Abstract
Background
Coronaviruses (CoVs) are distributed worldwide and have various susceptible hosts; CoVs infecting humans are called human coronaviruses (HCoVs). Although HCoV-specific drugs are still lacking, many potent targets for drug discovery are being explored, and many vigorously designed clinical trials are being carried out in an orderly manner. The aim of this review was to gain a comprehensive understanding of the current status of drug development against HCoVs, particularly severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Main text
A scoping review was conducted by electronically searching research studies, reviews, and clinical trials in PubMed and the CNKI. Studies on HCoVs and therapeutic drug discovery published between January 2000 and October 2020 and in English or Chinese were included, and the information was summarized. Of the 3248 studies identified, 159 publication were finally included. Advances in drug development against HCoV, especially SARS-CoV-2, are summarized under three categories: antiviral drugs aimed at inhibiting the HCoV proliferation process, drugs acting on the host's immune system, and drugs derived from plants with potent activity. Furthermore, clinical trials of drugs targeting SARS-CoV-2 are summarized.
Conclusions
During the spread of COVID-19 outbreak, great efforts have been made in therapeutic drug discovery against the virus, although the pharmacological effects and adverse reactions of some drugs under study are still unclear. However, well-designed high-quality studies are needed to further study the effectiveness and safety of these potential drugs so as to provide valid recommendations for better control of the COVID-19 pandemic.
Keywords: Human coronavirus, Drug discovery, Drug development, SARS-CoV-2
Background
Coronaviruses (CoVs), which consist of nucleoproteins (N), envelope proteins (E), matrix proteins (M), spike proteins (S), and many non-structural proteins, are linear single-stranded RNA viruses [1]. CoVs are a large family of viruses with various susceptible hosts, including humans and many other animal species, such as camels, cattle, cats, and bats [2]; those infecting humans are called human coronaviruses (HCoVs). HCoVs include HCoV-229E, NL63, OC43, HKU1, severe acute respiratory syndrome coronavirus (SARS-CoV), Middle East respiratory syndrome coronavirus (MERS-CoV) and SARS-CoV-2 and are recognized to be important causes of respiratory tract infection [3, 4]. The former four types are considered common HCoVs and usually lead to mild to moderate upper respiratory tract illnesses [4], while the other three types are different. After the outbreaks of SARS in 2002 and MERS in 2012, the world experienced the coronavirus disease 2019 (COVID-19) pandemic caused by SARS-CoV-2 in 2020. Similar to SARS-CoV, SARS-CoV-2 appeared unexpectedly and spread throughout the world rapidly, with 56 623 643 confirmed cases and 1 355 963 deaths [5]. Fever and cough are the most common symptoms of COVID-19; patients infected with SARS-CoV-2 can develop dyspnoea a week after onset, and critical patients usually die from uncontrollable sepsis, respiratory failure, acute respiratory distress syndrome (ARDS) and septic shock [6]. Therapeutic interventions excluding virus-specific drugs, are often experiential or anecdotal and have not been tested in an integrated trial to provide sufficient and widely accepted evidence. The most common interventions include a combination of antivirals (such as ribavirin and lopinavir/ritonavir) and interferons (IFNs), corticosteroids, COVID-19 convalescent plasma and supportive treatment for critical patients [7].
Previous studies have revealed the invasion mechanism of HCoVs. In brief, S1 binds to the relevant receptor and induces endocytosis, then the conformation of the S2 subunit changes. The viral envelope fuses with the endosomal membrane and releases the nucleocapsid or viral genome [8]. Genomic RNA (gRNA) serves as a translation template for polyproteins pp1a and pp1ab, which are automatically hydrolysed into various non-structural proteins (NSPs), such as papain-like protease (PLpro), 3C-like protease (Mpro), and RNA-dependent RNA polymerase (RdRp). Full-length gRNA is replicated by negative sense intermediates and transcribed into subgenomic RNA (sgRNA). sgRNA encodes the structural proteins of the virus (N, M, E, and S) as well as helper proteins (e.g., 3, 4a, 4B, 5, and 8b). Particle assembly occurs in the ER-Golgi intermediate compartment (ERGIC) and is then released in the vesicle via the secretory pathway [3]. Interruption of the proliferation process might help cure patients and disrupt transmission. As the understanding of both the biological characteristics and pathogenicity of HCoVs has deepened, many potent targets for drug discovery have been explored, such as inhibiting HCoV invasion and strengthening host immune defences. In addition, traditional Chinese medicine might be effective in the fight against HCoVs. To establish additional evidence supporting recommended treatment strategies, some drugs, such as remdesivir, favipiravir, lopinavir/ritonavir, arbidol/umifenovir, and hydroxychloroquine, have been tested in vigorously designed clinical trials. Herein, we review the progress in therapeutic drug discovery and development, including drugs that inhibit the CoV proliferation process (attachment and entry, replicase expression, replication, transcription and translation, assembly and release), antiviral drugs that affect the action of the host's immune system, and drugs derived from plants with potent activity, in order to accelerate drug discovery and development, especially during the current pandemic.
Main text
Methodology
Search strategy
We searched two databases: PubMed (https://www.ncbi.nlm.nih.gov/pubmed/) and CNKI (www.cnki.net). We searched for coronavirus (or HCoV) and important components (such as S protein, PLpro, Mpro, NSPs, RNA, N protein, E protein, host factors) and drugs (or Chinese medicine, plant derivates, or research or treatment). References of studies retrieved were cross checked as well. All the search results were evaluated. First, the titles and abstracts were screened to identify relevant studies; then, full texts were evaluated carefully to determine eligibility for inclusion. The complete search and selection processes were performed by two independent researchers. Any disagreements were resolved through consultation with a third researcher or team discussion until consensus was reached.
Inclusion criteria
(1) The target coronaviruses were HCoVs, with special attention to highly pathogenic HCoVs; (2) The studied drugs included newly developed targeted drugs, broad-spectrum antiviral drugs, small-molecule compounds, plant derivatives, etc.; (3) the research performed included in vivo or in vitro tests, clinical trials, or literature reviews; (4) the publication language was English or Chinese; (5) the literature type was an article, review, or clinical trial; and (6) the publication time was from January 1, 2000, to October 27, 2020.
Exclusion criteria
(1) Duplicate studies; (2) studies for which the full text was unavailable; (3) news, reports, interviews, comments, patents, letters, or case reports; and (4) reviews or studies with the aim of elucidating the impact of coronavirus infection on the underlying diseases and their treatment in a target population.
Data extraction, summary, and analysis
We classified the selected documents according to the following categories: (1) antiviral drugs intended to inhibit the HCoV proliferation process; (2) antiviral drugs that affect the action of the host's immune system; and (3) antiviral drugs derived from plants with potent activity. All articles were processed using NoteExpress V 3.0 (Beijing Aegean Technology Co., Ltd., Beijing, China).
Results
The scoping process
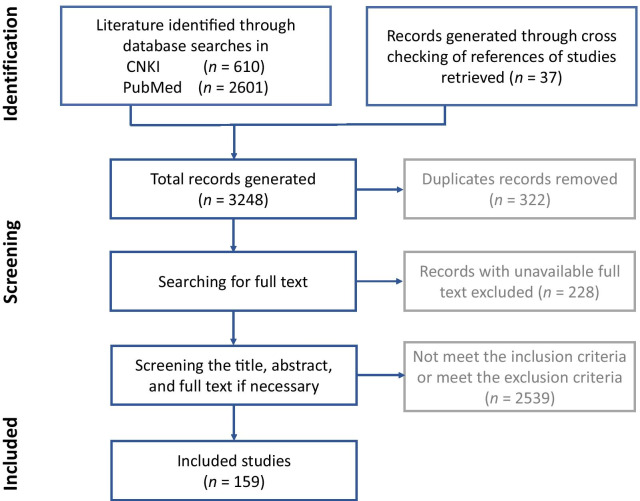
A total of 3248 records were retrieved. After excluding 322 duplicate records, 228 records with unavailable full texts, and 2539 records that met the exclusion criteria mentioned above, 159 records were finally included in this review. A flow diagram of the study selection process is shown in Fig. 1.
Fig. 1.
PRISMA flow diagram of the scoping review process
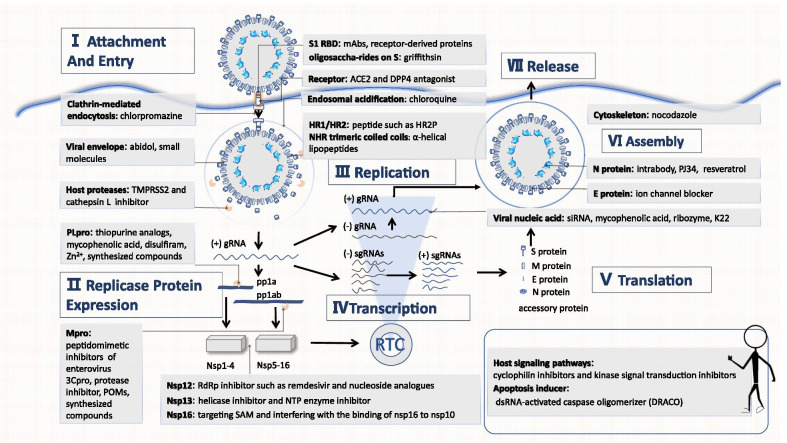
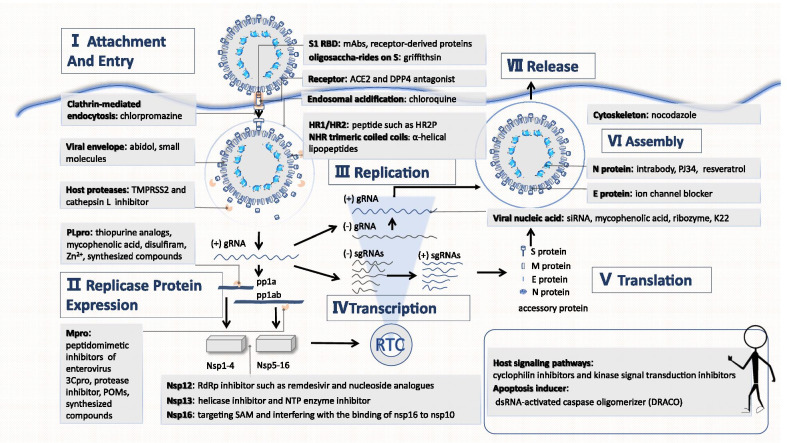
The proliferation process as well as the key targets of CoVs are presented (Fig. 2). A summary of the progress in therapeutic drug discovery and development targeting HCoVs is demonstrated below and is divided into three sections: drugs that inhibit HCoV proliferation (attachment and entry, replicase expression, replication, transcription and translation, assembly and release), antiviral drugs that affect the actions of the host's immune system, and drugs derived from plants with potent activity.
Fig. 2.
