Abstract
Background
Pregnant women with inflammatory bowel disease (IBD) may require biologic or thiopurine therapy to control disease activity. Lack of safety data has led to therapy discontinuation during pregnancy with health repercussions to mother and child.
Methods
Between 2007 and 2019, pregnant women with IBD were enrolled in a prospective, observational, multicenter study across the United States. The primary analysis was a comparison of five outcomes (congenital malformations, spontaneous abortions, preterm birth, low birth weight (LBW) and infant infections) among pregnancies exposed versus unexposed in utero to biologics, thiopurines or a combination. Bivariate analyses followed by logistic regression models adjusted for relevant confounders were utilized to determine the independent effects of specific drug classes on outcomes of interest.
Results
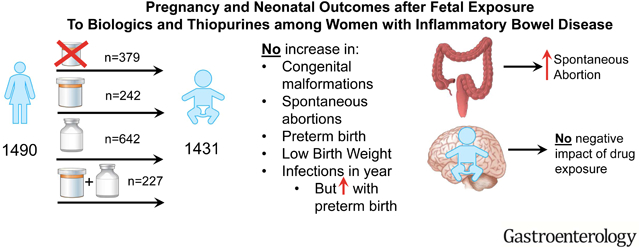
Among 1490 completed pregnancies, there were 1431 live births. One-year infant outcomes were available in 1010. Exposure was to thiopurines (242), biologics (642) or both (227) versus unexposed (379). Drug exposure did not increase the rate of congenital malformations, spontaneous abortions, preterm birth, LBW, and infections over the first year of life. Higher disease activity was associated with risk of spontaneous abortion (HR 3.41, 95% CI 1.51–7.69) and preterm birth with increased infant infection (OR 1.73, 95% CI 1.19–2.51).
Conclusions
Biologic, thiopurine, or combination therapy exposure during pregnancy was not associated with increased adverse maternal or fetal outcomes at birth or within the first year of life. Therapy with these agents can be continued throughout pregnancy in women with IBD to maintain disease control and reduce pregnancy related adverse events. (NCT00904878)
Keywords: Crohn’s disease, Ulcerative Colitis, Pregnancy
Graphical Abstract
Lay Summary:
Among 1491 pregnant women with IBD, there was no increase in harm to the pregnancy or the infants by drug exposure (biologics, thiopurines or both in combination).
Incidence of inflammatory bowel disease (IBD), Crohn’s disease (CD) and ulcerative colitis (UC), is highest during reproductive years.1 Compared with age-matched controls, pregnant women with IBD are more likely to experience spontaneous abortion (SAB), preterm birth, and complications during labor and delivery.2 Active disease further increases risk of adverse outcomes.3 Therefore, medical therapy is required prior to and during pregnancy to maintain remission.
Therapy includes thiopurines (azathioprine, 6-mercaptopurine); biologics (monoclonal antibody)-tumor necrosis factor (TNF) antagonists (infliximab, adalimumab, certolizumab pegol, golimumab), anti-integrins (vedolizumab, natalizumab), anti-interleukin 12/23 (ustekinumab); or combination of biologic and thiopurines. Fetal exposure of animals to supratherapeutic doses of thiopurines produce congenital malformations,4 but human studies have not shown clear risk.5, 6 Biologics are immunoglobulins (IgG) that are actively transported across the placenta by Fc receptors (FcRn) during the second and third trimester. They can persist in infants up to 9 months following birth.7, 8 The exception is certolizumab pegol, a Fab’ fragment that passively crosses the placenta and is present in trivial concentrations at birth.8 Despite current biologic safety data ,9 there remains hesitation about use during pregnancy. Primarily, concerns remain regarding risk of congenital malformations and consequences of placental transfer on immune function. Recent data from France demonstrated increased maternal complications in IBD women receiving anti-TNF therapy (OR 1.49, 95% CI 1.31–1.67). However, therapy discontinuation prior to week 24 gestation was associated with increased maternal disease but no reduction in maternal complications compared to continuation of therapy.10 Current North American guidelines11 and American Gastroenterological (AGA) Care Pathway12 recommend continuation of anti-TNF and thiopurine therapy throughout pregnancy, but acknowledge the low quality evidence. In contrast, European guidelines suggest stopping biologics as early as 22 weeks gestation13 despite 10–25% increased risk of disease flare14 or loss of response to the agent. We performed a prospective cohort study at selected United States centers to assess pregnancy outcomes after in utero exposure to thiopurines, biologics and combination therapy, and measured maternal-infant placental transfer of monoclonal antibodies and infant achievement of developmental milestones.
METHODS
Study Design
The Pregnancy in Inflammatory bowel disease And Neonatal Outcomes (PIANO) study is a prospective observational study that enrolled pregnant women with IBD at 30 U.S. centers between January 2007 and March 2019. (NCT00904878) We administered questionnaires at study intake (any point during pregnancy), each subsequent trimester, delivery, and 4, 9, and 12 months after birth. Offspring enrolled after 2010 were assessed for developmental milestones at 12, 24, 36, and 48 months after estimated due date.
