Abstract
Background
The long‐term incidence of acute myocardial infarction (AMI) in patients with acute ischemic stroke (AIS) has not been well defined in large cohort studies of various race‐ethnic groups.
Methods and Results
A prospective cohort of patients with AIS who were registered in a multicenter nationwide stroke registry (CRCS‐K [Clinical Research Collaboration for Stroke in Korea] registry) was followed up for the occurrence of AMI through a linkage with the National Health Insurance Service claims database. The 5‐year cumulative incidence and annual risk were estimated according to predefined demographic subgroups, stroke subtypes, a history of coronary heart disease (CHD), and known risk factors of CHD. A total of 11 720 patients with AIS were studied. The 5‐year cumulative incidence of AMI was 2.0%. The annual risk was highest in the first year after the index event (1.1%), followed by a much lower annual risk in the second to fifth years (between 0.16% and 0.27%). Among subgroups, annual risk in the first year was highest in those with a history of CHD (4.1%) compared with those without a history of CHD (0.8%). The small‐vessel occlusion subtype had a much lower incidence (0.8%) compared with large‐vessel occlusion (2.2%) or cardioembolism (2.4%) subtypes. In the multivariable analysis, history of CHD (hazard ratio, 2.84; 95% CI, 2.01–3.93) was the strongest independent predictor of AMI after AIS.
Conclusions
The incidence of AMI after AIS in South Korea was relatively low and unexpectedly highest during the first year after stroke. CHD was the most substantial risk factor for AMI after stroke and conferred an approximate 5‐fold greater risk.
Keywords: acute ischemic stroke, acute myocardial infarction, coronary heart disease, prospective cohort study, risk factors
Subject Categories: Epidemiology, Cerebrovascular Disease/Stroke, Risk Factors, Myocardial Infarction
Nonstandard Abbreviations and Acronyms
- AIS
acute ischemic stroke
- CRCS‐K
Clinical Research Collaboration for Stroke in Korea
- NHIS
National Health Insurance Service
- NOMAS
Northern Manhattan Study
- OxVasc
Oxford Vascular Study
- SPARCL
Stroke Prevention by Aggressive Reduction in Cholesterol Levels
Clinical Perspective
What Is New?
In a multicenter prospective registry of Korean patients with acute ischemic stroke, 5‐year cumulative incidence of acute myocardial infarction was 2%, and was highest during the first year after stroke onset.
Notably, patients with history of coronary heart disease showed a 5‐fold risk of acute myocardial infarction after stroke onset, and those with cardioembolism subtype had a higher risk than other subtypes.
What Are the Clinical Implications?
In general, the risk of acute myocardial infarction after ischemic stroke was low.
However, patients with certain risk factors, such as history of coronary heart disease, need attention during the first year after stroke event.
Ischemic stroke and coronary heart disease (CHD) are the major causes of death worldwide. 1 They share risk factors and strategies for secondary prevention, such as use of antithrombotics and statin therapy. 2 , 3 The coprevalence of CHD has been reported in one fifth of stroke patients. 4
Although the incidence of acute myocardial infarction (AMI) after acute ischemic stroke (AIS) has been reported in several studies, 5 , 6 changes in risk factor profiles and recent advances in secondary prevention strategies may have led to differences in the occurrence of AMI after stroke over time. 7 , 8 , 9 For example, there was no study focusing on the incidence of poststroke AMI after high‐intensity statin was introduced to the clinical practice guideline in year 2008, 10 whereas much lower incidence of AMI could be expected compared with previous reports, according to the use of high‐intensity statin in patients with AIS.
Furthermore, as ischemic stroke is a heterogeneous disorder with diverse causes, 11 patient characteristics, cardiovascular risk profiles, and poststroke prognoses may vary by ischemic stroke subtype. 12 , 13 Thus, one might expect that the risk of AMI in patients with stroke may differ by the aforementioned characteristics. However, there were only few studies with small number of subjects that explored the incidence of AMI according to stroke subtypes. 5 , 14
Determination of the risk and predictors of AMI after AIS is important for the establishment of prevention strategies for both AMI and stroke recurrence. In this context, using a large AIS cohort from a nationwide multicenter stroke registry in South Korea, we estimated the incidence of AMI after stroke, and explored factors that might heighten risk of poststroke AMI.
Methods
Study Population
This study was based on a multicenter prospective stroke registry, the CRCS‐K (Clinical Research Collaboration for Stroke in Korea) registry. 15 , 16 Patients with AIS who were admitted to 14 tertiary or academic hospitals between January 2011 and November 2013 and gave consent for a linkage to secondary administrative data were included. The CRCS‐K registry database was linked to a national claims database of the National Health Insurance Service (NHIS). 17 The NHIS has provided all citizens of South Korea with a universal health insurance program since 1989, and 97.1% of the Korean population is covered by this program. 17 The NHIS claims database contains information on beneficiaries’ demographic characteristics, diagnostic codes, procedures, and prescription records for hospitalization and outpatient care. The claims database was linked to the CRCS‐K registry database using the claim serial number that was generated for each claim at each hospital for the purpose of reimbursement. 18
After the linkage process, patients were selected according to the following inclusion criteria: (1) aged ≥18 years, (2) admission within 7 days after symptom onset, and (3) corresponding stroke lesions documented on brain imaging (magnetic resonance imaging or computed tomography). We excluded those whose stroke subtype was not specified.
