Abstract
Objective
Recent evidence has accumulated that the immune system is intimately intertwined with cancer development. Two key characteristics of carcinogens in which the immune system plays a central role are chronic inflammation and immunosuppression. In this systematic review, we investigated the association of chronic inflammatory and immunosuppressive outcomes with benzene, a widely used industrial chemical. Benzene has been confirmed to cause acute myeloid leukaemia and suspected to cause non-Hodgkin lymphoma, two cancers of the blood-forming system that affect immune cells.
Methods
We systematically searched PubMed and Embase for all relevant studies using a combination of Medical Subject Headings (MeSH) and selected key words. The detailed review protocol, including search strategy, was registered with PROSPERO, the international prospective register of systematic reviews (#CRD42019138611).
Results
Based on all human studies selected in the final review, we report new evidence of a benzene-induced immunosuppressive effect on the adaptive immune system and activation of the innate immune system to cause inflammation. In particular, benzene significantly lowers the number of white blood cells, particularly lymphocytes such as CD4+ T-cells, B-cells and natural killer cells, and increases proinflammatory biomarkers at low levels of exposure.
Conclusion
To the best of our knowledge, this is the first comprehensive review of benzene’s immunotoxicity in humans. Based on results obtained from this review, we propose two potential immunotoxic mechanisms of how benzene induces leukaemia/lymphoma: (1) cancer invasion caused by proinflammatory cytokine production, and (2) cancer promotion via impaired immunosurveillance. Further studies will be required to confirm the connection between benzene exposure and its effects on the immune system.
BACKGROUND AND OBJECTIVES
The immune system and cancer
Our immune system consists of a diverse group of cells and molecules that have evolved to protect against pathogens like bacteria, viruses and parasites, as well as tumour cells. This defence system has been divided into two branches: (1) innate immunity and (2) adaptive immunity.
Innate immunity represents the first line of defence against a pathogen. The response is rapid and non-specific with no immunological memory. Cells involved in the innate immune response include dendritic cells, neutrophils, macrophages, natural killer (NK) cells, basophils and eosinophils. The adaptive immune system is activated by innate immunity and has a slower, but more targeted response with immunological memory. The primary cells involved are B-lymphocytes and T-lymphocytes. The adaptive immune system can be further divided into two branches—humoral and cell mediated. The humoral system is mediated by macromolecules found in extracellular fluids such as secreted antibodies, complement proteins and antimicrobial peptides. The cell-mediated immune system involves cells such as phagocytes and T-lymphocytes which respond to antigens.
Most immune responses require the interplay of both innate and adaptive immunity. When one branch is suppressed or overactive, it creates the opportunity for chronic infection or cancer to occur due to reduced immunosurveillance or inflammation and subsequent tissue damage. In particular, a dysfunctional immune system is a known risk factor for leukaemia or lymphoma, cancers of the blood-forming system which makes immune cells to help fight infection.
Benzene as a human leukaemogen and ubiquitous environmental pollutant
The formation of these blood cancers has been linked to exposure to environmental carcinogens. For example, benzene, a widely used industrial chemical, has been confirmed to cause acute myeloid leukaemia and is suspected to cause non-Hodgkin lymphoma (NHL).1 Benzene is conclusively listed as a group 1 carcinogen by the International Agency for Research on Cancer (IARC)1 and designated as category A (known human carcinogen) by the U.S. Environmental Protection Agency.2 Although the relationship between benzene and cancer is confirmed, the effects of benzene on the immune system and subsequently, cancer development, still need to be clarified.
Despite recent regulations that attempt to limit human exposure to benzene, exposures still commonly occur within both occupational settings and the environment. Inhalation is the primary route of exposure to benzene, though dermal absorption or ingestion of contaminated water or foods can also occur. As a common industrial chemical, workers in a wide range of industries such as petroleum, rubber, shoe manufacturing, printing and painting, among others, are often exposed to benzene. Benzene is also present environmentally via gasoline fumes, automobile exhaust, factory emissions or cigarette smoke. Although environmental benzene exposure typically occurs at levels much lower than occupational exposure, considerably more people are exposed. Due to the substantial evidence of ongoing human exposure and its carcinogenicity, benzene remains a critical environmental health concern.
The key characteristics of carcinogens: benzene as an example
In order to identify and evaluate mechanistic evidence of carcinogens, like benzene, a set of 10 key characteristics (KCs) has been proposed.3 Known carcinogens typically exhibit at least one or more of these KCs. The 10 KCs approach has been applied using benzene as an example (online supplemental table 1). Our current study focuses on chronic inflammation (KC6) and immunosuppression (KC7), two outcomes that directly involve the immune system and have never been independently and systematically reviewed in relation to benzene.
IARC previously concluded that benzene is immunosuppressive but did not draw a conclusion on whether benzene induces chronic inflammation.1 With the application of a systematic review approach and a broader, more in-depth, and up-to-date analysis, we aim to: (1) collect more evidence for chronic inflammation (KC6) since less is known regarding its association with benzene, and (2) to confirm the immunosuppression (KC7) caused by benzene.
Importance of chronic inflammation (KC6) and immunosuppression (KC7) in relation to benzene-associated cancers
Chronic inflammation from persistent infection or chronic exposure to chemical agents such as silica or asbestos fibres has been associated with several forms of cancer.4 Similarly, immunosuppression presents a risk of cancer, especially for lymphoma and leukaemia.5 These two outcomes often coexist in the tumour microenvironment and are hypothesised to contribute to multiple aspects of cancer development.6 It is important to note, however, that KC6 and KC7 often overlap with other KCs as well (online supplemental table 1). For example, strong links exist between inflammation and the induction of oxidative stress (KC5) and genomic stability (KC3). Thus, it may be challenging to separate out the effects of each of these mechanisms.
Since immune deficiencies and dysfunction are known risk factors for blood cancer development, this review could offer further insight into benzene-induced carcinogenesis and how to bolster the immune system in its defence against blood cancers. Additionally, this review could help clarify the controversial association between benzene and NHL, since chronic inflammation, immune activation and acquired immunosuppression have all been reported to play a key role in the aetiology of NHL.7
CURRENT APPROACH AND ANALYSIS
Search strategy
The literature search was conducted according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).8 Two electronic databases, PubMed and Embase, were searched for all published studies that examined the relationship between benzene and outcomes related to chronic inflammation or immunosuppression. Each database was searched from inception to May 2019. The specific search strategy used can be referenced in our published protocol (PROSPERO, #CRD42019138611). To maximise the currency of our review, we conducted an updated search for newly added references on the topic in July 2020 before our final submission for publication. We focused our efforts on PubMed since its database was recently updated.
