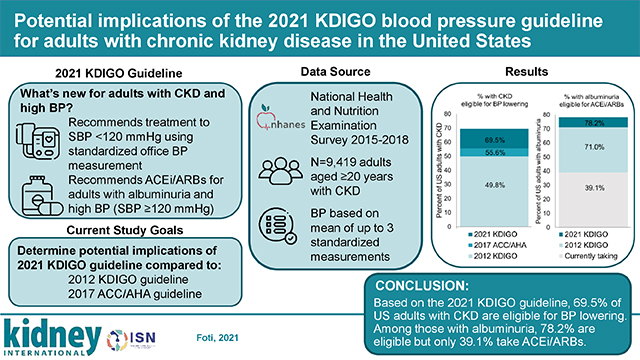
Abstract
The 2021 Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline on the Management of Blood Pressure in Chronic Kidney Disease (CKD) recommends a target systolic blood pressure under 120 mmHg based on standardized office blood pressure measurement. Here, we examined the potential implications of this new guideline for blood pressure lowering with antihypertensive medication among adults in the United States with CKD compared to the 2012 KDIGO guideline (target blood pressure 130/80 mmHg or under with albuminuria or 140/90 mmHg or under without albuminuria) and the 2017 American College of Cardiology/American Heart Association (target blood pressure under 130/80 mmHg) guideline. Additionally, we determined implications of the 2021 KDIGO guideline for angiotensin converting enzyme inhibitor (ACEi) or angiotensin II-receptor blocker (ARB) use for those with albuminuria (recommended at systolic blood pressure of 120 mmHg or over) compared to the 2012 KDIGO guideline (recommended at blood pressures over 130/80 mmHg). Data were analyzed from 1,699 adults with CKD (estimated glomerular filtration rate 15–59 ml/min/1.73m2 or a urinary albumin-to-creatinine ratio of 30 mg/g or more) in the 2015–2018 National Health and Nutrition Examination Survey and averaged up to three standardized blood pressure measurements. Among adults with CKD, 69.5% were eligible for blood pressure lowering according to the 2021 KDIGO guideline, compared with 49.8% as per 2012 KDIGO or 55.6% as per 2017 American College of Cardiology/American Heart Association guidelines. Among those with albuminuria, 78.2% were eligible for ACEi/ARB use by the 2021 KDIGO guideline compared with 71.0% by the 2012 KDIGO guideline. However, only 39.1% were taking an ACEi/ARB. Thus, our findings highlight opportunities to improve blood pressure management and reduce cardiovascular risk among adults in the United States with CKD.
Keywords: Hypertension, blood pressure, chronic kidney disease, albuminuria, clinical practice guidelines
Graphical Abstract
INTRODUCTION
The 2021 Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline on the Management of Blood Pressure (BP) in Chronic Kidney Disease (CKD)1 updated the KDIGO BP guideline published in 2012,2 and includes recommendations for BP management for individuals with non-dialysis CKD. In comparison to the systolic/diastolic blood pressure (SBP/DBP) targets of ≤130/≤80 mmHg for those with albuminuria and ≤140/≤90 mmHg for those without albuminuria in the 2012 KDIGO guideline, the 2021 guideline includes a recommendation that adults with CKD and high BP who are not kidney transplant recipients be treated to a target SBP <120 mmHg based on standardized office BP measurements (Table 1).1,2 Standardized office BP refers to measurements obtained following a guideline-recommended protocol and conducted by trained clinical staff.3
Table 1.
2012 KDIGO, 2021 KDIGO, and 2017 ACC/AHA blood pressure guideline recommendations for blood pressure goals and use of renin angiotensin aldosterone system inhibitors (ACEi or ARB)
| Recommendation | 2012 KDIGO | 2021 KDIGO | 2017 ACC/AHA |
|---|---|---|---|
| Blood pressure goals* | |||
| No albuminuria (A1) | SBP ≤140 mm Hg and DBP ≤90 mm Hg (1B) | SBP <120 mm Hg, when tolerated, using standardized office BP measurement (2B) | SBP <130 mm Hg and DBP < 80 mm Hg for all adults with CKD |
| Albuminuria (A2, A3),† No diabetes | SBP ≤130 mm Hg and DBP ≤80 mm Hg (A2: 2D, A3: 2C) | ||
| Albuminuria (A2, A3),† Diabetes | SBP ≤130 mm Hg and DBP ≤80 mm Hg (2D) | ||
| RASi (ACEi/ARB) use | |||
| No albuminuria (A1) | — | — | Treatment with ACEi (or ARB if not tolerated) may be reasonable in those with hypertension∥ and eGFR<60 ml/min/1.73m2 or eGFR ≥60 ml/min/1.73m2 with ACR ≥300 mg/g |
| Albuminuria (A2, A3),† No diabetes | Suggest (A2: 2D)/Recommend (A3: 1B) RASi when BP-lowering drugs are indicated‡ | Suggest (A2: 2C)/ Recommend (A3: 1B) starting RASi for those with high BP§ | |
| Albuminuria (A2, A3),† Diabetes | Suggest (A2: 2D)/Recommend (A3: 1B) RASi when BP-lowering drugs are indicated‡ | Recommend (A2, A3) RASi for people with high BP§ (1B) | |
Abbreviations: KDIGO, Kidney Disease: Improving Global Outcomes. ACC, American College of Cardiology. AHA, American Heart Association. RASi, Renin angiotensin aldosterone system inhibitors [defined as angiotensin-converting enzyme inhibitors (ACEi) or Angiotensin II-receptor blockers (ARB)]. SBP, systolic blood pressure. DBP, diastolic blood pressure. BP, blood pressure. CKD, chronic kidney disease. eGFr, estimated glomerular filtration rate.
Evidence grading: Level 1 “We recommend,” Level 2 “We suggest.” Quality of evidence: A “High,”, B “Moderate,” C “Low,” D “Very low.”
Recommend those whose blood pressure is consistently above the goal be treated with blood pressure-lowering drugs to maintain a blood pressure that is consistently below the goal
Albuminuria defined as albumin-to-creatinine ratio (ACR) ≥30 mg/g. A2: ACR 30–300 mg/g. A3: ACR>300 mg/g
BP above the goal of SBP ≤130 mm Hg and DBP ≤80 mm Hg for those with albuminuria
Blood pressure above the goal of SBP <120 mm Hg
Blood pressure above the goal of SBP <130 mm Hg and DBP <80 mmHg
The evidence supporting the lower treatment target comes primarily from the Systolic Blood Pressure Intervention Trial (SPRINT), which showed targeting SBP <120 mmHg as compared with <140 mmHg reduced the risk of cardiovascular disease by 25% and all-cause mortality by 27%.4 CKD was a pre-specified sub-group in SPRINT and the benefits of a target SBP <120 mm Hg versus a target <140 mm Hg were similar for participants with and without CKD.4 BP measurements in SPRINT were obtained using standardized measurement procedures and automated BP devices, the use of which are emphasized in the 2021 KDIGO guideline when applying the lower-recommended SBP target.1,4
Both the 2021 and 2012 KDIGO guidelines recommend use of angiotensin-converting enzyme inhibitor (ACEi) or Angiotensin II-receptor blocker (ARB) medications in patients with albuminuria and BP above the target who are not kidney transplant recipients.1 However, as the target is lower in the 2021 KDIGO guideline (SBP <120 mmHg) compared with the 2012 KDIGO guideline (SBP/DBP ≤130/≤80), individuals with albuminuria and SBP 120–129 mmHg are now recommended treatment with ACEi/ARBs.1
The 2021 KDIGO guideline has major implications for the management of BP in adults with CKD, a population with high cardiovascular disease (CVD) risk. Using nationally representative data from the 2015–2018 National Health and Nutrition Examination Survey (NHANES), the objective of the current analysis was to estimate the proportion of US adults with CKD eligible for BP lowering according to the KDIGO 2021 guideline and compare this to the proportion eligible based on the 2012 KDIGO guideline, as well as the 2017 American College of Cardiology (ACC)/American Heart Association (AHA) guideline which recommended a SBP/DBP target <130/<80 mmHg for adults with CKD treated with antihypertensive medication.5 Secondarily, we compared the proportion of adults with CKD and albuminuria recommended for treatment with an ACEi/ARB according to the 2021 and 2012 KDIGO guidelines. Finally, we described the number and classes of antihypertensive medication being taken among US adults with CKD.
