Abstract
Convalescent plasma is currently being used in the treatment of COVID-19. Recommendations regarding use convalescent plasma in COVID-19 requires systematic summaries of available evidence. We searched the databases Medline, Embase, Cochrane CENTRAL, Epistomonikos, Medrxiv and Biorxiv. Title/abstract screening, full text screening and data abstraction were carried out in duplicate by two reviewers. Pooled effect sizes and 95% confidence intervals were calculated using random effects meta-analysis. GRADE tool was used to rate the certainty of evidence. Twenty two studies were found eligible for inclusion: nine randomized controlled trials and thirteen cohort studies. Low certainty evidence from eight RCTs showed inconclusive effects of convalescent plasma on mortality at 28 days (OR 0.85, 95% CI 0.61 to 1.18). Low certainty evidence from thirteen cohort studies showed a reduction in mortality at 28 days (OR 0.66, 95% CI 0.53 to 0.82). The pooled OR for clinical improvement was 1.07 (95% CI 0.86 to 1.34) representing low certainty evidence. Evidence from three RCTs showed inconclusive effect of CP on the need for mechanical ventilation (OR 1.20, 95% CI 0.72 to 1.98). Four cohort studies reporting unadjusted estimates suggested a reduction in the need for mechanical ventilation with convalescent plasma (OR 0.80 95% CI 0.71 to 0.91, low certainty). Pooled estimates from 2 RCTs showed inconclusive effects of convalescent plasma on the proportion of patients with nondetectable levels of virus in nasopharyngeal specimens on day 3 (OR 3.62, 95% CI 0.43, 30.49, very low-quality evidence). The present review reports uncertain estimates on the efficacy of convalescent plasma in the treatment of COVID-19. There is low certainty evidence of a possible reduction in mortality and mechanical ventilation, a faster viral clearance and the absence of any serious adverse events. However, its efficacy for these outcomes requires evidence from good quality and adequately powered randomized controlled trials.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12288-021-01417-w.
Keywords: COVID-19 , Convalescent plasma, Plasma therapy, Meta-analysis, Systematic review
Introduction
The worldwide spread of COVID-19, the illness caused by SARS-CoV-2, poses an enormous threat to human health, and is a cause of major social and economic crises worldwide. As of January 2021, COVID-19 has resulted in more than 2 million deaths worldwide (1), and its transmission continues unabated in many parts of the globe.
The massive potential for adverse health consequences from this pandemic has led to a desperate need for interventions that can reduce the resulting morbidity and mortality. Research is ongoing to develop vaccines and identify therapeutics for COVID-19, including repurposing of medications [2]. A globally implemented, safe mass vaccination programme seems to be the only long term solution; [3]. Interventions with evidence of reducing morbidity and mortality from this disease are urgently required in order to form the bridge to the endgame of mass vaccination.
However, the use of medications without proven effectiveness may result in avoidable harm to patients, detract investment in other resources and erode public trust in the medical community [4, 5].
Convalescent plasma (CP) is currently being used in the treatment of COVID-19 with the rationale that it contains antibodies that potentially neutralize the virus and thus prevent the inflammatory cascade that ensues. It has been used historically for the treatment of infectious diseases and there is also some evidence of its usefulness in the treatment of other coronavirus infections like SARS and MERS [6, 7].
Recommendation regarding use convalescent plasma in COVID-19 requires systematic summaries of available evidence. At a time when research is being produced at an unprecedented pace, it is necessary to rigorously appraise the evidence to distinguish the trustworthy from the untrustworthy. Moreover, in the context of COVID-19, where the best evidence is constantly changing, it is required that we have the most updated summaries available for practice. Therefore, we conducted a systematic review and meta-analysis to study the efficacy and safety of convalescent plasma in patients with COVID-19.
Methodology
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) and Meta-analysis of observational studies in epidemiology (MOOSE) statements were adhered to in the present report [8, 9]. We developed a protocol before beginning this systematic review. The protocol for this review was registered in PROSPERO, with the registration number CRD42020227417.
Inclusion Criteria
The following inclusion criteria were used for eligibility:
Type of participants We included studies on patients with severe and non-severe COVID-19.
Type of intervention We included studies assessing intravenous convalescent or hyperimmune plasma as an intervention in addition to standard of care
Type of outcomes We included studies reporting the following outcomes:
Primary outcome: Overall mortality
Secondary outcomes:
Clinical recovery
Rate of ICU admission
Length of ICU stay
Length of hospital stay
Need for mechanical ventilation
Viral clearance
Adverse events: Intravascular volume overload related complications and transfusion-related acute lung injury, allergy or anaphylaxis, and any other patient-important safety outcomes that study reports
Type of Studies We included randomized controlled trials, cohort studies and case series that compared the use of convalescent plasma to treatment without convalescent plasma and reported on at least one of our outcomes of interest. We excluded case series in which all patients, or no patients, received convalescent plasma.
Data sources and Searches
We searched in the following databases: Medline (Ovid), Embase, Cochrane CENTRAL, Epistomonikos and ran a PubMed search for studies not yet indexed in Medline. We reviewed reference lists of all included studies and relevant systematic reviews for additional references. We searched medRxiv and biorxiv for any relevant pre-print previews.
No language restriction was imposed. The search strategy implemented is provided in Appendix 1. The search was updated till 15th January 2021.
Study Selection
Pairs of reviewers independently screened titles and abstracts, and reviewed the full texts of potential eligible studies to determine the final eligible studies. Disagreements were resolved by discussion or by referring to a third reviewer.
Data Extraction
Pairs of reviewers abstracted data. We abstracted surname of the first author, year of publication, country, region and hospital, population, interventions, and outcomes. For cohort studies, we also abstracted the method of adjustment used and covariates adjusted for in the analysis. Disagreements were resolved by discussion or, if necessary, by a third reviewer.
Risk of Bias Assessment
We assessed the risk of bias in randomized trials using the modified Cochrane tool that includes sequence generation, allocation sequence concealment, blinding, and missing outcome data. Each criterion was judged as definitely or probably low risk of bias, or probably or definitely high risk of bias [8]. We assessed risk of bias in cohort studies using the New Castle Ottawa scale for Cohort studies [9]. Two review authors independently assessed the study risk of bias with disagreements resolved by involving a third reviewer.
Data Synthesis or Analysis
We calculated pooled odds ratio (OR) and 95% confidence intervals (95% CI) for dichotomous outcomes. We used adjusted estimates from studies wherever reported, and used the random-effects models to pool study data. We used DerSimonian and Laird inverse variance random-effect models to pool adjusted odds ratios (ORs). We carried out all statistical analyses using Review Manager 5.3. We assessed heterogeneity in the meta-analyses by visual inspection of the forest plot and by the I2 statistic.
GRADE Assessment of the Overall Quality in the Body of Evidence by Outcome
We used the Grading of Recommendation, Assessment, Development, and Evaluation (GRADE) methodology to rate the certainty in evidence for each outcome as high, moderate, low, or very low [10]. The assessment included judgments addressing risk of bias, imprecision, inconsistency, indirectness, and publication bias.
We summarized the evidence both narratively and in GRADE evidence profiles.
