Abstract
Over the years, conventional cancer treatments, such as chemotherapy with only a limited specificity for tumors, have undergone significant improvement. Moreover, newer therapies such as immunotherapy have undergone a revolution to stimulate the innate as well as adaptive immune responses against the tumor. However, it has been found that tumors can be selectively colonized by certain bacteria, where they can proliferate, and exert direct oncolytic effects as well as stimulating the immune system. Bacterial-mediated cancer therapy (BMCT) is now one example of a hot topic in the antitumor field. Salmonella typhimurium is a Gram-negative species that generally causes self-limiting gastroenteritis in humans. This species has been designed and engineered in order to be used in cancer-targeted therapeutics. S. typhimurium can be used in combination with other treatments such as chemotherapy or radiotherapy for synergistic modification of the tumor microenvironment. Considerable benefits have been shown by using engineered attenuated strains for the diagnosis and treatment of tumors. Some of these treatment approaches have received FDA approval for early-phase clinical trials. This review summarizes the use of Salmonella bacteria for cancer therapy, which could pave the way towards routine clinical application. The benefits of this therapy include an automatic self-targeting ability, and the possibility of genetic manipulation to produce newly engineered attenuated strains. Nevertheless, Salmonella-mediated anticancer therapy has not yet been clinically established, and requires more research before its use in cancer treatment.
Keywords: bacteria-mediated cancer therapy, Salmonella species, cancer, drug delivery, therapy
Introduction
Recently, bacteria-mediated cancer therapy (BMCT) has attracted attention, in which different attenuated bacteria have been created using genetic engineering, and are capable of targeting tumors and exerting various antitumor effects (1). For more than a century bacteria have been investigated as a therapeutic approach to cancer (1). The bone surgeon William B. Coley was the first to report that injection of a preparation of heat-killed Streptococcus pyogenes into patients with inoperable bone and soft-tissue sarcomas could produce tumor regression (2). The treatment sometimes led to successful shrinkage of the tumors and extended the survival of the patients (3). Nevertheless, over the years, the lack of understanding of the basic mechanisms, and inadequate procedures and methods for diagnosis and quantification of the immune responses, resulted in a disappointing level of acceptance by the medical community. Nowadays, due to advances in medical technology (particularly in genetic engineering), BMCT has attracted more attention, and a range of different species of bacteria, including Streptococcus (4), Bifidobacterium (5), Clostridium (6), and Salmonella species (7, 8), have been investigated.
Salmonella enterica serovar Typhimurium (S. typhimurium) is capable of growth in both aerobic and anaerobic environments, and is therefore able to target and colonize both non-hypoxic and hypoxic tumors, along with metastatic tumor deposits which are accessible through the circulatory system. S. typhimurium can serve as a novel antitumor therapy to fill an existing gap, because radiotherapy and chemotherapy are known to be less effective in necrotic and hypoxic regions of tumors. Preferential accumulation and colonization of S. typhimurium takes place in tumors, leading to tumor-to-normal tissue ratios being measured, which can exceed 1,000–10,000 to 1. Salmonella species have shown the highest efficiency as an antitumor bacterial strain in the empirical models of cancer that have been tested up to now (9). Salmonella-based immunotherapy can be thought of as possessing “Trojan horse” properties, and can also be used in tumors, which have developed resistance to conventional treatments (1, 3, 10). Why do S. typhimurium bacterial strains possess tumor-targeting as well as tumor-destroying properties? The present review discusses these questions and provides some possible answers.
Moreover, S. typhimurium-based therapy can be effectively combined with radiotherapy (11) or chemotherapy (12). Salmonella-mediated antitumor therapy could be a new and effective approach in the treatment of different cancers.
Treatment of Cancer Using Bacteria
One of the challenges in conventional cancer therapy is that anticancer drugs generally show restricted penetration into tumor tissue. Not only chemotherapy, but also other biological treatments such as monoclonal antibodies and cytokines, are limited by the passive transport of the molecules into the tumor. This consideration not only limits their effectiveness, but if the doses are increased to compensate, it leads to higher risks of toxicity (13).
Recently, some new therapeutic approaches have been developed based on microorganisms. Talimogene laherparepvec (T-VEC; Imlygic™) was approved by the US FDA for the treatment of advanced melanoma. T-VEC is a genetically modified herpes simplex virus, type 1. T-VEC was attenuated by the deletion of the herpes neurovirulence viral genes and enhanced for immunogenicity by the deletion of the viral ICP47 gene (14).
However, bacterial therapy relies on the intrinsic properties of living organisms to penetrate and accumulate in tumors, which are not available with traditional methods, making bacterial therapy promising for the future (13).
In the 19th century, bacterial therapy was known for some adverse effects related to over-stimulation of the immune system, including fever, septic shock, and even death (15, 16). Advances in genetic engineering have resulted in the ability to use genetically modified bacteria, thus reducing their off-target toxicity for the treatment of cancer. The ability to manipulate the bacterial genome has made them a favorable option compared to other microorganisms (17).
BMCT has attracted much attention because of its considerable benefits. For instance, bacteria have the capability to penetrate into cancer tissue, showing selectivity towards tumor cells based on specific chemical signals that are enriched within the tumor microenvironment (18, 19). Moreover, bacteria can function as vectors for transport of therapeutic molecules and as drug delivery vehicles. Rationally designed bacteria can be controlled from the outside by administration of agents that only affect the microorganisms themselves and not the host tissue (20, 21). Interestingly, the concept of “artificial medical bacteria” could also contribute to the process of diagnosis (detection of molecules or tumor markers associated with specific diseases), and to the preparation of smart therapeutics (by responding to chemical stimuli by releasing therapeutic agents) (22). The design and construction of “living biological robots” may be possible with the use of synthetic biology to modify bacteria. A wide variety of agents can be integrated into laboratory-designed bacteria, such as genes, proteins, and drugs which could originally come from different organisms, but could now display satisfactory safety and efficacy against human cancer (23). Due to the tumor-tropic properties of bacteria they can be administered systemically. Accordingly, lower concentrations are required, and complicated procedures for purification or formulation may not be needed (22). Nevertheless, BMCT does have some limitations, such as biosafety, genetic instability, and the possible interaction of the bacteria with therapeutic drugs. The payload capacity of bacteria may be limited, leading to oncolytic viruses being preferred as a cancer therapy. Nevertheless, oncolytic viruses also have their own limitations, such as easy recognition by the immune system, and questions about an adequate level of biosafety (24, 25).
Salmonella Bacteria and Treatment of Cancer
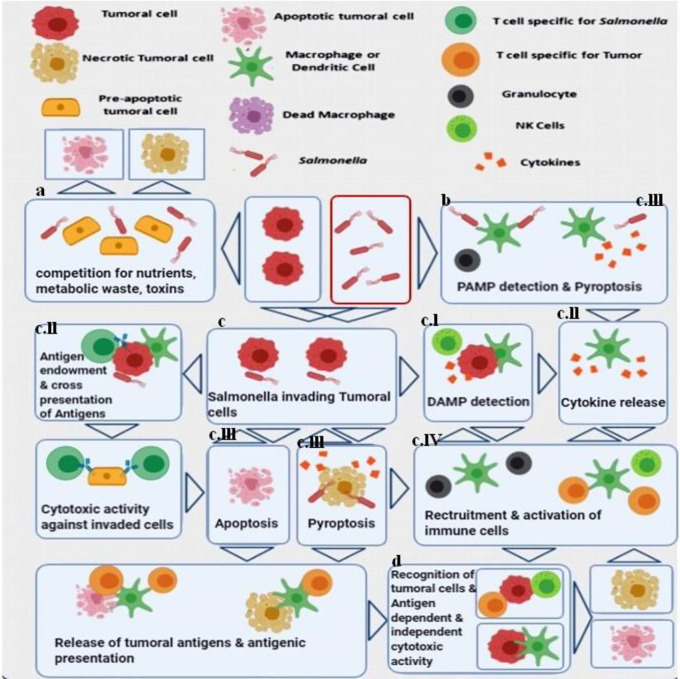
Research has been performed on different mutant strains of S. typhimurium that have been investigated for anti-cancer applications. Laboratory strains of S. typhimurium have been modified to reduce their toxicity to normal organs, while increasing their specific targeting properties for tumors along with enhancing their antitumor activity (26–28). Figure 1 displays a complicated series of processes that may be involved in the anti-tumor effectiveness of Salmonella species.
Figure 1.
Major antitumor mechanisms stimulated by Salmonella (S. typhimurium). Both bacteria-mediated direct cytotoxicity and immune system-mediated indirect tumor cell death have been proposed. (A) In the tumor microenvironment, bacterial infection inhibits the growth of the tumor and results in considerable cell death. (B) The recognition of pathogen-related molecular patterns (PAMP) on the bacterial cells by receptors expressed on immune cells, triggers the release of cytokines and the recruitment of leukocytes that can initiate anti-tumor immune responses (29–31). (C) S. typhimurium uses a Type III secretion system which leads to the release of different factors into cancer cells, and the bacteria can also be internalized and undergo intra-cellular replication (32, 33). (C.I) Cell stress responses are induced by invasive Salmonella cells mediated via danger-associated molecular patterns (DAMP) that also act as signals to the immune system. (C.II) At the same time, this process together with the released cytokines leads to the presentation of bacterial antigens as well as cancer specific antigens, to the adaptive immune system leading to the production of cancer-antigen specific T-cells that can recognize and kill the cancer cells as well as the bacteria (34). An immunological synapse occurs between the bacteria, the cancer cells and the host dendritic cells leading to cross-presentation of tumor antigens, resulting in an antigen-specific anti-cancer immune response (35, 36). (C.III) Salmonella bacteria have the potential to directly cause cancer cell death through induction of apoptosis or pyroptosis. Pyroptosis is a form of programmed inflammatory cell death, characterized by activation of caspase 1 and the inflammasomes, and secretion of IL-1β as well as IL-18. It is also characterized by cell rounding and detachment, reorganization of the cytoskeleton, deformation of the nucleus, together with rupture of the cell membrane and release of additional inflammatory signals (37–39). The description of pyroptosis first took place in macrophages that died rapidly under certain conditions, which is particularly important in cancer immunotherapy, since tumor-associated macrophages (TAM) are considered to possess immune-suppressive properties. Reduction of TAMs can be considered as another aspect of the S. typhimurium anti-tumor activity. The dead cancer cells can liberate tumor antigens, while the surrounding cancer cells are infected by the released bacteria. (C.IV) During pyroptosis, immune cell recruitment and activation can be triggered by the pro-inflammatory cytokines IL-1β and IL-18 (37, 38, 40). (D) The convergence of several different mechanisms enables recognition of tumor antigens, leading to stimulation of both antigen-dependent and antigen-independent cell killing. The proteasomal degradation of S. typhimurium proteins within the cancer cell cytosol, leads to production of bacterial peptides that can be presented to cytotoxic lymphocytes through MHC I (34, 41). This figure is adapted from (42).
Salmonella Bacteria as Delivery Systems for Anti-Tumor Agents
Ovarian cancer is a gynecological tumor with considerable morbidity and mortality (43, 44). Chemotherapy and surgery are the main treatments for ovarian cancer, but because of multidrug resistance (MDR) these approaches are not completely successful. Tumors develop MDR as a defense mechanism against cytotoxic drugs, which causes chemotherapy failure in many patients. The most important mechanism of MDR is the production of the plasma membrane glycoprotein (170 KDaP-gp) that binds to ATP and can then pump cytotoxic drugs out of the cells, thus reducing the concentration to a sublethal level. P-gp is a highly conserved membrane glycoprotein encoded by the MDR1 gene. In one study, Deng et al., assessed the effects of attenuated Salmonella typhi acting as a tumor-targeted vector to deliver small interfering RNA (siRNA) against the multidrug-resistance gene (MDR1) (45). This was tested in the cisplatin-resistant ovarian cancer cell line SKOV-3/DDP, to increase cisplatin sensitivity. For this purpose a MDR1 siRNA expression plasmid was constructed that contained short hairpin RNA (shRNA) against the MDR1 gene, which was loaded into cells of the S. typhi strain (SL7207). MDR1 gene expression was measured with real-time PCR and protein expression by Western blotting. BALB/c nude mice were subcutaneously injected with SKOV-3/DDP cells and orally inoculated with Salmonella containing MDR1 siRNA plasmid, and then intraperitoneally injected with cisplatin. It was found that the high level of MDR1 in SKOV-3/DDP cells was reduced by the bacteria, and the cisplatin resistance was reversed. The tumor growth in mice was slower when treated with DDP. Attenuated Salmonella bacteria could be used for delivery of RNA interference as a component of cancer treatment (45).
