Abstract
Background
Impairments in fetal oxygen delivery have been implicated in brain dysmaturation seen in congenital heart disease (CHD), suggesting a role for in utero transplacental oxygen therapy. We applied a novel imaging tool to quantify fetal cerebral oxygenation by measuring T2* decay. We compared T2* in fetuses with CHD with controls with a focus on cardiovascular physiologies (transposition or left‐sided obstruction) and described the effect of brief administration of maternal hyperoxia on T2* decay.
Methods and Results
This is a prospective study performed on pregnant mothers with a prenatal diagnosis of CHD compared with controls in the third trimester. Participants underwent a fetal brain magnetic resonance imaging scan including a T2* sequence before and after maternal hyperoxia. Comparisons were made between control and CHD fetuses including subgroup analyses by cardiac physiology. Forty‐four mothers (CHD=24, control=20) participated. Fetuses with CHD had lower total brain volume (238.2 mm3, 95% CI, 224.6–251.9) compared with controls (262.4 mm3, 95% CI, 245.0–279.8, P=0.04). T2* decay time was faster in CHD compared with controls (beta=−14.4, 95% CI, −23.3 to −5.6, P=0.002). The magnitude of change in T2* with maternal hyperoxia was higher in fetuses with transposition compared with controls (increase of 8.4 ms, 95% CI, 0.5–14.3, P=0.01), though between‐subject variability was noted.
Conclusions
Cerebral tissue oxygenation is lower in fetuses with complex CHD. There was variability in the response to maternal hyperoxia by CHD subgroup that can be tested in future larger studies. Cardiovascular physiology is critical when designing neuroprotective clinical trials in the fetus with CHD.
Keywords: brain imaging, congenital heart disease, fetal
Subject Categories: Clinical Studies
Nonstandard Abbreviations and Acronyms
- LSOL
left‐sided obstructive lesion
- MH
maternal hyperoxia
- TBV
total brain volume
- TGA
transposition of the great arteries
Clinical Perspective
What Is New?
Cerebral oxygenation is lower in fetuses with congenital heart disease compared with controls with similarities noted across physiologic subgroups; however, there is variable response to brief administration of maternal hyperoxia based on underlying cardiovascular physiology.
What Are the Clinical Implications?
T2* magnetic resonance imaging is a noninvasive prenatal tool that can provide efficient and quantitative assessment of fetal cerebral oxygenation, which can be used in future clinical trials studying in utero therapies to optimize brain development in congenital heart disease.
Multiple lines of evidence support the fact that brain development is delayed in the context of complex congenital heart disease (CHD) and that this delay originates in utero. 1 , 2 Brain immaturity is thought to be one of the contributors to acquired brain injury after birth, 3 which has been associated with impaired motor outcomes in infancy. 4 The cause of brain immaturity in utero is currently under investigation, with reports suggesting impaired oxygen delivery as the main driver, though many of these studies are limited to animal models 5 or human studies with heterogeneous groups of fetuses with varying types of CHD. 6 This has led to the hypothesis that supplemental oxygen administration to the fetus via transplacental passage may be beneficial for brain health and in utero growth. Administration of oxygen to the pregnant mother in late gestation has been shown to increase the partial pressure of oxygen in the fetus in both invasive and noninvasive studies. 7 , 8 However, proof of concept studies to test the influence of supplemental oxygen on the fetal brain using quantitative imaging techniques are lacking.
Deoxygenated hemoglobin is paramagnetic and will cause local dephasing of protons, reducing the tissue signals measured in magnetic resonance imaging (MRI). MRI T2* relaxation, which is the decay of transverse magnetization caused by spin‐spin relaxation and local field inhomogeneities, can be derived from the signal changes (ie, T2* decay time) for quantitatively assessing the tissue oxygenation level in an imaging voxel. 9 , 10 T2* MRI has been widely explored in humans for different clinical applications, such as functional, susceptibility‐weighted, perfusion, and iron‐deposition MRI, including studies focused on the brain. 11 , 12 , 13 This technique has also been applied to image the human fetal brain, providing estimates for fetal cerebral tissue oxygenation in normal fetuses. 14 Preliminary reports of T2* MRI in the fetus with CHD demonstrate decreased cerebral oxygenation compared with controls (ie, faster rate of T2* signal decay), though these studies were limited by small sample size and high rates of imaging and motion artifact, thereby prohibiting subgroup analyses. 15
In this study we have developed a novel imaging tool to achieve reliable and efficient whole fetal brain oxygenation assessment based on quantitative T2* measurements. The primary aim for this study was to compare T2* values in late gestation fetuses with CHD with those of controls, focusing on unique cardiovascular physiologies. We also sought to determine whether T2* values, as a measure of blood oxygenation in the fetal brain, are associated with brain maturity. Additionally, we sought to describe the effect of brief administration of maternal hyperoxia (MH) on T2* values for control and CHD fetuses.
Methods
Participants
The data that support the findings of this study are available from the corresponding author upon reasonable request. This is a prospective study conducted between June 2017 and December 2019. Pregnant mothers with a prenatal diagnosis of CHD requiring a neonatal intervention referred to the University of California San Francisco Fetal Cardiovascular Program were recruited to undergo a fetal brain MRI with brief administration of MH in the third trimester. The control group consisted of healthy pregnant women with normal fetal cardiovascular anatomy. These subjects were recruited from the low‐risk obstetric clinic at University of California San Francisco to undergo a voluntary fetal echocardiogram and fetal brain MRI with MH testing in the third trimester. Exclusion criteria consisted of prenatally diagnosed genetic or extracardiac abnormalities, twin gestation, growth restriction, or significant uteroplacental disease such as preeclampsia and maternal disease. Pregnancies were dated by last menstrual period and dates confirmed were by first trimester ultrasound. A detailed fetal echocardiogram was performed in the third trimester for both groups using standard clinical protocols. 16 , 17 Informed consent was obtained from all mothers. The institutional review board on human research at University of California San Francisco approved this study protocol. Detailed clinical maternal, fetal, and neonatal data were collected from both groups and all cardiac defects were confirmed after birth. Neonatal hemoglobin levels in the first 12 hours were recorded for the CHD groups but is not the routine practice for normal newborns.
MH Protocol
Subjects (mothers) underwent 2 components of imaging: baseline and during MH for both the MRI and fetal echocardiogram. Both a clinical routine MR protocol and a research imaging sequence (3D multi‐echo gradient echo acquisition) for a T2* measurement of the fetal brain were performed at baseline with mothers breathing ambient air. Then mothers were administered 8 L/min of oxygen via a nonrebreather mask at 100% Fio 2 for 5 minutes as previously described, 8 , 18 , 19 followed by the repeated research imaging sequence to measure T2* values of the fetal brain during MH. The results from MH during the fetal echocardiogram were previously published by our group 20 and are not included in this report.
