Summary
The leaf is a crucial organ evolved with remarkable morphological diversity to maximize plant photosynthesis. The leaf shape is a key trait that affects photosynthesis, flowering rates, disease resistance and yield. Although many genes regulating leaf development have been identified in the past years, the precise regulatory architecture underlying the generation of diverse leaf shapes remains to be elucidated. We used cotton as a reference model to probe the genetic framework underlying divergent leaf forms. Comparative transcriptome analysis revealed that the GhARF16‐1 and GhKNOX2‐1 genes might be potential regulators of leaf shape. We functionally characterized the auxin‐responsive factor ARF16‐1 acting upstream of GhKNOX2‐1 to determine leaf morphology in cotton. The transcription of GhARF16‐1 was significantly higher in lobed‐leaved cotton than in smooth‐leaved cotton. Furthermore, the overexpression of GhARF16‐1 led to the up‐regulation of GhKNOX2‐1 and resulted in more and deeper serrations in cotton leaves, similar to the leaf shape of cotton plants overexpressing GhKNOX2‐1. We found that GhARF16‐1 specifically bound to the promoter of GhKNOX2‐1 to induce its expression. The heterologous expression of GhARF16‐1 and GhKNOX2‐1 in Arabidopsis led to lobed and curly leaves, and a genetic analysis revealed that GhKNOX2‐1 is epistatic to GhARF16‐1 in Arabidopsis, suggesting that the GhARF16‐1 and GhKNOX2‐1 interaction paradigm also functions to regulate leaf shape in Arabidopsis. To our knowledge, our results uncover a novel mechanism by which auxin, through the key component ARF16‐1 and its downstream‐activated gene KNOX2‐1, determines leaf morphology in eudicots.
Keywords: leaf shape, auxin, cotton, transcriptional regulation, GhARF16‐1, GhKNOX2‐1
Introduction
The aerial parts of higher plants are derived from a group of pluripotent stem cells in the shoot apical meristem (SAM), which leads to the generation of new outgrowth, leaves and flowers (Du et al., 2018; Shi et al., 2018). In comparison to the vibrant and diverse colours of flowers, leaves are typically more monotonously coloured. Nonetheless, leaves display remarkable morphological diversity with highly diversified geometry. Leaves can be simple with smooth margins or small marginal protrusions (serrations) as in Arabidopsis thaliana, while some can be complicated with protrusions of different sizes and patterns (e.g. teeth or lobes, depending on the depth of sinuses). Variation in leaf morphology is due to environmental or genetic factors (Nicotra et al., 2011). However, the extraordinary diversity is mostly attributed to genetic control, through which gene regulatory networks or signalling pathways control leaf formation from a small bulge on the SAM to a fully developed lateral outgrowth with diverse shapes (Dkhar and Pareek, 2014).
Genetic studies have identified the KNOTTED1‐LIKE HOMEOBOX (KNOX) family genes, such as KNAT1, SHOOT MERISTEMLESS (STM) and BREVIPEDICELLUS (BP), as key regulators of leaf shape (Chuck et al., 1996; Lincoln et al., 1994; Spinelli et al., 2011). In Arabidopsis, KNAT2 is mainly expressed in the SAM and its overexpression induces highly lobed and curled rosette leaves in Arabidopsis (Chuck et al., 1996; Scofield et al., 2008). In A. thaliana, ASYMMETRIC LEAVES1 (AS1) and AS2 repress the activity of KNAT1 and KNAT2 in the leaves (Byrne et al., 2000; Byrne et al., 2002). Both as1 and as2 mutants display abnormal lobed leaves with ectopic expression of BP and KNAT2 (Byrne et al., 2000; Byrne et al., 2002). A transcriptional repressor complex POLYCOMB REPRESSIVE COMPLEX (PRC)2 interacts with the AS1‐AS2 protein complex to stably silence the KNAT1 and KNAT2 genes in leaf primordia (Lodha et al., 2013). Nonetheless, it is still unknown how the key factor KNOX is enlisted to determine leaf diversity during the diversification of plant species.
In addition to the KNOX family genes, the CUP‐SHAPED COTYLEDON (CUC) and REDUCED COMPLEXITY (RCO) genes are also important regulators in determining leaf morphology (Bar and Ori, 2014; Bilsborough et al., 2011; Streubel et al., 2018; Vlad et al., 2014). CUC2, a member of the NAC gene family, plays a key role in the development of serrated leaf margins (Nikovics et al., 2006), and its ectopic expression leads to greater sinus width and lobe appearance rate in Arabidopsis (Blein et al., 2013). In Cardamine hirsuta, which belongs to the Brassicaceae family and shares a very close common ancestor with Arabidopsis, the homeodomain‐leucine zipper (HD‐ZIP) transcription factor gene ChRCO is specifically expressed in developing leaves and promotes leaflet development by repressing growth at the flanks of protrusions generated by CUC‐auxin patterning (Sicard et al., 2014; Vlad et al., 2014).
Moreover, auxin is involved in the regulation of leaf morphology. The local peaks of auxin activity are mediated by the auxin efflux carrier PIN‐FORMED. PIN1 regulates the growth of the leaf margin to affect leaf morphology (Barkoulas et al., 2008; Mohammed et al., 2018), which is evidenced by the fact that a loss‐of‐function mutation of PIN1 leads to smooth margins in C. hirsuta. Auxin response factors (ARFs) regulate the expression of auxin‐responsive genes by directly binding to the auxin‐responsive element (AuxRE, TGTCTC) (Israeli et al., 2019). The ARF proteins contain two conserved domains, an N‐terminal DNA‐binding domain and a C‐terminal dimerization domain that mediates protein–protein interaction (Gray et al., 2001). In response to auxin, the auxin/indoleacetic acids (Aux/IAAs) are targeted for proteolysis through the ubiquitin‐mediated pathway, relieving ARFs, which regulate downstream gene transcription (Serino and Deng, 2003). Genetic analyses have shown that the ARF10, ARF16 and ARF17 genes regulate leaf morphology (Hendelman et al., 2012; Mallory et al., 2005; Wang et al., 2005). Interestingly, the ARF10, ARF16 and ARF17 mRNAs are targeted and negatively regulated by miR160 (Jones‐Rhoades et al., 2006; Natarajan and Banerjee, 2020; Sunkar et al., 2012). The expression of a miR160‐resistant form of ARF10/ARF16/ARF17 results in the interference of auxin signalling, leading to the changes of leaf shape (Rubio‐Somoza et al., 2009). Likewise, in cotton, ARF10, ARF16 and ARF17, were also targeted by miR160. Overexpression of miR160 led to significant decrease of ARF10 and ARF17, while down‐regulation of miR160 resulted in increased transcripts of ARF10 and ARF17 (Ding et al., 2017; Liu et al., 2020). Despite all these significant advances, the signalling pathways acting downstream of auxin to modulate leaf shape remain enigmatic. Furthermore, the genetic basis for the auxin‐mediated morphological diversity of leaves is still poorly understood.
In this study, we used Gossypium (cotton) species, one of the most important economic crops, to clarify the genetic underpinnings of the generation of divergent leaf forms. We show that the Gossypium auxin‐responsive factor ARF16‐1 acts as a key regulator that directly binds to the promoter of KNOX2‐1 to fine‐tune its expression levels and, consequently, determines leaf shape diversity among the genus Gossypium. Notably, the Gossypium ARF16‐KNOX2 interaction paradigm was also successfully implemented in Arabidopsis, which resulted in similar divergent leaf forms that were observed in Gossypium spp., suggesting that this regulatory mechanism is evolutionarily conserved in eudicots.
Results
Identification of candidate genes for divergent leaf shapes in Gossypium species
Gossypium species exhibit diverse morphological variations because of natural selection and artificial domestication. Here, we used three completely sequenced cotton species, including one allotetraploid species, G. hirsutum and two diploid species, G. arboreum and G. raimondii, as reference plants to study leaf shape diversity as well as the genetic basis of the divergent leaf morphology during the diversification of the genus Gossypium. We showed that the leaves of G. arboreum were typically lobed, whereas those of G. raimondii were classic smooth (Figure 1a). Interestingly, we observed both lobed and smooth leaves occurring simultaneously in the same G. hirsutum plant. The shape of a leaf is determined by the geometric form of its epidermal cells (Tao et al., 2013). Therefore, we analysed the leaf epidermal cell shape using scanning electron microscopy. The epidermal cells of mature smooth leaves of G. raimondii and G. hirsutum showed a jigsaw‐puzzle‐piece shape, whereas the much smaller epidermal cells of mature lobed leaves of G. arboreum and G. hirsutum displayed a homogeneous shape. Furthermore, we used three different leaf parameters to quantitatively analyse the leaf shape of the three Gossypium species: (i) leaf dissection index (perimeter2/4π × leaf area), (ii) number of teeth/leaf perimeter, and (iii) tooth area/leaf area (Bilsborough et al., 2011; Royer et al., 2008; Zheng et al., 2016). The results showed that G. arboreum had a more pronounced leaf margin serration than G. raimondii, and distinct serrations were also observed between the lobed and smooth leaves of G. hirsutum (Figure 1b–d). Moreover, we calculated the ratio of lobed to smooth leaves in G. hirsutum and quantified the number of teeth on each leaf. Our calculations showed that the lobed leaves were the dominant type in G. hirsutum (Figure S1), and the number of teeth on each leaf in G. raimondii was nearly the same as that on a smooth leaf of G. hirsutum. However, the numbers of teeth on the lobed leaves of G. arboreum and G. hirsutum were much higher than those on the smooth leaves of G. raimondii and G. hirsutum (Figure S2). These results revealed the diversity in leaf margins of the three different cotton species.
Figure 1.
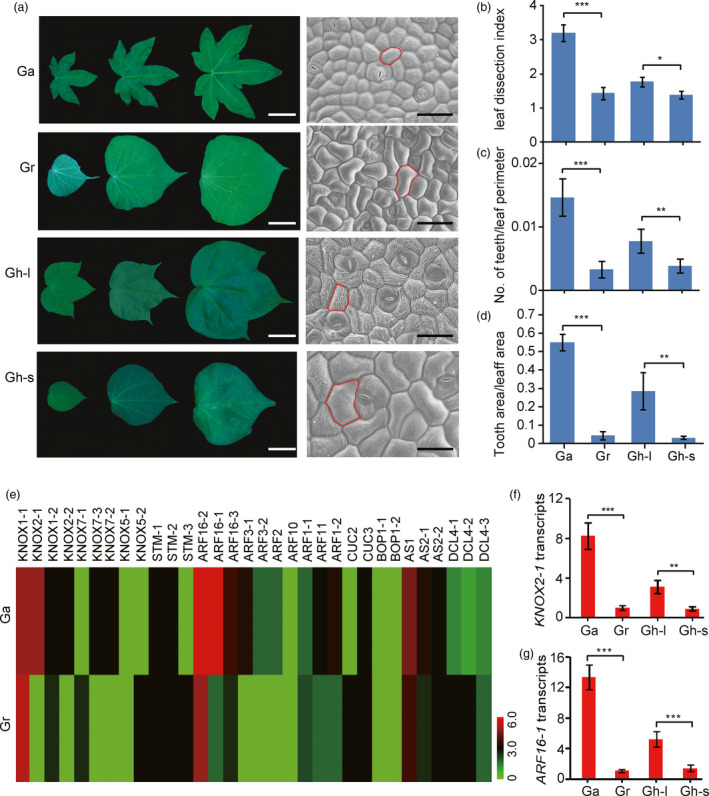
Candidate genes evolved to mediate leaf shape diversity among different Gossypium (cotton) species. (a) Left, morphological analysis of the leaves of Gossypium hirsutum (Gh), Gossypium arboreum (Ga), and Gossypium raimondii (Gr). From top to bottom, the first true leaf of G. arboreum, the first true leaf of G. raimondii, the fourth true leaf of G. hirsutum (Gh‐lobed, Gh‐l), and the first true leaf of G. hirsutum (Gh‐smooth Gh‐s). In each image, from left to right, the representative leaves from three different developmental stages (5, 15, and 30 days, respectively). Bars = 4 cm. The right column of the images shows scanning electron micrographs of the epidermal cells at the base of the abaxial side of mature leaves of Ga, Gr, Gh‐l, and Gh‐s, respectively, from top to bottom. Bars = 50 µm. (b) to (d) Quantitative comparisons of the leaf shapes of Ga, Gr, Gh‐l, and Gh‐s from (a) based on the leaf dissection index (perimeter2/4π × leaf area) (b), the number of teeth/leaf perimeter (c), and the tooth area/leaf area (d). A total of 10 leaves from each genotype were used for the measurement. Data are presented as mean ± SE. Error bars represent standard errors of the means from three independent experiments. Statistical significance was determined using one‐way analysis of variance (ANOVA) combined with Tukey's test: *P < 0.05, **P < 0.01, ***P < 0.001. (e) Expression profiles of the genes involved in the regulation of leaf shape in Ga and Gr. Scaled log2 expression values are shown from green to red, indicating low to high expression levels, respectively. (f) Quantitative real‐time PCR (qRT‐PCR) analysis of KNOX2‐1 transcriptional levels in leaf primordia of Ga, Gr, Gh‐l, and Gh‐s. The expression level of the KNOX2‐1 gene in Gr was set to 1.0. (g) qRT‐PCR analysis of the ARF16‐1 mRNA levels in the leaf primordia of Ga, Gr, Gh‐l, and Gh‐s. The expression level of the ARF16‐1 gene in Gr was set to 1.0. Each qRT‐PCR experiment was performed in three biological replicates, and the error bars represent standard errors of the means from three independent experiments. Statistical significance was determined using one‐way ANOVA combined with Tukey's test. **P < 0.01; ***P < 0.001.
