Abstract
Background
Despite the increasing interest in cardiac autonomic nervous activity, the normal development is not fully understood. The main aim was to determine the maturation of different cardiac sympathetic‐(SNS) and parasympathetic nervous system (PNS) activity parameters in healthy patients aged 0.5 to 20 years. A second aim was to determine potential sex differences.
Methods and Results
Five studies covering the 0.5‐ to 20‐year age range provided impedance‐ and electrocardiography recordings from which heart rate, different PNS‐parameters (eg, respiratory sinus arrhythmia) and an SNS‐parameter (pre‐ejection period) were collected. Age trends were computed in the mean values across 12 age‐bins and in the age‐specific variances. Age was associated with changes in mean and variance of all parameters. PNS‐activity followed a cubic trend, with an exponential increase from infancy, a plateau phase during middle childhood, followed by a decrease to adolescence. SNS‐activity showed a more linear trend, with a gradual decrease from infancy to adolescence. Boys had higher SNS‐activity at ages 11 to 15 years, while PNS‐activity was higher at 5 and 11 to 12 years with the plateau level reached earlier in girls. Interindividual variation was high at all ages. Variance was reasonably stable for SNS‐ and the log‐transformed PNS‐parameters.
Conclusions
Cardiac PNS‐ and SNS‐activity in childhood follows different maturational trajectories. Whereas PNS‐activity shows a cubic trend with a plateau phase during middle childhood, SNS‐activity shows a linear decrease from 0.5 to 20 years. Despite the large samples used, clinical use of the sex‐specific centile and percentile normative values is modest in view of the large individual differences, even within narrow age bands.
Keywords: autonomic nervous system, heart rate variability, sympathetic nerve activity, development, pediatrics
Subject Categories: Autonomic Nervous System, Physiology, Pediatrics
Nonstandard Abbreviations and Acronyms Abbreviations
- ABCD
Amsterdam Born Children and their Development study
- ANS
autonomic nervous system
- FemNAT‐CD
Neurobiology and Treatment of Adolescent Female Conduct Disorder Study
- HP
heart period
- HR
heart rate
- HRV
heart rate variability
- ICG
impedance cardiography
- MINDS
Mother‐Infant Neurodevelopment study
- NTR
Netherlands Twin Register
- PEP
pre‐ejection period
- PNS
parasympathetic nervous system
- RMSSD
root mean square of successive differences between normal sinus beats
- RSA
respiratory sinus arrhythmia
- SDNN
inter‐beat interval of normal sinus beats
- SNS
sympathetic nervous system
Clinical Perspective
What Is New?
To our knowledge, this is the first and largest multi‐cohort study simultaneously comparing cardiac sympathetic and parasympathetic activity in 4820 healthy subjects from childhood to late adolescence.
Impedance cardiography and ECG analysis showed a linear increase in sympathetic activity and a non‐linear pattern for parasympathetic activity with an exponential increase from infancy, a plateau phase during middle childhood, followed by a decrease to adolescence.
Parasympathetic nervous system maturation in boys peaked later than that in girls.
What Are the Clinical Implications?
Our study provides the longitudinal maturation of sympathetic and parasympathetic activity from childhood to adolescence in healthy children.
These maturational trajectories may be used as normative for different subjects in whom normal development may be precluded, eg, due to various disease states as cardiopulmonary, metabolic, or musculoskeletal diseases.
The clinical use of the age‐specific centile and percentile values for cardiac autonomic nervous system indices at a single point of the maturational trajectory is modest in view of the large individual differences even within narrow age ranges.
The autonomic nervous system (ANS) coordinates bodily functions to ensure homeostasis in response to the external and the internal environment. 1 This system can be subdivided into 2 branches; the parasympathetic nervous system (PNS), which prevails during periods of rest, and the sympathetic nervous system (SNS), which prevails when the body is active. 1 The effect of ANS activity on the heart rate (HR) can be measured using respiratory sinus arrhythmia (RSA) mainly reflecting PNS activity and the pre‐ejection period (PEP) reflecting SNS activity. RSA refers to heart rate variability (HRV) that is closely coupled to inspiratory and expiratory phases of the respiratory cycle and reflects cardiac vagal effects on the sinoatrial node. 2 PEP is the time interval between ventricular electrical depolarization and the beginning of ventricular ejection. 3 , 4 It has been shown to reflect β‐adrenergic inotropic drive to the left ventricle through a variety of manipulations of cardiac SNS activity by pharmacological (ant)agonists, exercise, and mental stress. 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22
Because of its immediate relevance for cardiovascular disease and mortality in adults the interest in cardiac ANS activity has grown exponentially. 23 The field further expanded with increasing evidence for cardiac ANS activity as a biomarker for healthy development of mental and physical functioning in children. 24 , 25 , 26 Although many studies addressed the maturation of the cardiac ANS from birth to adolescence using RSA or PEP, 24 , 39 most used short follow‐up times in a single developmental period 27 , 39 and relatively small samples of <500 children, 24 , 39 often selected to be at high risk for or diagnosed with psychopathology. 27 , 31 , 32 , 33 , 36 , 38 , 39 Moreover, studies simultaneously comparing PNS‐ and SNS‐activity, with PEP and RSA levels, in healthy children across the childhood and adolescent age ranges are rare. 27 , 31 , 32 This is unfortunate, as knowledge of the normal development of the cardiac ANS in childhood is essential if we want to use ANS activity to discriminate between health and disease.
Results of various studies, using different HRV parameters, have suggested substantial maturational changes in cardiac PNS activity. 40 , 41 , 42 , 43 Most of these studies investigated the SD of the inter‐beat interval of normal sinus beats (SDNN) or the root mean square of successive differences between normal sinus beats (RMSSD), both HRV parameters reflecting PNS activity in short‐term recordings. 44 A clear and steep increase in HRV in the breathing frequency range is seen in the first months of life, 33 congruent with an increase in the amount of myelinated fibers of the vagus nerve. 45 HRV then further increases rapidly in early childhood and levels off in late childhood, reaching its peak in adolescence. 28 , 40 , 42 , 43 , 46 Effects of sex on the maturation of HRV in children have not been consistent across studies. Higher HRV values in boys have been found in some studies, 29 , 41 , 47 , 48 , 49 while others have reported minimal or no differences. 24 , 28 , 32 , 34 , 39 , 43 , 46 , 50 , 51 In contrast to PNS maturation, the developmental trajectory across childhood and adolescence has been less frequently studied. The few studies so far showed that PEP increases from infancy to late adolescence with an inconsistent sex difference across the those studies. 27 , 30 , 32 , 35
An increase or decrease in individual differences of ANS activity can arise from age‐specific hormonal 52 and brain connectivity changes 53 relevant to ANS functioning. In addition, differential exposure across childhood to ANS affecting factors like psychosocial stress and lifestyle behaviors may act to increase the variance in ANS activity between individuals. 54 , 55 Many of the previous studies have implicitly assumed homogeneity of variance across childhood, when comparing means between age groups, whereas this assumption has rarely been tested explicitly.
The main aim of the present study was to investigate changes in PEP, RSA, RMSSD, and SDNN from ages 6 months to 20 years to evaluate normal maturation of cardiac PNS and SNS activity. A second aim was to determine if there were sex differences in maturation of cardiac ANS activity. The results of this study can provide normative values for the maturation of cardiac PNS and SNS activity in childhood, which would have substantial clinical use.
Methods
Study Populations
In this study we combined data from participants of 5 different cohorts and different ages. All study cohorts had received ethical approval. In all studies written informed consent was obtained from all participants and/or parent(s)/guardian(s) where appropriate. The data that support the findings of this study are available from the corresponding author upon reasonable request. Methods of cardiac ANS measurement were identical in all 5 cohorts with all studies using the same ambulatory impedance cardiography (ICG) and electrocardiography recording device for data collection. Data collection of every cohort is described separately below.
NTR (Netherlands Twin Register) is a large cohort consisting of multiples and their families from the Netherlands, enrolled in ongoing longitudinal survey studies. 56 From this cohort, twin pairs aged between 16 to 18 years as well as their siblings aged between 12 to 25 years were invited to participate in a study investigating determinants of adolescent exercise behavior. 4 A total of 549 healthy participants completed combined ICG and electrocardiography during a 4‐minute sitting baseline measurement. A random selection of one member per family yielded 285 non‐related participants for a sensitivity analysis testing the potential effects of clustering within family.
The FemNAT‐CD (Neurobiology and Treatment of Adolescent Female Conduct Disorder) study is a European project aimed at understanding the neurobiological and sex differences of conduct disorder. 57 The study therefore investigates autonomic nervous system activity in boys and girls with conduct disorder and in normally developing boys and girls. Participants were recruited in England, Germany, Switzerland, Spain, Greece, Hungary, and the Netherlands. A control group of 753 normally developing boys and girls between the ages of 9 to 18 years completed ICG and electrocardiography recording during a 5‐minute baseline assessment in a sitting position.
MINDS (Mother‐Infant Neurodevelopment Study) is a Dutch longitudinal study that is aimed at investigating factors of influence on emotional and behavioral problems in children. 58 Primiparas women between ages 17 to 25 years with uncomplicated pregnancies were recruited to participate. To obtain enough variance in children’s early behavioral problems, the study oversampled women from a high‐risk background (ie, due to the presence of ≥1 risk factors, eg, maternal psychopathology, substance use, and social adversity). A total of 86 healthy infants from low‐risk and 54 healthy infants from high‐risk mothers completed ICG and electrocardiography baseline measurements at the age of 6 months during a 2‐minute relaxing movie while lying on a blanket. Given that published data from the MINDS‐study showed that there were no differences in baseline measurements of PEP and RSA between the low‐ and high‐risk groups, 58 we found it justified to include the data from all 140 children.
Nederend et al. performed a study to improve stroke volume assessment from a spot‐electrode based impedance cardiogram in a pediatric population of both healthy children and children with a corrected congenital heart disease between ages 1 to18 years. 3 Here we use the former group of 117 healthy controls only. They completed the combined ICG and electrocardiography recording during a 4‐minute sitting baseline measurement.