Variables
Clinical information on demographics, vascular risk factors, stroke characteristics, and other potential risk factors of CHD was directly obtained from the CRCS‐K registry database. The following vascular risk factors were chosen for study: hypertension, diabetes mellitus, hyperlipidemia, history of CHD, atrial fibrillation, history of stroke or transient ischemia attack, and smoking status. Symptomatic carotid artery disease was defined as stenosis of >50% or occlusion of the proximal internal carotid artery or the common carotid artery ipsilateral to the ischemic lesion documented on magnetic resonance imaging, computed tomography, or conventional cerebral angiography. 19 Initial stroke severity was measured according to the National Institutes of Health Stroke Scale score, and functional status at discharge was assessed with the modified Rankin Scale. Stroke subtypes were determined by vascular neurologists in charge of patients’ treatment, according to the Trial of Org 10172 in Acute Stroke Treatment classification, with some modifications 20 ; the modifications were developed for the purpose of considering magnetic resonance imaging findings and the results of the reperfusion therapy for deciding the cause of ischemic stroke. 20 In addition, the following variables were collected: antiplatelet and statin therapy administration before the index stroke event and at discharge, baseline systolic blood pressure, and laboratory data, such as low‐density lipoprotein (LDL) cholesterol level, high‐density lipoprotein (HDL) cholesterol level, and glomerular filtration rate (GFR). 21
AMI was defined by the admission event claimed under the disease code (International Classification of Diseases, Tenth Revision [ICD‐10]) I21* after index stroke through linkage with the NHIS claims database. The AMI events were further specified as ST‐segment–elevation myocardial infarction (MI) (codes I21.0–I21.3), non–ST‐segment–elevation MI (code I21.4), and fatal MI, if a patient died within 28 days after an AMI event. 22 Data on mortality were obtained from national vital statistics reports.
Ethical Approval
The collection of clinical information and linkage of the information with secondary databases for the purpose of stroke research with informed consent were approved by the local ethic committees at all participating centers. The use of the CRCS‐K registry database and its linkage with the NHIS claims database for this study were approved further by the Institutional Review Board of Seoul National University Bundang Hospital (No. B‐1511/322–106).
The data will not be available to other researchers for the purpose of reproducing the results because of local legal regulations regarding access to patient‐level data.
Statistical Analysis
The baseline characteristics of study subjects were summarized as frequencies and percentages for categorical variables and as means with SDs for continuous variables. If a variable was recorded in form of a scale (eg, National Institutes of Health Stroke Scale or modified Rankin Scale) or skewed, it was summarized as the median and interquartile range.
The cumulative incidence of AMI up to 5 year after the index stroke was estimated using the cumulative incidence function, with mortality treated as a competing risk for AMI, and is displayed on a yearly basis. The cumulative incidence of AMI was determined for all study subjects according to predetermined subgroups: aged ≤70 versus >70 years, sex, history of hypertension, diabetes mellitus, CHD, and atrial fibrillation, presence of symptomatic carotid artery disease, and stroke subtype. The differences between subgroups were evaluated using the Grey test.
To determine the time trend of poststroke AMI incidence and to identify the point of the highest risk of AMI, the annual risk of AMI after index stroke was calculated on the basis of the survival estimates obtained using the Grey method for all study subjects and via a post hoc analysis in subgroups according to history of CHD, stroke subtype, history of atrial fibrillation, and presence of symptomatic carotid artery disease. 23 For the first year, the monthly risk was calculated using the same method.
A Cox proportional hazards regression analysis was performed to explore risk factors of AMI after AIS. The following predetermined covariates were entered into the models: age, sex, vascular risk factors listed above, antiplatelet and statin use before index stroke, systolic blood pressure, and laboratory data, including LDL cholesterol, HDL cholesterol, and GFR. To include a competing risk analysis into the Cox models, a subdistribution hazard model using the Fine and Grey method was adopted; a cause‐specific hazard model treating the competing events as censored observations is also presented as supporting data. 24 Age, LDL cholesterol, HDL cholesterol, GFR, and systolic blood pressure were dichotomized as follows: aged ≤70 versus >70 years, LDL cholesterol <100 versus ≥100 mg/dL, HDL cholesterol <40 versus ≥40 mg/dL, and GFR <60 versus ≥60 mL/min per 1.73 m2. Considering multicollinearity, stroke subtype and symptomatic carotid artery disease/atrial fibrillation were alternatively introduced into the multivariable models.
All tests were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC), and statistical significance was declared with a 2‐sided P value of <0.05.
Results
Of 15,114 patients with AIS who were registered in the database during the study period and provided consent, 11 720 were analyzed for the study (Figure S1). The mean age was 67 years, and 59% were men. Two thirds of the study subjects had hypertension, and one third had diabetes mellitus or hyperlipidemia. Of the subjects, 9% had a history of CHD and 11% had symptomatic carotid artery disease. As for stroke subtypes, 37% had large‐artery atherosclerosis, 18% had small‐vessel occlusion, and 21% had cardioembolism. About 14% of the patients were using statin before index stroke event, and 82% were prescribed statin at discharge (Table 1). 25
Table 1.
Baseline Characteristics of the Study Subjects (N=11 720)
| Characteristics | Values |
|---|---|
| Age, mean±SD, y | 67.53±12.87 |
| Men, N (%) | 6888 (58.8) |
| Hypertension, N (%) | 7917 (67.6) |
| Diabetes mellitus, N (%) | 3834 (32.7) |
| Hyperlipidemia, N (%) | 3776 (32.2) |
| Previous stroke or TIA, N (%) | 2254 (19.2) |
| Current smoking, N (%) | 3099 (26.4) |
| Symptomatic carotid artery disease, N (%) | 1268 (10.8) |
| Atrial fibrillation, N (%) | 2450 (20.9) |
| Coronary heart disease, N (%) | 1067 (9.1) |
| Stroke subtype, N (%) | |
| Large‐artery atherosclerosis | 4322 (36.9) |
| Small‐vessel occlusion | 2070 (17.7) |
| Cardioembolism | 2506 (21.4) |
| Other determined* | 254 (2.2) |
| Undetermined † | 2568 (21.9) |
| Initial NIHSS, median (IQR) | 3 (1–8) |
| Discharge mRS score, median (IQR) | 2 (1–4) |
| Previous antiplatelet use, N (%) | 3295 (28.1) |
| Previous statin use, N (%) | 1690 (14.4) |
| Antiplatelet use at discharge, N (%) | 9097 (77.6) |
| Statin use at discharge, N (%) | 9652 (82.4) |
| High‐intensity statin use at discharge, N (%) ‡ | 3153 (26.9) |
| SBP, mean±SD, mm Hg | 147.26±27.29 |
| LDL cholesterol, mean±SD, mg/dL | 111.62±36.43 |
| HDL cholesterol, mean±SD, mg/dL | 44.48±12.11 |
| GFR, mean±SD, mL/min per 1.73 m2 | 102.48±46.42 |
GFR indicates glomerular filtration rate; HDL, high‐density lipoprotein; IQR, interquartile range; LDL, low‐density lipoprotein; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; SBP, systolic blood pressure; and TIA, transient ischemic attack.