To uncover the genetic underpinnings of leaf margin variation in Gossypium, we firstly analysed the differentially expressed genes between G. arboreum and G. raimondii using the RNA‐Seq data (Data S1). Subsequently, we summarized all genes potentially involved in the regulation of leaf morphological differences in Arabidopsis (Table S1). Finally, we investigated the expression of the cotton genes homologous to these potential Arabidopsis genes. Our results showed that the KNOX2‐1 and AUXIN RESPONSE FACTOR16 (ARF16) genes were significantly up‐regulated in the lobed leaves of G. arboreum compared to the smooth leaves of G. raimondii (Figure 1e), indicating that these genes might be recruited to regulate leaf shape differences among the Gossypium species. Moreover, the quantitative real‐time PCR (qRT‐PCR) results showed predominantly higher expression levels of GhARF16‐1 and GhKNOX2‐1 in lobed leaves than in smooth leaves, further supporting the expression profiles of ARF16‐1 and KNOX2‐1 (Figure S1f,g and S3). To confirm that dissimilar leaf profiles were attributed to the differentiated expression profiles of ARF16‐1 and KNOX2‐1, we introduced another diploid Gossypium species, G. trilobum, whose genome constitution (D‐genome, DD) highly resembles that of G. raimondii. Compared to the smooth leaves of G. raimondii, the leaves of G. trilobum were lobed, as indicated by their greater values of leaf dissection index, number of teeth/leaf perimeter and tooth area/leaf area (Figure S4a–d). Consistent with the above‐mentioned phenotypic analysis results, the transcriptional levels of ARF16‐1 and KNOX2‐1 in G. trilobum leaves were much higher than those in G. raimondii leaves (Figure S4e,f), suggesting that ARF16‐1 and KNOX2‐1 might modulate leaf patterning during the diversification of these cotton species.
GhARF16‐1 and GhKNOX2‐1 are key regulators that control cotton leaf shape
To verify that the two candidate genes, GhARF16‐1 and GhKNOX2‐1, are the key regulators that modulate leaf shape variation in Gossypium species, we constructed several transgenic lines that overexpressed or down‐regulated GhARF16‐1 and GhKNOX2‐1 in G. hirsutum.
A sequence analysis of GhARF16‐1 showed that GhARF16‐1 had a high sequence similarity with Arabidopsis ARF16 (Figure S5), and GhARF16‐1 mRNA containing an miR160 complementary sequence (Figure S6). To obtain a GhARF16‐1 overexpressing line, a mutated version of GhARF16‐1 (GhmARF16‐1), which modifies only miRNA160 complementary sequences by synonymous substitutions without altering the protein sequence (Figure S6) (Wang et al., 2005), was generated and introduced into cotton plants under the control of its own native promoter (Table S2). After pedigree selection assisted with kanamycin and transgene PCR amplification, 16 and 21 independent homozygous transgenic lines (approximately 10 plants per line) were regenerated for pARF16::GhmARF16‐1 and GhmARF16‐1‐RNAi, respectively. Because the modification of miRNA160 disrupts its affinity for GhmARF16‐1, which results in the promotion of the stability of GhmARF16‐1, the expression level of GhARF16‐1 in the pARF16::GhmARF16‐1 lines was much higher than that in wild‐type G. hirsutum (Figure S7). As a result, these transgenic plants produced deeper lobed leaves as compared to the wild‐type leaves (Figure 2a,c,d). In contrast, the transgenic GhARF16‐1‐RNAi lines with reduced expression of GhARF16‐1 exhibited leaf‐type similar to that of the wild type (Figure 2b,d). The epidermal cell shape was polygonal in mature leaves of wild‐type and GhARF16‐1‐RNAi lines, but was more regular in mature lobed leaves of pARF16::GhmARF16‐1 (Figure 2a–c). The quantitative analysis of leaf morphology revealed that the pARF16::GhmARF16‐1 plants had significantly greater leaf dissection index, number of teeth/leaf perimeter, tooth area/leaf area, and teeth number compared to the wild‐type and GhARF16‐1‐RNAi line (Figure 2e–h). These results suggest an important role of GhARF16‐1 in regulating leaf shape in cotton.
Figure 2.
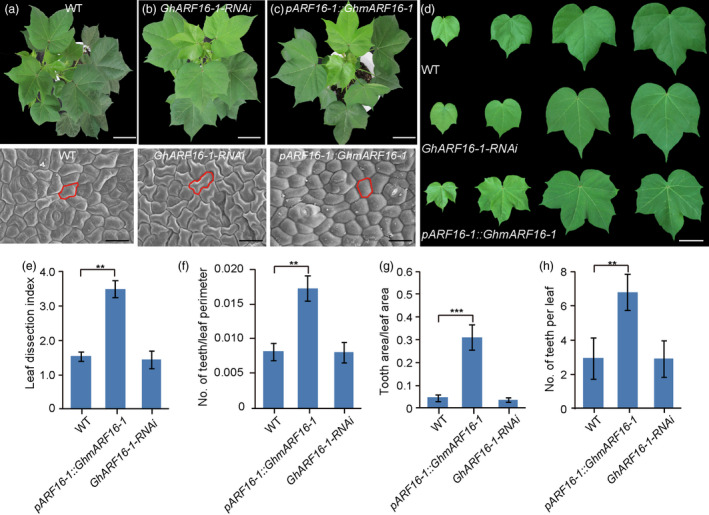
GhARF16‐1 is involved in regulating leaf shape in cotton. (a)–(c) Top, from left to right, 48‐d‐old phenotypes of wild‐type Gossypium hirsutum (a), GhARF16‐1 RNAi (b), and pARF16‐1::GhmARF16‐1 transgenic G. hirsutum plants (c). Bars = 4 cm. Bottom, from left to right, scanning electron micrographs of the leaf epidermal cells at the base of the abaxial side of mature leaves of wild‐type, GhARF16‐1 RNAi, and pARF16‐1::GhmARF16‐1 transgenic lines. Bars = 50 µm. (d) Close‐up view of the leaves of 48‐d‐old wild‐type, GhARF16‐1 RNAi, and pARF16‐1::GhmARF16‐1 transgenic plants. (e)–(g) Quantitative comparisons of the leaf shapes of wild‐type, GhARF16‐1 RNAi plants, and pARF16‐1::GhmARF16‐1 transgenic lines based on the leaf dissection index (perimeter2/4π × leaf area) (e), the number of teeth/leaf perimeter (f), and the tooth area/leaf area (g). (h) Statistical analysis of number of teeth per leaf of wild‐type, GhARF16‐1 RNAi, and pARF16‐1::GhmARF16‐1 transgenic plants. A total of 10 leaves from each line were used for each measurement. Data are presented as mean ± SE. Statistical significance was determined using one‐way analysis of variance combined with Tukey's test. **P < 0.01; ***P < 0.001.
To determine the role of GhKNOX2‐1 in cotton leaf development, we generated the GhKNOX2‐1 overexpression (35S::GhKNOX2‐1) and GhKNOX2‐1 knocked down (GhKNOX2‐1‐RNAi) transgenic plants. After pedigree selection assisted with kanamycin and transgene PCR amplification, 34 and 16 independent homozygous transgenic lines (8–12 plants per line) were regenerated for 35S::GhKNOX2‐1 and GhKNOX2‐1‐RNAi, respectively. The expression level of GhKNOX2‐1 in the 35S::GhKNOX2‐1 line was much higher than that in the wild‐type plants (Figure S8). Compared with the wild‐type plants, the 35S::GhKNOX2‐1 plants displayed deeper lobed leaves (Figure 3a,c,d), resembling the leaves of pARF16::GhmARF16‐1 (Figure 2a–d). Conversely, the GhKNOX2‐1‐RNAi line exhibited decreased GhKNOX2‐1 transcription (Figure S8) and smooth leaves (Figure 3b,d), a phenotype similar to that of the wild type. Furthermore, the epidermal cell patterning of mature leaves of both wild‐type and GhKNOX2‐1‐RNAi lines were polygonal (Figure 3a,b), whereas the cells of mature leaves of the 35S::GhKNOX2‐1 line showed a more homogeneous shape (Figure 3c). Compared to the wild‐type plants, the 35S::GhKNOX2‐1 transgenic plants exhibited significantly greater values of the leaf dissection index, number of teeth/leaf perimeter, tooth area/leaf area and number of teeth on each leaf (Figure 3e,h). These results suggest that GhKNOX2‐1 is also a key regulator that determines leaf shape in cotton.
Figure 3.
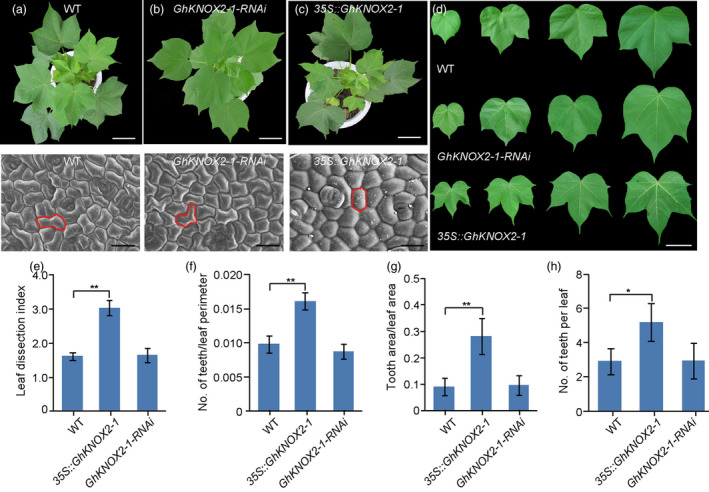
GhKNOX2‐1 regulates leaf shape in cotton. (a)–(d) Top, from left to right, phenotypic analysis of 48‐d‐old wild‐type Gossypium hirsutum (a), GhKNOX2‐1 RNAi (b), and 35S::GhKNOX2‐1 transgenic G. hirsutum plants (c). Bars = 4 cm. Bottom, from left to right, the scanning electron micrographs of the leaf epidermal cells at the base of the abaxial side of mature leaves of wild‐type, GhKNOX2‐1 RNAi and 35S::GhKNOX2‐1 transgenic plants. Bars = 50 µm. (d) Close‐up images of the leaves of 48‐d‐old wild‐type plant (top), GhKNOX2‐1 RNAi (middle) and 35S::GhKNOX2‐1 transgenic plants (bottom). Bars = 4 cm. (e)–(g) Quantitative comparisons of leaf shapes of wild‐type, GhKNOX2‐1 RNAi and 35S::GhKNOX2‐1 transgenic lines based on the leaf dissection index (perimeter2/4π × leaf area) (e), the number of teeth/ leaf perimeter (f) and the tooth area/leaf area (g). A total of 10 leaves from each line were used for each measurement. (h) Statistical analysis of number of teeth per leaf from the wild‐type, GhKNOX2‐1 RNAi and 35S::GhKNOX2‐1 transgenic plants. A total of 10 leaves from each line were used for ach measurement. Data are presented as mean ± SE. Statistical significance was determined using one‐way analysis of variance combined with Tukey's test. *P < 0.05; **P < 0.01. WT, wild type.
