Abstract
The regularity of the physical world and the biomechanics of the human body movements generate distributions of highly probable states that are internalized by the brain in the course of a lifetime. In Bayesian terms, the brain exploits prior knowledge, especially under conditions when sensory input is unavailable or uncertain, to predictively anticipate the most likely outcome of upcoming stimuli and movements. These internal models, formed during development, yet still malleable in adults, continuously adapt through the learning of novel stimuli and movements.
Traditionally, neural beta (β) oscillations are considered essential for maintaining sensorimotor and cognitive representations, and for temporal coding of expectations. However, recent findings show that fluctuations of β band power in the resting state strongly correlate between cortical association regions. Moreover, central (hub) regions form strong interactions over time with different brain regions/networks (dynamic core). β band centrality fluctuations of regions of the dynamic core predict global efficiency peaks suggesting a mechanism for network integration. Furthermore, this temporal architecture is surprisingly stable, both in topology and dynamics, during the observation of ecological natural visual scenes, whereas synthetic temporally scrambled stimuli modify it. We propose that spontaneous β rhythms may function as a long-term “prior” of frequent environmental stimuli and behaviors.
Keywords: neural oscillations, beta band, priors, spontaneous activity, natural vision
Intrinsic Cortical Oscillations as Long-Term “Priors” for Real-Life Events and Behaviors
A long-standing principle in neuroscience is that the nervous systems of animals process sensory inputs moment-by-moment in sensory cortices and that their output transfers to higher-order associative regions for decision making, action, and memory. The notion of serial bottom-up processing developed within the visual system (Van Essen and others 1992), then extended to somatosensory (Felleman and Van Essen 1991; Iwamura 1998) and auditory systems (Kaas and Hackett 1998). Sensory stimuli build up sensory representations that change over time as events unfold in the environment, and the content of these representations carried out by individual neurons and groups of neurons increases in complexity as one moves along the processing hierarchy.
In the early 2000s, an influential hypothesis posited that sensory and perceptual analyses do not depend only on “external” stimuli and bottom-up processes. Instead, they also reflect concurrent “internal” processes that code expected features of the environment and task goals, and that are active simultaneously to the sensory flow of information processing. The brain, then, is not a passive analyzer of sensory information, but it is always intrinsically active. It generates predictions about forthcoming stimuli and events through top-down control mechanisms from higher to lower brain areas that are active both during stimulus-evoked and spontaneous activity (Engel and others 2001).
As posited by the theory of predictive coding, sensory processing is, in turn, an inference problem, where the brain predicts the forthcoming sensory stimuli through internal representations, or prior expectations (Friston 2005). The theory predicts that higher cortical areas generate predictions transferred to sensory regions for comparison with incoming inputs. When there is no match, errors lead through feedback to the correction of behavior, and in turn, a modification of the prior. For example, driving in optimal conditions, such as, excellent visibility and low traffic, is almost an automatic behavior in which the neural signals related to the task and incoming sensory information perfectly match. By contrast, driving in the fog is a stressful situation that requires concentration. The influence of priors is especially evident under conditions of sensory uncertainty. To manage this condition, we use the prior experience of what is safe driving, for example, driving slowly, to manage when the sensory input is low quality. The mismatch between expectation and sensory inputs, for example, a truck emerging suddenly from the fog, so-called prediction error, leads to corrections (sudden stops, accelerations) that reflect the updating of our predictions through recurrent interactions between sensory, cognitive, and motor regions of the brain (Garrido and others 2009; Kiebel and others 2008).
However, internal models and priors must also be stable and long-term. In Bayesian terms, a prior represents a priori belief about the sensory environment that builds on the laws and the regularity of the physical world. Such regularities reduce the complexity of the natural environment, that is, the degrees of freedom of the real-world into a few highly probable states. These states, in turn, constrain our body positions and movements, actions, and perceptions. An example is the downward movement of an object due to gravity. Even in the absence of visual feedback, we can predict the direction of the movement, for example, the object will not move upward, and its precise timing based on our internal estimates of g (9.80 m/s2). In the absence of gravity (g = 0), when astronauts estimate the time-to-contact of a falling ball, they rely on an implicit prior model of gravity to supplement sensory information. This model assumes that descending targets accelerate at a certain speed by Earth’s gravity, and it produces an erroneous performance. The astronauts estimate the time of contact too early. Interestingly, even in adult life, these models can be slowly adapted. During microgravity exposure, this adaptation is slow. The reduction of the amplitude of the anticipatory motor response occurs after 10 to 14 days, despite available visual, vestibular, and proprioceptive online feedback. However, the readaptation of the model to Earth’s condition occurs almost immediately (McIntyre and others 2001). In other words, priors are stable, but remain malleable in adults, adapting through the interaction of the environment with the body. The adaptation of prior models is also evident in all sorts of visuo-motor learning paradigms, both long-term, in the matter of minutes or hours, for example, the prismatic adaptation paradigm (Helmholtz 1909), or short term, in the matter of seconds, for example, Lackner’s Pinocchio effect (Lackner 1988).
Priors form in the course of development as shown by studies in the ferret’s visual cortex where the tuning function of neurons “learn” the statistics of natural stimuli as the animals grow (Berkes and others 2011). Behavioral studies in infants show that humans are influenced by gravity effects early in life (Lacquaniti and others 2014). Electroencephalography studies in 12-month babies show that statistical regularities in sensory stimuli modulate neural responses both early and late in processing (Kouider and others 2015).
While anticipatory attention or working memory signals are traditionally considered the neural correlates of top-down predictions and the modulation of sensory responses reflect prediction errors, long-term models and priors must rely on a more stable set of neural signals. A possible candidate for this mechanism is the spontaneous activity of the brain, i.e. activity that is not evoked by stimuli, tasks, or responses. Thanks largely to functional neuroimaging studies in human subjects it is now evident that spontaneous activity is not noise, as postulated in traditional neurophysiological models (Shadlen and Newsome 1998), but correlated in space and time in large-scale networks of brain regions. These networks imaged with functional magnetic resonance imaging (fMRI) have a counterpart in slow frequency-specific power fluctuations (band-limited power, BLP) of the alpha and beta band power measured with electroencephalography/magnetoencephalography/electrocorticography (EEG/MEG/ECoG). It has been proposed that this intrinsic activity may function as a prior that constrains the recruitment of task-driven (see Box 1).
Box 1.
Spontaneous Brain Activity as Sampling from Prior Distributions.
| In awake resting humans and animals, the spontaneous slow-frequency fluctuations of the blood oxygen level–dependent (BOLD) signal maintain a high degree of temporal coupling across regions, also known as functional connectivity (Biswal and others 1995; Fox and others 2005; Raichle and others 2001). Regions showing strong coupling over time are functionally connected and form so-called resting-state networks (RSNs). Over the past two decades, several RSNs have been identified in cortex: sensory-motor networks (visual, auditory, somatomotor), and cognitive networks (dorsal attention, ventral attention, cingulo-opercular, frontoparietal). Cognitive networks, in turn, split along two functional axes, one related to external events/processes, e.g., spatial attention (dorsal attention, DAN), the other to internal events/processes, e.g., episodic memory (default mode, DMN). These signals define various functional parcellations of the human brain. MEG, EEG, and ECoG have measured similar patterns of topographically organized intrinsic activity fluctuations (e.g., Brookes and others 2011; Hacker and others 2017; Mantini and others 2007; Marzetti and others 2013), but also (Betti and others 2013; Betti and others 2018; de Pasquale and others 2010). The most consistent neurophysiological correlate of fMRI RSNs is the slow (0.1-1 Hz) BLP fluctuation in the alpha and beta bands, albeit frequency-specific RSNs for internal vs. external events have been described (de Pasquale and others 2010; Hacker and others 2017). RSNs are not monolithic functional systems but vary dynamically in time, splitting and coupling with other networks, or subsets thereof, with rules that are yet to define (Hutchison and others 2013). |
