To commemorate JEM's 125th anniversary, Kim and Cantor discuss the seminal work by Landsteiner and colleagues on human blood groups and reflect on how those findings have influenced our current understanding of immune recognition, clonal selection, and self-tolerance.
Abstract
Landsteiner’s definition of human blood groups and the genetic rules that govern blood transfusion represents a milestone in human genetics and a historic event in public health. His research into the specificity of serological reactions, although less well known, has had a critical influence on the development of contemporary views on immune recognition, clonal selection, and immunological self-tolerance.
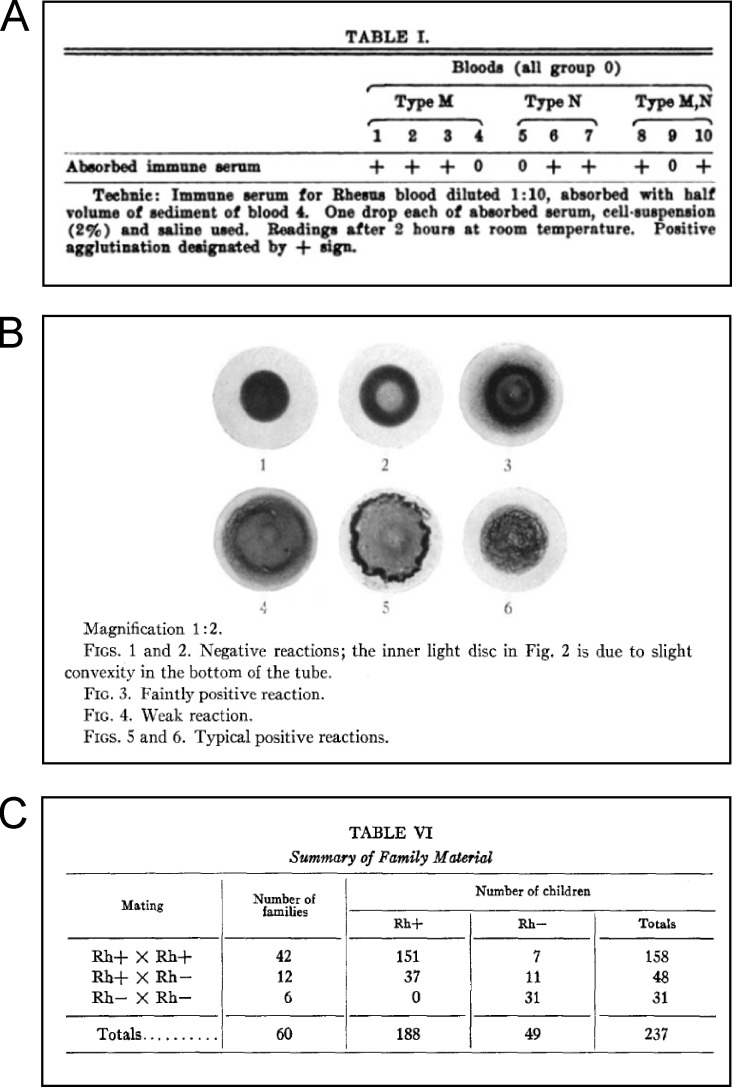
Landsteiner’s groundbreaking experiments in blood transfusion began with a simple analysis of the interaction between sera and red blood cells from healthy individuals. He found that specific agglutinating antibodies were limited to those individuals who lacked the blood type antigens to which these antibodies were directed (Landsteiner, 1900; Landsteiner, 1901). He explained these reactions by postulating two types of blood cell antigens, called A and B. Individuals that expressed neither A nor B, but whose sera contained antibodies to A and B, were called type O. Although widespread application of these findings (along with anticoagulants to prevent clotting of collected blood specimens) allowed safe and successful blood transfusions during the First World War, the problem of serious hemolytic reactions after recurrent transfusions between A-B-O–matched individuals remained. These unusual agglutination reactions led Landsteiner to postulate additional blood isoagglutinins that were distinct from the major blood A and B agglutinins and prompted a series of attempts to define them using antisera raised in animals against human RBCs. Immunization of guinea pigs with monkey RBCs produced immune sera that agglutinated a novel human blood factor, which was designated Rh to reflect the use of Rhesus RBCs as the source of immunizing erythrocytes (Landsteiner and Wiener, 1940). In their 1941 JEM paper, Landsteiner and Wiener reported that ∼85% of 448 individuals tested were Rh positive and 15% were Rh negative (Landsteiner and Wiener, 1941). Genetic studies of Rh factor revealed that it was inherited as a Mendelian dominant autosomal trait that segregated independently from previously defined A, B, M, and N factors (see figure). Further studies established that the Rh factor is expressed at the RBC membrane and is currently termed the erythrocyte D antigen. These studies marked the first systematic attempt to capture and define cell surface antigens by deliberate immunizations of animals—a general approach that was applied to the analysis of lymphocyte differentiation (Cantor and Boyse, 1977). In this case, Landsteiner’s discovery and characterization of an Rh factor provided an explanation for two medical mysteries: the immunological basis of hemolytic reactions in patients that had received blood from A-B-O–compatible donors and a potential genetic basis for an often-fatal hemolytic disease of infants called erythroblastosis fetalis.