METHODS
Data source
NHANES is a population-based survey conducted by the National Center for Health Statistics of the U.S. Centers for Disease Control and Prevention, which uses stratified, multistage probability sampling to produce nationally representative estimates of the health and nutritional status of the civilian, noninstitutionalized US population. Since 1999–2000, the survey has been conducted in 2-year cycles. For the present analysis, we pooled data from the 2015–2016 and 2017–2018 survey cycles to obtain stable prevalence estimates. NHANES was approved by the National Center for Health Statistics Institutional Review Board and written informed consent was obtained from all participants.
Data collection
NHANES data collection occurred during an in-home interview and a study visit at a mobile examination center where physical and laboratory measurements were conducted. Covariates included in this analysis and their methods of assessment are described in Table S1.
Study population
The study population included adults aged ≥20 years who completed the NHANES interview and examination, and had laboratory data (N=10,739). We excluded participants missing information on serum creatinine or urine albumin/creatinine ratio (N=838), those who did not have at least one valid blood pressure measurement (N=360), as well as those missing information on other covariates (N=122). The analytic sample included 9,419 participants.
Chronic kidney disease assessment
CKD was defined as having a single estimated glomerular filtration rate (eGFR) <60 ml/min/1.73m2 or as having albuminuria based on a single urinary albumin-to-creatinine ratio (ACR) ≥30 mg/g. We calculated creatinine-based eGFR using the CKD-EPI equation.6 We excluded participants with eGFR <15 ml/min/1.73m2 from the analysis as this was a small group and might not be representative of those individuals with eGFR in this range but not on dialysis. There were 1,699 participants with CKD in the analytic sample.
Blood pressure measurement
Blood pressure measurements were obtained by trained study physicians using a standardized protocol during the NHANES examination.7 After five minutes of seated rest, three BP measurements were taken using a mercury sphygmomanometer with an appropriately sized cuff. If a measurement was interrupted or incomplete, a fourth attempt was made. We used up to three measurements to calculate the mean SBP and DBP for each participant.8 Study physicians were certified to measure BP following the completion of a training program, which included practice listening to Korotkoff sounds using a standardized audio-video tape presentation and practice measuring BP on volunteers. Physicians were recertified quarterly.7
Antihypertensive medication use
Antihypertensive medication use was determined based on questionnaire responses and confirmed by a pill bottle review. Participants who responded yes to the questions, “Have you ever been told by a doctor or other healthcare professional that you had hypertension, also called high blood pressure?” and, “Are you now taking prescribed medication for high blood pressure?”, and had a prescription for one or more classes of antihypertensive medication were considered to be taking antihypertensive medication. Antihypertensive medication classes were determined using the Multum Lexicon 3-level nested category system that assigns a therapeutic classification to each drug and each ingredient of the drug. Combination antihypertensive medications were classified into individual antihypertensive medication classes.
Recommended BP goals and ACEi/ARB use
Recommended BP goals were based on the 2012 KDIGO guideline, 2021 KDIGO guideline, and 2017 ACC/AHA guideline (Table 1). Those with SBP ≥120 mmHg were considered to have BP above the 2021 KDIGO target. Those with albuminuria and SBP/DBP >130/80 mmHg and those without albuminuria and SBP/DBP >140/90 mmHg were considered to have BP above the 2012 KDIGO target. Those with SBP/DBP ≥130/80 mmHg were considered to have BP above the 2017 ACC/AHA target.
Individuals were considered eligible for ACEi/ARB use by the 2021 or 2012 KDIGO guidelines if they had albuminuria and met 1 of the 2 following conditions: 1) were currently taking an ACEi/ARB regardless of their BP; or 2) had BP above the respective KDIGO guideline target.
Analysis
We estimated the distribution of US adults with CKD across 6 groups with SBP <120 mmHg, 120–140 mmHg, and >140 mmHg, according to whether they were taking antihypertensive medication. We estimated the sociodemographic (age, sex, race/ethnicity, education, family income-to-poverty ratio) and clinical characteristics [eGFR category (G1/G2: eGFR ≥60, G3a: eGFR 45–59, G3b: eGFR 30–44, G4: eGFR 15–29 ml/min/1.73m2),9 ACR category (A1: ACR <30, A2: ACR 30–300, A3: ACR >300 mg/g),9 CKD awareness, diabetes, 10-year predicted ASCVD risk10 or history of CVD, 5-year predicted kidney failure risk11] of adults with CKD in each of these categories.
We estimated the SBP distribution among US adults with CKD, overall, and for those taking and not taking antihypertensive medication. We calculated the percent of US adults with CKD who had BP above the 2021 KDIGO, 2012 KDIGO, and 2017 ACC/AHA guideline recommended targets, as well as the difference in the percent with BP above the 2021 KDIGO guideline compared to the 2012 KDIGO and 2017 ACC/AHA guidelines. These calculations were performed for the overall population with CKD and for those with CKD by sociodemographic and clinical characteristics. We examined factors associated with BP above the 2021 KDIGO guideline goal (i.e., SBP ≥120 mm Hg) among US adults with CKD using negative binomial regression to calculate adjusted prevalence ratios.
We estimated the percent of US adults with CKD and albuminuria taking an ACEi/ARB and the percent recommended an ACEi/ARBs by the 2021 and 2012 KDIGO guidelines, separately. Calculations were conducted overall and by participant characteristics. We used negative binomial regression to calculate adjusted prevalence ratios for factors associated with ACEi/ARB use.
Finally, among those taking antihypertensive medication, we estimated the percent taking ACEi/ARB, beta-blockers, calcium channel blockers, diuretics, or other classes of medication. Also, we estimated the distributions of the number and classes of antihypertensive medications being taken.
All analyses were conducted using Stata version 15.1 (StataCorp, College Station, TX) using survey commands to account for the complex survey design (using strata and sampling units to identify participants) and sampling weights to produce nationally representative prevalence estimates for the noninstitutionalized US population.12,13 We estimated the number of US adults with CKD overall and by subgroup by adjusting the total weights for the NHANES participants with non-missing data to the 2018 US census population estimates.14 A 2-sided p-value of 0.05 was used to define statistical significance.
RESULTS
In 2015–2018, 40.9% of US adults with CKD were not taking antihypertensive medication, including 15.6% with SBP < 120 mm Hg, 14.8% with SBP of 120–140 mm Hg and 10.5% with SBP >140 mmHg (Table 2). Among those who were not taking antihypertensive medication, the majority had eGFR ≥60 ml/min/1.73m2 and met criteria for CKD due to the presence of albuminuria alone. Adults who were taking antihypertensive medication were more likely to be older, have diabetes, high 10-year predicted atherosclerotic cardiovascular disease (ASCVD) risk or prevalent CVD compared to those who were not taking antihypertensive medication. Among those who were and were not taking antihypertensive medication, individuals with SBP >140 mmHg were more likely to be non-Hispanic Black or non-Hispanic Asian, as well as to have lower family income-to-poverty ratio and lower education compared with their counterparts with SBP <120 mm Hg and 120–140 mm Hg.
