Abstract
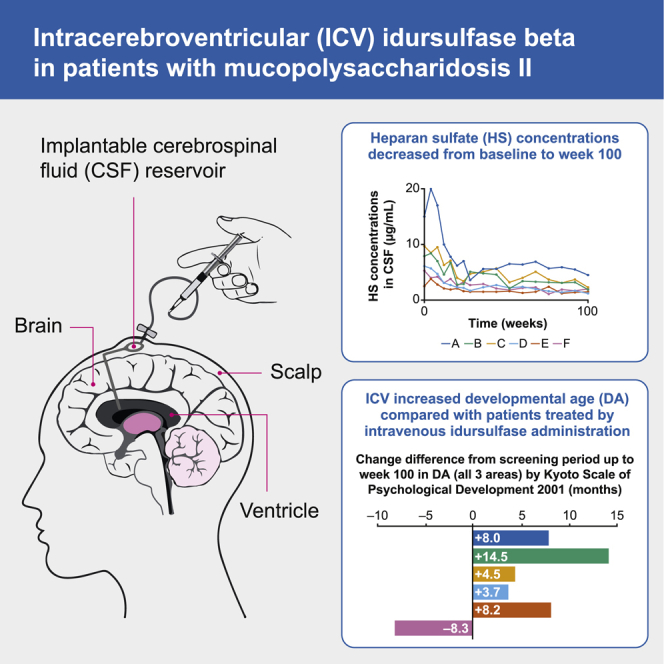
This open-label, phase 1/2 study (JMACCT CTR JMA-IIA00350) evaluated the efficacy and safety of intracerebroventricular idursulfase beta in patients with mucopolysaccharidosis II (MPS II). Herein, we report the 100-week results. Six patients with severe MPS II aged 23–65 months were enrolled. Idursulfase beta (increasing from 1 to 30 mg between weeks 0 and 24, followed by a 30-mg final dose) was administered intracerebroventricularly once every 4 weeks using an implanted cerebrospinal fluid (CSF) reservoir; intravenous administration of idursulfase was also continued throughout the study. Efficacy endpoints included developmental age by the Kyoto Scale of Psychological Development 2001 and heparan sulfate (HS) concentration in CSF (primary outcome). In all six patients, HS concentrations decreased (40%–80%) from baseline to week 100. For overall developmental age, the difference in change from baseline to week 100 in each patient compared with patients treated by intravenous idursulfase administration (n = 13) was +8.0, +14.5, +4.5, +3.7, +8.2, and –8.3 months (mean, +5.1 months). Idursulfase beta was well tolerated. The most common adverse events were pyrexia, upper respiratory tract infection, and vomiting. The results suggest that intracerebroventricular idursulfase beta is well tolerated and can be effective at preventing and stabilizing developmental decline in patients with neuronopathic MPS II.
Keywords: heparan sulfate, idursulfase beta, infusions, intracerebroventricular, mucopolysaccharidosis II, pediatrics, psychology, developmental age, Kyoto Scale of Psychological Development 2001 (KSPD), enzyme replacement therapy
Graphical abstract
Patients with neuronopathic mucopolysaccharidosis II (MPS II) develop severe cognitive impairment and progressive neurological decline. The results of this study by Seo et al. suggest that intracerebroventricular idursulfase beta treatment for 100 weeks is well tolerated and effective at preventing and stabilizing developmental decline in patients with neuronopathic MPS II.
Introduction
Mucopolysaccharidosis II (MPS II) (Hunter syndrome, OMIM 309900) is a rare X-linked lysosomal storage disorder caused by a deficiency of iduronate-2-sulfatase (IDS).1 IDS is an essential enzyme for the catabolism of glycosaminoglycans (GAGs), such as heparan sulfate (HS) and dermatan sulfate.1 IDS mutations lead to progressive lysosomal accumulation of GAGs in many organs and tissues, resulting in a wide spectrum of symptoms, including psychomotor developmental delay, hepatosplenomegaly, joint contracture, obstructive respiratory disease, and cardiac dysfunction.1 Patients with MPS II exhibit a number of neurological and behavioral problems associated with HS accumulation, including aggression, hyperactivity, sleep disturbances, and progressive decline of language and cognitive ability.2 In patients with MPS II with central nervous system (CNS) involvement, severe cognitive impairment and progressive neurological decline are observed.1 Patients are categorized according to the presence (neuronopathic MPS II) or absence (attenuated MPS II) of CNS involvement, with approximately two-thirds of patients having neuronopathic MPS II.3 Neuronopathic MPS II can be further divided into two groups based on the genetic mutation: group MS is characterized by missense mutations and is presumed to have slight residual enzyme activity, and group NT is considered to have null type mutations, such as deletions, recombination with pseudogene, and nonsense mutations.4 The patients in group NT show a more rapid decline than do those in group MS.4
Enzyme replacement therapy (ERT) with human recombinant idursulfase (Elaprase®) has been used since 2006 for the treatment of MPS II. Idursulfase is administered intravenously once a week and has been used for more than 120 Japanese patients since its approval in Japan in 2007.5 However, because intravenously administered idursulfase cannot penetrate the blood-brain barrier, it cannot reach the cerebral parenchyma. As such, CNS involvement in patients with neuronopathic MPS II, such as psychomotor developmental delay and neurological regressive episode, cannot be improved with current intravenous ERT. To improve the CNS symptoms, including cognitive decline, enzyme preparations that can reach the cerebral parenchyma have been sought. Non-clinical evidence has shown that idursulfase administered intrathecally or intracerebroventricularly (i.c.v.) rapidly reaches the brain.6 Consequently, global clinical studies of intrathecal idursulfase in patients with MPS II have been conducted. A phase 1/2 study of intrathecal idursulfase in children with MPS II conducted in the US and the UK supported the continued development of intrathecal administration as a potential therapy for the cognitive impairment caused by MPS II.7
Idursulfase beta (Hunterase®), developed by GC Pharma for ERT in patients with MPS II, was approved in South Korea for intravenous administration in 2012 and received orphan drug designation in the US in 2013. Idursulfase and idursulfase beta have identical amino acid sequences but differ slightly in their glycosylation patterns because they are produced in different cell lines using different manufacturing processes.8 Nevertheless, the structure, biological activity, and pharmacokinetics of the enzymes are almost the same. A recent non-clinical study in a mouse model of MPS II demonstrated that i.c.v. ERT with idursulfase beta significantly reduced HS concentrations in cerebrospinal fluid (CSF) and produced improvements in biochemical, histological, and memory/learning function parameters.9 i.c.v. ERT can be an effective and safe therapy for treating CNS symptoms, as demonstrated for cerliponase alfa (Brineura®), an approved treatment for neuronal ceroid lipofuscinosis type 2 disease.10 However, i.c.v. administration of idursulfase beta has not been evaluated in humans. This article presents the 100-week results of a multicenter, open-label, phase 1/2 clinical study to evaluate the efficacy and safety of i.c.v. idursulfase beta in patients with MPS II.