The CoV proliferation process. TMPRSS2: Transmembrane protease serine 2; Zn2+: Zinc ion; PLpro: Papain-like protease; Mpro: 3C-like protease; 3Cpro: 3C protease; POMs: Polyoxometalates; Nsps: non-structural proteins; NTP: Nucleoside triphosphate; SAM: S-adenosyl-l-methionine; RBD: Receptor binding domain; mAb: monoclonal antibodies; ACE2: Angiotensin converting enzyme 2; DPP4: dipeptidyl peptidase 4; HR1: Heptad repeat 1 domain; HR2: Heptad repeat 2 domain; gRNA: genomic RNA; sgRNA: subgenomic RNA; siRNA: small interfering RNA; NHR: N-terminal heptad repeat; dsRNA: Double-stranded RNA
Blocking the HCoV proliferation process is the key to identify effective drugs against the virus
The drugs that target the CoV proliferation process are summarized in Table 1.
Table 1.
Drugs aiming at the proliferation process
| Step | Target | Drugs | Type | Reference number | ||
|---|---|---|---|---|---|---|
| Category | Example | |||||
| Attachment and entry | Block receptor binding | S1-RBD | mAbs | m336, m337 and m338 | MERS-CoV | [9] |
| Human-derived SARS mAbs | SARS-CoV | [10, 11] | ||||
| Receptor-derived proteins | P4 and P5 peptides | SARS-CoV | [12] | |||
| Receptor | ACE2 antagonist | NAAE | SARS-CoV | [13] | ||
| DPP4 antagonist | Adenosine deaminase, anti-DPP4 mAbs | MERS-CoV | [14, 15] | |||
| Oligosaccharide-rides on S | Antiviral protein | Griffithsin | SARS-CoV, MERS-CoV, HCoV‑229E, HCoV‑OC43 | [16] | ||
| Block endocytosis | Host proteases that cleavage S protein | TMPRSS2 inhibitor | Camostat, nafamostat | SARS-CoV,; MERS-CoV | [17, 18] | |
| Cathepsin L inhibitor | Teicoplanin, dalbavancin | SARS-CoV, MERS-CoV, SARS-CoV-2 | [19] | |||
| HR1/HR2 | Peptide | HR2P and P1 peptide | MERS-CoV | [20, 21] | ||
| 229E-HR1P and 229E-HR2P | HCoV-229E | [22] | ||||
| OC43-HR2P | Broad spectrum | [23] | ||||
| Viral envelope | Antiviral drug | arbidol | Broad spectrum | [24] | ||
| Small molecules | LJ001 | [25] | ||||
| NHR trimeric coiled coils | α-helical lipopeptides | MERS-CoV | [26] | |||
| Endosomal acidification | Antimalarial | Chloroquine, chloroquine phosphate | SARS-CoV-2 | [30–32] | ||
| Clathrin-mediated endocytosis | Antagonist of DA receptor | Chlorpromazine | MERS-CoV | [30] | ||
| Replicase protein expression | PLpro; (Papain-like protease); (nsp3) | Thiopurine analogs | 6-mercaptopurine (6MP) and 6-thioguanine (6TG) | MERS-CoV | [33] | |
| Immunosuppressive drug | Mycophenolic acid | MERS-CoV | [33] | |||
| Alcohol-aversive drug | Disulfiram | SARS-CoV, MERS-CoV | [34] | |||
| Protease inhibitor | Zinc ion (Zn2+) and zinc conjugate inhibitors | SARS-CoV | [35] | |||
| Compound | F2124-0890 | SARS-CoV, MERS-CoV | [36] | |||
| Mpro; (3C-like protease); (nsp5) | Peptidomimetic inhibitors of enterovirus 3Cpro | 6b, 6c and 6d | SARS-CoV, MERS-CoV | [37] | ||
| 1,2,3-triazole derivatives | Compounds 14d, 14n, 14q, 18f and 18i | HCoV-229E | [38] | |||
| Protease inhibitor | Lopinavir/ritonavir | SARS-CoV, MERS-CoV, HCoV-229E | [30, 39, 46, 48, 49] | |||
| Darunavir/cobicistat | SARS-CoV-2 | NCT04252274 | ||||
| Protease inhibitor | Ti-containing polyoxometalates (POMs) | SARS-CoV | [40] | |||
| Derivates of pyrithiobac (PTB) | Compound 6–5 | SARS-CoV | [41] | |||
| Peptide; mimic inhibitor | N3 | Broad spectrum | [42] | |||
| Synthesized compounds | Derivates of isatin, piperazineare and phenylisoserine | SARS-CoV | [44, 45] | |||
| Derivates of piperidine | MERS-CoV | [43] | ||||
| Replication, transcription and translation | NSPs | NSP12 | RdRp inhibitor | Remdesivir | Broad spectrum | [50–54] |
| Doubly flexible nucleoside analogues such as compound 2 | Broad spectrum | [55] | ||||
| Galidesivir (synthetic adenosine analogue) | Broad spectrum | [56] | ||||
| 6′-fluorinated-aristeromycin analogues | Broad spectrum | [57] | ||||
| Favipiravir | broad spectrum | [58, 59] | ||||
| NSP13 | Helicase inhibitor | Aryl diketoacids (ADK), SSYA10-001, halogenated triazole compounds | SARS-CoV, MERS-CoV | [61, 62, 65] | ||
| NTP enzyme inhibitor | Bananins, 2,6-bis-arylmethyloxy-5-hydroxychromones | SARS-CoV, SARS-associated CoV | [63, 64] | |||
| NSP16 | Drug targeting SAM | S-adenosine-1 homocysteine | SARS-CoV, HCoV 229E | [66] | ||
| Paclitaxel | [66] | |||||
| Aurintricarboxylic acid (ATA) | [66] | |||||
| Drug interfering with the binding of NSP16 to NSP10 | The peptide chain reversely designed according to the sequence of nsp16′s binding domain | [66] | ||||
| Host signaling pathways | Cyclophilin inhibitors | Cyclosporine, alisporivir | SARS-CoV, MERS- CoV, HCoV-NL63 | [67–69] | ||
| Kinase signal transduction inhibitors | Trametinib, imatinib | MERS-CoV | [3, 70] | |||
| Viral nucleic acid or RNA synthesis complex | siRNA | Specific siRNAs targeting the S/M/E/N/accessory protein gene | SARS-CoV, MERS-CoV | [71–76] | ||
| Immunosuppressive drug | Mycophenolic acid | MERS-CoV | [77] | |||
| Ribozyme | Synthetic chimeric DNA–RNA hammerhead ribozyme | SARS-CoV | [78] | |||
| Synthesis | K22 (targeting membrane-bound viral RNA) | Broad spectrum | [79] | |||
| Apoptosis inducer | dsRNA-activated caspase oligomerizer (DRACO) | Broad spectrum | [80] | |||
| N protein | Compound | N-(6-oxo-5,6-dihydrophenanthridin-2-yl) (N,N-dimethylamino)acetamide hydrochloride (PJ34) | HCoV-OC43 | [81] | ||
| Intrabody | Fibronectin-based intrabodies | SARS-CoV | [82] | |||
| Inhibitor | Resveratrol | MERS-CoV | [83] | |||
| Assembly and release | E protein | Ion channel blocker | Hexamethylene amiloride | HCoV-229E | [84] | |
| Cytoskeleton | Filament depolymerizing drug | NOC (nocodazole) | HCoV-229E, HCoV-NL6 | [3, 85] | ||
RBD: receptor binding domain; mAbs: monoclonal antibodies; SARS: Severe acute respiratory syndrome; MERS: Middle East respiratory syndrome; ACE2: Angiotensin converting enzyme 2; DPP4: dipeptidyl peptidase 4; NHR: N-terminal heptad repeat; HR1: Heptad repeat 1 domain; HR2: Heptad repeat 2 domain; DA: dopamine; 6MP: 6-mercaptopurine; 6-TG: 6-thioguanine; Zn2+: zinc ion; POM: polyoxometalate; PTB: derivates of pyrithiobac; NAAE: N-(2-aminoethyl)-1-aziridineethanamine; TMPRSS2: transmembrane protease serine 2; Nsps: non-structural proteins; RdRp: RNA-dependent RNA polymerase; ADK: aryl diketoacids; NTP: nucleoside triphosphate; SAM: S-adenosyl-l-methionine; ATA: aurintricarboxylic acid; dsRNA: double-stranded RNA; DRACO: dsRNA-activated caspase oligomerizer; siRNA: small interfering RNAs; PJ34: N-(6-oxo-5,6-dihydrophenanthridin-2-yl) (N,N-dimethylamino) acetamide hydrochloride; NOC: nocodazole
Inhibition of attachment and entry
The process of invasion can be divided into receptor binding and endocytosis [2]. Drugs developed from viruses or host structures that participate in the above two processes have the potential to block virus invasion.
Prevention of receptor binding
The receptor binding domain (RBD) is the domain that binds to the receptor during HCoV invasion; RBDs have substantial diversity [2]. There have been many studies on therapeutic monoclonal antibodies (mAbs), including m336, m337 and m338, that target the RBD to prevent MERS-CoV invasion [9] and human-derived SARS-CoV [10, 11]. In addition to mAbs, receptor-derived proteins based on the ligand-binding domain, such as P4 and P5 peptides, can be utilized to competitively bind to the RBD [12].
To enter a cell, the RBD needs to bind to a receptor. Thus, in theory, drugs that compete with RBDs for receptor binding sites, such as N-(2-aminoethyl)-1-aziridineethanamine (NAAE) [13] and anti-dipeptidyl peptidase 4 (DPP4) mAbs, can block CoV invasion [14, 15]. However, considering that the receptors on the host cell surface also play an important role in the normal metabolism and function of the cell, the development of such drugs should take into account their impacts on the body, such as hypotensive and hypoglycaemic effects. Griffithsin (GRFT) can specifically bind to the glycosyl groups of protein S, thereby inhibiting virus invasion [16].
Prevention of endocytosis
Inhibitors of host proteases that cleave protein S, such as transmembrane protease serine 2 (TMPRSS2) inhibitors (camostat [17], nafamostat [18]) and cathepsin L inhibitors (teicoplanin, dalbavancin), can prevent exposure and insertion of the hydrophobic end of S2 into the endosomal membrane [19]. Drugs targeting HR1/HR2, such as HR2P, P1 peptide, 229E-HR1P, 229E-HR2P and OC43-HR2P peptide [20–23], can prevent the formation of the 6-helix bundle structure, thereby inhibiting the fusion of the viral envelope with the endosomal membrane. Furthermore, arbidol [24], LJ001 [25], and NHR trimeric coiled coil alpha-helical lipopeptides prevent enveloped virus-cell membrane fusion [26]. Arbidol was tested in a clinical trial and appeared to reduce the SARS-CoV-2 RNA load [27]; however, other studies revealed that Arbidol did not improve the clinical outcomes of patients or SARS-CoV-2 elimination [28, 29]. Attention should be paid to host factors that affect endocytosis. For instance, chloroquine inhibits endosomal acidification [30, 31], and chlorpromazine inhibits clathrin-mediated endocytosis [30]. Recently, chloroquine phosphate has been recommended by Chinese scholars for the treatment of SARS-CoV-2 [32], but some studies have shown that hydroxychloroquine induces cardiotoxicity [33, 34].