Participants
Institutional review boards at each center approved the study; written informed consent was obtained. Only women with IBD and singleton pregnancies were eligible for inclusion.
Exposures
Demographic and Disease Specific Exposures
Using patient questionnaires and medical records, we collected maternal demographic variables (age, marital status, smoking, substance use) and IBD-related variables (diagnosis, duration of disease, prior surgeries, disease location).
Medication use
Information was collected on medication use prior to conception, during pregnancy, and for 1 year postpartum. Exposure was defined as use of thiopurines or biologic in the 3 months prior to last menstrual period or any time during pregnancy. Women were assigned to one of four groups based on drug exposure: unexposed (includes mesalamine, corticosteroids, and antibiotics); thiopurine exposed; biologic exposed; and combination (both thiopurine and biologic) exposed. We repeated analyses evaluating only anti-TNFs, excluding other biologics.
Disease Activity
Disease activity was measured using Harvey-Bradshaw Index (HBI)15 for CD and Simple Clinical Colitis Activity Index (SCCAI) for UC.16 In additional analyses, remission or mild disease was considered inactive and moderate or severe disease was considered active. A flare was further defined as score consistent with active disease and at least one of the following: a) new medication added b) change in current prescription i.e. increased dose, c) IBD-related surgery, or d) IBD-related hospitalization.
Pregnancy Outcomes
We collected pregnancy outcomes and complications of labor including: SAB, preterm birth (<37 weeks), stillbirth, intrauterine growth restriction (IUGR), small for gestational age (SGA), low birth weight (LBW) (<2500 g), abruptio placenta, eclampsia/preeclampsia, cesarean section, and fetal distress.
Neonatal Outcomes
We collected infant outcomes by questionnaires. Height and weight percentiles were calculated at birth by Intergrowth-21st calculators, and then by World Health Organization (WHO) curves. Very low for height or weight was defined as <25th percentile. Infant intensive care unit (ICU) admission, congenital malformations and maternal reported infant infections were collected. Infections were categorized into serious infections (requiring hospitalization) or non-serious infection (any reported infection without hospitalization). Due to the frequency of otitis media in childhood, sensitivity analyses were repeated excluding this infection.
Developmental Milestones
Developmental milestones were assessed through the nationally validated Ages and Stages Questionnaire (ASQ3), Third Edition17 at 12, 24, 36 and 48 months of age for patient subsets who reached these additional age milestones in the registry. Specific categories of milestones evaluated included communication, fine motor skills, gross motor skills, personal social, and problem solving.
Placental Transfer
We collected maternal, cord and infant serum on the day of birth in a subset of maternal-child pairs on biologic agents. If the infant had detectable serum concentration at birth, additional samples were collected at months 3 and 6. Prometheus Biosciences or Sanquin laboratories determined serum concentrations of all drugs.18 As serum trough concentrations for various biologic agents are not well-established , for purposes of analysis, we considered a trough concentration of 0–3 to be low, 3–10 therapeutic, 10–20 high, and >20 very high. Drug concentration at birth was analyzed as both a continuous variable as well as by category, overall and for each biologic class.
Outcomes
The primary outcomes of the study were rates of congenital malformations, SAB, preterm birth, LBW, and infections (serious and non-serious) in the three exposure groups, relative to the unexposed group. We also aimed to determine differences in infant developmental milestones at 12 months of age by drug exposure and to correlate infant serum drug exposure data with infectious complications in the first year of life.
Statistical Analysis
We examined univariate and bivariate distributions of all exposures and outcomes. We compared incidence or birth prevalence of pregnancy and neonatal outcomes among women in the thiopurine, biologic, and combination therapy exposure groups relative to the same outcomes among women exposed neither to thiopurines or to biologics. We calculated crude odds ratios, and then adjusted for relevant confounders such as disease activity, maternal characteristics (age, smoking), prior SAB, and infant characteristics (preterm birth) if applicable. For analyses of SAB, we limited the cohort to those enrolled prior to 20 weeks’ gestation. We used Cox proportional hazard models to determine factors associated with SAB by 20 weeks, including medication exposures, maternal age, disease activity, and prior SAB. For analyses of infant infections (serious, non-serious, or any infection), we further divided biologics into anti-TNF treated women alone, and individually assessed the risk with the three most common agents - infliximab, adalimumab and certolizumab pegol. For developmental milestones, we compared mean scores on each milestone by drug exposure category to 1) unexposed IBD referent and 2) the general population mean by t-test. Bivariate statistics were used to determine whether maternal or infant exposures were associated with any one low scoring domain on developmental milestones. Weight and height percentiles were also compared across drug exposure groups using bivariate analyses. We calculated odds ratios of being <25% for length or weight by drug exposure class, adjusted for maternal age and highest disease activity during pregnancy. For the subset with placental transfer data available, we reported mean drug concentrations for cord, maternal and baby serum as well as distribution of concentrations. Spearman’s rho and Kruskal-Wallis chi-square test statistics were used to analyze associations between serum drug concentrations and outcomes of interest as continuous drug concentrations and by category. As missing data were rare, a complete case analysis is presented as the primary analysis. Data were analyzed using SAS 9.2 (Cary, NC).
