Abstract
Background
Severe coronavirus disease 2019 (COVID‐19) is characterized by a proinflammatory state with high mortality. Statins have anti‐inflammatory effects and may attenuate the severity of COVID‐19.
Methods and Results
An observational study of all consecutive adult patients with COVID‐19 admitted to a single center located in Bronx, New York, was conducted from March 1, 2020, to May 2, 2020. Patients were grouped as those who did and those who did not receive a statin, and in‐hospital mortality was compared by competing events regression. In addition, propensity score matching and inverse probability treatment weighting were used in survival models to examine the association between statin use and death during hospitalization. A total of 4252 patients were admitted with COVID‐19. Diabetes mellitus modified the association between statin use and in‐hospital mortality. Patients with diabetes mellitus on a statin (n=983) were older (69±11 versus 67±14 years; P<0.01), had lower inflammatory markers (C‐reactive protein, 10.2; interquartile range, 4.5–18.4 versus 12.9; interquartile range, 5.9–21.4 mg/dL; P<0.01) and reduced cumulative in‐hospital mortality (24% versus 39%; P<0.01) than those not on a statin (n=1283). No difference in hospital mortality was noted in patients without diabetes mellitus on or off statin (20% versus 21%; P=0.82). Propensity score matching (hazard ratio, 0.88; 95% CI, 0.83–0.94; P<0.01) and inverse probability treatment weighting (HR, 0.88; 95% CI, 0.84–0.92; P<0.01) showed a 12% lower risk of death during hospitalization for statin users than for nonusers.
Conclusions
Statin use was associated with reduced in‐hospital mortality from COVID‐19 in patients with diabetes mellitus. These findings, if validated, may further reemphasize administration of statins to patients with diabetes mellitus during the COVID‐19 era.
Keywords: COVID‐19, diabetes mellitus, hospitalization, statin
Subject Categories: Inflammation, Primary Prevention, Mortality/Survival
Nonstandard Abbreviations and Acronyms
- COVID‐19
coronavirus disease 2019
- IPTW
inverse probability treatment weighting
- PS
propensity score
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
Clinical Perspective
What Is New?
In this observational study that included 4252 patients admitted with coronavirus disease 2019, those with diabetes mellitus receiving statins had a 12% reduction in the adjusted risk of in‐hospital mortality.
Despite a higher comorbidity burden, patients with diabetes mellitus on statin therapy presented with lower markers of inflammation in comparison with those not receiving statins.
What Are the Clinical Implications?
These findings may further strengthen and refocus administration of statins to patients with diabetes mellitus in the coronavirus disease 2019 era.
Coronavirus disease 2019 (COVID‐19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has rapidly spread globally. 1 , 2 Although nearly 80% of cases recover, COVID‐19 still appears to retain an elevated mortality with the number of deaths escalating to over 250 000 worldwide through April 2020. 2 The severity of COVID‐19 is characterized by a proinflammatory state evident by elevated serum biomarkers of inflammation including C‐reactive protein, interleukin‐6, and ferritin. 3 , 4 These biomarkers are predictive of death during SARS‐CoV‐2 infection. 4
Statins have known anti‐inflammatory properties 5 and may represent an agent for modulating the host response during COVID‐19. 6 Pleotropic effects of statins also reduce reactive oxygen species and platelet reactivity, and acute administration improves survival in proinflammatory states such as myocardial infarction. 7 , 8 In addition, statins can limit viral endotheliitis, 9 which has been implicated during SARS‐CoV‐2 infection. 10 On the contrary, animal studies indicate that statins increase membrane‐bound angiotensin‐converting enzyme 2 receptor, 11 which is a port of entry for SARS‐CoV‐2, thereby raising concern for statin usage during the COVID‐19 pandemic. 12 As statins are one of the most commonly prescribed to patients with cardiovascular disease and diabetes mellitus, we sought to determine if statin use is associated with survival during COVID‐19 by evaluating the association of in‐hospital mortality and statin therapy.
Methods
The data that form the basis of the presented findings are available from the corresponding author upon reasonable request.
Study Population
A retrospective single‐center review of all patients who were admitted to Montefiore Medical Center in the Bronx, New York, with a confirmed COVID‐19 diagnosis from March 1, 2020 to May 2, 2020, was conducted. COVID‐19 was confirmed by a positive real‐time reverse transcriptase polymerase chain reaction assay. A sample for positive real‐time reverse transcriptase polymerase chain reaction was obtained by either nasopharyngeal or oropharyngeal swab. The follow‐up period was from March 1, 2020 to May 4, 2020. The study was approved by the Institutional Review Board (2020‐11308), and informed consent was waived.
Data Collection, Exposure, and Outcome
Baseline demographic, clinical, and laboratory variables were retrieved from the electronic medical record system. The exposure was statin administration. Patients were grouped as those who received and those who did not receive a statin during the hospitalization. The primary outcome was in‐hospital mortality during the follow‐up period.
