‐10 codes: F30–31), or severe depression (ICD‐10 codes: F32.2–.3 and F33.2–.3). If patients had more than one registered SMI diagnosis prior to the index date, patients were assigned to the lowest hierarchical diagnosis code regardless of the date of onset to comply with the diagnostic hierarchical order of the ICD‐10 system.
Patients were assigned as controls if they were without any prior mental illness (ICD‐10 code: F*) and had not filled any prescriptions for psychotropic medications within 180 days prior to the index date, which was identified using Anatomical Therapeutic Chemical codes in the Danish National Prescription Registry. 18
ECG Abnormalities
All standard 12‐lead ECGs were digitally recorded at rest and in the supine position, stored in the MUSE Cardiology Information System (GE Healthcare, Milwaukee, WI, USA), and processed using the Marquette 12SL algorithm version 23. 19 All ECGs have been over read by a consultant cardiologist. Using 12SL algorithm statements, we excluded ECGs of poor quality, ECGs with paced rhythms, or ECGs unsuitable for further interpretation, as described previously. 13
We reported data on continuous ECG measurements and defined corrected QT prolongation as >450 ms for males and >470 ms for females using the Fridericia formula. 20 Furthermore, ECG abnormalities were divided into minor (ie, first‐degree atrioventricular, incomplete bundle branch, and left fascicular blocks or corrected QT prolongation) and major (ie, left ventricular hypertrophy, atrial fibrillation/flutter, bundle branch block, intraventricular conduction disturbance, Q waves, or ST‐T deviations), in accordance with contemporary studies. 7 , 9 Moreover, we analyzed the heart rate based on cut offs of <60 and >90 beats per minute. When analyzing the association between ECG abnormalities and outcomes, patients with both minor and major ECG abnormalities were assigned as having major ECG abnormalities. Patients without any ECG abnormalities were considered to have no ECG abnormalities. See Table S1 for an overview of criteria used to define ECG abnormalities.
Covariates
We used the Danish National Patient Registry 21 to additionally exclude patients with a prior pacemaker or implantable cardioverter‐defibrillator implantation based on either ICD‐10 or Nordic Medico‐Statistical Committee Classification of Surgical Procedures codes, whichever came first. The Danish National Patient Registry was also used to identify prior diagnoses of heart failure, coronary artery disease including prior myocardial infarction, atrial fibrillation/flutter, valvular heart disease, hypertension, hyperlipidemia, diabetes mellitus, chronic obstructive pulmonary disease, and chronic kidney disease. Using Nordic Medico‐Statistical Committee Classification of Surgical Procedures codes, we also identified percutaneous coronary intervention or coronary artery bypass grafting for coronary artery disease, ablation for atrial fibrillation/flutter, aortic or mitral valve surgery for valvular heart disease, and renal replacement therapy for chronic kidney disease. We further identified filled prescriptions for cardiovascular medications within 180 days prior to the index date based on Anatomical Therapeutic Chemical codes. Because hypertension, hyperlipidemia, diabetes mellitus, and chronic obstructive pulmonary disease are often managed in primary care, patients may not necessarily have ICD codes registered. Accordingly, prior filled prescriptions for antihypertensives (at least dual therapy), lipid‐lowering medications, antidiabetics, and β‐adrenergic or anticholinergic inhalants were also used to define these conditions. Finally, we used CredibleMeds, an internet‐based registry of QT‐prolonging medications, to identify medications associated with known or possible QT prolongation risk. 22 See Tables S2 and S3 for an overview of used ICD, Nordic Medico‐Statistical Committee Classification of Surgical Procedures, and Anatomical Therapeutic Chemical codes.
Outcomes
Patients were followed from the index date until the occurrence of an outcome or censoring in case of emigration or end of study on December 31, 2017, whichever came first. The primary outcome fatal CVD was defined as death from any CVD (ICD‐10 code: I*). We also performed an additional analysis using all‐cause mortality as a secondary outcome.
Statistical Analysis
Continuous variables were reported as medians with 25th to 75th percentiles and categorical variables as counts with percentages. Between‐group differences were compared using Mann‐Whitney U and χ2 tests, as appropriate.
Outcomes were compared between patients with SMI and controls without mental illness, which served as the reference group, across similar levels of ECG abnormalities. Cumulative incidence curves of fatal CVD were generated using the Aalen‐Johansen method, with death from other causes being accounted for as a competing risk event, and event distributions were compared using Gray's test. Multivariable Cox regression analysis was used to compute hazard ratios (HRs) with 95% CIs. Furthermore, we performed an additional analysis using a dummy variable that combined SMI status with ECG abnormalities, in which controls without mental illness demonstrating no ECG abnormalities served as the reference. We also performed stratified analyses of subtype of SMI diagnosis and individual ECG abnormalities.
All models were adjusted for age, sex, heart failure, coronary artery disease, atrial fibrillation/flutter, valvular heart disease, hypertension, hyperlipidemia, diabetes mellitus, chronic obstructive pulmonary disease, chronic kidney disease, and QT‐prolonging medications.