The ABCD (Amsterdam Born Children and their Development) study is a Dutch prospective birth cohort study examining the association between prenatal and early‐life influences on later health. 59 The study population included 8266 pregnant women. At follow‐up, an electrocardiography and ICG during a 7‐minute sitting baseline measurement was completed in 3083 children at ages 5 to 7 years (ABCD 1 cohort) and 962 children at 11 to 12 years (ABCD 2 cohort). From these, 784 children completed the measurement at both follow‐up points, allowing us to compute temporal stability of the PEP and RSA across a 5‐year period. To avoid double use of the same children and introduce within‐subject correlation, only the second measurements for these 784 children were used for determination of the maturational effects across all age groups. Omission of their data had no effect on the ANS outcomes in the remaining ABCD 1 cohort. ANOVA for the HR, PEP, and RSA between the initial group of 3083 children at the age of 5 to 7 years versus the remaining 2299 children showed no significant differences (HR, mean 91.8 versus 91.8 bpm; P=0.909; PEP mean 73.4 versus 73.3 ms, P=0.639; RSA mean 108.2 versus 108.1 ms; P=0.916).
Age Grouping
All subjects from the previously mentioned cohorts were divided in 12 age groups, aged 6 months, 1 to 4, 5, 6, 7 to 10, 11, 12, 13 to 15, 16, 17, 18, and ≥19 years. This age grouping provided an optimal balance of having a sufficient sample size in each age group, while maintaining a good representation of the maturational trajectory across the entire 0.5‐ to 20‐year age span.
Data Collection
For all studies the following data were collected per individual subject: date of measurement , age, sex, and the mean HR, heart period (HP), PEP, RSA, RMSSD, and SDNN across the entire baseline measurement period of the study.
ICG and electrocardiography recording were conducted using the 5fs version of the VU Ambulatory Monitoring System (VU University, Amsterdam, The Netherlands, www.vu‐ams.nl). One lead electrocardiography was derived from 3 pre‐gelled Ag/AgCl (Kendal H124SG) spot electrodes on the chest. Thoracic impedance (Z) was conducted by introducing a small alternating current (50 kHz, 350 mA) through the thorax, also by the use of spot electrodes. 3 The VU Ambulatory Monitoring System records electrocardiography and ICG using a sample rate of respectively 1000 and 250 Hz. The whole electrocardiography/ICG recording of each separate study, ranging from 2 to 7 minutes, was used for analysis. All cohorts used the Vrije Universiteit Data Analysis and Management Software (also designed at the VU University) offline for data analysis and the analysts were trained by the same team. Ectopic beats and artefacts were removed in the Vrije Universiteit Data Analysis and Management Software program after an automated scoring of R‐peaks and artefact detection by the software and a final visual check of the electrocardiography.
PEP, a measure of SNS activity, 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 is defined as the time interval between the ventricular electrical depolarization (ie, Q‐wave onset in the electrocardiography signal) and the start of left ventricular outflow (ie, B‐point in the ICG signal). The Q‐point were checked manually in the Vrije Universiteit Data Analysis and Management Software. The ICG was ensemble averaged and automatically suggested candidate B‐points were visually inspected and corrected when needed. This procedure yields a mean intraclass correlation coefficient (ICC) of .75 for PEP across 7 different raters 60 and ICC of .57 with PEP derived from transthoracic echocardiography. 3 Increases in SNS activity are reflected in a shorter PEP. 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22
Furthermore, RSA, a measure of PNS activity, 2 was calculated using the peak valley method by combining the respiration signal and the inter‐beat interval time series. RSA is measured by subtracting the shortest inter‐beat interval during inhalation from the longest inter‐beat interval during exhalation. If no shortest or longest inter‐beat interval could be detected, RSA was set to zero for that particular breath. Increases in PNS activity are reflected in a higher RSA value. PNS activity was additionally measured by calculating RMSSD and SDNN. 44 SDNN refers to the SD of the inter‐beat interval of normal sinus beats and RMSSD to the root mean square of successive differences between normal sinus beats.
For all variables the mean of the whole recording of each included study was used for analysis.
ANS Adjustment
HRV variables are highly correlated to HR (or its inverse, the HP/inter‐beat interval). 2 , 61 Various previous studies on HRV maturation have resorted to some form of adjustment of HRV variables for HR, 43 although the value of such “correction” has been questioned. 2 Here, we have taken an empirical approach to this debate and present the results for the HRV measures with and without adjustment for HP. HR adjusted values were calculated by dividing the mean of RSA, RMSSD, SDNN, and PEP with the mean of HP of the whole recording of each study.
Statistical Analysis
SPSS (IBM, version 25) and R statistics software (R Core Team, 2019 62 ) were used for statistical analysis. Within each cohort separately (NTR, FemNAT‐CD, MINDS, Nederend, ABCD 1 and 2) outliers were identified by standardized Z‐scores >3.8 and removed. Distributional and QQ‐plots and assessment of skewness were used to detect deviations from normality and suggested that a natural log‐transformation of RSA, RMSSD, SDNN as well as their HP adjusted values were needed for parametric testing. However, to provide interpretable normative values we computed the sex‐ and age‐stratified values of the median and 2.5th and 97.5th percentiles of the untransformed variables. To test for sex differences in these values (or their appropriate transforms) at each age, we compared boys and girls across all 12 age groups with an age by sex ANOVA. A significant age by sex interaction was followed up by an ANOVA of the sex difference per age group.
We next tested a variety of trends to the developmental trajectory over age (linear, quadratic, cubic, power, exponential, compound, and logistic) and used the R‐squared value as a measure of model fit. Linear, quadratic, or cubic trends came out as the best fitting curves for all variables, untransformed, transformed, and HP‐adjusted. The ggplot 2 package (Wickham, 2016 63 ) was used to create scatterplots of all variables per sex (boys, girls) and the lm function with sex and, dependent on the best fitting model, age, age2, and age3 predictors were used to create centile as well as 2.5th and 97.5th percentile curves.
To visualize the age‐specific variance of the different cardiac ANS parameters, histograms of the SD per age group were produced, separately for boys and girls. To obtain an idea of the sampling distribution of the age‐specific SDs we performed a 1000‐fold resampling of 100% of the sample per age group and added the 95% CI to the histograms. For the HRV parameters, this was done for the SDs of the untransformed and the transformed parameters. Using age groups as the independent variable, Levene test provided a formal test of homogeneity of variance in these SDs across age. In the subset of children from the ABCD study who were measured twice across a 5.5‐year period, temporal stability of all variables, untransformed, natural log‐transformed, and HP‐adjusted were computed, using both Pearson correlation and ICC.
Results
Because of technical failures, artefacts, or excessive ectopic beats 266 (Nederend, 9; FemNAT‐CD, 25; ABCD first wave, 161; ABCD second wave, 64; MINDS, 2; NTR, 5) children were excluded for analysis. Therefore, a total of 4820 children from 5 different cohorts were included in the study (Table 1). Overall, 47% of the subjects were boys, varying from 33.6% to 53.6% in the individual cohorts. Age distribution of multiple cohorts overlapped. All recordings were performed between October 2008 and February 2018. Table 2 shows the number of subjects per age group and sex for the total study population. Most age groups contained >100 subjects, except for age groups 1 to 4 years (25 subjects) and ≥19 years (73 subjects). In young children from the MINDS‐cohort, the thorax impedance signal showed substantial clipping and was deemed not sufficient enough to quantify PEP and RSA in a way that would be comparable with the older children of the remaining cohorts. Therefore, PEP and RSA are missing for age group of 6 months.
Table 1.
Characteristics Per Study Cohort
| NTR | FemNAT‐CD | MINDS | Nederend |
ABCD Wave 1 |
ABCD Wave 2 |
|
|---|---|---|---|---|---|---|
| Total participants (n) | 549 | 753 | 140 | 117 | 2299 | 962 |
| Boys (%) | 261 (47.5%) | 253 (33.6%) | 75 (53.6%) | 61 (52.1%) | 1149 (50%) | 485 (50.4%) |
| Age (y) (±SD) |
17.36 (±1.26) |
14.76 (±2.48) |
0.54 (±0.04) |
10.16 (±4.92) |
5.74 (±0.50) |
11.83 (±0.38) |
| Period of testing |
02‐2012 ‒ 08‐2013 |
03‐2014 ‒ 02‐2018 |
02‐2012 – 02‐2015 |
02‐2014 ‒ 04‐2016 |
10‐2008 – 12‐2010 |
10‐2015 ‒ 03‐2016 |
Table 2.
Age and Sex Distribution of the Total Study Population
| Age Group | Boys (n) | Girls (n) | Total (n) |
|---|---|---|---|
| 6, mo | 75 | 65 | 140 |
| 1–4, y | 12 | 13 | 25 |
| 5, y | 804 | 792 | 1596 |
| 6, y | 336 | 337 | 673 |
| 7–10, y | 54 | 86 | 140 |
| 11, y | 347 | 333 | 680 |
| 12, y | 194 | 222 | 416 |
| 13–15, y | 116 | 212 | 328 |
| 16, y | 157 | 187 | 344 |
| 17, y | 107 | 186 | 293 |
| 18, y | 55 | 57 | 112 |
| ≥19, y | 27 | 46 | 73 |
| Total | 2285 | 2537 | 4822 |
Developmental Trajectories in Mean PEP and HRV
The cubic model had overall the highest R‐square and best fit according to Akaike Information Criterium for resting HR (R2, 0.55–0.58), RSA (R2, 0.07–0.11), RMSSD (R2, 0.07–0.11) and SDNN (R2, 0.10–0.13). Whereas the cubic model for PEP had a slightly lower Akaike Information Criterium, we chose the simple linear model as the difference in R2 was small (R2, 0.45–0.54 for cubic and 0.42–0.53 for linear). Figures 1 and 2 depict scatterplots of resting HR, PEP, RSA, RMSSD, and SDNN with fitted centile curves, separately for boys and girls. We display the raw data for ease of comparison to previous studies whereas statistical testing was always performed on the transformed data that better matched the assumptions of a normal distribution. The logarithmic transformation of the HRV variables did not change the pattern of the trajectories (Figure S1). As illustrated by Figure 1, resting HR declined almost linearly from infancy to middle childhood and reached a plateau towards adolescence. However, PEP increased gradually from infancy to adolescence. The maturation of the HRV parameters showed comparable curves with a clear increase from infancy to middle/late childhood followed by a plateau phase during middle/late childhood and a small decrease to late adolescence (see Figure 2). Moreover, in girls the plateau phase of the HRV parameters was reached earlier compared with boys.