RESULTS
Of 1712 patients enrolled, 1490 completed pregnancies with 1431 live births. One-year measurements were available in 1010 offspring. Table 1 reports maternal demographics. Among 30 centers, the estimated gestational age at enrollment was a median of 115 days, (IQR 70–172 days). Of 869 women with exposure to biologic (51 exposed to more than one) or combination therapy, 421 were infliximab, 279 adalimumab, 135 certolizumab pegol, 11 golimumab, 15 natalizumab, 41 vedolizumab, and 18 ustekinumab. Individuals with CD were less likely unexposed versus UC (16% vs. 41%, p<0.0001), and more likely to be on biologics (51% vs 30%, p<0.0001) or combination therapy (18% vs. 11%, p<0.001). There were no differences in rates of thiopurine use (15% vs. 18%, p=0.30) for CD versus UC.
Table 1:
Characteristics of entire cohort by drug exposure class
| Variable | Overall (n=1490) | None (n=379) | Biologics * (n=642) | Thiopurine# (n=242) | Combination** (n=227) | p |
|---|---|---|---|---|---|---|
| Maternal Age at Delivery in Years [mean (SD)] | 32.0 (4.6) | 32.5 (4.6) | 31.8 (4.5) | 31.9 (4.7) | 31.6 (4.5) | 0.07 |
| Total Pregnancies, including current [mean (SD)] | 2.1 (13) | 2.2 (1.3) | 2.1 (1.3) | 2.1 (1.4) | 2.0 (1.2) | 0.18 |
| Disease Duration [median years (IQR)] | 8.3 (4.4, 13.0) | 7.1(2.9, 12.6) | 8.7 (4.8, 13.3) | 8.5 (4.7, 12.4) | 8.7 (5.4, 13.6) | 0.001 |
| Body Mass Index^ [mean (SD)] | 24.9 (4.8) | 24.9 (4.3) | 25.0 (4.9) | 24.2 (4.5) | 25.2 (5.1) | 0.46 |
| Disease Status n (%) Crohn’s disease Ulcerative colitis IBD-unclassified | 921 (62%) 535 (36%) 34 (2%) |
147 (39%) 218 (58%) 14 (4%) |
471 (74%) 137 (23%) 8 (1%) |
137 (57) 98 (40) 7 (3) |
123 (71) 47 (27) 3 (2) |
<0.001 |
| Smoking Status n (%) Current Former (prior to pregnancy) Never | 23 (2%) 308 (22%) 1,057 (76%) |
4 (1%) 75 (21%) 272 (77%) |
11 (2%) 137 (23%) 451 (75%) |
5 (2%) 57 (25%) 163 (72%) |
3 (1%) 38 (18%) 169 (80%) |
0.53 |
| Alcohol Use n (%) Current Former (prior to pregnancy) Never | 146 (11%) 855 (62%) 384 (28%) |
28 (8%) 220 (63%) 103 (29%) |
59 (10%) 381 (64%) 156 (26%) |
22 (10%) 139 (61%) 65 (29%) |
37 (18%) 115 (55%) 57 (27%) |
0.02 |
| Recreationa 1 Drug Use n (%) Current Former (prior to pregnancy) Never | 1 (0.1%) 65 (5%) 1,321 (95%) |
1 (0.3%) 22 (6%) 327 (93%) |
0 (0%) 26 (4%) 573 (96%) |
0 (0%) 10 (4%) 216 (96%) |
0 (0%) 7 (3%) 202 (97%) |
0.42 |
Biologics defined as anti-TNF, anti-integrin, anti-IL 12/23
Thiopurine (azathioprine or 6-mercaptopurine)
Combination defined as biologic + thiopurine
Pre-pregnancy BMI as reported at intake
Pregnancy Outcomes
There were 133 (9%) infants with congenital malformations, 42 (3%) SABs, 91 (7%) LBWs, and 132 (10%) preterm births. There were 58 (4%) SGA, 30 (2%) IUGRs, 5 (0.30%) stillbirths, 613 (44%) cesarean sections, 137 (10%) neonatal ICU stays, and 280 (20%) patients with at least one self-reported pregnancy related complication (excluding cesarean section, IUGR or pre-term delivery). There were overall no differences in rates of pregnancy complications by drug class, although women on biologics and combination therapy had higher rates of cesarean sections as compared to the unexposed population (Table 2, Table S3). No pattern of congenital malformations suggests an association for a specific drug or disease type (CD or UC). (Table S6).