| The origin of RSNs, and their function, is complex and no single hypothesis accounts for the results. A critical component is the underlying anatomical structural connectivity (Vincent and others 2007). However, while the correlation between structural and functional connectivity topography at the group level is relatively high, r values ~ 0.4-0.6 (e.g., Deco and others 2013), at the level of a single subject the correlation is much lower, r values ~0.1 (Misic and others 2016). This observation is not surprising. |
| Functional connectivity reflects both mono-and polysynaptic pathways, and their dynamics, while structural connectivity measures mono-synaptic pathways. Another important observation is that the topography of RSNs resembles patterns of task activation (Smith and others 2009), and that is resilient not only to manipulations induced by sensory, motor, and cognitive paradigms (Betti and others 2013), but also (Cole and others 2014), and across levels of consciousness (e.g., Larson-Prior and others 2009). Specific task conditions gently modulate a stable RSN scaffolding (e.g., Cole and others 2014; Kim and others 2018). This observation, by now widely accepted, is also not surprising since any specific cognitive task modifies activity in a relatively small number of synapses, regions, and pathways, as compared to the work of maintaining the rest of the brain connected. Analogously, in learning paradigms, despite prolonged training on new tasks, relatively few pathways change their level of intrinsic correlation (Albert and others 2009; Lewis and others 2009). |
| The remarkable similarity between rest and task activity has led to the hypothesis that spontaneous activity patterns reflect the history of task coactivation of brain networks and that this architecture functions as a spatio-temporal “prior” for the recruitment of task networks (Deco and Corbetta 2010; Raichle 2011). In Bayesian terms, sensory stimuli occur probabilistically. The related neural activity represents samples from this “posterior distribution,” as well as a “prior” distribution that codes for a priori internal models about the sensory environment. In the absence of sensory stimulation, as in the resting state, or perhaps in conditions of high sensory uncertainty (the drive in the fog), neural activity collapses to the prior distribution, which, in turn, may explain the similarity between intrinsic and evoked activity (Fiser and others 2010). In behavioral terms, a prior shall encode information that is helpful to solve a task or carry out a behavior. Empirically, it is essential to separate spatial and temporal neural priors. Spatial priors may be evident in the topography of activity, while temporal priors reflect the temporal organization of activity. Biologically, these two dimensions are intrinsically linked, but it is convenient to keep them separate experimentally. |
| While this review focuses on temporal priors, here we provide some examples of what we mean by spatial prior. In terms of functional connectivity, a spatial prior is the topographic pattern of temporal correlation among brain regions. If the pattern at rest resembles that recorded during a task, then those connections are a spatial prior for task activity patterns. |
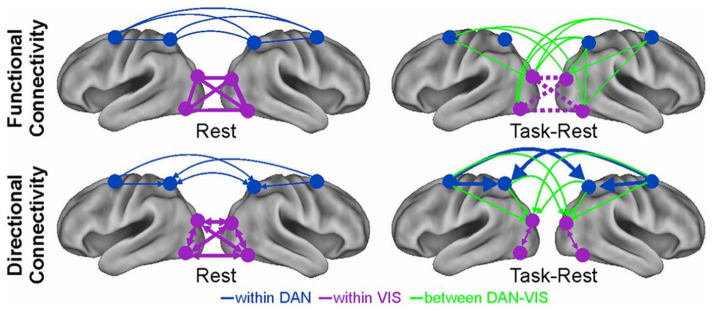
| In a recent fMRI study (Spadone and others 2015), observers performed a spatial attention task or maintained fixation (rest). In the visual system, the attention task modified both the strength and directionality of interaction between visual regions, and between visual regions and frontoparietal control regions. This result implies that the resting organization of the visual system, whatever codes at rest, was significantly modified to solve our artificial attention task. In contrast, frontoparietal regions of the dorsal attention network (DAN) not only maintained the same topography, but the same pattern of directional interactions (see Figure). |
|
| Spatial priors. The functional and directional functional connections within the dorsal attention network (blue) are not significantly different during fixation or a demanding visuospatial attention task. In contrast, connections within the visual system (pink), or between visual and dorsal attention networks (green) are modified. |
| There is a constant bias from frontal-to-parietal-to occipital regions at rest which significantly increases during attention (Sylvester and others 2012). And, we found that the strength of these inter-regional interactions across subjects correlates with performance. Therefore, the functional topography and directional connections in frontal and parietal cortex are tuned to maintain an attention stance, even “intrinsically” at rest, which biases performance. Another example is the intrinsic organization of functional connections in the occipital lobe that predicts across subjects the level of future performance on an orientation learning task (Baldassarre and others 2012). These are two examples of what we mean by a spatial prior in connectivity. Notably, this prior is coded long-term in slow patterns of functional connectivity. Slow not only for the slow temporal resolution of fMRI, but also because functional connectivity patterns are computed over minutes or tens of minutes. The rest of the review will deal with temporal patterns of activity that may function as priors. |
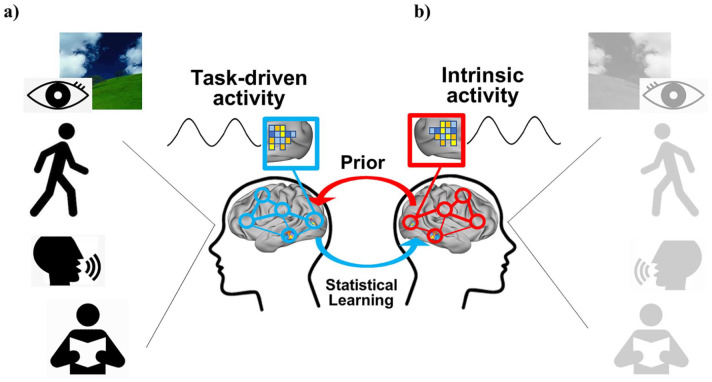
Oscillations are a fundamental property of all nervous systems (Buzsáki and others 2013). Temporal regularities embodied in oscillations may represent a mechanism to time, hence predict forthcoming sensory events (Engel and others 2001; Morillon and others 2015). Neuronal oscillations represent cycles of high and low excitability synchronized across large groups of neurons. When oscillations align to an input rhythm (entrainment) their high excitability phases coincide with events in the stream, resulting in the amplification of the neuronal response to the sensory input (Schroeder and others 2009). However, in natural conditions, most sensory events occur in the context of movements such as walking, speaking, and eye movements that are also rhythmic and evoke task-driven activity (Fig. 1a). Therefore, motor rhythms represent an ideal mechanism for timing and anticipating the flow of sensory events (Buzsáki 2019). We propose that the temporal structure of motor-sensory interactions during natural behavior is entrained during development and through experience into patterns of spontaneous cortical oscillations. Further, to be helpful ongoing patterns of spontaneous activity must maintain on-line a vast repertoire of natural behaviors and environmental events. Patterns of spontaneous activity bias the recruitment of task driven patterns (Fig. 1b). The problem of the high dimensionality of potential patterns to be encoded is helped by the covariance between events and body actions forced by the law of physics in the environment and the body (biomechanics). For instance, walking upright requires symmetrical leg movements, an assumption that the natural light comes from above, and a possible bias for the lower visual field. The potential high dimensionality of linear combinations of muscle activation in the two legs is summarized by one coordinated pattern of multi-joint movements (muscle synergies); similarly, the lower field bias for walking is coded in the organization of the visual pathways whereby the lower visual field project dorsally to the occipito-parietal cortex specialized in navigation. We argue later on that the correlation of events in the environment and body actions may be coded in “neural synergies” represented in patterns of correlated spontaneous activity. Online maintenance of spontaneous activity is metabolically expensive (Attwell and Laughlin 2001), but behaviorally efficient, because it allows the organism to anticipate and react to the most common environmental events and situations.
Figure 1.
(a) Many real-life events and behaviors such as walking, speaking, and eye movements have a rhythmic structure and evoke task-driven activity. The temporal structure of motor-sensory interactions during natural behavior is entrained during development and experience into patterns of spontaneous cortical oscillations, through statistical learning; (b) the idea then is that the statistics of common behaviors and real-life events are retained in the spontaneous brain activity (prior) that biases the recruitment of task driven patterns.
Since the dynamics of real-life events is at the timescale of seconds, we further propose that a plausible mechanism to preserve environmental and body statistics is the slow fluctuations of activity in higher-order cortical regions, specifically in the beta band. Many studies have associated beta-band oscillations both with predicting coding (Arnal and Giraud 2012) and the representation of temporal information (e.g., Fujioka and others 2012). Furthermore, beta-band activity is one of the main neurophysiological correlates of RSNs as seen in fMRI studies (Box 1).
The plan of the review is then to first consider the traditional role of beta oscillations in motor and cognitive processes, especially in the maintenance of sensorimotor and cognitive sets (Engel and Fries 2010). Then, we will consider beta oscillations in the resting brain, their stable topography and dynamics at rest and during natural vision conditions, and their relevance for behavior. The working hypothesis is that beta rhythms reflect internal models of highly probable states of the body and the environment.
Beta Band: What Is It?
The beta frequency band is traditionally considered the default rhythm of the sensorimotor system (Rolandic beta rhythm). Motor actions but also cognitive tasks dampen or elicit beta oscillations, as observed noninvasively on the scalp with EEG and MEG, or invasively with ECoG and local field potentials (LFPs), mostly through measures of power, coherence, and synchronization (Box 2).
Box 2.
The Study of Large-Scale Brain Dynamics.