Inhibition of replicase expression
In the replication cycle, CoV RNA is first translated into two polyproteins, pp1a and pp1ab, which are then hydrolysed to generate sixteen NSPs with various functions [2]. Certain NSPs are essential for virus replication and transcription.
Papain-like protease (namely, NSP3 and PLpro) and Achilles’ heel 3C-like protease (also known as NSP5 and Mpro) play a vital role in hydrolysing polyproteins to generate NSPs [2]. Hence, inhibitors of the two proteases can block the generation of NSPs.
PLpro
Drugs targeting PLpro include the thiopurine analogues 6-mercaptopurine (6MP) and 6-thioguanine (6TG), mycophenolic acid [35], disulfiram [36], zinc ion (Zn2+) and zinc conjugate inhibitors [37], as well as F2124-0890 [38].
Mpro
Drugs targeting Mpro include peptidomimetic inhibitors of enterovirus 3Cpro (6b, 6c and 6d) [39], a novel series of fused 1,2,3-triazoles [40], lopinavir/ritonavir, [30, 41], Ti-containing polyoxometalates (POMs) [42], compounds 6–5 derived from pyrithiobac (PTB) [43], some molecules such as N3 [44], and synthesized compounds (derived from isatin, piperazine, piperidine and phenylisoserine) [45–47]. According to a randomized control trial published in The Lancet, lopinavir–ritonavir was not associated with survival improvement or mortality reduction [48, 49], so the World Health Organization (WHO) terminated related experiments.
Inhibition of replication, transcription and translation
CoV replicase synthesizes the full-length antisense genome using gRNA as a template and then synthesizes new gRNA according to the sequence of the antisense RNA. Thereafter, with the help of RNA polymerase and certain transcription factors, the virus recognizes specific transcriptional regulatory sequences (TRSs) with "discontinuous transcription" and selectively transcribes all components that make up a mature mRNA. Finally, mRNA is translated into a variety of structural proteins (nucleocapsid protein N, membrane protein M, envelope protein E, and spike protein S) and accessory proteins (such as 3, 4a, 4b, 5 and 8b) [2].
Essential NSPs
A variety of NSPs play significant roles in the replication process, whereas drugs targeting them are limited.
NSP12 (RdRp inhibitors): The NSP12 (RdRp) inhibitor remdesivir prevents viral replication and thus reduces the viral load in patients [50]. However, two recent clinical trials have reached two different conclusions. A study published in the Lancet revealed that the drug is not effective [51–53], while a study published in New England Journal of Medicine showed that the drug shortened the length of hospitalization and virus removal time. However, this paper was withdrawn for many reasons [54]. Other drugs include a series of doubly flexible nucleoside analogues [55], galidesivir (BCX4430) [56], a novel synthetic adenosine analogue, 6′-fluorinated-aristeromycin analogues [57], and favipiravir. Favipiravir has been associated with improvement in chest CT findings [58, 59]. However, the broad-spectrum antiviral drug ribavirin had no significant effects on clinical outcomes when administered alone for the treatment of SARS [60].
NSP13: With the activity of both nucleotide helicase and nucleoside triphosphate (NTP) enzymes, NSP13 functions to unravel the dsRNA helix. Drugs targeting NSP13 not only alter helicase activity [such as aryl diketoacids (ADK) and SSYA10-001] [61, 62], but also affect NTP enzyme activity [such as bananins and 2,6-bis-arylmethyloxy-5-hydroxychromones] [63, 64]. Furthermore, molecular docking results showed that 16 halogenated triazole compounds could bind to NSP13, with inhibitory effects [65].
NSP16 [S-adenosyl-l-methionine (SAM)-dependent 2′O-MTase]: Drug action mechanisms can be divided into two types: direct termination of 2′O-MTase activity through the alteration of SAM (drugs that utilize this mechanism include S-adenosine-1 homocysteine, paclitaxel, and aurintricarboxylic acid (ATA) [66]) or alteration of 2′O-MTase activity by interfering with the binding of NSP16 to NSP10 (drugs that utilize this mechanism include complementary reverse peptides designed according to the sequence of the NSP16 binding domain [66]).
Host signalling pathways
Certain host signalling pathways are essential for viral replication [3]. The cyclophilin inhibitors cyclosporine and alisporivir regulate the interactions of cyclophilin with NSP1 and the calcineurin-NFAT pathway [67–69]. Kinase signal transduction inhibitors, such as trametinib and imatinib, block the ABL1, ERK–MAPK and PI3K–AKT–mTOR pathways, potentially preventing early virus invasion and resulting immune disorders [3, 70].
Viral nucleic acids and RNA synthesis complex
Various small interfering RNAs (siRNAs) can interfere with viral replication as well as the expression of structural proteins and accessory proteins [71–76]. Mycophenolic acid may inhibit viral nucleic acid synthesis [77], but it is advisable to combine it with an interferon since its immunosuppressive effect may create an environment amenable to virus replication and dissemination. In addition, a synthetic chimaeric DNA–RNA hammerhead ribozyme can suppress the expression of SARS-CoV RNA [78]. Moreover, K22 can suppress RNA synthesis by inhibiting the formation of double membrane vesicles (DMVs) [79]. Finally, given the existence of replication intermediates, dsRNA-activated caspase oligomerizer (DRACO) can selectively induce apoptosis in cells containing viral dsRNA [80].
Protein N
Newly generated RNA needs to bind to protein N to form a nucleocapsid for stability; protein N also plays an important role in the normal replication and transcription of gRNA [2]. Therefore, drugs targeting protein N, such as fibronectin-based intrabodies and the inhibitors PJ34 and resveratrol, may influence these processes [81–83].
Inhibition of assembly and release
Viral assembly occurs in the ERGIC, where proteins M and E play important roles [2]. Hexamethylene amiloride [84] blocks the E protein ion channel. CoV particles in ERGIC are transported through the secretory pathway in vesicles and released through exocytosis [2].
The interactions between the cytoskeleton and structural proteins are essential for the assembly and release of CoVs [3]. For example, nocodazole may reduce the amount of transmissible gastroenteritis virus (TGEV), which belongs to the genus α-CoV and shares a similar assembly and release mechanisms with HCoV-229E and HCoV-NL63, particles released from the body [85]. Nonetheless, the advantages and disadvantages must be considered before administering the drug due to the significant role of the cytoskeleton in the normal metabolism and functioning of cells.
Drugs that affect the action of the host's immune system could help relieve the symptoms
Innate immunity
Complement activation and IFNs are believed to play an active role in the innate immune response against HCoVs.
Complement activation
Inhibition of complement activation alleviates acute lung injury induced by SARS-CoV and MERS-CoV infection. For instance, anti-C5aR antibody treatment resulted in decreased viral replication in lung tissues in hDPP4-transgenic mice infected with MERS-CoV. SARS-CoV-infected C3−/− mice exhibited significantly less weight loss and less respiratory dysfunction despite an equivalent viral load in the lungs [86–88].
IFNs. IFN-α/β (IFN-1) is an important component of innate immune defence, which protects mammalian hosts from viral infection [89]. While mild HCoV infections, such as infection by HCoV-229E, typically induce a high level of IFN-I production [90], SARS-CoV and MERS-CoV were shown to suppress the activation of the host innate immune response by inhibiting interferon production or signalling. Several structural proteins (M and N) [91–93], NSPs (NSP1 and NSP3) [94–96], and accessory proteins of SARS-CoV and/or MERS-CoV were identified as IFN antagonists [92]. In addition to inhibiting CoV replication, drugs targeting these proteins may work by unblocking IFN suppression by the CoV. IFN has been clinically indicated to be effective for the treatment of SARS-CoV and MERS-CoV. In clinical treatment, the routine use of IFNs is not recommended for SARS-CoV treatment [97]. IFNs are usually administered in combination with other drugs, such as IFN-β-1b combined with lopinavir/ritonavir [98] or ribavirin and IFN-α combined with lopinavir/ritonavir [99], for MERS-CoV treatment. In severe to critical COVID-19 patients, early treatment with IFN-α2b can reduce in-hospital mortality, but it has no significant benefit in moderately ill patients [100].
Cell-mediated immunity
Lymphocytopenia is commonly observed in patients infected with SARS-CoV [97], MERS-CoV [101], or SARS-CoV-2 [102], but the mechanism remains unclear. Human T cells are highly susceptible to MERS-CoV infection. Studies have demonstrated that MERS-CoV persists in T cell-deficient mice but is cleared in B cell-deficient mice, suggesting that T cells play a critical role in MERS-CoV clearance [103]. SARS-CoV-specific T cells also play important roles in the recognition and clearance of infected cells [104].
Humoural immunity
Antibodies play an important role in preventing CoV infection. Antibody production against protein S was less in SARS-CoV-infected patients with fatal outcomes than in non-severe patients [105]. The level and presence of antibodies are related to the clinical severity of SARS and MERS [106, 107]. Experiments have shown that antibody therapy improves symptoms and promotes recovery. SARS-CoV-specific monoclonal antibodies include human mAb CR3014 [10], CR3022 [108], and 5H10 [109]. MERS-CoV-specific monoclonal antibodies include m336 [110], REGN3051, REGN3048 [111], 3B11-N [112], LCA60 [113], MCA1 [114], MERS-4, MERS-27 [115], MERS-GD27, and MERS-GD33 [116]. Serum cross-reaction is important for both detection and treatment. Studies have shown the absence of cross-reactivity between SARS-CoV and MERS-CoV. The SARS-CoV-specific human monoclonal antibody CR3022 can effectively bind to the RBD of SARS-CoV-2 [117].
Convalescent plasma
Convalescent plasma therapy may be beneficial for patients with early SARS infection because it provides antibodies from convalescent patients [118], but evidence of its efficacy in MERS-CoV patients is still lacking. It is recommended for the treatment of rapidly progressing, severe and critical cases of SARS-CoV-2 infection [99], but it is limited by safety concerns and inadequate sources. Trials indicate that convalescent plasma is most effective in reducing mortality when administered in the early stage of infection, but it does not significantly shorten the time to recovery [119, 120].
Glucocorticoids
Corticosteroids not only suppress lung inflammation but also inhibit immune responses and pathogen clearance. Available observational data suggest impaired clearance of SARS-CoV and MERS-CoV as well as increased complication rates in survivors receiving corticosteroid therapy. Therefore, it is not advisable to administer corticosteroid treatment in patients with SARS-CoV-2-associated lung injury or shock outside of a clinical trial setting [121]. Recent clinical trials suggest that early, low-dose methylprednisolone administered in the short term improved clinical outcomes and reduced mortality in severe COVID-19 patients [122–124]. Guidelines from China recommend that glucocorticoids should be used in the short term as appropriate in patients with progressive deterioration of the oxygenation index, rapid radiographic development, and excessive activation of the inflammatory response [99].