In one study, tumor-bearing nude mice that were orally administered recombinant Salmonella displayed a slower tumor growth and became more sensitive to DDP. Deng et al. established a cisplatin (DDP)-resistant ovarian cancer cell line SKOV-3/DDP by treatment with gradually increasing concentrations of cisplatin. They found that compared with the parental cell line, the DDP-resistant SKOV-3/DDP cells expressed a much higher level of MDR1. The expression of MDR1 in SKOV-3/DDP cells infected with the Salmonella strain bearing MDR1 siRNA plasmid in vitro was found to be down-regulated and the DDP tolerance of these cells was reversed (45).
Epidermal growth factor receptor (EGFR) is often over-expressed in tumor cells (46). Transforming growth factor alpha (TGFα) is a naturally occurring ligand for the EGFR. PE38 is a recombinant immunotoxin prepared as a conjugate between TGFα and a laboratory-engineered Pseudomonas exotoxin A. The PE38 immunotoxin is under investigation for EGFR-positive cancers, for example brain tumors (47, 48). Pseudomonas exotoxin A suppresses protein production in mammalian cells (49). PE38 binds to EGFR-expressing cancer cells due to the TGFα fragment present in the recombinant fusion protein. Studies have shown that PE38 has a toxic effect on EGFR-expressing tumor cells, both in vitro as well as against tumors in a mouse model (50, 51). However, systemic injection of TGFα-PE38 has shown dose-dependent hepatotoxicity (51). In this study, the ΔppGpp Salmonella mutant that expressed recombinant TGFα-PE38 was investigated. The ΔppGpp Salmonella mutant neither attacks nor proliferates within mammalian cells (52, 53), but exerts its anti-tumoral effects by inducing the expression of pro-inflammatory cytokines from macrophages and neutrophils, such as IL-1β and TNFα (54). In this study, a plasmid was constructed with DNA encoding TGFα-PE38 and inserted into Salmonella cells. The mouse tumor experiment employed colon or breast tumors with high levels of EGFR expression. They used an inducible system based on the PBAD promoter from E. coli (induced by intraperitoneal injection of L-arabinose) (55). In order to export the TGFα-PE38 recombinant protein from the Salmonella cells, an engineered phage lysis system (56) or a bacterial membrane transport signal fused to the protein were investigated. Both approaches appeared to be equally effective. They found that TGFα-PE38 produced by the bacteria slowed tumor growth compared to non-engineered Salmonella alone (56). Treatment of the tumor cells with TGFα-PE38 increased the expression of EGFR in the cells and induced tumor cell apoptosis. This research showed that the effectiveness of immunotoxins for tumor treatment could be significantly improved if they were delivered using bacteria (57).
Solid tumors are often highly hypoxic in comparison to healthy tissue, and are therefore readily colonized by attenuated S. typhimurium. Anti-tumor effects can be obtained if the bacteria express cytotoxic genes or by invasive bacterial infection leading to apoptosis or necrosis of cancer cells (58, 59). However the antitumor effects of S. typhimurium in human tumors have been relatively weak, compared to those seen in tumors in animal models (60, 61). This disparity is likely due to the considerable complexity of human immune system, and possibly to over-attenuation of S. typhimurium. Salmonella can also induce apoptosis or necrosis of endothelial cells within the tumor microvessels, as well as a decrease in the level of VEGF and the number of CD31 positive cells. This anti-angiogenesis effect can further increase the hypoxia within the tumor (59, 62). Therefore colonization of the tumor by Salmonella could create a positive feedback loop leading to the creation of a more complete anaerobic environment within the tumor. Research has shown that the use of Salmonella in combination with an angiogenesis inhibitor showed better performance in a mouse tumor model of pancreatic cancer patient-derived orthotopic xenografts (PDOX) (63).
Traditional Chinese medicine has used the medicinal herb called Tripterygium wilfordii Hook F for centuries to treat inflammatory and autoimmune diseases (64). This herb contains a diterpenoid triepoxide called Triptolide, which has anti-inflammatory and anti-angiogenesis properties. Triptolide has been investigated for suppressing the growth and metastasis of solid tumors such as melanoma (65–67). Moreover, combinations of Triptolide as an anti-angiogenic drug (68) with chemotherapy drugs, such as cisplatin have shown synergistic benefits (69). S. typhimurium VNP20009 has been tested in experimental models (60, 61), but did not show convincing therapeutic effects (60, 68). In one study, Chen et al. attempted to overcome the poor tumor colonization of attenuated Salmonella (70). They combined the VNP20009 Salmonella strain with administration of tryptolide against murine melanoma tumors. It was found that tryptolide combined with VNP20009 was superior to VNP20009 alone in reducing the number of neutrophils in the melanoma, and therefore improved the colonization of Salmonella and produced extensive necrosis. The combination therapy reduced VEGF expression and inhibited angiogenesis and melanoma growth. They concluded that combination therapy with triptolide enhanced the antitumor effects of VNP20009 by inhibiting angiogenesis and stimulating the host immune response (70).
5-Fluorocytosine (5-FC) is a non-toxic cytosine analog that can be converted into 5-fluorouracil (5-FU) by the action of the enzyme cytosine deaminase. The conversion of 5-FU into 5-F-dUMP inside the cell blocks thymidylate synthase activity. 5-FUTP and 5-FdUTP can be produced by the insertion of enzymes into RNA and DNA (71) thus leading to cell death (72, 73). In one study, Mesa-Pereir et al. investigated the cytotoxic activity of a Salmonella strain equipped with a salicylate-inducible expression apparatus, which could modulate the expression of cytosine deaminase (74). Firstly, they introduced a binding site for the T7 phage gene 10 ribosome sequence and replaced the original GUG start codon with AUG to allow the inclusion of an E. coli coda gene under the Pm promoter. A 5-FU resistant Salmonella strain was created to increase the production of cytosine deaminase within the bacteria. A purD mutation in the producer strain was further developed to control the intracellular proliferation in the presence of adenine as well as to prevent intracellular death of Salmonella. This approach allowed the use of cytosine deaminase produced by Salmonella strains to kill tumor cells in the presence of 5-FU (74).
In cancer cells, as well as blood vessels that express the αvβ3 integrin during angiogenesis, the RGD peptide can specifically bind to the αvβ3 integrin. Bacteria can be engineered to express the peptide RGD-4C associated with the outer membrane protein A (OmpA) on the bacterial cell surface to increase the efficiency of tumor targeting (75). In this study, the tumor tropism of S. typhimurium containing a RGD peptide sequence (ACDCRGDCFCG) in the external region of OmpA was investigated. Salmonella with the RGD sequence strongly bound to αvβ3 expressing tumor cells, whereas its binding to cells without αvβ3 was weak. In vivo studies showed that the use of RGD-expressing Salmonella could produce regression in αvβ3-over-expressing cancer xenografts, human breast cancer (MDA-MB-231) and human melanoma (MDA-MB-435), and prolong survival in mice. Therefore, the expression of RGD peptides on the surface of Salmonella could improve tumor targeting (75).
An enzyme called asparaginase (L-ASNase), derived from E. coli, has been used to treat acute lymphocytic leukemia (76). The function of L-ASNase is to catalyze the conversion of asparagine to aspartic acid, and to some extent glutamine to glutamate (77). Both reactions may be helpful for the treatment of cancer (78). When the asparagine concentration is lowered, uncharged tRNA activates the serine/threonine kinase GCN2 (79). eIF2á, is then phosphorylated by GCN2, and in turn inhibits the guanine nucleotide exchange factor eIF2B. This can block eIF2 recycling and inhibit global protein synthesis (80). If the resynthesis of asparagine is insufficient to keep the tRNA fully charged, the reduced rate of total protein synthesis triggers cell death due to apoptosis (81).
Kim et al. (82) devised a treatment for acute lymphoblastic leukemia using L-ASNase expressed in Salmonella bacteria. The araBAD E. coli inducible promoter was used to design Salmonella cells capable of delivering L-ASNase to tumor tissues (82). Table 1 lists various anti-tumor agents that have been targeted or delivered by Salmonella strains.
Table 1.
Anti-tumor agents that have been delivered or targeted by Salmonella strains.