Due to nonlinearity, age was grouped by quartiles. The proportional hazards assumption was assessed using Martingale residuals and was not violated. Interaction testing was based on introducing an interaction term in a Cox regression model and using a likelihood ratio test to compare this model with another without an interaction term. Specifically, we tested for an interaction between SMI and ECG abnormalities on outcomes.
The time‐dependent area under the receiver operating characteristics curve (AUC) was calculated to assess the added discriminative value of ECG abnormalities to a conventional risk model for the purpose of 10‐year risk prediction of fatal CVD. 23 The conventional risk model was based on age, sex, hypertension, hyperlipidemia, diabetes mellitus, and chronic obstructive pulmonary disease. Patients with SMI and controls without mental illness were stratified by the absence/presence of CVD at baseline, and data were split into training (63%) and test (37%) sets using bootstrap cross‐validation with 1000 bootstrap samples. Brier scores were calculated to evaluate model calibration. 24 Furthermore, we calculated the standardized 10‐year absolute risk of fatal CVD. 24
Data management and analysis were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA) and R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria). A P<0.05 was considered statistically significant for all analyses except for during interaction testing where an a prior decision was made to use P<0.01 to account for multiple testing.
Ethics
In Denmark, registry‐based studies do not require ethical committee approval or individual patient consent if the study is conducted for the sole purpose of statistics and scientific research, as defined in the Data Protection Act. Approval to use the data sources for research purposes was granted by the institute responsible for the data in the Capital Region of Denmark in accordance with the General Data Protection Regulation (approval number: P‐2019‐533).
Results
Patients and Characteristics
A total of 346 552 patients with digital ECGs were included, of whom 10 028 (3%) had SMI, and 336 524 (97%) were controls without mental illness. See Figure S1 for the flowchart. Patient characteristics are shown in Table 1. The majority of patients with SMI had a diagnosis of schizophrenia (45%). The median age at the time of ECG recording was 54 years (25th–75th percentiles, 42–66 years) among patients with SMI and 56 years (25th–75th percentiles, 41–69 years) among controls without mental illness. Approximately 45% and 48% of patients with SMI and controls without mental illness were male, respectively. Overall, patients with SMI had a higher cardiovascular comorbidity burden and were less likely to fill prescriptions for cardiovascular medications compared with controls without mental illness. Furthermore, 68% of patients with SMI filled prescriptions for QT‐prolonging medications, which in most cases were antipsychotics or antidepressants (61%).
Table 1.
Baseline Characteristics
| Patients With SMI |
Controls Without Mental Illness |
P Value | |
|---|---|---|---|
| n | 10 028 | 336 524 | NA |
| Age at ECG recording, y | 54 [42–66] | 56 [41–69] | <0.001 |
| Male | 4464 (44.5) | 161 282 (47.9) | <0.001 |
| Subtype of SMI diagnosis | NA | NA | |
| Schizophrenia | 4477 (44.6) | ||
| Bipolar disorder | 2571 (25.6) | ||
| Severe depression | 2980 (29.7) | ||
| SMI duration, y | 8 [3–13] | NA | NA |
| Heart failure | 421 (4.2) | 10 433 (3.1) | <0.001 |
| Coronary artery disease | 888 (8.9) | 29 232 (8.7) | 0.567 |
| Atrial fibrillation/flutter | 424 (4.2) | 14 903 (4.4) | 0.349 |
| Valvular heart disease | 123 (1.2) | 3690 (1.1) | 0.237 |
| Hypertension | 1479 (14.7) | 50 432 (15.0) | 0.521 |
| Hyperlipidemia | 1686 (16.8) | 49 201 (14.6) | <0.001 |
| Diabetes mellitus | 1222 (12.2) | 26 732 (7.9) | <0.001 |
| Chronic obstructive pulmonary disease | 1351 (13.5) | 29 093 (8.6) | <0.001 |
| Chronic kidney disease | 311 (3.1) | 6328 (1.9) | <0.001 |
| ACEIs/ARBs | 1401 (14.0) | 64 743 (19.2) | <0.001 |
| Beta‐blockers | 765 (7.6) | 32 384 (9.6) | <0.001 |
| Diuretics | 1467 (14.6) | 45 533 (13.5) | 0.002 |
| QT‐prolonging medications | 6819 (68.0) | 44 377 (13.2) | <0.001 |
Data are reported as median (25th–75th percentiles) or n (%). P values based on Mann‐Whitney U and χ2 tests, as appropriate. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; NA, not applicable; and SMI, severe mental illness.
ECG characteristics are shown in Table 2. The distribution of ECG abnormalities was overall similar between patients with SMI (no, 73%; minor, 7%; major, 21%) and controls without mental illness (no, 71%; minor, 6%; major, 24%).
Table 2.