Table 2.
Characteristics of US adults with chronic kidney disease by systolic blood pressure and antihypertensive medication use, NHANES 2015–2018
| Characteristic | Not Treated with Antihypertensive Medication |
Treated with Antihypertensive Medication |
||||
|---|---|---|---|---|---|---|
| SBP, mmHg | <120 | 120–140 | >140 | <120 | 120–140 | >140 |
| Unweighted N | 197 | 244 | 184 | 221 | 391 | 462 |
| Weighted % | 15.6% | 14.8% | 10.5% | 14.9% | 20.0% | 24.2% |
| eGFR (ml/min/1.73m2), Median (IQR) | 98.7 (76.5–117.7) | 87.4 (58.1–110.7) | 80.5 (53.5–109.3) | 56.1 (48.6–68.8) | 58.4 (49.8–88.7) | 59.4 (47.2–83.1) |
| eGFR category (ml/min/1.73m2), % (SE) | ||||||
| ≥60* | 82.5 (3.4) | 67.5 (4.6) | 65.9 (4.9) | 33.5 (6.4) | 45.0 (3.8) | 49.1 (3.6) |
| 45–59 | 16.5 (3.3) | 28.0 (5.0) | 24.1 (5.4) | 51.0 (5.3) | 38.5 (3.2) | 30.7 (3.4) |
| 30–44 | 1.0 (0.6) | 4.1 (1.5) | 7.8 (2.6) | 14.4 (2.5) | 12.0 (1.9) | 15.3 (2.4) |
| 15–29 | 0.0 (.) | 0.4 (0.3) | 2.2 (1.1) | 1.1 (0.5) | 4.5 (1.5) | 4.9 (1.1) |
| ACR (mg/g), Median (IQR) | 48.3 (34.8–114.5) | 50.9 (17.3–110.3) | 52.6 (30.6–149.3) | 16.9 (8.1–60.2) | 42.4 (11.0–88.0) | 54.4 (18.8–154.9) |
| ACR category (mg/g), % (SE) | ||||||
| <30* | 13.5 (2.6) | 28.5 (4.5) | 22.9 (5.7) | 53.4 (5.6) | 37.7 (3.2) | 28.8 (2.8) |
| 30–300 | 77.8 (3.3) | 60.6 (3.9) | 61.0 (5.9) | 39.1 (5.5) | 53.4 (3.3) | 54.7 (3.1) |
| >300 | 8.7 (2.6) | 11.0 (2.6) | 16.1 (4.1) | 7.5 (2.9) | 8.9 (1.5) | 16.5 (2.5) |
| Age (years), Mean (SE) | 43.6 (1.6) | 54.4 (1.8) | 58.2 (1.5) | 65.0 (0.9) | 66.9 (0.8) | 70.4 (0.8) |
| Age category (years), % (SE) | ||||||
| 20–44 | 55.3 (5.2) | 33.4 (5.2) | 21.0 (3.9) | 5.4 (2.0) | 5.7 (1.8) | 1.5 (0.6) |
| 45–64 | 28.8 (4.5) | 31.9 (4.2) | 39.4 (6.7) | 38.2 (6.1) | 30.2 (3.0) | 24.8 (3.1) |
| ≥65 | 15.9 (3.9) | 34.7 (3.8) | 39.6 (6.5) | 56.4 (5.7) | 64.1 (3.2) | 73.8 (3.2) |
| Female, % (SE) | 71.2 (4.1) | 49.6 (4.3) | 56.9 (7.1) | 47.3 (4.9) | 49.3 (3.7) | 59.7 (3.7) |
| Race/ethnicity, % (SE) | ||||||
| Non-Hispanic White | 61.7 (4.7) | 61.3 (5.3) | 62.0 (4.9) | 77.0 (3.9) | 67.3 (4.4) | 68.0 (3.4) |
| Mexican American | 10.5 (2.4) | 13.5 (3.1) | 7.4 (2.5) | 5.3 (1.6) | 5.5 (1.4) | 5.4 (1.4) |
| Non-Hispanic Black | 9.7 (2.4) | 8.9 (2.1) | 13.3 (3.2) | 7.1 (1.6) | 14.4 (3.0) | 14.6 (2.4) |
| Non-Hispanic Asian | 6.1 (1.7) | 5.7 (1.2) | 5.5 (1.6) | 1.7 (0.7) | 5.8 (1.3) | 4.9 (1.4) |
| Other | 11.9 (2.5) | 10.7 (2.2) | 11.8 (3.5) | 8.9 (2.6) | 7.0 (1.7) | 7.0 (1.5) |
| Income-to-poverty ratio, % (SE) | ||||||
| Tertile 1 | 24.0 (4.3) | 26.0 (3.1) | 25.8 (4.1) | 17.2 (3.0) | 22.6 (3.0) | 23.9 (3.3) |
| Tertile 2 | 30.9 (3.9) | 32.1 (4.6) | 33.4 (5.1) | 25.6 (4.4) | 28.8 (3.3) | 41.0 (3.6) |
| Tertile 3 | 36.8 (6.2) | 32.9 (5.0) | 27.8 (6.8) | 52.9 (4.6) | 38.8 (4.3) | 24.3 (3.9) |
| Missing | 8.3 (2.6) | 9.0 (2.2) | 13.0 (2.6) | 4.3 (2.2) | 9.8 (1.7) | 10.9 (1.7) |
| Education, % (SE) | ||||||
| <High school | 5.6 (1.7) | 7.1 (1.7) | 9.2 (2.1) | 3.9 (1.0) | 7.7 (1.3) | 8.0 (1.6) |
| High school degree | 33.6 (4.6) | 33.2 (3.6) | 42.5 (4.3) | 31.8 (3.5) | 35.9 (3.3) | 46.4 (3.3) |
| >High school degree | 60.9 (4.5) | 59.7 (4.1) | 48.3 (4.4) | 64.2 (3.5) | 56.5 (3.7) | 45.6 (3.3) |
| CKD awareness, % (SE) | 2.7 (1.2) | 7.6 (3.0) | 5.3 (1.3) | 14.5 (3.5) | 16.1 (2.3) | 16.3 (2.0) |
| Diabetes, % (SE) | 5.7 (1.8) | 20.9 (3.8) | 29.6 (5.0) | 46.9 (4.4) | 38.8 (3.2) | 38.4 (3.3) |
| SBP (mmHg), Mean (SE) | 108.8 (0.8) | 128.8 (0.5) | 160.3 (1.6) | 110.4 (0.8) | 130.2 (0.4) | 158.2 (0.8) |
| DBP (mmHg), Mean (SE) | 67.3 (0.7) | 72.3 (0.9) | 82.6 (1.1) | 63.4 (0.9) | 69.0 (0.8) | 73.0 (1.0) |
| 10-year predicted ASCVD risk, % (SE) | ||||||
| <5% | 76.6 (4.1) | 43.8 (4.5) | 26.8 (4.6) | 14.7 (3.5) | 8.6 (1.8) | 1.4 (0.6) |
| 5–<10% | 10.9 (3.3) | 16.2 (4.1) | 17.9 (5.4) | 14.9 (3.5) | 10.3 (2.8) | 8.6 (2.8) |
| 10–<20% | 5.7 (2.1) | 14.6 (3.4) | 17.6 (3.6) | 20.9 (4.0) | 22.1 (3.4) | 10.9 (2.4) |
| ≥20% | 2.7 (1.1) | 16.5 (2.8) | 25.8 (4.6) | 21.1 (4.0) | 28.7 (3.7) | 48.0 (3.8) |
| Prevalent CVD | 4.0 (1.3) | 8.9 (2.1) | 11.9 (2.7) | 28.5 (4.4) | 30.2 (2.8) | 31.0 (3.2) |
| 5-year predicted kidney failure risk†, % (SE) | ||||||
| <2% | 97.9 (1.1) | 97.2 (1.0) | 90.4 (3.1) | 92.4 (2.1) | 85.9 (2.2) | 84.6 (2.0) |
| 2–<5% | 1.3 (1.0) | 1.0 (0.5) | 4.9 (2.4) | 3.8 (1.4) | 6.5 (1.3) | 7.5 (1.6) |
| ≥5% | 0.8 (0.4) | 1.8 (1.0) | 4.7 (1.5) | 3.9 (1.1) | 7.6 (1.6) | 7.8 (1.5) |
| Number of antihypertensive medications | ||||||
| 1 | -- | -- | -- | 43.5 (4.8) | 36.6 (3.4) | 33.6 (3.7) |
| 2 | -- | -- | -- | 18.3 (3.2) | 27.9 (3.5) | 28.9 (3.4) |
| ≥3 | -- | -- | -- | 38.2 (3.9) | 35.6 (2.9) | 37.5 (3.5) |
Abbreviations: SBP, systolic blood pressure. SE, standard error. IQR, interquartile range. eGFR, estimated glomerular filtration rate. ACR, albumin-to-creatinine ratio. CKD, chronic kidney disease. DBP, diastolic blood pressure. ASCVD, atherosclerotic cardiovascular disease. CVD, cardiovascular disease.
