Abstract
Three chops from 20 pork carcasses were aged for 1, 8, and 21 days. Electrospray ionization-tandem mass spectrometry was used to comprehensively analyze profiles of phospholipids from each sample (n=60). Total phospholipid quantity decreased 4-folds (P < 0.01) from 1 to 21 days of aging in pork loins. Phosphatidylinositol (PI) and phosphatidylserine (PS) increased by 30% and 73%, respectively, from 1 to 21 days of aging in pork loins (P < 0.01). This increase was mainly due to relative percentage increase from PI 38:4 (18:0–20:4) and PS 36:2 (18:0–18:2; P < 0.01). The results also showed that the relative percentage of lysophosphatidylcholine increased by 35% after short term aging (8d), and phosphatidic acid increased by 10-folds after extended aging (21d; P < 0.01). These results documented that phospholipids undergo enzymatic hydrolysis during aging, but also indicated that lipid species containing 18:2 or 20:4 within PI and PS were slightly more resistant to enzymatic hydrolysis compared with the other phospholipids.
Keywords: lipidomics, pork, phospholipid, aging, hydrolysis
1. Introduction
Aging of fresh pork improves tenderness (Melody et al., 2004; Wheeler, Shackelford, & Koohmaraie, 2000), but decreases display life (Holmer et al., 2009; Zhu & Brewer, 1998). It is well established that the shortened display life from aged meat is the direct result of endogenous antioxidants depletion, thus leading to lipid oxidation (Fennema, Damodaran, & Parkin, 2017). Membrane phospholipid composition may play a critical role in subsequent lipid oxidation development in raw and cooked meat (Igene, Pearson, Dugan Jr, & Price, 1980; Pikul, Leszczynski, & Kummerow, 1984; Yin & Faustman, 1994). However, this hypothesis is difficult to further verify as past studies utilized gas chromatography with known standards or mass spectrometry (MS) to detect and identify total fatty acids in the samples (Wood et al., 2008). This method requires saponification and derivatization protocols in order to generate the volatile fatty acid analytes (Metcalfe, Schmitz, & Pelka, 1966), which result in loss of information about the original esterified structure (neutral or polar lipid).
The MS-based complete lipid species profiling is a new lipidomic methodology that utilizes multiple mass analyzers to separately detect the head groups and the fatty acids based on intact ion analyzed, mass of the ions and electrical field strength (Shiva et al., 2013). The technology is applied to many biochemical and medical sciences to characterize lipid profile changes associated with diseases (Braun et al., 2009; Panasenko, Vakhrusheva, Tretyakov, Spalteholz, & Arnhold, 2007; Sparkes, Slone, Roth, Welti, & Fleming, 2010). However, this MS approach has rarely been utilized to characterize lipid metabolism or postmortem lipid alteration in meat. Dannenberger et al. (2017) utilized a non-targeted MS profiling approach to characterize the effect of supplemental diets on lipid metabolites in the muscle of Landrace pigs. Trivedi et al. (2016) utilized this technique to detect changes in sphingomyelin in ground beef adulterated with ground pork. Bermingham et al. (2018) used this MS approach to characterize phospholipid species presented in different beef cuts of New Zealand pasture-fed Wagyu-dairy cross cattle. These results showed that the MS approach can be a useful tool to characterize membrane changes in phospholipid species in response to the meat aging process. However, like most other “omics”, untargeted MS approach in lipidomic research may reveal a high number (>1,000) of known and unknown lipid metabolites that were different between the treatment groups, which would make it difficult for the authors to draw meaningful conclusions from the results.
In the current study, we hypothesize that membrane phospholipid species will be hydrolyzed through extended aging from phospholipase A2 (PLA2) activity, resulting in an increase in phospholipid hydrolysis products. Knowing how the phospholipid species are altered during aging could be an important step toward better understanding the subsequent lipid oxidation and shelf-life of aged fresh meat. Therefore, the objective of this study was to utilize a targeted MS approach to quantitatively document membrane phospholipids and phospholipid hydrolysis product alterations at 3 different aging periods of fresh pork loins.
2. Materials and methods
2.1. Sample Collection
Paired fresh pork loins were collected 1 day postmortem from 20 carcasses (Duroc sired crossbreds) harvested at a commercial processing facility in the Midwest United States. Loins were vacuum packaged and transported to the Iowa State University Meat Laboratory for fabrication. Upon arrival at the meat laboratory, loins were fabricated and aged as described by Schulte et al. (2019). Loin chops (2.54 cm in thickness), containing only the longissimus muscle without fat and connective tissue, were vacuum packaged prior to aging. Three chops from each carcass were aged for 1, 8 and 21 days at 4° C. Samples from aging periods 1 day and 8 day were from the same center cut chop, cut in half, and aged accordingly. Samples from aging period 21 day was also from a chop that was the same anatomical location as aging periods 1 day and 8 day, but from the other side of the same animal. In addition, the sides of aging timepoints were randomized to minimize effects from different sides. The 3 chops from each carcass representing all 3 aging periods (100 g) were frozen in liquid nitrogen and pulverized. A small portion (~5 g) of each pulverized sample was shipped overnight with dry ice to Kansas State University Meat and Muscle Biology Laboratory.
2.2. Lipid Extraction
Total lipid was extracted from all 20 samples for each aging period (n=60) following the procedure described by Folch, Lees, and Sloane Stanley (1957) with modifications. Briefly, 1 g of powdered sample was weighed and transferred to a 50 mL polypropylene conical tube. Ultrapure water (3.2 mL) and chloroform/methanol (1:1 v/v; 16 mL) was added to the sample and vortexed for 5 s. The sample was allowed to sit at room temperature for 30 min and shaken on a wrist action shaker (Model 75, Burrell, Pittsburgh, PA, USA) for 4 min. Four mL of 0.74% potassium chloride in water (w/v) was added to the sample and vortexed for 5 s. The sample was centrifuged at 1,000 × g for 5 min. One mL of the lower (chloroform) layer was removed and transferred to a dried and preweighed 16 × 100 mm disposable glass culture tube. The chloroform was evaporated to dryness under nitrogen (Reacti-Vap, Thermo Scientific, Waltham, MA, USA) on a dry bath incubator set at 40 °C. The extracted lipid and glass tube were placed in a vacuum dryer (CentriVap DNA Vacuum Concentrator, Labconco, Kansas City, MO, USA) and dried for another 1 hr with no heat applied to remove any residual moisture. The dried glass tube with the extracted lipid was immediately weighed to determine lipid weight. After obtaining the lipid weight, 1 mL of chloroform was added to the glass tube, and the content was transferred to a 1.8 mL glass vial with PTFE-lined cap and stored at −80 °C until sample preparation.
2.3. Lipid Sample Preparation
An aliquot of 2 to 11 μL of the diluted lipid in chloroform, corresponding to approximately 9 μg of diluted lipid in chloroform, was added to each vial. One μL of phospholipid internal standards were added to each vial containing the following quantities: 0.66 nmol of di14:0-phosphatidylcholine (PC), 0.66 nmol of di24:1-PC, 0.66 nmol of 13:0- lysophosphatidylcholine (LPC), 0.66 nmol of 19:0-LPC, 0.36 nmol of di14:0- phosphatidylethanolamine (PE), 0.36 nmol of di24:1-PE, 0.36 nmol of 14:0- lysophosphatidylethanolamine (LPE), 0.36 nmol of 18:0-lysoPE, 0.36 nmol of di14:0-PG, 0.36 nmol of di24:1- phosphatidylglycerol (PG), 0.36 nmol of 14:0- lysophosphatidylglycerol (LPG), 0.36 nmol of 18:0-LPG, 0.36 nmol of di14:0- phosphatidic acid (PA), 0.36 nmol of di20:0(phytanoyl)-PA, 0.24 nmol of di14:0- phosphatidylserine (PS), 0.24 nmol of di20:0(phytanoyl)-PS, 0.20 nmol of 16:0–18:0- phosphatidylinositol (PI), and 0.16 nmol of di18:0-PI. The validated phospholipid internal standard mixture was purchased from Kansas Lipidomics Research Center, and the mixture was prepared using the methods outlined in Welti et al. (2002). The sample and internal standard mixture in each vial were combined with solvent (chloroform: methanol: 300 mM ammonium acetate in water, 300:665:35, v/v/v) with a final volume at 1.25 mL.
2.4. Electrospray Ionization (ESI)-Triple Quadrupole Mass Spectrometry
Prepared lipid samples were analyzed at Kansas Lipidomics Research Center. Samples were introduced by continuous infusion into the ESI source on a triple quadrupole MS/MS (API4000, Applied Biosystems, Foster City, CA, USA) using an autosampler (LC MiniPAL, CTC Analytics AG, Zwingen, Switzerland) fitted with the required injection loop for the acquisition time and presented to the ESI needle at 300 μL min−1. The scan speed was 100 μ sec−1. The collision gas pressure was set at 2 (arbitrary units). The mass analyzers were adjusted to a resolution of 0.7 μ full width at half height. For each spectrum, 9 to 150 continuum scans were averaged in multiple channel analyzer (MCA) mode. The source temperature (heated nebulizer) was 100 °C. The interface heater was on, and +5.5 kV or −4.5 kV was applied to the electrospray capillary. The collision gas was set at “low”. The curtain gas was set at 20 (arbitrary units), and the two ion source gases were set at 45 (arbitrary units).
