Abstract
Certain viruses and parasites can cause persistent infections that often co-occur and have been associated with substantial morbidity and mortality. Separate lines of research indicate exposures to per- and polyfluoroalkyl substances (PFAS) suppress the immune system. We hypothesized that PFAS exposures might systematically increase susceptibility to persistent infections resulting in a higher pathogen burden. We used data from 8,778 individuals (3,189 adolescents, 5,589 adults) in the nationally-representative U.S. National Health and Nutrition Examination Survey (NHANES) 1999–2016 to examine cross-sectional associations between serum concentrations of four highly detected PFAS (PFOS, PFOA, PFHxS, PFNA) with the presence of antibodies to cytomegalovirus, Epstein Barr virus, hepatitis C and E, herpes simplex 1 and 2, HIV, T. gondii, and Toxocara spp. Seropositivity was summed to calculate a pathogen burden score reflecting the total number of infections. Separate survey-weighted multivariable regression models were fitted to analyze PFAS individually and quantile g-computation was used to analyze PFAS mixtures. Among adolescents, 38.7% had at least one persistent infection while 14.9% had two or more; among adults, these percentages were 48.0% and 19.7%. Each PFAS was individually associated with significantly higher pathogen burdens and the most pronounced associations were observed in adolescents [e.g., among adolescents, a doubling of PFOS was associated with 30% (95% CI: 25-36%) higher pathogen burden]. Quantile g-computation revealed PFAS mixtures as a whole were also associated with higher pathogen burdens. Taken together, these results suggest PFAS exposure may increase susceptibility to and foster the clustering of persistent infections, particularly among adolescents. Since persistent infections are important contributors to long-term health, prospective data are needed to confirm these findings.
Keywords: PFAS, infectious disease, pathogens, chemical mixtures
Graphical Abstract

Capsule:
Using nationally-representative National Health and Nutrition Examination Survey 1999-2016 data, we find that perfluoroalkyl substances (PFAS) are associated with higher burdens of persistent infections. This finding was consistent for both individual PFAS and PFAS mixtures, with adolescents appearing particularly susceptible.
Background
Per- and polyfluoroalkyl substances (PFAS) are a class of synthetic chemicals that contain an alkyl chain with at least one fully fluorinated carbon atom.1 They have been manufactured and used in many industries since the 1940s.2 More than 4,700 PFAS exist and are commonly found in consumer products such as nonstick cookware, stain repellants, waxes, paints, and cleaning products.2 Perfluorooctanesulfonic acid (PFOS), perflurooctanoic acid (PFOA), perfluorohexane sulfonate (PFHxS), and perfluorononanoic acid (PFNA), are the most extensively produced and studied of these chemicals.3 PFOS and PFOA are no longer manufactured in the U.S. due to the phase-out of these chemicals by the Environmental Protection Agency in 2009, but are still produced internationally and imported.4 Recently, extensive biomonitoring by the U.S. National Health and Nutrition Examination Survey (NHANES) has detected these compounds in the blood of more than 98% of Americans.5 Such widespread human exposures along with long clearance half-lives and persistence in the environment have made PFAS a concerning and actively researched class of environmental contaminants.6
Existing literature surrounding the health effects of PFAS indicates that exposure can result in numerous health consequences such as reduced fertility, preeclampsia, birth defects, liver damage, thyroid disease, and cancer.3 Importantly, a number of studies have reported PFAS-associated immunotoxicity in both humans and animals.7-11 A systematic review by the U.S. National Toxicology Program concluded that both PFOS and PFOA are hazards to the human immune system based on a high level of evidence from animal studies and a moderate level of evidence from epidemiological investigations.4 In these studies, PFAS immunotoxicity primarily manifested as suppression of immune responses; we have summarized the existing epidemiologic literature on PFAS immunosuppression in Table 1. Previous epidemiologic studies of the immune system consequences of PFAS exposure have focused largely on vaccine-induced antibody responses. For instance, PFAS exposures during the prenatal period have been associated with lower concentrations of vaccine-induced diphtheria, tetanus, and rubella antibodies in childhood.12,13 Exposures to certain PFAS have also been inversely associated with vaccine-induced rubella, mumps, and A/H3N2 influenza virus antibodies in studies of adults.14-17 These findings generally indicate that PFAS exposures suppress the humoral immune response, rendering vaccines less effective.
Table 1.
Epidemiologic Studies of PFAS Exposure and Immune Suppression
| Study | Country | Study Design | Sample Size | Age Group | Exposure | Result Summary |
|---|---|---|---|---|---|---|
| Pilkerton et al., 201814 | USA | Cross-sectional | N=1202, adults N=1012, youth |
Adults (19-49 years) Youth (12-18 years) |
PFOS, PFOA |
Lower rubella IgG titer in men only No significant association with rubella IgG |
| Stein et al., 201515 | USA | Cross-sectional | N=1191 | Adolescents (12-19 years) | PFOS, PFOA, PFHxS PFNA |
Lower rubella and mumps IgG Lower rubella IgG No significant associations with measles, mumps, or rubella IgG |
| Grandjean et al., 201213 | Faroe Islands | Birth cohort | N=587 | Children (5-7 years) | PFOS, PFOA |
Lower diphtheria and tetanus IgG |
| Granum et al., 201312 | Norway | Birth cohort | N=99 | Children (0-3 years) | PFOS, PFOA, PFHxS, PFNA, PFOS, PFOA, PFHxS, PFNA |
Lower rubella IgG More frequent episodes of common cold More frequent episodes of gastroenteritis |
| Looker et al., 201416 | Ohio, USA | Cross-sectional | N=411 | Adults (>18 years) | PFOS PFOA |
Lower antibody titer rise to A/H3N2 influenza virus No significant association with antibody titer rise to A/H3N2 influenza virus |
| Zeng et al., 201917 | Guangzhou, China | Birth cohort | N=201 | Infants (3 months) | 17 linear PFAS and 10 PFAS isomers | Lower antibody concentrations to two hand, foot, and mouth viruses |
| Fei et al., 201085 | Denmark | Birth cohort | N=1400 | Children (5-10 years) | PFOS, PFOA |
No significant association with hospitalizations due to infections |
| Leonard et al., 200886 | West Virginia, USA | Retrospective occupationally-exposed cohort | N=6,027 | Adults (23-68 years) | PFOA | No significant association with mortality due to infectious and parasitic diseases and influenza and pneumonia |
| Okada et al., 201287 | Sapporo, Japan | Birth cohort | N=343 | Infants (0-18 months) | PFOS, PFOA |
No significant association with otitis media |
However, there are many pathogens that pose serious health threats for which vaccines do not exist. Of particular concern are pathogens that evade the immune system’s clearance mechanisms to establish persistent infections.18 Despite often being asymptomatic, persistent infections and especially co-infections have been implicated as risk factors for all-cause mortality.19 Given compelling evidence that PFAS exposures subdue innate immunity in animal models,20-22 we reasoned that exposures might do the same in humans, thereby increasing susceptibility to persistent infections. Compared to adaptive arm of the immune system that produces antibodies when triggered by specific antigens, the innate arm of the immune system is more primitive. It is comprised of anatomical barriers as well as a variety of biochemical and chemical responses that together act as the first line of defense against invading pathogens.23 Impaired innate immune function is therefore of critical importance to host resistance. We hypothesized that if human PFAS exposures disrupt the integrity of anatomical barriers or suppress the action of phagocytic or natural killer cells,20,21 which are responsible for identifying and eliminating pathogens, we would expect systematically reduced resistance to persistent pathogens. Thus, we examined PFAS exposures in relation to several viral and parasitic infections, individually and combined as a “pathogen burden,” which may capture immune system-wide effects of PFAS in addition to distal effects of immunomodulatory pathogens on secondary infections.24
Materials and Methods
Study Population
In this study, we analyzed publicly available data from eight cycles of the continuous NHANES (1999-2000, 2003-2004, 2005-2006, 2007-2008, 2009-2010, 2011-2012, 2013-2014, 2015-2016). NHANES is a nationally-representative survey conducted by the National Center for Health Statistics. It is designed to assess the health and nutritional status of adults and children in the United States through a combination of in-home interviews and medical examinations performed in a mobile examination center (MEC).25 All study protocols were approved by the National Center for Health Statistics institutional review board and all participants gave written informed consent.