Table 2:
Pregnancy related complications by drug exposure, controlling for maternal age, steroid use and disease activity (Odds Ratio (95% Confidence Interval))
| Event | No Exposure (n=379) | Biologics* (n=642) | Thiopurine# (n=242) | Combination** (n=227) |
|---|---|---|---|---|
| Any Pregnancy Complication^ | 1.0 (Ref) | 1.2 (0.8, 1.7) | 1.3 (0.8, 2.0) | 0.8 (0.5, 1.3) |
| Spontaneous Abortion (Only Gestation Ages <= 140 Days) | 1.0 (Ref) | 1.3 (0.5, 3.3) | 1.4 (0.4, 4.2) | 1.2, (0.4, 3.8) |
| Spontaneous Abortion (All Gestation Ages) | 1.0 (Ref) | 1.3 (0.5, 3.0) | 1.3 (0.4, 3.8) | 1.1 (0.3, 3.3) |
| Preterm Birth (<37 weeks) | 1.0 (Ref) | 0.9 (0.5, 15) | 1.4 (0.8, 2.6) | 1.8 (1.0, 3.3) |
| Small for Gestational Age | 1.0 (Ref) | 1.1 (0.5, 2.0) | 0.5 (0.2, 15) | 0.7 (0.3, 1.8) |
| Low Birth Weight (<2500 g) | 1.0 (Ref) | 1.0 (0.5, 18) | 0.6 (0.3, 15) | 1.2 (0.6, 2.5) |
| Intrauterine Growth Restriction | 1.0 (Ref) | 0.6 (0.2, 14) | 0.3 (0.07, 15) | 0.7 (0.2, 2.3) |
| Cesarean Section | 1.0 (Ref) | 1.3 (1.0, 18) | 1.3 (0.9, 19) | 1.7 (1.1, 2.5) |
| NICU at Birth | 1.0 (Ref) | 1.1 (0.7, 19) | 1.2 (0.6, 2.2) | 1.5 (0.8, 2.8) |
| Congenital Malformations | 1.0 (Ref) | 1.5 (0.9, 2.5) | 1.4 (0.8, 2.7) | 1.6 (0.8, 3.1) |
| Any of The Above | 1.0 (Ref) | 1.5 (1.1, 2.0) | 1.6 (1.1, 2.3) | 1.4 (0.9, 2.0) |
| Any of the Above w/o Considering Cesarean Section | 1.0 (Ref) | 1.2 (0.9, 16) | 1.4 (1.0, 2.0) | 1.2 (0.8, 1.8) |
Biologics defined as anti-TNF, anti-integrin, anti-IL 12/23
Thiopurine (azathioprine or 6-mercaptopurine)
Combination defined as biologic + thiopurine
Defined as any self-reported pregnancy complication (excludes intrauterine growth restriction, cesarean section or pre-term delivery)
Logistic regression models controlling for maternal age, steroid use, and disease activity
Analyzing those entering the cohort prior to 20 weeks, the rate of SAB was 41/944 (4%). In a Cox model for SAB censored at 20 weeks’ gestation, maternal age (HR 1.03, 95% CI 0.96–1.11) and drug class were not predictive of SAB [biologic HR 1.20 (95% CI 0.50–2.90), thiopurine HR 0.96 (95% CI 0.28–3.33), combination HR 0.96 (95% CI 0.28–3.33)]. Active disease (HR 3.41, 95% CI 1.51–7.69) and prior SAB (HR 2.17, 95% CI 1.05–4.49) were independently associated with SAB prior to 20 weeks.
Infections
We found no increase in serious, non-serious or any infection in the first year of life. (Table 3) No significant difference in infectious outcomes was found when analyses were repeated excluding otitis media, preterm birth, limiting biologic exposure to only anti-TNF agents or when each anti-TNF agent was individually excluded. Infection rates did not differ by individual biologic agent. (Table S7) Controlling for preterm birth, maternal age and disease activity, use of biologic (OR 0.92, 95% CI 0.70–1.20), thiopurine (OR 0.90, 95% CI 0.64–1.28) or combination therapy (OR 0.93, 95% CI 0.66–1.32) was not associated with increased risk of any infection in the first year of life. Preterm birth was the only independent risk factor for infection (OR 1.73, 95% CI 1.19–2.51). The majority of infections were non-serious, consisting primarily of otitis media and upper respiratory infections. Serious infections were rare, consisting of febrile illnesses requiring hospitalization and antibiotics, or sepsis.
