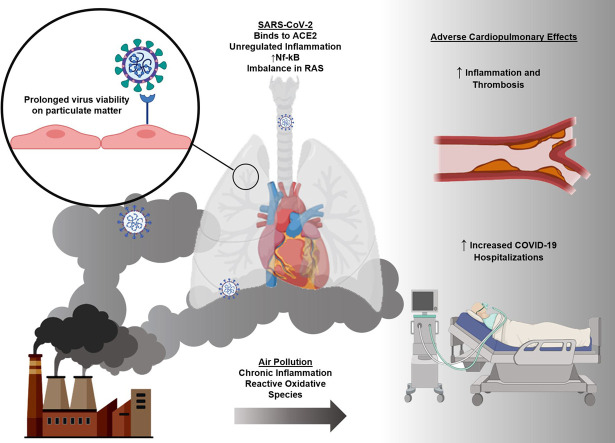
Abstract
Ambient air pollution contributes to 7 million premature deaths annually. Concurrently, the ongoing coronavirus disease 2019 (COVID-19) pandemic, complicated with S-protein mutations and other variants, caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has resulted in over 2.5 million deaths globally. Chronic air pollution-mediated cardiopulmonary diseases have been associated with an increased incidence of hospitalization and mechanical ventilation following COVID-19 transmission. While the underlying mechanisms responsible for this association remain elusive, air pollutant-induced vascular oxidative stress and inflammatory responses have been implicated in amplifying COVID-19-mediated cytokine release and vascular thrombosis. In addition, prolonged exposure to certain types of particulate matter (PM2.5, d < 2.5 μm) has also been correlated with increased lung epithelial and vascular endothelial expression of the angiotensin-converting enzyme-2 (ACE2) receptors to which the SARS-CoV-2 spike glycoproteins (S) bind for fusion and internalization into host cells. Emerging literature has linked high rates of SARS-CoV-2 infection to regions with elevated levels of PM2.5, suggesting that COVID-19 lockdowns have been implicated in regional reductions in air pollutant-mediated cardiopulmonary effects. Taken together, an increased incidence of SARS-CoV-2-mediated cardiopulmonary diseases seems to overlap with highly polluted regions. To this end, we will review the redox-active components of air pollutants, the pathophysiology of SARS-CoV-2 transmission, and the key oxidative mechanisms and ACE2 overexpression underlying air pollution-exacerbated SARS-CoV-2 transmission.
Keywords: Air pollution, COVID-19, SARS-CoV-2, Cardiopulmonary effects
Graphical abstract
1. Introduction
Ambient air pollution affects millions of people daily as one of the leading causes of morbidity and mortality worldwide (Rajagopalan et al., 2018) (Table 1 ). In parallel, the coronavirus disease 2019 (COVID-19) represents the worst infectious outbreak of the century, infecting cardiovascular, pulmonary, and other organ systems with the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The global death toll from COVID-19 infection has risen beyond 2.5 million, as reported by the World Health Organization (WHO) (“Coronavirus Disease (COVID-19)”, 2021).
Table 1.
Size and composition of air pollutants.
| Pollutant | Source | US Levels | US Air Quality Standard | Reference |
|---|---|---|---|---|
| PM2.5 | Combustion sources (e.g. vehicle emissions and industrial processes) Natural causes (e.g. wildfires) Atmospheric photochemical reactions with gaseous pollutants such as NOx, O3, SO2, CO, and VOCs |
10.40 μg/m3 (2000–2019) 8.57 μg/m3 (2010–2019) |
12 μg/m3 (annual mean) 25 μg/m3 (24-hour mean) |
Brandt et al. (2020) Health effects of particulate matter (2013) |
| PM10 | 79.49 μg/m3 (2000–2019) 75.13 μg/m3 (2010–2019) |
No annual mean 150 μg/m3 (24-hour mean) |
Brandt et al. (2020) California Air Resources Board (2020) |
|
| PM0.1 (UFPs) | 4730 particles/cm3 | 10,760 particles/cm3 |
Brandt et al. (2020) Morawska et al. (2008) |
|
| NO2 and other NOx | Combustion sources Conversion from NO by atmospheric O3 |
44.45 ppb (2000–2019) 37.29 ppb (2010–2019) |
40 μg/m3 (annual mean) 200 μg/m3 (1-hour mean) |
“Basic Information about NO2” (2016) Brook et al. (2004) U.S. Environmental Protection Agency (2016) |
| O3 | Atmospheric photochemical reactions with oxygen, NOx, and reactive hydrocarbons in sunlight | 0.074 ppm (2000–2019) 0.068 ppm (2010–2019) |
100 μg/m3 (8-hour mean) | U.S. Environmental Protection Agency (2015) |
| CO | Vehicle emissions Incomplete combustion of organic fuels (e.g. gasoline, oil, and coal from vehicles and other fossil fuel combustion sources) |
1.88 ppm (2000–2019) 1.26 ppm (2010–2019) |
9 ppm (8-hr mean) |
“Carbon Monoxide Trends” (2016) U.S. Environmental Protection Agency (2016) |
| SO2 | Burning of fuel containing sulfur (e.g. coal and oil in power plants, vehicles, and volcanoes) Reacts with water to form sulfuric acid |
47.33 ppb (2000–2019) 25.84 ppb (2010–2019) |
20 μg/m3 (annual mean) 500 μg/m3 (10-minute mean) |
U.S. Environmental Protection Agency (2016) |
| VOC (e.g. formaldehyde and benzene) | Mostly indoor burning of fuels, organic chemicals in household products | 0.1–1 ppb | N/A | Pankow et al. (2003) |
Only the most representative citations are given. (PM, particulate matter; PM2.5, particles with a diameter ≤ 2.5 μm; PM10, particles with a diameter ≤ 10 μm; PM0.1, particles with a diameter ≤ 2.5 μm; NO2, nitrogen dioxide; NOx, nitrogen oxides; O3, ozone; CO, carbon monoxide; SO2, sulfur dioxide; VOC, volatile organic compounds; μg/m3, micrograms per meter cubed; ppm, parts per million; ppb, parts per billion.)
Industrial regions with the highest levels of pollutants are experiencing particularly high mortality rates from SARS-CoV-2 transmission (X. Wu et al., 2020; Ali and Islam, 2020; Comunian et al., 2020; Mukherjee et al., 2021). The first case of COVID-19 in Wuhan City in China and subsequently, in the Po Valley in Italy, are examples of the geographical link with SARS-CoV-2 infection (Fig. 1 ) (Conticini et al., 2020; Hui et al., 2020; Jiang et al., 2020; Remuzzi and Remuzzi, 2020; Coccia, 2021a, Coccia, 2021b, Coccia, 2021c). The Po Valley spans the cities of Lodi, Cremora, Bergamo, and Brescia, known as the four Italian cities with the highest pollution levels (Frontera et al., 2020a). These highly industrialized regions were found to have a two-fold higher mortality rate from COVID-19. However, in other regions of Italy with low pollution, 40–50% of the population had positive COVID-19 swabs but were asymptomatic. Thus, there seems to be a correlation between COVID-19 patients having more severe symptoms and living in an area with higher air pollution (X. Wu et al., 2020).
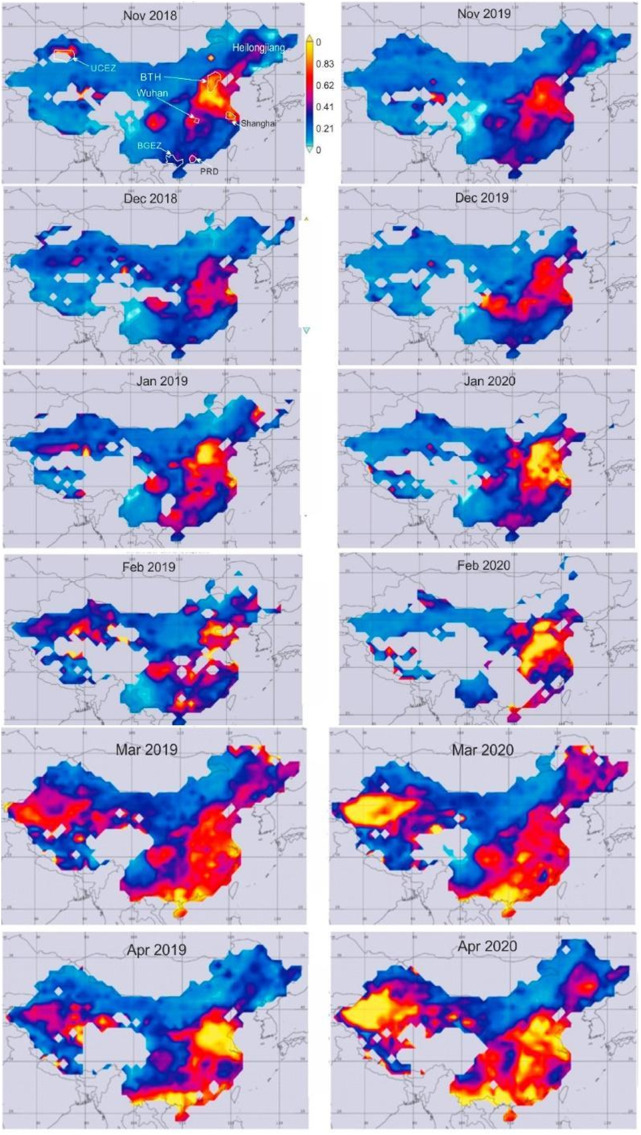
Fig. 1.
Maps of the geographical distribution of Aerosol Optical Depth (AOD) across China, which represents tropospheric particulate concentrations. COVID-19 cases and air pollution concentrations were concentrated in industrial regions. Wuhan, China is one of these regions. Figure from Nichol et al. in Remote Sens. 2020 under the Creative Commons license (Nichol et al., 2020).
While ambient particulate matter is widely recognized as a contributor to the underlying cardiopulmonary diseases, recent epidemiological findings support the emerging association between elevated levels of air pollution and COVID-19 outbreaks and mortality (Martelletti and Martelletti, 2020; Petroni et al., 2020). Patients with pre-existing diseases that are correlated with chronic exposure to air pollution, such as atherosclerosis, chronic obstructive pulmonary disease (COPD), cystic fibrosis (CF), diabetes, and obesity, fare worse clinically after contracting SARS-CoV-2. These patients develop an increased risk of death and are in need of mechanical ventilation (Chen et al., 2020a; Colombo et al., 2020; Ji et al., 2020; F. Wu et al., 2020; Mohammad et al., 2021). Emergency department admissions due to exacerbation of COPD or CF have been attributed to 10 μg/m3 increases of particulate matter (PM) in the air (Zanobetti and Schwartz, 2005).
Aggressive measures to contain COVID-19 outbreaks through public lockdowns and home quarantines led to a reduction in air pollution from transportation and industrial emissions (Chen et al., 2020b; Huang et al., 2020). In the Yangtze River Delta region, including Shanghai, in China, concentrations of particulate matter with a diameter of 2.5 μm (PM2.5) were reduced by 22.9% as compared to pre-lockdown levels (Huang et al., 2020). Daily premature mortality related to PM2.5 exposure during the lockdown period was estimated to be 895 (95% confidence interval: 637–1081). This mortality was 43.3% lower than the pre-lockdown period and 46.5% lower than averages for 2017–2019 (Huang et al., 2020). Thus, the substantial health benefits, such as a lowered incidence of premature deaths due to cardiopulmonary diseases in Wuhan, China, suggest an association with the reduced emission levels from reduced human and industrial activities (Chen et al., 2020a; Giani et al., 2020; Han and Hong, 2020; Xu et al., 2020).