For our study, we did not include data collected by the 2001-2002 cycle because PFAS were measured in pooled rather than individual serum samples. We further restricted our analyses to participants aged 12-49 years with complete serum PFAS measurements, complete information on important sociodemographic and physical characteristics, and unequivocal serologic test results for at least one persistent pathogen of interest [cytomegalovirus (CMV), Epstein Barr virus (EBV), hepatitis virus types C and E (HCV, HEV), human immunodeficiency virus (HIV), herpes simplex virus types 1 and 2 (HSV-1, HSV-2), Toxoplasma gondii (T. gondii), and Toxocara canis and Toxocara cati (Toxocara spp.)]. We also excluded pregnant women on the basis of either self-report or a positive urine pregnancy test, as pregnancy increases susceptibility to infectious diseases.26 The final analytic sample was comprised of 8,778 non-pregnant individuals (3,189 adolescents aged 12-19 years and 5,589 adults aged 20-49 years; see flow diagram in Supplemental Figure 1).
PFAS Exposure Assessment
Solid phase extraction-high performance liquid chromatography-turboionspray ionization-tandem mass spectrometry was used to measure PFAS concentrations in serum samples. Detailed descriptions of the analytic methods have been published previously.27,28 We focused our investigation on four highly detected PFAS: PFOS, PFOA, PFHxS, and PFNA. Non-detectable concentrations were substituted with the respective detection limit divided by the square root of two (Supplemental Table 1). Typically, total concentrations of PFOS and PFOA were measured. However, the 2013-2014 and 2015-2016 cycles measured linear and branched isomers, which we summed to calculate “total” PFOS and PFOA concentrations. Participants aged 12 years and older were eligible for measurement of PFAS concentrations in serum samples, but their selection changed over time. Specifically, in the 1999-2000 cycle, PFAS were measured only among individuals with sufficient quantities of surplus sera (approximately 17.8% of participants) whereas for all subsequent cycles, PFAS were measured in a random one-third sub-sample.
Pathogen Assessment
The prevalence of infections by CMV, EBV, HCV, HEV, HIV, HSV-1, HSV-2, T. gondii, and Toxocara spp. were determined by serological testing for immunoglobulin G (IgG) antibodies. IgG antibodies are typically produced a short time after initial infection. Their presence indicates that an individual was infected at some point in their life, but not when the initial infection occurred. Details regarding the methods used to detect IgG specific to each pathogen and the individuals eligible for testing are provided in Supplemental Table 2. For each pathogen, any individual found to have an equivocal serologic test result was excluded from our analyses. We examined pathogens in two different ways. First, we analyzed individual pathogens, excluding any pathogens with a seroprevalence <1.0% to avoid data sparseness. Second, we followed the approach of several prior NHANES analyses29-32 by constructing a “pathogen burden” score. For these analyses, we summed the number of pathogens for which an individual was seropositive (including any pathogens with a seroprevalence <1.0%).
Covariates
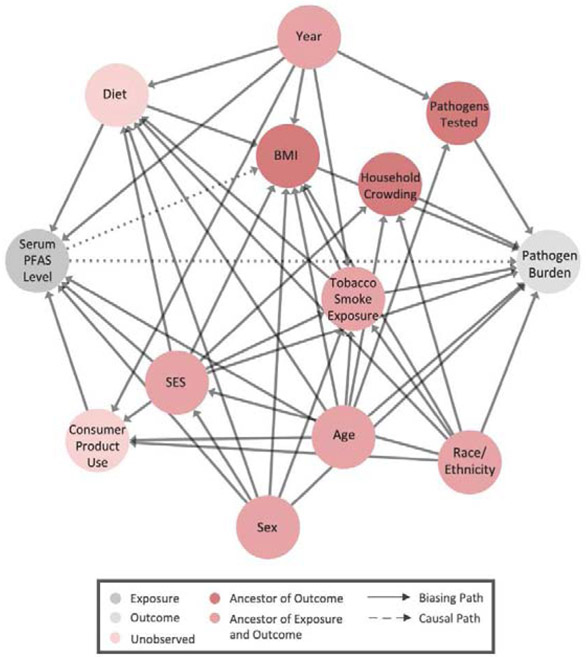
We used a directed acyclic graph (DAG) to identify sociodemographic, lifestyle, and survey-specific factors that could potentially bias observational associations of PFAS exposures with individual pathogens or pathogen burden (Figure 1). Diet and the use of select consumer products have been identified as sources of PFAS exposure and could influence susceptibility to infections.33,34 In our analyses, we did not evaluate diet, as associations with infectious disease appear to mostly be related to extreme cases of malnutrition.35 Furthermore, the dietary information collected by NHANES is quite limited, capturing only 48-hours’ worth of intakes. Similarly, we did not evaluate consumer product use because of the limited information available for this variable. In constructing our DAG, we therefore considered both diet and consumer product use to be latent. Among variables ascertained through household interviews, we reasoned that age,28,36 gender,37-40 race/ethnicity,41-43 and socioeconomic status36,44,45 were potential confounders, as each has previously been linked to variations in PFAS exposures and persistent infections. We operationalized socioeconomic status using two variables: 1.) the ratio of the total family income to the federal poverty threshold; and 2.) educational attainment. For individuals under the age of 20, we used the educational attainment of the household reference person (defined as the adult who owns or rents the residence where members of the household reside) rather than that of the individual who may have been too young to have completed schooling.
Figure 1.
Directed Acyclic Graph of Hypothesized Associations between PFAS Exposures and Pathogen Burden
We also considered the role of certain lifestyle factors (tobacco smoke exposure, household crowding, and body mass index [BMI]) in associations of PFAS exposures with infections. Previous research has established tobacco smoke exposure increases susceptibility to infectious disease.46 As a marker of tobacco smoke exposure, we used serum concentrations of cotinine—a major metabolite of nicotine—to incorporate both active and passive smoke exposures. Cotinine was measured in serum samples by isotope dilution-high performance liquid chromatography/atmospheric pressure chemical ionization tandem mass spectrometry. Living in a crowded household increases the risk of exposure to pathogens.47 Therefore, we calculated a household crowding index by dividing the reported total number of people to reside in the household by the number of rooms in the home. It has been suggested that PFAS are endocrine-disruptors and exposures have been shown to promote weight gain.48 Since the literature also suggests being underweight or obese are risk factors for infection,49 we conceptualized BMI as a mediator. BMI was calculated from anthropometric measurements taken in the mobile examination center as weight (kg) divided by height squared (m2).
Finally, we considered the influence of temporal trends and the study design of NHANES. Exposures to PFAS have changed over time as a result of some long-chain PFAS (namely PFOS and PFOA) being voluntarily phased out of U.S. manufacturing in favor of short-chain PFAS (e.g., PFHxS) and alternatives (e.g., GenX) which are thought to be less toxic.5 The design of NHANES has also changed over time, which affects the number and types of pathogens to which IgG antibodies were measured (Supplemental Table 3).
