Abstract
Background
Bisphenol A (BPA) is a non-persistent chemical with endocrine disrupting abilities used in a variety of consumer products. Fetal exposure to BPA is of concern due to the elevated sensitivity, which particularly relates to the developing brain. Several epidemiological studies have investigated the association between prenatal BPA exposure and neurodevelopment, but the results have been inconclusive.
Objective
To assess the association between in utero exposure to BPA and Attention Deficit/Hyperactivity Disorder (ADHD-) symptoms and symptoms of Autism Spectrum Disorder (ASD) in 2 and 5-year old Danish children.
Method
In the prospective Odense Child Cohort, BPA was measured in urine samples collected in gestational week 28 and adjusted for osmolality. ADHD and ASD symptoms were assessed with the use of the ADHD scale and ASD scale, respectively, derived from the Child Behaviour Checklist preschool version (CBCL/1½-5) at ages 2 and 5 years. Negative binomial and multiple logistic regression analyses were performed to investigate the association between maternal BPA exposure (continuous ln-transformed or divided into tertiles) and the relative differences in ADHD and ASD problem scores and the odds (OR) of an ADHD and autism score above the 75th percentile adjusting for maternal educational level, maternal age, pre-pregnancy BMI, parity and child age at evaluation in 658 mother-child pairs at 2 years of age for ASD-score, and 427 mother-child pairs at 5 years of age for ADHD and ASD-score.
Results
BPA was detected in 85.3% of maternal urine samples even though the exposure level was low (median 1.2 ng/mL). No associations between maternal BPA exposure and ASD at age 2 years or ADHD at age 5 years were found. Trends of elevated Odds Ratios (ORs) were seen among 5 year old children within the 3rd tertile of BPA exposure with an ASD-score above the 75th percentile (OR = 1.80, 95% CI 0.97,3.32), being stronger for girls (OR = 3.17, 95% CI 1.85,9.28). A dose-response relationship was observed between BPA exposure and ASD-score at 5 years of age (p-trend 0.06) in both boys and girls, but only significant in girls (p-trend 0.03).
Conclusion
Our findings suggest that prenatal BPA exposure even in low concentrations may increase the risk of ASD symptoms which may predict later social abilities. It is therefore important to follow-up these children at older ages, measure their own BPA exposure, and determine if the observed associations persist.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12940-021-00709-y.
Keywords: Bisphenol A, ADHD, Autism Spectrum Disorder, Neurodevelopment, Endocrine disruptor
Introduction
Attention Deficit Hyperactivity Disorder (ADHD) and Autism Spectrum Disorders (ASD) are two of the most common neurodevelopmental disorders in children [1]. Both have a large impact on the lives of the affected individuals and are associated with reduced everyday life functioning, academic performance and a decreased quality of life [2, 3]. The global prevalence of ADHD is estimated to be between 5.3–7.2% [4, 5] and the European prevalence of ASD reported range from 4.2/1000–17.4/1000 with high variability in prevalence estimates worldwide [6]. Although genetic factors play an important role in the occurrence of these disorders, knowledge of the underlying causes are scarce, and environmental toxicants are suspected to contribute to their occurrence [7–10].
During fetal life, the brain development is particular sensitive, and prenatal exposure to environmental chemicals is therefore of concern [11]. Bisphenol A (BPA) is a non-persistent chemical used in the production of polycarbonate plastics and epoxy resins. It is a known endocrine disrupter, which possesses estrogenic, anti-estrogenic, anti-androgenic and anti-thyroid properties [12]. It is present in a variety of consumer products, including; microwave ovenware, food and liquid storage containers, children’s toys, protective inner lining in food cans, dental sealants, and thermal paper receipts [13]. Exposure to BPA occurs through oral ingestion of BPA contaminated food, through dermal contact or inhalation [14]. BPA is present in urine samples of 95% of the general population in the US [15], and > 80% of samples of the general population in Denmark [16], thus BPA exposure is ubiquitous. Several human studies have investigated associations between maternal BPA exposure and behavioural alterations in the offspring e.g. externalizing and internalizing behaviour, hyperactivity, inattention, anxiety, aggression and autistic symptoms [17–31]. However, the results have been inconclusive, and most cohort studies have reported generally larger effect size in boys [20–24, 27]. Furthermore, a recent study has provided evidence that prenatal BPA exposure has an adverse effect on the development of white matter microstructure in some brain regions which might mediate behavioural problems in children [29]. Three studies found no association between prenatal BPA exposure and behaviour [30–32]. The inconsistencies may be explained by methodological differences between studies, the time window of exposure assessment, the specific behavioural domains tested, the matrix of exposure assessment (urine sample or cord blood), child age (2–10 years), exposure level, and sociodemographic characteristics of the study participants [33–35].
A previous study in the Odense Child Cohort (OCC) found an association between prenatal BPA exposure and delayed language development, but no association with ADHD related symptoms at 2 years of age among 658 children [32]. The lack of an association might be attributed to the low prevalence of ADHD symptoms at 2 years, and thus a follow up at 5 years might detect symptoms that were not recognized at 2 years. We therefore aimed to investigate, if maternal exposure to BPA was associated with increased symptoms of ASD at 2 and 5 years, and with increased ADHD symptoms at 5 years in 427 mother-child pairs from the OCC.
Materials and methods
Study population
From 2010 to 2012, women residing in the municipality of Odense with a newly diagnosed pregnancy before 16 weeks of gestation were invited to participate in the prospective OCC, and 2874 agreed to participate [36]. Twins and mothers of non-western origin (N = 332) were excluded from this study.
BPA exposure assessment
BPA was measured in maternal urine samples. An aliquot of 10–12 mL fasting urine was collected in the morning at approximately week 28 of pregnancy (range: week 26–34). Samples were stored in freezers at − 80 °C until chemical analysis [36, 37]. The first 196 samples were selected randomly from women enrolled in OCC between September 2010 and June 2011. The remaining samples were selected based on availability of questionnaire and clinical data. Urine samples were deconjugated by enzymatic hydrolysis and then analysed by an isotope dilution Turboflow-LC-MS/MS method for simultaneous determination of BPA and other phenols [16, 38]. Samples were analysed in 17 batches (5 batches in 2011 and 11 in the end of 2012) all including standards for calibration curves, about 35 cohort samples, two blanks and two control pools spiked with high and low level bisphenol A standards. The inter-day variation was ≤14%. The limit of detection (LOD) for urinary BPA was 0.12 ng/mL. There was no difference in the spiked control material between the two measuring periods [16].
To account for urinary dilution, all BPA concentrations were adjusted for osmolality. In contrast to urinary creatinine adjustment, urine osmolality is directly related to the number of particles in solution and is unaffected by the molecular weight and size of these particles [39]. Osmolality was measured by freezing point depression method with automatic cryoscopic osmometer (Osmomat®030 from Gonotec, Berlin, Germany). BPA concentrations >LOD were adjusted for urinary dilution by multiplying the individual BPA urine concentration (ng/mL) with the median osmolality for all urine samples (0.62 osm/kg) and dividing it with the osmolality (osm/kg) of the individual urine sample. The BPA concentrations below the LOD were not osmolality adjusted but were substituted by LOD/√2.
Child behaviour checklist for ages 1½-5
The Child Behaviour Checklist; 1½-5 (CBCL/1½-5) is a parent rated questionnaire measuring emotional and behavioural problems in children between 1½ and 5 years of age. CBCL/1½-5 consists of 100 problem questions, which the parents are asked to rate on a 3-point Likert scale based on the preceding 2 months as: 0 = not true, 1 = somewhat/sometimes true or 2 = very true/ often true. A standardized version of CBCL/1½-5 translated into Danish [40] was sent to the parents in the OCC, when children were between 1.9–4.0 (median 2.7) and 4.9–7.0 (median 5.2) years of age.