| Salmonella species | Type of cancer | Anti-tumor agent | Anti-cancer effect | Model | Type of cell line | Ref |
|---|---|---|---|---|---|---|
| Salmonella typhi SL7207 | Ovarian cancer | RNA interference | Reduction of MDR1 | SKOV-3/DDP tumor-bearing mice | SKOV-3/DDP | (83) |
| Salmonella typhimurium | shIDO | Apoptosis of tumor | Mice bearing B16F10 tumors | (84) | ||
| Salmonella typhimurium VNP20009 | Lung cancer | Sox2shRNA | Anti-angiogenesis | Mice bearing A549 tumors | (85) | |
| Salmonella typhimurium | Tumor | PLK1 | Nude mice bearing human MDA-MB-231 xenografts | (86) | ||
| Salmonella typhimurium | Prostate tumor | Stat3-specific | Treatment of primary and metastatic cancer | C57BL6 mice bearing an implanted prostate tumor | (87) | |
| Salmonella typhimurium | Hepatocellular carcinoma | Stat3-shRNA | Ectopic transplanted model of C57BL6 mice | (88) | ||
| Salmonella typhimurium SC36 | Melanomas or pulmonary tumor | PNR | Apoptosis | Mice bearing melanomas or pulmonary tumors | (89) | |
| Salmonella typhimurium SC36 | Mammary carcinoma | PNR | Suicide gene/prodrug therapy | Mice bearing mammary carcinoma | (90) | |
| Salmonella typhimurium VNP20009 | Melanoma | PNR | Delayed tumor growth; increased CD8(+) T-cell infiltration | Tumor-implanted mice | (70) | |
| Salmonella typhimurium Dam(-), AroA(-) | Breast cancer | Legumain | Suppressing tumor angiogenesis | BALB/c mice | D2F2 | (91) |
| Salmonella typhimurium -lux | Hepatocellular and colon cancer | Mouse alpha-fetoprotein (AFP) gene | Promote protective immunity | BALB/c mice C57/J mice |
CT26 | (92) |
| Salmonella typhimurium SL7207 | Prostate cancer | Prostate stem cell antigen (PCSA) | Generated specific antitumor immune responses | C57 BL/6 mice | TRAMPC1 | (93) |
| Salmonella typhimurium | Lung carcinoma | DNA vaccine (pcDNA3.1-FLK1(ECD) | Prevented recurrence and metastasis | Lewis lung carcinoma model in mice | (94) | |
| Salmonella typhimurium SL7207 | Neuroblastoma | DNA vaccines | Improved cellular anti-NB immune response | Mice | (95) | |
| Salmonella typhimurium, MvP728 (purD/htrA) | Colon carcinoma and orthotopic DBT glioblastoma | Survivin | Regulated T3SS of Salmonella and NKT ligands | Female BALB/c mice | CT26 | (96) |
| Salmonella SL3261 | Colorectal cancer | 4-1BBL | Enhanced T cell immunity | Male Sprague Dawley (SD) rat model of colorectal tumor | (97) | |
| Salmonella | Lung adenocarcinoma | RBM5 | Apoptosis | BALB/c nude mice bearing A549 tumors | A549 | (98) |
| Salmonella typhimurium | Mammary carcinoma | TRAIL | Reduced tumor growth | Mice | 4T1 | (99) |
| Salmonella choleraesuis | Melanoma and bladder tumor | Endostatin | Decreased intra tumoral microvessel density, reduced VEGF, CD8(+) T cell infiltration | BALB/c mice bearing 4T1 tumors | (100) | |
| Salmonella typhimurium | Hepatocarcinoma | Stat3-siRNA and endostatin | Reduced cell proliferation, increased apoptosis, inhibited angiogenesis | HCC model in C57BL/6 mice | (101) | |
| Salmonella typhimurium | Gastric cancer | Apoptin | Inhibited tumor growth | Mice | (102) | |
| Salmonella typhimurium | Tumor | Diphtheria toxin A chain (DTA) | PLK1 reduction | Nude mice bearing human MDA-MB-231 xenografts | (103) | |
| Salmonella typhimurium | B-cell lymphoma | CD40L | Prevented tumor growth | BALB/c mice | (104) | |
| Salmonella | Melanoma | IL-18 | Production of IFN gamma | Melanoma‐bearing mice | (105) | |
| Salmonella | Melanoma | IL-4 | Production of IFN gamma | Melanoma‐bearing mice | (105) | |
| Salmonella typhimurium SL3261 | Tumor | hGM-CSF | Increased cytotoxic T cells | BALB/c and C57BL/6 mice | (106) | |
| Salmonella typhimurium SL3261 | Tumor | hIL-12& mIL-12 | Increased cytotoxic T cells | BALB/c and C57BL/6 mice | (106) | |
| Salmonella typhimurium | Lymphoma | Herpes simplex virus thymidine kinase | Lymphoma reduced | Tumor-bearing C57/Bl6J-OlaHsd mice | (107) | |
| Salmonella | Tumor | Cytosine deaminase | Inhibition of tumor growth | (74) | ||
| Salmonella typhimurium TAPET-CD | Tumor | Cytosine deaminase | Inhibition of tumor growth | Tumor-bearing mice | (108) | |
| Salmonella typhimurium | Cervical cancer | SipB160/HPV16 E7 | Secretion of INF-γ and TNF-α | Mice bearing TC-1 tumors | TC-1 | (109) |
| Salmonella typhimurium VNP20009 | Tumor | EGFR-targeted cytotoxic proteins | Induce apoptosis | Mice | (110) | |
| Salmonella typhimurium | Colon or breast tumor cells | TGFα-PE38 | Inhibition of solid tumor growth | Mice bearing colon or breast tumors | (57) | |
| Salmonella typhimurium | Lymphoma cells | CD20 | Tumor-specific response | Tumor-bearing C57/Bl6J-OlaHsd mice | (107) | |
| Salmonella typhimurium | Human breast cancer and melanoma | RGD peptide | Targeting and therapeutic effects | Mice bearing human lymphomas | (MDA-MB-231)& (MDA-MB-435) | (75) |
| Salmonella typhimurium VNP20009 | Tumors | Anti-CEA- scFv | Tumor accumulation of CD3(+) T cells and CD11b(+) macrophages | CEA transgenic mice | MC38CEA | (111) |
| Salmonella typhimurium ST8 | Colon cancer | ST8/pSEndo | Necrosis and anti-angiogenesis | Tumor-bearing immunocompetent mice | CT26 | (112) |
| Salmonella typhimurium S634 | Colon carcinoma and melanoma | S636/pES | Apoptosis and anti-angiogenesis | Tumor-bearing mice | CT26 & B16F10 | (113) |
| Salmonella typhimurium | Lymphoblastic leukemia | L-asparaginase | Tumor-bearing mice | (82) | ||
| Salmonella typhimurium | Colon cancer | FlaB | Activation of M1 macrophages and suppression ofM2 | TLR4 and MyD88 knockout mice | (TLR5)-negative | (114) |
| Salmonella typhimurium | Breast and colon carcinoma | FasL | Reduced pulmonary metastases | BALB/c mice injected with D2F2 breast carcinoma cells | D2F2 or CT-26 | (115) |
| Salmonella typhimurium | Mammary carcinoma | TRAIL | Apoptosis | Mice | 4T1 | (99) |
| Salmonella typhimurium | Colon and breast tumor | TGFα-PE38 | Inhibition of tumor growth | Mice bearing colon or breast tumors | (57) | |
| Salmonella typhimurium | Colon carcinoma | Noxa | Anti-cancer effect | Mice with CT26 colon carcinoma | CT26 | (56) |
| Salmonella typhimurium | Tumor | Cytolysin A | Expression of reporter genes | BALB/c athymic nu−/nu− mice | (CT-26) | (55) |
| Salmonella typhimurium | Melanoma | IFN-γ | induced cytotoxicity | C57BL/6 mice bearing B16F10 melanoma | B16F10 | (116) |
| Salmonella typhimurium | Multi-drug-resistant carcinomas | CCL21 | Anti-tumor activity | Mice | (117) | |
| Salmonella typhimurium | Tumors | LIGHT | Primary tumor inhibition and fewer pulmonary metastases | BALB/c mice bearing CT-26 colon carcinoma | (118) | |
| Salmonella typhimurium | Subcutaneous tumors | IL-18 | Primary tumor inhibition and fewer pulmonary metastases | BALB/c mice | (119) | |
| Salmonella typhimurium | Melanoma | IL2 | Decreased angiogenesis and increased necrosis | Mice | B16F1 | (120) |
| Salmonella typhimurium | Osteosarcoma | IL-2 | Reduced metastases | Female Balb/c mice | ATCC K7M2 | (121) |
Salmonella Bacteria in Combination Therapies
Chemotherapy can block tumor cell division by interfering with microtubule assembly, disrupting cellular metabolism, or inhibiting DNA repair or replication (122). Several chemotherapy drugs, such as anthracyclines, also work by an immunologic mechanism by causing so-called “immunologic tumor cell death.” Moreover, chemotherapy may be able to inhibit immunosuppressive pathways occurring in the tumor thus releasing the immune attack to be more effective (123).
Saltzman et al. investigated the efficacy of bacterial therapy combined with chemotherapy against tumors (124) (Table 2). They used BALB-neu T mice with orthotopic mammary tumors resembling invasive human breast cancer driven by the Her2 oncogene. An attenuated strain of S. typhimurium was used in combination with doxorubicin at two doses, high (5 mg/kg) and low (1.25 mg/kg). S. typhimurium was injected intravenously on day 0 and doxorubicin days 0, 7, and 14. Mammary pad tumors were evaluated weekly up to day 35 to determine the efficacy, and mice were weighed for toxicity assessment. At day 35, the high dose of doxorubicin limited tumor growth to 1.4-fold, but the body weight had fallen by 25% due to severe toxicity. A single dose of S. typhimurium plus low dose doxorubicin (1.25 mg/kg) restricted the tumor growth to a somewhat lesser degree, but with only 5% weight loss showing no clinical toxicity (124).
Table 2.
Various Salmonella strains that have been used in the combination therapy of cancer.
| Salmonella species | Type of cancer | Combine with | Anti-cancer effect | Model | Type of cell line | Ref |
|---|---|---|---|---|---|---|
| Salmonella typhimurium A1-R | Human colon cancer | Bright-light surgery (BLS) | Increase survival | Athymic nu/nu nude mice | HT-29 | (140) |
| Salmonella typhimurium A1-R | Mammary adenocarcinoma | Surgery | Inhibiting surgery-induced metastasis | Mice | 4T1-RFP | (141) |
| Salmonella typhimurium | Melanoma | γ-radiation | Apoptosis | Tumor-bearing mice | (142) | |
| Salmonella typhimurium ΔppGpp | Colon tumor | Radiotherapy | Reduced tumor growth | Colon tumor (CT26) model of BALB/c mice | CT26 | (11) |
| Salmonella ˗Lipid A | Melanomas | X-rays | Produced supra-additive antitumor effects | Mice bearing B16F10 or Cloudman S91 melanomas | B16F10 or Cloudman S91 |
(143) |
| Salmonella | Mammary tumors | Lipid A | Robust intratumoral accumulation of Salmonella | Mice with 4T1 mammary tumors | 4T1 | (144) |
| Salmonella typhimurium | Breast cancer | Low dose chemotherapy | Decreases tumor burden and less toxic | BALB-neuT mice | Her2-driven | (124) |
| Salmonella typhimurium LVR01 | Melanoma | Imiquimod | Enhancement the pro-inflammatory cytokines and chemokines | B16F1 melanoma-bearing mice | B16F1 | (145) |
| SalmonellatyphiSL7207 | Ovarian cancer | RNA interference | Slow tumor growth | SKOV-3/DDP tumor-bearing mice | SKOV-3/DDP | (45) |
| Salmonella typhimurium A1-R | Cancers | Cisplatinum (CDDP) or paclitaxel (PTX) | Prevented tumor growth | Nude mice | (146) | |
| Salmonella typhimurium A1-R | Osteosarcoma | Recombinant methioninase (rMETase) | Inhibited tumor growth | Athymic nu/nu nude mice | PDOX | (126) |
| Salmonella typhimurium A1-R | Sarcoma | Doxorubicin (DOX) | Inhibited tumor growth | Athymic nu/nu nude mice | PDOX | (147) |
| Salmonella typhimurium A1-R | Melanoma | Temozolomide (TEM) | Inhibited tumor growth | Athymic nu/nu nude mice | PDOX | (148) |
| Salmonella typhimurium A1-R | Pancreatic cancer | Anti-vascular endothelial growth factor (VEGF) therapy | Reduced tumor weight | Male athymic (nu/nu) nude mice | MiaPaCa-2-GFP | (63) |
| Salmonella typhimurium VNP20009 | Melanoma | Anti-angiogenesis therapy (triptolide | Necrosis and modulation of angiogenesis | Female C57BL/6 mice | (69) | |
| Salmonella typhimurium VNP20009 | Melanoma | (ABCB5) | Delay tumor growth | Mice | B16F10 | (132) |
| Salmonella typhimurium VNP20009 | Melanoma | Chemotherapy cyclophosphamide (CTX) | Decrease in tumor microvessel density and (VEGF) level | Murine melanoma model | (62) | |
| Salmonella typhimurium VNP20009 | Lung cancer | Sox2 shRNA | Inhibition of angiogenesis | B16F10 mice model | A549 | (137) |
Shi et al. designed a S. typhimurium strain for the transfer of plasmid vectors (103). This vector was designed to allow dual transcription of therapeutic molecules, which could either be cytotoxic proteins or short hairpin RNAs, within the nucleus or the cytoplasm of eukaryotic cells. The expression of the foreign gene in the bacteria was driven using the T7 RNA polymerase enzyme and dual promoters, and was continuously propagated using an autocatalytic feedback loop. They used attenuated Salmonella to express the gene coding for diphtheria toxin A chain within the hypoxic regions of the tumor, thereby eradicating the tumor in a mouse model. The results showed that 26% (n = 5/19) of the mice were cured and the remainder survived to the termination of the experiment. In a different experiment, nude mice with human MDA-MB-231 xenograft tumors were treated with another strain of S. typhimurium containing a shRNA-encoding plasmid that targeted a cell cycle-associated protein, polo-like kinase 1 (PLK1). Tests showed that, in tumors, the levels of PLK1 transcript were 62.5 ± 18.6% (p = 0.015) lower compared to the control group 3 weeks after injection of 5 × 10(6) CFU of ST4/pIKT-shPlk. They named this approach “inter-kingdom gene delivery” (103).
