Abstract
The Centers for Disease Control and Prevention (CDC) recognizes Neisseria gonorrhoeae as an urgent-threat Gram-negative bacterial pathogen. Additionally, resistance to frontline treatment (dual therapy with azithromycin and ceftriaxone) has led to the emergence of multidrug-resistant N. gonorrhoeae, which has caused a global health crisis. The drug pipeline for N. gonorrhoeae has been severely lacking as new antibacterial agents have not been approved by the FDA in the last twenty years. Thus, there is a need for new chemical entities active against drug-resistant N. gonorrhoeae. Trifluoromethylsulfonyl (SO2CF3), trifluoromethylthio (SCF3), and pentafluorosulfanyl (SF5) containing N-(1,3,4-oxadiazol-2-yl)benzamides are novel compounds with potent activities against Gram-positive bacterial pathogens. Here, we report the discovery of new N-(1,3,4-oxadiazol-2-yl)benzamides (HSGN-237 and -238) with highly potent activity against N. gonorrhoeae. Additionally, these new compounds were shown to have activity against clinically important Gram-positive bacteria, such as methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), and Listeria monocytogenes (minimum inhibitory concentrations (MICs) as low as 0.25 µg/mL). Both compounds were highly tolerable to human cell lines. Moreover, HSGN-238 showed an outstanding ability to permeate across the gastrointestinal tract, indicating it would have a high systemic absorption if used as an anti-gonococcal therapeutic.
Keywords: Neisseria gonorrhoeae; 1,3,4-oxadiazole; antibiotic; antimicrobial resistance
1. Introduction
Drug-resistant bacterial infections have become a serious global threat. Neisseria gonorrhoeae is a Gram-negative bacterial pathogen which causes gonorrhea, a sexually transmitted infection (STI) [1]. N. gonorrhoeae infects a variety of mucosal surfaces (i.e., the urethra, endocervix, pharynx, and rectum) [2] and, if left untreated, can cause drastic complications, such as pelvic inflammatory disease, ectopic pregnancy, and increased susceptibility to HIV infections [3]. The Centers for Disease Control and Prevention (CDC) considers N. gonorrhoeae an urgent threat, as it accounts for 550,000 infections per year and $133.4 million dollars in medical costs in the United States alone [4]. Globally, N. gonorrhoeae is also devastating. The World Health Organization (WHO) listed N. gonorrhoeae as a priority 2 (high) pathogen, as it is has caused 87 million new cases as well as an estimated total treatment cost of $5 billion dollars [5,6,7,8].
Efforts to develop novel antibiotics against urgent threat pathogens, especially N. gonorrhoeae, have intensified [9]. For instance, N. gonorrhoeae is now resistant to former front-line therapies such as penicillin, fluoroquinolones, and cefixime, which are now deemed ineffective as treatment options [10]. This increased resistance rate prompted a global health scare, leading the CDC to recommend treating N. gonorrhoeae with dual therapy involving ceftriaxone and azithromycin [1,11]. Yet, resistance to this dual therapy has been reported, leading to the rise of multidrug-resistant N. gonorrhoeae (commonly referred to as super gonorrhea) [2]. To make matters worse, no new classes of antibiotics to treat N. gonorrhoeae have been FDA-approved over the last two decades, warranting the public health concern that once easily treated gonorrhea infections will soon become deadly [12,13,14]. Therefore, the rise in multidrug-resistant N. gonorrhoeae infections necessitates intense research efforts to identify and develop new antibiotics.
Our program focuses on the development of N-(1,3,4-oxadiazol-2-yl)benzamides to treat drug-resistant bacterial pathogens [15,16]. We recently reported the discovery of trifluoromethylsulfonyl (SO2CF3), trifluoromethylthio (SCF3), and pentafluorosulfanyl (SF5) containing N-(1,3,4-oxadiazol-2-yl)benzamides that exhibited potent antibacterial activities against clinically important Gram-positive bacterial pathogens [17]. These agents were found to be active against clinical isolates of drug-resistant Gram-positive bacteria, were non-toxic to mammalian cells, and effectively reduced the burden of intracellular methicillin-resistant Staphylococcus aureus (MRSA) [17]. Here, we describe a new generation of N-(1,3,4-oxadiazol-2-yl)benzamides with potent activity against N. gonorrhoeae. The antibacterial activity against N. gonorrhoeae, cytotoxicity against mammalian cells, and bi-directional Caco-2 permeability were investigated.
2. Results and Discussion
2.1. Synthesis and Antigonococcal Activity of N-(1,3,4-oxadiazol-2-yl)benzamides:
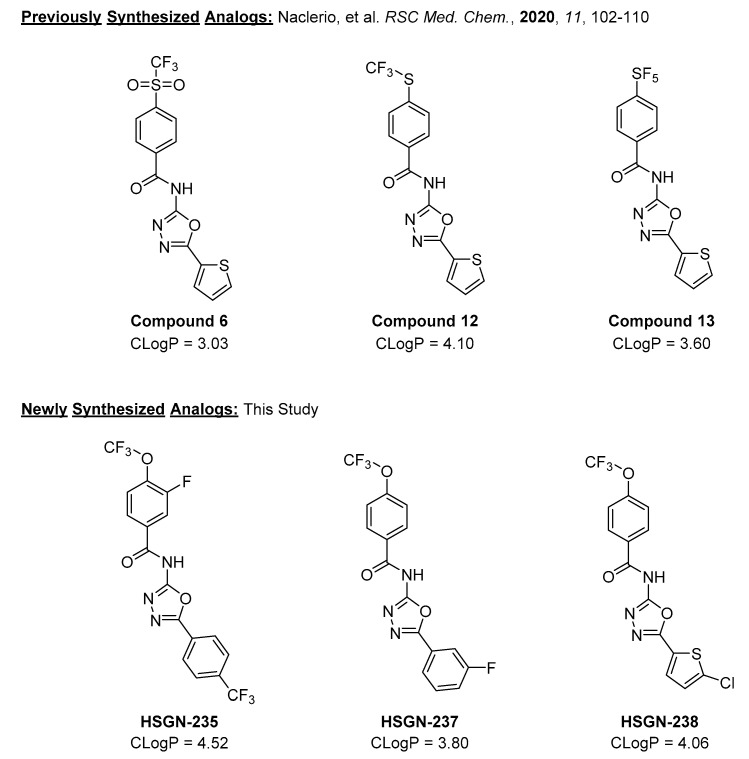
We previously reported that trifluoromethylsulfonyl (SO2CF3), trifluoromethylthio (SCF3), and pentafluorosulfanyl (SF5) containing N-(1,3,4-oxadiazol-2-yl)benzamides (compounds 6, 12, and 13, respectively) were potent against a panel of drug-resistant Gram-positive bacteria [17]. We wondered if these compounds would be active against N. gonorrhoeae and discovered that compounds 6, 12, and 13 have quite potent activity against N. gonorrhoeae strain 181, with minimum inhibitory concentrations (MICs) of 0.5, 0.06, and 0.06 µg/mL, respectively (see Table 1). While all three compounds have favorable CLogP values, they also contain an unsubstituted thiophene moiety (Figure 1) which can cause toxicity. For example, the cytochrome P450-mediated oxidation of thiophene moeities can lead to reactive metabolites such as thiophene epoxides [18], thiophene-S oxides [18,19], and sulphenic acids [20], which can react with nucleophiles such as glutathione and/or water [21]. However, since Compounds 6, 12, and 13 have shown excellent activities against N. gonorrhoeae as well as adequate CLogP values, we desired to further optimize these compounds via the synthesis of new analogs. We proceeded to use computational methods to guide our synthetic strategy. We began to substitute the benzamide ring with the trifluoromethoxy (OCF3) group due to its importance in medicinal chemisty [22,23]. For instance, it was reported that the electronegativity of the OCF3 group allows for enhanced in vivo uptake and transport in biological systems [22]. Thus, utilizing this strategy has led to the synthesis of HSGN-235, which contained a fluoro atom ortho to the OCF3 group as well as a trifluoromethyl phenyl. Yet, HSGN-235 was found to contain a much larger CLogP value compared to previously synthesized analogs (Figure 1). Since LogP shows a positive correlation between low aqueous solubility and compromising bioavailability (an extremely important attribute when creating antibacterial agents against N. gonorrhoeae) [24], we replaced the thiophene moiety with a substituted thiophene or phenyl group as unsubstituted thiophene could be a toxicophore, as mentioned above. Considering that the addition of halogens to compounds has been shown to improve drug properties and metabolic stability [25,26,27,28,29], our new analogs were made up of compounds with halogen substitutions to a phenyl ring (Figure 1).