Statistical Analysis
Continuous data are displayed as mean±SD or median 25% to 75% interquartile range (IQR), and characteristics were compared between statin users and nonusers using the Student t test, or Wilcoxon rank‐sum test. Categorical data are shown as percent and were compared by the chi‐squared test. As hospital discharge is a competing event with in‐hospital mortality, instead of Kaplan–Meier estimates, we used competing events analysis to estimate the cumulative incidence of in‐hospital mortality since admission. 13 Patients who remained in the hospital at the end of the follow‐up period on May 4, 2020, were censored. The difference in the cumulative incidence of in‐hospital mortality was compared between statin users and nonusers separately among patients with and without diabetes mellitus by Fine and Gray's method. 14
Multivariable Regression Modeling
A multivariable regression model with subdistribution hazard ratios (HRs) 13 , 14 was used to calculate the adjusted HR of in‐hospital mortality only for patients with diabetes mellitus that were on a statin in comparison with those not receiving statins. A full model was determined with demographics and clinical and laboratory variables that were independently associated with statin use or in‐hospital mortality at a P<0.2. Then a reduced model was derived with all covariates that had a P<0.05. This reduced model included statin use (yes or no), age, sex, history of atherosclerotic heart disease, Charlson comorbidity index, presenting diastolic blood pressure, respiratory rate, pulse oximetry measurement, serum glucose, serum lactic acid, serum creatinine, and intravenous antibiotic use during hospitalization.
Propensity Score Analyses
To limit potential residual confounding by indication, we also used propensity score (PS) matching and inverse probability treatment weighting (IPTW) to examine the association between statin use and in‐hospital mortality in a Cox proportional hazards model among patients with diabetes mellitus. 15 Covariate selection for PS matching and IPTW was done in accordance with criteria described previously. 16 , 17 Variables included for PS matching were true confounders, or those that were associated with the outcome of interest (ie, in‐hospital death). We did not include variables that were associated only with exposure (ie, statin use) and not the outcome (ie, in‐hospital death), since it has been demonstrated that these covariates increase the variance of the estimated exposure effect without decreasing bias. 16 The covariates in the propensity analyses included age, sex, body mass index, days of symptoms before admission, history of atherosclerotic heart disease, Charlson comorbidity index, presenting diastolic blood pressure, respiratory rate, pulse oximetry measurement, heart rate, serum glucose, lactic acid, serum creatinine, serum troponin levels, usage of angiotensin‐converting enzymes inhibitors, usage of angiotensin receptor blockers, serum troponin levels, and intravenous antibiotics during hospitalization. PS matching was carried out through a 1:1 greedy matching algorithm, with a caliper width of 0.1 SD, and through IPTW. The PS and IPTW analyses were carried out using treatment effects in Stata (StataCorp, College Station, TX).
Missing Data
The proportion of patients with diabetes mellitus and missing data for a covariate that was included in multivariable competing risk regression and propensity score models was <5%, with the exception of glucose (9.2%), lactic acid (9.1%), and serum troponin (15.4%). Since troponin was missing in >10% of the patients with diabetes mellitus, we created a categorical variable using quartile cutoff point with an additional category for missing values and added this variable in the PS models. Given a skewed distribution, quartiles 1 and 2 were collapsed as a single category and served as a reference for quartile 3 (intermediate‐level troponin), quartile 4 (high‐level troponin), and missing troponin categories. The final data set for the multivariable competing risks regression model included 2039 (90%) of the 2266 patients with diabetes mellitus, while the models using PS matching and IPTW included 1902 (84%) of patients with diabetes mellitus.
A P<0.05 was considered statistically significant. Stata 16 (StataCorp) and R (R Foundation for Statistical Computing, Vienna, Austria) software was used for all statistical analysis.
Results
Patient Characteristics
Overall, 4252 patients (65±16 years old; 47% female) were admitted with COVID‐19. Thirty‐seven percent (n=1570) were Hispanic. Diabetes mellitus (53%), hypertension (72%), and atherosclerotic heart disease (26%) were highly prevalent comorbidities (Table 1). On average, patients presented 3 (IQR, 0–7) days after onset of symptoms and had elevated inflammatory markers including C‐reactive protein (10.6; IQR, 4.5–18.9 mg/dL) and ferritin (785; IQR, 369–1607 ng/mL).