GhKNOX2‐1 is the direct target of GhARF16‐1
The lobed leaves of the 35S::GhKNOX2‐1 line are reminiscent of the leaf shape of the pARF16::GhmARF16‐1 transgenic G. hirsutum, implying that GhARF16‐1 and GhKNOX2‐1 might regulate leaf shape in a common regulatory network. To preliminarily investigate a functional relationship between the GhARF16‐1 and GhKNOX2‐1 genes, we analysed the transcriptional level of GhKNOX2‐1 in the pARF16::GhmARF16‐1 and GhARF16‐1‐RNAi transgenic plants. The GhKNOX2‐1 transcripts were up‐regulated in G. hirsutum plants expressing GhmARF16‐1 and were markedly down‐regulated in the GhARF16‐1‐RNAi line compared to the wild‐type control plants (Figure 4a), suggesting that GhKNOX2‐1 may act as a downstream target gene of GhARF16‐1. In line with this hypothesis, the transcriptional levels of GhARF16‐1 in 35S::GhKNOX2‐1 and GhKNOX2‐1‐RNAi transgenic plants were comparable to its transcriptional levels in the wild‐type plants (Figure 4b).
Figure 4.
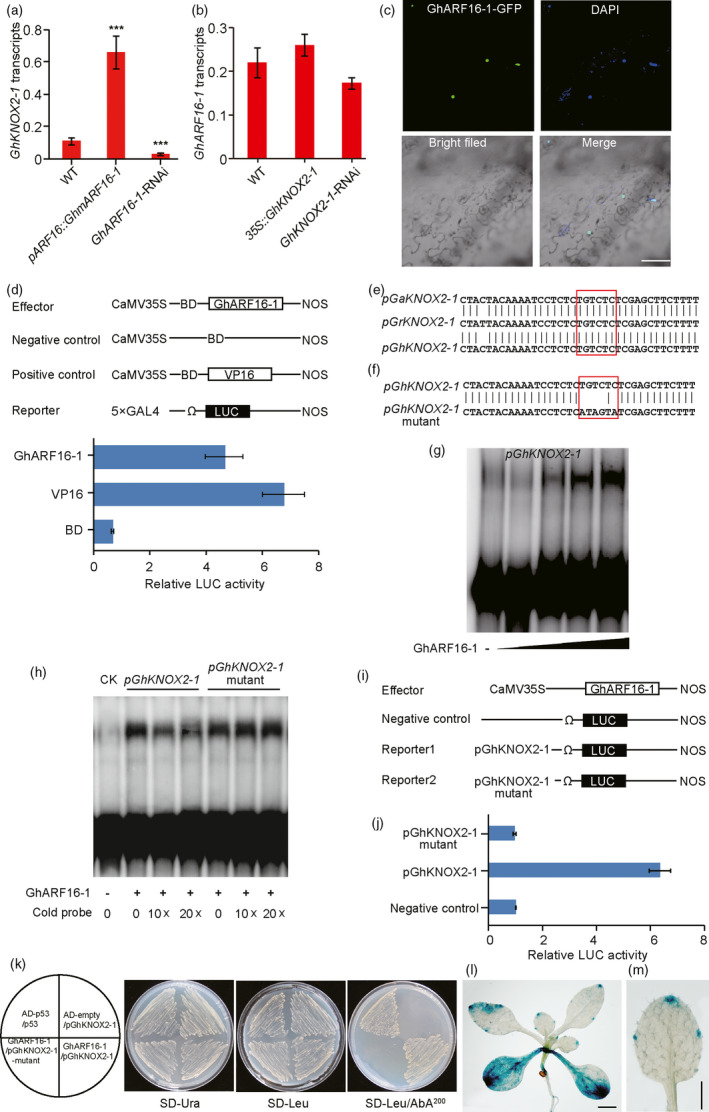
GhKNOX2‐1 is targeted by GhARF16‐1. (a) qRT‐PCR analysis of the GhKNOX2‐1 mRNA levels in wild‐type, GhARF16‐1 RNAi and pARF16::GhmARF16‐1 transgenic plants. (b) qRT‐PCR analysis of the GhARF16‐1 mRNA levels in wild‐type, GhKNOX2‐1 RNAi and 35S::GhKNOX2‐1 transgenic plants. Each qRT‐PCR experiment in (a) and (b) was performed in three biological replicates, and the error bars represent the standard errors of the means from three independent experiments. Statistical significance was determined using one‐way analysis of variance (ANOVA) combined with Tukey's test. ***P < 0.001. WT, wild type. (c) The subcellular localization of GhARF16‐1 in Nicotiana benthamiana leaf cells. Nuclei were counterstained with 4′,6′‐diamidino‐2‐phenylindole (DAPI). The merged images of green fluorescent protein (GFP), DAPI and bright‐field microscopy. (d) Transcriptional ability of GhARF16‐1 in Arabidopsis protoplasts. Upper panel, schematic diagrams of various constructs used in the transient expression assay. Lower panel, transcription activity of GhARF16‐1. The empty GAL4 DNA‐binding domain (BD) and BD‐VP16 vectors were used as negative and positive controls, respectively. The transcriptional assay was performed in three biological replicates, and the error bars represent the standard errors of the means from three independent experiments. (e) An ARF‐binding site was identified in the KNOX2‐1 promoter region. The red rectangle indicates the predicted ARF‐binding sequence in G. hirsutum (Gh), G. arboreum (Ga) and G. raimondii Gr. (f) to (h) Electrophoretic mobility shift assay (EMSA) showed that GhARF16‐1 binds directly to the ARF‐binding site of the GhKNOX2‐1 promoter. The 32P‐labelled DNA fragments of the GhKNOX2‐1 promoter containing the intact (upper) or mutated (lower) ARF‐binding site (h) were incubated with the gradient concentrations of maltose‐binding protein (MBP)‐GhARF16‐1 fusion protein (g). The 32P‐labelled DNA fragments were incubated with MBP‐GhARF16‐1 to compete with different concentrations of the cold probes (without 32P‐labelling) of intact or mutated ARF‐binding site (h). (i) Schematic diagrams of various constructs used in the transient expression assay. (j) Transient expression assay of GhKNOX2‐1 transcriptional activity modulated by GhARF16‐1 in Arabidopsis protoplasts. Either pGhKNOX2‐1‐LUC or pGhKNOX2‐1 mutant‐LUC was co‐transformed with the effector or empty vector (negative control) into Arabidopsis protoplasts. Transient expression assays were performed using three biological replicates, and the error bars represent the standard errors of the means from three independent experiments. (k) Yeast one‐hybrid assay of protein–DNA interaction. The 3x fragments of the GhKNOX2‐1 promoter described in (e) and (f) were used. (l) and (m) GUS staining of the 25‐d‐old Arabidopsis seedlings expressing pGhKNOX2‐1::GUS. Bars = 0.25 cm in (l) and 0.5 cm (m).
To investigate whether GhARF16‐1 and GhKNOX2‐1 are transcription factors, we analysed their subcellular localization. Our results showed that both GhARF16‐1 and GhKNOX2‐1 were localized to the nucleus (Figure 4c, Figure S9). A dual‐luciferase reporter assay was performed to further examine the transcriptional activity of GhARF16‐1 and GhKNOX2‐1 in Arabidopsis protoplasts. The results showed that the luciferase activities of GhARF16‐1 and GhKNOX2‐1 were much higher than those of the negative control (Figure 4d, Figure S10), indicating that both GhARF16‐1 and GhKNOX2‐1 have transcriptional ability in vivo.
Coincidently, the cis‐element analysis of the GhKNOX2‐1 promoter (−1425 bp from the start codon ATG) showed that an AuxRE (TGTCTC) element was located at a region spanning from nucleotide −379 to −374 relative to the ATG start codon (Tables S3), which is a reported binding site for ARF transcription factor (Cherenkov et al., 2018). To confirm the interaction between the transcription factor GhARF16‐1 and the GhKNOX2‐1 promoter, the GhKNOX2‐1 promoter fragments containing either native or mutated ARF‐binding site were used separately for an electrophoretic mobility shift assay (EMSA) (Figure 4e,f). The results revealed that the recombinant GhARF16‐1 protein had significant affinity to the GhKNOX2‐1 promoter fragment containing the AuxRE motif (Figure 4g). Moreover, the binding efficiency was gradually reduced by the addition of increasing amounts of unlabelled native fragment probes (cold probe) (Figure 4h). However, the application of the unlabelled mutated fragment probes did not affect the binding ability of GhARF16‐1 to the 32P‐label native GhKNOX2‐1 promoter fragments. To further examine the DNA‐binding activity and transcriptional activity of GhARF16‐1 bound to the GhKNOX2‐1 promoter, we performed a dual‐luciferase reporter assay, in which GhARF16‐1, driven by the CaMV 35S promoter, was coexpressed with a luciferase reporter driven by the GhKNOX2‐1 promoter containing either a native or mutated ARF‐binding site (Figure 4i). Significant luciferase activity was detected only when GhARF16‐1 was coexpressed with the GhKNOX2‐1 promoter containing the native ARF‐binding site (Figure 4j), suggesting that the AuxRE sequence in the GhKNOX2‐1 promoter is essential for GhARF16‐1‐mediated transcriptional activation of GhKNOX2‐1. Additionally, a yeast one‐hybrid assay showed that GhARF16‐1 was able to bind to the GhKNOX2‐1 promoter with the native but not the mutated AuxRE site (Figure 4k), which further confirmed the interplay between GhARF16‐1 and GhKNOX2‐1 promoter in vivo.
Furthermore, to understand how the GhKNOX2‐1 promoter regulates its gene expression in the leaf, a 1501‐bp GhKNOX2‐1 promoter fragment upstream of the ATG initiation codon was fused with a β‐glucuronidase (GUS) reporter gene to generate the pGhKNOX2‐1::GUS construct. After transferring this construct into Arabidopsis, the expression patterns of the GhKNOX2‐1 gene in Arabidopsis leaves were examined. Our results showed that a strong GUS activity was observed at the leaf margins (Figure 4l,m). Taken together, our results suggest that GhARF16‐1 binds to the AuxRE in the GhKNOX2‐1 promoter and subsequently activates the expression of GhKNOX2‐1 at the leaf margin, thus regulating leaf shape.