IL-6 receptor inhibitors
IL-6 plays an important role in the development of a cytokine storm. As an IL-6 receptor inhibitor, tocilizumab does not prevent the disease from progressing, but it can reduce the symptoms of serious infection [125–127].
Clinical trials of drugs targeting SARS-CoV-2 are summarized in Table 2.
Table 2.
Published clinical trials of drugs against SARS-CoV-2
| Step | Target | Drug | Result | Population | Methodology | ID | Reference number | |
|---|---|---|---|---|---|---|---|---|
| Positive | Negative | |||||||
| Attachment and entry | Viral envelope | Arbidol/umifenovir | N/A | Umifenovir did not improve the prognosis or accelerate SARS-CoV-2 clearance in non-ICU patients | 81 moderates to severe COVID-19 patients (umifenovir vs control = 45: 36) | Single-centre, retrospective | N/A | [29] |
| The ability of arbidol to reduce SARS-CoV-2 RNA load is better than lopinavir–ritonavir | N/A | 50 COVID-19 patients (lopinavir/ritonavir vs arbidol = 34:16) | N/A | N/A | [27] | |||
| Endosomal acidification | Hydroxychloroquine | N/A | Hydroxychloroquine did not induce SARS-CoV-2 negative conversion. It has cardiotoxicity | 150 patients with mainly persistent mild to moderate COVID-19 (hydroxychloroquine + standard of care vs standard of care alone = 75:75) | Multicenter, open label, randomized controlled | ChiCTR2000029868 | [33] | |
| Chloroquine diphosphate | N/A | Chloroquine diphosphate did not reduce mortality and may extend QT intervals | 81 patients (high-dosage vs low-dosage group = 41:40) | Parallel, double-masked, randomized, phase IIb | N/A | [34] | ||
| Replicase protein expression | Mpro (3C-like protease) (nsp5) | Lopinavir/ritonavir | N/A | Lopinavir–ritonavir did not reduce mortality or the time to clinical improvement | 199 COVID-19 adults (lopinavir–ritonavir:standard-care = 99:100) | Randomized, controlled, open-label | ChiCTR2000029308 | [49] |
| N/A | Lopinavir–ritonavir did not reduce mortality or prevent progression | 5040 (lopinavir/ritonavir:usual care = 1616:3424) | Randomised, controlled, open-label, platform | NCT04381936 | [48] | |||
| Replication, transcription and translation | Nsp12 | Remdesivir | By day 28, 10-day remdesivir group had a better clinical status distribution than standard care group | Remdesivir did not reduce the length of hospitalization or oxygen therapy | 584 moderate COVID-19 patients (10-day remdesivir:5-day remdesivir:standard care = 197:199:200) | Randomized, controlled, open-label | NCT04292730 | [53] |
| Remdesivir reduces the duration of hospitalization and infection | N/A | 1059 COVID-19 adults with lower respiratory tract infection (remdesivir:placebo = 538:521) | Double-blind, randomized, placebo-controlled | NCT04280705 | [54] | |||
| N/A | There is no significant clinical difference between a 5-day course and a 10-day course of remdesivir | 397 severe COVID-19 patients (5-day remdesivir:10-day remdesivir = 200:197) | Randomized, open-label | NCT04292899 | [52] | |||
| N/A | Remdesivir did not reduce time to clinical improvement | 237 COVID-19 adults (10- day remdesivir vs placebo = 158:79) | Randomised, double-blind, placebo-controlled, multicentre | NCT04257656 | [51] | |||
| Favipiravir | Favipiravir leads to faster viral clearance and better chest CT changes than patients treated with lopinavir/ritonavir | N/A | 80 COVID-19 patients (favipiravir vs lopinavir/ritonavir = 35:45) | Open-label comparative controlled | ChiCTR2000029600 | [59] | ||
| Immunology | IFN supplement | IFN-α2b | Among severe to critical COVID-19 patients, early treatment with IFN-α2b reduced in-hospital mortality | IFN-α2b did not benefit significantly in moderately ill patients | 242 (IFN + LPV/r, IFN + UFV, IFN alone) of 446 COVID-19 patients received IFN-α2b | Retrospective, multicenter | [100] | |
| Protective antibody supplement | Convalescent plasma | Convalescent plasma is most effective in early application and reduces mortality | N/A | 39 patients with severe to life-threatening COVID-19 (convalescent plasma vs controls = 1:4 and 1:2 ratios) | Retrospective, propensity score–matched case–control | N/A | [119] | |
| Convalescent plasma was associated with antiviral activity | Convalescent plasma did not significantly reduce time to the clinical improvement | 103 patients with severe to life-threatening COVID-19 (Convalescent plasma in addition to standard treatment vs standard treatment alone = 52:51) | Open-label, multicenter, randomized, prospective | ChiCTR2000029757 | [120] | |||
| Cytokine storm | Corticosteroids | Early, low-dose and short-term application of methylprednisolone helped reach better clinical outcomes in severe patients with COVID-19 pneumonia | N/A | 46 severe patients with COVID-19 pneumonia (26 of them received extra low-dose and short-term methylprednisolone treatment) | Retrospective | N/A | [122] | |
| For severe COVID-19 patients, methylprednisolone pulse promoted clinical improvements and reduced mortality | N/A | 68 severe COVID-19 patients (methylprednisolone vs standard care alone = 34:34) | Single-blind, randomized, controlled | N/A | [123] | |||
| IL-6R | Tocilizumab | N/A | Tocilizumab showed no benefit on disease progression compared with standard care. (early shutdown) | 126 adults with COVID-19 pneumonia and PaO2/FIO2 ratio between 200 and 300 mmHg (tocilizumab vs supportive care = 60:66) | Prospective, open-label, randomized | NCT04346355 | [127] | |
| N/A | Tocilizumab did not promote clinical improvements or reduced mortality | 131 COVID-19 patients with moderate or severe pneumonia requiring oxygen but without ventilation or admission to the ICU (tocilizumab vs usual care alone = 64:67) | Multicenter, open-label, Bayesian randomized | NCT04331808 | [126] | |||
| Tocilizumab reduced serious infections | Tocilizumab did not prevent intubation or death in moderately ill hospitalized patients with COVID-19 | 243 hospitalized COVID-19 patients (standard care + tocilizumabvs vs standard care + placebo = 162:81) | Randomized, double-blind, placebo-controlled | NCT04356937 | [125] | |||
N/A: not applicable; ICU: intensive care unit; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; COVID-19: Coronavirus disease 2019; CT: computed tomography; IFN: Interferon; IL: interleukin; PaO2/FIO2: OXYGENATION index
Plant-derived Chinese medicine might have antiviral effect
Single Chinese medicines and their associated active ingredients
SARS-CoV-2
The traditional Chinese medicine components that might block the binding regions of grid3 and grid4 between angiotensin converting enzyme 2 (ACE2) and viral protein S include Folium mori, Atractylodes lancea, Fritillaria, Zingiber officinale, Lonicerae japonicae flos, Forsythia suspensa, and Amomum tsao-ko [143]. SARS-CoV-2 leads to the downregulation of ACE2 upon binding to the receptor, thus disrupting normal regulation of the ACE-Ang II and ACE2-Ang-(1–7) axes, consequently inducing multiple organ damage. Astragalus, Panax ginseng, Dioscorea spp., and arecae semen, which are major components of traditional Chinese medicine preparations for COVID-19 pneumonia, have shown a regulatory effect on the renin–angiotensin–aldosterone system (RAAS) [144]. Quercetin and its derivatives have strong binding ability to ACE2 and IL-6R and have the potential to inhibit the cytokine storm by blocking SARS-CoV-2 and IL-6 binding. In addition, licorice, ephedra, Bupleurum root, etc., also have different IL-6R binding abilities [145–147]. Saikoside A and saikoside D had good affinity with Mpro and ACE2 of SARS-CoV-2 [148]. The binding strengths of baicalein and SARS-CoV-2 Mpro are the same as those of lopinavir and remdesivir, and the bond to ACE2 is relatively stable [149]. Liquiritin apioside, iridin, liquiritin, forsythiaside, procyanidin B-5,3′-o-gallate and saikosaponin C are latent active RdRp inhibitors, and their flavonoid structures may be potential active groups that induce RdRp inhibition [150]. Aster pentapeptide A, ligustrazine, salvianolic acid B, etc., have potential inhibitory effects on SARS-COV-2 Mpro, while gingerol, ginnol, ferulic acid, etc., have potential inhibitory effects on SARS-COV-2 PLpro [151]. Hypericin and baicalein can bind to SARS-CoV-2 NSP14 and interact with key amino acid residues in the active centre [152].
SARS-CoV
Glycyrrhizin [128, 129] is capable of inhibiting the invasion and replication of SARS-CoV in vitro, and various derivatives [130] (such as the introduction of 2-acetamide-glucan amine into the glycyrrhizin chain) may account for increased anti-SARS-CoV activity along with enhanced cytotoxicity. Lycorine from Lycoris radiata and ZZ-1 [131, 132] may inhibit SARS-CoV replication. Polysaccharides and ethyl acetate extracts from Houttuynia cordata act on the body's immune system with anti-complement activity, among which afzerin and quercetin also have antipyretic effects [133]. Houttuynia cordata also promotes the inhibition of RdRp [134]. Lung injury caused by SARS-CoV is associated with inflammation due to cytokine storms and neutrophil infiltration. Thus, inhibiting cAMP-PDE, which plays a key role in the inflammatory response, may help prevent inflammation. Rhizoma phragmitis, Folium isatidis, honeysuckle, forsythia, perilla leaf, mint and Astragalus significantly inhibit cAMP-PDE activity [135]. Multiflorum and Rheum rhabarbarum, specifically its extract-derived component emodin, affect virus invasion [136]. Protease inhibitors of natural origin include 3CLpro inhibitors (such as quinone-methide triterpenes extracted from Tripterygium regelii [137], dieckol from Ecklonia cava [138], and extracts of Houttuynia cordata and Rheum rhabarbarum [134, 136]) and PLpro inhibitors (such as diarylheptanoids from Alnus japonica [139], and phenolic phytochemicals from the seeds of Psoralea corylifolia [140]). Finally, chalcone 6 from Angelica keiskei and tanshinones from Salvia miltiorrhiza are capable of inhibiting both 3CLpro and PLpro [141, 142].
MERS-CoV
Silvestrol [153], an inhibitor of eIF4A, can inhibit viral mRNA cap-dependent translation. In addition, research on MERS-CoV 3CLpro suggests that flavonoids such as herbacetin, isobavachalcone, quercetin 3-β-D-glucoside and helichrysetin [154] can act as inhibitors.