Other determined cause was defined as rare causes of ischemic stroke with diverse entities (details in study by Ko et al 20 ).
Undetermined cause was defined as follows: (1) not being classified as large‐artery atherosclerosis, small‐vessel disease, or cardioembolism and no cardioembolic source despite comprehensive workups; (2) no workup for stroke cause; or (3) coexistence of >1 stroke cause.
According to the American College of Cardiology/American Heart Association Guideline on Treatment of Blood Cholesterol (2013) (≥50% reduction in LDL cholesterol).
The median follow‐up duration was 4.0 years (interquartile range, 3.2–4.9 years), and AMI occurred in 218 patients (1.9%), while 3270 patients (27.9%) died during this period. The cumulative incidence of AMI was 1.1% at 1 year and 2.0% at 5 years (Table 2 and Figure S2). Among the AMI events, there were 69 (31.7%) ST‐segment–elevation MIs and 52 (23.9%) non–ST‐segment–elevation MIs, whereas other 97 (44.5%) were not specified as either ST‐segment–elevation MI or non–ST‐segment–elevation MI. AMI events were fatal in 33 patients (15.1%) (Table S1). Ninety‐six (44.0%) patients were admitted to the same hospital, where they were treated for index stroke. The subgroup analysis showed the greatest difference in 5‐year cumulative incidence between those with and without a history of CHD (5.3% versus 1.6%; P<0.001), followed by those with and without symptomatic carotid artery disease (3.1% versus 1.8%; P=0.007). There was no significant difference between men and women. The patients with cardioembolism (2.38%), undetermined cause (2.23%), and large‐artery atherosclerosis (2.18%) had a similar 5‐year cumulative incidence, whereas those with small‐vessel occlusion had a much lower cumulative incidence (0.84%; P=0.001) (Table 2 and Figure 1). The 5‐year cumulative incidence of AMI was higher in ex‐smokers (2.91%) than in current smokers (1.92%) and nonsmokers (1.79%; P=0.012) (Figure S3).
Table 2.
Cumulative Incidences of AMI After AIS for All Subgroups
| Variable | Cumulative Incidence, % (95% CI) | P Value* | ||||
|---|---|---|---|---|---|---|
| 1 y | 2 y | 3 y | 4 y | 5 y | ||
| Total all patients | 1.07 (0.89–1.27) | 1.29 (1.10–1.51) | 1.56 (1.35–1.80) | 1.81 (1.58–2.07) | 1.97 (1.72–2.26) | |
| Aged ≤70 y | 0.85 (0.65–1.11) | 1.05 (0.82–1.33) | 1.26 (1.00–1.56) | 1.42 (1.15–1.75) | 1.61 (1.30–1.98) | 0.0022 |
| Aged >70 y | 1.31 (1.03–1.63) | 1.56 (1.26–1.91) | 1.90 (1.57–2.29) | 2.25 (1.87–2.67) | 2.38 (1.98–2.83) | |
| Women | 1.08 (0.81–1.40) | 1.24 (0.96–1.59) | 1.47 (1.16–1.84) | 1.67 (1.33–2.07) | 1.85 (1.47–2.29) | 0.41 |
| Men | 1.06 (0.84–1.32) | 1.32 (1.07–1.61) | 1.63 (1.35–1.95) | 1.91 (1.60–2.26) | 2.06 (1.73–2.44) | |
| No history of hypertension | 0.58 (0.38–0.86) | 0.71 (0.48–1.02) | 0.89 (0.63–1.23) | 1.06 (0.76–1.43) | 1.23 (0.89–1.67) | <0.001 |
| History of hypertension | 1.30 (1.07–1.57) | 1.57 (1.31–1.86) | 1.88 (1.60–2.20) | 2.17 (1.86–2.52) | 2.33 (2.00–2.70) | |
| No history of diabetes mellitus | 0.82 (0.64–1.04) | 0.95 (0.76–1.18) | 1.18 (0.96–1.44) | 1.40 (1.16–1.69) | 1.51 (1.24–1.82) | <0.001 |
| History of diabetes mellitus | 1.56 (1.21–2.00) | 1.98 (1.58–2.46) | 2.35 (1.90–2.86) | 2.65 (2.17–3.20) | 2.65 (2.17–3.20) | |
| No history of CHD | 0.76 (0.61–0.94) | 1.00 (0.82–1.20) | 1.24 (1.04–1.46) | 1.46 (1.24–1.71) | 1.64 (1.39–1.92) | <0.001 |
| History of CHD | 4.12 (3.05–5.44) | 4.22 (3.13–5.54) | 4.78 (3.61–6.18) | 5.32 (4.07–6.79) | 5.32 (4.07–6.79) | |
| Stroke subtype | 0.001 | |||||
| Large‐artery atherosclerosis | 0.97 (0.71–1.30) | 1.13 (0.85–1.49) | 1.46 (1.13–1.85) | 1.82 (1.45–2.26) | 2.18 (1.73–2.71) | |
| Small‐vessel occlusion | 0.29 (9.12–0.61) | 0.53 (0.29–0.93) | 0.73 (0.43–1.17) | 0.84 (0.51–1.32) | 0.84 (0.51–1.32) | |
| Cardioembolism | 1.56 (1.13–2.10) | 1.84 (1.36–2.42) | 2.08 (1.57–2.69) | 2.31 (1.77–2.97) | 2.38 (1.82–3.04) | |
| Other determined | 0.79 (0.16–2.62) | 0.79 (0.16–2.62) | 1.18 (0.33–3.20) | 1.18 (0.33–3.20) | 1.18 (0.33–3.20) | |
| Undetermined | 1.40 (1.00–1.92) | 1.67 (1.23–2.23) | 1.95 (1.47–2.54) | 2.15 (1.63–2.77) | 2.23 (1.69–2.88) | |
| No symptomatic carotid artery disease | 0.98 (0.80–1.18) | 1.21 (1.01–1.43) | 1.47 (1.26–1.72) | 1.70 (1.46–1.97) | 1.84 (1.58–2.13) | 0.007 |
| Symptomatic carotid artery disease | 1.81 (1.18–2.67) | 1.97 (1.31–2.85) | 2.29 (1.57–3.22) | 2.73 (1.93–3.75) | 3.06 (2.16–4.20) | |
| No history of atrial fibrillation | 0.90 (0.72–1.10) | 1.11 (0.91–1.34) | 1.37 (1.15‐1.62) | 1.64 (1.39‐1.92) | 1.83 (1.55‐2.14) | 0.01 |
| History of atrial fibrillation | 1.71 (1.26‐2.29) | 1.96 (1.47‐2.57) | 2.29 (1.75‐2.94) | 2.47 (1.90‐3.14) | 2.53 (1.96‐3.22) | |
AIS indicates acute ischemic stroke; AMI, acute myocardial infarction; and CHD, coronary heart disease.
