Abstract
The default mode network (DMN) is classically considered an ‘intrinsic’ system, specializing in internally oriented cognitive processes such as daydreaming, reminiscing and future planning. In this Perspective, we suggest that the DMN is an active and dynamic ‘sense-making’ network that integrates incoming extrinsic information with prior intrinsic information to form rich, context-dependent models of situations as they unfold over time. We review studies that relied on naturalistic stimuli, such as stories and movies, to demonstrate how an individual’s DMN neural responses are influenced both by external information accumulated as events unfold over time and by the individual’s idiosyncratic past memories and knowledge. The integration of extrinsic and intrinsic information over long timescales provides a space for negotiating a shared neural code, which is necessary for establishing shared meaning, shared communication tools, shared narratives and, above all, shared communities and social networks.
Making sense of the world is a dynamic, ever-changing process requiring the integration of incoming information with prior knowledge about the situation over multiple timescales. For example, while listening to a debate, a listener must integrate their own prior thoughts and beliefs about the debate topics with the current and past statements of the speakers. Thus, our mindsets and our actions at each moment in time are shaped by at least three factors: what is happening now in the world (incoming sensory input); what happened in the world in the preceding moments (recent active memories that further contextualize and shape the present); and who we are and what we bring to the moment (that is, our long-term memories, conditional responses, beliefs and emotions that shape the way we process the incoming inputs). The ongoing integration of these three factors happens effortlessly in our brains, almost by default.
In this Perspective, we propose a key role for the default mode network (DMN) in dynamically integrating these three factors over extended timescales in order to make sense of a novel, constantly evolving situation. The DMN, which includes the posterior medial cortex, medial prefrontal cortex and temporoparietal junction1–3, was classically viewed as an ‘intrinsic’ system, showing decreased activity during experimental tasks. Later work updated this view to suggest that the DMN is specialized for internally oriented or non-stimulus-induced tasks, such as ‘mind-wandering’, recall of past events and internal simulation of future events4–7. Although a recent review further divided the DMN into multiple interleaved networks1, researchers have often made a distinction between this ‘intrinsic’ system and ‘extrinsic’ systems that process incoming sensory information or outgoing motor outputs4,5,8–14 (see Box 1).
Box 1 |. Alternative accounts of default mode network function
The function of the default mode network (DMN) is a matter of substantial interest and debate. in this box, we aim to highlight several prominent lines of studies that expose the richness and diversity of functions associated with the DMN. Furthermore, we aim to outline how such diversity of functions can be unified by our theoretical perspective.
Baseline activation for healthy function
The DMN was initially conceptualized as a ‘task-negative’ network, active only when the brain is not occupied by a task4,8,123,124. Later, it was discovered that regions of the DMN show spontaneous fluctuations in activity that are highly correlated across regions, suggesting that the DMN forms a functional network11. these findings led to the hypothesis that the activity and connectivity of the DMN have an important role in the offline intrinsic activity that is required to maintain balanced and steady internal states during rest2,125.
Mind-wandering
Follow-up studies aimed to further characterize the cognitive functions associated with periods of rest, or when the mind is disengaged from processing external information. as a result, researchers found that DMN activity is increased during periods of ‘mind-wandering’126–129 and that an individual’s tendency to mind wander is correlated with the DMN response130. Moreover, the content of mind-wandering may modulate DMN activity131 (for example, thinking about what one needs to advance their research project versus thinking about how one’s bedroom looks) and the connectivity of the DMN is different for different components of mind-wandering (for example, memory, social cognition and planning)132.
Autobiographical memory
The first study to detect DMN responses associated with internally generated autobiographical memories in the absence of external stimuli was exploring brain mechanisms underlying personal memory133. since then, various neuroimaging studies have demonstrated that DMN activity and connectivity are modulated by tasks that require the retrieval of past events and by spontaneous autobiographical memory128,134–136. it was suggested that autobiographical memory is one of the prominent processes that shows the involvement of the DMN in mental representations generated using intrinsic information, independent of extrinsic information1.
Prospective memory
In addition to the retrieval of episodic memories, participants are often occupied with thinking about future events and planning ahead while resting in the Mri scanner137. intriguingly, memory ‘for the future’, like memory for the past, activates the DMN and shapes its connectivity131,136,138,139.
Social cognition
Many studies have found extensive overlap between the DMN and regions involved in social cognition that are collectively known as the ‘social brain’140–148. in addition to being involved in self-referential processes such as thinking about self-mental states149,150, the DMN is involved in thinking about other people’s beliefs, intentions and motivations53,136,151, and in priming the intentional stance152. importantly, thinking about other people is not confined to moments of disengagement but is also, and perhaps even primarily, evident as people are engaged with other social agents in the real world, for example during telling (speaking) and listening to (comprehending) real-life personal stories33,46,153 as well as watching and listening to fictional stories37,42,47.
Where intrinsic meets extrinsic
Taken together, the studies described here suggest that, on the one hand, the DMN is active during internal-related thoughts that are self-generated in the absence of external stimuli, as in the case of mind-wandering, and therefore can be considered intrinsic; and, on the other, the response of the DMN is locked to external stimuli, especially during social interactions, and thus can be considered extrinsic. thus, in this Perspective, we combine many of these findings to suggest that the DMN is at the nexus of the interaction between the external and internal worlds.
Here, we challenge the view of the DMN as an intrinsic system by pointing to the active and dynamic role of the DMN in synthesizing extrinsic and intrinsic information over long timescales. In a previous review, we argued that memory is an integral component of any processing act15 and that the DMN resides at the top of this ‘process–memory’ hierarchy, playing a unique and central role in processing events as they dynamically unfold over time. Building on this model, we make three related arguments. First, we propose that activity in the DMN is modulated by incoming external information (top arrow in Fig. 1a), which is integrated over many seconds to many minutes (horizontal arrow in Fig. 1a). Therefore, the DMN should not be considered an intrinsic system active only during internally oriented or stimulus-independent processes. Second, we argue that activity in the DMN is nonetheless sensitive to intrinsic information (long-term memories, conditional responses, beliefs, emotions and so on), which influences and interacts with the interpretation and processing of incoming external information (bottom arrow in Fig. 1a). Therefore, the DMN should not be considered an extrinsic system, sensitive only to incoming sensory information. Last, we combine these points to propose that the DMN is an active and dynamic ‘sense-making’ network that integrates incoming extrinsic information with prior intrinsic information over long timescales to form rich, context-dependent, idiosyncratic models of the situation as it unfolds over time.
Fig. 1 |. A new view of the default mode network.
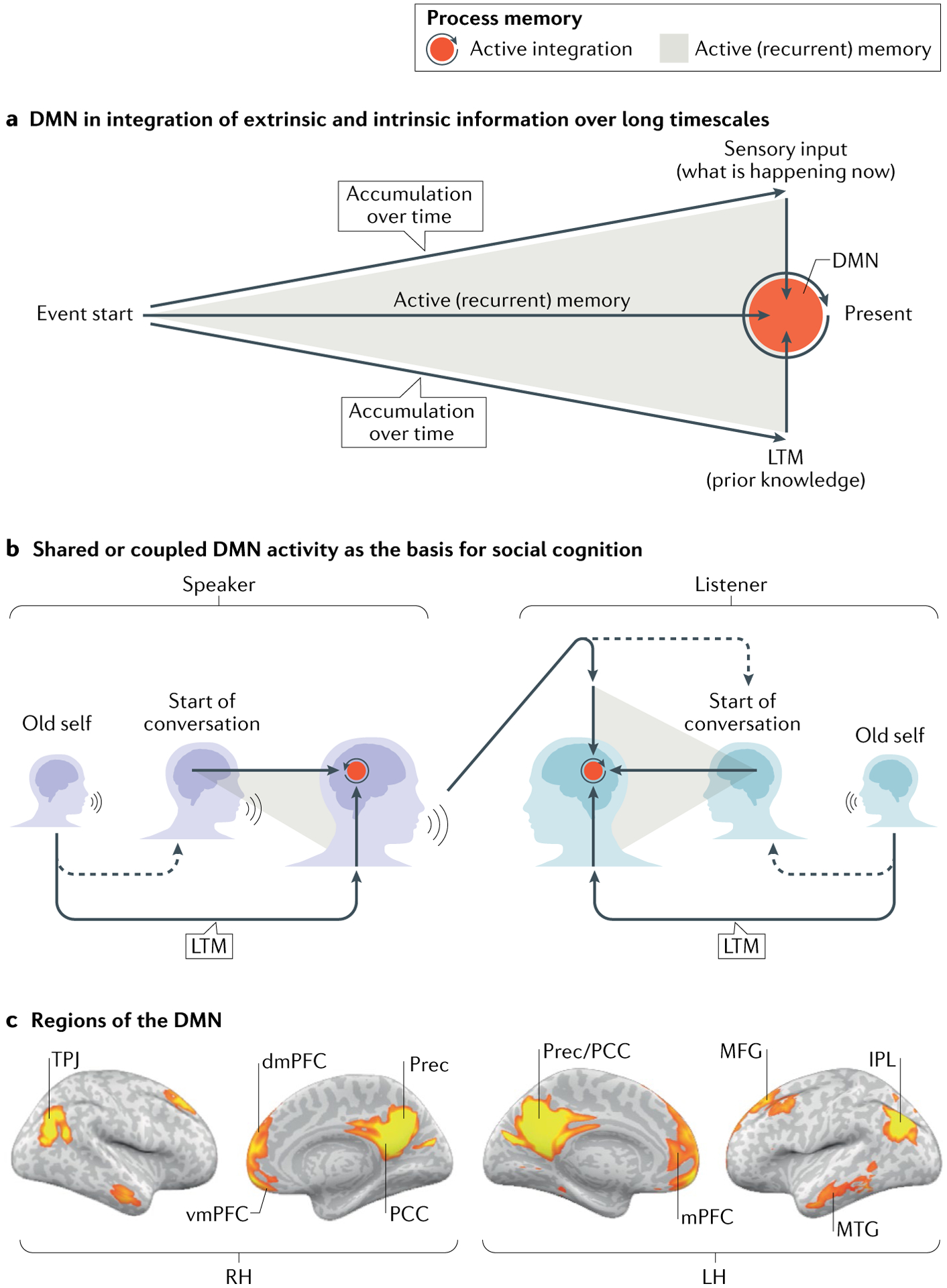
a | Activity in the default mode network (DMN) is modulated by incoming external information (top arrow), which is actively accumulated (grey expanding triangle) and integrated (red circle) over hundreds of seconds (horizontal arrow) with our intrinsic information (long-term memories (LTMs), conditional responses, beliefs and so on, represented by the bottom arrow) to form a rich, context-dependent, dynamic model of the unfolding situation. b | Our thoughts, feelings and actions are constantly being shaped by the actions, memories and stories of others. At the same time, our LTMs shape the way we process the external input. This unique interplay between the extrinsic and intrinsic forces provides a space for negotiating a shared neural code necessary for establishing shared meanings, shared communication tools, shared narratives and, importantly, shared communities and social networks. c | Regions of the DMN defined by functional connectivity analysis. These regions include the posterior cingulate cortex (PCC) and precuneus (Prec), the ventromedial prefrontal cortex (vmPFC) and dorsomedial prefrontal cortex (dmPFC), and the bilateral temporoparietal junction (TPJ). IPL, inferior parietal lobule; LH, left hemisphere; RH, right hemisphere; MFG, middle frontal gyrus; MTG, middle temporal gyrus. Part c adapted from REF.26, CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/).