Insights from Hye-Jung Kim and Harvey Cantor.
Landsteiner performed these studies in an era that was dominated by Paul Ehrlich’s chemical models to explain the interactions of antibody, antigen, and complement (Ehrlich, 1900). Ehrlich’s “side-chain theory” held that cells expressed a variety of side-chains (antibodies) at their surface that are released after infection and bind to potential pathogens that neutralize microbial toxins while sparing the organism’s own tissues. According to this model, aberrant production of self-reactive side-chains would give rise to “horror autotoxicus,” a kind of immunological self-poisoning. However, this rule seemed to be violated by a number of observations, including experiments from Landsteiner’s laboratory. His studies of paroxysmal nocturnal hemoglobinuria (with Donath) established that this disorder was mediated by antibodies specific for the patient’s hemoglobin (Donath and Landsteiner, 1904). Landsteiner’s development of more precise and quantitative methods to raise and characterize antibody represents a cornerstone in immunochemistry. They also would have an impact on theories of antibody formation and diversity.
Studies on an agglutinogen (Rh) in human blood reacting with anti-Rhesus sera and with human isoantibodies. (A) Results from the original experiment that detected Rh factor in human blood using sera from rabbits immunized against Rhesus blood cells. Landsteiner and Wiener revealed a factor of human blood—Rh—that was independent of previously identified blood types M and N (from Landsteiner and Wiener, 1940, with permission of SAGE Publications, Ltd.). (B) Agglutination of fresh blood samples by guinea pig sera obtained following immunization with Rhesus blood cells (Landsteiner and Wiener, 1941). (C) Results of Landsteiner’s original 1941 Rh phenotype experiment using guinea pig immune sera and post-transfusion human serum. This table shows that the Rh factor is inherited as a simple Mendelian dominant trait (Landsteiner and Wiener, 1941).
Antibody specificity and diversity. A second series of experiments from Landsteiner’s laboratory provided a more severe challenge to Ehrlich’s theory that preformed antibodies (side-chains) were sufficient to defend against the antigenic universe. Landsteiner used hapten-conjugated proteins to induce and characterize antibodies that bound to the immunizing epitope and to closely related molecules (Landsteiner and van der Scheer, 1924; Landsteiner and van der Scheer, 1936). Since these chemically synthesized molecules had never existed in nature, their ability to elicit robust antibody responses seemed incompatible with the biologically fixed repertoire postulated by Ehrlich and were initially used as evidence for “instructive” mechanisms of antibody diversity (Landsteiner, 1933). However, increasing evidence for remarkably high levels of antibody cross-reactivity provided by Landsteiner and others using several systems opened the possibility that individual antibodies might bind to a very large array of antigens and might suffice for clonal selection models. Landsteiner was not drawn into these theoretical arguments. His focus was on developing new immunochemical methods for measuring complex antibody responses.