| Brain functioning is associated with primary electric and volume currents generated by the postsynaptic potentials in the cortical pyramidal neurons. These neuronal currents give rise to both voltage differences measurable on the scalp using EEG and magnetic fields detectable outside the head by using MEG. Since both EEG and MEG measure brain signals at high temporal resolution (milliseconds vs. seconds with fMRI), they represent the best tools to study the topographic distribution and the temporal dynamics of brain oscillations in humans (de Tommaso and others 2020). |
| The EEG instrumentation is considerably simpler and cheaper than MEG; however, its spatial resolution is worse than MEG (from few centimeters to 4-5 mm). This is due to the solution of inverse problems typically performed by different methods (Mosher and others 1999). The drawback of the EEG/MEG low spatial resolution is overcome by invasive microelectrode recordings of local field potentials (LFP), which record signals directly from the cortical surface and thus can identify the neuronal sources with a high spatial resolution. However, this technique is invasive, has limited spatial coverage and leads to significant discomfort for the subject (generally primates). |
| Once the source activity is obtained, it is possible to study how the temporal dynamics of oscillatory power is modulated by task, as revealed by the analysis of the event-related desynchronization/synchronization (ERD/ERS) (see Pfurtscheller and others 1999, and the main text). For each frequency bin, ERD/ERS are defined as the percentage decrement/increment of post- trigger power relative to the baseline power. Commonly, the baseline is estimated as the mean power over the time-interval before the trigger onset. Alternatively, it can be estimated over the whole duration of an experimental block or of a trial. ERD/ERS represents a macroscopic effect of the involvement of neurons in task processing. Specifically, in the post-trigger epoch, the neurons processing the stimuli change their oscillatory phase with respect to the other neurons not involved in the task, and thus desynchronize, reducing the spectral power density—ERD. Usually, ERD is followed by an increment of power, which is produced by an increased synchronization—ERS. |
| The activity of brain regions can be functionally coupled through links that are spectrally specific and that change slowly over time (O’Neill and others 2018). Different measures of functional connectivity can be applied to study large-scale interactions, and electrophysiological data allow to analyze functional connectivity at different timescales, from the slow band-limited power (BLP) to the fast signal. BLP is the time course of the oscillatory power in a given frequency band and has been linked to the BOLD signal using simultaneous fMRI and LFP in primates (Logothetis and others 2001). The most popular measure of pairwise signal similarity is the Pearson correlation coefficient whose static and dynamic versions have been widely applied during rest and task conditions on BLP (see (Betti and others 2013; Betti and others 2018) in the main text) and on the fast signal. Its frequency counterpart is coherency, which is defined as the normalized cross-spectrum between two signals. Coherency is a complex quantity and either the norm or the imaginary part can be used to estimate functional connectivity. This measure is less prone to the signal leakage effects (e.g., see Marzetti and others 2013, and Box 1). Nonlinear measures as the phase locking value (Lachaux and others 1999) and the phase lag index (Stam and others 2007), quantify the phase lag stability between fast signal pairs. |
| Functional networks can be built based on the above measures of connectivity and their topology, that is, the role of the network elements in the communication architecture can be addressed by the graph theory (Bullmore and others 2009). |
The beta frequency band (13-30 Hz) is at the boundaries between alpha (8-12 Hz) and gamma oscillations (>30 Hz), although differences in topography and reactivity to different tasks characterize at least two frequency bands, low beta (13-20 Hz) and high beta (20-30 Hz). However, the view of beta as rhythmically sustained oscillations may change given recent studies showing spontaneous short burst-like or intermittent periods of high beta oscillations in nonaveraged data (Sherman and others 2016; van Ede and others 2018). Along the sensorimotor pathway, beta oscillations (~20 Hz) are particularly prominent in almost all structures involved in movement and processing of somatic information including muscles (Baker 2007; Kilner and others 1999), dorsal root ganglia (Baker and others 2006), basal ganglia (Leventhal and others 2012), and cortex.
This widespread localization begs the question of the locus of beta activity generators. One leading hypothesis suggests that beta emerges in the neocortex from the deep infragranular layers (van Kerkoerle and others 2014), but it is dependent on driving signals originating from the basal ganglia and the thalamus (Sherman and others 2016).
At the cortical level, beta-band activity is not spatially confined to the sensorimotor system, but these oscillations have been observed in various cortical areas, and linked to different cognitive functions (visual attention, perception, emotion, working memory) (Miller and others 2018; Wang 2010). They have been also considered a general mechanism for long-range synchronization between regions linked through long conduction delays (Kopell and others 2000). While working memory and visual attention may still depend on motor processes, as for instance in the well-known link between visuospatial attention and eye movements (Corbetta 1998; Rizzolatti and others 1987), beta band modulations also occur in emotion and long-term memory tasks (Miller and others 2018), and are more difficult to classify as motor-related. Herein, we will review the interpretation of beta rhythms modulations in the sensory-motor domain, as a stepping stone toward a novel theoretical framework.
Beta Band: What It Does?
Sensorimotor Beta Oscillations for Maintenance of Internal Models Related to Body Movements
There are multiple hypotheses about the functional role of beta oscillations in sensory-motor functions. Most studies have focused on movement. The most consistent result is a decrement of beta band power during motor planning and execution (event-related desynchronization, ERD), and then an increase (event-related synchronization, ERS) from 300 to 1000 ms after the end of the movement (Jurkiewicz and others 2006; Pfurtscheller and others 1999) (Fig. 2a and b). The mere observation of movements also produces similar effects (Sebastiani and others 2014). Whereas the ERD may reflect the asynchronous excitatory activation of the motor cortex, the ERS has been long considered rebound inhibition (or idling) of the motor system, which is interrupted during the movement and enhanced after the movement (Pfurtscheller and others 1996).
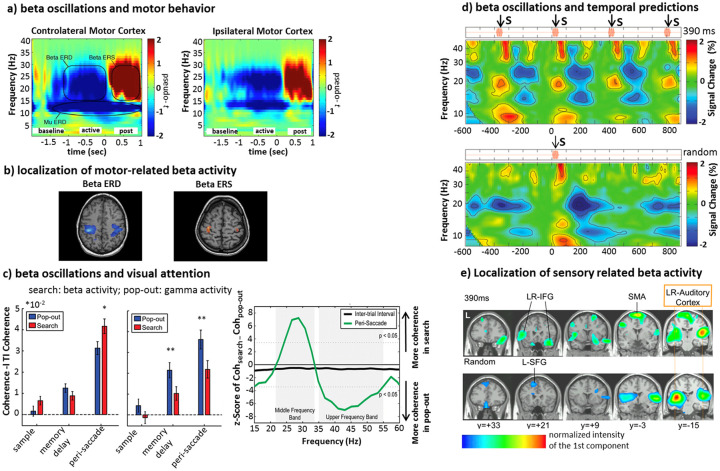
Figure 2.
(a, b) Movement-related beta activity. Voluntary movements produce typical beta responses in the sensorimotor cortex. This time-frequency representation depicts decrement—event-related desynchronization (ERD; in blue color scales) and increment—event-related synchronization (ERS; in red color scales) in power, in the contralateral (left) and ipsilateral (right) motor cortex. ERD begins before movement (baseline) and lasts throughout the movement (active), followed by a strong ERS that starts shortly after movement termination (post). (b) Localization of beta ERD/ERS over the sensorimotor regions (modified from Jurkiewicz and others 2006). (c) Attention-related beta activity. Paradigms of serial versus pop-out visual search produce different modulations of beta and gamma rhythms. The beta band coherence between frontal and parietal regions occurs during top-down serial attention; by contrast, pop-out search induces stronger gamma coherence (adapted from Buschman and Miller 2007). (d, e) Temporal predictability and beta band activity. Sensory stimuli that are predictable in time produce beta ERD/ERS similar to that observed during voluntary movement. This time-frequency representation shows a decrement of beta power that occurs within 200 ms after the stimulus onset. Only in the periodic stimulus condition, the beta ERS reaches the maximum around the onset of the following expected tone. This is not the case for the irregular stimulus. (e) Brain areas in which the beta activity was modulated by the auditory stimuli (d and e, modified from Fujioka and others 2012).
This classic view, however, must be reconciled with results showing that brief enhancement of beta power/coherence correlate with active processes in motor control. For example, in monkey motor cortex, transient bursts of beta activity appear in the local field potential (LFP), and are associated with increased spikes, during precise sensorimotor voluntary tasks that require fine finger movements and focused attention. Such beta oscillations (sharp waves) are instead less frequent during routine movements, such as flexion and extension movements at the wrist (Murthy and Fetz 1992). In humans, during a speeded visuospatial attention task, increases in beta sharp waves correspond to faster reaction times (Chacko and others 2018).
However, one leading hypothesis is that beta activity synchronization is related to the maintenance of tonic activity at the expense of voluntary movements (Baker and others 1997; Baker and others 1999; Kilner and others 1999). These findings, based on the estimation of the cortico-muscular coherence, support the view that raised physiological levels of beta activity act in anti-kinetic fashion (Jenkinson and Brown 2011), for example, they produce slowness of movements (Khanna and Carmena 2017) or signal the tendency of the sensorimotor system to maintain current task goals or stimulus features (i.e., the status quo) over new signals (Engel and Fries 2010). This maintenance can be instrumental to preserve prior expectations about the sensory environment.
Consistent with the Bayesian framework, in which the sensorimotor system provides a generative model to predict the sensory consequence of a movement (Wolpert and Miall 1996), recent studies have suggested that modulations of beta power measured over the sensorimotor cortex may represent the precision of forward internal models, prior to and following a movement (Palmer and others 2019; Tan and others 2016; Tzagarakis and others 2010). For example, Tan and coworkers (2016) show that, when the uncertainty of the prediction is high, the amplitude of the post-movement beta rebound is low. Hence, beta oscillations do not merely index a movement per se, but the confidence in internal models associated to body movements.
To be effective internal models must incorporate sensory signals on the prior state of the body and must be widely available across the cortex. In studies of passive finger movements, normal ERD/ERS are observed in healthy subjects. This indicates that beta power modulation is not only strictly related to motor signals, but also the integration of proprioceptive afferents during planning as in feedforward control, and during movement as in sensory feedback. In Parkinson’s disease, while the ERD component to passive finger movements is normal, the ERS component is significantly attenuated. The ERS attenuation may indicate deterioration of proprioceptive afferents that lead to imprecise movements (Vinding and others 2019). For a more extensive discussion on the role of beta activity and Parkinson’s disease, see (Jenkinson and Brown 2011).
Moreover, beta oscillations increase in central and post-central regions during motor maintenance tasks (Brovelli and others 2004), and across many cortical-subcortical regions in both humans and animals (Baker 2007; Brovelli and others 2004; Kilner and others 2000; Ohara and others 2001; Takei and Seki 2008). This may indicate a role of this rhythm in long-range coordination of distant brain regions.
In summary, current evidence clearly indicates a link between modulations of beta power/coherence, and planning-maintenance of body movements, not only in motor cortex, but also in the somatic system. There is also evidence of abnormal integration of somatosensory signals into motor plans, which would be consistent with alterations of internal models during planning, and error signals after movement onset.