FIGURE 1. Scatterplots of age‐related heart rate and pre‐ejection period for boys and girls with cubic and linear smoothing of median.
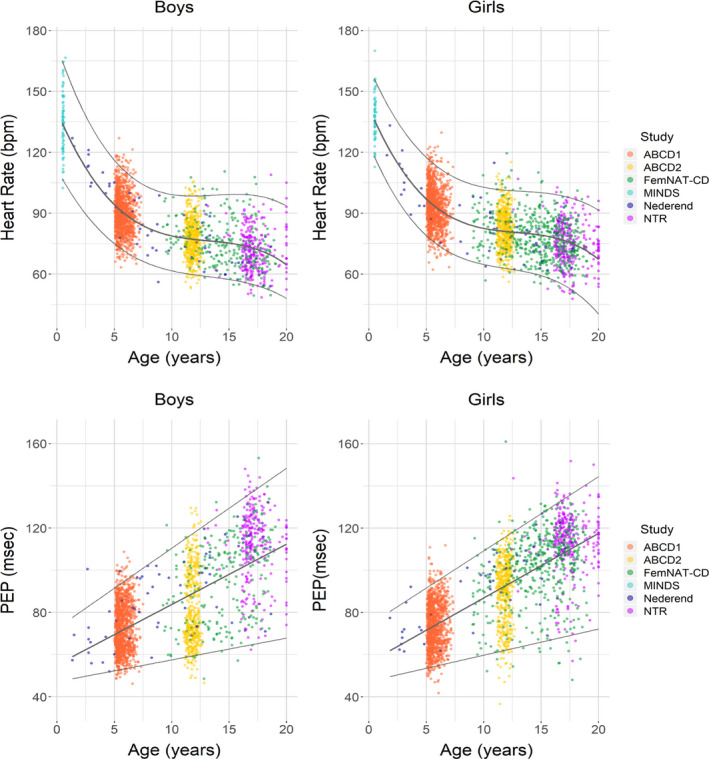
ABCD indicates the Amsterdam Born Children and their Development study; FemNAT‐CD, the Neurobiology and Treatment of Adolescent Female Conduct Disorder study; MINDS, the Mother‐Infant Neurodevelopment Study; and NTR, Netherlands Twin Register; and PEP, pre‐ejection period.
FIGURE 2. Scatterplots of age‐related heart rate variability with cubic smoothing of median.
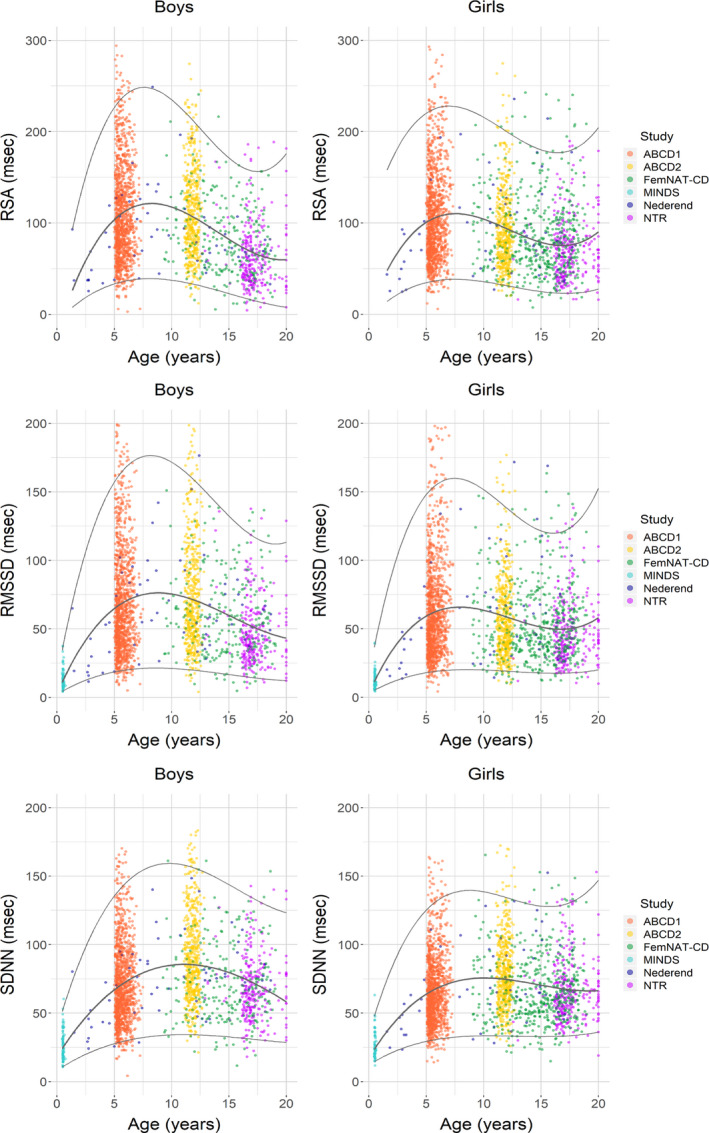
ABCD indicates the Amsterdam Born Children and their Development study; FemNAT‐CD, the Neurobiology and Treatment of Adolescent Female Conduct Disorder study; MINDS, the Mother‐Infant Neurodevelopment Study; NTR, Netherlands Twin Register; RSA, respiratory sinus arrhythmia; RMSSD, root mean square of successive differences between normal sinus beats; and SDNN, SD of the inter‐beat interval of normal sinus beats.
The median values and the 2.5th and 97.5th percentile per age group and sex for all variables are shown in Tables 3 and 4. We compared the means of boys and girls with an ANOVA using age and sex as factors. A significant interaction between sex and age was found for HR (F[11,4787]=2.37, P=0.006), PEP (F[10,4539]=6.31, P<0.001), RSA (F[10,4605]=5.42, P<0.001, RMSSD (F[11,4747]=4.31, P<0.001) and SDNN (F[11,4777]=3.76, P<0.001). When boys and girls were compared separately for each of the age groups, less than half of the age groups for HR and a quarter of the age groups of PEP, RSA, RMSSD, and SDNN showed a significant difference. Girls had overall higher values of HR at all ages while PEP was only higher in girls at ages 11, 12, and 13 to 15 years (see Table 3) and RSA, RMSSD, and SDNN lower in girls at ages 5, 11, and 12 years (see Table 4).
Table 3.
Age‐Related Heart Rate (bpm) and Pre‐Ejection Period (ms) Per Sex and Age Group
| Age, y | HR (bpm) | PEP (ms) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boys | Girls | Boys | Girls | |||||||||
| Median | Percentile | Median | Percentile | Median | Percentile | Median | Percentile | |||||
| 2.5th | 97.5th | 2.5th | 97.5th | 2.5th | 97.5th | 2.5th | 97.5th | |||||
| 0.5 | 134.9 | 109.6 | 164.3 | 137.9 | 118.0 | 155.8 | NA | NA | NA | NA | NA | NA |
| 1–4 | 111.4 | 100.8 | 125.8 | 105.1 | 90.6 | 129.5 | 66.6 | 53.1 | 95.0 | 71.2 | 61.8 | 90.1 |
| 5 | 90.8 | 72.6 | 111.4 | 94.4* | 77.0 | 113.9 | 70.3 | 53.7 | 93.7 | 73.1 | 54.3 | 94.1 |
| 6 | 88.2 | 71.2 | 110.0 | 90.4 | 72.4 | 109.6 | 74.2 | 54.6 | 96.2 | 75.3 | 54.8 | 95.6 |
| 7–10 | 82.8 | 69.5 | 99.0 | 86.4 | 64.1 | 108.3 | 83.9 | 54.8 | 108.8 | 86.2 | 60.1 | 109.8 |
| 11 | 77.8 | 60.0 | 99.3 | 83.0* | 65.0 | 102.6 | 75.8 | 56.1 | 115.0 | 92.0* | 58.1 | 112.6 |
| 12 | 77.5 | 59.6 | 99.0 | 81.5* | 61.9 | 104.3 | 80.1 | 57.6 | 121.1 | 96.3* | 61.7 | 118.5 |
| 13‐15 | 74.2 | 57.4 | 97.5 | 76.7 | 59.0 | 98.8 | 102.4 | 65.6 | 125.4 | 105.4* | 66.9 | 128.5 |
| 16 | 71.0 | 55.8 | 92.2 | 76.8* | 59.4 | 95.5 | 112.1 | 71.9 | 138.4 | 112.5 | 74.4 | 134.0 |
| 17 | 69.5 | 54.0 | 91.6 | 73.3 | 51.2 | 94.7 | 115.8 | 72.2 | 138.2 | 112.8 | 70.4 | 133.0 |
| 18 | 68.6 | 53.3 | 95.9 | 75.8* | 59.1 | 97.4 | 110.3 | 71.4 | 136.0 | 111.1 | 68.8 | 130.6 |
| ≥19 | 68.9 | 55.8 | 100.1 | 72.5 | 55.5 | 95.5 | 111.8 | 77.2 | 126.3 | 115.0 | 88.7 | 135.9 |
that per variable tests were conducted in 12 age groups and that Bonferroni correction of a nominal alpha of 0.05 would yield a p‐value threshold of 0.004. HR indicates heart rate; NA, Not applicable; and PEP, pre‐ejection period.
P<0.001 for difference between boys and girls.
Table 4.