The basis for antibody diversity was not resolved until it was reformulated at a cellular level. After Nossal and Lederberg showed that a single cell produces a single antibody that carries a unique specificity (Nossal and Lederberg, 1958), the antibody diversity problem could be viewed as a biological property of B cell clones. The demonstration of rearrangements of Ig heavy and light chain V region genes in individual B cell clones provided a robust genetic mechanism for a highly diverse B cell repertoire (Tonegawa et al., 1974).
Although Landsteiner’s definition of the rules that governed production of antibodies to RBCs represent an early example of self-tolerance, the mechanisms that ensure B cell tolerance awaited analyses of genetically manipulated mice. These experiments revealed early B cell deletional mechanisms that were followed by B cell receptor editing to remove self-reactive B cells during B cell activation and expansion in germinal centers (Wardemann and Nussenzweig, 2007). What about T cells?
T cell recognition and tolerance. Although analysis of antibody specificity was at the core of Landsteiner’s research, his work also provided early insight into the specificity of cell-mediated immunity. Landsteiner produced chemically active forms of haptens that were able to conjugate to host proteins in skin and promote robust contact sensitivity reactions. These cutaneous reactions were highly specific, accompanied by strong immunological cell infiltrates, and could be transferred by cells but not sera from sensitized donors (Landsteiner and Chase, 1942). The specificity of these cell-mediated responses (contact sensitivity and delayed-type hypersensitivity) differed markedly from classical antibody responses. Recognition of hapten-protein conjugates required larger portions of the haptenic molecule than antibody recognition, as judged by the requirements for successful immunological challenge, and skin reactions were evoked equally well by heat-denatured and native proteins, unlike antibody responses (Gell and Benacerraf, 1959). These reactions were, of course, early experimental examples of cell-mediated immunity that were specifically controlled by T cells. Subsequent findings that guinea pig responses to protein antigens were controlled by a single autosomal dominant genetic locus mapping to the MHC region (Ellman et al., 1970; McDevitt, 2000) led to well-known experiments that revealed T cell recognition of (peptide) antigens in association with MHC products. The subsequent definition of the T cell receptor genes and their products provided a clear molecular distinction between T cell and B cell recognition, as well as a genetic mechanism that ensured a very large T cell repertoire (Chien et al., 1984).
Landsteiner’s original observation that “corresponding antigens and antibodies do not physiologically co-exist in the same individual’s blood” represents a landmark finding that stimulated extensive research efforts into immunological tolerance. Landsteiner and Chase’s contact sensitivity system was used to investigate immunological unresponsiveness (Battisto and Chase, 1963; Chase, 1946; Landsteiner and Chase, 1941). Unresponsiveness could not be overcome by injections of lymphoid cells and could not easily be accounted for by deletional mechanisms (Chase, 1982). Increased understanding of dominant tolerance came from the definition of regulatory T cells belonging to the CD4+ T cell lineage (Rudensky, 2011; Sakaguchi et al., 1985). There is emerging evidence that CD8 T cells also include a regulatory cell lineage that inhibits expansion of self-reactive CD4 T cells and prevents autoimmunity (Kim et al., 2011; Saligrama et al., 2019).
The 1941 paper with Wiener that defined the Rh factor represented the result of decades-long research into blood groups and is widely recognized as the foundational paper in this field. However, Landsteiner’s second line of research into antibody specificity may hold more interest for contemporary immunologists. These studies built upon his early realization that immunological reactions were essentially chemical in nature. The culmination of this work is probably the classic paper with van der Scheer of the fine specificity of antibodies (Landsteiner and van der Scheer, 1939). This study, published in 1939, depended only on the use of precipitin and absorption tests. Analysis of antibody specificity for defined pentapeptides revealed that the order of each amino acid in the peptide could be distinguished by antibodies. The results had clear implications for models of antibody diversity and structure. They also suggested that antibodies might be capable of capturing and distinguishing the receptors that decorate the surface of human cells. This remarkable finding that antibodies could distinguish between peptides that differed only by a single change in amino acid sequence represents the first indication of the discriminating power of antibodies. Landsteiner’s description of the remarkable specificity of antibody was well ahead of its time and has had far-reaching consequences for theoretical and applied immunology.