eGFR ≥60 ml/min/1.73m2 indicates individuals defined as having CKD based on the presence of albuminuria. ACR <30 mg/g indicates individuals defined as having CKD based on reduced eGFR without albuminuria.
Among those with eGFR 15–59 ml/min/1.73m2
Among the 35.3 million US adults with CKD, 69.5% had SBP ≥120 mmHg, 49.8% had SBP >130 mmHg, and 34.7% had SBP >140 mmHg (Figure 1). Among the 14.4 million US adults with CKD not taking antihypertensive medication, 61.8% had SBP ≥120 mmHg, 39.5% had SBP >130 mmHg, and 25.6% had SBP >140 mmHg. Among the 20.9 million US adults with CKD taking antihypertensive medication, 74.8%, 57.0%, and 40.9% had SBP ≥120 mmHg, >130 mmHg, and >140 mmHg, respectively.
Figure 1 |.
Distribution of systolic blood pressure (SBP) in US adults with chronic kidney disease (CKD) overall and by antihypertensive medication use, National Health and Nutrition Examination Survey, 2015–2018. This figure shows the distribution of SBP overall among US adults with CKD, among those not taking antihypertensive medication, and among those taking antihypertensive medication. The SBP cut points shown in the figures reflect the percentage with SBP above the thresholds used in the 2021 (<120 mm Hg) and 2012 (≤130 mm Hg with albuminuria, ≤140 mm Hg without albuminuria) Kidney Disease: Improving Global Outcomes guidelines. M, million.
Abbreviations: M. million. CKD. chronic kidney disease. SBP. systolic blood pressure.
Overall, 69.5% (24.5 million) of US adults with CKD had BP above the 2021 KDIGO target compared with 49.8% (17.6 million) who had BP above the KDIGO 2012 target (Table 3), an increase of 19.6% or approximately 6.9 million US adults. The percent of US adults with BP above the 2021 KDIGO target was higher in lower eGFR categories and in higher ACR categories, among those who were non-Hispanic Black, non-Hispanic Asian versus non-Hispanic white, had lower income or education, were older, had diabetes, had higher ASCVD risk or prevalent CVD, or had higher kidney failure risk. Overall, 55.6% (19.6 million) of adults with CKD had BP above the 2017 ACC/AHA BP guideline target (Table S2). Overall, 13.9% or approximately 4.9 million US adults with CKD had BP above the target in the 2021 KDIGO guideline but not the 2017 AHA/ACC guideline.
Table 3.
Percent (SE) and millions (SE) of US adults with chronic kidney disease and blood pressure above the KDIGO 2021 and KDIGO 2012 guideline targets*, NHANES 2015–2018
| Characteristic | Weighted N, millions† | Blood pressure above 2021 KDIGO target % (SE) | Blood pressure above 2012 KDIGO target % (SE) | Difference between 2021 and 2012 KDIGO targets % (SE) |
|---|---|---|---|---|
| Unweighted N | 1281 | 930 | 1699 | |
| Overall | 35.3 (1.5) | 69.5 (1.7) | 49.8 (1.7) | 19.6 (1.0) |
| eGFR category (ml/min/1.73m2) | ||||
| ≥60‡ | 19.6 (0.7) | 67.7 (2.4) | 55.9 (2.6) | 11.8 (1.2) |
| 45–59 | 11.3 (0.6) | 68.0 (3.9) | 37.0 (3.5) | 31.0 (2.1) |
| 30–44 | 3.5 (0.3) | 76.4 (3.8) | 49.9 (4.7) | 26.5 (3.2) |
| 15–29 | 0.9 (0.2) | 93.4 (2.9) | 70.0 (8.5) | 23.4 (5.4) |
| ACR category (mg/g) | ||||
| <30† | 11.0 (0.6) | 67.7 (3.5) | 31.1 (3.1) | 36.7 (2.2) |
| 30–300 | 20.2 (0.6) | 68.6 (2.5) | 56.9 (2.5) | 11.7 (1.2) |
| >300 | 4.1 (0.4) | 78.6 (4.3) | 65.7 (5.0) | 12.9 (2.5) |
| Age category (years) | ||||
| 20–44 | 6.4 (0.5) | 47.7 (3.9) | 35.4 (4.2) | 12.3 (2.8) |
| 45–64 | 11.0 (0.7) | 67.2 (2.4) | 53.0 (3.2) | 14.2 (1.7) |
| ≥65 | 17.9 (0.6) | 78.6 (2.6) | 53.0 (2.4) | 25.5 (1.4) |
| Sex | ||||
| Female | 19.7 (0.7) | 67.4 (2.4) | 47.5 (2.2) | 19.9 (1.4) |
| Male | 15.6 (0.7) | 72.0 (2.5) | 52.7 (2.9) | 19.3 (1.5) |
| Race/ethnicity | ||||
| Non-Hispanic White | 23.5 (1.0) | 68.3 (2.2) | 47.7 (2.2) | 20.6 (1.6) |
| Mexican American | 2.7 (0.5) | 68.0 (4.0) | 49.3 (4.1) | 18.7 (2.8) |
| Non-Hispanic Black | 4.1 (0.6) | 78.0 (2.3) | 59.5 (2.7) | 18.5 (2.2) |
| Non-Hispanic Asian | 1.8 (0.3) | 75.8 (4.6) | 59.9 (3.8) | 15.9 (3.2) |
| Other | 3.2 (0.3) | 64.9 (5.0) | 47.9 (4.2) | 17.0 (2.8) |
| Income-to-poverty ratio tertile | ||||
| Tertile 1 | 8.2 (0.5) | 72.7 (2.4) | 53.4 (3.0) | 19.3 (1.8) |
| Tertile 2 | 11.5 (0.5) | 73.4 (2.6) | 55.5 (3.2) | 17.9 (1.8) |
| Tertile 3 | 12.4 (1.0) | 61.1 (3.5) | 39.6 (3.9) | 21.4 (2.2) |
| Missing | 3.2 (0.4) | 79.0 (4.8) | 59.5 (4.8) | 19.5 (2.9) |
| Education | ||||
| <High school | 2.4 (0.3) | 79.0 (4.4) | 59.9 (4.3) | 19.0 (2.9) |
| High school degree | 13.3 (0.5) | 73.5 (2.2) | 54.1 (2.6) | 19.4 (1.7) |
| >High school degree | 19.5 (0.6) | 65.5 (2.5) | 45.6 (2.2) | 19.9 (1.5) |
| CKD awareness | ||||
| No | 31.2 (0.4) | 68.4 (1.9) | 49.3 (1.9) | 19.1 (1.1) |
| Yes | 4.0 (0.4) | 77.3 (4.3) | 53.1 (4.7) | 24.2 (2.7) |
| Diabetes | ||||
| No | 24.3 (0.6) | 67.1 (2.3) | 46.2 (2.4) | 20.9 (1.3) |
| Yes | 11.0 (0.6) | 74.6 (2.5) | 57.9 (2.8) | 16.7 (1.7) |