HCoV-229E
3β-Friedelanol [155], a triterpenoid extracted from the leaves of Euphorbia neriifolia, showed stronger antiviral activity than actinomycin D, the positive control. Furthermore, silvestrol [153], an eIF4A inhibitor, affects the translation of HCoV-229E.
HCoV-NL63
Caffeic acid, which is related to the ethanol extract of Sambucus FormosanaNakai [156], has been confirmed to have a significant inhibitory effect on the invasion of HCoV-NL63, possibly by directly interfering with the binding of HCoV-NL63 to ACE2 and co-receptors, such as heparin sulphate proteoglycan.
Compound traditional Chinese medicines
SARS-CoV-2
Based on the Chinese COVID-19 diagnosis and treatment scheme, ageratum upright capsules (in the form of pills, water, or oral liquid), Jinhuaqinggan particles, Lianhuaqingwen capsules (particles) and Shufengjiedu capsules (particles) are recommended during the SARS-CoV-2 medical observation period, while Qingfeipaidu soup (including Maxingshigan soup, Sheganmahuang soup, Xiaochaihu soup, Wuling powder), Xiyanping injection, Xuebijing injection, Reduning injection, Tanreqing injection, Xingnaojing injection, Shenfu injection, Shengmai injection and Shenmai injection are recommended in the clinical phase [99].
SARS-CoV
The Ministry of Science and Technology of China has announced eight Chinese medicines that have been clinically confirmed to improve symptoms in SARS patients: Qingkailing injection, Houttuynia cordata injection, Radix isatidis granules, Xinxue granules, Jinlian Qingre granules, Dengzhanxixin injection, compound Kuh-seng injection and Xiangdan injection. In addition, Qingqi Liangying oral liquid and Qingwen oral liquid, and Jiedu pills, as well as anti-SARS I and anti-SARS II showed effective inhibitory effects on SARS-CoV [157] (Table 3).
Table 3.
Chinese medicine with active ingredients against HCoV
| Type | Chinese medicine | Active ingredients | Mechanism | Reference number |
|---|---|---|---|---|
| SARS-CoV | Glycyrrhiza radix | Glycyrrhizin and derivatives from it | Inhibit the invasion and replication | [128, 129] |
| Lycoris radiata | Lycorine | N/A | [132] | |
| N/A | ZZ-1 | N/A | [131] | |
| Houttuynia cordata | Polysaccharides and ethyl acetate extracts, such as afzerin and quercetin | Act on the body's immune system with anti-complement activity, and inhibits 3CLpro and RdRp activity | [133, 134, 136] | |
| Rheum rhabarbarum | Emodin | Suppress the interaction between S protein and ACE2 and inhibits 3clpro activity | [135] | |
| Polygonum multiflorum | N/A | Suppress the interaction between S protein and ACE2 | [136] | |
| Tripterygium regelii | Quinone-methide triterpenes | Inhibit the enzymatic activity of 3CLpro | [137] | |
| Ecklonia cava | Dieckol | Inhibit the enzymatic activity of 3CLpro | [138] | |
| Alnus japonica | Diarylheptanoids | Inhibit the enzymatic activity of 3CLpro | [139] | |
| Seeds of Psoralea corylifolia | Phenolic phytochemical | Inhibit the enzymatic activity of 3CLpro | [140] | |
| Angelica keiskei | Chalcone 6 | Inhibit the enzymatic activity of both 3CLpro and PLpro | [141] | |
| Salvia miltiorrhiza | Tanshinones | Inhibit the enzymatic activity of both 3CLpro and PLpro | [142] | |
| MERS-CoV | Fruits and twigs of Aglaia foveolate | Silvestrol | Inhibit viral mRNA cap-dependent translation | [153] |
| N/A | Flavonoids such as herbacetin, isobavachalcone, quercetin-3-beta-O-d-glucoside and helichrysetin | Inhibit the enzymatic activity of 3CLpro | [154] | |
| SARS-CoV-2 | Folium mori, Atractylodes lancea, Fritillaria, Zingiber officinale, Lonicerae japonicae flos, Forsythia suspensa, and Amomum tsao-ko | N/A | Block the binding between ACE2 and viral S protein | [143] |
| Astragalus, Panax ginseng, Dioscorea spp., and arecae semen | N/A | Regulate RAAS | [144] | |
| N/A | Quercetin and its derivatives | Block the binding between ACE2 and viral S protein, IL-6R and IL-6 | [147] | |
| Licorice, Ephedra, Bupleurum root | N/A | Block the binding between IL-6R and IL-6 | [145, 146] | |
| Bupleurum | Saikoside A and saikoside D | Bind to 3CLpro and block the binding between ACE2 and viral S protein | [148] | |
| Scutellaria baicalensis | Baicalein | Bind to 3CLpro and block the binding between ACE2 and viral S protein | [149] | |
| N/A | Liquiritin apioside, iridin, liquiritin, forsythiaside, procyanidin B-5, 3′ -o-gallate and saikosaponin C | Inhibit RdRp | [150] | |
| Aster, Ligusticum chuanxiong, Salvia miltiorrhiza | Aster pentapeptide A, ligustrazine, salvianolic acid B | Inhibit the enzymatic activity of 3CLpro | [151] | |
| Ginger, Ginkgo, Chuanxiong | Gingerol, ginnol, ferulic acid | Inhibit the enzymatic activity of PLpro | [151] | |
| N/A | Hypericin and baicalein | Bind to nsp14 | [152] | |
| HCoV-229E | Leaves of Euphorbia neriifolia | 3β-Friedelanol | N/A | [155] |
| Fruits and twigs of Aglaia foveolate | Silvestrol | Inhibit viral mRNA cap-dependent translation | [153] | |
| HCoV-NL63 | Sambucus FormosanaNakai | Ethanol extract-related caffeic acid | Interfere with the binding of HCoV-NL63 to the receptor of ACE2 and co-receptors such as heparin sulfate proteoglycan | [156] |
SARS-CoV: Severe acute respiratory syndrome coronavirus; MERS-CoV: Middle East respiratory syndrome coronavirus; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; N/A: not applicable; 3CLpro: 3C-like protease; PLpro: Papain-like protease; RdRp: RNA-dependent RNA polymerase; ACE2: Angiotensin converting enzyme 2; RAAS: Renin–angiotensin–aldosterone system; IL: interleukin; Nsp: non-structural protein; mRNA: messenger RNA
Discussion
As of November 13, 2020, SARS-CoV-2 had infected 53 218 786 people worldwide and killed a total of 1 301 631 people. Unfortunately, the epidemic is still not under control in many countries. Despite a lack of HCoV-specific drugs, many potent targets for drug discovery have been explored, and many vigorously designed clinical trials are being carried out in an orderly manner. In the present study, we analysed the pathogenesis of and drug therapy targeting seven HCoVs, including four common types (HCoV-229E, -OC43, -NL63, -HKU1) and three highly pathogenic types (SARS-CoV, MERS-CoV, SARS-CoV-2); special attention was given to SARS-CoV-2.
Among the highly pathogenic CoVs, SARS-CoV transmission has been rare since 2004, so clinical trials of drugs and vaccines are difficult to carry out. To date, there are no specific drugs or vaccines against MERS-CoV. mABs, such as m336 [9], lopinavir/ritonavir [30], IFN [98, 99], etc., are potential antiviral drugs against MERS-CoV, but additional evidence is needed to determine their efficacy.
Because COVID-19 is a new, acute, severe infectious disease, the anti-SARS-CoV-2 drug development strategies are to screen existing drugs to identify potentially effective drugs, to expand indications and to develop a vaccine. The safety of conventional drugs has been mostly verified; if effective, they can be quickly applied in clinical practice. To date, thousands of clinical trials of SARS-CoV-2 have been registered worldwide. Hot topics include antiviral drugs such as RaRp inhibitors [51–54, 59], Mpro inhibitors [48, 49], chloroquine and its derivatives [33, 34], viral envelope inhibitors, arbidol [27, 29], and immunotherapy drugs such as IFNs [100] and cytokine storm inhibitors [122, 123, 125–127]. Usually, the duration from initial experimental research to clinical trial completion is long. However, due to the COVID-19 pandemic, many drugs have been entered into clinical trials that are not randomized, controlled, or double-blinded. Their efficacy, toxicity, and side effects are discovered during application. For example, it was previously reported that hydroxychloroquine and chloroquine acted against coronavirus, and the synergistic use of hydroxychloroquine and azithromycin reduced the viral load and improved clinical results. However, later studies found that the heart-related side effects of these drugs included extension of the QT interval, so the WHO terminated the studies [33, 34]. Clinical trials found that lopinavir/ritonavir had a poor effect on COVID-19, while others, such as arbidol [27, 29], remdesivir [51–54], favipiravir [59], IFN-α2b [100], convalescent plasma [119, 120], corticosteroids [122, 123] and tocilizumab [125–127], had different and even opposite results, which can be further validated by experimental evaluation and clinical experience. When the production of inflammatory factors is increased, convalescent plasma, corticosteroids, and tocilizumab should be used early and in appropriate amounts. Because most traditional Chinese medicines are compounds and few single drugs or single active ingredients are used, it is difficult to determine which ingredients are effective in clinical trials. It is hard to differentiate the compounds associated with the mechanism. In a laboratory study of a single active ingredient, glycyrrhizin had a strong inhibitory effect on SARS-CoV-2 [128, 129], which is of great significance for further clinical study. The future research direction for traditional Chinese medicine is to identify and modify a single potent drug or active ingredient and adjust the compound dose and administration method.
This review summarized the conventional and potential drugs at according to each action site, which can improve clinicians’ understanding of the results of current clinical studies to guide clinical decisions. It also enables researchers to understand drug action sites to discover potential effective drugs.
Conclusions
This review summarized the progress in drugs that inhibit the HCoV proliferation, affect the action of the host's immune system as well as plant-derived Chinese medicines, which not only provides researchers a more comprehensive understanding of the current status of drug development against HCoVs, but also provides directions for further exploration. However, the pharmacological effects and adverse reactions of some drugs under study are still unclear, and hence well-designed high-quality studies are needed to further study the effectiveness and safety of these potential drugs in order to accelerate drug development targeting SARS-CoV-2 and thus promote progress towards ending the pandemic.
Acknowledgements
We thank Dr. Bo Cui for editing and helpful advice.