Table 1.
MICs (µg/mL) of the previously reported analogs (compounds 6, 12, and 13) and the new compounds (HSGN-235, -237, and -238) against N. gonorrhoeae strain 181. The experiment was repeated 3 independent times and the same MIC values were obtained. Compounds tested are in bold while control drugs are in regular script.
| Compound/Control Drug | N. gonorrhoeae Strain 181 |
| Compound 6 | 0.5 |
| Compound 12 | 0.06 |
| Compound 13 | 0.06 |
| HSGN-235 | 16 |
| HSGN-237 | 0.125 |
| HSGN-238 | 0.125 |
| Azithromycin | 256 |
| Tetracycline | 2 |
Figure 1.
Previously reported analogs as well as newly synthesized N-(1,3,4-oxiadizol-2-yl)benzamides for this study. Note: CLogP was calculated using SwissADME.
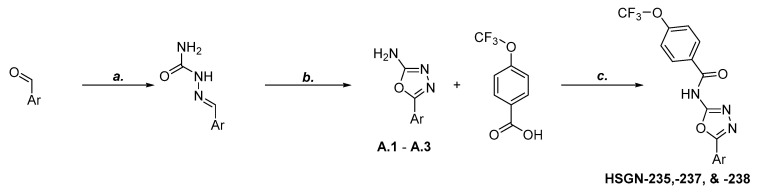
The synthesis of these compounds started with a substituted aryl aldehyde, followed by the addition of semicarbazide and sodium acetate to give the corresponding semicarbazone. Then, using bromine and sodium acetate, the semicarbazone was converted into the subsequent aryl 1,3,4-oxadizol-2-amine. Amide coupling between the aryl 1,3,4-oxadiazol-2-amine and 4-trifluoromethoxy benzoic acid using benzotriazol-1-yloxytris(dimethylamino)phosphonium hexafluorophosphate (BOP) reagent gave the desired N-(1,3,4-oxadiazol-2-yl)benzamides (Scheme 1).
Scheme 1.
Synthesis of N-(1,3,4-oxadiazol-2-yl)benzamides. Reagents and Conditions: (a) Semicarbazide hydrochloride, NaOAc, MeOH:H2O (1:1), rt, 30 min. (b) Bromine, NaOAc, AcOH, 60°C, 1 h. (c) BOP Reagent, DIPEA, DMF, rt, 12 h.
Trifluoromethoxy containing (1,3,4-oxadiazol-2-yl)benzamides with the substitution of the thiophene moiety with a fluorophenyl (HSGN-237) or chlorothiophenyl (HSGN-238) groups had potent activity against N. gonorrhoeae strain 181 with MICs of 0.125 µg/mL (Table 1). Interestingly, the substitution of the 4-trifluoromethoxy phenyl group with a fluorine, as well as the substitution of the thiophene moiety with trifluoromethylphenyl (HSGN-235) only had moderate activity when tested against N. gonorrhoeae strain 181 (Table 1). Since both HSGN-237 and HSGN-238 contained aromatic rings bearing a halogen atom, we speculate that the loss of activity for HSGN-235 is due to the addition of the fluorine atom ortho to the trifluoromethoxy group (see Figure 1 and Table 1 for comparisons).
After the initial screening against N. gonorrhoeae 181, the anti-gonococcal activity of HSGN-235, -237, and -238 was explored against a panel drug-resistant pathogenic N. gonorrhoeae strains, including one WHO reference strain (N. gonorrhoeae WHO L) which has a well-characterized antibiogram and phenotypic and genetic markers [30]. As depicted in Table 2, HSGN-237 and -238 exhibited potent activity against the tested strains, with inhibitions of their growth at concentrations ranging from 0.03 to 0.125 µg/mL. Both were superior to azithromycin and tetracycline against the tested isolates. On the other hand, HSGN 235 inhibited the growth of the tested strains at concentrations ranging from 1 to 2 µg/mL. Interestingly, the minimum bactericidal concentration (MBC) values of HSGN235, -237, and -238 were the same as or one-fold higher than their corresponding MIC values, indicating that the compounds exhibit bactericidal activity against the tested N. gonorrhoeae strains.
Table 2.