GhARF16‐GhKNOX2 signalling module regulates leaf shape in Arabidopsis
With respect to sequence similarity, the GhKNOX2‐1 and GhARF16‐1 proteins shared 71.12% and 62.14% identity with the AtKNAT2 and AtARF16 proteins, respectively. To clarify the GhARF16‐1‐mediated GhKNOX2‐1 function in leaf morphology, we used the model flowering plant Arabidopsis to verify the working machinery. First, we expressed the gain‐of‐function GhmARF16‐1 mutation into Arabidopsis under the control of its native promoter. A qRT‐PCR analysis revealed that the GhARF16‐1 transcripts were strongly expressed in the pARF16::GhmARF16‐1 transgenic Arabidopsis plants (Figure S11). Compared to the wild‐type and arf16 mutant plants, the transgenic Arabidopsis plants carrying the pGhARF16::GhmARF16‐1 construct displayed lobed leaves (Figure 5a–d, Figure S12), and their leaf epidermal cells were regular in shape. Moreover, the leaf patterning analysis indicated that the leaves of the pGhARF16::GhmARF16 transgenic Arabidopsis plants displayed significantly higher leaf dissection index, number of teeth/leaf perimeter, tooth area/leaf area and teeth number (Figure 5e–h) than those of the wild‐type and arf16 mutant plants. These results suggest that the heterologous expression of cotton GhARF16 also promotes leaf serration in Arabidopsis. Notably, in Arabidopsis, expression of mARF17 under its own promoter rather than mARF16 phenocopied pARF16::GhmARF16‐1 leaf shape, suggesting that cotton GhARF16‐1 showed similar function to Arabidopsis ARF17. The reason might be that in Arabidopsis, ARF16 and ARF17 originated from a common ancestor gene, and slightly undergo the functional divergence during the evolution process. However, ARF16 and ARF17 genes are functional conserved in cotton, leading to the similar function between GhARF16 and Arabidopsis ARF17.
Figure 5.
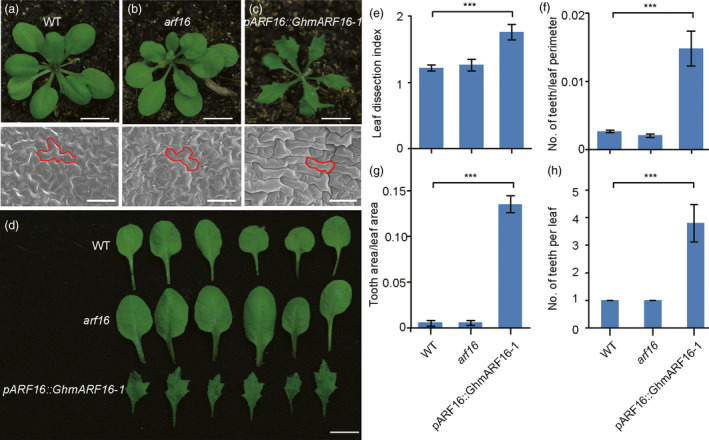
Cotton GhARF16‐1 also functions in modulating leaf shape in Arabidopsis thaliana. (a)–(c) Top, from left to right, 21‐d‐old phenotypes of wild‐type (a), arf16 mutant (b) and pARF16::GhmARF16‐1 transgenic Arabidopsis plants (c). Bars = 4 mm. Bottom, from left to right, scanning electron micrographs of the leaf epidermal cells at the base of the abaxial side of mature leaves of wild‐type, arf16 mutant and pARF16::GhmARF16‐1 transgenic Arabidopsis plants. Bars = 50 µm. (d) Close‐up images of the leaves of 21‐d‐old wild‐type (top), arf16 mutant (middle) and pARF16::GhmARF16‐1 transgenic plants (bottom). (e)–(g) Quantitative comparisons of the leaf shapes of wild‐type, arf16 mutant and pARF16::GhmARF16‐1 transgenic plants based on the leaf dissection index (perimeter2/4π × leaf area) (e), number of teeth/leaf perimeter (f) and tooth area/leaf area (g). (h) Statistical analysis of the number of teeth per leaf from wild‐type, arf16 mutant and pARF16::GhmARF16‐1 transgenic plants. A total of 10 leaves from each line were used for each measurement. Data are presented as mean ± SE. Statistical significance was determined using one‐way analysis of variance combined with Tukey's test. ***P < 0.001. WT, wild type.
Likewise, we generated the GhKNOX2‐1‐overexpressing Arabidopsis transgenic line 35::GhKNOX2‐1. A qRT‐PCR analysis revealed that GhKNOX2‐1 was highly expressed in the 35::GhKNOX2‐1 transgenic Arabidopsis plants (Figure S13). Compared with the leaves of the wild‐type plants, the null mutant knat2 exhibited a similar leaf phenotype with smooth margins, while the 35S::GhKNOX2‐1 line produced lobed leaves (Figure 6a–d, Figure S14). Similar to the observations in Gossypium, the epidermal cells of mature leaves of both wild‐type and knat2 mutant Arabidopsis plants were polygonal, while the cells of mature lobed leaves of the 35S::GhKNOX2‐1 transgenic Arabidopsis plants showed a more homogeneous form. In contrast to the wild‐type leaves, the 35S::GhKNOX2‐1 transgenic Arabidopsis leaves showed greater values of leaf dissection index, number of teeth/leaf perimeter, tooth area/leaf area and number of teeth (Figure 6e–h). These results suggest that GhKNOX2‐1, which regulates Gossypium leaf patterning, can also function in the modification of Arabidopsis leaf shape.
Figure 6.
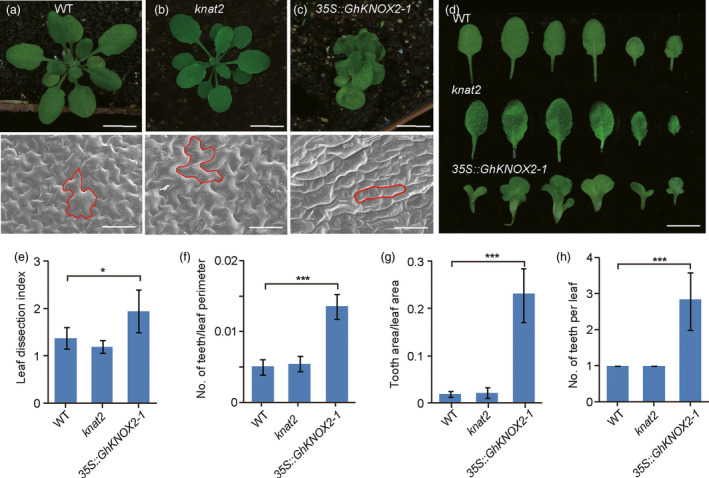
Ectopic expression of GhKNOX2‐1 leads to lobed leaves in Arabidopsis thaliana. (a)–(d) Top, from left to right, 21‐d‐old phenotypes of wild‐type A. thaliana (a), knat2 mutant (b) and 35S::GhKNOX2‐1 transgenic Arabidopsis (c). Bars = 4 mm. Bottom, from left to right, scanning electron micrographs of the leaf epidermal cells at the base of the abaxial side of mature leaves of wild‐type, knat2 mutant and 35S::GhKNOX2‐1 transgenic Arabidopsis. Bars = 50 µm. (d) Close‐up images of the leaves of 21‐d‐old wild‐type (top), knat2 mutant (middle) and 35S::GhKNOX2‐1 transgenic Arabidopsis (bottom). (e)–(g) Quantitative comparisons of the leaf shapes of wild‐type, knat2 mutant and 35S::GhKNOX2‐1 transgenic lines based on the leaf dissection index (perimeter2/4π × leaf area) (e), number of teeth/leaf perimeter (f) and tooth area/leaf area (g). (h) Statistical analysis of the number of teeth per leaf of wild‐type, knat2 mutant and 35S::GhKNOX2‐1 transgenic Arabidopsis. A total of 10 leaves of each phenotype were used for each measurement. Data are presented as the mean ± SE. Statistical significance was determined using one‐way analysis of variance combined with Tukey's test. *P < 0.05; ***P < 0.001. WT, wild type.
GhKNOX2‐1 functions epistatically to GhARF16‐1 to regulate leaf shape in Arabidopsis
Although the overexpression of the G. hirsutum genes GhKNOX2‐1 and GhARF16‐1 in Arabidopsis resulted in a phenotype similar to that in Gossypium, it is still unclear whether the GhARF16‐GhKNOX2 interaction paradigm is also conserved in Arabidopsis. To elucidate this, we first generated the arf16/35S::GhKNOX2‐1 transgenic Arabidopsis line overexpressing GhKNOX2‐1 in the arf16 mutant. As a result, the arf16/35S::GhKNOX2‐1 Arabidopsis produced irregularly shaped lobed leaves (Figure 7a,e), resembling the phenotype of the 35S::GhKNOX2‐1 line rather than the phenotype of the arf16 mutant. In the transgenic Arabidopsis line knat2/pARF16::GhmARF16‐1, in which the gain‐of‐function GhmARF16‐1 was heterologously expressed in the Arabidopsis knat2 mutant under the control of its native promoter, the leaf patterning was similar to that shown by the knat2 mutant with smooth and undivided margins, and its leaf epidermal cells were polygonal‐shaped. Overall, these characteristics of the transgenic line knat2/pARF16::GhmARF16‐1 differed from those of pARF16::GhmARF16‐1 (Figure 7b,e). These results suggest that GhKNOX2‐1 can act as a downstream target of GhARF16‐1 to regulate leaf morphology in Arabidopsis.
Figure 7.
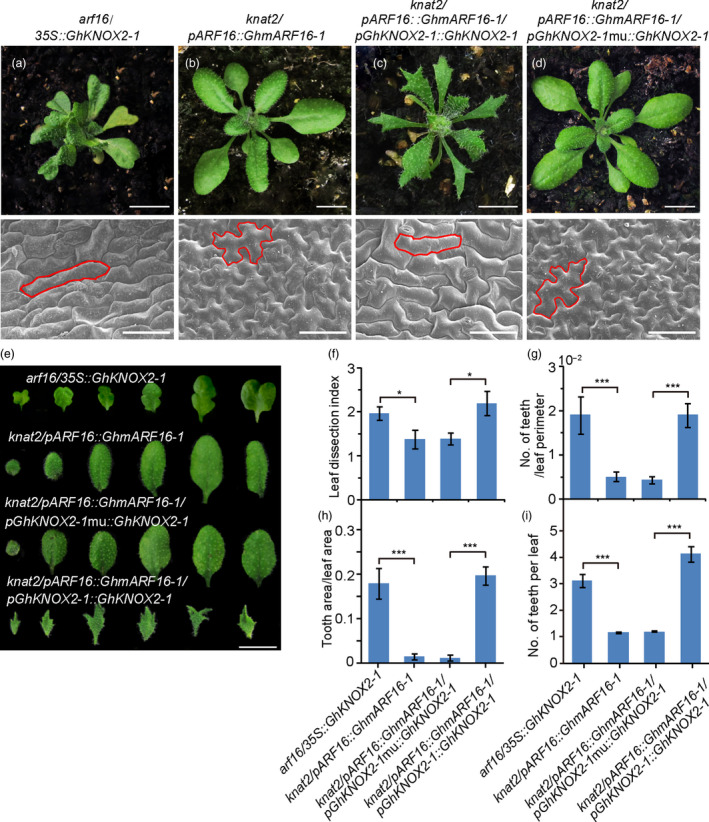
GhKNOX2‐1 is functionally epistatic to GhARF16‐1 in regulating leaf morphology. (a)–(d) Top, from left to right, 21‐d‐old phenotypes of the arf16/35S::GhKNOX2‐1 transgenic Arabidopsis line overexpressing GhKNOX2‐1 in the arf16 mutant (a), knat2/pARF16::GhmARF16‐1 line overexpressing GhmARF16‐1 in the knat2 mutant (b), knat2/pARF16::GhmARF16‐1/pGhKNOX2‐1::GhKNOX2‐1 line overexpressing GhARF16‐1 and GhKNOX2‐1 in the knat2 mutant under the control of their respective native promoters (c) and knat2/pARF16::GhmARF16‐1/pGhKNOX2‐1mu::GhKNOX2‐1 line overexpressing GhARF16‐1 under the control of its native promoter and GhKNOX2‐1 under the control of its mutated promoter (pGhKNOX2‐1mu; designated in Figure 4f) in the knat2 mutant (d). Bars = 4 mm. Bottom, from left to right, scanning electron micrographs of the leaf epidermal cells at the base of the abaxial side of mature leaves of transgenic lines (a)–(d). Bars = 50 µm. (e) Close‐up images of the leaves of 21‐d‐old Arabidopsis transgenic lines (a)–(d). Bars = 4 mm. (f)–(h) Quantitative comparisons of the leaf shapes of (a)–(d) plants based on the leaf dissection index (perimeter2/4π × leaf area) (f), number of teeth/leaf perimeter (g) and tooth area/leaf area (h). (i) Statistical analysis of the number of teeth per leaf of transgenic plants (a)–(d). A total of 10 leaves from each phenotype were used for each measurement. Data are mean ± SE. Statistical significance was determined using one‐way analysis of variance combined with Tukey's test. *P < 0.05; ***P < 0.001.