A study conducted by Nguyen et al. (2010) described a strain of attenuated S. typhimurium that was defective in guanosine 5’-diphosphate-3’-diphosphate, and engineered to express cytolysin A (a 34-kDa pore-forming hemolytic protein) as well as the bacterial luciferase gene lux to allow in vivo imaging (55). In order to avoid undesirable expression in the liver and spleen after tail vein injection, the PBAD promoter from the E. coli arabinose operon, which can be activated by the sugar l-arabinose was employed. Mice with CT-26 tumors could be successfully imaged, some tumors were eradicated, and metastases were reduced upon cytolysin gene induction (55).
Liu et al. combined the identical engineered S. typhimurium strain (ΔppGpp) with radiotherapy (RT) to treat CT26 tumors in BALB/c mice (11). Mice were subjected to RT treatment after S. typhimurium ΔppGpp/pBAD-ClyA injection, using a single dose of 7 Gy at days 1, 4, and 7 for a total of 21 Gy. The combination treatment reduced tumor growth compared with either treatment used alone, and furthermore the radiation was shown not to affect the actual bacteria themselves (11).
It has been shown that recombinant methioninase (rMETase) can act as an anti-tumor agent by targeting the altered methionine metabolism typical of cancer. rMETase could overcome gemcitabine-resistance and lead to regression in a patient-derived orthotopic xenograft (PDOX) nude mouse model of pancreatic cancer (125). The clinical survival rate in metastatic osteosarcoma is less than 20%. Researchers had previously designed a PDOX osteosarcoma mouse model using a lung metastasis from a patient with osteosarcoma who failed CDDP therapy (126). The tumor-targeting S. typhimurium strainA1-R is auxotrophic for the amino-acids leucine and arginine, and has been used to treat mouse xenografts of prostate, breast cancer, osteosarcoma, and lung metastases (127). AR-1 is able to induce chemoresistant cancer cells to move from the G0/G1 phase of the cell cycle into the S/G2/Mphase (128). Intravenously injected S. typhimurium AR-1 could be beneficially combined with orally administered rMETase, because rMETase can selectively act against cells in the S/G2 phase of the cell cycle. A study tested this approach in the PDOX model of CDDP-resistant metastatic osteosarcoma. Different groups of PDOX mice were exposed to the following treatments 14 days after inoculation: G1, control with no treatment; G2, CDDP (6 mg/kg, i.p. daily for 2 weeks); G3, rMETase (100 unit/mouse, i.p., daily for 2 weeks); G4, S. typhimurium A1-R (5 × 10(7) CFU/100 µl, i.v., weekly, for 2 weeks); G5, S. typhimuriumA1-R combined with rMETase; G6, S. typhimuriumA1-R combined with rMETase and CDDP. Except for CDDP alone, all the other treatments inhibited growth of tumor in comparison with G1 control. The triple combination was the most effective.
Yano et al. investigated whether S. typhimurium A1-R could decoy quiescent cancer cells in the cell cycle from G0/G1 to S/G2/M phase as demonstrated by fluorescent imaging mediated by a fluorescence ubiquitination cell cycle indicator (FUCCI) (125). They used sequential treatment of a model of FUCCI-expressing stomach cancer MKN45 in vivo in mice with S. typhimurium A1-R plus rMETase to selectively trap the decoyed cancer cells in S/G2 phase. This was followed by additional administration of cisplatin (CDDP) or paclitaxel (PTX) chemotherapy to kill the decoyed and trapped cancer cells. The combination led to the complete regression of tumors, thus demonstrating the effectiveness of this “decoy, trap and shoot” chemotherapy.
Melanoma is known as one of the most deadly types of skin cancer (129, 130). Although some chemotherapy drugs such as dacarbazine, cyclophosphamide, and temozolomide show some therapeutic benefit, other drugs such as nitrosoureas, taxanes, vinca alkaloids or platinum-related drugs have not shown any positive effects in randomized trials, and do not extend survival (131). Melanoma tumors contain cancer stem cells, with a long-lasting renewable capacity and expression of a multidrug resistance pump called ATP-binding cassette sub-family B member 5 (ABCB5). In one study by Zhang et al., the researchers treated melanoma-bearing mice with an attenuated Salmonella strain (VNP20009) that was engineered to express short hairpin RNA targeting the ABCB5 gene (132). They combined this treatment with the chemostherapy drug cyclophosphamide (CTX) which is a known substrate of ABCB5. Concomitant use of VNP20009-shABCB5 and CTX compared with VNP20009 alone, could synergistically delay tumor growth and extend survival in the B16F10 mouse model (132).
In one study, Chen et al. compared the effects of conventional VNP20009 monotherapy, highly attenuated Salmonella strain, and a combination therapy that used both triptolide and VNP20009 in a mouse melanoma model. They showed that triptolide improved the tumor colonization with VNP20009 through decreasing the numbers of infiltrated neutrophils, which led to a larger necrotic area in the melanoma. Furthermore, the combination therapy could suppress angiogenesis of the tumor by decreasing the VEGF expression in a synergistic manner, and retarding the growth of the melanoma (133).
Lung cancer is a leading cause of cancer death, and 85% of cases are non-small cell lung carcinomas (NSCLC) with an average 5-year survival of only 4% (134). SOX2 (sex determining region Y)-box 2 is a transcription factor that is essential for maintaining the pluripotency of undifferentiated embryonic stem cells and lung cancer stem cells (135). Regulating Sox2 suppresses the metastasis and growth of many cancer cells, so Sox2 is a potential target in cancer treatment (136).
In one study by Zhao et al., a S. typhimurium VNP20009 strain carrying a Sox2 shRNA was designed (137). They also used a polypeptide called HM-3, which is an 18-amino acid peptide generated by the fusion of the Arg-Gly-Asp (RGD) sequence to the C-terminus of an endostatin-derived peptide. The RGD domain in HM3 specifically recognized the αvβ3 integrin, which is over-expressed in tumor vasculature. The combination of HM-3 and S. typhimurium VNP20009 carrying shRNA targeting Sox2 was tested in vitro and in vivo. Cell invasion and colony formation assays using A549 human lung cancer cells showed that shRNA against Sox2 could reduce migration, and trigger apoptosis by increasing Bax expression, cleaving caspase 3, and decreasing Bcl2. A mouse xenograft model of A549 was used to show that the combination therapy of HM-3 and VNP20009 Sox2 shRNA, could inhibit angiogenesis and slow lung cancer growth (137).
Gao et al. developed an oxygen tolerant attenuated Salmonella strain (KST0650) using radiation mutation technology. Results showed that the oxytolerant KST0650 strain possessed 20-times higher replication activity in CT26 cancer cells and was less virulent than wild type Salmonella. Furthermore, KST0650 could migrate effectively into tumor tissues in mice. KST0650 was further equipped with a plasmid harboring a spliced form of the intracellular pro-apoptotic protein sATF6, and the expression of sATF6 was controlled by the radiation-inducible recN promoter. The new strain was named KST0652, in which sATF6 protein expression was induced in response to radiation in a dose-dependent manner. This strain was effectively delivered to cancer cells and tumor tissues via the Salmonella type III secretion system (T3SS). In addition, combination treatment with KST0652 plus radiation showed a synergistic anti-tumor effect in the murine tumor model with complete inhibition of tumor growth and protected against mouse death (138).
Miyake et al. developed the tumor-targeting bacteria S. typhimurium A1-R. In this study cervical-cancer tumor fragments were implanted orthotopically into the uterine cervix of nude mice. They showed that nab-paclitaxal combined with S. typhimurium A1-R significantly suppressed tumor growth compared to the untreated control group, while the nab-PTX alone and S. typhimurium A1-R alone groups did not show significant efficacy as monotherapy compared to the control group (139).
Conclusions
The pathophysiology of solid tumors imposes severe barriers that prevent the penetration and accumulation of anti-tumor chemotherapy drugs. The application of therapeutic bacteria to treat cancer has a long history, but may only recently have been taken seriously. A variety of oncolytic bacteria have been described including Mycobacterium bovis (BCG), Streptococcus and Serratia species, Listeria monocytogenes, as well as Salmonella species as discussed in the present review. Salmonella species have been often studied because of the availability of tumor-selective strains that have been significantly attenuated in their virulence, so that they do not pose a major threat of systemic infection or collateral damage. Moreover the bacteria can be controlled after their administration, by using compounds that only affect the bacterial cells and not the host cells. According to many studies, Salmonella-mediated antitumor therapy has significantly helped to suppress tumors and achieve higher rates of survival in animal models. The benefits of this therapy include automatic self-targeting, as well as the possibility of genetic manipulation to produce newly engineered attenuated strains. Nevertheless, Salmonella-mediated anticancer therapy has not yet been clinically established, and is still faced with numerous practical challenges. For instance, somewhat unsatisfactory results were obtained in the phase I clinical trials that have so far been conducted. The changes in the tumor microenvironment produced by Salmonella are still not fully understood. The complicated interactions between Salmonella, inflammatory mediators, as well as the effects on host immunity are difficult to figure out Combinations of Salmonella-mediated approaches together with other types of tumor treatment could show synergistic benefits. BMCT alone will probably not be a complete substitute for traditional cancer therapy, but it may take part in new combination approaches to improve outcomes.
Author Contributions
HM and MRH contributed in conception, design, statistical analysis and drafting of the manuscript. FB, MaS, KM, VT, MG, SAT, AK, MoH, MM-T, and AJ contributed in data collection and manuscript drafting. All authors contributed to the article and approved the submitted version.
Conflict of Interest
MH declares the following potential conflicts of interest. Scientific Advisory Boards: Transdermal Cap Inc, Cleveland, OH; BeWell Global Inc, Wan Chai, Hong Kong; Hologenix Inc. Santa Monica, CA; LumiTheraInc, Poulsbo, WA; Vielight, Toronto, Canada; Bright Photomedicine, Sao Paulo, Brazil; Quantum Dynamics LLC, Cambridge, MA; Global Photon Inc, Bee Cave, TX; Medical Coherence, Boston MA; NeuroThera, Newark DE; JOOVV Inc, Minneapolis-St. Paul MN; AIRx Medical, Pleasanton CA; FIR Industries, Inc. Ramsey, NJ; UVLRx Therapeutics, Oldsmar, FL; Ultralux UV Inc, Lansing MI; Illumiheal&Petthera, Shoreline, WA; MB Lasertherapy, Houston, TX; ARRC LED, San Clemente, CA; Varuna Biomedical Corp. Incline Village, NV; Niraxx Light Therapeutics, Inc, Boston, MA. Consulting; Lexington Int, Boca Raton, FL; USHIO Corp, Japan; Merck KGaA, Darmstadt, Germany; Philips Electronics Nederland B.V. Eindhoven, Netherlands; Johnson & Johnson Inc, Philadelphia, PA; Sanofi-Aventis Deutschland GmbH, Frankfurt am Main, Germany. Stockholdings: Global Photon Inc, Bee Cave, TX; Mitonix, Newark, DE.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviation
5-FC, 5-Fluorocytosine; 5-FdUMP, 5-fluorodeoxyuridine monophosphate; 5-FdUTP, 5-fluorodeoxyuridine triphosphate; 5-FU, 5-fluorouracil; 5-FUTP, 5-fluorouridine triphosphate; ABCB5, ATP-binding cassette sub-family B member 5; AFP, fetoprotein; Bax, Bcl-2-associated X protein; BLS, bright-light surgery; BMCT, Bacterial-mediated cancer therapy; CCL21, Chr. Chemokine (C-C motif) ligand 21; CD31, cluster of differentiation 31; CDDP, cisplatinum; CEA, carcinoembryonic antigen; CTX, cyclophosphamide; DAMP, danger-associated molecular patterns; DOX, Doxorubicin; DTA, Diphtheria toxin A chain; EGFR, Epidermal growth factor receptor; eIF2B, Eukaryotic translation initiation factor 2B;FasL, fas ligand; FLK1, Fetal Liver Kinase 1; FUCCI, fluorescence ubiquitination cell cycle indicator; GCN2, general control nonderepressible 2; Her2, Human epidermal growth factor receptor 2; hGM-CSF, Human Granulocyte Macrophage Colony Stimulating Factor; hIL-12, human IL-12; HPV, human papilloma virus; IL, interleukin; INF-γ, interferon gamma; L-ASNase, asparaginase; MDR, multidrug resistance; MDR1, multidrug-resistance gene; MHC, Major histocompatibility complex; NSCLC, non-small cell lung carcinomas; OmpA, outer membrane protein A; P-gp, permeability glycoprotein; PAMP, pathogen-related molecular patterns; PCR, polymerase chain reaction; PCSA, Prostate stem cell antigen; PDOX, pancreatic cancer patient-derived orthotopic xenografts; PLK1, polo like kinase 1; PTX, paclitaxel; RBM5, RNA‐binding motif protein 5; RGD, Arg-Gly-Asp; RGD, Arginylglycylaspartic acid; rMETase, recombinant methioninase; RT, radiotherapy; scFv, single-chain variable fragment; shRNA, short hairpin RNA; siRNA, small interfering RNA; Sox2, sex determining region Y; Stat3, Signal transducer and activator of transcription 3; TAM, tumor-associated macrophages; TEM, Temozolomide; TGFα, Transforming growth factor alpha; TNFα, Tumor necrosis factor; TRAIL, TNF-related apoptosis-inducing ligand; tRNA, transfer ribonucleic acid; VEGF, Vascular endothelial growth factor; αvβ3, alpha-v beta-3.