Results
Study Selection
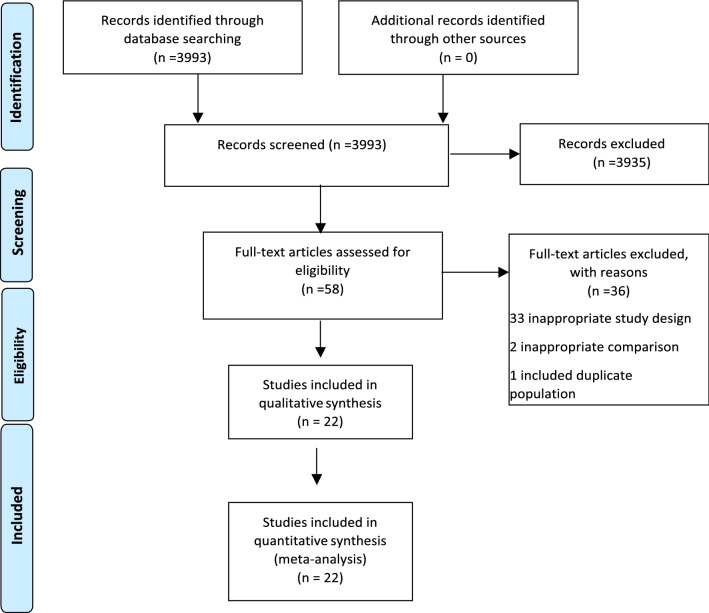
Our search yielded 3993 titles and abstracts - all were identified from the electronic database search. We excluded 3935 articles based on a review of the title and abstract, leaving 58 articles for full review. Of these, 36 were excluded- 33 for having an inappropriate study design, two for having inappropriate comparison, one for including a duplicate population. Twenty two studies were found eligible on full text screening. Nine of these were pre-print articles that had not been peer reviewed [12–16, 20, 24, 28, 32]. These 22 studies were included in the systematic review and meta-analyses. [11–32] (Fig. 1).
Fig. 1.
PRISMA Flow diagram of selection of studies for inclusion in meta-analysis
Study Characteristics and Estimates Reported
The studies included were nine randomized controlled trials (1254 patients) [11–16, 26–28] and thirteen cohort studies (8368 patients) [16–24, 29–32]. All studies included patients hospitalized with COVID-19 (Tables 1 and 2). Most studies included patients with severe COVID-19 except one study that included moderately ill patients [12].
Table 1.
Characteristics of included randomized controlled trials
| Study/year (country)(Reference) | Setting/inclusion criteria | Median age (IQR), % males | Time since symptom onset | Intervention | Control group | Sample size | Outcomes reported |
|---|---|---|---|---|---|---|---|
| Li 2020 China Multicenter[11] | Hospitalized adult 18 years or older, Positive PCR 72 hours or less prior to randomization with pneumonia on Chest radiograph and clinical symptoms consistent with severe or life threatening COVID-19 and S protein–RBD-specific (receptor binding domain) IgG antibody not ≥1:640CP: Severe disease=23/52; life threatening=29/52SOC: severe disease 22/51 life threatening 29/52 | CP: 70 (62–80), 52% malesStandard of care group: 69 (63–76), 65% males | CP: 27 days (22–39)SOC: 30 days (19–38) | S-RBD–specific IgG titer of at least 1:640 were used for this study. | Standard of care including one or more of antivirals, antibiotics, steroids, IVIg, Chinese herbal medications and supportive care | CP group=52, Control group=51 |
Time to clinical improvement (discharge or 2 point reduction on a 6 point severity scale) within a 28-day period. 28-day mortality, including analysis of time from randomization to death. Duration of hospitalization, including analyses of time from randomization to discharge, Time from admission to discharge, and 28-day discharge rates Conversion of nasopharyngeal swab viral PCR results from positive at baseline to negative at follow-up assessed at 24, 48, and 72 hours. |
| Agarwal 2020 India Multicentre[12] | Hospitalized adult 18 years or older; moderate COVID-19 (RR ≥24/min SpO2≤ 93% on room air or PaO2/FiO2 200–300 mm Hg; critically ill patients and those on vasopressors with MAP < 65 excluded; antibody titre not done in recipients prior to transfusion; done in donors and recipients after donation and NAb (1: 20 or more) found in 80% in SOC and 86% in CP arm | CP: 52(42–60) 75% malesStandard of care: 52(41–60) 77% males | CP: 8(6–11) daysSOC: 8 (6–11 days) | Plasma 200 mL 24 hours apart extracted from males or nulliparous females aged between 18 and 65 years, weighingover 50 kg, who had received a diagnosis of COVID-19 confirmed with a positive RT-PCR test, suffered from symptomatic COVID-19 with at least fever and cough, which had completely resolved for a period of 28 consecutive days prior to donation or a period of 14 days prior to donation with two negative SARS-CoV-2 RT-PCR tests from nasopharyngeal swabs collected 24 hours apart. | Standard of care which could be one or more of antivirals (Hydroxychloroquine, Remdesivir, Lopinavir/Ritonavir, Oseltamivir), broadspectrum antibiotics, immunomodulators (steroids, Tocilizumab) and supportive management (oxygen via nasal cannula, face mask, non-rebreathing face mask; non-invasive or invasive mechanical ventilation; awake proning) | CP+SOC=235, SOC=229 |
Composite measure of progress to severe disease (PaO2/FiO2 ratio <100) any time within 28 days of enrolment or all-cause mortality at 28 days. Clinical improvement and symptom resolution on day 7 Variation in fraction of inspired oxygen (Fio2%) on days 1, 3, 5, 7 and 14; Total duration of respirator support during hospitalization and post enrolment Duration of respiratory support till day 28 or discharge whichever is earlier; Negative conversion of SARS-CoV-2 viral RNA on days 3 and 7; Requirement of vasopressor support Clinical improvement on WHO ordinal scale on day 0,1,3,5,7,14 and 28 days. Frequency of minor and serious adverse event (death and invasive mechanical ventilation, hemodynamic instability) within 6 hours of CP transfusion. |
| Gharbharan 2020 Netherlands Multicentre [13] | Hospitalized patients atleast 18 years, COVID-19 disease proven by a positive SARS-CoV-2 reverse transcriptase polymerase chain reaction (RT-PCR) test in the previous 96 hours. Patients on mechanical ventilation for >96 hours excluded | CP+SOC: 61 (56–70) 61% malesSOC: 63 (55–77) 77% males | CP+SOC: 9 (7–13 days)SOC: 11 (6–16 days) | 300 mL single dose plasma; Plasma was extracted from patients with RT-PCR confirmed SARS-CoV-2 infection and be asymptomatic for at least 14 days. Only plasma with antiSARS-CoV-2 neutralizing antibodies confirmed by a SARS-COV-2 plaque reduction neutralization test (PRNT) and a PRNT50 titer of at least 1:80 was used. | Standard of care chloroquine, azithromycin, lopinavir/ritonavir, tocilizumab, anakinra) | CP+SOC=43, SOC=43 |
Overall mortality until discharge from the hospital or a maximum of 60 days after admission whichever came first. improvement on the 8-point WHO COVID-19 disease severity scale from inclusion to day 15, Hospital length of stay and Safety |