Table 1.
Characteristics of Patients Admitted With COVID‐19
| Characteristic |
All Patients (n=4252) |
Statin (n=1355) |
No Statin (n=2897) |
P Value |
|---|---|---|---|---|
| Age, y | 65±16 | 69±12 | 63±17 | <0.001 |
| Female, n (%) | 1997 (47) | 618 (46) | 1379 (47) | 0.22 |
| Race/Ethnicity, n (%) | 0.54 | |||
| Non‐Hispanic Black | 1561 (37) | 510 (38) | 1051 (36) | |
| Non‐Hispanic White | 351 (8) | 114 (8) | 237 (8) | |
| Hispanic | 1570 (37) | 502 (37) | 1068 (37) | |
| Other*/Unknown | 770 (18) | 229 (17) | 541 (19) | |
| Body mass index, kg/m2 | 28.5 (24.7–33.2) | 28 (25–33) | 29 (25–33) | 0.028 |
| Past medical diagnosis, n (%) | ||||
| Diabetes mellitus | 2266 (53) | 983 (73) | 1283 (44) | <0.001 |
| Hypertension | 3060 (72) | 1175 (86) | 1885 (65) | <0.001 |
| ASHD | 1111 (26) | 583 (43) | 528 (18) | <0.001 |
| Lung disease | 1203 (28) | 458 (34) | 745 (26) | <0.001 |
| Charlson comorbidity index | 3 (2–7) | 5 (3–8) | 3 (1–5) | <0.001 |
| Days from symptoms to presentation | 3 (0–7) | 2 (0–5) | 3 (0–7) | 0.015 |
| Vital signs at presentation | ||||
| Systolic blood pressure, mm Hg | 131 (115–148) | 133 (117–151) | 129 (113–146) | <0.001 |
| Diastolic blood pressure, mm Hg | 75 (65–84) | 74 (65–83) | 75 (65–85) | 0.042 |
| Heart rate, beats/min | 98 (86–112) | 95 (82–108) | 100 (87–114) | <0.001 |
| Oxygen saturation, % | 95 (90–98) | 95 (92–98) | 95 (90–98) | <0.001 |
| Respiratory rate, breaths/min | 20 (18–22) | 20 (18–22) | 20 (18–23) | <0.001 |
| Temperature, ˚F | 98.9 (98.2–100.1) | 98.8 (98.2–100) | 99 (98.2–100.1) | 0.012 |
| Laboratory markers | ||||
| Alanine transaminase, U/L | 27 (17–44) | 24 (16–38) | 29 (18–47) | <0.001 |
| Neutrophil count, k/μL | 5.7 (3.9–8.4) | 5.4 (3.7–7.7) | 5.8 (4–8.7) | <0.001 |
| Lymphocyte count, k/μL | 1 (0.7–1.4) | 1 (0.7–1.3) | 1 (0.7–1.4) | <0.002 |
| Ferritin, ng/mL | 785 (369–1607) | 710 (355–1464) | 816 (389–1703) | 0.015 |
| Lactate dehydrogenase, U/L | 397 (292–547) | 370 (285–505) | 413 (298–576) | <0.001 |
| C‐reactive protein, mg/L | 10.6 (4.5–18.9) | 9.9 (4–17.4) | 11.1 (4.7–19.5) | 0.005 |
| d‐dimer, μg/mL | 1.84 (0.94–4.2) | 1.83 (0.92–3.61) | 1.86 (0.94–4.55) | 0.17 |
| Procalcitonin, ng/mL | 0.1 (0.3–0.9) | 0.2 (0.1–0.7) | 0.3 (0.1–1.1) | 0.003 |
| Lactic acid, mmole/L | 2.1 (1.6–3) | 2.1 (1.5–2.8) | 2.1 (1.6–3.2) | 0.001 |
| ProBNP, pg/mL | 480 (114–1983) | 611 (151–2724) | 415 (95–1661) | <0.001 |
| Creatinine, mg/dL | 1.2 (0.8–2) | 1.3 (0.92–2.4) | 1.1 (0.8–1.8) | <0.001 |
| Glucose, mg/dL | 141 (114–207) | 153 (117–229) | 138 (133–195) | <0.001 |
| Troponin T, mg/mL | 0.01 (0.01–0.04) | 0.01 (0.01–0.05) | 0.01 (0.01–0.03) | <0.001 |
| Inpatient medications, n (%) | ||||
| ACE inhibitors | 282 (6.6) | 163 (12) | 119 (4) | <0.001 |
| ARB | 241 (5.7) | 124 (9) | 117 (4) | <0.001 |
| Hydroxycholoroquine | 3031 (71) | 1007 (74) | 2024 (70) | 0.003 |
| Antibiotics | 3352 (76) | 1026 (76) | 2226 (77) | 0.42 |
| Intravenous steroids | 947 (22) | 315 (23) | 632 (22) | 0.30 |
| Statin type, % | ||||
| Atorvastatin | 1024 (76) | |||
| Pravastatin | 74 (5) | |||
| Rosuvastatin | 10 (1) | |||
| Simvastatin | 247 (18) | |||
The following were the available samples sizes for variables with >5% missing data: alanine transaminase (n=3901), ferritin (n=1663), lactate dehydrogenase (n=2635), C‐reactive protein (n=2083), d‐dimer (n=1857), procalcitonin (n=1537), lactic acid (n=3705), creatinine (n=4108), glucose (n=3703), and troponin (n=3384). ACE indicates angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; ASHD, atherosclerotic heart disease; BNP, B‐type natriuretic peptide; and COVID‐19, coronavirus disease 2019.
Other includes Asian, Pacific Islander, American Indian, Alaskan Native, or Other.
Statin Use and In‐Hospital Mortality
Within all admitted patients, 32% (n=1355) received a statin. Those on statin therapy were older (69±12 versus 63±17 years; P<0.01) and had a higher Charlson comorbidity index (5; IQR, 3–8 versus 3; IQR, 1–5; P<0.01). Despite a higher comorbidity burden, we observed a lower cumulative in‐hospital mortality in those receiving a statin (23% versus 27%; P<0.01). Seventy‐six percent of the patients received atorvastatin. Further stratification analysis revealed that this effect was modified by diabetes mellitus. Patient with diabetes mellitus (n=2266) on a statin had a lower cumulative in‐hospital mortality (24% versus 39%; P<0.01; Figure [A]), while no difference was noted in patients without diabetes mellitus (20% versus 21%; P=0.82; Figure [B]).
Figure 1. Cumulative incidence of in‐hospital mortality during COVID‐19 in statin users and nonusers stratified by presence (A) or absence (B) of diabetes mellitus.