References
- 1. Mi Z, Feng Z-C, Li C, Yang X, Ma M-T, Rong P-F. Salmonella-Mediated Cancer Therapy: An Innovative Therapeutic Strategy. J Cancer (2019) 10:4765–76. 10.7150/jca.32650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Coley W. Contribution to the knowledgeofsarcoma. Am Surg (1891) 14:190. 10.1097/00000658-189112000-00015 [DOI] [Google Scholar]
- 3. Duong MT-Q, Qin Y, You S-H, Min J-J. Bacteria-cancer interactions: bacteria-based cancer therapy. Exp Mol Med (2019) 51:1–15. 10.1038/s12276-019-0297-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maletzki C, Linnebacher M, Kreikemeyer B, Emmrich J. Pancreatic cancer regression by intratumoural injection of live Streptococcus pyogenes in a syngeneic mouse model. Gut (2008) 57:483–91. 10.1136/gut.2007.125419 [DOI] [PubMed] [Google Scholar]
- 5. Zhu H, Li Z, Mao S, Ma B, Zhou S, Deng L, et al. Antitumor effect of sFlt-1 gene therapy system mediated by Bifidobacterium Infantis on Lewis lung cancer in mice. Cancer Gene Ther (2011) 18:884–96. 10.1038/cgt.2011.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Agrawal N, Bettegowda C, Cheong I, Geschwind JF, Drake CG, Hipkiss EL, et al. Bacteriolytic therapy can generate a potent immune response against experimental tumors. Proc Natl Acad Sci U S A (2004) 101:15172–7. 10.1073/pnas.0406242101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang Y, Chen J, Tang B, Zhang X, Hua ZC. Systemic administration of attenuated Salmonella typhimurium in combination with interleukin-21 for cancer therapy. Mol Clin Oncol (2013) 1:461–5. 10.3892/mco.2013.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yoon W, Yoo Y, Chae YS, Kee SH, Kim BM. Therapeutic advantage of genetically engineered Salmonella typhimurium carrying short hairpin RNA against inhibin alpha subunit in cancer treatment. Ann Oncol (2018) 29:2010–7. 10.1093/annonc/mdy240 [DOI] [PubMed] [Google Scholar]
- 9. Eisenstark A. A Geneticist’s View of Prostate Cancer: Prostate Cancer Treatment Considerations. Adv Exp Med Biol (2018) 1095:125–9. 10.1007/978-3-319-95693-0_8 [DOI] [PubMed] [Google Scholar]
- 10. Na HS, Kim HJ, Lee HC, Hong Y, Rhee JH, Choy HE. Immune response induced by Salmonella typhimurium defective in ppGpp synthesis. Vaccine (2006) 24:2027–34. 10.1016/j.vaccine.2005.11.031 [DOI] [PubMed] [Google Scholar]
- 11. Liu X, Jiang S, Piao L, Yuan F. Radiotherapy combined with an engineered Salmonella typhimurium inhibits tumor growth in a mouse model of colon cancer. Exp Anim (2016) 65:413–8. 10.1538/expanim.16-0033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang CJ, Chang WW, Lin ST, Chen MC, Lee CH. Salmonella Overcomes Drug Resistance in Tumor through P-glycoprotein Downregulation. Int J Med Sci (2018) 15:574–9. 10.7150/ijms.23285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Laliani G, Ghasemian Sorboni S, Lari R, Yaghoubi A, Soleimanpour S, Khazaei M, et al. Bacteria and cancer: Different sides of the same coin. Life Sci (2020) 246:117398. 10.1016/j.lfs.2020.117398 [DOI] [PubMed] [Google Scholar]
- 14. Bommareddy PK, Patel A, Hossain S, Kaufman HL. Talimogene Laherparepvec (T-VEC) and Other Oncolytic Viruses for the Treatment of Melanoma. Am J Clin Dermatol (2017) 18:1–15. 10.1007/s40257-016-0238-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sedighi M, Zahedi Bialvaei A, Hamblin MR, Ohadi E, Asadi A, Halajzadeh M, et al. Therapeutic bacteria to combat cancer; current advances, challenges, and opportunities. Cancer Med (2019) 8:3167–81. 10.1002/cam4.2148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cao Z, Liu J. Bacteria and bacterial derivatives as drug carriers for cancer therapy. J Controll Release (2020) 326:396–407. 10.1016/j.jconrel.2020.07.009 [DOI] [PubMed] [Google Scholar]
- 17. Schmidt CK, Medina-Sánchez M, Edmondson RJ, Schmidt OG. Engineering microrobots for targeted cancer therapies from a medical perspective. Nat Commun (2020) 11:1–18. 10.1038/s41467-020-19322-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shrivastava A. Single-celled bacteria as tool for cancer therapy, Evolutionary diversity as a source for anticancer molecules. Amsterdam, Netherlands: Elsevier; (2020). p. 103–26. 10.1016/B978-0-12-821710-8.00005-9 [DOI] [Google Scholar]
- 19. Guo Y, Chen Y, Liu X, Min J-J, Tan W, Zheng JH. Targeted cancer immunotherapy with genetically engineered oncolytic Salmonella typhimurium. Cancer Lett (2020) 469:102–10. 10.1016/j.canlet.2019.10.033 [DOI] [PubMed] [Google Scholar]
- 20. Shanmugaraj B, Priya LB, Mahalakshmi B, Subbiah S, Hu R-M, Velmurugan BK, et al. Bacterial and viral vectors as vaccine delivery vehicles for breast cancer therapy. Life Sci (2020), 117550. 10.1016/j.lfs.2020.117550 [DOI] [PubMed]
- 21. Ullah N. Use of Tumor Targeting Bacteria as Cancer Therapeutic Agents and Drug Delivery Vehicles: A Conceptual Approach. EC Microbiol (2019) 15:565–74. [Google Scholar]
- 22. Claesen J, Fischbach MA. Synthetic microbes as drug delivery systems. ACS Synth Biol (2015) 4:358–64. 10.1021/sb500258b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abil Z, Xiong X, Zhao H. Synthetic biology for therapeutic applications. Mol Pharm (2015) 12:322–31. 10.1021/mp500392q [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marintcheva B. Chapter 9 - Virus-Based Therapeutic Approaches. In: Marintcheva B, editor. Harnessing the Power of Viruses. Amsterdam, Netherlands: Academic Press; (2018). p. 243–76. 10.1016/B978-0-12-810514-6.00009-X [DOI] [Google Scholar]
- 25. Cao G-D, He X-b, Sun Q, Chen S, Wan K, Xu X, et al. The Oncolytic Virus in Cancer Diagnosis and Treatment. Front Oncol (2020) 10:1786. 10.3389/fonc.2020.01786 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26. Ning BT, Yu B, Chan S, Chan JL, Huang JD, Chan GC. Treatment of Neuroblastoma with an Engineered “Obligate” Anaerobic Salmonella typhimurium Strain YB1. J Cancer (2017) 8:1609–18. 10.7150/jca.18776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Drees JJ, Mertensotto MJ, Augustin LB, Schottel JL, Saltzman DA. Vasculature Disruption Enhances Bacterial Targeting of Autochthonous Tumors. J Cancer (2015) 6:843–8. 10.7150/jca.12491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang CZ, Kazmierczak RA, Eisenstark A. Strains, Mechanism, and Perspective: Salmonella-Based Cancer Therapy. Int J Microbiol (2016) 2016:5678702. 10.1155/2016/5678702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Laursen MF, Kofod-Olsen E, Agger R. Activation of dendritic cells by targeted DNA: a potential addition to the armamentarium for anti-cancer immunotherapy. Cancer Immunol Immunother (2019) 68:1875–80. 10.1007/s00262-019-02400-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gong Y, Chang C, Liu X, He Y, Wu Y, Wang S, et al. Stimulator of Interferon Genes Signaling Pathway and its Role in Anti-tumor Immune Therapy. Curr Pharm Des (2020) 26:3085–95. 10.2174/1381612826666200610183048 [DOI] [PubMed] [Google Scholar]
- 31. Watkins-Schulz R, Tiet P, Gallovic MD, Junkins RD, Batty C, Bachelder EM, et al. A microparticle platform for STING-targeted immunotherapy enhances natural killer cell-and CD8+ T cell-mediated anti-tumor immunity. Biomaterials (2019) 205:94–105. 10.1016/j.biomaterials.2019.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Taylor SJ, Winter SE. Salmonella finds a way: Metabolic versatility of Salmonella enterica serovar Typhimurium in diverse host environments. PloS Pathog (2020) 16:e1008540. 10.1371/journal.ppat.1008540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Habenstein B, El Mammeri N, Tolchard J, Lamon G, Tawani A, Berbon M, et al. Structures of type III secretion system needle filaments. Curr Top Microbiol Immunol (2020) 427:109–31. 10.1007/82_2019_192 [DOI] [PubMed] [Google Scholar]
- 34. Avogadri F, Martinoli C, Petrovska L, Chiodoni C, Transidico P, Bronte V, et al. Cancer immunotherapy based on killing of Salmonella-infected tumor cells. Cancer Res (2005) 65:3920–7. 10.1158/0008-5472.CAN-04-3002 [DOI] [PubMed] [Google Scholar]
- 35. Zhu F-J, Tong Y-L, Sheng Z-Y, Yao Y-M. Role of dendritic cells in the host response to biomaterials and their signaling pathways. Acta Biomater (2019) 94:132–44. 10.1016/j.actbio.2019.05.038 [DOI] [PubMed] [Google Scholar]
- 36. Escoll P, Buchrieser C. Metabolic reprogramming: an innate cellular defence mechanism against intracellular bacteria? Curr Opin Immunol (2019) 60:117–23. 10.1016/j.coi.2019.05.009 [DOI] [PubMed] [Google Scholar]
- 37. Wang Q, Imamura R, Motani K, Kushiyama H, Nagata S, Suda T. Pyroptotic cells externalize eat-me and release find-me signals and are efficiently engulfed by macrophages. Int Immunol (2013) 25:363–72. 10.1093/intimm/dxs161 [DOI] [PubMed] [Google Scholar]
- 38. Knodler LA, Vallance BA, Celli J, Winfree S, Hansen B, Montero M, et al. Dissemination of invasive Salmonella via bacterial-induced extrusion of mucosal epithelia. Proc Natl Acad Sci U S A (2010) 107:17733–8. 10.1073/pnas.1006098107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fink SL, Cookson BT. Pyroptosis and host cell death responses during Salmonella infection. Cell Microbiol (2007) 9:2562–70. 10.1111/j.1462-5822.2007.01036.x [DOI] [PubMed] [Google Scholar]
- 40. Zhao M, Suetsugu A, Ma H, Zhang L, Liu F, Zhang Y, et al. Efficacy against lung metastasis with a tumor-targeting mutant of Salmonella typhimurium in immunocompetent mice. Cell Cycle (2012) 11:187–93. 10.4161/cc.11.1.18667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Saccheri F, Pozzi C, Avogadri F, Barozzi S, Faretta M, Fusi P, et al. Bacteria-induced gap junctions in tumors favor antigen cross-presentation and antitumor immunity. Sci Transl Med (2010) 2:44ra57. 10.1126/scitranslmed.3000739 [DOI] [PubMed] [Google Scholar]