| Sola 2020 Spain Multicentre [14] | Patients hospitalized for laboratory-confirmed SARS-CoV-2 infection (RT-PCR) with either radiographic evidence of pulmonary infiltrates or clinical evidence plus SpO2 ≤94% on room air, and within 12 days from the onset of symptoms (fever or cough). Patients were excluded if already on mechanical ventilation (invasive or non-invasive) or high flow oxygen devices | CP+SOC: 61 (46–74), 53% malesSOC: 58 (51-73), 56% males | CP+SOC: 8 days (7–9)SOC: 8 (6-9 days) | 200-300 mL single dose Plasma was extracted from CP donors who had laboratory confirmed SARSCoV-2 infection, anti-SARS-CoV-2 IgG (ratio ≥1.1 with the Euroimmun ELISA test; Euroimmun, Lübeck, Germany) and were asymptomatic for at least 14 days. Titres were not used to select donors or recipientsHowever all units had titers >1:80, median titer 1:292, IQR 238–451; pseudovirus neutralizing ID50 assay: median titer 1:327, IQR 168–882, two CP units had ID50 titer <1:80). | SOC included all supportive and specific treatments with off-label marketed medicines used according to local or national recommendations | CP+SOC=38, SOC=43 |
Proportion of patients in categories 5, 6 or 7 at day 15 of the study. Time to improvement of one category on the ordinal scale; Mean change in the ordinal scale from baseline to days 3, 5, 8, 11, 15 and 29; Proportion of patients in categories 5, 6 or 7 at day 29; Mortality at days 15 and 29; Duration of hospital stay; number of days alive and free from oxygen support; Number of days alive and free from mechanical ventilation. Serial naso/oropharyngeal swabs and blood samples were collected at days 3, 5, 8, 11, 15 and 29, and tested for SARS-CoV- 2 RNA by RT-PCR assay. Serious adverse events (AE), grade 3 or 4 AE and infusion related AE (within 24 hours after administration |
| Bajpai 2020 India [15] | SARS-CoV-2 infection (positive by real-time PCR assay) patient, with severe COVID-19 [respiratory rate (RR)30/min, oxygen saturation level less than 93% in resting state, the partial pressure of oxygen (PaO2)/oxygen concentration (FiO2) 300 mmHg, lung infiltrates >50% within 24 to 48 hours]. | CP: mean age±SD 48.1 ± 9.1, 78.6% maleFFP: mean age±SD 48.3 ± 10.8, 73.3% male | NR | Median volume of plasma collected 500ml, Donor median neutralizing antibody titre >80, Donor median S1 RBD IgG antibody titre> 640 | FFP | CP: 14, FFP: 15 |
Proportion of patients remaining free of mechanical ventilation Mortality at day 8and 28 Improvement in PaO2/FiO2 SOFA scores reduction at 48 hours and day 7 Duration of hospital stay, duration of Intensive Care Unit stay, Requirements of vasopressors and days free of dialysis up to 28 days from randomization |
| Al Qahtani 2020 Bahrain (16) | Hospitalized, aged at least 21 years; COVID-19 diagnosis based on polymerase chain reaction (PCR) testing; Hypoxia (Oxygen saturation of89 less than or equal 92% on air, or PO2 < 60mmHg in arterial blood gas, or arterial partial pressure90 of oxygen (PaO2)/fraction of inspired oxygen (FIO) of 300 or less) and patient requiring oxygen91 therapy, pneumonia confirmed by chest imaging. | CP: mean age±SD 52.6 ± 14.9, 85% maleControl: mean age±SD 50.7 ± 12.5, 75% male | NR | Dosage of CP: 400 mL, given as 200ml over 2hrs over 2 successive days | Paracetamol antiviral medications, Tocilizumab and antibacterial medication. | CP:20, Standard of care: 20 |
Requirement for invasive or non-invasive ventilation Duration of ventilation. C-reactive protein, procalcitonin, lactate dehydrogenase Troponin, ferritin, D-Dimer, brain natriuretic peptide, lactate changes 28 day mortality rate |
| Simonovich 2020, 12 sites in Argentina (26) | Hospitalized 18 year or older patients with RT-PCR positive for SARS-CoV-2, and radiologically confirmed pneumonia, with at least one of the following severity criteria: oxygen saturation (SaO2) below 93% while they were at rest and breathing ambient air, PaO2:FiO2<300, or a Sequential Organ Failure Assessment (SOFA) or modified SOFA (mSOFA) score of two or more points above baseline status (scores range from 0 to 24, with higher scores indicating more severe disease). | CP 62.5(53–72.5 years) 70.6% malesSOC 62 (49–71 years) males 61% | 8 days (5–10 days) | Covid-19 convalescent Convalescent plasma was from a single donor or from a pool of two to five donors. Specific SARS-CoV-2 IgG antibody titer was measured in each convalescent plasma pool before transfusion. The total antibody titer goal in convalescent plasma was above 1:800 in all cases along with standard of care | Placebo: Normal saline along with standard of care including antiviral agents, glucocorticoids, or both according to the standard of care at the provider health care institution. | CP: 228, placebo: 105 |
Patient’s clinical status 30 days after the intervention, as measured on a six-point ordinal scale ranging from total recovery to death Mortality at 30 days Antibody titre on day 2 after intervention |
| Libster 2020 Argentina [27] | Patients with RTPCR proven COVID-19 who were 75 years of age or older, irrespective of current coexisting conditions, or between 65 and 74 years of age with at least one coexisting condition including hypertension, diabetes, obesity, chronic renal failure, cardiovascular disease, and COPD. Exclusion criteria included severe respiratory disease | The mean (±SD) age of the patients was 77.2±8.6 years, and 100 patients (62%) were women. 45% patients were 65 to 74 years of age and 55% were 75 years of age or older. | CP arm 39.6±13.9 hours Placebo arm 38.3±14.3 hours | 250 ml of convalescent plasma with an IgG titer greater than 1:1000 against SARS-CoV-2 spike (S) protein (COVIDAR IgG, Instituto Leloir, Argentina). | 250 ml of placebo (0.9% normal saline) along with standard of care | CP: 80, Placebo: 80 |
Severe respiratory disease Life threatening respiratory disease Critical systemic illness Death |
| Ray 2020, Single centre, Kolkata, India [28] | RT-PCR proven COVID-19 with severe disease (fever or suspected respiratory infection, plus one of the following; respiratory rate >30 breaths/min, severe respiratory distress, SpO2< 90% at room air) with mild ARDS, defined as PaO2)/FiO2) 200-300 mmHg or moderate ARDS, defined as PaO2/FiO2 100-200 mmHg, not on mechanical ventilation | Female 61.43±11.33 years; Male: 61.36±12.17 years71.25% males | 4.2±2.21 days for CP arm3.85±2.63 days for SOC arm | 2 doses of 200 mL of convalescent plasma with neutralizing antibody value of 1.5 for the ratio optical density between the sample and calibrator | Standard of care including Hydroxychloroquine 400 mg BD on first day followed by 400 mg OD for four days, Azithromycin 500mg OD for 5 days, Ivermectin 12 mg OD for 5days and Doxycyclin 100 mg BD for 10 days, corticosteroids and anticoagulation | CP: 40 SOC: 40 | All cause mortality at 30 days |
NR: Not reported, SOC: standard of care
Table 2.