Despite the rising mortality rates in the highly polluted regions, the relationship between air pollutant-mediated cardiopulmonary diseases and exacerbation of COVID-19-associated comorbidities has yet to be determined (Giani et al., 2020). High rates of hospitalization from COVID-19 outbreaks have occurred in regions with elevated levels of pollutants (Benmarhnia, 2020; Bianconi et al., 2020; Bontempi, 2020; Frontera et al., 2020b; Giani et al., 2020). To further study the overlapping relationship between the impact of air pollution and COVID-19 on the cardiopulmonary system, we hereby conducted this critical review. This emerging domain of study covers the intersection of COVID-19 and the environmental impacts on the heart, lungs, and vasculature, thereby providing an epidemiological basis for future basic and clinical research. Our review highlights the inflammatory responses, and overexpression of angiotensin-converting enzyme 2 (ACE2) receptors underlying the cumulative effects of ambient PM2.5-exposure and SARS-CoV-2 transmission on exacerbating cardiopulmonary outcomes.
2. Research methodology
We conducted a literature search on the PubMed database for keywords (“COVID-19” OR “SARS-CoV-2”) AND (“air pollution” OR “environment”) AND (“cardiovascular” OR “cardiopulmonary”) to identify articles that were relevant to COVID-19 and the intersection between cardiopulmonary effects and air pollution. These articles were manually selected for further comparison. Each article was inserted into a Microsoft Excel document where the title, year, keywords, methodology, key findings, authors, and journal names were documented. Through an iterative process, the articles were coded into different themes, followed by classification into three categories in the ensuing Results section. The articles under each category were analyzed for critical reflection and future research directions.
3. Results
Cumulative effects from the exposure to air pollution and COVID-19 on the cardiopulmonary system are not fully understood. From our literature search, we identified three overarching research categories that relate the cumulative effects of COVID-19 and air pollution on the cardiopulmonary systems that require future studies: (1) the direct physical impact of air pollution and COVID-19 on cardiopulmonary organs and tissues, (2) subsequent activation of immune system and imbalance in inflammatory responses, and (3) the indirect and direct effects of air pollution on the transmission of SARS-CoV-2.
3.1. Cumulative effects from direct physical impact of air pollutants and COVID-19 on the cardiopulmonary system
Air pollutants and COVID-19 both enter the human body via inhalation through the lungs, and the cumulated effects may accentuate COVID-19-mediated transmission and symptoms. Patients with existing cardiopulmonary comorbidities are predisposed to cytokine storms and subsequent need for mechanical ventilation following COVID-19 infection (Ejaz et al., 2020; Sanyaolu et al., 2020; Vaughan et al., 2021; Silverio et al., 2021). This may be due to the direct physical impact that both air pollution and COVID-19 impart on the cells at the respiratory interface and the cardiovascular level. The primary ingestion of air pollutant particles is through the lungs, where small gaseous air pollutants and soluble PM deposit (Rao et al., 2018). Some atmospheric ultrafine particles (UFP, d < 0.1–0.2 μm) are reported to enter the digestive system via inhalation (Li et al., 2015). Larger particles, including PM10, tend to deposit in the upper airways, whereas smaller particles, including PM2.5 and UFP, have the potential to reach the depths of the alveolar sacs where these particles cross the alveolar epithelial and capillary endothelial tight junctions into the bloodstream (Daigle et al., 2003; Nemmar et al., 2002). A chronic, low-grade inflammatory response due to ineffective mucociliary clearance of the particles is also implicated in the increased risk of cardiopulmonary disease (Lawal, 2017) (Table 2 ).
Table 2.
Redox mechanisms of air pollutants and cardiopulmonary diseases.
| Pollutant | Mechanisms | Physiological outcomes | Reference |
|---|---|---|---|
| PM2.5, PM10, & PM0.1 (UFPs) | Enters bloodstream Promotion of atherosclerotic progression Oxidative stress Acute conduit artery vasoconstriction |
Increased presence of hypertension, blood coagulation, and reactive hyperemia Increased risk for exacerbations of congestive heart failure and respiratory diseases |
Briet et al. (2007) Brook et al. (2004) California Air Resources Board. (2020) Gold et al. (2000) Lucking et al. (2008) Morawska et al. (2008) Mutlu et al. (2007) Peters et al. (2001) Suwa et al. (2002) Wellenius et al. (2005) |
| NO2 and other NOx | Oxidative Stress ROS production causing lung tissue damage Inflammation Disrupts endothelial function |
Exacerbation of respiratory diseases (e.g. COPD) Trigger/aggravator of cardiovascular conditions (e.g. acute coronary episodes, arrhythmia) |
Briet et al. (2007) Brook et al. (2004) Peters et al. (2001) |
| O3 | Airway inflammation Disrupts endothelial function Oxidative stress Acute conduit artery vasoconstriction |
Increased presence of hypertension and blood packet activation, increasing risk for CVD Aggravates CVD (e.g. coronary artery disease, stroke) |
Briet et al. (2007) Rajagopalan et al. (2018) |
| CO | Body cell and tissue hypoxia by binding to hemoglobin Disrupts endothelial function |
Damage to heart and lung tissue Worsens respiratory and cardiac function Increased embolisms and thrombotic changes |
Blumenthal (2001) Briet et al. (2007) Hoek et al. (2001) |
| SO2 | Oxidative stress Produced sulfuric acid causes irritation to eyes, mucous membranes, and skin |
Disrupts respiratory function and exacerbates pre-existing respiratory conditions Increased morbidity and mortality of CVD (heart failure, arrythmia) Increased hypertension and thrombotic events |
Hoek et al. (2001) Ibald-Mulli et al. (2001) Lipsett (2001) |
| VOC | May combine to form harmful pollutants (tropospheric ozone and smog) May increase CRP plasma levels |
Damage to heart and lung tissue Worsens respiratory and cardiac function Increased embolisms and thrombotic changes |
Pankow et al. (2003) U.S. Environmental Protection Agency (2014) |
Only the most representative citations are given. (PM, particulate matter; PM2.5, particles with a diameter ≤ 2.5 μm; PM10, particles with a diameter ≤ 10 μm; PM0.1, particles with a diameter ≤ 2.5 μm; NO2, nitrogen dioxide; NOx, nitrogen oxides; O3, ozone; CO, carbon monoxide; SO2, sulfur dioxide; VOC, volatile organic compounds; μg/m3, micrograms per meter cubed; ppm, parts per million; CVD, cardiovascular disease; ROS, reactive oxygen species; CRP, C-reactive protein.)
The disease at large, COVID-19, is a respiratory infection with systemic effects. As upper airway epithelial cells are the first to be infected, they further contribute to viral shedding and consequently, transmission, as observed during the early phase of infection, when patients' symptoms resemble a routine upper respiratory infection (Wölfel et al., 2020).
3.1.1. Particulate matter and ultrafine particles
Atmospheric pollutants, including components within PM, are well-recognized to induce a systemic inflammatory response and oxidative stress. The compositions and seasonal variations in air pollutants further modulate the overexpression of inflammatory cytokines (Kumarathasan et al., 2018) (Table 2). A host of data supports that exposure to PM promotes cardiomyocyte injury, cardiac sodium channel dysfunction, and decreased cardiomyocyte mitochondrial function (Liu et al., 2015; Nichols et al., 2015; Wang et al., 2012).
PM2.5 exposure has been associated with acceleration of atherosclerosis and subsequent vascular calcification, as well as exacerbation of chronic respiratory diseases (Dominici et al., 2006; Kaufman et al., 2016; Rajagopalan et al., 2018; Sun et al., 2005). Recently, studies have shown that inhalation of ambient air particles, especially those from combustion-related sources, imparts far-reaching cardiopulmonary sequelae and mortality in humans. These types of PM consist of a combination of organic and inorganic components in the form of solid and liquid particles of varying sizes and chemical compositions. The PM are categorized in terms of size: (1) PM10 particles are less than 10 μm, (2) PM2.5 particles are less than 2.5 μm, and (3) PM0.1 particles, or ultrafine particles (UFPs), are less than 0.1–0.2 μm in diameter (Conticini et al., 2020).
Due to their small size, PM2.5 particles are widely studied epigenetic factors for cardiovascular morbidity and mortality (Kaufman et al., 2016). Compared to PM10, these particles harbor a higher probability of evading the mucociliary clearance to reach the alveoli, accentuating the severity of cardiopulmonary diseases (Sun et al., 2010). Other inhaled smaller particles, such as UFP, may further induce NF-κB-mediated vascular oxidative stress and inflammatory responses, alter the diversity of gut microbiota, and elevate circulating lipid metabolites (Li et al., 2010, Li et al., 2015, Li et al., 2017; Salim et al., 2014) (Table 2).
3.1.2. Gaseous pollutants
The gaseous pollutants in the atmosphere, including nitrogen oxides (NOx), sulfur dioxide (SO2), ozone (O3), carbon monoxide (CO), volatile organic compounds (VOCs), and polycyclic aromatic hydrocarbons, are considered redox-active (Manisalidis et al., 2020). Akin to PM pollution, these gaseous pollutants gain entry into the circulatory system primarily via inhalation. They can also be absorbed through the skin, causing direct damage in other organ systems (Ghorani-Azam et al., 2016) (Table 2). The relatively low concentration of ambient SO2 reacts with water to form sulfuric acid (Lipsett, 2001). Due to hemoglobin having a higher affinity for CO rather than for oxygen, CO binds to hemoglobin upon inhalation, resulting in reduced oxygen perfusion to tissues and organs (Blumenthal, 2001). Some VOCs such as formaldehyde and benzene are highly redox-active, undergoing photochemical reaction with other atmospheric gases. This can adversely affect the cardiopulmonary system upon inhalation. However, VOC concentrations tend to be so low that they impart little or no adversarial effects on human health (“Volatile Organic Compounds' Impact on Indoor Air Quality”, 2014).
3.1.3. Air pollutants and COVID-19: histology and tissue deterioration
The findings in lung histology of COVID-19 patients resemble those of injured microscopic structures in air pollutant exposure. A histological study of admitted COVID-19 patients revealed viral penetration of liver and small intestine endothelial cells, as well as endothelial inflammation in the small intestine, vascular, lung, heart, liver, and kidney cells. These are all organ systems that are also affected by chronic air pollution (Varga et al., 2020). Although preliminary, animal models exposed to chronic PM2.5 report increased fibrosis in the alveolar walls (Sun et al., 2005). Current histological findings of COVID-19 patients reveal diffuse infiltration of alveolar walls by lymphocytes and edema, and patients with severe symptoms developed extensive intra-alveolar fibrin deposits in association with inflammatory or deteriorating hyaline membranes, suggestive of early onset acute respiratory distress syndrome (ARDS) (Bezzio et al., 2020). Tissue biopsies revealed extravasation of red blood cells in both the lung and skin issues such as the mid-dermis (Jimenez-Cauhe et al., 2020; Mayor-Ibarguren et al., 2020). Vascular injury was also a prominent feature in association with endothelial dysfunction, micro-thrombus formation, and cellular inflammation (Fig. 2 ). Furthermore, a study in Germany found that lung histology from COVID-19 patients showed consistent diffuse alveolar injury in association with activated pneumocytes, protein-enriched edema, and microvascular thromboemboli (Wichmann et al., 2020). Heart tissue biopsies further revealed interstitial mononuclear inflammatory infiltrates consistent with an elevated level of serum troponin and cardiac arrhythmias; however, the molecular mechanisms remain undefined (Lindner et al., 2020). In addition to cardiopulmonary injury, gastrointestinal involvement is reported in up to 26% of patients (Z. Zhou et al., 2020). These COVID-19-infected tissues run in parallel with the organ systems as reported in populations living in areas with high air pollutant concentrations.