MICs and MBCs (µg/mL) of HSGN-235, -237, and -238 against N. gonorrhoeae clinical isolates. The experiment was repeated three independent times and the same MIC values were obtained.
| Bacterial Strains | HSGN-235 | HSGN-237 | HSGN-238 | Azithromycin | Tetracycline | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| N. gonorrhoeae 165 | 2 | 2 | 0.06 | 0.125 | 0.125 | 0.25 | 1 | 4 | 4 | 8 |
| N. gonorrhoeae 166 | 2 | 2 | 0.06 | 0.06 | 0.125 | 0.25 | 0.5 | 1 | 2 | 8 |
| N. gonorrhoeae 194 | 1 | 1 | 0.03 | 0.06 | 0.125 | 0.125 | 0.25 | 0.5 | 1 | 4 |
| N. gonorrhoeae 197 | 1 | 2 | 0.03 | 0.06 | 0.125 | 0.125 | 0.5 | 2 | 2 | 4 |
| N. gonorrhoeae 200 | 2 | 2 | 0.06 | 0.06 | 0.125 | 0.125 | 0.5 | 0.5 | 2 | 8 |
| N. gonorrhoeae WHO L | 1 | 2 | 0.06 | 0.06 | 0.06 | 0.125 | 0.5 | 1 | 0.5 | 2 |
2.2. Antibacterial Activity of N-(1,3,4-oxadiazol-2-yl)benzamides against Other Bacterial Species:
While the focus of these new N-(1,3,4-oxadiazol-2-yl)benzamides is towards N. gonorrhoeae, we proceeded to test their activity against other Gram-positive and Gram-negative pathogens. Intriguingly, HSGN-235, HSGN-237, and HSGN-238 had potent activity against the tested Gram-positive bacterial pathogens. For instance, all three compounds had potent activity against the staphylococcal strains, with MICs ranging from 0.25 to 1 µg/mL (Table 3). Furthermore, HSGN-235, HSGN-237, and HSGN-238 maintained potent activity against clinically relevant Gram-positive bacterial pathogens such as vancomycin-resistant enterococci (VRE) and Listeria monocytogenes (Table 3). Additionally, we moved to test if HSGN-235, HSGN-237, and HSGN-238 were active against other Gram-negative bacterial pathogens. These compounds were found to be inactive against E. coli BW25113. This lack of activity against Gram-negative bacteria appears to be due to HSGN-235, HSGN-237, and HSGN-238 being a substrate for efflux. This can be seen by the shift in the MICs observed for HSGN-235, HSGN-237, and HSGN-238 against wild-type E. coli BW25113 (MIC >8 µg/mL for all compounds; Table 3) in comparison to a mutant strain (E. coli JW55031), where the AcrAB-TolC multidrug-resistant efflux pump is knocked out (MIC for HSGN-235, HSGN-237, and HSGN-238 improves to 4, 0.25, and 0.06 µg/mL, respectively; Table 3). A similar result was observed with linezolid, an antibiotic known to be a substrate for the AcrAB-TolC efflux pump in Gram-negative bacteria, as reported in previous reports [31,32]. Interestingly, HSGN-235, HSGN-237, and HSGN-238 appeared to be bacteriostatic agents, as their MBCs were more than three-fold higher than their corresponding MICs against the tested bacterial strains (Table 3).
Table 3.
MICs (µg/mL) and minimum bactericidal concentrations (MBCs, in µg/mL) of HSGN-235, HSGN-237, and HSGN-238 and control drugs (vancomycin, linezolid, and gentamicin) against a panel of clinically important Gram-positive and Gram-negative bacterial pathogens including: Staphylococcus aureus, methicillin-resistant Staphylococcus aureus (MRSA), Enterococcus faecalis, Enterococcus faecium, Listeria monocytogenes, and Escherichia coli. The experiment was repeated three independent times and the same MIC values were obtained.
| HSGN-235 | HSGN-237 | HSGN-238 | Vancomycin | Linezolid | Gentamicin | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacterial Strains | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC |
| S. aureus ATCC 25923 | 1 | >64 | 0.25 | >64 | 0.25 | >64 | 1 | 1 | 2 | 64 | NT | NT |
| MRSA USA300 | 0.5 | 64 | 0.25 | 32 | 0.25 | 16 | 1 | 2 | 1 | 16 | NT | NT |
|
E. faecalis ATCC 29212 |
4 | 32 | 1 | >64 | 1 | 32 | 1 | 1 | 2 | 64 | NT | NT |
| VRE. faecalisATCC 51575 | 2 | >64 | 1 | >64 | 1 | 32 | >64 | >64 | 2 | 64 | NT | NT |
| VRE. faecalis ATCC 51299 | 1 | 64 | 0.5 | 16 | 0.25 | 8 | >64 | >64 | 1 | 32 | NT | NT |
| VRE. faecium ATCC 700221 | 1 | 32 | 0.5 | 8 | 0.25 | 8 | 32 | 32 | 2 | 64 | NT | NT |
|
L. monocytogenes ATCC 19115 |
1 | 64 | 0.5 | 64 | 0.5 | 32 | 1 | 1 | 2 | 64 | NT | NT |
|
E. coli
BW25113 (wild-type strain) |
>8 | >8 | >8 | >8 | >8 | >8 | >64 | >64 | >64 | >64 | 0.25 | 0.25 |
|
E. coli
JW55031 (TolC Mutant) |
4 | >64 | 0.25 | 16 | 0.06 | 32 | >64 | >64 | 8 | >64 | 0.25 | 0.25 |
NT: Not tested.
2.3. Antibacterial Activity of N-(1,3,4-oxadiazol-2-yl)benzamides against N. gonorrhoeae in Presence of Serum:
An increase in MIC due to antibiotics being highly protein-bound has been documented in several classes of antibiotics [33,34,35]. Therefore, we evaluated our compounds’ activity against N. gonorrhoeae in the presence of different concentrations of fetal bovine serum (FBS). As presented in Table S2, the activity of HSGN-235, -237, and -238 was reduced in the presence of FBS. The addition of 1%, 5%, and 10% FBS to the media increased the MIC of HSGN-237 to 0.125, 1, and 4 µg/mL, respectively (see Table S2). Similarly, the MIC of HSGN-238 also changed to 0.25 and 1 µg/mL (still considered good potency) in the presence of 1% and 5% FBS, respectively, but stayed at 1 µg/mL with the addition of 10% FBS (Table S2). The MIC of HSGN-235 did not change in the presence of 1% FBS, but increased to 4 and 8 µg/mL in the presence of 5% and 10% FBS, respectively (Table S2). Hydrophobic antibiotics such as antimicrobial peptides and lipopeptides have been found to show an increase in MIC upon the addition of serum to media [36,37]. We predict that the hydrophobicity of these N-(1,3,4-oxadiazol-2-yl)benzamides contributes to the rise in MIC in the presence of FBS. Future studies will attempt to develop analogs thereof with fewer protein-binding properties.