Characteristics of included cohort studies
| Study | Country, region and hospital | Study design | Population | Interventions | Adjustment | Outcomes |
|---|---|---|---|---|---|---|
| Rasheed 2020 [17] | Baghdad, Iraq; 3 centres | Cohort study | 21 patients; critically ill COVID admitted in RCU for less than 3 days; severe organ dysfunction excluded; late ARDS excluded. 28 patients who did not receive CP | Plasma recovered from moderate COVID-19 patients two weeks previously recovered patients from COVID-19 2 weeks prior to donation younger than 50 years, healthy, non-pregnant females, with no comorbidities. Only the donors with IgG index equal or more than 1.25 on ELISA for SARS-CoV-2 were selected | Not done | Allergic reaction to CP at 3 hours; Recovery time from critical illness; Mortality rate; duration of infection |
| Abolghasemi 2020 [18] | Iran; multiple centres | Cohort study | 115 patients Age ≥ 18 years 2 Confirmed COVID-19 infection through laboratory (RT-qPCR) and/ or lung involvement confirmed with chest imaging (CT scan) 3 Presence of some or all of disease clinical symptoms such as shortness of breath (dyspnea), respiratory frequency ≥ 20/min, fever and cough 4 Hospitalized with a blood oxygen saturation (SPO2) ≤93 % at rest on room air 5 ≤7 days since illness onset 6 Willingness to participate in the trail and sign the consent form Exclusion Criteria - Patients with either of following criteria excluded from the trail: 1 Intubated patients or patients on mechanical ventilation. 2 Severe liver or kidney diseaseSeptic Shock 6 Known hypersensitivity to plasma. Compared with 74 controls | 500 mL Convalescent plasma was extracted from clinically and laboratory-confirmed recovered patients of COVID-19 who were between 18–60 years old. To prevent transfusion related acute lung injury (TRALI) female donors with a history of pregnancy were excluded. Selected donors had negative qRT-PCR for COVID-19 and no symptoms for atleast 14 days and other standard virology tests at the time of donation while their test results had been previously positive by qRT-PCR for COVID-19. Donated plasmas contained antibody titer cut off index higher than 1.1by ELISA VsStandard of care | Not adjusted for baseline variables; disease severity scores; other co-interventions | All cause mortality; length of hospital stay; patients discharged less than 5 days from hospitalization; Intubation |
| Omrani 2020 [19] | Qatar; multiple centres | Cohort study | 40 patients RT PCR proven severe COVID-19 (any one or more of the following is present: respiratory rate >30/min, oxygen saturation ≤90% while in ambient room air, partial pressure of oxygen–oxygen concentration (PaO2/FiO2) ≤ 300 mmHg, hypotension, or any organ failure). 40 patients who did not receive CP | 400 mL convalescent plasma derived from recovered adult COVID‐19 patients symptoms resolved more than 2 weeks ago, with documented negative upper airway SARS‐CoV‐2 RT‐PCR and negative serological tests for syphilis and blood‐borne viruses;Administered at a median of 10(IQR 9-10) days after symptom onset Antibody titre not done in donor plasma | Adjusted for invasive mechanical ventilation; use of methylprednisolone and APACHE II score at baseline; not adjusted for other co-interventions such as TCZ, antivirals, anticoagulation, HCQ, vasopressor use; dialysis requirement etc | Improvement in respiratory support; all cause mortality at 28 days; viral clearance |
| Rogers 2020 [20] | Rhode Island, USA; 3 centres | Cohort study | 64 patients who received CP a median of 7 days after symptom onset COVID-19 symptom onset ≤ 10 days prior, requiring supplemental oxygen (but not invasive ventilation), no evidence of current hypercoagulability (D-dimer > 1000 µg/L, clinical signs of thrombosis); control group of 177 patients | 1 or 2 units of plasma Donor characteristics not mentioned; antibody levels probably not checked | Age, gender, race, baseline oxygen requirements, remdesivir use, and corticosteroid use; AI of plasmaNot adjusted for TCZ, anticoagulation, HCQ use | Primary outcome of this study was the impact of CP treatment on all cause in-hospital mortality; the secondary outcome was the impact of CP treatment on the time to hospital discharge. All outcomes were censored at day 28 |
| Salazar E 2020 [21] | Houston Methodist hospital; eight Centres in USA | Cohort study | 136 patients with Severe and life threatening COVID-19 disease Severe disease was defined as one or more of the following: shortness of breath (dyspnea), respiratory rate 30/minute, blood oxygen saturation 93% (on room air), partial pressure of arterial oxygen/fraction of inspired oxygen ratio <300, and/or pulmonary infiltrates >50% within 24 to 48 hours (of screening assessment). Life-threatening disease was defined as one or more of the following: respiratory failure, septic shock, and/or multiple organ dysfunction or failure. 251 non transfused controls | One or 2 units (vol not mentioned) Convalescent plasma was extracted from SARS-CoV-2 RT-PCR positive donors 18-65 years had recovered and were asymptomatic for >14 days and tested negative for SARS-CoV-2 at the time of plasmapheresisTitres were not criteria for donor | Adjusted for steroids, remdesivir and TCZPropensity matching for respiratory status | Mortality |
| Xia 2020 [22] | Wuhan, China; single centre | Cohort | 138 patients with severe or critical COVID-191430 non transfused controls | Convalescent plasma extracted from patients >3 weeks after symptom onset agedbetween 18-55 4–5 mL/kg of Convalescent plasma with titre >1:160 were transfused | Not adjusted; no mention of other therapy that might affect prognosis | Mortality; clinical improvement on six point scale |
| Liu 2020 [23] | USA; Mount Sinai Hospital | Cohort study | Adult patients (39 patients) with severe to life threatening COVID-19 who received CP were compared with propensity score matched (1:4 and 1:2) | Volume of plasma: not mentioned; Only donors with an MSH-ELISA serum IgG titer of ≥1:320 (n=25) were referred for plasmapheresis | Standard of care | Oxygen requirement at d14; survival |
| Salazar M 2020 [24] | Argentina; muticentre | Cohort | 868 consecutive patients ≥18 years diagnosed with SARSCoV-2 with RT-PCR, with lung infiltrates, plus one of the following:- Dyspnea with respiratory rate ≥ 30 breaths/minute- Oxygen saturation ≤93%- Oxygen requirement- PaO2FIO2 <300 mmHg- Increase in lung infiltrates >50% during the previous 24-48 hours- Alteration in consciousness- Multiple organ dysfunction- Age >65 years | 200–400 mL of convalescent plasma with Ig-G antibody titer ≥1:400 | Age, ICU admission, mechanical ventilation; diabetes and preexisting cardiovascular disease | 28 day mortality; duration of ICU stay |
| Altuntas 2020 [25] | Turkey; multiple centres | Cohort | Severe or critically ill COVID-19 patients who received anti-SARS-CoV-2 antibody-containing CP along with the antiviral treatment (n = 888)Anti-SARS-CoV-2 Ig G antibodies were not routinely screened in COVID-19 patients before CP treatment | 200–600 mL of plasma collected from patients with COVID-19 documented by a laboratory test (b) Resolution of symptoms at least 14 days prior to donation and negative results for COVID-19. All CP donors were screened for the presence of anti-SARS-CoV-2 Ig G antibodies Titer of neutralizing antibody was not routinely performed | Age-gender, comorbidity, and other COVID-19 treatments (favipravir, lopinavir + ritonavir, hydroxychloroquine, high dose vitamin C, azithromycin) matched severe or critically ill COVID-19 patients at 1:1 ratio (n = 888) were used for comparison | Duration of hospital/ICU stay; mechanical ventilation rate; vasopressor requirement; case fatality rate |
| Hegerova 2020 [29] | Seattle, 5 hospitals | Cohort | Twenty patients with severe or critical COVID-19 diagnosed using RT-PCR were treated with one unit of CP | Plasma collected from COVID-19–recovered donors aged from 29 to 79 years after atleast 28 days past their symptom onset. Anti–SARS-CoV-2 immunoglobulin G serology, as determined by the Abbott ARCHITECT, was positive in all but 1 donor | Control patients were well matched with regard to age, number of comorbidities, WHO score, sequential organ failure assessment score, and severity of illness. | WHO ordinal clinical score; mortality; Length of hospitalization |
| Jiang 2020 [30] | Nanjing. China | Cohort | 163 cases received CP and 163 matched controls received the standard treatment diagnosed as COVID-19 were included. 43.56% were male. Mean age 64.07 ± 13.37 years; 65.64% > 60 years old. 48.47% cases and 77 47.24% controls had hyperlipidemia, diabetes mellitus, coronary heart disease or tumor | Volume of CP infused and antibody titres have not been mentioned | Propensity score matched controls were enrolled. Matching parameters not mentioned | DeathDuration of Hospitalization |
| Alsharidah 2020 [31] | Kuwait; 4 hospitals | Cohort | 135 patients received CP and 233 controls with laboratory diagnosis of SARS-CoV-2 infection and moderate or severe COVID-19 according to the WHO classification at admission | 200 mL of plasma collected from donors with positive EUA-approved qualitative serological test for SARS-CoV-2 IgG antibodies after recovery. Donors who tested negative or were positive only for SARS-CoV-2 IgM were excluded | For each patient who received CCP the first two patients with the same disease severity strata admitted on that calendar date from the same participating center were included as control; Adjusted for age, baseline oxygen saturation < 88%, lymphocyte count, and C-reactive protein | Clinical recovery at 30 days; time to clinical recovery |
| Yoon 2020 [32] | New York single hospital | Cohort | 90 patients with serious or life-threatening COVID-19 | 200 mL CP with SARS-CoV-2 spike protein IgG endpoint titers Median IgG, IgM and IgA titers were, respectively, 1:47,385 (interquartile range [IQR], 21,870 – 65,610; n = 46), 1:810 (IQR, 810 – 2,430; n = 43) and 1:90 (IQR, 90 – 270; n = 43) | 258 Propensity score matched controls | Clinical status and mortality on day 28 post-transfusion |
Risk of Bias Assessment
All trials were assessed to have low risk of bias for adequate random sequence generation and allocation concealment. Risk of bias for selective outcome reporting and missing outcome data was assessed to be low as most studies reported important outcomes and very less loss to follow up. However, only two trials were blinded and four were stopped early [11, 13, 14, 27]. Three trials reported stopping early because the epidemic had considerably diminished and the trial saw low enrolment [11, 14, 27]; while one reported stopping early since a high proportion of patients were found to have SARS-CoV-2 antibodies at baseline [13]. All trials were also assessed to be high risk of bias due to imbalance of co-interventions amongst the two arms.