To obtain the final set of relevant studies included in the review, there were two stages of paper selection: (1) a title and abstract screening, and (2) a full-text review. Both reviewers (HG, SA) independently selected articles against the predetermined inclusion and exclusion criteria (shown in online supplemental table 2). Disagreements were resolved by consensus with a third reviewer (LZ).
Identifying relevant studies: screening process
To screen retrieved papers, we used Sysrev, an open-access web-based platform designed for the collaborative extraction of data from academic articles. Our entire screening process and results are shown in figure 1 in accordance with PRISMA guidelines.8 From the initial database searches, we retrieved 1782 studies from PubMed and 2723 studies from Embase. After removal of duplicates using Endnote, 3458 studies were screened by title and abstract. After title and abstract screening, we identified 141 human studies. Then, following a full-text review based on our inclusion and exclusion criteria (online supplemental table 2), we selected a total of 85 final studies that reported on inflammatory or immunosuppressive effects in benzene-exposed populations. The included studies consisted of 68 cross-sectional studies, 15 cohort studies and 2 case reports (online supplemental tables 3 and 4) with most studies including either a control group or comparator group with lower exposure to benzene. From our updated search in July 2020, we reviewed 182 further studies and identified an additional 6 studies to be included in our review (online supplemental table 5).
Figure 1.
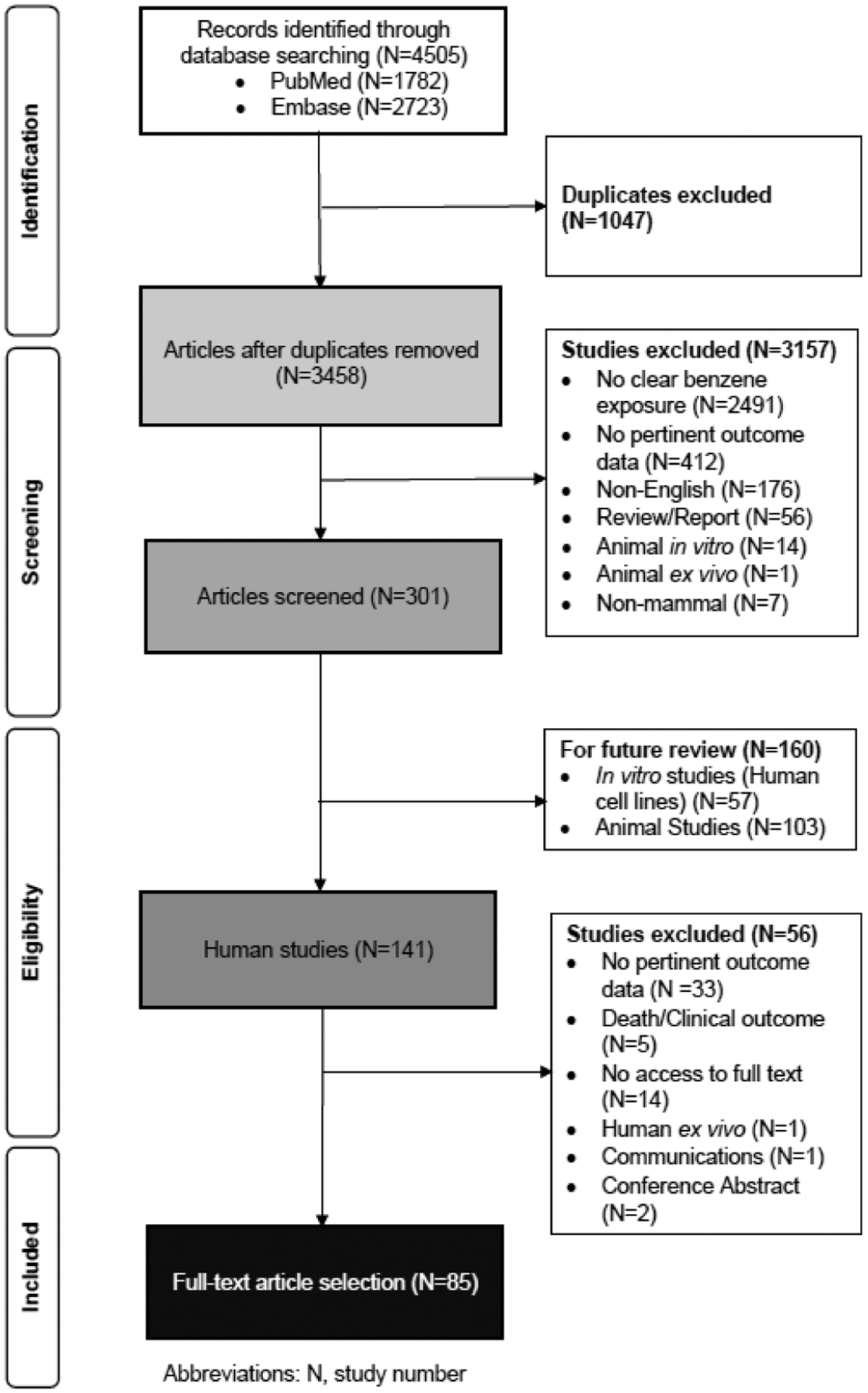
Study selection and screening process using Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.
Data collection and extraction
Our results offer a broad consideration of the published mechanistic evidence regarding chronic inflammation and immunosuppression outcomes associated with benzene exposure. The methodological heterogeneity observed across studies prevented the undertaking of a meta-analysis. Thus, all findings are presented in a qualitative, narrative format. Data extracted from reviewed studies include the author, year published, country, study population and size, exposure assessment methods, level of exposure and study outcomes. Detailed information and outcomes for each individual study, relevant to KC6, KC7 or both, can be found in online supplemental tables 3–5, respectively. Main findings for KC6 and KC7 are summarised in tables 1–2. For ease of viewing all results, a Venn Diagram is provided in online supplemental figure 1.
Table 1.