Beta Band in the Top-Down Neocortical Processing
A different line of research has proposed a role of beta oscillations in mediating top-down modulation of visual attention. For example, in monkeys trained to detect targets amongst a set of distractors, either through a serial or pop-out search, an enhancement of beta coupling (coherence) between frontal and parietal regions occurs more strongly in the serial (top-down) condition. In contrast, coupling was more prominent in the gamma band during pop-out search (bottom-up) (Buschman and Miller 2007) (Fig. 2c). Specifically, beta oscillations are important for establishing and maintaining top-down influences on stimulus relevant/irrelevant representation in visual regions (Bastos and others 2015) through long-range synchronization (Kopell and others 2000). In the predictive coding scheme, beta oscillations represent internal models while gamma oscillations reflect prediction errors and their propagation from sensory to associative regions (Arnal and Giraud 2012; Bastos and others 2012).
It is important to emphasize that while these studies interpret beta oscillatory modulations as attention mechanisms, both behavioral (e.g., Rizzolatti and others 1987) and human neuroimaging data (e.g., Corbetta and others 1998) clearly show that visuospatial attention mechanisms are tightly linked to premotor oculomotor mechanisms, especially during exogenous, not endogenous, orienting (Smith and Schenk 2012). Therefore, top-down beta oscillations during attention likely correspond to plans for exploratory eye movements and their modulation on sensory regions.
Beyond Sensorimotor Control: The Role of the Beta Band in Temporal Predictability
A final trend in the literature is the relationship between beta rhythms and temporal prediction of sensory events even in the absence of overt movements. For example, in MEG recordings during passive listening of regular tone sequences, a decrement of beta power occurs within 200 ms after tone onset in bilateral auditory cortex. Beta power returns, then, to the baseline before the onset of the following expected tone (beta rebound). This pattern is similar to that observed in motor cortex (see Fig. 2a). In the case of the randomized stimulation, the beta ERD/ERS is aperiodic and transient in its nature (Fujioka and others 2012) (Fig. 2d and e). These results indicate that beta ERS do not only reflect a state of nonspecific activation/inhibition of cortex, but a more refined signal that predicts sensory events and keeps tracks of modification in sensory stimulation. Although more recent findings claimed that for longer intertrial intervals beta ERS does not closely follow timing mechanisms related to the upcoming stimulation (Meijer and others 2016), phasic increments of beta coherence also occurs in the auditory and motor system and modulate on the interstimulus interval peaking just before the occurrence of the predicted sound (Fujioka and others 2012). Similar modulations occur during temporal prediction tasks (e.g., Iversen and others 2009). These effects strongly suggest a link between beta band oscillations and the short-term structure of a specific task.
In summary, low β activity (~20 Hz) is associated with the generation and maintenance of temporal predictions for sensory stimuli. The origin of these rhythms is in the motor cortex (Jurkiewicz and others 2006), but are also recorded in task-relevant sensory areas, for example, auditory cortex during tone discrimination tasks (Morillon and Baillet 2017), and visual cortex during quasi-periodic or isochronous visual stimulation (Keitel and others 2017). Finally, low motor β oscillations are also observed during speech processing, where beta rhythms may provide a temporal structure for the extraction of linguistic information from sensory regions (Arnal and Giraud 2012).
This review indicates that the original framework of excitation/inhibition of motor cortex during voluntary movements must be modified to take into account the apparent role of beta rhythms in coordinating sensory-motor systems during movement, selection of sensory stimuli during visuospatial attention, and temporal predictions of sensory stimuli. These apparently different interpretations can be reconciled by the idea that beta rhythms originating from motor circuitries reflect a temporal model for sampling, hence predicting, environmental stimuli. While previous work has focused on task-evoked activity, next, we review novel evidence that shows how beta rhythms are important for maintaining long-term predictions about the sensory environment. First, we show the importance of beta rhythms in maintaining the spontaneous architecture of brain networks. Next, this intrinsic (resting) architecture and related dynamics are similar to those observed during natural vision, but not during artificial visual stimulation.
New Perspectives on the Functional Significance of Beta Oscillations
The Beta Band as a Spectral Signature of Dynamic Integration at Rest
It is now well established that the spontaneous activity of the brain is not random but organized in networks of brain regions that correlate in space and time (resting-state networks, RSNs). The most consistent correlates of fMRI RSNs are the slow fluctuations of alpha and beta band BLP. RSNs are not static but dynamically change over time (Box 1). Specifically, functional links among regions that belong to a specific RSN alternate between epochs of weak and robust values (e.g., Fig. 3a, the correlation between left and right pIPS and FEF of the DAN). We describe three main aspects of the dynamic organization of RSNs, in which beta band power fluctuations play an essential role in the integration of functional systems at rest.
Figure 3.
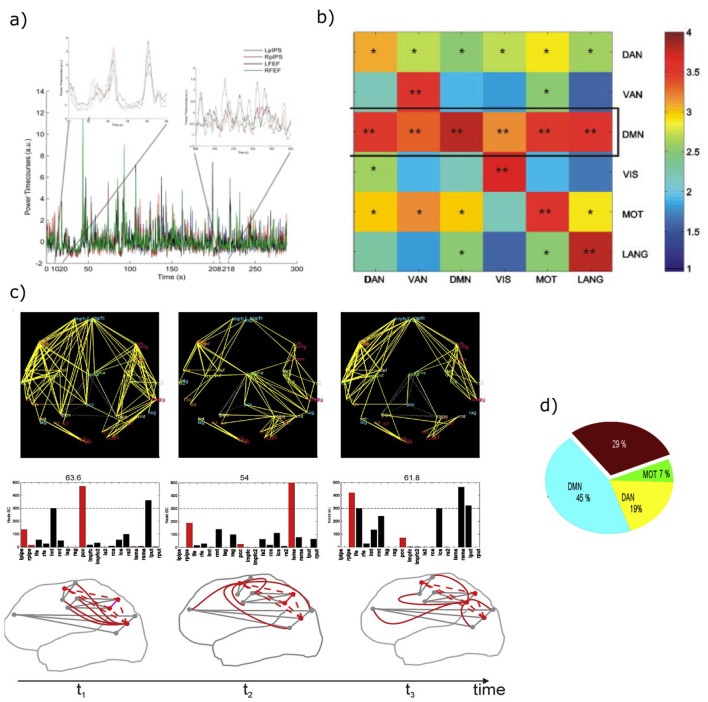
(a) Magnetoencephalography band-limited power (BLP) based resting-state networks shows evidence of temporal dynamics, as an example the BLP fluctuations of nodes from the dorsal attention network (DAN) alternate epochs of high (left panel) and low (right panel) internal coupling (modified from (de Pasquale and others 2010). (b) During epochs of high internal coupling, the default mode network (DMN) acts as a core of across-network integration in the beta band. The represented quantity is the connectivity across networks quantified through z-scores based on the Pearson correlation coefficient (see de Pasquale and others 2010 for details). For every pair of networks, the integration is obtained by averaging the set of z-scores obtained for every possible pairs of nodes from the two networks. Statistical significance: *P < 0.01; **P < 0.05 (modified from de Pasquale and others 2012). (c) In the beta band, functional hubs such as posterior cingulate cortex (PCC), left supplementary motor area (lSMA), and right posterior intraparietal sulcus (rPIPS) act as way stations of integration in the brain over time, as shown by the large number of connections established with other nodes (top panel), alternate their centrality (BC reported in the middle panel) and form a dynamic core network (bottom panel). (d) This strategy corresponds to an optimal criterion of integration as measured via the global efficiency (GE). Epochs of high integration through the dynamic core networks cover around 70% of GE peaks (c and d are modified from de Pasquale and others 2018).
First, the coupling between regions that belong to the same RSN (within-network) fluctuates over time, and this fluctuation allows for the occurrence of interactions between networks (de Pasquale and others 2012). In time intervals in which regions of an RSN strongly couple, regions that belong to other RSNs show weak interactions, and some regions synchronize with the strongly coupled RSN. The default mode network (DMN) and the posterior cingulate cortex (PCC), in particular, exhibit the highest degree of transient correlation with other networks, specifically in the beta frequency range (de Pasquale and others 2012)(Figure 3b). Other regions show strong within and across-network coupling: the left posterior intraparietal sulcus (lPIPS), a region of the DAN, and the supplementary motor area (SMA) in the somatomotor network (SMN) (de Pasquale and others 2016). Other measures of centrality confirm that all these regions are central during the epoch of high within-network coupling (de Pasquale and others 2016) as they tend to be the way of stations among networks. However, their centrality is not static, as in the case of structural connectivity hubs (van den Heuvel and Sporns 2013), but varies over time, hence the term “dynamic hubs” (Fig. 3c).
Second, these central regions not only connect with regions of other RSNs but also become central more frequently, particularly in the beta band (for a recent review, see de Pasquale and others 2018). This property is analogous to the concept of “rich club” derived from the graph analysis of structural connection patterns. Rich-club regions are central (hubs) and strongly interconnected (van den Heuvel and Sporns 2013). Similarly, our dynamic hubs, whose topography overlaps with the rich-club regions of the structural connectome, form a core of functional dynamic hubs, hence the term “dynamic core” (de Pasquale and others 2018). Third, dynamic network coupling and related variations in hub centrality correspond to fluctuations of global efficiency. Efficiency is inversely related to the average path length; the latter is the minimum number of edges necessary to connect every possible pair of nodes (Bullmore and Sporns 2009). Hence, periods of high efficiency correspond to a configuration where nodes of a network can easily connect with other nodes. Efficiency is thus a global measure of information transfer in a network (Bullmore and Sporns 2009; de Pasquale and others 2016). We found that fluctuations of the dynamic core in the beta band BLP, that is, the peaks of the centrality of regions of the dynamic core, predict a large percentage of global efficiency peaks (around 70%) (Fig. 3c and d). In other words, periods of high efficiency correspond to moments of high within- and across- network correlation, that is, moments in which different RSN communicate (de Pasquale and others 2016; de Pasquale and others 2018).