ECG Characteristics
| Patients With SMI | Controls Without Mental Illness | P Value | |
|---|---|---|---|
| n | 10 028 | 336 524 | NA |
| Heart rate, bpm | 75 [66–86] | 69 [61–78] | <0.001 |
| Missing | 686 | 30 361 | |
| P‐wave duration, ms | 108 [100–116] | 108 [100–116] | <0.001 |
| Missing | 686 | 30 361 | |
| PR interval, ms | 156 [142–172] | 156 [144–174] | 0.604 |
| Missing | 686 | 30 361 | |
| QRS duration, ms | 90 [84–98] | 92 [84–100] | <0.001 |
| Missing | 686 | 30 361 | |
| QT interval, ms | 388 [366–410] | 396 [376–416] | <0.001 |
| Missing | 686 | 30 361 | |
| QTcF interval, ms | 417 [404–431] | 414 [402–427] | <0.001 |
| Missing | 686 | 30 361 | |
| No ECG abnormality | 7295 (72.7) | 237 397 (70.5) | <0.001 |
| Minor ECG abnormality | 660 (6.6) | 18 541 (5.5) | <0.001 |
| First‐degree atrioventricular block | 384 (3.8) | 13 477 (4.0) | 0.391 |
| Incomplete bundle branch block | 210 (2.1) | 8504 (2.5) | 0.007 |
| Incomplete right bundle branch block | 192 (1.9) | 7680 (2.3) | 0.016 |
| Incomplete left bundle branch block | 18 (0.2) | 824 (0.2) | 0.227 |
| Left fascicular block | 177 (1.8) | 4504 (1.3) | <0.001 |
| Left anterior fascicular block | 102 (1.0) | 2743 (0.8) | 0.031 |
| Left posterior fascicular block | 75 (0.7) | 1761 (0.5) | 0.003 |
| QTcF prolongation | 324 (3.2) | 5831 (1.7) | <0.001 |
| Major ECG abnormality | 2073 (20.7) | 80 586 (23.9) | <0.001 |
| Left ventricular hypertrophy | 850 (8.5) | 35 832 (10.6) | <0.001 |
| Atrial fibrillation/flutter | 234 (2.3) | 12 561 (3.7) | <0.001 |
| Bundle branch block | 302 (3.0) | 11 550 (3.4) | 0.024 |
| Right bundle branch block | 217 (2.2) | 7803 (2.3) | 0.326 |
| Left bundle branch block | 85 (0.8) | 3747 (1.1) | 0.014 |
| Intraventricular conduction disturbance | 76 (0.8) | 2786 (0.8) | 0.479 |
| Q waves | 541 (5.4) | 17 064 (5.1) | 0.152 |
| ST‐T deviations | 381 (3.8) | 15 283 (4.5) | <0.001 |
Data are reported as median (25th–75th percentiles) or n (%). P values based on Mann‐Whitney U and χ2 tests, as appropriate. bpm indicates beats per minute; QTcF, Fridericia‐corrected QT; and SMI, severe mental illness.
See Tables S4 and S5 for patient and ECG characteristics stratified by subtype of SMI diagnosis.
Associations Between SMI, ECG Abnormalities, and Adverse Outcomes
During a median follow‐up of 6 years (25th–75th percentiles, 3–10 years), 23% of patients with SMI and 17% of controls without mental illness died, with fatal CVD accounting for 10% among patients with SMI and 8% among controls without mental illness. Across all levels of ECG abnormalities, patients with SMI had the highest rate of fatal CVD (P<0.001) (Figure 1).
Figure 1. Cumulative incidence curves of fatal CVD among patients with SMI and controls without mental illness across no (A), minor (B), and major (C) ECG abnormalities.
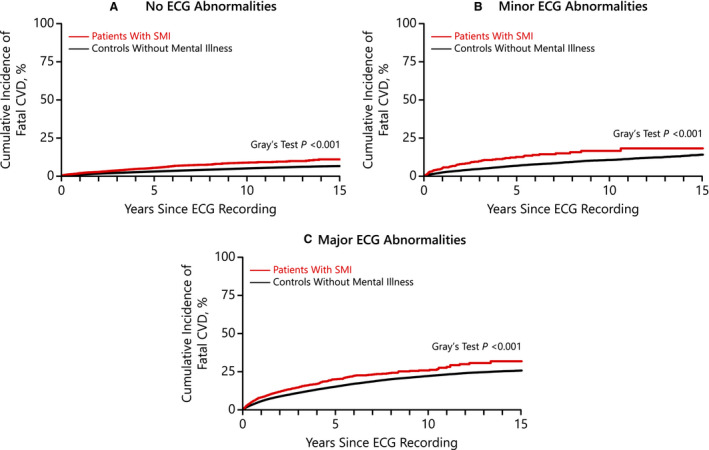
CVD indicates cardiovascular disease; and SMI, severe mental illness.
We observed an interaction between SMI and ECG abnormalities on fatal CVD (P<0.001) as well as all‐cause mortality (P<0.001). Severe mental illness was associated with fatal CVD across no (HR, 2.17; 95% CI, 1.95–2.43), minor (HR, 1.90; 95% CI, 1.49–2.42), and major (HR, 1.40; 95% CI, 1.26–1.55) ECG abnormalities compared with controls without mental illness (Figure 2A). Overall, similar results were observed with all‐cause mortality (Figure 2B). Furthermore, when using a dummy variable that combined SMI status and ECG abnormalities, fatal CVD rate was increased among patients with SMI demonstrating no (HR, 2.11; 95% CI, 1.89–2.35), minor (HR, 2.78; 95% CI, 2.22–3.48), or major (HR, 3.23; 95% CI, 2.91–3.58) ECG abnormalities compared with controls without mental illness demonstrating no ECG abnormalities. Similar but less pronounced rates were observed among controls without mental illness demonstrating minor (HR, 1.37; 95% CI, 1.30–1.45) or major (HR, 2.26; 95% CI, 2.20–2.33) ECG abnormalities (Figure S2A). As in the main analysis, similar results were obtained with all‐cause mortality (Figure S2B).