MRI Protocol
All subjects underwent a fetal MRI during the third trimester using the same imaging protocol on a 3.0‐T MRI system equipped with a 32‐channel cardiac coil (GE Medical Systems, Waukesha, WI). Quantitative T2* measurements are typically performed using a 2D/3D spoiled gradient echo sequence with multiple echo acquisition (ie, multi‐echo gradient‐echo acquisition). However, the lengthy acquisition time is a challenge for achieving good image quality, especially for fetal brain imaging that is prone to fetal movement as well as maternal motion. Therefore, in this study we developed an efficient 3D imaging method to overcome this challenge. Data S1 demonstrates numerical simulations to achieve the optimal length of echo time to provide accurate T2* measurements while maintaining high scan efficiency (Figure S1). Furthermore, highly accelerated dynamic 3D (4D) fetal imaging with multi‐echo gradient‐echo acquisition was utilized to measure fetal cerebral T2*. A previously developed continuous data acquisition scheme with a specific sampling strategy allows for accelerated dynamic 3D imaging for a variety of applications, resulting in high frame rate imaging with motion robustness in a relatively short scan time. 21 , 22 , 23 The 4D MRI based on multi‐echo spoiled gradient echo acquisition has the following parameters: field of view=32.0 × 32.0 cm2, slice thickness=6 mm, image matrix=160 × 160 × 30, time of repetition=24.0–24.2 ms, 8 echo times=2.0 to 22.0 ms with 2.9 ms incensement, flip angle=20°, and readout bandwidth=±125 kHz with a scan time of 140 s. The continuously acquired data were reconstructed into a 3D image at 10 time frames (14 s per frame) using a multicoil compressed sensing method (k‐t SPARSE‐SENSE) 24 , 25 with an acceleration factor of ≈8.
MRI Postprocessing
To ensure the accuracy of the fetal brain oxygenation evaluation, we developed a new image pipeline to process the dynamic 3D fetal images. First, we identified 1 time frame that had the least motion artifact for image processing and final measurement. A semi‐automatic segmentation tool was developed for efficiently segmenting the whole fetal brain based on the multi‐echo MR acquisition, as described in detail in Data S1 and Figure S2. An example of whole fetal brain segmentation is shown in Figure 1A. The segmented fetal brain allows us to calculate total brain volume (TBV, mm3) as a measure of brain maturity.. This process was applied at both time points (baseline and MH), resulting in <5% difference and suggesting adequate reproducibility of the segmentation tool.
Figure 1. T2* fetal brain MRI.
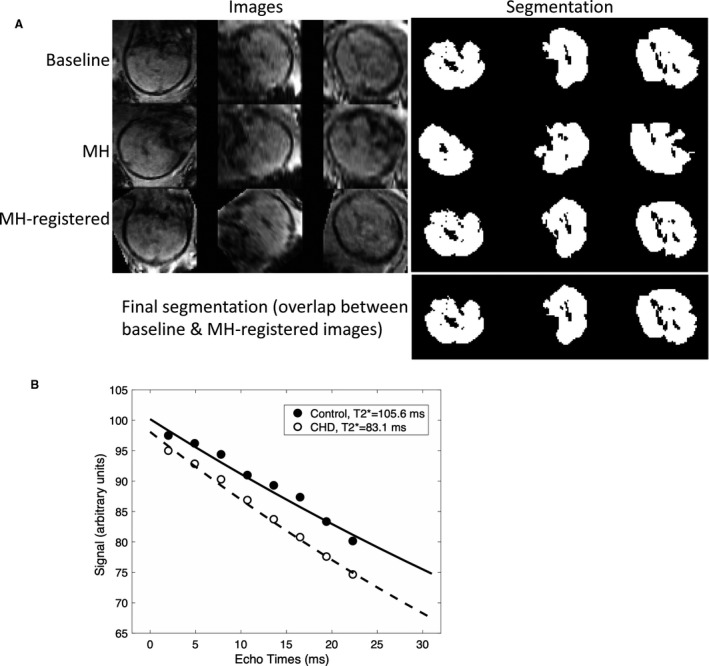
A, Images from 3 orthogonal plans were segmented and registered to obtain the final segmentation for T2* measurements at baseline and with MH; B, T2* decay curves for a control and CHD subject with hypoplastic left heart syndrome. The gestational age at fetal MRI was 33 3/7 weeks in the control subject and 34 2/7 weeks in the CHD subject. CHD indicates congenital heart disease; MH, maternal hyperoxia; and MRI, magnetic resonance imaging.
The averaged signal intensity in the segmented whole brain at each echo time was obtained and used to fit the exponential decay formula, si=s0·exp(‐TEi/T2*), where si denotes the measured signals from ith echo time (TEi), to derive the T2* value in ms (Figure 1B).
Statistical Analysis
Baseline characteristics of the control and CHD groups were compared using descriptive statistics including t test for continuous variables and 2 χ2 or Fisher’s exact test for categorical variables. First, the effect of gestational age on T2* as the dependent variable was determined by linear regression. Comparisons were made in rate of change in T2* by gestational age of fetal MRI between the CHD and control groups using repeated‐measures analysis. Second, to determine the effect of our independent variable of group (control versus CHD) on our dependent variable (T2*), linear regression was used to compare T2* between the control and CHD group at each time point (baseline and MH) with adjustment for gestational age at scan and fetal sex. To describe the differences in T2* based on cardiovascular physiology, T2* at each time point was compared between the control group and each CHD subgroup (transposition of the great arteries, right‐sided obstructive lesion, left‐sided obstructive lesion) using linear regression with adjustment for gestational age at scan.
Within each group, a paired t test was performed to assess the change in T2* from baseline to MH. Given that each subject had 2 measurements for the T2* value (baseline and MH), a repeated‐measures analysis was performed using generalized estimating equations with an interaction term for cardiac group and the condition (baseline or MH) to compare the magnitude of change in T2* between each CHD group and the control group. The model was adjusted for gestational age at scan. P values <0.05 were considered statistically significant. All analyses were performed using STATA version 14.2 (Stata Statistical Software: Release 14; College Station, TX: Stata Corp LP).