Abbreviations
- CoV
Coronavirus
- HCoVs
Human coronaviruses
- SARS
Severe acute respiratory syndrome
- MERS
Middle East respiratory syndrome
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- SARS-CoV
Severe acute respiratory syndrome coronavirus
- MERS-CoV
Middle East respiratory syndrome coronavirus
- COVID-19
Coronavirus disease 2019
- ARDS
Acute respiratory distress syndrome
- IFNs
Interferons
- gRNA
Genomic RNA
- NSPs
Non-structural proteins
- PLpro
Papain-like protease
- Mpro
3C-like protease
- RdRp
RNA-dependent RNA polymerase
- sgRNA
Subgenomic RNA
- ERGIC
ER-Golgi intermediate compartment
- RBD
Receptor binding domain
- mABs
Monoclonal antibodies
- NAAE
N-(2-Aminoethyl)-1-aziridineethanamine
- DPP4
Dipeptidyl peptidase 4
- GRFT
Griffithsin
- TMPRSS2
Transmembrane protease serine 2
- HR1
Heptad repeat 1 domain
- HR2
Heptad repeat 2 domain
- NHR
N-terminal heptad repeat
- 6MP
6-Mercaptopurine
- 6TG
6-Thioguanine
- POMs
Polyoxometalates
- PTB
Pyrithiobac
- WHO
World Health Organization
- TRS
Transcriptional regulatory sequence
- mRNA
Messenger RNA
- NTP
Nucleoside triphosphate
- dsRNA
Double-stranded RNA
- ADK
Aryl diketoacids
- SAM
S-adenosyl-l-methionine
- ATA
Aurintricarboxylic acid
- siRNA
Small interfering RNAs
- DMV
Double-membrane vesicles
- DRACO
DsRNA-activated caspase oligomerizer
- TGEV
Transmissible gastroenteritis virus
- ACE2
Angiotensin converting enzyme 2
- RAAS
Renin–angiotensin–aldosterone system
Authors' contributions
L-GS, Q-XX and H-LL were responsible for study selection and data extraction and drafted the manuscript. L-GS and Z-YL designed the study and revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by Grants from the Major Science and Technology Program of Hainan Province (Grant No. ZDKJ202003), the National Natural Science Foundation of China (Grant Nos. 81572023 and 81371836), the Guangdong Natural Science Foundation (Grant No. 2019A1515011541), the Science and Technology Planning Project of Guangdong Province (Grant No. 2019B030316025), the National Parasitic Resources Center of China (Grant No. NPRC-2019-194-30), the Open Foundation of Key Laboratory of Tropical Translational Medicine of the Ministry of Education, Hainan Medical University (Grant No. 2020TTM007), the 111 Project (Grant No. B12003), and the Teaching Reform Project of Guangdong Province (Grant No. 2017001).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Lan-Gui Song, Qing-Xing Xie and Hui-Lin Lao contributed equally to this work
Contributor Information
Lan-Gui Song, Email: songlg5@mail.sysu.edu.cn.
Zhi-Yue Lv, Email: lvzhiyue@mail.sysu.edu.cn.
References
- 1.Zumla A, Chan JF, Azhar EI, Hui DS, Yuen KY. Coronaviruses-drug discovery and therapeutic options. Nat Rev Drug Discov. 2016;15:327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maier HJ, Bickerton E, Editors PB. Coronaviruses: methods and orotocols. New York: Springer; 2015. [Google Scholar]
- 3.Fung TS, Liu DX. Human coronavirus: Host-pathogen interaction. Annu Rev Microbiol. 2019;73:529–557. doi: 10.1146/annurev-micro-020518-115759. [DOI] [PubMed] [Google Scholar]
- 4.Su S, Wong G, Shi W, Liu J, Lai ACK, Zhou J, et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24:490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO Coronavirus Disease (COVID-19) dashboard. https://covid19.who.int/. Accessed 2 June 2020.
- 6.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zumla A, Chan JFW, Azhar EI, Hui DSC, Yuen K. Coronaviruse-drug discovery and therapeutic options. Nat Rev Drug Discov. 2016;15:327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Huan DSAL. Advances in the study of coronavirus entry pathways. J Virol. 2019;06:964–971. [Google Scholar]
- 9.Ying T, Du L, Ju TW, Prabakaran P, Lau CC, Lu L, et al. Exceptionally potent neutralization of Middle East respiratory syndrome coronavirus by human monoclonal antibodies. J Virol. 2014;88:7796–7805. doi: 10.1128/JVI.00912-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ter Meulen J, Bakker AB, van den Brink EN, Weverling GJ, Martina BE, Haagmans BL, et al. Human monoclonal antibody as prophylaxis for SARS coronavirus infection in ferrets. Lancet. 2004;363:2139–2141. doi: 10.1016/S0140-6736(04)16506-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roberts A, Thomas WD, Guarner J, Lamirande EW, Babcock GJ, Greenough TC, et al. Therapy with a severe acute respiratory syndrome-associated coronavirus—neutralizing human monoclonal antibody reduces disease severity and viral burden in golden Syrian hamsters. J Infect Dis. 2006;193:685–692. doi: 10.1086/500143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han DP, Penn-Nicholson A, Cho MW. Identification of critical determinants on ACE2 for SARS-CoV entry and development of a potent entry inhibitor. Virology. 2006;350:15–25. doi: 10.1016/j.virol.2006.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huentelman MJ, Zubcevic J, Hernandez PJ, Xiao X, Dimitrov DS, Raizada MK, et al. Structure-based discovery of a novel angiotensin-converting enzyme 2 inhibitor. Hypertension. 2004;44:903–906. doi: 10.1161/01.HYP.0000146120.29648.36. [DOI] [PubMed] [Google Scholar]
- 14.Ohnuma K, Haagmans BL, Hatano R, Raj VS, Mou H, Iwata S, et al. Inhibition of Middle East respiratory syndrome coronavirus infection by anti-CD26 monoclonal antibody. J Virol. 2013;87:13892–13899. doi: 10.1128/JVI.02448-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raj VS, Smits SL, Provacia LB, van den Brand JM, Wiersma L, Ouwendijk WJD, et al. Adenosine deaminase acts as a natural antagonist for dipeptidyl peptidase 4-mediated entry of the Middle East respiratory syndrome coronavirus. J Virol. 2014;88:1834–1838. doi: 10.1128/JVI.02935-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Keefe BR, Giomarelli B, Barnard DL, Shenoy SR, Chan PK, McMahon JB, et al. Broad-spectrum in vitro activity and in vivo efficacy of the antiviral protein griffithsin against emerging viruses of the family Coronaviridae. J Virol. 2010;84:2511–2521. doi: 10.1128/JVI.02322-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Y, Vedantham P, Lu K, Agudelo J, Carrion RJ, Nunneley JW, et al. Protease inhibitors targeting coronavirus and filovirus entry. Antivir Res. 2015;116:76–84. doi: 10.1016/j.antiviral.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamamoto M, Matsuyama S, Li X, Takeda M, Kawaguchi Y, Inoue J, et al. Identification of nafamostat as a potent inhibitor of Middle East respiratory syndrome coronavirus S protein-mediated membrane fusion using the split-protein-based cell-cell fusion assay. Antimicrob Agents Chemother. 2016;60:6532–6539. doi: 10.1128/AAC.01043-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang H, Liu C, Li Q, Zhang J, Zhang X, Bai C, et al. Glycopeptide antibiotics potently inhibit cathepsin L in the late endosome/lysosome and block the entry of Ebola virus, Middle East respiratory syndrome coronavirus (MERS-CoV), and severe acute respiratory syndrome coronavirus (SARS-CoV) J Biol Chem. 2016;291:9218–9232. doi: 10.1074/jbc.M116.716100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao J, Lu G, Qi J, Li Y, Wu Y, Deng Y, et al. Structure of the fusion core and inhibition of fusion by a heptad repeat peptide derived from the S protein of Middle East respiratory syndrome coronavirus. J Virol. 2013;87:13134–13140. doi: 10.1128/JVI.02433-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Channappanavar R, Lu L, Xia S, Du L, Meyerholz DK, Perlman S, et al. Protective effect of intranasal regimens containing peptidic Middle East respiratory syndrome coronavirus fusion inhibitor against MERS-CoV infection. J Infect Dis. 2015;212:1894–1903. doi: 10.1093/infdis/jiv325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xia S, Xu W, Wang Q, Wang C, Hua C, Li W, et al. Peptide-based membrane fusion inhibitors targeting HCoV-229E spike protein HR1 and HR2 domains. Int J Mol Sci. 2018;19:487. doi: 10.3390/ijms19020487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xia S, Yan L, Xu W, Agrawal AS, Algaissi A, Tseng CK, et al. A pan-coronavirus fusion inhibitor targeting the HR1 domain of human coronavirus spike. Sci Adv. 2019;5:v4580. doi: 10.1126/sciadv.aav4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blaising J, Polyak SJ, Pecheur E. Arbidol as a broad-spectrum antiviral: an update. Antivir Res. 2014;107:84–94. doi: 10.1016/j.antiviral.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fontanes V, Moscona A, Hong PW, Grock A, Zhang TH, et al. A broad-spectrum antiviral targeting entry of enveloped viruses. Proc Natl Acad Sci USA. 2010;107:3157–3162. doi: 10.1073/pnas.0909587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang C, Zhao L, Xia S, Zhang T, Cao R, Liang G, et al. De novo design of alpha-helical lipopeptides targeting viral fusion proteins: a promising strategy for relatively broad-spectrum antiviral drug discovery. J Med Chem. 2018;61:8734–8745. doi: 10.1021/acs.jmedchem.8b00890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu Z, Lu Z, Xu T, Chen C, Yang G, Zha T, et al. Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19. J Infect. 2020;81:e21–e23. doi: 10.1016/j.jinf.2020.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang D, Yu H, Wang T, Yang H, Yao R, Liang Z. Efficacy and safety of umifenovir for coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. J Med Virol. 2020 doi: 10.1002/jmv.26256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lian N, Xie H, Lin S, Huang J, Zhao J, Lin Q. Umifenovir treatment is not associated with improved outcomes in patients with coronavirus disease 2019: a retrospective study. Clin Microbiol Infect. 2020;26:917–921. doi: 10.1016/j.cmi.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Wilde AH, Jochmans D, Posthuma CC, Zevenhoven-Dobbe JC, van Nieuwkoop S, Bastebroer TM, et al. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob Agents Chemother. 2014;58:4875–4884. doi: 10.1128/AAC.03011-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schrezenmeier E, Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. 2020;16:155–166. doi: 10.1038/s41584-020-0372-x. [DOI] [PubMed] [Google Scholar]
- 32.Yao X, Cui C, Liu D, Li H. Clinical pharmacology progress of chloroquine in the treatment of corona virus disease 2019. Clin Med J. 2020;02:30–33. [Google Scholar]