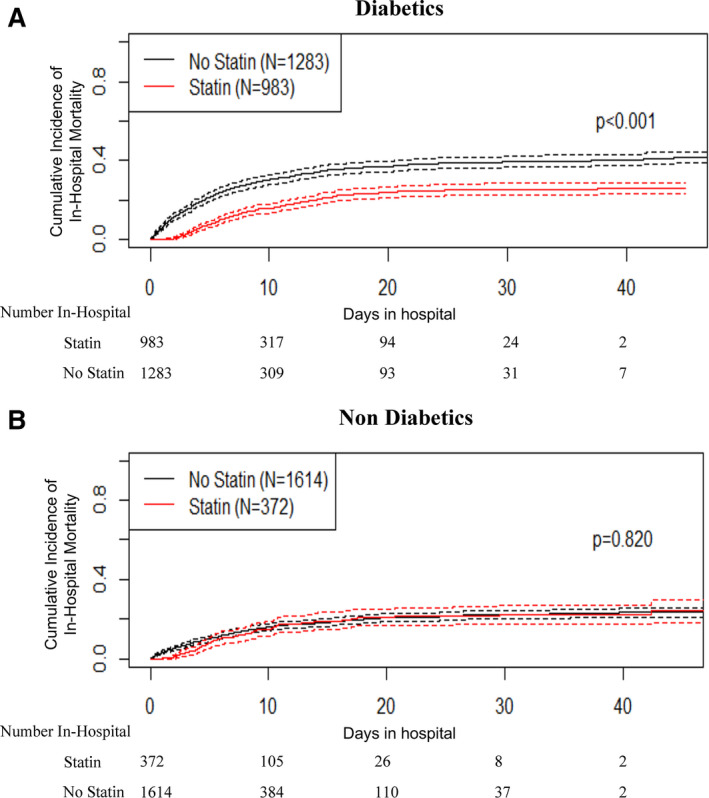
COVID‐19 indicates coronavirus disease 2019.
Diabetes Mellitus and In‐Hospital Mortality
Patients with diabetes mellitus admitted for COVID‐19 were 68±13 years old, 48% were female, 36% classified as Hispanic, 87% had a history of hypertension, and 36% had atherosclerotic heart disease. Their blood glucose at presentation was 180 (IQR, 120–274 mg/dL), and the glycosylated hemoglobin if available within 3 months before admission was 7.8 (IQR, 6.5%–9.7%; n=507).
Overall, patients with diabetes mellitus had an in‐hospital mortality of 32% over a follow‐up period of 5.9 (IQR, 3.4–10.8) days. Those who died in the hospital were older (74±12 versus 67±13 years; P<0.01), were more frequently men (57% versus 50%, P<0.01), and had a greater proportion of known atherosclerotic heart disease (44% versus 32%; P<0.01). Nonsurvivors had lower blood pressure, reduced oxygen saturation at presentation, and higher levels of inflammatory biomarkers (Table 2).
Table 2.
Characteristics of Survivors and Nonsurvivors Among Patients With Diabetes Mellitus and COVID‐19
| Characteristic |
All Patients With Diabetes Mellitus (n=2266) |
Survivors (n=1537) |
Nonsurvivors (n=729) |
P Value* |
|---|---|---|---|---|
| Age, y | 68±13 | 67±13 | 74±12 | <0.001 |
| Female, n (%) | 1077 (48) | 766 (49) | 311 (43) | 0.001 |
| Race/Ethnicity, n (%) | 0.46 | |||
| Non‐Hispanic Black | 887 (39) | 612 (40) | 275 (38) | |
| Non‐Hispanic White | 164 (7) | 104 (7) | 60 (8) | |
| Hispanic | 882 (36) | 561 (37) | 261 (36) | |
| Other † /Unknown | 393 (17) | 260 (17) | 133 (18) | |
| Body mass index, kg/m2 | 28.6 (24.8–33.3) | 28.9 (24.9–33.7) | 28.2 (24.5–32.6) | 0.026 |
| Past medical diagnosis, n (%) | ||||
| Hypertension | 1980 (87) | 1348 (88) | 632 (87) | 0.23 |
| ASHD | 820 (36) | 496 (32) | 324 (44) | <0.001 |
| Lung disease | 761 (34) | 504 (33) | 257 (35) | 0.58 |
| Charlson comorbidity index | 5 (3–9) | 4 (2–8) | 7 (4–10) | <0.001 |
| Days from symptoms to presentation | 3 (0–6) | 3 (0–7) | 2 (0–5) | <0.001 |
| Vital signs at presentation | ||||
| Systolic blood pressure, mm Hg | 133 (116–151) | 134 (118–151) | 129 (107–150) | <0.001 |
| Diastolic blood pressure, mm Hg | 74 (64–84) | 75 (66–84) | 71 (58–83) | <0.001 |
| Heart rate, beats/min | 98 (85–112) | 97 (85–110) | 101 (86–117) | 0.001 |
| Oxygen saturation (%) | 95 (90–98) | 95 (92–98) | 93 (86–97) | <0.001 |
| Respiratory rate, breaths/min | 20 (18–23) | 20 (18–22) | 22 (18–26) | <0.001 |
| Temperature, ˚F | 98.9 (98.2–100) | 98.9 (98.2–100) | 98.9 (98.1–100.3) | 0.02 |
| Laboratory markers | ||||
| Alanine transaminase, U/L | 25 (16–40) | 25 (16–39) | 26 (17–42) | 0.050 |
| Neutrophil count, k/μL | 5.8 (4–8.4) | 5.6 (3.8–7.9) | 6.5 (4.6–9.9) | <0.001 |
| Lymphocyte count, k/μL | 1 (0.7–1.3) | 1 (0.7–1.4) | 0.9 (0.6–1.3) | <0.001 |
| Ferritin, ng/mL | 733 (757–1581) | 701 (335–1452) | 911 (504–2097) | <0.001 |
| Lactate dehydrogenase, U/L | 401 (300–549) | 372 (285–492) | 506 (359–693) | <0.001 |
| C‐reactive protein, mg/L | 11.6 (5.1–20.1) | 10 (4.4–18.2) | 15.7 (8.6–24) | <0.001 |
| d‐dimer, μg/mL | 2 (1–4.5) | 1.8 (0.9–3.6) | 3 (1.5–8.5) | <0.001 |
| Procalcitonin, ng/mL | 0.3 (0.1–1.1) | 0.2 (0.1–0.7) | 0.9 (0.3–3.4) | <0.001 |
| Lactic acid, mmole/L | 2.2 (1.6–3.1) | 2.1 (1.6–2.8) | 2.6 (1.9–4) | <0.001 |
| ProBNP, pg/mL | 581 (149–2857) | 354 (104–1479) | 1645 (547–8487) | <0.001 |
| Creatinine, mg/dL | 1.3 (0.9–2.5) | 1.2 (0.9–2.1) | 1.8 (1.2–3.4) | <0.001 |
| Glucose, mg/dL | 180 (120–274) | 172 (124–266) | 197 (138–304) | <0.001 |