For all outcomes in the cohort studies, risk of bias was low for selection of exposed and non-exposed population and assessment of exposure. All cohort studies were assessed to have low risk of bias from outcome being present at the start of the study. However, adequate adjustment and assessment of prognostic factors were not carried out by three studies. Follow up was adequate for all outcomes in the cohort studies; however, no study documented similar co-interventions in both groups (Tables 3 and 4).
Table 3.
Risk of bias of included randomized controlled trials
| Study (Reference) | Sequence generation | Allocation sequence concealment | Blinding | Missing outcome data | Selective outcome reporting | Other BIAS |
|---|---|---|---|---|---|---|
| Li 2020 [11] | Definitely low | Definitely low | Definitely high | Definitely low | Definitely low | Probably high1,2 |
| Agarwal 2020 [12] | Definitely low | Definitely low | Definitely high | Probably low | Definitely low | Probably high2 |
| Gharbharan 2020 [13] | Definitely low | Definitely low | Definitely high | Probably low | Definitely low | Probably high1,2 |
| Sola 2020 [14] | Probably low | Probably low | Definitely high | Definitely low | Definitely low | Probably high1,2 |
| Bajpai 2020 [15] | Probably low | Probably low | Definitely high | Definitely low | Definitely low | Probably high2 |
| AlQahtani 2020 [16] | Definitely low | Unclear | Definitely high | Definitely low | Definitely low | Probably high2 |
| Simonovich 2020 [26] | Definitely low | Definitely low | Definitely low | Definitely low | Definitely low | Definitely low |
| Libster 2020 [27] | Definitely low | Definitely low | Definitely low | Definitely low | Definitely low | Probably high1 |
| Ray 2020 [28] | Unclear | Unclear | Definitely high | Definitely low | Definitely low | Probably low |
1 Study terminated early
2 Imbalance in cointerventions
Table 4.
Risk of bias of included cohort studies
| Study (Reference) | From the same population | Assessment of exposure | Outcome present at start | Adjustment | Assessment of prognostic factors | Assessment of outcome | Adequate follow-up | Co-Interventions similar |
|---|---|---|---|---|---|---|---|---|
| Rasheed 2020 [17] | Definitely low | Definitely low | Definitely low | Definitely high | Probably high | Probably high | Probably low | Probably high |
| Abolghasemi 2020 [18] | Definitely low | Definitely low | Definitely low | Definitely high | Definitely high | Definitely high | Probably low | Probably high |
| Omrani 2020 [19] | Definitely low | Definitely low | Definitely low | Definitely lowa | Definitely low | Probably highb | Probably low | Probably high |
| Rogers 2020 [20] | Definitely low | Definitely low | Definitely low | Definitely lowe | Definitely low | Definitely low | Probably low | Definitely high |
| Salazar E 2020 [21] | Definitely low | Definitely low | Definitely low | Definitely lowf | Definitely low | Probably high | Probably low | Definitely high |
| Xia 2020 [22] | Definitely low | Definitely low | Definitely low | Definitely high | Definitely high | Probably highg | Probably low | Definitely high |
| Liu 2020 [23] | Definitely low | Definitely low | Definitely low | Definitely lowc | Definitely low | Definitely lowd | Probably low | Definitely high |
| Salazar M 2020 [24] | Definitely low | Definitely low | Definitely low | Definitely lowh | Probably highj | Probably highg | Probably low | Definitely high |
| Altuntas 2020 [25] | Definitely low | Definitely low | Definitely low | Definitely lowi | Probably highk | Probably highg | Probably low | Definitely high |
| Hegerova 2020 [29] | Definitely low | Definitely low | Definitely low | Definitely low | Probably low | Probably high | Probably low | Definitely low |
| Jiang 2020 [30] | Definitely low | Definitely low | Definitely low | Probably low | Probably low | Probably high | Probably low | Definitely high |
| Alsharidah 2020 [31] | Definitely low | Definitely low | Definitely low | Definitely low | Probably high | Probably high | Probably low | Definitely high |
| Yoon 2020 [32] | Probably low | Probably low | Definitely low | Probably low | Probably low | Probably low | Probably low | Probably high |
a Adjusted for invasive mechanical ventilation; use of methylprednisolone and APACHE II score at baseline; hight adjusted for other co-interventions such as TCZ, antivirals, anticoagulation, HCQ, vasopressor use; dialysis requirement etc
b high risk of bias for respiratory support and viral clearance; low risk for survival
c Propensity matched controls
d Hight blinded; high risk for O2 requirement; low risk for survival
e Age, gender, race, baseline oxygen requirements, remdesivir use, and corticosteroid use; AI of plasma, hight adjusted for TCZ, anticoagulation, HCQ use
f Adjusted for steroids, remdesivir and TCZ, propensity matching for respiratory status
g low risk of bias for mortality; high for SCSS
h Adjusted for age, ICU admission, mechanical ventilation; diabetes and preexisting cardiovascular disease
i Adjusted for age, gender, comorbidity, and other COVID-19 treatments (favipravir, lopinavir + ritonavir, hydroxychloroquine, high dose vitamin C, azithromycin)
j Other interventions with potential to change outcome have hight been collected or compared between the groups
k Baseline severity has hight been compared between CP arm and standard of care arm. Other interventions with potential to change outcome were matched when selecting controls
Pooled Effects of Convalescent Plasma on Safety and Efficacy Outcomes
Mortality
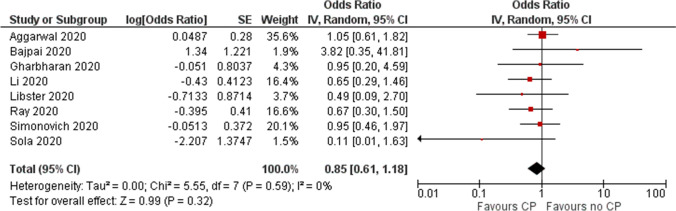
Low certainty evidence from 8 RCTs (n = 1374) (11−15, 26-28) showed inconclusive effects of convalescent plasma on mortality at 28 days with a possible but uncertain reduction in mortality (OR 0.85, 95% CI 0.61 to 1.18, I2=0%) (Fig. 2 and Table 5).
Fig. 2.
Effect of convalescent plasma on mortality: evidence from RCTs
Table 5.