As the activity in the DMN is shaped by our unique history, it is by nature idiosyncratic. However, at the same time, our knowledge, memories and beliefs are shaped by the people we are connected to and the world in which we are immersed (Fig. 1b). Thus, in the last section of this Perspective, we argue that the DMN provides a space for social ‘others’ (the extrinsic people we interact with) to shape the self (our set of intrinsic memories and beliefs), which in turn can enable us to shape the memories and beliefs of others. This unique interplay between the extrinsic and intrinsic forces provides a mechanism for negotiating a shared neural code to facilitate learning and communication via shared common ground. Our view of the DMN is informed by numerous studies using naturalistic stimuli and inter-subject analyses that map shared neural responses across participants (Fig. 2).
Fig. 2 |. Using inter-subject correlation analysis to map shared responses across subjects.
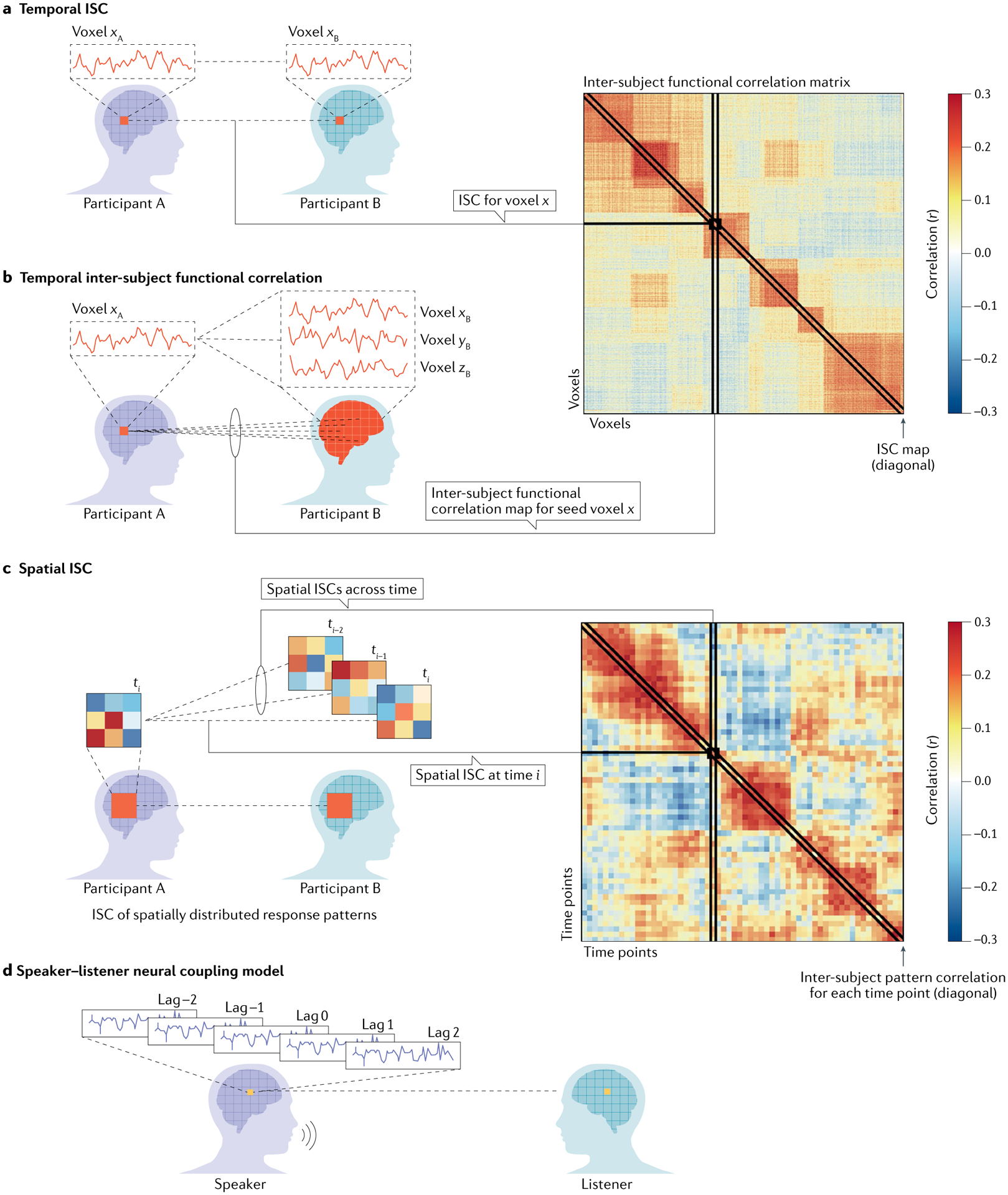
Inter-subject correlation (ISC) analyses allow researchers to measure neural responses that are shared across participants and locked to the structure of the external input. ISC can be computed in the following ways (for more details, see REF.122). a,b | Temporal ISC within and between brain areas. In this basic form, the neural response to a continuous natural stimulus (as a story or a movie) in a given brain area is correlated with the responses in the same brain region in another participant (part a). This simple analysis allows us to measure the consistency of neural responses to complex naturalistic stimuli and to identify neural responses shared across individuals. The ISC method can be extended to correlate the response time course between each voxel in one participant’s brain and all other voxels in another participant’s brain to yield a voxel by a voxel inter-subject functional correlation matrix (part b). The diagonal values of this matrix reflect the conventional ISC map in which correlations are computed only between homologous targets in the participants’ brains. A single value on the diagonal corresponds to the ISC for a given area. A single column (or row) of this matrix represents the functional connectivity map for one seed voxel. c | Inter-subject pattern correlation. ISCs can also be computed across spatially distributed response patterns. For a given region, the correlation between voxels’ response patterns at a given moment (as illustrated by the coloured squares) is correlated across participants. Inter-subject pattern correlations can also be computed across time points to capture the evolution of response patterns over time (for example, if a particular pattern recurs at multiple time points). d | Speaker–listener neural coupling model. ISCs can also be extended to model the direct interaction between a speaker’s brain and a listener’s brain during communication (that is, not induced by shared external input). The speaker–listener coupling model uses the speaker’s brain activity as a model for predicting the brain activity in each listener. To capture the temporal structure of the speaker–listener interaction, the speaker’s time courses are shifted by time intervals backwards and forwards relative to the moment of vocalization. These shifted speaker time courses are combined with linear weights to build a predictive model for the listener brain dynamics. This method enabled investigation of whether the speaker’s neural activity during speech production is coupled over time with the listener’s neural activity during speech comprehension. Adapted with permission from REF.122, CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/).
Extrinsic responses of the DMN
We begin by proposing that the DMN integrates moment-by-moment external information with prior information over seconds to minutes, as events unfold over time.
DMN activity is influenced by context and incoming inputs.
Research in visual and auditory perception has revealed the influence of context on neural activity even in early sensory areas. In the primary visual cortex of non-human primates, for example, the response of a simple cell to a vertical line will change as a function of lines in its extra-visual field, or its spatial context16,17. Regions in higher-order cortex then integrate these responses, resulting in sensitivity over larger spatial contexts18,19. This hierarchy of visual integration across the visual cortex is captured by spatial receptive fields: early visual areas process local parts of the visual field and so have small spatial receptive fields, whereas higher-order visual areas integrate information over larger parts of the visual field and thus have larger spatial receptive fields.
Analogously, temporal receptive windows (TRWs) capture the influence of context over time. The TRW is the window of time in which past information affects neural processing of new information. Studies using naturalistic stimuli have identified a topographical hierarchy of processing timescales extending from early sensory cortex to higher-order areas including the DMN7,20–23. TRWs are short in sensory areas and become gradually longer towards higher-order areas. The responses in early sensory areas such as the early auditory and visual cortex are influenced by the temporal structure over tens of milliseconds20,21. These findings are consistent with research22 suggesting that early sensory areas process fast-changing, low-level stimulus features, such as sound amplitude or visual edges. By contrast, neural responses in mid-level areas, which include linguistic regions along temporal cortex, are affected by information integrated over a few seconds (for example, the preceding words in the sentence). At the apex of the processing hierarchy, the neural response of the DMN to each sentence is influenced by prior information accumulated over many minutes15,20,21,23. In addition to the modulation of DMN activity by information accumulated over many seconds, in the next section we discuss evidence for the modulation of DMN connectivity by information accumulated over many seconds.
DMN connectivity is influenced by context and incoming inputs.
