Abstract
Absent in melanoma 2 (AIM2) is a cytoplasmic sensor that upon recognizing double-stranded DNA assembles with apoptosis-associated speck-like protein containing a CARD (ASC) and procaspase-1 to form the multi-protein complex AIM2 inflammasome. Double-stranded DNA from bacterial, viral, or host cellular origins triggers AIM2 inflammasome assembly and activation, ultimately resulting in secretion of proinflammatory cytokines and pyroptotic cell death in order to eliminate microbial infection. Many pathogens therefore evade or suppress AIM2 inflammasome to establish infection. On the other hand, AIM2 activation is tightly controlled by multiple cellular factors to prevent autoinflammation. Extensive structural studies have captured the molecular details of multiple steps in AIM2 inflammasome assembly. The structures collectively revealed a nucleated polymerization mechanism that not only pervades each step of AIM2 inflammasome assembly, but also underlies assembly of other inflammasomes and complexes in immune signaling. In this article, we briefly review the identification of AIM2 as a cytoplasmic DNA sensor, summarize the importance of AIM2 inflammasome in infections and diseases, and discuss the molecular mechanisms of AIM2 assembly, activation, and regulation using recent cellular, biochemical, and structural results.
Keywords: AIM2 inflammasome, DNA binding, death domain, PYD, HIN domain, helical filament, nucleated polymerization
Inflammasomes are high molecular weight multimeric protein complexes present in the cytosol of stimulated immune cells that serve as the molecular platforms for activating proinflammatory caspases-1 and-11 (Kayagaki et al., 2011; Martinon et al., 2002). Activated caspase-1 then proceeds to cleave cytokines such as pro-interleukin 1β (pro-IL-1β) and pro-IL-18, leading to their maturation and eventual secretion (Van Opdenbosch and Lamkanfi, 2019). Caspase-1 and caspase-11 also cleave gasdermin family proteins to induce pyroptosis, a highly inflammatory form of cell death (Van Opdenbosch and Lamkanfi, 2019). Inflammasome activation is an integral component of innate immune response that is critical for pathogen clearance or damaged cell removal. Multiple inflammasomes have been identified since the pioneering work of Tschopp in 2002 (Martinon et al., 2002). Each inflammasome is defined by a unique pattern recognition receptor that can recognize and respond to pathogen-associated molecular patterns (PAMPs) or endogenous danger-associated molecular patterns (DAMPs) in the host cell cytosol. Depending on the structural features, the inflammasome sensors are grouped into: (1) nucleotide-binding domain-like receptors (NLRs), (2) absent in melanoma 2 (AIM2)-like receptors (ALRs), and (3) pyrin. Upon recognizing the ligand, the sensor is activated, which then oligomerizes and recruits an adaptor protein, often the apoptosis-associated speck-like protein containing a CARD (ASC). ASC consists of two death domains, a pyrin domain (PYD) at the N-terminus and a caspase recruitment domain (CARD) at the C-terminus. ASC then recruits procaspase-1, leading to its proximity-induced auto-processing to produce active caspase-1. Dysregulated activation of inflammasome is linked to cancer, autoimmunity, metabolic diseases, and neurodegenerative disorders (de Zoete et al., 2014; Guo et al., 2015; Lamkanfi and Dixit, 2012; Man et al., 2016a; Van Opdenbosch and Lamkanfi, 2019). Hence, inflammasome activation is tightly regulated to provide defense against pathogenic insults yet to avoid damage to the host tissues.
This review focuses on the founding member of the ALR family, absent in melanoma 2 (AIM2). AIM2 belongs to the PYHIN family that contains one pyrin domain (PYD) at the N-terminus and one or two hematopoietic, interferon inducible, and nuclear (HIN) domains at the C-terminus (Figure 1). In addition to AIM2, PYHIN family also includes interferon-inducible protein 16 (IFI16) in human and p204 in mouse. AIM2 was initially discovered as an interferon-inducible tumor suppressor (DeYoung et al., 1997) but was later identified as a cytosolic double-stranded DNA (dsDNA) sensor that can assemble into inflammasome with ASC and procaspase-1 (Burckstummer et al., 2009; Fernandes-Alnemri et al., 2009; Hornung et al., 2009; Roberts et al., 2009). AIM2 binds to dsDNA via its C-terminal HIN domain, which frees up the N-terminal PYD domain to engage ASC. ASC then recruits procaspase-1, forming the AIM2 inflammasome. The AIM2 inflammasome core structure is the first inflammasome structure studied in atomic details (Lu et al., 2014b), serving as a prototype to understanding assembly and activation of other inflammasomes. Surprisingly, AIM2 inflammasome components do not assemble in a simple stoichiometric way, but rather through filament formation nucleated by upstream molecules. Briefly, binding of AIM2 to dsDNA via the HIN domain relieves it from an autoinhibitory state or lowers the concentration threshold for AIM2 oligomerization (Jin et al., 2012; Morrone et al., 2015). The proximity of multiple AIM2 molecules on dsDNA facilitates formation of AIM2 PYD helical filament (Lu et al., 2015; Morrone et al., 2015). The AIM2 PYD filament then serves as the “seed” to nucleate ASC helical filaments via the interaction between AIM2 PYD and ASC PYD domains (Lu et al., 2014b). The CARD domains in ASC subsequently self organize into filaments, nucleate the filamentation of procaspase-1, and activates caspase-1 via induced proximity (Lu et al., 2016). This “nucleated polymerization” has been shown to be prevalent in other inflammasome and immune complexes in general. In this article, we are going to review our current understanding on AIM2 inflammasome from both biological and structural perspectives.
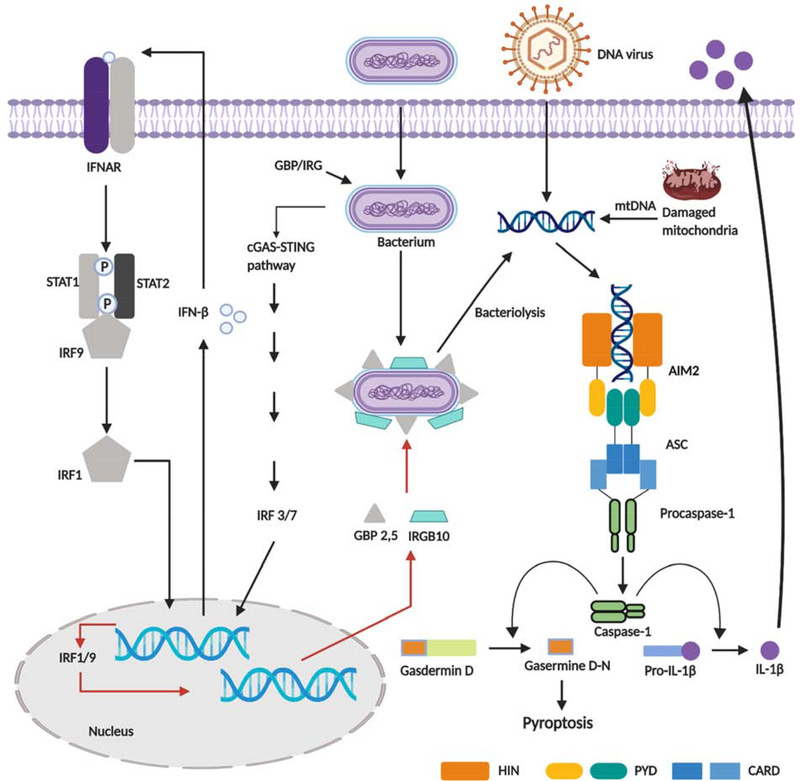
Figure 1.
Diagram of AIM2 inflammasome activation. Double-stranded DNA released from bacteria, viruses, or damaged mitochondria binds to AIM2, inducing assembly of AIM2 inflammasome that consists of AIM2, ASC, and procaspase-1. Activated caspase-1 then cleaves pro-interleukin-1β and gasdermins to mediate inflammation and pyroptosis, respectively. Release of bacterial DNA into the cytoplasm relies on bacteriolysis mediated by interferon-inducible GTPases, whose expression is activated by cGAS-STING axis, also activated by cytoplasmic dsDNA.
1. AIM2 forms inflammasome and activates caspase-1 upon dsDNA sensing in cytosol
The first hint of a dsDNA sensing inflammasome came from studies on virus triggered proinflammatory responses. It was found that transfected cytosolic bacterial, viral, and mammalian (host) DNA results in inflammasome activation (Muruve et al., 2008). Such activation depends on ASC but not NLRP3, a well-known inflammasome component. Furthermore, this dsDNA sensing inflammasome formation functions independently of Toll-like receptor 9 (TLR9), another well characterized DNA sensor, albeit working in the endolysosomes.
In 2009, four groups independently identified AIM2 as a cytosolic dsDNA sensor that forms inflammasome with ASC, activates caspase-1, and eventually leads to maturation of pro-IL-1β or cell death (Burckstummer et al., 2009; Fernandes-Alnemri et al., 2009; Hornung et al., 2009; Roberts et al., 2009). The researchers adopted similar approaches, namely searching for proteins that contain both DNA binding domains and the PYD domain for homotypic interaction with ASC (Fernandes-Alnemri et al., 2009; Hornung et al., 2009; Roberts et al., 2009) or are transcriptionally regulated by IFN-β (Burckstummer et al., 2009). Although several other proteins from the PYHIN family meet both criteria, later experiments pinpointed AIM2 as the only one that both interacts with ASC (Fernandes-Alnemri et al., 2009) and is exclusively cytoplasmic (Hornung et al., 2009). AIM2 was found to prefer DNA over RNA, and dsDNA over single-stranded DNA (ssDNA) (Burckstummer et al., 2009; Roberts et al., 2009). Moreover, the dsDNA recognized by AIM2 does not have to be sequence specific, as poly (dA:dT) is capable of binding to AIM2 and inducing formation of “ASC speck”, the hallmark of inflammasome, in an AIM2-dependent way (Fernandes-Alnemri et al., 2009; Hornung et al., 2009). It was further identified that the C-terminal HIN domain of AIM2 is responsible to interact with dsDNA while the N-terminal PYD domain interacts with ASC (Fernandes-Alnemri et al., 2009; Hornung et al., 2009). It is later found that though no specific DNA sequence is required to activate AIM2 inflammasome, the length of DNA does play a role. The length to achieve optimal AIM2-mediated inflammatory responses is ~ 80 base pairs (Jin et al., 2012).