Sequential precursor and neutral loss scans of the samples produce a series of spectra with each spectrum revealing a set of lipid species containing a common head group or fatty acids fragment. The intact ion analyzed, fragment type, scan mode, polarity, collision energy, declustering potentials, entrance potentials and exit potentials to detect the phospholipid species used in this study were the same as described in Xiao et al., 2010 (table 1). The sphingomyelin (SM) was determined from the same mass spectrum as PC and by comparison with PC internal standards using a conversion factor for SM in comparison with PC (determined experimentally to be 0.39). Ether-linked PCs and PEs (ePCs and ePEs) were determined in relation (less in masses compared to their ester-linked counterparts) to the same standards as other PC and PE species. In addition, the identification of the most abundant phospholipid molecular species (total acyl carbons: total double bonds) based on their mass to charge ratio within a specific head group is summarized in table 2. It is important to note that all phospholipid molecular species were detected using the scans previously described and validated (Brügger, Erben, Sandhoff, Wieland, & Lehmann, 1997; Welti et al., 2002; Xiao et al., 2010).
Table 1.
Mass spectrometry analysis parameters used in this study.
| Class | PA | PC, LPC and SM | PE and LPE | PI | PS |
|---|---|---|---|---|---|
| Intact ion analyzed | (M + NH4)+ | (M + H)+ | (M + H)+ | (M + NH4)+ | (M + H)+ |
| Fragment Type | Head group | Head group | Head group | Head group | Head group |
| Scan mode | Neutral Loss of 115.00 | Precursors of 184.07 | Neutral Loss of 141.02 | Neutral Loss of 277.06 | Neutral Loss of 185.01 |
| Polarity | + | + | + | + | + |
| Mass/charge range | 500–850 | 450–960 | 420–920 | 790–950 | 600–920 |
| Collision energy (V) | 25 | 40 | 28 | 25 | 25 |
| Declustering potential (V) | 100 | 100 | 100 | 100 | 100 |
| Entrance potential (V) | 14 | 14 | 15 | 14 | 14 |
| Exit potential (V) | 14 | 14 | 11 | 14 | 14 |
PA = phosphatidic acid; PC = phosphatidylcholine; LPC= lysophosphatidylcholine; SM=sphingomyelin; PE = phosphatidylethanolamine; LPE= lysophosphatidylethanolamine; PI = phosphatidylinositol; PS = phosphatidylserine.
Table 2.
Identification of the mass/charge ratio of the most abundant phospholipid molecular species.
| Class of phospholipid | Ion | Scan Mode | Total acyl carbons: total double bonds |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 34:1 | 34:2 | 36:1 | 36:2 | 38:4 | 16:0 | 18:0 | 18:2 | 22:0 | |||
| PC | (M + H)+ | Precursors of 184.07 | 760.6 | 758.6 | 788.6 | 786.6 | 810.6 | ||||
| ePC | (M + H)+ | Precursors of 184.07 | 774.6 | 744.6 | 774.6 | 772.6 | 796.6 | ||||
| PE | (M + H)+ | Neutral Loss of 141.02 | 718.5 | 716.5 | 746.6 | 744.5 | 768.5 | ||||
| ePE | (M + H)+ | Neutral Loss of 141.02 | 704.6 | 702.5 | 732.6 | 730.6 | 754.6 | ||||
| PI | (M + NH4)+ | Neutral Loss of 277.06 | 854.5 | 852.5 | 882.6 | 880.6 | 904.6 | ||||
| PS | (M + H)+ | Neutral Loss of 185.01 | 762.5 | 760.5 | 790.6 | 788.5 | 812.5 | ||||
| SM | (M + H)+ | Precursors of 184.07 | 703.6 | 731.6 | N/A | 787.7 | |||||
| LPC | (M + H)+ | Precursors of 184.07 | 496.3 | 524.4 | 520.3 | N/A | |||||
| LPE | (M + H)+ | Neutral Loss of 141.02 | 454.3 | N/A | 478.3 | N/A | |||||
PC = phosphatidylcholine; ePC= ether-linked PC; PE = phosphatidylethanolamine; ePE= ether-linked PE; PI = phosphatidylinositol; PS = phosphatidylserine; SM=sphingomyelin; LPC= lysophosphatidylcholine; LPE= lysophosphatidylethanolamine.
The background of each spectrum was subtracted, and peak areas were integrated using a custom script in the Applied Biosystems Analyst software (Applied Biosystems). The lipid peak areas in each phospholipid class was uploaded to an online processing software for direct-infusion mass spectral data for lipid profile (LipidomeDB Data Calculation Environment; Zhou et al., 2011). The LipidomeDB software corrected signals for isotopic overlap as the presence of a double bond in a lipid species may create more than 1 peak area. The second peak of one species may produce a signal at the same mass as the main peak of another (more saturated) lipid species. Therefore, the contribution of signal from the second peak must be subtracted. A linear calibration curve (signal vs. mass/charge) was fitted to the signals of the internal standards and was used to correct for mass-dependent variation in instrument response. Finally, the corrected signals for the target lipids were converted to nmol based on the signals from the 2 internal standards and the corresponding internal standard amounts specified by the user (Zhou et al., 2011). Each lipid class was normalized to the dried lipid weight for each sample and expressed as nmol lipid class/mg lipid to quantify amount of phospholipid throughout the aging periods. The data were also expressed as mole percent (mol%; distribution of each phospholipid species in relative % of total phospholipid) of total lipid analyzed. To calculate mol%, we multiplied each nanomolar value × 100% and divided by the total of the nanomolar amounts of the lipids analyzed.
An “internal standards only” sample was inserted for every 10 samples, and the mass spectra were acquired on the “internal standards only” samples to correct for chemical or instrumental noise in the samples. The molar amount of each lipid metabolite detected in the “internal standards only” samples was treated as contaminations and used to subtract the molar amount of each lipid metabolite calculated for the next 10 samples. Furthermore, 14 quality control (QC) samples were prepared for this study. The QC samples were prepared by pooling a 4 μL of lipid extract from each sample. The values for the first three QC samples were eliminated due to potential instrument instability when the instrument was first started. Lipid analytes in which the variation (standard deviation divided by mean of the amount of the analyte in the QC) was greater than 30% were removed from the data. The average variation of retained lipids for this study was 2.5%. Finally, each apparent lipid molecular species is displayed as total acyl carbons: total double bonds.
2.5. Fatty acid groups identification
Further characterization of the following major phospholipid species was performed using MS/MS product-ion analysis to reveal the true identity of the molecular species: PC 34:2, PC 36:4, PE 36:2, PE 38:4, PI 36:2, PI 38:3, PI 38:4, PS 36:1 and PS 36:2. Fatty acids anions from the phospholipids of pooled samples were identified using the appropriate negative ion precursors: PI, PE, and PS were analyzed as [M - H]− ions, and PC was analyzed as [M+OAc]−. Specific running conditions and masses used for product-ion analysis were described by Devaiah et al. (2006). This method is effective in identifying the specific fatty acids on the sn1 and sn2 position not only because fatty acids can be differentiated by their fragmentation pattern, but also based on the fact that for phospholipids, the sn2 position generally fragments more readily than sn1 position (personal communication with Dr. Ruth Welti- Director of Kansas Lipidomics Research Center). These phospholipid species were selected for product-ion analysis because either they represent a significant portion of total phospholipid or their contents were altered throughout the aging periods.
2.6. Unsaturation index
Unsaturation index (UI) refers to the number of double bonds in a lipid, such that the greater the UI, the greater is the unsaturation of that lipid. The UI of each lipid molecular species was calculated as the product of the mol% of the lipid species and the average number of double bonds per acyl chain. The average number of double bonds per acyl chain was calculated by dividing the number of double bonds in the lipid species by the number of acyl chains. Finally, the UI of a phospholipid class was calculated as the sum of the UI of individual lipid species in that class (Hong et al., 2002).