Major inflammatory outcomes from human studies of benzene exposure
| Results | |||
|---|---|---|---|
| KC6 outcomes | Increase | No association | Decrease |
| Cytokines | |||
| IL-1β | Guo et al13 | ||
| IL-6 | Dutta et al,14 Jorgensen et al17 | Rothman et al59 | |
| IL-8 | Dutta et al,14 Elango et al,15 Moro et al,16 Guo et al13 | ||
| TNF-α | Dutta et al,14 Samadi et al18 | Rothman et al59 | Haro-Garcia et al60 |
| IFNB1 | Jorgensen et al17 | ||
| IFN-γ | Samadi et al18 | ||
| CXCL-16 | Forrest et al,19 McHale et al20 | ||
| Serum proteins | |||
| Alpha-1-antitrypsin | Zhang et al22 | ||
| Alpha-1-antichymotrypsin | Zhang et al22 | ||
| Caspase-1 | Guo et al13 | ||
| Haptoglobin | Zhang et al22 | ||
| Eosinophil cationic protein | Elango et al15 | ||
| Leukotriene B4 | Elango et al15 | ||
| NF-kB | Fenga et al23 | ||
| Phospho-lkB | Fenga et al23 | ||
| STAT3 | Fenga et al,23 Yang et al24 | ||
| Cellular biomarkers | |||
| Alveolar macrophages | Dutta et al14 | ||
| Band neutrophils | Bogadi-Sare et al,25 Ray et al26 | ||
| CD3+ T-cells | Lehmann et al27 | ||
CXCL-16, CXC chemokine ligand 16; IFN-γ, interferon-gamma; IFNB1, interferon beta; IL, interleukin; NF-kB, nuclear factor kappa-light-chain-enhancer of activated B-cells; STAT3, signal transducer and activators of transcription 3; TNF-α, tumour necrosis factor alpha.
Table 2.
Major immunosuppressive outcomes from human studies of benzene exposure
| KC7 outcomes | Results | ||
|---|---|---|---|
| Increase | No association | Decrease | |
| Innate immunity | |||
| CD56+ NK cells | Ray et al,26 Boscolo et al31 | Santiago et al30 | |
| Complement factor | Zhang et al22 | Smolik et al,33 Sauer et al34 | |
| CSF3R promoter methylation | Ren et al35 | ||
| Humoral immunity | |||
| Immunoglubulin production | |||
| IgM and IgA | Kirkeleit et al37 | ||
| IgG | Bogadi-Sare et al38 | Uzma et al36 | |
| PAIgs | Huang et al39 | ||
| Autoantibodies against heat shock protein 71 | Wu et al40 | ||
| B-cell activation | |||
| SCD27 and sCD30 | Bassig et al41 | ||
| Cell-mediated immunity | |||
| T-cell function | |||
| TCRVβ | Li et al42 | ||
| CD3 gene family | Li et al43 | ||
| CD80 (B7.1), CD86 (B7.2) | Moro et al,16 Sauer et al34 | ||
| T-cell subsets | |||
| HLA-DR+/CD3+ | Biro et al44 | ||
| CD71+/CD19+ | Biro et al44 | ||
| CD62L+/CD3+ | Biro et al44 | ||
| CD4+/CD25+ | Baiz et al45 | ||
| TRECs | Lan et al47 | Li et al46 | |
| Cytokine/chemokine production | |||
| PF4 | Forrest et al,19 McHale et al,20 Vermeulen et al48 | ||
| CXCL7 (CTAP III) | Vermeulen et al48 | ||
CSF3R, colony-stimulating factor 3 receptor; CTAP III, connective tissue-activating peptide III; CXCL7, CXC chemokine ligand 7; HLA-DR, human leucocyte antigen-DR isotype; Ig, immunoglobulin; NK cells, natural killer cells; PAIgs, platelet-associated immunoglobulins; PF4, platelet factor 4; sCD27, soluble CD27; sCD30, soluble CD30; TCRVβ, variable β-domain of T-cell receptors; TRECs, T-cell receptor excision circles.
BENZENE AND CHRONIC INFLAMMATION (KC6)
The link between chronic inflammation and cancer
Inflammation is a critical function of the innate immune system that protects against pathogens and consists of an immediate response to tissue damage caused by potentially harmful stimuli. Initially, inflammatory agents elicit an acute inflammatory response in which the immune system recognises and removes harmful stimuli to begin the healing process. Unfortunately, if the resolution is not adequate or the stimulus persists, chronic inflammation can occur, partly as a consequence of the activation of adaptive immunity. Inflammation has been recognised as an enabling characteristic of cancer9 and the induction of chronic inflammation is a KC of carcinogens.3
Chronic inflammation associated with the development of cancer is a prolonged response to persistent infections or irritants that inflict tissue injury and death followed by deregulated cell proliferation and aberrant repair.10 Environmental contaminants, such as cadmium, have been shown to be non-infectious causes of inflammation.11 It is thus possible that benzene, similar to cadmium, could also induce chronic inflammation and subsequently promote cancer development.
Known or proposed biomarkers of inflammation
Chronic inflammation is characterised by a continual recruitment of innate and adaptive immune cells, which produce high levels of proinflammatory molecules. This generates a harmful environment, resulting in tissue damage and increased risk of cancer initiation and progression. Inflammatory biomarkers include cellular factors, such as the infiltration of inflammatory cells (macrophages, lymphocytes, plasma cells) in the tissue site, or molecular factors, such as the production of inflammatory cytokines, growth factors and/or enzymes.12
In our review of 85 total studies, we found 29 studies that included outcomes related to inflammation. Each individual study is summarised in detail in online supplemental table 3. An additional study13 identified from our updated July 2020 search is summarised in online supplemental table 5. A condensed list of the main outcomes and corresponding studies is shown in table 1.
Molecular biomarkers
Cytokine production
Inflammation is mediated by a variety of soluble factors, including a group of signalling molecules called cytokines. Several studies report an increased serum level of proinflammatory cytokines interleukin (IL)-6, IL-8, tumour necrosis factor (TNF)-α, interferon (IFN)-γ, and IFNB1, or increased expression in their corresponding genes, even at low levels of benzene exposure.13–18 These proinflammatory cytokines induce blood vessels to become more permeable, recruit other immune cells such as neutrophils, basophils and T-cells to sites of inflammation, and raise the temperature in infected tissue. Similarly, a recently published study reports that benzene at ~1 ppm exposure (the current U.S. occupational standard) increased expression of caspase-1 (a serum protein) and IL-1β, accompanied with elevated serum IL-1β.13 The finding indicates an increased pyroptosis—an inherently proinflammatory form of cell death mediated by the activation of caspase-1, a protease that also activates inflammatory cytokine IL-1β. Additionally, upregulation of proinflammatory chemokine CXCL16 has also been reported in association with benzene exposure.19,20 Increased expression of CXCL16 has been linked to inflammation-associated cancers.21
Serum proteins
An increase or decrease in certain serum proteins can often indicate inflammatory conditions in the body. In particular, acute phase proteins change their serum concentration significantly in response to inflammatory cytokines and are often used as a diagnostic tool in the clinic to monitor inflammation. In a cross-sectional study of 75 chronic benzene poisoning patients compared with 90 normal controls, the significant upregulation of two acute phase proteins alpha-1-antitrypsin and alpha-1-antichymotrypsin was observed, as well as a downregulation of haptoglobin.22
Other proinflammatory mediators that have been observed to increase in response to benzene exposure include eosinophil cationic protein and leukotriene B4.15,17 Activation of the NF-kB proinflammatory signalling pathway has been indicated by evidence of increased NF-kB and phospho-IkB levels as well as altered STAT3 levels,23,24 a protein that acts as a liaison in the promotion of the NF-kB pathway.