| Troponin T, mg/mL | 0.01 (0.01–0.05) | 0.01 (0.01–0.03) | 0.03 (0.01–0.1) | <0.001 |
| Inpatient medications, n (%) | ||||
| ACE inhibitors | 196 (8.7) | 158 (10) | 38 (5.2) | <0.001 |
| ARB | 165 (7.4) | 127 (8.3) | 38 (5.2) | 0.009 |
| Hydroxycholoroquine | 1652 (73) | 1130 (74) | 522 (72) | <0.001 |
| Antibiotics | 1758 (78) | 1096 (71) | 662 (91) | <0.001 |
| Intravenous steroids | 557 (25) | 299 (20) | 258 (35) | 0.12 |
ACE indicates angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; ASHD, atherosclerotic heart disease; BNP, B‐type natriuretic peptide; and COVID‐19, coronavirus disease 2019.
Survivors vs nonsurvivors.
Other includes Asian, Pacific Islander, American Indian, Alaskan Native, or Other.
Diabetes Mellitus and Statin Use
Among patients with diabetes mellitus, 983 (43%) received and 1283 (57%) did not receive a statin. Those on a statin were older (69±11 versus 67±14 years; P<0.01), and more of them had a history of hypertension (91% versus 84%; P<0.01), atherosclerotic heart disease (46% versus 28%; P<0.01), and a higher Charlson comorbidity index (6; IQR, 3–9 versus 4; IQR, 2–8; P<0.01). Time from initial symptoms to presentation was similar between those on and off statins (3; IQR, 0–7 versus 2; IQR, 0–5 days; P=0.28). At presentation, those receiving a statin had lower inflammatory markers including C‐reactive protein (10.2; IQR, 4.5–18.4 versus 12.9; IQR, 5.9–21.4 mg/dL; P<0.01) and ferritin (683; IQR. 340–1486 versus 786; IQR, 368–1702 ng/mL; P=0.048) but similar blood glucose (176; IQR, 128–260 versus 183; IQR, 129–283 mg/dL; P=0.16) in comparison with those who did not receive a statin (Table 3).
Table 3.
Characteristics of Statin Users and Nonusers Among Patients With and Without Diabetes Mellitus Hospitalized With COVID‐19
| Characteristic | Patients With Diabetes Mellitus (n=2266) | Patients Without Diabetes Mellitus (n=1986) | ||||
|---|---|---|---|---|---|---|
| Statin (n=983) | No Statin (n=1283) | P Value* | Statin (n=372) | No Statin (n=1614) | P Value* | |
| Age, y | 69±11 | 67±14 | <0.003 | 70±12 | 59±18 | <0.001 |
| Female, n (%) | 456 (46%) | 621 (49%) | 0.34 | 162 (44) | 758 (47) | 0.23 |
| Race/Ethnicity, n (%) | 0.29 | 0.54 | ||||
| Non‐Hispanic Black | 375 (38) | 512 (40) | 135 (36) | 539 (33) | ||
| Non‐Hispanic White | 69 (7) | 95 (7) | 45 (12) | 142 (9) | ||
| Hispanic | 378 (38) | 444 (35) | 124 (33) | 624 (39) | ||
| Other † /Unknown | 161 (16) | 161 (16) | 68 (18) | 309 (19) | ||
| Body mass index, kg/m2 | 28.5 (24.8–32.8) | 28.7 (24.9–33.7) | 0.32 | 27.3 (23.8–32.1) | 28.9 (24.6–33.2) | 0.002 |
| Past medical diagnosis, n (%) | ||||||
| Hypertension | 898 (91) | 1082 (84) | <0.001 | 277 (74) | 903 (50) | <0.001 |
| ASHD | 456 (46) | 364 (28) | <0.001 | 127 (34) | 162 (10) | <0.001 |
| Lung disease | 358 (36) | 403 (31) | 0.012 | 100 (27) | 342 (21) | 0.017 |
| Charlson comorbidity index | 6 (3–9) | 4 (2–8) | <0.001 | 4 (2–6) | 2 (0–4) | <0.001 |
| Days from symptoms to presentation | 2 (0–5) | 3 (0–6) | 0.28 | 2 (0–6) | 3 (0–7) | 0.033 |
| Vital signs at presentation | ||||||
| Systolic blood pressure, mm Hg | 135 (118–153) | 132 (113–149) | <0.001 | 130 (113–148) | 128 (114–144) | 0.10 |
| Diastolic blood pressure, mm Hg | 74 (64–83) | 75 (63–85) | 0.26 | 75 (66–84) | 75 (66–85) | 0.60 |
| Heart rate, beats//min | 95 (82–108) | 101 (87–115) | <0.001 | 95 (82–107) | 99 (86.8–113.3) | <0.001 |
| Oxygen saturation (%) | 95 (92–98) | 94 (89–97) | <0.001 | 96 (92–98) | 95 (91–98) | 0.71 |
| Respiratory rate, breaths/min | 20 (18–22) | 20 (18–24) | <0.001 | 20 (18–22) | 20 (18–22) | 0.037 |