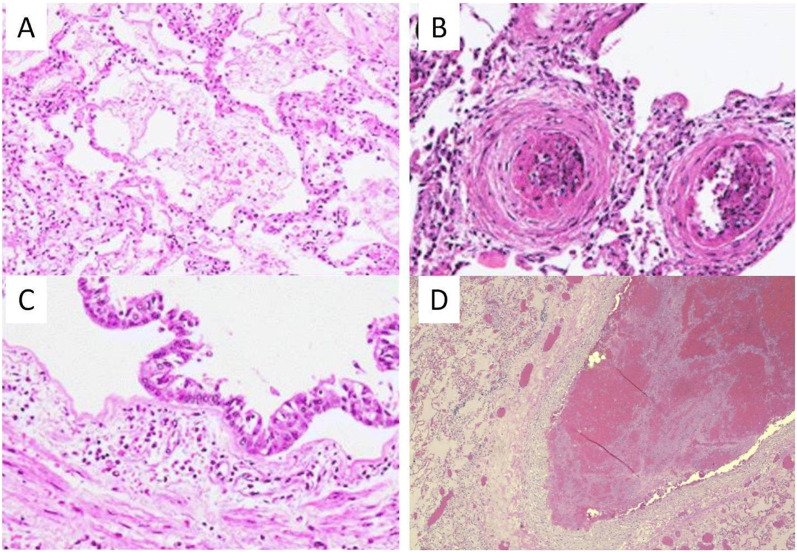
Fig. 2.
Histopathologic images from COVID-19 patients showing endothelial injury and thrombus, which is similar to the histopathology seen in populations living in regions with high air pollution. (A) Histopathologic findings from COVID-19 patients show acute lung injury with hyaline membrane in the alveolar space. (B) Vascular damage with microthrombi in lung small vessels. (C) Cases show, airway inflammation in trachea sections with polymorphous inflammatory infiltrate of submucosal layers. (D) Pulmonary thromboembolus is also seen in many COVID-19 patients. Photos A–C from Calabrese et al. in Virchows Arch. 2020 and D from Vasquez-Bonilla et al. in Hum. Pathol. 2020 under the Creative Commons license (Calabrese et al., 2020; Vasquez-Bonilla et al., 2020).
3.1.4. Future directions
While air pollution-mediated inflammatory effects often start in the pulmonary system, subsequent effects cascade throughout the circulatory system to induce oxidative stress and deterioration in the heart, lungs, and vasculature. In recent decades, exposure to PM2.5 pollution has been associated with increased hospitalization and mortality, especially in patients with congestive heart failure or arrhythmia (Dominici et al., 2006; Mann et al., 2002; Pope et al., 2002). Statistics on COVID-19 mortality corroborate that comorbidities, including coronary artery diseases, hypertension, diabetes, and congestive heart disease, worsen the severity of symptoms and patient outcomes (Harrison et al., 2020; Ssentongo et al., 2020). In the face of increased COVID-19 cases in air pollutant-affected regions, epidemiological data supports chronic air pollution-induced oxidative stress and inflammatory response, mediating the direct impact on the cardiopulmonary system from chronic air pollution exposure.
Additionally, industrial and urban regions harbor higher PM2.5 and PM10 concentrations compared to rural areas. For example, rural locations in the Midwestern United States exhibited lower PM2.5 concentrations (8.4–10.4 μg/m3) as compared to urban locations (9.5–11.6 μg/m3) (Kundu and Stone, 2014). Thus, investigating the local impact of industrial areas on cardiopulmonary health is essential to elucidate the subsequent impact on the severity of COVID-19 infections.
3.2. Activation of multiple inflammatory mechanisms in response to both chronic air pollution exposure and COVID-19 infection
Populations exposed to long-term high concentrations of PM and gaseous pollutants develop chronic inflammation in association with the pathogenesis of cardiopulmonary diseases. Similarly, a severe immune response has been reported in approximately 15% of COVID-19 patients that present with intravascular cytokine release, and, in more severe cases, microvascular thrombosis (Lippi et al., 2020). One such response is the cytokine release syndrome (CRS) as characterized by the systemic release of cytokines, specifically, interleukin-6 (IL-6) (P.P. Liu et al., 2020; Hirawat et al., 2021). The pro-thrombotic properties of cytokines promote both microvascular and macrovascular thrombosis (P.P. Liu et al., 2020). In combination with raised baseline inflammation levels of populations living in areas of high air pollution concentration, the additive inflammation caused by COVID-19 further magnifies the rate of COVID-19-associated acute respiratory distress syndrome (ARDS), myocarditis, cardiac arrhythmia, and heart failure (P.P. Liu et al., 2020). If left unchecked, these conditions lead to inflammatory infiltration and destruction of alveolar septae and cardiac injury, largely being responsible for patient death due to lung and heart failure (Wichmann et al., 2020). As outlined by this section, the effects of short- and long-term exposure to air pollution create a heightened inflammatory state with symptoms that mirror those of COVID-19 patients. These consequences may be additive and exacerbate cardiopulmonary symptoms in COVID-19 patients, increasing their risk of mortality.
3.2.1. Cardiopulmonary inflammation due to exposure to pollutants increases susceptibility to respiratory infections
Effects of pollutants include pulmonary and systemic oxidative stress that alter vascular homeostasis (Roy et al., 2014). These oxidative effects come through pollutants affecting the lipids and proteins or indirectly through the activation of intracellular oxidant pathways (Daellenbach et al., 2020; Lodovici and Bigagli, 2011). At the molecular level, exposure to pollutants may activate cell signaling membrane receptors, intracellular phosphatases and kinases, and transcription factors that regulate inflammatory responses (Glencross et al., 2020).
Exposure to common air pollutants is well-recognized to alter host immunity to viral respiratory infections by suppression of the host's defenses (Ciencewicki and Jaspers, 2007). Long-term exposure to air pollution is linked to elevated blood pressure, ventricular diastolic dysfunction, reduced coronary flow reserve, and myocardial fibrosis (Rao et al., 2018; Wold et al., 2012). In addition, an altered cardiac autonomic nervous system, including increased mean heart rate and heart rate variability, develop in response to prolonged exposure to air pollution (Park et al., 2005, Park et al., 2008). However, short-term exposure to PM2.5 mediates vascular endothelial dysfunction and increased blood viscosity and circulating fibrinogen to promote a hypercoagulable state, predisposing patients to hypertension, acute coronary events, heart failure, and stroke (Donaldson et al., 2001; Peters et al., 2000; Ghio et al., 2000; Nelin et al., 2012; Rajagopalan et al., 2018). These conditions are implicated in the cumulative effects of COVID-19-patients in whom cardiac injury is evidenced by elevated serum troponin and cytokine levels in association with cardiac arrhythmia, myocarditis, and contractile dysfunction (Bonow et al., 2020; Driggin et al., 2020; Yang and Jin, 2020).
3.2.2. The role of ubiquitous ACE2 receptor and inflammation
Chronic exposure to air pollution has been associated with the expression of ACE2 in lung endothelial cells (Aztatzi-Aguilar et al., 2015; Paital and Agrawal, 2020). ACE2 is the receptor for the SARS-CoV-2 spike protein for virus internalization into the host (Lv et al., 2020). In mammalian models, chronic exposure to PM2.5 has also been known to increase epithelial and endothelial ACE2 expression (Lindner et al., 2020). In addition to binding to the ACE2 receptor, transmembrane protease serine 2 (TMPRSS2) and potentially another common protease, furin, participate in the internalization of SARS-CoV-2 to the alveolar type 2 cells in the lung (Ackermann et al., 2020; Walls et al., 2020; Sajuthi et al., 2020). Thus, COVID-19 infection may be correlated with air pollution-mediated ACE2 expression (Hamouche et al., 2020).
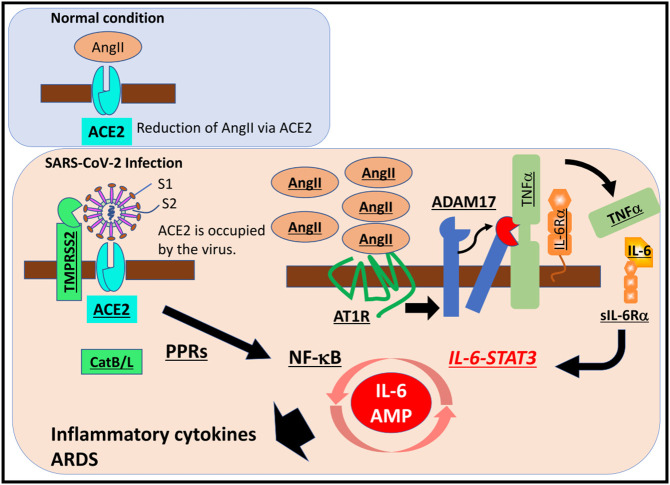
ACE2 is a key part of the lung renin-angiotensin system (RAS), an inflammatory response balancing act. ACE2 cleaves Angiotensin II (Ang-II) to form [Ang(1-7)] which binds to the Mas receptor. The ACE2/Ang1-7/Mas receptor axis activation is a response to PM2.5 exposure. Mas activation occurs as a result and suppresses STAT3 and ERK, exerting an anti-inflammatory response. The other axis is the ACE/Ang-II/AT1R axis that leads to the release of proinflammatory cytokines (Chamsi-Pasha et al., 2014). When the SARS-CoV-2 virus binds to ACE2 receptors of epithelial cells, there is an increase in Ang-II in the systemic circulation which primes the ACE/Ang-II/AT1R axis activation (Fig. 3 ). ACE2 and TMPRSS2 are ubiquitous in lung alveolar type 2 cells where their upregulation can lead to increased susceptibility to SARS-CoV-2 binding.
Fig. 3.
ACE2 receptor binding to the SARS-CoV-2 spike protein leads to an imbalance of the RAS system and a subsequent inflammatory response. SARS-CoV-2 binding to ACE2 receptors on the surface of endothelial cells leads to the activation of multiple pathways that ultimately result in massive cytokine release. Figure from Hirano and Murakami in Immunity. 2020 under the Creative Commons license (Hirano and Murakami, 2020).
3.2.3. Inflammation and predisposition to cytokine storm
Atmospheric contaminants modulate the host inflammatory response, leading to overexpression of inflammatory cytokines and chemokines. The cytokine storm that is present in severely ill COVID-19 patients may also be worse in populations exposed to chronic air pollution.
The recently coined “cytokine storm” is a symptom seen in patients with severe COVID-19 infection and is characterized by the release of inflammatory cytokines (Hu et al., 2020; P. Zhou et al., 2020). Interleukin-6 (IL-6), interferon-gamma (IFN-γ), and tumor necrosis factor-alpha (TNF-α) are dominant cytokines released by lung epithelium upon interaction with PM (Pope et al., 2016). When the inflammatory response “spills over” into systemic circulation, a cellular inflammatory response and platelet activation are implicated in the abundant microthrombi found in COVID-19 patients (Brook and Rajagopalan, 2010). Another major route of inflammation is through the subsequent activation of the NF-κB pathway (Deng et al., 2018). NF-κB activation after coronavirus infection occurs via pattern recognition receptors in the MyD88 pathway, resulting in cytokine induction (DeDiego et al., 2014). Furthermore, receptor CD40, expressed by both immune and non-immune cells, binds to ligand CD40L, temporarily expressed on T cells and is involved with recruitment of TNF-α receptor-associated factors (Kawabe et al., 2011). This interaction further leads to the release of inflammatory cytokines (Bai and Sun, 2016). IL-6 is also a major marker of cellular senescence, supporting the notion of susceptibility to COVID-19 infection and complication from the age-dependent increase in IL-6 (Hirano and Murakami, 2020; Moccia et al., 2020). Taken together, both ambient PM exposure and COVID-19 infection prime the host inflammatory state via the NF-κB signaling pathway in association with elevated IL-6 levels, and COVID-19 infection further potentiates the progression to cytokine storms (Kim et al., 2016).