Cellular biomarkers
In general, chronic inflammation is characterized by the presence of macrophages, monocytes, and lymphocytes, which produce inflammatory cytokines and enzymes that can cause lasting damage to cells. This change in the composition of white blood cells (WBCs) is often accompanied by the proliferation of blood vessels and connective tissue.
Increased levels of alveolar macrophages have been reported in benzene-exposed women.14 Two studies reported an increase in band (immature) neutrophils in benzene-exposed workers,25,26 a phenomenon that typically accompanies infectious and inflammatory conditions and indicates an increase in the production of WBCs by the bone marrow. Exposure to benzene has also been associated with higher percentages of IL-4 producing CD3+ T-cells,27 which can induce the differentiation and expansion of naïve CD4+ T-cells into mature Th2 effector cells.28 The Th2 response has been suggested to drive chronic inflammation in patients with cancer with metastatic melanoma,29 indicating it could perform a similar function in benzene-associated cancers.
Overall, however, the data for benzene-associated cellular changes indicating chronic inflammation are limited. The majority of studies reviewed indicate evidence of immunosuppression in the form of decreased T-lymphocyte counts, specifically CD4+ and/or CD8+ lymphocytes (see the Haematotoxicity section).
BENZENE AND IMMUNOSUPPRESSION (KC7)
Immunosuppression
Immunosuppression is characterised as a decreased capacity to neutralise external agents which may result in repeated, more severe or prolonged infections, as well as an increased susceptibility to cancer development.3 Immunosuppression can be evident in the adaptive and innate immune systems, manifesting as abnormal blood cell counts, abnormal levels of immunoglobulins or altered immune cell function. There is a large body of evidence suggesting that the bone marrow immunosuppressive microenvironment plays a major role in leukaemogenesis. Since benzene is an established leukaemogen, it is possible that benzene exposure can promote immune dysfunction and thus cancer via its haematotoxic effects on the blood-forming system and immune cells.
Among the 85 total studies included in our review, 76 studies (~89%) addressed outcomes related, either directly or indirectly, to immunosuppression. A detailed summary of each individual study can be found in online supplemental table 4. Additional studies related to KC7, identified in our updated July 2020 search, are summarised in online supplemental table 5. A condensed list of the main outcomes and corresponding studies is shown in table 2. Beyond benzene’s effects on components of both the innate and adaptive immune system branches, many of the study findings indicated evidence of haematotoxicity or altered blood cell populations (see the Haematotoxicity section). online supplemental table 6
Innate immunity
Altered levels of CD56+ NK cells and abnormal CD16 expression have been reported in response to benzene exposure.26,30,31 In healthy donors, less than 10% of all peripheral blood NK cells are usually CD56+.32 Expansion above this low percentage and the abnormal CD16 expression suggests immune system irregularity.
Impairment of the complement system, a cascade of plasma proteins that work to opsonise pathogens and induce inflammatory responses, could also impact the ability of the immune system to eliminate disease-causing agents. Decreased serum complement levels in benzene-exposed workers were reported in two studies,33,34 although a separate study has reported increased expression of complement factor C3 in chronic benzene-poisoned patients.22
A recent study published in 2020 has additionally revealed that benzene-exposed individuals have a higher frequency of promoter methylation in the colony-stimulating factor 3 receptor (CSF3R) gene, which is necessary for the production of neutrophils.35 This high methylation rate was correlated with significant neutrophil reduction in the benzene-exposed participants.
Adaptive immunity
Humoral immunity
Multiple studies have investigated immunoglobulin production in benzene-exposed humans. Two studies have demonstrated a reduction in circulating immunoglobulins, such as IgM, IgA and IgG.36,37 In other cases, however, higher levels of antibodies have been detected in humans exposed to benzene such as IgG, antibodies against heat shock protein 71, as well as platelet-associated immunoglobulins (PAIgs).38–40 The presence of autoantibodies against heat shock protein 71 and PAIgs detected in benzene-exposed humans indicate a degree of autoimmunity.
There is also evidence that B-cell function and activation may be negatively affected by benzene exposure as decreased levels of two soluble B-cell activation markers, sCD27 and sCD30, have been observed in the peripheral blood of 250 benzene-exposed shoe factory workers.41
Cell-mediated immunity
T-cell function
A number of studies have reported evidence of diminished T-cell function. Skewed gene expression ratios of molecules that serve as crucial components of the T-cell receptor such as the T-cell receptor variable β-chain (TCRVβ) and CD3 gene family suggest impaired T-cell receptor signalling in benzene-exposed workers.42,43 Additionally, suppression of T-cell activity is suggested by the decrease in CD80 (B7.1) and CD86 (B7.2) proteins and downregulation of their corresponding genes observed in benzene-exposed gas station workers from Brazil.16,34 These two molecules are required costimulatory molecules expressed on the surface of antigen-presenting cells to effectively activate T-cells.
T-cell subsets
Unique populations of specific T-cell subsets have also been observed in workers occupationally exposed to benzene, such as an increase in HLA-DR+/CD3+ T-cells and CD71+/CD19+ lymphocytes, and a decrease in CD62L+/CD3+ lymphocytes.44 Additionally, a decrease in CD4+/CD25+ T-cells, known as regulatory T-cells (Tregs), has been observed in association with personal exposure to benzene in a sample of non-smoking women.45 These findings indicate an overall increase in number of activated lymphocytes in response to benzene exposure.
TRECs
Two studies have also been performed on T-cell receptor excision circles (TRECs), the presence of which indicates T-cell maturity. Using real-time PCR, one study reported decreased TRECs in peripheral blood mononuclear cells among benzene-exposed workers,46 whereas a separate study found no significant difference in TREC levels between the exposed and control groups.47
Cytokine and chemokine production
Cytokines play an important role in cell signalling within the immune system. PF4 (also known as CXCL4), a gene responsible for encoding a chemokine involved in platelet aggregation and inhibition of haematopoiesis and T-cell function, has been reported to be consistently downregulated in benzene-exposed workers.19,20 This downregulation was confirmed at the proteome level with the observation of a decrease in PF4 (CXCL4) protein as well as a decrease in CXCL7 (CTAPIII) serum levels, a platelet-associated chemokine that stimulates inflammation.48
Haematotoxicity
Many studies investigating the association between benzene exposure and altered blood cell counts demonstrate evidence of haematological suppression in exposed humans. Since the haematotoxicity of benzene exposure is well-characterised,49 we have summarised only major findings in brief below. A more comprehensive summary of the haematotoxicity outcomes, along with study citations, can be found in online supplemental table S6 and Benzene-induced Haematotoxicity Outcomes in the online supplemental.