2. Activation of AIM2 inflammasome by cytosolic DNA of various origins
Initial identification of AIM2 as a cytosolic dsDNA sensor was carried out using synthetic dsDNA. It is quickly confirmed that AIM2 responds to cytosolic dsDNA from natural sources. Cytoplasm is in theory a space devoid of dsDNA, therefore, any dsDNA may bind to AIM2 and activate AIM2 inflammasome. Such dsDNA may derive from invading microbes including bacteria and viruses, or from damaged nucleus and mitochondria.
2.1. AIM2 detects pathogens in the cytoplasm
2.1.1. AIM2 in response to bacterial infection
Some bacteria potently activate AIM2 inflammasome, which produces mature IL-1β and IL-18 to suppress bacteria proliferation. Francisella tularensis (Fernandes-Alnemri et al., 2010; Jones et al., 2010) and listeria monocytogenes (Rathinam et al., 2010; Warren et al., 2010) were the first live bacteria found to activate AIM2 inflammasome. Streptococcus pneumoniae, species of Mycobaterium as well as Legionella pneumophila and Staphylococcus aureus have been found to activate the AIM2 inflammasome pathway as well (Man et al., 2016a; Zhu et al., 2019). In order to activate AIM2 inflammasome, these vacuolar bacteria need to first escape the vacuoles to get into the cytoplasm, then undergo bacteriolysis to expose bacterial DNA to the cytoplasmic sensors. In the case of Francisella novicida infection, AIM2 inflammasome activation relies on type I interferons (Belhocine and Monack, 2012; Man et al., 2015a). Although AIM2 is constitutively expressed in the cell, the concentration of F. novicida DNA accidentally released into the cytoplasm is not sufficient to induce AIM2 inflammasome formation. It is postulated that the low-level bacterial DNA first induces type I interferon production via other cytoplasmic dsDNA sensors such as cyclic GMP-AMP synthase (cGAS). Type I interferons then act in an autocrine manner to activate transcription factors IRF9 and IRF1, driving the transcription of interferon-stimulated genes (ISGs), in particular a family of interferon-inducible GTPases including guanylate-binding proteins (GBPs) (Man et al., 2015a) (Figure 1). Two of the GTPases, GBP2 and GBP5, associate with cytoplasmic Francisella novicida and trigger bacteriolysis that leads to massive release of bacterial DNA into the cytoplasm for detection by AIM2 (Man et al., 2015a; Meunier et al., 2015). Transfection of dsDNA into cytoplasm directly activates AIM2 inflammasome without priming by interferons, validating the DNA sensor role of AIM2 (Man et al., 2015a). GBP2 and GBP5 may mediate the lysis of F. novicida by recruiting a third interferon-inducible GTPase IRGB10 (Man et al., 2016b).
2.1.2. AIM2 in response to viral infection
AIM2 inflammasome is crucial for the protection of the host against certain DNA virus infections (Hornung et al., 2009; Rathinam et al., 2010). Mouse cytomegalovirus (MCMV) and vaccinia virus infection induces caspase-1 activation and IL-1β secretion in mouse macrophages and dendritic cells in an AIM2-dependent manner (Hornung et al., 2009; Rathinam et al., 2010). Human papillomavirus (HPV) 16-infected human skin displayed elevated levels of AIM2, active caspase-1, and processed IL-1β, implicating an AIM2 inflammasome response to HPV16 in keratinocytes (Reinholz et al., 2013). A recent study with human glomerular mesangial cell line infected with hepatitis B virus indicates that silencing the gene encoding AIM2 by siRNA leads to reduced expression of IL-1β, IL-18 and caspase-1 (Zhen et al., 2014). Epstein-Barr virus infection induces AIM2 inflammasome activation in THP-1 cell line and likely in primary human monocytes as well (Torii et al., 2017). Still, AIM2 inflammasome may not respond to all DNA viruses. IL-1β maturation and secretion upon herpes simplex virus 1 (HSV-1) infection is normal in AIM2 deficient thioglycollate-elicited macrophages (Rathinam et al., 2010). This lack of AIM2-dependent response may be attributed to the fact that HSV-1 DNA is protected from AIM2 recognition by a viral capsid (Paludan et al., 2011) or that some DNA viruses strategically inhibit AIM2 activation. Indeed, a later study identified HSV-1 tegument protein VP22 as an inhibitor for AIM2 inflammasome activation in mouse macrophages. Without VP22, HSV-1 infection activates AIM2 inflammasome and IL-1β secretion, which substantially suppresses HSV-1 replication (Maruzuru et al., 2018). Other studies reported the role for AIM2 in driving IL-1β secretion in response to RNA viruses. Human dermal fibroblasts infected with the RNA viruses Chikungunya virus or West Nile virus showed reduced proteolytic cleavage of caspase-1 and release of IL-1β when the gene encoding AIM2 was silenced (Ekchariyawat et al., 2015). Similarly, enterovirus A71 infection upregulates AIM2 expression and increases caspase-1 activation in SK-N-SH neuroblastoma cells. Knockdown of AIM2 suppresses pyroptosis of EV-A71 infected cells, resulting in substantial increase in viral infection (Yogarajah et al., 2017). Although AIM2 inflammasome is activated in response to RNA virus infection, it is unclear how AIM2 senses such infection.
2.2. AIM2 detects self-DNA in cytoplasm and nucleus
Genomic dsDNA does not usually elicit AIM2-dependent inflammatory responses as it is nuclear, hence physically segregated from cytoplasmic AIM2. Furthermore, mammalian nuclear DNA usually associates with DNA-binding proteins such as histones that shield nuclear DNA from AIM2 detection. Loss of nuclear envelope integrity results in exchange of materials between the nucleus and cytoplasm. Consequently, presence of nuclear DNA in the cytoplasm leads to activation of AIM2 inflammasome (Di Micco et al., 2016). Linker DNA between adjacent nucleosomes, ranging from 20 to 90 base pairs, poses as a probable agonist to activate AIM2 inflammasome. Perturbing nuclear envelope integrity activates cGAS-dependent interferon responses (Di Micco et al., 2016), further supporting the presence of “naked” dsDNA in nuclear genomic DNA. AIM2 inflammasome has not been reported to be activated during cell division, when nuclear envelope completely disintegrates. The lack of activation likely resides in the highly condensed nature of chromosomes during cell division, where nucleosomes are further packed into chromatin fibers and higher-order structures, leaving little unprotected DNA that is long enough to activate AIM2 inflammasome. AIM2 also senses self-DNA delivered by exosomes. Cytotoxic agent irinotecan (CPT-11) triggers massive release of genomic DNA from intestinal cells. Exosomes deliver such nuclear genomic DNA into the cytoplasm of innate immune cells, inducing AIM2 inflammasome assembly and secretion of IL-1β and IL-18 (Lian et al., 2017). Although AIM2 was first thought to be exclusively cytoplasmic, later studies found AIM2 detects irradiation-induced DNA damage and assembles into inflammasome within the nucleus (Hu et al., 2016). Drugs like doxorubicin that promote double-strand breaks in DNA induce AIM2-mediated cell death but UV radiations that promote single-strand breaks in DNA do not (Hu et al., 2016). In this case, AIM2 is likely recruited to the site of DNA damage by the phosphorylated histone gamma-H2AX (Hu et al., 2016). Chromatin structure is highly dynamic at the sites of DNA damage. Nucleosomes are evicted and histones are degraded (Hauer and Gasser, 2017; Hauer et al., 2017; Price and D’Andrea, 2013) to allow DNA repair machinery gaining access to naked DNA. The relationship between AIM2 and DNA repair machinery at the site of double-strand break may be competitive. Indeed, nuclear cGAS has been shown to suppress DNA repair, especially homologous recombination, providing an example of competition between DNA repair machinery and innate immune dsDNA sensing (Jiang et al., 2019; Liu et al., 2018). A recent study uncovered the developmental importance of AIM2 inflammasome surveillance on DNA damage. Developing neurons that accumulate too many DNA damage activate AIM2 inflammasome, and the consequent pyroptosis removes such defective neurons to ensure proper neurodevelopment (Lammert et al., 2020).
3. Regulation of AIM2
AIM2 inflammasome assembly and activation release large amounts of proinflammatory cytokines, even commit the infected or damaged cells to irreversible death. Both outcomes potently suppress pathogen proliferation and spread. To ensure infection and proliferation, pathogens develop their own strategies to evade or suppress AIM2 inflammasome responses. On the other hand, production of cytokines and cell death may cause damage to innocent bystander host cells. Therefore, regulating the activation, intensity, and duration of AIM2 response is critical to optimize the inflammation so that it has minimum deleterious side effect.