| Number of antihypertensive medications | ||||
| 0 | 14.4 (0.6) | 61.8 (2.8) | 41.7 (3.4) | 20.2 (1.7) |
| 1 | 7.7 (0.7) | 70.4 (3.6) | 53.2 (3.6) | 17.2 (2.3) |
| 2 | 5.4 (0.3) | 82.1 (3.3) | 62.8 (3.9) | 19.3 (2.4) |
| ≥3 | 7.7 (0.5) | 74.0 (2.9) | 52.7 (2.9) | 21.2 (2.0) |
| Any ACEi or ARB | ||||
| No | 20.3 (0.5) | 67.6 (2.6) | 47.1 (3.0) | 20.5 (1.4) |
| Yes | 14.9 (0.5) | 72.0 (2.5) | 53.5 (2.8) | 18.5 (1.5) |
| 10-year predicted ASCVD risk | ||||
| <5% | 9.0 (0.5) | 44.5 (2.9) | 30.4 (3.3) | 14.1 (2.2) |
| 5–<10% | 4.3 (0.5) | 68.2 (5.6) | 53.8 (6.3) | 14.4 (3.5) |
| 10–<20% | 5.3 (0.4) | 73.4 (4.6) | 48.6 (5.0) | 24.8 (2.8) |
| ≥20% | 9.2 (0.5) | 86.3 (2.7) | 64.1 (3.1) | 22.2 (1.9) |
| Prevalent CVD | 7.4 (0.4) | 76.8 (3.5) | 54.3 (3.8) | 22.5 (2.0) |
| 5-year predicted kidney failure risk§ | ||||
| <2% | 31.9 (0.3) | 67.9 (1.9) | 48.5 (1.9) | 19.4 (1.1) |
| 2–<5% | 1.6 (0.2) | 83.1 (4.9) | 60.7 (5.6) | 22.4 (4.4) |
| ≥5% | 1.7 (0.2) | 85.7 (3.3) | 64.0 (5.7) | 21.7 (3.4) |
Abbreviations: SE, standard error. KDIGO, Kidney Disease: Improving Global Outcomes. eGFR, estimated glomerular filtration rate. ACR, albumin-to-creatinine ratio. CKD, chronic kidney disease. ACEi, angiotensin-converting enzyme inhibitor. ARB, Angiotensin II-receptor blocker. ASCVD, atherosclerotic cardiovascular disease. CVD, cardiovascular disease.
BP control by the 2021 KDIGO guideline: SBP <120 mmHg. BP control by the 2012 KDIGO guideline: ≤130/80 mmHg for those with albuminuria, ≤140/90 mmHg for those without albuminuria.
Based on 2018 US Census population estimates.
eGFR ≥60 ml/min/1.73m2 indicates individuals defined as having CKD based on the presence of albuminuria. ACR <30 mg/g indicates individuals defined as having CKD based on reduced eGFR without albuminuria.
Among those with eGFR 15–59 ml/min/1.73m2
After adjustment for sociodemographic and clinical characteristics, US adults with eGFR 15–29 vs 45–59 ml/min/1.73m2 [Prevalence Ratio (PR): 1.19, 95% Confidence Interval (CI): 1.03–1.38)], with ACR >300 mg/g vs <30 mg/g (PR: 1.20, 95% CI: 1.01–1.41), and who were non-Hispanic Black (PR: 1.20, 95% CI: 1.08–1.32) or non-Hispanic Asian (PR: 1.12, 95% CI: 1.02–1.23) vs non-Hispanic white were more likely to have BP above the 2021 KDIGO target (Table S3).
Among US adults with CKD and albuminuria, 39.1% were currently taking an ACEi/ARB (Table 4). Overall, 78.2% of US adults with albuminuria were eligible for an ACEi/ARB according to the KDIGO 2021 guideline compared with 71.0% according to KDIGO 2012 guideline. ACEi/ARB use was less common, and a lower proportion was recommended for ACEi/ARB treatment, among those with eGFR ≥60 ml/min/1.73m2 or 15–29 ml/min/1.73m2 versus with eGFR of 30–59 ml/min/1.73 m2, those 20–44 versus ≥ 45 years of age, who were non-white, in the first or second tertile of family income-to-poverty ratio, who were not aware of their CKD, and without diabetes. ACEi/ARB use was higher among men and among those with ACR >300 mg/g, but in the difference in recommended and current ACEi/ARB use was greater than for women and those with ACR 30–300 mg/g.
Table 4.
Percentage (SE) of US adults with chronic kidney disease and albuminuria currently taking ACEi/ARBs and recommended ACEi/ARBs by the 2021 and 2012 KDIGO guidelines, NHANES 2015–2018
| Characteristic | Taking ACEi/ARBs, % (SE) | Recommended ACEi/ARBs, % (SE)* |
Difference between % recommended by 2021 guideline and % currently taking ACEi/ARBs, % (SE) | |
|---|---|---|---|---|
| By 2021 guideline | By 2012 guideline | |||
| Overall | 39.1 (1.4) | 78.2 (2.0) | 71.0 (2.2) | 39.1 (1.4) |
| eGFR category (ml/min/1.73m2) | ||||
| ≥60 | 34.6 (1.7) | 74.8 (2.4) | 67.0 (2.6) | 40.3 (1.6) |
| 45–59 | 60.8 (6.0) | 88.9 (5.1) | 85.9 (4.8) | 28.1 (4.0) |
| 30–44 | 64.3 (6.6) | 94.9 (2.6) | 88.8 (3.3) | 30.6 (5.0) |
| 15–29 | 46.0 (9.2) | 98.3 (1.7) | 95.8 (2.7) | 52.3 (7.7) |
| ACR category (mg/g) | ||||
| 30–300 | 38.6 (1.6) | 76.8 (2.5) | 70.1 (2.6) | 38.2 (1.6) |
| >300 | 41.4 (3.9) | 85.1 (4.0) | 75.8 (5.1) | 43.7 (3.3) |
| Age category (years) | ||||
| 20–44 | 10.6 (2.4) | 50.9 (4.0) | 41.3 (4.3) | 40.4 (3.2) |
| 45–64 | 43.8 (3.5) | 82.4 (2.8) | 76.1 (3.5) | 38.6 (2.4) |
| ≥65 | 53.1 (2.6) | 91.9 (2.6) | 85.6 (2.7) | 38.7 (2.1) |
| Sex | ||||
| Female | 35.8 (2.3) | 72.1 (2.3) | 65.8 (2.6) | 36.4 (2.0) |
| Male | 43.0 (2.8) | 85.3 (2.6) | 77.3 (3.0) | 42.3 (2.0) |
| Race/ethnicity | ||||
| Non-Hispanic White | 41.7 (2.0) | 78.5 (3.0) | 71.5 (3.0) | 36.8 (2.4) |
| Mexican American | 32.4 (4.1) | 75.5 (3.5) | 64.0 (5.1) | 43.1 (3.4) |
| Non-Hispanic Black | 38.1 (2.6) | 83.4 (2.3) | 77.0 (2.5) | 45.3 (3.0) |
| Non-Hispanic Asian | 34.3 (5.0) | 78.1 (4.5) | 72.8 (4.7) | 43.8 (4.3) |
| Other | 34.6 (5.0) | 72.5 (4.4) | 66.4 (4.8) | 37.9 (3.7) |
| Income-to-poverty ratio tertile | ||||
| Tertile 1 | 34.5 (2.5) | 78.2 (2.5) | 68.2 (3.2) | 43.7 (2.4) |