We measured ADHD related symptoms at the DSM-oriented ADHD problem scale, extracted from CBCL/1½-5. It contains 6 questions (cannot concentrate, hyperactive, cannot stand waiting, demands met immediately, gets into everything, quickly shifts) with a scale-score between 0 and 12 points. Autistic symptoms were measured at the DSM-oriented ASD problem scale (also called Pervasive Developmental Problem (PDP) scale), extracted from CBCL/1½-5. It contains 13 questions (afraid to try new things, avoids eye contact, can’t stand having things out of place, disturbed by any change in routine, does not answer, does not get along with other children, repeatedly rocks head or body, seems unresponsive to affection, shows little affection toward people, speech problem, strange behaviour, upset by new people or situations, withdrawn) with a possible scale-score between 0 and 26 points. Results were kept as raw scores and dichotomized at the 75th percentile to identify a subclinical population, and at the 90th percentile, since a score on the DSM-ADHD scale derived from CBCL/1½-5 above the 90th percentile is a predictor of a later ADHD diagnosis [41].
Covariates
Data on maternal education and Body Mass Index (BMI) and smoking habits were obtained through questionnaires filled in during pregnancy. Birth information, including; maternal age, parity, birth weight, gestational age, and child sex was extracted from obstetric journals. Data on child’s health including duration of breastfeeding were obtained through questionnaires filled in, when the children were 3 and 18 months of age. Maternal ethnicity was obtained through data from the municipality. Finally, information regarding parental psychiatric diagnosis was extracted from the Danish National Patient Registry.
Statistical analysis
ADHD- and ASD scores were not normally distributed and scores of respectively below and above the 75th and 90th percentile was calculated for both scales. The 75th and 90th percentile dichotomization of the ADHD score corresponded to a score of ≥4 and ≥ 5, respectively, at 5 years of age. The 75th and 90th percentile dichotomization of the ASD score corresponded to a score of ≥3 and ≥ 4, respectively, at 2 years of age, and a score of ≥4 and ≥ 5, respectively, at 5 years. At 2 years of age, 26% of children had an ASD score ≥ 3, and were dichotomized into </≥ 75th percentile. At 5 years of age respectively 20 and 26% of children had a score of ≥4 on the ASD and ADHD scale. Therefore, the children were dichotomized into ≤/> the 75th for ASD score and </≥ 75th percentile for ADHD score. BPA concentrations were not normally distributed, and 15% of concentrations were below LOD. Thus, we both divided BPA concentrations into tertiles, and kept it as an ln-transformed continuous variable. However, it should be noted that residuals did not fit the logistic model with ln-transformed continuous BPA, and thus results should be interpreted with caution. The characteristics of the included participants were compared to the excluded participants by the use of Chi2 test. Differences in urinary BPA concentration, ADHD and ASD scores (below and above 75th percentile) were examined according to maternal and child characteristics using non-parametric Kruskal Wallis test (BPA) and Chi2 test (ADHD and ASD).
First, we used multiple logistic regression to calculate the Odds Ratio (OR) and 95% confidence intervals (95% CI) of having an ADHD or ASD problem score > 75th and ≥ 90th percentile across increasing maternal BPA exposure (continuous ln-transformed or divided into tertiles) and to test the trend across tertiles. Second, ASD- and ADHD-scores were analysed as ordinal data using negative binomial regression to estimate the relative change in ASD or ADHD-score expressed as ratio of risk between exposure groups. In addition, a sub-analysis was undertaken to calculate the odds ratio of having an ASD-score above the 75% percentile at both 2 and 5 years of age. Confounders were selected based on careful review of the literature, and if they varied by exposure and outcome status. Furthermore, covariates were included, if they changed the effect estimate of the independent variable with ≥10%. Possible confounders included in the final analyses were maternal educational level, parity, pre-pregnancy BMI and maternal age. Furthermore, we adjusted for child age at evaluation, as small differences in age could affect neuropsychological status. In addition, the unstratified analyses were also adjusted for child sex. A sensitivity analyses was performed for the 75th percentile additionally adjusting for the possible mediators breastfeeding and birth weight, as we were interested in the direct rather than the total effect of BPA on ADHD and ASD symptoms. Since previous research has found BPA to cause sexually dimorphic alterations in child behaviour [33, 34], all regression analyses were also performed for boys and girls separately, and we tested, if the associations were modified by sex by inserting interaction terms (BPA x child sex) in the regression models. No significant interaction was found between maternal BPA exposure and child sex.
To evaluate the fit of the logistic regression models, goodness of fit was tested using Hosmer-Lemeshow test and accepted for all models. Results are presented with an Odds Ratio (OR) 95% confidence interval (CI), and a p-value < 0.05 was considered statistically significant.
Results
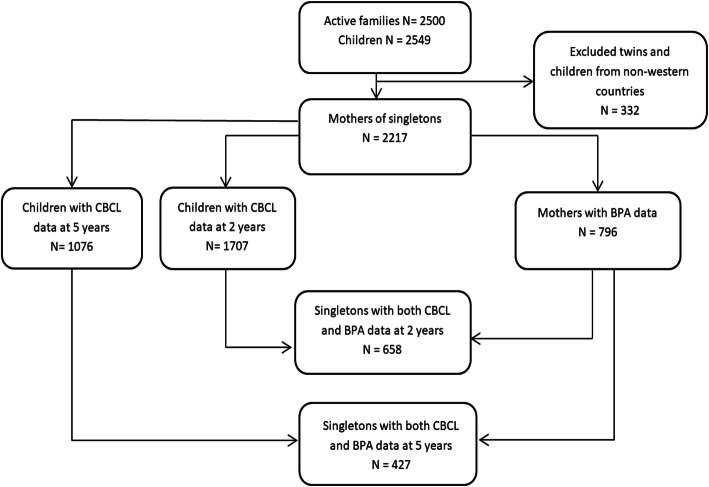
A total of 2217 mother-child pairs were enrolled in the OCC at 27 months of age. Of these, 1707 responded to CBCL1½-5 at 2 years of age, 1076 participants responded at 5 years of age, and 796 mothers had BPA measured in urine. A total of 658 participants had both BPA and CBCL1½-5 data available at 2 years of age, and 427 at 5 years of age, and were therefore included in this study (Fig. 1). Compared to the excluded participants (N = 1559), the included participants were less often smokers and breastfeed for a longer period of time (Supplementary Table 1).
Fig. 1.
Flowchart presenting the selection of the 658 and 427 study participants from the Odense Child Cohort
BPA was detected in 85.3% of samples with a median (25–75 percentile) concentration of 1.2 ng/mL (0.5–2.6) (Table 1). BPA concentrations were significantly higher among overweight women. Furthermore, BPA concentrations were higher among nulliparous women, among less educated women and among children born at term. Women who did not breastfeed their children exclusively also had higher BPA concentration than women who breastfed, though these differences were not statistically significant (Table 1).
Table 1.