3.1. Regulation by DNA availability
AIM2 inflammasome is activated by cytoplasmic dsDNA. Compartmentalization is one way to sequester dsDNA from AIM2. DNA is normally present in the nucleus or mitochondria of healthy cells, hence inaccessible to largely cytosolic AIM2. However, several studies have indicated AIM2 can localize to the nucleus under specific circumstances (See 2.2) (Hu et al., 2016). A second mechanism is to degrade aberrant DNA in cytoplasm. Endonucleases such as DNase III and TREX1 make sure self-DNA does not accumulate beyond the threshold of AIM2 inflammasome activation. Interestingly, telomeric DNA sequences such as TTAGGG repeat inhibit AIM2 inflammasome activation, presumably by competing with dsDNA for AIM2 binding and sequestering AIM2 in an unproductive form (Kaminski et al., 2013).
3.2. Regulation by decoy proteins
Inflammasome component proteins interact with each other via PYD: PYD or CARD: CARD interactions. Exploiting PYD: PYD and CARD: CARD homotypic interactions is thus a way to interfere with inflammasome assembly and activation. PYD- and CARD-only proteins have been found to inhibit inflammasome assembly and signaling, presumably by competing for available binding sites.
3.2.1. PYD-only proteins (POPs)
Identification of viral PYD-only proteins (POPs) led to the idea that POPs negatively regulate inflammasomes (Johnston et al., 2005).Three POP genes have been identified in human genome: POP1 (also known as ASC2), POP2, and POP3 (Dorfleutner et al., 2007; Khare et al., 2014; Stehlik et al., 2003). POP1 is localized on human chromosome 16p11.2, adjacent to ASC gene. POP3 is localized on human chromosome 1q23 in an interferon-inducible gene cluster containing AIM2 (Khare et al., 2014). POP1 and POP2 proteins interact with ASC PYD to interfere with inflammasome assembly. Instead, POP3 binds directly to AIM2 PYD to prevent the interaction between AIM2 and ASC and subsequently blocks the release of IL-1β. In this sense, POP3 is a specific inhibitor to AIM2 inflammasome while POP1 and POP2 are wide-spectrum inflammasome inhibitors (Khare et al., 2014).
3.2.2. CARD-only proteins (COPs)
Human genome encodes three CARD-only proteins: pseudo-ICE (also known as COP and CARD16), INCA (CARD17), and ICEBERG (CARD18) (Druilhe et al., 2001; Humke et al., 2000; Lamkanfi et al., 2004; Lee et al., 2001). The genes encoding COPs are on chromosome 11q22, adjacent to the gene cluster encoding inflammatory caspases. All three human COPs share significant sequence similarity to caspase-1 CARD domain (Dorfleutner et al., 2015; Matusiak et al., 2015). Although they all interact with caspase-1 CARD domain (Casp1 CARD), INCA and ICEBERG function differently to inhibit inflammasome assembly. INCA is monomeric in solution. It caps the ends of Casp1 CARD filament (See 6.3 for more information), preventing its further elongation. ICEBERG can be incorporated into Casp1 CARD filaments, thus “diluting” the caspase-1 catalytic domains and reducing its proximity-induced autoactivation (Lu et al., 2016).
3.2.3. Decoy proteins belonging to the ALR family
Since most POPs and COPs interact with ASC or caspase-1 (with the notable exception of POP3 discussed in 3.2.1), the common denominators of all inflammasomes, they function as pan-inflammasome inhibitors. In contrast, regulators of the ALR family specifically target AIM2 inflammasome. Mouse AIM2-like protein p202, a protein with two HIN domains but no PYD domain, inhibits AIM2 activation (Roberts et al., 2009).The inhibition is achieved partly through competition for dsDNA via the first HIN domain of p202, but more specifically through direct interaction with AIM2 HIN domain using the second HIN domain of p202 (Yin et al., 2013). As human genome does not encode a p202 homolog, the mechanism is initially thought to be specific to mice. However, a recent study identified a HIN domain-only IFI16 isoform in human, IFI16-β, that inhibits AIM2 similarly via competition for dsDNA and interaction with AIM2 (Wang et al., 2018), suggesting the inhibitory mechanism may be more prevalent.
3.3. Regulation by AIM2 stability
AIM2 inflammasome may be regulated directly at protein level. Tripartite motif protein 11 (TRIM11), upon activation, interacts with AIM2 and recruits the autophagic cargo receptor p62 that delivers AIM2 for degradation via selective autophagy (Liu et al., 2016).
3.4. Regulation by pathogens
Bacterial escape from the vacuole and subsequent release of DNA into the cytoplasm are necessary for activating AIM2, a predominantly cytoplasmic protein. Bacteria have evolved to encode virulence factors that assist in evading AIM2 detection and/or suppressing AIM2 activation, much in the same way that host cell modulates AIM2 activation. A virulent F. tularensis subspecies SchuS4 is resistant to bacteriolysis induced by mitochondrial reactive oxygen species (Crane et al., 2014), thus limiting the cytoplasmic availability of bacterial DNA. F. novicida uses the CRISPR-Cas system to strengthen the bacterial envelope and prevent recognition by AIM2 (Sampson et al., 2014). A putative lipid flippase MviN in F. tularensis suppresses AIM2 inflammasome activation, thus increase its virulence, but the mechanism is unknown (Ulland et al., 2010). Viruses encode inhibitors of AIM2 inflammasome as well. Herpesviruses shield viral genomic DNA in capsids to evade AIM2 recognition in cytoplasm (Paludan et al., 2011). HSV-1 tegument protein VP22 inhibits AIM2 oligomerization, preventing its activation (Maruzuru et al., 2018). Human cytomegalovirus (HCMV) protein pUL83 has been shown to interact with AIM2 and inhibit its activation (Huang et al., 2017). Poxvirus encodes a viral PYD-only protein to interfere with inflammasome assembly (Johnston et al., 2005). Furthermore, viruses may regulate AIM2 activation indirectly by modulating type I interferon signaling pathways.
4. AIM2 in non-infectious diseases
4.1. AIM2 in inflammation, autoimmunity and other pathological conditions
Inflammation can be induced by accumulation of host DNA in the cytosol due to uptake of DNA from damaged neighboring cells or impairment in DNA degradation and clearance. Psoriasis is characterized by DNA-induced AIM2-dependent release of IL-1β in skin cells (Dombrowski et al., 2011). Male patients suffering from systemic lupus erythematosus show increased expression of AIM2 in macrophages; in contrast, female patients have decreased AIM2 expression level but elevated dsDNA autoantibody (Kimkong et al., 2009; Yang et al., 2015). DNA-dependent AIM2 inflammasome activation is also widely implicated in a wide range of pathologies, including chronic kidney diseases, diabetes, atherosclerosis, and neuronal diseases (Sharma et al., 2019).
4.2. AIM2 in cancer progression
The gene encoding AIM2 was initially identified from human melanoma cell lines as a tumor suppressor gene (DeYoung et al., 1997). Later studies indicated anti-tumorigenesis role of AIM2 in multiple tumor types. Frameshift mutations in the AIM2 gene have been associated with colon, gastric, endometrial, and small bowel cancers (Schulmann et al., 2005; Woerner et al., 2007). Hypermethylation in the AIM2 promoter region and downregulation of AIM2 are observed in several colorectal cancer lines (Choubey, 2016; Dihlmann et al., 2014; Schulmann et al., 2005; Wilson et al., 2015; Woerner et al., 2007). Upregulation of AIM2 correlates with better prognosis in EBV-associated nasopharyngeal carcinoma patients (Chen et al., 2012). Decreased expression of AIM2 has been observed in prostate cancer cells and hepatocellular carcinoma (Chen et al., 2017; Ma et al., 2016; Ponomareva et al., 2013). Hepatitis B virus X protein (HBx) contributes to AIM2 downregulation in hepatocellular carcinoma patients infected with hepatitis B virus (Chen et al., 2017). However, such tumor suppressive property of AIM2 may arise from its role in modulating cell cycle, proliferation, and metabolism, rather than assembly into inflammasome (Ma et al., 2016; Man et al., 2015b; Patsos et al., 2010; Wilson et al., 2015).
Pro-tumorigenesis activities have also been reported for AIM2. Human cutaneous squamous carcinoma cells show higher expression of AIM2 with respect to the normal keratinocytes and AIM2 expression can be correlated with the grade of the tumors (Farshchian et al., 2017). In human papillomavirus (HPV)-associated cervical cancer, the promoter region of AIM2 is demethylated and AIM2 expression level upregulated (Milutin Gasperov et al., 2014). AIM2 inflammasome is also upregulated in non-small cell lung cancer (Kong et al., 2015). Together, these studies demonstrate that AIM2 has an important role in regulating tumorigenesis, but the mechanisms are still poorly understood.
5. Crosstalk between AIM2 inflammasome and other cytosolic DNA sensors
Several other DNA sensors co-exist with AIM2 in the cytoplasm, including cGAS and IFI16 (Wu and Chen, 2014). They crosstalk to each other to amplify, dampen, or finetune the DNA-elicited inflammatory responses, including production of IL-1β, type I interferons, and pyroptosis. As all known dsDNA sensors bind to dsDNA without sequence preference, it is conceivable that they all compete for the same pool of dsDNA. Therefore, signaling strength of individual pathway depends on expression levels of sensors and their binding affinities to cytoplasmic dsDNA. The best studied relationship between cytoplasmic dsDNA sensors is between AIM2 and cGAS-stimulator of interferon genes (STING) axis.