Figure 2. Multivariable Cox regression of the association between SMI and fatal CVD (A) and all‐cause mortality (B) across no, minor, and major ECG abnormalities.
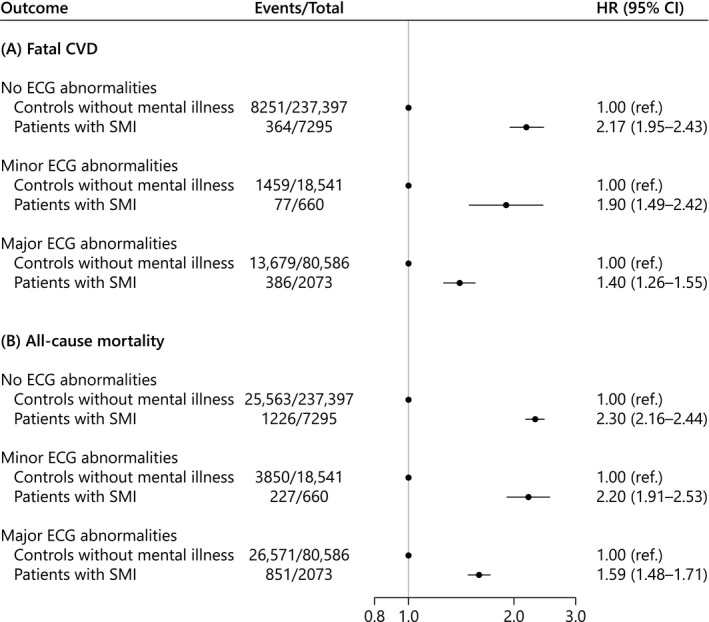
Adjusted for age, sex, heart failure, coronary artery disease, atrial fibrillation/flutter, valvular heart disease, hypertension, hyperlipidemia, diabetes mellitus, chronic obstructive pulmonary disease, chronic kidney disease, and QT‐prolonging medications. CVD indicates cardiovascular disease; HR, hazard ratio; and SMI, severe mental illness.
When stratifying by subtype of SMI diagnosis, the interaction was driven by schizophrenia (P<0.001) rather than bipolar disorder (P=0.628) or severe depression (P=0.150), and patients with schizophrenia also had the worst prognosis (Figure S3).
Most of the individual minor and major ECG abnormalities as well as heart rate <60 or >90 beats per minute conferred a poorer prognosis among patients with SMI compared with controls without mental illness (Figure 3). In particular, an increased rate of fatal CVD was associated with patients with SMI demonstrating incomplete bundle branch block (HR, 2.29; 95% CI, 1.47–3.56), intraventricular conduction disturbance (HR, 2.00; 95% CI, 1.15–3.47), heart rate <60 beats per minute (HR, 1.64; 95% CI, 1.20–2.24), Q waves (HR, 1.57; 95% CI, 1.28–1.94), or heart rate >90 beats per minute (HR, 1.50; 95% CI, 1.25–1.79).
Figure 3. Multivariable Cox regression of the association between individual ECG abnormalities and fatal CVD for patients with SMI compared with controls without mental illness.
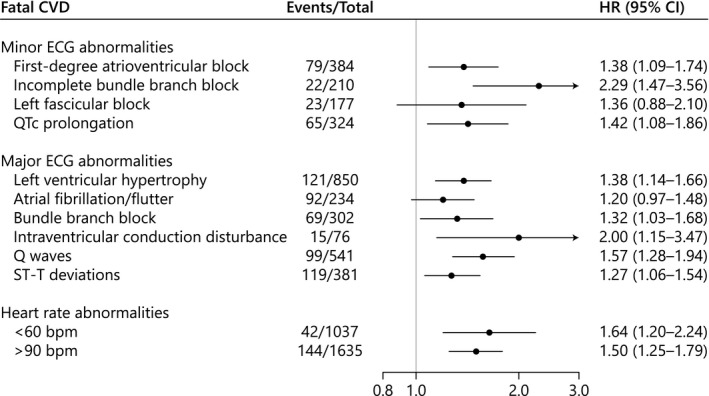
Adjusted for age, sex, heart failure, coronary artery disease, atrial fibrillation/flutter, valvular heart disease, hypertension, hyperlipidemia, diabetes mellitus, chronic obstructive pulmonary disease, chronic kidney disease, and QT‐prolonging medications. bpm indicates beats per minute; CVD, cardiovascular disease; HR, hazard ratio; QTc, corrected QT; and SMI, severe mental illness.