In particular, WBC counts are reported to be the most affected with a majority of studies reporting lower overall WBCs in benzene-exposed individuals. Additionally, many studies have documented lower absolute lymphocyte levels in benzene-exposed humans with decreased numbers of circulating B-lymphocytes and T-lymphocytes. CD4 + T-lymphocytes were observed to decrease in all studies reviewed. Several studies also reported a decrease in the CD4+/CD8+ T-cell ratio, a test that is most commonly used to indicate suppressed immune function in AIDS (acquired immunodeficiency syndrome).
DISCUSSION AND CONCLUSION
Application of the KCs framework
Using the KCs approach in this review has provided a systematic and uniform way to identify and categorise the scientific findings in literature. A significant advantage of this framework is that a specific mechanism of action is not assumed and thus, we can provide a broad, holistic consideration of the published mechanistic evidence instead of focusing in on specific pathways and hypotheses.
As the tool is still under development, however, questions have emerged about which endpoints, assays and biomarkers represent accurate measures of each of the KCs.50 The lack of biomarkers for chronic inflammation and immunosuppression represents a limitation of our review and could have resulted in the possible exclusion of relevant data as well as the potential inclusion of irrelevant data. At present, there are no standard biomarkers in use that distinguish health-damaging chronic inflammation from acute inflammation.51 Thus, for the purposes of our review, all markers used as indications of chronic inflammation are general inflammatory markers. Efforts are ongoing to identify and develop new approaches and methods to evaluate evidence of the KCs.50
Association of benzene with inflammation and immunosuppression
Although the studies included in this review reported on heterogeneous outcomes such as gene expression, cytokine/chemokine production, serum protein levels, blood cell counts, humoral immunity, and so on, there were a few apparent trends in the data collected and analysed.
First, it is evident that benzene exposure is associated with an immunosuppressive effect on the production of WBCs, specifically lymphocytes such as CD4+ T-cells, B-cells and NK cells. A deficiency of CD4+ T-lymphocytes is especially harmful since helper T-cells are required for almost all adaptive immune responses, activating not only B-cells to produce antibodies, but also CD8+ T-cells and macrophages to destroy infected target cells. This depletion of CD4+ T-cells and impaired cellular immunity is similar to what is observed in AIDS, which can be life threatening and results in susceptibility to a host of other illnesses. Second, a considerable number of studies reported an increase in inflammatory cytokines or other proinflammatory molecules. Although these proinflammatory markers are more conclusive of generalised inflammation, we cannot exclude the potential that generalised or acute inflammation caused by benzene could lead to chronic inflammation downstream. Lastly, the deregulation of soluble factors (ie, TNF-α, IL-6) and promotion of local inflammation caused by benzene could disturb major functions involved in tissue homeostasis, leading to cancer initiation, immune evasion and eventual tumour dissemination.
Thus, the data collected and synthesised in this review provide a mechanistic link between benzene exposure and immunosuppression and suggest a potential association of benzene with chronic inflammation. Generally, benzene appears to activate the innate immune system to cause inflammation, but suppresses the adaptive immune system, which is critical for long-lasting defence and protection against recurrent exposure to irritants such as benzene. Due to the limited amount of studies reporting on each outcome, however, further studies will be required to confirm the connection between benzene exposure and its effects on the immune system.
Inter-relationship between inflammation and immunosuppression
Based on results derived from our systematic review, it is apparent that the effects of KC6 and KC7 are deeply intertwined and cannot be clearly separated. Chronic inflammatory environments form the optimal environment for developing tumours and can induce local immunosuppression.6 Thus, it is possible that we see less evidence of inflammation due to the immunosuppressant effects of benzene on T-lymphocytes, the primary cells that produce proinflammatory cytokines and molecules. However, it is also possible that we see more evidence of inflammation due to potentially higher rates of infection in benzene-exposed populations due to suppressed immune systems. In this case, causality is hard to determine between these two outcomes, similar to ‘the chicken or the egg’ dilemma.
Proposed mechanisms of benzene-induced carcinogenesis based upon review findings
Evidence supports that both chronic inflammatory conditions and immunosuppression can contribute to tumour growth.6 Here, we propose potential mechanisms of how benzene exposure may promote carcinogenesis based on outcomes from the reviewed studies (figure 2).
Figure 2.
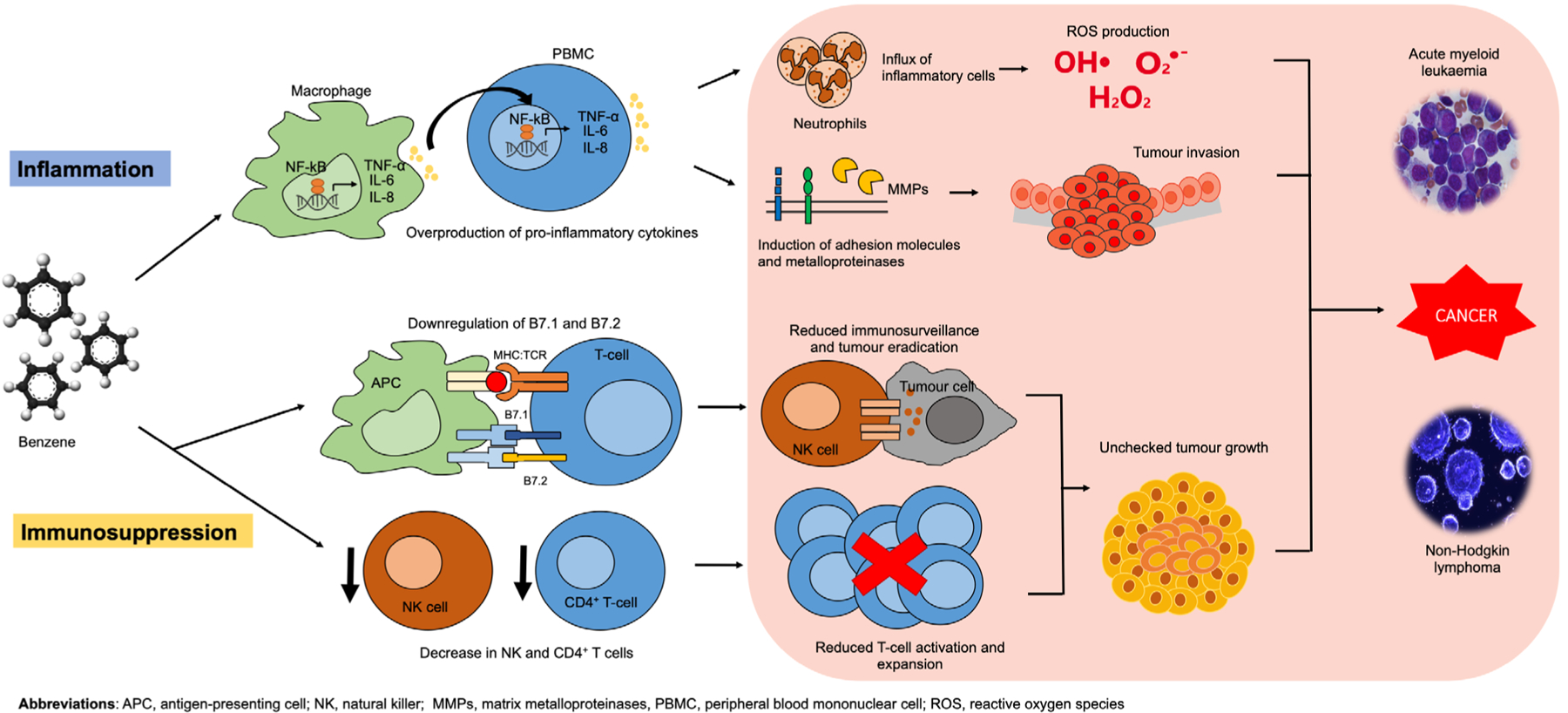
Proposed mechanism of benzene-facilitated carcinogenesis via chronic inflammation and immunosuppression.