GRADE assessment and summary of findings
| Certainty assessment | Summary of findings | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Participants (studies) | Risk of bias | Inconsistency | Indirectness | Imprecision | Other bias | Overall certainty of evidence | Study event rates (%) | Relative effect (95 % CI) | Anticipated absolute effects | ||
| With no CP | With CP | Risk with no CP | Risk difference with CP | ||||||||
| Mortality- RCTs | |||||||||||
| 1374 (8 RCTs) | serio usa | not serious | not serious | serious | none | ◯◯ LOW | 87/62 5 (13.9 %)c | 93/74 9 (12.4 %) | OR 0.85 (0.61 to 1.18) | 139 per 1,00 0 | 18 fewer per 1,000 (from 49 fewer to 21 more) |
| Mortality - Cohort | |||||||||||
| 8368 (13 observa tional studies) | serio usb | not serious | not serious | not serious | all plausible residual confounding would reduce the demonstrated effect | ◯◯ LOW | 1548/ 5692 (27.2 %)c | 536/2 676 (20%) | OR 0.66 (0.53 to 0.82) | 272 per 1,00 0 | 74 fewer per 1,000 (from 107 fewer to 37 fewer) |
| Viral clearance- day 3 | |||||||||||
| 609 (3 RCTs) | serio us | seriousd | not serious | serious | none | ◯ ◯◯ VERY LOW | 94/29 9 (31.4 %)c | 142/3 10 (45.8 %) | OR 3.37 (0.89 to 12.7 3) | 314 per 1,00 0 | 293 more per 1,000 (from 25 fewer to 539 more) |
| Clinical improvement | |||||||||||
| 522 (3 RCTs) | serio us | seriouse | not serious | not serious | none | ◯◯ LOW | 107/1 99 (53.8 %) | 207/3 23 (64.1 %) | OR 1.07 (0.86 to 1.34) | 538 per 1,00 0 | 17 more per 1,000 (from 38 fewer to 71 more) |
| LOS | |||||||||||
| 2269 (2 RCTs and one observa tional study) | serio us | seriousf | not serious | serious | all plausible residual confounding would reduce the demonstrated effect | ◯ ◯◯ VERY LOW | 1132 | 1137 | – | The median LOS was 13 day s g | MD 0.97 days lower (3.28 lower to 1.33 higher) |
| Mechanical ventilation - RCT | |||||||||||
| 522 (3 RCTs) | serio us | not serious | not serious | serious | none | ◯◯ LOW | 29/20 0 (14.5 %) | 66/32 2 (20.5 %) | OR 1.20 (0.72 to 1.98) | 145 per 1,00 0 | 24 more per 1,000 (from 36 fewer to 106 more) |
| Mechanical ventilation - Cohort studies | |||||||||||
| 5206 (4 observa tional studies) | serio us | not serious | not serious | not serious | all plausible residual confounding would reduce the demonstrated effec | ◯◯ LOW | 1091/ 3318 (32.9 %)c | 648/1 888 (34.3 %) | OR 0.80 (0.71 to 0.91) | 329 per 1,00 0 | 47 fewer per 1,000 (from 71 fewer to 20 fewer) |
CI: Confidence interval; OR: Odds ratio; MD: Mean difference
a Included trials were unblinded and reported unbalanced co-interventions. Three trials were stopped early
b Included unadjusted estimates from four cohort studies
c Baseline risk was obtained from COVID-19 patients that had not been administered CP in studies included in the meta-analysis for the outcome.
d I 2=87%
e Clinical improvement was measured on two different ordinal scales in the included trials
f I 2=82%
g Baseline risk from a study of the COVID-19 patients without CP use: Guan W et al. https://doi.org/10.1056/NEJMoa2002032
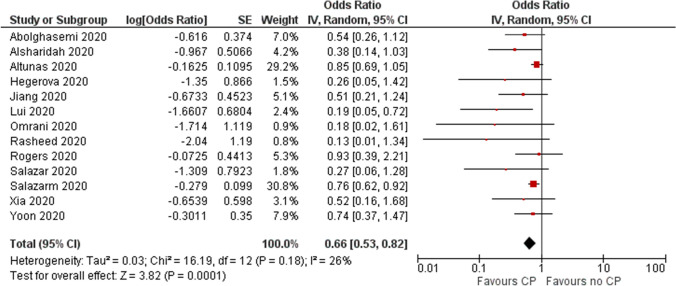
Low certainty evidence from 13 cohort studies [17–25, 29–32] (n=8368) showed a reduction in mortality at 28 days (OR 0.66, 95% CI 0.53 to 0.82, I2=26%). (Fig. 3 and Table 5).
Fig. 3.
Effect of convalescent plasma on mortality: evidence from cohort studies
Clinical Improvement
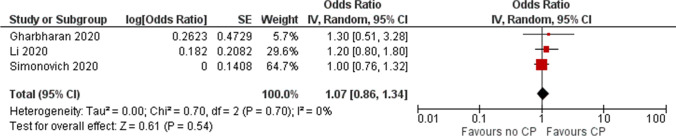
Two RCTs reported ordinal outcomes relating to extent of clinical improvement at 30 days and one RCT at 14 days. The pooled OR for clinical improvement (522 patients) [11, 13, 26] was OR 1.07 (95% CI 0.86 to 1.34) representing low certainty evidence (Fig. 4 and Table 5).
Fig. 4.
Effect of convalescent plasma on clinical improvement
Duration of Hospital Stay
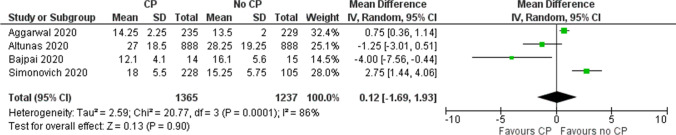
Evidence from 3 RCTs and one cohort study [12, 15, 25, 26] suggested uncertain effect of convalescent plasma on decreasing length of hospital stay (MD 0.12, 95% CI –1.69 to 1.93) (Fig. 5 and Table 5).
Fig. 5.
Effect of convalescent plasma on length of stay
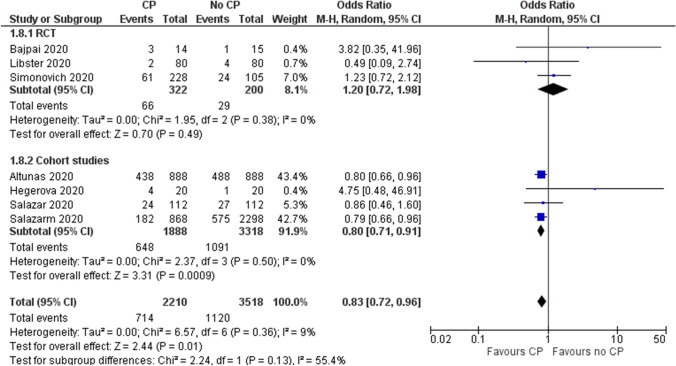
Need for Mechanical Ventilation
Evidence from three RCTs (n=522) [12, 16, 26] showed inconclusive effect of CP on need for mechanical ventilation (OR 1.20, 95% CI 0.72 to 1.98, I2=0%, low certainty). However, four cohort studies reporting unadjusted estimates suggested a reduction in the need for mechanical ventilation with convalescent plasma (OR 0.80 95% CI 0.71 to 0.91, I2=0%, low certainty) (Fig. 6 and Table 5).
Fig. 6.
Effect of convalescent plasma on need for mechanical ventilation
Viral Clearance
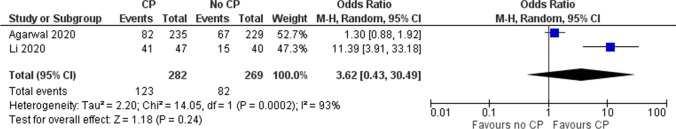
One RCT (n=522) [12] showed higher proportion of patients with undetectable virus on day 7 (OR 1.52, 95% CI 1.06, 2.20).
Pooled estimates from 2 RCTs (n = 609) [11, 12] showed inconclusive effects of convalescent plasma on the proportion of patients with nondetectable levels of virus in nasopharyngeal specimens on day 3 (OR 3.62, 95% CI 0.43, 30.49, very low-quality evidence) (Fig. 7 and Table 5).
Fig. 7.
Effect of convalescent plasma on viral clearance at day 3
There were very few adverse events reported in all included studies, with most trials and cohort studies not reporting any adverse events.