P values determined with the Grey test.
Figure 1. Cumulative incidence of acute myocardial infarction after ischemic stroke among subgroups.
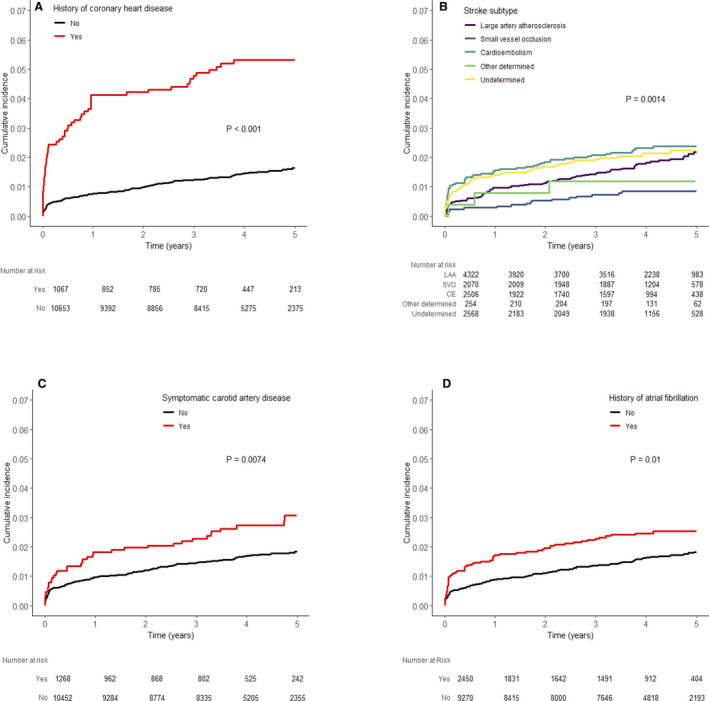
A, History of coronary artery disease. B, Stroke subtype. C, Symptomatic carotid artery disease. D, Atrial fibrillation. CE, cardioembolism; LAA, large artery atherosclerosis; SVO, small vessel occlusion.
The annual risk was highest in the first year after the index stroke: 1.1% in the first year, followed by 0.2%, 0.3%, 0.3%, and 0.2% in the second, third, fourth, and fifth years, respectively. When we focused on the first year, the monthly risk was highest in the first month (0.51%), followed by the second month (0.13%), and then was <0.1% in the third month and afterwards (Figure S4). The annual risk in patients with history of CHD in the first year was 4.1%, which was much higher than the risk in the following years and in those without a history of CHD. The annual risk in the first year was lowest in patients with small‐vessel occlusion (0.3%) compared with those with cardioembolism (1.6%), undetermined stroke cause (1.4%), and large‐artery occlusion (1.0%). Also, those with symptomatic carotid artery disease (1.8%) and atrial fibrillation (1.7%) had higher annual risk in the first year than those without the conditions (Figure 2).
Figure 2. Annual risk of acute myocardial infarction after index stroke event.
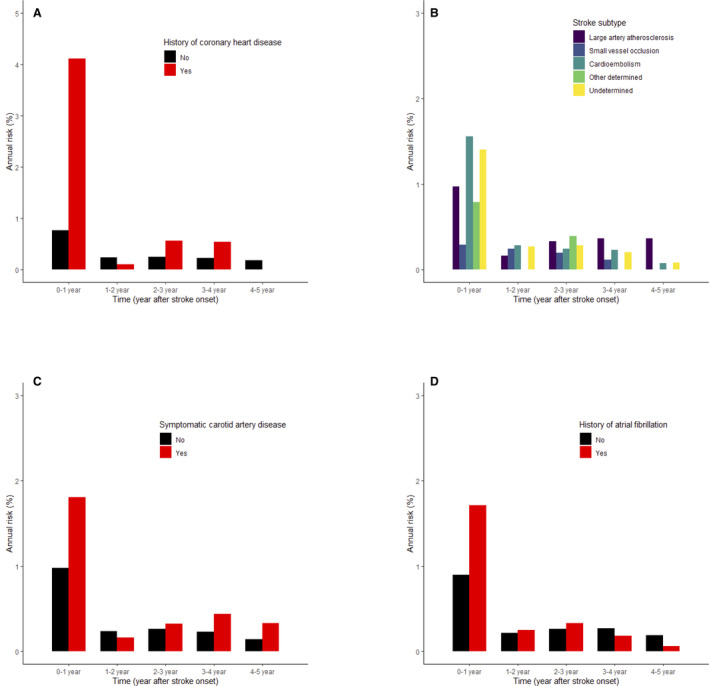
A, History of coronary artery disease. B, Stroke subtype. C, Symptomatic carotid artery disease. D, Atrial fibrillation.