2.7. Lipid Oxidation Assay
Lipid oxidation was determined with the 2-thiobarbituric acid reactive substances protocol (TBARS) as described by Ahn et al. (1998) with modifications. Briefly, 1 g of powdered sample was weighed into 50 mL conical tubes to which 4 mL of ultrapure water was added. After samples were homogenized (Model 850, Fisher Sci, Hampton, NH, USA) for 15 s, the samples were centrifuged (2,000 × g for 5 min). Four hundred μL of the supernatant was transferred to a screw cap microcentrifuge tube (1.5 mL) and 0.8 mL of thiobarbituric acid / trichloroacetic acid (TBA/TCA) solution (15% TCA and 20 mM TBA in deionized distilled water) was added. The tubes were vortexed for 5 s before placing samples in a forced air oven (95°C for 1 hr). After cooling samples on ice for 5 min, samples were centrifuged (2,000 × g for 5 min). Six hundred μL of supernatant were transferred to another microcentrifuge tube. Six hundred μL of n-butanol were added to the tube to increase sensitivity and reduce potential sugar interference (Shlafer and Shepard, 1984). The microcentrifuge tube containing the sample and n-butanol mixture were vortexed and centrifuged (2,000 × g for 5 min). Duplicates of 200 μL of supernatant (n-butanol layer) were transferred to black 96-well plates and read on a multi-mode microplate reader using the fluorescent mode at excitation: 540 nm and emission: 590 nm (Synergy HTX, Biotek, Winooski, VT). All 96-well plates had standards (a series of diluted 1,1,3,3-tetraethoxypropane extracted with n-butanol) to calculate standard curves, and each sample was calculated as mg of malonaldehyde per kg of tissue using the standard curve from each plate.
2.8. Statistical Analysis
All analyses were analyzed as a randomized complete block design in SAS (version 9.4, Cary, NC) using the procedure GLIMMIX. Day was used as a fixed effect and individual carcass as a random effect. Tukey and Tukey-Kramer adjustments were used for multiple comparisons at P < 0.05. More than 300 phospholipid species were identified in the data, and a SAS macro was created for performing all the analyses automatically and repeatedly. Throughout the statistical analysis, only significant results (P < 0.05) were reported and presented in this paper.
3. Results and discussion
3.1. Overall lipidomic analysis of the molecular composition of phospholipids
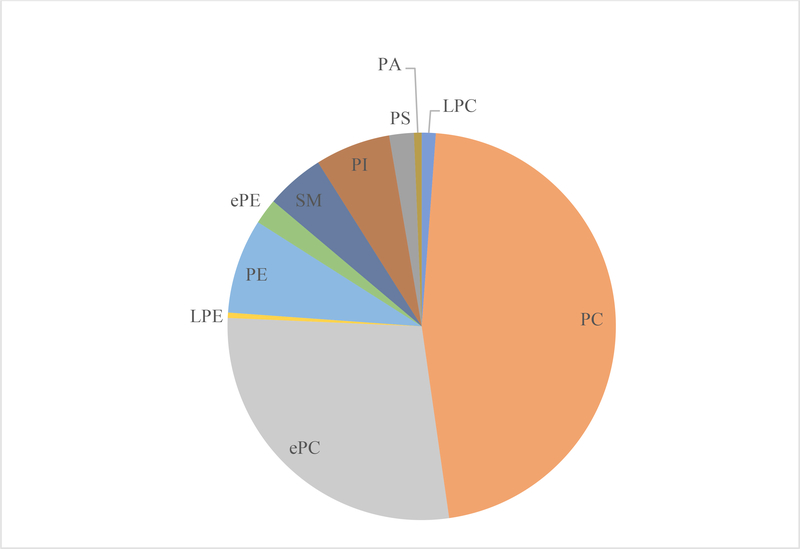
Using ESI MS/MS, the characterization of pork loins membrane lipids revealed 352 different polar lipid species with the major classes being LPC, PC, ePC, SM, LPE, PE, ePE, PI, PS, and PA (Figure 1). The pooled lipid from all 60 pork loin chops samples showed that PC and ePC were the two largest phospholipid classes making up approximately 46.6 and 28.0 mol% respectively of all the classes analyzed in the pork loins across 3 different aging periods. The LPC, PE, ePE, SM, PI and PS classes represented 1.2, 7.9, 2.1, 4.9, 6.3 and 2.1 mol% respectively, while other classes such as LPE and PA were presented in lesser amount (<1 mol%).
Figure 1.
Overall phospholipid and phospholipid hydrolysis product profile (as a % of total phospholipid) of lipid mixture from all 60 pork chops (m. longissimus lumborum; aged for 1, 8, and 21 d) from this study. LPC= lysophosphatidylcholine; PC = phosphatidylcholine; ePC= ether-linked PC; LPE= lysophosphatidylethanolamine; PE = phosphatidylethanolamine; ePE= ether-linked PE; SM=sphingomyelin; PI = phosphatidylinositol; PS = phosphatidylserine; PA = phosphatidic acid.
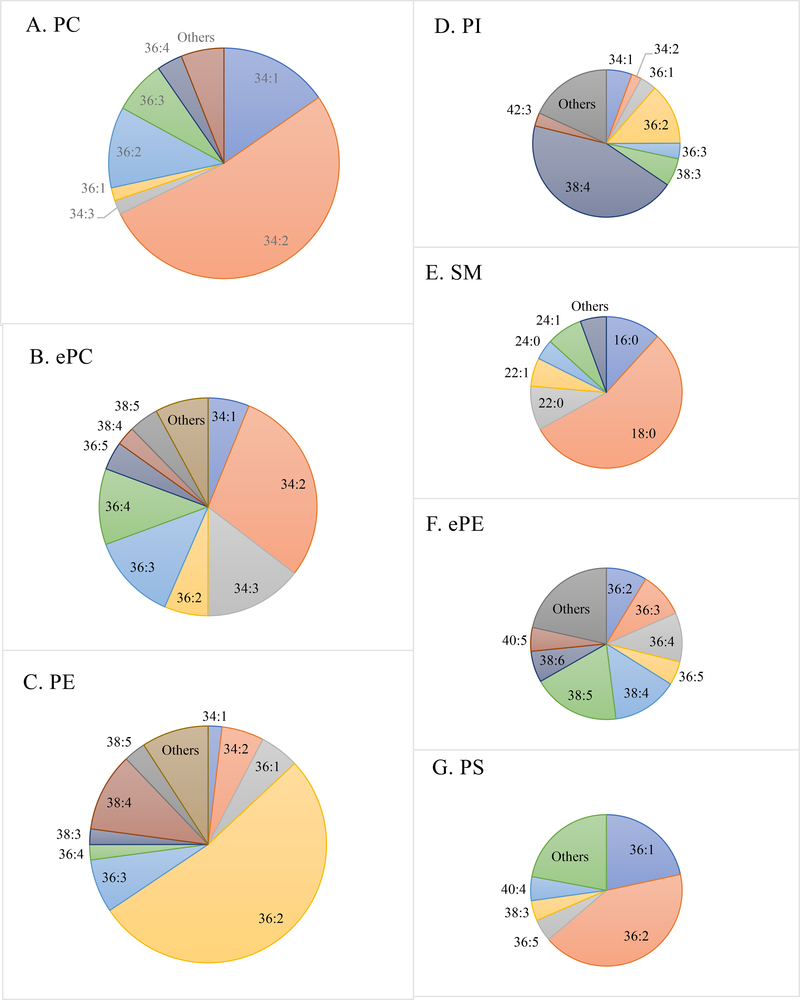
The fatty acid compositions of the phospholipid classes were different (Figure 2). In PC, species containing a total of 34 and 36 acyl carbons were most abundant. In particular, the 34:2 species of PC represented 52.4% of the total PC. The ePC followed the same trend as its counterpart (PC), but contained more species with high unsaturation indices such as 36:4, 36:5, 38:4, 38:5 and 38:6. Again, the 34:2 species in ePC represented 29.3% of the total ePC. On the other hand, PE species containing 36 and 38 carbons were most abundant, and the 36:2 species in PE represented 52.5% of the total PE. Same as ePC, the ePE contained more species with high UI than its counterpart (PE). There is not a single species that dominated the rest of ePE species, with 38:4 and 38:5 combined to represent 32.8% of all ePE species. The SM species had very few double bonds, and the 18:0 species represented 55.1% of the all SM species. Similar to PE, the PI species containing 36 and 38 carbons were most abundant, and the 38:4 species represented nearly half (44.4%) of the total PI. Finally, species containing 36 acyl carbons are the most abundant in PS with 36:2 representing 42.1% of all PS species.
Figure 2.
Composition of various lipid molecular species (total acyl carbons: total double bonds) as a % of total (A) phosphatidylcholine (PC); (B) ether-linked PC (ePC); (C) phosphatidylethanolamine (PE); (D) phosphatidylinositol (PI); (E) sphingomyelin (SM); (F) ether-linked PE (ePE); (G) phosphatidylserine (PS) of lipid mixture from all 60 pork chops (m. longissimus lumborum; aged for 1, 8, and 21 d) from this study.
The fatty acid combinations for the major diacyl molecular species are presented in Table 3. The product-ion analysis revealed that the most common molecular species in PC, 34:2, was almost exclusively (98%) comprised of 16:0 and 18:2, and the most common PE molecular species was 36:2, which was mostly (89%) comprised of 18:0 and 18:2. Finally, the most common molecular species in PI, 38:4, was mostly (92%) comprised of 18:0 and 20:4, and the most common molecular species in PS is 36:2, which was mostly (92%) comprised of 18:0 and 18:2.
Table 3.