Cytoplasmic dsDNA activates both AIM2 inflammasome and cGAS-STING axis. While AIM2 inflammasome leads to secretion of IL-1β/IL-18 and pyroptosis, cGAS-STING eventually leads to production of type I interferons. Upon DNA binding, cGAS synthesizes the second messenger 2’,3’-cGAMP (cGAMP) that acts as the high affinity ligand for the adaptor protein STING. cGAMP binding induces STING conformational change, oligomerization, and translocation that allow it to interact with two downstream molecules, TANK-binding kinase 1 (TBK1) and interferon regulatory transcription factor (IRF3). TBK1 and IRF3 are phosphorylated, leading to IRF3 translocation into the nucleus and consequent production of type I interferons. As AIM2 expression is induced by interferons, the cGAS-STING axis naturally upregulates AIM2 activation. In addition to the “priming” of AIM2, cGAMP also activates AIM2 inflammasome likely through induced colocalization of inflammasome components (Swanson et al., 2017). AIM2 inflammasome, however, negatively impacts the cGAS-STING axis in dendritic cells and macrophages (Corrales et al., 2016; Yan et al., 2018; Yin et al., 2013). Aside from competition for dsDNA, activation of AIM2 inflammasome induces potent pyroptosis of the infected or DNA-stimulated cells, permanently terminating cGAS-STING signaling and interferon production in these cells (Corrales et al., 2016; Yin et al., 2013). Inhibition of caspase-1 protects cells from pyroptosis and restores cGAS-STING signaling and interferon production upon DNA stimulation (Corrales et al., 2016). Additionally, overexpression of ASC in murine macrophages inhibits phosphorylation of TBK1 and IRF3 and reduces STING-induced interferon production (Yan et al., 2018). Coimmunoprecipitation assays in HEK293T cells show the CARD domain of ASC associates with the C-terminal tail of STING, a region known for STING-TBK1 interaction. Indeed, expression of ASC decreases the interaction between STING and TBK1 in a dose-dependent manner (Yan et al., 2018). It is then proposed that ASC disrupts the interaction between STING and TBK1, abrogating TBK1 and IRF3 phosphorylation, and terminating signaling events leading to interferon production.
6. Molecular Basis of AIM2 Inflammasome Assembly
The bipartite organization of AIM2 naturally suggests its working mechanism: the HIN domain is responsible for DNA binding, while the PYD domain interacts with ASC through PYD: PYD homotypic interaction (Broz and Dixit, 2016). Multiple crystallographic and electron microscopic structures have identified the structural basis of DNA recognition and PYD: PYD interactions (Wang and Yin, 2017; Wang et al., 2019). It is quickly recognized that domain polymerization plays a critical role throughout the assembly of AIM2 inflammasome. AIM2 PYD domain (AIM2PYD), although not directly contacts dsDNA, promotes AIM2 binding to dsDNA through oligomerization of AIM2. AIM2 HIN domain also contributes to AIM2 oligomerization upon dsDNA binding (Morrone et al., 2015). Clustering of AIM2 on dsDNA leads to filamentous polymerization of AIM2PYD, which in turn serves as the “nucleus” to polymerize ASC into filamentous structures with ASC PYD domains (ASCPYD) forming the “stalk” of the filaments and the CARD domains (ASCCARD) protruding out (Lu et al., 2014b). The elevated local concentration of ASCCARD induces its own filamentous polymerization and nucleation of caspase-1 polymerization (Lu et al., 2016). The structures and assembly mechanisms of AIM2 have been reviewed recently (Wang and Yin, 2017; Wang et al., 2019), therefore, in this article, we will briefly summarize the overall structures of AIM2 inflammasome and focus more on the interactions not covered previously.
6.1. DNA recognition by HIN domain
HIN domain features in the protein family of AIM2-like DNA-recognizing innate receptors (also known as PYHIN family receptors), which includes AIM2, IFI16 and p202 (Broz and Dixit, 2016). A HIN domain contains two subdomains of oligonucleotide/oligosaccharide binding (OB) fold, which is a small structural motif with 70 to 150 amino acids in length for nucleic acid binding, mostly single-stranded DNA (Flynn and Zou, 2010; Theobald et al., 2003). Despite low sequence similarity, the canonical OB folds share a very conserved three-dimensional structure: a core β barrel composed of five highly coiled antiparallel β strands capped at one end by an α helix. By convention, the proximal and distal OB folds in HIN domain are named as OB1 and OB2, respectively (Figure 2A). Although OB1 and OB2 are connected by a long linker of helix-loop-helix structure, extensive hydrophobic interactions between the two subdomains hold them together strongly as a single unit as shown by IFI16 HIN structures (Liao et al., 2011).
Figure 2.
Structural basis of AIM2 inflammasome assembly. A. Cartoon representation of human AIM2 HIN domain showing the tandem OB-fold structure (PDB ID 3RLN); α helices are colored in cyan, β strands in magenta, and loops in pink. OB1, OB2, and the connecting α-helical linker are labeled. B. DNA binding mode of human AIM2 HIN (PDB ID 3RN2); AIM2 HIN is shown as electrostatic surface with blue representing positive charges, red representing negative charges, and white representing neutral surface; OB1 and OB2 are indicated. C. DNA binding mode of mouse p202 HIN1 (PDB ID 4L5R); p202 HIN is shown in electrostatic surface colored the same way as in B; OB1 and OB2 are labeled. Superposing structures in B and C places AIM2-bound DNA (gray ribbon) at the back side of p202 HIN1. D. Superposition of human AIM2 PYD fused with MBP (yellow, PDB ID 3VD8) and human ASC PYD in filament (green, PDB ID 3J63). For clarity the MBP portion is not shown. N- and C-termini and the six α helices are labeled; Red arrow: α2-α3 loop; red arrowhead: the conserved Lys residue in AIM2 PYD α2; E. Side and top views of human ASCPYD filament structure (PDB ID 3J63); the three strands in C3 symmetry are colored in teal, cyan, and pale cyan, respectively; C-termini of three PYD subunits are labeled to the right. F. A schematic diagram of ASCPYD filament structure colored in the same way as in E; each hexagon represents a PYD subunit. G. A schematic diagram of six types of asymmetric surfaces and three pairs of interfaces in ASCPYD filament structure. Interfaces examined in detail in H-J are labeled in bold. H-J. Detailed type I (H), type II (I), and type III (J) interfaces in the ASCPYD filament structure.
Currently, the known HIN domain structures include human AIM2 (hAIM2) HIN (Jin et al., 2012), mouse AIM2 (mAIM2) HIN (Ru et al., 2013), human IFI16 HIN1 and HIN2 (Jin et al., 2012; Liao et al., 2011; Ni et al., 2016), mouse p202 HIN1 and HIN2 (Ru et al., 2013; Yin et al., 2013), and mouse p204 HIN1 (Tian and Yin, 2019). Although the topological arrangement among HIN domains is highly conserved, OB1 and OB2 in both HIN domains show clear divergences comparing to canonical OB fold. In subdomain OB1, each of three strands (β1, β4, β5) of canonical OB fold is observed as two corresponding short β strands (β’ and β), and similarly in subdomain OB2, the β5 of canonical OB fold is split into β5’ and β5 in some HIN structures (Figure 2A). The superposition of all HIN domain structures reveals significant flexibility in the loops that connect β1 and β2 strands (L12 loop) and β4 and β5 strands (L45 loop) in both OB subdomains (Wang et al., 2019), which are known to contribute to oligonucleotide binding (Theobald et al., 2003).
To date, four structures of HIN domain in complex with dsDNA have been reported, including HIN domains from human AIM2 (Jin et al., 2012), mouse AIM2 (Ru et al., 2013), human IFI16 HIN2 (Jin et al., 2012), and mouse p202 HIN1 (Ru et al., 2013; Yin et al., 2013). In all these structures, both OB folds directly engage the dsDNA, underlying the fact that the two OB subdomains connecting by the linker function as a single unit. Both strands of dsDNA contact the HIN domain (Figures 2B–C). The primary nature of HIN: DNA interaction is electrostatic (Figures 2B–C), but polar interactions contribute as well. Positively charged amino acids on the HIN domain interact with the backbone phosphates and riboses and no significant interactions with nucleotide bases are observed. This is consistent with the findings that recognition of dsDNA is sequence-independent (Roberts et al., 2009).
Based on the DNA binding interface, HIN could be divided into two different classes among the four HIN: dsDNA structures. AIM2 and IFI16 HIN2 employ the OB1-OB2 linker and surrounding residues on both OB folds to engage dsDNA, while p202 use a completely opposite surface to the linker to interact with dsDNA (Figures 2B–C). In AIM2 HIN: dsDNA structure, the DNA runs almost orthogonal to the OB1-OB2 axis (Figure 2B). The DNA binding residues of AIM2 and IFI16 are primarily located on the OB1-OB2 linker, but the surrounding OB1 and OB2 residues contribute to a certain extent as well. These residues are largely conserved, except that two lysine residues in β1- β1’ corner of OB1 of hAIM2 HIN are absent in mAIM2 and IFI16 HIN2. The reduced contribution of OB1 in mAIM2 and IFI16 HIN2 is likely to cause the different orientations of DNA molecules. In both HIN: dsDNA structures of mAIM2 and IFI16 HIN2, DNA slightly tilts away from OB1, comparing to that of hAIM2 (Jin et al., 2012; Ru et al., 2013). Interestingly, hAIM2 HIN domains from different crystal forms also use slightly different sets of residues for DNA binding, suggesting the DNA binding surface is intrinsically plastic, likely to accommodate a wider variety of DNA species. In contrast, the DNA binding interface of p202 HIN1 is on the opposite side of the OB1-OB2 linker, and the dsDNA is parallel to the OB1-OB2 axis (Figure 2C). The residues involved in DNA binding in p202 HIN1 are located at L12 and L45 loops in both OB folds, the same region used for single-stranded DNA binding in canonical OB domains. Different from both AIM2 HIN and p202 HIN1, p202 HIN2 completely sheds DNA binding capacity. HIN2 in p202 forms a tetrameric platform to promote full-length p202 binding to dsDNA via avidity. Moreover, p202 HIN2 specifically interacts with AIM2 HIN, thus preventing AIM2 oligomerization on dsDNA and subsequent activation (Yin et al., 2013). The different interaction capabilities of HIN domains likely arise from their rapid evolution under selection pressure (Cridland et al., 2012).