We used DAGitty,50 which relies on graph theory,51,52 to identify the smallest set of covariates for which adjustment would sufficiently block all back-door paths. There was no set of covariates that could be adjusted for to estimate the “total effect” of PFAS exposure on pathogen burden. This was because BMI, which was hypothesized to be an intermediate, was also influenced by (unmeasured) diet, making it a collider (Figure 1). Thus, our analyses instead attempted to estimate the “direct effect” of PFAS exposure with pathogen burden, that is, the portion of the effect that occurs through mechanisms other than weight gain. Note that while we are using the term “effect” to maintain consistency with the causal mediation literature, we will use the term “association” when reporting these results as the underlying data are cross-sectional and are therefore temporally ambiguous.
Statistical Analyses
All the analyses were conducted in R version 4.0 (R Foundation for Statistical Computing, Vienna, Austria). Since we combined eight NHANES cycles, we rescaled the appropriate sampling weights (the 2-year MEC exam weight for the 1999-2000 cycle and the PFAS sub-sample weights for all subsequent cycles) by multiplying by 1/8, as recommended by NHANES. Unless otherwise specified, all statistical analyses incorporated the rescaled sampling weights and estimated robust variances by Taylor series linearization to account for the complex sampling design. We stratified all analyses by age group, 12-19 years or 20-49 years, because an individual’s age dictated eligibility for select serologic tests (as described in Supplemental Tables 2 and 3). For instance, only individuals under the age of 20 were tested for IgG antibodies to Epstein Barr Virus.
Individual PFAS and Individual Pathogen Analyses
To estimate direct associations of PFAS exposures with the prevalence of individual infections, we fit multivariable Poisson regression models to estimate prevalence ratios with 95% confidence intervals (95% CI).53 Serum PFAS concentrations were modeled as log2-transformed to reduce the influence of outliers. Separate models were fit for each PFAS-pathogen combination. Models were adjusted for age (years), race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, other Hispanic, other race), sex (male, female), the ratio of family income to the federal poverty threshold (unitless), educational attainment (less than high school diploma, high school diploma, some college, college graduate), serum cotinine concentrations (ng/mL), and BMI (kg/m2). Age, the ratio of family income to the federal poverty threshold, serum cotinine concentrations, and BMI were modeled flexibly by fitting restricted cubic splines with 3 equally-spaced knots,54 whereas race/ethnicity, sex, and educational attainment were modeled with indicator variables.
Individual PFAS and Pathogen Burden Analyses
To understand how PFAS exposures were directly related to pathogen burden, we again used Poisson regression models, but this time the dependent variable was the summed seropositivity score (a count ranging from 0 to a maximum possible value of 2 to 7 depending on age group and survey cycle, see Supplemental Table 3 for details). These models controlled for the same covariates as the single-pathogen models with the addition of an offset term for the natural logarithm of the number of pathogens to which IgG antibodies were measured. We included of cross-product terms of log2-transformed PFAS concentrations with BMI and tested these with Wald tests to ensure there was no exposure-mediator interaction, an assumption that is required to validly estimate direct effects in regression models containing mediating variables.55
Quantile G-Computation Analysis of PFAS Mixtures and Pathogen Burden
We evaluated how correlated the PFAS were with one another and how joint exposures were related to persistent infections. We first calculated survey-weighted Spearman rank correlation coefficients for all possible pairs of serum PFAS concentrations. Next, we used quantile g-computation to evaluate the joint association of the four correlated PFAS with pathogen burden.56 This method is a parametric, generalized linear model-based implementation of g-computation.57,28In this context, quantile g-computation estimated the difference in pathogen burden expected when increasing all serum PFAS concentrations by one quantile, simultaneously, conditional on covariates. To implement this method, we converted each PFAS concentration to a common ordinal scale (here, quintiles). Each PFAS was then assigned a weight by fitting a multivariable Poisson regression model of summed seropositivity that included the same covariates used in individual PFAS models. The weights can either be positive or negative and reflect the individual contribution of each PFAS to the overall mixture effect (called ψ). The derivation of weights and the overall mixture effect, ψ, incorporated the rescaled NHANES sampling weights but not the primary sampling units or strata as the R package qgcomp is unable to accommodate nested clustering at this time.58 As a result, the confidence intervals are likely overly precise but the point estimates are expected to be unbiased.
Time-Stratified Analyses of Individual PFAS and PFAS Mixtures with Pathogen Burden
Finally, due to dramatic declines in exposures to several PFAS in recent years,28,59 we repeated several analyses to allow for heterogeneous associations over time. Specifically, we re-ran the models of pathogen burden with PFAS individually and as a mixture, stratifying across both age group (adolescents and adults) and time period (1999-2008 and 2009-2016).
Results
Descriptive Statistics
Population characteristics and geometric mean concentrations of the four PFAS measured in serum are presented in Supplemental Table 4. The population was about half female and mostly non-Hispanic white. Socioeconomic status was varied with approximately one-third of individuals reporting a family income less than 1.3 times that of the appropriate federal poverty threshold and another one-third reporting a family income more than 3.5 times that amount. Similarly, 20.3% of adolescents and 15.6% of adults had low educational attainment (i.e., either themselves or their household reference person had less than a high school diploma) whereas 23.0% of adolescents and 29.5% of adults were considered to have high educational attainment. The majority had serum cotinine concentrations below 1 ng/mL, indicative of no tobacco smoke exposure. Among adolescents, about one-third were classified as overweight or obese (BMI >25 kg/m2) while among adults, about two-thirds were overweight or obese. Of the four PFAS, PFOS was the most abundant in serum samples followed by PFOA, PFHxS, and PFNA. For all PFAS, adults tended to have higher serum concentrations than adolescents.
As shown in Supplemental Table 5, the most common infection among adolescents was Epstein Barr virus (72.6% seropositive) while the most common infection among adults was herpes simplex virus 1 (56.8% seropositive). Distributions of summed seropositivity, or “pathogen burden,” are provided in Table 2. Among adolescents, 38.7% were infected by only one persistent pathogen and 14.9% were co-infected by two or more persistent pathogens. Among adults, infections were more common with 48.0% infected by one persistent pathogen and 19.7% co-infected by two or more. Serum PFAS concentrations were crudely associated with higher pathogen burdens, and this pattern was most apparent in adolescents (Table 2).
Table 2.
Geometric Mean Serum PFAS Concentrations by Pathogen Burden Among Adolescents and Adults
| Aged 12 to 19 Years (N=3,189) | Aged 20 to 49 Years (N=5,589) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PFOS (ng/mL) |
PFOA (ng/mL) |
PFHxS (ng/mL) |
PFNA (ng/mL) |
PFOS (ng/mL) |
PFOA (ng/mL) |
PFHxS (ng/mL) |
PFNA (ng/mL) |
|||
| Pathogen Burden | N (%) | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | N (%) | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE |
| None (uninfected) | 1,131 (46.4) | 5.58 ± 0.26 | 2.16 ± 0.08 | 1.51 ± 0.05 | 0.72 ± 0.03 | 1,446 (32.3) | 8.14 ± 0.31 | 2.63 ± 0.05 | 1.53 ± 0.03 | 0.82 ± 0.03 |
| 1 pathogen | 1,278 (38.7) | 8.91 ± 0.39 | 2.81 ± 0.09 | 1.98 ± 0.05 | 0.84 ± 0.03 | 2,691 (48.0) | 8.56 ± 0.29 | 2.71 ± 0.04 | 1.37 ± 0.03 | 0.89 ± 0.03 |
| 2 pathogens | 661 (13.0) | 11.94 ± 0.50 | 3.30 ± 0.13 | 1.93 ± 0.07 | 0.89 ± 0.04 | 1,134 (15.4) | 9.21 ± 0.38 | 2.66 ± 0.04 | 1.24 ± 0.04 | 0.85 ± 0.03 |
| 3 or more pathogens | 119 (1.9) | 16.14 ± 2.15 | 3.30 ± 0.41 | 1.93 ± 0.21 | 0.89 ± 0.21 | 318 (4.3) | 12.69 ± 0.88 | 2.75 ± 0.08 | 1.35 ± 0.06 | 0.93 ± 0.06 |
| ptrend | <0.01 | <0.01 | <0.01 | <0.01 | 0.02 | 0.61 | <0.01 | 0.08 | ||
Models of Individual PFAS and Individual Pathogens
Given the low prevalence of hepatitis C (0.1%) and E viruses (0.4%) in adolescents and of HIV (0.3%) in adults, we did not fit models for these specific infections. Adolescents with higher serum concentrations of PFOS had a higher prevalence of herpes simplex virus 1, Toxoplasma gondii, and Toxocara spp., but 95% confidence intervals included the null value (Table 3). We also observed positive, although imprecise, associations of PFOA, PFHxS, and PFNA with Toxocara spp. in adolescents. Among adults, higher serum PFAS concentrations were generally associated with seropositivity to herpes simplex viruses 1 and 2 and with Toxocara spp. The strongest association was observed for PFOS with Toxocara spp., for which each doubling of serum PFOS concentrations was associated with a 57% (95% CI: 26-96%) higher prevalence.