Discussion
The results of our meta-analysis show that there is very low to low certainty evidence to suggest that CP does not reduce mortality, hospital stay, need for mechanical ventilation or improvement in clinical status. Based on a single study, whether CP improves viral clearance is not clear.
The theory behind using CP for treatment goes back to the early days of antimicrobial therapy when it was used for pneumococcal pneumonia [6]. More recently, it has been used in the treatment for various viral diseases like SARS, MERS and Ebola [7]. The rationale is that antibodies in CP have the capacity to work in two different ways: first is the potential to act on the microbes causing the infection, and the second is that they can exert immunomodulatory therapy, thus boosting the immunity of the patient.
The included studies demonstrated a very good safety profile for CP. There is a theoretical possibility of antibody dependent enhancement [33], though there are no reports of this from trials.
However, the use of convalescent plasma as therapy for COVID-19 is not without challenges. Only limited quantities of plasma can be obtained at a time and its collection consumes resources. There is considerable difference in the titres, antibody response and type of antibodies amongst people. Besides this, though most studies did not report many adverse events, there are safety issues related to transfusion such as acute lung injury, transfusion related circulatory overload and anaphylaxis that need to be managed at times [34]. Risk of transmission of blood borne infections is also a potential threat to recipients of CP.
In March 2020, the US FDA approved convalescent plasma as an investigational new drug in the expanded access program. The unadjusted analysis of data from the EAP, though not comparing CP with no CP, suggested that treatment given early after diagnosis (within 3 days) is associated with lower 7-day and 30-day mortality, and transfusion of convalescent plasma with high SARS-CoV-2 IgG antibody levels compared to medium or low IgG levels was furthermore associated with lower mortality. This could be an explanation as to why other studies investigating convalescent plasma may not be able to detect a clinically relevant difference, although these findings should be confirmed in RCTs before it can be translated into practice [35].
To the best of our knowledge, there is no previous systematic review that has included all the studies included in the present review. Some previous systematic reviews and meta-analyses have evaluated the efficacy of CP in the treatment of COVID-19. Chai et al in a Cochrane review that included two trials and eight NRSIs, concluded that there is uncertain evidence of the effect of CP on mortality (risk ratio (RR) 0.55, 95% CI 0.22 to 1.34, low-certainty evidence) and clinical improvement (RR 0.98, 95% CI 0.30 to 3.19, low-certainty evidence) [36]. This conclusion is consistent with what the present study found. The systematic review by Shao et al evaluated the effect of convalescent blood products (CBPs) for patients with severe acute respiratory infections of all viral aetiologies, and concluded that the all-cause mortality in the RCTs showed no difference between the interventional group and the control group (OR 0.82; 95% CI 0.57 to 1.19; P = 0.30). Using CBPs earlier, compared with using CBPs later, was associated with a significant reduction in all-cause mortality (OR 0.18; 95% CI 0.08-0.40; P < 0.0001) [37]. Sarkar et al identified two RCTs and five cohort in their systematic review on role of CP in COVID-19. They concluded that there is very low- to low quality evidence that CP reduces mortality (OR 0.44, 95% CI 0.25 to 0.77) from seven studies and increases clinical improvement (OR 2.06, 95% CI 0.8 to 4.9) from five studies [38]. The review by Devasenapathy et al provided indirect evidence of the role of CP from studies on SARS, MERS, Influenza and Ebola. However, they reported very low-quality evidence that raised the possibility that CP has minimal or no benefit in the treatment of COVID-19 and low-quality evidence that it does not cause serious adverse events [7].
The present systematic review incorporates a robust search strategy with a comprehensive search of three major databases as well as preprint servers. Screening of titles, abstracts and full texts was done independently by two reviews in duplicate, as was the data abstraction including risk of bias assessments. Lastly, we used the GRADE methodology to rate the certainty in the evidence, thus enabling us to pay close attention to methodological issues like inconsistency, imprecision and risk of bias.
The limitations of the present review are largely due to the limitations of the included primary studies, most of which yielded imprecise estimates. Imprecision persisted for most outcomes in the meta-analyses since evidence from large RCTs are not yet available. Though we used the adjusted estimates wherever reported, many studies failed to report adjusted estimates and were assessed as having serious risk of bias due to confounding. Another consideration is that this review includes evidence from pre-print articles, the quality of which has not been peer-reviewed and the results of which are subject to change following the peer review process. The number of included studies were few, precluding the possibility of subgroup analyses to explore the heterogeneity observed in the meta-analyses of some outcomes. However, the difference in severity of patients included amongst trials could possibly explain the heterogeneity. For instance, Agarwal et al [12] enrolled patients that were less severe than the other trials in the meta-analyses. Clinical improvement scales were different between the various included trials. Differences in the interventions with respect to titres, volume, etc could also be a source of heterogeneity. Clinical heterogeneity is evident from the different inclusion criteria used by studies. There is limited knowledge so far with regard to what level of antibody titre is protective and three of the included studies did not measure antibody titres of donors [19, 21, 25]. There is very limited reporting of adverse events in the included studies. Many studies excluded patients with moderate to severe ARDS and this precluded studying the effect of severity of disease on the efficacy of CP.
Uncertainty remains regarding the role of convalescent plasma in the treatment of COVID-19. There is low certainty evidence of a possible reduction in mortality and mechanical ventilation, a faster viral clearance and the absence of any serious adverse events. However, its efficacy for these outcomes requires evidence from good quality and adequately powered randomized controlled trials.
Supplementary Information
Below is the link to the electronic supplementary material.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Manya Prasad, Email: manya.2311@gmail.com.
Tulika Seth, Email: drtulikaseth@gmail.com.
Arunmozhimaran Elavarasi, Email: arun_ela@yahoo.com.