| Temperature, ˚F | 98.8 (98.2–100) | 98.9 (98.2–100.1) | 0.16 | 98.8 (98.2–100) | 99.0 (98.3–100.1) | 0.065 |
| Laboratory markers | ||||||
| Alanine transaminase, U/L | 23 (16–36) | 27 (17–44) | <0.001 | 28 (18–43.5) | 31 (19–49) | 0.031 |
| Neutrophil count, k/μL | 5.5 (3.9–7.9) | 6.1 (4.2–8.8) | <0.001 | 5.2 (3.4–7.4) | 5.6 (3.7–8.6) | 0.001 |
| Lymphocyte count, k/μL | 0.9 (0.7–1.3) | 1 (0.7–1.4) | 0.32 | 1 (0.7–1.4) | 1.1 (0.7–1.5) | 0.079 |
| Ferritin, ng/mL | 683 (340–1486) | 786 (368–1702) | 0.048 | 785 (455.5–1425) | 854.5 (408–1709) | 0.34 |
| Lactate dehydrogenase, U/L | 367 (282–487) | 434 (323–616) | <0.001 | 381 (295–546) | 393 (285–543.5) | 0.89 |
| C‐reactive protein, mg/L | 10.2 (4.5–18.4) | 12.9 (5.9–21.4) | <0.001 | 8.4 (3–16.8) | 9.7 (3.7–18.2) | 0.14 |
| d‐dimer, μg/mL | 1.8 (0.9–3.7) | 2.2 (1.1–5) | <0.001 | 1.9 (0.9–3.6) | 1.6 (0.9–4) | 0.56 |
| Procalcitonin, ng/mL | 0.2 (0.1–0.8) | 0.4 (0.1–1.5) | <0.001 | 0.2 (0.1–0.6) | 0.2 (0.1–0.8) | 0.32 |
| Lactic acid, mmole/L | 2.1 (1.6–2.8) | 2.3 (1.7–3.4) | <0.001 | 1.9 (1.4–2.6) | 2 (1.5–2.9) | 0.028 |
| ProBNP, pg/mL | 617 (174–3467) | 572 (137–2519) | 0.14 | 598 (128–1848) | 257 (66–1240.5) | <0.001 |
| Creatinine, mg/dL | 1.4 (1–2.8) | 1.3 (0.9–2.3) | <0.001 | 1.1 (0.9–1.6) | 1 (0.8–1.4) | <0.001 |
| Glucose, mg/dL | 176 (128–260) | 183 (129–283) | 0.16 | 123 (109–143) | 123 (108–145) | 0.76 |
| Troponin T, mg/mL | 0.01 (0.01–0.06) | 0.01 (0.01–0.05) | 0.10 | 0.01 (0.01–0.03) | 0.01 (0.01–0.02) | 0.009 |
| Inpatient medications, n (%) | ||||||
| ACE inhibitors | 127 (12.9) | 69 (5.4) | <0.001 | 36 (9.7) | 50 (3.1) | <0.001 |
| ARB | 102 (10.4) | 63 (4.9) | <0.001 | 22 (5.9) | 54 (3.3) | 0.020 |
| Hydroxycholoroquine | 734 (74) | 918 (72) | 0.10 | 273 (73) | 1106 (69) | 0.67 |
| Antibiotics | 739 (75) | 1019 (79) | 0.016 | 287 (77) | 1207 (75) | 0.34 |
| Intravenous steroids | 245 (25) | 312 (24) | 0.74 | 70 (19) | 320 (20) | 0.66 |
| Statin type, % | ||||||
| Atorvastatin | 751 (76) | 273 (73) | ||||
| Pravastatin | 51 (5) | 23 (6) | ||||
| Rosuvastatin | 8 (1) | 2 (1) | ||||
| Simvastatin | 173 (18) | 74 (20) | ||||
ACE indicates angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; ASHD, atherosclerotic heart disease; BNP, B‐type natriuretic peptide; and COVID‐19, coronavirus disease 2019.
Statin vs no statin.
Other includes Asian, Pacific Islander, American Indian, Alaskan Native, or Other.
Statin Use and In‐Hospital Mortality in Patients With Diabetes Mellitus
There was a 49% reduction in hospital mortality (HR, 0.51; 95% CI, 0.43–0.61) associated with stain use among patients with diabetes mellitus admitted with COVID‐19 in the multivariable competing risk regression model (Table 4).
Table 4.
Association Between Statin Use and In‐Hospital Mortality in Patients With Diabetes Mellitus by Crude Analysis, Multivariable Analysis, and Propensity‐Matching Methods
| HR (95% CI) | P Value | |
|---|---|---|
| Regression models with competing risks | ||
| Crude | 0.54 (0.46–0.63) | <0.001 |
| Age adjusted | 0.50 (0.43–0.58) | <0.001 |
| Multivariable adjusted* | 0.51 (0.43–0.61) | <0.001 |
| Propensity analyses † | ||
| Caliper matching | ||
| ATE | 0.87 (0.83–0.91) | <0.001 |
| ATT | 0.88 (0.83–0.94) | <0.001 |
| IPTW | ||
| ATE | 0.88 (0.84–0.91) | <0.001 |
| ATT | 0.88 (0.84–0.92) | <0.001 |
ATE indicates average treatment effect of statins in patients with diabetes mellitus admitted with COVID‐19; ATT, average treatment effect in patients with diabetes mellitus treated with a statin; and IPTW, inverse probability treatment weighing.