Combinations of fatty acids identified by product-ion analysis of the most common lipid molecular species in pork loins arranged roughly from most to least abundant.
| Apparent Lipid molecular species (total acyl carbons: total double bonds) | Fatty acid combinations identified | Relative Abundance (%) |
|---|---|---|
| PC 34:2 |
||
| 16:0/18:2 | 98.35 | |
| 16:1/18:1 | 1.30 | |
| PC 36:4 |
||
| 16:0/20:4 | 50.76 | |
| 18:2/18:2 | 36.38 | |
| PE 36:2 |
||
| 18:0/18:2 | 89.83 | |
| 18:1/18:1 | 3.38 | |
| PE 38:4 |
||
| 18:0/20:4 | 84.91 | |
| PI 36:2 |
||
| 18:0/18:2 | 74.42 | |
| 18:1/18:1 | 16.32 | |
| PI 38:3 |
||
| 18:0/20:3 | 70.66 | |
| PI 38:4 |
||
| 18:0/20:4 | 92.97 | |
| PS 36:1 |
||
| 18:0/18:1 | 52.38 | |
| PS 36:2 |
||
| 18:0/18:2 | 91.93 |
PC = phosphatidylcholine; PE = phosphatidylethanolamine; PI = phosphatidylinositol; PS = phosphatidylserine
Similar phospholipid profiles were obtained by Pérez-Palacios, Antequera, Muriel, Martín, and Ruiz (2007) and Pérez-Palacios, Ruiz, Dewettinck, Trung Le, and Antequera (2010) using solid phase extraction (SPE) and high-performance liquid chromatography (HPLC) in various muscles of rats and Iberian pigs. Unfortunately, the SPE method used to separate the phospholipid classes in those studies was not capable of separating the ether-linked phospholipids from the conventional ester-linked phospholipids. Ether-linked phospholipids constitute approximately 20% of the total phospholipid in various mammalian tissues (Braverman & Moser, 2012). On top of the conventional structural roles, ether-linked phospholipids are thought to function as endogenous antioxidants and may be involved in cell differentiation and signaling pathways (Dean & Lodhi, 2018). However, there is a wide knowledge gap regarding the structures and roles of ether-linked phospholipids in meat quality. A recent study by Broniec et al. (2017) using synthetic ePC in model membrane systems suggested that ePC had an antioxidant effect that could protect unsaturated membrane lipids from oxidation by singlet oxygen. However, this antioxidant effect was still not fully understood as the effect depended on the degree of unsaturation of the sn1 and sn2 chains, the length of the carbon chains, and their conformation in the membrane.
Again, similar to the findings from this study, Sparkes et al. (2010) found that 34:2, 36:2, 38:4 and 36:2 were the prominent species in PC, PE, PI and PS, respectively in mouse intestines utilizing a similar ESI-MS/MS approach. This was further supported by stereospecific analysis of phospholipid by Pérez-Palacios et al. (2007) and Pérez-Palacios et al. (2010) showing that the highest proportion of 16:0 and 18:2 (potentially representing 34:2 as shown by product-ion analysis from this study) were found in PC, and the highest proportion of 20:4 (potentially representing the sn2 position of 38:4 as shown by product-ion analysis from this study) were found in PI from both rat and Iberian pig muscles. A few studies have demonstrated that the phospholipid fraction of meat is the major contributor to lipid oxidation in meat, and they hypothesized that this is mostly due to phospholipids’ known association with polyunsaturated fatty acids (PUFA; Igene et al., 1980; Mottram & Edwards, 1983; Pikul et al., 1984). It is also well established that unsaturated fatty acids esterified to phospholipids are major targets for non-enzymatic free radical attack (Reis & Spickett, 2012). Phospholipid classes containing greater concentration of PUFA such ePE and PI as demonstrated in this study may be more prone to lipid oxidation than the others.
3.2. Influence of Extended Aging on Quantity, Composition and Unsaturation Index of Phospholipids in Pork Loins
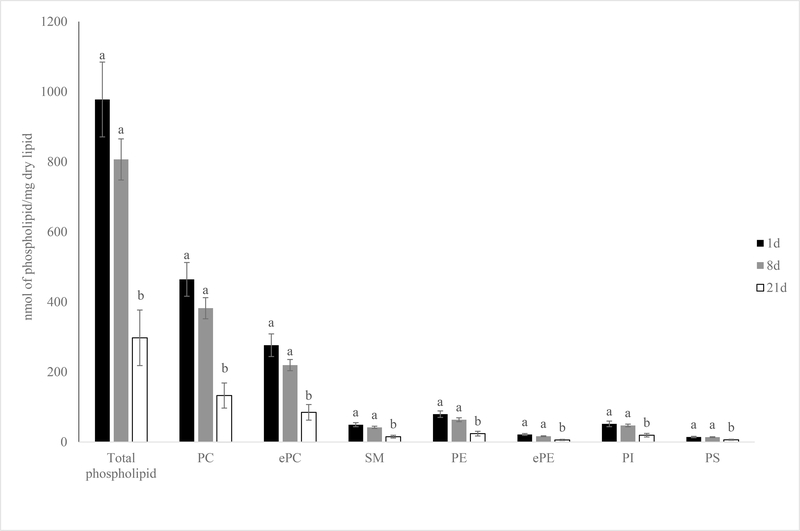
After extended aging, the total amount of phospholipids in 21 days aged pork loins dropped 70.1% (977.7 vs. 297.5 nmol phospholipid/mg lipid) from the level of 1-day aged pork loins (Figure 3; P < 0.01). The drop in total phospholipids amount was mainly due to the decrease in the levels of all major phospholipid classes including PC, ePC, SM, PE, ePE, PI and PS from 1-day aged pork loins to 21 day aged pork loins (Figure 3; P < 0.01). It was interesting to note that the level of total phospholipid and individual phospholipid classes quantity were stable for the most part between 1- and 8-day aged samples (977.7 vs. 806.6 nmol phospholipid/mg lipid; P > 0.05).
Figure 3.
Effects of aging time on phospholipid and lysophospholipid classes amount (nmol of phospholipid class/mg dry lipid) of pork chops aged for 1, 8, or 21 d. Each bar represents the mean ± standard error; n = 60 (three aging periods and 20 replications). Means with different letters within a lipid class are different at P < 0.05. PC = phosphatidylcholine; ePC= ether-linked PC; PE = phosphatidylethanolamine; ePE= ether-linked PE; SM=sphingomyelin; PI = phosphatidylinositol; PS = phosphatidylserine.
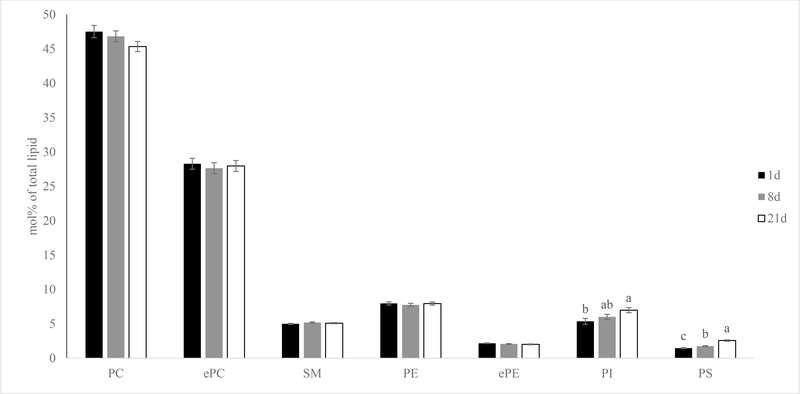
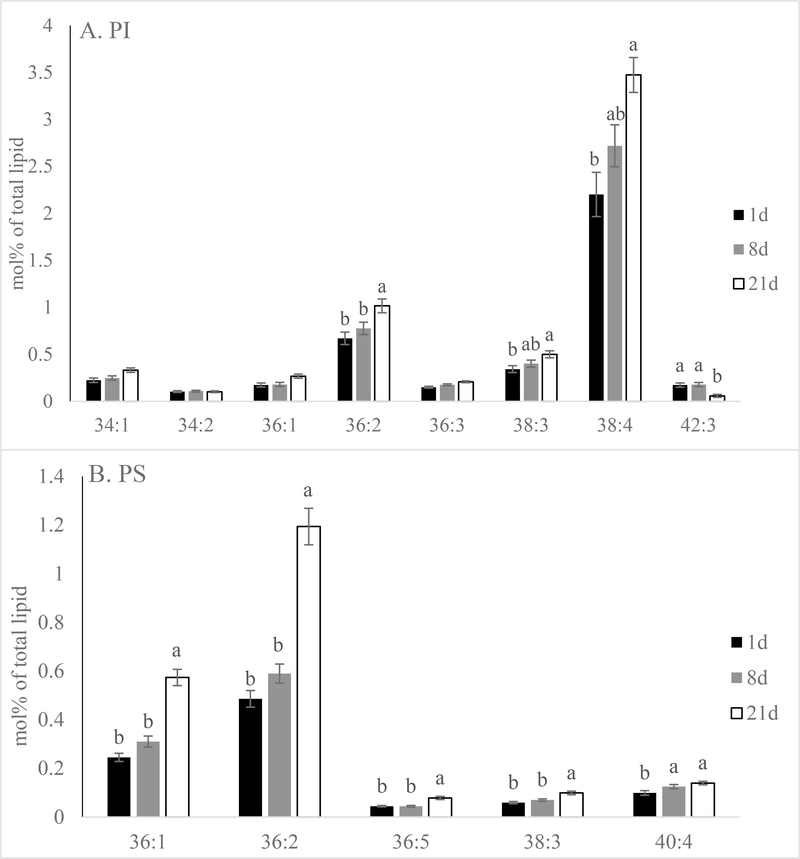
The mol% data revealed that both PI and PS increased in mol% following extended aging in pork loins (Figure 4; P < 0.01). This is mainly due to the total mol% of PI 38:4 and PS 36:2 increased from 2.2 % and 0.5% in 1-day aged samples to 3.5% and 1.2% in 21-day aged samples, respectively (Figure 5; P < 0.01). In addition, many major lipid species in PI and PS such as PI 36:2 (18:0–18:2 as revealed by product-ion analysis) increased in mol%. The only exception was PI 42:3 (quantity was too low to be determined by product-ion analysis), which decreased in mol% following extended aging (P < 0.01). Extended aging did not alter total PC, ePC, SM, PE or ePE mol% nor the majority of the lipid species in these phospholipid classes (data not shown; P > 0.05).