Table 3:
Rates of infection over the first 12 months of life by drug exposure among live births
| Variable | Overall (n=1,431) | No Exposure (n=366) | Biologics* (n=616) | Thiopurines# (n=233) | Combination** (n=216) | p |
|---|---|---|---|---|---|---|
| Infection at birth | ||||||
| Serious | 29 / 1,384 (2%) | 3 / 348 (1%) | 15 / 601 (2%) | 6 / 227 (3%) | 5/ 208 (2%) | 0.27 |
| Infection by 4 months of life | ||||||
| Serious | 37 / 1,103 (3%) | 7 / 278 (3%) | 19 / 479 (4%) | 8/ 183 (4%) | 3 / 163 (2%) | 0.41 |
| Non-Serious | 175 / 1,103 (16) | 38 / 278 (14) | 89 / 479 (19) | 26 / 183 (14) | 22 / 163 (13) | 0.19 |
| Anyinfection^ | 191 / 1,103 (17) | 42 / 278 (15) | 95 / 479 (20) | 32 / 183 (17) | 22 / 163 (13) | 0.19 |
| Infection in the first year of life | ||||||
| Serious | 79/1431 (6) | 18/366 (5) | 35/616 (6) | 18 /233(8) | 8/216 (4) | 0.28 |
| Non-Serious | 637/1431 (43) | 165/366 (44) | 268/616 (43) | 103/233 (44) | 102/216 (47) | 0.79 |
| Anyinfection^ | 658/1431 (46) | 173/366 (47) | 277/616 (45) | 107/233 (46) | 102/216 (47) | 0.87 |
Biologics defined as anti-tumor necrosis factor alpha, anti-integrin, anti-IL 12/23
Thiopurines (azathioprine or 6-mercaptopurine)
Combination defined as biologic + thiopurine
Any infection defined as any serious (infection requiring hospitalization) or non-serious infection (maternal reported infection not requiring hospitalization)
Disease Activity
UC mothers had significantly lower rates of remission per trimester compared to CD mothers (p=0.002 trimester 1, p<0.0001 trimesters 2,3). Additionally, there were significantly higher rates of flares for UC compared to CD. There were no differences in post-partum flares. When evaluating flare by pregnancy trimester, a significantly greater percentage of women with IBD who flared in the 1st and 3rd trimester and postpartum were not on biologic agents or immunomodulators. (Table S8) For women who were on biologics during pregnancy, we also evaluated timing of biologic agents and subsequent flares. Discontinuation of a biologic in the 3rd trimester was not associated with an increased risk of subsequent flare post-partum. (Table S3D) A significantly higher percentage of women exposed to more than one biologic experienced a flare during pregnancy compared to those on only a single biologic (47% vs. 20%, p=.001). The vast majority of women (81%) exposed to more than one biologic switched within the anti-TNF class.
Infant Growth
Infants of mothers receiving thiopurines or combination therapy had significantly increased birthweight. Otherwise, there were no differences in height or weight outcomes by drug exposure (Table S9), or in odds of being very low for length or weight, controlling for preterm birth and maternal disease activity (Table S10).
Serum Drug Concentrations
In the subset with biologic serum concentration data (Table S2A), the greatest number (n=99) were exposed to infliximab, followed by adalimumab (n=66), certolizumab pegol (n=33), vedolizumab (n=22), ustekinumab (n=7), natalizumab (n=4) and golimumab (n=4). The highest infant and cord serum concentrations at birth was with infliximab, with certolizumab pegol having the lowest. For all biologics except certolizumab pegol, infants had detectable concentrations at delivery, most at levels higher than the mother. (Table 4) By a median of 129.5 days (range 81–242) after delivery, at time of first subsequent infant drug level, 86.8% had a concentration <2.0 mcg/ml. We evaluated risk of serious infection or any infection in the first 12 months by birth serum drug concentration and found no differences. (Table S11).
Table 4:
Subset with maternal, cord and infant serum drug concentrations at time of delivery, median [mcg/ml] and range (n=235)
| Variable | Infliximab | Adalimumab | Certolizumabpegol | Golimumab | Vedolizumab | Natalizumab | Ustekinumab |
|---|---|---|---|---|---|---|---|
| n | 99 | 66 | 33 | 4 | 22 | 4 | 7 |
| Infant Concentration Median μg/ml (range)] | 27.1 (0.1 103.1) | 9.4 (2.5, 26.0) | 0.0 (0.0, 0.0) | 2.2 (0.0, 4.1) | 8.2 (0.0, 22.0) | 3.8 (3.7, 3.9) | 5.0 (0.6, 8.7) |
| Infant or Cord Concentration [median μg/ml (range)] * | 28.5 (0.0,110.1) | 9.1 (0.0, 26.0) | 0.0 (0.0, 5.1) | 3.4 (1.1, 4.1) | 8.9 (3.0, 22.0) | 1.8 (0.0, 3.9) | 5.0 (0.6, 40.0) |
| Maternal Concentration [median μg/ml (range)] | 12.0 (0.0,129.3) | 8.2 (0.0, 39.8) | 25.0 (0.0, 56.4) | 2.2 (0.5, 3.7) | 13.0 (0.4, 44.0) | 2.5 (0.0, 5.5) | 4.0 (0.1, 18.9) |
| Infant or Cord/Maternal Concentration Ratio (median (range)) ** | 2.4 (0.7, 8.0) | 1.3 (0.4, 5.4) | 0.0 (0.0, 0.1) | 1.5 (0.0, 2.2) | 0.5 (0.0, 1.7) | 0.7 (0.7, 0.7) | 1.4 (0.7, 13.7) |
| Days Since Last Maternal Dose [median (range)] | 50.0 (6.0, 133.0) | 14.0 (1.0, 150.0) | 13.0 (2.0, 30.0) | 21.0 (18.0, 28.0) | 29.0 (1.0, 84.0) | 32.5 (6.0, 141.0) | 35.0 (7.0, 74.0) |
| Infant Concentration >10 μg/ml [n (%)] *** | 85 /98 (87) | 20 / 65 (31) | 0 / 33 (0) | 0 / 4 (0) | 9 / 22 (41) | 9 / 22 (41) | 1/7 (14) |
| Maternal Concentration >10 μg/ml [n (%)] *** | 54 /95 (57) | 20 / 65 (31) | 30 / 33 (91) | 0 / 4 (0) | 12 / 22 (55) | 0 / 4 (0) | 1/7(14) |
| Maternal Concentration >10 μg/ml despite held dose [n (%)] **** | 7/32 (22) | 3/23 (13) | 1 / 1 (100) | -- | 0/ 3 (0) | 0 / 2 (0) | 1/ 3 (33) |
| Infant Concentration at Subsequent Blood A Draw^ [median μg/ml (range)] | 0.6 (0.0,7. 0) | 0.0 (0.0, 2.2) | 0.0 (0.0, 0.0) | -- | 0.0 (0.0, 0.0) | -- | 0.1 (0.0, 0.2) |
Infant concentration; if missing, then cord concentration.