Age‐Related RSA (ms), RMSSD (ms), and SDNN (ms) Per Sex and Age
| Age, y | RSA (ms) | RMSSD (ms) | SDNN (ms) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boys | Girls | Boys | Girls | Boys | Girls | |||||||||||||
| Median | Percentile | Median | Percentile | Median | Percentile | Median | Percentile | Median | Percentile | Median | Percentile | |||||||
| 2.5th | 97.5th | 2.5th | 97.5th | 2.5th | 97.5th | 2.5th | 97.5th | 2.5th | 97.5th | 2.5th | 97.5th | |||||||
| 0.5 | NA | NA | NA | NA | NA | NA | 11.6 | 4.9 | 34.1 | 11.1 | 5.2 | 31.8 | 24.5 | 11.5 | 49.8 | 23.6 | 15.0 | 47.9 |
| 1–4 | 43.0 | 27.8 | 112.0 | 57.5 | 24.8 | 99.0 | 26.4 | 12.7 | 62.1 | 34.8 | 14.3 | 74.0 | 43.0 | 25.5 | 78.1 | 46.7 | 23.8 | 71.0 |
| 5 | 99.1 | 36.0 | 235.5 | 92.7* | 38.0 | 223.3 | 58.2 | 19.9 | 172.4 | 51.6* | 19.1 | 156.4 | 64.5 | 29.4 | 144.4 | 60.6* | 30.1 | 130.7 |
| 6 | 102.7 | 36.3 | 213.2 | 102.6 | 37.2 | 227.6 | 63.2 | 19.4 | 156.5 | 59.1 | 19.3 | 157.9 | 67.4 | 28.9 | 135.8 | 66.2 | 31.4 | 136.0 |
| 7–10 | 92.8 | 40.9 | 206.7 | 102.0 | 38.3 | 190.4 | 61.6 | 24.3 | 137.6 | 63.0 | 20.4 | 145.1 | 67.9 | 29.9 | 133.3 | 69.4 | 31.5 | 131.4 |
| 11 | 103.3 | 39.2 | 224.7 | 87.3* | 35.3 | 198.4 | 64.4 | 21.5 | 176.5 | 54.0* | 20.5 | 137.1 | 85.3 | 40.6 | 162.2 | 74.9* | 38.4 | 131.4 |
| 12 | 105.2 | 35.1 | 210.4 | 84.0* | 25.6 | 206.5 | 65.8 | 22.4 | 151.2 | 48.3* | 14.2 | 130.0 | 84.2 | 36.5 | 154.8 | 72.6* | 30.6 | 137.1 |
| 13–15 | 74.9 | 18.7 | 166.8 | 74.2 | 21.4 | 186.7 | 56.7 | 13.6 | 131.3 | 45.6 | 18.4 | 130.0 | 69.8 | 26.4 | 126.8 | 61.1 | 27.8 | 130.3 |
| 16 | 55.3 | 15.5 | 151.7 | 68.3 | 27.1 | 150.4 | 42.1 | 14.6 | 112.9 | 43.0 | 17.6 | 109.3 | 64.7 | 29.3 | 125.0 | 60.4 | 32.6 | 122.3 |
| 17 | 58.5 | 21.2 | 147.0 | 70.7 | 29.8 | 192.0 | 42.5 | 17.8 | 112.8 | 47.6 | 20.2 | 128.1 | 65.2 | 33.2 | 117.8 | 65.9 | 38.4 | 132.0 |
| 18 | 66.0 | 17.9 | 143.3 | 75.2 | 20.4 | 177.8 | 52.0 | 15.3 | 114.2 | 46.4 | 14.2 | 116.1 | 67.0 | 34.8 | 138.5 | 60.8 | 36.8 | 120.1 |
| ≥19 | 51.3 | 17.4 | 166.1 | 70.5 | 24.1 | 139.4 | 38.4 | 13.5 | 112.6 | 45.6 | 15.3 | 97.9 | 68.5 | 31.4 | 135.4 | 65.3 | 33.9 | 120.1 |
Note that tests for respiratory sinus arrhythmia, root mean square of successive differences, and SD of inter‐beat intervals were conducted in 12 age groups and as all these variables are highly correlated, their results can be regarded as a within‐study replication. Therefore, the Bonferroni correction of a nominal alpha of 0.05 would yield a P value threshold of 0.001.
NA indicated not applicable; RMSSD, root mean square of successive differences; RSA, respiratory sinus arrhythmia (set at zero); and SDNN, SD of the inter‐beat intervals of normal sinus beats.
P<0.001 for difference between Boys and Girls after LN transformation.
Repeating the analyses using only unrelated children form the NTR study did not change this pattern of results.
Adjustment for Heart Rate
The HR adjusted ANS parameters, stratified by sex, are shown as scatterplots in Figures S2 and S3. Similar to the unadjusted variables, the cubic model had the highest R square and best fit for the natural log of RSA/HP, RMSSD/HP, and SDNN/HP and the linear model was again chosen for PEP/HP. The trajectories of RSA/HP, RMSSD/HP, and SDNN/HP corresponded with the trajectories of the unadjusted RSA, RMSSD, and SDNN (Figure 2), with an increase from infancy to middle/late childhood flowed by a plateau phase with a small decrease during adolescence. Although the trajectory of PEP/HP also corresponded with the trajectory of the uncorrected PEP, the gradual increase of PEP/HP was less steep compared with the unadjusted PEP.
Developmental Changes in the Variance of SNS and PNS Parameters
The variances of the ANS parameters are summarized as SD with 95% CIs per age group and sex in Figure 3. The variance in resting HR was stable across the entire age range, and Levene test confirmed homogeneity of variance in girls (F[11,2520]=1.21, P=0.272). For boys, the variances for resting HR were not comparable (F[11,2267]=3360, P<0.001), but this was entirely attributable to age group, 6 months. Removing this measurement rendered the Levene test non‐significant (F[10,2193]=1.15, P=0.324). In contrast to HR, the variances for PEP, RSA, RMSSD, and SDNN were not comparable across all age groups (P values of Levene tests, all <0.001). The variance of PEP gradually increased until age 12 and plateaued towards adolescence. RSA, RMSSSD, and SDNN showed a steep increase from 0.5 to 5 years, and then plateaued followed by a slight decrease around age 11, stabilizing at the end of adolescence. Homogeneity of variance was largely restored by correcting for skewness in the HRV values by logarithmic transformation (Figure S4) with only RMSSD and SDNN in girls not being equal across age groups (RMSSD F[11,2502]=1.88, P=0.038 and SDNN F[11,2517]=2.38, P=0.006).
FIGURE 3. Histograms of SDs per age group for heart rate, pre‐ejection period, respiratory sinus arrhythmia, root mean square of successive differences between normal sinus beats and SD of the inter‐beat interval of normal sinus beats.
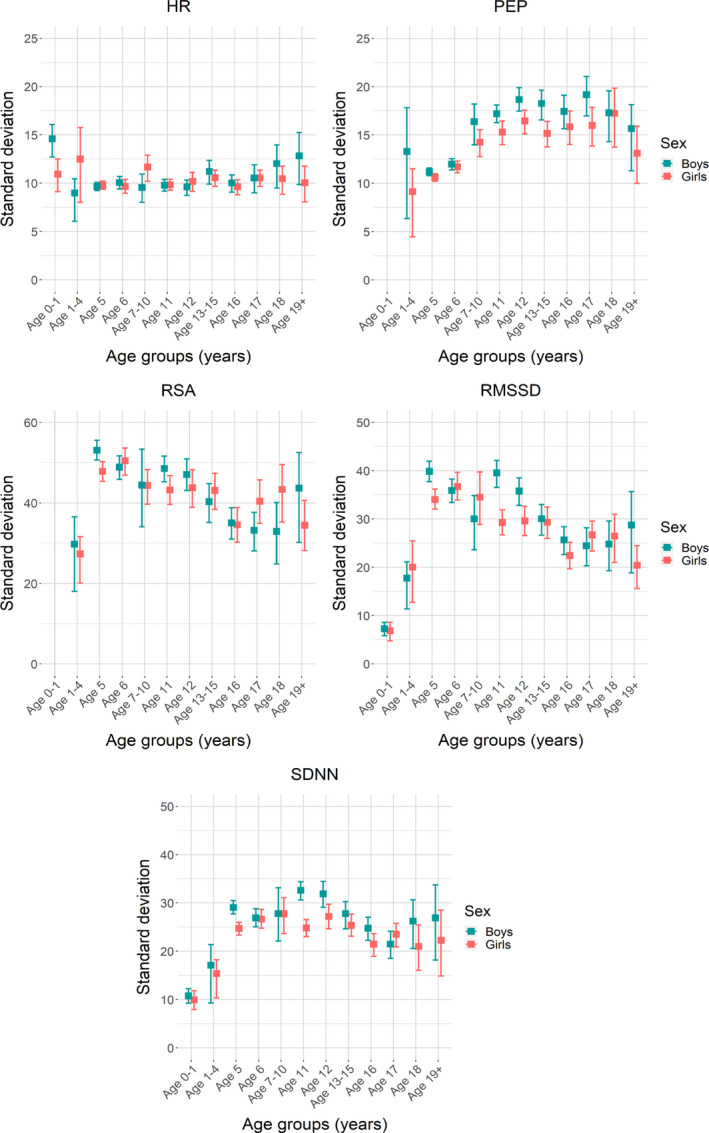
HR indicates heart rate; PEP, pre‐ejection period; RMSSD, root mean square of successive differences; RSA, respiratory sinus arrhythmia (set at zero); and SDNN, SD of the inter‐beat intervals of normal sinus beats.
Temporal Stability from Age 6 to Age 11.5 Years
In the subset of 784 children from the ABCD study measured twice across a 5.5‐year period, temporal stability computed as the Pearson correlation coefficient for boys and girls is shown in Table 5. Moderate temporal stability was found for PEP and good stability for HR, RSA, RMSSD, and SDNN (Pearson, 0.26<r<0.58; ICC, 0.25<r<0.57). Log transformation of HRV variables barely changed these correlations (Pearson and ICC, 0.42<r<0.53) despite significant longitudinal changes in mean values with age, all of which were compatible with the cross‐sectional comparisons of the relevant age groups. Furthermore, adjustment for HP, which adds temporal instability in the HP itself, barely influenced the stability of the ANS parameters (Pearson, 0.28<r< 0.53; ICC, 0.27<r<0.54).
Table 5.
