Abstract
BACKGROUND AND PURPOSE:
The present prognostic models for open globe injuries have a limited ability to predict visual outcome before a comprehensive ophthalmologic examination or operation because they depend on the data derived from the ophthalmologic examination and intraoperative findings. The purpose of our study was to determine the specific CT and preoperative clinical data that can predict the prognosis of open globe injury.
MATERIALS AND METHODS:
We analyzed the relationship of 29 variables derived from clinical and CT data from 97 globe injuries with visual acuity at 1 month. A prediction model was derived from 49 globe injuries by regression analysis, followed by receiver operating characteristic curve analysis of the best CT predictor.
RESULTS:
Four variables with significance on a regression model were the following: posterior segment hemorrhage (β = −0.93, P < .0001), presenting visual acuity (β = 0.28, P = .042), orbital emphysema (β = 0.46, P = .0018), and complex facial fracture (β = −0.43, P = .009). Receiver operating characteristic analysis of the posterior segment hemorrhage predicted profound vision loss (light perception or no light perception) with an area under the curve of 0.97. The receiver operating characteristic table indicated that grade III posterior segment hemorrhage has a strong positive predictive value of 100% for profound vision loss. On the other hand, the absence of posterior segment hemorrhage has a strong positive predictive value of 93% for mild-to-severe vision loss (visual acuity better than light perception).
CONCLUSIONS:
Radiologists, with the help of CT and preoperative clinical data, can predict visual acuity after open globe injury.
Open globe injury is described as a full-thickness injury of the eye wall resulting from either blunt or penetrating trauma.1 It is a vision-threatening injury, and primary surgical repair is the standard practice to restore the structural and physiologic integrity of the globe, regardless of the extent of the injury and the presenting visual acuity (VA).2,3 Prompt diagnosis and surgical repair of the injury are crucial to optimize visual outcome.4,5
There are only 2 predictive models of poor visual outcome after open globe injury, the Ocular Trauma Score system6 and the Classification and Regression Tree model, that were developed to guide both clinicians and patients in clinical decision-making.7 The Ocular Trauma Score is derived from presenting VA, globe rupture, endophthalmitis, perforating injury, retinal detachment, and relative afferent pupillary defect (RAPD) as predictors of poor visual outcome. The Classification and Regression Tree model identifies RAPD, presenting VA, lid laceration, and posterior wound location at surgery as predictors of poor outcome. Both models depend on data derived from a comprehensive ophthalmologic examination and intraoperative findings. This requirement limits the ability of the models to consistently provide prognostic information before ophthalmologic examination or an operation. The time between the injury and surgery is crucial because that is when clinicians need the most prognostic information to reduce the patient's anxiety and assist in informed decision-making regarding treatment choices.6 A comprehensive ophthalmologic assessment is challenging and is often delayed in the acute trauma setting due to periorbital soft-tissue swelling, poor patient cooperation, and altered mental status due to concomitant head trauma or the use of mind-altering medications.5,8 Subjecting patients with globe rupture to an aggressive ophthalmologic examination can even worsen the initial injury.9
In contemporary trauma care, CT has evolved as the imaging technique of choice for evaluating orbital trauma, especially in patients with difficult ophthalmologic examinations.4,5,8–10 Various studies have shown the sensitivities and specificities of CT, ranging from 56% to 76% and 79% to 100%, respectively, in detecting open globe injuries.4,5,8–10 CT offers the additional benefit of detecting concomitant intraorbital soft-tissue injuries and orbitofacial fractures.8 Objective preoperative prognostic data that can be derived from CT findings and limited clinical data obtainable in acute trauma settings would be of great clinical value. Such information could facilitate effective communication and counseling of patients.
Our purpose was to determine the specific CT and preoperative clinical data that can predict the prognosis of open globe injury. VA at 1 month after surgery was used as the reference standard for visual outcome in this study.
Materials and Methods
The study was compliant with the Health Insurance Portability and Accountability Act, and permission was obtained from the University of Maryland, Baltimore (UMB) institutional review board. The study was conducted at a level 1 trauma center. The inclusion criteria for this retrospective study were the following: 1) a history of open globe injury and subsequent surgical repair between July 2005 and January 2014, 2) CT of the face performed before surgical repair, 3) subject at least 18 years of age, and 4) clinical follow-up with determination of VA at 1 month after globe repair.