Figure 4.
Effects of aging time on phospholipid classes mol% [(nmol of phospholipid class/nmol of total phospholipid) x100] of pork chops aged for 1, 8, or 21 d. Each bar represents the mean ± standard error; n = 60 (three aging periods and 20 replications). Means with different letters within a lipid class are different at P < 0.05. PC = phosphatidylcholine; ePC= ether-linked PC; PE = phosphatidylethanolamine; ePE= ether-linked PE; SM=sphingomyelin; PI = phosphatidylinositol; PS = phosphatidylserine.
Figure 5.
Effects of aging time on lipid molecular species (total acyl carbons: total double bonds) mol% [(nmol of lipid molecular species/nmol of total phospholipid) x100] of (A) phosphatidylinositol (PI) and (B) phosphatidylserine (PS) of pork chops aged for 1, 8, or 21 d. Each bar represents the mean ± standard error; n = 60 (three aging periods and 20 replications). Means with different letters within a lipid molecular species are different at P < 0.05.
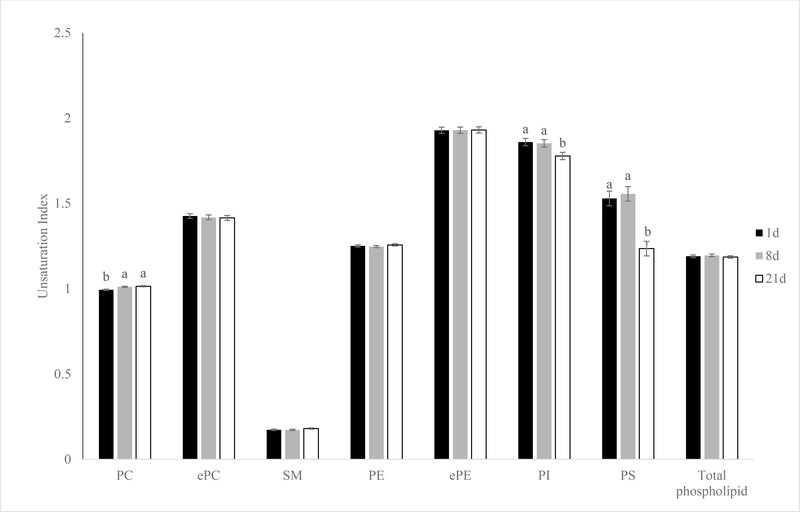
Surprisingly, UI revealed the exact opposite trend as the mol% data (Figure 6). The UI of PI and PS decreased (P < 0.01) from 1 to 21 days of aging in pork loins. There was a slight increase of PC UI after 8 days of aging in pork loins mainly due to the increase of PC 36:4 (P < 0.01). Product-ion analysis revealed that PC 36:4 is comprised of 16:0–20:4 (51%) or 18:2–18:2 (36%). The UI for ePC, SM, PE, ePE and total phospholipid were not altered in any of the aging periods (P > 0.05).
Figure 6.
Effects of aging time on unsaturation index of phospholipid classes of pork chops aged for 1, 8, or 21 d. Each bar represents the mean ± standard error; n = 60 (three aging periods and 20 replications). Means with different letters within a lipid molecular species are different at P < 0.05. PC = phosphatidylcholine; ePC= ether-linked PC; PE = phosphatidylethanolamine; ePE= ether-linked PE; SM=sphingomyelin; PI = phosphatidylinositol; PS = phosphatidylserine.
Ahn, Shimada, and Takahashi (2003) and Shimada, Ahn, and Takahashi (1998) found similar results that the amount of total phospholipid decreased after 72 hours, 10 days, 21 days of postmortem aging in chicken, pork and beef, respectively. Furthermore, Ji and Takahashi (2006) reported that phospholipids were released from sarcoplasmic reticulum (SR) during the aging of pork and beef, and they hypothesized that calcium ions leaked into the sarcoplasm through channels formed by the disappearance of phospholipids. What is interesting about this finding is that phospholipid hydrolyzing enzyme-PLA2 activity is also calcium-dependent (Rangl et al., 2017). It is possible this destabilization of SR membrane from phospholipid hydrolysis was caused by calcium influx activating PLA2, which can also lead to additional exposure of PLA2 throughout the aging process, resulting in less intact phospholipid and more phospholipid hydrolysis products. Kagan (1989) demonstrated that vitamin E can protect membrane against the damaging action of PLA2. Another study by Chao, Domenech-Perez, and Calkins (2017) showed that vitamin E supplementation decelerated the tenderization process (measured by troponin-T degradation), which could be the direct result of membrane protection effect from vitamin E. In contrast, Nutt (1963) showed that the relative percentage of phospholipid in total lipid extracted was not influenced by aging time. Unfortunately, none of those studies differentiated individual phospholipid classes to allow for class to class comparison to the results from this study.
Pérez-Palacios et al. (2010) speculated that PE would be the most susceptible to oxidation, followed by PC and PI, with PS being the least prone to oxidation based on their fatty acid profile. However, PI and PS showed greater overall UI (more double bonds) compared to PC and PE in this current study. This disagreement can be explained by the differences between how this study and Pérez-Palacios et al. (2010) interpreted fatty acid unsaturation. Pérez-Palacios et al. (2010) looked at the proportion of total PUFA within each phospholipid class but did not take into account of the total number of double bonds in each individual PUFA. The PI and PS classes contain many molecular species with very long chain carbon backbone and multiple double bonds such as PI 42:10 and PS 44:10 (Hicks, DeLong, Thomas, Samuel, & Cui, 2006). These very long chain molecular species are not as abundant as major diacyl species like 36:2 and 38:4, but they contributed to the UI of PI and PS in this study due to their high number of double bonds.
In this study, many of these very long chain PUFA within the PI and PS species decreased or were no longer detectable in samples aged 21 days resulting in a decrease in PI and PS UI. On the other hand, more abundant molecular species like PI 38:4 and PS 36:2 retained well throughout extended aging, which contributed to the increase in overall mol% of PI and PS. Other studies have also shown that PI was the least affected phospholipid class when it came to influence of diet (Dannenberger, Nuernberg, Scollan, Ender, & Nuernberg, 2006; McHaney et al., 2013). This is probably due to the role of PI as a secondary messenger in cell signal transduction, and the maintenance of PI composition is particularly important to membrane hemostasis (Williams & Maunder, 1992).
Revealing the specific fatty acid compositions of these phospholipids provided greater granularity and was beneficial to elucidating what happened during aging. There was an observable trend that phospholipid species containing PUFA 18:2 and 20:4 in PC, PI and PS were less likely to be hydrolyzed after extended aging. Alma M. Astudillo, Balboa, and Balsinde (2019) pointed out that multiple PLA2 enzymes co-exist in a single cell, and each PLA2 type exhibits different headgroup and/or fatty acid preferences for hydrolysis, The availability of free fatty acid (FFA) is well established to serve as a limiting factor for additional PLA2 activity (Alma M Astudillo, Balgoma, Balboa, & Balsinde, 2012; Pérez-Chacón, Astudillo, Balgoma, Balboa, & Balsinde, 2009). The typical U.S. commercial swine diet consists of high proportions of corn, soybean meal and/or dried distillers grains, which is known to contain a significant amount (>50%) of 18:2 (Kerr, Kellner, & Shurson, 2015). In addition, 20:4 is synthesized from 18:2 (Norris & Carr, 2007). Perhaps, there was an accumulation of 18:2 and 20:4 in the form of FFA from various sources in the pork loins, which restricted specific PLA2 activity to hydrolyze intact phospholipids that contain 18:2 and 20:4.