- 33.Tang W, Cao Z, Han M, Wang Z, Chen J, Sun W, et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369:m1849. doi: 10.1136/bmj.m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borba M, Val F, Sampaio VS, Alexandre M, Melo GC, Brito M, et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw Open. 2020;3:e208857. doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng K, Cheng S, Chen W, Lin M, Chuang S, Cheng I, et al. Thiopurine analogs and mycophenolic acid synergistically inhibit the papain-like protease of Middle East respiratory syndrome coronavirus. Antivir Res. 2015;115:9–16. doi: 10.1016/j.antiviral.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng S, Moses DC, Sun C, Lin M, Hsieh C, Chen Y, et al. Disulfiram can inhibit MERS and SARS coronavirus papain-like proteases via different modes. Antivir Res. 2018;150:155–163. doi: 10.1016/j.antiviral.2017.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baez-Santos YM, St JS, Mesecar AD. The SARS-coronavirus papain-like protease: structure, function and inhibition by designed antiviral compounds. Antivir Res. 2015;115:21–38. doi: 10.1016/j.antiviral.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clasman JR, Baez-Santos YM, Mettelman RC, O'Brien A, Baker SC, Mesecar AD, et al. X-ray structure and enzymatic activity profile of a core papain-like protease of MERS coronavirus with utility for structure-based drug design. Sci Rep. 2017;7:40292. doi: 10.1038/srep40292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar V, Shin JS, Shie JJ, Ku KB, Kim C, Go Y, et al. Identification and evaluation of potent Middle East respiratory syndrome coronavirus (MERS-CoV) 3CL(Pro) inhibitors. Antivir Res. 2017;141:101–106. doi: 10.1016/j.antiviral.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karypidou K, Ribone SR, Quevedo MA, Persoons L, Pannecouque C, Helsen C, et al. Synthesis, biological evaluation and molecular modeling of a novel series of fused 1,2,3-triazoles as potential anti-coronavirus agents. Bioorg Med Chem Lett. 2018;28:3472–3476. doi: 10.1016/j.bmcl.2018.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smolders EJ, Te BL, Burger DM. SARS-CoV-2 and HIV protease inhibitors: why lopinavir/ritonavir will not work for COVID-19 infection. Antivir Ther. 2020 doi: 10.3851/IMP3365. [DOI] [PubMed] [Google Scholar]
- 42.Hu D, Shao C, Guan W, Su Z, Sun J. Studies on the interactions of Ti-containing polyoxometalates (POMs) with SARS-CoV 3CL(pro) by molecular modeling. J Inorg Biochem. 2007;101:89–94. doi: 10.1016/j.jinorgbio.2006.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu RJ, Zhou KX, Yang H, Song GQ, Li YH, Fu JX, et al. Chemical synthesis, crystal structure, versatile evaluation of their biological activities and molecular simulations of novel pyrithiobac derivatives. Eur J Med Chem. 2019;167:472–484. doi: 10.1016/j.ejmech.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ren Z, Yan L, Zhang N, Guo Y, Yang C, Lou Z, et al. The newly emerged SARS-Like coronavirus HCoV-EMC also has an "Achilles’ heel": current effective inhibitor targeting a 3C-like protease. Protein Cell. 2013;4:248–250. doi: 10.1007/s13238-013-2841-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang K, Kankanamalage ACG, Fehr AR, Mehzabeen N, Battaile KP, Lovell S, et al. Structure-guided design of potent and permeable inhibitors of MERS coronavirus 3CL protease that utilize a piperidine moiety as a novel design element. Eur J Med Chem. 2018;150:334–346. doi: 10.1016/j.ejmech.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Konno H, Onuma T, Nitanai I, Wakabayashi M, Yano S, Teruya K, et al. Synthesis and evaluation of phenylisoserine derivatives for the SARS-CoV 3CL protease inhibitor. Bioorg Med Chem Lett. 2017;27:2746–2751. doi: 10.1016/j.bmcl.2017.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Y, Zheng TF, Jin F, Zhou L, Liu ZM, Wei P, et al. Design and bioassay of non-peptidic inhibitors of SARS coronavirus 3C-like proteinase. Acta Chim Sin. 2007;16:1707–1712. [Google Scholar]
- 48.RECOVERY Collaborative Group Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2020;396:1345–1352. doi: 10.1016/S0140-6736(20)32013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020;19:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sheahan TP, Sims AC, Graham RL, Menachery VD, Gralinski LE, Case JB, et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017;9:eaal3653. doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goldman JD, Lye D, Hui DS, Marks KM, Bruno R, Montejano R, et al. Remdesivir for 5 or 10 days in patients with severe COVID-19. N Engl J Med. 2020;383:1827–1837. doi: 10.1056/NEJMoa2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spinner CD, Gottlieb RL, Criner GJ, Arribas LJ, Cattelan AM, Viladomiu AS, et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA. 2020;324:1048–1057. doi: 10.1001/jama.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beigel JH, Tomashek KM, Dodd LE. Remdesivir for the treatment of COVID-19-preliminary report. N Engl J Med. 2020;383:994. doi: 10.1056/NEJMoa2007764. [DOI] [PubMed] [Google Scholar]
- 55.Peters HL, Jochmans D, de Wilde AH, Posthuma CC, Snijder EJ, Neyts J, et al. Design, synthesis and evaluation of a series of acyclic fleximer nucleoside analogues with anti-coronavirus activity. Bioorg Med Chem Lett. 2015;25:2923–2926. doi: 10.1016/j.bmcl.2015.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Warren TK, Wells J, Panchal RG, Stuthman KS, Garza NL, Tongeren SAV, et al. Protection against filovirus diseases by a novel broad-spectrum nucleoside analogue BCX4430. Nature. 2014;508:402. doi: 10.1038/nature13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yoon JS, Kim G, Jarhad DB, Kim HR, Shin YS, Qu S, et al. Design, synthesis, and anti-rna virus activity of 6'-fluorinated-aristeromycin analogues. J Med Chem. 2019;62:6346–6362. doi: 10.1021/acs.jmedchem.9b00781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shrestha DB, Budhathoki P, Khadka S, Shah PB, Pokharel N, Rashmi P. Favipiravir versus other antiviral or standard of care for COVID-19 treatment: a rapid systematic review and meta-analysis. Virol J. 2020;17:141. doi: 10.1186/s12985-020-01412-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cai Q, Yang M, Liu D, Chen J, Shu D, Xia J, et al. Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering (Beijing) 2020;6:1192–1198. doi: 10.1016/j.eng.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Booth CM, Matukas LM, Tomlinson GA, Rachlis AR, Rose DB, Dwosh HA, et al. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289:2801–2809. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- 61.Adedeji AO, Singh K, Kassim A, Coleman CM, Elliott R, Weiss SR, et al. Evaluation of SSYA10-001 as a replication inhibitor of severe acute respiratory syndrome, mouse hepatitis, and Middle East respiratory syndrome coronaviruses. Antimicrob Agents Chemother. 2014;58:4894–4898. doi: 10.1128/AAC.02994-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee C, Lee JM, Lee NR, Jin BS, Jang KJ, Kim DE, et al. Aryl diketoacids (ADK) selectively inhibit duplex DNA-unwinding activity of SARS coronavirus NTPase/helicase. Bioorg Med Chem Lett. 2009;19:1636–1638. doi: 10.1016/j.bmcl.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tanner JA, Zheng BJ, Zhou J, Watt RM, Jiang JQ, Wong KL, et al. The adamantane-derived bananins are potent inhibitors of the helicase activities and replication of SARS coronavirus. Chem Biol. 2005;12:303–311. doi: 10.1016/j.chembiol.2005.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim MK, Yu MS, Park HR, Kim KB, Lee C, Cho SY, et al. 2,6-Bis-arylmethyloxy-5-hydroxychromones with antiviral activity against both hepatitis C virus (HCV) and SARS-associated coronavirus (SCV) Eur J Med Chem. 2011;46:5698–5704. doi: 10.1016/j.ejmech.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zaher NH, Mostafa MI, Altaher AY. Design, synthesis and molecular docking of novel triazole derivatives as potential CoV helicase inhibitors. Acta Pharm. 2020;70:145–159. doi: 10.2478/acph-2020-0024. [DOI] [PubMed] [Google Scholar]
- 66.Menachery VD, Debbink K, Baric RS. Coronavirus non-structural protein 16: evasion, attenuation, and possible treatments. Virus Res. 2014;194:191–199. doi: 10.1016/j.virusres.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pfefferle S, Schopf J, Kogl M, Friedel CC, Muller MA, Carbajo-Lozoya J, et al. The SARS-coronavirus-host interactome: identification of cyclophilins as target for pan-coronavirus inhibitors. PLoS Pathog. 2011;7:e1002331. doi: 10.1371/journal.ppat.1002331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de Wilde AH, Raj VS, Oudshoorn D, Bestebroer TM, van Nieuwkoop S, Limpens RWAL, et al. MERS-coronavirus replication induces severe in vitro cytopathology and is strongly inhibited by cyclosporin A or interferon-α treatment. J Gen Virol. 2013;94:1749–1760. doi: 10.1099/vir.0.052910-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carbajo-Lozoya J, Ma-Lauer Y, Malešević M, Theuerkorn M, Kahlert V, Prell E, et al. Human coronavims NL63 replication is cyclophilin A-dependent and inhibited by non-immunosuppressive cyclosporine A-derivatives Including Alisporivir. Virus Res. 2014;184:44–53. doi: 10.1016/j.virusres.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dyall J, Coleman CM, Hart BJ, Venkataraman T, Holbrook MR, Kindrachuk J, et al. Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrob Agents Chemother. 2014;58:4885–4893. doi: 10.1128/AAC.03036-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Y, Li T, Fu L, Yu C, Li Y, Xu X, et al. Silencing SARS-CoV Spike protein expression in cultured cells by RNA interference. FEBS Lett. 2004;560:141–146. doi: 10.1016/S0014-5793(04)00087-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 72.Wu CJ, Huang HW, Liu CY, Hong CF, Chan YL. Inhibition of SARS-CoV replication by siRNA. Antivir Res. 2005;65:45–48. doi: 10.1016/j.antiviral.2004.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.He ML, Zheng BJ, Chen Y, Wong KL, Huang JD, Lin MC, et al. Development of interfering RNA agents to inhibit SARS-associated coronavirus infection and replication. Hong Kong Med J. 2009;15:28–31. [PubMed] [Google Scholar]