3.2.4. Future directions
The precise mechanism underlying air pollution-mediated inflammation and COVID-19-mediated cardiovascular diseases remains elusive. A potential mechanism is the ACE2 receptor and disrupted activation of the RAS in the myocardium in association with the pathogenesis of cardiovascular disease (Kuba et al., 2010). As shown by a murine model, the first generation of the SARS virus affected the pulmonary system, triggering an ACE2 dependent myocardial infarction (Kassiri et al., 2009). Additional cellular injury due to elevated IL-6, D-dimer, ferritin, other inflammatory cytokines, and hypoxia-induced excessive intracellular calcium are also implicated in cardiac myocyte apoptosis. However, the precise mechanisms must be investigated further (Clerkin et al., 2020).
Viral entry to the host cell occurs by binding between the S1 region of the virus spike (S) protein to the ACE2 receptor on the cell surface (Lv et al., 2020). SARS-CoV-2 binds to ACE2 for entry, and TMPRSS2 and endosomal cysteine proteases cathepsin B and L (CatB/L) prime the S protein (Hoffmann et al., 2020b). Interestingly, SARS-CoV-2 also harbors a multibasic cleavage site at the S1/S2 boundary that is absent in SARS-CoV and other closely related animal coronaviruses (Walls et al., 2020). This unique cleavage site can be recognized by furin, essential for SARS-CoV-2 infection of human cells (Hoffmann et al., 2020a). While TMPRSS2 is highly expressed in the lungs, furin is expressed in many other organs, which may contribute to SARS-CoV-2's deleterious effects on multiple organs. In this context, developing inhibitors for both TMPRSS2 and furin may represent a promising therapeutic approach targeting SARS-CoV-2 in the setting of exposure to air pollution (Barile et al., 2020).
3.3. Air pollution exposure-mediated transmission of COVID-19 and overlap
SARS-CoV-2 is the seventh documented coronavirus to infect humans, targeting the ACE-2 receptor for entry into the host cell (Andersen et al., 2020). While SARS-CoV-2 may not be as lethal as previous coronavirus outbreaks, its transmission rate is higher, with R0 being between 3.6 and 4 (Chen et al., 2020a, Chen et al., 2020b). Respiratory viruses, including SARS-CoV-2, have been understood to undergo transmission via direct contact (person to person) and droplet (bodily fluid to person) routes (Shiu et al., 2019). Controversy remains regarding the spread of viral particles suspended in the atmosphere, otherwise known as aerosol spread (Tellier et al., 2019). Furthermore, other routes of viral transmission relevant to the current pandemic, such as wastewater, have been implicated in its spread (D. Liu et al., 2020).
Air pollution and weather patterns have been recently studied for their physical contribution to the transmission of SARS-CoV-2. Similarly, components of air pollution, which are distributed by weather patterns, may act as viral distributors by acting as a surface to prolong viral survival. SARS-CoV-2 also survives for a longer period of time on dry surfaces than in an aerosol form (van Doremalen et al., 2020). One study analyzing air particles in two Wuhan hospitals found SARS-CoV-2 genetic material in sampled PM in the 0.25 to 1.0 μm range (Y. Liu et al., 2020). This may point to PM2.5 and smaller particles acting as transporters for SARS-CoV-2 particles. These PM-virus aggregates can be more easily distributed in the alveoli and upper respiratory tract and facilitate delivery and virus binding to the pulmonary epithelium (Farhangrazi et al., 2020).
Additionally, as with other viruses, the coronaviruses have been observed to survive outside host cells for longer periods at lower temperatures and humidity (Farhangrazi et al., 2020). However, the impact of meteorological conditions on virus transmission remains less understood. One study conducted in a tropical climate revealed that higher temperatures and increased solar radiation limited the effect on COVID-19 transmission (Rosario et al., 2020). A similar study conducted in Italy, a Mediterranean climate, reported that temperature and humidity were negatively correlated with COVID-19 transmission (Lolli et al., 2020). Lastly, a more-comprehensive study using spatial and temporal models found that weather had a non-influential effect on COVID-19 transmission when compared with other factors such as homestay and urban density (Jamshidi et al., 2020). While meteorological factors such as temperature and wind speed have been shown to potentiate the ability of coronavirus to survive outside of a host, they are unlikely to be the singular reason for the extreme variability in COVID-19 infection rates across political borders (Coccia, 2021a, Coccia, 2021b, Coccia, 2021c).
3.3.1. Future directions
The precise mechanism whereby the environment modulates SARS-CoV-2 transmission warrants further investigation. Several studies have noted the seasonal relationship between various respiratory virus infections and meteorological variables, including temperature and humidity (Moriyama et al., 2020; Srivastava, 2021). Furthermore, simulated sunlight deactivates SARS-CoV-2 in minutes (Schuit et al., 2020). While PM pollution is a contributor to atmospheric haze, its impact on SARS-CoV-2 transmissibility warrants further study. Whether PM2.5 and smaller particles have the capacity to transport SARS-CoV-2 remains to be explored. A previous outdoor study comprehensively isolated and categorized viral and microbial genetic material from PM (Cao et al., 2014), suggesting some viral material was able to associate with the pollution particles. The three most-represented samples were DNA-based viruses; however, SARS-CoV-2 is an RNA virus, and its RNA is unstable under most extracellular conditions. Thus, whether the mere presence of viral RNA indicates infectivity also requires further validation. In addition, volcano ash emission, along with the heavy metals, may contribute to SARS-CoV-2 transmission and/or atmospheric persistence of the viral particle. Both the Po Valley and Wuhan City are relatively close to active volcanoes and vents, possibly providing a link to the severe infection and adverse clinical outcomes observed in those areas (Raciti and Calabrò, 2020).
4. Discussion: pre-existing cardiopulmonary diseases mediated by air pollution and COVID-19 infection
Increasing evidence suggests that chronic exposure to air pollution leads to exacerbation and hospitalization of patients with COVID-19. As explained in the aforementioned redox-active mechanisms (Table 2), PM and air pollutants deposited in the lung reach the deep alveolar spaces to induce cytokine release and oxidative stress. These redox-active reactions are implicated in the exacerbation of COVID-19 infection and hospitalizations. A study in Italy supported the correlation between the Po Valley regions with 4-year exposure to high levels of NO2, PM2.5 and PM10 and the regions with the most hospitalizations of COVID-19 patients (Fattorini and Regoli, 2020). The northern regions of Italy, in particular, reflected up to 80 days of exceedance per year of the regulatory limits (Fattorini and Regoli, 2020). These same areas are regions in Italy that have experienced a high number of COVID-19 cases.
In the SARS-CoV-2 predecessors, SARS and MERS, the pre-existing cardiovascular diseases (CVDs) were reported to increase the risk of death, similar to the COVID-19 patients (Badawi and Ryoo, 2016; Booth et al., 2003; Chan et al., 2003). In Wuhan City, where there are high levels of air pollutants, hospitalizations were significantly prevalent among the COVID-19 patients with cardiopulmonary diseases (F. Zhou et al., 2020). In particular, these Wuhan residents with coronary heart disease, congestive heart failure, hypertension, and/or diabetes develop increased susceptibility to the severity of COVID-19 infection and death (Gold et al., 2000; Mutlu et al., 2007). Thus, patients living in polluted regions with pre-existing cardiopulmonary conditions, which may arise from exposure to air pollution, were likely to develop high rates of hospitalization when saddled with a COVID-19 infection (Hamouche et al., 2020).
The relationship between COVID-19 and underlying cardiopulmonary diseases and impact of air pollution is an emerging topic. More studies and comprehensive reviews are necessary to further determine the interrelationships between the deleterious effects of air pollution exposure and the severity of COVID-19 infections.
5. Conclusion
This review presented overarching themes found in current literature that suggest exposure to pollution increases susceptibility to COVID-19 infection, creating a pre-inflammatory state in patients. Populations that are more at-risk for pollution-related CVDs, such as the elderly living in urban areas, may also be more at-risk to COVID-19 (Dockery et al., 1993). Inhalation of PM has been associated with respiratory and cardiovascular events. Therefore, as air pollutants affect respiratory and cardiovascular health, COVID-19 prognosis and mortality are impacted by the presence of respiratory and cardiovascular comorbidities. Air pollution may also negatively impact COVID-19 outcomes.
Current literature broadly finds that chronic exposure to air pollution results in patients with higher compensatory ACE2 receptor expression, which may also lead to a lower barrier to entry for the SARS-CoV-2 virus. Targeting this pathway alongside pharmaceutical agents to reduce inflammation may ultimately mitigate the severity of symptoms in patients from areas of high air pollution. Furthermore, considering the environmental factors surrounding a COVID-19 patient is essential for effective intervention and prevention.
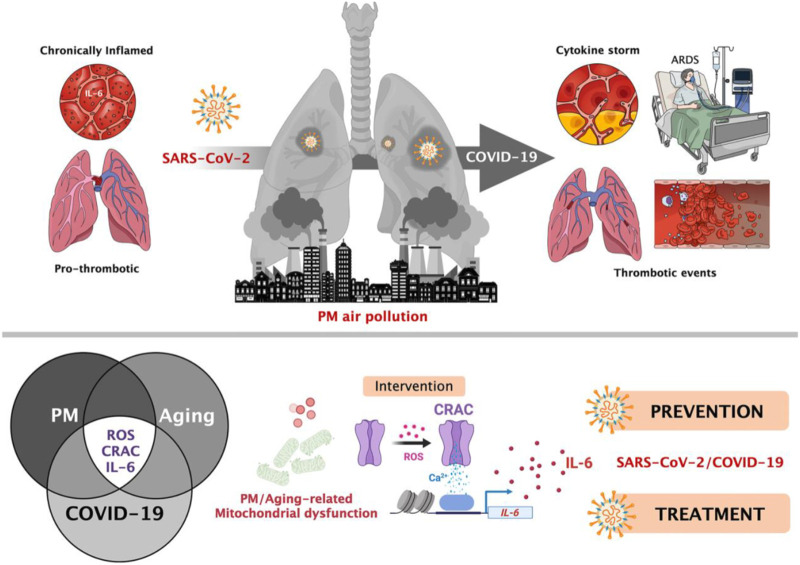
Ambient pollutants are increasingly recognized as the epigenetic cofactors in the current COVID-19 pandemic and inform potential intervention and prevention (Fig. 4 ). Chronic exposure to air pollution increases inflammation in populations that are thus more susceptible to contracting the virus. The signaling pathways underlying inflammatory responses can potentially be therapeutic targets to attenuate the cardiopulmonary manifestation from SARS-CoV-2 infection. The RECOVERY Trial is assessing whether existing drugs can be repurposed to treat COVID-19 by enrolling 104 patients for a relatively low dose of 6 mg of dexamethasone, a steroid for inflammation, for 10 days (RECOVERY Collaborative Group et al., 2020). The RECOVERY trial is also evaluating several experimental COVID-19 therapies, including the HIV drug combination Kaletra, convalescent plasma, and the controversial antimalarial drug hydroxychloroquine.
Fig. 4.
Relationship between particulate matter (PM) air pollution, SARS-CoV-2 infection, and COVID-19 prognosis and a potential therapy for the prevention and treatment of disease. PM air pollution largely targets the lung, triggering signaling pathways that have also been found to be caused by SARS-CoV-2. These signals include the release of inflammatory cytokines including IL-6, reactive oxygen species (ROS), and calcium-release activation calcium (CRAC) channels and a consequent rise in thrombotic events. Patients who already experience this response may be at risk for a higher severity of COVID-19 disease and increased risk of mortality. Figure from Menendez in Aging. 2020 under the Creative Commons license (Menendez, 2020).