2.4. N-(1,3,4-Oxadiazol-2-yl)benzamides Are Highly Tolerable to Human Cell Lines:
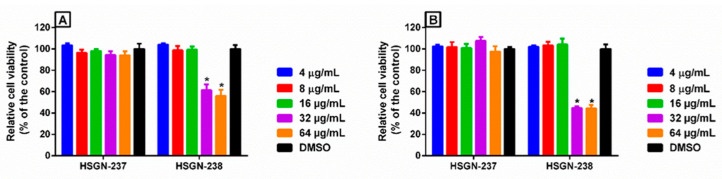
Prokaryotic cell selectivity is highly important for an antibiotic candidate. Therefore, since HSGN-237 and -238 were found to be the most potent analogs against N. gonorrhoeae, they were assessed for toxicity to mammalian cells over 24- and 48-h periods (Figure 2A,B). Both compounds showed excellent safety profiles against human colorectal cells (Caco-2). For instance, HSGN-237 was non-toxic at concentrations higher than 64 µg/mL, which is 512-times higher than the compound‘s corresponding MIC values against N. gonorrhoeae (Figure 2A,B). Additionally, HSGN-238 was non-toxic at concentrations of up to 16 µg/mL which is 128 times higher than the compound‘s corresponding MIC values against N. gonorrhoeae (Figure 2A,B).
Figure 2.
In vitro cytotoxicity assessment of HSGN-237 and -238 (tested in triplicate) against human colorectal cells (Caco-2) after (A) 24 h, and (B) 48 h, using the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay. Results are presented as percent viable cells relative to DMSO (negative control). Error bars represent standard deviation values. A two-way ANOVA, with post hoc Dunnet’s multiple comparisons test, determined the statistical difference between the values obtained for the compound and DMSO. Asterisks denote statistically significant differences between treatments of cells with either HSGN-237 or -238 as compared to DMSO-treated cells. The experiment was repeated 3 independent times.
2.5. HSGN-238 Demonstrates High Intestinal Permeability:
Oral bioavailability is a highly important consideration when developing bioactive molecules as therapeutic agents [38]. A critical factor of oral bioavailability is human intestinal absorption. The Caco-2 bidirectional permeability assay is the most widely used in vitro model for predicting if a bioactive molecule can have adequate systemic absorption [39,40]. Thus, we selected HSGN-238 to act as a model to analyze the drug-like properties of newly synthesized N-(1,3,4-oxadiazol-2-yl)benzamides. The assay demonstrated that HSGN-238 showed an outstanding ability to permeate across Caco-2 bilayers (apparent permeability, Papp = 82.3 × 10−6 cm s−1 from the apical to basolateral and Papp = 32.9 × 10−6 cm s−1 from the basolateral to apical; see Table 4). This permeability is comparable to propranolol (Papp = 37.2 × 10−6 cm s−1 from the apical to basolateral and Papp = 22.7 × 10−6 cm s−1 from the basolateral to apical (Table 4)), a drug that is known to have a high permeability across Caco-2 bilayers. Ranitidine was used as a low-permeability control, as its Papp = 0.5 × 10−6 cm s−1 from the apical to basolateral and Papp = 1.3 × 10−6 cm s−1 from the basolateral to apical. Therefore, the Caco-2 permeability results indicate that HSGN-238 has a high potential to be strongly absorbed after being administered orally.
Table 4.
Caco-2 permeability analysis for HSGN-238 and control drugs.
| Compound/Control Drug | Mean A → B Papp (cm s−1) | Mean B → A Papp (cm s−1) | Notes |
| HSGN-238 | 82.3 × 10−6 | 32.9 × 10−6 | High Permeability |
| Ranitidine | 0.5 × 10−6 | 1.3 × 10−6 | Low Permeability Control |
| Propranolol | 37.2 × 10−6 | 22.7 × 10−6 | High Permeability Control |
3. Materials and Methods
3.1. Chemistry
General considerations: All reagents and solvents were purchased from commercial sources. The 1H, 13C, and 19F NMR spectra were acquired in DMSO-d6 as solvent using a 500 MHz spectrometer with Me4Si as an internal standard. Chemical shifts are reported in parts per million (δ) and were calibrated using residual undeuterated solvent as an internal reference. Data for 1H NMR spectra are reported as follows: chemical shift (δ ppm) (multiplicity, coupling constant (Hz), integration). Multiplicities are reported as follows: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, or combinations thereof. High resolution mass spectra (HRMS) were obtained using electron spray ionization (ESI) technique and as TOF mass analyzer. New compounds were characterized by 1H NMR, 13C NMR, 19F NMR, and HRMS data.
3.2. Synthesis of 1,3,4-Oxadiazol-2-Amines [A.1-A.3]
The synthesis of A.1-A.3 was performed following a literature-reported procedure [41]. 1H, 13C, and 19F NMR spectra were in agreement with the literature-reported data.
3.3. Amide Coupling Procedure for Synthesis of Compounds
A 20 mL screw-capped vial, charged with the corresponding acid (1 eq.), amine (1 eq.), BOP reagent (2.7 eq.), and diisopropylethylamine (23 eq.) in DMF solvent (3 mL), was stirred at room temperature for 16 h. After completion, the reaction mixture was concentrated under reduced pressure, followed by flash column chromatography (hexanes:ethyl acetate 80:20 to 60:40) to give the desired product.
3.4. 3-Fluoro-4-(trifluoromethoxy)-N-(5-(4-(trifluoromethyl)phenyl)-1,3,4-oxadiazol-2-yl)benzamide (HSGN-235)
Off-white solid (34 mg, 18%). 1H NMR (500 MHz, DMSO-d6) δ 8.1 (m, 2H), 8.0 (m, 2H), 7.8 (m, 2H), 7.5 (m, 1H). 13C NMR (126 MHz, DMSO-d6) δ 161.9, 160.1, 158.4, 153.0 (d, J = 258.3 Hz), 136.3 (d, J = 12.6 Hz), 132.0 (q, J = 32.8 Hz), 129.7, 127.5, 127.4, 126.9 (d, J = 3.78 Hz), 126.8, 126.0 (d, J = 5.04 Hz), 125.3, 123.1 (q, J = 288.5 Hz), 121.5 (q, J = 259.6 Hz). 19F NMR (471 MHz, DMSO-d6) δ −59.0 (s, 3F), −62.9 (s, 3F), −132.1 (s, 1F). HRMS (ESI) m/z calcd for C17H9F7N3O3 [M + H]+ 436.0532, found 436.0531.