Results
Six patients with a confirmed diagnosis of severe MPS II having significant developmental delay (group MS or NT) provided informed consent and were enrolled in the study. All patients had tolerated ≥24 weeks of treatment with intravenous idursulfase before the start of this study. During the study, all patients received idursulfase beta for 100 weeks via a CSF reservoir implanted under the patient’s scalp for i.c.v. administration; intravenous administration of idursulfase was also continued throughout the study. One patient initially did not receive i.c.v. idursulfase beta owing to fever observed 1 day before the planned day of reservoir placement; however, this patient later re-consented and re-enrolled in the study. All six patients were included in both the full analysis set and the safety analysis set. All patients were Japanese and male, with a mean age (range) at screening of 42.2 (23–65) months (Table 1). Five patients were in group NT and one patient was in group MS; genetic mutation was confirmed in all six patients. The disease-specific findings of peculiar facies and bone deformity were observed in all six patients. All patients received 26 injections of idursulfase beta during the 100-week period and are continuing at a final dose of 30 mg, as determined by the data monitoring committee for each patient (Table 1).
Table 1.
Demographic and characteristics of patients
| Patient no. | Sex | Group by gene mutationa | Details of gene mutation | Developmental retardation | Age (months) |
|||
|---|---|---|---|---|---|---|---|---|
| Diagnosis | Screening | 0 weeks of injection (baseline) | 100 weeks of injection | |||||
| A | M | NT | c.2_3C>G | yes | 13 | 23 | 25 | 48 |
| B | M | NT | c.1349_1350insGA (p.D450Efsx12) | yes | 26 | 36 | 36 | 59 |
| C | M | NT | c.1349_1350insGA (p.D450Efsx12) | yes | 26 | 36 | 36 | 59 |
| D | M | NT | c.1139_1140insA (p.Y380X) | yes | 38 | 47 | 48 | 70 |
| E | M | MS | c.419G>T (p.G140V) | yes | 28 | 65 | 65 | 88 |
| F | M | NT | c814C>T (pQ272∗) | yes | 39 | 47 | 48 | 70 |
| Mean age | 28.3 | 42.2 | 43.0 | 65.7 | ||||
Group MS is characterized by missense mutations and is presumed to have slight residual enzyme activity; group NT is considered to have null mutations, such as deletions, recombination with pseudogene, and a nonsense mutation.
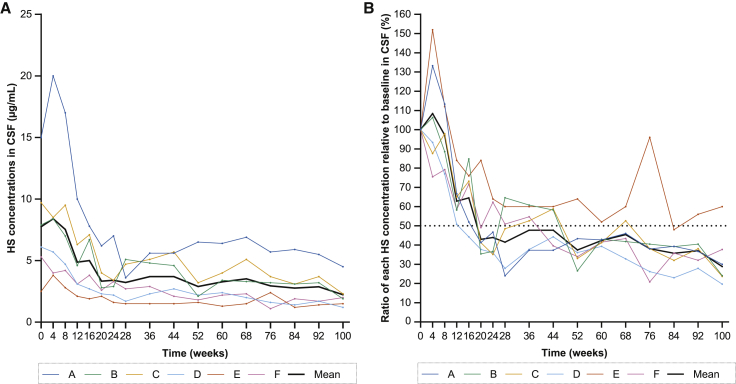
HS concentrations in CSF (primary endpoint) decreased in all patients following i.c.v. idursulfase beta administration (Figure 1). A rapid decrease was observed in the first 20 weeks, and concentrations were maintained throughout 100 weeks of treatment. The mean HS concentration was 7.75 μg/mL at baseline, 2.90 μg/mL at week 52, and 2.23 μg/mL at week 100 (Figure 1A). The mean reduction in the HS concentration relative to baseline was 62.6% at week 52 and 71.2% at week 100 (Figure 1B). The HS concentration in CSF at week 100 was lower than at baseline in all patients. Five patients had a ≥50% decrease from baseline at weeks 52 and 100.
Figure 1.
Change in HS in the CSF of patients with MPS II from the start of idursulfase beta treatment up to week 100
(A) HS concentrations and (B) ratio of HS concentrations relative to baseline are presented for each patient and as the mean of six patients. Baseline was defined as between placement of the reservoir for intracerebroventricular (i.c.v.) administration of idursulfase beta and the initial administration. A rapid decrease was observed in the first 20 weeks, and concentrations were maintained throughout 100 weeks of treatment. Five patients had a ≥50% decrease from baseline at weeks 52 and 100. CSF, cerebrospinal fluid; HS, heparan sulfate; MPS, mucopolysaccharidosis.