Targeting endothelial inflammation and using anti-inflammatory drugs already proven in clinical use could be particularly relevant for vulnerable patients with pre-existing endothelial dysfunction (Varga et al., 2020). Additionally, medicine targeting ACE2 receptors may also reduce viral entry into the alveolar space. Despite concerns that CVD patients taking ACE inhibitors and angiotensin receptor blockers (ARBs) may be more-susceptible to COVID-19, due to resulting ACE2R up-regulation, a global observational study with 169 sites and 8910 patients found no increased risk of in-hospital death (Mehra and Ruschitzka, 2020). In addition, mitigating the long-term cardiopulmonary effects of air pollution would require concerted public health actions to help protect residents in highly polluted regions. While urban air pollution seems to have decreased in the United States during the COVID-19 pandemic, more efforts must be made to maintain lower levels even after business returns to normal (Berman and Ebisu, 2020).
CRediT authorship contribution statement
AL, RO, and MC contributed equally to this review by conducting a literature review and writing the manuscript. JAS and CZ contributed to editing. TKH conceived the review paper and edited the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The present work was funded by AHA COVID-19 Rapid Response (TKH), NIH grants National Institutes of Health R01HL083015 (TKH), R01HL111437 (TKH), R01HL129727 (TKH), R01HL118650 (TKH), R01HL139614 (JAS), K01HL130650 (JAS), R01ES023470 (CZ), and R01HL131925 (CZ), T32HL139450-01 (AL), and Veterans Affairs Merit Award I01BX004356 (TKH).
Editor: Jay Gan
References
- Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., Vanstapel A., Werlein C., Stark H., Tzankov A., Li W.W., Li V.W., Mentzer S.J., Jonigk D. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali N., Islam F. The effects of air pollution on COVID-19 infection and mortality-a review on recent evidence. Front. Public Health. 2020;8:580057. doi: 10.3389/fpubh.2020.580057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020;26(4):450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aztatzi-Aguilar O.G., Uribe-Ramírez M., Arias-Montaño J.A., Barbier O., De Vizcaya-Ruiz A. Acute and subchronic exposure to air particulate matter induces expression of angiotensin and bradykinin-related genes in the lungs and heart: angiotensin-II type-I receptor as a molecular target of particulate matter exposure. Particle and Fibre Toxicology. 2015;12:17. doi: 10.1186/s12989-015-0094-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawi A., Ryoo S.G. Prevalence of diabetes in the 2009 influenza A (H1N1) and the middle east respiratory syndrome coronavirus: a systematic review and meta-analysis. Journal of Public Health Research. 2016;5(3):733. doi: 10.4081/jphr.2016.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y., Sun Q. Fine particulate matter air pollution and atherosclerosis: mechanistic insights. Biochim. Biophys. Acta. 2016;1860(12):2863–2868. doi: 10.1016/j.bbagen.2016.04.030. [DOI] [PubMed] [Google Scholar]
- Barile E., Baggio C., Gambini L., Shiryaev S.A., Strongin A.Y., Pellecchia M. Potential therapeutic targeting of coronavirus spike glycoprotein priming. Molecules. 2020;25(10):2424. doi: 10.3390/molecules25102424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basic Information about NO2 United States Environmental Protection Agency; 2016. Overviews and factsheets. https://www.epa.gov/no2-pollution/basic-information-about-no2 (July 6)
- Benmarhnia T. Linkages between air pollution and the health burden from COVID-19: methodological challenges and opportunities. Am. J. Epidemiol. 2020 doi: 10.1093/aje/kwaa148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman J.D., Ebisu K. Changes in U.S. air pollution during the COVID-19 pandemic. Science of the Total Environment. 2020;739 doi: 10.1016/j.scitotenv.2020.139864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzio C., Saibeni S., Variola A., Allocca M., Massari A., Gerardi V., Casini V., Ricci C., Zingone F., Amato A., Caprioli F., Lenti M.V., Viganò C., Ascolani M., Bossa F., Castiglione F., Cortelezzi C., Grossi L., Milla M.…Fiorino G. Outcomes of COVID-19 in 79 patients with IBD in Italy: an IG-IBD study. Gut. 2020;69(7):1213–1217. doi: 10.1136/gutjnl-2020-321411. [DOI] [PubMed] [Google Scholar]
- Bianconi V., Bronzo P., Banach M., Sahebkar A., Mannarino M.R., Pirro M. Particulate matter pollution and the COVID-19 outbreak: results from Italian regions and provinces. Archives of Medical Science : AMS. 2020;16(5):985–992. doi: 10.5114/aoms.2020.95336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal I. Carbon monoxide poisoning. J. R. Soc. Med. 2001;94(6):270–272. doi: 10.1177/014107680109400604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonow R.O., Fonarow G.C., O’Gara P.T., Yancy C.W. Association of coronavirus disease 2019 (COVID-19) with myocardial injury and mortality. JAMA Cardiol. 2020;5(7):751–753. doi: 10.1001/jamacardio.2020.1105. [DOI] [PubMed] [Google Scholar]
- Bontempi E. First data analysis about possible COVID-19 virus airborne diffusion due to air particulate matter (PM): the case of Lombardy (Italy) Environ. Res. 2020;186:109639. doi: 10.1016/j.envres.2020.109639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth C.M., Matukas L.M., Tomlinson G.A., Rachlis A.R., Rose D.B., Dwosh H.A., Walmsley S.L., Mazzulli T., Avendano M., Derkach P., Ephtimios I.E., Kitai I., Mederski B.D., Shadowitz S.B., Gold W.L., Hawryluck L.A., Rea E., Chenkin J.S., Cescon D.W.…Detsky A.S. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289(21):2801–2809. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- Brandt E.B., Beck A.F., Mersha T.B. Air pollution, racial disparities, and COVID-19 mortality. J. Allergy Clin. Immunol. 2020;146(1):61–63. doi: 10.1016/j.jaci.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briet M., Collin C., Laurent S., Tan A., Azizi M., Agharazii M., Jeunemaitre X., Alhenc-Gelas F., Boutouyrie P. Endothelial function and chronic exposure to air pollution in normal male subjects. Hypertension (Dallas, Tex.: 1979) 2007;50(5):970–976. doi: 10.1161/HYPERTENSIONAHA.107.095844. [DOI] [PubMed] [Google Scholar]
- Brook R.D., Rajagopalan S. Particulate matter air pollution and atherosclerosis. Curr. Atheroscler. Rep. 2010;12(5):291–300. doi: 10.1007/s11883-010-0122-7. [DOI] [PubMed] [Google Scholar]
- Brook R.D., Franklin B., Cascio W., Hong Y., Howard G., Lipsett M., Luepker R., Mittleman M., Samet J., Smith S.C., Tager I., Expert Panel on Population and Prevention Science of the American Heart Association Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004;109(21):2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- Calabrese F., Pezzuto F., Fortarezza F., Hofman P., Kern I., Panizo A., von der Thüsen J., Timofeev S., Gorkiewicz G., Lunardi F. Pulmonary pathology and COVID-19: lessons from autopsy. The experience of European Pulmonary Pathologists. Virchows Archiv. 2020:1–14. doi: 10.1007/s00428-020-02886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao C., Jiang W., Wang B., Fang J., Lang J., Tian G., Jiang J., Zhu T.F. Inhalable microorganisms in Beijing’s PM2.5 and PM10 pollutants during a severe smog event. Environmental Science & Technology. 2014;48(3):1499–1507. doi: 10.1021/es4048472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon Monoxide (CO) Standards—Risk and Exposure Assessments from Current Review United States Environmental Protection Agency; 2016. Reports and assessments. https://www.epa.gov/naaqs/carbon-monoxide-co-standards-risk-and-exposure-assessments-current-review (October 24)
- Carbon Monoxide Trends United States Environmental Protection Agency; 2016. Data and tools. https://www.epa.gov/air-trends/carbon-monoxide-trends (May 4)
- Chamsi-Pasha M.A.R., Shao Z., Tang W.H.W. Angiotensin-converting enzyme 2 as a therapeutic target for heart failure. Current Heart Failure Reports. 2014;11(1):58–63. doi: 10.1007/s11897-013-0178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J., Ng C., Chan Y., Mok T., Lee S., Chu S., Law W., Lee M., Li P. Short term outcome and risk factors for adverse clinical outcomes in adults with severe acute respiratory syndrome (SARS) Thorax. 2003;58(8):686–689. doi: 10.1136/thorax.58.8.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K., Wang M., Huang C., Kinney P.L., Anastas P.T. Air pollution reduction and mortality benefit during the COVID-19 outbreak in China. The Lancet. Planetary Health. 2020;4(6):e210–e212. doi: 10.1016/S2542-5196(20)30107-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K., Wang M., Kinney P.L., Anastas P.T. Reduction in air pollution and attributable mortality due to COVID-19 lockdown—authors’ reply. The Lancet. Planetary Health. 2020;4(7) doi: 10.1016/S2542-5196(20)30149-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciencewicki J., Jaspers I. Air pollution and respiratory viral infection. Inhal. Toxicol. 2007;19(14):1135–1146. doi: 10.1080/08958370701665434. [DOI] [PubMed] [Google Scholar]
- Clerkin K.J., Fried J.A., Raikhelkar J., Sayer G., Griffin J.M., Masoumi A., Jain S.S., Burkhoff D., Kumaraiah D., Rabbani L., Schwartz A., Uriel N. COVID-19 and cardiovascular disease. Circulation. 2020;141(20):1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- Coccia M. Effects of the spread of COVID-19 on public health of polluted cities: results of the first wave for explaining the dejà vu in the second wave of COVID-19 pandemic and epidemics of future vital agents. Environmental Science and Pollution Research International. 2021:1–8. doi: 10.1007/s11356-020-11662-7. (Advance online publication) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia M. How do low wind speeds and high levels of air pollution support the spread of COVID-19? Atmospheric Pollution Research. 2021;12(1):437–445. doi: 10.1016/j.apr.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia M. The effects of atmospheric stability with low wind speed and of air pollution on the accelerated transmission dynamics of COVID-19. Int. J. Environ. Stud. 2021;78(1):1–27. doi: 10.1080/00207233.2020.1802937. [DOI] [Google Scholar]