Proinflammatory cytokine production and cancer invasion
One potential mechanism of benzene-induced carcinogenesis is based on the link between chronic inflammation and cytokine production. Proinflammatory cytokines are typically considered beneficial since they stimulate the immune system to respond to foreign invaders. Once infection is cleared, the production of proinflammatory cytokines stops. This new mechanism proposes that benzene acts as an inflammatory trigger enabling the activator signal of the cytokine cascade to stay in the ‘on’ mode, resulting in persistent cytokine production. The resulting proinflammatory cytokines that are produced attract neutrophils which are key players in the production of reactive oxygen species, which are carcinogenic in high concentrations.52 Additionally, the cytokines induce matrix metalloproteinases to degrade extracellular matrices and adhesion molecules to facilitate tumour cell release and spread.52 The result is the promotion of tumour invasion.
In the case of benzene, chronically exposed individuals experience a prolonged inflammatory response which dysregulates the production of cytokines and leads to the influx of inflammatory cells, as described above. Normal cells are subsequently damaged, exacerbating tissue destruction. Two key contributing factors, TNF-α and NF-kB, are discussed next.
Cytokine TNF-α
TNF-α, a highly inflammatory cytokine, has been shown to be induced in response to benzene exposure,14,18 and is frequently detected in human cancers as an endogenous tumour promoter.53 The critical molecular link between TNF-α and tumour promotion is activation of the NF-kB pathway, which has been suggested as crucial for malignant conversion.54
NF-kB pathway
54Evidence suggests that the NF-kB pathway is also promoted in benzene-exposed individuals.23,24 Selective deletion of NF-kB or IKKβ, a key intermediary of NF-kB, has been shown to reduce incidence of liver or intestinal tumours, as well as decrease mRNA expression for several proinflammatory cytokines.55 Thus, the NF-kB pathway seems to play a dual role in tumour promotion: preventing death of cells with malignant potential and stimulating production of proinflammatory cytokines in infiltrating lymphoid and myeloid cells. The increased transcription of proinflammatory genes such as IL-1, IL-6, IL-8, TNF-α, COX-2, nitric oxide synthase and vascular endothelial growth factor (VEGF) from the activated NF-kB pathway results in a positive feedback loop that generates a cancer-supporting microenvironment allowing for tumour formation, growth and progression.56
Impaired immunosurveillance and cancer promotion
Reduced immunosurveillance could be another mechanism of benzene-induced cancer promotion. There is abundant experimental evidence supporting the antitumour properties of CD4+ T-cells.57 Additionally, the observed downregulation of important costimulatory ligands such as B7.1 and B7.2 on antigen-presenting cells in response to benzene exposure16,34 suggests overall decreased T-cell activation and expansion. NK cells are also critical for detecting and controlling early signs of cancer. In fact, the first study to coin their name as ‘natural’ killer cells recognised their ability to kill tumour cells without any priming or prior activation.58 Since both cell types are critical for tumour eradication, the decreased number of CD4+ T-cells and NK cells observed in response to benzene exposure suggests an impaired host ability to fight off cancer.
Overall, both potential mechanisms described above play a likely role in benzene-facilitated carcinogenesis; however, areas of uncertainty still exist. It is unclear whether or not benzene causes angiogenesis and whether immune suppressor cells such as myeloid-derived suppressor cells or Tregs accumulate in response to benzene exposure. Therefore, further studies focused on benzene’s effects on the immune system are urgently needed to test these hypotheses.
Future directions
For future studies, we have three recommendations. First, in order to accurately assess evidence of chronic inflammation and immunosuppression, more assays need to be developed and new biomarkers elucidated. Second, additional primary research studies are needed to test the association between benzene and the immune system comprehensively. Few of the studies reviewed in this paper had immune system outcomes as their primary outcome, suggesting that this needs to be more of a priority in future studies. Lastly, it is critical to further explore how the immune system response differs with acute versus chronic exposure to benzene.
In summary, our review has confirmed immunosuppression to be a KC of benzene and provided new, suggestive evidence for chronic inflammation. Based on the findings from this review, we have proposed a potential pathway (figure 2) for how benzene-induced inflammation and immunosuppression connect to cancer. To prove this hypothesis, however, more empirical data from experimental studies are needed.
Supplementary Material
Key messages.
What is already known about this subject?
Previous reviews on benzene, a confirmed leukaemogen, focus on genotoxicity and haematotoxicity as mechanisms of action.
What are the new findings?
Our review indicates that benzene appears to activate the innate immune system to cause inflammation, but suppresses the adaptive immune system.
Benzene induces an immunosuppressive effect on the production of white blood cells, particularly lymphocytes such as CD4+ T-cells, B-cells and natural killer cells.
An increase in proinflammatory biomarkers has been observed in association with benzene exposure even at low doses, implying a potential role of benzene in inducing chronic inflammation.
How might this impact on policy or clinical practice in the foreseeable future?
This review demonstrates that benzene is not only carcinogenic, but also immunotoxic.