Subjects
A search of the trauma data base of our institution from July 2005 to January 2014 yielded 132 patients with 138 globe repairs; 6 patients sustained bilateral injuries. Only 1 globe was randomly selected from patients with bilateral injuries. One-month VA was available in 97 globes and constituted the study group. CT of the face was performed in 85 of the 97 patients. Figure 1 shows the patient selection flowchart. In the study group, there were 72 men and 25 women (mean age, 41.4 years; range, 18–94 years). The mechanism of injury was blunt trauma in 44% (43 of 97), gunshot wound in 25% (24 of 97), and stab wounds/penetrating injuries by sharp objects in 15% (15 of 97); and in 15% (15 of 97) of patients, the mechanism could not be determined.
Fig 1.
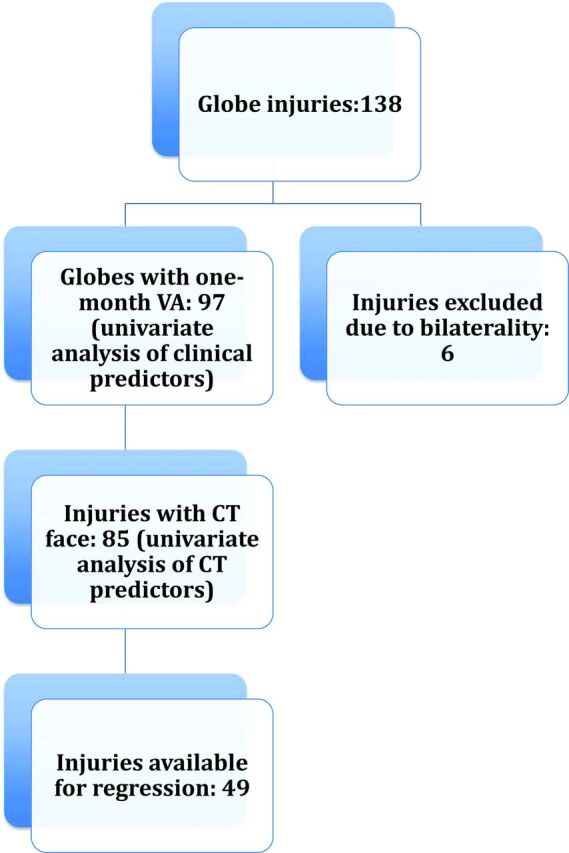
Flowchart shows the patient selection process.
Clinical information regarding the complete ophthalmic/pupil examination, if performed, was obtained. The data included presenting VA, RAPD, and intraocular pressure (IOP) measurements. Clinical information was obtained from the initial consultation note, progress notes, and operative reports. A senior ophthalmology resident (J.A.M.) reviewed the medical records.
Imaging Technique
Protocols for multidetector row CT are shown in On-line Table 1. Admission multidetector row CT was performed with a 16-, 40-, or 64 section CT system (Brilliance 16-, 40-, and 64-channel system; Philips Healthcare, Best, the Netherlands). Facial CT was performed from the frontal sinuses through the mental symphysis. The CT images included 2-mm axial sections and reformatted sagittal and coronal images (2-mm thickness, obtained at 1-mm intervals through the face).
Variable Construction
We analyzed the relationship of 29 study variables derived from demographic, clinical, and CT imaging data (Table 1 and On-line Table 2). Predictors of visual outcome identified in the literature were also included, in addition to the CT variables most commonly related to intraorbital soft-tissue injuries and craniofacial fractures.8,12
Table 1:
Correlation between continuous predictor variables and VA at 1 month
| Variable | No. of Globes with Variable | Spearman ρ | P Value |
|---|---|---|---|
| Age | 97/97 | −0.02 | .84 |
| IOP | 29/97 | −0.48 | .008a |
| Presenting VA | 59/97 | 0.84 | <.0001a |
| Fractional decrease in globe volume | 53/85 | −0.36 | .0074a |
| Fractional increase in globe volume | 32/85 | 0.4 | .026a |
Statistical significance (P < .05).