Table 3.
Adjusted Prevalence Ratios with 95% Confidence Intervals for Persistent Infections According to a Per-Doubling Increase in Serum PFAS (ng/mL) Concentration
| Aged 12 to 19 Years | Aged 20 to 49 Years | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pathogen | PFAS | N | Cases | PR (95% CI) | p-value | N | Cases | PR (95% CI) | p-value |
| Cytomegalovirus | PFOS | 968 | 484 | 0.92 (0.77, 1.09) | 0.36 | 1,005 | 642 | 0.99 (0.92, 1.05) | 0.70 |
| PFOA | 0.87 (0.70, 1.08) | 0.24 | 0.98 (0.91, 1.05) | 0.57 | |||||
| PFHxS | 0.99 (0.88, 1.12) | 0.86 | 0.96 (0.92, 1.02) | 0.19 | |||||
| PFNA | 0.99 (0.85, 1.15) | 0.91 | 1.00 (0.94, 1.07) | 0.99 | |||||
| Epstein Barr Virus | PFOS | 1,646 | 299 | 1.01 (0.96, 1.05) | 0.74 | - | - | - | - |
| PFOA | 0.99 (0.94, 1.05) | 0.83 | - | - | |||||
| PFHxS | 1.01 (0.98, 1.04) | 0.38 | - | - | |||||
| PFNA | 0.99 (0.95, 1.04) | 0.78 | - | - | |||||
| Hepatitis C Virus | PFOS | 2,530 | 2 | - | - | 4,027 | 54 | 0.96 (0.71, 1.29) | 0.77 |
| PFOA | - | - | 0.89 (0.62, 1.29) | 0.54 | |||||
| PFHxS | - | - | 0.97 (0.71, 1.34) | 0.87 | |||||
| PFNA | - | - | 0.91 (0.72, 1.14) | 0.41 | |||||
| Hepatitis E Virus | PFOS | 919 | 7 | - | - | 2,305 | 97 | 1.00 (0.83, 1.20) | 0.99 |
| PFOA | - | - | 1.01 (0.78, 1.31) | 0.92 | |||||
| PFHxS | - | - | 0.97 (0.81, 1.15) | 0.70 | |||||
| PFNA | - | - | 0.98 (0.75, 1.29) | 0.89 | |||||
| Herpes Simplex Virus 1 | PFOS | 2,499 | 1,040 | 1.05 (0.99, 1.11) | 0.13 | 5,551 | 3,508 | 1.04 (1.01, 1.06) | <0.01 |
| PFOA | 1.02 (0.93, 1.11) | 0.75 | 1.03 (1.01, 1.06) | 0.01 | |||||
| PFHxS | 0.98 (0.93, 1.04) | 0.57 | 1.00 (0.98, 1.02) | 0.95 | |||||
| PFNA | 1.01 (0.93, 1.10) | 0.79 | 1.05 (1.02, 1.08) | <0.01 | |||||
| Herpes Simplex Virus 2 | PFOS | - | - | - | - | 5,537 | 1,187 | 1.04 (0.99, 1.09) | 0.10 |
| PFOA | - | - | 1.11 (1.05, 1.17) | <0.01 | |||||
| PFHxS | - | - | 1.03 (0.99, 1.09) | 0.18 | |||||
| PFNA | - | - | 1.04 (0.99, 1.11) | 0.14 | |||||
| Toxoplasma gondii | PFOS | 1,489 | 61 | 1.15 (0.90, 1.48) | 0.27 | 2,816 | 336 | 1.10 (0.97, 1.26) | 0.15 |
| PFOA | 0.99 (0.68, 1.42) | 0.94 | 1.03 (0.89, 1.18) | 0.70 | |||||
| PFHxS | 0.90 (0.75, 1.08) | 0.28 | 0.97 (0.89, 1.06) | 0.53 | |||||
| PFNA | 0.99 (0.71, 1.39) | 0.95 | 1.06 (0.91, 1.23) | 0.45 | |||||
| Toxocara spp. | PFOS | 614 | 23 | 1.12 (0.66, 1.91) | 0.68 | 1,439 | 91 | 1.57 (1.26, 1.96) | <0.01 |
| PFOA | 1.21 (0.56, 2.65) | 0.63 | 1.23 (1.00, 1.51) | 0.08 | |||||
| PFHxS | 1.16 (0.77, 1.76) | 0.48 | 1.21 (1.06, 1.37) | 0.01 | |||||
| PFNA | 1.10 (0.72, 1.70) | 0.66 | 1.40 (1.13, 1.73) | 0.01 | |||||
Note: Models were adjusted for age (years, restricted cubic spline), race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, other Hispanic, other race), sex (male, female), the ratio of family income to the federal poverty threshold (unitless, restricted cubic spline), educational attainment (less than high school diploma, high school diploma, some college, college graduate), serum cotinine concentrations (ng/mL, restricted cubic spline), and BMI (kg/m2, restricted cubic spline).
Models of Individual PFAS and Pathogen Burden
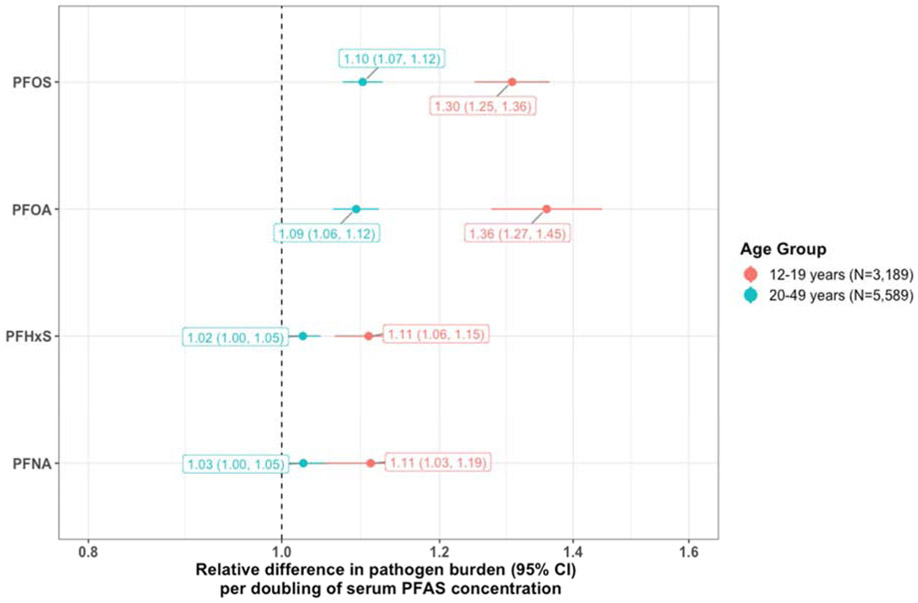
Regression models for individual serum PFAS concentrations with pathogen burden scores suggested associations did not differ by BMI (i.e., there was no exposure-mediator interaction, all pinteraction > 0.4). Point estimates with 95% confidence intervals for the direct associations of serum PFAS concentrations with pathogen burden are depicted in Figure 2. A doubling of each PFAS was associated with higher pathogen burdens in adolescents. The strongest association was observed for PFOA such that a doubling of serum concentrations was associated with a 36% higher pathogen burden (95% CI: 27-45%). In adults, only PFOS and PFOA were associated with pathogen burden, albeit at smaller magnitudes than seen in adolescents.