6.2. PYD, CARD, and filament formation
PYD and CARD domains conform to the canonical six-helix bundle fold of death domain (DD) superfamily (Ferrao and Wu, 2012; Fesik, 2000; Huang et al., 1996; Park et al., 2007). Death domain superfamily includes death domain (DD), death effector domain (DED), caspase recruitment domain (CARD), and PYD subfamilies (Park et al., 2007). DD fold takes its role as a protein interaction module in mediating homotypic interactions for assembling functional signaling complexes involved in the biological processes of inflammation or apoptosis (Park et al., 2007). Previous complex structures of DD superfamily defined three types of asymmetric interactions, type I, II, and III that include three interaction pairs and totally six interaction surfaces (Ia, Ib; IIa, IIb; IIIa, IIIb) despite low sequence similarity (Ferrao and Wu, 2012; Weber and Vincenz, 2001).
Structure studies of isolated PYD domains from both AIM2 and ASC have revealed the flexible characteristic of helix α3 and the preceding loop of α2-α3 (Liepinsh et al., 2003; Natarajan et al., 2006) (Figure 2D), indicating that the conformational change in this region may be very important for homotypic interactions. Of note, both AIM2PYD and ASCPYD tend to self-associate in solution, severely hindering crystallographic studies before the advent of cryo-EM. Despite the tendency of self-association, isolated AIM2 PYD and ASC PYD structures have been determined in several studies through efforts of fusion with solubility tag maltose-binding protein (Jin et al., 2013), mutation of a surface bulky hydrophobic residue (Lu et al., 2014a), or purification at low pH (Hou and Niu, 2015; Liepinsh et al., 2003). AIM2PYD adopts a globular structure of six-helix bundle, canonical of the death domain superfamily, but the flexible region of α3 helix and α2-α3 loop are shortened comparing to typical death domains, which is also the common feature of other members of PYD subfamily (Kersse et al., 2011). α2-α3 loop and the short α3 helix are also the regions that display most structural divergence among the AIM2 PYD structures (red arrow in Figure 2D). The dynamic feature suggests conformational changes upon AIM2 inflammasome assembly via PYD: PYD homotypic interaction. Another distinct feature of AIM2PYD structure is the presence of a highly-conserved lysine residue (Lys26 in AIM2 and Lys21 in ASC, arrowhead in Figure 2D) at the α2 helix that stabilizes the variable α3 helix (Jin et al., 2013). The surface of AIM2PYD shows a bipolar distribution pattern of electrostatic charges with one side of dominant acidic residues at the α1 and α2 helix and an opposite side of primarily basic residues at the α5 and α6 helix, which likely mediate PYD: HIN association for auto-inhibition and PYD: PYD interaction for filament formation and inflammasome assembly (Jin et al., 2012).
To elucidate the molecular mechanism of AIM2 inflammasome assembly, cryo-electron microscopy structures of GFP-hAIM2PYD and hASCPYD helical filaments have been determined at 5 Å and 3.8 Å resolution, respectively (Lu et al., 2015; Lu et al., 2014b). Both PYD filament reconstructions revealed the similar shape of hollow tubes with an inner diameter of ~20 Å and an outer diameter of ~90 Å (Figure 2E). However, the filament symmetry is largely different in two structures. GFP-hAIM2PYD filament is a one-start right-handed rotation helix, in contrast to the three-start right-handed C3 point group symmetry in hASCPYD filament (Figures 2E–F). In GFP-hAIM2PYD filament, the rotation angle and axial rise between adjacent subunits are 138.9° and 6.0 Å respectively, while those of hASCPYD filament are correspondingly 52.9° and 13.9 Å (Lu et al., 2015; Lu et al., 2014b; Sborgi et al., 2015). Though the symmetries are different, GFP-hAIM2PYD is capable of nucleating filament formation of ASCPYD (Lu et al., 2015), implying the plasticity of subunit interaction and filament assembly. The cryo-EM reconstruction of mouse ASCPYD displayed very similar structure to hASCPYD filament (Sborgi et al., 2015), suggesting structural and functional conservation in the two species.
In ASCPYD filament structure, all C-termini of ASCPYD point outwards, easily accommodating the CARD domains in full-length ASC (Lu et al., 2014b) (Figure 2E). ASCCARD interacts with the CARD domain of procaspase-1 (Casp1CARD) via homotypic CARD: CARD interactions. ASCCARD and Casp1CARD filamentous structures have been determined by cryo-EM at 4.8 and 5.1 Å resolution, respectively (Li et al., 2018; Lu et al., 2016). Briefly, ASCCARD and Casp1CARD filament structures are hollowed tubes like PYD filaments but slimmer: the inner and outer diameters are < 20 Å and ~ 80 Å, respectively. Another difference is they are left-handed in contrast to the right-handed helices seen in PYD filaments. ASCCARD and Casp1CARD filaments share strikingly similar helical symmetry and subunit arrangements, further corroborated the nucleated polymerization model observed for PYD helical filaments. We have amassed considerable knowledge on the structural features of helical filaments in AIM2 inflammasome, but little is known in the kinetics of filament nucleation and elongation. It is not clear the minimal number of subunits required for filament nucleation. In an elongating filament, it is also not known whether subunits are added one by one or in the form of small oligomers.
PYD structures are largely conserved in filament assemblies when compared to their monomeric forms. Again, the most structural divergence of AIM2PYD and ASCPYD upon filament formation is observed in the region encompassing helix α2, α2-α3 loop, and α3 helix (Figure 2D). In ASCPYD filament structure, the α2-α3 loop and following α3 helix are ordered and clear in cryo-EM density, while in isolated ASCPYD the same region is disordered (Liepinsh et al., 2003; Lu et al., 2014b), consistent with the dynamic nature of this region and its role in forming ASC filament. Indeed, this region participates in all three types of DD interactions in the filament (Lu et al., 2014b) (Figures 2G–J). Despite the difference in filament symmetry, the six interaction surfaces that pair to form the three asymmetry interfaces are primarily conserved between the AIM2PYD filament and ASCPYD filament structures, especially for the orientation and position. Type I asymmetry interface comprises residues in α1 and α4 (type Ia) of one subunit, and residues in α2 and α3 (type Ib) of another subunit; type II interface includes residues at the α4-α5 corner (IIa) and α5-α6 corner (IIb); type III interface is consisted of residues at α2-α3 corner (IIIa) and α1-α2 corner (IIIb) (Figures 2H–J). Type I interface is the largest one among the three types in both filaments. Notably, the interaction of type I interface in ASCPYD is majorly electrostatic (Arg3, Arg5, Leu9, Asp54, Leu50, Asp48, Asp51 of Ia; Lys26, Ser29, leu28, Arg41, Leu25, Lys21, Lys22 of Ib), in contrast to the hydrophobic nature in AIM2PYD type I interface (Ser3, Lys6, Leu10, Leu11, Asp15 and Ile46 of Ia; Arg24, Phe27, Phe28 and Asp31 of Ib). CARD filaments are assembled utilizing the same type I, II, III interactions even though sequence identity is low.
7. Conclusion
AIM2 inflammasome is the first inflammasome with a defined ligand. The prevalence of dsDNA, the ligand of AIM2, in living organisms and its compartmentalization in eukaryotic cells position AIM2 as a crucial cytoplasmic sensor of disruption of cellular homeostasis as well as pathogen invasion. Once bound to dsDNA, AIM2 assembles into inflammasome, eventually releasing potent proinflammatory cytokines and inducing cell pyroptosis to combat infection. Recent studies expand AIM2’s role to nuclear detection of DNA damage. Dysregulation of AIM2 inflammasome leads to autoimmunity, and both anti- and pro-tumorigenesis functions have been reported for AIM2. The molecular basis of AIM2 inflammasome activation has been studied extensively, from initial interaction with dsDNA to final recruitment and activation of procaspase-1. The unifying theme is helical assemblies nucleated by upstream molecules. Upstream proteins often “nucleate” unidirectional polymerization of downstream proteins via homotypic interactions between PYD or CARD domains. Structurally, downstream helical assemblies often follow the symmetry, subunit packing, and charge distribution patterns of their upstream nucleating filaments, but certain degree of flexibility is allowed. The nucleated helical assembly mechanism is found in other death domain-containing immune signaling complexes including NLRP3 and NLRC4 inflammasomes and cytoplasmic sensing of viral RNAs (Lu et al., 2015; Lu et al., 2014b; Lu et al., 2016; Wu et al., 2014). Helical filament formation provides an effective way to mobilize multiple proteins in a short period of time for rapid and robust activation of signaling, for signal amplification, and for reduction of stochastic activation (Wu, 2013).