10‐Year Risk Prediction
Adding ECG abnormalities to a conventional risk model increased the AUC for the 10‐year risk prediction of fatal CVD only among the subset of patients with SMI without CVD at baseline (difference in AUC, 2.33%; 95% CI, 0.17%–4.18%). No model improvement was observed among the subset of patients with SMI and CVD at baseline. Contrarily, model improvement was observed among controls without mental illness regardless of baseline CVD status. See Figure S4 and Table S6 for AUC results including Brier scores.
As model improvement with ECG abnormalities was most pronounced in the non‐CVD population, we calculated the standardized 10‐year absolute risk of fatal CVD for levels of ECG abnormalities stratified by age groups, sex, and SMI status, as shown in Figure 4. Patterns of increasing risk with minor and major ECG abnormalities were observed among patients with SMI.
Figure 4. Risk chart showing the standardized 10‐year absolute risk of fatal CVD among patients with SMI and controls without mental illness free of CVD at baseline across ECG abnormalities and age‐ and sex‐specific subgroups.
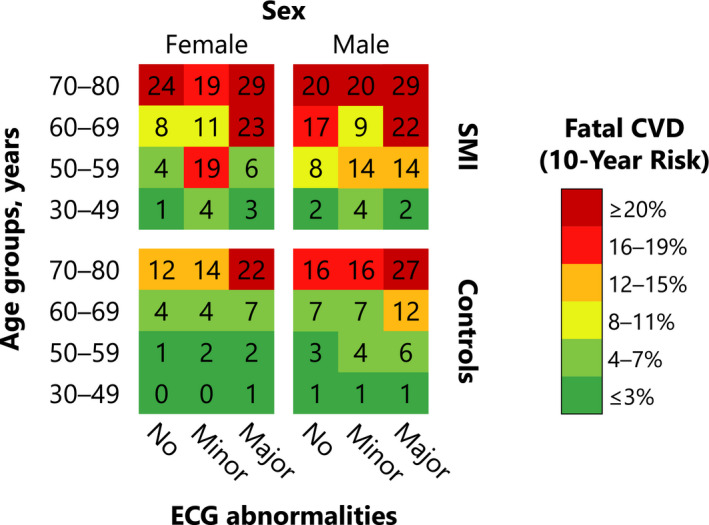
CVD indicates cardiovascular disease; and SMI, severe mental illness.
Discussion
In this large‐scale study, we report several key findings underscoring a differential association between ECG abnormalities and adverse outcomes among primary care patients with SMI compared with controls without mental illness. First, patients with SMI demonstrating minor or major ECG abnormalities had a poorer prognosis compared with the control group. Second, we observed clinically relevant differences in fatal CVD associated with individual ECG abnormalities among patients with SMI, where particularly incomplete bundle branch block, intraventricular conduction disturbance, and Q waves conferred a poorer prognosis. Finally, among patients with SMI but no CVD at baseline, adding ECG abnormalities to a conventional risk model improved 10‐year risk prediction of fatal CVD.
Several prior studies have reported associations between minor and major ECG abnormalities and adverse outcomes, 7 , 8 , 9 , 10 , 11 , 12 and the magnitude of risk associated with abnormal ECGs varied across different populations. This is emphasized in clinical guideline recommendations for the utilization of the ECG as a screening tool among asymptomatic adults. 25 , 26 However, considering that the distribution of ECG abnormalities varies among patients with SMI compared with a psychiatrically healthy population, 13 and that no studies have investigated their prognostic significance, evidence to suggest clear clinical ECG recommendations in the setting of SMI is lacking. This underscores the importance of our comprehensive study and adds to the evidence of the potential benefit of the ECG as a cardiovascular risk stratification tool among patients with SMI. However, the optimal frequency and cost‐effectiveness of using the ECG to screen for CVD during routine clinical care of patients with SMI need to be explored.
Our finding of an association between ECG abnormalities and adverse outcomes that was particularly important among patients with SMI, as identified by interaction testing, has been underrecognized and not previously described. Moreover, considering the excess cardiovascular morbidity and mortality as well as poor cardiovascular care across other mental illnesses and patients treated with psychotropic medications only, 27 ECG abnormalities may also prove valuable in assessing cardiovascular risk among these patients. However, further studies are warranted to explore this. Although several common major ECG abnormalities conferred a poorer prognosis among patients with SMI in our study, clinicians should also pay attention to those who are demonstrating minor ECG abnormalities, as they are at high cardiovascular risk. Overall, this suggests that CVD may have a more severe course in the setting of SMI, which may be due to a multifactorial interplay of genetic risk, immune system alterations, unhealthy lifestyle, adverse effects of psychotropic medications, lack and neglect of timely cardiovascular care, cognitive impairment, and social deprivation. 1 , 28 Furthermore, a majority of patients with SMI experience sudden cardiac death as the first manifestation of CVD. 29 In most cases, this may be due to undetected or silent myocardial infarction, as identified by Q waves on the ECG, which occurs in up to 75% of patients with schizophrenia. 30 In our recent work, we observed an overrepresentation of Q waves among patients with SMI compared with a psychiatrically healthy population, 13 and the current study demonstrated that Q waves are not only more prevalent but also associated with an increase in mortality over time (HR of 1.57).