Figure 2.
Adjusted Associations of Individual Serum PFAS Concentrations with Pathogen Burden
Note: Adjusted for age (years, restricted cubic spline), race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, other Hispanic, other race), sex (male, female), the ratio of family income to the federal poverty threshold (unitless, restricted cubic spline), educational attainment (less than high school diploma, high school diploma, some college, college graduate), serum cotinine concentrations (ng/mL, restricted cubic spline), BMI (kg/m2, restricted cubic spline) with an offset term for the (log-transformed) number of pathogens to which IgG antibodies were tested.
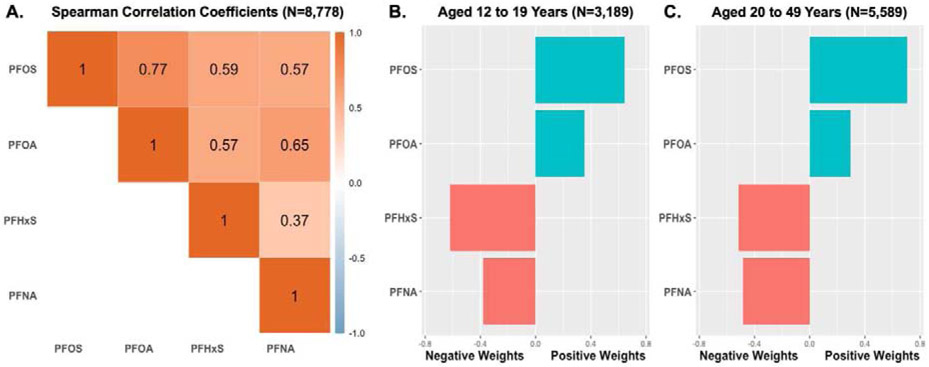
Quantile G-Computation Results for PFAS Mixtures with Pathogen Burden
All PFAS were moderately to strongly correlated with one another with correlation coefficients ranging from 0.37 to 0.77 (Figure 3). Quantile g-computation revealed that, after adjustment for covariates, increasing serum concentrations of all PFAS by one quartile was associated with a 28% (95% CI: 27-28%) higher pathogen burden in adolescents and a 7% (95% CI: 6-7%) higher pathogen burden in adults. Within these models, PFHxS and PFNA were weighted negatively whereas PFOS and PFOA were weighted positively amongst both adolescents and adults (Figure 3). The negative weights for PFHxS and PFNA are indicative of inverse associations with pathogen burden. In contrast, the assignment of positive weights to PFOS and PFOA suggests these compounds in particular are important drivers of the overall positive association between the PFAS mixture and elevated pathogen burdens.
Figure 3.
Correlations between Serum PFAS Concentrations and Quantile G-Computation Weights in Relation to Pathogen Burden
Time-Stratified Models of Individual PFAS and PFAS Mixtures with Pathogen Burden
Serum concentrations of PFOS, PFOA, and PFHxS generally declined over time while PFNA remained relatively stable (Supplemental Figure 2). When we stratified the models for individual serum PFAS concentrations with pathogen burden, we observed positive associations for PFOS, PFOA, and PFNA among adolescents only during 2009-2016 (Supplemental Figure 3). Associations among adults and during 1999-2008 were largely null. Similarly, when we performed stratified quantile g-computation, we found PFAS mixtures were only associated with adolescent pathogen burdens during 2009-2016; PFHxS negatively contributed whereas PFOS and PFOA positively contributed for an overall positive association with pathogen burdens (Supplemental Table 6).
Discussion
In this cross-sectional study, we analyzed associations of PFAS exposures with several persistent infections and pathogen burden in a nationally-representative sample of adolescents and adults. We found that serum PFAS concentrations, individually and as mixtures, were associated with a higher burden of persistent infections. These associations were independent of differences in sociodemographic characteristics and lifestyle factors including body mass index, which we hypothesized as a mediator, indicating PFAS exposures may increase susceptibility to persistent infections through mechanisms other than changes in weight. Among the four PFAS we evaluated, PFOS and PFOA were the most strongly tied to higher pathogen burdens – a finding that was consistent across traditional, single PFAS regression models and novel quantile g-computation. Both of these substances have largely been phased out of production in the U.S., but they remain in circulation from imported goods such as carpet, textiles, furniture, automobile parts, electronics, and household appliances,60 and persist in the environment. PFAS accumulate in the human body over time and indeed, we found adults tended to have higher serum concentrations than adolescents. Despite this, we consistently observed stronger associations in adolescents as compared to adults, suggesting adolescents may be uniquely susceptible to adverse effects of PFAS exposures on the immune system.
PFAS exposures have been studied with respect to human immune function although most epidemiologic investigations have focused on antibody-mediated immunity. Data from animal studies, however, suggest PFAS exposures also suppress non-specific immune responses. For example, mice exposed to PFOS have been shown to have damaged gastrointestinal tract barriers.22 PFAS are often ingested via contaminated foods and water, and certain persistent pathogens (e.g., hepatitis E virus, Toxocara spp., Toxoplasma gondii) are also commonly ingested making the gastrointestinal tract a plausible mechanistic target. Other animal studies have shown PFOS reduces natural killer cells activity,20 PFOA decreases neutrophil migration responses,61 and PFOS and PFOA both lower macrophage counts.21 These cell types are responsible for killing virally infected cells and engulfing and destroying parasites,62,63 and as such, impaired functionality and deficient quantities can increase susceptibility to infectious disease.
The results of this study demonstrated PFOS and PFOA were associated with higher pathogen burdens across individuals aged 12-49 years but suggest adolescents may be particularly at risk. One possible explanation for this finding is the immaturity of the adolescent innate immune response. There are several aspects of innate immunity that increase with age and do not develop fully until adulthood.64 For instance, the gastrointestinal wall increases in thickness by about 20-40 μm annually from birth until age 20,65 forming a first line of defense against pathogens. Circulating cells of the innate immune system also exhibit qualitative and quantitative differences with age. One such example is neutrophilic leukocytes, which are the first cells to migrate from the bloodstream to infected sites and can eliminate many pathogens via phagocytosis.23 Neutrophil proportions increase linearly with age to become the predominant leukocyte subtype in adulthood.64 The capability of neutrophils to migrate to infected tissue (referred to as neutrophil chemotaxis) also matures with age, with adolescents reaching adults levels of neutrophil chemotaxis around the age of 15 years.66 Therefore, differences in innate immune function between adolescents and adults may explain heterogeneous associations of PFAS exposures with pathogen burden and are worthy of future study.