To further investigate the crucial role of the AuxRE binding site in the GhKNOX2‐1 promoter of the GhARF16‐GhKNOX2 regulatory machinery, we expressed GhKNOX2‐1 in knat2/pARF16::GhmARF16‐1 Arabidopsis using the native (pGhKNOX2‐1) or mutated (pGhKNOX2‐1mu) promoter (without AuxRE, Figure 4f). Compared to its strong expression in the knat2/pGhKNOX2‐1::GhKNOX2‐1/pARF16::GhmARF16‐1 transgenic line with the native promoter pGhKNOX2‐1, the GhKNOX2‐1 gene showed barely detectable transcription levels in the knat2/pGhKNOX2‐1mu::GhKNOX2‐1/pARF16::GhmARF16‐1 transgenic Arabidopsis plants whose leaves displayed a smooth margin comparable to those of the knat2 mutant (Figure 7c,d, Figure S15). An electron microscopic analysis revealed that the regular shape of epidermal cells was observed only in the lobed leaves of the transgenic line with the native promoter pGhKNOX2‐1 (Figure 7c), but not in the line with the mutated promoter pGhKNOX2‐1mu (Figure 7d). Additionally, the knat2/pGhKNOX2‐1::GhKNOX2‐1/pARF16::GhmARF16‐1 transgenic line carrying the native promoter pGhKNOX2‐1 had significant positive effects on the leaf dissection index, number of teeth/leaf perimeter, number of teeth per leaf and tooth area/leaf area, compared to the knat2/pGhKNOX2‐1mu::GhKNOX2‐1/pARF16::GhmARF16‐1 transgenic Arabidopsis with the mutated promoter pGhKNOX2‐1mu (Figure 7f–i) These results strongly suggest that GhARF16‐1 regulates GhKNOX2‐1 via the AuxRE motif in the GhKNOX2‐1 promoter, and the GhARF16‐GhKNOX2 interaction paradigm can also regulate leaf shape in Arabidopsis.
Discussion
Understanding the genetic basis of auxin signalling‐mediated leaf shape diversity provides an attractive opportunity to reveal the morphological diversity of a plant form during evolution. Recently, leaf morphological differences have been illustrated in numerous reference plants, including Pelargonium sp., grape vine (Vitis sp.), tomato (Solanum lycopersicum) and species of the poppy family (Papaveraceae), demonstrating that small genetic or hormonal changes can yield significantly different patterns (Bar and Ori, 2014; Scarpella et al., 2010; Scarpella et al., 2006). The divergent leaf shapes were also studied at the molecular level in a comparative context with two related plant species from the family Brassicaceae: A. thaliana, which has simple leaves with nearly smooth margins, and C. hirsuta, which has compound leaves with individual leaflets (Kierzkowski et al., 2019). Here, we used different species of the genus Gossypium, belonging to the mallow family Malvaceae, which is phylogenetically adjacent to the family Brassicaceae, as reference plants to illustrate differentiated leaf forms and reveal the genetic underpinnings of leaf shape diversity among Gossypium species. The KNOX family genes have been identified as the key regulators of leaf shape diversity in distinct plant species (Kierzkowski et al., 2019). Our work revealed that distinct amounts of ARF16‐1 proteins that bind to the AuxRE motif in the KNOX2‐1 promoter can fine‐tune the expression levels of KNOX2‐1 in different Gossypium species and, thus, determine their diverse leaf morphologies (Figure 8). Generally, this auxin signalling module mediating leaf shape might apply to other plant species in eudicots.
Figure 8.
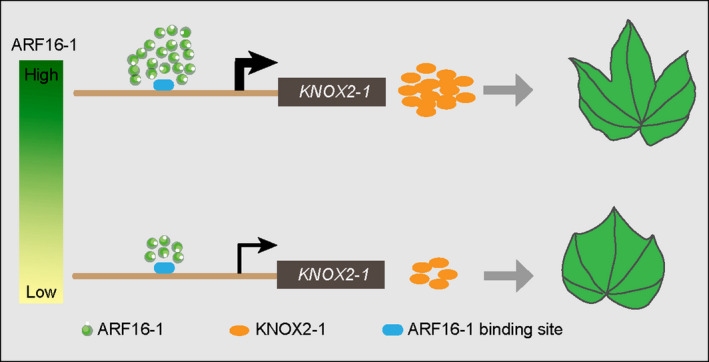
Working model for ARF16‐KNOX2 interaction paradigm in regulating diverse leaf shapes. In the lobed leaves, where ARF16‐1 is highly expressed, a large amount of ARF16‐1 protein binds directly to the ARF‐binding site [Auxin‐responsive element (AuxRE)] in the KNOX2‐1 promoter regions to activate an abundance of KNOX2‐1 transcripts that result in lobed leaves. In the smooth leaves, where ARF16‐1 is weakly expressed, a deficiency of GhARF16‐1 transcripts leads to a low expression level of KNOX2‐1 that results in the production of smooth leaves.
ARF transcription factors are able to be negatively regulated by different miRNAs. It has been reported that the ARF10, ARF16 and ARF17 were specifically targeted by miR160. ARF10, ARF16 and ARF17 shared very high sequence similarities and formed a subgroup (Figure S5) in the phylogenetic tree of ARF family members, suggesting that the miR160‐targeted ARFs are conserved for the ARF members in the same subgroup. In Arabidopsis, expression of a miR160‐resistant version of ARF17 under its native promoter resulted in rosette and cauline leaf margin serration (Mallory et al., 2005), whereas expression of a miR160‐resistant version of ARF16 resulted in upcurled and occasionally serrated leaves (Wang et al., 2005). Similarly, in cotton, ARF10, ARF16 and ARF17 were also identified, and they were predicted to be targeted by miR160 (Liu et al., 2020). The previous work confirmed that overexpression of miR160 in cotton resulted in significant decrease of ARF10 and ARF17 (Ding et al., 2017). Our work further revealed that transgenic Arabidopsis carrying the pGhARF16::GhmARF16‐1 construct displayed upcurled and occasionally serrated leaves (Figure 5), which phenotype was more similar to that in Arabidopsis ARF16 overexpressing plants. Moreover, in tomato, constitutive expression of miR160‐resistant ARF10 caused a deeply divided leaf phenotype with completely separated stripe‐like lobes (Hendelman et al., 2012). In addition, overexpression of miR160 gene under a constitutive CaMV‐35S promoter in tomato resulted in an entire leaflet blade phenotype compared to the wild‐type leaves. Meanwhile the expression level of the tomato Knotted 2 protein (Tkn2) was negatively correlated with miR160 levels and positively correlated with ARF gene expression levels, such as ARF16 (Damodharan et al., 2018). Consistently, in our study, expression of a miR160‐resistant GhARF16 gene in Arabidopsis and cotton led to significantly increased GhKNOX2‐1 transcripts and deeply serrated leaf margins. We speculate that miR160 may cleave the ARF16 transcripts and repress the expression of ARF16, which resulted in the down‐regulation of KNOX2 gene. By elevating cytokinin levels and repressing gibberellin levels, which in turn promoted cell division and repressed cell differentiation, ARF‐KNOX2 module participated in the establishment of leaf morphogenesis (Hay and Tsiantis, 2010; Jasinski et al., 2005; Yanai et al., 2005). Combing these studies, therefore we proposed that the miR160‐targeted ARF‐KNOX2 module was conserved in leaf development.
In A. thaliana and C. hirsuta, researchers determined that two key genes, STM (KNOX family) and RCO, worked in combination to regulate variation in leaf shape (Hay and Tsiantis, 2010; Rast‐Somssich et al., 2015; Vlad et al., 2014; Vuolo et al., 2016). Here, we revealed that the Gossypium proteins KNOX2‐1 and ARF16‐1 likely act as two key regulators of leaf shape variation during the evolution or artificial domestication of Gossypium spp. By co‐manipulating STM (KNOX family member) and RCO in Arabidopsis leaves, Kierzkowski et al. (2019) produced a dissected leaf pattern resembling that of C. hirsuta (Kierzkowski et al., 2019). Likewise, by manipulating the expression of GhKNOX2‐1 and GhARF16‐1 in G. hirsutum, we were able to modify its original leaf form to resemble that of the other Gossypium species. Additionally, other KNOX or ARF members might be functionally redundant to the GhKNOX2 and GhARF16 proteins in the regulation of Gossypium leaf shape because the GhARF16‐1 and GhKNOX2‐1 RNAi transgenic lines showed no obvious alteration of leaf shapes.
Previous studies have indicated that the LATE MERISTEM IDENTITY 1 (LMI1)‐like transcription factor plays an important role in the regulation of cotton leaf shape (Andres et al., 2017; Chang et al., 2016). Silencing the LMI1‐like gene with the virus‐induced gene silencing (VIGS) method led to a change in cotton leaf patterning from okra to broad. The overexpression of the LMI1‐like gene significantly enhanced the depth of the leaf lobes in Arabidopsis. In Arabidopsis, LMI1 was reported to directly activate WEE1, a conserved mitosis blocker, to regulate stipule proportions (Vuolo et al., 2018). Although the LMI1‐like gene was found to coordinate with the KNOX gene to regulate leaf development in plants (Chang et al., 2019), it remains to be investigated whether the Gossypium LMI1‐like gene is integrated into the GhARF16‐GhKNOX2 network or it works in parallel with the ARF16‐KNOX2 pathway to collaboratively regulate the complexities of leaf morphology in Gossypium.
The allotetraploid cotton, G. hirsutum (AADD genome), speciated through an allopolyploidization event in which an A‐genome species resembling G. arboreum (AA) and a D‐genome species resembling G. raimondii (DD) were reunited approximately 1 to 2 million years ago (Chen et al., 2020; Guan et al., 2014). We postulated that the Gossypium ARF16‐KNOX2 interaction paradigm might have evolved prior to the formation of the allotetraploid cotton because the diploid cotton species G. trilobum (D‐genome) and G. raimondii (D‐genome) displayed divergent leaf shapes – lobed in G. trilobum and smooth in G. raimondii (Figure S4). Moreover, the differential expression levels of ARF16‐1 and KNOX2‐1 were also observed between the two diploid cotton species.
Our work not only provides new insights into the genetic basis of auxin‐regulated morphological diversity during plant evolution, but also offers a potential theoretical basis for breeding better varieties of crops. Leaf shape, one of most important characteristics of plants, directly affects crop growth and yield (Blein et al., 2008; Tsukaya, 2018). Leaf morphology can affect fibre yield, timing of crop maturation, disease resistance and even efficiency of foliar chemical application (Andres et al., 2014). Identifying genes involved in leaf shape and revealing the mechanisms of leaf morphogenesis will enrich our toolkit for agronomic trait improvement and horticultural applications in the future.