| Tertile 2 | 38.6 (3.3) | 79.9 (2.6) | 75.0 (3.0) | 41.3 (2.5) |
| Tertile 3 | 42.7 (4.2) | 74.6 (5.3) | 67.5 (5.4) | 32.0 (3.0) |
| Missing | 41.4 (5.3) | 83.8 (4.4) | 76.7 (4.7) | 42.4 (4.1) |
| Education | ||||
| <High school | 42.3 (4.3) | 84.6 (4.6) | 75.9 (4.6) | 42.3 (3.7) |
| High school degree | 39.4 (2.4) | 80.5 (2.5) | 72.9 (2.3) | 41.2 (2.3) |
| >High school degree | 38.4 (2.6) | 75.6 (3.3) | 69.0 (3.4) | 37.2 (2.0) |
| Diabetes | ||||
| No | 27.1 (1.7) | 69.6 (2.9) | 61.5 (2.8) | 42.5 (1.9) |
| Yes | 61.9 (2.6) | 94.4 (1.6) | 89.2 (2.0) | 32.6 (2.1) |
| CKD awareness | ||||
| No | 37.0 (1.5) | 76.6 (2.1) | 69.1 (2.4) | 39.6 (1.5) |
| Yes | 54.8 (5.3) | 90.6 (4.3) | 86.6 (4.3) | 35.8 (3.8) |
| Number of antihypertensive medications in addition to ACEi/ARB | ||||
| 0 | 20.4 (2.5) | 66.2 (2.6) | 56.1 (3.1) | 45.8 (2.0) |
| 1 | 53.7 (3.7) | 91.0 (3.3) | 87.4 (3.4) | 37.3 (3.2) |
| ≥2 | 80.5 (3.0) | 98.3 (0.7) | 95.9 (1.2) | 23.5 (2.3) |
| 10-year predicted ASCVD risk | ||||
| <5% | 12.6 (2.2) | 49.9 (3.0) | 40.0 (3.4) | 37.2 (2.8) |
| 5–<10% | 39.3 (8.0) | 82.2 (5.1) | 75.1 (5.6) | 42.9 (4.8) |
| 10–<20% | 44.9 (5.6) | 88.1 (5.7) | 85.6 (5.8) | 43.1 (3.8) |
| ≥20% | 57.0 (3.6) | 98.3 (0.7) | 91.4 (2.3) | 41.4 (2.7) |
| Prevalent CVD | 60.8 (4.0) | 94.8 (1.8) | 88.8 (2.9) | 34.0 (2.9) |
| 5-year predicted kidney failure risk† | ||||
| <2% | 37.3 (1.5) | 76.4 (2.2) | 69.1 (2.4) | 39.1 (1.5) |
| 2–<5% | 67.7 (7.9) | 93.4 (5.2) | 88.4 (5.6) | 25.7 (5.8) |
| ≥5% | 44.7 (5.7) | 92.8 (2.6) | 86.2 (5.1) | 48.1 (4.6) |
Abbreviations: SE, standard error. ACEi, angiotensin-converting enzyme inhibitor. ARB, Angiotensin II-receptor blocker. KDIGO, Kidney Disease: Improving Global Outcomes. eGFR, estimated glomerular filtration rate. ACR, albumin-to-creatinine ratio. CKD, chronic kidney disease. ASCVD, atherosclerotic cardiovascular disease. CVD, cardiovascular disease.
Includes those currently taking ACE/ARBs and those who have BP above goal. Recommended to take ACE/ARBs by 2021 KDIGO guideline: currently taking ACE/ARBs or (ACR≥30 mg/g and SBP >120 mmHg). Recommended to take ACE/ARBs by 2012 KDIGO guideline: currently taking ACE/ARBs or [ACR≥30 mg/g and (SBP >130 mmHg or DBP >80 mmHg)].
Among those with eGFR 15–59 ml/min/1.73m2
After adjustment for sociodemographic and clinical characteristics, ACEi/ARB use was lower among US adults with albuminuria who had eGFR 15 to 29 ml/min per 1.73 m2 versus those with eGFR of 30 to 59 ml/min per 1.73 m2 (PR, 0.60; 95% CI, 0.37–0.96) and who were aged ≥65 versus 45 to 64 years (PR: 0.66, 95% CI: 0.46–0.96) (Table S4). ACEi/ARB use was more common among adults with albuminuria who were in the highest versus lowest tertile of the income-to-poverty ratio (PR: 1.37, 95% CI: 1.07–1.76), who were aware of their CKD (PR: 1.32, 95% CI: 1.07–1.64), and who had diabetes (PR: 1.91, 95% CI: 1.55–2.35).
Among US adults with CKD taking antihypertensive medication, 40.5% were taking an ACEi, 31.7% an ARB, 44.3% a beta-adrenergic antagonist, 41.8% a diuretic (loop, thiazide-like, K-sparing), 32.3% a calcium channel blocker, and 0.9% were taking other medication classes (Table S5).
DISCUSSION
According to NHANES 2015–2018, 69.5% or approximately 24.5 million US adults with CKD, had BP above the 2021 KDIGO target. This represents an increase of 19.6% (~6.9M US adults) with above target BP compared to the 2012 KDIGO guideline and 13.9% (~4.9M US adults) compared to the 2017 ACC/AHA BP guideline. Also, 78.2% of US adults with CKD and albuminuria were recommended an ACEi/ARB by the 2021 KDIGO guideline, with only 39.1% actually taking these classes of medications. Although there was a large difference between eligible and actual ACEi/ARB use, 71.0% of US adults were previously recommended an ACEi/ARB by the 2012 KDIGO guideline.
The 2021 KDIGO guideline includes a recommendation that adults with CKD and high BP be treated to a target SBP <120 mmHg based on standardized office BP measurement, if tolerated by the patient, based on the cardiovascular, survival, and potential cognitive benefits of more intensive BP lowering.1 Meta-analyses of randomized trials of antihypertensive medication have suggested that treating SBP to <120 mmHg is associated with reduced CVD risk.15 For individuals with CKD, the evidence supporting the SBP target <120 mmHg is largely derived from SPRINT, which included 2,646 patients with CKD, defined as eGFR <60 ml/min/1.73m2, as a pre-specified subgroup.4 SPRINT found that the effect of intensive SBP lowering on the primary cardiovascular outcome and all-cause death was similar in those with and without CKD.4 However, SPRINT excluded certain important subgroups, such as those with SBP <130 mmHg, diabetes mellitus, orthostatic hypotension, frailty and heavy proteinuria (>1g/day).