- 42. Kramer MG, Masner M, Ferreira FA, Hoffman RM. Bacterial Therapy of Cancer: Promises, Limitations, and Insights for Future Directions. Front Microbiol (2018) 9:16. 10.3389/fmicb.2018.00016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Razavi ZS, Tajiknia V, Majidi S, Ghandali M, Mirzaei HR, Rahimian N, et al. Gynecologic cancers and non-coding RNAs: Epigenetic regulators with emerging roles. Crit Rev Oncol Hematol (2020), 103192. 10.1016/j.critrevonc.2020.103192 [DOI] [PubMed]
- 44. Shabaninejad Z, Vafadar A, Movahedpour A, Ghasemi Y, Namdar A, Fathizadeh H, et al. Circular RNAs in cancer: new insights into functions and implications in ovarian cancer. J Ovarian Res (2019) 12:84. 10.1186/s13048-019-0558-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Deng J, Guo Y, Jiang Z, Yang M, Li H, Wang J. Enhancement of ovarian cancer chemotherapy by delivery of multidrug-resistance gene small interfering RNA using tumor targeting Salmonella. J Obstet Gynaecol Res (2015) 41:615–22. 10.1111/jog.12598 [DOI] [PubMed] [Google Scholar]
- 46. Grandis JR, Tweardy DJ. Elevated levels of transforming growth factor alpha and epidermal growth factor receptor messenger RNA are early markers of carcinogenesis in head and neck cancer. Cancer Res (1993) 53:3579–84. [PubMed] [Google Scholar]
- 47. Phillips PC, Levow C, Catterall M, Colvin OM, Pastan I, Brem H. Transforming growth factor-alpha-Pseudomonas exotoxin fusion protein (TGF-alpha-PE38) treatment of subcutaneous and intracranial human glioma and medulloblastoma xenografts in athymic mice. Cancer Res (1994) 54:1008–15. [PubMed] [Google Scholar]
- 48. Rubin Grandis J, Chakraborty A, Melhem MF, Zeng Q, Tweardy DJ. Inhibition of epidermal growth factor receptor gene expression and function decreases proliferation of head and neck squamous carcinoma but not normal mucosal epithelial cells. Oncogene (1997) 15:409–16. 10.1038/sj.onc.1201188 [DOI] [PubMed] [Google Scholar]
- 49. Weldon JE, Pastan I. A guide to taming a toxin–recombinant immunotoxins constructed from Pseudomonas exotoxin A for the treatment of cancer. FEBS J (2011) 278:4683–700. 10.1111/j.1742-4658.2011.08182.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Siegall CB, FitzGerald DJ, Pastan I. Selective killing of tumor cells using EGF or TGF alpha-Pseudomonas exotoxin chimeric molecules. Semin Cancer Biol (1990) 1:345–50. [PubMed] [Google Scholar]
- 51. Wright SE, Rewers-Felkins KA, Quinlin I, Chowdhury NI, Ahmed J, Eldridge PW, et al. TGFalpha-PE38 enhances cytotoxic T-lymphocyte killing of breast cancer cells. Oncol Lett (2014) 7:2113–7. 10.3892/ol.2014.1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Song M, Kim H-J, Ryu S, Yoon H, Yun J, Choy HE. ppGpp-mediated stationary phase induction of the genes encoded by horizontally acquired pathogenicity islands and cob/pdu locus in Salmonella enterica serovar Typhimurium. J Microbiol (2010) 48:89–95. 10.1007/s12275-009-0179-6 [DOI] [PubMed] [Google Scholar]
- 53. Song M, Kim HJ, Kim EY, Shin M, Lee HC, Hong Y, et al. ppGpp-dependent stationary phase induction of genes on Salmonella pathogenicity island 1. J Biol Chem (2004) 279:34183–90. 10.1074/jbc.M313491200 [DOI] [PubMed] [Google Scholar]
- 54. Kim JE, Phan TX, Nguyen VH, Dinh-Vu HV, Zheng JH, Yun M, et al. Salmonella typhimurium Suppresses Tumor Growth via the Pro-Inflammatory Cytokine Interleukin-1beta. Theranostics (2015) 5:1328–42. 10.7150/thno.11432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nguyen VH, Kim HS, Ha JM, Hong Y, Choy HE, Min JJ. Genetically engineered Salmonella typhimurium as an imageable therapeutic probe for cancer. Cancer Res (2010) 70:18–23. 10.1158/0008-5472.CAN-09-3453 [DOI] [PubMed] [Google Scholar]
- 56. Jeong JH, Kim K, Lim D, Jeong K, Hong Y, Nguyen VH, et al. Anti-tumoral effect of the mitochondrial target domain of Noxa delivered by an engineered Salmonella typhimurium. PloS One (2014) 9:e80050. 10.1371/journal.pone.0080050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lim D, Kim KS, Kim H, Ko KC, Song JJ, Choi JH, et al. Anti-tumor activity of an immunotoxin (TGFalpha-PE38) delivered by attenuated Salmonella typhimurium. Oncotarget (2017) 8:37550–60. 10.18632/oncotarget.17197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Low KB, Ittensohn M, Le T, Platt J, Sodi S, Amoss M, et al. Lipid A mutant Salmonella with suppressed virulence and TNFalpha induction retain tumor-targeting in vivo. Nat Biotechnol (1999) 17:37–41. 10.1038/5205 [DOI] [PubMed] [Google Scholar]
- 59. Chen J, Wei D, Zhuang H, Qiao Y, Tang B, Zhang X, et al. Proteomic screening of anaerobically regulated promoters from Salmonella and its antitumor applications. Mol Cell Proteomics (2011) 10:M111.009399. 10.1074/mcp.M111.009399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Toso JF, Gill VJ, Hwu P, Marincola FM, Restifo NP, Schwartzentruber DJ, et al. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J Clin Oncol (2002) 20:142–52. 10.1200/JCO.2002.20.1.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nemunaitis J, Cunningham C, Senzer N, Kuhn J, Cramm J, Litz C, et al. Pilot trial of genetically modified, attenuated Salmonella expressing the E. coli cytosine deaminase gene in refractory cancer patients. Cancer Gene Ther (2003) 10:737–44. 10.1038/sj.cgt.7700634 [DOI] [PubMed] [Google Scholar]
- 62. Jia LJ, Wei DP, Sun QM, Jin GH, Li SF, Huang Y, et al. Tumor-targeting Salmonella typhimurium improves cyclophosphamide chemotherapy at maximum tolerated dose and low-dose metronomic regimens in a murine melanoma model. Int J Cancer (2007) 121:666–74. 10.1002/ijc.22688 [DOI] [PubMed] [Google Scholar]
- 63. Hiroshima Y, Zhang Y, Murakami T, Maawy A, Miwa S, Yamamoto M, et al. Efficacy of tumor-targeting Salmonella typhimurium A1-R in combination with anti-angiogenesis therapy on a pancreatic cancer patient-derived orthotopic xenograft (PDOX) and cell line mouse models. Oncotarget (2014) 5:12346–57. 10.18632/oncotarget.2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tang W, Zuo JP. Immunosuppressant discovery from Tripterygium wilfordii Hook f: the novel triptolide analog (5R)-5-hydroxytriptolide (LLDT-8). Acta Pharmacol Sin (2012) 33:1112–8. 10.1038/aps.2012.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yang S, Chen J, Guo Z, Xu XM, Wang L, Pei XF, et al. Triptolide inhibits the growth and metastasis of solid tumors. Mol Cancer Ther (2003) 2:65–72. [PubMed] [Google Scholar]
- 66. Wang C, Liu B, Xu X, Zhuang B, Li H, Yin J, et al. Toward targeted therapy in chemotherapy-resistant pancreatic cancer with a smart triptolide nanomedicine. Oncotarget (2016) 7:8360–72. 10.18632/oncotarget.7073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Jiang QW, Cheng KJ, Mei XL, Qiu JG, Zhang WJ, Xue YQ, et al. Synergistic anticancer effects of triptolide and celastrol, two main compounds from thunder god vine. Oncotarget (2015) 6:32790–804. 10.18632/oncotarget.5411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cunningham C, Nemunaitis J. A phase I trial of genetically modified Salmonella typhimurium expressing cytosine deaminase (TAPET-CD, VNP20029) administered by intratumoral injection in combination with 5-fluorocytosine for patients with advanced or metastatic cancer. Protocol no: CL-017. Version: April 9, 2001. Hum Gene Ther (2001) 12:1594–6. [PubMed] [Google Scholar]
- 69. Chen J, Qiao Y, Tang B, Chen G, Liu X, Yang B, et al. Modulation of Salmonella Tumor-Colonization and Intratumoral Anti-angiogenesis by Triptolide and Its Mechanism. Theranostics (2017) 7:2250–60. 10.7150/thno.18816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chen G, Tang B, Yang BY, Chen JX, Zhou JH, Li JH, et al. Tumor-targeting Salmonella typhimurium, a natural tool for activation of prodrug 6MePdR and their combination therapy in murine melanoma model. Appl Microbiol Biotechnol (2013) 97:4393–401. 10.1007/s00253-012-4321-8 [DOI] [PubMed] [Google Scholar]
- 71. Meyers M, Hwang A, Wagner MW, Bruening AJ, Veigl ML, Sedwick WD, et al. A role for DNA mismatch repair in sensing and responding to fluoropyrimidine damage. Oncogene (2003) 22:7376–88. 10.1038/sj.onc.1206941 [DOI] [PubMed] [Google Scholar]
- 72. Polak A, Scholer HJ. Mode of action of 5-fluorocytosine and mechanisms of resistance. Chemotherapy (1975) 21:113–30. 10.1159/000221854 [DOI] [PubMed] [Google Scholar]
- 73. Damon LE, Cadman E, Benz C. Enhancement of 5-fluorouracil antitumor effects by the prior administration of methotrexate. Pharmacol Ther (1989) 43:155–85. 10.1016/0163-7258(89)90117-4 [DOI] [PubMed] [Google Scholar]
- 74. Mesa-Pereira B, Medina C, Camacho EM, Flores A, Santero E. Improved cytotoxic effects of Salmonella-producing cytosine deaminase in tumour cells. Microb Biotechnol (2015) 8:169–76. 10.1111/1751-7915.12153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Park SH, Zheng JH, Nguyen VH, Jiang SN, Kim DY, Szardenings M, et al. RGD Peptide Cell-Surface Display Enhances the Targeting and Therapeutic Efficacy of Attenuated Salmonella-mediated Cancer Therapy. Theranostics (2016) 6:1672–82. 10.7150/thno.16135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wriston JC, Jr., Yellin TO. L-asparaginase: a review. Adv Enzymol Relat areas Mol Biol (1973) 39:185–248. 10.1002/9780470122846.ch3 [DOI] [PubMed] [Google Scholar]
- 77. Willems L, Jacque N, Jacquel A, Neveux N, Maciel TT, Lambert M, et al. Inhibiting glutamine uptake represents an attractive new strategy for treating acute myeloid leukemia. Blood (2013) 122:3521–32. 10.1182/blood-2013-03-493163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ehsanipour EA, Sheng X, Behan JW, Wang X, Butturini A, Avramis VI, et al. Adipocytes cause leukemia cell resistance to L-asparaginase via release of glutamine. Cancer Res (2013) 73:2998–3006. 10.1158/0008-5472.CAN-12-4402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hao S, Sharp JW, Ross-Inta CM, McDaniel BJ, Anthony TG, Wek RC, et al. Uncharged tRNA and sensing of amino acid deficiency in mammalian piriform cortex. Science (New York NY) (2005) 307:1776–8. 10.1126/science.1104882 [DOI] [PubMed] [Google Scholar]