The effects of benzene on the immune system, in particular its reduction on both B-cell and T-cell proliferation, suggest that benzene exposure needs to be more stringently monitored and regulated, both in occupational settings and in the environment.
Acknowledgements
The authors thank Sarah Stanley, PhD from the School of Public Health, UC Berkeley for intellectual review and helpful comments.
Funding This project was partially supported by the UC Berkeley Superfund Research Program (P42ES004705 to LZ) and the Society of Toxicology Undergraduate Faculty Research Grant (HG and LZ).
Footnotes
Competing interests None declared.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.IARC. Benzene. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans 2018;120:307. [Google Scholar]
- 2.Epa. benzene: EPA, 2016. Available: https://www.epa.gov/sites/production/files/2016-09/documents/benzene.pdf [Google Scholar]
- 3.Smith MT, Guyton KZ, Gibbons CF, et al. Key characteristics of carcinogens as a basis for organizing data on mechanisms of carcinogenesis. Environ Health Perspect 2016;124:713–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gale RP, Opelz G. Commentary: does immune suppression increase risk of developing acute myeloid leukemia? Leukemia 2012;26:422–3. [DOI] [PubMed] [Google Scholar]
- 6.Wang D, DuBois RN. Immunosuppression associated with chronic inflammation in the tumor microenvironment. Carcinogenesis 2015;36:1085–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Makgoeng SB, Bolanos RS, Jeon CY, et al. Markers of immune activation and inflammation, and non-Hodgkin lymphoma: a meta-analysis of prospective studies. JNCI Cancer Spectr 2018;2:pky082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- 10.Karin M, Clevers H. Reparative inflammation takes charge of tissue regeneration. Nature 2016;529:307–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Angelis C, Galdiero M, Pivonello C, et al. The environment and male reproduction: the effect of cadmium exposure on reproductive function and its implication in fertility. Reprod Toxicol 2017;73:105–27. [DOI] [PubMed] [Google Scholar]
- 12.Meirow Y, Baniyash M. Immune biomarkers for chronic inflammation related complications in non-cancerous and cancerous diseases. Cancer Immunol Immunother 2017;66:1089–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo X, Zhong W, Chen Y, et al. Benzene metabolites trigger pyroptosis and contribute to haematotoxicity via TET2 directly regulating the Aim2/Casp1 pathway. EBioMedicine 2019;47:578–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dutta A, Roychoudhury S, Chowdhury S, et al. Changes in sputum cytology, airway inflammation and oxidative stress due to chronic inhalation of biomass smoke during cooking in premenopausal rural Indian women. Int J Hyg Environ Health 2013;216:301–8. [DOI] [PubMed] [Google Scholar]
- 15.Elango N, Kasi V, Vembhu B, et al. Chronic exposure to emissions from photocopiers in copy shops causes oxidative stress and systematic inflammation among photocopier operators in India. Environ Health 2013;12:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moro AM, Brucker N, Charão MF, et al. Early hematological and immunological alterations in gasoline station attendants exposed to benzene. Environ Res 2015;137:349–56. [DOI] [PubMed] [Google Scholar]
- 17.Jørgensen KM, Færgestad Mosleth E, Hovde Liland K, et al. Global gene expression response in peripheral blood cells of petroleum workers exposed to Sub-Ppm benzene levels. Int J Environ Res Public Health 2018;15. doi: 10.3390/ijerph15112385. [Epub ahead of print: 27 Oct 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samadi MT, Shakerkhatibi M, Poorolajal J, et al. Association of long term exposure to outdoor volatile organic compounds (BTXS) with pro-inflammatory biomarkers and hematologic parameters in urban adults: a cross-sectional study in Tabriz, Iran. Ecotoxicol Environ Saf 2019;180:152–9. [DOI] [PubMed] [Google Scholar]
- 19.Forrest MS, Lan Q, Hubbard AE, et al. Discovery of novel biomarkers by microarray analysis of peripheral blood mononuclear cell gene expression in benzene-exposed workers. Environ Health Perspect 2005;113:801–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McHale CM, Zhang L, Lan Q, et al. Changes in the peripheral blood transcriptome associated with occupational benzene exposure identified by cross-comparison on two microarray platforms. Genomics 2009;93:343–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Darash-Yahana M, Gillespie JW, Hewitt SM, et al. The chemokine CXCL16 and its receptor, CXCR6, as markers and promoters of inflammation-associated cancers. PLoS One 2009;4:e6695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Z, Li P, Lin D, et al. Proteome analysis of the potential serum biomarkers for chronic benzene poisoning. Environ Toxicol Pharmacol 2018;60:157–64. [DOI] [PubMed] [Google Scholar]
- 23.Fenga C, Gangemi S, Giambò F, et al. Low-Dose occupational exposure to benzene and signal transduction pathways involved in the regulation of cellular response to oxidative stress. Life Sci 2016;147:67–70. [DOI] [PubMed] [Google Scholar]
- 24.Yang J, Bai W, Niu P, et al. Aberrant hypomethylated STAT3 was identified as a biomarker of chronic benzene poisoning through integrating DNA methylation and mRNA expression data. Exp Mol Pathol 2014;96:346–53. [DOI] [PubMed] [Google Scholar]
- 25.Bogadi-Sare A, Turk R, Karacić V, et al. Red blood cell glycerol lysis and hematologic effects in occupational benzene exposure. Toxicol Ind Health 1997;13:485–94. [DOI] [PubMed] [Google Scholar]
- 26.Ray MR, Roychoudhury S, Mukherjee S, et al. Occupational benzene exposure from vehicular sources in India and its effect on hematology, lymphocyte subsets and platelet P-selectin expression. Toxicol Ind Health 2007;23:167–75. [DOI] [PubMed] [Google Scholar]
- 27.Lehmann I, Rehwagen M, Diez U, et al. Enhanced in vivo IgE production and T cell polarization toward the type 2 phenotype in association with indoor exposure to VOC: results of the LARS study. Int J Hyg Environ Health 2001;204:211–21. [DOI] [PubMed] [Google Scholar]
- 28.Chen L, Grabowski KA, Xin J-P, et al. Il-4 induces differentiation and expansion of Th2 cytokine-producing eosinophils. J Immunol 2004;172:2059–66. [DOI] [PubMed] [Google Scholar]