To map DMN functional connectivity, analyses sample the temporal response in one node of the DMN and use this as a seed to search for co-varying fluctuations in neural activity across other nodes of the network within an individual brain (Fig. 3a). This analysis, although effective in outlining the anatomical boundaries of the DMN, is unable to distinguish subtle task-related fluctuations in connectivity across various experimental conditions including, for example, lying at rest in a scanner, listening to a list of scrambled words and listening to an intact story several minutes long24–26 (Fig. 3a). The stability of the averaged connectivity maps across numerous conditions has frequently been used to support the claim of the intrinsic nature of the DMN5,27. Such interpretation, however, was called into question by the realization that within-brain functional connectivity patterns are heavily influenced by direct and indirect anatomical wiring, but are less sensitive for isolating spontaneous activity (for example, during rest) from stimulus-locked activity that propagates through the network as during processing of extrinsic information26,28.
Fig. 3 |. Isolating stimulus-locked brain connectivity using inter-subject functional correlation.
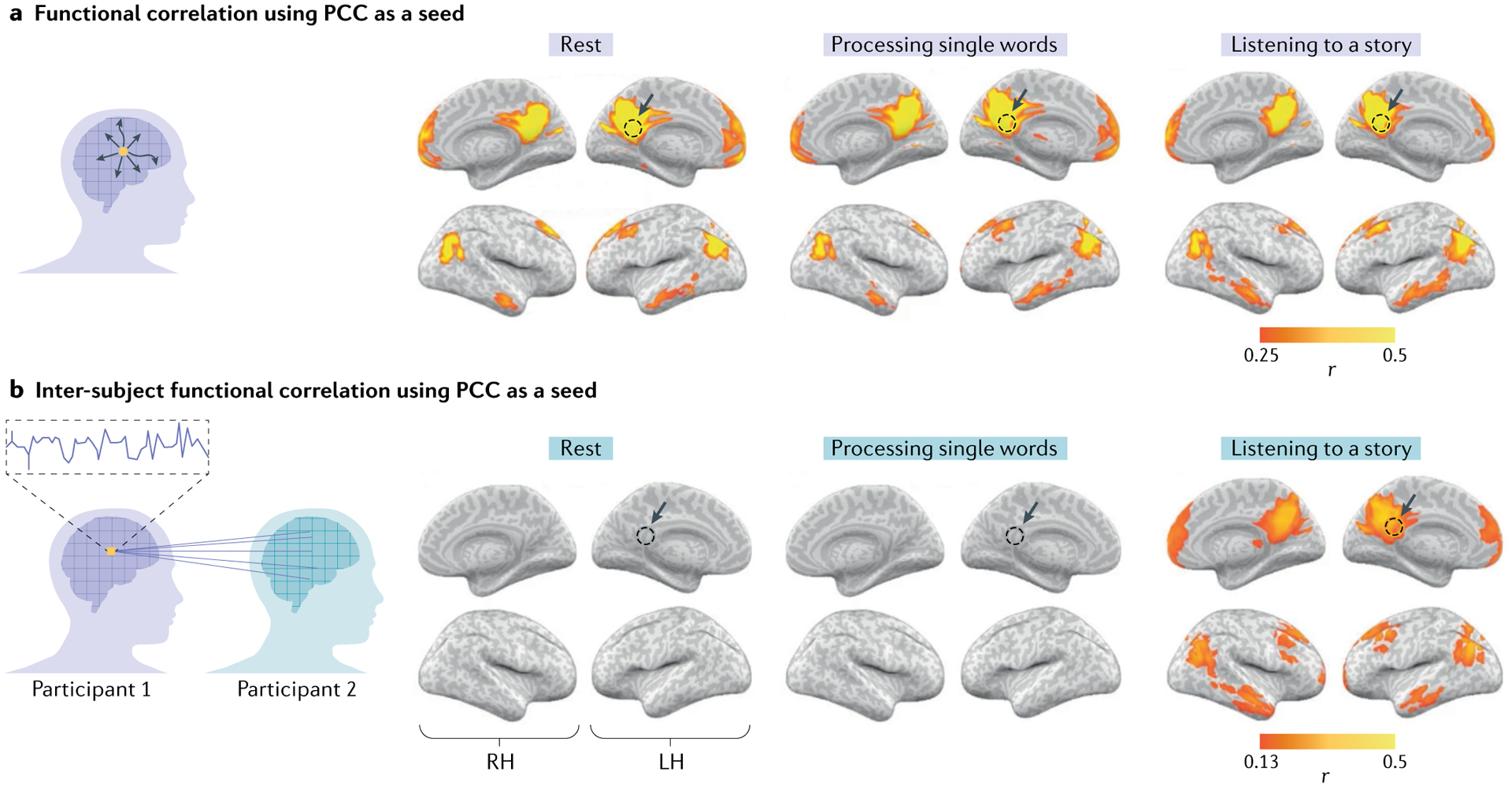
a | Within-participant functional correlation maps between the posterior cingulate cortex (PCC) seed (yellow voxel in the schematic; dashed circles in the brain maps) and the whole-brain neural activity. The functional correlation analysis delineates nodes of the default mode network (DMN) in which the activity fluctuates together (co-varies) in a given participant, owing to the direct or indirect anatomical connections during rest (left panel), processing of single words (middle panel) and listening to a coherent story (right panel). b | Inter-subject functional correlation maps between the PCC seed and the whole-brain neural activity observed in other participants. This analysis can filter out spontaneous intrinsic neural facilitation, and as such reveals no substantial stimulus-locked correlations in the DMN during rest (left panel) or during the processing of single words (middle panel). By contrast, however, inter-subject functional correlation exposed stimulus-locked shared responses across participants in the DMN as subjects listen to and process a spoken story minutes long (right panel). LH, left hemisphere; RH, right hemisphere. Adapted from REF.26, CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/).
Task-related connectivity patterns can be isolated from spontaneous neural fluctuations by switching to a multi-brain perspective that characterizes the shared response across brains during the processing of temporally extended naturalistic stimuli. We recently developed such an approach: inter-subject functional correlation analysis26. Inter-subject functional correlation differs from standard functional connectivity analysis in one crucial way: it calculates the functional correlation across brains, as opposed to within brains (Fig. 2). Intrinsic neural dynamics during rest, as well as non-neuronal artefacts (such as those associated with respiration and head motion), can only influence the pattern of correlations within each brain, but cannot induce correlations between brains. By contrast, neural processes that are locked to the structure of the stimulus and shared across participants can be correlated across brains.
Using inter-subject functional correlation, we observed shared connectivity patterns within the DMN that are locked to the stimulus26 (Fig. 3b). Crucially, we observed these stimulus-locked connectivity patterns only during processing of an intact, coherent and temporally extended spoken story. There are no shared connectivity patterns during rest or while participants listened to temporally incoherent stimuli such as scrambled words (Fig. 3b). The finding of stimulus-locked responses in the DMN only for intact, but not temporally scrambled, narratives highlights the importance of integration of information over long timescales in the DMN. This temporal dependency over long timescales makes the DMN more context-dependent than early sensory brain regions, in which responses are less affected by temporal structure of stimuli over such long timescales. These findings also highlight the importance of studying the brain’s dynamics under temporally extended, ecological conditions29.
Integration in the DMN depends on the content, not form, of external input.
DMN responses are locked to the content of the stimuli over long timescales. At the same time, they are relatively invariant to even dramatic changes in low-level stimulus properties that do not change the interpretation (meaning) of the stimuli. Despite substantial differences in low-level properties, the DMN shows similar temporal responses to the same narrative presented using: different modalities, such as written text versus speech30,31 or audiovisual movies versus verbal descriptions32–34; different synonyms35; different languages, such as English versus Russian36; and different levels of abstraction, such as abstract Heidel and Simmel-like shape animation versus concrete verbal description of the same story37. A similar pattern of results was identified in the DMN using encoding models across written and spoken narratives38. In these studies, a model trained to predict neural responses to a narrative in one modality accurately predicted neural responses to the same narrative presented in a different modality. Other researchers have trained decoding models, showing that the specific story a participant is reading can be decoded from DMN neural responses, even across languages39. The DMN also has a role in integrating information across sensory modalities40; for example, the precuneus and bilateral temporoparietal junction have been shown to integrate sensory and motor information to represent word concepts41.
Nevertheless, a relatively small change to the stimulus can produce increasingly large differences in neural responses across the timescale hierarchy when it changes the interpretation of the stimulus. For example, manipulation of a small number of words in a spoken story (for example, changing ‘laughing’ to ‘sobbing’), resulting in two very different stories35, led to differences in the neural responses elicited by the two stories that correlated with the size of the TRW. As such, early auditory cortex showed relatively little difference in its response to the two stories, reflecting the relatively intact low-level acoustic properties of the stimuli. By contrast, DMN regions (which are on the top of the timescale hierarchy) showed large differences, reflecting the large difference in story meaning.
Integration in the DMN is modulated by attention.
Evidence suggests that the DMN is also modulated by attention. Stimuli that are more engaging elicit greater shared neural responses across participants in DMN regions such as the medial prefrontal cortex42, and DMN regions of listeners who are more engaged and attentive show greater shared brain responses across participants43,44. Intriguingly, attention may facilitate propagation of information along the cortical processing timescale hierarchy. In one study45, two unrelated narratives were presented simultaneously, with one in written form and one in spoken form. By manipulating attention to one or the other modality, processing of unattended stimuli was shown to be primarily restricted to sensory cortex, whereas attended stimuli went on to be processed by the DMN and dorsal attention networks.
Crucially, although attention can modulate responses in the DMN, it cannot, in isolation, account for the results described above. Between-subject multivoxel pattern analysis (Fig. 2) within each node of the DMN revealed that the multidimensional spatial patterns of responses are aligned across participants watching a movie46. Furthermore, using a shared response model, the same study reported at least 20 orthogonal dimensions in the DMN’s spatial response patterns that are shared across participants, suggesting that no single univariate dimension, such as attention or arousal, can fully account for the complex multidimensional aligned responses in the DMN46.