Methods
Plant materials and growth conditions
Gossypium hirsutum ‘Xuzhou 142,’ G. arboreum ‘Shixiya 1,’ G. raimondii ‘CMD10,’ and G. trilobum (Mocono & Sesse ex DC) were used in this study and grown in a climate‐controlled greenhouse with a 16‐h light and 8‐h dark cycle at 30 °C (Xiao et al., 2018). Arabidopsis thaliana plants were grown on soil in growth chambers with a 16‐h light and 8‐h dark cycle at 23 °C. The Arabidopsis mutant lines with disrupted KNAT2 (knat2‐5, SALK_099837) and ARF16 (arf16‐2, SALK_ 021448) were T‐DNA insertion alleles obtained from the SALK collections (Arabidopsis Biological Resource Center: Columbus, OH; http://signal.salk.edu). Seeds were surface sterilized with 0.1% HgCl2, germinated on Murashige and Skoog (MS) medium for 2 weeks, transferred to soil and grown in a fully automated control system (Percival) with a 16‐h light and 8‐h dark cycle at 23 °C in 70% humidity (Zhang et al., 2015).
Cotton transformation
All overexpression and RNAi constructs were introduced into the Agrobacterium strain LBA4404. Seeds (G. hirsutum cv. YZ1) were sterilized and cultured in a chamber without light for 6 days at 30 °C. The Agrobacterium‐mediated cotton transformation was performed using hypocotyl segments as explants, as described previously (Jin et al., 2006; Li et al., 2009). After cutting into 5‐mm segments, the explants were immersed in the Agrobacterium tumefaciens suspension (OD = 0.2–0.4) for 15 min. The infected explants were transferred onto a callus‐induced medium. After callus induction, proliferation, embryogenic callus induction, embryo differentiation and plantlet regeneration, the putative transgenic plants were transferred into pots and grown in the greenhouse with a 14‐h light and 10‐h dark condition at 25 °C.
RNA extraction and quantitative RT‐PCR (qRT‐PCR) analysis
For cotton samples, the plant material was frozen in liquid nitrogen and then ground to a fine powder with a mortar and pestle using a modified method (He et al., 2018). For Arabidopsis samples, total RNA was isolated from rosette leaves and seedlings at 0, 2, 4, 8, 12 and 20 days after germination. Total RNA was extracted using the PureLink™ RNA Mini Kit (Lot no. 1687455; Invitrogen: Waltham, MA) according to the manufacturer’s instructions, and cDNA was reverse‐transcribed from 5 μg of the total RNA as described previously (Xiao et al., 2018). The cDNA sequences of G. KNOX and ARF family genes were obtained from the Cotton Functional Genomics Database (https://cottonfgd.org/). The accession numbers and IDs of the identified genes are given in Tables S4 and S5. In the qRT‐PCR experiments, each gene was run in three biological and three technical replicates with the following reaction parameters: 95 °C for 10 min, followed by 40 cycles of 95 °C for 10 s and 56 °C for 30 s. A melting curve was generated from 65 to 95 °C. The SigmaStat software was used to statistically analyse data by one‐way analysis of variance (ANOVA). The housekeeping genes GhUBQ7 and AtUBQ5 were used as internal controls for cotton and Arabidopsis, respectively.
RNA‐Seq analysis
The leaf primordia of G. arboreum and G. raimondii leaf were used for RNA extraction and RNA‐Seq. Total RNA was isolated using the PureLinkTM RNA Mini Kit (Invitrogen: Waltham, MA). The RNA was digested with RNase‐free DNase (Qiagen: Germantown, MD) and checked for integrity by capillary gel electrophoresis and Agilent 2100 Bioanalyzer (Agilent: Santa Clara, CA). The RNA [with RNA integrity number (RIN) ≥8] was pooled from the three biological replicates of each sample and then submitted to Novogene Bioinformatics Technology Co., Ltd. (Beijing, China) for high‐throughput sequencing via the Illumina HiSeq2000 sequencer. For RNA library construction, mRNA was enriched using oligo(dT) magnetic beads and then immediately broken into short segments in a fragmentation buffer. Subsequently, double‐stranded cDNA was synthesized and purified, followed by adaptor ligation. After the libraries were sequenced to generate > 30 million 125‐bp paired‐end (PE) reads for each sample, high‐quality reads were obtained after several steps of quality checks, which included trimming, removal of adaptor/primer sequences and low‐quality reads.
We used HISAT2 and Cufflinks software for the RNA‐seq expression analysis. In brief, RNA‐seq reads were mapped to the corresponding each cotton genome using HISAT2 (Version 2.1.0). Then, the expression of each gene was calculated using RPKM (Reads per kilobase of gene per million mapped reads) with Cufflinks (Version 2.2.1). The values for the heatmap in Figure 1e were displayed by log2RPKM. The differentially expressed genes between the two sets of samples were obtained by inputting read counts of each genes as expression matrix into the edgeR package. Only the genes with (P value <= 0.01 and fold change >= 2.0) were considered as significantly differential expressed.
Transcription factor activity assay
The transcription factor assay was performed on Arabidopsis protoplasts (Wang et al., 2013). The reporter plasmid contained the firefly luciferase gene under the control of the 35S promoter with five GAL4‐binding elements upstream of the gene. The effector plasmids containing 35S‐BD and 35S‐BD‐VP16 were used as the negative and positive controls, respectively. The coding regions of GhARF16‐1 and GhKNOX2‐1 were amplified by PCR using the primers listed in Table S6, digested with Spe I and EcoR I, and cloned into the pRT‐BD vector to generate effector plasmids containing the 35S‐BD‐GhARF16‐1 and 35S‐BD‐GhKNOX2‐1 constructs, respectively. The reporter, effector and control plasmids were co‐transfected into Arabidopsis protoplasts by polyethylene glycol transformation. Luciferase assays were performed using the dual‐luciferase reporter assay system and a luminometer (GloMax20‐20, Promega: Madison, WI, USA).
GUS staining and microscopy
Histochemical GUS staining was carried out as described previously (Tao et al., 2013). Tissues from the GhKNOX2‐1‐GUS transgenic lines were immersed in the staining buffer. Samples were vacuumed for 5 min and incubated overnight at 37 °C, after which the staining buffer was removed. Samples were stored in 70% ethanol before microscopic observation.
Scanning electron microscopy was conducted as described previously (Tao et al., 2013). The 5th leaf was sampled from the 25‐d‐old Arabidopsis plants and from 40‐d‐old cotton plants. The fifth‐leaf samples were fixed in FAA buffer (50% ethanol, 6% glacial acetic acid and 5% formaldehyde) for 4 h at 25 °C. After serial ethanol dehydration and isoamyl acetate substitution, the samples were dried in liquid carbon dioxide and then analysed using scanning electron microscopy (Hitachi S‐3400N, Hitachi, Tokyo, Japan).
Transcriptional activation assay
The dual‐luciferase reporter assay was performed as described previously (Wang et al., 2013). The native coding region of GhARF16‐1 was amplified by PCR using the primers listed in Table S6, digested with Spe I and EcoR I and cloned into the pRT‐BD vector driven by the constitutive 35S promoter to generate an effector plasmid. The native and mutated GhKNOX2‐1 promoter sequences were separately amplified by PCR using the primers listed in Table S6, digested with HindIII and Pst I, and inserted into a pGreen‐LUC vector to drive the firefly luciferase reporter gene as reporter plasmids. The plasmid containing the Renilla luciferase gene, driven by the 35S promoter, was the control plasmid. The effector, reporter and internal control plasmids were mixed at a ratio of 6:6:1. These mixtures were then co‐transfected into Arabidopsis protoplasts by the polyethylene glycol transformation method (Zhang et al., 2015). After culturing for 16 h, the transcriptional activation assay was conducted by determining the luciferase activity using the dual‐luciferase reporter assay system according to the manufacturer's manual. The assay was performed with three biological replicates, and the error bars represent the standard errors of the means from three independent experiments.
Electrophoretic mobility shift assay (EMSA)
The EMSA assay was performed using the chemiluminescent EMSA kit according to a previously described method (Hou et al., 2014). The promoter fragment of GhKNOX2‐1 containing the native or mutant AuxRE element, was labelled with biotin on both ends of the probe. Sequences of DNA probes are as follows: Native probe, 5′–TAGTCATTAGCAGAGAGCATTAATTCTCACTTTCTATTACAAAATCCTCTCTGTCT CTCGAGCTTCTTTTGCACTTGTTTGGAAGAAAAGAAAGACAGAA‐3′, mutant probe, 5′‐TAGTCATTAGCAGAGAGCATTAATTCTCACTTTCTATTACAAAATCCTCTCATA GTATCGAGCTTCTTTTGCACTTGTTTGGAAGAAAAGAAAGACAGAA‐3′. The assay was conducted by incubating the labelled probe with purified GhARF16‐1 proteins at 25 °C for 1 h and separated by 8% native PAGE in 0.5X TBE buffer. Non‐labelled probes were used as cold competitors.
Yeast one‐hybrid assay
The yeast one‐hybrid (Y1H) assay was performed as previously described (Xiao et al., 2018). Briefly, the ORFs of GhARF16‐1 was constructed into the pGADT7 vector using homologous recombination. The 1425 bp sequence of GhKNOX2‐1 promoter at the upstream of ATG (Tables S3) was amplified and inserted into the pAbAi vector with the Sac I and Kpn I. Y1HGold yeast strain cells were transformed with a pGADT7 prey vector carrying the ORF of GhARF16‐1 and a constructed pAbAi bait vector described above. The transformed yeast cell suspension was dropped on SD [DDO for –Leu, with or without Aureobasidin A (AbA)] medium plates and cultured at 30 °C for 3 days.
Leaf margin analysis
Leaf shape was recorded for transgenic cotton at the six‐leaf stage and then confirmed at the flowering stage. A leaf margin analysis was conducted as described previously (Zheng et al., 2016). Individual leaves were photographed using a camera (D3‐U3., Nikon, Tokyo, Japan). The leaf dissection index (perimeter2/4π × leaf area), number of teeth/leaf perimeter and/tooth area/leaf area were used to quantify leaf margins (Bilsborough et al., 2011). Leaf area and perimeter were measured using the ImageJ software. A total of 10 leaves each from wild‐type and mutant plants were chosen for analysis. Statistical significance was determined by one‐way ANOVA using the SigmaStat 3.5 software combined with Tukey's test.
Vector construction and retro‐transformation
The full‐length sequences (from the start codon to the stop codon) of GhKNOX2‐1 and GhARF16‐1 were amplified from the cDNA using the primers listed in Table S6. For GhmARF16‐1, the miR160‐binding site of the GhARF16‐1 gene was mutated to generate GhmARF16‐1 according to a previous report (Wang et al., 2005). The product of each gene was digested with KpnI and XbaI, then cloned into the digested pQG110 vector driven by the constitutive cauliflower mosaic virus 35S promoter, and the construct was further introduced into A. tumefaciens strain GV3101 for the transformation of A. thaliana via the floral dip method. The transgenic plants were selected on solid half‐strength MS medium plates containing 50 mg/mL of appropriate antibiotics (Zhang et al., 2015) and were further validated by genomic PCR. The expression levels of GhKNOX2‐1 and GhARF16‐1 were also investigated in transgenic seedlings. Leaves from wild‐type and transgenic plants were used for further analysis.
Conflict of interest
The authors declare that they have no conflict of interest.
Author contributions
G.X. conceived the project and designed the experiments. P.H., Y.Z., X.F. and H.L. performed the experiments and analysed the data. H.S., C.Z. and J.F. wrote the manuscript with input from all authors.
Supporting information
Figure S1 Statistical analysis of the percentages of the lobed and smooth leaves in Gossypium hirsutum.