We propose that the dynamic core mechanism optimizes the efficiency of communication among distinct functional domains. The three primary nodes of the dynamic core belong to three different RSNs: PCC (DMN), PIPS (DAN), and SMA (SMN), which in turn form the principal axes of the functional brain architecture. Several studies have shown that at the highest level, the brain divides into three functional domains. Sensory-motor regions, for processing sensory stimuli and planning motor responses; cognitive regions related to external processes, such as perception and attention; and cognitive regions for internal processes, like memory and emotion (Doucet and others 2011; Glasser and others 2016; Hacker and others 2013). At rest, the patterns of functional interactions among brain regions shift between moments in which PCC (DMN) is the pivot point, to moments in which IPS (DAN) or SMA (SMN), or a combination of these hubs, become central in the brain graph. We have proposed (de Pasquale and others 2016; de Pasquale and others 2018) that this temporal mechanism allows for interaction in the resting state of the brain’s principal functional systems.
Brain Beta Rhythms as Temporal Priors for Natural Vision
Now that we have described the role of spontaneous beta oscillations in the dynamic functional architecture of the brain at rest, we will consider whether this architecture is modified or not during ecological tasks to evaluate the hypothesis that spontaneous activity may serve as a mechanism for learning and generating prior representations reflecting the statistics of the environment.
Cognitive and neural processes not only occur along a hierarchy of spatial but also temporal scales. For instance, neurons in regions close to the periphery are optimized for rapid transient responses to stimuli, that is, they integrate information over short periods of time. Association regions of parietal and frontal cortex instead accumulate information over longer timescales (Hasson and others 2008). These regions, especially in the frontal lobes, display slow ECoG power dynamics, and weak coupling to low-level stimulus properties, which likely encode more abstract information (Honey and others 2012). Transient responses in visual occipital regions, and more sustained responses in frontal and posterior parietal cortex have been shown in human fMRI, during a cue period of a visuospatial attention task (Corbetta and others 2000). This implies that different temporal scales of neuronal responses are not stimulus driven but reflect intrinsic connectivity.
Brain regions that function as hubs in the human brain exhibit power fluctuations in the order of seconds, and these fluctuations correspond to variations in the level of integration and global efficiency of the entire brain network (de Pasquale and others 2016; de Pasquale and others 2018).
We think that these slow dynamics represent a suitable candidate to represent the slow-varying structure of real-life events. For instance, in human language, sentence comprehension requires inferences about upcoming words, and from one sentence to the next during a conversation. This process implies an integration of the auditory input on a scale of many seconds. In experiments of sentence processing that require inference about lexical or syntactic binding, increments of beta coherence are found in a network of frontal, temporal, and parietal regions. By contrast, language production or processing of action words, yet not involving motor components, produce decrements of beta power, likely in accordance with the role of beta oscillations in action semantics (for a review, see Weiss and Mueller 2012). Overall, these results suggest that beta enhancements are integral to higher-order representations, especially when the comprehension requires integration over many seconds. Similar timescales are compatible with the understanding of natural visual events, such as movie clip watching in an experimental setting.
These observations lead to a straightforward hypothesis. If the dynamics of integration of central regions (hubs) in the resting state recapitulates the statistics of natural visual stimuli, then they shall be modified by artificial stimuli, but shall remain unchanged when viewing natural scenes. Moreover, if this function is carried out by beta rhythms, then both topography and dynamics of resting beta band connectivity, but not other frequencies, shall resemble that of natural movies.
We recorded BLP fluctuations measure with MEG in regions of a set of RSN involved in movie watching. We estimated static and time-varying functional connectivity when observers were either fixating or watching movie clips that were, in turn, either played regularly or temporally scrambled (Betti and others 2018). Functional connectivity in the alpha band sharply decreased both in the visual system (within RSN) and between visual and DAN (between RSN), both for scrambled and natural movies. This result is consistent with previous observations indicating that alpha BLP reflects idling networks that reconfigure during tasks (Betti and others 2013). In contrast, in the beta band, functional connectivity decreased only for scrambled, not for normal movie stimuli. In particular, the spatial pattern of functional connections in beta did not significantly change during the observation of regular movie clips (Fig. 4a). Hence the topography of beta band connectivity is already set up at rest in a way congenial to the processing of natural stimuli, and as such represent a potential neural correlate of a spatial prior, similarly to the functional connectivity of the DAN during visuospatial attention (Box 1). Next, we measured fluctuations of centrality, that is, moments in which core regions are more connected with other regions of the brain. Figure 4b shows the time course of centrality fluctuations for two dynamic core regions. Note that these fluctuations are slow (~1 every 10-20 seconds) and joint. Figure 4c shows the time course for all regions of the dynamic core. We statistically tested the similarity of joint dynamics for each couple of hub regions in the beta and alpha bands, comparing fixation (rest) to regular versus scrambled movies. In the alpha band, fixation and movie conditions were different. In contrast, in the beta band, the dynamics between fixation and regular movies were similar, while they were different between fixation scrambled movies. Therefore, not only the topology of functional connections in the beta band but also the slow fluctuations of centrality in regions of the dynamic core were similar between rest (fixation) and natural vision.
Figure 4.
(a) In the alpha band, the audiovisual stimulation reorganizes the overall intrinsic network topology. By contrast, in the beta band, the comparison between movie versus fixation does not change the overall topology and, for this reason, is not shown. (b) Time course of the betweenness centrality (dynamic BC) in the beta band during fixation, or the observation of natural and scrambled movie clips. The pink shadows represent temporal epochs of joint centrality for two hubs, in a representative subject. (c) Spatial location of hubs regions of the core network in the beta band. The figure is adapted by Betti and others (2018).
In summary, we propose that joint fluctuations of beta-band BLP between dynamic hub regions of association cortex reflect a mechanism for coding a temporal prior during natural vision. A possible caveat is that these effects might reflect a general preference of BLP correlation for slow- versus fast-changing stimuli, as in the case of regular versus scrambled movie stimuli. Of note, because our studies considered nearly the whole beta band (14-25 Hz), further studies are needed to understand a different a potential role of low versus high beta bands.
Overall, these findings suggest that the spatiotemporal pattern of intrinsic fluctuations in the beta band resemble those observed during natural vision. A possible explanation is that through development and experience the statistics of the spontaneous activity have been tuned to the statistics of the environment. This idea has been proposed in previous animal studies of early visual cortex to explain the similarity between spontaneous and stimulus-driven activity (Berkes and others 2011; Kenet and others 2003). More recent observations have shown that the spontaneous activity of the brain encodes behaviorally relevant motor patterns (Stringer and others 2019). Our studies in human association cortex show similar effects during cognitive tasks (spatial attention, natural vision) (Betti and others 2018; Spadone and others 2015).
It is important to highlight that the relationship between spontaneous and task-evoked activity is a two-way relationship. Spontaneous activity is an intrinsic property of neural tissue, even when entirely disconnected (Sanchez-Vives and McCormick 2000). In the course of development, the statistics of sensory stimuli and the biomechanical properties of the body shape the brain’s developing network topology and dynamics (Byrge and others 2014). This occurs because the repeated exposure to sensory or motor signals produces patterns of brain activity that reverberate in the post-stimulus period and leave a trace in the spontaneous activity (Yao and others 2007). Similar mechanisms have been hypothesized to explain learning-related changes of connectivity. At the same time spontaneous activity patterns form a scaffolding, spatial and temporal, for how the brain can respond to stimuli or move the body. These constraints explain the strong similarity in patterns of rest and task connectivity, and that task activation patterns represent relatively small adjustments and reorganization of patterns of resting connectivity (see Box 1).
An important issue is why do beta rhythms, and not alpha or gamma, are important for maintaining spatial and temporal priors in spontaneous activity? We propose that rapid environmental changes, and error predictions, are coded in early sensory regions, specifically at gamma frequencies. In contrast, the slower temporal structure of natural sensory events and motor sequences may be computed through, slower beta coupling fluctuations. This integration is slow, not only because sensory information is integrated along the cortical hierarchy but also because it consists of a weighted integration of incoming information and prior (statistical) inferences. An efficient brain must be able to use both anticipatory predictions and signals that code for errors and can change the model if needed. For instance, as we prepare to give a lecture in a new lecture hall, we may have strong models of what a lecture hall may look like: a large room with tens or hundreds of seats, usually with a large screen and desk on one side, and a suffused light. However, the specific details of the room: the color of the seats or the floor, must be filled in with sensory information.
Theoretical and empirical studies indicate that gamma oscillations carry information about stimulus features and are important for synaptic plasticity. Indeed, the critical temporal window of plasticity corresponds to the length of the gamma cycle (Buzsáki and Draguhn 2004). In contrast, beta rhythms influence the activation of relevant cell assemblies, through feedback influences, without producing structural changes in the neural circuitry underlying the assemblies (Zheng and Colgin 2015). Another principle is that feedforward signals are carried by gamma (and theta) rhythms, whereas feedback signals by beta synchronization (Bastos and others 2012). Therefore, beta oscillations may mediate the functional coupling of neurons or regions over much longer distances whereas high gamma promotes local processing (Kopell and others 2000).