Intrinsic responses of the DMN
We next review research demonstrating how the activity in the DMN is affected not only by external events unfolding over many minutes but also by an individual’s long-term memories, schemas and belief systems acquired over the years (Fig. 1a).
DMN responses are modulated by prior beliefs.
Context is not narrowly confined by the structure of the unfolding event. Information acquired prior to events can substantially change their interpretations. In one intriguing study47, two groups of participants were presented with the same short story by J. D. Salinger, Pretty Mouth and Green My Eyes, in which a husband calls his friend at night asking for the whereabouts of his wife. Before listening to the story, the two groups were biased with different contextual information. One group was led to believe that the wife was unfaithful, whereas the other group was led to believe that the husband was paranoid and jealous. DMN regions showed stronger alignment of neural responses within a group that shared an interpretation than between groups with differing interpretations. This suggests that participants’ prior beliefs (acquired just before the story started) changed the way the DMN processed the same story, which unfolded over many minutes. Similar findings have been observed following manipulations of perspective48,49 or focus50, as well as when context has led participants to interpret a situation as ironic51, reflecting an attempt to ‘save face’52 or intentionally harmful53. Moreover, a recent study suggests that regions in the DMN are involved in reinstating the context of a storyline, which facilitates the convergence of the plot of two storylines into one54. Together, these findings suggest that DMN activity is influenced not only by the structure of the incoming events, but also by mindset and prior knowledge.
The DMN is sensitive to long-term memories.
Context extends to the days and even years before an event, as information gathered throughout a lifetime is used to contextualize an event. For example, an individual’s experience of watching episode 5 in season 3 of the series Game of Thrones both depends on and is influenced by internally stored knowledge of events and characters in episodes that aired years ago. Indeed, researchers have exploited stimuli such as television shows or stories to demonstrate how the activity in the DMN is influenced by information acquired over days.
For example, one study55 used functional MRI (fMRI) to scan participants as they watched the second half of an episode of the Twilight Zone. One group of participants watched the first half of the episode a day before they watched the second part (long-term context); a second group watched the entire episode without a break (short-term context); and a third group watched only the second half of the episode (no context). Inter-subject correlation in the DMN was greater among participants who had the background knowledge to interpret the clip than among participants who did not. Crucially, responses in the DMN were similar across the short-term context and the long-term context groups (although DMN regions showed increased connectivity to the hippocampus in the long-term context group compared with in the short-term context group). This result suggests that the responses in the DMN can be shaped by information accumulated across many minutes during uninterrupted viewing (short-term context) as well as by information gathered over days (as in the long-term context group) — and, conceivably, even years.
The ability of the DMN to use long-term context is probably related to its anatomical and functional connections to the medial temporal lobe and hippocampus, which are central to learning and memory56–59. At the same time, the ability of the DMN to accumulate information over many minutes (short-term context) may explain why individuals with amnesia can often sustain coherent conversations over a few minutes60 or recall coherent narratives61 during uninterrupted conversations, while showing profound impairments in tasks that require the retrieval of long-term memories60. Consistent with such behavioural results, a recent neuroimaging study involving an individual with hippocampal amnesia suggests that integration of information over several minutes in the DMN can be sustained even after hippocampal lesion62. In this study, the participant with amnesia showed typical response patterns in the DMN while listening to an intact narrative, as seen in neurotypical controls. Moreover, as in neurotypical controls, the DMN activity of the participant with amnesia was disrupted by temporally scrambling the paragraphs of the narrative, suggesting that the DMN otherwise successfully integrated information over windows of at least 10–30 s. Further research is needed to better characterize the role of the hippocampus in supporting the integration of information over long timescales in the DMN.
The DMN represents acquired schemas.
The ability to integrate information over days is necessary for building schemas, a mental codification of experience. Neuroimaging studies suggest that instantiation and reinstatement of schemas are mediated by the interaction among regions within the DMN — namely, the ventromedial prefrontal cortex, precuneus, bilateral temporoparietal junction and hippocampus63. One study demonstrated the involvement of the DMN in schema representation by measuring brain responses to different schemas (‘eating at a restaurant’ or ‘going through the airport’) presented as stories that varied widely in terms of their characters and storylines, and in terms of format (audiovisual clips or spoken narration)64. Regions including the posterior medial cortex and medial prefrontal cortex exhibited schema-associated event patterns that generalized across stories, participants and modalities. Moreover, the DMN was found to be involved in updating schemas when events violated expectations or were inconsistent with the established schema65,66, as in the case of surprising events during a movie (for example, an unexpected plot twist). A recent review highlights the role of the DMN in representing and reinstating schemas while processing narratives67. Thus, the DMN is involved in generating and retrieving schemas in order to dynamically interpret an external situation.
The DMN is sensitive to individual differences in interpretation.
The finding that responses in the DMN are shaped by our long-term memories and beliefs raises the intriguing possibility that individual differences in the way we perceive the world can account for individual differences in DMN responses. Indeed, several studies have linked similarities in spontaneous, idiosyncratic interpretations of narratives to neural similarities. In one study37, participants were scanned using fMRI while watching an ambiguous animation that conveyed a complex social narrative using only the motion of simple geometric shapes, in the vein of work by Heider and Simmel68. Greater similarity in the interpretation of the ambiguous shape animation (as reported by each of the participants) was associated with greater similarity in neural response in DMN regions across participants. Similar studies have reported that increased alignment in neural response across subjects in the DMN is correlated with the similarity in interpretation of spoken narratives69, similarity in emotional response over time during spoken narratives70, similarity in humour perception over time71, similarity in socio-sexual desire while watching erotic movies72 and similarity in moral decision-making73,74.
Individual differences in behaviour or interpretation of a narrative that are linked to differences in DMN activity have been shown to be associated with trait-level personality differences, demographic factors or cultural differences75,76. Several recent studies have shown that individual differences in personality traits, including levels of paranoia77, memory load75 and a tendency to hold an analytic versus holistic perspective78, may modulate the level of shared responses in regions of the DMN. Several researchers have also identified correlations between neural similarity in regions of the DMN and measures of mental health in clinical populations, including depression79,80 and schizophrenia81.
An active sense-making network
The evidence reviewed in this Perspective suggests that activity in the DMN is neither solely intrinsic nor solely extrinsic. Rather, the activity in the DMN is shaped by both the unfolding dynamical structure of real-life events (including what other social agents say and do in those events) over long timescales as well as our idiosyncratic prior dispositions, including learned schemas, prior knowledge and beliefs that shape our responses to a given context (Fig. 1b). In other words, we suggest that the DMN is an active and dynamic sense-making network that integrates incoming extrinsic social information with prior intrinsic information to form rich, context-dependent models of the situation as they unfold over time.
Perhaps one of the most intriguing findings across these studies is the discovery of neural patterns in the DMN that are shared across participants and aligned to the abstract structure and interpretation of the external events. In other words, the results suggest that participants who understand the situation in the same way will have similar neural patterns in nodes of the DMN, irrespective of considerable differences in the low-level stimulus properties of the sensory input. At the same time, subtle differences in DMN neural response patterns across participants seem to correlate with subtle differences in their interpretation. Together, these studies suggest that, through the interaction between the intrinsic self and the extrinsic world, the DMN develops a shared neural code.
The DMN in social interaction
A fundamental question is why similar understandings or interpretations of high-level concepts produce shared neural activity across individuals in the DMN. It was recently suggested that a crucial aspect of interpersonal interactions is a sense of dyadic shared reality82. Here, we propose that the shared representation in the DMN arises from dynamical social interaction between the self and others. In other words, through reciprocal and dynamical social interaction, other people’s brains can shape your brain responses and, at the same time, be shaped by your brain’s responses to generate a shared reality (Fig. 1b). Indeed, many studies suggested an extensive overlap between the DMN and the ‘social brain’ — the brain regions involved in social cognition (Box 1).
Throughout the day, DMN neural responses are shaped by the actions and gestures of other social agents. Even when isolated at home or lying down in a dark fMRI scanner during experiments, an individual’s brain responses are still shaped by the actions of others, as conveyed by audiovisual movies, spoken words or written text. The switch from a single-brain perspective to a multi-brain perspective83 opens the way to studying how the responses of one brain (the sender) shape the responses of other brains (the receivers). Reciprocally, the verbal and non-verbal actions of receivers’ brains can also shape the neural responses of the sender in a coupled dynamical system84,85. Although we review evidence supporting our claim that DMN response patterns are shaped by the actions of other social agents, we note that this notion does not preclude the possibility that additional genetic factors on longer evolutionary timescales also shape responses in the DMN.
Brain–brain coupling between speakers and listeners.
Brain–brain coupling was first reported in a study that correlated the responses in the speaker’s brain recorded while telling a personal story in the fMRI scanner with the listeners’ brain responses while listening to the story86. The speaker’s neural responses were coupled (that is, correlated, with or without a short time lag; Fig. 2d) to the listeners’ neural responses in brain regions at various levels of the timescale processing hierarchy. Speaker–listener coupling in early auditory regions reflected shared processing of low-level acoustic properties of the stimulus, such as the audio amplitude envelope. By contrast, in language areas and the DMN, the responses in the listeners’ brains lagged behind the responses in the speaker’s brain, suggesting that responses in the speaker’s brain causally shaped the responses in the listeners’ brains. Furthermore, speaker–listener neural coupling in higher-order areas, including the DMN, reflected communication and shared understanding of the narrative. This study along with further studies revealed that speaker–listener coupling in the DMN, but not early auditory regions, is disrupted when communication is disrupted by either speaking nonsense words86,87, telling a story in a language that the listener does not understand86 or temporally scrambling the stimulus88. Moreover, speaker–listener coupling in the DMN predicted the quality of communication: the better the listener understood the speaker, the closer their brain responses matched the speaker’s brain responses86–89. The speaker–listener coupling was observed in the temporal fluctuations of the response time courses within each node of the DMN86–89 (for example, the time course of neural responses in the speaker’s precuneus was correlated with that of the listener) as well as in the within-area alignment of spatial patterns across participants (for example, the spatial voxel pattern in the speaker’s precuneus was correlated with that of the listeners)33.