Maternal and child characteristics according to median (M) and 25–75 percentiles (25–75%) BPA concentrations in maternal urine; and percentage of children with an ADHD- or ASD- score above the 75th percentile at 2 or 5 years of age
| Maternal and child characteristics | N (%) | BPA ng/mL M (25–75%) |
ASD-score > 75% 2 years Percent |
ASD-score > 75% 5 years Percent |
ADHD-score > 75% 5 years Percent |
|---|---|---|---|---|---|
| 658 (100) | 1.2 (0.5–2.6) | ||||
| Parity | |||||
| Nulliparous | 371 (56) | 1.37 (0.47–2.88) | 32 | 42 | 29 |
| Multiparous | 287 (44) | 1.20 (0.49–2.26) | 18 | 24 | 22 |
| P-valuea | 0.07 | < 0.001* | < 0.001* | 0.08 | |
| Pre-pregnancy BMI (kg/m2) | |||||
| > 18.5- < 25 | 412 (62) | 1.03 (0.30–2.32) | 27 | 32 | 24 |
| 25–30 | 169 (26) | 1.82 (0.90–2.77) | 24 | 33 | 28 |
| > 30 | 77 (12) | 1.17 (0.55–2.97) | 24 | 49 | 33 |
| P-valuea | 0.002* | 0.65 | 0.06 | 0.35 | |
| Smoking | |||||
| No | 639 (97) | 1.23 (0.47–2.54) | 25 | 32 | 25 |
| Yes | 19 (3) | 1.21 (0.63–2.68) | 37 | 73 | 33 |
| P-valuea | 0.19 | 0.29 | 0.001* | 0.49 | |
| Education b | |||||
| high school or less | 177 (27) | 1.53 (0.55–2.66) | 32 | 54 | 39 |
| high school + 1–4 years | 336 (53) | 1.20 (0.49–2.73) | 25 | 29 | 22 |
| high school + > 4 years | 131 (20) | 1.35 (0.33–2.24) | 18 | 21 | 18 |
| P-valuea | 0.34 | 0.04* | < 0.001* | < 0.001* | |
| Age | |||||
| < 25 | 61 (9) | 1.08 (0.39–3.34) | 40 | 35 | 44 |
| 25–34 | 438 (67) | 1.23 (0.47–2.49) | 26 | 35 | 25 |
| > 34 | 159 (24) | 1.28 (0.55–2.52) | 19 | 30 | 21 |
| P-valuea | 0.82 | 0.006* | 0.06 | 0.03* | |
| Psychiatric predisposition | |||||
| None | 565 (86) | 1.27 (0.50–2.52) | 24 | 33 | 24 |
| Predisposition from parents | 93 (14) | 1.14 (0.38–2.97) | 36 | 42 | 33 |
| P-valuea | 0.88 | 0.01* | 0.16 | 0.14 | |
| Birth weight (gram) | |||||
| ≤ 3560 | 332 (51) | 1.24 (0.44–2.89) | 27 | 42 | 27 |
| > 3560 | 326 (49) | 1.23 (0.52–2.38) | 25 | 26 | 24 |
| P-valuea | 0.13 | 0.52 | < 0.001* | 0.5 | |
| Exclusive breastfeeding (weeks)c | |||||
| 0 | 101 (16) | 1.96 (0.79–3.72) | 23 | 45 | 36 |
| 1–12 | 256 (42) | 1.20 (0.47–2.38) | 25 | 34 | 31 |
| > 12 | 259 (42) | 1.24 (0.41–2.42) | 27 | 32 | 18 |
| P-valuea | 0.22 | 0.65 | 0.18 | 0.005* | |
| Gestation | |||||
| < 37 + 0 | 24 (4) | 0.54 (0.00–2.73) | 42 | 62 | 31 |
| > 37 + 0 | 634 (96) | 1.23 (0.49–2.54) | 25 | 33 | 26 |
| P-valuea | 0.84 | 0.07 | 0.03* | 0.67 | |
aP-value < 0.05 with Kruskal wallis test (BPA) or Chi2 test (Autistic- or ADHD symptoms)
b 4 observations missing
c42 observations missing
The median score (25–75%) on the ASD-scale was 1 (0–2) for girls, and 1 (0–3) for boys at 2 years of age and 1 (0–3) for girls, and 2 (1–3) for boys at 5 years of age. The median score (25–75%) on the ADHD scale was 2 (0–3) for girls, and 2 (1–4) for boys at 5 years of age. Higher ADHD/ASD scores were found among children of mothers, who were nulliparous, smokers, less educated, younger, among children with a psychiatric predisposition, children who had been exclusively breastfed for a shorter duration, children with lower birth weight, and children who were born prematurely (Table 1).
At 2 years of age, no monotonic-trend was observed across tertiles of BPA for the categorized and continuous ASD-score (Table 2, Table 3). In addition, no association was found with continuous ln-transformed BPA (Table 3). At 5 years of age, children within the 3rd tertile of BPA exposure had a 23% increase in ASD-score (IRR: 1.23 (95% CI 0.98,1.53)), compared to those in the 1st tertile (p-trend = 0.07), although not significant (Table 2). Furthermore, children within the 3rd tertile of prenatal BPA exposure had an OR of 1.80 (95% CI 0.97,3.32) scoring above the ASD-score > 75th percentile compared to those within the 1st tertile of exposure (p-trend = 0.06), and each doubling in BPA exposure (ng/mL) was associated with a 25% higher odds of an ASD-score score > 75th percentile (OR: 1.25 (95% CI 1.03,1.52)). The sex-stratified analysis showed that the association was stronger in girls, and girls in the 3rd tertile of BPA exposure had an OR of 3.17 (95% CI 1.85,9.28) and boys had an OR of 1.42 (95% CI 0.64,3.13) compared to those within the 1st tertile of exposure, and a similar direction of association was found with continuous ln-transformed BPA (Table 3). The association was even stronger for the 90th percentile cut-off in girls, (OR: 5.03 (95% CI 0.97–26.08)) (p-trend 0.04) but the confidence interval was also wider (Table 3).
Table 2.
Negative binomial regression analysis of the association between osmolality adjusted maternal BPA exposure divided into tertiles and the incidence rate ratio (IRR) and 95% confidence intervals (CI 95%) of ADHD-score in boys and girls at 2 and 5 years of age
| Osmolality adjusted BPA (ng/mL) |
Adjusteda IRR (CI 95%) All (N = 654) |
Adjusteda IRR (CI 95%) Boys (N = 347) | Adjusteda IRR (CI 95%) Girls (N = 307) |
| ASD-score at 2 years | |||
| 1th tertile (≤0.87) | Reference | Reference | Reference |
| 2nd tertile (0.88–1.96) | 0.87 (0.71–1.07) | 0.85 (0.65–1.10) | 0.89 (0.64–1.24) |
| 3rd tertile (≥ 1.97) | 1.02 (0.84–1.24) | 1.08 (0.84–1.39) | 0.91 (0.66–1.25) |
| p-trendb | 0.80 | 0.48 | 0.54 |
| Osmolality adjusted BPA (ng/mL) |
Adjusteda IRR (CI 95%) All (N = 425) |
Adjusteda IRR (CI 95%) Boys (N = 223) | Adjusteda IRR (CI 95%) Girls (N = 202) |
| ASD-score 5 years | |||
| 1st tertile (≤0.87) | Reference | Reference | Reference |
| 2nd tertile (0.88–1.96) | 1.13 (0.89–1.42) | 1.09 (0.80–1.51) | 1.18 (0.58–5.54) |
| 3rd tertile (≥ 1.97) | 1.23 (0.98–1.53) | 1.20 (0.88–1.63) | 1.29 (0.93–1.78) |
| p-trendb | 0.07 | 0.24 | 0.13 |
| Osmolality adjusted BPA (ng/mL) |
Adjusteda IRR (CI 95%) All (N = 425) |
Adjusteda IRR (CI 95%) Boys (N = 223) | Adjusteda IRR (CI 95%) Girls (N = 202) |
| ADHD-score at 5 years | |||
| 1st tertile (≤0.87) | Reference | Reference | Reference |
| 2nd tertile (0.88–1.96) | 0.96 (0.76–1.22) | 0.88 (0.66–1-17) | 1.11 (0.75–1.65) |
| 3rd tertile (≥ 1.97) | 1.08 (0.86–1.35) | 0.98 (0.75–1.30) | 1.26 (0.87–1.85) |
| p-trendb | 0.86 | 0.43 | 0.76 |
aAnalyses adjusted for maternal education, maternal age, pre-pregnancy BMI, child age at evaluation, parity. The sex-combined analyses were additionally adjusted for child sex
bTrend across tertiles tested by inserting the tertile osmolality adjusted BPA as an ordinal indicator variable (0,1,2)
* P-value < 0.05
Table 3.