Results
A total of 52 subjects were enrolled in the study (CHD=29; Control=23), of which 51 had a fetal brain MRI performed during the third trimester. Three scans were discarded because of poor image quality and 3 because of maternal intolerance of completing the MRI examination. One control subject was removed from the analysis after developing preeclampsia, resulting in a total of 44 MRIs available for analysis (CHD=24; Control=20) (Figure 2). Table 1 demonstrates detailed clinical data including cardiac anatomy, postnatal surgical management, and outcomes of the CHD fetuses. Eleven subjects underwent whole exome genetic testing as part of a separate research protocol, of which 5 were found to have a genetic abnormality after enrollment into the current study (all 11 subjects had a normal microarray). CHD subjects were categorized by fetal cardiac physiologic differences and included the following: left‐sided obstructive lesions (LSOL, eg, hypoplastic left heart syndrome) (n=15); right‐sided obstructive lesions (eg, pulmonary atresia) (n=2); and transposition of the great arteries (TGA) (n=7). Among the LSOL subjects, 11 underwent a Norwood operation for hypoplastic left heart syndrome. Given the small number of right‐sided obstructive lesion subjects, subgroup analyses were limited to the TGA and LSOL groups. Baseline maternal, fetal, and neonatal demographics did not differ between CHD and control groups (Table 2). In particular, mean gestational age at fetal MRI was similar between groups (Control, 33.9 weeks, 95% CI, 33.5–34.4; CHD, 33.8 weeks, 95% CI, 33.4–34.2; P=0.64).
Figure 2. Flowchart of participants included in the study.
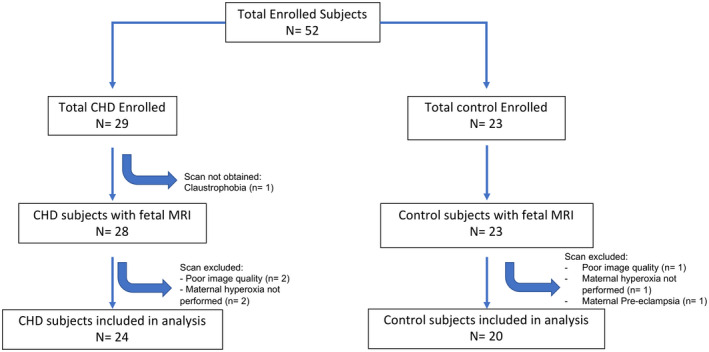
CHD indicates congenital heart disease; and MRI, magnetic resonance imaging.
Table 1.
Clinical Characteristics of CHD Fetuses (Median Age of Follow‐Up 6.2 Months)
| Diagnosis | Associated Anomaly | Genetic Abnormality by Exome Testing* | Management Approach | Outcome |
|---|---|---|---|---|
| HLHS (MA/AA) | None | NA | Norwood | Alive and well |
| d‐TGA/IVS | Dysmorphic facies | Yes | Arterial switch operation | Alive with developmental delay |
| HLHS (MA/AA) | None | No | Norwood | Interstage death |
| d‐TGA/VSD | None | NA | Arterial switch operation/VSD closure | Alive and well |
| Critical aortic stenosis | None | NA |
‐ Fetal balloon aortic valvuloplasty ‐ Neonatal Ross/Konno |
Alive and well |
| HLHS (MA/AA) | None | No | Norwood | Alive and well |
| d‐TGA/VSD | None | NA | Arterial switch operation/VSD closure | Alive and well |
| Tricuspid atresia, malposed great arteries, interrupted aortic arch | None | NA | Norwood | Alive and well |
| HLHS (MA/AA), restricted atrial septum, PAPVC | None | NA | Comfort care | Death before surgery |
| Heterotaxy, unbalanced CAVC, pulmonary atresia, infradiaphragmatic TAPVC | None | NA | TAPVC repair, RV‐PA conduit | Alive and well |
| d‐TGA/VSD | None | No | Arterial switch operation/VSD closure | Alive and well |
| Unbalanced CAVC, aortic arch hypoplasia | None | Yes | Aortic arch repair, PA band | Alive and well |
| HLHS (MA/AA) | None | No | Norwood | Alive and well |
| HLHS (MA/AA) | None | NA | Norwood | Alive and well |
| d‐TGA/IVS | Dysmorphic facies | Yes | Arterial switch operation | Alive and well |
| DILV, aortic arch hypoplasia | None | NA | Norwood | Alive and well |
| HLHS (MA/AA) | None | No | Norwood | Alive and well |
| Heterotaxy, unbalanced CAVC, supracardiac TAPVC | None | NA | TAPVC repair, RV‐PA conduit | Alive and well |
| Aortic coarctation | None | No | Coarctation repair | Alive and well |
| HLHS (MA/AA) | Dysmorphic facies | Yes | Norwood | Alive and well |
| d‐TGA/VSD | None | NA | Arterial switch operation/VSD closure | Alive and well |
| Unbalance CAVC, aortic arch hypoplasia | None | Yes | Norwood | Alive and well |
| d‐TGA/IVS | None | NA | Arterial switch operation | Alive and well |
| DORV, aortic valve and arch hypoplasia/coarctation | None | NA | Aortic arch repair/VSD closure | Alive and well |
AA/MA indicates aortic atresia/mitral atresia; CAVC, common atrioventricular canal; CHD, congenital heart disease; d‐TGA, d‐transposition of the great arteries; DILV, double inlet left ventricle; DORV, double outlet right ventricle; HLHS, hypoplastic left heart syndrome; IVS, intact ventricular septum; PAPVC, partial anomalous pulmonary venous connection; TAPVC, total anomalous pulmonary venous connection; VSD, ventricular septal defect.
A subset of participants underwent genetic testing by whole exome analysis as part of a separate research protocol (all had a normal microarray). Those who did not participate in the exome study are denoted as NA (not applicable).
Table 2.
Maternal, Fetal, and Neonatal Characteristics of Study Population
| Control (n=20) | CHD (n=24) | P Value* | |
|---|---|---|---|
| Maternal | |||
| Age (y), mean (95% CI) | 32.4 (31.4, 33.4) | 32.8 (30.1, 35.5) | 0.80 |
| Race/ethnicity, N (%) | |||
| Non‐Hispanic White | 11 (55.0%) | 10 (41.7%) | 0.13 |
| Hispanic | 3 (15%) | 8 (33.3%) | |
| Black | 0 | 2 (8.3%) | |
| Asian | 6 (30%) | 3 (12.5%) | |
| Other | 0 | 1 (4.2%) | |
| Nulliparous, N (%) | 13 (65%) | 6 (25%) | 0.01 |
| Fetal | |||
| GA at MRI, mean (95% CI) | 33.9 (33.5, 34.4) | 33.8 (33.4–34.2) | 0.64 |
| Male sex, N (%) | 7 (35%) | 13 (59.1%) | 0.2 |
| EFW (g), mean (95% CI) | 2268.8 (2147.4, 2390.1) | 2283.7 (2139.6, 2427.8) | 0.86 |
| Head circumference (cm), mean (95% CI) | 311.6 (305.8, 317.3) | 308.8 (302.8, 314.7) | 0.48 |
| Neonatal | |||
| GA birth (wks ), mean (95% CI) | 39.3 (38.7, 39.9) | 39.0 (38.7, 39.4) | 0.41 |
| Birthweight (kg), mean (95% CI) | 3.16 (2.97,3.36) | 3.36 (3.17, 3.54) | 0.15 |
| Head circumference (cm), mean (95% CI) | 34.3 (33.8, 34.9) | 33.9 (33.1, 34.6) | 0.35 |
| Hemoglobin (first 12 h), mean (95% CI) | … | 16.8 (15.9, 17.8) | NA |
CHD indicates congenital heart disease; EFW, estimated fetal weight; GA, gestational age; MRI, magnetic resonance imaging; and NA, not applicable.