The multivariable competing risk regression model was adjusted for age, sex, history of atherosclerotic heart disease, Charlson comorbidity index, presenting diastolic blood pressure, respiratory rate, pulse oximetry measurement, serum glucose, serum lactic acid, serum creatinine, and intravenous antibiotic use during hospitalization.
Covariates in the propensity analyses (caliper matching and IPTW) included: age, sex, body mass index, days of symptoms before admission, history of atherosclerotic heart disease, Charlson comorbidity index, presenting diastolic blood pressure, respiratory rate, pulse oximetry measurement, heart rate, serum glucose, lactic acid, serum creatinine, serum troponin levels, usage of angiotensin converting enzymes inhibitors, usage of angiotensin receptor blockers, serum troponin level, and intravenous antibiotics during hospitalization.
Through PS caliper matching, statin recipients and nonrecipients had similar distributions of PS (Figure S1), and standardized average differences among covariates were greatly reduced (Figure S2). PS analyses with either caliper matching (average treatment effect on the treated: HR, 0.88; 95% CI, 0.83–0.94; P<0.001) or IPTW (average treatment effect on the treated: HR, 0.88; 95% CI, 0.84–0.92; P<0.001) showed a statistically significant 12% reduced risk of in‐hospital mortality in patients with diabetes mellitus receiving statin therapy (Table 4).
Discussion
In this analysis involving a large cohort of hospitalized patients with COVID‐19, statin use was associated with reduced in‐hospital mortality in patients with diabetes mellitus. This observation was made despite older age and higher prevalence of hypertension and atherosclerotic heart disease in statin users with diabetes mellitus. Patients with diabetes mellitus either on or off statin therapy presented to the hospital in a similar time frame from the onset of symptoms; however, those on a statin had lower inflammatory markers. After matching and weighing confounding clinical covariables and indication, there remained an association between statin therapy and reduced in‐hospital mortality.
There is a paucity of studies that have assessed the association between statin use and in‐hospital mortality during COVID‐19. An observational analysis from China reported an association between statin use and improved survival among hospitalized patients with COVID‐19. 18 Unlike this study, our investigation focuses on a cohort of sicker hospitalized patients within the United States, which had notably greater overall in‐hospital mortality. Moreover, in our cohort, we observed higher survival within patients with diabetes mellitus, who may carry mitigating characteristics for statin therapy.
Type 1 diabetes mellitus is as an autoimmune disease with destruction of pancreatic beta cells. During type 1 diabetes mellitus, there is persistent inflammation mediated through interferon gamma, tumor necrosis factor alpha, and interleukin‐1 beta. 19 Type 2 diabetes mellitus is characterized by a chronic low‐grade inflammatory state promoted by a variety of pathways including immune dysregulation, alterations in gut microorganisms, and metabolic syndrome. 20 Patients with diabetes mellitus can have an initial delay in the adaptive immune response to viral infection and may subsequently experience an exaggerated hyperinflammatory response to SARS‐CoV‐2 infection. 21 Indeed, mortality during COVID‐19 is disproportionally elevated in patients with diabetes mellitus, who incur a 2‐ to 3‐fold greater adjusted risk of death, 22 , 23 and early studies have noted higher levels of interleukin‐6 and C‐reactive protein with COVID‐19. 24
Statins are known to have anti‐inflammatory effects that are clinically evident through a reduction in inflammatory biomarkers such as C‐reactive protein, 5 and these effects are also apparent in patients with diabetes mellitus. 25 In our cohort, patients with diabetes mellitus in the statin group presented with lower inflammatory markers and had reduced mortality. In contrast, there was no difference in inflammatory markers at presentation in patients without diabetes mellitus either on or off statins (Table 3). Presuming that in‐hospital statin administration was indicative of outpatient usage and adherence, one might speculate that statin therapy blunted the COVID‐19 inflammatory response before admission as well as during hospitalization, and improved survival was related to ongoing chronic statin use. This hypothesis would have to be prospectively tested. Guidelines recommend statin therapy for adults 40 to 75 years of age with diabetes mellitus to reduce cardiovascular disease, 26 but these agents remain underused. 27 Usage of statins in patients with diabetes mellitus is reported to be low, in the 40% to 50% range, with further underusage in minorities, 27 which was consistent within our cohort. Our findings, if validated, may further strengthen and refocus administration of statins to patients with diabetes mellitus during the COVID‐19 era.
Limitations
This investigation has several limitations. First, given the retrospective nature of our investigation and admission of patients not necessarily followed chronically at our institution, we were unable to firmly differentiate patients who received a statin into subcategories of prehospital user or in‐hospital user and those with cessation of chronic statin use. However, it has not been our institutional practice to initiate new statin therapy in patients with acute COVID‐19 illness. In addition, typical clinical parameters that might lead to discontinuation of statin therapy, such as a significant elevation in liver enzymes including alanine aminotransferase, was neither apparent nor clinically meaningfully different between those who did and did not receive statins. Patients on statins may have unmeasured confounders that could not be assessed through matching and weighting methods. However, such unmeasured confounders would also be present in statin users without diabetes mellitus, who did not show any difference in hospital mortality in comparison to nonusers. The fidelity of the data may be limited by the accuracy and level of documentation within the electronic medical records. Because of the single‐center design, generalizability may be limited. These observational findings do not assess the efficacy of statins as a therapeutic agent for COVID‐19 in patients with diabetes mellitus, which can be accomplished only through a randomized control trial.