Image Analysis and Definitions
CT studies were loaded onto the thin-client server of our institution (IntelliSpace Portal; Philips Healthcare) to facilitate postprocessing of multiplanar reconstruction images in additional planes. Three trauma attending radiologists (reviewer 1, 5 years of experience; reviewer 2, 8 years of experience; reviewer 3, 10 years of experience), blinded to the clinical data, performed independent reviews of each CT study on the PACS of our institution with additional use of the thin-client software. For all the CT variables, discrepancies between the assessments of 2 reviewers were resolved by adjudication by a third reviewer.
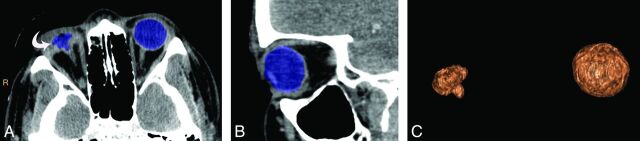
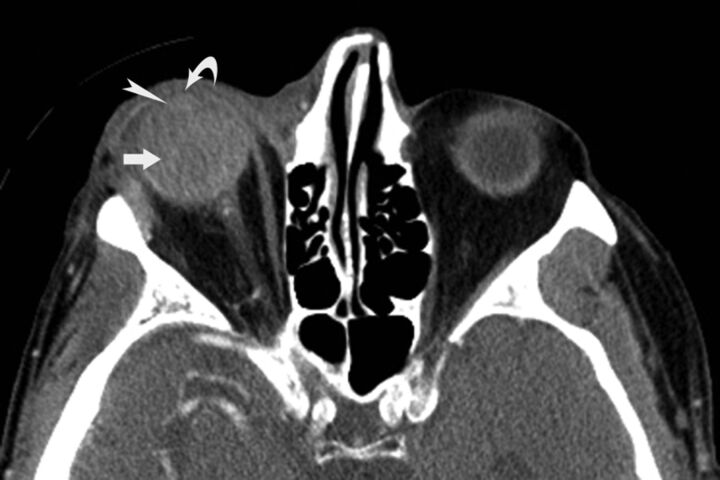
The reviewers assessed and recorded the presence of the CT variables of globe and orbitofacial trauma. All the variables were given nominal scores based on the presence (score of 1) versus the absence (score of 0) of each variable. Apart from the qualitative analysis, a quantitative analysis was performed for posterior segment hemorrhage, fractional decrease in globe volume, and fractional increase in globe volume. Reviewer 3 measured globe volumes on the thin-client server by using semiautomated 3D segmentation. ROIs were drawn on axial sections with the use of sagittal sections to exclude unwanted surrounding bone and soft tissues from the ROIs (Fig 2). Volumes of both globes were obtained in all patients. In patients with lens destruction or severe intraocular hemorrhage too dense to allow visualization of the lens on CT, evaluation for lens subluxation and dislocation and assessment of the anterior chamber depth were not performed. It is quite difficult to distinguish hemorrhage into the vitreous chamber and subretinal or suprachoroidal subtypes in globes with severe injury. Hence, we considered it more appropriate to refer to them collectively as posterior segment hemorrhage (Fig 3).
Fig 2.
A 30-year-old man with a stab wound to the right eye. Axial (A) and coronal (B) CT images demonstrate the ROI drawn by 3D segmentation on the thin-client server. The ruptured right globe resulted in decompression (curved arrow) due to vitreous and uveal prolapse. C, Segmented volume-rendered image of both the ruptured globe and the normal contralateral globe. Based on the calculated volumes, there was a fractional decrease in globe volume of 0.89.
Fig 3.
CT image shows a grade III posterior segment hemorrhage (arrow) in a 77-year-old man with right-sided open globe injury after blunt force trauma. The image shows intraocular hemorrhage that was too dense; however, the lens can be delineated from the hemorrhage (arrowhead) along with shallow anterior chamber (curved arrow). The right eye had a relative afferent pupillary defect with a measured intraocular pressure (IOP = 44 mm Hg) and a fractional increase in globe volume of 0.27.
Study Term Definitions
Grade of Posterior Segment Hemorrhage.
Posterior segment hemorrhage was graded from 0 to III, based on the amount of blood in the posterior segment: grade 0 (no posterior hemorrhage), grade I (<25% of posterior segment filled with blood), grade II (25%–75% filled), and grade III (>75% filled) (Fig 3).
Fractional Change in Globe Volume.
Fractional increase or fractional decrease in the globe volume was obtained by using the contralateral normal globe volume as a reference (Fig 2). In patients with bilateral globe injuries, the mean volume of all the normal globes included in the study was used as reference.