Multiple logistic regression analysis of the association between osmolality adjusted maternal BPA exposure divided into tertiles and as continuous ln-transformed and the odds ratio (OR) and 95% confidence intervals (CI 95%) of an ASD-score > 75th percentile compared to < 75th percentile (reference) and ≥ 90th percentile compared to <90th percentile (reference) in boys and girls at 2 and or 5 years of age
|
≥75th percentile Adjusted OR (CI 95%) |
≥90th percentile Adjusted OR (CI 95%) |
|||||
| ASD-score 2 years | ||||||
| Osmolality adjusted BPA (ng/mL) |
All (N = 166/488) |
Boys (N = 97/250) |
Girls (N = 69/238) |
All (N = 98/556) |
Boys (N = 64/283) |
Girls (N = 34/273) |
| 1th tertile (≤0.87) | Reference | Reference | Reference | Reference | Reference | Reference |
|
2nd tertile (0.88–1.96) |
0.81 (0.47–1.20) | 0.86 (0.47–1.59) | 0.71 (0.35–1.43) | 0.84 (0.47–1.48) | 0.80 (0.39–1.66) | 0.89 (0.35–2.27) |
|
3rd tertile (≥ 1.97) |
1.06 (0.70–1.68) | 1.07 (0.60–1.94) | 1.05 (0.54–2.31) | 1.18 (0.69–2.00) | 1.37 (0.70–2.68) | 0.83 (0.34–2.06) |
| p-trendb | 0.65 | 0.78 | 0.86 | 0.49 | 0.31 | 0.69 |
|
Continuous lnBPAc |
1.08 (0.94–1.24) | 1.11 (0.93–1.34) | 1.02 (0.83–1.25) | 1.10 (0.93–1.31) | 1.17 (0.94–1.45) | 0.97 (0.73–1.29) |
|
>75th percentile Adjusted OR (CI 95%) |
≥90th percentile Adjusted OR (CI 95%) |
|||||
| ASD-score 5 years | ||||||
| Osmolality adjusted BPA (ng/mL) |
All (N = 86/339) |
Boys (N = 54/169) |
Girls (N = 32/170) |
All (N = 59/366) |
Boys (N = 34/189) |
Girls (N = 25/177) |
| 1th tertile (≤0.87) | Reference | Reference | Reference | Reference | Reference | Reference |
|
2nd tertile (0.88–1.96) |
1.22 (0.63–2.36) | 1.02 (0.43–2.40) | 1.80 (0.58–5.54) | 0.86 (0.36–1.99) | 0.68 (0.24–1.90) | 1.96 (0.32–11.83) |
|
3rd tertile (≥ 1.97) |
1.80 (0.97–3.32) | 1.42 (0.64–3.13) | 3.17 (1.85–9.28)* | 1.65 (0.78–3.49) | 1.20 (0.47–2.99) | 5.03 (0.97–26.08) |
| p-trendb | 0.06 | 0.37 | 0.03* | 0.15 | 0.69 | 0.04* |
|
Continuous lnBPAc |
1.25 (1.03–1.52)* | 1.16 (0.91–1.49) | 1.48 (1.04–2.12)* | 1.20 (0.94–1.53) | 1.10 (0.83–1.47) | 1.61 (0.96–2.69) |
aAnalyses adjusted for maternal education, maternal age, pre-pregnancy BMI, child age at evaluation, parity. The sex-combined analyses were additionally adjusted for child sex
bTrend across tertiles tested by inserting the tertile osmolality adjusted BPA as an ordinal indicator variable (0,1,2)
cBPA inserted as a continuous variable transformed by the natural logarithm
* P-value < 0.05
Forty children had an ASD-score ≥ 75th percentile at both 2 and 5 years. Increased odds of an ASD-score ≥ 75th percentile was found among children in the 3rd tertile of prenatal BPA exposure, with an OR of 2.05 (95% CI 0.90,4.64) (p-trend = 0.06). For each doubling in BPA exposure the odds of a score ≥ 75th percentile increased with 38% (OR: 1.38 (95% CI 1.02,1.84)) compared to a score < 75th percentile (Table 4).
Table 4.
Multiple logistic regression analysis of the association between osmolality adjusted maternal BPA exposure divided into tertiles and as continuous ln-transformed and the odds ratio (OR) and 95% confidence intervals (CI 95%) of an ASD-score ≥ 75th percentile compared to < 75th percentile (reference) in boys and girls at 2 and 5 years of age
| Osmolality adjusted BPA (ng/ml) | All (N = 38/376) Adjusteda OR (CI 95%) |
Boys (N = 25/197) Adjusteda OR (CI 95%) |
Girls (N = 13/179) Adjusteda OR (CI 95%) |
|---|---|---|---|
| ASD-score ≥ 75th percentile at both 2 and 5 years | |||
| 1th tertile (≤0.87) | Reference | Reference | Reference |
| 2nd tertile (0.88–1.96) | 0.72 (0.26–2.01) | 0.58 (0.15–2.17) | 0.95 (0.17–5.52) |
| 3rd tertile (≥ 1.97) | 2.05 (0.90–4.64) | 2.17 (0.79–6.01) | 1.94 (0.44–8.57) |
| p-trendb | 0.06 | 0.09 | 0.33 |
| Continuous lnBPA c | 1.38 (1.02–1.84)* | 1.42 (0.99–2.02) | 1.34 (0.79–2.25) |
aAnalyses adjusted for maternal education, maternal age, pre-pregnancy BMI, child age at evaluation, parity. The sex-combined analyses were additionally adjusted for child sex
bTrend across tertiles tested by inserting the tertile osmolality adjusted BPA as an ordinal indicator variable (0,1,2)
cBPA inserted as a continuous variable transformed by the natural logarithm
* P-value < 0.05
An increase in OR was found among children in the 3rd tertile of BPA exposure and ADHD symptoms at age 5 years at the 75th percentile and at the 90th percentile cut-off (Table 5), although modest in magnitude and not statistically significant.
Table 5.
Multiple logistic regression analysis of the association between osmolality adjusted maternal BPA exposure divided into tertiles and as continuous ln-transformed and the odds ratio (OR) and 95% confidence intervals (CI 95%) of an ADHD-score ≥ 75% compared to < 75% (reference) and ≥ 90th percentile compared to <90th percentile (reference) in boys and girls at 5 years of age
| Osmolality adjusted BPA (ng/mL) | All chidren (N = 109/316) Adjusteda OR (CI 95%) |
Boys (N = 66/157) Adjusteda OR (CI 95%) |
Girls (N = 43/159) Adjusteda OR (CI 95%) |
|---|---|---|---|
| ADHD-score 5 years ≥ 75th percentile | |||
| 1st tertile (≤0.87) | Reference | Reference | reference |
| 2nd tertile (0.88–1.96) | 0.81 (0.45–1.45) | 0.80 (0.37–1-73) | 0.88 (0.36–2.20) |
| 3rd tertile (≥ 1.97) | 1.09 (0.64–1.87) | 1.06 (0.51–2.17) | 1.29 (0.57–2.99) |
| P-trendb | 0.71 | 0.86 | 0.52 |
| Continuous lnBPAc | 1.05 (0.88–1.25) | 1.01 (0.80–1.26) | 1.14 (0.85–1.50) |
| Osmolality adjusted BPA (ng/mL) |
All chidren (N = 64/361) Adjusteda OR (CI 95%) |
Boys (N = 39/184) Adjusteda OR (CI 95%) |
Girls (N = 25/177) Adjusteda OR (CI 95%) |
| ADHD-score 5 years ≥ 90th percentile | |||
| 1st tertile (≤0.87) | Reference | Reference | Reference |
| 2nd tertile (0.88–1.96) | 0.92 (0.42–1.93) | 0.60 (0.21–1.69) | 1.78 (0.55–5.73) |
| 3rd tertile (≥ 1.97) | 1.33 (0.68–2.61) | 1.45 (0.61–3.45) | 1.29 (0.46–4.64) |
| p-trendb | 0.37 | 0.32 | 0.56 |
| Continuous lnBPAc | 1.17 (0.93–1.46) | 1.17 (0.88–1.56) | 1.25 (0.85–1.85) |
aAnalyses adjusted for maternal education, maternal age, pre-pregnancy BMI, child age at evaluation, parity. The sex-combined analyses were additionally adjusted for child sex
bTrend across tertiles tested by inserting the tertile osmolality adjusted BPA as an ordinal indicator variable (0,1,2)
cBPA inserted as a continuous variable transformed by the natural logarithm
* P-value < 0.05
In the sensitivity analysis additionally adjusted for breastfeeding and birth weight the ORs did not change substantially (supplementary Table 2).