With regards to alpha rhythms, studies report feedback modulations in the alpha rhythm (van Kerkoerle and others 2014), and sensory sampling timed to the alpha cycle (VanRullen 2016). It has been proposed that alpha and beta oscillations constitute distinct classes of rhythms, both involved in top-down processing (Bressler and Richter 2015). In our MEG experiments, we find alpha and beta BLP connectivity are both strong at rest with a topography similar to that of fMRI networks. However, during natural vision, alpha BLP connectivity significantly decreases, as compared to rest, and task-specific BLP connections form in different frequencies (beta, theta, gamma). Similarly, the dynamics of alpha BLP connectivity is modified during movie observation (Betti and others 2013). Moreover, compared with rest, both natural and scrambled vision cause a significant reorganization of the topology and the dynamics of hub regions in the alpha band (Betti and others 2018). Hence in our hands, alpha BLP connectivity represents an idling rhythm at rest that must be desynchronized for task processing to take place. This is consistent with a large literature on attention in which alpha power decrements reflect activation of visual cortex, while alpha power increments reflect inhibition (Thut and others 2006). To conclude, accordingly with the idea that alpha and beta band have a different functional role, the evidence reviewed above using visual stimuli indicates that the alpha band might reflect an idling rhythm or the inactivity of the system not currently engaged in specific tasks.
Synergistic Coding of Real-Life Events
If the human brain implements predicting strategy to infer the timing of real-world events, it requires an organizing principle to transmit packages of information to downstream or upstream regions. The idea that perception and cognition are periodic is based on the idea that the brain samples sensory information discontinuously thus creating a temporal code, and different rhythms from theta to low beta, have been proposed as the relevant timing mechanism in the visual (VanRullen 2016), somatosensory (Baumgarten and others 2015), and auditory systems (VanRullen and others 2014).
A prior coding for real-world events, be they sensory or motor, must include both spatial, that is the combination of neural events representing the spatial features of the event, and temporal information, that is, the timing of how the event unfolds. In the motor domain, a recent view posits that sensorimotor beta oscillations rather than playing a direct role in generating movement per se maintain representations of muscle synergy in the primary motor cortex (Aumann and Prut 2015). The term “synergy” indicates a control strategy to solve the computational problem of the high dimensionality of the degrees of freedom of arm and hand movements. Although theoretically arm and hand movements can be planned through a very high number of combined muscle activation and joint movements, many studies have shown that in real-life conditions a small set of correlated muscle activations across multiple joints explains the majority of arm movements. This is because in real life the statistics of movements are very correlated in space and time, and across individuals (d’Avella and Bizzi 2005; Ingram and others 2008). As an example, we always reach with the arm ipsilateral to the location of the target and mostly near the body (Howard and others 2010). Only rarely we cross the midline or reach behind our backs.
There is also evidence for low dimensionality of other functions within and across subjects. For instance, exploratory eye movements are similar across tasks, and one factor explains the majority of inter-individual variability, which is partly genetically determined, hence intrinsic not task-evoked (Kennedy and others 2017).
Similarly, low dimensionality at the population level has been observed when the brain is hit by lesions. In the case of stroke across hundreds of focal lesions distributed across the brain, three components of correlated deficits explain the majority of variance (Corbetta and others 2015; Siegel and others 2016). Our interpretation is that this low dimensionality of behavior reflects some intrinsic low dimensionality of macroscale neural patterns. We have proposed the term “cognitive synergies” to indicate patterns of highly frequent behaviors or information states that decrease the dimensionality of neural states and that correlate behavioral variables within/across subjects (Corbetta and others 2018). Therefore, we would like to propose is that intrinsic beta rhythms, especially in central hub regions of the brain, may be one of the neural correlates of these cognitive synergies. Joint fluctuations of the core network hubs in the beta band might represent a synergistic code that represents features that are highly probable in the natural environment. Fluctuations of the core network transmit samples of information by top-down biasing the functional coupling between other network regions. By contrast, this mechanism does not occur in other bands, for example, the alpha band. The idea of synergies may also be coded in resting-state activity thus forming prior representations (Corbetta and others 2018). Intrinsic beta oscillations maintain discrete sensorimotor and cognitive representations of real-life events, that in turn, facilitate action and perception, because they permit to predict what is likely to happen in the environment, based on the recent history. As such, for highly predictable behaviors, for example, the movement of the arm that grasps an object, the outcome can be inferred only based on the observation of the first stages of the movement.
Conclusions
The article has reviewed evidence for the role of beta rhythms slow and fast in the anticipatory coding of motor and sensory events. We have shown that beta rhythms are important for maintaining an intrinsic functional organization in most central regions of the brain. These regions form a dynamic core whose fluctuations correlate with variations in overall brain’s centrality and global efficiency. The fluctuating nature of information exchange suggests that a pulsatile mode is also important for motor and sensory processing. Interestingly, similar fluctuations occur in the resting state, and that both the topography of functional connections as well as the dynamics at rest are in-tune with the statistics of natural visual stimuli, but not with those of artificial stimuli. We propose that intrinsic beta rhythms may be one of the mechanisms of spatiotemporal neural and behavioral priors used to improve processing in the face of potentially high dimensionality of sensory and motor events.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (Grant Agreement No. 759651 to VB). MC was funded by NIH RO1 NS095741; FLAG-ERA JTC; BIAL Foundation; Dipartimento di Eccellenza, ITALIAN MINISTRY OF RESEARCH (MIUR); CARIPARO FOUNDATION Padova; MINISTRY OF HEALTH ITALY; CELEGHIN FOUNDATION Padova.
ORCID iD: Viviana Betti  https://orcid.org/0000-0003-3429-2104
https://orcid.org/0000-0003-3429-2104
References
- Albert NB, Robertson EM, Miall RC. 2009. The resting human brain and motor learning. Curr Biol 19(12):1023–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnal LH, Giraud AL. 2012. Cortical oscillations and sensory predictions. Trends Cogn Sci 16(7):390–8. [DOI] [PubMed] [Google Scholar]
- Attwell D, Laughlin SB. 2001. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab 21(10):1133–45. [DOI] [PubMed] [Google Scholar]
- Aumann TD, Prut Y. 2015. Do sensorimotor beta-oscillations maintain muscle synergy representations in primary motor cortex? Trends Neurosci 38(2):77–85. [DOI] [PubMed] [Google Scholar]
- Baker SN. 2007. Oscillatory interactions between sensorimotor cortex and the periphery. Curr Opin Neurobiol 17(6):649–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SN, Chiu M, Fetz EE. 2006. Afferent encoding of central oscillations in the monkey arm. J Neurophysiol 95(6):3904–10. [DOI] [PubMed] [Google Scholar]
- Baker SN, Kilner JM, Pinches EM, Lemon RN. 1999. The role of synchrony and oscillations in the motor output. Exp Brain Res 128(1–2): 109–17. [DOI] [PubMed] [Google Scholar]
- Baker SN, Olivier E, Lemon RN. 1997. Coherent oscillations in monkey motor cortex and hand muscle EMG show task-dependent modulation. J Physiol 501(pt 1): 225–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldassarre A, Lewis CM, Committeri G, Snyder AZ, Romani GL, Corbetta M. 2012. Individual variability in functional connectivity predicts performance of a perceptual task. Proc Natl Acad Sci U S A 109(9):3516–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos AM, Usrey WM, Adams RA, Mangun GR, Fries P, Friston KJ. 2012. Canonical microcircuits for predictive coding. Neuron 76(4):695–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos AM, Vezoli J, Bosman CA, Schoffelen JM, Oostenveld R, Dowdall JR, and others. 2015. Visual areas exert feedforward and feedback influences through distinct frequency channels. Neuron 85(2):390–401. [DOI] [PubMed] [Google Scholar]
- Baumgarten TJ, Schnitzler A, Lange J. 2015. Beta oscillations define discrete perceptual cycles in the somatosensory domain. Proc Natl Acad Sci U S A 112(39):12187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkes P, Orban G, Lengyel M, Fiser J. 2011. Spontaneous cortical activity reveals hallmarks of an optimal internal model of the environment. Science 331(6013):83–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betti V, Corbetta M, de Pasquale F, Wens V, Della Penna S. 2018. Topology of functional connectivity and hub dynamics in the beta band as temporal prior for natural vision in the human brain. J Neurosci 38(15):3858–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betti V, Della Penna S, de Pasquale F, Mantini D, Marzetti L, Romani GL, and others. 2013. Natural scenes viewing alters the dynamics of functional connectivity in the human brain. Neuron 79(4):782–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. 1995. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34(4):537–41. [DOI] [PubMed] [Google Scholar]
- Brookes MJ, Woolrich M, Luckhoo H, Price D, Hale JR, Stephenson MC, and others. 2011. Investigating the electrophysiological basis of resting state networks using magnetoencephalography. Proc Natl Acad Sci U S A 108(40):16783–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler SL, Richter CG. 2015. Interareal oscillatory synchronization in top-down neocortical processing. Curr Opin Neurobiol 31:62–6. [DOI] [PubMed] [Google Scholar]