Intraorbital Hemorrhage and Emphysema.
Intraorbital hemorrhage and emphysema were further divided into extraconal and intraconal components.
Facial and Orbital Fractures.
Simple facial fractures were defined as fractures without involvement of facial buttresses. Complex facial fractures, defined as buttress fractures, included naso-orbitoethmoid fractures, zygomaticomaxillary complex fractures, and Le Fort fractures. Orbital fractures included isolated wall fractures and also those commonly associated with posterior propagation of complex midfacial fractures.
Perforating Injury.
Injuries caused by stab wounds and gunshot wounds were divided into penetrating injury (only entry wound) and perforating injury (both entry and exit wounds present).1
Profound Vision Loss.
Profound vision loss was defined as VA of light perception (LP) or no light perception (NLP). Mild-to-severe vision loss was further defined as VA better than LP.
Statistical Analysis
Statistical analysis was performed by K.S., by using statistical software (R statistical and computing software, Version 3.3.1; http://www.r-project.org/; and JMP 12 software; SAS Institute, Cary, North Carolina). Univariate analysis was performed by the Spearman rank correlation for continuous variables. Categoric variables were compared by 1-way ANOVA. After testing for homogeneous variance (Levene test), a post hoc analysis was performed by using the Welch and Wilcoxon tests. All significant predictor variables in univariate analysis were incorporated into a multivariate logistic regression model. A backward selection model was used. Variables thought to be of infrequent incidence were excluded to result in a model with less overfitting and greater generalizability. Missing data were dealt with by pair-wise deletion for univariate analysis and by a list-wise deletion for multivariate analysis. The regression equation was derived from a continuous dependent variable (ie, VA). The variance inflation factor and adjusted R2 were also obtained from regression analysis. A P value of .05 was significant. To examine the degree of overfitting of the prediction model, we performed a k-fold × 10 cross-validation. First, the sample was partitioned into 10 equal-sized subsamples. Of the 10 subsamples, 9 were used to develop the regression model and the resulting prediction equation was applied to the left-out sample. This procedure was repeated 10 times, each time rotating the cross-validation subset to derive the root mean square error and standard error for the root mean square error.
VA was converted into a logarithm of the minimum angle of resolution (logMAR) units (On-line Table 3) to provide a numeric scale for statistical analysis.11,12 Diagnostic performance of the strongest CT predictor derived from the regression analysis was further analyzed by receiver operating characteristic curves after converting the visual outcome into a binary variable (VA ≤ LP and VA ≥ LP). The data excluded from regression analysis were used as a test set to validate the results because all the patients had admission facial CT scans. Contingency tables were used to obtain sensitivities, specificities, positive predictive values (PPVs), and negative predictive values. The κ statistic was used to test interobserver reliability in assessing CT variables by the radiologists.
Results
Of the 97 globe injuries that constituted the study group, IOP could be measured in 29 globe injuries, only 41 eyes could be evaluated for the presence of RAPD, and preoperative VA could be measured in 59 eyes. Patients who had IOP, RAPD, and presenting VA measured on the initial examination were compared with those who did not. Patients with measured IOP had a mean logMAR of −3.38 (difference = 0.2, P = .5), the RAPD evaluated had a mean logMAR of −3.1 (difference = 0.68, P = .06), and those with presenting VA had a logMAR of −3.1 (difference = 1.15, P = .0002). The data indicate that patients with poor outcomes were less likely to have had a complete ophthalmologic examination, mainly a VA test.
Univariate Analysis
Univariate analysis showed that penetrating injuries with stabs/sharp objects predicted a favorable VA at 1 month (P = .03), while penetrating injuries caused by gunshot wounds predicted unfavorable VA (P < .0001), with no significant difference in outcome between penetrating and perforating injuries (P = .4) (On-line Table 2).
The ophthalmologic examination data associated with poor VA include higher IOP (Spearman ρ = −0.48, P = .008), the presence of RAPD (P = .008), and poor presenting VA (Spearman ρ = 0.84, P < .0001) (Table 1 and On-line Table 2).