P value represents comparison between control and CHD groups using either t test for continuous variables and χ2 or Fisher’s exact test for categorical variables.
We first explored the relationship between T2* and gestational age at fetal MRI. For both CHD and control groups, T2* declined with advancing gestational age (Control=7.1 ms, 95% CI, −14.8 to 0.72; CHD=8.2 ms, 95% CI, −14.5 to −1.8). The rate of decline was similar in CHD and control groups, suggesting no significant interaction in the relationship between gestational age and T2* with the study group. At baseline, for every 1‐week increase in gestational age, T2* declined by 1 ms more in the CHD group compared with the control group (coefficient, −1.1 ms, 95% CI, −9.9 to 7.7; P=0.81), which was not significant. The change in T2* with MH was not associated with gestational age at fetal MRI for either the CHD or control groups (Figures S3A and B).
TBV was significantly lower in the CHD group compared with the control group (Control: 262.4 mm3, 95% CI, 245.0–279.8; CHD, 238.2 mm3, 95% CI, 224.6–251.9, P=0.04) (Table 3). When analyzing the subgroups of CHD, fetuses with LSOL had the lowest TBV (235.1 mm3, 95% CI, 215.2–252.7).
Table 3.
T2* Values at Baseline and With MH in Control and CHD Subjects. Values Listed by CHD Subgroup as Well
|
Control N=20 |
CHD N=24 |
LSOL N=15 |
RSOL N=2 |
TGA N=7 |
P Value + | |
|---|---|---|---|---|---|---|
|
GA scan, wks Mean, SD |
33.9 (33.6, 34.4) |
33.8 (33.4, 34.2) |
33.6 (33.2, 34.1) |
34.1 (32.1, 36.2) |
34.2 (33.5, 34.9) |
0.64 |
|
EFW, g Mean, SD |
2268.8 (2150.7, 2386.8) |
2283.7 (2144.1, 2423.4) |
2161.7 (2020.5, 2302.9) | 2324.5 (1860.7, 2788.3) | 2534.7 (2257.6, 2811.6) | 0.86 |
|
TBV, mL Mean, SD |
262.4 (245.0, 279.8) |
238.2 (224.6, 251.9) |
235.1 ‡ (215.2, 252.7) |
256.3 (215.2, 252.7) |
241.6 (214.7, 259.3) |
0.04 |
|
T2* base, ms Mean, 95% CI |
99.7 (93.0, 106.4) |
84.8 (78.9, 90.7) |
84.5 ‡ (77.4, 91.6) |
81.6 (65.0, 98.2) |
86.3 ‡ (70.6, 102.0) |
0.002 |
|
T2* MH, ms Mean, 95% CI |
99.5 (94.1, 104.8) |
88.9 § (81.4, 96.5) |
87.4 (77.3, 97.5) |
84.8 (84.7, 85.0) |
93.6 § (79.9, 107.2) |
0.06 |
|
T2* change, ms Mean, 95% CI |
−0.2 (−7.3, 6.8) |
4.2 (0.5, 7.8) |
2.9 (−2.4, 8.1) |
3.2 (−13.2, 19.7) |
7.2 (4.6, 9.8) |
0.1 |
CHD indicates congenital heart disease; EFW, estimated fetal weight; GA, gestational age; LSOL, left‐sided obstructive lesion; MH, maternal hyperoxia; RSOL, right‐sided obstructive lesion; TBV, total brain volume; and TGA, transposition of the great arteries.
P value represents comparison between control and CHD group using linear regression with adjustment for gestational age at scan and fetal sex.
P value represents comparison between control group and each subtype of congenital heart disease using a linear regression model with adjustment for gestational age at scan. Those with the symbol are significantly different from the control group (P<0.05).
Paired t test within each group, symbol denotes significant change of T2* from baseline to MH (P<0.05).
At baseline, T2* was significantly lower in the CHD group compared with the control group after adjusting for gestational age at MRI and fetal sex (Beta, −14.4, 95% CI, −23.3 to −5.6, P=0.002). Among the CHD subjects, baseline T2* was similar between the LSOL and TGA groups and both were significantly lower compared with the control group (Figure 3). In particular, after adjusting for gestational age at MRI, T2* was on average 12 ms lower in the TGA group (95% CI, −24.4 to 0.4; P=0.05) and 15 ms lower in the LSOL group (95% CI, −25.3 to −5.5; P=0.003) compared with the control group. There was no difference in TBV or baseline T2* between CHD fetuses with and without a genetic abnormality (P=0.48). There was no correlation noted between TBV and T2* (r = −0.11, P=0.47) (Figure S4).
Figure 3. T2* values at baseline for the control, LSOL, and TGA groups with mean and 95% CI.
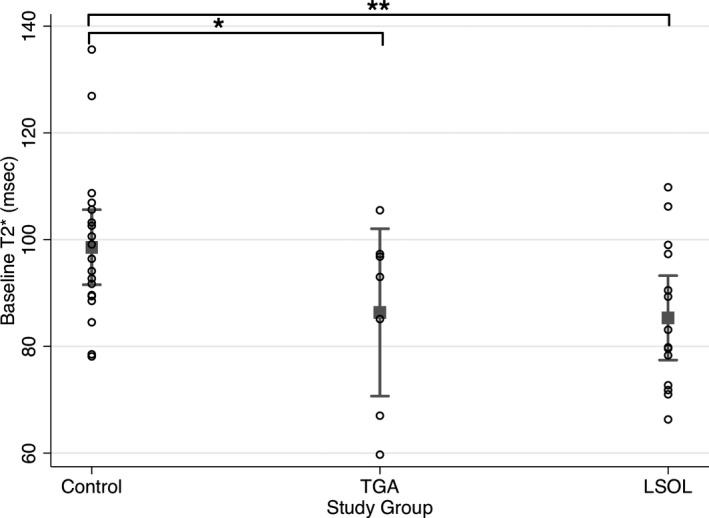
At baseline, cerebral tissue oxygenation (T2*) is significantly lower in LSOL (**coeff:−15.4, 95% CI, −25.3 to −5.5, P=0.003) and TGA (*coeff: −12.0, 95% CI, −24.4 to 0.4, P=0.05) compared with the control group after adjusting for gestational age at MRI. LSOL indicates left‐sided obstructive lesions; MRI, magnetic resonance imaging; and TGA, transposition of the great arteries.