- Brovelli A, Ding M, Ledberg A, Chen Y, Nakamura R, Bressler SL. 2004. Beta oscillations in a large-scale sensorimotor cortical network: directional influences revealed by Granger causality. Proc Natl Acad Sci U S A 101(26):9849–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. 2009. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10(3):186–98. [DOI] [PubMed] [Google Scholar]
- Buschman TJ, Miller EK. 2007. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science 315(5820):1860–2. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. 2019. The brain from inside out. Oxford, England: Oxford University Press. [Google Scholar]
- Buzsáki G, Draguhn A. 2004. Neuronal oscillations in cortical networks. Science 304(5679):1926–9. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Logothetis N, Singer W. 2013. Scaling brain size, keeping timing: evolutionary preservation of brain rhythms. Neuron 80(3):751–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrge L, Sporns O, Smith LB. 2014. Developmental process emerges from extended brain-body-behavior networks. Trends Cogn Sci 18(8):395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacko RV, Kim B, Jung SW, Daitch AL, Roland JL, Metcalf NV, and others. 2018. Distinct phase- amplitude couplings distinguish cognitive processes in human attention. Neuroimage 175:111–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Bassett DS, Power JD, Braver TS, Petersen SE. 2014. Intrinsic and task-evoked network architectures of the human brain. Neuron 83(1):238–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M. 1998. Frontoparietal cortical networks for directing attention and the eye to visual locations: identical, independent, or overlapping neural systems? Proc Natl Acad Sci U S A 95(3):831–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, and others. 1998. A common network of functional areas for attention and eye movements. Neuron 21(4):761–73. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. 2000. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat Neurosci 3(3):292–7. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Ramsey L, Callejas A, Baldassarre A, Hacker CD, Siegel JS, and others. 2015. Common behavioral clusters and subcortical anatomy in stroke. Neuron 85(5):927–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Siegel JS, Shulman GL. 2018. On the low dimensionality of behavioral deficits and alterations of brain network connectivity after focal injury. Cortex 107:229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Avella A, Bizzi E. 2005. Shared and specific muscle synergies in natural motor behaviors. Proc Natl Acad Sci U S A 102(8):3076–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pasquale F, Corbetta M, Betti V, Della Penna S. 2018. Cortical cores in network dynamics. Neuroimage 180(pt B):370–82. [DOI] [PubMed] [Google Scholar]
- de Pasquale F, Della Penna S, Snyder AZ, Lewis C, Mantini D, Marzetti L, and others. 2010. Temporal dynamics of spontaneous MEG activity in brain networks. Proc Natl Acad Sci U S A 107:6040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pasquale F, Della Penna S, Snyder AZ, Marzetti L, Pizzella V, Romani GL, and others. 2012. A cortical core for dynamic integration of functional networks in the resting human brain. Neuron 74:753–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pasquale F, Della Penna S, Sporns O, Romani GL, Corbetta M. 2016. A dynamic core network and global efficiency in the resting human brain. Cereb Cortex 26(10):4015–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Tommaso M, Betti V, Bocci T, Bolognini N, Di Russo F, Fattapposta F, and others. 2020. Pearls and pitfalls in brain functional analysis by event–related potentials: a narrative review by Italian Psychophysiology and Cognitive Neuroscience Society on methodological limits and clinical reliability—Part I. Neurol Sci. Epub May 9. doi: 10.1007/s10072-020-04420-7 [DOI] [PubMed] [Google Scholar]
- Deco G, Corbetta M. 2010. The dynamical balance of the brain at rest. Neuroscientist 17(1):107–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco G, Ponce-Alvarez A, Mantini D, Romani GL, Hagmann P, Corbetta M. 2013. Resting-state functional connectivity emerges from structurally and dynamically shaped slow linear fluctuations. J Neurosci 33(27):11239–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet G, Naveau M, Petit L, Delcroix N, Zago L, Crivello F, and others. 2011. Brain activity at rest: a multiscale hierarchical functional organization. J Neurophysiol 105(6):2753–63. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P. 2010. Beta-band oscillations—signalling the status quo? Curr Opin Neurobiol 20(2):156–65. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P, Singer W. 2001. Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci 2(10):704–16. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. 1991. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex 1(1):1–47. [DOI] [PubMed] [Google Scholar]
- Fiser J, Berkes P, Orban G, Lengyel M. 2010. Statistically optimal perception and learning: from behavior to neural representations. Trends Cogn Sci 14(3):119–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. 2005. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A 102(27):9673–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K. 2005. A theory of cortical responses. Philos Trans R Soc Lond B Biol Sci 360(1456):815–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka T, Trainor LJ, Large EW, Ross B. 2012. Internalized timing of isochronous sounds is represented in neuromagnetic beta oscillations. J Neurosci 32(5):1791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido MI, Kilner JM, Stephan KE, Friston KJ. 2009. The mismatch negativity: a review of underlying mechanisms. Clin Neurophysiol 120(3):453–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, and others. 2016. A multi-modal parcellation of human cerebral cortex. Nature 536(7615):171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker CD, Laumann TO, Szrama NP, Baldassarre A, Snyder AZ, Leuthardt EC, and others. 2013. Resting state network estimation in individual subjects. Neuroimage 82:616–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker CD, Snyder AZ, Pahwa M, Corbetta M, Leuthardt EC. 2017. Frequency-specific electrophysiologic correlates of resting state fMRI networks. Neuroimage 149:446–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U, Yang E, Vallines I, Heeger DJ, Rubin N. 2008. A hierarchy of temporal receptive windows in human cortex. J Neurosci 28(10):2539–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmholtz HEFv. 1909. Treatise on physiological optic. In Southhall JPC. (translator, editor). Rochester, NY: Optical Society of America. [Google Scholar]
- Honey CJ, Thesen T, Donner TH, Silbert LJ, Carlson CE, Devinsky O, and others. 2012. Slow cortical dynamics and the accumulation of information over long timescales. Neuron 76(2):423–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard IS, Ingram JN, Wolpert DM. 2010. Context-dependent partitioning of motor learning in bimanual movements. J Neurophysiol 104(4):2082–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison RM, Womelsdorf T, Allen EA, Bandettini PA, Calhoun VD, Corbetta M, and others. 2013. Dynamic functional connectivity: promise, issues, and interpretations. Neuroimage 80:360–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram JN, Kording KP, Howard IS, Wolpert DM. 2008. The statistics of natural hand movements. Exp Brain Res 188(2):223–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen JR, Repp BH, Patel AD. 2009. Top-down control of rhythm perception modulates early auditory responses. Ann NY Acad Sci 1169:58–73. [DOI] [PubMed] [Google Scholar]
- Iwamura Y. 1998. Hierarchical somatosensory processing. Curr Opin Neurobiol 8(4):522–8. [DOI] [PubMed] [Google Scholar]
- Jenkinson N, Brown P. 2011. New insights into the relationship between dopamine, beta oscillations and motor function. Trends Neurosci 34(12):611–8. [DOI] [PubMed] [Google Scholar]
- Jurkiewicz MT, Gaetz WC, Bostan AC, Cheyne D. 2006. Post-movement beta rebound is generated in motor cortex: evidence from neuromagnetic recordings. Neuroimage 32(3):1281–9. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Hackett TA. 1998. Subdivisions of auditory cortex and levels of processing in primates. Audiol Neurootol 3(2–3): 73–85. [DOI] [PubMed] [Google Scholar]
- Keitel C, Thut G, Gross J. 2017. Visual cortex responses reflect temporal structure of continuous quasi-rhythmic sensory stimulation. Neuroimage 146:58–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenet T, Bibitchkov D, Tsodyks M, Grinvald A, Arieli A. 2003. Spontaneously emerging cortical representations of visual attributes. Nature 425(6961):954–6. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, D’Onofrio BM, Quinn PD, Bolte S, Lichtenstein P, Falck-Ytter T. 2017. Genetic influence on eye movements to complex scenes at short timescales. Curr Biol 27(22):3554–60 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna P, Carmena JM. 2017. Beta band oscillations in motor cortex reflect neural population signals that delay movement onset. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiebel SJ, Daunizeau J, Friston KJ. 2008. A hierarchy of time-scales and the brain. PLoS Comput Biol 4(11):e1000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner JM, Baker SN, Salenius S, Hari R, Lemon RN. 2000. Human cortical muscle coherence is directly related to specific motor parameters. J Neurosci 20(23):8838–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner JM, Baker SN, Salenius S, Jousmaki V, Hari R, Lemon RN. 1999. Task-dependent modulation of 15-30 Hz coherence between rectified EMGs from human hand and forearm muscles. J Physiol 516(pt 2):559–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Kay K, Shulman GL, Corbetta M. 2018. A new modular brain organization of the BOLD signal during natural vision. Cereb Cortex 28(9):3065–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopell N, Ermentrout GB, Whittington MA, Traub RD. 2000. Gamma rhythms and beta rhythms have different synchronization properties. Proc Natl Acad Sci U S A 97(4):1867–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouider S, Long B, Le Stanc L, Charron S, Fievet AC, Barbosa LS, and others. 2015. Neural dynamics of prediction and surprise in infants. Nat Commun 6:8537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachaux JP, Rodriguez E, Martinerie J, Varela FJ. 1999. Measuring phase synchrony in brain signals. Hum Brain Mapp 8(4):194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner JR. 1988. Some proprioceptive influences on the perceptual representation of body shape and orientation. Brain 111(pt 2):281–97. [DOI] [PubMed] [Google Scholar]