Correlation of All Variables, Untransformed, Natural Log Transformed, and Heart Period Adjusted Between 5 to 7 Years and 11 to 12 Years in Children Measured Twice
| Boys (n=398) | Girls (n=386) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Mean 5–7, y |
±SD |
Mean 11–12, y |
±SD | Pearson Correlation | Intraclass Correlation |
Mean 5–7, y |
±SD |
Mean 11–12, y |
±SD | Pearson Correlation | Intraclass Correlation | |
| HR, bpm | 90.0 | 9.9 | 77.7 † | 9.7 | 0.46 † | 0.47 † | 93.7 | 9.8 | 82.3 † | 9.8 | 0.46 † | 0.46 † |
| PEP, ms | 73.3 | 12.4 | 81.3 † | 17.5 | 0.33 † | 0.31 † | 74.3 | 11.3 | 89.2 † | 15.4 | 0.26 † | 0.25 † |
| RSA, ms | 110.5 | 48.7 | 113.4 | 48.7 | 0.49 † | 0.50 † | 105.0 | 48.3 | 95.1 † | 44.5 | 0.47 † | 0.47 † |
| RMSSD, ms | 69.1 | 36.2 | 75.2 † | 38.4 | 0.53 † | 0.55 † | 60.7 | 32.3 | 58.2 | 29.1 | 0.46 † | 0.47 † |
| SDNN, ms | 71.0 | 27.0 | 92.0 † | 33.0 | 0.58 † | 0.57 † | 66.1 | 25.7 | 80.0 † | 26.2 | 0.49 † | 0.49 † |
| LN RSA | 4.61 | 0.46 | 4.64 | 0.46 | 0.49 † | 0.50 † | 4.56 | 0.47 | 4.46 † | 0.46 | 0.43 † | 0.43 † |
| LN RMSSD | 4.12 | 0.54 | 4.20* | 0.53 | 0.53 † | 0.53 † | 3.99 | 0.52 | 3.97 | 0.49 | 0.42 † | 0.42 † |
| LN SDNN | 4.21 | 0.38 | 4.47 † | 0.37 | 0.53 † | 0.53 † | 4.14 | 0.38 | 4.34 † | 0.32 | 0.45 † | 0.45 † |
| PEP/HP | 10.9 | 2.1 | 10.4 † | 2.6 | 0.38 † | 0.37 † | 11.5 | 2.0 | 12.1 † | 2.6 | 0.28 † | 0.27 † |
| LN RSA/HP | 2.76 | 0.37 | 2.65 † | 0.37 | 0.48 † | 0.49 † | 2.75 | 0.37 | 2.54 † | 0.37 | 0.44 † | 0.44 † |
| LN RMSSD/HP | 2.30 | 0.41 | 2.25* | 0.39 | 0.53 † | 0.54 † | 2.22 | 0.40 | 2.09 † | 0.36 | 0.43 † | 0.43 † |
| LN SDNN/HP | 2.38 | 0.28 | 0.28 † | 0.27 | 0.50 † | 0.51 † | 2.35 | 0.28 | 2.42 † | 0.24 | 0.43 † | 0.42 † |
HP indicates heart period; HR, heart rate; LN, natural log transformation; PEP, pre‐ejection period; RMSSD, root mean square of successive differences; RR, respiration rate; RSA, respiratory sinus arrhythmia (set at zero); and SDNN, SD of inter‐beat intervals.
P value <0.05 for difference between the mean at 11 to 12 years vs 5 to 7 years for both boys and girls or Pearson an intraclass correlation.
P value <0.001.
Discussion
To our knowledge, this study is the first and largest multi‐cohort study which simultaneously compared HR, PEP, and RSA to investigate the maturation of resting cardiac ANS activity in healthy children from infancy to late adolescence. Between the ages of 0.5 to 20 years, resting HR decreased linearly from infancy to the beginning of late childhood (aged 10 years) after which it plateaued with only a minimal further decrease towards the end of adolescence (aged 19 years). PEP linearly increased in both boys and girls throughout all age groups which implies a linear decrease of the cardiac effects of SNS activity with age. Cardiac PNS activity showed the most complex pattern, a clear increase from infancy to middle (girls) or late (boys) childhood, a plateau phase during middle and late childhood, followed by a slight decrease throughout adolescence. Furthermore, whereas the variance in HR was largely stable throughout childhood and adolescence, the variance of the SNS and PNS parameters increased from infancy to early childhood and plateaued or slightly decreased towards adolescence. For PNS, the often‐used natural log‐transformation largely removed this heterogeneity of variance.
A main limitation of this study is the cohort approach in which age groups were compared rather than repeated measurements in the same individuals across the entire 0.5‐ to 20‐age span. For this reason, the ‘developmental’ trends in Figures 1 and 2 should be interpreted with caution. Furthermore, because of substantial clipping of the thorax impedance PEP and RSA were missing in the age group of 6 months, an important data point for early development. In addition, the RMSSD and SDNN results of this MINDS‐cohort should be interpreted with caution acknowledging that body position differed from the other cohorts in this study, lying in supine position instead of sitting. It is known that body position influences cardiac ANS parameters. 8 These limitations are balanced by the larger sample size of healthy children available, with the exception of ages 1 to 4 years, compared with previous studies, and the highly comparable and well‐validated methodology used to assess PEP and RSA in all age groups. However, substantial caution is needed as PEP is known to be sensitive to preload and afterload effects, which may change across childhood and adolescence in view of the changes in eg, stature and body composition, the size of the heart, HR, arterial stiffness, and mean arterial pressure. Also, as reviewed below, striking resemblance was found between our multi‐cohort results and those from longitudinal studies that, although using narrower age ranges, together still cover the entire 0.5‐ to 20‐age span.
A clear and steep increase in HRV 33 , 40 , 49 and an increase in the amount of myelinated fibers of the vagus nerve 45 in the first months of life suggest that cardiac PNS activity starts to increase after birth. HRV then continues to increase from the first months to early and middle childhood 28 , 31 , 37 , 39 , 40 , 41 , 42 , 46 and levels off in late childhood/early adolescence (≈age 11) and does not change or decrease during adolescence. 24 , 29 , 34 , 36 , 40 , 42 , 46 , 50 Such a pattern is highly compatible with the course of maturation seen in our own study and also resembles the pattern reported by the multicohort study, 38 where RSA peaked at age 7 to 8 years, even independently of the definition of the respiratory frequency band used to define the spectral RSA measure. However, another study that compared PNS activity across multiple age cohorts 43 found that the increase in SDNN and RMSSD values from a 10‐second electrocardiography strip (uncorrected for HR, their Figure 5 and 6) leveled off around the age of 20 years, which is much later than in our study (≈11 years). Future research must establish whether this reflects differences in measurement strategy or baseline tasks used to obtain resting values.
For the maturation of cardiac SNS activity, the time course and direction of maturation was still largely unknown. Some longitudinal studies have used the ratio of low frequency to high frequency power as a measure of sympathetic over parasympathetic dominance. These studies showed a decrease of the ratio of low frequency to high frequency power during infancy after which it levels off and may not change or even increase slightly to late adolescence. 40 , 50 However, the use of the ratio of low frequency to high frequency power to index SNS activity is not unanimously supported 64 , 65 and the PEP is considered to be a better index in theory, which has been borne out in empirical validation studies. 11 , 66 , 67
Most studies investigating developmental trends in PEP showed an increase of mean PEP from infancy to adolescence. 27 , 30 , 32 , 35 Matthews et al. 35 showed an increase of mean PEP from 8 to 10 years and higher values of PEP in children aged 15 to 17 years compared with the younger children, but no significant increase of mean PEP from 15 to 17 years, indicating that the increase may level off towards late adolescence. Results from our study suggest a clear maturational effect on mean PEP with a linearly increase from early childhood to late adolescence (Figure 1), meaning that SNS activity decreases over age. However, some caution is needed as PEP is known to be sensitive to preload and afterload effects, which may change across childhood and adolescence in view of the changes in blood pressure and HR. Also, an age‐related β‐adrenergic desensitization in the elderly has been observed compared with the young. 68 If this β‐adrenergic desensitization starts already in childhood, the decrease in PEP may partly reflect reduced receptor responsiveness rather than decreases in SNS activity.
All previous studies which reported a significant sex difference in cardiac PNS activity, described a higher PNS‐activity in boys compared with girls, 47 , 48 , 49 although some studies reported the difference in one specific age group only 41 or in a subset of HRV values. 42 Many other studies did not show any sex differences in PNS activity. 24 , 28 , 32 , 34 , 39 , 43 , 46 , 50 , 51 Likewise, there is no consensus in the literature with regards to sex differences in cardiac SNS. A higher mean PEP in boys compared with girls in the ages 8 to 10 and 15 to 17 years was found by some 32 , 35 and a lower mean PEP in boys aged 9 to 11 years by others. 30
Our study offers an explanation for these discrepant findings on sex differences in ANS activity. We showed that the peak in PNS activity is reached earlier in girls compared with boys, indicating a difference in maturation; this is likely because of the difference in hormonal changes and their downstream biological effects. An association between HRV and reproductive life stages has been described before, 69 and girls are known to enter puberty earlier compared with boys. 70 Because of the modulatory effects of sex on the developmental trajectory of the ANS parameters, detection of sex differences may be rather dependent on the exact composition of the age groups studied. In our study, when considering the entire age range, the PEP was lower in boys compared with girls and RSA, RMSSD, and SDNN were higher in boys <13 years. However, not all of these parameters were significantly different across all age groups. Again, the exact age range used to compare boys and girls can determine the outcome of the test for sex differences.
We discovered a significant change in the variance of ANS parameters during childhood in both boys and girls which has not been described earlier. However, homogeneity of variance is an assumption of the ANOVA tests often used to compare age groups. The variance of PEP, RSA, and the 2 HRV parameters increased from 6 months to 7 years, peaked in middle childhood, and gradually decreased towards the end of adolescence. The peak in variation during middle childhood may be attributed to differences in maturational speed that will be most manifest in this age range. When applying the often‐used natural log transformation on RSA, RMSSD, and SDNN, the homogeneity of variance assumption started to hold well across age groups. This is in concordance with previous studies where log‐transformed variables with 95% CI curves showed no clear peak in variance. 24 , 43
A major aim of the present study was to provide normative absolute values for the sex‐specific maturation of cardiac PNS and SNS activity in childhood, which could have substantial clinical use for detecting children at risk for cardiovascular or psychiatric problems. Results dictate substantial modesty about this aim. The overarching message of Figures 1 and 2 is that there are large interindividual differences in children of the same age and sex. The range is somewhat reduced after natural log transformation and/or adjustment for HP but even then, the variance of same‐aged children for both boys and girls remains striking (see Figures S1 and S3). Hence, the normative values or PEP and RSA/HRV, as presented in Tables 3 and 4, will prove useful to detect clearly outlying values that would require further clinical pediatric follow‐up, but their clinical use of detecting more subtle deviations in ANS functioning, as is potentially the case in child psychiatric conditions, is hampered by the large individual differences that remain even after stratifying for age and sex.