During MH testing, when comparing within groups, T2* did not change significantly in the control group whereas it increased significantly in the CHD group (increase of 4.2 ms, P=0.04). Among the CHD subgroups, fetuses with TGA had the greatest increase in T2* with MH testing (increase of 7.2 ms, 95% CI, 4.6–9.8) (Table 3). Given that each subject had 2 measures, a repeated‐measures analysis was performed comparing the LSOL and TGA subgroups with the control group after adjusting for gestational age at MRI. Compared with the control group, T2* had a much greater magnitude of change from baseline to MH in the TGA group (increase of 8.4 ms, 95% CI, 0.5–14.3, P=0.01) (Figure 4). The magnitude of change in the LSOL group was minimal and similar to the control group (increase of 3.3 ms, 95% CI, −5.1 to 11.2, P=0.44). However, there was significant variability noted in response to MH within the LSOL group. While all the TGA subjects demonstrated an increase in T2* with MH, among the LSOL subjects, 8 demonstrated an increase in T2* with MH while 7 were unchanged.
Figure 4. The magnitude of change in T2* from baseline to MH testing for the control (black line: mean; gray lines: individual patients), transposition of the great arteries (TGA) (blue line: mean; light blue lines: individual patients), and LSOL (red line: mean; light red lines: individual patients).
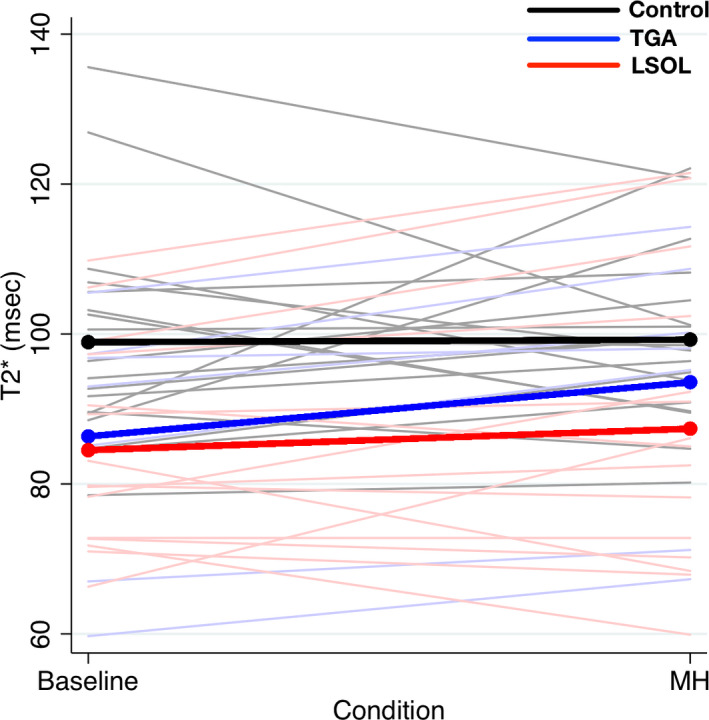
LSOL indicates left‐sided obstructive lesions; MH, maternal hyperoxia; and TGA, transposition of the great arteries.
Discussion
In this prospective study using novel MRI techniques in the fetus, we demonstrate that cerebral tissue oxygenation is lower in fetuses with critical CHD compared with controls. Although we found an overall increase in cerebral tissue oxygenation with brief administration of MH, we identified significant variability in the response by CHD subgroup, bringing to light several physiologic considerations that are unique to the fetus with complex CHD. Importantly, we identify a noninvasive prenatal tool to test the response of the fetal brain to supplemental oxygen.
Quantitative T2* imaging, a relatively novel tool for fetal MRI, has the ability to estimate fetal blood oxygenation in tissue by measuring relative levels of deoxyhemoglobin.10 T2* measurements can also be affected by tissue composition and vascularity of the organ. Higher levels of deoxyhemoglobin in the tissue typically result in faster rates of decay in the T2* signal. We found that CHD fetuses overall have lower cerebral tissue oxygenation compared with controls. This is similar to a recent study, which investigated a small group of fetuses with varying types of CHD using T2* MRI at variable gestational ages 15 ; however, given the small number of CHD fetuses studied because of imaging and motion artifact, subgroup analyses were not possible. In our study we utilized a novel MRI acquisition method and a robust semiautomatic segmentation tool allowing for accurate quantitative T2* measurements with very few cases (3 out of 51) being discarded because of poor image quality. Our 4D highly accelerated imaging method allows for high spatial resolution, which allows fetal brain evaluation with less partial volume effect. Additionally, the acceleration scheme allows for high time frame rate (14‐s footprint), which largely mitigates the artifacts caused by fetal motion. Moreover, performing the study on a 3‐T scanner (as opposed to 1.5 T) also allows for much shorter echo times for achieving the same level of accuracy for T2* measurements.
Our sample size enabled subgroup analyses demonstrating similar degrees of impairment in cerebral tissue oxygenation in the TGA and LSOL groups. Many studies have shown that these 2 groups have similar degrees of fetal and early neonatal (ie, preoperative) delays in brain development. 1 , 26 In fetal lamb models, hypoplastic left heart syndrome (HLHS) results in abnormalities of both perfusion (because of retrograde flow from the ductus arteriosus) and oxygenation of cerebral blood flow (because of mixing). 27 In contrast, those with TGA have abnormalities of oxygenation of cerebral blood flow because of preferential streaming of deoxygenated blood to the right ventricle and ascending aorta. Though mechanisms have been suggested to compensate for this desaturation via local changes in cerebral vasculature to increase cerebral blood flow, 28 , 29 both lesions result in decreased cerebral oxygen delivery and consumption as identified by phase‐contrast and T2 mapping MRI in the fetus, 6 suggesting inadequate compensatory mechanisms.