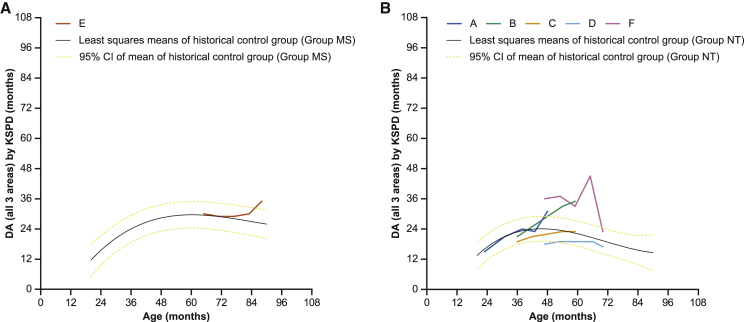
The mean overall developmental age (DA), determined by the Kyoto Scale of Psychological Development 2001 (KSPD),11 increased from the screening period (23.2 months) up to week 76 (28.8 months), then decreased slightly (27.3 months) at week 100. DAs in each patient were compared with 13 Japanese patients with neuronopathic MPS II treated with intravenous idursulfase (n = 13).4 In the one patient in group MS (patient E), the DA from the screening period up to week 52 was similar to the least-squares means of the historical control group but then increased up to week 100 (Figure 2A). In the patients in group NT (other than patient F), the DA either was similar to or increased above the least-squares means for the historical control group (Figure 2B). Patient F, in group NT, started the i.c.v. treatment at the age of 47 months. He had increases in overall DA from the screening period (36 months) up to week 76 (45 months), followed by a marked decrease at week 100 (23 months). However, because the person who conducted the KSPD commented that the patient was distracted and disconnected during the observation at week 100, the numerical DA value should be interpreted carefully.
Figure 2.
Score change in overall DA by KSPD in patients with MPS II from the screening period up to week 100
(A and B) Neuronopathic MPS II can be further divided into two groups based on the genetic mutation: group MS is characterized by missense mutations and is presumed to have slight residual enzyme activity, and group NT is considered to have null mutations, such as deletions, recombination with pseudogene, and a nonsense mutation. The scores in patients with MPS II (A) group MS and (B) group NT for each patient and the least-squares means (95% confidence interval [CI]) of the age-matched historical control group (n = 13) who received only intravenous idursulfase are presented. The DA of patient E in group MS from the screening period up to week 52 was similar to the least-squares means of the historical control group, but then increased up to week 100. The DA of patients in group NT (other than patient F) either was similar to or increased above the least-squares means for the historical control group. DA, developmental age; KSPD, Kyoto Scale of Psychological Development 2001; MPS, mucopolysaccharidosis.
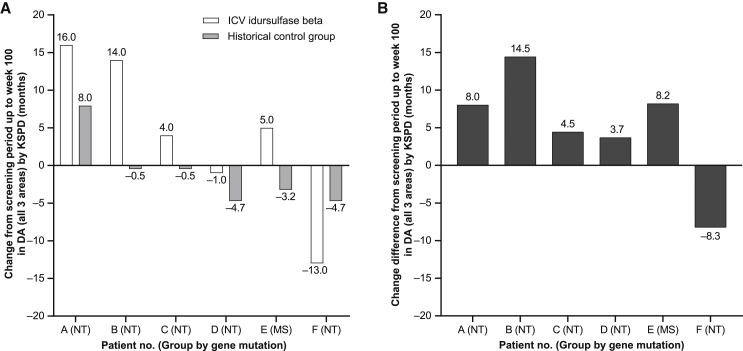
The differences in overall DA from the screening period to week 100 in the six patients (patients A–F) receiving i.c.v. idursulfase beta were +16.0, +14.0, +4.0, −1.0, +5.0, and −13.0 months (Figure 3). The corresponding least-squares means of the DA increase in the historical control group (n = 13) were +8.0, −0.5, −0.5, −4.7, −3.2, and −4.7 months, respectively (Figure 3). The mean difference in the change in DA from the screening period to week 100 between the six patients and the historical control group was 5.1 months. In the comparison with the historical control group, the developmental improvement was confirmed in five of six patients (all except patient F) in the study group that received i.c.v. administration.
Figure 3.
Comparison with the historical control data in patients with MPS II
(A) Change in DA (all areas) by KSPD from the screening period up to week 100 in six patients (patients A–F) receiving i.c.v. idursulfase beta (open bars) and the corresponding least-squares means of the DA increase in an age-matched historical control group (n = 13) who received only intravenous idursulfase (filled bars). (B) Change difference in DA (all areas) by KSPD between each patient and the historical control group. When compared with the historical control group, the DA increase was confirmed in five patients. DA, developmental age; i.c.v., intracerebroventricular; KSPD, Kyoto Scale of Psychological Development 2001; MPS, mucopolysaccharidosis.
Through week 100, adverse events (AEs) occurring from placement of the reservoir for i.c.v. administration of idursulfase beta were observed in three patients (Table 2); all AEs were mild. Two AEs (pyrexia and restlessness) in one patient were suspected as being related to the procedure. AEs occurring after i.c.v. administration of idursulfase beta were observed in all patients. All patients reported mild (n = 6 patients) or moderate (n = 3 patients) AEs. The most common AEs reported were pyrexia, upper respiratory tract infection, and vomiting (n = 6 patients, 100% each). AEs suspected as being related to the study drug were observed in all six patients (vomiting [n = 6, 100%], pyrexia [n = 3, 50.0%], procedural nausea [n = 2, 33.3%], and blood bilirubin increase and urticaria [n = 1 each, 16.7%]). No deaths or discontinuations owing to AEs occurred during the study. Eleven serious AEs (SAEs), all designated as being because of hospitalization or prolongation of hospitalization, were reported in four patients. One patient experienced six SAEs (three asthma, one vomiting, one respiratory syncytial virus infection, and one inguinal hernia); one patient experienced two SAEs (pyrexia and gastroenteritis norovirus); one patient experienced two SAEs (pyrexia and viral pharyngitis); and one patient had one SAE (carious tooth). Two SAEs (pyrexia and vomiting) were suspected as being related to the study drug. One 4-year-old patient (patient D) experienced vomiting after drug administration at week 68. Another 4-year-old patient (patient F) experienced moderate pyrexia and vomiting 6 h after the start of the first study drug administration. Because CSF examination showed mild inflammation, intravenous antibacterial infusion was administered. The patient’s fever was resolved after 2 days and he was discharged the next day. The pyrexia was reported as an SAE. There were no clinically meaningful changes from baseline in laboratory values, vital signs, or electrocardiogram findings over time.