Causes for the large differences in cardiac SNS and PNS measures can partly be found in between‐subject differences in a variety of factors including ethnicity, maturational changes in stature and body composition, the size of the heart, sensitivity of the baroreflex and lung stretch reflexes and respiratory behaviors (frequency and tidal volume), adopted lifestyle patterns (smoking, dietary habits, and physical activity), and psychosocial stress exposures. In addition to these between‐subject factors, there is a slew of factors in the experimental design that can impact on absolute values of the ANS measures. Time of day, previous physical activity, posture, exact shaping of resting baseline conditions, analytic strategy to quantify SNS and PNS cardiac activity, and ensuing statistical transformations or adjustments are just some of the many factors that impact on reported RSA and PEP levels. We note that this concern mostly pertains to absolute values. It does not disqualify these ANS markers as potential biomarkers of developmental processes when comparing relative ranking of children tested within a fixed experimental design. This is further supported by the good temporal stability of the cardiac ANS activity in the 735 children participating in the ABCD study twice across a 5‐year period.
The test‐retest correlation of cardiac ANS activity in the 6‐ to 11‐year age range was good for RSA (r=0.47–0.50) and comparable with previous reports of RSA stability in this age range. 29 , 31 , 32 , 36 Studies performed in infants showed moderate to good stability of RSA in the first years of life, 27 , 28 , 37 but Dollar et al. 24 show moderate long‐term stability in RSA from age 2 to 15 years. In our study, stability was moderate for PEP (r=0.25–0.33) and these estimates were somewhat lower compared with those previously reported for the narrower 5‐ to 8‐year, 31 8‐ to 11‐year, 30 , 32 , 35 and 15‐ to 17‐year periods 35 in which good stability was described. Moreover, a good stability for PEP has even been described in the younger age ranges from 6 months to 5 years. 27 In short, the parameters used here to index cardiac ANS activity can be considered to be a stable individual trait over time, at least with regard to the relative ranking of a child compared with others of the same age and provided that it is measured using the same experimental/analytical approach.
Conclusions
This study provides maturation curves of resting cardiac PNS and SNS activity in healthy children aged 6 months to 20 years. It shows a differential maturation of the PNS and SNS, with PNS activity increasing rapidly directly after birth, leveling off during middle childhood, and decreasing at the end of adolescence. The SNS, in contrast, shows a monotonic decrease across all ages. Trajectories differ between boys and girls, with the latter showing earlier ANS maturation. Despite the large samples used, the clinical use of the sex‐specific centile and percentile values is modest in view of the large individual differences present, even within narrow age bands.
Appendix
FemNAT‐CD consortium
Helena Oldenhof (Department of Child and Adolescent Psychiatry, VU University Medical Center, Amsterdam, Netherlands), Martin Prätzlich (Department of Child and Adolescent Psychiatry, Basel, Switzerland), Katharina Ackermann (Department of Child and Adolescent Psychiatry, Psychosomatics and Psychotherapy, University Hospital Frankfurt, Goethe University, Frankfurt am Main, Germany), Rosalind Baker (School of Psychology, University of Birmingham, Birmingham, England), Molly Batchelor (Department of Psychology, University of Southampton, Southampton, England), Sarah Baumann (Department of Child and Adolescent Psychiatry, Psychosomatics and Psychotherapy, University Hospital of the RWTH Aachen, Germany), Anka Bernhard (Department of Child and Adolescent Psychiatry, Psychosomatics and Psychotherapy, University Hospital Frankfurt, Goethe University, Frankfurt am Main, Germany), Roberta Clanton (School of Psychology, University of Birmingham, Birmingham, England), Dimitris Dikeos (Children and Adolescents Mental Health Unit of Athens University, Athens, Greece), Roberta Dochnal (Szeged University, Faculty of Medicine, Child and Adolescent Psychiatry Department of the Child Health Center, SU, Szeged, Hungary), Lynn Valérie Fehlbaum (Department of Child and Adolescent Psychiatry, Basel, Switzerland), Aranzazu Fernández‐Rivas (Basurto University Hospital, Bilbao, Spain), Eco J.C. de Geus (Department of Biological Psychology, VU University, Amsterdam, The Netherlands), Karen Gonzalez (Department of Psychology, University of Southampton, Southampton, England), Maider González de Artaza‐Lavesa (Basurto University Hospital, Bilbao, Spain), Silvina Guijarro (Child and Adolescent Mental Health Unit, University Hospital Mutua Terrassa, Barcelona, Spain), Malou Gundlach (Department of Child and Adolescent Psychiatry, Psychosomatics and Psychotherapy, University Hospital of the RWTH Aachen, Germany), Beate Herpertz‐Dahlmann (Department of Child and Adolescent Psychiatry, Psychosomatics and Psychotherapy, University Hospital of the RWTH Aachen, Germany), Amaia Hervas (Child and Adolescent Mental Health Unit, University Hospital Mutua Terrassa, Barcelona, Spain), Lucres M.C. Jansen (Department of Child and Adolescent Psychiatry, VU University Medical Center, Amsterdam, Netherlands), Linda Kersten (Department of Child and Adolescent Psychiatry, Basel, Switzerland), Gregor Kohls (Department of Child and Adolescent Psychiatry, Psychosomatics and Psychotherapy, University Hospital of the RWTH Aachen, Germany), Angeliki Konsta (Children and Adolescents Mental Health Unit of Athens University, Athens, Greece), Helen Lazaratou (Children and Adolescents Mental Health Unit of Athens University, Athens, Greece), Iñaki Kerexeta‐Lizeaga (Basurto University Hospital, Bilbao, Spain), Anne Martinelli (Department of Child and Adolescent Psychiatry, Psychosomatics and Psychotherapy, University Hospital Frankfurt, Goethe University, Frankfurt am Main, Germany), Tisse van Nimwegen (Department of Child and Adolescent Psychiatry, VU University Medical Center, Amsterdam, Netherlands), Ignazio Puzzo (Broadmoor High Secure Hospital, West London Mental Health NHS Trust, Crowthorne, England), Nora Maria Raschle (Department of Child and Adolescent Psychiatry, Basel, Switzerland), Jack Rogers (School of Psychology, University of Birmingham, Birmingham, England), Réka Siklósi (Szeged University, Faculty of Medicine, Child and Adolescent Psychiatry Department of the Child Health Center, SU, Szeged, Hungary), Areti Smaragdi (Department of Psychology, University of Southampton, Southampton, England), Martin Steppan (Department of Child and Adolescent Psychiatry, Basel, Switzerland), Stephane De Brito (School of Psychology, University of Birmingham, Birmingham, England), Graeme Fairchild (Department of Psychology, University of Bath, Bath, England), Meinhard Kieser (Institute of Medical Biometry and Informatics, Ruprecht‐Karls University, Heidelberg, Germany), Kerstin Konrad (Department of Child and Adolescent Psychiatry, Psychosomatics and Psychotherapy, University Hospital of the RWTH Aachen, Germany), Christine Freitag (Department of Child and Adolescent Psychiatry, Psychosomatics and Psychotherapy, University Hospital Frankfurt, Goethe University, Frankfurt am Main, Germany), Christina Stadler (Department of Child and Adolescent Psychiatry, Basel, Switzerland), Arne Popma (Department of Child and Adolescent Psychiatry, VU University Medical Center, Amsterdam, Netherlands).
Sources of Funding
The NTR study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases under grant number RO1DK092127 and the Netherlands Organization for Scientific Research under grant number 022.003.010. The study from Nederend et al. was funded by the VU University Ambulatory Monitoring System fund. MINDS‐Leiden was funded by grant number 056‐23‐001 from the National Initiative for Brain and Cognition Research supported and coordinated by the Netherlands Organization for Scientific Research (NWO). The FemNAT‐CD consortium (coordinated by Christine M. Freitag) collaborative project is funded by the European Commission under the 7th Framework Health Program with Grant Agreement no. 602407. The ABCD study was supported by the Netherlands Organization for Health Research and Development (ZonMw) and the Dutch Heart Foundation.
Disclosures
None.
Supporting information
Figures‐S1‐S4
Acknowledgments
The NTR study thanks the members of the twin families registered with The Netherlands Twin Register for continued support of scientific research. The MINDS‐Leiden study thanks all families for their participation and the research assistants who contributed to the data collection. The ABCD study thanks the participating mothers and their children, and all other persons and institutions who contributed to the ABCD study: obstetric care providers, primary schools, students, and youth healthcare centers in Amsterdam, The Netherlands.
(J Am Heart Assoc.2021;10:e017405. DOI: 10.1161/JAHA.120.017405.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.017405
For Sources of Funding and Disclosures, see page 14.
Contributor Information
Lisette M. Harteveld, Email: L.M.Harteveld@LUMC.nl.