References
- 1.COVID-19 Map [Internet]. Johns Hopkins coronavirus resource center. [cited 2020 Sep 30]. Available from: https://coronavirus.jhu.edu/map.html
- 2.Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 COVID-19. A review. JAMA. 2020 doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 3.O'Callaghan KP, Blatz AM, Offit PA. Developing a SARS-CoV-2 vaccine at warp speed. JAMA. 2020;324(5):437–438. doi: 10.1001/jama.2020.12190. [DOI] [PubMed] [Google Scholar]
- 4.Goodman JL, Borio L. Finding effective treatments for COVID-19: scientific integrity and public confidence in a time of crisis. JAMA. 2020 doi: 10.1001/jama.2020.6434. [DOI] [PubMed] [Google Scholar]
- 5.Juurlink DN. Safety considerations with chloroquine, hydroxychloroquine and azithromycin in the management of SARS-CoV-2 infection. CMAJ Can Med Assoc J J Assoc Medicale Can. 2020;192(17):E450–3. doi: 10.1503/cmaj.200528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xi Y. Convalescent plasma therapy for COVID-19: a tried-and-true old strategy? Signal Transduct Target Ther. 2020;5(1):203. doi: 10.1038/s41392-020-00310-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devasenapathy N, Ye Z, Loeb M, Fang F, Najafabadi BT, Xiao Y, et al. Efficacy and safety of convalescent plasma for severe COVID-19 based on evidence in other severe respiratory viral infections: a systematic review and meta-analysis. CMAJ. 2020;192(27):E745–E755. doi: 10.1503/cmaj.200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guyatt GH, Busse JW. (2020) Modification of cochrane tool to assess risk of bias in randomized trials. [Internet]. [cited 2020 Sep 30]. Available from: https://www.evidencepartners.com/resources/methodological-resources/
- 9.Busse JW, Guyatt GH. (2020) Tool to assess risk of bias in cohort studies. [Internet]. [cited 2020 Sep 30]. Available from https://www.evidencepartners.com/resources/methodological-resources/
- 10.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li L, Zhang W, Hu Y, Tong X, Zheng S, Yang J, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020;324(5):460–70. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agarwal A, Mukherjee A, Kumar G, Chatterjee P, Bhatnagar T, Malhotra P, et al. (2020) Convalescent plasma in the management of moderate COVID-19 in India: An open-label parallel-arm phase II multicentre randomized controlled trial (PLACID Trial). medRxiv. 10;09.03.20187252
- 13.Gharbharan A, Jordans CCE, GeurtsvanKessel C, den Hollander JG, Karim F, Mollema FPN, et al. Convalescent plasma for COVID-19 A randomized clinical trial. medRxiv. 2020;07(01):20139857. [Google Scholar]
- 14.Avendano-Sola C, Ramos-Martinez A, Munez-Rubio E, Ruiz-Antoran B, Malo de Molina R, Torres F, et al. (2020) Convalescent Plasma for COVID-19: A multicenter, randomized clinical trial. medRxiv. 1(08):26-20182444
- 15.Bajpai M, Kumar S, Maheshwari A, Chabra K, Kale P, Gupta A, et al. (2020) Efficacy of Convalescent Plasma Therapy compared to Fresh Frozen Plasma in Severely ill COVID-19 Patients A Pilot Randomized Controlled Trial. medRxiv. 27(10): 25-20219337
- 16.AlQahtani M, Abdulrahman A, Almadani A, Alali SY, Al Zamrooni AM, Hejab AH, et al. (2020) Randomized controlled trial of convalescent plasma therapy against standard therapy in patients with severe COVID-19 disease. medRxiv. 1(11): 02-20224303 [DOI] [PMC free article] [PubMed]
- 17.Rasheed AM, Ftak DF, Hashim HA, Maulood MF, Kabah KK, Almusawi YA, et al. (2020) The therapeutic effectiveness of Convalescent plasma therapy on treating COVID-19 patients residing in respiratory care units in hospitals in Baghdad, Iraq. medRxiv. 1(06): 24-20121905 [PubMed]
- 18.Abolghasemi H, Eshghi P, Cheraghali AM, Imani Fooladi AA, Bolouki Moghaddam F, Imanizadeh S, et al. Clinical efficacy of convalescent plasma for treatment of COVID-19 infections: Results of a multicenter clinical study. Transfus Apheresis Sci: Off J World Apheresis Assoc: Off J Eur Soc Haemapheresis. 2020 doi: 10.1016/j.transci.2020.102875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Omrani AS, Zaqout A, Baiou A, Daghfal J, Elkum N, Alattar RA, et al. Convalescent plasma for the treatment of patients with severe coronavirus disease 2019: a preliminary report. J Med Virol. 2020;93(3):1678–1686. doi: 10.1002/jmv.26537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rogers R, Shehadeh F, Mylona E, Rich J, Neill M, Touzard-Romo F, et al. (2020) Convalescent plasma for patients with severe COVID-19: a matched cohort study. medRxiv. 1(08): 18-20177402 [DOI] [PMC free article] [PubMed]
- 21.Salazar E, Christensen PA, Graviss EA, Nguyen DT, Castillo B, Chen J, et al. (2020) Treatment of COVID-19 Patients with Convalescent Plasma Reveals a Signal of Significantly Decreased Mortality. The American journal of pathology [Internet].; Available from: http://www.epistemonikos.org/documents/29ee31cf41f2718027546e3b0493e08db5f18c71 [DOI] [PMC free article] [PubMed]
- 22.Xia X, Li K, Wu L, Wang Z, Zhu M, Huang B, et al. Improved clinical symptoms and mortality among patients with severe or critical COVID-19 after convalescent plasma transfusion. Blood. 2020;136(6):755–9. doi: 10.1182/blood.2020007079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu STH, Lin H-M, Baine I, Wajnberg A, Gumprecht JP, Rahman F, et al. Convalescent plasma treatment of severe COVID-19 a propensity score-matched control study. Nat Med. 2020;26(11):1708–1713. doi: 10.1038/s41591-020-1088-9. [DOI] [PubMed] [Google Scholar]
- 24.Salazar MR, González SE, Regairaz L, Ferrando NS, González Martínez VV, Carrera Ramos PM, et al. (2020) Effect of convalescent plasma on mortality in patients with Covid-19 pneumonia. medRxiv. 1(10): 08-20202606
- 25.Altuntas F, Ata N, Yigenoglu TN, Bascı S, Dal MS, Korkmaz S, et al. Convalescent plasma therapy in patients with COVID-19. Transfus Apher Sci. 2020;19:102955–102955. doi: 10.1016/j.transci.2020.102955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simonovich VA, Burgos Pratx LD, Scibona P, Beruto MV, Vallone MG, Vázquez C, et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2020 doi: 10.1056/NEJMoa2031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Libster R, Pérez Marc G, Wappner D, Coviello S, Bianchi A, Braem V, et al. Early High-titer plasma therapy to prevent severe Covid-19 in older adults. N Engl J Med. 2021 doi: 10.1056/NEJMoa2033700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ray Y, Paul SR, Bandopadhyay P, D’Rozario R, Sarif J, Lahiri A, et al. (2020) Clinical and immunological benefits of convalescent plasma therapy in severe COVID-19: insights from a single center open label randomised control trial. medRxiv. 111(25): 20237883
- 29.Hegerova L, Gooley TA, Sweerus KA, Maree C, Bailey N, Bailey M, et al. Use of convalescent plasma in hospitalized patients with COVID-19: case series. Blood. 2020;136(6):759–762. doi: 10.1182/blood.2020006964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang W, Li W, Xiong L, Wu Q, Wu J, He B, et al. Clinical efficacy of convalescent plasma therapy on treating COVID-19 patients: Evidence from matched study and a meta-analysis. Clin Transl Med. 2020;10(8):e259. doi: 10.1002/ctm2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alsharidah S, Ayed M, Ameen RM, Alhuraish F, Rouheldeen NA, Alshammari FR, et al. COVID-19 convalescent plasma treatment of moderate and severe cases of SARS-CoV-2 infection: a multicenter interventional study. Int J Infect Dis. 2020;4(103):439–446. doi: 10.1016/j.ijid.2020.11.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoon HA, Bartash R, Gendlina I, Rivera J, Nakouzi A, Bortz RH, et al. Treatment of severe COVID-19 with convalescent plasma in the Bronx NYC. medRxiv. 2020 doi: 10.1101/2020.12.02.20242909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arvin AM, Fink K, Schmid MA, Cathcart A, Spreafico R, Havenar-Daughton C, et al. A perspective on potential antibody-dependent enhancement of SARS-CoV-2. Nature. 2020;584(7821):353–363. doi: 10.1038/s41586-020-2538-8. [DOI] [PubMed] [Google Scholar]
- 34.Joyner MJ, Wright RS, Fairweather D, Senefeld JW, Bruno KA, Klassen SA, et al. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J Clin Invest. 2020;130(9):4791–4797. doi: 10.1172/JCI140200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joyner MJ, Senefeld JW, Klassen SA, Mills JR, Johnson PW, Theel ES, et al. (2020) Effect of convalescent plasma on mortality among hospitalized patients with COVID-19: initial Three-month experience. medRxiv. 1(08): 12-20169359
- 36.Chai KL, Valk SJ, Piechotta V, Kimber C, Monsef I, Doree C, et al. (2020) Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review. Cochrane Database Syst Rev 12(10): CD013600 [DOI] [PMC free article] [PubMed]
- 37.Shao S, Wang Y, Kang H, Tong Z. Effect of convalescent blood products for patients with severe acute respiratory infections of viral etiology: a systematic review and meta-analysis. Int J Infect Dis. 2020;S1201–9712(20):32159–7. doi: 10.1016/j.ijid.2020.09.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarkar S, Soni KD, Khanna P. Convalescent plasma is a clutch at straws in COVID-19 management! A systematic review and meta-analysis. J Med Virol. 2020 doi: 10.1002/jmv.26408. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.