3.3. Influence of Extended Aging on Phospholipid Hydrolysis Products Content and Composition, and Lipid Oxidation in Pork Loins
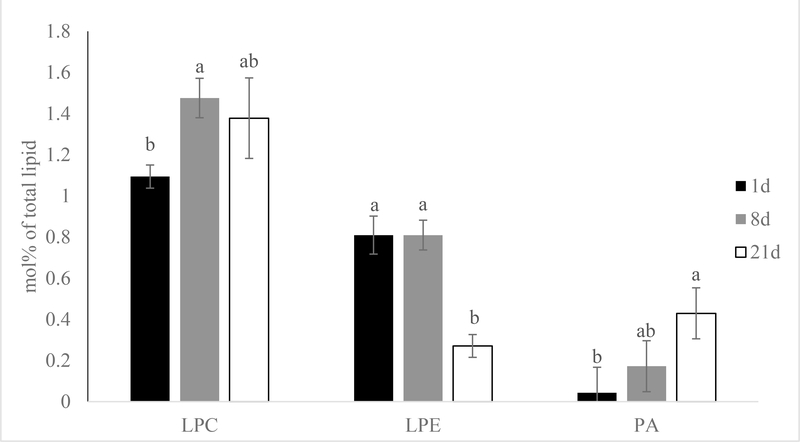
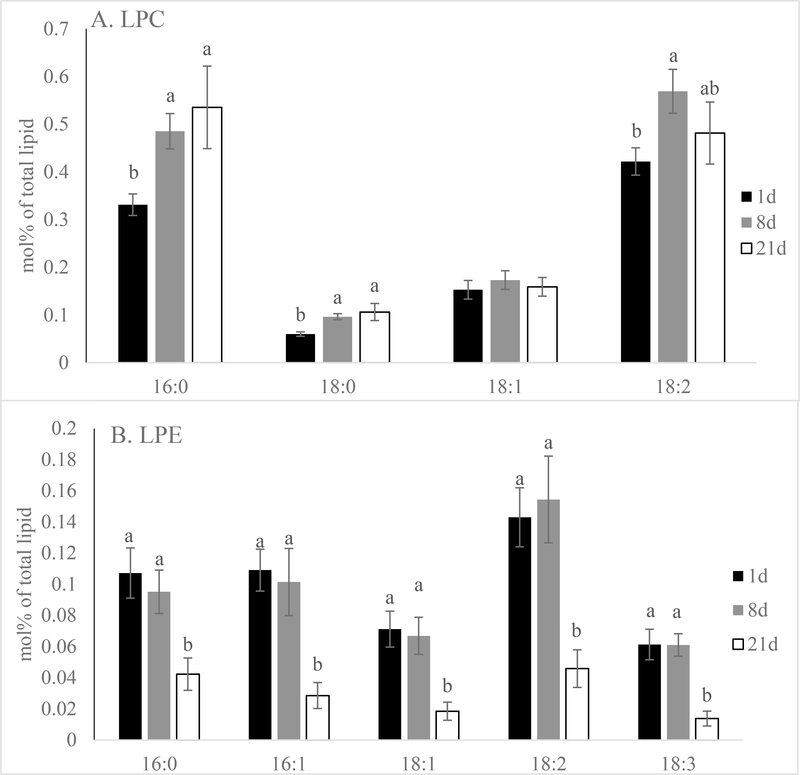
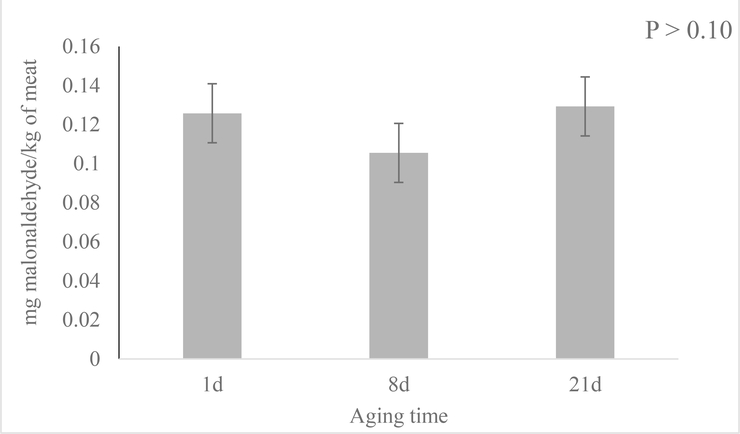
The mol% data also showed that phospholipid hydrolysis product LPC mol% rose quickly after short term aging (8 days) and remained constant through the rest of the aging period (Figure 7; P < 0.01). Molecular species like LPC 16:0, 18:0 and 18:2 followed the same trend which increased after 8 days of aging and stayed constant for the rest of the aging period (Figure 8; P < 0.01). Conversely, overall LPE mol% was unaltered after short term aging, but decreased between 8- and 21-day aged pork loins (Figure 6; P < 0.01). All the major molecular species like LPE 16:0, 16:1, 18:1, 18:2, 18:3 again followed the same trend as the total LPE (Figure 7; P < 0.01). Although there was very little PA presented in the samples, the mol% of PA increased from 1 to 21-day aged pork loins. Unfortunately, the quantity of PA was insufficient to accurately determine the mol% of each molecular species. Although significant phospholipid alteration was documented in aged pork loins in this study, malonaldehyde in muscle tissue level remained low (0.12 mg malonaldehyde/kg muscle tissue) and was not influenced by aging (Figure 9; P > 0.10).
Figure 7.
Effects of aging time on phospholipid hydrolysis product mol% [(nmol of phospholipid hydrolysis product/nmol of total phospholipid) x100] of pork chops aged for 1, 8, or 21 d. Each bar represents the mean ± standard error; n = 60 (three aging periods and 20 replications). Means with different letters within a lipid class are different at P < 0.05. LPC= lysophosphatidylcholine; LPE= lysophosphatidylethanolamine; PA = phosphatidic acid.
Figure 8.
Effects of aging time on lipid molecular species mol% [(nmol of lipid molecular species/nmol of total phospholipid) x100] of (A) lysophosphatidylcholine (LPC) and (B) lysophosphatidylethanolamine (LPE) of pork chops aged for 1, 8, or 21 d. Each bar represents the mean ± standard error; n = 60 (three aging periods and 20 replications). Means with different letters within a lipid molecular species are different at P < 0.05.
Figure 9.
Effects of aging time on lipid oxidation (malonaldehyde mg/kg of meat) of pork chops aged for 1, 8, or 21 d. Each bar represents the mean ± standard error; n = 60 (three aging periods and 20 replications.
Similar to this current study, several lipidomic studies focusing on diseases and phospholipid profile interactions denoted a strong negative correlation of intact phospholipids and phospholipid hydrolysis products (Braun et al., 2009; Sparkes et al., 2010). Braun et al. (2009) found an increase in LPC to PC ratio in ulcerative colitis patients, while Sparkes et al. (2010) showed that mouse model of ischemia/reperfusion increased LPC and free fatty acids with an associated decrease in PC. Like free fatty acids, LPC is a direct product of PLA2 enzyme activity on the hydrolysis of an acyl group from the sn1 position of PC (Yeagle, 2016). The increase of LPC mol% documented in this study (8 days postmortem) is an evidence of PLA2 activity. However, LPC level stayed constant for the rest of the aging period, and we are uncertain if this phenomenon was caused by product inhibition from increased level of LPC or lipid extraction inefficiency. In this experiment, we took a small portion of total extracted lipid from the bottom chloroform layer instead of the traditional quantitative collection of the entire bottom chloroform layer for phospholipid analysis, and we did not utilize phospholipid internal standards to compensate for potential differences in phospholipid solubility in the solvent matrices. Future studies in this area should include phospholipid internal standards such as phospholipid labeled with stable isotopes or with unique fatty acid compositions to ensure lipid extraction efficiency.
On the other hand, this present study reported a decrease in LPE and an increase in PA mol% after extended aging of pork loin. The finding is interesting as other studies have found PLD using PC as a substrate to generate PA, and PLD activity was inhibited by LPE (Ryu, Karlsson, Özgen, & Palta, 1997; Yu & Rasenick, 2012). It is possible that an unknown mechanism has taken place to further breakdown LPE after extended aging of pork loin, which resulted in the increase of PLD activity and PA production.
Finally, malonaldehyde, an end product of lipid peroxidation that mainly occurs through the action of reactive oxygen species, is a well-defined marker for oxidative damage in meat and meat products (Min & Ahn, 2005). However, pork loin malonaldehyde content was not altered during the aging process in this study. This result was expected as many other studies have shown similar lipid oxidation results for pork loin chops (Kim, Kim, Seo, Setyabrata, & Kim, 2018) and beef striploin steaks (Hart, Ribeiro, Henriott, Herrera, & Calkins, 2019) aged in vacuum packaging without going through a retail display process. Sun et al. (2018) described phospholipid deterioration as the combined effect of phospholipid activity (enzymatic) and phospholipid oxidation (non-enzymatic). The secondary lipid oxidation results suggested that the phospholipid alteration found in this study was likely not generated by non-enzymatic oxidation, but mainly through enzymatic activities. However, primary oxidation products (i.e. hydroperoxides) were not measured, and their assumed low levels will need to be confirmed in future studies.