FemNAT‐CD collaborators:
Martin Prätzlich, Katharina Ackermann, Rosalind Baker, Molly Batchelor, Sarah Baumann, Anka Bernhard, Roberta Clanton, Dimitris Dikeos, Roberta Dochnal, Lynn Valérie Fehlbaum, Aranzazu Fernández‐Rivas, Karen Gonzalez, Maider González de Artaza‐Lavesa, Silvina Guijarro, Malou Gundlach, Beate Herpertz‐Dahlmann, Amaia Hervas, Linda Kersten, Gregor Kohls, Angeliki Konsta, Helen Lazaratou, Iñaki Kerexeta‐Lizeaga, Anne Martinelli, Tisse van Nimwegen, Ignazio Puzzo, Nora Maria Raschle, Jack Rogers, Réka Siklósi, Areti Smaragdi, Martin Steppan, Stephane De Brito, Graeme Fairchild, Meinhard Kieser, Kerstin Konrad, Christine Freitag, and Christina Stadler
References
- 1. Gibbons CH. Basics of autonomic nervous system function. Handb Clin Neurol. 2019;160:407–418. [DOI] [PubMed] [Google Scholar]
- 2. de Geus EJC, Gianaros PJ, Brindle RC, Jennings JR, Berntson GG. Should heart rate variability be "corrected" for heart rate? Biological, quantitative, and interpretive considerations. Psychophysiol. 2019;56:e13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nederend I, Ten Harkel ADJ, Blom NA, Berntson GG, de Geus EJC. Impedance cardiography in healthy children and children with congenital heart disease: Improving stroke volume assessment. Int J Psychophysiol. 2017;120:136–147. [DOI] [PubMed] [Google Scholar]
- 4. Nederend I, Schutte NM, Bartels M, Ten Harkel AD, de Geus EJ. Heritability of heart rate recovery and vagal rebound after exercise. Eur J Appl Physiol. 2016;116:2167–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berntson GG, Cacioppo JT, Quigley KS. Autonomic cardiac control. I. Estimation and validation from pharmacological blockades. Psychophysiol. 1994;31:572–585. [DOI] [PubMed] [Google Scholar]
- 6. Goedhart AD, Kupper N, Willemsen G, Boomsma DI, de Geus EJ. Temporal stability of ambulatory stroke volume and cardiac output measured by impedance cardiography. Biol Psychol. 2006;72:110–117. [DOI] [PubMed] [Google Scholar]
- 7. Harris WS, Schoenfeld CD, Weissler AM. Effects of adrenergic receptor activation and blockade on the systolic preejection period, heart rate, and arterial pressure in man. J Clin Invest. 1967;46:1704–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Houtveen JH, Groot PF, Geus EJ. Effects of variation in posture and respiration on RSA and pre‐ejection period. Psychophysiol. 2005;42:713–719. [DOI] [PubMed] [Google Scholar]
- 9. Krzemiński K, Kruk B, Nazar K, Ziemba AW, Cybulski G, Niewiadomski W. Cardiovascular, metabolic and plasma catecholamine responses to passive and active exercises. J Physiol Pharmacol. 2000;51:267–278. [PubMed] [Google Scholar]
- 10. Kupper N, Willemsen G, Boomsma DI, de Geus EJ. Heritability of indices for cardiac contractility in ambulatory recordings. J Cardiovasc Electrophysiol. 2006;17:877–883. [DOI] [PubMed] [Google Scholar]
- 11. Mezzacappa ES, Kelsey RM, Katkin ES. The effects of epinephrine administration on impedance cardiographic measures of cardiovascular function. Int J Psychophysiol. 1999;31:189–196. DOI: 10.1016/S0167-8760(98)00058-0. [DOI] [PubMed] [Google Scholar]
- 12. Miyamoto Y, Higuchi J, Abe Y, Hiura T, Nakazono Y, Mikami T. Dynamics of cardiac output and systolic time intervals in supine and upright exercise. J Appl Physiol Respir Environ Exerc Physiol. 1983;55:1674–1681. DOI: 10.1152/jappl.1983.55.6.1674. [DOI] [PubMed] [Google Scholar]
- 13. Nelesen RA, Shaw R, Ziegler MG, Dimsdale JE. Impedance cardiography‐derived hemodynamic responses during baroreceptor testing with amyl nitrite and phenylephrine: a validity and reliability study. Psychophysiol. 1999;36:105–108. DOI: 10.1017/S0048577299971500. [DOI] [PubMed] [Google Scholar]
- 14. Newlin DB, Levenson RW. Pre‐ejection period: measuring beta‐adrenergic influences upon the heart. Psychophysiol. 1979;16:546–553. DOI: 10.1111/j.1469-8986.1979.tb01519.x. [DOI] [PubMed] [Google Scholar]
- 15. Richter M, Gendolla GH. The heart contracts to reward: monetary incentives and preejection period. Psychophysiol. 2009;46:451–457. DOI: 10.1111/j.1469-8986.2009.00795.x. [DOI] [PubMed] [Google Scholar]
- 16. Schachinger H, Weinbacher M, Kiss A, Ritz R, Langewitz W. Cardiovascular indices of peripheral and central sympathetic activation. Psychosom Med. 2001;63:788–796. DOI: 10.1097/00006842-200109000-00012. [DOI] [PubMed] [Google Scholar]
- 17. Sherwood A, Allen MT, Obrist PA, Langer AW. Evaluation of beta‐adrenergic influences on cardiovascular and metabolic adjustments to physical and psychological stress. Psychophysiol. 1986;23:89–104. DOI: 10.1111/j.1469-8986.1986.tb00602.x. [DOI] [PubMed] [Google Scholar]
- 18. Smith JJ, Muzi M, Barney JA, Ceschi J, Hayes J, Ebert TJ. Impedance‐derived cardiac indices in supine and upright exercise. Ann Biomed Eng. 1989;17:507–515. [DOI] [PubMed] [Google Scholar]
- 19. Svedenhag J, Martinsson A, Ekblom B, Hjemdahl P. Altered cardiovascular responsiveness to adrenaline in endurance‐trained subjects. Acta Physiol Scand. 1986;126:539–550. DOI: 10.1111/j.1748-1716.1986.tb07853.x. [DOI] [PubMed] [Google Scholar]
- 20. Vrijkotte TG, van Doornen LJ, de Geus EJ. Overcommitment to work is associated with changes in cardiac sympathetic regulation. Psychosom Med. 2004;66:656–663. DOI: 10.1097/01.psy.0000138283.65547.78. [DOI] [PubMed] [Google Scholar]
- 21. Williams PD, Puddey IB, Beilin LJ, Vandongen R. Genetic influences on plasma catecholamines in human twins. J Clin Endocrinol Metab. 1993;77:794–799. [DOI] [PubMed] [Google Scholar]
- 22. Winzer A, Ring C, Carroll D, Willemsen G, Drayson M, Kendall M. Secretory immunoglobulin a and cardiovascular reactions to mental arithmetic, cold pressor, and exercise: effects of beta‐adrenergic blockade. Psychophysiol. 1999;36:591–601. DOI: 10.1111/1469-8986.3650591. [DOI] [PubMed] [Google Scholar]
- 23. Billman GE. Heart rate variability ‐ a historical perspective. Front Physiol. 2011;2:86. DOI: 10.3389/fphys.2011.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dollar JM, Calkins SD, Berry NT, Perry NB, Keane SP, Shanahan L, Wideman L. Developmental patterns of respiratory sinus arrhythmia from toddlerhood to adolescence. Dev Psychol. 2020;56:783–794. DOI: 10.1037/dev0000894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gower AL, Crick NR. Baseline autonomic nervous system arousal and physical and relational aggression in preschool: The moderating role of effortful control. Int J Psychophysiol. 2011;81:142–151. DOI: 10.1016/j.ijpsycho.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 26. Ohuchi H, Negishi J, Miyake A, Sakaguchi H, Miyazaki A, Yamada O. Long‐term prognostic value of cardiac autonomic nervous activity in postoperative patients with congenital heart disease. Int J Cardiol. 2011;151:296–302. DOI: 10.1016/j.ijcard.2010.05.062. [DOI] [PubMed] [Google Scholar]
- 27. Alkon A, Boyce WT, Davis NV, Eskenazi B. Developmental changes in autonomic nervous system resting and reactivity measures in latino children from 6 to 60 months of age. J Dev Behav Pediatr. 2011;32:668–677. DOI: 10.1097/DBP.0b013e3182331fa6. [DOI] [PubMed] [Google Scholar]
- 28. Bornstein MH, Suess PE. Child and mother cardiac vagal tone: Continuity, stability, and concordance across the first 5 years. Dev Psychol. 2000;36:54–65. DOI: 10.1037/0012-1649.36.1.54. [DOI] [PubMed] [Google Scholar]
- 29. El‐Sheikh M. Stability of respiratory sinus arrhythmia in children and young adolescents: a longitudinal examination. Dev Psychobiol. 2005;46:66–74. DOI: 10.1002/dev.20036. [DOI] [PubMed] [Google Scholar]
- 30. El‐Sheikh M, Hinnant JB, Philbrook LE. Trajectories of sleep and cardiac sympathetic activity indexed by pre‐ejection period in childhood. J Sleep Res. 2017;26:578–586. DOI: 10.1111/jsr.12491. [DOI] [PubMed] [Google Scholar]
- 31. Gatzke‐Kopp L, Ram N. Developmental dynamics of autonomic function in childhood. Psychophysiol. 2018;55:e13218. [DOI] [PubMed] [Google Scholar]
- 32. Hinnant JB, Elmore‐Staton L, El‐Sheikh M. Developmental trajectories of respiratory sinus arrhythmia and preejection period in middle childhood. Dev Psychobiol. 2011;53:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jewell SL, Suk HW, Luecken LJ. Respiratory sinus arrhythmia: modeling longitudinal change from 6 weeks to 2 years of age among low‐income Mexican Americans. Dev Psychobiol. 2018;60:232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Longin E, Dimitriadis C, Shazi S, Gerstner T, Lenz T, Konig S. Autonomic nervous system function in infants and adolescents: impact of autonomic tests on heart rate variability. Pediatr Cardiol. 2009;30:311–324. [DOI] [PubMed] [Google Scholar]
- 35. Matthews KA, Salomon K, Kenyon K, Allen MT. Stability of children's and adolescents' hemodynamic responses to psychological challenge: a three‐year longitudinal study of a multiethnic cohort of boys and girls. Psychophysiol. 2002;39:826–834. [DOI] [PubMed] [Google Scholar]