Figure S2 Statistical analysis of the number of teeth per leaf from Gossypium arboreum (Ga), G. raimondii (Gr), Gh‐lobed (Gh‐l), and Gh‐smooth (Gh‐s).
Figure S3 qRT‐PCR analysis of KNOX1‐1, KNOX1‐2, KNOX2‐2, ARF16‐2, and ARF16‐3 mRNA levels from Gossypium arboreum (Ga), G. raimondii (Gr), Gh‐lobed (Gh‐l), and Gh‐smooth (Gh‐s).
Figure S4 Analysis of leaf morphology and transcriptional levels of KNOX2‐1 and ARF16‐1 from Gossypium raimondii and Gossypium trilobum.
Figure S4 A phylogenetic tree was constructed with MEGA 6.0 using the Maximum Likelihood (ML) method with 1000 bootstrap replicates based on a multiple alignment of the amino acid sequences of the Arabidopsis ARF proteins.
Figure S5 Sequences of GhARF16‐1, GhmARF16‐1, and miR160.
Figure S6 qRT‐PCR analysis of GhARF16‐1 mRNA levels in wild‐type, GhARF16‐1 RNAi, and pARF16::GhmARF16‐1 transgenic plants.
Figure S7 qRT‐PCR analysis of GhKNOX2‐1 mRNA levels in wild‐type, GhKNOX2‐1 RNAi, and 35S::GhKNOX2‐1 transgenic plants.
Figure S8 Subcellular localization of GhKNOX2‐1 using Nicotiana benthamiana leaves.
Figure S9 Transcriptional ability of GhKNOX2‐1 in Arabidopsis protoplasts.
Figure S10 qRT‐PCR analysis of GhARF16‐1 mRNA levels in pARF16::GhmARF16‐1 transgenic Arabidopsis.
Figure S11 Scanning electron micrographs of leaf epidermal cells at the base of the abaxial side of mature leaves of wild‐type, arf16 mutant, and pARF16::GhmARF16‐1 transgenic plants.
Figure S12 qRT‐PCR analysis of GhKNOX2‐1 mRNA levels in 35::GhKNOX2‐1 transgenic Arabidopsis.
Figure S13 Scanning electron micrographs of leaf epidermal cells at the base of the abaxial sides of mature leaves of wild‐type, knat2 mutant, and 35S::GhKNOX2‐1 transgenic plants.
Figure S14 qRT‐PCR analysis of GhKNOX2‐1 mRNA levels in leaves from individual lines shown in Figure 5A to 5D.
Table S1 List of genes related to leaf shape development in Arabidopsis.
Table S2 Promoter sequence of GhARF16‐1.
Table S3. Promoter sequence of GhKNOX2‐1.
Table S4 Analysis of Gossypium hirsutum KNOX gene family and its orthologs in AA and DD cotton genomes.
Table S5 Analysis of Gossypium hirsutum ARF gene family and its orthologs in AA and DD cotton genomes.
Table S6 List of primers used in this study.
Data S1 Differentially expressed genes between Gossypium arboreum and Gossypium raimondi.
Acknowledgements
We are thankful to Professor Yuxian Zhu from Wuhan University for his extremely valuable remarks and helpful comments on the manuscript. This work was supported by the Shaanxi Natural Science Foundation (2019JQ‐062 and 2020JQ‐410), Shaanxi Youth Entrusted Talents Program (20190205), China Postdoctoral Science Foundation (2018M640947, 2020T130394), Shaanxi Postdoctoral Project (2018BSHYDZZ76), Natural Science Basic Research Plan in Shaanxi Province of China (2018JZ3006), Fundamental Research Funds for the Central Universities (GK201903064, GK201901004, GK202002005 and GK202001004), and State Key Laboratory of Cotton Biology Open Fund (CB2020A12).
He, P. , Zhang, Y. , Li, H. , Fu, X. , Shang, H. , Zou, C. , Friml, J. and Xiao, G. (2021) GhARF16‐1 modulates leaf development by transcriptionally regulating the GhKNOX2‐1 gene in cotton. Plant Biotechnol J, 10.1111/pbi.13484
References
- Andres, R.J. , Bowman, D.T. , Kaur, B. and Kuraparthy, V. (2014) Mapping and genomic targeting of the major leaf shape gene (L) in Upland cotton (Gossypium hirsutum L.). Theor. Appl. Genet. 127, 167–177. [DOI] [PubMed] [Google Scholar]
- Andres, R.J. , Coneva, V. , Frank, M.H. , Tuttle, J.R. , Samayoa, L.F. , Han, S.W. , Kaur, B. et al. (2017) Modifications to a LATE MERISTEM IDENTITY1 gene are responsible for the major leaf shapes of Upland cotton (Gossypium hirsutum L.). Proc. Natl. Acad. Sci. U. S. A. 114, E57–E66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar, M. and Ori, N. (2014) Leaf development and morphogenesis. Development 141, 4219–4230. [DOI] [PubMed] [Google Scholar]
- Barkoulas, M. , Hay, A. , Kougioumoutzi, E. and Tsiantis, M. (2008) A developmental framework for dissected leaf formation in the Arabidopsis relative Cardamine hirsuta . Nat. Genet. 40, 1136–1141. [DOI] [PubMed] [Google Scholar]
- Bilsborough, G.D. , Runions, A. , Barkoulas, M. , Jenkins, H.W. , Hasson, A. , Galinha, C. , Laufs, P. et al. (2011) Model for the regulation of Arabidopsis thaliana leaf margin development. Proc. Natl. Acad. Sci. U. S. A. 108, 3424–3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blein, T. , Pautot, V. and Laufs, P. (2013) Combinations of mutations sufficient to alter Arabidopsis leaf dissection. Plants, 2, 230–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blein, T. , Pulido, A. , Vialette‐Guiraud, A. , Nikovics, K. , Morin, H. , Hay, A. , Johansen, I.E. et al. (2008) A conserved molecular framework for compound leaf development. Science, 322, 1835–1839. [DOI] [PubMed] [Google Scholar]
- Byrne, M.E. , Barley, R. , Curtis, M. , Arroyo, J.M. , Dunham, M. , Hudson, A. and Martienssen, R.A. (2000) Asymmetric leaves1 mediates leaf patterning and stem cell function in Arabidopsis . Nature, 408, 967–971. [DOI] [PubMed] [Google Scholar]
- Byrne, M.E. , Simorowski, J. and Martienssen, R.A. (2002) ASYMMETRIC LEAVES1 reveals knox gene redundancy in Arabidopsis . Development, 129, 1957–1965. [DOI] [PubMed] [Google Scholar]
- Chang, L.J. , Fang, L. , Zhu, Y.J. , Wu, H.T. , Zhang, Z.Y. , Liu, C.X. , Li, X.H. et al. (2016) Insights into interspecific hybridization events in allotetraploid cotton formation from characterization of a gene‐regulating leaf shape. Genetics, 204, 799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, L.J. , Mei, G.F. , Hu, Y. , Deng, J.Q. and Zhang, T.Z. (2019) LMI1‐like and KNOX1 genes coordinately regulate plant leaf development in dicotyledons. Plant Mol. Biol. 99, 449–460. [DOI] [PubMed] [Google Scholar]
- Chen, Z.J. , Sreedasyam, A. , Ando, A. , Song, Q. , De Santiago, L.M. , Hulse‐Kemp, A.M. , Ding, M. et al. (2020) Genomic diversifications of five Gossypium allopolyploid species and their impact on cotton improvement. Nat. Genet. 52, 525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherenkov, P. , Novikova, D. , Omelyanchuk, N. , Levitsky, V. , Grosse, I. , Weijers, D. and Mironova, V. (2018) Diversity of cis‐regulatory elements associated with auxin response in Arabidopsis thaliana . J. Exp. Bot. 69, 329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuck, G. , Lincoln, C. and Hake, S. (1996) KNAT1 induces lobed leaves with ectopic meristems when overexpressed in Arabidopsis . Plant Cell, 8, 1277–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damodharan, S. , Corem, S. , Gupta, S.K. and Arazi, T. (2018) Tuning of SlARF10A dosage by sly‐miR160a is critical for auxin‐mediated compound leaf and flower development. Plant J. 96, 855–868. [DOI] [PubMed] [Google Scholar]
- Ding, Y.H. , Ma, Y.Z. , Liu, N.A. , Xu, J. , Hu, Q. , Li, Y.Y. , Wu, Y.L. et al. (2017) microRNAs involved in auxin signalling modulate male sterility under high‐temperature stress in cotton (Gossypium hirsutum). Plant J. 91, 977–994. [DOI] [PubMed] [Google Scholar]
- Dkhar, J. and Pareek, A. (2014) What determines a leaf's shape? EvoDevo, 5, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, F. , Guan, C.M. and Jiao, Y.L. (2018) Molecular mechanisms of leaf morphogenesis. Mol. Plant, 11, 1117–1134. [DOI] [PubMed] [Google Scholar]
- Gray, W.M. , Kepinski, S. , Rouse, D. , Leyser, O. and Estelle, M. . (2001) Auxin regulates SCFTIR1‐dependent degradation of AUX/IAA proteins. Nature, 414, 271–276. [DOI] [PubMed] [Google Scholar]
- Guan, X. , Song, Q. and Chen, Z.J. (2014) Polyploidy and small RNA regulation of cotton fiber development. Trends Plant Sci. 19, 516–528. [DOI] [PubMed] [Google Scholar]
- Hay, A. and Tsiantis, M. (2010) KNOX genes: versatile regulators of plant development and diversity. Development, 137, 3153–3165. [DOI] [PubMed] [Google Scholar]
- He, P. , Xiao, G. , Liu, H. , Zhang, L. , Zhao, L. , Tang, M. , Huang, S. et al. (2018) Two pivotal RNA editing sites in the mitochondrial atp1 mRNA are required for ATP synthase to produce sufficient ATP for cotton fiber cell elongation. New Phytol. 218, 167–182. [DOI] [PubMed] [Google Scholar]
- Hendelman, A. , Buxdorf, K. , Stav, R. , Kravchik, M. and Arazi, T. (2012) Inhibition of lamina outgrowth following Solanum lycopersicum AUXIN RESPONSE FACTOR 10 (SlARF10) derepression. Plant Mol. Biol. 78, 561–576. [DOI] [PubMed] [Google Scholar]
- Hou, X. , Zhou, J. , Liu, C. , Liu, L. , Shen, L. and Yu, H. (2014) Nuclear factor Y‐mediated H3K27me3 demethylation of the SOC1 locus orchestrates flowering responses of Arabidopsis . Nat. Commun. 5, 4601. [DOI] [PubMed] [Google Scholar]
- Israeli, A. , Capua, Y. , Shwartz, I. , Tal, L. , Meir, Z. , Levy, M. , Bar, M. et al. (2019) Multiple auxin‐response regulators enable stability and variability in leaf development. Current Biol. 29(1746–1759), e1745. [DOI] [PubMed] [Google Scholar]
- Jasinski, S. , Piazza, P. , Craft, J. , Hay, A. , Woolley, L. , Rieu, I. , Phillips, A. et al. (2005) KNOX action in Arabidopsis is mediated by coordinate regulation of cytokinin and gibberellin activities. Curr. Biol. 15, 1560–1565. [DOI] [PubMed] [Google Scholar]
- Jin, S. , Liang, S. , Zhang, X. , Nie, Y. and Guo, X. (2006) An efficient grafting system for transgenic plant recovery in cotton (Gossypium hirsutum L.). Plant Cell Tissue Organ Cult. 85, 181–185. [Google Scholar]
- Jones‐Rhoades, M.W. , Bartel, D.P. and Bartel, B. (2006) MicroRNAS and their regulatory roles in plants. Annu. Rev. Plant Biol. 57, 19–53. [DOI] [PubMed] [Google Scholar]
- Kierzkowski, D. , Runions, A. , Vuolo, F. , Strauss, S. , Lymbouridou, R. , Routier‐Kierzkowska, A.L. , Wilson‐Sanchez, D. et al. (2019) A growth‐based framework for leaf shape development and diversity. Cell, 177, 1405–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, F.F. , Wu, S.J. , Chen, T.Z. , Zhang, J. , Wang, H.H. , Guo, W.Z. and Zhang, T.Z. (2009) Agrobacterium‐mediated co‐transformation of multiple genes in upland cotton. Plant Cell Tissue Organ Cult. 97, 225–235. [Google Scholar]