- 80. Clemens MJ. Initiation factor eIF2 alpha phosphorylation in stress responses and apoptosis. Prog Mol Subcell Biol (2001) 27:57–89. 10.1007/978-3-662-09889-9_3 [DOI] [PubMed] [Google Scholar]
- 81. Ueno T, Ohtawa K, Mitsui K, Kodera Y, Hiroto M, Matsushima A, et al. Cell cycle arrest and apoptosis of leukemia cells induced by L-asparaginase. Leukemia (1997) 11:1858–61. 10.1038/sj.leu.2400834 [DOI] [PubMed] [Google Scholar]
- 82. Kim K, Jeong JH, Lim D, Hong Y, Lim HJ, Kim GJ, et al. L-Asparaginase delivered by Salmonella typhimurium suppresses solid tumors. Mol Ther Oncolytics (2015) 2:15007. 10.1038/mto.2015.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Deng J, Guo Y, Jiang Z, Yang M, Li H, Wang J. Enhancement of ovarian cancer chemotherapy by delivery of multidrug-resistance gene small interfering RNA using tumor targeting S almonella. J Obstet Gynaecol Res (2015) 41:615–22. 10.1111/jog.12598 [DOI] [PubMed] [Google Scholar]
- 84. Blache CA, Manuel ER, Kaltcheva TI, Wong AN, Ellenhorn JD, Blazar BR, et al. Systemic delivery of Salmonella typhimurium transformed with IDO shRNA enhances intratumoral vector colonization and suppresses tumor growth. Cancer Res (2012) 72:6447–56. 10.1158/0008-5472.CAN-12-0193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zhao C, He J, Cheng H, Zhu Z, Xu H. Enhanced therapeutic effect of an antiangiogenesis peptide on lung cancer in vivo combined with salmonella VNP20009 carrying a Sox2 shRNA construct. J Exp Clin Cancer Res (2016) 35:107. 10.1186/s13046-016-0381-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Shi L, Yu B, Cai C-H, Huang W, Zheng B-J, Smith DK, et al. Combined prokaryotic–eukaryotic delivery and expression of therapeutic factors through a primed autocatalytic positive-feedback loop. J Controll Release (2016) 222:130–40. 10.1016/j.jconrel.2015.12.005 [DOI] [PubMed] [Google Scholar]
- 87. Zhang L, Gao L, Zhao L, Guo B, Ji K, Tian Y, et al. Intratumoral delivery and suppression of prostate tumor growth by attenuated Salmonella enterica serovar typhimurium carrying plasmid-based small interfering RNAs. Cancer Res (2007) 67:5859–64. 10.1158/0008-5472.CAN-07-0098 [DOI] [PubMed] [Google Scholar]
- 88. Tian Y, Guo B, Jia H, Ji K, Sun Y, Li Y, et al. Targeted therapy via oral administration of attenuated Salmonella expression plasmid-vectored Stat3-shRNA cures orthotopically transplanted mouse HCC. Cancer Gene Ther (2012) 19:393–401. 10.1038/cgt.2012.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Fu W, Lan H, Li S, Han X, Gao T, Ren D. Synergistic antitumor efficacy of suicide/ePNP gene and 6-methylpurine 2’-deoxyriboside via Salmonella against murine tumors. Cancer Gene Ther (2008) 15:474–84. 10.1038/cgt.2008.19 [DOI] [PubMed] [Google Scholar]
- 90. Fu W, Lan H, Liang S, Gao T, Ren D. Suicide gene/prodrug therapy using salmonella-mediated delivery of Escherichia coli purine nucleoside phosphorylase gene and 6-methoxypurine 2’-deoxyriboside in murine mammary carcinoma 4T1 model. Cancer Sci (2008) 99:1172–9. 10.1111/j.1349-7006.2008.00808.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lewen S, Zhou H, Hu HD, Cheng T, Markowitz D, Reisfeld RA, et al. A Legumain-based minigene vaccine targets the tumor stroma and suppresses breast cancer growth and angiogenesis. Cancer Immunol Immunother (2008) 57:507–15. 10.1007/s00262-007-0389-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Chou CK, Hung JY, Liu JC, Chen CT, Hung MC. An attenuated Salmonella oral DNA vaccine prevents the growth of hepatocellular carcinoma and colon cancer that express alpha-fetoprotein. Cancer Gene Ther (2006) 13:746–52. 10.1038/sj.cgt.7700927 [DOI] [PubMed] [Google Scholar]
- 93. Ahmad S, Casey G, Cronin M, Rajendran S, Sweeney P, Tangney M, et al. Induction of effective antitumor response after mucosal bacterial vector mediated DNA vaccination with endogenous prostate cancer specific antigen. J Urol (2011) 186:687–93. 10.1016/j.juro.2011.03.139 [DOI] [PubMed] [Google Scholar]
- 94. Zuo SG, Chen Y, Wu ZP, Liu X, Liu C, Zhou YC, et al. Orally administered DNA vaccine delivery by attenuated Salmonella typhimurium targeting fetal liver kinase 1 inhibits murine Lewis lung carcinoma growth and metastasis. Biol Pharm Bull (2010) 33:174–82. 10.1248/bpb.33.174 [DOI] [PubMed] [Google Scholar]
- 95. Berger E, Soldati R, Huebener N, Hohn O, Stermann A, Durmus T, et al. Salmonella SL7207 application is the most effective DNA vaccine delivery method for successful tumor eradication in a murine model for neuroblastoma. Cancer Lett (2013) 331:167–73. 10.1016/j.canlet.2012.12.026 [DOI] [PubMed] [Google Scholar]
- 96. Xiong G, Husseiny MI, Song L, Erdreich-Epstein A, Shackleford GM, Seeger RC, et al. Novel cancer vaccine based on genes of Salmonella pathogenicity island 2. Int J Cancer (2010) 126:2622–34. 10.1002/ijc.24957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ye J, Li L, Zhang Y, Zhang X, Ren D, Chen W. Recombinant Salmonella-based 4-1BBL vaccine enhances T cell immunity and inhibits the development of colorectal cancer in rats: in vivo effects of vaccine containing 4-1BBL. J BioMed Sci (2013) 20:8. 10.1186/1423-0127-20-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Shao C, Yang B, Zhao L, Wang S, Zhang J, Wang K. Tumor suppressor gene RBM5 delivered by attenuated Salmonella inhibits lung adenocarcinoma through diverse apoptotic signaling pathways. World J Surg Oncol (2013) 11:123. 10.1186/1477-7819-11-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ganai S, Arenas RB, Forbes NS. Tumour-targeted delivery of TRAIL using Salmonella typhimurium enhances breast cancer survival in mice. Br J Cancer (2009) 101:1683–91. 10.1038/sj.bjc.6605403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lee CH, Wu CL, Shiau AL. Endostatin gene therapy delivered by Salmonella choleraesuis in murine tumor models. J Gene Med (2004) 6:1382–93. 10.1002/jgm.626 [DOI] [PubMed] [Google Scholar]
- 101. Jia H, Li Y, Zhao T, Li X, Hu J, Yin D, et al. Antitumor effects of Stat3-siRNA and endostatin combined therapies, delivered by attenuated Salmonella, on orthotopically implanted hepatocarcinoma. Cancer Immunol Immunother (2012) 61:1977–87. 10.1007/s00262-012-1256-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Cao HD, Yang YX, Lu L, Liu SN, Wang PL, Tao XH, et al. Attenuated Salmonella typhimurium carrying TRAIL and VP3 genes inhibits the growth of gastric cancer cells in vitro and in vivo. Tumori (2010) 96:296–303. 10.1177/030089161009600218 [DOI] [PubMed] [Google Scholar]
- 103. Shi L, Yu B, Cai CH, Huang W, Zheng BJ, Smith DK, et al. Combined prokaryotic-eukaryotic delivery and expression of therapeutic factors through a primed autocatalytic positive-feedback loop. J Controll Release (2016) 222:130–40. 10.1016/j.jconrel.2015.12.005 [DOI] [PubMed] [Google Scholar]
- 104. Urashima M, Suzuki H, Yuza Y, Akiyama M, Ohno N, Eto Y. An oral CD40 ligand gene therapy against lymphoma using attenuated Salmonella typhimurium. Blood (2000) 95:1258–63. 10.1182/blood.V95.4.1258.004k18_1258_1263 [DOI] [PubMed] [Google Scholar]
- 105. Agorio C, Schreiber F, Sheppard M, Mastroeni P, Fernandez M, Martinez MA, et al. Live attenuated Salmonella as a vector for oral cytokine gene therapy in melanoma. J Gene Med (2007) 9:416–23. 10.1002/jgm.1023 [DOI] [PubMed] [Google Scholar]
- 106. Yuhua L, Kunyuan G, Hui C, Yongmei X, Chaoyang S, Xun T, et al. Oral cytokine gene therapy against murine tumor using attenuated Salmonella typhimurium. Int J Cancer (2001) 94:438–43. 10.1002/ijc.1489 [DOI] [PubMed] [Google Scholar]
- 107. Massa PE, Paniccia A, Monegal A, de Marco A, Rescigno M. Salmonella engineered to express CD20-targeting antibodies and a drug-converting enzyme can eradicate human lymphomas. Blood (2013) 122:705–14. 10.1182/blood-2012-12-474098 [DOI] [PubMed] [Google Scholar]
- 108. King I, Bermudes D, Lin S, Belcourt M, Pike J, Troy K, et al. Tumor-targeted Salmonella expressing cytosine deaminase as an anticancer agent. Hum Gene Ther (2002) 13:1225–33. 10.1089/104303402320139005 [DOI] [PubMed] [Google Scholar]
- 109. Yoon W, Choi JH, Kim S, Park YK. Engineered Salmonella typhimurium expressing E7 fusion protein, derived from human papillomavirus, inhibits tumor growth in cervical tumor-bearing mice. Biotechnol Lett (2014) 36:349–56. 10.1007/s10529-013-1370-8 [DOI] [PubMed] [Google Scholar]
- 110. Quintero D, Carrafa J, Vincent L, Bermudes D. EGFR-targeted Chimeras of Pseudomonas ToxA released into the extracellular milieu by attenuated Salmonella selectively kill tumor cells. Biotechnol Bioeng (2016) 113:2698–711. 10.1002/bit.26026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Bereta M, Hayhurst A, Gajda M, Chorobik P, Targosz M, Marcinkiewicz J, et al. Improving tumor targeting and therapeutic potential of Salmonella VNP20009 by displaying cell surface CEA-specific antibodies. Vaccine (2007) 25:4183–92. 10.1016/j.vaccine.2007.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Shi L, Yu B, Cai CH, Huang JD. Angiogenic inhibitors delivered by the type III secretion system of tumor-targeting Salmonella typhimurium safely shrink tumors in mice. AMB Express (2016) 6:56. 10.1186/s13568-016-0226-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Liang K, Liu Q, Li P, Han Y, Bian X, Tang Y, et al. Endostatin gene therapy delivered by attenuated Salmonella typhimurium in murine tumor models. Cancer Gene Ther (2018) 25:167–83. 10.1038/s41417-018-0021-6 [DOI] [PubMed] [Google Scholar]
- 114. Zheng JH, Nguyen VH, Jiang SN, Park SH, Tan W, Hong SH, et al. Two-step enhanced cancer immunotherapy with engineered Salmonella typhimurium secreting heterologous flagellin. Sci Trans Med (2017) 9(376):eaak9537. 10.1126/scitranslmed.aak9537 [DOI] [PubMed] [Google Scholar]