- Colombo C., Burgel P.-R., Gartner S., van Koningsbruggen-Rietschel S., Naehrlich L., Sermet-Gaudelus I., Southern K.W. Impact of COVID-19 on people with cystic fibrosis. Lancet Respir. Med. 2020;8(5):e35–e36. doi: 10.1016/S2213-2600(20)30177-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comunian S., Dongo D., Milani C., Palestini P. Air pollution and Covid-19: the role of particulate matter in the spread and increase of Covid-19’s morbidity and mortality. Int. J. Environ. Res. Public Health. 2020;17(12):4487. doi: 10.3390/ijerph17124487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conticini E., Frediani B., Caro D. Can atmospheric pollution be considered a co-factor in extremely high level of SARS-CoV-2 lethality in Northern Italy? Environmental Pollution (Barking, Essex: 1987) 2020;261:114465. doi: 10.1016/j.envpol.2020.114465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronavirus disease (COVID-19) 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- Daellenbach K.R., Uzu G., Jiang J., Cassagnes L.-E., Leni Z., Vlachou A., Stefenelli G., Canonaco F., Weber S., Segers A., Kuenen J.J.P., Schaap M., Favez O., Albinet A., Aksoyoglu S., Dommen J., Baltensperger U., Geiser M., El Haddad I.…Prévôt A.S.H. Sources of particulate-matter air pollution and its oxidative potential in Europe. Nature. 2020;587(7834):414–419. doi: 10.1038/s41586-020-2902-8. [DOI] [PubMed] [Google Scholar]
- Daigle C.C., Chalupa D.C., Gibb F.R., Morrow P.E., Oberdörster G., Utell M.J., Frampton M.W. Ultrafine particle deposition in humans during rest and exercise. Inhal. Toxicol. 2003;15(6):539–552. doi: 10.1080/08958370304468. [DOI] [PubMed] [Google Scholar]
- DeDiego M.L., Nieto-Torres J.L., Jimenez-Guardeño J.M., Regla-Nava J.A., Castaño-Rodriguez C., Fernandez-Delgado R., Usera F., Enjuanes L. Coronavirus virulence genes with main focus on SARS-CoV envelope gene. Virus Res. 2014;194:124–137. doi: 10.1016/j.virusres.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L., Zeng Q., Wang M., Cheng A., Jia R., Chen S., Zhu D., Liu M., Yang Q., Wu Y., Zhao X., Zhang S., Liu Y., Yu Y., Zhang L., Chen X. Suppression of NF-κB activity: a viral immune evasion mechanism. Viruses. 2018;10(8) doi: 10.3390/v10080409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockery D.W., Pope C.A., Xu X., Spengler J.D., Ware J.H., Fay M.E., Ferris B.G., Speizer F.E. An association between air pollution and mortality in six U.S. cities. N. Engl. J. Med. 1993;329(24):1753–1759. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- Dominici F., Peng R.D., Bell M.L., Pham L., McDermott A., Zeger S.L., Samet J.M. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA : The Journal of the American Medical Association. 2006;295(10):1127–1134. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson K., Stone V., Seaton A., MacNee W. Ambient particle inhalation and the cardiovascular system: potential mechanisms. Environ. Health Perspect. 2001;109(Suppl. 4):523–527. doi: 10.1289/ehp.01109s4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driggin E., Madhavan M.V., Bikdeli B., Chuich T., Laracy J., Biondi-Zoccai G., Brown T.S., Der Nigoghossian C., Zidar D.A., Haythe J., Brodie D., Beckman J.A., Kirtane A.J., Stone G.W., Krumholz H.M., Parikh S.A. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J. Am. Coll. Cardiol. 2020;75(18):2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejaz H., Alsrhani A., Zafar A., Javed H., Junaid K., Abdalla A.E., Abosalif K.O.A., Ahmed Z., Younas S. COVID-19 and comorbidities: deleterious impact on infected patients. Journal of Infection and Public Health. 2020;13(12):1833–1839. doi: 10.1016/j.jiph.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhangrazi Z.S., Sancini G., Hunter A.C., Moghimi S.M. Airborne particulate matter and SARS-CoV-2 partnership: virus hitchhiking, stabilization and immune cell targeting — a hypothesis. Frontiers in Immunology. 2020;11 doi: 10.3389/fimmu.2020.579352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattorini D., Regoli F. Role of the chronic air pollution levels in the Covid-19 outbreak risk in Italy. Environ. Pollut. 2020;264:114732. doi: 10.1016/j.envpol.2020.114732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontera A., Cianfanelli L., Vlachos K., Landoni G., Cremona G. Severe air pollution links to higher mortality in COVID-19 patients: the “double-hit” hypothesis. J. Infect. 2020;81(2):255–259. doi: 10.1016/j.jinf.2020.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontera A., Martin C., Vlachos K., Sgubin G. Regional air pollution persistence links to COVID-19 infection zoning. J. Infect. 2020;81(2):318–356. doi: 10.1016/j.jinf.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghio A.J., Kim C., Devlin R.B. Concentrated ambient air particles induce mild pulmonary inflammation in healthy human volunteers. Am. J. Respir. Crit. Care Med. 2000;162(3 Pt 1):981–988. doi: 10.1164/ajrccm.162.3.9911115. [DOI] [PubMed] [Google Scholar]
- Ghorani-Azam A., Riahi-Zanjani B., Balali-Mood M. Effects of air pollution on human health and practical measures for prevention in Iran. Journal of Research in Medical Sciences : The Official Journal of Isfahan University of Medical Sciences. 2016:21. doi: 10.4103/1735-1995.189646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giani P., Castruccio S., Anav A., Howard D., Hu W., Crippa P. Short-term and long-term health impacts of air pollution reductions from COVID-19 lockdowns in China and Europe: a modelling study. The Lancet. Planetary Health. 2020;4(10):e474–e482. doi: 10.1016/S2542-5196(20)30224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glencross D.A., Ho T.-R., Camiña N., Hawrylowicz C.M., Pfeffer P.E. Air pollution and its effects on the immune system. Free Radic. Biol. Med. 2020;151:56–68. doi: 10.1016/j.freeradbiomed.2020.01.179. [DOI] [PubMed] [Google Scholar]
- Gold D.R., Litonjua A., Schwartz J., Lovett E., Larson A., Nearing B., Allen G., Verrier M., Cherry R., Verrier R. Ambient pollution and heart rate variability. Circulation. 2000;101(11):1267–1273. doi: 10.1161/01.CIR.101.11.1267. [DOI] [PubMed] [Google Scholar]
- Ground-level Ozone Pollution United States Environmental Protection Agency; 2015. Other policies and guidance. https://www.epa.gov/ground-level-ozone-pollution (May 15)
- Hamouche W., Bisserier M., Brojakowska A., Eskandari A., Fish K., Goukassian D.A., Hadri L. Pathophysiology and pharmacological management of pulmonary and cardiovascular features of COVID-19. Journal of Molecular and Cellular Cardiology. 2020;153:72–85. doi: 10.1016/j.yjmcc.2020.12.009. (Advance online publication) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C., Hong Y.-C. Decrease in ambient fine particulate matter during COVID-19 crisis and corresponding health benefits in Seoul, Korea. Int. J. Environ. Res. Public Health. 2020;17(15) doi: 10.3390/ijerph17155279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison S.L., Fazio-Eynullayeva E., Lane D.A., Underhill P., Lip G.Y.H. Comorbidities associated with mortality in 31,461 adults with COVID-19 in the United States: a federated electronic medical record analysis. PLoS Med. 2020;17(9) doi: 10.1371/journal.pmed.1003321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health effects of particulate matter . 2013. Policy implications for countries in eastern Europe, Caucasus and central Asia.https://www.euro.who.int/en/health-topics/environment-and-health/air-quality/publications/2013/health-effects-of-particulate-matter.-policy-implications-for-countries-in-eastern-europe,-caucasus-and-central-asia-2013 Retrieved August 6, 2020, from. [Google Scholar]
- Hirano T., Murakami M. COVID-19: a new virus, but a familiar receptor and cytokine release syndrome. Immunity. 2020;52(5):731–733. doi: 10.1016/j.immuni.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirawat R., Saifi M.A., Godugu C. Targeting inflammatory cytokine storm to fight against COVID-19 associated severe complications. Life Sci. 2021;267:118923. doi: 10.1016/j.lfs.2020.118923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek G., Brunekreef B., Fischer P., van Wijnen J. The association between air pollution and heart failure, arrhythmia, embolism, thrombosis, and other cardiovascular causes of death in a time series study. Epidemiology (Cambridge, Mass.) 2001;12(3):355–357. doi: 10.1097/00001648-200105000-00017. [DOI] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Pöhlmann S. A multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells. Molecular Cell. 2020;78(4):779–784. doi: 10.1016/j.molcel.2020.04.022. (e5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. (e8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B., Huang S., Yin L. The cytokine storm and COVID-19. J. Med. Virol. 2020 doi: 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Liu Z., Li H., Wang Y., Li Y., Zhu Y., Ooi M.C.G., An J., Shang Y., Zhang D., Chan A., Li L. The silver lining of COVID-19: estimation of short-term health impacts due to lockdown in the Yangtze River Delta region, China. GeoHealth. 2020 doi: 10.1029/2020GH000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui D.S., Azhar E.I., Madani T.A., Ntoumi F., Kock R., Dar O., Ippolito G., Mchugh T.D., Memish Z.A., Drosten C., Zumla A., Petersen E. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—the latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibald-Mulli A., Stieber J., Wichmann H.E., Koenig W., Peters A. Effects of air pollution on blood pressure: a population-based approach. Am. J. Public Health. 2001;91(4):571–577. doi: 10.2105/ajph.91.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inhalable Particulate Matter and Health (PM2.5 and PM10). (n.d.). Retrieved October 28, 2020, from https://ww2.arb.ca.gov/resources/inhalable-particulate-matter-and-health.