3.5. N-(5-(3-Fluorophenyl)-1,3,4-oxadiazol-2-yl)-4-(trifluoromethoxy)benzamide (HSGN-237)
Off-white solid (42 mg, 24%). 1H NMR (500 MHz, DMSO-d6) δ 8.2 (dd, J = 8.5, 3.6 Hz, 2H), 7.8 (dd, J = 7.9, 3.6 Hz, 1H), 7.7 – 7.6 (m, 2H), 7.5 (ddd, J = 34.6, 10.1, 6.0 Hz, 3H). 13C NMR (126 MHz, DMSO-d6) δ 165.0, 163.7 (d, J = 245.7 Hz), 160.2, 158.8, 151.9, 132.4 (d, J = 8.82 Hz), 132.2, 131.3, 125.9 (d, J = 8.82 Hz), 122.8, 121.5 (q, J = 259.6 Hz), 121.1, 119.3 (d, J = 21.4 Hz), 113.3 (d, J = 23.9 Hz). 19F NMR (471 MHz, DMSO-d6) δ −57.8 (s, 3F), −112.5 (q, J = 8.1 Hz, 1F). HRMS (ESI) m/z calcd for C16H10F4N3O3 [M + H]+ 368.0658, found 368.0659.
3.6. N-(5-(5-Chlorothiophen-2-yl)-1,3,4-oxadiazol-2-yl)-4-(trifluoromethoxy)benzamide (HSGN-238)
Off-white solid (45 mg, 24%). 1H NMR (500 MHz, DMSO-d6) δ 8.1 (d, J = 8.5 Hz, 2H), 7.6 (d, J = 4.1 Hz, 1H), 7.5 (m, 2H), 7.3 (m, 1H). 13C NMR (126 MHz, DMSO-d6) δ 164.8, 158.0, 156.5, 151.9, 133.5, 131.9, 131.3, 129.9, 129.1, 123.7, 121.4 (q, J = 258.3 Hz), 121.1. 19F NMR (471 MHz, DMSO-d6) δ -57.8 (s, 3F). HRMS (ESI) m/z calcd for C14H8ClF3N3O3S [M + H]+ 389.9927, found 389.9925.
3.7. Bacterial Strains, Media, Reagents and Cell Lines
Neisseria gonorrhoeae clinical isolates (Table S1) used in this study were obtained from the CDC. S. aureus, MRSA, E. faecalis, E. faecium, and L. monocytogenes strains were obtained from the American Type Culture Collection (ATCC). E. coli BW25113 and JW25113 were obtained from the Coli Genetic Stock Center (CGSC), Yale University, USA. Media and reagents were purchased from commercial vendors: Brucella broth, chocolate II agar, cation-adjusted Mueller Hinton broth, tryptic soy broth (TSB), and tryptic soy agar (TSA) (Becton, Dickinson and Company, Cockeysville, MD, USA); yeast extract and dextrose (Fisher Bioreagents, Fairlawn, NJ, USA), proteose-peptone, nicotinamide adenine dinucleotide (NAD), agarose and tetracycline (Sigma-Aldrich, St. Louis, MO, USA); hematin, Tween 80, pyridoxal, linezolid and gentamicin sulfate (Chem-Impex International, Wood Dale, IL, USA); Dulbecco’s Modified Eagle Medium (DMEM), fetal bovine serum (FBS) and phosphate-buffered saline (PBS) (Corning, Manassas, VA, USA); and azithromycin (TCI America, Portland, OR, USA). Human colorectal adenocarcinoma epithelial cells (Caco-2) (ATCC HTB-37) was obtained from the American Type Culture Collection (ATCC) (Manassas, VA, USA). Compounds were synthesized from commercial sources in our laboratory.
3.8. Determination of the MICs of Compounds and Control Drugs against N. gonorrhoeae Strains
The MICs of the tested compounds and control drugs; azithromycin, and tetracycline were determined using the broth microdilution as described previously [42,43,44]. Briefly, bacteria were grown overnight on chocolate agar II at 37° C in the presence of 5% CO2. Afterwards, a bacterial suspension equivalent to 1.0 McFarland standard was prepared and diluted in brucella broth supplemented with yeast extract, neopeptone, hematin, pyridoxal, and NAD. Test agents were added in the first row of the 96-well plates and serially diluted along the plates. Plates were then incubated at 37° C in the presence of 5% CO2 for 24 h. The MICs reported in Table 1 are the minimum concentrations of the compounds and control drugs that could completely inhibit the visual growth of bacteria. The minimum bactericidal concentration (MBC) of these drugs was tested by plating 4 µL from wells with no growth onto chocolate agar II plates. Plates were then incubated at 37 ° C in the presence of 5% CO2 for 24 h. The MBC was categorized as the lowest concentration that reduced bacterial growth by 99.9% [45,46,47].
3.9. Determination of the MICs and MBCs of Compounds and Control Drugs against Clinically Important Gram-Positive and Gram-Negative Bacteria
The minimum inhibitory concentrations (MICs) of the tested compounds and control drugs, linezolid, vancomycin, and gentamicin, were determined using the broth microdilution method according to the guidelines outlined by the Clinical and Laboratory Standards Institute (CLSI) [48] against clinically relevant bacterial (Staphylococcus aureus, MRSA, Escherichia coli, Enterococcus faecalis and Enterococcus faecium strains. S. aureus, MRSA, E. coli, Enterococcus faecalis and Enterococcus faecium were grown aerobically overnight on tryptone soy agar (TSA) plates at 37° C. Afterwards, a bacterial solution equivalent to 0.5 McFarland standard was prepared and diluted in cation-adjusted Mueller–Hinton broth (CAMHB) (for S. aureus, MRSA, and E. coli) to achieve a bacterial concentration of about 5 × 105 CFU/mL. Enterococcus faecalis and Enterococcus faecium 0.5 McFarland standard solution was diluted in tryptone soy broth (TSB) to achieve a bacterial concentration of about 5 × 105 CFU/mL. Compounds and control drugs were added in the first row of 96-well plates and serially diluted with the corresponding media containing bacteria. Plates were then incubated as previously described. MICs reported in Table 2 are the minimum concentration of the compounds and control drugs that could completely inhibit the visual growth of bacteria. The minimum bactericidal concentration (MBC) was tested by spotting 4 µL from wells with no growth onto TSA plates. Plates were incubated at 37 ° C for at least 18 h before recording the MBC. The MBC was categorized as the lowest concentration that reduced bacterial growth by 99.9% [45,46,47].
3.10. In Vitro Cytotoxicity Analysis of HSGN-237 and -238 against Human Colorectal Cells
HSGN-237 and -238 were assayed for potential cytotoxicity against a human colorectal adenocarcinoma (Caco-2) cell line, as described previously [16,49,50]. Briefly, tested compounds were incubated with caco-2 cells for 24 and 48 h. Then, the cells were incubated with MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) reagent for 4 h before measuring the absorbance values (OD490).