These findings demonstrate that comprehension-based processes in a listener’s brain are driven by, or shaped by, production-based processes in the speaker’s brain. Speaker–listener coupling has been observed using various stimuli and imaging modalities, including fMRI33,70,88,90,91, functional near-infrared spectroscopy (fNIRS)92–94 and electroencephalography95. Sender–receiver coupling has also been reported during non-verbal communications such as gestures96,97 and facial expressions98.
The communication cycle.
The full circle of experience transmission from the speaker’s brain to the listener’s brain has also been demonstrated33 (Fig. 4a). In this study, participants were scanned using fMRI while watching an episode of a television series. Participants were then scanned while describing the episode out loud in their own words. A second group of participants, who had never seen the television episode before, were then scanned while listening to one of the viewer participants’ descriptions. Strikingly, participants who had never seen the episode before showed spatio-temporally similar DMN activity to the speaker both as the speaker recalled the episode and as the speaker watched the episode (Fig. 4b,c). Classification analysis showed that similarities in the DMN responses between the movie viewers, the speaker and the listeners were scene-specific and invariant to low-level sensory features (such as differences between the audiovisual movie and its spoken description), suggesting that these shared responses reflected the transmission of information. Moreover, listener comprehension was better predicted by the unique alignment between the listeners’ and the speaker’s response patterns as the speaker encoded the movie to memory, relative to the alignment to the responses of other participants who watched the movie but did not share their experience with the listeners. This tight alignment between the speaker and their audience may reveal the mechanism by which we share our idiosyncratic perspectives and memories with other brains who never directly experienced them.
Fig. 4 |. Transmission of experience and the similarity of neural responses among friends.
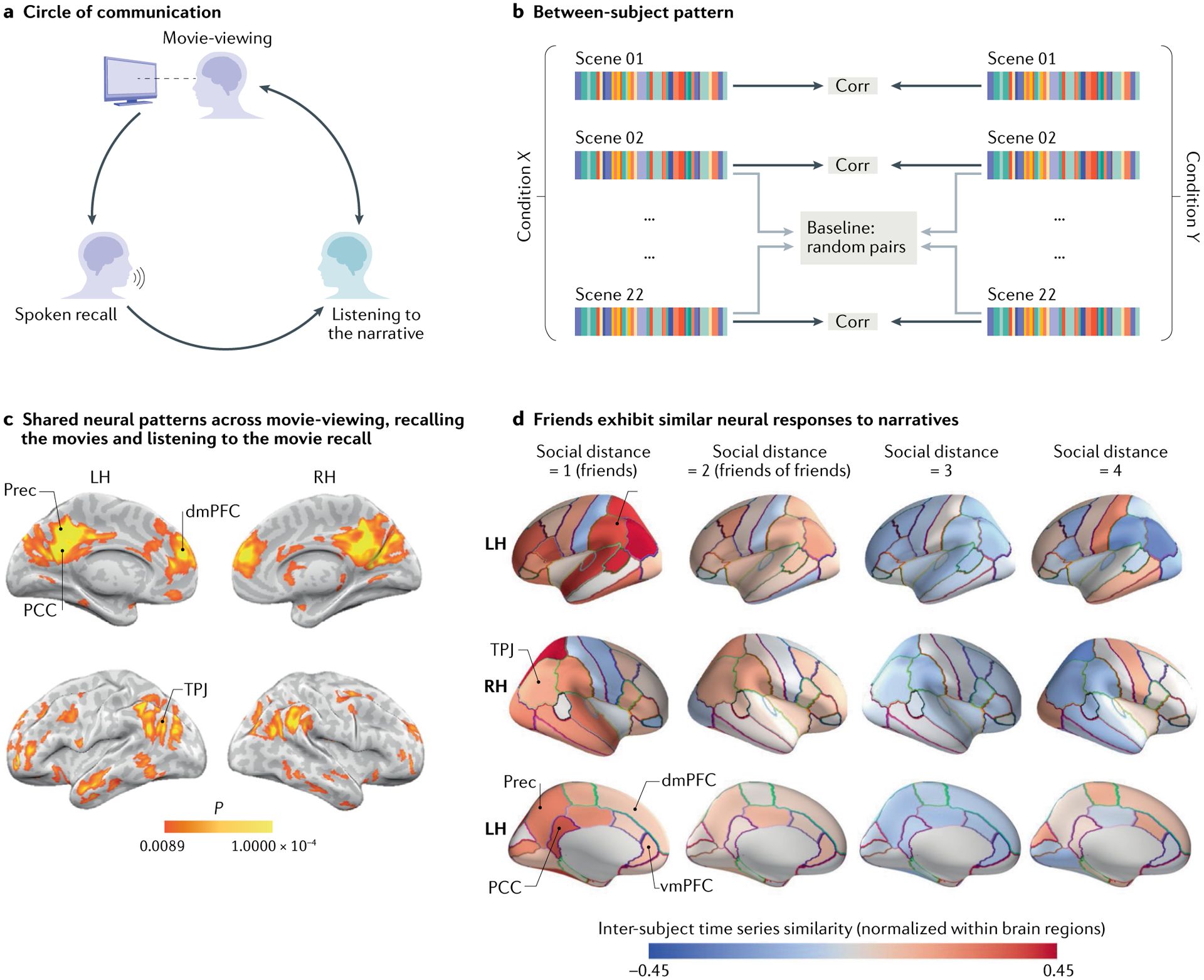
a | An experimental paradigm for measuring how we share our memories with other brains. First, a participant was scanned while watching an episode of the television series Merlin (encoding information to memory). Next, the participant was scanned as they used words to recall and share their memories with a new group of participants who had never seen the television episode before. b | Neural coupling between groups was assessed using a between-subject pattern similarity spotlight analysis. An average spatial pattern of activity was calculated for each participant for each scene of the movie (coloured bars). Spatial patterns for each scene were then correlated (corr) across participants in different groups and averaged. c | Participants who had never seen the episode before and listened to the description of the episode had a similar pattern of neural response within the default mode network (DMN) to the participant who recounted the episode from memory. d | The social network of a cohort of students at a business school was reconstructed based on a survey of the students. From this network, 42 students participated in a functional MRI (fMRI) study and watched a collection of short movie clips while in the scanner. Similarities in fMRI response while watching these clips were calculated according to the social distance (defined by the social network) between a dyad. These were calculated by correlating the time series of response for each participant to that of every other participant in a given brain region and taking the mean of the resulting correlations. Warmer colours indicate relatively similar responses for a given brain region; cooler colours indicate relatively dissimilar responses for that brain region. Note the higher degree of inter-subject alignment of the neural responses in regions of the DMN (posterior cingulate cortex, PCC; precuneus, Prec; ventromedial prefrontal cortex, vmPFC; dorsomedial prefrontal cortex, dmPFC; and temporoparietal junction, TPJ) and nearby networks to the same movie clips among dyads that are social closer. LH, left hemisphere; RH, right hemisphere. Parts a–c adapted from REF.33. Part d adapted from REF.117, CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/).
One-way versus two-way communication.
Although unidirectional communication through television, movies, social media and even online learning is increasingly common (Fig. 1b), much of communication is bidirectional and requires mutual adaptation and coupling between individuals who act simultaneously as senders and receivers84. Investigating these dynamical processes requires a shift to simultaneous scanning of multiple brains, which is sometimes called hyperscanning99. Emerging work using hyperscanning is uncovering the bidirectional flow of information during free dialogue and other naturalistic, complex tasks. For example, fNIRS was used to simultaneously scan dyads engaged in free dialogue; this approach showed that face-to-face communication elicits greater brain–brain coupling than does back-to-back communication100, suggesting a role of non-verbal signals in enhancing communication and neural alignment. A later study using fNIRS identified conversational ‘leaders’ that emerge naturally in group discussions and used Granger causality analysis to show that the leader’s neural activity more strongly predicted the followers’ neural activity than vice versa101. Other work has focused on groups of individuals completing complex, real-life tasks that require substantial goal-directed coordination among group members102–106, finding increased neural alignment — both spatial and temporal — during periods of the task that required heightened coordination and communication. Another electroencephalography study tested neural alignment between romantic dyads and unacquainted dyads, and found elevated coupling in the DMN during mutual gaze and smiling107. It was suggested that rudimentary non-verbal social cues such as mutual gaze and smiling, which are used by caregivers to initially establish coupling with children, can be used later on to facilitate neural coupling routinely throughout life.
The emergence of shared understanding.
There is no period in life when our brain responses are more strongly shaped by others than during the early years of child development. This is especially apparent during the acquisition of a shared linguistic code in early childhood, which enables the sharing of semantic representations between a child and their caregivers. Emerging fNIRS studies in which caregivers and children were simultaneously scanned during communication and interactive play shows coupled neural responses between caregivers and children (ages 8–12 years108) and between caregivers and infants as young as 9–10 months old1–3. This coupling was evident in a few DMN regions, mainly in the prefrontal cortex. Infant–caregiver coupling was most strongly associated with mutual gaze (eye contact), infant smiling and joint attention to objects109, supporting a mutual influence of neural dynamics. Together, these findings suggest that that caregiver–child DMN coupling emerges early in development and likely plays a role in learning and language acquisition.
The development of shared understanding of concepts is also the hallmark of teaching and learning in formal education. In such contexts, novel, sometimes jargonized, information must be transmitted from an expert’s brain to novices’ brains. Here, we conceptualize teaching as a process of building a shared understanding of new information, supported by new or changed neural representations. For example, taking a computer science class might lead to the development of a new understanding of computer-science concepts and terms such as ‘recursion’ or ‘multiplexor’, as well as the contextual adjustment and modification of already familiar concepts. It might also require flexibly changing the interpretation of words depending on the context: for example, ‘string’ in common parlance refers to a length of thread, whereas ‘string’ in computer science refers to a sequence of characters. Several recent studies have identified teacher–student DMN coupling during teaching and learning in both real-life classrooms110,111 and simulated classrooms88,112–116. Notably, teacher–student neural coupling predicts student learning outcomes88,110,113,114,116.