Persistent pathogens establish chronic infections and some are never fully cleared from the human body. Due to their lack of acute health effects, many persistent pathogens have been understudied. However, recent research has begun to suggest links between infection with these pathogens and chronic disease. For example, cytomegalovirus and herpes simplex virus type 1 have each been associated with cardiovascular disease67 whereas Toxoplasma gondii has been associated with impaired memory.68 Simultaneous infections by multiple persistent pathogens (i.e., pathogen burden) may have even more relevance for long-term health, with several studies identifying cumulative health effects beyond those of infections by a single pathogen.19,69,70 By systematically evaluating individual pathogens and total pathogen burden, our study contributes to our understanding of whether PFAS exposures modulate susceptibility to and foster the clustering of persistent infections.
There are notable limitations to the present study. Foremost is the cross-sectional nature of the NHANES data. Although PFOS and PFOA have long half-lives in humans (estimated to be approximately 5 years),6 without prospective data, we have no way of knowing whether exposures preceded initial infections. An additional concern is outcome misclassification. In response to cytomegalovirus,71 Epstein Barr virus,72 herpes simplex virus types 1 and 2,73 hepatitis virus types C and E,74 human immunodeficiency virus,75 T. gondii,76 and Toxocara spp.,77 IgG is produced within the first few months following primary infection and generally persists for life. Without additional serological data (e.g., serial IgG measurements, IgG avidity, or immunoglobulin M),71 it is possible that some very recently acquired infections were missed in our analyses although we expect this number to be small. At the same time, it is possible that some symptomatic cases were also excluded, especially any cases who were hospitalized thus precluding NHANES eligibility. While this scenario may have introduced bias, we expect the influence to be minimal given that the majority of the infections we examined tend to manifest without symptoms.78-84 Another limitation was that we only investigated four legacy PFAS. Interestingly, time-stratified analyses revealed positive associations with adolescent pathogen burdens only within NHANES 2009-2016, when serum concentrations of PFOS and PFOA were markedly lower than in earlier years. It is possible that differences in associations over time are due to residual confounding by newer replacement PFAS such as GenX,5 for which high-quality biomonitoring data were unavailable. Finally, a lack of data on long-term diet (a strong contributor to PFAS exposure)33 meant we could only assess how PFAS exposures were directly related to persistent infections without capturing indirect effects mediated by body composition. Therefore, the total effect of PFAS exposures on pathogen burden may be even larger than we were able to estimate. We were, however, able to account for several other determinants of PFAS exposure that may have confounded associations and were also able to address confounding by PFAS co-exposures through quantile g-computation. The consistency of results from this novel statistical method with our traditional multivariable models strengthens the internal validity of our findings. Other important strengths of our study include its large sample size, inclusion of a study sample representative of the non-institutionalized civilian U.S. population, and use of objective biomarkers of PFAS exposure.
Conclusions
In summary, we are the first to evaluate environmental PFAS exposures in relation to persistent infections. We observed higher exposures to PFOS and PFOA were associated with higher numbers of infections or pathogen burden, a composite measure that appears to increase the risk for subsequent chronic disease and mortality. While confirmation of our findings with prospective data is necessary, lowering exposures to PFAS exposures, particularly during youth, may decrease infectious disease susceptibility and long-term consequences of persistent infections.
Supplementary Material
Highlights.
We examined the relationship between serum PFAS levels and persistent infections.
We analyzed four common PFAS, each on their own and together as a mixture.
As outcomes, we evaluated seropositivity to several viral and parasitic infections.
PFAS were positively associated with pathogen burden, especially in adolescents.
Acknowledgments
Funding: This research was supported in part by the National Institute of Environmental Health Sciences (P42ES031007).
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Competing Financial Interests: The authors declare they have no actual or potential competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Basic Information on PFAS, <https://www.epa.gov/pfas/basic-information-pfas> (
- 2.Buck RC et al. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag 7, 513–541, doi: 10.1002/ieam.258 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agency for Toxic Substances & Disease Registry. Toxicological Profile for Perfluoroalkyls. (2018). [PubMed]
- 4.NTP. Monograph on immunotoxicity associated with exposure to perfluorooctanoic acid or perfluorooctane sulfonate. (National Toxicology Program., 2016). [Google Scholar]
- 5.Calafat AM et al. Legacy and alternative per- and polyfluoroalkyl substances in the U.S. general population: Paired serum-urine data from the 2013-2014 National Health and Nutrition Examination Survey. Environ Int 131, 105048, doi: 10.1016/j.envint.2019.105048 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olsen GW et al. Half-life of serum elimination of perfluorooctanesulfonate,perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect 115, 1298–1305, doi: 10.1289/ehp.10009 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fairley KJ, Purdy R, Kearns S, Anderson SE & Meade B Exposure to the immunosuppressant, perfluorooctanoic acid, enhances the murine IgE and airway hyperreactivity response to ovalbumin. Toxicol Sci 97, 375–383, doi: 10.1093/toxsci/kfm053 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Ryu MH et al. Chronic exposure to perfluorinated compounds: Impact on airway hyperresponsiveness and inflammation. Am J Physiol Lung Cell Mol Physiol 307, L765–774, doi: 10.1152/ajplung.00100.2014 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh TS, Lee S, Kim HH, Choi JK & Kim SH Perfluorooctanoic acid induces mast cell-mediated allergic inflammation by the release of histamine and inflammatory mediators. Toxicol Lett 210, 64–70, doi: 10.1016/j.toxlet.2012.01.014 (2012). [DOI] [PubMed] [Google Scholar]