Contemporary risk prediction algorithms for CVD have been shown to underestimate the risk among patients with SMI. 31 Accordingly, to assess if ECG abnormalities improved risk prediction on an individual level compared with a conventional risk model, we calculated measures of discrimination of 10‐year risk of fatal CVD and observed improvement with ECG abnormalities in the AUC by ≈2% points among patients with SMI but no CVD at baseline. Furthermore, ECG abnormalities conferred very high standardized 10‐year absolute risks of fatal CVD. For example, we predicted a 55‐year‐old female with SMI but no CVD at baseline who demonstrated a minor ECG abnormality to have a 19% 10‐year absolute risk of fatal CVD compared with 4% if she had no ECG abnormality or 1% if she had no SMI. Based on our findings, we suggest that primary care patients with SMI, not already treated for or diagnosed with CVD, merit close monitoring and follow‐up, and clinicians should incorporate a multidisciplinary approach to caring for patients with SMI and newly detected ECG abnormalities.
Limitations
Our study has several limitations. Both unmeasured and unknown confounding including cardiovascular symptoms, cardiovascular family history, and lifestyle factors including smoking may affect findings, but such data were not available in our registries. However, we indirectly accounted for smoking by adjusting analyses for a diagnosis of chronic obstructive pulmonary disease or filled prescriptions for inhalants. Furthermore, the use of large sample sizes may reduce variation in data, and by performing several between‐group comparisons, statistically significant differences that are not necessarily clinically meaningful may appear. In particular, this was the case with most of the continuous ECG measurements. Although most patients with SMI undergo routine ECG examinations in primary care, particularly for screening for corrected QT prolongation, data on ECG indications were unfortunately not available. However, the proportional hazards assumption was not violated, indicating that the observed associations were not driven by accumulation of events in close proximity to the ECG recording. Finally, fatal CVD was used as our primary outcome, but the number of medicolegal autopsies has decreased over recent years in Denmark. Therefore, in some cases, the cause of death was based on a subjective judgement by clinicians, and caution should be taken when interpreting data. Therefore, we used all‐cause mortality as a secondary outcome, which for no ECG abnormalities showed a comparable rate (HR of 2.30) to fatal CVD (HR of 2.17), suggesting that although patients with SMI do not have established risk factors or recognized CVD, they still have excess cardiovascular mortality. For minor and major ECG abnormalities, we observed increased rates of all‐cause mortality compared with fatal CVD, suggesting that patients with ECG abnormalities may be more comorbid than if they had a normal ECG and thus have an overall poorer prognosis.
Conclusions
In a large contemporary primary care population, ECG abnormalities conferred a poorer prognosis among patients with SMI compared with controls without mental illness. SMI patients with ECG abnormalities but no CVD at baseline represent a high‐risk population that may benefit from greater surveillance and cardiovascular risk management.
Sources of Funding
This study was supported by Departmental Sources, the Danish Heart Foundation (grant number: 18‐R125‐A8382‐22086), Eva and Henry Frænkel Memorial Foundation, and Overlæge dr. med. Einar Geert‐Jørgensen og Hustrus Forskningslegat. The funding sources had no influence on study design; collection, analysis, or interpretation of data; writing of the manuscript; or decision to submit the manuscript for publication.
Disclosures
Dr Polcwiartek reported receiving speaking fees from Lundbeck Pharma A/S. Dr Atwater reported receiving speaking fees from Medtronic; research grants from Abbott and Boston Scientific; and acting as adviser to Abbott, Biotronik, and Medtronic. Dr Kragholm reported receiving speaking fees from Novartis and a research grant from the Laerdal Foundation. Dr Friedman reported receiving salary support from the National Institutes of Health; research grants from Abbott, Biosense Webster, Boston Scientific, and the National Cardiovascular Data Registry; and educational grants from Abbott, Biotronik, Boston Scientific, and Medtronic. Dr Søgaard reported receiving research grants from Biotronik and GE Healthcare and acting as adviser to Biotronik. Dr Torp‐Pedersen reported receiving speaking fees from Bayer and research grants from Bayer and Biotronik. Dr Jensen reported receiving a research grant from the Obel Family Foundation. The remaining authors have no disclosures to report.
Supporting information
(J Am Heart Assoc.2021;10:e019416. DOI: 10.1161/JAHA.120.019416.)
For Sources of Funding and Disclosures, see page 10.