Table 2.
Summary of AEs
| Preferred term | 52 weeks n (%) | 100 weeks n (%) | |
|---|---|---|---|
| Total N | 6 | 6 | |
| AEs associated with placement procedure of implantable reservoir | 3 (50.0) | 3 (50.0) | |
| constipation | 1 (16.7) | 1 (16.7) | |
| contusion | 1 (16.7) | 1 (16.7) | |
| post-procedural haemorrhage | 1 (16.7) | 1 (16.7) | |
| pyrexia | 1 (16.7) | 1 (16.7) | |
| restlessness | 1 (16.7) | 1 (16.7) | |
| wheezing | 1 (16.7) | 1 (16.7) | |
| AEs after study drug administrationa | 6 (100.0) | 6 (100.0) | |
| pyrexia | 5 (83.3) | 6 (100.0) | |
| upper respiratory tract infection | 5 (83.3) | 6 (100.0) | |
| vomiting | 5 (83.3) | 6 (100.0) | |
| eczema | 3 (50.0) | 4 (66.7) | |
| gastroenteritis | 2 (33.3) | 4 (66.7) | |
| urticaria | 3 (50.0) | 3 (50.0) | |
| arthropod sting | 0 (0.0) | 2 (33.3) | |
| blood urine present | 2 (33.3) | 2 (33.3) | |
| dermatitis diaper | 1 (16.7) | 2 (33.3) | |
| eye discharge | 0 (0.0) | 2 (33.3) | |
| faeces soft | 1 (16.7) | 2 (33.3) | |
| injection site extravasation | 2 (33.3) | 2 (33.3) | |
| procedural nausea | 1 (16.7) | 2 (33.3) |
AE, adverse event; n, number of patients with AE.
AEs after study drug administration in two or more patients (100 weeks) are presented.
From the safety point of view, we measured anti-IDS antibodies in serum and CSF. Anti-IDS antibodies in serum were detected in three patients (A, C, and F) at baseline and in three patients (B, C, and F) at 24 and 52 weeks. These antibodies might be a result of previous intravenous idursulfase treatment, as all patients had been treated with idursulfase before and throughout this study. All patients were negative for anti-IDS antibodies in serum at week 100. Anti-IDS antibodies in CSF were not detected in all patients during this study.
Discussion
This study was the first to evaluate the efficacy and safety of i.c.v. administration of idursulfase beta in Japanese patients with severe MPS II. At data cutoff, six patients had received idursulfase beta (maximum 30 mg) once every 4 weeks for 100 weeks. Five patients had a >50% decrease from baseline in HS concentration in CSF at week 52, which was maintained throughout the study. The reduction in the HS concentration relative to baseline was 62.6% at week 52 and 71.2% at week 100. In addition, all but one patient had a greater increase in DA at week 100 compared with historical controls, with an average difference for all six patients of +5.1 months. Thus, unlike intravenously administered idursulfase and idursulfase beta, which cannot penetrate the blood-brain barrier,1,12 i.c.v. administration of idursulfase beta may be expected to improve CNS manifestations.
The concentration of HS in CSF was selected as the primary endpoint of this study because HS accumulation is considered to be the cause of CNS symptoms, such as psychomotor developmental delay, in patients with MPS II.13 Impairment in the morphogenesis of nerve cell dendrites and inflammatory nerve cell death owing to the accumulation of the GM2 ganglioside, as well as neurodegeneration-mediated oxidative stress, have been reported as mechanisms underlying CNS symptoms resulting from HS accumulation.14, 15, 16 It has been suggested that patients with MPS II with cognitive disorders tend to have high HS concentrations in the CSF.17 Furthermore, the concentrations of HS in CSF correlated with those in brain tissues of the mice treated by i.c.v. administration of idursulfase beta.9 These results suggest that HS concentrations in CSF reflect HS concentrations in brain tissue. The precise mechanism of the enzyme transfer from CSF to brain parenchyma is still unclear, and short-time injection may have facilitated driving idursulfase beta from cephalad toward the brain parenchyma. HS concentrations in CSF are an informative biomarker for evaluating neurodegeneration in MPS II.
As sufficient data of HS concentrations in CSF of normal children are not available, we only evaluated the decrease of HS concentration in CSF in each treated patient. In this study, monthly i.c.v. administration of idursulfase beta reduced the HS concentration in the CSF at week 52 in all patients, which was maintained through week 100. Recently, it was reported that weekly intravenous administration of JR-141, a new IDS fused with anti-human transferrin receptor antibody, for 3 weeks reduced HS concentrations in the CSF of patients with MPS II; however, the study did not have DA data, and the reduction in the HS concentration in the CSF was lower (25.1% and 31.5% in patients treated with 1 [n = 6] or 2 [n = 5] mg/kg/week, respectively) than in this study.18 More research is needed to confirm whether HS concentrations could be directly correlated with a reduction in CNS symptoms.