In the multivariable analysis, a history of CHD was the strongest predictor of poststroke AMI (Table 3). Also, among stroke subtypes, cardioembolism, followed by undetermined cause and large‐artery atherosclerosis, was associated with a higher risk of poststroke AMI compared with small‐vessel occlusion. Other cardiovascular risk factors, such as hypertension, diabetes mellitus, and history of stroke or transient ischemia attack, also increased the risk of poststroke AMI. Age >70 years increased the risk in the cause‐specific hazard model, but not in the subdistribution hazard model (Table S2). In addition, when atrial fibrillation and symptomatic carotid artery disease were entered into the models as an alternative to stroke subtype, they increased the risk in the cause‐specific hazard models, but did not in the subdistribution hazard models (Table S3). The results of when age, systolic blood pressure, LDL cholesterol, HDL cholesterol, and GFR systolic are treated as continuous variables are provided in Tables S4 and S5.
Table 3.
Predictors of AMI Up to 5 Years After AIS
| Variable | Hazard Ratio (95% CI) | P Value* |
|---|---|---|
| Aged >70 y | 1.33 (0.995‐1.78) | 0.054 |
| Male sex | 1.16 (0.85‐1.58) | 0.36 |
| Hypertension | 1.50 (1.05‐2.14) | 0.03 |
| Diabetes mellitus | 1.72 (1.31‐2.26) | <.001 |
| Previous stroke or TIA | 1.41 (1.03‐1.93) | 0.06 |
| Coronary heart disease | 2.84 (2.01‐3.93) | <.001 |
| Current smoker | 1.07 (0.75‐1.53) | 0.69 |
| Stroke subtype | 0.015 | |
| Large‐artery atherosclerosis | 2.03 (1.20‐3.44) | |
| Small‐vessel occlusion | Reference | |
| Cardioembolism | 2.60 (1.44‐4.70) | |
| Other determined | 1.76 (0.51‐6.04) | |
| Undetermined | 2.55 (1.47‐4.41) | |
| Antiplatelet use at discharge | 1.15 (0.79‐1.68) | 0.45 |
| Statin use at discharge | 1.20 (0.82‐1.75) | 0.35 |
| LDL‐C ≥100 mg/dL | 1.18 (0.88‐1.58) | 0.27 |
| HDL‐C <40 mg/dL | 1.23 (0.94‐1.61) | 0.13 |
| SBP ≥140 mm Hg | 0.81 (0.62‐1.06) | 0.13 |
| GFR <60 mL/min per 1.73 m2 | 1.21 (0.85‐1.70) | 0.29 |
AIS indicates acute ischemic stroke; AMI, acute myocardial infarction; GFR, glomerular filtration rate; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; and TIA, transient ischemic attach.
Subdistribution hazard model by Fine and Grey.
Discussion
Using a recent large cohort of patients with AIS from a nationwide multicenter stroke registry in South Korea, we report on the 5‐year incidence of poststroke AMI. Among various subgroups, those of older age and those with vascular risk factors, especially a history of CHD, had a higher cumulative incidence up to 5 years after AIS. The incidence also differed according to stroke subtype; those with small‐vessel occlusion had lower incidence compared with those with cardioembolism, undetermined cause, and large‐artery atherosclerosis. The risk of AMI was highest in the first year after AIS.
Several previous studies have reported incidence of AMI after stroke. 5 , 6 However, these studies were limited by small sample size or not being confined to patients with AIS. 14 , 26 , 27 , 28 A recent meta‐analysis of >130 000 patients showed that the annual risk of poststroke AMI is 1.67%. 5 However, this meta‐analysis reported on patient data from observational studies and clinical trials of diverse populations, and follow‐up durations and definitions of AMI across those studies differed. In addition, the study could not examine whether the incidence of AMI varied by stroke subtype because of a lack of such data. In contradistinction, our study analyzed the incidence of poststroke AMI using real‐world data starting at stroke onset, and the analysis was stratified by various subgroups, including the following factors: demographics, vascular risks, and stroke subtype.
We found a generally low risk of AMI, with the annual risk <1%, except in study subjects with known CHD during the first year after AIS. This is lower than what was reported in previous studies. 6 , 14 , 26 , 29 The recent meta‐analysis, however, showed a continuous decrease in the incidence of poststroke AMI, 5 which might be attributed to improvement in poststroke management, including administration of statin. It is noteworthy that our study was conducted after the SPARCL (Stroke Prevention by Aggressive Reduction in Cholesterol Levels) trial, which demonstrated the beneficial effect of high‐intensity statin in a predominantly AIS population. 30 More than 80% of our study subjects were on statin therapy at hospital discharge, even though only 30% were diagnosed as having hyperlipidemia. There was a 68% increase in statin use after stroke; only 14% were on statin before index stroke (Table 1). A secondary analysis of the SPARCL trial showed that patients randomized to high‐intensity statin experienced coronary events less frequently compared with those randomized to placebo, regardless of stroke subtype, and the reduction in coronary events was greater than that for stroke events. 31
Thus, it may not be surprising that our study showed a much lower incidence of AMI than previous studies. For example, the aforementioned meta‐analysis included only 5 of 58 studies that enrolled study subjects during the period after the publication of the SPARCL trial in 2006. 5 Despite the overall low incidence of poststroke AMI in our study, the high risk of AMI in the first year after AIS and in those with a history of CHD suggests that it may be prudent to focus surveillance and preventative cardiovascular therapy for AMI on those with a history of CHD and certain vascular risk factors or specific stroke subtypes, as noted in our study.