3.11. Caco-2 Permeability Assay
Assay and data analysis were performed by Eurofins Panlabs (MO, USA) according to a previously reported protocol [51,52]. The apparent permeability coefficient (Papp) of the tested agents was calculated using the equation below:
where VR is the volume of the receiver chamber. CR,end is the concentration of the test compound in the receiver chamber at the end time point, Δt is the incubation time, and A is the surface area of the cell monolayer. CD,mid is the calculated mid-point concentration of the test compound in the donor side, which is the mean value of the donor concentration at 0 minutes and the donor concentration at the end time point. CR,mid is the mid-point concentration of the test compound in the receiver side, which is one half of the receiver concentration at the end time point. Concentrations of the test compound were expressed as peak areas of the test compound.
4. Conclusions
We have identified promising N-(1,3,4-oxadiazol-2-yl)benzamides with potent antibacterial activity against N. gonorrhoeae. Furthermore, HSGN-237 and -238 exhibited ha ighly acceptable tolerability to human colon cells. Moreover, when assessed using a Caco-2 bidirectional permeability assay, HSGN-238 showed a remarkable ability to cross Caco-2 bilayers, indicating that it would have favorable systemic absorption. Thus, the potent antibacterial profiles of these N-(1,3,4-oxadiazol-2-yl)benzamides warrants further investigation and exploration as potential therapeutics to treat drug-resistant N. gonorrhoeae infections. OCF3-modified N-(1,3,4-oxadiazol-2-yl)benzamides can be added to the list of novel antibacterial agents with novel scaffolds that we have reported [53,54,55,56].
Supplementary Materials
The following are available online at https://www.mdpi.com/1422-0067/22/5/2427/s1.
Author Contributions
H.O.S. and M.N.S. supervised the study. H.O.S. and G.A.N. wrote the manuscript. N.S.A. and M.N.S. edited the manuscript. G.A.N., M.A. performed the synthesis of analogs. G.A.N. and N.S.A. performed minimum inhibition concentration (MIC) assays. N.S.A., M.A. performed cytotoxicity assays. H.O.S., M.N.S., G.A.N., and N.S.A. analyzed and interpreted the data. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Purdue University and the National Institute of Allergy And Infectious Diseases of the National Institutes of Health under Award Number T32AI148103.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Unemo M., A Nicholas R. Emergence of multidrug-resistant, extensively drug-resistant and untreatable gonorrhea. Futur. Microbiol. 2012;7:1401–1422. doi: 10.2217/fmb.12.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hill S.A., Masters T.L., Wachter J. Gonorrhea—An evolving disease of the new millennium. Microb. Cell. 2016;3:371–389. doi: 10.15698/mic2016.09.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burnett A.M., Anderson C.P., Zwank M.D. Laboratory-confirmed gonorrhea and/or chlamydia rates in clinically di-agnosed pelvic inflammatory disease and cervicitis. Am. J. Emerg. Med. 2012;30:1114–1117. doi: 10.1016/j.ajem.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 4.CDC Antibiotic Resistance Threats in the United States. [(accessed on 7 October 2020)]; Available online: www.cdc.gov/DrugResistance/Biggest-Threats.html.
- 5.Kirkcaldy R.D., Harvey A., Papp J.R., Del Rio C., Soge O.O., Holmes K.K., Torrone E. Neisseria gonorrhoeae Antimicrobial Susceptibility Surveillance—The Gonococcal Isolate Surveillance Project, 27 Sites, United States, 2014. MMWR Surveill. Summ. 2016;65:1–19. doi: 10.15585/mmwr.ss6507a1. [DOI] [PubMed] [Google Scholar]
- 6.Newman L.M., Rowley J.T., Hoorn S.V., Wijesooriya N.S., Unemo M., Low N., Stevens G.A., Gottlieb S.L., Kiarie J., Temmerman M. Global Estimates of the Prevalence and Incidence of Four Curable Sexually Transmitted Infections in 2012 Based on Systematic Review and Global Reporting. PLoS ONE. 2015;10:e0143304. doi: 10.1371/journal.pone.0143304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirkcaldy R.D., Weston E., Segurado A.C., Hughes G. Epidemiology of gonorrhoea: A global perspective. Sex. Heal. 2019;16:401–411. doi: 10.1071/SH19061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organisation WHO Publishes List of Bacteria for which New Antibiotics are Urgently Needed. 27 February 2017, News Release, Geneva. [(accessed on 25 October 2018)]; Available online: https://www.who.int/news-room/detail/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed.
- 9.Naclerio G.A., Sintim H.O. Multiple ways to kill bacteria via inhibiting novel cell wall or membrane targets. Futur. Med. Chem. 2020;12:1253–1279. doi: 10.4155/fmc-2020-0046. [DOI] [PubMed] [Google Scholar]
- 10.Unemo M., Shafer W.M. Antimicrobial Resistance in Neisseria gonorrhoeae in the 21st Century: Past, Evolution, and Future. Clin. Microbiol. Rev. 2014;27:587–613. doi: 10.1128/CMR.00010-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Workowski K.A., Bolan G.A., Prevention C.F.D.C.A. Sexually transmitted diseases treatment guidelines, 2015. MMWR. Recomm. Rep. 2015;64:1–137. [PMC free article] [PubMed] [Google Scholar]
- 12.Wise R., BSAC Working Party on The Urgent Need: Regenerating Antibacterial Drug Discovery and Development. Blaser M., Carrs O., Cassell G., Fishman N., White T. The urgent need for new antibacterial agents. J. Antimicrobe Chemother. 2011;66:1939–1940. doi: 10.1093/jac/dkr261. [DOI] [PubMed] [Google Scholar]
- 13.Blomquist P.B., Miari V.F., Biddulph J.P., Charalambous B.M. Is gonorrhea becoming untreatable? Futur. Microbiol. 2014;9:189–201. doi: 10.2217/fmb.13.155. [DOI] [PubMed] [Google Scholar]