Acknowledgments
We thank the members of Yin lab for constructive criticism. This work was supported by a National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases (NIAID) grant R01AI146330 and startup funds from Florida State University (to Q.Y.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE
- Belhocine K, Monack DM, 2012. Francisella infection triggers activation of the AIM2 inflammasome in murine dendritic cells. Cell Microbiol 14, 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broz P, Dixit VM, 2016. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol 16, 407–420. [DOI] [PubMed] [Google Scholar]
- Burckstummer T, Baumann C, Bluml S, Dixit E, Durnberger G, Jahn H, Planyavsky M, Bilban M, Colinge J, Bennett KL, Superti-Furga G, 2009. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol 10, 266–272. [DOI] [PubMed] [Google Scholar]
- Chen LC, Wang LJ, Tsang NM, Ojcius DM, Chen CC, Ouyang CN, Hsueh C, Liang Y, Chang KP, Chen CC, Chang YS, 2012. Tumour inflammasome-derived IL-1beta recruits neutrophils and improves local recurrence-free survival in EBV-induced nasopharyngeal carcinoma. EMBO molecular medicine 4, 1276–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SL, Liu LL, Lu SX, Luo RZ, Wang CH, Wang H, Cai SH, Yang X, Xie D, Zhang CZ, Yun JP, 2017. HBx-mediated decrease of AIM2 contributes to hepatocellular carcinoma metastasis. Molecular oncology 11, 1225–1240. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Choubey D, 2016. Absent in melanoma 2 proteins in the development of cancer. Cell Mol Life Sci 73, 4383–4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales L, Woo SR, Williams JB, McWhirter SM, Dubensky TW Jr., Gajewski TF, 2016. Antagonism of the STING Pathway via Activation of the AIM2 Inflammasome by Intracellular DNA. J Immunol 196, 3191–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane DD, Bauler TJ, Wehrly TD, Bosio CM, 2014. Mitochondrial ROS potentiates indirect activation of the AIM2 inflammasome. Front Microbiol 5, 438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cridland JA, Curley EZ, Wykes MN, Schroder K, Sweet MJ, Roberts TL, Ragan MA, Kassahn KS, Stacey KJ, 2012. The mammalian PYHIN gene family: phylogeny, evolution and expression. BMC evolutionary biology 12, 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zoete MR, Palm NW, Zhu S, Flavell RA, 2014. Inflammasomes. Cold Spring Harbor perspectives in biology 6, a016287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeYoung KL, Ray ME, Su YA, Anzick SL, Johnstone RW, Trapani JA, Meltzer PS, Trent JM, 1997. Cloning a novel member of the human interferon-inducible gene family associated with control of tumorigenicity in a model of human melanoma. Oncogene 15, 453–457. [DOI] [PubMed] [Google Scholar]
- Di Micco A, Frera G, Lugrin J, Jamilloux Y, Hsu ET, Tardivel A, De Gassart A, Zaffalon L, Bujisic B, Siegert S, Quadroni M, Broz P, Henry T, Hrycyna CA, Martinon F, 2016. AIM2 inflammasome is activated by pharmacological disruption of nuclear envelope integrity. Proc Natl Acad Sci U S A 113, E4671–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dihlmann S, Tao S, Echterdiek F, Herpel E, Jansen L, Chang-Claude J, Brenner H, Hoffmeister M, Kloor M, 2014. Lack of Absent in Melanoma 2 (AIM2) expression in tumor cells is closely associated with poor survival in colorectal cancer patients. Int J Cancer 135, 2387–2396. [DOI] [PubMed] [Google Scholar]
- Dombrowski Y, Peric M, Koglin S, Kammerbauer C, Goss C, Anz D, Simanski M, Glaser R, Harder J, Hornung V, Gallo RL, Ruzicka T, Besch R, Schauber J, 2011. Cytosolic DNA triggers inflammasome activation in keratinocytes in psoriatic lesions. Sci Transl Med 3, 82ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfleutner A, Chu L, Stehlik C, 2015. Inhibiting the inflammasome: one domain at a time. Immunol Rev 265, 205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfleutner A, Bryan NB, Talbott SJ, Funya KN, Rellick SL, Reed JC, Shi X, Rojanasakul Y, Flynn DC, Stehlik C, 2007. Cellular pyrin domain-only protein 2 is a candidate regulator of inflammasome activation. Infection and immunity 75, 1484–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druilhe A, Srinivasula SM, Razmara M, Ahmad M, Alnemri ES, 2001. Regulation of IL-1beta generation by Pseudo-ICE and ICEBERG, two dominant negative caspase recruitment domain proteins. Cell Death Differ 8, 649–657. [DOI] [PubMed] [Google Scholar]
- Ekchariyawat P, Hamel R, Bernard E, Wichit S, Surasombatpattana P, Talignani L, Thomas F, Choumet V, Yssel H, Despres P, Briant L, Misse D, 2015. Inflammasome signaling pathways exert antiviral effect against Chikungunya virus in human dermal fibroblasts. Infect Genet Evol 32, 401–408. [DOI] [PubMed] [Google Scholar]
- Farshchian M, Nissinen L, Siljamaki E, Riihila P, Piipponen M, Kivisaari A, Kallajoki M, Grenman R, Peltonen J, Peltonen S, Quint KD, Bavinck JNB, Kahari VM, 2017. Tumor cell-specific AIM2 regulates growth and invasion of cutaneous squamous cell carcinoma. Oncotarget 8, 45825–45836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES, 2009. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 458, 509–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes-Alnemri T, Yu JW, Juliana C, Solorzano L, Kang S, Wu J, Datta P, McCormick M, Huang L, McDermott E, Eisenlohr L, Landel CP, Alnemri ES, 2010. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat Immunol 11, 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrao R, Wu H, 2012. Helical assembly in the death domain (DD) superfamily. Current opinion in structural biology 22, 241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesik SW, 2000. Insights into programmed cell death through structural biology. Cell 103, 273–282. [DOI] [PubMed] [Google Scholar]
- Flynn RL, Zou L, 2010. Oligonucleotide/oligosaccharide-binding fold proteins: a growing family of genome guardians. Crit Rev Biochem Mol Biol 45, 266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Callaway JB, Ting JP, 2015. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nature medicine 21, 677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauer MH, Gasser SM, 2017. Chromatin and nucleosome dynamics in DNA damage and repair. Genes & development 31, 2204–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauer MH, Seeber A, Singh V, Thierry R, Sack R, Amitai A, Kryzhanovska M, Eglinger J, Holcman D, Owen-Hughes T, Gasser SM, 2017. Histone degradation in response to DNA damage enhances chromatin dynamics and recombination rates. Nature structural & molecular biology 24, 99–107. [DOI] [PubMed] [Google Scholar]
- Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA, 2009. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458, 514–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X, Niu X, 2015. The NMR solution structure of AIM2 PYD domain from Mus musculus reveals a distinct alpha2-alpha3 helix conformation from its human homologues. Biochemical and biophysical research communications 461, 396–400. [DOI] [PubMed] [Google Scholar]
- Hu B, Jin C, Li HB, Tong J, Ouyang X, Cetinbas NM, Zhu S, Strowig T, Lam FC, Zhao C, Henao-Mejia J, Yilmaz O, Fitzgerald KA, Eisenbarth SC, Elinav E, Flavell RA, 2016. The DNA-sensing AIM2 inflammasome controls radiation-induced cell death and tissue injury. Science 354, 765–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Eberstadt M, Olejniczak ET, Meadows RP, Fesik SW, 1996. NMR structure and mutagenesis of the Fas (APO-1/CD95) death domain. Nature 384, 638–641. [DOI] [PubMed] [Google Scholar]
- Huang Y, Ma D, Huang H, Lu Y, Liao Y, Liu L, Liu X, Fang F, 2017. Interaction between HCMV pUL83 and human AIM2 disrupts the activation of the AIM2 inflammasome. Virol J 14, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humke EW, Shriver SK, Starovasnik MA, Fairbrother WJ, Dixit VM, 2000. ICEBERG: a novel inhibitor of interleukin-1beta generation. Cell 103, 99–111. [DOI] [PubMed] [Google Scholar]
- Jiang H, Xue X, Panda S, Kawale A, Hooy RM, Liang F, Sohn J, Sung P, Gekara NO, 2019. Chromatin-bound cGAS is an inhibitor of DNA repair and hence accelerates genome destabilization and cell death. The EMBO journal 38, e102718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin T, Perry A, Smith P, Jiang J, Xiao TS, 2013. Structure of the absent in melanoma 2 (AIM2) pyrin domain provides insights into the mechanisms of AIM2 autoinhibition and inflammasome assembly. J Biol Chem 288, 13225–13235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin T, Perry A, Jiang J, Smith P, Curry JA, Unterholzner L, Jiang Z, Horvath G, Rathinam VA, Johnstone RW, Hornung V, Latz E, Bowie AG, Fitzgerald KA, Xiao TS, 2012. Structures of the HIN domain:DNA complexes reveal ligand binding and activation mechanisms of the AIM2 inflammasome and IFI16 receptor. Immunity 36, 561–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JB, Barrett JW, Nazarian SH, Goodwin M, Ricciuto D, Wang G, McFadden G, 2005. A poxvirus-encoded pyrin domain protein interacts with ASC-1 to inhibit host inflammatory and apoptotic responses to infection. Immunity 23, 587–598. [DOI] [PubMed] [Google Scholar]