The incidence of AMI was highest in the first year and especially during the first 2 months after AIS (Figure S4). Higher recurrence rate during the early period after index event is shown in patients with AMI or stroke. 12 , 32 , 33 Not sufficiently controlled vascular risk factors or comorbidities during the early period after index event and ongoing disease process, such as atherosclerosis or inflammation, provoked by index event could be explainable mechanisms. 32 , 34 , 35 Also, poststroke cardiac arrhythmias could be another possible cause of AMI after AIS. 36
The incidence of AMI in East Asia, for example, may be lower than in traditional Western countries. 37 The estimated age‐standardized incidence rate of hospitalized MI was 39 cases per 100 000 person‐years in 2011 in Korea, 38 which was much lower than that of 677 per 100 000 person‐years in the same year in the United States. 39 Differences in dietary and lifestyle factors may explain, at least in part, the lower incidence of AMI in East Asian countries. 37 Our results provide valuable race‐ethnic observations on East Asian patients with stroke.
Current smokers seemed not to have higher incidence of AMI after AIS in our study (Table 3 and Figure S3). This could be explained in line with the well‐known “smokers’ paradox” of better prognosis in smokers than nonsmokers. 40 This phenomenon is usually explained as current smokers are much younger and healthier than nonsmokers at the time of index event, and we were also able to find in our study that current smokers were much younger compared with ex‐smokers or nonsmokers (age, 60.6±12.4 versus 69.2±11.2 versus 70.2±12.3 years in current smokers, ex‐smokers, and nonsmokers, respectively).
Our study results are similar to those of earlier work. First, the 10‐year risk of AMI after ischemic stroke or transient ischemia attack in the OxVasc (Oxford Vascular Study) cohort, with a median follow‐up duration of 5.6 years, was much higher in those with CHD. 29 However, the 10‐year risk in those without CHD was about 8%, which may not be concordant with our results. This might be explained by the older age of the non‐CHD population (mean age, 73 years) and the earlier enrollment epoch (from 2002) in the OxVasc. Second, patients with embolic stroke had a significantly higher risk of poststroke AMI in the NOMAS (Northern Manhattan Study). 14 , 26 Atrial fibrillation is known to be a risk factor of AMI, as atrial fibrillation may cause thromboembolism, and high heart rates evoked by atrial fibrillation might impair coronary perfusion and lead to demand (type 2) MI. 41 The comparable risk in those with stroke of undetermined cause could be explained by the coexistence of large‐artery atherosclerosis and cardioembolic sources and the potential for the existence of undetected (ie, subclinical) AF in this stroke subtype. 11 The recent widespread use of non–vitamin K oral anticoagulants seemed to have a little effect on the AMI incidence in this study as they were not allowed for preventing atrial fibrillation–related stroke in most cases until the middle of 2015 in Korea. 42 Third, the risk of poststroke AMI was highest in the first year after index stroke in a study performed by the Virtual International Stroke Trials Archive collaboration, 34 although the aforementioned recent meta‐analysis did not show any time trend. 5 This discrepancy may be explained by the fact that our study and the study by the Virtual International Stroke Trials Archive collaboration included only patients with AIS and started to capture AMI from the early period after stroke onset, whereas, among 58 studies included in the recent meta‐analysis, 15 studies confined study subjects to AIS and 9 to those enrolled within 7 days from onset.
Our study has limitations. First, as AMI events were captured using the diagnosis code of the claims data, the incidence of AMI could be underestimated or overestimated. Previous validation studies in South Korea, however, reported that the accuracy of the diagnosis code for AMI was >70%. 43 , 44 Second, the study subjects were South Koreans who were admitted to academic or tertiary stroke centers, which may limit the generalizability of the study results. Third, AMI events occurring during hospitalization and associated with the index stroke were not included. Fourth, because of the Personal Information Protection Act, only the patients who gave their informed consent for a linkage between the registry and the secondary administrative databases were included in this study. Although the consent rate was as high as 92.4%, 45 there could be a possible selection bias, such as the fact that those who were more disabled or died during hospitalization were not included in our study.
To date, our study is the single largest one reporting on the incidence of AMI in a single race‐ethnic group over a relatively long period of time after AIS. Although the general incidence of poststroke AMI seems to be low, individuals with a history of CHD have a higher risk and thus may require more surveillance and preventative attention, especially during the first year after stroke.
Sources of Funding
This report was supported partially by funding from the research of Korea Centers for Disease Control and Prevention (code 2017ER620102).
Disclosures
None.
Supporting information
CRCS‐K Registry Investigators
Tables S1–S5
Figures S1–S4
Acknowledgments
We would like to thank the National Health Insurance Service (NHIS) for giving us the opportunity to analyze the claims data (national health information data; NHIS‐2015‐4‐022‐1) for this study.
(J Am Heart Assoc. 2021;10:e018807. DOI: 10.1161/JAHA.120.018807.)
For Sources of Funding and Disclosures, see page 9.