Discussion
In this low exposed mother child cohort, maternal exposure to BPA was associated with an increased odds of an ASD-score > 75th percentile among children at 5 years of age. No association between maternal BPA exposure and ASD or ADHD scores at respectively age 2 and 5 years was found. It is important to note, that none of these children were assessed or diagnosed with ASD or ADHD and CBCL/1½-5 is not a diagnostic tool. However, the scores obtained from the scales are in accordance with the Danish reference norms [40], and previous research has established that the CBCL derived DSM-ADHD and DSM-ASD scales are both valid assessors of ADHD or ASD psychopathology [41, 42]. The lack of an association at age 2 years may be explained by CBCL/1½-5 ASD scale does not capture autism specific symptoms as well at age 2 years compared to age 5 years.
The association between maternal BPA exposure and child neurodevelopment has been investigated by several studies (reviewed in [33–35]). To the best of our knowledge, five studies have investigated the association between prenatal exposure to BPA and social impairment or autism-symptoms [26, 27, 30, 31, 43]. Two North American prospective cohort studies and one North American prospective high-ASD risk cohort found no association between maternal BPA exposure and ASD or non-typical development at 3 years [43], autistic symptoms in preschool children [30] or social impairment in school-aged children [31], which is in contrast to our findings. However, in the latter study of school aged children Miodovnik et al. (2011) [31] a significant association between prenatal BPA exposure and total SRS-score was found when 6 outliers were removed. Our findings are in accordance with a Korean prospective cohort with 304 mother-child pairs, which found decreased social communication skills in 4-year old girls with prenatal BPA exposure > 3.0 μg/g (creatinine adjusted) [26]. However, the American and Korean studies included multiethnic or Korean participants most with higher BPA exposure, whereas our participants were homogeneous and most of Western origin. Since ethnic origin may impact how parents rate child behaviour [44, 45], our findings are not directly comparable, but are in the same direction. A Canadian cohort study comparable to ours in terms of sociodemographic characteristics of the study population and BPA exposure [27] found an association between prenatal BPA exposure and poorer reciprocal social behaviours in 537 children, strongest in boys, measured with the Social Responsiveness Scale 2 (SRS-2) at 3 years [27]. They measured BPA at 12 weeks of gestation with a median concentration of 0.8 ng/mL, whereas we measured BPA at 28 weeks of gestation, and the median BPA concentration was 1.2 ng/mL. We found stronger associations in girls, whereas Braun et al. (2017) [27] found strongest associations in boys. ASD is several times more frequent in boys compared to girls [46], and in animal studies, BPA exposure has been shown to affect sexual differentiation of the brain and can either increase, decrease or eliminate sex-differences [33]. During fetal life sex steroids are crucial for the sexual differentiation of the brain, and the most vulnerable time for the sexual differentiation is assumed to be between week 8–24 of gestation [33, 47]. The difference in the sex-specific direction of the association between our study and the study by Braun et al. (2017) [27] might be attributed to the difference in time of exposure assessment, as early pregnancy might impact behavior in a different way as opposed to mid pregnancy. Furthermore, Lim et al. (2017) [26] also found stronger associations in girls and measured BPA exposure between 14 and 27 (median 20 weeks) of gestation. In our study, only 33 girls scored above the 75th percentile, which reduced power. In addition, different tests were used.
In a previous publication from our cohort, no association between maternal BPA exposure and ADHD symptoms at age 2 years was found [32]. These findings were confirmed at age 5 years in the current work, and are in contrast to the results of a large exposome study including data from 5 European cohorts that found prenatal BPA to be associated with worse externalizing behavior problems measured with the Strength and Difficulties Questionnaire between 3 and 7 years of age [48]. In addition, four studies comparable to our have investigated prenatal BPA exposure and ADHD related symptoms, and the results were conflicting [20, 21, 25, 27]. A French [25] cohort study found an association between prenatal BPA exposure and ADHD symptoms in boys at age 5 years, measured by the Strength and Difficulties Questionnaire, and a Spanish [20] cohort study found increased inattention in boys and decreased inattention in girls at 4 years of age measured with the Criteria of Diagnostic and Statistical Manual of Mental Disorders-4th Edition (ADHD-DSM-IV). BPA exposure was, however, higher than in our cohort, which could explain, why we found no associations [20, 25]. A Canadian [27] and an American study [21] with BPA exposure concentrations comparable to ours found no association between prenatal BPA exposure and ADHD symptoms at age 3 years measured with the Behaviour Assessment System for Children 2 (BASC-2), and at 6–10 years of age as measured with CBCL (school age version), respectively, which is in accordance with our findings. Furthermore in a Canadian [29] birth cohort maternal BPA exposure measured in urine samples in the 2nd trimester (median 1.5 ng/mL) was associated with increased internalizing but not externalizing behaviour problems measured with the CBCL at 2–5 years of age in 56 mother child pairs [29]. The study is not directly comparable to ours, as they measured internalizing and externalizing problems, and we only measured specific ADHD- and ASD symptoms, but our results are in the same direction. In addition, Grohs et al (2020) [29] suggested that the association was mediated by less developed white matter microstructure in the splenium measured with diffusion magnetic resonance imaging (MRI) scan in the 56 children who provided both prenatal BPA and CBCL data.
The ability of in utero BPA exposure to alter behaviour has been confirmed in several animal studies (reviewed in [49]). In rodents, gestational or perinatal exposure to BPA have been associated with increased anxiety-like behaviour in male [50] and female mice [51], increased hyperactivity [52] and increased anxiety-like behaviour in both male and female mice [53]. The exact mechanism by which BPA impact behaviour is not completely understood, but in vitro and in vivo studies have found that exposure to BPA may modify normal brain development [54–57] Neurodevelopment is a complex process that starts early in the embryonic stage, and disruption of the critical developmental processes such as cell proliferation, neural migration, differentiation and synaptogenesis might cause adverse effects on the developing brain and could result in neurobehavioural disorders later in life [58]. A recent overview of the adverse effects of BPA exposure suggest that by disruption of thyroid and estrogenic pathways BPA may alter Brain-Derived Neurotropic Factor (BDNF) levels, which could be an important link between BPA exposure and altered neurodevelopment [59]. BDNF is a member of the neurotrophin family of proteins and is involved in modulating neurite outgrowth, synapse plasticity, and the promotion of neuronal survival and protection [59]. In utero BPA exposure has been linked to DNA methylation changes in the transcriptionally relevant region of the BDNF gene in mice [60], and in vitro, alone and in co-exposure with other chemicals BPA increases BDNF-levels which may be linked to cellular changes (increased number of neurons and altered synaptogenises) seen in children with ASD [61]. Furthermore, elevated blood BDNF levels are seen in children with ASD and thus BDNF is a possible biomarker for ASD [62].
Our study presents several strengths, including the relatively large study population and the prospective design. Furthermore, we were able to adjust for potential confounding factors. However, some limitations need mentioning. First, only a single urine sample was used as a proxy for fetal exposure to BPA. BPA is rapidly conjugated in the human body and almost fully excreted in urine within 24 h, and therefore temporal and diurnal variation in exposure patterns and excretion rates may occur [63–65]. BPA has a high within-subject variability [65, 66]. Thus, timing of urine collection may influence the observed BPA concentration, and a single spot urine sample may not reflect average gestational exposure [66]. Obtaining more than one urine sample could have improved precision. Furthermore, we measured BPA in fasting morning samples, which may have contributed to lower BPA concentrations. Misclassification of BPA exposure is, however, not likely to be differentially associated with CBCL results, as the women were unaware of their BPA exposure, when they responded to the CBCL questionnaire, and the strengths of the associations might therefore be underestimated, which could explain the non-significant findings. Likewise, the use of a parent rated questionnaire (CBCL/1½-5) to assess ASD and ADHD symptoms may cause parental reporting bias, as parents may under- or overestimate the severity of symptoms. Misclassification of ADHD- and autistic symptoms is, however, also likely non-differential, as women were unaware of their BPA exposure when responding to the questionnaires, thereby likely making the association go towards the null-hypothesis and explain the lack of statistically significant findings in our study.