The CT findings that predicted poor 1-month VA included the presence of posterior segment hemorrhage (P < .0001) (Fig 3), fractional decrease in globe volume (P = .007) (Fig 2), fractional increase in globe volume (P = .026) (Fig 3), hyphema (P < .0001), intraorbital hemorrhage (P = .0001), extraconal hemorrhage (P < .0001), intraconal hemorrhage (P < .0001), intraorbital emphysema (P < .0001), extraconal emphysema (P < .0001), intraconal emphysema (P < .0001), intraocular gas (P < .0001), lens subluxation (P = .0018), orbital fractures (P < .0001), complex facial fractures (P < .0001), and a shallow anterior chamber (P = .04) (Fig 3). A visible lens (P = .0054) on CT predicted a favorable VA at 1 month (Table 1 and On-line Table 2).
There was fair-to-very good agreement among the reviewers (On-line Table 2), except for traumatic cataract and lens subluxation, which had poor agreement.
Multivariate Analysis
Statistically significant variables such as IOP, RAPD, lens subluxation, and shallow anterior chamber were excluded from multivariate analysis as part of a list-wise deletion and due to their infrequent availability; this elimination resulted in a model with less overfitting and greater generalizability. Forty-nine globe injuries were selected to derive the regression model. The remaining 36 globe injuries, which were excluded from regression analysis due lack of adequate clinical data, were retained as test samples to validate the value of the strongest CT variable. The regression model identified 4 predictor variables with significance (Table 2): posterior segment hemorrhage, presenting VA, orbital emphysema, and complex facial fractures. The 4 variables resulted in an adjusted R2 of 0.827 and P < .0001 (root mean square error = 0.71). The variance inflation factor was <4 for the variables. On internal k-fold cross-validation with 10-fold analysis, the results were consistent in all the folds and the average root mean square error was 0.75 (standard error, 0.02).
Table 2:
Logistic regression analysis for the variables related to VA
| Variable | β Regression Coefficient | Standard Error | Accumulated Model Adjusted R2 | P Value |
|---|---|---|---|---|
| Posterior segment hemorrhage | −0.93 | 0.15 | 0.627 | <.0001 |
| Presenting VA | 0.28 | 0.13 | 0.789 | .042 |
| Orbital emphysema | 0.46 | 0.14 | 0.8 | .0018 |
| Complex facial fracture | −0.43 | 0.16 | 0.827 | .009 |
Receiver operating characteristic analysis of posterior segment hemorrhage in predicting profound vision loss yielded an area under the curve value of 0.97 in the derivation sample of 49 globes. The result was further validated on the test sample of 36 globes, which yielded a comparable area under the curve of 0.98. The addition of presenting VA to the model already containing posterior segment hemorrhage increased the area under the curve from 0.97 to 0.98 in the derivation sample.
The sensitivities, specificities, positive predictive values, and negative predictive values of various grades of posterior segment hemorrhage in predicting profound vision loss (LP or NLP) are shown in Table 3. The absence of posterior segment hemorrhage predicted a 1-month VA better than LP (mild-to-severe vision loss) with sensitivity, specificity, PPV, and negative predictive values of 64%, 98%, 93%, and 89%, respectively. The results indicate that grade III posterior segment hemorrhage has a strong PPV for profound vision loss (LP or NLP) at 1-month follow-up, with all 49 injured globes with grade III hemorrhage resulting in profound vision loss. On the other hand, the absence of posterior segment hemorrhage has a strong PPV for VA better than LP (mild-to-severe vision loss), with 14 of 15 patients without hemorrhage having a VA of better than LP on follow-up.
Table 3:
Sensitivities, specificities, PPVs, and NPVs of various grades of posterior segment hemorrhage in predicting profound vision loss
| Posterior Segment Hemorrhage | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95%CI) | NPV (95% CI) |
|---|---|---|---|---|
| PSH ≥ grade I | 98 (62/63) (91–100) | 64 (14/22) (41–83) | 89 (62/70) (19–95) | 93 (14/15) (68–100) |
| PSH ≥ grade II | 86 (54/63) (75–93) | 91 (20/22) (71–99) | 96 (54/56) (88–100) | 69 (20/29) (49–85) |
| PSH grade III | 78 (49/63) (65–87) | 100 (22/22) (85–100) | 100 (49/49) (93–100) | 61 (22/36) (43,77) |
Note:—PSH indicates posterior segment hemorrhage; NPV, negative predictive value.