- 29.Nevala WK, Vachon CM, Leontovich AA, et al. Evidence of systemic Th2-driven chronic inflammation in patients with metastatic melanoma. Clin Cancer Res 2009;15:1931–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santiago F, Lima S, Pinheiro T, et al. Benzene poisoning, clinical and blood abnormalities in two Brazilian female gas station attendants: two case reports. BMC Res Notes 2017;10:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boscolo P, Di Gioacchino M, Sabbioni E, et al. Lymphocyte subpopulations, cytokines and trace elements in asymptomatic atopic women exposed to an urban environment. Life Sci 2000;67:1119–26. [DOI] [PubMed] [Google Scholar]
- 32.Poli A, Michel T, Thérésine M, et al. CD56bright natural killer (NK) cells: an important NK cell subset. Immunology 2009;126:458–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smolik R, Grzybek-Hryncewicz K, Lange A, et al. Serum complement level in workers exposed to nebzene, toluene and xylene. Int Arch Arbeitsmed 1973;31:243–7. [DOI] [PubMed] [Google Scholar]
- 34.Sauer E, Gauer B, Nascimento S, et al. The role of B7 costimulation in benzene immunotoxicity and its potential association with cancer risk. Environ Res 2018;166:91–9. [DOI] [PubMed] [Google Scholar]
- 35.Ren J-C, Wang T, Wu H, et al. Promoter hypermethylation in CSF3R induces peripheral neutrophil reduction in benzene-exposure poisoning. Environ Mol Mutagen 2020. doi: 10.1002/em.22382. [Epub ahead of print: 23 Apr 2020]. [DOI] [PubMed] [Google Scholar]
- 36.Uzma N, Kumar BS, Hazari MAH. Exposure to benzene induces oxidative stress, alters the immune response and expression of p53 in gasoline filling workers. Am J Ind Med 2010;53:1264–70. [DOI] [PubMed] [Google Scholar]
- 37.Kirkeleit J, Ulvestad E, Riise T, et al. Acute suppression of serum IgM and IgA in tank workers exposed to benzene. Scand J Immunol 2006;64:690–8. [DOI] [PubMed] [Google Scholar]
- 38.Bogadi-Sare A, Zavalic M, Trosić I, et al. Study of some immunological parameters in workers occupationally exposed to benzene. Int Arch Occup Environ Health 2000;73:397–400. [DOI] [PubMed] [Google Scholar]
- 39.Huang J, Zhao M, Wang P, et al. Effects of low concentrations of benzene exposure on levels of platelet-associated antibodies and platelet parameters. J Occup Environ Med 2014;56:e92–7. [DOI] [PubMed] [Google Scholar]
- 40.Wu T, Yuan Y, Wu Y, et al. Presence of antibodies to heat stress proteins in workers exposed to benzene and in patients with benzene poisoning. Cell Stress Chaperones 1998;3:161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bassig BA, Zhang L, Vermeulen R, et al. Comparison of hematological alterations and markers of B-cell activation in workers exposed to benzene, formaldehyde and trichloroethylene. Carcinogenesis 2016;37:692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li B, Li Y, Chen S, et al. The T-cell receptor Vbeta gene repertoire and clonal expansion from peripheral blood T cells in benzene-exposed workers in China. Hematology 2009;14:106–10. [DOI] [PubMed] [Google Scholar]
- 43.Li B, Niu Y, Liu S, et al. A change in CD3γ, CD3δ, CD3ϵ, and CD3ζ gene expression in T-lymphocytes from benzene-exposed and benzene-poisoned workers. J Immunotoxicol 2012;9:160–7. [DOI] [PubMed] [Google Scholar]
- 44.Biró A, Pállinger E, Major J, et al. Lymphocyte phenotype analysis and chromosome aberration frequency of workers occupationally exposed to styrene, benzene, polycyclic aromatic hydrocarbons or mixed solvents. Immunol Lett 2002;81:133–40. [DOI] [PubMed] [Google Scholar]
- 45.Baïz N, Slama R, Béné M-C, et al. Maternal exposure to air pollution before and during pregnancy related to changes in newborn’s cord blood lymphocyte subpopulations. The EDEN study cohort. BMC Pregnancy Childbirth 2011;11:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li B, Li YQ, Yang LJ, et al. Decreased T-cell receptor excision DNA circles in peripheral blood mononuclear cells among benzene-exposed workers. Int J Immunogenet 2009;36:107–11. [DOI] [PubMed] [Google Scholar]
- 47.Lan Q, Zhang L, Hakim F, et al. Lymphocyte toxicity and T cell receptor excision circles in workers exposed to benzene. Chem Biol Interact 2005;153–154:111–5. [DOI] [PubMed] [Google Scholar]
- 48.Vermeulen R, Lan Q, Zhang L, et al. Decreased levels of CXC-chemokines in serum of benzene-exposed workers identified by array-based proteomics. Proc Natl Acad Sci U S A 2005;102:17041–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith MT. Advances in understanding benzene health effects and susceptibility. Annu Rev Public Health 2010;31:133–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith MT, Guyton KZ, Kleinstreuer N, et al. The key characteristics of carcinogens: relationship to the hallmarks of cancer, relevant biomarkers, and assays to measure them. Cancer Epidemiol Biomarkers Prev 2020:cebp.1346.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Furman D, Campisi J, Verdin E, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med 2019;25:1822–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dinarello CA. The paradox of pro-inflammatory cytokines in cancer. Cancer Metastasis Rev 2006;25:307–13. [DOI] [PubMed] [Google Scholar]
- 53.Balkwill F Tumor necrosis factor or tumor promoting factor? Cytokine Growth Factor Rev 2002;13:135–41. [DOI] [PubMed] [Google Scholar]
- 54.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 2005;7:211–7. [DOI] [PubMed] [Google Scholar]
- 55.Pikarsky E, Porat RM, Stein I, et al. Nf-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature 2004;431:461–6. [DOI] [PubMed] [Google Scholar]
- 56.Hendrayani S-F, Al-Harbi B, Al-Ansari MM, et al. The inflammatory/cancer-related IL-6/STAT3/NF-κB positive feedback loop includes AUF1 and maintains the active state of breast myofibroblasts. Oncotarget 2016;7:41974–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Corthay A, Skovseth DK, Lundin KU, et al. Primary antitumor immune response mediated by CD4+ T cells. Immunity 2005;22:371–83. [DOI] [PubMed] [Google Scholar]
- 58.Kiessling R, Klein E, Wigzell H. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol 1975;5:112–7. [DOI] [PubMed] [Google Scholar]
- 59.Rothman N, Smith MT, Hayes RB, et al. An epidemiologic study of early biologic effects of benzene in Chinese workers. Environ Health Perspect 1996;104 Suppl 6:1365–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haro-García LC, Juárez-Pérez CA, Aguilar-Madrid G, et al. Production of IL-10, TNF and IL-12 by peripheral blood mononuclear cells in Mexican workers exposed to a mixture of benzene-toluene-xylene. Arch Med Res 2012;43:51–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