References
- 1. Nielsen RE, Banner J, Jensen SE. Cardiovascular disease in patients with severe mental illness. Nat Rev Cardiol. 2020. Oct 30 [epub ahead of print]. DOI: 10.1038/s41569-020-00463-7 [DOI] [PubMed] [Google Scholar]
- 2. Lomholt LH, Andersen DV, Sejrsgaard‐Jacobsen C, Øzdemir CM, Graff C, Schjerning O, Jensen SE, Straszek SPV, Licht RW, Grøntved S, et al. Mortality rate trends in patients diagnosed with schizophrenia or bipolar disorder: a nationwide study with 20 years of follow‐up. Int J Bipolar Disord. 2019;7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Correll CU, Solmi M, Veronese N, Bortolato B, Rosson S, Santonastaso P, Thapa‐Chhetri N, Fornaro M, Gallicchio D, Collantoni E, et al. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large‐scale meta‐analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry. 2017;16:163–180. DOI: 10.1002/wps.20420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bodén R, Molin E, Jernberg T, Kieler H, Lindahl B, Sundström J. Higher mortality after myocardial infarction in patients with severe mental illness: a nationwide cohort study. J Intern Med. 2015;277:727–736. DOI: 10.1111/joim.12329 [DOI] [PubMed] [Google Scholar]
- 5. Smith DJ, Langan J, McLean G, Guthrie B, Mercer SW. Schizophrenia is associated with excess multiple physical‐health comorbidities but low levels of recorded cardiovascular disease in primary care: cross‐sectional study. BMJ Open. 2013;3:e002808. DOI: 10.1136/bmjopen-2013-002808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heiberg IH, Jacobsen BK, Balteskard L, Bramness JG, Næss O, Ystrom E, Reichborn‐Kjennerud T, Hultman CM, Nesvåg R, Høye A. Undiagnosed cardiovascular disease prior to cardiovascular death in individuals with severe mental illness. Acta Psychiatr Scand. 2019;139:558–571. DOI: 10.1111/acps.13017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Auer R, Bauer DC, Marques‐Vidal P, Butler J, Min LJ, Cornuz J, Satterfield S, Newman AB, Vittinghoff E, Rodondi N. Association of major and minor ECG abnormalities with coronary heart disease events. JAMA. 2012;307:1497–1505. DOI: 10.1001/jama.2012.434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jørgensen PG, Jensen JS, Marott JL, Jensen GB, Appleyard M, Mogelvang R. Electrocardiographic changes improve risk prediction in asymptomatic persons age 65 years or above without cardiovascular disease. J Am Coll Cardiol. 2014;64:898–906. DOI: 10.1016/j.jacc.2014.05.050 [DOI] [PubMed] [Google Scholar]
- 9. Denes P, Larson JC, Lloyd‐Jones DM, Prineas RJ, Greenland P. Major and minor ECG abnormalities in asymptomatic women and risk of cardiovascular events and mortality. JAMA. 2007;297:978–985. 10.1001/jama.297.9.978 [DOI] [PubMed] [Google Scholar]
- 10. Gorodeski EZ, Ishwaran H, Blackstone EH, Lauer MS. Quantitative electrocardiographic measures and long‐term mortality in exercise test patients with clinically normal resting electrocardiograms. Am Heart J. 2009;158:61–70.e1. DOI: 10.1016/j.ahj.2009.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hari KJ, Singleton MJ, Ahmad MI, Soliman EZ. Relation of minor electrocardiographic abnormalities to cardiovascular mortality. Am J Cardiol. 2019;123:1443–1447. DOI: 10.1016/j.amjcard.2019.01.050 [DOI] [PubMed] [Google Scholar]
- 12. Polcwiartek C, Kragholm K, Friedman DJ, Atwater BD, Graff C, Nielsen JB, Holst AG, Struijk JJ, Pietersen A, Svendsen JH, et al. Long‐term prognostic value of less‐stringent electrocardiographic Q waves and Fourth Universal Definition of Myocardial Infarction Q waves. Am J Med. 2020;133:582–589.e7. DOI: 10.1016/j.amjmed.2019.08.056 [DOI] [PubMed] [Google Scholar]
- 13. Polcwiartek C, Kragholm K, Hansen SM, Atwater BD, Friedman DJ, Barcella CA, Graff C, Nielsen JB, Pietersen A, Nielsen J, et al. Electrocardiogram characteristics and their association with psychotropic drugs among patients with schizophrenia. Schizophr Bull. 2020;46:354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nielsen JB, Graff C, Pietersen A, Lind B, Struijk JJ, Olesen MS, Haunsø S, Gerds TA, Svendsen JH, Køber L, et al. J‐shaped association between QTc interval duration and the risk of atrial fibrillation: results from the Copenhagen ECG study. J Am Coll Cardiol. 2013;61:2557–2564. DOI: 10.1016/j.jacc.2013.03.032 [DOI] [PubMed] [Google Scholar]
- 15. Pedersen CB. The Danish civil registration system. Scand J Public Health. 2011;39:22–25. DOI: 10.1177/1403494810387965 [DOI] [PubMed] [Google Scholar]
- 16. Helweg‐Larsen K. The Danish register of causes of death. Scand J Public Health. 2011;39:26–29. DOI: 10.1177/1403494811399958 [DOI] [PubMed] [Google Scholar]
- 17. Mors O, Perto GP, Mortensen PB. The Danish psychiatric central research register. Scand J Public Health. 2011;39:54–57. DOI: 10.1177/1403494810395825 [DOI] [PubMed] [Google Scholar]
- 18. Kildemoes HW, Sørensen HT, Hallas J. The Danish national prescription registry. Scand J Public Health. 2011;39:38–41. DOI: 10.1177/1403494810394717 [DOI] [PubMed] [Google Scholar]
- 19. GE Healthcare . Marquette 12SL ECG analysis program physician's guide. 2015. https://www.gehealthcare.com/en‐GB/products/diagnostic‐cardiology/marquette‐12sl. Accessed November 12, 2020.