- Jamshidi S., Baniasad M., Niyogi D. Global to USA county scale analysis of weather, urban density, mobility, homestay, and mask use on COVID-19. Int. J. Environ. Res. Public Health. 2020;17(21) doi: 10.3390/ijerph17217847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H.-L., Zhao R., Matalon S., Matthay M.A. Elevated plasmin(ogen) as a common risk factor for COVID-19 susceptibility. Physiol. Rev. 2020;100(3):1065–1075. doi: 10.1152/physrev.00013.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Wu X.-J., Guan Y.-J. Effect of ambient air pollutants and meteorological variables on COVID-19 incidence. Infect. Control Hosp. Epidemiol. 2020:1–5. doi: 10.1017/ice.2020.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Cauhe J., Ortega-Quijano D., Carretero-Barrio I., Suarez-Valle A., Saceda-Corralo D., Moreno-Garcia Del Real C., Fernandez-Nieto D. Erythema multiforme-like eruption in patients with COVID-19 infection: clinical and histological findings. Clin. Exp. Dermatol. 2020 doi: 10.1111/ced.14281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassiri Z., Zhong J., Guo D., Basu R., Wang X., Liu P.P., Scholey J.W., Penninger J.M., Oudit G.Y. Loss of angiotensin-converting enzyme 2 accelerates maladaptive left ventricular remodeling in response to myocardial infarction. Circ. Heart Fail. 2009;2(5):446–455. doi: 10.1161/CIRCHEARTFAILURE.108.840124. [DOI] [PubMed] [Google Scholar]
- Kaufman J.D., Adar S.D., Barr R.G., Budoff M., Burke G.L., Curl C.L., Daviglus M.L., Roux A.V.D., Gassett A.J., Jacobs D.R., Kronmal R., Larson T.V., Navas-Acien A., Olives C., Sampson P.D., Sheppard L., Siscovick D.S., Stein J.H., Szpiro A.A., Watson K.E. Association between air pollution and coronary artery calcification within six metropolitan areas in the USA (the Multi-Ethnic Study of Atherosclerosis and Air Pollution): a longitudinal cohort study. Lancet. 2016;388(10045):696–704. doi: 10.1016/S0140-6736(16)00378-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabe T., Matsushima M., Hashimoto N., Imaizumi K., Hasegawa Y. CD40/CD40 ligand interactions in immune responses and pulmonary immunity. Nagoya J. Med. Sci. 2011;73(3–4):69–78. [PMC free article] [PubMed] [Google Scholar]
- Kim J.A., Cho J.H., Park I.-H., Shin J.-M., Lee S.-A., Lee H.-M. Diesel exhaust particles upregulate interleukins IL-6 and IL-8 in nasal fibroblasts. PLoS One. 2016;11(6) doi: 10.1371/journal.pone.0157058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Imai Y., Ohto-Nakanishi T., Penninger J.M. Trilogy of ACE2: a peptidase in the renin–angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol. Ther. 2010;128(1):119–128. doi: 10.1016/j.pharmthera.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumarathasan P., Vincent R., Blais E., Bielecki A., Guénette J., Filiatreault A., Brion O., Cakmak S., Thomson E.M., Shutt R., Kauri L.M., Mahmud M., Liu L., Dales R. Cardiovascular and inflammatory mechanisms in healthy humans exposed to air pollution in the vicinity of a steel mill. Particle and Fibre Toxicology. 2018:15. doi: 10.1186/s12989-018-0270-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu S., Stone E.A. Composition and sources of fine particulate matter across urban and rural sites in the Midwestern United States. Environmental Science. Processes & Impacts. 2014;16(6):1360–1370. doi: 10.1039/c3em00719g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawal A.O. Air particulate matter induced oxidative stress and inflammation in cardiovascular disease and atherosclerosis: the role of Nrf2 and AhR-mediated pathways. Toxicol. Lett. 2017;270:88–95. doi: 10.1016/j.toxlet.2017.01.017. [DOI] [PubMed] [Google Scholar]
- Li R., Ning Z., Majumdar R., Cui J., Takabe W., Jen N., Sioutas C., Hsiai T. Ultrafine particles from diesel vehicle emissions at different driving cycles induce differential vascular pro-inflammatory responses: implication of chemical components and NF-κB signaling. Particle and Fibre Toxicology. 2010;7:6. doi: 10.1186/1743-8977-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Navab K., Hough G., Daher N., Zhang M., Mittelstein D., Lee K., Pakbin P., Saffari A., Bhetraratana M., Sulaiman D., Beebe T., Wu L., Jen N., Wine E., Tseng C.-H., Araujo J.A., Fogelman A., Sioutas C.…Hsiai T.K. Effect of exposure to atmospheric ultrafine particles on production of free fatty acids and lipid metabolites in the mouse small intestine. Environ. Health Perspect. 2015;123(1):34–41. doi: 10.1289/ehp.1307036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Yang J., Saffari A., Jacobs J., Baek K.I., Hough G., Larauche M.H., Ma J., Jen N., Moussaoui N., Zhou B., Kang H., Reddy S., Henning S.M., Campen M.J., Pisegna J., Li Z., Fogelman A.M., Sioutas C.…Hsiai T.K. Ambient ultrafine particle ingestion alters gut microbiota in association with increased atherogenic lipid metabolites. Sci. Rep. 2017;7(1):42906. doi: 10.1038/srep42906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner D., Fitzek A., Brauninger H., Aleshcheva G., Meissner K., Scherschel K., Kirchhof P., Escher F., Schultheiss H., Blankenberg S., Puschel K., Westermann D. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi G., Sanchis-Gomar F., Henry B.M. COVID-19: unravelling the clinical progression of nature’s virtually perfect biological weapon. Annals of Translational Medicine. 2020;8(11) doi: 10.21037/atm-20-3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsett M. In: Clinical Environmental Health and Toxic Exposures. 2nd ed. Sullivan J., Krieger G., editors. Lippincott Williams & Wilkins; 2001. Ozone; pp. 806–818. [Google Scholar]
- Liu Y., Goodson J.M., Zhang B., Chin M.T. Air pollution and adverse cardiac remodeling: clinical effects and basic mechanisms. Front. Physiol. 2015;6:162. doi: 10.3389/fphys.2015.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P.P., Blet A., Smyth D., Li H. The science underlying COVID-19: implications for the cardiovascular system. Circulation. 2020;142(1):68–78. doi: 10.1161/CIRCULATIONAHA.120.047549. [DOI] [PubMed] [Google Scholar]
- Liu Y., Ning Z., Chen Y., Guo M., Liu Y., Gali N.K., Sun L., Duan Y., Cai J., Westerdahl D., Liu X., Xu K., Ho K., Kan H., Fu Q., Lan K. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582(7813):557–560. doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- Liu D., Thompson J.R., Carducci A., Bi X. Potential secondary transmission of SARS-CoV-2 via wastewater. Sci. Total Environ. 2020;749:142358. doi: 10.1016/j.scitotenv.2020.142358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodovici M., Bigagli E. Oxidative stress and air pollution exposure. Journal of Toxicology. 2011;2011:487074. doi: 10.1155/2011/487074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lolli S., Chen Y.-C., Wang S.-H., Vivone G. Impact of meteorological conditions and air pollution on COVID-19 pandemic transmission in Italy. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-73197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucking A.J., Lundback M., Mills N.L., Faratian D., Barath S.L., Pourazar J., Cassee F.R., Donaldson K., Boon N.A., Badimon J.J., Sandstrom T., Blomberg A., Newby D.E. Diesel exhaust inhalation increases thrombus formation in man. Eur. Heart J. 2008;29(24):3043–3051. doi: 10.1093/eurheartj/ehn464. [DOI] [PubMed] [Google Scholar]
- Lv Z., Deng Y.-Q., Ye Q., Cao L., Sun C.-Y., Fan C., Huang W., Sun S., Sun Y., Zhu L., Chen Q., Wang N., Nie J., Cui Z., Zhu D., Shaw N., Li X.-F., Li Q., Xie L.…Wang X. Structural basis for neutralization of SARS-CoV-2 and SARS-CoV by a potent therapeutic antibody. Science. 2020;369(6510):1505–1509. doi: 10.1126/science.abc5881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manisalidis I., Stavropoulou E., Stavropoulos A., Bezirtzoglou E. Environmental and health impacts of air pollution: a review. Front. Public Health. 2020;8 doi: 10.3389/fpubh.2020.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann J.K., Tager I.B., Lurmann F., Segal M., Quesenberry C.P., Lugg M.M., Shan J., Van Den Eeden S.K. Air pollution and hospital admissions for ischemic heart disease in persons with congestive heart failure or arrhythmia. Environ. Health Perspect. 2002;110(12):1247–1252. doi: 10.1289/ehp.021101247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelletti L., Martelletti P. Air pollution and the novel Covid-19 disease: a putative disease risk factor. Sn Comprehensive Clinical Medicine. 2020:1–5. doi: 10.1007/s42399-020-00274-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor-Ibarguren A., Feito-Rodriguez M., Quintana Castanedo L., Ruiz-Bravo E., Montero Vega D., Herranz-Pinto P. Cutaneous small vessel vasculitis secondary to COVID-19 infection: a case report. J. Eur. Acad. Dermatol. Venereol. 2020;34(10):e541–e542. doi: 10.1111/jdv.16670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra M.R., Ruschitzka F. COVID-19 illness and heart failure: a missing link? JACC. Heart Failure. 2020;8(6):512–514. doi: 10.1016/j.jchf.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez J.A. Metformin and SARS-CoV-2: mechanistic lessons on air pollution to weather the cytokine/thrombotic storm in COVID-19. Aging (Albany NY) 2020;12(10):8760–8765. doi: 10.18632/aging.103347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moccia F., Gerbino A., Lionetti V., Miragoli M., Munaron L.M., Pagliaro P., Pasqua T., Penna C., Rocca C., Samaja M., Angelone T. COVID-19-associated cardiovascular morbidity in older adults: a position paper from the Italian Society of Cardiovascular Researchers. GeroScience. 2020;42(4):1021–1049. doi: 10.1007/s11357-020-00198-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad S., Aziz R., Al Mahri S., Malik S.S., Haji E., Khan A.H., Khatlani T.S., Bouchama A. Obesity and COVID-19: what makes obese host so vulnerable? Immun. Ageing. 2021;18(1):1. doi: 10.1186/s12979-020-00212-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawska L., Ristovski Z., Jayaratne R., Keogh D., Ling X. Ambient nano and ultrafine particles from motor vehicle emissions: characteristics, ambient processing and implications on human exposure. Atmos. Environ. 2008;42(35):8113–8138. [Google Scholar]
- Moriyama M., Hugentobler W.J., Iwasaki A. Seasonality of respiratory viral infections. Annual Review of Virology. 2020;7(1):83–101. doi: 10.1146/annurev-virology-012420-022445. [DOI] [PubMed] [Google Scholar]
- Mukherjee S., Boral S., Siddiqi H., Mishra A., Meikap B.C. Present cum future of SARS-CoV-2 virus and its associated control of virus-laden air pollutants leading to potential environmental threat - a global review. Journal of Environmental Chemical Engineering. 2021;9(2):104973. doi: 10.1016/j.jece.2020.104973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutlu G., Green D., Lo A., Baker C., Burgess Z., Rajamannan N., Christman J., Foiles N., Kamp D., Ghio A., Chandel N., Dean D., Sznajder J., Budinger G.R. Ambient particulate matter accelerates coagulation via an IL-6-dependent pathway. J. Clin. Invest. 2007;117:2952–2961. doi: 10.1172/JCI30639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelin T.D., Joseph A.M., Gorr M.W., Wold L.E. Direct and indirect effects of particulate matter on the cardiovascular system. Toxicol. Lett. 2012;208(3):293–299. doi: 10.1016/j.toxlet.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemmar A., Hoet P.H.M., Vanquickenborne B., Dinsdale D., Thomeer M., Hoylaerts M.F., Vanbilloen H., Mortelmans L., Nemery B. Passage of inhaled particles into the blood circulation in humans. Circulation. 2002;105(4):411–414. doi: 10.1161/hc0402.104118. [DOI] [PubMed] [Google Scholar]
- Nichol J.E., Bilal M., Ali M.A., Qiu Z. Air pollution scenario over China during COVID-19. Remote Sens. 2020;12(13):2100. doi: 10.3390/rs12132100. [DOI] [Google Scholar]
- Nichols C.E., Shepherd D.L., Knuckles T.L., Thapa D., Stricker J.C., Stapleton P.A., Minarchick V.C., Erdely A., Zeidler-Erdely P.C., Alway S.E., Nurkiewicz T.R., Hollander J.M. Cardiac and mitochondrial dysfunction following acute pulmonary exposure to mountaintop removal mining particulate matter. American Journal of Physiology. Heart and Circulatory Physiology. 2015;309(12):H2017–H2030. doi: 10.1152/ajpheart.00353.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paital B., Agrawal P.K. Air pollution by NO2 and PM2.5 explains COVID-19 infection severity by overexpression of angiotensin-converting enzyme 2 in respiratory cells: a review. Environmental Chemistry Letters. 2020:1–18. doi: 10.1007/s10311-020-01091-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankow J.F., Luo W., Bender D.A., Isabelle L.M., Hollingsworth J.S., Chen C., Asher W.E., Zogorski J.S. Concentrations and co-occurrence correlations of 88 volatile organic compounds (VOCs) in the ambient air of 13 semi-rural to urban locations in the United States. Atmospheric Environment. 2003;37(36):24. doi: 10.1016/j.atmosenv.2003.08.006. [DOI] [Google Scholar]