Though TBV, a measure of brain development, was significantly lower in the CHD group compared with controls, we found no significant correlation between baseline cerebral tissue oxygenation and TBV at this single time point. The lack of correlation does not suggest that impaired oxygen delivery plays no role in brain immaturity in the CHD fetus. As mentioned, T2* is a measure of blood saturation and does not take into account other factors that can influence overall oxygen delivery and oxygen consumption. Human fetal studies have shown a direct correlation between oxygen consumption and brain size, 6 however; it is difficult to separate cause and effect in this relationship. Animal studies have shown that chronic hypoxia alters neuronal and glial protein expression in the fetal brain 5 , 30 and impairs the generation and migration of neural progenitors destined to become forebrain interneurons. 31 In addition, the cross‐sectional nature of this study did not allow us to assess the rate of brain growth over time, which may be influenced by cerebral tissue oxygenation. However, other factors may contribute to delayed brain development in the context of CHD such as genetic mutations in genes expressed in the heart and brain 32 and environmental factors (ie, maternal stress). 33 Finally, delivery of nutrients (eg, glucose) to the brain is impaired which, in concert with hypoxia, can result in impairment of 2 important substrates to the developing fetal brain. 27
The influence of brief administration of MH on cerebral tissue oxygenation was also examined. In normal fetuses, there was very little change in cerebral tissue oxygenation with MH testing. This likely reflects simultaneous effects on both arterial content of oxygen and cerebral blood flow. Our prior study used transcranial Doppler to measure cerebral vascular impedance in the middle cerebral artery in response to MH in normal and CHD fetuses. 20 We demonstrated increased cerebral vascular impedance in normal fetuses in response to MH. Thus, although arterial oxygen content increases with MH, the increase in cerebral vascular impedance likely reflects a decline in cerebral blood flow to maintain a steady level of oxygenation.
Cerebral tissue oxygenation exhibited an overall increase among all CHD subjects. Mechanisms to explain this finding include the relationship between the arterial concentration of oxygen and oxygen saturation in blood as depicted in the oxyhemoglobin dissociation curve, 34 assuming unchanged cerebral oxygen consumption between the 2 states (baseline and MH). Fetuses with CHD have lower baseline cerebral oxygen saturations; thus increases in the partial pressure of oxygen (Pao 2) can potentially lead to significant increases in arterial blood saturations in the brain reflected in the T2* measurement. Interestingly, the increase in CHD subjects appeared to be largely driven by the TGA subgroup. This may reflect a unique physiologic phenomenon in TGA where supplemental oxygen not only results in direct delivery of more oxygen but also leads to flow redistribution. Specifically, oxygen can increase pulmonary blood flow and pulmonary venous return to the left atrium, decrease right to left shunting at the foramen ovale, and increase the flow of highly oxygenated umbilical venous blood across the aorta to the cerebral vasculature. This redistribution of blood may lead to more highly saturated blood reaching the fetal brain, thus increasing the T2* measurement.
In contrast to TGA, LSOL fetuses exhibited minimal change of cerebral T2* in response to MH. In this subgroup, there is complete intracardiac mixing; thus, streaming patterns should not be particularly altered in response to MH. We and others have shown that overall cardiac output is diminished in the fetus with HLHS. 6 , 20 , 35 This, in combination with retrograde blood flow to the cerebral vasculature, suggests lack of adequate perfusion and pulsatility to the brain, in addition to decreased oxygenation. This severe combination may lead to an adaptive response to a chronic hypoxic and low output state, with decreased cerebral oxygen consumption resulting in lack of response to supplemental oxygen. Indeed, we also demonstrated this phenomenon in fetuses with HLHS and aortic atresia, which exhibited no change in cerebral vascular impedance in response to MH. 20 However, it is important to note the significant variability in the T2* response to MH among patients in the LSOL subgroup. The variability may be explained by different starting points in arterial oxygen saturations or different degrees of adaptation in cerebral metabolism and compensation for a low cardiac output state. Placental delivery of oxygen may also vary significantly between subjects in the setting of placental pathology, as reported in fetal cases of HLHS. 36 , 37 To this point, a recent study utilizing similar imaging techniques suggested that fetuses with single‐ventricle CHD may benefit from transplacental oxygen therapy to increase o xygen supply to the brain by increasing the partial pressure of oxygen in arterial blood. 38 Our study differed in that our subgroup analysis included only patients with LSOLs, the vast majority of which had HLHS; thus, it was a more homogeneous group with respect to cardiac physiology. In addition, we studied TGA as a separate group rather than combining with other biventricular congenital heart defects. Our study highlights the crucial role of underlying cardiovascular physiology in understanding cerebral hemodynamics, oxygenation, and potential therapies to optimize outcomes.
It is important to discuss the influence of changes in proportional arterial and venous blood volume on the T2* measurement. T2* is thought to be a volume‐weighted average of both arterial and venous oxygenation (ie, relative proportions of highly saturated arterial and desaturated venous blood). This is similar to cerebral oximetry measured by near‐infrared spectroscopy, which is derived from direct measurements of changes in the absorption of near infrared light by oxy‐ and deoxyhemoglobin. We have previously shown that in TGA fetuses there is vasoconstriction of the middle cerebral artery with MH 20 ; thus it is possible that there is a redistribution of venous and arterial blood volumes within the brain such that the volume of arterial blood is decreased in proportion to venous blood. In a piglet model, researchers induced systemic hypotension leading to cerebral vasodilation with an increase in arterial blood volume relative to venous blood volume. 39 In this experiment, they compared co‐oximetry with direct measurements of known proportions of arterial and venous blood to demonstrate that cerebral tissue oxygenation measured by near‐infrared spectroscopy (similar to T2*) overestimated the direct oximetry measurement during cerebral vasodilation. Extrapolating from these observations, our T2* measurements in fetuses with TGA during MH in the setting of cerebral vasoconstriction may actually underestimate the increase in cerebral oxygenation.
Our results provide important data for development of MH as a therapy in the fetus with CHD. Acute MH appears safe and without adverse hemodynamic changes to fetal circulation. However, we find significant variability in the fetal cerebral oxygenation response, with clear differences based on underlying cardiovascular physiology. Although all TGA fetuses exhibited an acute increase in T2* with MH, these results should be interpreted with caution as transplacental oxygen therapy for a fetus with TGA may become detrimental if excessive reduction in pulmonary vascular resistance leads to increased pulmonary blood flow at the expense of cerebral perfusion. Our findings do not address potential chronic changes to pulmonary circulation or placental function with MH. These findings have implications for clinical trials of in utero oxygen therapy to optimize prenatal brain development. T2* MRI could provide both pragmatic study design for longitudinal clinical MH trials with predictive enrichment based upon acute T2* response and longitudinal safety monitoring. A correlation between brain growth and T2* response would inform therapeutic mechanisms and provide an early outcome measure before long‐term neurodevelopmental testing.