References
- 1. Roth GA, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, Abbastabar H, Abd‐Allah F, Abdela J, Abdelalim A, et al. Global, regional, and national age‐sex‐specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. DOI: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, Fang MC, Fisher M, Furie KL, Heck DV, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160–2236. DOI: 10.1161/STR.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 3. Smith SC, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, Gibbons RJ, Grundy SM, Hiratzka LF, Jones DW, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124:2458–2473. DOI: 10.1161/CIR.0b013e318235eb4d. [DOI] [PubMed] [Google Scholar]
- 4. Calvet D, Touzé E, Varenne O, Sablayrolles JL, Weber S, Mas JL. Prevalence of asymptomatic coronary artery disease in ischemic stroke patients: the Precoris Study. Circulation. 2010;121:1623–1629. DOI: 10.1161/CIRCULATIONAHA.109.906958. [DOI] [PubMed] [Google Scholar]
- 5. Boulanger M, Béjot Y, Rothwell PM, Touzé E. Long‐term risk of myocardial infarction compared to recurrent stroke after transient ischemic attack and ischemic stroke: systematic review and meta‐analysis. J Am Heart Assoc. 2018;7:e007267. DOI: 10.1161/JAHA.117.007267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Touzé E, Varenne O, Chatellier G, Peyrard S, Rothwell PM, Mas JL. Risk of myocardial infarction and vascular death after transient ischemic attack and ischemic stroke: a systematic review and meta‐analysis. Stroke. 2005;36:2748–2755. DOI: 10.1161/01.STR.0000190118.02275.33. [DOI] [PubMed] [Google Scholar]
- 7. Rosenson RS, Farkouh ME, Mefford M, Bittner V, Brown TM, Taylor B, Monda KL, Zhao H, Dai Y, Muntner P. Trends in use of high‐intensity statin therapy after myocardial infarction, 2011 to 2014. J Am Coll Cardiol. 2017;69:2696–2706. [DOI] [PubMed] [Google Scholar]
- 8. Otite FO, Liaw N, Khandelwal P, Malik AM, Romano JG, Rundek T, Sacco RL, Chaturvedi S. Increasing prevalence of vascular risk factors in patients with stroke: a call to action. Neurology. 2017;89:1985–1994. DOI: 10.1212/WNL.0000000000004617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bogiatzi C, Hackam DG, McLeod AI, Spence JD. Secular trends in ischemic stroke subtypes and stroke risk factors. Stroke. 2014;45:3208–3213. DOI: 10.1161/STROKEAHA.114.006536. [DOI] [PubMed] [Google Scholar]
- 10. Adams RJ, Albers G, Alberts MJ, Benavente O, Furie K, Goldstein LB, Gorelick P, Halperin J, Harbaugh R, Johnston SC, et al. Update to the AHA/ASA recommendations for the prevention of stroke in patients with stroke and transient ischemic attack. Stroke. 2008;39:1647–1652. DOI: 10.1161/STROKEAHA.107.189063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE. Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial: TOAST: Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. DOI: 10.1161/01.STR.24.1.35. [DOI] [PubMed] [Google Scholar]
- 12. Kang K, Park TH, Kim N, Jang MU, Park SS, Park JM, Ko Y, Lee SJ, Lee KB, Lee J, et al. Recurrent stroke, myocardial infarction, and major vascular events during the first year after acute ischemic stroke: the multicenter prospective observational study about recurrence and its determinants after acute ischemic stroke I. J Stroke Cerebrovasc Dis. 2016;25:656–664. [DOI] [PubMed] [Google Scholar]
- 13. Grau AJ, Weimar C, Buggle F, Heinrich A, Goertler M, Neumaier S, Glahn J, Brandt T, Hacke W, Diener HC. Risk factors, outcome, and treatment in subtypes of ischemic stroke: the German stroke data bank. Stroke. 2001;32:2559–2566. DOI: 10.1161/hs1101.098524. [DOI] [PubMed] [Google Scholar]
- 14. Dhamoon MS, Tai W, Boden‐Albala B, Rundek T, Paik MC, Sacco RL, Elkind MSV. Risk of myocardial infarction or vascular death after first ischemic stroke: the northern Manhattan study. Stroke. 2007;38:1752–1758. DOI: 10.1161/STROKEAHA.106.480988. [DOI] [PubMed] [Google Scholar]
- 15. Kim BJ, Park JM, Kang K, Lee SJ, Ko Y, Kim JG, Cha JK, Kim DH, Nah HW, Han MK, et al. Case characteristics, hyperacute treatment, and outcome information from the clinical research center for stroke‐fifth division registry in South Korea. J Stroke. 2015;17:38–53. DOI: 10.5853/jos.2015.17.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim BJ, Han MK, Park TH, Park SS, Lee KB, Lee BC, Yu KH, Cha JK, Kim DH, Lee J, et al. Current status of acute stroke management in Korea: a report on a multicenter, comprehensive acute stroke registry. Int J Stroke. 2014;9:514–518. DOI: 10.1111/ijs.12199. [DOI] [PubMed] [Google Scholar]
- 17. Seong SC, Kim YY, Khang YH, Park JH, Kang HJ, Lee H, Do CH, Song JS, Bang JH, Ha S, et al. Data resource profile: the national health information database of the national health insurance service in South Korea. Int J Epidemiol. 2017;46:799–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee KB, Lee JG, Kim BJ, Kim JY, Lee KJ, Han MK, Park JM, Kang K, Cho YJ, Park HK, et al. The epidemiology of fracture in patients with acute ischemic stroke in Korea. J Korean Med Sci. 2019;34:e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Anderson GB, Ashforth R, Steinke DE, Ferdinandy R, Findlay JM. CT angiography for the detection and characterization of carotid artery bifurcation disease. Stroke. 2000;31:2168–2174. DOI: 10.1161/01.STR.31.9.2168. [DOI] [PubMed] [Google Scholar]
- 20. Ko Y, Lee SJ, Chung JW, Han MK, Park JM, Kang K, Park TH, Park SS, Cho YJ, Kong KS, et al. MRI‐based algorithm for acute ischemic stroke subtype classification. J Stroke. 2014;16:161. DOI: 10.5853/jos.2014.16.3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D; Modification of Diet in Renal Disease Study Group . A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 22. Asaria P, Elliott P, Douglass M, Obermeyer Z, Soljak M, Majeed A, Ezzati M. Acute myocardial infarction hospital admissions and deaths in England: a national follow‐back and follow‐forward record‐linkage study. Lancet Public Health. 2017;2:e191–e201. DOI: 10.1016/S2468-2667(17)30032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rutten‐Jacobs LCA, Maaijwee NAM, Arntz RM, Schoonderwaldt HC, Dorresteijn LD, Van der Vlugt MJ, Van Dijk EJ, De Leeuw FE. Long‐term risk of recurrent vascular events after young stroke: the FUTURE study. Ann Neurol. 2013;74:592–601. [DOI] [PubMed] [Google Scholar]