- 14.Barbee L.A. Preparing for an era of untreatable gonorrhea. Curr. Opin. Infect. Dis. 2014;27:282–287. doi: 10.1097/QCO.0000000000000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naclerio G.A., Karanja C.W., Opoku-Temeng C., Sintim H.O. Antibacterial Small Molecules That Potently Inhibit Staphylococcus aureus Lipoteichoic Acid Biosynthesis. ChemMedChem. 2019;14:1000–1004. doi: 10.1002/cmdc.201900053. [DOI] [PubMed] [Google Scholar]
- 16.Opoku-Temeng C., Naclerio G.A., Mohammad H., Dayal N., Abutaleb N.S., Seleem M.N., Sintim H.O. N-(1,3,4-oxadiazol-2-yl)benzamide analogs, bacteriostatic agents against methicillin- and vancomycin-resistant bacteria. Eur. J. Med. Chem. 2018;155:797–805. doi: 10.1016/j.ejmech.2018.06.023. [DOI] [PubMed] [Google Scholar]
- 17.Naclerio G.A., Abutaleb N.S., Onyedibe K.I., Seleem M.N., Sintim H.O. Potent trifluoromethoxy, trifluoromethylsulfonyl, trifluoromethylthio and pentafluorosulfanyl containing (1,3,4-oxadiazol-2-yl)benzamides against drug-resistant Gram-positive bacteria. RSC Med. Chem. 2020;11:102–110. doi: 10.1039/C9MD00391F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dansette P.M., Bertho G., Mansuy D. First evidence that cytochrome P450 may catalyze both S-oxidation and epoxidation of thiophene derivatives. Biochem. Biophys. Res. Commun. 2005;338:450–455. doi: 10.1016/j.bbrc.2005.08.091. [DOI] [PubMed] [Google Scholar]
- 19.Valadon P., Dansette P.M., Girault J.P., Amar C., Mansuy D. Thiophene sulfoxides as reactive metabolites: Formation upon microsomal oxidation of a 3-aroylthiophene and fate in the presence of nucleophiles in vitro and in vivo. Chem. Res. Toxicol. 1996;9:1403–1413. doi: 10.1021/tx9601622. [DOI] [PubMed] [Google Scholar]
- 20.Mansuy D., Dansette P.M. Sulfenic acids as reactive intermediates in xenobiotic metabolism. Arch. Biochem. Biophys. 2011;507:174–185. doi: 10.1016/j.abb.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 21.Gramec D., Mašič L.P., Dolenc M.S. Bioactivation Potential of Thiophene-Containing Drugs. Chem. Res. Toxicol. 2014;27:1344–1358. doi: 10.1021/tx500134g. [DOI] [PubMed] [Google Scholar]
- 22.Feng P., Lee K.N., Lee J.W., Zhan C., Ngai M.Y. Access to a new class of synthetic building blocks via trifluorometh-oxylation of pyridines and pyrimidines. Chem. Sci. 2016;7:424–429. doi: 10.1039/C5SC02983J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leroux F.R., Manteau B., Vors J.P., Pazenok S. Trifluoromethyl ethers--synthesis and properties of an unusual sub-stituent. Beilstein J. Org. Chem. 2008;4:13. doi: 10.3762/bjoc.4.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van de Waterbeemd H., Testa B., Mannhold R., Kubinyi H., Folkers G. Drug Bioavailability: Estimation of Solubility, Permeability, Absorption and Bioavailability. 2nd ed. Wiley; Hoboken, NJ, USA: 2008. p. 649. [Google Scholar]
- 25.Gentry C.L., Egleton R.D., Gillespie T., Abbruscato T.J., Bechowski H.B., Hruby V.J., Davis T.P. The effect of halo-genation on blood-brain barrier permeability of a novel peptide drug. Peptides. 1999;20:1229–1238. doi: 10.1016/S0196-9781(99)00127-8. [DOI] [PubMed] [Google Scholar]
- 26.Hernandes M.Z., Cavalcanti S.M.T., Moreira D.R.M., Junior W.F.D.A., Leite A.C.L. Halogen Atoms in the Modern Medicinal Chemistry: Hints for the Drug Design. Curr. Drug Targets. 2010;11:303–314. doi: 10.2174/138945010790711996. [DOI] [PubMed] [Google Scholar]
- 27.Gerebtzoff G., Li-Blatter X., Fischer H., Frentzel A., Seelig A. Halogenation of Drugs Enhances Membrane Binding and Permeation. ChemBioChem. 2004;5:676–684. doi: 10.1002/cbic.200400017. [DOI] [PubMed] [Google Scholar]
- 28.Mendez L., Henriquez G., Sirimulla S., Narayan M. Looking Back, Looking Forward at Halogen Bonding in Drug Discovery. Molecules. 2017;22:1397. doi: 10.3390/molecules22091397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilcken R., Zimmermann M.O., Lange A., Joerger A.C., Boeckler F.M. Principles and Applications of Halogen Bonding in Medicinal Chemistry and Chemical Biology. J. Med. Chem. 2013;56:1363–1388. doi: 10.1021/jm3012068. [DOI] [PubMed] [Google Scholar]
- 30.Unemo M., Golparian D., Sanchez-Buso L., Grad Y., Jacobsson S., Ohnishi M., Lahra M.M., Limnios A., Sikora A.E., Wi T., et al. The novel 2016 WHO Neisseria gonorrhoeae reference strains for global quality assurance of laboratory investigations: Phenotypic, genetic and reference genome characterization. J. Antimicrob. Chemother. 2016;71:3096–3108. doi: 10.1093/jac/dkw288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piddock L.J.V. Multidrug-resistance efflux pumps? not just for resistance. Nat. Rev. Genet. 2006;4:629–636. doi: 10.1038/nrmicro1464. [DOI] [PubMed] [Google Scholar]
- 32.Opperman T.J., Nguyen S.T. Recent advances toward a molecular mechanism of efflux pump inhibition. Front. Microbiol. 2015;6:421. doi: 10.3389/fmicb.2015.00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li J., Xie S., Ahmed S., Wang F., Gu Y., Zhang C., Chai X., Wu Y., Cai J., Cheng G. Antimicrobial Activity and Re-sistance: Influencing Factors. Front Pharmacol. 2017;8:364. doi: 10.3389/fphar.2017.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaplan N., Awrey D., Bardouniotis E., Berman J., Yethon J., Pauls H.W., Hafkin B. In vitroactivity (MICs and rate of kill) of AFN-1252, a novel FabI inhibitor, in the presence of serum and in combination with other antibiotics. J. Chemother. 2013;25:18–25. doi: 10.1179/1973947812Y.0000000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeitlinger M.A., Derendorf H., Mouton J.W., Cars O., Craig W.A., Andes D., Theuretzbacher U. Protein Binding: Do We Ever Learn? Antimicrob. Agents Chemother. 2011;55:3067–3074. doi: 10.1128/AAC.01433-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang W.-H., Wang C.-F., Liao Y.-D. Fetal bovine serum albumin inhibits antimicrobial peptide activity and binds drug only in complex with α1-antitrypsin. Sci. Rep. 2021;11:1–13. doi: 10.1038/s41598-020-80540-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schneider E.K., Huang J.X., Carbone V., Han M., Zhu Y., Nang S., Khoo K.K., Mak J., Cooper M.A., Li J., et al. Plasma Protein Binding Structure–Activity Relationships Related to the N-Terminus of Daptomycin. ACS Infect. Dis. 2017;3:249–258. doi: 10.1021/acsinfecdis.7b00015. [DOI] [PubMed] [Google Scholar]