- Jones JW, Kayagaki N, Broz P, Henry T, Newton K, O’Rourke K, Chan S, Dong J, Qu Y, Roose-Girma M, Dixit VM, Monack DM, 2010. Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis. Proc Natl Acad Sci U S A 107, 9771–9776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski JJ, Schattgen SA, Tzeng TC, Bode C, Klinman DM, Fitzgerald KA, 2013. Synthetic oligodeoxynucleotides containing suppressive TTAGGG motifs inhibit AIM2 inflammasome activation. J Immunol 191, 3876–3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, Zhang J, Lee WP, Roose-Girma M, Dixit VM, 2011. Non-canonical inflammasome activation targets caspase-11. Nature 479, 117–121. [DOI] [PubMed] [Google Scholar]
- Kersse K, Verspurten J, Vanden Berghe T, Vandenabeele P, 2011. The death-fold superfamily of homotypic interaction motifs. Trends Biochem Sci 36, 541–552. [DOI] [PubMed] [Google Scholar]
- Khare S, Ratsimandresy RA, de Almeida L, Cuda CM, Rellick SL, Misharin AV, Wallin MC, Gangopadhyay A, Forte E, Gottwein E, Perlman H, Reed JC, Greaves DR, Dorfleutner A, Stehlik C, 2014. The PYRIN domain-only protein POP3 inhibits ALR inflammasomes and regulates responses to infection with DNA viruses. Nat Immunol 15, 343–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimkong I, Avihingsanon Y, Hirankarn N, 2009. Expression profile of HIN200 in leukocytes and renal biopsy of SLE patients by real-time RT-PCR. Lupus 18, 1066–1072. [DOI] [PubMed] [Google Scholar]
- Kong H, Wang Y, Zeng X, Wang Z, Wang H, Xie W, 2015. Differential expression of inflammasomes in lung cancer cell lines and tissues. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine 36, 7501–7513. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Dixit VM, 2012. Inflammasomes and their roles in health and disease. Annual review of cell and developmental biology 28, 137–161. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Denecker G, Kalai M, D’Hondt K, Meeus A, Declercq W, Saelens X, Vandenabeele P, 2004. INCA, a novel human caspase recruitment domain protein that inhibits interleukin-1beta generation. J Biol Chem 279, 51729–51738. [DOI] [PubMed] [Google Scholar]
- Lammert CR, Frost EL, Bellinger CE, Bolte AC, McKee CA, Hurt ME, Paysour MJ, Ennerfelt HE, Lukens JR, 2020. AIM2 inflammasome surveillance of DNA damage shapes neurodevelopment. Nature 580, 647–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Stehlik C, Reed JC, 2001. Cop, a caspase recruitment domain-containing protein and inhibitor of caspase-1 activation processing. J Biol Chem 276, 34495–34500. [DOI] [PubMed] [Google Scholar]
- Li Y, Fu TM, Lu A, Witt K, Ruan J, Shen C, Wu H, 2018. Cryo-EM structures of ASC and NLRC4 CARD filaments reveal a unified mechanism of nucleation and activation of caspase-1. Proc Natl Acad Sci U S A 115, 10845–10852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian Q, Xu J, Yan S, Huang M, Ding H, Sun X, Bi A, Ding J, Sun B, Geng M, 2017. Chemotherapy-induced intestinal inflammatory responses are mediated by exosome secretion of double-strand DNA via AIM2 inflammasome activation. Cell Res 27, 784–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao JC, Lam R, Brazda V, Duan S, Ravichandran M, Ma J, Xiao T, Tempel W, Zuo X, Wang YX, Chirgadze NY, Arrowsmith CH, 2011. Interferon-Inducible Protein 16: Insight into the Interaction with Tumor Suppressor p53. Structure 19, 418–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepinsh E, Barbals R, Dahl E, Sharipo A, Staub E, Otting G, 2003. The death-domain fold of the ASC PYRIN domain, presenting a basis for PYRIN/PYRIN recognition. J Mol Biol 332, 1155–1163. [DOI] [PubMed] [Google Scholar]
- Liu H, Zhang H, Wu X, Ma D, Wu J, Wang L, Jiang Y, Fei Y, Zhu C, Tan R, Jungblut P, Pei G, Dorhoi A, Yan Q, Zhang F, Zheng R, Liu S, Liang H, Liu Z, Yang H, Chen J, Wang P, Tang T, Peng W, Hu Z, Xu Z, Huang X, Wang J, Li H, Zhou Y, Liu F, Yan D, Kaufmann SHE, Chen C, Mao Z, Ge B, 2018. Nuclear cGAS suppresses DNA repair and promotes tumorigenesis. Nature 563, 131–136. [DOI] [PubMed] [Google Scholar]
- Liu T, Tang Q, Liu K, Xie W, Liu X, Wang H, Wang RF, Cui J, 2016. TRIM11 Suppresses AIM2 Inflammasome by Degrading AIM2 via p62-Dependent Selective Autophagy. Cell reports 16, 1988–2002. [DOI] [PubMed] [Google Scholar]
- Lu A, Kabaleeswaran V, Fu T, Magupalli VG, Wu H, 2014a. Crystal structure of the F27G AIM2 PYD mutant and similarities of its self-association to DED/DED interactions. J Mol Biol 426, 1420–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A, Li Y, Yin Q, Ruan J, Yu X, Egelman E, Wu H, 2015. Plasticity in PYD assembly revealed by cryo-EM structure of the PYD filament of AIM2. Cell discovery 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A, Magupalli VG, Ruan J, Yin Q, Atianand MK, Vos MR, Schroder GF, Fitzgerald KA, Wu H, Egelman EH, 2014b. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell 156, 1193–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A, Li Y, Schmidt FI, Yin Q, Chen S, Fu TM, Tong AB, Ploegh HL, Mao Y, Wu H, 2016. Molecular basis of caspase-1 polymerization and its inhibition by a new capping mechanism. Nature structural & molecular biology 23, 416–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Guo P, Qiu Y, Mu K, Zhu L, Zhao W, Li T, Han L, 2016. Loss of AIM2 expression promotes hepatocarcinoma progression through activation of mTOR-S6K1 pathway. Oncotarget 7, 36185–36197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man SM, Karki R, Kanneganti TD, 2016a. AIM2 inflammasome in infection, cancer, and autoimmunity: Role in DNA sensing, inflammation, and innate immunity. Eur J Immunol 46, 269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man SM, Karki R, Malireddi RK, Neale G, Vogel P, Yamamoto M, Lamkanfi M, Kanneganti TD, 2015a. The transcription factor IRF1 and guanylate-binding proteins target activation of the AIM2 inflammasome by Francisella infection. Nat Immunol 16, 467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man SM, Karki R, Sasai M, Place DE, Kesavardhana S, Temirov J, Frase S, Zhu Q, Malireddi RKS, Kuriakose T, Peters JL, Neale G, Brown SA, Yamamoto M, Kanneganti TD, 2016b. IRGB10 Liberates Bacterial Ligands for Sensing by the AIM2 and Caspase-11-NLRP3 Inflammasomes. Cell 167, 382–396 e317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man SM, Zhu Q, Zhu L, Liu Z, Karki R, Malik A, Sharma D, Li L, Malireddi RK, Gurung P, Neale G, Olsen SR, Carter RA, McGoldrick DJ, Wu G, Finkelstein D, Vogel P, Gilbertson RJ, Kanneganti TD, 2015b. Critical Role for the DNA Sensor AIM2 in Stem Cell Proliferation and Cancer. Cell 162, 45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon F, Burns K, Tschopp J, 2002. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 10, 417–426. [DOI] [PubMed] [Google Scholar]
- Maruzuru Y, Ichinohe T, Sato R, Miyake K, Okano T, Suzuki T, Koshiba T, Koyanagi N, Tsuda S, Watanabe M, Arii J, Kato A, Kawaguchi Y, 2018. Herpes Simplex Virus 1 VP22 Inhibits AIM2-Dependent Inflammasome Activation to Enable Efficient Viral Replication. Cell host & microbe 23, 254–265 e257. [DOI] [PubMed] [Google Scholar]
- Matusiak M, Van Opdenbosch N, Lamkanfi M, 2015. CARD- and pyrin-only proteins regulating inflammasome activation and immunity. Immunol Rev 265, 217–230. [DOI] [PubMed] [Google Scholar]
- Meunier E, Wallet P, Dreier RF, Costanzo S, Anton L, Ruhl S, Dussurgey S, Dick MS, Kistner A, Rigard M, Degrandi D, Pfeffer K, Yamamoto M, Henry T, Broz P, 2015. Guanylate-binding proteins promote activation of the AIM2 inflammasome during infection with Francisella novicida. Nat Immunol 16, 476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milutin Gasperov N, Farkas SA, Nilsson TK, Grce M, 2014. Epigenetic activation of immune genes in cervical cancer. Immunol Lett 162, 256–257. [DOI] [PubMed] [Google Scholar]
- Morrone SR, Matyszewski M, Yu X, Delannoy M, Egelman EH, Sohn J, 2015. Assembly-driven activation of the AIM2 foreign-dsDNA sensor provides a polymerization template for downstream ASC. Nat Commun 6, 7827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muruve DA, Petrilli V, Zaiss AK, White LR, Clark SA, Ross PJ, Parks RJ, Tschopp J, 2008. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature 452, 103–107. [DOI] [PubMed] [Google Scholar]
- Natarajan A, Ghose R, Hill JM, 2006. Structure and dynamics of ASC2, a pyrin domain-only protein that regulates inflammatory signaling. J Biol Chem 281, 31863–31875. [DOI] [PubMed] [Google Scholar]