Discussion
In this study, we systematically analyzed the CT findings of open globe injuries, intraorbital soft-tissue injuries, orbitofacial skeletal injuries, and limited clinical data that are possible to obtain in the acute trauma setting to determine the predictors of VA at 1 month. According to our results, posterior segment hemorrhage, presenting VA, orbital emphysema, and complex facial fractures are the independent predictors of poor VA. The developed model has a good predictive performance, and the strengths of the model are the following: 1) Most of the predictors can be assessed on admission CT, 2) it requires a minimum amount of clinical data, and 3) the prediction model does not depend on surgical findings or postsurgical outcomes. Posterior segment hemorrhage was found to be the strongest predictor of VA at 1 month. Moreover, grade III posterior segment hemorrhage had a strong PPV (100%) for profound vision loss (LP or NLP) at 1 month, while absence of hemorrhage had a strong PPV (93%) for mild-to-severe vision loss (VA better than LP).
The Ocular Trauma Score model predicted profound vision loss with a sensitivity of 100% and specificity of 91%, respectively.13 On the other hand, Classification and Regression Tree analysis has shown a sensitivity of 82% and specificity of 86%.13 Our results showed that grade III posterior segment hemorrhage, which can be easily derived from facial CT, alone has a sensitivity of 78% and specificity of 100% in predicting profound vision loss. The absence of posterior segment hemorrhage, on the other hand, had a sensitivity of 64% and a specificity of 98% in predicting mild-to-severe vision loss. The best predictor from Classification and Regression Tree analysis was RAPD. In contrast, RAPD was not incorporated into the logistic regression model due to list-wise deletion, though RAPD was significant on univariate analysis. The other disadvantage of depending on RAPD as a predictor was that only 42% of our injuries could be evaluated for the presence of RAPD in the acute trauma setting.
Joseph et al9 have reported that nearly 90% of globes with moderate-to-severe deformity had poor visual outcomes and 64% underwent enucleation, while no globes without scleral deformity underwent enucleation.9 Yuan et al5 showed that scleral deformities are associated with a decompressed globe in 36% of their studied globe ruptures and 15% showed enlarged globes. Decompression of the globe results from vitreous or uveal prolapse through the defect.4,5,9,14 Enlargement of the globe has been ascribed to intraocular hemorrhage, which can also contribute to increased IOP.3–5,9,14 To increase the quantitative precision of the degree of scleral deformity, we measured the globe volumes and calculated the fractional decrease or fractional increase in the volume of the ruptured globe. Our results showed that neither fractional decrease nor fractional increase in the globe volume was significant on regression analysis, though both variables were significant on univariate analysis. Margo et al,3 in a recent study, showed a correlation between high IOP and poor visual prognosis. Increased IOP, which had collinearity with posterior segment hemorrhage (Spearman ρ = 0.015), was associated with poor visual outcome. Joseph et al9 showed that 60% of patients with vitreous hemorrhage end up with enucleation and 75% of those with blood occupying >50% of the vitreous chamber end up with enucleation. Although our study did not evaluate the rates of enucleation, the results confirmed high sensitivity (98%; 95% CI, 91–100) of posterior segment hemorrhage in predicting profound vision loss.
Limitations
Our study has several limitations. First, it is a retrospective design with inherent biases. Second, some significant CT variables may have been excluded from analysis because CT variable selection was performed on the basis of the previously identified risk factors and some of the variables were selected by the study team members on the basis of their clinical experience. Simultaneous review of all signs may have resulted in some degree of bias in determining their individual performance. Finally, a direct comparison of our model with either the Ocular Trauma Score or the Classification and Regression Tree model was not performed because we did not record the surgical data that were the major components of those models, in keeping with the purpose of our study (ie, to derive a predictive model before surgery).
Conclusions
Previously known prognostic prediction models after open globe injury depend on data derived from ophthalmologic examination and surgical findings. However, ophthalmologic examination or even testing for RAPD, the best predictor in the Classification and Regression Tree model, may not be possible or reliable in some patients as seen from our results, due to nonreactive pupils frequently encountered in patients with associated traumatic brain injury, coma, or raised intracranial pressures. In this context, our results may have clinical implications when radiologists, with the help of CT and limited preoperative clinical data that is realistically possible to obtain in acute trauma settings, can predict VA after open globe injury. Such information reduces anxiety in patients and helps informed decision-making regarding treatment choices.