References
- Battisto, J.R., and Chase M.W.. 1963. J. Exp. Med. 10.1084/jem.118.6.1021 [DOI] [Google Scholar]
- Cantor, H., and Boyse E.A.. 1977. Immunol. Rev. 10.1111/j.1600-065X.1977.tb00364.x [DOI] [PubMed] [Google Scholar]
- Chase, M.W. 1946. Proc. Soc. Exp. Biol. Med. 10.3181/00379727-61-15294P [DOI] [Google Scholar]
- Chase, M.W. 1982. Ann. N. Y. Acad. Sci. 10.1111/j.1749-6632.1982.tb36110.x [DOI] [Google Scholar]
- Chien, Y.H., et al. 1984. Nature. 10.1038/309322a0 [DOI] [Google Scholar]
- Donath, J.K., and Landsteiner K.. 1904. Munch. Med. Wochenschr. 51:1590–1593. [Google Scholar]
- Ehrlich, P. 1900. Proc. R. Soc. Lond. 10.1098/rspl.1899.0121 [DOI] [Google Scholar]
- Ellman, L., et al. 1970. Proc. Natl. Acad. Sci. USA. 10.1073/pnas.66.2.322 [DOI] [Google Scholar]
- Gell, P.G., and Benacerraf B.. 1959. Immunology. 2:64–70. [PMC free article] [PubMed] [Google Scholar]
- Kim, H.J., et al. 2011. Proc. Natl. Acad. Sci. USA. 10.1073/pnas.1018974108 [DOI] [Google Scholar]
- Landsteiner, K. 1900. Zentralbl. Bakteriol. Orig. 27:357–362. [Google Scholar]
- Landsteiner, K. 1933. Die Spezifizität der Serologischen Reaktionen. 10.1007/978-3-662-33113-2 [DOI] [Google Scholar]
- Landsteiner, K. 1901. Wien. Klin. Wochenschr. 14:1132–1134. [PubMed] [Google Scholar]
- Landsteiner, K., and Chase M.W.. 1941. J. Exp. Med. 10.1084/jem.73.3.431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsteiner, K., and Chase M.W.. 1942. Exp. Biol. 10.3181/00379727-49-13670 [DOI] [Google Scholar]
- Landsteiner, K., and van der Scheer J.. 1924. J. Exp. Med. 10.1084/jem.40.1.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsteiner, K., and van der Scheer J.. 1936. J. Exp. Med. 10.1084/jem.63.3.325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsteiner, K., and van der Scheer J.. 1939. J. Exp. Med. 10.1084/jem.69.5.705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsteiner, K., and Wiener A.S.. 1940. Exp. Biol. Med. 10.3181/00379727-43-11151 [DOI] [Google Scholar]
- Landsteiner, K., and Wiener A.S.. 1941. J. Exp. Med. 10.1084/jem.74.4.309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt, H.O. 2000. Annu. Rev. Immunol. 10.1146/annurev.immunol.18.1.1 [DOI] [PubMed] [Google Scholar]
- Nossal, G.J., and Lederberg J.. 1958. Nature. 10.1038/1811419a0 [DOI] [Google Scholar]
- Rudensky, A.Y. 2011. Immunol. Rev. 10.1111/j.1600-065X.2011.01018.x [DOI] [Google Scholar]
- Sakaguchi, S., et al. 1985. J. Exp. Med. 10.1084/jem.161.1.72 [DOI] [Google Scholar]
- Saligrama, N., et al. 2019. Nature. 10.1038/s41586-019-1467-x [DOI] [Google Scholar]
- Tonegawa, S., et al. 1974. Proc. Natl. Acad. Sci. USA. 10.1073/pnas.71.10.4027 [DOI] [Google Scholar]
- Wardemann, H., and Nussenzweig M.C.. 2007. Adv. Immunol. 10.1016/S0065-2776(07)95003-8 [DOI] [PubMed] [Google Scholar]