Ratio only computed when maternal concentration is greater than 0
Defined as >10 mcg/ml - note that this is the level used for analysis for all biologics
Only for those with days since last maternal dose as follows: Greater than 14 for Adalimumab, greater than 28 for Natalizumab, Certolizumab pegol and Golimumab, and greater than 56 for Infliximab, Vedolizumab and Ustekinumab.
Among those with infant samples available, first subsequent level at a median of 129.5 days after delivery
Developmental Milestones
Infant developmental milestones by drug exposure are reported in Table 5. There were no differences in developmental milestones in the first year of life by exposure status within the cohort or compared to validated population norms (ASQ3). Developmental milestones to 48 months are in Table S12.
Table 5:
Comparison of Drug Exposure During Pregnancy on Childhood Developmental Milestones at 12 months^ (n=411)
| Milestone | Unexposed | Biologics* | Thiopurines& | Combination** | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | mean | n | mean | delta | p | n | mean | delta | p | n | Mean | delta | p | |
| Communication | 92 | 50.6 | 206 | 52.5 | 1.9 | 0.12 | 52 | 50.7 | 0.1 | 0.97 | 62 | 52.8 | 2.2 | 0.17 |
| Fine Motor | 92 | 55.7 | 205 | 56.3 | 0.6 | 0.46 | 52 | 57.1 | 1.4 | 0.15 | 62 | 55.9 | 0.2 | 0.87 |
| Gross Motor | 92 | 49.8 | 206 | 53.0 | 3.2 | 0.06 | 52 | 50.0 | 0.2 | 0.96 | 61 | 51.3 | 1.5 | 0.52 |
| Personal-Social | 92 | 48.6 | 205 | 52.1 | 3.5 | 0.01 | 52 | 49.7 | 1.1 | 0.58 | 62 | 51.3 | 2.7 | 0.12 |
| Problem Solving | 92 | 51.0 | 204 | 53.2 | 2.2 | 0.10 | 52 | 53.1 | 2.1 | 0.20 | 62 | 53.0 | 2.0 | 0.24 |
Calculated using Ages and Stages Questionnaire; premature infants were only included if their questionnaire window correlated with the window for their adjusted age (based on due date) rather than birth date.
Biologics defined as anti-TNF, anti-integrin, anti-IL 12/23
Thiopurine (azathioprine or 6-mercaptopurine)
Combination defined as biologic + thiopurine
DISCUSSION
In this prospective cohort of pregnant women with IBD, the use of biologic and thiopurine therapy, alone or in combination, was not associated with an increase in congenital malformations, SAB, preterm birth, LBW, or infections in the first year of life. However, maternal disease activity was associated with SAB, and preterm birth with infant infections.
Multiple smaller studies support our findings, with no reported increase in congenital malformations with anti-TNF use in pregnancy.19–22 The rate of SAB in our cohort was lower than the general population. However, SAB is inherently difficult to study, as it can occur early in pregnancy before the woman is aware of the pregnancy or reports it to her provider. Maternal disease activity and prior SAB were significant risk factors for SAB in this cohort. Other studies have reported preterm birth to be increased with maternal disease activity.23 In our cohort, preterm birth was associated with increased infant infections. Overall, these findings emphasize the need to control disease activity during pregnancy as well as the lack of harm associated with the use of biologic and thiopurine therapy.
As noted in other studies,14 UC patients had significantly increased disease activity compared to CD during each trimester. This may be due to production of pro-inflammatory cytokines by the placenta that activate UC,24or because UC activity is undetected and undertreated in pregnant women. In this cohort, 43% of UC women were unexposed to biologics or thiopurines despite higher rates of disease activity, whereas only 19% of women with CD were unexposed. Women who were unexposed to biologics or immunomodulators were more likely to flare during the course of pregnancy. This demonstrates the need for therapy to maintain control of inflammation throughout pregnancy. Pre-conception counseling on medication utilization during pregnancy could improve these outcomes. Additionally, women exposed to more than one biologic during pregnancy had higher rates of disease flare. While the directionality of this cannot be determined from the data, it is presumed that therapy was changed because of active disease, rather than that disease activity was a result of a change in therapy.