DA was set as the secondary endpoint to evaluate the effect of i.c.v. idursulfase beta on psychomotor developmental delay in pediatric patients. To date, only one other study directly evaluated the improvement in CNS symptoms in patients with MPS II who were administered intrathecal idursulfase.7 This study showed that monthly i.c.v. administration of idursulfase beta maintained or increased DA in five of six patients compared with the historical control group receiving intravenous idursulfase. At 100 weeks (about 2 years) after starting this study, six patients who received i.c.v. idursulfase beta had a 5.1-month increase in mean DA compared with 13 historical control patients who received only intravenous administration of idursulfase. Comparing patient ages when starting i.c.v. idursulfase beta, three patients who started at age <3 years increased DA by 9.0 months, whereas the other three patients who started at age ≥3 years increased DA by only 1.2 months. i.c.v. idursulfase beta may be expected to improve DA in patients at an early age when DA is still increasing; for older patients in whom DA is starting to decrease, i.c.v. idursulfase beta could increase DA slightly or slow the rate of DA decline. This suggests the importance of early initiation of i.c.v. administration because of the irreversible nature of CNS disease. In general, the first step in the diagnosis of MPS II is to identify the clinical manifestations characteristic of the disorder.19 However, children begin to present these characteristic manifestations at 3–4 years of age, by which time intellectual retardation is already advanced. In exceptional cases, children with a family history may be diagnosed before disease onset.20 Recently, neonatal screening for MPS II initiated in some countries and territories has increased the number of children diagnosed before intellectual retardation occurs.21,22 Thus, i.c.v. administration of idursulfase beta may be initiated for children before progression of intellectual retardation, and this will be a new treatment option for patients.
On the basis of the frequency and severity of AEs, including AEs associated with placement of the implantable reservoir, idursulfase beta was well tolerated during the long-term study period. This may be related to short-time (1 min) injection, which may have helped to avoid the risk of bacterial infection. The CSF reservoir we used in this study was a well-practiced device, and we did not have any trouble related to the device. From a safety point of view, short-time injection and the CSF reservoir were good methods and materials.
Our study has some limitations that must be acknowledged. As MPS II is a rare disease, the number of eligible patients was limited. Early treatment affects prognosis and the patient’s subsequent medical condition. Consequently, the study was designed to be open label with no placebo control group, because of an acceptable risk/benefit ratio as with device placement and i.c.v. administration of an inactive comparator, although a historical control group receiving intravenous idursulfase was used for DA comparison. Additionally, we used the KSPD for assessment of DA. This is another limitation of our study because KSPD is only applicable to Japan, although it is strongly correlated with the Bayley III,23 which is applicable to many other countries.
In conclusion, monthly i.c.v. administration of idursulfase beta using the implantable CSF reservoir for 100 weeks in Japanese pediatric patients with severe MPS II reduced CSF HS concentrations, maintained DA, and appeared to be well tolerated. These results suggest that i.c.v. idursulfase beta penetrates the brain and improves CNS manifestations. This ongoing study will further evaluate the long-term efficacy and safety outcomes associated with idursulfase beta; however, the results obtained to date support the continued development of idursulfase beta as a potential therapy for MPS II.
Materials and methods
Study design and participants
This was a phase 1/2, open-label, non-controlled, investigator-initiated clinical study (JMACCT CTR JMA-IIA00350, date of registration June 4, 2018). A placebo control was not considered because of an unacceptable risk/benefit ratio as with device placement and i.c.v. administration of an inactive comparator. The study was initiated at two clinical sites in Japan in July 2016 and is ongoing until March 2021. This analysis includes data collected up to February 2019 (100-week data cutoff). The study was conducted in compliance with the Declaration of Helsinki and the International Council for Harmonisation Guideline for Good Clinical Practice. The protocol and patient informed consent form were reviewed and approved by the Institutional Review Boards of the National Center for Child Health and Development and the Osaka City University Graduate School of Medicine. All legally acceptable representatives of patients provided signed informed consent at the screening period.
Male patients aged 1.5 to <15 years with a confirmed diagnosis of severe MPS II having significant developmental delay (group MS or NT) were eligible for this study if they had never received hematopoietic stem cell transplantation (HSCT) and tolerated ≥24 weeks of treatment with intravenous idursulfase (administered ≥20 times during the 24 weeks before the start of this study). Confirmed diagnosis of severe MPS II was defined as IDS activity in leukocytes that is low or below the quantitation limit, a urinary uronic acid value that exceeds the reference value, and having genetic variants observed exclusively in neuronopathic MPS II.24, 25, 26 Patients were excluded for any of the following: prior HSCT, previous intrathecal administration of idursulfase, urinary uronic acid level ≥50-fold the upper limit of the reference value by age, ventricular/intraperitoneal shunt, end-stage organ dysfunction or other serious diseases, malignant neoplasm, participation in other clinical studies within 6 months before the study start, or history of anaphylactic shock from any component of the study drug.
Procedures
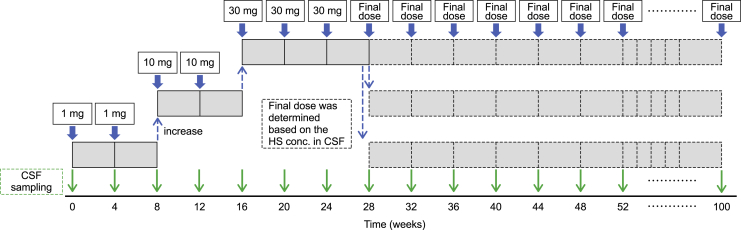
For enrolled patients, a CSF reservoir was implanted under the patient’s scalp for i.c.v. administration. Idursulfase beta i.c.v. injection (15 mg/mL), provided by GC Pharma (Yongin, South Korea), was used for this study. The appropriate dose of idursulfase beta was diluted with normal saline under sterile conditions, and 2 mL was administered for 1 min. The first dose was administered in a hospital setting to allow detailed observation of the patient. Idursulfase beta was i.c.v. administered once every 4 weeks: 1 mg at weeks 0 (baseline) and 4; 10 mg at weeks 8 and 12; 30 mg at weeks 16, 20, and 24; and 42 administrations from weeks 28 to 100 at the final dose as decided by the data monitoring committee based on changes in the HS concentration in the CSF and the safety evaluation (Figure 4). CSF samples were drawn immediately before administration of each idursulfase beta i.c.v. dose. Intravenous administration of idursulfase (0.5 mg/kg/week) was continued throughout the study; an interval of ≥24 h was set between intravenous idursulfase and i.c.v. idursulfase beta. In order to evaluate the efficacy and safety, HS concentration, urinary uronic acid, biochemistry tests, vital signs, immunogenicity tests (IDS antibody, anti-IDS antibody), a head computed tomography or magnetic resonance imaging scan, AEs, KSPD, and other tests were measured and checked for all patients at regular intervals during the clinical trial period (Table 3).