Social networks shape shared understanding.
An old proverb says “Tell me who your friends are, and I will tell you who you are”. Emerging neuroscientific research provides evidence that neural alignment across subjects is influenced and shaped by the people with whom we are connected in our real and virtual social networks. A striking recent study117,118 mapped the close-knit social network of a class of students at a business school and found that pairs of people who have closer social relationships have more similar neural responses to narratives than do pairs of people who are less close (Fig. 4d).
Moreover, recent work from the same group measured brain responses of participants from the same social network as they watched ambiguous movie clips119. The participants then gathered in groups to with the aim of coming to a consensus about the narrative interpretation of each movie clip. Consensus-building conversation changed the minds of the participants and led to the alignment of their subsequent DMN neural responses as they watched the clip again. The increase in neural alignment following the consensus-building task persisted over novel clips sampled from the same movie. Participants who were central in their real-world social networks played an outsized role in building group alignment. Group-level differences in neural responses between cultures have also been observed during processing of culturally relevant stimuli, such as stories describing protected collectivist values, that may be related to the degree of identification with the culture120,121.
Conclusions
Our thoughts, feelings and actions are constantly being shaped by the actions, memories and stories of others. At the same time, our actions, memories and stories shape and influence the thoughts, feelings and actions of the people to whom we are connected. In this Perspective, we argue that we are able to shape and be shaped by others in part because activity in the DMN is capable of both shaping other brains’ responses and being shaped by the actions of other brains during social interaction. Shared neural activity at the sensory level naturally arises from the tendency of these areas to align with the low-level perceptual properties of the external stimuli. Shared neural activity at the top of the processing hierarchy, in the DMN, naturally arises from the tendency of social brains to align thoughts and actions. The same situation can be described using spoken words, written text or abstract animated shapes. Thus, the DMN representation must be invariant to changes in low-level perceptual properties that are not associated with changes in meaning or action. Conversely, the same situation can have markedly different meanings associated with different actions across different contexts. Thus, DMN representations must differ between people who perceive and act differently in a given situation. Shared language, shared memories and shared schemas allow us to better align and couple our DMN responses. Sharing stories and building collaborations further enhance such coupling.
Developing shared ways to understand and act in the world is an evolving communal effort in which we synthesize our intrinsic idiosyncratic perspectives and actions with the extrinsic perspectives and actions of others. In a world that is more polarized by the day, the need for rebuilding a shared common ground is perhaps more urgent than ever. The Ubuntu expression states “I am because we are”. “Are” in this insightful expression can refer to ‘us but not them’ or to ‘us as all people’. The synthesis of different perspectives in the DMN depends primarily on the structure and nature of our social interactions and social connections. In this Perspective, we argue that the DMN is ‘default’ not because it is engaged when we are looking inward, nor because it is shaped by others. Rather, we suggest that the DMN is ‘default’ because it is central for integrating external and internal information, allowing for shared communication and alignment tools, shared meanings, shared narratives and, above all, shared communities and social networks. This is what people continuously and naturally do by default.
Acknowledgements
The authors thank S. Nastase, R. Malach and E. Simony for helpful discussion and comments on the manuscript. This work was supported by the US National Institutes of Health (NIH) under award numbers DP1HD091948 (U.H.) and R01MH112566-01 (M.N.).
Footnotes
Competing interests
The authors declare no competing interests.
Peer review information
Nature Reviews Neuroscience thanks D. Bzdok, Y. Hu and C. Lu for their contribution to the peer review of this work.
References
- 1.Buckner RL & DiNicola LM The brain’s default network: updated anatomy, physiology and evolving insights. Nat. Rev. Neurosci 20, 593–608 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Raichle ME The brain’s default mode network. Annu. Rev. Neurosci 38, 433–447 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R & Buckner RL Functional-anatomic fractionation of the brain’s default network. Neuron 65, 550–562 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raichle ME et al. A default mode of brain function. Proc. Natl Acad. Sci. USA 98, 676–682 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Golland Y et al. Extrinsic and intrinsic systems in the posterior cortex of the human brain revealed during natural sensory stimulation. Cereb. Cortex 17, 766–777 (2006). [DOI] [PubMed] [Google Scholar]
- 6.Andrews-Hanna JR, Saxe R & Yarkoni T Contributions of episodic retrieval and mentalizing to autobiographical thought: evidence from functional neuroimaging, resting-state connectivity, and fMRI meta-analyses. NeuroImage 91, 324–335 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Konishi M, McLaren DG, Engen H & Smallwood J Shaped by the past: the default mode network supports cognition that is independent of immediate perceptual input. PLoS ONE 10, e0132209 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shulman GL et al. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J. Cognit. Neurosci 9, 648–663 (1997). [DOI] [PubMed] [Google Scholar]
- 9.McKiernan KA, Kaufman JN, Kucera-Thompson J & Binder JR A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J. Cogn. Neurosci 15, 394–408 (2003). [DOI] [PubMed] [Google Scholar]
- 10.Gusnard DA, Akbudak E, Shulman GL & Raichle ME Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc. Natl Acad. Sci. USA 98, 4259–4264 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greicius MD, Krasnow B, Reiss AL & Menon V Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl Acad. Sci. USA 100, 253–258 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braga RM & Buckner RL Parallel interdigitated distributed networks within the individual estimated by intrinsic functional connectivity. Neuron 95, e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schurz M, Radua J, Aichhorn M, Richlan F & Perner J Fractionating theory of mind: a meta-analysis of functional brain imaging studies. Neurosci. Biobehav. Rev 42, 9–34 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Kernbach JM et al. Subspecialization within default mode nodes characterized in 10,000 UK Biobank participants. Proc. Natl Acad. Sci. USA 115, 12295–12300 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hasson U, Chen J & Honey CJ Hierarchical process memory: memory as an integral component of information processing. Trends Cogn. Sci 19, 304–313 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li W, Piëch V & Gilbert CD Contour saliency in primary visual cortex. Neuron 50, 951–962 (2006). [DOI] [PubMed] [Google Scholar]
- 17.Jones HE, Wang W & Sillito AM Spatial organization and magnitude of orientation contrast interactions in primate V1. J. Neurophysiol 88, 2796–2808 (2002). [DOI] [PubMed] [Google Scholar]
- 18.Hubel DH & Wiesel TN Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. J. Physiol 160, 106–154.2 (1962). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serre T in Encyclopedia of Computational Neuroscience (eds Jaeger D & Jung R) 1–12 (Springer, 2013). [Google Scholar]
- 20.Hasson U, Yang E, Vallines I, Heeger DJ & Rubin N A hierarchy of temporal receptive windows in human cortex. J. Neurosci 28, 2539–2550 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lerner Y, Honey CJ, Silbert LJ & Hasson U Topographic mapping of a hierarchy of temporal receptive windows using a narrated story. J. Neurosci 31, 2906–2915 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hubel DH Eye, Brain, and Vision (Scientific American Library, 1988). [Google Scholar]
- 23.Ames DL, Honey CJ, Chow MA, Todorov A & Hasson U Contextual alignment of cognitive and neural dynamics. J. Cogn. Neurosci 27, 655–664 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Honey CJ et al. Predicting human resting-state functional connectivity from structural connectivity. Proc. Natl Acad. Sci. USA 106, 2035–2040 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greicius MD, Supekar K, Menon V & Dougherty RF Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb. Cortex 19, 72–78 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simony E et al. Dynamic reconfiguration of the default mode network during narrative comprehension. Nat. Commun 7, 12141 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrison BJ et al. Consistency and functional specialization in the default mode brain network. Proc. Natl Acad. Sci. USA 105, 9781–9786 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nastase SA, Liu Y-F, Hillman H, Norman KA & Hasson U Leveraging shared connectivity to aggregate heterogeneous datasets into a common response space. NeuroImage 15, 116865 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nastase SA, Goldstein A & Hasson U Keep it real: rethinking the primacy of experimental control in cognitive neuroscience. NeuroImage 222, 117254 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Regev M, Honey CJ, Simony E & Hasson U Selective and invariant neural responses to spoken and written narratives. J. Neurosci 33, 15978–15988 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson SM, Bautista A & McCarron A Convergence of spoken and written language processing in the superior temporal sulcus. NeuroImage 171, 62–74 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baldassano C et al. Discovering event structure in continuous narrative perception and memory. Neuron 95, 709–721.e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zadbood A, Chen J, Leong YC, Norman KA & Hasson U How we transmit memories to other brains: constructing shared neural representations via communication. Cereb. Cortex 27, 4988–5000 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tikka P, Kauttonen J & Hlushchuk Y Narrative comprehension beyond language: Common brain networks activated by a movie and its script. PLoS ONE 13, e0200134 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yeshurun Y, Nguyen M & Hasson U Amplification of local changes along the timescale processing hierarchy. Proc. Natl Acad. Sci. USA 114, 9475–9480 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Honey CJ, Thompson CR, Lerner Y & Hasson U Not lost in translation: neural responses shared across languages. J. Neurosci 32, 15277–15283 