Even though we adjusted for potential confounders, the possibility of additional confounding from e.g. maternal IQ cannot be dismissed. Furthermore, it is possible that other environmental chemicals in co-exposure with BPA could impact the findings. Finally, BPA was measured in gestational week 28, and brain development may be more vulnerable during early or late pregnancy or even during childhood, as studies have found childhood (preschool) BPA exposure is associated with behavioural alterations in children [18, 19, 22–24]. Thus, the association between BPA exposure and child behaviour could be dependent on the developmental time-window of exposure assessment. Unfortunately, we did not measure postnatal BPA exposure in children from the OCC. Future studies are needed to identify periods of heightened vulnerability to BPA exposure during fetal and childhood development.
Conclusions
In conclusion, in this low exposed mother-child cohort, children of mothers within the highest tertile of BPA exposure had suggestive higher odds of having an ASD-score > 75th percentile at 5-years of age whereas no association was found at age 2 years. High scores may predict later ASD symptoms which may influence social and learning abilities, and it is therefore important to follow-up these children and measure their own BPA exposure to determine if these findings persist.
Supplementary Information
Additional file 1: Supplementary table 1. Maternal and child characteristics according to included participants with BPA and CBCL1½-5 data at 2 years (N=658) and excluded participants (N=1559). Supplementary table 2. Multiple logistic regression analysis of the association between osmolality adjusted maternal BPA exposure divided into tertiles and the odds ratio (OR) and 95% confidence intervals (CI 95%) of an ASD and ADHD-score >75% compared to <75% (reference) at 2 and/or 5 years of age.
Acknowledgements
We thank the parents and children from the Odense Child Cohort for their participation. We thank the midwives for their help in recruitment and collection of samples, and the staff at Hans Christians Andersen’s Children’s hospital for their examination of the children. The laboratory technicians at the Department of Growth and Reproduction are acknowledged for their analysis of BPA.
Abbreviations
- °C
Degrees celcius
- ADHD
Attention Deficit Hyperactivity Disorder
- ASD
Autism Spectrum Disorder
- BMI
Body Mass Index
- BPA
Bisphenol A
- CBCL
Child Behaviour Checklist
- CI
Confidence interval
- IRR
Incidence rate ratio
- LC-MS/MS
Liquid chromatography and triple quadrupole mass spectrometry
- LOD
Limit of Detection
- mL
mililitre
- ng
nano gram
- OCC
Odense Child Cohort
- OR
Odds ratio
Authors’ contributions
This study was designed by TKJ, NB and JBH. JBH performed the analysis and wrote the manuscript. TKJ, NB and CAGT contributed to the data analysis and interpretation of the data. All authors contributed in editing the manuscript and have approved the final manuscript.
Funding
This work was supported by the Danish Center for Hormone Disrupting Chemicals (cend.dk), the Danish Council for Independent Research, medical sciences (4004-00352B_FSS and 8020-00123B_FSS), the Novo Nordisk Foundation (Grant no. NNF19OC000587266, NNF17OC0029404 and NNF19OC0058266), Odense University Hospital and Region of Southern Denmark, Municipality of Odense, the Mental Health Service of the Region of Southern Denmark, The National Board of Social Services, Odense University Hospital Research Foundation and Odense Patient data Explorative Network (OPEN), Helsefonden, Human Biomonitoring for EU (HBM4EU) and The Region of Southern Denmark. The LC-MS/MS equipment was financially supported by the Velux Foundation.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was performed in accordance with the second Helsinki Declaration, with written, informed consent and approved by the regional Ethical Committee (Project ID S-20090130).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Julie Bang Hansen, Email: juliebanghansen@gmail.com.
Niels Bilenberg, Email: niels.bilenberg@rsyd.dk.
Clara Amalie Gade Timmermann, Email: atimmermann@health.sdu.dk.
Richard Christian Jensen, Email: rcjensen@health.sdu.dk.
Hanne Frederiksen, Email: Hanne.Frederiksen@regionh.dk.
Anna-Maria Andersson, Email: Anna-Maria.Andersson@regionh.dk.
Henriette Boye Kyhl, Email: henriette.kyhl@rsyd.dk.
Tina Kold Jensen, Email: tkjensen@health.sdu.dk.
References
- 1.Erskine HE, Baxter AJ, Patton G, Moffitt TE, Patel V, Whiteford HA, Scott JG. The global coverage of prevalence data for mental disorders in children and adolescents. Epidemiol Psychiatry Sci. 2017;26(4):395–402. doi: 10.1017/S2045796015001158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma SR, Gonda X, Tarazi FI. Autism Spectrum disorder: classification, diagnosis and therapy. Pharmacol Ther. 2018;190:91–104. doi: 10.1016/j.pharmthera.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Escobar R, Soutullo CA, Hervas A, Gastaminza X, Polavieja P, Gilaberte I. Worse quality of life for children with newly diagnosed attention-deficit/hyperactivity disorder, compared with asthmatic and healthy children. Pediatrics. 2005;116(3):e364–e369. doi: 10.1542/peds.2005-0386. [DOI] [PubMed] [Google Scholar]
- 4.Thomas R, Sanders S, Doust J, Beller E, Glasziou P. Prevalence of attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Pediatrics. 2015;135(4):e994–1001. doi: 10.1542/peds.2014-3482. [DOI] [PubMed] [Google Scholar]
- 5.Polanczyk GV, Salum GA, Sugaya LS, Caye A, Rohde LA. Annual research review: a meta-analysis of the worldwide prevalence of mental disorders in children and adolescents. J Child Psychol Psychiatry. 2015;56(3):345–365. doi: 10.1111/jcpp.12381. [DOI] [PubMed] [Google Scholar]
- 6.Chiarotti F, Venerosi A. Epidemiology of autism spectrum disorders: a review of worldwide prevalence estimates since 2014. Brain Sci. 2020;10(5):274. doi: 10.3390/brainsci10050274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banerjee TD, Middleton F, Faraone SV. Environmental risk factors for attention-deficit hyperactivity disorder. Acta Paediatr. 2007;96(9):1269–1274. doi: 10.1111/j.1651-2227.2007.00430.x. [DOI] [PubMed] [Google Scholar]
- 8.Tick B, Bolton P, Happé F, Rutter M, Rijsdijk F. Heritability of autism spectrum disorders: a meta-analysis of twin studies. J Child Psychol Psychiatry. 2016;57(5):585–595. doi: 10.1111/jcpp.12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oh J, Bennett DH, Calafat AM, Tancredi D, Roa DL, Schmidt RJ, Hertz-Picciotto I, Shin HM. Prenatal exposure to per- and polyfluoroalkyl substances in association with autism spectrum disorder in the MARBLES study. Environ Int. 2020;147:106328. doi: 10.1016/j.envint.2020.106328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shin HM, Schmidt RJ, Tancredi D, Barkoski J, Ozonoff S, Bennett DH, Hertz-Picciotto I. Prenatal exposure to phthalates and autism spectrum disorder in the MARBLES study. Environ Health. 2018;17(1):85. doi: 10.1186/s12940-018-0428-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grandjean P, Landrigan PJ. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014;13(3):330–338. doi: 10.1016/S1474-4422(13)70278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacKay H, Abizaid A. A plurality of molecular targets: the receptor ecosystem for bisphenol-a (BPA) Horm Behav. 2018;101:59–67. doi: 10.1016/j.yhbeh.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Mikołajewska K, Stragierowicz J, Gromadzińska J. Bisphenol a - application, sources of exposure and potential risks in infants, children and pregnant women. Int J Occup Med Environ Health. 2015;28(2):209–241. doi: 10.13075/ijomeh.1896.00343. [DOI] [PubMed] [Google Scholar]