- 24. Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133:601–609. DOI: 10.1161/CIRCULATIONAHA.115.017719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd‐Jones DM, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:1–45. DOI: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 26. Dhamoon MS, Sciacca RR, Rundek T, Sacco RL, Elkind MSV. Recurrent stroke and cardiac risks after first ischemic stroke: the Northern Manhattan Study. Neurology. 2006;66:641–646. DOI: 10.1212/01.wnl.0000201253.93811.f6. [DOI] [PubMed] [Google Scholar]
- 27. Amarenco P, Lavallée PC, Labreuche J, Ducrocq G, Juliard JM, Feldman L, Cabrejo L, Meseguer E, Guidoux C, Adraï V, et al. Coronary artery disease and risk of major vascular events after cerebral infarction. Stroke. 2013;44:1505–1511. DOI: 10.1161/STROKEAHA.111.000142. [DOI] [PubMed] [Google Scholar]
- 28. Venketasubramanian N, Röther J, Bhatt DL, Pasquet B, Mas JL, Alberts MJ, Hill MD, Aichner F, Steg PG. Two‐year vascular event rates in patients with symptomatic cerebrovascular disease: the reach registry. Cerebrovasc Dis. 2011;32:254–260. [DOI] [PubMed] [Google Scholar]
- 29. Boulanger M, Li L, Lyons S, Lovett NG, Kubiak MM, Silver L, Touzé E, Rothwell PM. Essen risk score in prediction of myocardial infarction after transient ischemic attack or ischemic stroke without prior coronary artery disease. Stroke. 2019;50:3393–3399. DOI: 10.1161/STROKEAHA.119.025831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Amarenco P, Bogousslavsky J, Callahan A, Goldstein LB, Hennerici M, Rudolph AE, Sillesen H, Simunovic L, Szarek M, Welch KMA, et al. High‐dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–559. [DOI] [PubMed] [Google Scholar]
- 31. Amarenco P, Goldstein LB, Sillesen H, Benavente O, Zweifler RM, Callahan A, Hennerici MG, Zivin JA, Welch KMA. Coronary heart disease risk in patients with stroke or transient ischemic attack and no known coronary heart disease: findings from the stroke prevention by aggressive reduction in cholesterol levels (SPARCL) trial. Stroke. 2010;41:426–430. DOI: 10.1161/STROKEAHA.109.564781. [DOI] [PubMed] [Google Scholar]
- 32. Smolina K, Wright FL, Rayner M, Goldacre MJ. Long‐term survival and recurrence after acute myocardial infarction in England, 2004 to 2010. Circ Cardiovasc Qual Outcomes. 2012;5:532–540. [DOI] [PubMed] [Google Scholar]
- 33. Chen Y, Wright N, Guo Y, Turnbull I, Kartsonaki C, Yang L, Bian Z, Pei P, Pan D, Zhang Y, et al. Mortality and recurrent vascular events after first incident stroke: a 9‐year community‐based study of 0·5 million Chinese adults. Lancet Glob Health. 2020;8:e580–e590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Prosser J, MacGregor L, Lees KR, Diener HC, Hacke W, Davis S. Predictors of early cardiac morbidity and mortality after ischemic stroke. Stroke. 2007;38:2295–2302. DOI: 10.1161/STROKEAHA.106.471813. [DOI] [PubMed] [Google Scholar]
- 35. Wang H, Eitzman DT. Acute myocardial infarction leads to acceleration of atherosclerosis. Atherosclerosis. 2013;229:18–22. DOI: 10.1016/j.atherosclerosis.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kallmünzer B, Breuer L, Kahl N, Bobinger T, Raaz‐Schrauder D, Huttner HB, Schwab S, Köhrmann M. Serious cardiac arrhythmias after stroke: incidence, time course, and predictors‐a systematic, prospective analysis. Stroke. 2012;43:2892–2897. DOI: 10.1161/STROKEAHA.112.664318. [DOI] [PubMed] [Google Scholar]
- 37. Ueshima H, Sekikawa A, Miura K, Turin TC, Takashima N, Kita Y, Watanabe M, Kadota A, Okuda N, Kadowaki T, et al. Cardiovascular disease and risk factors in Asia: a selected review. Circulation. 2008;118:2702–2709. DOI: 10.1161/CIRCULATIONAHA.108.790048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim RB, Kim HS, Kang DR, Choi JY, Choi NC, Hwang S, Hwang JY. The trend in incidence and case‐fatality of hospitalized acute myocardial infarction patients in Korea, 2007 to 2016. J Korean Med Sci. 2019;34:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sacks NC, Ash AS, Ghosh K, Rosen AK, Wong JB, Rosen AB. Trends in acute myocardial infarction hospitalizations: are we seeing the whole picture? Am Heart J. 2015;170:1211–1219. DOI: 10.1016/j.ahj.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Aune E, Røislien J, Mathisen M, Thelle DS, Otterstad JE. The “smoker’s paradox” in patients with acute coronary syndrome: a systematic review. BMC Med. 2011;9:97. DOI: 10.1186/1741-7015-9-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Soliman EZ, Safford MM, Muntner P, Khodneva Y, Dawood FZ, Zakai NA, Thacker EL, Judd S, Howard VJ, Howard G, et al. Atrial fibrillation and the risk of myocardial infarction. JAMA Intern Med. 2014;174:107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ko YJ, Kim S, Park K, Kim M, Yang BR, Kim MS, Lee J, Park BJ. Impact of the health insurance coverage policy on oral anticoagulant prescription among patients with atrial fibrillation in Korea from 2014 to 2016. J Korean Med Sci. 2018;33:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim H, Yun JE, Lee SH, Jang Y, Jee SH. Validity of the diagnosis of acute myocardial infarction in Korean National Medical Health Insurance claims data: the Korean Heart Study. Korean Circ J. 2012;42:10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ryu SY, Park JK, Suh I, Jee SH, Park J, Kim CB, Kim KS. The accuracy of myocardial infarction diagnosis in medical insurance claims. Yonsei Med J. 2000;41:570–576. [DOI] [PubMed] [Google Scholar]
- 45. Kim JY, Lee KJ, Kang J, Kim BJ, Han MK, Kim SE, Lee H, Park JM, Kang K, Lee SJ, et al. Development of stroke identification algorithm for claims data using the multicenter stroke registry database. PLoS One. 2020;15:1–15. DOI: 10.1371/journal.pone.0228997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CRCS‐K Registry Investigators
Tables S1–S5
Figures S1–S4