- 10.Pennings JL et al. Cord blood gene expression supports that prenatal exposure to perfluoroalkyl substances causes depressed immune functionality in early childhood. J Immunotoxicol 13, 173–180, doi: 10.3109/1547691X.2015.1029147 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Routti H et al. Contaminants in Atlantic walruses in Svalbard Part 2: Relationships with endocrine and immune systems. Environ Pollut 246, 658–667, doi: 10.1016/j.envpol.2018.11.097 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Granum B et al. Pre-natal exposure to perfluoroalkyl substances may be associated with altered vaccine antibody levels and immune-related health outcomes in early childhood. J Immunotoxicol 10, 373–379, doi: 10.3109/1547691X.2012.755580 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Grandjean P et al. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA 307, 391–397, doi: 10.1001/jama.2011.2034 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pilkerton CS, Hobbs GR, Lilly C & Knox SS Rubella immunity and serum perfluoroalkyl substances: Sex and analytic strategy. PLoS One 13, e0203330, doi: 10.1371/journal.pone.0203330 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stein CR, McGovern KJ, Pajak AM, Maglione PJ & Wolff MS Perfluoroalkyl and polyfluoroalkyl substances and indicators of immune function in children aged 12-19 y: National Health and Nutrition Examination Survey. Pediatr Res 79, 348–357, doi: 10.1038/pr.2015.213 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Looker C et al. Influenza vaccine response in adults exposed to perfluorooctanoate and perfluorooctanesulfonate. Toxicol Sci 138, 76–88, doi: 10.1093/toxsci/kft269 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeng XW et al. Prenatal exposure to perfluoroalkyl substances is associated with lower hand, foot and mouth disease viruses antibody response in infancy: Findings from the Guangzhou Birth Cohort Study. Sci Total Environ 663, 60–67, doi: 10.1016/j.scitotenv.2019.01.325 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Meneghin A & Hogaboam CM Infectious disease, the innate immune response, and fibrosis. J Clin Invest 117, 530–538, doi: 10.1172/JCI30595 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu J et al. Prospective study of pathogen burden and risk of myocardial infarction or death. Circulation 103, 45–51, doi: 10.1161/01.cir.103.1.45 (2001). [DOI] [PubMed] [Google Scholar]
- 20.Dong GH et al. Chronic effects of perfluorooctanesulfonate exposure on immunotoxicity in adult male C57BL/6 mice. Arch Toxicol 83, 805–815, doi: 10.1007/s00204-009-0424-0 (2009). [DOI] [PubMed] [Google Scholar]
- 21.Qazi MR et al. High-dose, short-term exposure of mice to perfluorooctanesulfonate (PFOS) or perfluorooctanoate (PFOA) affects the number of circulating neutrophils differently, but enhances the inflammatory responses of macrophages to lipopolysaccharide (LPS) in a similar fashion. Toxicology 262, 207–214, doi: 10.1016/j.tox.2009.06.010 (2009). [DOI] [PubMed] [Google Scholar]
- 22.Wang G et al. Intestinal environmental disorders associate with the tissue damages induced by perfluorooctane sulfonate exposure. Ecotoxicol Environ Saf 197, 110590, doi: 10.1016/j.ecoenv.2020.110590 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Janeway C Immunobiology 5 : the immune system in health and disease. 5th edn, (Garland Pub., 2001). [Google Scholar]
- 24.Damania B & Dittmer DP What lies within: coinfections and immunity. Cell Host Microbe 16, 145–147, doi: 10.1016/j.chom.2014.07.014 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson CL et al. National health and nutrition examination survey: analytic guidelines, 1999-2010. Vital Health Stat 2, 1–24 (2013). [PubMed] [Google Scholar]
- 26.Sappenfield E, Jamieson DJ & Kourtis AP Pregnancy and susceptibility to infectious diseases. Infect Dis Obstet Gynecol 2013, 752852, doi: 10.1155/2013/752852 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calafat AM et al. Serum concentrations of 11 polyfluoroalkyl compounds in the u.s. population: data from the national health and nutrition examination survey (NHANES). Environ Sci Technol 41, 2237–2242, doi: 10.1021/es062686m (2007). [DOI] [PubMed] [Google Scholar]
- 28.Kato K, Wong LY, Jia LT, Kuklenyik Z & Calafat AM Trends in exposure to polyfluoroalkyl chemicals in the U.S. Population: 1999-2008. Environ Sci Technol 45, 8037–8045, doi: 10.1021/es1043613 (2011). [DOI] [PubMed] [Google Scholar]
- 29.Dowd JB, Zajacova A & Aiello A Early origins of health disparities: burden of infection, health, and socioeconomic status in U.S. children. Soc Sci Med 68, 699–707, doi: 10.1016/j.socscimed.2008.12.010 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simanek AM, Dowd JB, Zajacova A & Aiello AE Unpacking the 'black box' of total pathogen burden: is number or type of pathogens most predictive of all-cause mortality in the United States? Epidemiol Infect 143, 2624–2634, doi: 10.1017/S0950268814003215 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zajacova A, Dowd JB & Aiello AE Socioeconomic and race/ethnic patterns in persistent infection burden among U.S. adults. J Gerontol A Biol Sci Med Sci 64, 272–279, doi: 10.1093/gerona/gln012 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simanek AM, Dowd JB & Aiello AE Persistent pathogens linking socioeconomic position and cardiovascular disease in the US. Int J Epidemiol 38, 775–787, doi: 10.1093/ije/dyn273 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Susmann HP, Schaider LA, Rodgers KM & Rudel RA Dietary Habits Related to Food Packaging and Population Exposure to PFASs. Environ Health Perspect 127, 107003, doi: 10.1289/EHP4092 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kotthoff M, Muller J, Jurling H, Schlummer M & Fiedler D Perfluoroalkyl and polyfluoroalkyl substances in consumer products. Environ Sci Pollut Res Int 22, 14546–14559, doi: 10.1007/s11356-015-4202-7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katona P & Katona-Apte J The interaction between nutrition and infection. Clin Infect Dis 46, 1582–1588, doi: 10.1086/587658 (2008). [DOI] [PubMed] [Google Scholar]
- 36.Aiello AE et al. Socioeconomic and psychosocial gradients in cardiovascular pathogen burden and immune response: the multi-ethnic study of atherosclerosis. Brain Behav Immun 23, 663–671, doi: 10.1016/j.bbi.2008.12.006 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu CH, Riker CD, Lu SE & Fan ZT Biomonitoring of emerging contaminants, perfluoroalkyl and polyfluoroalkyl substances (PFAS), in New Jersey adults in 2016-2018. Int J Hyg Environ Health 223, 34–44, doi: 10.1016/j.ijheh.2019.10.008 (2020). [DOI] [PubMed] [Google Scholar]
- 38.McQuillan GM et al. Racial and ethnic differences in the seroprevalence of 6 infectious diseases in the United States: data from NHANES III, 1988-1994. Am J Public Health 94, 1952–1958, doi: 10.2105/ajph.94.11.1952 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Staras SA et al. Seroprevalence of cytomegalovirus infection in the United States, 1988-1994. Clin Infect Dis 43, 1143–1151, doi: 10.1086/508173 (2006). [DOI] [PubMed] [Google Scholar]
- 40.Schillinger JA et al. National seroprevalence and trends in herpes simplex virus type 1 in the United States, 1976-1994. Sex Transm Dis 31, 753–760, doi: 10.1097/01.olq.0000145852.43262.c3 (2004). [DOI] [PubMed] [Google Scholar]
- 41.Park SK, Peng Q, Ding N, Mukherjee B & Harlow SD Determinants of per- and polyfluoroalkyl substances (PFAS) in midlife women: Evidence of racial/ethnic and geographic differences in PFAS exposure. Environ Res 175, 186–199, doi: 10.1016/j.envres.2019.05.028 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bangma J et al. Identifying Risk Factors for Levels of Per- and Polyfluoroalkyl Substances (PFAS) in the Placenta in a High-Risk Pregnancy Cohort in North Carolina. Environ Sci Technol 54, 8158–8166, doi: 10.1021/acs.est.9b07102 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stebbins RC et al. Persistent socioeconomic and racial and ethnic disparities in pathogen burden in the United States, 1999-2014. Epidemiol Infect 147, e301, doi: 10.1017/S0950268819001894 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buekers J et al. Socio-Economic Status and Health: Evaluation of Human Biomonitored Chemical Exposure to Per- and Polyfluorinated Substances across Status. Int J Environ Res Public Health 15, doi: 10.3390/ijerph15122818 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sagiv SK et al. Sociodemographic and Perinatal Predictors of Early Pregnancy Per- and Polyfluoroalkyl Substance (PFAS) Concentrations. Environ Sci Technol 49, 11849–11858, doi: 10.1021/acs.est.5b02489 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bagaitkar J, Demuth DR & Scott DA Tobacco use increases susceptibility to bacterial infection. Tob Induc Dis 4, 12, doi: 10.1186/1617-9625-4-12 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.World Health Organization. in WHO Housing and Health Guidelines WHO Guidelines Approved by the Guidelines Review Committee (2018).