Our study has a number of strengths including our imaging methods, a dedicated control group as well as relatively homogeneous subgroups (though with limited sample size) to report these measurements by underlying cardiac physiology, though there was some modest variability in final circulatory outcome in the LSOL group. However, there are some notable limitations. First, as shown, gestational age influences T2* in the brain. Although the gestational age at the time of fetal MRI was similar between our CHD and control groups, we adjusted for gestational age in our analyses. Second, higher hemoglobin levels could lead to a lower T2* measurement because of higher levels of deoxyhemoglobin at the same saturation level. However, the mean hemoglobin levels at birth in the CHD group were within normal range; thus we expect that hemoglobin levels had a minimal influence on our findings when comparing CHD with control fetuses in late gestation. Our findings may have been strengthened with multiple acquisitions for the T2* measurement to ensure minimal measurement error. Third, there is a higher percentage of nulliparous mothers in the control group; however, we would not expect this to influence our findings as particular attention was paid to excluding patients with maternal comorbidities (ie, hypertension, preeclampsia, and diabetes mellitus). Although we excluded mothers with other comorbidities, future studies with a larger sample size could assess the influence of these factors on brain health in the context of CHD. There was a trend towards more male fetuses in the CHD group, though this did not reach significance and was included in the final regression model. Given our sample size, we were unable to fully account for all possible confounders. Finally, a small number of fetuses were found to have genetic abnormalities on whole exome sequencing (all had a normal microarray) as part of a separate study, though we did not identify any differences in total brain volume or baseline T2* between CHD fetuses with a genetic abnormality versus those without.
In conclusion, fetuses with complex CHD have evidence of decreased cerebral tissue oxygenation. These findings hold true for fetuses with LSOL and TGA to the same degree. We demonstrate significant variability in the response of the fetal brain to MH. In the era of in utero therapy to optimize outcomes in patients with CHD such as chronic MH, our findings support the use of T2* MRI, as a quantitative and relatively quick MRI tool, in clinical trials to stratify fetuses by initial response to MH in order to better understand its influence on neurodevelopmental outcomes over time.
Sources of Funding
This study is funded by K23 NS099422 (SP), P01 NS082330 (PSM), R21CA238137 (JL), from the National Institute of Health (NINDS).
Disclosures
None.
Supporting information
Data S1
Figures S1–S4
Reference 40
(J Am Heart Assoc. 2021;10:e018777. DOI: 10.1161/JAHA.120.018777.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.018777
For Sources of Funding and Disclosures, see page 10.
References
- 1. Miller SP, McQuillen PS, Hamrick S, Xu D, Glidden DV, Charlton N, Karl T, Azakie A, Ferriero DM, Barkovich AJ, et al. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. 2007;357:1928–1938. [DOI] [PubMed] [Google Scholar]
- 2. Limperopoulos C, Tworetzky W, McElhinney DB, Newburger JW, Brown DW, Robertson RL, Guizard N, McGrath E, Geva J, Annese D, et al. Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation. 2010;121:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goff DA, Shera DM, Tang S, Lavin NA, Durning SM, Nicolson SC, Montenegro LM, Rome JJ, Gaynor JW, Spray TL. Risk factors for preoperative periventricular leukomalacia in term neonates with hypoplastic left heart syndrome are patient related. J Thorac Cardiovasc Surg. 2014;147:1312–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Peyvandi S, Chau V, Guo T, Xu D, Glass H, Synnes A, Poskitt K, Barkovich AJ, Miller S, McQuillen P. Neonatal brain injury and timing of neurodevelopmental assessment in patients with congenital heart disease. J Am Coll Cardioll. 2018;71:1986–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lawrence KM, McGovern PE, Mejaddam A, Rossidis AC, Baumgarten H, Kim A, Grinspan JB, Licht DJ, Didier RA, Vossough A, et al. Chronic intrauterine hypoxia alters neurodevelopment in fetal sheep. J Thorac Cardiovasc Surg. 2019;157:1982–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sun L, Macgowan CK, Sled JG, Yoo S‐J, Manlhiot C, Porayette P, Grosse‐Wortmann L, Jaeggi E, McCrindle BW, Kingdom J, et al. Reduced fetal cerebral oxygen consumption is associated with smaller brain size in fetuses with congenital heart disease. Circulation. 2015;131:1313–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nicolaides KH, Campbell S, Bradley RJ, Bilardo CM, Soothill PW, Gibb D. Maternal oxygen therapy for intrauterine growth retardation. Lancet. 1987;1:942–945. [DOI] [PubMed] [Google Scholar]
- 8. Rasanen J, Wood DC, Debbs RH, Cohen J, Weiner S, Huhta JC. Reactivity of the human fetal pulmonary circulation to maternal hyperoxygenation increases during the second half of pregnancy: a randomized study. Circulation. 1998;97:257–262. [DOI] [PubMed] [Google Scholar]
- 9. Chavhan GB, Babyn PS, Singh M, Vidarsson L, Shroff M. MR imaging at 3.0 T in children: technical differences, safety issues, and initial experience. Radiographics. 2009;29:1451–1466. [DOI] [PubMed] [Google Scholar]
- 10. Chavhan GB, Babyn PS, Thomas B, Shroff MM, Haacke EM. Principles, techniques, and applications of T2*‐based MR imaging and its special applications. Radiographics. 2009;29:1433–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Peters AM, Brookes MJ, Hoogenraad FG, Gowland PA, Francis ST, Morris PG, Bowtell R. T2* measurements in human brain at 1.5, 3 and 7 T. Magn Reson Imaging. 2007;25:748–753. [DOI] [PubMed] [Google Scholar]
- 12. Li W, Xu X, Liu P, Strouse JJ, Casella JF, Lu H, van Zijl P, Qin Q. Quantification of whole‐brain oxygenation extraction fraction and cerebral metabolic rate of oxygen consumption in adults with sickle cell anemia using individual T2 ‐based oxygenation calibrations. Magn Reson Med. 2020;83:1066–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nagao M, Yamasaki Y, Kawanami S, Kamitani T, Sagiyama K, Higo T, Ide T, Takemura A, Ishizaki U, Fukushima K, et al. Quantification of myocardial oxygenation in heart failure using blood‐oxygen‐level‐dependent T2* magnetic resonance imaging: comparison with cardiopulmonary exercise test. Magn Reson Imaging. 2017;39:138–143. [DOI] [PubMed] [Google Scholar]