Figure 4.
Injection schedule
Idursulfase beta was i.c.v. administered every 4 weeks: 1 mg at weeks 0 (baseline) and 4; 10 mg at weeks 8 and 12; 30 mg at weeks 16, 20, and 24; and 42 administrations from weeks 28 to 100 at the final dose as decided by the Data Monitoring Committee based on changes in the HS concentration in the CSF and the safety evaluation. CSF samples were drawn immediately before administration of each idursulfase beta i.c.v. dose. CSF, cerebrospinal fluid; HS, heparan sulfate; i.c.v., intracerebroventricular.
Table 3.
Schedule of clinical trial
| Tests | Time (weeks) |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −5 to −1a | 0 | 1 | 2 | 4 | 8 | 10 | 12 | 16 | 18 | 20 | 24 | 28 | 32 | 36 | 40 | 44 | 48 | 52 | +4 | 100 | |
| Implantation of CSF reservoir | X | ||||||||||||||||||||
| Administration | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ~ | X | |||||
| Injection dose (mg)b | 1 | 1 | 10 | 10 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | ~ | 30 | |||||
| HS concentration in CSFc | X | X | X | X | X | X | X | X | X | X | X | X | ~ | X | |||||||
| Urinary uronic acid | X | X | X | X | X | X | X | X | ~ | X | |||||||||||
| KSPD | X | X | X | ~ | X | ||||||||||||||||
| Biochemistry tests | X | X | X | X | X | X | X | X | X | X | X | X | ~ | X | |||||||
| Vital signs | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ~ | X |
| Immunogenicity tests | X | X | X | X | ~ | X | |||||||||||||||
| Head CT or MRI scan | X | X | ~ | X | |||||||||||||||||
| Adverse events check | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ~ | X |
Screening period.
Idursulfase beta was intracerebroventricularly administered once every 4 weeks: 1 mg at weeks 0 (baseline) and 4; 10 mg at weeks 8 and 12; 30 mg at weeks 16, 20, and 24; and a final dose (all patients received 30 mg) from weeks 28 to 100.
CSF samples were drawn immediately before administration.
Outcomes
The primary endpoint was the HS concentration in the CSF. CSF samples were collected every 4 weeks from baseline and every 8 weeks from week 28 prior to each drug administration, and HS concentrations were measured by Toray Research Center (Kamakura, Japan).27,28 The CSF sample (20 μL) was transferred to a glass tube and evaporated to dryness under nitrogen at 40°C. Methanolic hydrochloric acid (3 M, 200 μL) and 2,2-dimethoxy propane (20 μL) were added, and the solution was mixed, sonicated, heated (at 65°C for 75 min), and evaporated. The residue was reconstituted with 200 μL of internal standard working solution and further mixed and sonicated. The solution was centrifuged at 10,000 × g at 4°C for 3 min, and the resultant filtrate was placed into an autosampler vial and analyzed. A UPLC® system (Waters, Milford, MA, USA) and a triple quadrupole mass spectrometer API5000 (AB SCIEX, Framingham, MA, USA) were used for liquid chromatography with tandem mass spectrometry analysis. Analyst v1.5.1 (AB SCIEX, Framingham, MA, USA) was used for data processing.
The secondary endpoint was DA determined by the KSPD, which is an individualized face-to-face test to assess a child’s development in the following three areas: postural-motor, cognitive-adaptive, and language-social.11 The KSPD is a standardized tool that is widely used in Japan for developmental assessment in all age groups. The Bayley Scales of Infant Development III (BSID-III), which is used globally to assess the developmental/cognitive function of young children, has not been standardized in Japanese.23 It has been reported that the developmental quotients of the KSPD are strongly correlated with the corresponding composite score of the BSID-III.23
Safety measures included incidence and severity of AEs, vital signs, electrocardiograms, and clinical laboratory tests. AEs were separately collected as: (1) AEs occurring from placement of the reservoir until i.c.v. administration of idursulfase beta, and (2) AEs occurring after i.c.v. administration of idursulfase beta. AEs were classified by system organ class and preferred term using the Medical Dictionary for Regulatory Activities version 18.1. Immunogenicity tests, including anti-idursulfase beta antibodies in serum and CSF, were performed using the BioNote IDS Ab ELISA kit (BioNote, Gyeonggi, South Korea).
Statistical analysis
A planned sample size of at least four patients was set in consideration of the number of eligible pediatric patients. The efficacy analysis set was defined as patients who had received idursulfase beta and for whom data were available for at least one time point after administration of idursulfase beta. The safety analysis set included all patients who had received idursulfase beta. The primary endpoint, HS concentration in CSF, is presented at each time point for each patient and as the mean of all patients. In addition, the HS concentrations relative to baseline were calculated, and the percentage of patients whose HS values at week 100 decreased by >50% compared with baseline were reported. The secondary endpoint, DA in a total of three areas, is presented at each time point for each patient and as the mean of all patients. DAs in each patient were compared with 13 Japanese patients with neuronopathic MPS II treated with intravenous idursulfase,4 defined as patients with severe MPS II and etiological mutation of the IDS gene who received only intravenous idursulfase and had two or more KSPD evaluations collected before the study. Least-squares means calculated by a linear mixed-effects model in the reference group were used for comparison with patients in this study. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Data availability
The data for this study contain personal information that is not suitable for sharing in its current format. Appropriately de-identified datasets for the current study can be made available by the corresponding author upon reasonable request.