Practitioners have generally become more comfortable using biologics in the first trimester of pregnancy. However, as significant placental transfer occurs, there have been lingering concerns about infection and altered immune development in infants when biologics are used in the third trimester. The European guidelines13 recommend cessation of biologics as early as week 22 gestation, despite the risk of increased disease activity in the mother10. We found that neither combination therapy nor monotherapy with any biologic increased the risk of infections at one year after accounting for disease activity and preterm birth. Preterm delivery was the only factor independently associated with increased infant infections. This study supports the recent AGA Clinical Care Pathway recommendation to continue biologic therapy through pregnancy.12 There is no strong rationale to withhold biologic therapy in any pregnant IBD patient based on available evidence from PIANO and other international studies.
In a subset of infants followed for up to 48 months, drug exposure during pregnancy was not associated with differences in developmental milestones as measured by ASQ3, suggesting biologic and thiopurine exposure does not adversely affect infant neurodevelopment. Socioeconomic factors are influential in developmental milestones and our cohort has high income and education levels. However, within the exposure groups in the cohort, there was no reduction in achievement of milestones. There are a number of strengths to this study. The large sample of women with IBD followed prospectively throughout pregnancy and the first four years of the infant’s life increases the precision of our estimates. Detailed prospective data collection allowed for documentation of disease activity, medication and disease state changes and complications to both mother and child. The limitations include the self-reported nature of the data and lack of objective markers of disease activity. It is possible that there is misclassification bias as data are reported by mothers rather than ascertained from the medical record. However, self-report is the gold standard for instruments such as ASQ3 and the basis of patient reported outcome measures of disease activity in IBD. It is also unlikely that this bias would be differential by specific class of therapy. Objective measures were used whenever possible, such as calculation of LBW based on infant’s actual weight and preterm birth based on gestational age. Given the observational nature of the data, selection bias is possible due to loss of follow-up. However, loss of follow-up was not different by drug class, suggesting that this would be a non-differential bias, if present.
CONCLUSIONS
Biologic, thiopurine, and combination therapy exposure during pregnancy in women with IBD was not associated with increased maternal or infant adverse events in the first year of life. Maternal disease activity was an independent risk factor for SAB and preterm birth increased the risk of infant infections. Practitioners should continue biologic and/or thiopurine therapy throughout pregnancy given no evidence of increase in harm from drug exposure and the clear association of active disease with adverse events.
Supplementary Material
What you need to know:
Background and Context:
Active inflammatory bowel disease in pregnancy can lead to adverse pregnancy and neonatal events including pregnancy loss and preterm birth. To maintain remission, medications need to be continued, however, the safety of biologics is not well established. This has led to drug discontinuation in pregnancy with subsequent increased flares and adverse neonatal outcomes. The aim of this study was to compare outcomes among pregnancies exposed versus unexposed in utero to biologics, thiopurines or a combination. Placental transfer was measured and developmental milestones among infants was assessed.
New Findings:
This was the largest prospective study of biologic safety in pregnancy with 1490 pregnant women, including 869 biologic exposed. There was no increase in adverse events based on drug exposure during pregnancy or placental transfer of biologics. Our data confirmed that disease activity increased spontaneous abortion and preterm birth increased infections. Developmental milestones out to one year were unaffected by drug exposure.
Limitations:
The limitations include the self-reported nature of the data and lack of objective markers of disease activity. However, for many endpoints, patient reported outcomes are standard and the sites had access to medical records to confirm findings.
Impact:
The findings of the study strongly support continuing biologic therapy throughout pregnancy given no increase in harm from drug exposure and clear evidence of adverse events with disease activity.