Supplementary Material
ABBREVIATIONS
- IOP
intraocular pressure
- LP
light perception
- logMAR
logarithm of the minimum angle of resolution
- NLP
no light perception
- PPV
positive predictive value
- RAPD
relative afferent pupillary defect
- VA
visual acuity
Footnotes
Disclosures: Osamah Saeedi—UNRELATED: Grant: National Institutes of Health, Comments: K23 National Institutes of Health career development award.* *Money paid to the institution.
REFERENCES
- 1. Kuhn F, Morris R, Witherspoon CD. Birmingham Eye Trauma Terminology (BETT): terminology and classification of mechanical eye injuries. Ophthalmol Clin North Am 2002;15:139–43, v 10.1016/S0896-1549(02)00004-4 [DOI] [PubMed] [Google Scholar]
- 2. Rahman I, Maino A, Devadson D, et al. Open globe injuries: factors predictive of poor outcome. Eye (Lond) 2006;20:1336–41 10.1038/sj.eye.6702099 [DOI] [PubMed] [Google Scholar]
- 3. Margo JA, Feldman S, Addis H, et al. Open globe injuries presenting with normal or high intraocular pressure. Eye Contact Lens 2016;42:256–61 10.1097/ICL.0000000000000188 [DOI] [PubMed] [Google Scholar]
- 4. Kim SY, Lee JH, Lee YJ, et al. Diagnostic value of the anterior chamber depth of a globe on CT for detecting open-globe injury. Eur Radiol 2010;20:1079–84 10.1007/s00330-009-1653-6 [DOI] [PubMed] [Google Scholar]
- 5. Yuan WH, Hsu HC, Cheng HC, et al. CT of globe rupture: analysis and frequency of findings. AJR Am J Roentgenol 2014;202:1100–07 10.2214/AJR.13.11010 [DOI] [PubMed] [Google Scholar]
- 6. Kuhn F, Maisiak R, Mann L, et al. The Ocular Trauma Score (OTS). Ophthalmol Clin North Am 2002;15:163–65, vi 10.1016/S0896-1549(02)00007-X [DOI] [PubMed] [Google Scholar]
- 7. Schmidt GW, Broman AT, Hindman HB, et al. Vision survival after open globe injury predicted by classification and regression tree analysis. Ophthalmology 2008;115:202–09 10.1016/j.ophtha.2007.04.008 [DOI] [PubMed] [Google Scholar]
- 8. Bodanapally UK, Van der Byl G, Shanmuganathan K, et al. Traumatic optic neuropathy prediction after blunt facial trauma: derivation of a risk score based on facial CT findings at admission. Radiology 2014;272:824–31 10.1148/radiol.14131873 [DOI] [PubMed] [Google Scholar]
- 9. Joseph DP, Pieramici DJ, Beauchamp NJ. Computed tomography in the diagnosis and prognosis of open-globe injuries. Ophthalmology 2000;107:1899–906 10.1016/S0161-6420(00)00335-3 [DOI] [PubMed] [Google Scholar]
- 10. Arey ML, Mootha VV, Whittemore AR, et al. Computed tomography in the diagnosis of occult open-globe injuries. Ophthalmology 2007;114:1448–52 10.1016/j.ophtha.2006.10.051 [DOI] [PubMed] [Google Scholar]
- 11. Bodanapally UK, Shanmuganathan K, Shin RK, et al. Hyperintense optic nerve due to diffusion restriction: diffusion-weighted imaging in traumatic optic neuropathy. AJNR Am J Neuroradiol 2015;36:1536–41 10.3174/ajnr.A4290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reddy RP, Bodanapally UK, Shanmuganathan K, et al. Traumatic optic neuropathy: facial CT findings affecting visual acuity. Emerg Radiol 2015;22:351–56 10.1007/s10140-014-1292-3 [DOI] [PubMed] [Google Scholar]
- 13. Yu Wai Man C, Steel D. Visual outcome after open globe injury: a comparison of two prognostic models—the Ocular Trauma Score and the Classification and Regression Tree. Eye 2010;24:84–89 10.1038/eye.2009.16 [DOI] [PubMed] [Google Scholar]
- 14. Weissman JL, Beatty RL, Hirsch WL, et al. Enlarged anterior chamber: CT finding of a ruptured globe. AJNR Am J Neuroradiol 1995;16:936–38 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.