- Ni X, Ru H, Ma F, Zhao L, Shaw N, Feng Y, Ding W, Gong W, Wang Q, Ouyang S, Cheng G, Liu ZJ, 2016. New insights into the structural basis of DNA recognition by HINa and HINb domains of IFI16. Journal of molecular cell biology 8, 51–61. [DOI] [PubMed] [Google Scholar]
- Paludan SR, Bowie AG, Horan KA, Fitzgerald KA, 2011. Recognition of herpesviruses by the innate immune system. Nat Rev Immunol 11, 143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HH, Lo YC, Lin SC, Wang L, Yang JK, Wu H, 2007. The death domain superfamily in intracellular signaling of apoptosis and inflammation. Annual review of immunology 25, 561–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsos G, Germann A, Gebert J, Dihlmann S, 2010. Restoration of absent in melanoma 2 (AIM2) induces G2/M cell cycle arrest and promotes invasion of colorectal cancer cells. Int J Cancer 126, 1838–1849. [DOI] [PubMed] [Google Scholar]
- Ponomareva L, Liu H, Duan X, Dickerson E, Shen H, Panchanathan R, Choubey D, 2013. AIM2, an IFN-inducible cytosolic DNA sensor, in the development of benign prostate hyperplasia and prostate cancer. Mol Cancer Res 11, 1193–1202. [DOI] [PubMed] [Google Scholar]
- Price BD, D’Andrea AD, 2013. Chromatin remodeling at DNA double-strand breaks. Cell 152, 1344–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinam VA, Jiang Z, Waggoner SN, Sharma S, Cole LE, Waggoner L, Vanaja SK, Monks BG, Ganesan S, Latz E, Hornung V, Vogel SN, Szomolanyi-Tsuda E, Fitzgerald KA, 2010. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol 11, 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinholz M, Kawakami Y, Salzer S, Kreuter A, Dombrowski Y, Koglin S, Kresse S, Ruzicka T, Schauber J, 2013. HPV16 activates the AIM2 inflammasome in keratinocytes. Arch Dermatol Res 305, 723–732. [DOI] [PubMed] [Google Scholar]
- Roberts TL, Idris A, Dunn JA, Kelly GM, Burnton CM, Hodgson S, Hardy LL, Garceau V, Sweet MJ, Ross IL, Hume DA, Stacey KJ, 2009. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science 323, 1057–1060. [DOI] [PubMed] [Google Scholar]
- Ru H, Ni X, Zhao L, Crowley C, Ding W, Hung LW, Shaw N, Cheng G, Liu ZJ, 2013. Structural basis for termination of AIM2-mediated signaling by p202. Cell Res 23, 855–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson TR, Napier BA, Schroeder MR, Louwen R, Zhao J, Chin CY, Ratner HK, Llewellyn AC, Jones CL, Laroui H, Merlin D, Zhou P, Endtz HP, Weiss DS, 2014. A CRISPR-Cas system enhances envelope integrity mediating antibiotic resistance and inflammasome evasion. Proc Natl Acad Sci U S A 111, 11163–11168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sborgi L, Ravotti F, Dandey VP, Dick MS, Mazur A, Reckel S, Chami M, Scherer S, Huber M, Bockmann A, Egelman EH, Stahlberg H, Broz P, Meier BH, Hiller S, 2015. Structure and assembly of the mouse ASC inflammasome by combined NMR spectroscopy and cryo-electron microscopy. Proc Natl Acad Sci U S A 112, 13237–13242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulmann K, Brasch FE, Kunstmann E, Engel C, Pagenstecher C, Vogelsang H, Kruger S, Vogel T, Knaebel HP, Ruschoff J, Hahn SA, Knebel-Doeberitz MV, Moeslein G, Meltzer SJ, Schackert HK, Tympner C, Mangold E, Schmiegel W, German HC, 2005. HNPCC-associated small bowel cancer: clinical and molecular characteristics. Gastroenterology 128, 590–599. [DOI] [PubMed] [Google Scholar]
- Sharma BR, Karki R, Kanneganti TD, 2019. Role of AIM2 inflammasome in inflammatory diseases, cancer and infection. European journal of immunology 49, 1998–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehlik C, Krajewska M, Welsh K, Krajewski S, Godzik A, Reed JC, 2003. The PAAD/PYRIN-only protein POP1/ASC2 is a modulator of ASC-mediated nuclear-factor-kappa B and pro-caspase-1 regulation. Biochem J 373, 101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson KV, Junkins RD, Kurkjian CJ, Holley-Guthrie E, Pendse AA, El Morabiti R, Petrucelli A, Barber GN, Benedict CA, Ting JP, 2017. A noncanonical function of cGAMP in inflammasome priming and activation. J Exp Med 214, 3611–3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theobald DL, Mitton-Fry RM, Wuttke DS, 2003. Nucleic acid recognition by OB-fold proteins. Annu Rev Biophys Biomol Struct 32, 115–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Yin Q, 2019. Structural analysis of the HIN1 domain of interferon-inducible protein 204. Acta crystallographica. Section F, Structural biology communications 75, 455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii Y, Kawada JI, Murata T, Yoshiyama H, Kimura H, Ito Y, 2017. Epstein-Barr virus infection-induced inflammasome activation in human monocytes. PLoS One 12, e0175053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulland TK, Buchan BW, Ketterer MR, Fernandes-Alnemri T, Meyerholz DK, Apicella MA, Alnemri ES, Jones BD, Nauseef WM, Sutterwala FS, 2010. Cutting edge: mutation of Francisella tularensis mviN leads to increased macrophage absent in melanoma 2 inflammasome activation and a loss of virulence. J Immunol 185, 2670–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Opdenbosch N, Lamkanfi M, 2019. Caspases in Cell Death, Inflammation, and Disease. Immunity 50, 1352–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Yin Q, 2017. AIM2 inflammasome activation and regulation: A structural perspective. Journal of structural biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Tian Y, Yin Q, 2019. AIM2 Inflammasome Assembly and Signaling. Advances in experimental medicine and biology 1172, 143–155. [DOI] [PubMed] [Google Scholar]
- Wang PH, Ye ZW, Deng JJ, Siu KL, Gao WW, Chaudhary V, Cheng Y, Fung SY, Yuen KS, Ho TH, Chan CP, Zhang Y, Kok KH, Yang W, Chan CP, Jin DY, 2018. Inhibition of AIM2 inflammasome activation by a novel transcript isoform of IFI16. EMBO Rep 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren SE, Armstrong A, Hamilton MK, Mao DP, Leaf IA, Miao EA, Aderem A, 2010. Cutting edge: Cytosolic bacterial DNA activates the inflammasome via Aim2. J Immunol 185, 818–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber CH, Vincenz C, 2001. The death domain superfamily: a tale of two interfaces? Trends Biochem Sci 26, 475–481. [DOI] [PubMed] [Google Scholar]
- Wilson JE, Petrucelli AS, Chen L, Koblansky AA, Truax AD, Oyama Y, Rogers AB, Brickey WJ, Wang Y, Schneider M, Muhlbauer M, Chou WC, Barker BR, Jobin C, Allbritton NL, Ramsden DA, Davis BK, Ting JP, 2015. Inflammasome-independent role of AIM2 in suppressing colon tumorigenesis via DNA-PK and Akt. Nature medicine 21, 906–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woerner SM, Kloor M, Schwitalle Y, Youmans H, Doeberitz M, Gebert J, Dihlmann S, 2007. The putative tumor suppressor AIM2 is frequently affected by different genetic alterations in microsatellite unstable colon cancers. Genes Chromosomes Cancer 46, 1080–1089. [DOI] [PubMed] [Google Scholar]
- Wu B, Peisley A, Tetrault D, Li Z, Egelman EH, Magor KE, Walz T, Penczek PA, Hur S, 2014. Molecular imprinting as a signal-activation mechanism of the viral RNA sensor RIG-I. Mol Cell 55, 511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, 2013. Higher-order assemblies in a new paradigm of signal transduction. Cell 153, 287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Chen ZJ, 2014. Innate immune sensing and signaling of cytosolic nucleic acids. Annu Rev Immunol 32, 461–488. [DOI] [PubMed] [Google Scholar]
- Yan S, Shen H, Lian Q, Jin W, Zhang R, Lin X, Gu W, Sun X, Meng G, Tian Z, Chen ZW, Sun B, 2018. Deficiency of the AIM2-ASC Signal Uncovers the STING-Driven Overreactive Response of Type I IFN and Reciprocal Depression of Protective IFN-gamma Immunity in Mycobacterial Infection. J Immunol 200, 1016–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CA, Huang ST, Chiang BL, 2015. Sex-dependent differential activation of NLRP3 and AIM2 inflammasomes in SLE macrophages. Rheumatology (Oxford) 54, 324–331. [DOI] [PubMed] [Google Scholar]
- Yin Q, Sester DP, Tian Y, Hsiao YS, Lu A, Cridland JA, Sagulenko V, Thygesen SJ, Choubey D, Hornung V, Walz T, Stacey KJ, Wu H, 2013. Molecular mechanism for p202-mediated specific inhibition of AIM2 inflammasome activation. Cell reports 4, 327–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogarajah T, Ong KC, Perera D, Wong KT, 2017. AIM2 Inflammasome-Mediated Pyroptosis in Enterovirus A71-Infected Neuronal Cells Restricts Viral Replication. Sci Rep 7, 5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen J, Zhang L, Pan J, Ma S, Yu X, Li X, Chen S, Du W, 2014. AIM2 mediates inflammation-associated renal damage in hepatitis B virus-associated glomerulonephritis by regulating caspase-1, IL-1beta, and IL-18. Mediators Inflamm 2014, 190860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Zu X, Liu S, Zhang H, 2019. The absent in melanoma 2 (AIM2) inflammasome in microbial infection. Clin Chim Acta 495, 100–108. [DOI] [PubMed] [Google Scholar]