- Larson-Prior LJ, Zempel JM, Nolan TS, Prior FW, Snyder AZ, Raichle ME. 2009. Cortical network functional connectivity in the descent to sleep. Proc Natl Acad Sci U S A 106(11):4489–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacquaniti F, Bosco G, Gravano S, Indovina I, La Scaleia B, Maffei V, and others. 2014. Multisensory integration and internal models for sensing gravity effects in primates. Biomed Res Int 2014:615854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal DK, Gage GJ, Schmidt R, Pettibone JR, Case AC, Berke JD. 2012. Basal ganglia beta oscillations accompany cue utilization. Neuron 73(3):523–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CM, Baldassarre A, Committeri G, Romani GL, Corbetta M. 2009. Learning sculpts the spontaneous activity of the resting human brain. Proc Natl Acad Sci U S A 106:17558–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. 2001. Neurophysiological investigation of the basis of the fMRI signal. Nature 412(6843):150–7. [DOI] [PubMed] [Google Scholar]
- Mantini D, Perrucci MG, Del Gratta C, Romani GL, Corbetta M. 2007. Electrophysiological signatures of resting state networks in the human brain. Proc Natl Acad Sci U S A 104:13170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzetti L, Della Penna S, Snyder AZ, Pizzella V, Nolte G, de Pasquale F, and others. 2013. Frequency specific interactions of MEG resting state activity within and across brain networks as revealed by the multivariate interaction measure. Neuroimage 79:172–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre J, Zago M, Berthoz A, Lacquaniti F. 2001. Does the brain model Newton’s laws? Nat Neurosci 4(7):693–4. [DOI] [PubMed] [Google Scholar]
- Meijer D, Te Woerd E, Praamstra P. 2016. Timing of beta oscillatory synchronization and temporal prediction of upcoming stimuli. Neuroimage 138:233–41. [DOI] [PubMed] [Google Scholar]
- Miller EK, Lundqvist M, Bastos AM. 2018. Working memory 2.0. Neuron 100(2):463–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misic B, Betzel RF, de Reus MA, van den Heuvel MP, Berman MG, McIntosh AR, and others. 2016. Network-level structure-function relationships in human neocortex. Cereb Cortex 26(7):3285–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morillon B, Baillet S. 2017. Motor origin of temporal predictions in auditory attention. Proc Natl Acad Sci U S A 114(42):E8913–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morillon B, Hackett TA, Kajikawa Y, Schroeder CE. 2015. Predictive motor control of sensory dynamics in auditory active sensing. Curr Opin Neurobiol 31:230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher JC, Leahy RM, Lewis PS. 1999. EEG and MEG: forward solutions for inverse methods. IEEE Trans Biomed Eng 46(3):245–59. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Fetz EE. 1992. Coherent 25- to 35-Hz oscillations in the sensorimotor cortex of awake behaving monkeys. Proc Natl Acad Sci U S A 89(12):5670–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara S, Mima T, Baba K, Ikeda A, Kunieda T, Matsumoto R, and others. 2001. Increased synchronization of cortical oscillatory activities between human supplementary motor and primary sensorimotor areas during voluntary movements. J Neurosci 21(23):9377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill GC, Tewarie P, Vidaurre D, Liuzzi L, Woolrich MW, Brookes MJ. 2018. Dynamics of large-scale electrophysiological networks: a technical review. Neuroimage 180(pt B):559–76. [DOI] [PubMed] [Google Scholar]
- Palmer CE, Auksztulewicz R, Ondobaka S, Kilner JM. 2019. Sensorimotor beta power reflects the precision-weighting afforded to sensory prediction errors. Neuroimage 200:59–71. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da, Silva FH. 1999. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol 110(11):1842–57. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Stancak A, Jr, Neuper C. 1996. Post-movement beta synchronization. A correlate of an idling motor area? Electroencephalogr Clin Neurophysiol 98(4):281–93. [DOI] [PubMed] [Google Scholar]
- Raichle ME. 2011. The restless brain. Brain Connect 1:3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. 2001. A default mode of brain function. Proc Natl Acad Sci U S A 98(2):676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Riggio L, Dascola I, Umilta C. 1987. Reorienting attention across the horizontal and vertical meridians: evidence in favor of a premotor theory of attention. Neuropsychologia 25(1A):31–40. [DOI] [PubMed] [Google Scholar]
- Sanchez-Vives MV, McCormick DA. 2000. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat Neurosci 3(10):1027–34. [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Lakatos P. 2009. Low-frequency neuronal oscillations as instruments of sensory selection. Trends Neurosci 32(1):9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastiani V, de Pasquale F, Costantini M, Mantini D, Pizzella V, Romani GL, and others. 2014. Being an agent or an observer: different spectral dynamics revealed by MEG. Neuroimage 102(pt 2):717–28. [DOI] [PubMed] [Google Scholar]
- Shadlen MN, Newsome WT. 1998. The variable discharge of cortical neurons: implications for connectivity, computation, and information coding. J Neurosc 18(10):3870–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman MA, Lee S, Law R, Haegens S, Thorn CA, Hamalainen MS, and others. 2016. Neural mechanisms of transient neocortical beta rhythms: converging evidence from humans, computational modeling, monkeys, and mice. Proc Natl Acad Sci U S A 113(33):E4885–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JS, Ramsey LE, Snyder AZ, Metcalf NV, Chacko RV, Weinberger K, and others. 2016. Disruptions of network connectivity predict impairment in multiple behavioral domains after stroke. Proc Natl Acad Sci U S A 113(30): E4367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester CM, Corbetta M, Raichle ME, Rodebaugh TL, Schlaggar BL, Sheline YI, and others. 2012. Functional network dysfunction in anxiety and anxiety disorders. Trends Neurosci 35(9):527–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DT, Schenk T. 2012. The Premotor theory of attention: time to move on? Neuropsychologia 50(6):1104–14. [DOI] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, and others. 2009. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A 106:13040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadone S, Della Penna S, Sestieri C, Betti V, Tosoni A, Perrucci MG, and others. 2015. Dynamic reorganization of human resting-state networks during visuospatial attention. Proc Natl Acad Sci U S A 112(26):8112–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam CJ, Nolte G, Daffertshofer A. 2007. Phase lag index: assessment of functional connectivity from multi channel EEG and MEG with diminished bias from common sources. Hum Brain Mapp 28(11):1178–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer C, Pachitariu M, Steinmetz N, Reddy CB, Carandini M, Harris KD. 2019. Spontaneous behaviors drive multidimensional, brainwide activity. Science 364(6437):255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei T, Seki K. 2008. Spinomuscular coherence in monkeys performing a precision grip task. J Neurophysiol 99(4):2012–20. [DOI] [PubMed] [Google Scholar]
- Tan H, Wade C, Brown P. 2016. Post-movement beta activity in sensorimotor cortex indexes confidence in the estimations from internal models. J Neuroscience 36(5):1516–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut G, Nietzel A, Brandt SA, Pascual-Leone A. 2006. Alpha-band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. J Neurosci 26(37):9494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzagarakis C, Ince NF, Leuthold AC, Pellizzer G. 2010. Beta-band activity during motor planning reflects response uncertainty. J Neuroscience 30(34):11270–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O. 2013. Network hubs in the human brain. Trends Cogn Sci 17(12):683–96. [DOI] [PubMed] [Google Scholar]
- van Ede F, Quinn AJ, Woolrich MW, Nobre AC. 2018. Neural oscillations: sustained rhythms or transient burst-events? Trends Neurosci 41(7):415–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Anderson CH, Felleman DJ. 1992. Information processing in the primate visual system: an integrated systems perspective. Science 255(5043):419–23. [DOI] [PubMed] [Google Scholar]
- van Kerkoerle T, Self MW, Dagnino B, Gariel-Mathis MA, Poort J, van der Togt C, and others. 2014. Alpha and gamma oscillations characterize feedback and feedforward processing in monkey visual cortex. Proc Natl Acad Sci U S A 111(40):14332–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanRullen R. 2016. Perceptual cycles. Trends Cogn Sci 20(10):723–35. [DOI] [PubMed] [Google Scholar]
- VanRullen R, Zoefel B, Ilhan B. 2014. On the cyclic nature of perception in vision versus audition. Philos Trans R Soc Lond B Biol Sci 369(1641):20130214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder aZ, Baker JT, Van Essen DC, and others. 2007. Intrinsic functional architecture in the anaesthetized monkey brain. Nature 447:83–6. [DOI] [PubMed] [Google Scholar]
- Vinding MC, Tsitsi P, Piitulainen H, Waldthaler J, Jousmaki V, Ingvar M, and others. 2019. Attenuated beta rebound to proprioceptive afferent feedback in Parkinson’s disease. Sci Rep 9(1):2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ. 2010. Neurophysiological and computational principles of cortical rhythms in cognition. Physiol Rev 90(3):1195–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S, Mueller HM. 2012. “Too many betas do not spoil the broth”: The role of beta brain oscillations in language processing. Front Psychol 3:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert DM, Miall RC. 1996. Forward models for physiological motor control. Neural Netw 9(8):1265–79. [DOI] [PubMed] [Google Scholar]
- Yao H, Shi L, Han F, Gao H, Dan Y. 2007. Rapid learning in cortical coding of visual scenes. Nat Neurosci 10(6):772–8. [DOI] [PubMed] [Google Scholar]
- Zheng C, Colgin LL. 2015. Beta and gamma rhythms go with the flow. Neuron 85(2):236–7. [DOI] [PMC free article] [PubMed] [Google Scholar]