- Park S.K., O’Neill M.S., Vokonas P.S., Sparrow D., Schwartz J. Effects of air pollution on heart rate variability: the VA normative aging study. Environ. Health Perspect. 2005;113(3):304–309. doi: 10.1289/ehp.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.K., O’Neill M.S., Vokonas P.S., Sparrow D., Wright R.O., Coull B., Nie H., Hu H., Schwartz J. Air pollution and heart rate variability: effect modification by chronic lead exposure. Epidemiology (Cambridge, Mass.) 2008;19(1):111–120. doi: 10.1097/EDE.0b013e31815c408a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A., Liu E., Verrier R.L., Schwartz J., Gold D.R., Mittleman M., Baliff J., Oh J.A., Allen G., Monahan K., Dockery D.W. Air pollution and incidence of cardiac arrhythmia. Epidemiology (Cambridge, Mass.) 2000;11(1):11–17. doi: 10.1097/00001648-200001000-00005. [DOI] [PubMed] [Google Scholar]
- Peters A., Fröhlich M., Döring A., Immervoll T., Wichmann H.E., Hutchinson W.L., Pepys M.B., Koenig W. Particulate air pollution is associated with an acute phase response in men; results from the MONICA-Augsburg Study. Eur. Heart J. 2001;22(14):1198–1204. doi: 10.1053/euhj.2000.2483. [DOI] [PubMed] [Google Scholar]
- Petroni M., Hill D., Younes L., Barkman L., Howard S., Howell I.B., Mirowsky J., Collins M.B. Hazardous air pollutant exposure as a contributing factor to COVID-19 mortality in the United States. Environmental Research Letters. 2020;15(9) doi: 10.1088/1748-9326/abaf86. [DOI] [Google Scholar]
- Pope C.A., Burnett R.T., Thun M.J., Calle E.E., Krewski D., Ito K., Thurston G.D. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287(9):1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope C.A., Bhatnagar A., McCracken J.P., Abplanalp W., Conklin D.J., O’Toole T. Exposure to fine particulate air pollution is associated with endothelial injury and systemic inflammation. Circ. Res. 2016;119(11):1204–1214. doi: 10.1161/CIRCRESAHA.116.309279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raciti L., Calabrò R.S. Can volcanic trace elements facilitate Covid-19 diffusion? A hypothesis stemming from the Mount Etna area, Sicily. Med. Hypotheses. 2020;144:110058. doi: 10.1016/j.mehy.2020.110058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan S., Al-Kindi S.G., Brook R.D. Air pollution and cardiovascular disease. J. Am. Coll. Cardiol. 2018;72(17):2054. doi: 10.1016/j.jacc.2018.07.099. [DOI] [PubMed] [Google Scholar]
- Rao X., Zhong J., Brook R.D., Rajagopalan S. Effect of particulate matter air pollution on cardiovascular oxidative stress pathways. Antioxid. Redox Signal. 2018;28(9):797–818. doi: 10.1089/ars.2017.7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., Staplin N., Brightling C., Ustianowski A., Elmahi E., Prudon B., Green C., Felton T., Chadwick D., Rege K., Fegan C., Chappell L.C., Faust S.N.…Landray M.J., RECOVERY Collaborative Group Dexamethasone in hospitalized patients with Covid-19—preliminary report. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remuzzi A., Remuzzi G. COVID-19 and Italy: what next? Lancet. 2020;395(10231):1225–1228. doi: 10.1016/S0140-6736(20)30627-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario D.K.A., Mutz Y.S., Bernardes P.C., Conte-Junior C.A. Relationship between COVID-19 and weather: case study in a tropical country. Int. J. Hyg. Environ. Health. 2020;229:113587. doi: 10.1016/j.ijheh.2020.113587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A., Gong J., Thomas D.C., Zhang J., Kipen H.M., Rich D.Q., Zhu T., Huang W., Hu M., Wang G., Wang Y., Zhu P., Lu S.-E., Ohman-Strickland P., Diehl S.R., Eckel S.P. The cardiopulmonary effects of ambient air pollution and mechanistic pathways: a comparative hierarchical pathway analysis. PLoS One. 2014;9(12) doi: 10.1371/journal.pone.0114913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajuthi S.P., DeFord P., Li Y., Jackson N.D., Montgomery M.T., Everman J.L., Rios C.L., Pruesse E., Nolin J.D., Plender E.G., Wechsler M.E., Mak A.C.Y., Eng C., Salazar S., Medina V., Wohlford E.M., Huntsman S., Nickerson D.A., Germer S.…Seibold M.A. Type 2 and interferon inflammation regulate SARS-CoV-2 entry factor expression in the airway epithelium. Nat. Commun. 2020;11(1):5139. doi: 10.1038/s41467-020-18781-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim S.Y., Kaplan G.G., Madsen K.L. Air pollution effects on the gut microbiota: a link between exposure and inflammatory disease. Gut Microbes. 2014;5(2):215–219. doi: 10.4161/gmic.27251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyaolu A., Okorie C., Marinkovic A., Patidar R., Younis K., Desai P., Hosein Z., Padda I., Mangat J., Altaf M. Comorbidity and its impact on patients with COVID-19. SN Comprehensive Clinical Medicine. 2020:1–8. doi: 10.1007/s42399-020-00363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuit M., Ratnesar-Shumate S., Yolitz J., Williams G., Weaver W., Green B., Miller D., Krause M., Beck K., Wood S., Holland B., Bohannon J., Freeburger D., Hooper I., Biryukov J., Altamura L.A., Wahl V., Hevey M., Dabisch P. Airborne SARS-CoV-2 is rapidly inactivated by simulated sunlight. J. Infect. Dis. 2020;222(4):564–571. doi: 10.1093/infdis/jiaa334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu E.Y.C., Leung N.H.L., Cowling B.J. Controversy around airborne versus droplet transmission of respiratory viruses: implication for infection prevention. Curr. Opin. Infect. Dis. 2019;32(4):372–379. doi: 10.1097/QCO.0000000000000563. [DOI] [PubMed] [Google Scholar]
- Silverio A., Di Maio M., Citro R., Esposito L., Iuliano G., Bellino M., Baldi C., De Luca G., Ciccarelli M., Vecchione C., Galasso G. Cardiovascular risk factors and mortality in hospitalized patients with COVID-19: systematic review and meta-analysis of 45 studies and 18,300 patients. BMC Cardiovasc. Disord. 2021;21(1):23. doi: 10.1186/s12872-020-01816-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A. COVID-19 and air pollution and meteorology-an intricate relationship: a review. Chemosphere. 2021;263:128297. doi: 10.1016/j.chemosphere.2020.128297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ssentongo P., Ssentongo A.E., Heilbrunn E.S., Ba D.M., Chinchilli V.M. Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: a systematic review and meta-analysis. PLoS One. 2020;15(8) doi: 10.1371/journal.pone.0238215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q., Wang A., Jin X., Natanzon A., Duquaine D., Brook R.D., Aguinaldo J.-G.S., Fayad Z.A., Fuster V., Lippmann M., Chen L.C., Rajagopalan S. Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. JAMA. 2005;294(23):3003–3010. doi: 10.1001/jama.294.23.3003. [DOI] [PubMed] [Google Scholar]
- Sun Q., Hong X., Wold L.E. Cardiovascular effects of ambient particulate air pollution exposure. Circulation. 2010;121(25):2755–2765. doi: 10.1161/CIRCULATIONAHA.109.893461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwa T., Hogg J.C., Quinlan K.B., Ohgami A., Vincent R., van Eeden S.F. Particulate air pollution induces progression of atherosclerosis. J. Am. Coll. Cardiol. 2002;39(6):935–942. doi: 10.1016/s0735-1097(02)01715-1. [DOI] [PubMed] [Google Scholar]
- Tellier R., Li Y., Cowling B.J., et al. Recognition of aerosol transmission of infectious agents: a commentary. BMC Infect Dis. 2019;19(101) doi: 10.1186/s12879-019-3707-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., Lloyd-Smith J.O., de Wit E., Munster V.J. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet (London, England) 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez-Bonilla W.O., Orozco R., Argueta V., Sierra M., Zambrano L.I., Muñoz-Lara F., López-Molina D.S., Arteaga-Livias K., Grimes Z., Bryce C., Paniz-Mondolfi A., Rodríguez-Morales A.J. A review of the main histopathological findings in coronavirus disease 2019. Hum. Pathol. 2020 doi: 10.1016/j.humpath.2020.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan L., Veruttipong D., Shaw J.G., Levy N., Edwards L., Winget M. Relationship of socio-demographics, comorbidities, symptoms and healthcare access with early COVID-19 presentation and disease severity. BMC Infect. Dis. 2021;21(1):40. doi: 10.1186/s12879-021-05764-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volatile Organic Compounds'’ Impact on Indoor Air Quality United States Environmental Protection Agency; 2014. Overviews and factsheets. https://www.epa.gov/indoor-air-quality-iaq/volatile-organic-compounds-impact-indoor-air-quality (August 18)
- Walls A.C., Park Y.-J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281–292. doi: 10.1016/j.cell.2020.02.058. (e6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Lang G.D., Moreno-Vinasco L., Huang Y., Goonewardena S.N., Peng Y.-J., Svensson E.C., Natarajan V., Lang R.M., Linares J.D., Breysse P.N., Geyh A.S., Samet J.M., Lussier Y.A., Dudley S., Prabhakar N.R., Garcia J.G.N. Particulate matter induces cardiac arrhythmias via dysregulation of carotid body sensitivity and cardiac sodium channels. Am. J. Respir. Cell Mol. Biol. 2012;46(4):524–531. doi: 10.1165/rcmb.2011-0213OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellenius G.A., Bateson T.F., Mittleman M.A., Schwartz J. Particulate air pollution and the rate of hospitalization for congestive heart failure among medicare beneficiaries in Pittsburgh, Pennsylvania. Am. J. Epidemiol. 2005;161(11):1030–1036. doi: 10.1093/aje/kwi135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann D., Sperhake J.-P., Lütgehetmann M., Steurer S., Edler C., Heinemann A., Heinrich F., Mushumba H., Kniep I., Schröder A.S., Burdelski C., de Heer G., Nierhaus A., Frings D., Pfefferle S., Becker H., Bredereke-Wiedling H., de Weerth A., Paschen H.-R.…Kluge S. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann. Intern. Med. 2020 doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold L.E., Ying Z., Hutchinson K.R., Velten M., Gorr M.W., Velten C., Youtz D.J., Wang A., Lucchesi P.A., Sun Q., Rajagopalan S. Cardiovascular remodeling in response to long-term exposure to fine particulate matter air pollution. Circulation. Heart Failure. 2012;5(4):452–461. doi: 10.1161/CIRCHEARTFAILURE.112.966580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., Niemeyer D., Jones T.C., Vollmar P., Rothe C., Hoelscher M., Bleicker T., Brünink S., Schneider J., Ehmann R., Zwirglmaier K., Drosten C., Wendtner C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- Wu F., Zhao S., Yu B., Chen Y.-M., Wang W., Song Z.-G., Hu Y., Tao Z.-W., Tian J.-H., Pei Y.-Y., Yuan M.-L., Zhang Y.-L., Dai F.-H., Liu Y., Wang Q.-M., Zheng J.-J., Xu L., Holmes E.C., Zhang Y.-Z. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Nethery R.C., Sabath B.M., Braun D., Dominici F. Exposure to air pollution and COVID-19 mortality in the United States: a nationwide cross-sectional study. MedRxiv (2020.04.05.20054502) 2020 doi: 10.1101/2020.04.05.20054502. [DOI] [Google Scholar]
- Xu K., Cui K., Young L.-H., Hsieh Y.-K., Wang Y.-F., Zhang J., Wan S. Impact of the COVID-19 event on air quality in Central China. Aerosol Air Qual. Res. 2020;20(5):915–929. doi: 10.4209/aaqr.2020.04.0150. [DOI] [Google Scholar]
- Yang C., Jin Z. An acute respiratory infection runs into the most common noncommunicable epidemic—COVID-19 and cardiovascular diseases. JAMA Cardiol. 2020;5(7):743. doi: 10.1001/jamacardio.2020.0934. [DOI] [PubMed] [Google Scholar]
- Zanobetti A., Schwartz J. The effect of particulate air pollution on emergency admissions for myocardial infarction: a multicity case-crossover analysis. Environ. Health Perspect. 2005;113(8):978–982. doi: 10.1289/ehp.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L., Chen H.-D., Chen J., Luo Y., Guo H., Jiang R.-D., Liu M.-Q., Chen Y., Shen X.-R., Wang X.…Shi Z.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet (London, England) 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Zhao N., Shu Y., Han S., Chen B., Shu X. Effect of gastrointestinal symptoms in patients with COVID-19. Gastroenterology. 2020;158(8):2294–2297. doi: 10.1053/j.gastro.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]