- 48.Liu G et al. Perfluoroalkyl substances and changes in body weight and resting metabolic rate in response to weight-loss diets: A prospective study. PLoS Med 15, e1002502, doi: 10.1371/journal.pmed.1002502 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dobner J & Kaser S Body mass index and the risk of infection - from underweight to obesity. Clin Microbiol Infect 24, 24–28, doi: 10.1016/j.cmi.2017.02.013 (2018). [DOI] [PubMed] [Google Scholar]
- 50.Textor J, van der Zander B, Gilthorpe MS, Liskiewicz M & Ellison GT Robust causal inference using directed acyclic graphs: the R package 'dagitty'. Int J Epidemiol 45, 1887–1894, doi: 10.1093/ije/dyw341 (2016). [DOI] [PubMed] [Google Scholar]
- 51.Pearl J Causal inference from indirect experiments. Artif Intell Med 7, 561–582, doi: 10.1016/0933-3657(95)00027-3 (1995). [DOI] [PubMed] [Google Scholar]
- 52.Greenland S, Pearl J & Robins JM Causal diagrams for epidemiologic research. Epidemiology 10, 37–48 (1999). [PubMed] [Google Scholar]
- 53.Zou GY & Donner A Extension of the modified Poisson regression model to prospective studies with correlated binary data. Stat Methods Med Res 22, 661–670, doi: 10.1177/0962280211427759 (2013). [DOI] [PubMed] [Google Scholar]
- 54.Howe CJ et al. Splines for trend analysis and continuous confounder control. Epidemiology 22, 874–875, doi: 10.1097/EDE.0b013e31823029dd (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaufman JS, Maclehose RF & Kaufman S A further critique of the analytic strategy of adjusting for covariates to identify biologic mediation. Epidemiol Perspect Innov 1, 4, doi: 10.1186/1742-5573-1-4 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keil AP et al. A Quantile-Based g-Computation Approach to Addressing the Effects of Exposure Mixtures. Environ Health Perspect 128, 47004, doi: 10.1289/EHP5838 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Robins J A new approach to causal inference in mortality studies with a sustained exposure period—application to control of the healthy worker survivor effect. Mathematical Modelling 7, 1393–1512, doi: 10.1016/0270-0255(86)90088-6 (1986). [DOI] [Google Scholar]
- 58.Keil AP, B. J, O’Brien KM, Ferguson KK, Zhao S, White AJ. The qgcomp package: g-computation on exposure quantiles., <https://cran.r-project.org/web/packages/qgcomp/vignettes/qgcomp-vignette.html> (2020). [Google Scholar]
- 59.Dong Z et al. Using 2003-2014 U.S. NHANES data to determine the associations between per- and polyfluoroalkyl substances and cholesterol: Trend and implications. Ecotoxicol Environ Saf 173, 461–468, doi: 10.1016/j.ecoenv.2019.02.061 (2019). [DOI] [PubMed] [Google Scholar]
- 60.Environmental Protection Agency. (2020).
- 61.Pecquet AM, Maier A, Kasper S, Sumanas S & Yadav J Exposure to perfluorooctanoic acid (PFOA) decreases neutrophil migration response to injury in zebrafish embryos. BMC Res Notes 13, 408, doi: 10.1186/s13104-020-05255-3 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Orange JS Natural killer cell deficiency. J Allergy Clin Immunol 132, 515–525, doi: 10.1016/j.jaci.2013.07.020 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rolot M & Dewals BG Macrophage Activation and Functions during Helminth Infection: Recent Advances from the Laboratory Mouse. J Immunol Res 2018, 2790627, doi: 10.1155/2018/2790627 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Valiathan R, Ashman M & Asthana D Effects of Ageing on the Immune System: Infants to Elderly. Scand J Immunol 83, 255–266, doi: 10.1111/sji.12413 (2016). [DOI] [PubMed] [Google Scholar]
- 65.Haber HP & Stern M Intestinal ultrasonography in children and young adults: bowel wall thickness is age dependent. J Ultrasound Med 19, 315–321 (2000). [PubMed] [Google Scholar]
- 66.Al-Nakeeb S & Thompson EN Assessment of neutrophil chemotaxis and random migration in childhood. Comparison between leading-front and lower surface count methods. Arch Dis Child 55, 296–298, doi: 10.1136/adc.55.4.296 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sorlie PD et al. A prospective study of cytomegalovirus, herpes simplex virus 1, and coronary heart disease: the atherosclerosis risk in communities (ARIC) study. Arch Intern Med 160, 2027–2032, doi: 10.1001/archinte.160.13.2027 (2000). [DOI] [PubMed] [Google Scholar]
- 68.Wyman CP et al. Association between Toxoplasma gondii seropositivity and memory function in nondemented older adults. Neurobiol Aging 53, 76–82, doi: 10.1016/j.neurobiolaging.2017.01.018 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Elkind MS et al. Infectious burden and risk of stroke: the northern Manhattan study. Arch Neurol 67, 33–38, doi: 10.1001/archneurol.2009.271 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rupprecht HJ et al. Impact of viral and bacterial infectious burden on long-term prognosis in patients with coronary artery disease. Circulation 104, 25–31, doi: 10.1161/hc2601.091703 (2001). [DOI] [PubMed] [Google Scholar]
- 71.Prince HE & Lape-Nixon M Role of cytomegalovirus (CMV) IgG avidity testing in diagnosing primary CMV infection during pregnancy. Clin Vaccine Immunol 21, 1377–1384, doi: 10.1128/CVI.00487-14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hess RD Routine Epstein-Barr virus diagnostics from the laboratory perspective: still challenging after 35 years. J Clin Microbiol 42, 3381–3387, doi: 10.1128/JCM.42.8.3381-3387.2004 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.LeGoff J, Pere H & Belec L Diagnosis of genital herpes simplex virus infection in the clinical laboratory. Virol J 11, 83, doi: 10.1186/1743-422X-11-83 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Centers for Disease Control and Prevention (U.S.). Division of Viral Hepatitis, N. C. f. H. A., Viral Hepatitis, STD, and TB Prevention. (2015).
- 75.Branson BM, O. S, Wesolowski LG, Bennett B, Werner BG, Wroblewski KE, Pentella MA. Laboratory testing for the diagnosis of HIV infection: updated recommendations. (United States, 2014). [Google Scholar]
- 76.Robert-Gangneux F & Darde ML Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev 25, 264–296, doi: 10.1128/CMR.05013-11 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ybanez RHD, Ybanez AP & Nishikawa Y Review on the Current Trends of Toxoplasmosis Serodiagnosis in Humans. Front Cell Infect Microbiol 10, 204, doi: 10.3389/fcimb.2020.00204 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Amin MM, Bialek SR, Dollard SC & Wang C Urinary Cytomegalovirus Shedding in the United States: The National Health and Nutrition Examination Surveys, 1999-2004. Clin Infect Dis 67, 587–592, doi: 10.1093/cid/ciy143 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abbott RJ et al. Asymptomatic Primary Infection with Epstein-Barr Virus: Observations on Young Adult Cases. J Virol 91, doi: 10.1128/JVI.00382-17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Groves MJ Genital Herpes: A Review. Am Fam Physician 93, 928–934 (2016). [PubMed] [Google Scholar]
- 81.Seymour CA Asymptomatic infection with hepatitis C virus. BMJ 308, 670–671, doi: 10.1136/bmj.308.6930.670 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Horvatits T, Schulze Zur Wiesch J, Lutgehetmann M, Lohse AW & Pischke S The Clinical Perspective on Hepatitis E. Viruses 11, doi: 10.3390/v11070617 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Halonen SK & Weiss LM Toxoplasmosis. Handb Clin Neurol 114, 125–145, doi: 10.1016/B978-0-444-53490-3.00008-X (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen J et al. Toxocariasis: a silent threat with a progressive public health impact. Infect Dis Poverty 7, 59, doi: 10.1186/s40249-018-0437-0 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fei C, McLaughlin JK, Lipworth L & Olsen J Prenatal exposure to PFOA and PFOS and risk of hospitalization for infectious diseases in early childhood. Environ Res 110, 773–777, doi: 10.1016/j.envres.2010.08.004 (2010). [DOI] [PubMed] [Google Scholar]
- 86.Leonard RC, Kreckmann KH, Sakr CJ & Symons JM Retrospective cohort mortality study of workers in a polymer production plant including a reference population of regional workers. Ann Epidemiol 18, 15–22, doi: 10.1016/j.annepidem.2007.06.011 (2008). [DOI] [PubMed] [Google Scholar]
- 87.Okada E et al. Prenatal exposure to perfluorinated chemicals and relationship with allergies and infectious diseases in infants. Environ Res 112, 118–125, doi: 10.1016/j.envres.2011.10.003 (2012). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.