BP in SPRINT and virtually all major outcome trials16 was measured using a standardized approach and averaged multiple readings. Standardized office BP measurements can be obtained following a protocol such as the one from the AHA by trained clinical staff.3 The AHA BP measurement protocol includes guidance on having the patient rest before BP is measured, the proper positioning of the patient, selecting appropriate equipment, and not talking during the procedure. On average, BP is substantially higher when measured in routine clinical practice as compared with following a standardized protocol.17,18 However, given the high degree of variability in the difference between routine office-measured BP and standardized BP, it is not possible to apply a correction factor to estimate standardized office BP from a routine BP measurement.17,18 Most systematic errors in BP measurement can be avoided if a protocol is followed.19 For these and other reasons, the 2021 KDIGO guideline emphasizes the importance of obtaining BP measurements following a recommended protocol and individualization of BP management, including consideration of the patient’s characteristics, tolerability, and preferences when considering the optimal SBP target.1
Among adults with CKD and high BP, the 2021 KDIGO guideline provides a strong recommendation for ACEi/ARB use in adults who have diabetes and those without diabetes who have severely increased albuminuria. Also, the guideline provides a weak recommendation for ACEi/ARB use in those without diabetes with moderately increased albuminuria. A post-hoc analysis of SPRINT found that the benefits of intensive BP lowering on the primary cardiovascular outcome and all-cause mortality were similar among patients with and without albuminuria.20 Although albuminuria is a potent risk factor for adverse kidney and cardiovascular outcomes, screening for it remains suboptimal, particularly in patients with hypertension.21–23 When albuminuria is measured, higher ACR values are associated with greater likelihood of ACEi/ARB initiation.24 Thus, increasing adherence to recommended albuminuria testing has the potential to impact BP management among US adults with CKD.24 In the current study, CKD awareness was low, underscoring the need to educate patients and providers. Albuminuria testing is critical early in the course of CKD to detect damage before substantial loss of GFR.
The current analysis identified several antihypertensive medication treatment gaps for adults with CKD. For example, we estimated that 8.9 million of the 24.5 million adults with CKD who had BP above the 2021 KDIGO target were not taking any antihypertensive medication. Of these individuals, 6.0 million had CKD based on albuminuria but an eGFR ≥60 mL/min/1.73m2, a group where the CKD diagnosis rate is low and the diagnosis cannot be made without albuminuria measurement.25 Approximately one-third of US adults with CKD who had treated SBP ≥120 mmHg, an estimated 5.5 million individuals, were taking only a single antihypertensive medication. The SPRINT treatment algorithm for the intensive group began with a 2 or 3 drug therapy (thiazide-type diuretic and/or an ACEi or ARB and/or a calcium channel blocker) and multiple drugs26 may be needed to achieve the 2021 KDIGO target. Beta-blockers, considered a second line antihypertensive medication, were used more frequently in the current study than thiazide diuretics and calcium channel blockers, indicating that appropriate drug selection may be another critical point of intervention to improve BP management.
In the current study, BP control and ACEi/ARB use among those with albuminuria were lower among US adults who were non-white, had lower family income relative to poverty, and lower education regardless of which guideline criteria were used. Differences by sociodemographic factors in recommended BP management among adults with CKD contributes to disparities in cardiovascular and kidney disease outcomes. When implementing the 2021 KDIGO guideline recommendations, it is important to focus on health equity as a priority.
Reducing BP to the lower target recommended by the 2021 KDIGO guideline will require concerted effort, especially in the context of worsening trends in BP control in the general adult population.27 In October 2021, the U.S. Surgeon General released a Call to Action focused on hypertension control.28 This document provided evidence-based approaches for improving BP control including the use of standardized treatment approaches, promoting health care teams to manage hypertension and empowering and equipping patients to use self-measured BP monitoring and medication adherence strategies.28 Health care teams can improve BP control by implementing protocols to ensure proper BP measurement, developing evidence-based treatment algorithms that prioritize use of recommended antihypertensive medications, including combination medications where needed,29 and partnering with patients, families, and communities to implement both pharmacologic and non-pharmacologic approaches.30
The current analysis has several strengths. NHANES followed standardized procedures for examination and laboratory measurements and enrolled a large sample size. The selection of participants for NHANES allowed for the generation of nationally representative estimates. Finally, we used the two most recent survey cycles to provide contemporary data on BP management in adults with CKD. This analysis has several limitations. Though multiple standardized measurements were obtained, BP was measured only on one occasion, whereas the KDIGO 2021 BP guideline indicates an average of ≥2 readings obtained on ≥2 occasions should be used to estimate an individual’s BP.1 We relied on single measurements of serum creatinine and urinary albumin, which may result in misclassification of CKD, particularly when albuminuria exclusively is used to define CKD.31 Clinical practice guidelines define CKD based on reduced eGFR or presence of albuminuria for at least 3 months.9 However, ACEi/ARB use in our study was similar across the range of elevated ACR. Finally, we excluded individuals with eGFR <15 ml/min/1.73m2 not on dialysis due to concerns about generalizability to the broader patient population with these characteristics.
Conclusion
Improving BP control is a priority for reducing CVD risk in adults with CKD. A majority of the 35 million US adults with CKD have SBP ≥120 mmHg based on standardized measurement procedures in NHANES and are recommended for initiation or intensification of antihypertensive medication by the 2021 KDIGO guideline. Additionally, almost 80% of US adults with albuminuria are recommended an ACEi/ARB but less than 40% were taking one. If implemented successfully, reducing BP to the target recommended by the KDIGO 2021 guideline and appropriate use of ACEi/ARB among those with albuminuria has the potential to prevent a large number of cardiovascular events and deaths32 and slow kidney disease progression among US adults with CKD.
Supplementary Material
Table S1. Variables included the current analysis and methods of ascertainment in the National Health and Nutrition Examination Survey, 2015–2018
Table S2. Percent (SE) and millions (SE) of US adults with chronic kidney disease and blood pressure above the 2021 KDIGO and 2017 ACC/AHA guideline targets, NHANES 2015–2018
Table S3. Characteristics associated with systolic blood pressure ≥120 mmHg in US adults with chronic kidney disease, NHANES 2015–2018
Table S4. Characteristics associated with ACEi/ARB use in US adults with albuminuria, NHANES 2015–2018
Table S5. Patterns of antihypertensive medication use among adults with chronic kidney disease currently taking antihypertensive medication, NHANES 2015–2018
Acknowledgments
Funding support
K.F. is supported by National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI) grant T32 HL007024.
A.R.C. is supported NIH/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant K23 DK106515.
E.S. is supported by NIH/NHLBI grant K24 HL152440.
Footnotes
DISCLOSURE STATEMENT
T.I.C., M.J.S., and P.M. served as members of the 2021 KDIGO Blood Pressure Work Group.
M.J.S. attended a Bayer Advisory Board in May 2019 and serves as consultant to Cardurian. He is on the Akebia Steering Committee with funds payed to Tufts Medical Center.
T.I.C has received funding paid by Janssen Pharmaceuticals to Stanford University; has served as a consultant for Bayer, Janssen Pharmaceuticals, Novo Nordisk, Fresenius Medical Care, Tricida, Gilead and AstraZeneca; and has received grant support from Satellite Healthcare.
P.M. receives grant support and consulting fees from Amgen Inc.
J.C. is an advisor to Healthy.io and receives grants from the National Kidney Foundation which receives industry support for epidemiologic research.
Implications of the 2021 KDIGO BP Guideline
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kathryn E. Foti, Johns Hopkins Bloomberg School of Public Health, Department of Epidemiology.
Dan Wang, Johns Hopkins Bloomberg School of Public Health, Department of Epidemiology.
Alexander R. Chang, Geisinger Health, Kidney Health Research Institute.
Elizabeth Selvin, Johns Hopkins Bloomberg School of Public Health, Department of Epidemiology.
Mark J. Sarnak, Division of Nephrology, Tufts Medical Center, Tufts University School of Medicine.
Tara I. Chang, Stanford University School of Medicine, Department of Medicine, Division of Nephrology.