- 115. Loeffler M, Le’Negrate G, Krajewska M, Reed JC. Inhibition of tumor growth using salmonella expressing Fas ligand. J Natl Cancer Inst (2008) 100:1113–6. 10.1093/jnci/djn205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Yoon W, Park YC, Kim J, Chae YS, Byeon JH, Min SH, et al. Application of genetically engineered Salmonella typhimurium for interferon-gamma-induced therapy against melanoma. Eur J Cancer (Oxford Engl 1990) (2017) 70:48–61. 10.1016/j.ejca.2016.10.010 [DOI] [PubMed] [Google Scholar]
- 117. Loeffler M, Le’Negrate G, Krajewska M, Reed JC. Salmonella typhimurium engineered to produce CCL21 inhibit tumor growth. Cancer Immunol Immunother (2009) 58:769–75. 10.1007/s00262-008-0555-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Loeffler M, Le’Negrate G, Krajewska M, Reed JC. Attenuated Salmonella engineered to produce human cytokine LIGHT inhibit tumor growth. Proc Natl Acad Sci U S A (2007) 104:12879–83. 10.1073/pnas.0701959104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Loeffler M, Le’Negrate G, Krajewska M, Reed JC. IL-18-producing Salmonella inhibit tumor growth. Cancer Gene Ther (2008) 15:787–94. 10.1038/cgt.2008.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. al-Ramadi BK, Fernandez-Cabezudo MJ, El-Hasasna H, Al-Salam S, Bashir G, Chouaib S. Potent anti-tumor activity of systemically-administered IL2-expressing Salmonella correlates with decreased angiogenesis and enhanced tumor apoptosis. Clin Immunol (Orlando Fla) (2009) 130:89–97. 10.1016/j.clim.2008.08.021 [DOI] [PubMed] [Google Scholar]
- 121. Sorenson BS, Banton KL, Frykman NL, Leonard AS, Saltzman DA. Attenuated Salmonella typhimurium with IL-2 gene reduces pulmonary metastases in murine osteosarcoma. Clin Orthop Relat Res (2008) 466:1285–91. 10.1007/s11999-008-0243-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Wang YJ, Fletcher R, Yu J, Zhang L. Immunogenic effects of chemotherapy-induced tumor cell death. Genes Dis (2018) 5:194–203. 10.1016/j.gendis.2018.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Zhang Z, Yu X, Wang Z, Wu P, Huang J. Anthracyclines potentiate anti-tumor immunity: A new opportunity for chemoimmunotherapy. Cancer Lett (2015) 369:331–5. 10.1016/j.canlet.2015.10.002 [DOI] [PubMed] [Google Scholar]
- 124. Saltzman D, Augustin L, Leonard A, Mertensotto M, Schottel J. Low dose chemotherapy combined with attenuated Salmonella decreases tumor burden and is less toxic than high dose chemotherapy in an autochthonous murine model of breast cancer. Surgery (2018) 163:509–14. 10.1016/j.surg.2017.09.036 [DOI] [PubMed] [Google Scholar]
- 125. Yano S, Takehara K, Zhao M, Tan Y, Han Q, Li S, et al. Tumor-specific cell-cycle decoy by Salmonella typhimurium A1-R combined with tumor-selective cell-cycle trap by methioninase overcome tumor intrinsic chemoresistance as visualized by FUCCI imaging. Cell Cycle (Georgetown Tex) (2016) 15:1715–23. 10.1080/15384101.2016.1181240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Igarashi K, Kawaguchi K, Kiyuna T, Miyake K, Miyake M, Li S, et al. Tumor-targeting Salmonella typhimurium A1-R combined with recombinant methioninase and cisplatinum eradicates an osteosarcoma cisplatinum-resistant lung metastasis in a patient-derived orthotopic xenograft (PDOX) mouse model: decoy, trap and kill chemotherapy moves toward the clinic. Cell Cycle (2018) 17:801–9. 10.1080/15384101.2018.1431596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Hoffman RM. Tumor-targeting amino acid auxotrophic Salmonella typhimurium. Amino Acids (2009) 37:509–21. 10.1007/s00726-009-0261-8 [DOI] [PubMed] [Google Scholar]
- 128. Murakami T, Hiroshima Y, Miyake K, Kiyuna T, Endo I, Zhao M, et al. Efficacy of Tumor-Targeting Salmonella typhimurium A1-R against Malignancies in Patient-Derived Orthotopic Xenograft (PDOX) Murine Models. Cells (2019) 8(6):599. 10.3390/cells8060599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Davoodvandi A, Darvish M, Borran S, Nejati M, Mazaheri S, Reza Tamtaji O, et al. The therapeutic potential of resveratrol in a mouse model of melanoma lung metastasis. Int Immunopharmacol (2020) 88:106905. 10.1016/j.intimp.2020.106905 [DOI] [PubMed] [Google Scholar]
- 130. Mardani R, Hamblin MR, Taghizadeh M, Banafshe HR, Nejati M, Mokhtari M, et al. Nanomicellar-curcumin exerts its therapeutic effects via affecting angiogenesis, apoptosis, and T cells in a mouse model of melanoma lung metastasis. Pathol Res Pract (2020) 216:153082. 10.1016/j.prp.2020.153082 [DOI] [PubMed] [Google Scholar]
- 131. Fujimura T, Kambayashi Y, Ohuchi K, Muto Y, Aiba S. Treatment of Advanced Melanoma: Past, Present and Future. Life (Basel Switzerland) (2020) 10(9):208. 10.3390/life10090208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Zhang X, Cheng X, Lai Y, Zhou Y, Cao W, Hua ZC. Salmonella VNP20009-mediated RNA interference of ABCB5 moderated chemoresistance of melanoma stem cell and suppressed tumor growth more potently. Oncotarget (2016) 7:14940–50. 10.18632/oncotarget.7496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Chen J, Qiao Y, Tang B, Chen G, Liu X, Yang B, et al. Modulation of Salmonella Tumor-Colonization and Intratumoral Anti-angiogenesis by Triptolide and Its Mechanism. Theranostics (2017) 7:2250–60. 10.7150/thno.18816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Rezaei S, Mahjoubin-Tehran M, Aghaee-Bakhtiari SH, Jalili A, Movahedpour A, Khan H, et al. Autophagy-related MicroRNAs in chronic lung diseases and lung cancer. Crit Rev Oncol Hematol (2020) 153:103063. 10.1016/j.critrevonc.2020.103063 [DOI] [PubMed] [Google Scholar]
- 135. Nakatsugawa M, Takahashi A, Hirohashi Y, Torigoe T, Inoda S, Murase M, et al. SOX2 is overexpressed in stem-like cells of human lung adenocarcinoma and augments the tumorigenicity. Lab Invest (2011) 91:1796–804. 10.1038/labinvest.2011.140 [DOI] [PubMed] [Google Scholar]
- 136. Malinee M, Kumar A, Hidaka T, Horie M, Hasegawa K, Pandian GN, et al. Targeted suppression of metastasis regulatory transcription factor SOX2 in various cancer cell lines using a sequence-specific designer pyrrole-imidazole polyamide. Bioorg Med Chem (2020) 28:115248. 10.1016/j.bmc.2019.115248 [DOI] [PubMed] [Google Scholar]
- 137. Zhao C, He J, Cheng H, Zhu Z, Xu H. Enhanced therapeutic effect of an antiangiogenesis peptide on lung cancer in vivo combined with salmonella VNP20009 carrying a Sox2 shRNA construct. J Exp Clin Cancer Res (2016) 35:107. 10.1186/s13046-016-0381-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Gao S, Jung J-H, Lin S-M, Jang AY, Zhi Y, Bum Ahn K, et al. Development of Oxytolerant Salmonella typhimurium Using Radiation Mutation Technology (RMT) for Cancer Therapy. Sci Rep (2020) 10:3764. 10.1038/s41598-020-60396-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Miyake K, Murata T, Murakami T, Zhao M, Kiyuna T, Kawaguchi K, et al. Tumor-targeting Salmonella typhimurium A1-R overcomes nab-paclitaxel resistance in a cervical cancer PDOX mouse model. Arch Gynecol Obstet (2019) 299:1683–90. 10.1007/s00404-019-05147-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Murakami T, Hiroshima Y, Zhao M, Zhang Y, Chishima T, Tanaka K, et al. Adjuvant treatment with tumor-targeting Salmonella typhimurium A1-R reduces recurrence and increases survival after liver metastasis resection in an orthotopic nude mouse model. Oncotarget (2015) 6:41856–62. 10.18632/oncotarget.6170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Zhang Y, Zhang N, Hoffman RM, Zhao M. Salmonella typhimurium A1-R inhibits surgically induced breast cancer metastasis. San Diego, CA, USA: AACR; (2014). 10.1158/1538-7445.AM2014-712 [DOI] [Google Scholar]
- 142. Yoon WS, Kim S, Seo S, Park Y. Salmonella typhimurium with gamma-radiation induced H2AX phosphorylation and apoptosis in melanoma. Biosci Biotechnol Biochem (2014) 78:1082–5. 10.1080/09168451.2014.905173 [DOI] [PubMed] [Google Scholar]
- 143. Platt J, Sodi S, Kelley M, Rockwell S, Bermudes D, Low KB, et al. Antitumour effects of genetically engineered Salmonella in combination with radiation. Eur J Cancer (Oxford Engl 1990) (2000) 36:2397–402. 10.1016/S0959-8049(00)00336-1 [DOI] [PubMed] [Google Scholar]
- 144. Zhang M, Swofford CA, Forbes NS. Lipid A controls the robustness of intratumoral accumulation of attenuated Salmonella in mice. Int J Cancer (2014) 135:647–57. 10.1002/ijc.28700 [DOI] [PubMed] [Google Scholar]
- 145. Vola M, Monaco A, Bascuas T, Rimsky G, Agorio CI, Chabalgoity JA, et al. TLR7 agonist in combination with Salmonella as an effective antimelanoma immunotherapy. Immunotherapy (2018) 10:665–79. 10.2217/imt-2017-0188 [DOI] [PubMed] [Google Scholar]
- 146. Yano S, Takehara K, Zhao M, Tan Y, Han Q, Li S, et al. Tumor-specific cell-cycle decoy by Salmonella typhimurium A1-R combined with tumor-selective cell-cycle trap by methioninase overcome tumor intrinsic chemoresistance as visualized by FUCCI imaging. Cell Cycle (2016) 15:1715–23. 10.1080/15384101.2016.1181240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Murakami T, DeLong J, Eilber FC, Zhao M, Zhang Y, Zhang N, et al. Tumor-targeting Salmonella typhimurium A1-R in combination with doxorubicin eradicate soft tissue sarcoma in a patient-derived orthotopic xenograft (PDOX) model. Oncotarget (2016) 7:12783. 10.18632/oncotarget.7226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Kawaguchi K, Igarashi K, Murakami T, Chmielowski B, Kiyuna T, Zhao M, et al. Tumor-targeting Salmonella typhimurium A1-R combined with temozolomide regresses malignant melanoma with a BRAF-V600E mutation in a patient-derived orthotopic xenograft (PDOX) model. Oncotarget (2016) 7:85929. 10.18632/oncotarget.13231 [DOI] [PMC free article] [PubMed] [Google Scholar]