- 14.Corrales J, Kristofco LA, Steele WB, Yates BS, Breed CS, Williams ES, Brooks BW. Global assessment of Bisphenol a in the environment: review and analysis of its occurrence and bioaccumulation. Dose Response. 2015;13(3):1559325815598308. doi: 10.1177/1559325815598308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LaKind JS, Naiman DQ. Temporal trends in bisphenol a exposure in the United States from 2003-2012 and factors associated with BPA exposure: spot samples and urine dilution complicate data interpretation. Environ Res. 2015;142:84–95. doi: 10.1016/j.envres.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 16.Frederiksen H, Jensen TK, Jørgensen N, Kyhl HB, Husby S, Skakkebæk NE, Main KM, Juul A, Andersson AM. Human urinary excretion of non-persistent environmental chemicals: an overview of Danish data collected between 2006 and 2012. Reproduction. 2014;147(4):555–565. doi: 10.1530/REP-13-0522. [DOI] [PubMed] [Google Scholar]
- 17.Braun JM, Yolton K, Dietrich KN, Hornung R, Ye X, Calafat AM, Lanphear BP. Prenatal bisphenol a exposure and early childhood behavior. Environ Health Perspect. 2009;117(12):1945–1952. doi: 10.1289/ehp.0900979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braun JM, Kalkbrenner AE, Calafat AM, Yolton K, Ye X, Dietrich KN, Lanphear BP. Impact of early-life bisphenol a exposure on behavior and executive function in children. Pediatrics. 2011;128(5):873–882. doi: 10.1542/peds.2011-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stacy SL, Papandonatos GD, Calafat AM, Chen A, Yolton K, Lanphear BP, Braun JM. Early life bisphenol a exposure and neurobehavior at 8years of age: identifying windows of heightened vulnerability. Environ Int. 2017;107:258–265. doi: 10.1016/j.envint.2017.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casas M, Forns J, Martínez D, Avella-García C, Valvi D, Ballesteros-Gómez A, Luque N, Rubio S, Julvez J, Sunyer J, et al. Exposure to bisphenol a during pregnancy and child neuropsychological development in the INMA-Sabadell cohort. Environ Res. 2015;142:671–679. doi: 10.1016/j.envres.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 21.Evans SF, Kobrosly RW, Barrett ES, Thurston SW, Calafat AM, Weiss B, Stahlhut R, Yolton K, Swan SH. Prenatal bisphenol a exposure and maternally reported behavior in boys and girls. Neurotoxicology. 2014;45:91–99. doi: 10.1016/j.neuro.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harley KG, Gunier RB, Kogut K, Johnson C, Bradman A, Calafat AM, Eskenazi B. Prenatal and early childhood bisphenol a concentrations and behavior in school-aged children. Environ Res. 2013;126:43–50. doi: 10.1016/j.envres.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perera F, Vishnevetsky J, Herbstman JB, Calafat AM, Xiong W, Rauh V, Wang S. Prenatal bisphenol a exposure and child behavior in an inner-city cohort. Environ Health Perspect. 2012;120(8):1190–1194. doi: 10.1289/ehp.1104492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roen EL, Wang Y, Calafat AM, Wang S, Margolis A, Herbstman J, Hoepner LA, Rauh V, Perera FP. Bisphenol a exposure and behavioral problems among inner city children at 7-9 years of age. Environ Res. 2015;142:739–745. doi: 10.1016/j.envres.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Philippat C, Nakiwala D, Calafat AM, Botton J, De Agostini M, Heude B, Slama R. Prenatal exposure to nonpersistent endocrine disruptors and behavior in boys at 3 and 5 years. Environ Health Perspect. 2017;125(9):097014. doi: 10.1289/EHP1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim YH, Bae S, Kim BN, Shin CH, Lee YA, Kim JI, Hong YC. Prenatal and postnatal bisphenol a exposure and social impairment in 4-year-old children. Environ Health. 2017;16(1):79. doi: 10.1186/s12940-017-0289-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braun JM, Muckle G, Arbuckle T, Bouchard MF, Fraser WD, Ouellet E, Séguin JR, Oulhote Y, Webster GM, Lanphear BP. Associations of prenatal urinary Bisphenol a concentrations with child behaviors and cognitive abilities. Environ Health Perspect. 2017;125(6):067008. doi: 10.1289/EHP984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minatoya M, Araki A, Nakajima S, Sasaki S, Miyashita C, Yamazaki K, Yamamoto J, Matumura T, Kishi R. Cord blood BPA level and child neurodevelopment and behavioral problems: the Hokkaido study on environment and Children's health. Sci Total Environ. 2017;607-608:351–356. doi: 10.1016/j.scitotenv.2017.06.060. [DOI] [PubMed] [Google Scholar]
- 29.Grohs MN, Reynolds JE, Liu J, Martin JW, Pollock T, Lebel C, Dewey D. Prenatal maternal and childhood bisphenol a exposure and brain structure and behavior of young children. Environ Health. 2019;18(1):85. doi: 10.1186/s12940-019-0528-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braun JM, Kalkbrenner AE, Just AC, Yolton K, Calafat AM, Sjödin A, Hauser R, Webster GM, Chen A, Lanphear BP. Gestational exposure to endocrine-disrupting chemicals and reciprocal social, repetitive, and stereotypic behaviors in 4- and 5-year-old children: the HOME study. Environ Health Perspect. 2014;122(5):513–520. doi: 10.1289/ehp.1307261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miodovnik A, Engel SM, Zhu C, Ye X, Soorya LV, Silva MJ, Calafat AM, Wolff MS. Endocrine disruptors and childhood social impairment. Neurotoxicology. 2011;32(2):261–267. doi: 10.1016/j.neuro.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jensen TK, Mustieles V, Bleses D, Frederiksen H, Trecca F, Schoeters G, Andersen HR, Grandjean P, Kyhl HB, Juul A, et al. Prenatal bisphenol a exposure is associated with language development but not with ADHD-related behavior in toddlers from the Odense child cohort. Environ Res. 2019;170:398–405. doi: 10.1016/j.envres.2018.12.055. [DOI] [PubMed] [Google Scholar]
- 33.Mustieles V, Pérez-Lobato R, Olea N, Fernández MF. Bisphenol a: human exposure and neurobehavior. Neurotoxicology. 2015;49:174–184. doi: 10.1016/j.neuro.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 34.Ejaredar M, Lee Y, Roberts DJ, Sauve R, Dewey D. Bisphenol a exposure and children's behavior: a systematic review. J Expo Sci Environ Epidemiol. 2017;27(2):175–183. doi: 10.1038/jes.2016.8. [DOI] [PubMed] [Google Scholar]
- 35.Mustieles V, Fernández MF. Bisphenol a shapes children’s brain and behavior: towards an integrated neurotoxicity assessment including human data. Environ Health. 2020;19(1):66. doi: 10.1186/s12940-020-00620-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kyhl HB, Jensen TK, Barington T, Buhl S, Norberg LA, Jørgensen JS, Jensen DF, Christesen HT, Lamont RF, Husby S. The Odense child cohort: aims, design, and cohort profile. Paediatr Perinat Epidemiol. 2015;29(3):250–258. doi: 10.1111/ppe.12183. [DOI] [PubMed] [Google Scholar]
- 37.Lassen TH, Frederiksen H, Kyhl HB, Swan SH, Main KM, Andersson AM, Lind DV, Husby S, Wohlfahrt-Veje C, Skakkebæk NE, et al. Prenatal Triclosan exposure and anthropometric measures including Anogenital distance in Danish infants. Environ Health Perspect. 2016;124(8):1261–1268. doi: 10.1289/ehp.1409637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frederiksen H, Aksglaede L, Sorensen K, Nielsen O, Main KM, Skakkebaek NE, Juul A, Andersson AM. Bisphenol a and other phenols in urine from Danish children and adolescents analyzed by isotope diluted TurboFlow-LC-MS/MS. Int J Hyg Environ Health. 2013;216(6):710–720. doi: 10.1016/j.ijheh.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 39.Middleton DR, Watts MJ, Lark RM, Milne CJ, Polya DA. Assessing urinary flow rate, creatinine, osmolality and other hydration adjustment methods for urinary biomonitoring using NHANES arsenic, iodine, lead and cadmium data. Environ Health. 2016;15(1):68. doi: 10.1186/s12940-016-0152-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kristensen S, Henriksen TB, Bilenberg N. The child behavior checklist for ages 1.5-5 (CBCL/1(1/2)-5): assessment and analysis of parent- and caregiver-reported problems in a population-based sample of Danish preschool children. Nord J Psychiatry. 2010;64(3):203–209. doi: 10.3109/08039480903456595. [DOI] [PubMed] [Google Scholar]