Paul Muntner, University of Alabama at Birmingham School of Public Health, Department of Epidemiology.
Josef Coresh, Johns Hopkins Bloomberg School of Public Health, Department of Epidemiology.
REFERENCES
- 1.KDIGO CLINICAL PRACTICE GUIDELINE ON THE MANAGEMENT OF BLOOD PRESSURE IN CHRONIC KIDNEY DISEASE. 2021. [Update with final citation.]
- 2.Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease KDIGO Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney inter, Suppl. 2012;2:337–414. [Google Scholar]
- 3.Muntner P, Shimbo D, Carey RM, et al. Measurement of Blood Pressure in Humans: A Scientific Statement From the American Heart Association. Hypertension. 2019;73(5). doi: 10.1161/HYP.0000000000000087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The SPRINT Research Group. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med 2015;373(22):2103–2116. doi: 10.1056/NEJMoa1511939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whelton PK, Carey RM, Aronow WS, et al. 2017. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Pr. Hypertens (Dallas, Tex 1979). November 2017:HYP.0000000000000065. doi: 10.1161/HYP.0000000000000065 [DOI] [Google Scholar]
- 6.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey (NHANES) Physician Examination Procedures Manual. Hyattsville, MD; 2015. [Google Scholar]
- 8.Crim MT, Yoon SS, Ortiz E, et al. National Surveillance Definitions for Hypertension Prevalence and Control Among Adults. Circ Cardiovasc Qual Outcomes. 2012;5(3):343–351. doi: 10.1161/CIRCOUTCOMES.111.963439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney inter, Suppl. 2013;3(1): 1–150. [Google Scholar]
- 10.Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk. Circulation. 2014;129(Suppl 2):S49–S73. doi: 10.1161/01.cir.0000437741.48606.98 [DOI] [PubMed] [Google Scholar]
- 11.Tangri N, Grams ME, Levey AS, et al. Multinational assessment of accuracy of equations for predicting risk of kidney failure ameta-analysis. JAMA - J Am Med Assoc. 2016;315(2): 164–174. doi: 10.1001/jama.2015.18202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.StataCorp. Stata Statistical Software: Release 15. 2017. College Station, TX; StataCorp LLC. [Google Scholar]
- 13.StataCorp. Stata 15 Stata Survey Data Reference Manual. 2017. College Station, TX; Stata Press. [Google Scholar]
- 14.United States Census Bureau. 2019. Population Estimates by Age, Sex, Race and Hispanic Origin. https://www.census.gov/newsroom/press-kits/2020/population-estimates-detailed.html. Accessed October 13, 2020.
- 15.Bundy JD, Li C, Stuchlik P, et al. Systolic Blood Pressure Reduction and Risk of Cardiovascular Disease and Mortality. JAMA Cardiol. 2017;2(7):775. doi: 10.1001/jamacardio.2017.1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drawz PE, Ix JH. BP Measurement in Clinical Practice: Time to SPRINT to Guideline-Recommended Protocols. J Am SocNephrol. 2018;29(2):383–388. doi: 10.1681/ASN.2017070753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drawz PE, Agarwal A, Dwyer JP, et al. Concordance Between Blood Pressure in the Systolic Blood Pressure Intervention Trial and in Routine Clinical Practice. JAMA Intern Med. October 2020. doi: 10.1001/jamaintemmed.2020.5028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agarwal R Implications of blood pressure measurement technique for implementation of Systolic Blood Pressure Intervention Trial (SPRINT). J Am Heart Assoc. 2017;6(2). doi: 10.1161/JAHA.116.004536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kallioinen N, Hill A, Horswill MS, Ward HE, Watson MO. Sources of inaccuracy in the measurement of adult patients’ resting blood pressure in clinical settings. J Hypertens. 2017;35(3):421–441. doi: 10.1097/HJH.0000000000001197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang AR, Kramer H, Wei G, et al. Effects of intensive blood pressure control in patients with and without albuminuria. Post hoc analyses from SPRINT. Clin J Am Soc Nephrol. 2020;15(8): 1121–1128. doi: 10.2215/CJN.12371019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peralta CA, Livaudais-toman J, Stebbins M, et al. Electronic decision support for management of CKD in primary care: A pragmatic randomized trial. AJKD. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peralta CA, Frigaard M, Rubinsky AD, et al. Implementation of a pragmatic randomized trial of screening for chronic kidney disease to improve care among non-diabetic hypertensive veterans. BMC Nephrol. 2017;18(1):132. doi: 10.1186/s12882-017-0541-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Litvin CB, Madison Hyer J, Ornstein SM. Use of clinical decision support to improve primary care identification and management of Chronic Kidney Disease (CKD). J Am Board Fam Med. 2016;29(5):604–612. doi: 10.3122/jabfm.2016.05.160020 [DOI] [PubMed] [Google Scholar]
- 24.Qiao Y, Shin J-I, Chen TK, et al. Association of Albuminuria Levels With the Prescription of Renin-Angiotensin System Blockade. Hypertension. 2020:1–7. doi: 10.1161/hypertensionaha.120.15956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirst JA, Ordóñez Mena JM, Taylor CJ, et al. Prevalence of chronic kidney disease in the community using data from OxRen: A UK population-based cohort study. Br J Gen Pract. 2020;70(693):E285–E293. doi: 10.3399/bjgp20X708245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheung AK, Rahman M, Reboussin DM, et al. Effects of intensive BP control in CKD. JAm Soc Nephrol. 2017;28(9):2812–2823. doi: 10.1681/ASN.2017020148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muntner P, Hardy ST, Fine LJ, et al. Trends in Blood Pressure Control Among US Adults With Hypertension, 1999–2000 to 2017–2018. JAMA. September 2020. doi: 10.1001/jama.2020.14545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hypertension C The Surgeon General’s Call to Action To.
- 29.Jaffe MG, Young JD. The Kaiser Permanente Northern California Story: Improving Hypertension Control From 44% to 90% in 13 Years (2000 to 2013). J Clin Hypertens. 2016;18(4):260–261. doi: 10.1111/jch.12803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boonyasai RT, Rakotz MK, Lubomski LH, et al. Measure accurately, Act rapidly, and Partner with patients: An intuitive and practical three-part framework to guide efforts to improve hypertension control. J Clin Hypertens. 2017;19(7):684–694. doi: 10.1111/jch.12995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of Chronic Kidney Disease and Decreased Kidney Function in the Adult US Population: Third National Health and Nutrition Examination Survey. AJKD Am J Kidney Dis Am J Kidney Dis. 2003;41(1): 1–12. doi: 10.1053/ajkd.2003.50007 [DOI] [PubMed] [Google Scholar]
- 32.Bress AP, Colantonio LD, Cooper RS, et al. Potential Cardiovascular Disease Events Prevented with Adoption of the 2017 American College of Cardiology/American Heart Association Blood Pressure Guideline. Circulation. 2019;139(1):24–36. doi: 10.1161/CIRCULATIONAHA.118.035640 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Variables included the current analysis and methods of ascertainment in the National Health and Nutrition Examination Survey, 2015–2018
Table S2. Percent (SE) and millions (SE) of US adults with chronic kidney disease and blood pressure above the 2021 KDIGO and 2017 ACC/AHA guideline targets, NHANES 2015–2018
Table S3. Characteristics associated with systolic blood pressure ≥120 mmHg in US adults with chronic kidney disease, NHANES 2015–2018
Table S4. Characteristics associated with ACEi/ARB use in US adults with albuminuria, NHANES 2015–2018
Table S5. Patterns of antihypertensive medication use among adults with chronic kidney disease currently taking antihypertensive medication, NHANES 2015–2018