4. Conclusions
Using ESI-MS/MS, this study revealed phospholipid profile alterations of pork loins from 3 aging periods. The data also indicated that the majority of phospholipids in pork loins may maintain integrity over short period aging (1–8 days). Among the phospholipid classes, PI and PS were more resistant to the hydrolysis compared with the others due to their ability to preserve lipid species containing 18:2 and 20:4. In this current study, we speculated that the phospholipid alteration during aging is the result of PLA2 activity activated by postmortem calcium influx from the SR, which resulted a surge of LPC as seen in this study. This possible aging-induced phospholipid profile alterations might shed light on a previously unknown mechanism of meat aging, and additional investigations are necessary to define the role of phospholipid modifications in fresh pork quality, shelf-life and potential influence on human health.
5. Acknowledgements
The authors would like to thank Dr. Ruth Welti and Mary Roth for the technical assistance. The lipid analyses described in this work were performed at the Kansas Lipidomics Research Center Analytical Laboratory. Instrument acquisition and lipidomics method development was supported by the National Science Foundation, K-IDeA Networks of Biomedical Research Excellence (INBRE) of National Institute of Health (P20GM103418), and Kansas State University. Partial funding from this research was provided from the Iowa Agricultural and Home Economics Experiment Station project no. 3721.
Literature Cited
- Ahn DH, Shimada K, & Takahashi K (2003). Relationship between weakening of Z‐disks and liberation of phospholipids during postmortem aging of pork and beef. Journal of Food Science, 68(1), 94–98. [Google Scholar]
- Ahn DU, Olson DG, Jo C, Chen X, Wu C, & Lee JI (1998). Effect of muscle type, packaging, and irradiation on lipid oxidation, volatile production, and color in raw pork patties. Meat Science, 49(1), 27–39. doi:Doi 10.1016/S0309-1740(97)00101-0 [DOI] [PubMed] [Google Scholar]
- Astudillo AM, Balboa MA, & Balsinde J (2019). Selectivity of phospholipid hydrolysis by phospholipase A2 enzymes in activated cells leading to polyunsaturated fatty acid mobilization. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids, 1864(6), 772–783. doi: 10.1016/j.bbalip.2018.07.002 [DOI] [PubMed] [Google Scholar]
- Astudillo AM, Balgoma D, Balboa MA, & Balsinde J (2012). Dynamics of arachidonic acid mobilization by inflammatory cells. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids, 1821(2), 249–256. [DOI] [PubMed] [Google Scholar]
- Bermingham EN, Reis MG, Subbaraj AK, Cameron-Smith D, Fraser K, Jonker A, & Craigie CR (2018). Distribution of fatty acids and phospholipids in different table cuts and co-products from New Zealand pasture-fed Wagyu-dairy cross beef cattle. Meat Science, 140, 26–37. doi: 10.1016/j.meatsci.2018.02.012 [DOI] [PubMed] [Google Scholar]
- Braun A, Treede I, Gotthardt D, Tietje A, Zahn A, Ruhwald R, … Erben G (2009). Alterations of phospholipid concentration and species composition of the intestinal mucus barrier in ulcerative colitis: a clue to pathogenesis. Inflammatory Bowel Diseases, 15(11), 1705–1720. [DOI] [PubMed] [Google Scholar]
- Braverman NE, & Moser AB (2012). Functions of plasmalogen lipids in health and disease. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, 1822(9), 1442–1452. doi: 10.1016/j.bbadis.2012.05.008 [DOI] [PubMed] [Google Scholar]
- Broniec A, Żądło A, Pawlak A, Fuchs B, Kłosiński R, Thompson D, & Sarna T (2017). Interaction of plasmenylcholine with free radicals in selected model systems. Free Radical Biology and Medicine, 106, 368–378. [DOI] [PubMed] [Google Scholar]
- Brügger B, Erben G, Sandhoff R, Wieland FT, & Lehmann WD (1997). Quantitative analysis of biological membrane lipids at the low picomole level by nano-electrospray ionization tandem mass spectrometry. Proceedings of the National Academy of Sciences, 94(6), 2339–2344. doi: 10.1073/pnas.94.6.2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MD, Domenech-Perez KI, & Calkins CR (2017). Feeding vitamin E may reverse sarcoplasmic reticulum membrane instability caused by feeding wet distillers grains plus solubles to cattle. The Professional Animal Scientist, 33, 12–23. [Google Scholar]
- Dannenberger D, Nuernberg G, Nuernberg K, Will K, Schauer N, & Schmicke M (2017). Effects of diets supplemented with n–3 or n–6 PUFA on pig muscle lipid metabolites measured by non-targeted LC–MS lipidomic profiling. Journal of Food Composition and Analysis, 56, 47–54. doi: 10.1016/j.jfca.2016.11.015 [DOI] [Google Scholar]
- Dannenberger D, Nuernberg G, Scollan N, Ender K, & Nuernberg K (2006). Diet alters the fatty acid composition of individual phospholipid classes in beef muscle. Journal of Agricultural and Food Chemistry, 55(2), 452–460. doi: 10.1021/jf061793x [DOI] [PubMed] [Google Scholar]
- Dean JM, & Lodhi IJ (2018). Structural and functional roles of ether lipids. Protein & cell, 9(2), 196–206. doi: 10.1007/s13238-017-0423-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaiah SP, Roth MR, Baughman E, Li M, Tamura P, Jeannotte R, … Wang X (2006). Quantitative profiling of polar glycerolipid species from organs of wild-type Arabidopsis and a phospholipase Dα1 knockout mutant. Phytochemistry, 67(17), 1907–1924. doi: 10.1016/j.phytochem.2006.06.005 [DOI] [PubMed] [Google Scholar]
- Fennema OR, Damodaran S, & Parkin KL (2017). Introduction to food chemistry. Fennema’s Food Chemistry (Fifth Edition). (pp. 1–16): CRC Press. [Google Scholar]
- Folch J, Lees M, & Sloane Stanley GH (1957). A simple method for the isolation and purification of total lipides from animal tissues. Journal of Biological Chemistry, 226(1), 497–509. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/13428781 [PubMed] [Google Scholar]
- Hart KB, Ribeiro FA, Henriott ML, Herrera NJ, & Calkins CR (2019). Quality effects on beef strip steaks from cattle fed high-protein corn distillers grains and other ethanol by-products. Journal of Animal Science, 97(5), 2087–2098. doi: 10.1093/jas/skz086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks AM, DeLong CJ, Thomas MJ, Samuel M, & Cui Z (2006). Unique molecular signatures of glycerophospholipid species in different rat tissues analyzed by tandem mass spectrometry. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids, 1761(9), 1022–1029. doi: 10.1016/j.bbalip.2006.05.010 [DOI] [PubMed] [Google Scholar]
- Holmer SF, McKeith RO, Boler DD, Dilger AC, Eggert JM, Petry DB, … Killefer J (2009). The effect of pH on shelf-life of pork during aging and simulated retail display. Meat Science, 82(1), 86–93. doi: 10.1016/j.meatsci.2008.12.008 [DOI] [PubMed] [Google Scholar]
- Hong MY, Chapkin RS, Barhoumi R, Burghardt RC, Turner ND, Henderson CE, … Murphy MEJC (2002). Fish oil increases mitochondrial phospholipid unsaturation, upregulating reactive oxygen species and apoptosis in rat colonocytes. Journal of Carcinogenesis, 23(11), 1919–1926. [DOI] [PubMed] [Google Scholar]
- Igene J, Pearson A, Dugan L Jr, & Price J (1980). Role of triglycerides and phospholipids on development of rancidity in model meat systems during frozen storage. Journal of Food Chemistry, 5(4), 263–276. [Google Scholar]
- Ji JR, & Takahashi K (2006). Changes in concentration of sarcoplasmic free calcium during post-mortem ageing of meat. Meat Science, 73(3), 395–403. doi: 10.1016/j.meatsci.2005.09.010 [DOI] [PubMed] [Google Scholar]
- Kagan VE (1989). Tocopherol stabilizes membrane against phospholipase A, free fatty acids, and lysophospholipids A. Annals of the New York Academy of Sciences, 570(1), 121–135. [DOI] [PubMed] [Google Scholar]
- Kerr BJ, Kellner TA, & Shurson GC (2015). Characteristics of lipids and their feeding value in swine diets. Journal of Animal Science and Biotechnology, 6(1), 30–30. doi: 10.1186/s40104-015-0028-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HW, Kim JH, Seo JK, Setyabrata D, & Kim YH (2018). Effects of aging/freezing sequence and freezing rate on meat quality and oxidative stability of pork loins. Meat Science, 139, 162–170. doi: 10.1016/j.meatsci.2018.01.024 [DOI] [PubMed] [Google Scholar]