- 20. Goldenberg I, Moss AJ, Zareba W. QT interval: how to measure it and what is "normal". J Cardiovasc Electrophysiol. 2006;17:333–336. DOI: 10.1111/j.1540-8167.2006.00408.x [DOI] [PubMed] [Google Scholar]
- 21. Lynge E, Sandegaard JL, Rebolj M. The Danish national patient register. Scand J Public Health. 2011;39:30–33. DOI: 10.1177/1403494811401482 [DOI] [PubMed] [Google Scholar]
- 22. CredibleMeds . Drugs that prolong QT & induce Torsades de Pointes (TdP). 2020. https://www.CredibleMeds.org/. Accessed September 01, 2020.
- 23. Blanche P, Kattan MW, Gerds TA. The c‐index is not proper for the evaluation of t‐year predicted risks. Biostatistics. 2019;20:347–357. DOI: 10.1093/biostatistics/kxy006 [DOI] [PubMed] [Google Scholar]
- 24. Gerds TA, Scheike TH, Andersen PK. Absolute risk regression for competing risks: interpretation, link functions, and prediction. Stat Med. 2012;31:3921–3930. DOI: 10.1002/sim.5459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, Foster E, Hlatky MA, Hodgson JM, Kushner FG, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2010;122:2748–2764. DOI: 10.1161/CIR.0b013e3182051bab [DOI] [PubMed] [Google Scholar]
- 26. Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, Davidson KW, Doubeni CA, Epling JW Jr, Kemper AR, Kubik M, et al. Screening for cardiovascular disease risk with electrocardiography: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:2308–2314. DOI: 10.1001/jama.2018.6848 [DOI] [PubMed] [Google Scholar]
- 27. Barcella CA, Mohr GH, Kragholm KH, Gerds TA, Jensen SE, Polcwiartek C, Wissenberg M, Lippert FK, Torp‐Pedersen C, Kessing LV, et al. Out‐of‐hospital cardiac arrest in patients with and without psychiatric disorders: differences in use of coronary angiography, coronary revascularization, and implantable cardioverter‐defibrillator and survival. J Am Heart Assoc. 2019;8:e012708. DOI: 10.1161/JAHA.119.012708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Henderson DC, Vincenzi B, Andrea NV, Ulloa M, Copeland PM. Pathophysiological mechanisms of increased cardiometabolic risk in people with schizophrenia and other severe mental illnesses. Lancet Psychiatry. 2015;2:452–464. DOI: 10.1016/S2215-0366(15)00115-7 [DOI] [PubMed] [Google Scholar]
- 29. Koponen H, Alaräisänen A, Saari K, Pelkonen O, Huikuri H, Raatikainen MJ, Savolainen M, Isohanni M. Schizophrenia and sudden cardiac death: a review. Nord J Psychiatry. 2008;62:342–345. DOI: 10.1080/08039480801959323 [DOI] [PubMed] [Google Scholar]
- 30. Nielsen J, Juel J, Alzuhairi KS, Friis R, Graff C, Kanters JK, Jensen SE. Unrecognised myocardial infarction in patients with schizophrenia. Acta Neuropsychiatr. 2015;27:106–112. DOI: 10.1017/neu.2014.41 [DOI] [PubMed] [Google Scholar]
- 31. Osborn DPJ, Hardoon S, Omar RZ, Holt RIG, King M, Larsen J, Marston L, Morris RW, Nazareth I, Walters K, et al. Cardiovascular risk prediction models for people with severe mental illness: results from the prediction and management of cardiovascular risk in people with severe mental illnesses (PRIMROSE) research program. JAMA Psychiatry. 2015;72:143–151. DOI: 10.1001/jamapsychiatry.2014.2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hancock EW, Deal BJ, Mirvis DM, Okin P, Kligfield P, Gettes LS, Bailey JJ, Childers R, Gorgels A, Josephson M, et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part V: electrocardiogram changes associated with cardiac chamber hypertrophy: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. Circulation. 2009;119:e251–e261. DOI: 10.1161/CIRCULATIONAHA.108.191097 [DOI] [PubMed] [Google Scholar]
- 33. Goldenberg I, Moss AJ, Zareba W. QT interval: how to measure it and what is “normal”. J Cardiovasc Electrophysiol. 2006;17:333–336. DOI: 10.1111/j.1540-8167.2006.00408.x [DOI] [PubMed] [Google Scholar]
- 34. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD. Fourth Universal Definition of Myocardial Infarction (2018). Circulation. 2018;138:e618–e651. [DOI] [PubMed] [Google Scholar]
- 35. Deshpande A, Birnbaum Y. ST‐segment elevation: distinguishing ST elevation myocardial infarction from ST elevation secondary to nonischemic etiologies. World J Cardiol. 2014;6:1067–1079. DOI: 10.4330/wjc.v6.i10.1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rasmussen PV, Nielsen JB, Pietersen A, Graff C, Lind B, Struijk JJ, Olesen MS, Haunsø S, Køber L, Svendsen JH, et al. Electrocardiographic precordial ST‐segment deviations and the risk of cardiovascular death: results from the Copenhagen ECG Study. J Am Heart Assoc. 2014;3:e000549. DOI: 10.1161/JAHA.113.000549 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