Conclusions
In summary, in this observational analysis, statin administration to patients with diabetes mellitus was associated with a reduced risk of in‐hospital mortality during COVID‐19. Further study to validate our findings and investigate underlying mechanisms is needed.
Sources of Funding
Dr Saeed is supported by grants from the National Institutes for Health/National Heart, Lung, and Blood Institute (K23HL145140) and the National Center for Advancing Translational Science Clinical and Translational Science Award at Einstein‐Montefiore (UL1TR001073). Dr Rivas‐Lasarte is supported by the Spanish Society of Cardiology. Dr Jorde is supported by the McAdam Family Foundation.
Disclosures
None.
Supporting information
Figures S1–S2
(J Am Heart Assoc. 2020;9:e018475. DOI: 10.1161/JAHA.120.018475.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.018475
For Sources of Funding and Disclosures, see page 8.
References
- 1. Fauci AS, Lane HC, Redfield RR. Covid‐19—navigating the uncharted. N Engl J Med. 2020;382:1268–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. John Hopkins University . COVID‐19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). 2020.
- 3. Velavan TP, Meyer CG. Mild versus severe COVID‐19: laboratory markers. Int J Infect Dis. 2020;95:304–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG. Reduction in C‐reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet. 2009;373:1175–1182. [DOI] [PubMed] [Google Scholar]
- 6. Fedson DS, Opal SM, Rordam OM. Hiding in plain sight: an approach to treating patients with severe COVID‐19 infection. MBio. 2020;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oesterle A, Laufs U, Liao JK. Pleiotropic effects of statins on the cardiovascular system. Circ Res. 2017;120:229–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stenestrand U, Wallentin L; Care SRoCI . Early statin treatment following acute myocardial infarction and 1‐year survival. JAMA. 2001;285:430–436. [DOI] [PubMed] [Google Scholar]
- 9. Potena L, Frascaroli G, Grigioni F, Lazzarotto T, Magnani G, Tomasi L, Coccolo F, Gabrielli L, Magelli C, Landini MP, et al. Hydroxymethyl‐glutaryl coenzyme a reductase inhibition limits cytomegalovirus infection in human endothelial cells. Circulation. 2004;109:532–536. [DOI] [PubMed] [Google Scholar]
- 10. Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID‐19. Lancet. 2020;395:1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tikoo K, Patel G, Kumar S, Karpe PA, Sanghavi M, Malek V, Srinivasan K. Tissue specific up regulation of ACE2 in rabbit model of atherosclerosis by atorvastatin: role of epigenetic histone modifications. Biochem Pharmacol. 2015;93:343–351. [DOI] [PubMed] [Google Scholar]
- 12. South AM, Diz DI, Chappell MC. COVID‐19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol. 2020;318:H1084–H1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Satagopan J, Ben‐Porat L, Berwick M, Robson M, Kutler D, Auerbach A. A note on competing risks in survival data analysis. Br J Cancer. 2004;91:1229–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gray RJ. A class of K‐sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 15. Austin PC, Fine JP. Propensity‐score matching with competing risks in survival analysis. Stat Med. 2019;38:751–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Stürmer T. Variable selection for propensity score models. Am J Epidemiol. 2006;163:1149–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garrido MM, Kelley AS, Paris J, Roza K, Meier DE, Morrison RS, Aldridge MD. Methods for constructing and assessing propensity scores. Health Serv Res. 2014;49:1701–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang X‐J, Qin J‐J, Cheng X, Shen L, Zhao Y‐C, Yuan Y, Lei F, Chen M‐M, Yang H, Bai L. In‐hospital use of statins is associated with a reduced risk of mortality among individuals with COVID‐19. Cell Metab. 2020;32:176–187.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tsalamandris S, Antonopoulos AS, Oikonomou E, Papamikroulis GA, Vogiatzi G, Papaioannou S, Deftereos S, Tousoulis D. The role of inflammation in diabetes: current concepts and future perspectives. Eur Cardiol. 2019;14:50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guzmán‐Flores JM, López‐Briones S. Cells of innate and adaptive immunity in type 2 diabetes and obesity. Gac Med Mex. 2012;148:381–389. [PubMed] [Google Scholar]
- 21. Muniyappa R, Gubbi S. COVID‐19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318:E736–E741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang I, Lim MA, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID‐19 pneumonia—a systematic review, meta‐analysis, and meta‐regression. Diabetes Metab Syndr. 2020;14:395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guo W, Li M, Dong Y, Zhou H, Zhang Z, Tian C, Qin R, Wang H, Shen Y, Du K. Diabetes is a risk factor for the progression and prognosis of COVID‐19. Diabetes Metab Res Rev. 2020;36:e3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sommeijer DW, MacGillavry MR, Meijers JC, Van Zanten AP, Reitsma PH, Ten Cate H. Anti‐inflammatory and anticoagulant effects of pravastatin in patients with type 2 diabetes. Diabetes Care. 2004;27:468–473. [DOI] [PubMed] [Google Scholar]
- 26. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd‐Jones D, McEvoy JW, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;74:1376–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gu A, Kamat S, Argulian E. Trends and disparities in statin use and low‐density lipoprotein cholesterol levels among US patients with diabetes, 1999–2014. Diabetes Res Clin Pract. 2018;139:1–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1–S2