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nguyen M, Vanderwal T & Hasson U Shared understanding of narratives is correlated with shared neural responses. NeuroImage 184, 161–170 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deniz F, Nunez-Elizalde AO, Huth AG & Gallant JL The representation of semantic information across human cerebral cortex during listening versus reading is invariant to stimulus modality. J. Neurosci 39, 7722–7736 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dehghani M et al. Decoding the neural representation of story meanings across languages. Hum. Brain Mapp 38, 6096–6106 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Margulies DS et al. Situating the default-mode network along a principal gradient of macroscale cortical organization. Proc. Natl Acad. Sci. USA 113, 12574–12579 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fernandino L et al. Concept representation reflects multimodal abstraction: a framework for embodied semantics. Cerebral. Cortex 26, 2018–2034 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmälzle R, Häcker FEK, Honey CJ & Hasson U Engaged listeners: shared neural processing of powerful political speeches. Soc. Cognit. Affect. Neurosci 10, 1137–1143 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cohen SS & Parra LC Memorable audiovisual narratives synchronize sensory and supramodal neural responses. eNeuro 10.1523/ENEURO.0203-16.2016 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ki JJ, Kelly SP & Parra LC Attention strongly modulates reliability of neural responses to naturalistic narrative stimuli. J. Neurosci 36, 3092–3101 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Regev M et al. Propagation of information along the cortical hierarchy as a function of attention while reading and listening to stories. Cereb. Cortex 29, 4017–4034 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen J et al. Shared memories reveal shared structure in neural activity across individuals. Nat. Neurosci 20, 115–125 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yeshurun Y et al. Same story, different story: the neural representation of interpretive frameworks. Psychological Sci. 28, 307–319 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lahnakoski JM et al. Synchronous brain activity across individuals underlies shared psychological perspectives. NeuroImage 100, 316–324 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bacha-Trams M et al. Differential inter-subject correlation of brain activity when kinship is a variable in moral dilemma. Sci. Rep 7, 14244 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cooper EA, Hasson U & Small SL Interpretation-mediated changes in neural activity during language comprehension. NeuroImage 55, 1314–1323 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uchiyama HT et al. Distinction between the literal and intended meanings of sentences: a functional magnetic resonance imaging study of metaphor and sarcasm. Cortex 48, 563–583 (2012). [DOI] [PubMed] [Google Scholar]
- 52.Bašnáková J, Weber K, Petersson KM, van Berkum J & Hagoort P Beyond the language given: the neural correlates of inferring speaker meaning. Cereb. Cortex 24, 2572–2578 (2014). [DOI] [PubMed] [Google Scholar]
- 53.Koster-Hale J & Saxe R Theory of mind: a neural prediction problem. Neuron 79, 836–848 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chang CHC, Lazaridi C, Yeshurun Y, Norman KA & Hasson U Relating the past with the present: information integration and segregation during ongoing narrative processing. Preprint at bioRxiv 10.1101/2020.01.16.908731 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen J et al. Accessing real-life episodic information from minutes versus hours earlier modulates hippocampal and high-order cortical dynamics. Cereb. Cortex 26, 3428–3441 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vincent JL, Kahn I, Snyder AZ, Raichle ME & Buckner RL Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J. Neurophysiol 100, 3328–3342 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aggleton JP Multiple anatomical systems embedded within the primate medial temporal lobe: implications for hippocampal function. Neurosci. Biobehav. Rev 36, 1579–1596 (2012). [DOI] [PubMed] [Google Scholar]
- 58.Kaplan R et al. Hippocampal sharp-wave ripples influence selective activation of the default mode network. Curr. Biol 26, 686–691 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khodagholy D, Gelinas JN & Buzsáki G Learning-enhanced coupling between ripple oscillations in association cortices and hippocampus. Science 358, 369–372 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scoville WB & Milner B Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosurg. Psychiatry 20, 11–21 (1957). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baddeley A & Wilson BA Prose recall and amnesia: implications for the structure of working memory. Neuropsychologia 40, 1737–1743 (2002). [DOI] [PubMed] [Google Scholar]
- 62.Zuo X et al. Temporal integration of narrative information in a hippocampal amnesic patient. NeuroImage 213, 116658 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gilboa A & Marlatte H Neurobiology of schemas and schema-mediated memory. Trends Cogn. Sci 21, 618–631 (2017). [DOI] [PubMed] [Google Scholar]
- 64.Baldassano C, Hasson U & Norman KA Representation of real-world event schemas during narrative perception. J. Neurosci 38, 9689–9699 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brandman T, Malach R & Simony E The surprising role of the default mode network. Preprint at bioRxiv http://biorxiv.org/lookup/doi/10.1101/2020.05.18.101758 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dohmatob E, Dumas G & Bzdok D Dark control: the default mode network as a reinforcement learning agent. Hum. Brain Mapp 41, 3318–3341 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee H, Bellana B & Chen J What can narratives tell us about the neural bases of human memory? Curr. Opin. Behav. Sci 32, 111–119 (2020). [Google Scholar]
- 68.Heider F & Simmel M An experimental study of apparent behavior. Am. J. Psychol 57, 243–259 (1944). [Google Scholar]
- 69.Saalasti S et al. Inferior parietal lobule and early visual areas support elicitation of individualized meanings during narrative listening. Brain Behav. 9, e01288 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Smirnov D et al. Emotions amplify speaker–listener neural alignment. Hum. Brain Mapp 40, 4777–4788 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jääskeläinen IP et al. Brain hemodynamic activity during viewing and reviewing of comedy movies explained by experienced humor. Sci. Rep 6, 27741 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen P-HA, Jolly E, Cheong JH & Chang LJ Inter-subject representational similarity analysis reveals individual variations in affective experience when watching erotic movies. NeuroImage 216, 116851 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van Baar JM, Chang LJ & Sanfey AG The computational and neural substrates of moral strategies in social decision-making. Nat. Commun 10, 1483 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tei S et al. Inter-subject correlation of temporoparietal junction activity is associated with conflict patterns during flexible decision-making. Neurosci. Res 144, 67–70 (2019). [DOI] [PubMed] [Google Scholar]
- 75.Finn E et al. Idiosynchrony: from shared responses to individual differences during naturalistic neuroimaging. NeuroImage 215, 116828 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nummenmaa L, Lahnakoski JM & Glerean E Sharing the social world via intersubject neural synchronisation. Curr. Opin. Psychol 24, 7–14 (2018). [DOI] [PubMed] [Google Scholar]
- 77.Finn ES, Corlett PR, Chen G, Bandettini PA & Constable RT Trait paranoia shapes inter-subject synchrony in brain activity during an ambiguous social narrative. Nat. Commun 9, 1–13 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bacha-Trams M et al. A drama movie activates brains of holistic and analytical thinkers differentially. Soc. Cogn. Affect. Neurosci 13, 1293–1304 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gruskin DC, Rosenberg MD & Holmes AJ Relationships between depressive symptoms and brain responses during emotional movie viewing emerge in adolescence. NeuroImage, 216, 116217 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guo CC, Nguyen VT, Hyett MP, Parker GB & Breakspear MJ Out-of-sync: disrupted neural activity in emotional circuitry during film viewing in melancholic depression. Sci. Rep 5, 11605 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang Z et al. Individualized psychiatric imaging based on inter-subject neural synchronization in movie watching. NeuroImage 216, 116227 (2020). [DOI] [PubMed] [Google Scholar]
- 82.Rossignac-Milon M Merged Minds: Generalized Shared Reality in Interpersonal Relationships (Columbia University, 2019). [DOI] [PubMed] [Google Scholar]
- 83.Hasson U, Ghazanfar AA, Galantucci B, Garrod S & Keysers C Brain-to-brain coupling: a mechanism for creating and sharing a social world. Trends Cogn. Sci 16, 114–121 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hasson U & Frith CD Mirroring and beyond: coupled dynamics as a generalized framework for modelling social interactions. Philos. Trans. R. Soc. B Biol. Sci 371, 20150366 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Friston KJ et al. Generative models, linguistic communication and active inference. Neurosci. Biobehav. Rev 118, 42–64 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stephens GJ, Silbert LJ & Hasson U Speaker–listener neural coupling underlies successful communication. Proc. Natl Acad. Sci. USA 107, 14425–14430 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Silbert LJ, Honey CJ, Simony E, Poeppel D & Hasson U Coupled neural systems underlie the production and comprehension of naturalistic narrative speech. Proc. Natl Acad. Sci. USA 111, E4687–E4696 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nguyen ML, Chang A, Micciche E, Meshulam M & Nastase SA Teacher–student neural coupling during teaching and learning. Preprint at bioRxiv 10.1101/2020.05.07.082958 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dikker S, Silbert LJ, Hasson U & Zevin JD On the same wavelength: predictable language enhances speaker–listener brain-to-brain synchrony in posterior superior temporal gyrus. J. Neurosci 34, 6267–6272 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.AbdulSabur NY et al. Neural correlates and network connectivity underlying narrative production and comprehension: a combined fMRI and PET study. Cortex 57, 107–127 (2014). [DOI] [PubMed] [Google Scholar]
- 91.Heidlmayr K, Weber K, Takashima A & Hagoort P No title, no theme: the joined neural space between speakers and listeners during production and comprehension of multi-sentence discourse. Cortex 130, 111–126 (2020). [DOI] [PubMed] [Google Scholar]