- 36. Pang KC, Beauchaine TP. Longitudinal patterns of autonomic nervous system responding to emotion evocation among children with conduct problems and/or depression. Dev Psychobiol. 2013;55:698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Patriquin MA, Lorenzi J, Scarpa A, Bell MA. Developmental trajectories of respiratory sinus arrhythmia: associations with social responsiveness. Dev Psychobiol. 2014;56:317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shader TM, Gatzke‐Kopp LM, Crowell SE, Jamila Reid M, Thayer JF, Vasey MW, Webster‐Stratton C, Bell Z, Beauchaine TP. Quantifying respiratory sinus arrhythmia: effects of misspecifying breathing frequencies across development. Dev Psychopathol. 2018;30:351–366. [DOI] [PubMed] [Google Scholar]
- 39. Sheinkopf SJ, Levine TP, McCormick CEB, Puggioni G, Conradt E, Lagasse LL, Lester BM. Developmental trajectories of autonomic functioning in autism from birth to early childhood. Biol Psychol. 2019;142:13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Massin M, von Bernuth G. Normal ranges of heart rate variability during infancy and childhood. Pediatr Cardiol. 1997;18:297–302. [DOI] [PubMed] [Google Scholar]
- 41. Michels N, Clays E, De Buyzere M, Huybrechts I, Marild S, Vanaelst B, De Henauw S, Sioen I. Determinants and reference values of short‐term heart rate variability in children. Eur J Appl Physiol. 2013;113:1477–1488. DOI: 10.1007/s00421-012-2572-9. [DOI] [PubMed] [Google Scholar]
- 42. Silvetti MS, Drago F, Ragonese P. Heart rate variability in healthy children and adolescents is partially related to age and gender. Int J Cardiol. 2001;81:169–174. DOI: 10.1016/S0167-5273(01)00537-X. [DOI] [PubMed] [Google Scholar]
- 43. van den Berg ME, Rijnbeek PR, Niemeijer MN, Hofman A, van Herpen G, Bots ML, Hillege H, Swenne CA, Eijgelsheim M, Stricker BH, et al. Normal values of corrected heart‐rate variability in 10‐second electrocardiograms for all ages. Front Physiol. 2018;10:424. DOI: 10.3389/fphys.2018.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health. 2017;5:258. DOI: 10.3389/fpubh.2017.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pereyra PM, Zhang W, Schmidt M, Becker LE. Development of myelinated and unmyelinated fibers of human vagus nerve during the first year of life. J Neurol Sci. 1992;110:107–113. DOI: 10.1016/0022-510X(92)90016-E. [DOI] [PubMed] [Google Scholar]
- 46. Lenard Z, Studinger P, Mersich B, Kocsis L, Kollai M. Maturation of cardiovagal autonomic function from childhood to young adult age. Circulation. 2004;110:2307–2312. DOI: 10.1161/01.CIR.0000145157.07881.A3. [DOI] [PubMed] [Google Scholar]
- 47. Gasior JS, Sacha J, Jelen PJ, Pawlowski M, Werner B, Dabrowski MJ. Interaction between heart rate variability and heart rate in pediatric population. Front Physiol. 2015;6:385. DOI: 10.3389/fphys.2015.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jarrin DC, McGrath JJ, Poirier P, Seguin L, Tremblay RE, Montplaisir JY, Paradis G, Seguin JR. Short‐term heart rate variability in a population‐based sample of 10‐year‐old children. Pediatr Cardiol. 2015;36:41–48. DOI: 10.1007/s00246-014-0962-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Patural H, Pichot V, Flori S, Giraud A, Franco P, Pladys P, Beuchee A, Roche F, Barthelemy JC. Autonomic maturation from birth to 2 years: normative values. Heliyon. 2019;5:e01300. DOI: 10.1016/j.heliyon.2019.e01300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fukuba Y, Sato H, Sakiyama T, Yamaoka Endo M, Yamada M, Ueoka H, Miura A, Koga S. Autonomic nervous activities assessed by heart rate variability in pre‐ and post‐adolescent Japanese. J Physiol Anthropol. 2009;28:269–273. DOI: 10.2114/jpa2.28.269. [DOI] [PubMed] [Google Scholar]
- 51. Seppala S, Laitinen T, Tarvainen MP, Tompuri T, Veijalainen A, Savonen K, Lakka T. Normal values for heart rate variability parameters in children 6–8 years of age: the panic study. Clin Physiol Funct Imaging. 2014;34:290–296. [DOI] [PubMed] [Google Scholar]
- 52. Coupal KE, Heeney ND, Hockin BCD, Ronsley R, Armstrong K, Sanatani S, Claydon VE. Pubertal hormonal changes and the autonomic nervous system: potential role in pediatric orthostatic intolerance. Front Neurosci. 2019;13:1197. DOI: 10.3389/fnins.2019.01197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gao Y, Raine A, Dawson ME, Venables PH, Mednick SA. Development of skin conductance orienting, habituation, and reorienting from ages 3 to 8 years: a longitudinal latent growth curve analysis. Psychophysiol. 2007;44:855–863. DOI: 10.1111/j.1469-8986.2007.00564.x. [DOI] [PubMed] [Google Scholar]
- 54. Gutin B, Howe C, Johnson MH, Humphries MC, Snieder H, Barbeau P. Heart rate variability in adolescents: relations to physical activity, fitness, and adiposity. Med Sci Sports Exerc. 2005;37:1856–1863. DOI: 10.1249/01.mss.0000175867.98628.27. [DOI] [PubMed] [Google Scholar]
- 55. Hu MX, Lamers F, de Geus EJ, Penninx BW. Influences of lifestyle factors on cardiac autonomic nervous system activity over time. Prev Med. 2017;94:12–19. DOI: 10.1016/j.ypmed.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 56. van Beijsterveldt CEM, Groen‐Blokhuis M, Hottenga JJ, Franić S, Hudziak JJ, Lamb D, Huppertz C, de Zeeuw E, Nivard M, Schutte N, et al. The Young Netherlands Twin Register (YNTR): longitudinal twin and family studies in over 70,000 children. Twin Res Hum Genet. 2013;16:252–267. DOI: 10.1017/thg.2012.118. [DOI] [PubMed] [Google Scholar]
- 57. Freitag CM, Konrad K, Stadler C, De Brito SA, Popma A, Herpertz SC, Herpertz‐Dahlmann B, Neumann I, Kieser M, Chiocchetti AG, et al. Conduct disorder in adolescent females: current state of research and study design of the FemNAT‐CD consortium. Eur Child Adolesc Psychiatry. 2018;27:1077–1093. DOI: 10.1007/s00787-018-1172-6. [DOI] [PubMed] [Google Scholar]
- 58. Suurland J, van der Heijden KB, Smaling HJA, Huijbregts SCJ, van Goozen SHM, Swaab H. Infant autonomic nervous system response and recovery: associations with maternal risk status and infant emotion regulation. Dev Psychopathol. 2017;29:759–773. DOI: 10.1017/S0954579416000456. [DOI] [PubMed] [Google Scholar]
- 59. de Beer M, van Eijsden M, Vrijkotte TG, Gemke RJ. Early growth patterns and cardiometabolic function at the age of 5 in a multiethnic birth cohort: the ABCD study. BMC Pediatr. 2009;10:23. DOI: 10.1186/1471-2431-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Riese H, Groot PF, van den Berg M, Kupper NH, Magnee EH, Rohaan EJ, Vrijkotte TG, Willemsen G, de Geus EJ. Large‐scale ensemble averaging of ambulatory impedance cardiograms. Behav Res Methods Instrum Comput. 2003;35:467–477. DOI: 10.3758/BF03195525. [DOI] [PubMed] [Google Scholar]
- 61. Schaffer L, Burkhardt T, Tomaske M, Schmidt S, Luzi F, Rauh M, Leone A, Beinder E. Effect of antenatal betamethasone administration on neonatal cardiac autonomic balance. Pediatr Res. 2010;68:286–291. DOI: 10.1203/PDR.0b013e3181ed0cf2. [DOI] [PubMed] [Google Scholar]
- 62. R Development Core Team . R: A language and Environment for Statistical Computing. 2019. [Google Scholar]
- 63. Wickham H. Ggplot2: Elegant Graphics for Data Analysis. New York: Springer; 2016. [Google Scholar]
- 64. Goedhart AD, Willemsen G, Houtveen JH, Boomsma DI, De Geus EJ. Comparing low frequency heart rate variability and preejection period: two sides of a different coin. Psychophysiol. 2008;45:1086–1090. DOI: 10.1111/j.1469-8986.2008.00710.x. [DOI] [PubMed] [Google Scholar]
- 65. Reyes del Paso GA, Langewitz W, Mulder LJ, van Roon A, Duschek S. The utility of low frequency heart rate variability as an index of sympathetic cardiac tone: a review with emphasis on a reanalysis of previous studies. Psychophysiol. 2013;50:477–487. DOI: 10.1111/psyp.12027. [DOI] [PubMed] [Google Scholar]
- 66. Cacioppo JT, Berntson GG, Binkley PF, Quigley KS, Uchino BN, Fieldstone A. Autonomic cardiac control. II. Noninvasive indices and basal response as revealed by autonomic blockades. Psychophysiol. 1994;31:586–598. DOI: 10.1111/j.1469-8986.1994.tb02351.x. [DOI] [PubMed] [Google Scholar]
- 67. Sherwood A, Allen MT, Fahrenberg J, Kelsey RM, Lovallo WR, van Doornen LJ. Methodological guidelines for impedance cardiography. Psychophysiol. 1990;27:1–23. DOI: 10.1111/j.1469-8986.1990.tb02171.x. [DOI] [PubMed] [Google Scholar]
- 68. Ferrara N, Komici K, Corbi G, Pagano G, Furgi G, Rengo C, Femminella GD, Leosco D, Bonaduce D. Beta‐adrenergic receptor responsiveness in aging heart and clinical implications. Front Physiol. 2014;4:396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. von Holzen JJ, Capaldo G, Wilhelm M, Stute P. Impact of endo‐ and exogenous estrogens on heart rate variability in women: a review. Climacteric. 2016;19:222–228. DOI: 10.3109/13697137.2016.1145206. [DOI] [PubMed] [Google Scholar]
- 70. Brix N, Ernst A, Lauridsen LLB, Parner E, Stovring H, Olsen J, Henriksen TB, Ramlau‐Hansen CH. Timing of puberty in boys and girls: a population‐based study. Paediatr Perinat Epidemiol. 2019;33:70–78. DOI: 10.1111/ppe.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures‐S1‐S4