- Lincoln, C. , Long, J. , Yamaguchi, J. , Serikawa, K. and Hake, S. (1994) A knotted1‐like homeobox gene in Arabidopsis is expressed in the vegetative meristem and dramatically alters leaf morphology when overexpressed in transgenic plants. Plant Cell, 6, 1859–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, N. , Wu, S. , Li, Z. , Khan, A.Q. , Hu, H. , Zhang, X. and Tu, L. (2020) Repression of microRNA 160 results in retarded seed integument growth and smaller final seed size in cotton. Crop J. 8, 602–612. [Google Scholar]
- Lodha, M. , Marco, C.F. and Timmermans, M.C. (2013) The ASYMMETRIC LEAVES complex maintains repression of KNOX homeobox genes via direct recruitment of Polycomb‐repressive complex2. Genes Dev. 27, 596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallory, A.C. , Bartel, D.P. and Bartel, B. (2005) MicroRNA‐directed regulation of Arabidopsis AUXIN RESPONSE FACTOR17 is essential for proper development and modulates expression of early auxin response genes. Plant Cell, 17, 1360–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed, B. , Bilooei, S.F. , Doczi, R. , Grove, E. , Railo, S. , Palme, K. , Ditengou, F.A. et al. (2018) Converging light, energy and hormonal signaling control meristem activity, leaf initiation, and growth. Plant Physiol. 176, 1365–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan, B. and Banerjee, A.K. (2020) MicroRNA160 regulates leaf curvature in potato (Solanum tuberosum L. cv. Desiree). Plant Signal Behav. 15, 1744373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicotra, A.B. , Leigh, A. , Boyce, C.K. , Jones, C.S. , Niklas, K.J. , Royer, D.L. and Tsukaya, H. (2011) The evolution and functional significance of leaf shape in the angiosperms. Funct. Plant Biol. 38, 535–552. [DOI] [PubMed] [Google Scholar]
- Nikovics, K. , Blein, T. , Peaucelle, A. , Ishida, T. , Morin, H. , Aida, M. and Laufs, P. (2006) The balance between the MIR164A and CUC2 genes controls leaf margin serration in Arabidopsis . Plant Cell, 18, 2929–2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rast‐Somssich, M.I. , Broholm, S. , Jenkins, H. , Canales, C. , Vlad, D. , Kwantes, M. , Bilsborough, G. et al. (2015) Alternate wiring of a KNOXI genetic network underlies differences in leaf development of A. thaliana and C. hirsuta . Genes Dev. 29, 2391–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer, D.L. , McElwain, J.C. , Adams, J.M. and Wilf, P. (2008) Sensitivity of leaf size and shape to climate within Acer rubrum and Quercus kelloggii . New Phytol. 179, 808–817. [DOI] [PubMed] [Google Scholar]
- Rubio‐Somoza, I. , Cuperus, J.T. , Weigel, D. and Carrington, J.C. (2009) Regulation and functional specialization of small RNA‐target nodes during plant development. Curr. Opin. Plant Biol. 12, 622–627. [DOI] [PubMed] [Google Scholar]
- Scarpella, E. , Barkoulas, M. and Tsiantis, M. (2010) Control of leaf and vein development by auxin. Cold Spring Harb. Perspect. Biol. 2, a001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpella, E. , Marcos, D. , Friml, J. and Berleth, T. (2006) Control of leaf vascular patterning by polar auxin transport. Genes Dev. 20, 1015–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield, S. , Dewitte, W. and Murray, J.A. (2008) A model for Arabidopsis class‐1 KNOX gene function. Plant Signal Behav. 3, 257–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serino, G. and Deng, X.W. (2003) The COP9 signalosome: regulating plant development through the control of proteolysis. Annu. Rev. Plant Biol. 54, 165–182. [DOI] [PubMed] [Google Scholar]
- Shi, B.H. , Guo, X.L. , Wang, Y. , Xiong, Y.Y. , Wang, J. , Hayashi, K. , Lei, J.Z. et al. (2018) Feedback from lateral organs controls shoot apical meristem growth by modulating auxin transport. Dev. Cell, 44, 204–216. [DOI] [PubMed] [Google Scholar]
- Sicard, A. , Thamm, A. , Marona, C. , Lee, Y.W. , Wahl, V. , Stinchcombe, J.R. , Wright, S.I. et al. (2014) Repeated evolutionary changes of leaf morphology caused by mutations to a homeobox gene. Curr. Biol. 24, 1880–1886. [DOI] [PubMed] [Google Scholar]
- Spinelli, S.V. , Martin, A.P. , Viola, I.L. , Gonzalez, D.H. and Palatnik, J.F. (2011) A mechanistic link between STM and CUC1 during Arabidopsis development. Plant Physiol. 156, 1894–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streubel, S. , Fritz, M.A. , Teltow, M. , Kappel, C. and Sicard, A. (2018) Successive duplication‐divergence mechanisms at the RCO locus contributed to leaf shape diversity in the Brassicaceae. Development, 145, dev164301. [DOI] [PubMed] [Google Scholar]
- Sunkar, R. , Li, Y.F. and Jagadeeswaran, G. (2012) Functions of microRNAs in plant stress responses. Trends Plant Sci. 17, 196–203. [DOI] [PubMed] [Google Scholar]
- Tao, Q. , Guo, D. , Wei, B. , Zhang, F. , Pang, C. , Jiang, H. , Zhang, J. et al. (2013) The TIE1 transcriptional repressor links TCP transcription factors with TOPLESS/TOPLESS‐RELATED corepressors and modulates leaf development in Arabidopsis . Plant Cell, 25, 421–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukaya, H. (2018) Leaf shape diversity with an emphasis on leaf contour variation, developmental background, and adaptation. Semin. Cell Dev. Biol. 79, 48–57. [DOI] [PubMed] [Google Scholar]
- Vlad, D. , Kierzkowski, D. , Rast, M.I. , Vuolo, F. , Dello Ioio, R. , Galinha, C. , Gan, X.C. et al. (2014) Leaf shape evolution through duplication, regulatory diversification, and loss of a homeobox gene. Science, 343, 780–783. [DOI] [PubMed] [Google Scholar]
- Vuolo, F. , Kierzkowski, D. , Runions, A. , Hajheidari, M. , Mentink, R.A. , Das Gupta, M. , Zhang, Z.J. et al. (2018) LMI1 homeodomain protein regulates organ proportions by spatial modulation of endoreduplication. Genes Dev. 32, 1361–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuolo, F. , Mentink, R.A. , Hajheidari, M. , Bailey, C.D. , Filatov, D.A. and Tsiantis, M. (2016) Coupled enhancer and coding sequence evolution of a homeobox gene shaped leaf diversity. Genes Dev. 30, 2370–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J.W. , Wang, L.J. , Mao, Y.B. , Cai, W.J. , Xue, H.W. and Chen, X.Y. (2005) Control of root cap formation by MicroRNA‐targeted auxin response factors in Arabidopsis . Plant Cell, 17, 2204–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, M.Y. , Zhao, P.M. , Cheng, H.Q. , Han, L.B. , Wu, X.M. , Gao, P. , Wang, H.Y. et al. (2013) The cotton transcription factor TCP14 functions in auxin‐mediated epidermal cell differentiation and elongation. Plant Physiol. 162, 1669–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, G. , He, P. , Zhao, P. , Liu, H. , Zhang, L. , Pang, C. and Yu, J. (2018) Genome‐wide identification of the GhARF gene family reveals that GhARF2 and GhARF18 are involved in cotton fibre cell initiation. J. Exp. Bot. 69, 4323–4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanai, O. , Shani, E. , Dolezal, K. , Tarkowski, P. , Sablowski, R. , Sandberg, G. , Samach, A. et al. (2005) Arabidopsis KNOXI proteins activate cytokinin biosynthesis. Curr. Biol. 15, 1566–1571. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Jiao, Y. , Liu, Z. and Zhu, Y.X. (2015) ROW1 maintains quiescent centre identity by confining WOX5 expression to specific cells. Nat. Commun. 6, 6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, M. , Liu, X. , Liang, S. , Fu, S. , Qi, Y. , Zhao, J. , Shao, J. et al. (2016) Chloroplast translation initiation factors regulate leaf variegation and development. Plant Physiol. 172, 1117–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Statistical analysis of the percentages of the lobed and smooth leaves in Gossypium hirsutum.
Figure S2 Statistical analysis of the number of teeth per leaf from Gossypium arboreum (Ga), G. raimondii (Gr), Gh‐lobed (Gh‐l), and Gh‐smooth (Gh‐s).
Figure S3 qRT‐PCR analysis of KNOX1‐1, KNOX1‐2, KNOX2‐2, ARF16‐2, and ARF16‐3 mRNA levels from Gossypium arboreum (Ga), G. raimondii (Gr), Gh‐lobed (Gh‐l), and Gh‐smooth (Gh‐s).
Figure S4 Analysis of leaf morphology and transcriptional levels of KNOX2‐1 and ARF16‐1 from Gossypium raimondii and Gossypium trilobum.
Figure S4 A phylogenetic tree was constructed with MEGA 6.0 using the Maximum Likelihood (ML) method with 1000 bootstrap replicates based on a multiple alignment of the amino acid sequences of the Arabidopsis ARF proteins.
Figure S5 Sequences of GhARF16‐1, GhmARF16‐1, and miR160.
Figure S6 qRT‐PCR analysis of GhARF16‐1 mRNA levels in wild‐type, GhARF16‐1 RNAi, and pARF16::GhmARF16‐1 transgenic plants.
Figure S7 qRT‐PCR analysis of GhKNOX2‐1 mRNA levels in wild‐type, GhKNOX2‐1 RNAi, and 35S::GhKNOX2‐1 transgenic plants.
Figure S8 Subcellular localization of GhKNOX2‐1 using Nicotiana benthamiana leaves.
Figure S9 Transcriptional ability of GhKNOX2‐1 in Arabidopsis protoplasts.
Figure S10 qRT‐PCR analysis of GhARF16‐1 mRNA levels in pARF16::GhmARF16‐1 transgenic Arabidopsis.
Figure S11 Scanning electron micrographs of leaf epidermal cells at the base of the abaxial side of mature leaves of wild‐type, arf16 mutant, and pARF16::GhmARF16‐1 transgenic plants.
Figure S12 qRT‐PCR analysis of GhKNOX2‐1 mRNA levels in 35::GhKNOX2‐1 transgenic Arabidopsis.
Figure S13 Scanning electron micrographs of leaf epidermal cells at the base of the abaxial sides of mature leaves of wild‐type, knat2 mutant, and 35S::GhKNOX2‐1 transgenic plants.
Figure S14 qRT‐PCR analysis of GhKNOX2‐1 mRNA levels in leaves from individual lines shown in Figure 5A to 5D.
Table S1 List of genes related to leaf shape development in Arabidopsis.
Table S2 Promoter sequence of GhARF16‐1.
Table S3. Promoter sequence of GhKNOX2‐1.
Table S4 Analysis of Gossypium hirsutum KNOX gene family and its orthologs in AA and DD cotton genomes.
Table S5 Analysis of Gossypium hirsutum ARF gene family and its orthologs in AA and DD cotton genomes.
Table S6 List of primers used in this study.
Data S1 Differentially expressed genes between Gossypium arboreum and Gossypium raimondi.