Acknowledgements:
Jessica Lim CRC
Funding sources: [No role in design, implementation or interpretation of study]
Crohn’s Colitis Foundation Senior Research Award
Lisa and Douglas Goldman Foundation
Lata and Vaibhav Goel Foundation
UNC: National Institutes of Health (P30 DK034987)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Registration: NCT00904878
References:
- 1.Ananthakrishnan AN KG, Ng SC. Changing Global Epidemiology of Inflammatory Bowel Diseases-Sustaining Healthcare Delivery into the 21st Century. Clin Gastroenterol Hepatol 2020. [DOI] [PubMed] [Google Scholar]
- 2.Mahadevan U, Sandborn WJ, Li DK, et al. Pregnancy outcomes in women with inflammatory bowel disease: a large community-based study from Northern California. Gastroenterology 2007;133:1106–12. [DOI] [PubMed] [Google Scholar]
- 3.Norgard B, Hundborg HH, Jacobsen BA, et al. Disease Activity in Pregnant Women With Crohn’s Disease and Birth Outcomes: A Regional Danish Cohort Study. Am J Gastroenterol 2007;102:1947–54. [DOI] [PubMed] [Google Scholar]
- 4.Polifka JE, Friedman JM. Teratogen update: azathioprine and 6-mercaptopurine. Teratology 2002;65:240–61. [DOI] [PubMed] [Google Scholar]
- 5.Casanova MJ, Chaparro M, Domenech E, et al. Safety of thiopurines and anti-TNF-alpha drugs during pregnancy in patients with inflammatory bowel disease. The American journal of gastroenterology 2013;108:433–40. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein LH, Dolinsky G, Greenberg R, et al. Pregnancy outcome of women exposed to azathioprine during pregnancy. Birth Defects Res A Clin Mol Teratol 2007;79:696–701. [DOI] [PubMed] [Google Scholar]
- 7.Julsgaard M, Christensen LA, Gibson PR, et al. Concentrations of Adalimumab and Infliximab in Mothers and Newborns, and Effects on Infection. Gastroenterology 2016;151:110–9. [DOI] [PubMed] [Google Scholar]
- 8.Mahadevan U, Wolf DC, Dubinsky M, et al. Placental transfer of anti-tumor necrosis factor agents in pregnant patients with inflammatory bowel disease. Clin Gastroenterol Hepatol 2013;11:286–92; quiz e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lichtenstein GR, Rutgeerts P, Sandborn WJ, et al. A pooled analysis of infections, malignancy, and mortality in infliximab- and immunomodulator-treated adult patients with inflammatory bowel disease. Am J Gastroenterol 2012;107:1051–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luu M, Benzenine E, Doret M, et al. Continuous Anti-TNFalpha Use Throughout Pregnancy: Possible Complications For the Mother But Not for the Fetus. A Retrospective Cohort on the French National Health Insurance Database (EVASION). Am J Gastroenterol 2018;113:1669–1677. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen GC, Seow CH, Maxwell C, et al. The Toronto Consensus Statements for the Management of Inflammatory Bowel Disease in Pregnancy. Gastroenterology 2016;150:734–757 e1. [DOI] [PubMed] [Google Scholar]
- 12.Mahadevan U, Robinson C, Bernasko N, et al. Inflammatory Bowel Disease in Pregnancy Clinical Care Pathway: A Report From the American Gastroenterological Association IBD Parenthood Project Working Group. Gastroenterology 2019;156:1508–1524. [DOI] [PubMed] [Google Scholar]
- 13.van der Woude CJ, Ardizzone S, Bengtson MB, et al. The second European evidenced-based consensus on reproduction and pregnancy in inflammatory bowel disease. J Crohns Colitis 2015;9:107–24. [DOI] [PubMed] [Google Scholar]
- 14.Pedersen N, Bortoli A, Duricova D, et al. The course of inflammatory bowel disease during pregnancy and postpartum: a prospective European ECCO-EpiCom Study of 209 pregnant women. Alimentary pharmacology & therapeutics 2013;38:501–12. [DOI] [PubMed] [Google Scholar]
- 15.Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet 1980;1:514. [DOI] [PubMed] [Google Scholar]
- 16.Walmsley RS, Ayres RC, Pounder RE, et al. A simple clinical colitis activity index. Gut 1998;43:29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Squires JTE, Bricker D, Potter L. ASQ-3 User’s Guide. Baltimore: Paul H Brookes Publishing Co, 2009. [Google Scholar]
- 18.Wang SL, Ohrmund L, Hauenstein S, et al. Development and validation of a homogeneous mobility shift assay for the measurement of infliximab and antibodies-to-infliximab levels in patient serum. Journal of immunological methods 2012;382:177–88. [DOI] [PubMed] [Google Scholar]
- 19.Marchioni RM, Lichtenstein GR. Tumor necrosis factor-alpha inhibitor therapy and fetal risk: a systematic literature review. World J Gastroenterol 2013;19:2591–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nielsen OH, Loftus EV Jr., Jess T Safety of TNF-alpha inhibitors during IBD pregnancy: a systematic review. BMC Med 2013;11:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schnitzler F, Fidder H, Ferrante M, et al. Outcome of pregnancy in women with inflammatory bowel disease treated with antitumor necrosis factor therapy. Inflammatory bowel diseases 2011;17:1846–54. [DOI] [PubMed] [Google Scholar]
- 22.Broms G, Granath F, Ekbom A, et al. Low Risk of Birth Defects for Infants Whose Mothers Are Treated With Anti-Tumor Necrosis Factor Agents During Pregnancy. Clin Gastroenterol Hepatol 2016;14:234–41 e1–5. [DOI] [PubMed] [Google Scholar]
- 23.Kammerlander H, Nielsen J, Kjeldsen J, et al. The Effect of Disease Activity on Birth Outcomes in a Nationwide Cohort of Women with Moderate to Severe Inflammatory Bowel Disease. Inflamm Bowel Dis 2017;23:1011–1018. [DOI] [PubMed] [Google Scholar]
- 24.Nasef NA, Ferguson LR. Inflammatory bowel disease and pregnancy: overlapping pathways. Transl Res 2012;160:65–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