Acknowledgments
The authors would like to thank all study participants. This research was supported by the Japan Agency for Medical Research and Development (AMED) under grant no. JP17lk0103012 and GC Pharma. The study drug used for this investigator-initiated study was provided by GC Pharma, manufacturer/licensee of idursulfase beta. Medical writing assistance funded by GC Pharma was provided by Hiroko Ebina, BPharm, Ph, MBA, CMPP and Rebecca Lew, PhD, CMPP of ProScribe – Envision Pharma Group. ProScribe’s services complied with international guidelines for Good Publication Practice (GPP3). The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Author contributions
All authors were involved in the investigation and participated in the writing, review, editing, and approval of the final version of the manuscript. J-H.S. was involved in the data curation and formal analysis. T.O. was involved in the conceptualization, funding acquisition, and supervision.
Declaration of interests
T.H. has received research grants from Amicus Therapeutics, JCR Pharmaceuticals, and Sanofi Genzyme. T.O. has received research grants from GC Pharma, Sanofi, and Sumitomo Dainippon and is the Principal Investigator for enzyme replacement therapy clinical trials for mucopolysaccharidosis II sponsored by GC Pharma and JCR Pharmaceuticals. The remaining authors declare no competing interests.
References
- 1.Wraith J.E., Scarpa M., Beck M., Bodamer O.A., De Meirleir L., Guffon N., Meldgaard Lund A., Malm G., Van der Ploeg A.T., Zeman J. Mucopolysaccharidosis type II (Hunter syndrome): a clinical review and recommendations for treatment in the era of enzyme replacement therapy. Eur. J. Pediatr. 2008;167:267–277. doi: 10.1007/s00431-007-0635-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shapiro E.G., Jones S.A., Escolar M.L. Developmental and behavioral aspects of mucopolysaccharidoses with brain manifestations—neurological signs and symptoms. Mol. Genet. Metab. 2017;122S:1–7. doi: 10.1016/j.ymgme.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Stapleton M., Kubaski F., Mason R.W., Yabe H., Suzuki Y., Orii K.E., Orii T., Tomatsu S. Presentation and treatments for mucopolysaccharidosis type II (MPS II; Hunter syndrome) Expert Opin. Orphan Drugs. 2017;5:295–307. doi: 10.1080/21678707.2017.1296761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seo J.-H., Okuyama T., Shapiro E., Fukuhara Y., Kosuga M. Natural history of cognitive development in neuronopathic mucopolysaccharidosis type II (Hunter syndrome): contribution of genotype to cognitive developmental course. Mol. Genet. Metab. Rep. 2020;24:100630. doi: 10.1016/j.ymgmr.2020.100630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Japanese Society for Inherited Metabolic Diseases . Practical Guideline for the Management of Mucopolysaccharidosis (MPS) Type II 2019 [Japanese] Shindan to Chiryo Sha; 2019. [Google Scholar]
- 6.Calias P., Papisov M., Pan J., Savioli N., Belov V., Huang Y., Lotterhand J., Alessandrini M., Liu N., Fischman A.J. CNS penetration of intrathecal-lumbar idursulfase in the monkey, dog and mouse: implications for neurological outcomes of lysosomal storage disorder. PLoS ONE. 2012;7:e30341. doi: 10.1371/journal.pone.0030341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muenzer J., Hendriksz C.J., Fan Z., Vijayaraghavan S., Perry V., Santra S., Solanki G.A., Mascelli M.A., Pan L., Wang N. A phase I/II study of intrathecal idursulfase-IT in children with severe mucopolysaccharidosis II. Genet. Med. 2016;18:73–81. doi: 10.1038/gim.2015.36. [DOI] [PubMed] [Google Scholar]
- 8.Kim C., Seo J., Chung Y., Ji H.J., Lee J., Sohn J., Lee B., Jo E.C. Comparative study of idursulfase beta and idursulfase in vitro and in vivo. J. Hum. Genet. 2017;62:167–174. doi: 10.1038/jhg.2016.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sohn Y.B., Ko A.R., Seong M.R., Lee S., Kim M.R., Cho S.Y., Kim J.S., Sakaguchi M., Nakazawa T., Kosuga M. The efficacy of intracerebroventricular idursulfase-beta enzyme replacement therapy in mucopolysaccharidosis II murine model: heparan sulfate in cerebrospinal fluid as a clinical biomarker of neuropathology. J. Inherit. Metab. Dis. 2018;41:1235–1246. doi: 10.1007/s10545-018-0221-0. [DOI] [PubMed] [Google Scholar]
- 10.Schulz A., Ajayi T., Specchio N., de Los Reyes E., Gissen P., Ballon D., Dyke J.P., Cahan H., Slasor P., Jacoby D., Kohlschütter A., CLN2 Study Group Study of intraventricular cerliponase alfa for CLN2 disease. N. Engl. J. Med. 2018;378:1898–1907. doi: 10.1056/NEJMoa1712649. [DOI] [PubMed] [Google Scholar]
- 11.Ikuzawa M., Iwachidou S., Oogami R. In: The Guide of Kyoto Scale of Psychological Development 2001 [Japanese]. Ikuzawa M., Matsushita Y., Nakase A., editors. Kyoto Kokusai Shakaifukushi Center; 2001. [Google Scholar]