- 38.Hou T.J., Zhang W., Xia K., Qiao X.B., Xu X.J. ADME evaluation in drug discovery. 5. Correlation of Caco-2 permeation with simple molecular properties. J. Chem. Inf. Comput. Sci. 2004;44:1585–1600. doi: 10.1021/ci049884m. [DOI] [PubMed] [Google Scholar]
- 39.Grès M., Julian B., Bourrié M., Meunier V., Roques C., Berger M., Boulenc X., Berger Y., Fabre G. Correlation Between Oral Drug Absorption in Humans, and Apparent Drug Permeability in TC-7 Cells, A Human Epithelial Intestinal Cell Line: Comparison with the Parental Caco-2 Cell Line. Pharm. Res. 1998;15:726–733. doi: 10.1023/A:1011919003030. [DOI] [PubMed] [Google Scholar]
- 40.Artursson P. Cell cultures as models for drug absorption across the intestinal mucosa. Crit. Rev. Ther. Drug Carr. Syst. 1991;8:305–330. [PubMed] [Google Scholar]
- 41.Kaur J., Soto-Velasquez M., Ding Z., Ghanbarpour A., Lill M.A., Van Rijn R.M., Watts V.J., Flaherty D.P. Optimization of a 1,3,4-oxadiazole series for inhibition of Ca2+/calmodulin-stimulated activity of adenylyl cyclases 1 and 8 for the treatment of chronic pain. Eur. J. Med. Chem. 2019;162:568–585. doi: 10.1016/j.ejmech.2018.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alhashimi M., Mayhoub A., Seleem M.N. Repurposing Salicylamide for Combating Multidrug-Resistant Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 2019;63 doi: 10.1128/AAC.01225-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elkashif A., Seleem M.N. Investigation of auranofin and gold-containing analogues antibacterial activity against mul-tidrug-resistant Neisseria gonorrhoeae. Sci. Rep. 2020;10:5602. doi: 10.1038/s41598-020-62696-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seong Y.J., Alhashimi M., Mayhoub A., Mohammad H., Seleem M.N. Repurposing Fenamic Acid Drugs to Combat Multidrug-Resistant Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 2020;64:7. doi: 10.1128/AAC.02206-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abutaleb N.S., Seleem M.N. Repurposing the Antiamoebic Drug Diiodohydroxyquinoline for Treatment of Clostridi-oides difficile Infections. Antimicrob. Agents Chemother. 2020;64 doi: 10.1128/AAC.02115-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elsebaei M.M., Mohammad H., Samir A., Abutaleb N.S., Norvil A.B., Michie A.R., Moustafa M.M., Samy H., Gowher H., Seleem M.N., et al. Lipophilic efficient phenylthiazoles with potent undecaprenyl pyrophosphatase in-hibitory activity. Eur. J. Med. Chem. 2019;175:49–62. doi: 10.1016/j.ejmech.2019.04.063. [DOI] [PubMed] [Google Scholar]
- 47.Kotb A., Abutaleb N.S., Hagras M., Bayoumi A., Moustafa M.M., Ghiaty A., Seleem M.N., Mayhoub A.S. tert-Butylphenylthiazoles with an oxadiazole linker: A novel orally bioavailable class of antibiotics exhibiting antibiofilm activity. RSC Adv. 2019;9:6770–6778. doi: 10.1039/C8RA10525A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cockerill F.R., Hindler J.A. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. 9th ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2012. [Google Scholar]
- 49.Dokla E.M., Abutaleb N.S., Milik S.N., Li D., El-Baz K., Shalaby M.-A.W., Al-Karaki R., Nasr M., Klein C.D., Abouzid K.A., et al. Development of benzimidazole-based derivatives as antimicrobial agents and their synergistic effect with colistin against gram-negative bacteria. Eur. J. Med. Chem. 2020;186:111850. doi: 10.1016/j.ejmech.2019.111850. [DOI] [PubMed] [Google Scholar]
- 50.Hammad S.G., El-Gazzar M.G., Abutaleb N.S., Li D., Ramming I., Shekhar A., Abdel-Halim M., Elrazaz E.Z., Seleem M.N., Bilitewski U., et al. Synthesis and antimicrobial evaluation of new halogenated 1,3-Thiazolidin-4-ones. Bioorganic Chem. 2020;95:103517. doi: 10.1016/j.bioorg.2019.103517. [DOI] [PubMed] [Google Scholar]
- 51.Obach R.S., Baxter J.G., E Liston T., Silber B.M., Jones B.C., MacIntyre F., Rance D.J., Wastall P. The prediction of human pharmacokinetic parameters from preclinical and in vitro metabolism data. J. Pharmacol. Exp. Ther. 1997;283:46–58. [PubMed] [Google Scholar]
- 52.Hammad A., Abutaleb N.S., Elsebaei M.M., Norvil A.B., Alswah M., Ali A.O., Abdel-Aleem J.A., Alattar A., Bayoumi S.A., Gowher H., et al. From Phenylthiazoles to Phenylpyrazoles: Broadening the Antibacterial Spectrum toward Carbapenem-Resistant Bacteria. J. Med. Chem. 2019;62:7998–8010. doi: 10.1021/acs.jmedchem.9b00720. [DOI] [PubMed] [Google Scholar]
- 53.Opoku-Temeng C., Dayal N., Miller J., Sintim H.O. Hydroxybenzylidene-indolinones, c-di-AMP synthase inhibitors, have antibacterial and anti-biofilm activities and also re-sensitize resistant bacteria to methicillin and vancomycin. RSC Adv. 2017;7:8288–8294. doi: 10.1039/C6RA28443D. [DOI] [Google Scholar]
- 54.Opoku-Temeng C., Onyedibe K.I., Aryal U.K., Sintim H.O. Proteomic analysis of bacterial response to a 4-hydroxybenzylidene indolinone compound, which re-sensitizes bacteria to traditional antibiotics. J. Proteom. 2019;202:103368. doi: 10.1016/j.jprot.2019.04.018. [DOI] [PubMed] [Google Scholar]
- 55.Dayal N., Opoku-Temeng C., Mohammad H., Abutaleb N.S., Hernandez D., Onyedibe K.I., Wang M., Zeller M., Seleem M.N., Sintim H.O. Inhibitors of Intracellular Gram-Positive Bacterial Growth Synthesized via Povarov–Doebner Reactions. ACS Infect. Dis. 2019;5:1820–1830. doi: 10.1021/acsinfecdis.9b00022. [DOI] [PubMed] [Google Scholar]
- 56.Naclerio G.A., Abutaleb N.S., Li D., Seleem M.N., Sintim H.O. Ultrapotent Inhibitor of Clostridioides difficile Growth, Which Suppresses Recurrence In Vivo. J. Med. Chem. 2020;63:11934–11944. doi: 10.1021/acs.jmedchem.0c01198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.