- 41.Aebi M, Winkler Metzke C, Steinhausen HC. Accuracy of the DSM-oriented attention problem scale of the child behavior checklist in diagnosing attention-deficit hyperactivity disorder. J Atten Disord. 2010;13(5):454–463. doi: 10.1177/1087054708325739. [DOI] [PubMed] [Google Scholar]
- 42.Rescorla LA, Adams A, Ivanova MY. The CBCL/1½-5's DSM-ASD scale: confirmatory factor analyses across 24 societies. J Autism Dev Disord. 2020;50(9):3326–3340. doi: 10.1007/s10803-019-04189-5. [DOI] [PubMed] [Google Scholar]
- 43.Barkoski JM, Busgang SA, Bixby M, Bennett D, Schmidt RJ, Barr DB, Panuwet P, Gennings C, Hertz-Picciotto I. Prenatal phenol and paraben exposures in relation to child neurodevelopment including autism spectrum disorders in the MARBLES study. Environ Res. 2019;179(Pt A):108719. doi: 10.1016/j.envres.2019.108719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Donohue MR, Childs AW, Richards M, Robins DL. Race influences parent report of concerns about symptoms of autism spectrum disorder. Autism. 2019;23(1):100–111. doi: 10.1177/1362361317722030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pastor PN, Reuben CA. Racial and ethnic differences in ADHD and LD in young school-age children: parental reports in the National Health Interview Survey. Public Health Rep. 2005;120(4):383–392. doi: 10.1177/003335490512000405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bacon EC, Courchesne E, Barnes CC, Cha D, Pence S, Schreibman L, Stahmer AC, Pierce K. Rethinking the idea of late autism spectrum disorder onset. Dev Psychopathol. 2018;30(2):553–569. doi: 10.1017/S0954579417001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berenbaum SA, Beltz AM. Sexual differentiation of human behavior: effects of prenatal and pubertal organizational hormones. Front Neuroendocrinol. 2011;32(2):183–200. doi: 10.1016/j.yfrne.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 48.Jedynak P, Maitre L, Guxens M, Gützkow KB, Julvez J, López-Vicente M, Sunyer J, Casas M, Chatzi L, Gražulevičienė R, et al. Prenatal exposure to a wide range of environmental chemicals and child behaviour between 3 and 7 years of age - an exposome-based approach in 5 European cohorts. Sci Total Environ. 2020;763:144115. doi: 10.1016/j.scitotenv.2020.144115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Inadera H. Neurological effects of Bisphenol a and its analogues. Int J Med Sci. 2015;12(12):926–936. doi: 10.7150/ijms.13267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsuda S, Matsuzawa D, Ishii D, Tomizawa H, Sutoh C, Nakazawa K, Amano K, Sajiki J, Shimizu E. Effects of perinatal exposure to low dose of bisphenol a on anxiety like behavior and dopamine metabolites in brain. Prog Neuro-Psychopharmacol Biol Psychiatry. 2012;39(2):273–279. doi: 10.1016/j.pnpbp.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 51.Gioiosa L, Parmigiani S, Vom Saal FS, Palanza P. The effects of bisphenol a on emotional behavior depend upon the timing of exposure, age and gender in mice. Horm Behav. 2013;63(4):598–605. doi: 10.1016/j.yhbeh.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 52.Komada M, Itoh S, Kawachi K, Kagawa N, Ikeda Y, Nagao T. Newborn mice exposed prenatally to bisphenol a show hyperactivity and defective neocortical development. Toxicology. 2014;323:51–60. doi: 10.1016/j.tox.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 53.Ohtani N, Suda K, Tsuji E, Tanemura K, Yokota H, Inoue H, Iwano H. Late pregnancy is vulnerable period for exposure to BPA. J Vet Med Sci. 2018;80(3):536–543. doi: 10.1292/jvms.17-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim K, Son TG, Kim SJ, Kim HS, Kim TS, Han SY, Lee J. Suppressive effects of bisphenol a on the proliferation of neural progenitor cells. J Toxicol Environ Health A. 2007;70(15–16):1288–1295. doi: 10.1080/15287390701434216. [DOI] [PubMed] [Google Scholar]
- 55.Kim K, Son TG, Park HR, Kim SJ, Kim HS, Kim HS, Kim TS, Jung KK, Han SY, Lee J. Potencies of bisphenol a on the neuronal differentiation and hippocampal neurogenesis. J Toxicol Environ Health A. 2009;72(21–22):1343–1351. doi: 10.1080/15287390903212501. [DOI] [PubMed] [Google Scholar]
- 56.Nakamura K, Itoh K, Sugimoto T, Fushiki S. Prenatal exposure to bisphenol a affects adult murine neocortical structure. Neurosci Lett. 2007;420(2):100–105. doi: 10.1016/j.neulet.2007.02.093. [DOI] [PubMed] [Google Scholar]
- 57.Itoh K, Yaoi T, Fushiki S. Bisphenol a, an endocrine-disrupting chemical, and brain development. Neuropathology. 2012;32(4):447–457. doi: 10.1111/j.1440-1789.2011.01287.x. [DOI] [PubMed] [Google Scholar]
- 58.Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108(Suppl 3):511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mustieles V, D'Cruz SC, Couderq S, Rodríguez-Carrillo A, Fini JB, Hofer T, Steffensen IL, Dirven H, Barouki R, Olea N, et al. Bisphenol a and its analogues: a comprehensive review to identify and prioritize effect biomarkers for human biomonitoring. Environ Int. 2020;144:105811. doi: 10.1016/j.envint.2020.105811. [DOI] [PubMed] [Google Scholar]
- 60.Kundakovic M, Gudsnuk K, Herbstman JB, Tang D, Perera FP, Champagne FA. DNA methylation of BDNF as a biomarker of early-life adversity. Proc Natl Acad Sci U S A. 2015;112(22):6807–6813. doi: 10.1073/pnas.1408355111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pistollato F, de Gyves EM, Carpi D, Bopp SK, Nunes C, Worth A, Bal-Price A. Assessment of developmental neurotoxicity induced by chemical mixtures using an adverse outcome pathway concept. Environ Health. 2020;19(1):23. doi: 10.1186/s12940-020-00578-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saghazadeh A, Rezaei N. Brain-derived Neurotrophic factor levels in autism: a systematic review and meta-analysis. J Autism Dev Disord. 2017;47(4):1018–1029. doi: 10.1007/s10803-016-3024-x. [DOI] [PubMed] [Google Scholar]
- 63.Thayer KA, Doerge DR, Hunt D, Schurman SH, Twaddle NC, Churchwell MI, Garantziotis S, Kissling GE, Easterling MR, Bucher JR, et al. Pharmacokinetics of bisphenol a in humans following a single oral administration. Environ Int. 2015;83:107–115. doi: 10.1016/j.envint.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lassen TH, Frederiksen H, Jensen TK, Petersen JH, Main KM, Skakkebæk NE, Jørgensen N, Kranich SK, Andersson AM. Temporal variability in urinary excretion of bisphenol a and seven other phenols in spot, morning, and 24-h urine samples. Environ Res. 2013;126:164–170. doi: 10.1016/j.envres.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 65.Braun JM, Kalkbrenner AE, Calafat AM, Bernert JT, Ye X, Silva MJ, Barr DB, Sathyanarayana S, Lanphear BP. Variability and predictors of urinary bisphenol a concentrations during pregnancy. Environ Health Perspect. 2011;119(1):131–137. doi: 10.1289/ehp.1002366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vernet C, Philippat C, Agier L, Calafat AM, Ye X, Lyon-Caen S, Hainaut P, Siroux V, Schisterman EF, Slama R. An empirical validation of the within-subject biospecimens pooling approach to minimize exposure misclassification in biomarker-based studies. Epidemiology. 2019;30(5):756–767. doi: 10.1097/EDE.0000000000001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary table 1. Maternal and child characteristics according to included participants with BPA and CBCL1½-5 data at 2 years (N=658) and excluded participants (N=1559). Supplementary table 2. Multiple logistic regression analysis of the association between osmolality adjusted maternal BPA exposure divided into tertiles and the odds ratio (OR) and 95% confidence intervals (CI 95%) of an ASD and ADHD-score >75% compared to <75% (reference) at 2 and/or 5 years of age.
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.