- 12.Ngu L.H., Ong Peitee W., Leong H.Y., Chew H.B. Case report of treatment experience with idursulfase beta (Hunterase) in an adolescent patient with MPS II. Mol. Genet. Metab. Rep. 2017;12:28–32. doi: 10.1016/j.ymgmr.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hendriksz C.J., Muenzer J., Vanderver A., Davis J.M., Burton B.K., Mendelsohn N.J., Wang N., Pan L., Pano A., Barbier A.J. Levels of glycosaminoglycans in the cerebrospinal fluid of healthy young adults, surrogate-normal children, and Hunter syndrome patients with and without cognitive impairment. Mol. Genet. Metab. Rep. 2015;5:103–106. doi: 10.1016/j.ymgmr.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohmi K., Greenberg D.S., Rajavel K.S., Ryazantsev S., Li H.H., Neufeld E.F. Activated microglia in cortex of mouse models of mucopolysaccharidoses I and IIIB. Proc. Natl. Acad. Sci. USA. 2003;100:1902–1907. doi: 10.1073/pnas.252784899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villani G.R.D., Gargiulo N., Faraonio R., Castaldo S., Gonzalez Y Reyero E., Di Natale P. Cytokines, neurotrophins, and oxidative stress in brain disease from mucopolysaccharidosis IIIB. J. Neurosci. Res. 2007;85:612–622. doi: 10.1002/jnr.21134. [DOI] [PubMed] [Google Scholar]
- 16.Walkley S.U., Siegel D.A., Dobrenis K. GM2 ganglioside and pyramidal neuron dendritogenesis. Neurochem. Res. 1995;20:1287–1299. doi: 10.1007/BF00992503. [DOI] [PubMed] [Google Scholar]
- 17.Hendriksz C.J., Muenzer J., Burton B.K., Pan L., Wang N., Naimy H., Pano A., Barbier A.J. A cerebrospinal fluid collection study in pediatric and adult patients with Hunter syndrome. J. Inborn Errors Metab. Screen. 2015;3:1–5. [Google Scholar]
- 18.Okuyama T., Eto Y., Sakai N., Minami K., Yamamoto T., Sonoda H., Yamaoka M., Tachibana K., Hirato T., Sato Y. Iduronate-2-sulfatase with anti-human transferrin receptor antibody for neuropathic mucopolysaccharidosis II: a phase 1/2 trial. Mol. Ther. 2019;27:456–464. doi: 10.1016/j.ymthe.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scarpa M., Almássy Z., Beck M., Bodamer O., Bruce I.A., De Meirleir L., Guffon N., Guillén-Navarro E., Hensman P., Jones S., Hunter Syndrome Europena Expert Council Mucopolysaccharidosis type II: European recommendations for the diagnosis and multidisciplinary management of a rare disease. Orphanet J. Rare Dis. 2011;6:72. doi: 10.1186/1750-1172-6-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tajima G., Sakura N., Kosuga M., Okuyama T., Kobayashi M. Effects of idursulfase enzyme replacement therapy for mucopolysaccharidosis type II when started in early infancy: comparison in two siblings. Mol. Genet. Metab. 2013;108:172–177. doi: 10.1016/j.ymgme.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 21.Chuang C.K., Lin H.Y., Wang T.J., Huang Y.H., Chan M.J., Liao H.C., Lo Y.T., Wang L.Y., Tu R.Y., Fang Y.Y. Status of newborn screening and follow up investigations for mucopolysaccharidoses I and II in Taiwan. Orphanet J. Rare Dis. 2018;13:84. doi: 10.1186/s13023-018-0816-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donati M.A., Pasquini E., Spada M., Polo G., Burlina A. Newborn screening in mucopolysaccharidoses. Ital. J. Pediatr. 2018;44(Suppl 2):126. doi: 10.1186/s13052-018-0552-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kono Y., Yonemoto N., Kusuda S., Hirano S., Iwata O., Tanaka K., Nakazawa J. Developmental assessment of VLBW infants at 18 months of age: A comparison study between KSPD and Bayley III. Brain Dev. 2016;38:377–385. doi: 10.1016/j.braindev.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 24.Kosuga M., Mashima R., Hirakiyama A., Fuji N., Kumagai T., Seo J.H., Nikaido M., Saito S., Ohno K., Sakuraba H., Okuyama T. Molecular diagnosis of 65 families with mucopolysaccharidosis type II (Hunter syndrome) characterized by 16 novel mutations in the IDS gene: genetic, pathological, and structural studies on iduronate-2-sulfatase. Mol. Genet. Metab. 2016;118:190–197. doi: 10.1016/j.ymgme.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Sohn Y.B., Ki C.-S., Kim C.-H., Ko A.-R., Yook Y.-J., Lee S.-J., Kim S.J., Park S.W., Yeau S., Kwon E.-K. Identification of 11 novel mutations in 49 Korean patients with mucopolysaccharidosis type II. Clin. Genet. 2012;81:185–190. doi: 10.1111/j.1399-0004.2011.01641.x. [DOI] [PubMed] [Google Scholar]
- 26.Vafiadaki E., Cooper A., Heptinstall L.E., Hatton C.E., Thornley M., Wraith J.E. Mutation analysis in 57 unrelated patients with MPS II (Hunter’s disease) Arch. Dis. Child. 1998;79:237–241. doi: 10.1136/adc.79.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Auray-Blais C., Lavoie P., Zhang H., Gagnon R., Clarke J.T.R., Maranda B., Young S.P., An Y., Millington D.S. An improved method for glycosaminoglycan analysis by LC-MS/MS of urine samples collected on filter paper. Clin. Chim. Acta. 2012;413:771–778. doi: 10.1016/j.cca.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 28.Zhang H., Young S.P., Auray-Blais C., Orchard P.J., Tolar J., Millington D.S. Analysis of glycosaminoglycans in cerebrospinal fluid from patients with mucopolysaccharidoses by isotope-dilution ultra-performance liquid chromatography-tandem mass spectrometry. Clin. Chem. 2011;57:1005–1012. doi: 10.1373/clinchem.2010.161141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data for this study contain personal information that is not suitable for sharing in its current format. Appropriately de-identified datasets for the current study can be made available by the corresponding author upon reasonable request.