- McHaney AM, Welti R, Roth MR, Dinnetz JM, Furtney SR, Pendergraft JS, … Minton JE (2013). Omega-3 fatty acid supplementation affects selected phospholipids in peripheral white blood cells and in plasma of full-sized and miniature mares. Journal of Equine Veterinary Science, 33(10), 779–786. doi: 10.1016/j.jevs.2012.12.008 [DOI] [Google Scholar]
- Melody J, Lonergan S, Rowe L, Huiatt T, Mayes M, & Huff-Lonergan E (2004). Early postmortem biochemical factors influence tenderness and water-holding capacity of three porcine muscles. Journal of Animal Science, 82(4), 1195–1205. [DOI] [PubMed] [Google Scholar]
- Metcalfe LD, Schmitz AA, & Pelka JR (1966). Rapid preparation of fatty acid esters from lipids for gas chromatographic analysis. Analytical Chemistry, 38(3), 514–515. [Google Scholar]
- Min B, & Ahn D (2005). Mechanism of lipid peroxidation in meat and meat products–A review. Food Science and Biotechnology, 14(1), 152–163. [Google Scholar]
- Mottram D, & Edwards R (1983). The role of triglycerides and phospholipids in the aroma of cooked beef. Journal of the Science of Food and Agriculture, 34(5), 517–522. [Google Scholar]
- Norris DO, & Carr JA (2013). Synthesis, Metabolism, and Actions of Bioregulators. Vertebrate Endocrinology (Fifth Edition) (pp. 41–91). San Diego: Academic Press. [Google Scholar]
- Nutt SM (1963). Effect of Finish and Aging Time on the Phospholipid Constituents of Beef. Masters Thesis. University of Tennessee - Knoxville, Retrieved from https://trace.tennessee.edu/utk_gradthes/3023 [Google Scholar]
- Panasenko OM, Vakhrusheva T, Tretyakov V, Spalteholz H, & Arnhold J (2007). Influence of chloride on modification of unsaturated phosphatidylcholines by the myeloperoxidase/hydrogen peroxide/bromide system. Chemistry Physics of Lipids, 149(1–2), 40–51. [DOI] [PubMed] [Google Scholar]
- Pérez-Chacón G, Astudillo AM, Balgoma D, Balboa MA, & Balsinde J (2009). Control of free arachidonic acid levels by phospholipases A2 and lysophospholipid acyltransferases. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids, 1791(12), 1103–1113. [DOI] [PubMed] [Google Scholar]
- Pérez-Palacios T, Antequera T, Muriel E, Martín D, & Ruiz J (2007). Stereospecific analysis of phospholipid classes in skeletal muscle from rats fed different fat sources. Journal of Agricultural and Food Chemistry, 55(15), 6191–6197. doi: 10.1021/jf071354d [DOI] [PubMed] [Google Scholar]
- Pérez-Palacios T, Ruiz J, Dewettinck K, Trung Le T, & Antequera T (2010). Individual phospholipid classes from iberian pig meat as affected by diet. Journal of Agricultural and Food Chemistry, 58(3), 1755–1760. doi: 10.1021/jf9029805 [DOI] [PubMed] [Google Scholar]
- Pikul J, Leszczynski DE, & Kummerow FA (1984). Relative role of phospholipids, triacylglycerols, and cholesterol esters on malonaldehyde formation in fat extracted from chicken meat. Journal of Food Science, 49(3), 704–708. [Google Scholar]
- Rangl M, Rima L, Klement J, Miyagi A, Keller S, & Scheuring S (2017). Real-time visualization of phospholipid degradation by outer membrane phospholipase A using high-speed atomic force microscopy. Journal of Molecular Biology, 429(7), 977–986. doi: 10.1016/j.jmb.2017.03.004 [DOI] [PubMed] [Google Scholar]
- Reis A, & Spickett CM (2012). Chemistry of phospholipid oxidation. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1818(10), 2374–2387. [DOI] [PubMed] [Google Scholar]
- Ryu SB, Karlsson BH, Özgen M, & Palta JP (1997). Inhibition of phospholipase D by lysophosphatidylethanolamine, a lipid-derived senescence retardant. Proceedings of the National Academy of Sciences, 94(23), 12717–12721. doi: 10.1073/pnas.94.23.12717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte MD, Johnson LG, Zuber EA, Patterson BM, Outhouse AC, Fedler CA, … Lonergan SM (2019). Influence of postmortem aging and post-aging freezing on pork loin quality attributes. Meat and Muscle Biology, 3(1), 313–323. doi: 10.22175/mmb2019.05.0015 [DOI] [Google Scholar]
- Shimada K. i., Ahn DH, & Takahashi K (1998). Liberation of phospholipids from z-disks of chicken skeletal muscle myofibrils by 0.1 mm calcium ions: weakening mechanism for z-disks during post-mortem aging of meat. Bioscience, Biotechnology, and Biochemistry, 62(5), 919–926. doi: 10.1271/bbb.62.919 [DOI] [PubMed] [Google Scholar]
- Shiva S, Vu HS, Roth MR, Zhou Z, Marepally SR, Nune DS, … Welti R (2013). Lipidomic Analysis of Plant Membrane Lipids by Direct Infusion Tandem Mass Spectrometry. Methods in Molecular Biology, 1009. 79–91. doi: 10.1007/978-1-62703-401-2_9 [DOI] [PubMed] [Google Scholar]
- Sparkes BL, Slone EEA, Roth M, Welti R, & Fleming SD (2010). Intestinal lipid alterations occur prior to antibody-induced prostaglandin E2 production in a mouse model of ischemia/reperfusion. Biochimica et Biophysica Acta -Molecular Cell Biology of Lipids, 1801(4), 517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun GY, Simonyi A, Fritsche KL, Chuang DY, Hannink M, Gu Z, … Beversdorf DQ (2018). Docosahexaenoic acid (DHA): An essential nutrient and a nutraceutical for brain health and diseases. Prostaglandins, Leukotrienes and Essential Fatty Acids, 136, 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi DK, Hollywood KA, Rattray NJ, Ward H, Trivedi DK, Greenwood J, … Goodacre R (2016). Meat, the metabolites: an integrated metabolite profiling and lipidomics approach for the detection of the adulteration of beef with pork. Analyst, 141(7), 2155–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welti R, Li W, Li M, Sang Y, Biesiada H, Zhou HE, … Wang X (2002). Profiling membrane lipids in plant stress responses: Role of phospholipase dα in freezing-induced lipid changes in arabidopsis. Journal of Biological Chemistry, 277(35), 31994–32002. doi: 10.1074/jbc.M205375200 [DOI] [PubMed] [Google Scholar]
- Wheeler T, Shackelford S, & Koohmaraie M (2000). Variation in proteolysis, sarcomere length, collagen content, and tenderness among major pork muscles. Journal of Animal Science, 78(4), 958–965. [DOI] [PubMed] [Google Scholar]
- Williams CM, & Maunder K (1992). Effect of dietary fatty acid composition on inositol-, choline- and ethanolamine-phospholipids of mammary tissue and erythrocytes in the rat. British Journal of Nutrition, 68(1), 183–193. doi: 10.1079/BJN19920076 [DOI] [PubMed] [Google Scholar]
- Wood JD, Enser M, Fisher AV, Nute GR, Sheard PR, Richardson RI, … Whittington FM (2008). Fat deposition, fatty acid composition and meat quality: A review. Meat Science, 78(4), 343–358. doi:DOI 10.1016/j.meatsci.2007.07.019 [DOI] [PubMed] [Google Scholar]
- Xiao S, Gao W, Chen Q-F, Chan S-W, Zheng S-X, Ma J, … Chye M-L (2010). Overexpression of Arabidopsis acyl-CoA binding protein ACBP3 promotes starvation-induced and age-dependent leaf senescence. The Plant Cell, 22(5), 1463–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeagle PL (2016). Membrane Proteins. The Membranes of Cells (Third Edition) (pp. 219–268). Boston: Academic Press. [Google Scholar]
- Yin M-C, & Faustman C (1994). The influence of microsomal and cytosolic components on the oxidation of myoglobin and lipid in vitro. Journal of Food Chemistry, 51(2), 159–164. [Google Scholar]
- Yu J-Z, & Rasenick MM (2012). Chapter 2 - Receptor signaling and the cell biology of synaptic transmission. In Aminoff MJ, Boller F, & Swaab DF (Eds.), Handbook of Clinical Neurology (Vol. 106, pp. 9–35): Elsevier. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Marepally SR, Nune DS, Pallakollu P, Ragan G, Roth MR, … Welti R (2011). LipidomeDB Data Calculation Environment: Online processing of direct-infusion mass spectral data for lipid profiles. Lipids, 46(9), 879–884. doi: 10.1007/s11745-011-3575-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu LG, & Brewer MS (1998). Metmyoglobin reducing capacity of fresh normal, pse, and dfd pork during retail display. Journal of Food Science, 63(3), 390–393. doi:doi: 10.1111/j.1365-2621.1998.tb15749.x [DOI] [Google Scholar]