- 14. Blazejewska AI, Seshamani S, McKown SK, Caucutt JS, Dighe M, Gatenby C, Studholme C. 3D in utero quantification of T2* relaxation times in human fetal brain tissues for age optimized structural and functional MRI. Magn Reson Med. 2017;78:909–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lauridsen MH, Uldbjerg N, Henriksen TB, Petersen OB, Stausbøl‐Grøn B, Matthiesen NB, Peters DA, Ringgaard S, Hjortdal VE. Cerebral oxygenation measurements by magnetic resonance imaging in fetuses with and without heart defects. Circulation: Cardiovascular Imaging. 2017;10:e006459. 10.1161/CIRCIMAGING.117.006459 [DOI] [PubMed] [Google Scholar]
- 16. AIUM . AIUM Practice Guideline for the Performance of Fetal Echocardiography. J Ultrasound Med. 2013;32:1067–1082. [DOI] [PubMed] [Google Scholar]
- 17. Donofrio MT, Moon‐Grady AJ, Hornberger LK, Copel JA, Sklansky MS, Abuhamad A, Cuneo BF, Huhta JC, Jonas RA, Krishnan A, et al. Diagnosis and treatment of fetal cardiac disease: a scientific statement from the American Heart Association. Circulation. 2014;129:2183–2242. [DOI] [PubMed] [Google Scholar]
- 18. Szwast A, Tian Z, McCann M, Donaghue D, Rychik J. Vasoreactive response to maternal hyperoxygenation in the fetus with hypoplastic left heart syndrome. Circulation: Cardiovascular Imaging. 2010;3:172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Szwast A, Putt M, Gaynor JW, Licht DJ, Rychik J. Cerebrovascular response to maternal hyperoxygenation in fetuses with hypoplastic left heart syndrome depends on gestational age and baseline cerebrovascular resistance. Ultrasound Obstet Gynecol. 2018;52:473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hogan WJ, Moon‐Grady AJ, Zhao Y, Cresalia NM, Nawaytou H, Quezada E, Brook MM, McQuillen P, Peyvandi S. Fetal cerebral vascular response to maternal hyperoxia in congenital heart disease: effects of cardiac physiology. Ultrasound Obstet Gynecol. 2020;1–30. DOI: 10.1002/uog.22024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu J, Saloner D. Accelerated MRI with CIRcular Cartesian UnderSampling (CIRCUS): a variable density Cartesian sampling strategy for compressed sensing and parallel imaging. Quant Imaging Med Surg. 2014;4:57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu J, Koskas L, Faraji F, Kao E, Wang Y, Haraldsson H, Kefayati S, Zhu C, Ahn S, Laub G, et al. Highly accelerated intracranial 4D flow MRI: evaluation of healthy volunteers and patients with intracranial aneurysms. MAGMA. 2018;31:295–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu J, Wang Y, Wen Z, Feng L, Lima APS, Mahadevan VS, Bolger A, Saloner D, Ordovas K. Extending cardiac functional assessment with respiratory‐resolved 3D cine MRI. Sci Rep. 2019;9:11563–11610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Otazo R, Kim D, Axel L, Sodickson DK. Combination of compressed sensing and parallel imaging for highly accelerated first‐pass cardiac perfusion MRI. Magn Reson Med. 2010;64:767–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Feng L, Srichai MB, Lim RP, Harrison A, King W, Adluru G, Dibella E, Sodickson D, Otazo R, Kim D. Highly accelerated real‐time cardiac cine MRI using k‐t SPARSE‐SENSE. Magn Reson Med. 2013;70:64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Peyvandi S, Kim H, Lau J, Barkovich AJ, Campbell A, Miller S, Xu D, McQuillen P. The association between cardiac physiology, acquired brain injury, and postnatal brain growth in critical congenital heart disease. J Thorac Cardiovasc Surg. 2017;155:291–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rudolph AM. Impaired cerebral development in fetuses with congenital cardiovascular malformations: Is it the result of inadequate glucose supply? Pediatr Res. 2016;80:172–177. [DOI] [PubMed] [Google Scholar]
- 28. Kaltman JR, Di H, Tian Z, Rychik J. Impact of congenital heart disease on cerebrovascular blood flow dynamics in the fetus. Ultrasound Obstet Gynecol. 2005;25:32–36. [DOI] [PubMed] [Google Scholar]
- 29. Donofrio MT, Bremer YA, Schieken RM, Gennings C, Morton LD, Eidem BW, Cetta F, Falkensammer CB, Huhta JC, Kleinman CS. Autoregulation of cerebral blood flow in fetuses with congenital heart disease: the brain sparing effect. Pediatr Cardiol. 2003;24:436–443. [DOI] [PubMed] [Google Scholar]
- 30. Pearce W. Hypoxic regulation of the fetal cerebral circulation. J Appl Physiol. 2006;100:731–738. [DOI] [PubMed] [Google Scholar]
- 31. Morton PD, Korotcova L, Lewis BK, Bhuvanendran S, Ramachandra SD, Zurakowski D, Zhang J, Mori S, Frank JA, Jonas RA, et al. Abnormal neurogenesis and cortical growth in congenital heart disease. Sci Transl Med. 2017;9(eaah7029). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Homsy J, Zaidi S, Shen Y, Ware JS, Samocha KE, Karczewski KJ, DePalma SR, McKean D, Wakimoto H, Gorham J, et al. De novo mutations in congenital heart disease with neurodevelopmental and other congenital anomalies. Science. 2015;350:1262–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wu Y, Kapse K, Jacobs M, Niforatos‐Andescavage N, Donofrio MT, Krishnan A, Vezina G, Wessel D, du Plessis A, Limperopolous C. Association of maternal psychological distress with in utero brain development in fetuses with congenital heart disease. JAMA Pediatr. 2020;174(3):e195316–e195326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Avni R, Golani O, Akselrod‐Ballin A, Cohen Y, Biton I, Garbow JR, Neeman M. MR Imaging‐derived oxygen‐hemoglobin dissociation curves and fetal‐placental oxygen‐hemoglobin affinities. Radiology. 2016;280:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nafisi Al B, van Amerom JFP, Forsey J, Jaeggi E, Grosse‐Wortmann L, Yoo S‐J, Macgowan CK, Seed M. Fetal circulation in left‐sided congenital heart disease measured by cardiovascular magnetic resonance: a case‐control study. J Cardiovasc Magn Reson. 2013;15:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schlatterer SD, Murnick J, Jacobs M, White L, Donofrio MT, Limperopoulos C. Placental pathology and neuroimaging correlates in neonates with congenital heart disease. Sci Rep. 2019;9:4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rychik J, Goff D, McKay E, Mott A, Tian Z, Licht DJ, Gaynor JW. Characterization of the placenta in the newborn with congenital heart disease: distinctions based on type of cardiac malformation. Pediatr Cardiol. 2018;39:1165–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. You W, Andescavage NN, Kapse K, Donofrio MT, Jacobs M, Limperopoulos C. Hemodynamic responses of the placenta and brain to maternal hyperoxia in fetuses with congenital heart disease by using blood oxygen‐level dependent MRI. Radiology. 2020;294:141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rasmussen MB, Eriksen VR, Andresen B, Hyttel‐Sørensen S, Greisen G. Quantifying cerebral hypoxia by near‐infrared spectroscopy tissue oximetry: the role of arterial‐to‐venous blood volume ratio. J Biomed Opt. 2017;22:025001. [DOI] [PubMed] [Google Scholar]
- 40. Wang Y, Zhang Y, Xuan W, Kao E, Cao P, Tian B, Ordovas K, Saloner D, Liu J, et al. Fully automatic segmentation of 4D MRI for cardiac functional measurements. Med Phys. 2019;46:180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Figures S1–S4
Reference 40