- 92.Liu Y et al. Measuring speaker–listener neural coupling with functional near infrared spectroscopy. Sci. Rep 7, 43293 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hirsch J, Adam Noah J, Zhang X, Dravida S & Ono SY A cross-brain neural mechanism for human-to-human verbal communication. Soc. Cogn. Affect. Neurosci 13, 907–920 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dai R et al. Holistic cognitive and neural processes: a fNIRS-hyperscanning study on interpersonal sensorimotor synchronization. Soc. Cogn. Affect. Neurosci 13, 1141–1154 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kuhlen AK, Allefeld C & Haynes J-D Content-specific coordination of listeners’ to speakers’ EEG during communication. Front. Hum. Neurosci 6, 266 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schippers MB, Roebroeck A, Renken R, Nanetti L & Keysers C Mapping the information flow from one brain to another during gestural communication. Proc. Natl Acad. Sci. USA 107, 9388–9393 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schippers MB, Gazzola V, Goebel R & Keysers C Playing charades in the fMRI: are mirror and/or mentalizing areas involved in gestural communication? PLoS ONE 4, e6801 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Anders S, Heinzle J, Weiskopf N, Ethofer T & Haynes J-D Flow of affective information between communicating brains. NeuroImage 54, 439–446 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Montague P Hyperscanning: simultaneous fMRI during linked social interactions. NeuroImage 16, 1159–1164 (2002). [DOI] [PubMed] [Google Scholar]
- 100.Jiang J et al. Neural synchronization during face-to-face communication. J. Neurosci 32, 16064–16069 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jiang J et al. Leader emergence through interpersonal neural synchronization. Proc. Natl Acad. Sci. USA 112, 4274–4279 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Astolfi L et al. Imaging the social brain by simultaneous hyperscanning during subject interaction. IEEE Intell. Syst 26, 38–45 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stevens RH & Galloway TL Modeling the neurodynamic organizations and interactions of teams. Soc. Neurosci 11, 123–139 (2016). [DOI] [PubMed] [Google Scholar]
- 104.Dodel S et al. in Foundations of Augmented Cognition. Directing the Future of Adaptive Systems (eds Schmorrow DD & Fidopiastis CM) 288–297 (Springer, 2011). [Google Scholar]
- 105.Fishburn FA et al. Putting our heads together: interpersonal neural synchronization as a biological mechanism for shared intentionality. Soc. Cogn. Affect. Neurosci 13, 841–849 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yang J, Zhang H, Ni J, De Dreu CKW & Ma Y Within-group synchronization in the prefrontal cortex associates with intergroup conflict. Nat. Neurosci 23, 754–760 (2020). [DOI] [PubMed] [Google Scholar]
- 107.Kinreich S, Djalovski A, Kraus L, Louzoun Y & Feldman R Brain-to-brain synchrony during naturalistic social interactions. Sci. Rep 7, 17060 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Miller JG et al. Inter-brain synchrony in mother-child dyads during cooperation: an fNIRS hyperscanning study. Neuropsychologia 124, 117–124 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Piazza EA, Hasenfratz L, Hasson U & Lew-Williams C Infant and adult brains are coupled to the dynamics of natural communication. Psychol. Sci 12, 6–17 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bevilacqua D et al. Brain-to-brain synchrony and learning outcomes vary by student–teacher dynamics: evidence from a real-world classroom electroencephalography study. J. Cognit. Neurosci 31, 401–411 (2019). [DOI] [PubMed] [Google Scholar]
- 111.Dikker S et al. Brain-to-brain synchrony tracks real-world dynamic group interactions in the classroom. Curr. Biol 27, 1375–1380 (2017). [DOI] [PubMed] [Google Scholar]
- 112.Pan Y et al. Instructor–learner brain coupling discriminates between instructional approaches and predicts learning. NeuroImage 211, 116657 (2020). [DOI] [PubMed] [Google Scholar]
- 113.Davidesco I et al. Brain-to-brain synchrony between students and teachers predicts learning outcomes. Preprint at bioRxiv 10.1101/644047 (2019). [DOI] [PubMed] [Google Scholar]
- 114.Zheng L et al. Enhancement of teaching outcome through neural prediction of the students’ knowledge state. Hum. Brain. Mapp 39, 3046–3057 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cohen SS et al. Neural engagement with online educational videos predicts learning performance for individual students. Neurobiol. Learn. Mem 155, 60–64 (2018). [DOI] [PubMed] [Google Scholar]
- 116.Meshulam M et al. Think like an expert: neural alignment predicts understanding in students taking an introduction to computer science course. Preprint at bioRxiv 10.1101/2020.05.05.079384 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Parkinson C, Kleinbaum AM & Wheatley T Similar neural responses predict friendship. Nat. Commun 9, 1532 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hyon R, Kleinbaum AM & Parkinson C Social network proximity predicts similar trajectories of psychological states: evidence from multi-voxel spatiotemporal dynamics. NeuroImage 216, 116492 (2020). [DOI] [PubMed] [Google Scholar]
- 119.Sievers B, Welker C, Hasson U, Kleinbaum AM & Wheatley T How consensus-building conversation changes our minds and aligns our brains. Preprint at PsyArXiv 10.31234/osf.io/562z7 (2020). [DOI] [Google Scholar]
- 120.Kaplan JT et al. Processing narratives concerning protected values: a cross-cultural investigation of neural correlates. Cereb. Cortex 27, 1428–1438 (2017). [DOI] [PubMed] [Google Scholar]
- 121.Levy J et al. Adolescents growing up amidst intractable conflict attenuate brain response to pain of outgroup. Proc. Natl Acad. Sci. USA 113, 13696–13701 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nastase SA, Gazzola V, Hasson U & Keysers C Measuring shared responses across subjects using intersubject correlation. Soc. Cogn. Affect. Neurosci 14, 667–685 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Buckner RL et al. Functional anatomical studies of explicit and implicit memory retrieval tasks. J. Neurosci 15, 12–29 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Buckner RL The serendipitous discovery of the brain’s default network. NeuroImage 62, 1137–1145 (2012). [DOI] [PubMed] [Google Scholar]
- 125.Raichle ME The restless brain: how intrinsic activity organizes brain function. Philos. Trans. R. Soc. Lond. B Biol. Sci 370, 20140172 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Buckner RL, Andrews-Hanna JR & Schacter DL The brain’s default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci 1124, 1–38 (2008). [DOI] [PubMed] [Google Scholar]
- 127.Fox KCR, Spreng RN, Ellamil M, Andrews-Hanna JR & Christoff K The wandering brain: meta-analysis of functional neuroimaging studies of mind-wandering and related spontaneous thought processes. NeuroImage 111, 611–621 (2015). [DOI] [PubMed] [Google Scholar]
- 128.Andrews-Hanna JR, Reidler JS, Huang C & Buckner RL Evidence for the default network’s role in spontaneous cognition. J. Neurophysiol 104, 322–335 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Smallwood J & Schooler JW The science of mind wandering: empirically navigating the stream of consciousness. Annu. Rev. Psychol 66, 487–518 (2015). [DOI] [PubMed] [Google Scholar]
- 130.Mason MF et al. Wandering minds: the default network and stimulus-independent thought. Science 315, 393–395 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Preminger S, Harmelech T & Malach R Stimulus-free thoughts induce differential activation in the human default network. NeuroImage 54, 1692–1702 (2011). [DOI] [PubMed] [Google Scholar]
- 132.Poerio GL et al. The role of the default mode network in component processes underlying the wandering mind. Soc. Cogn. Affect. Neurosci 12, 1047–1062 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Andreasen NC et al. Remembering the past: two facets of episodic memory explored with positron emission tomography. Am. J. Psychiatry 152, 1576–1585 (1995). [DOI] [PubMed] [Google Scholar]
- 134.Maguire EA Neuroimaging studies of autobiographical event memory. Philos. Trans. R Soc. Lond. B Biol. Sci 356, 1441–1451 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cabeza R & St Jacques P Functional neuroimaging of autobiographical memory. Trends Cogn. Sci 11, 219–227 (2007). [DOI] [PubMed] [Google Scholar]
- 136.Spreng RN, Mar RA & Kim ASN The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J. Cogn. Neurosci 21, 489–510 (2009). [DOI] [PubMed] [Google Scholar]
- 137.Delamillieure P et al. The resting state questionnaire: an introspective questionnaire for evaluation of inner experience during the conscious resting state. Brain Res. Bull 81, 565–573 (2010). [DOI] [PubMed] [Google Scholar]
- 138.Schacter DL, Addis DR & Buckner RL Remembering the past to imagine the future: the prospective brain. Nat. Rev. Neurosci 8, 657–661 (2007). [DOI] [PubMed] [Google Scholar]
- 139.Schacter DL, Addis DR & Buckner RL Episodic simulation of future events: concepts, data, applications. Ann. N. Y. Acad. Sci 1124, 39–60 (2008). [DOI] [PubMed] [Google Scholar]
- 140.Adolphs R The social brain: neural basis of social knowledge. Annu. Rev. Psychol 60, 693–716 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Meyer ML Social by default: characterizing the social functions of the resting brain. Curr. Dir. Psychol. Sci 28, 380–386 (2019). [Google Scholar]
- 142.Mars RB et al. On the relationship between the “default mode network” and the “social brain”. Front. Hum. Neurosci 6, 189 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Amodio DM & Frith CD Meeting of minds: the medial frontal cortex and social cognition. Nat. Rev. Neurosci 7, 268–277 (2006). [DOI] [PubMed] [Google Scholar]
- 144.Schilbach L, Eickhoff SB, Rotarska-Jagiela A, Fink GR & Vogeley K Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the “default system” of the brain. Conscious. Cognition 17, 457–467 (2008). [DOI] [PubMed] [Google Scholar]
- 145.Lieberman MD Social cognitive neuroscience: a review of core processes. Annu. Rev. Psychol 58, 259–289 (2007). [DOI] [PubMed] [Google Scholar]
- 146.Alcalá-López D et al. Computing the social brain connectome across systems and states. Cereb. Cortex 28, 2207–2232 (2018). [DOI] [PubMed] [Google Scholar]
- 147.Mitchell JP Social psychology as a natural kind. Trends Cogn. Sci 13, 246–251 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Krienen FM, Tu P-C & Buckner RL Clan mentality: evidence that the medial prefrontal cortex responds to close others. J. Neurosci 30, 13906–13915 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Qin P & Northoff G How is our self related to midline regions and the default-mode network? NeuroImage 57, 1221–1233 (2011). [DOI] [PubMed] [Google Scholar]
- 150.Northoff G et al. Self-referential processing in our brain — a meta-analysis of imaging studies on the self. NeuroImage 31, 440–457 (2006). [DOI] [PubMed] [Google Scholar]
- 151.Saxe R & Powell LJ It’s the thought that counts: specific brain regions for one component of theory of mind. Psychol. Sci 17, 692–699 (2006). [DOI] [PubMed] [Google Scholar]
- 152.Spunt RP, Meyer ML & Lieberman MD The default mode of human brain function primes the intentional stance. J. Cognit. Neurosci 27, 1116–1124 (2015). [DOI] [PubMed] [Google Scholar]
- 153.Stephens GJ, Honey CJ & Hasson U A place for time: the spatiotemporal structure of neural dynamics during natural audition. J. Neurophysiol 110, 2019–2026 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]