- 74.Nur SM, Hasan MA, Amin MA, Hossain M, Sharmin T. Design of potential RNAi (miRNA and siRNA) molecules for Middle East respiratory syndrome coronavirus (MERS-CoV) gene silencing by computational method. Interdiscip Sci. 2015;7:257–265. doi: 10.1007/s12539-015-0266-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Akerstrom S, Mirazimi A, Tan YJ. Inhibition of SARS-CoV replication cycle by small interference RNAs silencing specific SARS proteins, 7a/7b, 3a/3b and S. Antivir Res. 2007;73:219–227. doi: 10.1016/j.antiviral.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tang Q, Li B, Woodle M, Lu PY. Application of siRNA against SARS in the rhesus macaque model. Methods Mol Biol. 2008;442:139–158. doi: 10.1007/978-1-59745-191-8_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hart BJ, Dyall J, Postnikova E, Zhou H, Kindrachuk J, Johnson RF, et al. Interferon-beta and mycophenolic acid are potent inhibitors of Middle East respiratory syndrome coronavirus in cell-based assays. J Gen Virol. 2014;95:571–577. doi: 10.1099/vir.0.061911-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fukushima A, Fukuda N, Lai Y, Ueno T, Moriyama M, Taguchi F, et al. Development of a chimeric DNA-RNA hammerhead ribozyme targeting SARS virus. Intervirology. 2009;52:92–99. doi: 10.1159/000215946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lundin A, Dijkman R, Bergstrom T, Kann N, Adamiak B, Hannoun C, et al. Targeting membrane-bound viral RNA synthesis reveals potent inhibition of diverse coronaviruses including the Middle East respiratory syndrome virus. PLoS Pathog. 2014;10:e1004166. doi: 10.1371/journal.ppat.1004166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rider TH, Zook CE, Boettcher TL, Wick ST, Pancoast JS, Zusman BD. Broad-spectrum antiviral therapeutics. PLoS ONE. 2011;6:e22572. doi: 10.1371/journal.pone.0022572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lin SY, Liu CL, Chang YM, Zhao J, Perlman S, Hou MH. Structural basis for the identification of the N-terminal domain of coronavirus nucleocapsid protein as an antiviral target. J Med Chem. 2014;57:2247–2257. doi: 10.1021/jm500089r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liao HI, Olson CA, Hwang S, Deng H, Wong E, Baric RS, et al. mRNA display design of fibronectin-based intrabodies that detect and inhibit severe acute respiratory syndrome coronavirus nucleocapsid protein. J Biol Chem. 2009;284:17512–17520. doi: 10.1074/jbc.M901547200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lin SC, Ho CT, Chuo WH, Li S, Wang TT, Lin CC. Effective inhibition of MERS-CoV infection by resveratrol. BMC Infect Dis. 2017;17:144. doi: 10.1186/s12879-017-2253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wilson L, Gage P, Ewart G. Hexamethylene amiloride blocks E protein ion channels and inhibits coronavirus replication. Virology. 2006;353:294–306. doi: 10.1016/j.virol.2006.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ruediger A, Mayrhofer P, Ma-Lauer Y, Pohlentz G, Muething J, von Brunn A, et al. Tubulins interact with porcine and human S proteins of the genus Alphacoronavirus and support successful assembly and release of infectious viral particles. Virology. 2016;497:185–197. doi: 10.1016/j.virol.2016.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jiang Y, Zhao G, Song N, Li P, Chen Y, Guo Y, et al. Blockade of the C5a–C5aR axis alleviates lung damage in hDPP4-transgenic mice infected with MERS-CoV. Emerg Microbes Infect. 2018;7:77. doi: 10.1038/s41426-018-0063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sun S, Zhao G, Liu C, Wu X, Guo Y, Yu H, et al. Inhibition of complement activation alleviates acute lung injury induced by highly pathogenic avian influenza H5N1 virus infection. Am J Respir Cell Mol Biol. 2013;49:221–230. doi: 10.1165/rcmb.2012-0428OC. [DOI] [PubMed] [Google Scholar]
- 88.Gralinski LE, Sheahan TP, Morrison TE, Menachery VD, Jensen K, Leist SR, et al. Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. mBio. 2018;9:e1718–e1753. doi: 10.1128/mBio.01753-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kindler E, Thiel V, Weber F. Interaction of SARS and MERS coronaviruses with the antiviral interferon response. Adv Virus Res. 2016;96:219. doi: 10.1016/bs.aivir.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mesel-Lemoine M, Millet J, Vidalain P, Law H, Vabret A, Lorin V, et al. A human coronavirus responsible for the common cold massively kills dendritic cells but not monocytes. J Virol. 2012;86:7577–7587. doi: 10.1128/JVI.00269-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Siu K, Kok K, Ng MJ, Poon VKM, Yuen K, Zheng BJ, et al. Severe acute respiratory syndrome coronavirus M protein inhibits type I interferon production by impeding the formation of TRAF3.TANK.TBK1/IKKepsilon complex. J Biol Chem. 2009;284:16202–16209. doi: 10.1074/jbc.M109.008227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang Y, Zhang L, Geng H, Deng Y, Huang B, Guo Y, et al. The structural and accessory proteins M, ORF 4a, ORF 4b, and ORF 5 of Middle East respiratory syndrome coronavirus (MERS-CoV) are potent interferon antagonists. Protein Cell. 2013;4:951–961. doi: 10.1007/s13238-013-3096-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kopecky-Bromberg SA, Martínez-Sobrido L, Frieman M, Baric RA, Palese P. Severe acute respiratory syndrome coronavirus open reading frame (ORF) 3b, ORF 6, and nucleocapsid proteins function as interferon antagonists. J Virol. 2007;81:548–557. doi: 10.1128/JVI.01782-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lokugamage KG, Narayanan K, Nakagawa K, Terasaki K, Ramirez SI, Tseng CT. Middle East respiratory syndrome coronavirus nsp1 inhibits host gene expression by selectively targeting mRNAs transcribed in the nucleus while sparing mRNAs of cytoplasmic origin. J Virol. 2015;89:10970–10981. doi: 10.1128/JVI.01352-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tanaka T, Kamitani W, DeDiego ML, Enjuanes L, Matsuura Y. Severe acute respiratory syndrome coronavirus nsp1 facilitates efficient propagation in cells through a specific translational shutoff of host mRNA. J Virol. 2012;86:11128–11137. doi: 10.1128/JVI.01700-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang X, Chen X, Bian G, Tu J, Xing Y, Wang Y, et al. Proteolytic processing, deubiquitinase and interferon antagonist activities of Middle East respiratory syndrome coronavirus papain-like protease. J Gen Virol. 2014;95:614–626. doi: 10.1099/vir.0.059014-0. [DOI] [PubMed] [Google Scholar]
- 97.Chinese Medical Association. China Association of Chinese Medicine Consensus of the management of severe acute respiratory syndrome. Natl Med J Chin. 2003;83:1731–1752. [PubMed] [Google Scholar]
- 98.Channappanavar R, Fehr AR, Zheng J, Wohlford-Lenane C, Abrahante JE, Mack M, et al. IFN-I response timing relative to virus replication determines MERS coronavirus infection outcomes. J Clin Invest. 2019;130:3625–3639. doi: 10.1172/JCI126363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chinese Medical Association. China Association of Chinese Medicine Guidelines on diagnosis and treatment of novel coronavirus pneumonia (Trial sixth edition) Chin J Infect Contl. 2020;19:192–195. [Google Scholar]
- 100.Wang N, Zhan Y, Zhu L, Hou Z, Liu F, Song P, et al. Retrospective multicenter cohort study shows early interferon therapy is associated with favorable clinical responses in COVID-19 patients. Cell Host Microbe. 2020;28:455–464. doi: 10.1016/j.chom.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhou J, Chu H, Chan JF, Yuen K. Middle East respiratory syndrome coronavirus infection: virus-host cell interactions and implications on pathogenesis. Virol J. 2015;12:218. doi: 10.1186/s12985-015-0446-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhao J, Li K, Wohlford-Lenane C, Agnihothram SS, Fett C, Zhao J, et al. Rapid generation of a mouse model for Middle East respiratory syndrome. Proc Natl Acad Sci USA. 2014;111:4970–4975. doi: 10.1073/pnas.1323279111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu WJ, Zhao M, Liu K, Xu K, Wong G, Tan W, et al. T-cell immunity of SARS-CoV: implications for vaccine development against MERS-CoV. Antivir Res. 2017;1:82–92. doi: 10.1016/j.antiviral.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cameron MJ, Ran L, Xu L, Danesh A, Bermejo-Martin JF, Cameron CM, et al. Interferon-mediated immunopathological events are associated with atypical innate and adaptive immune responses in patients with severe acute respiratory syndrome. J Virol. 2007;81:8692–8706. doi: 10.1128/JVI.00527-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Alshukairi AN, Khalid I, Ahmed WA, Dada AM, Bayumi DT, Malic LS, et al. Antibody response and disease severity in healthcare worker MERS survivors. Emerg Infect Dis. 2016;22:1113–1115. doi: 10.3201/eid2206.160010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tang F, Quan Y, Xin Z, Wrammert J, Ma M, Lv H, et al. Lack of peripheral memory B cell responses in recovered patients with severe acute respiratory syndrome: a six-year follow-up study. J Immunol. 2011;186:7264–7268. doi: 10.4049/jimmunol.0903490. [DOI] [PubMed] [Google Scholar]
- 108.ter Meulen J, van den Brink EN, Poon LLM, Marissen WE, Leung CSW, Cox F, et al. Human monoclonal antibody combination against SARS coronavirus: synergy and coverage of escape mutants. PLoS Med. 2006;3:e237. doi: 10.1371/journal.pmed.0030237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Miyoshi-Akiyama T, Ishida I, Fukushi M, Yamaguchi K, Matsuoka Y, Ishihara T, et al. Fully human monoclonal antibody directed to proteolytic cleavage site in severe acute respiratory syndrome (SARS) coronavirus S protein neutralizes the virus in a rhesus macaque SARS model. J Infect Dis. 2011;203:1574–1581. doi: 10.1093/infdis/jir084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Houser KV, Gretebeck L, Ying T, Wang Y, Vogel L, Lamirande EW, et al. Prophylaxis with a Middle East respiratory syndrome coronavirus (MERS-CoV)—specific human monoclonal antibody protects rabbits from MERS-CoV infection. J Infect Dis. 2016;213:1557–1561. doi: 10.1093/infdis/jiw080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pascal KE, Coleman CM, Mujica AO, Kamat V, Badithe A, Fairhurst J, et al. Pre- and postexposure efficacy of fully human antibodies against Spike protein in a novel humanized mouse model of MERS-CoV infection. Proc Natl Acad Sci USA. 2015;112:8738–8743. doi: 10.1073/pnas.1510830112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Johnson RF, Bagci U, Keith L, Tang X, Mollura DJ, Zeitlin L, et al. 3B11-N, a monoclonal antibody against MERS-CoV, reduces lung pathology in rhesus monkeys following intratracheal inoculation of MERS-CoV Jordan-n3/2012. Virology. 2016;490:49–58. doi: 10.1016/j.virol.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Corti D, Zhao J, Pedotti M, Simonelli L, Agnihothram S, Fett C, et al. Prophylactic and postexposure efficacy of a potent human monoclonal antibody against MERS coronavirus. Proc Natl Acad Sci USA. 2015;112:10473–10478. doi: 10.1073/pnas.1510199112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen Z, Bao L, Chen C, Zou T, Xue Y, Li F, et al. Human neutralizing monoclonal antibody inhibition of Middle East respiratory syndrome coronavirus replication in the common marmoset. J Infect Dis. 2017;215:1807–1815. doi: 10.1093/infdis/jix209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jiang L, Wang N, Zuo T, Shi X, Poon KM, Wu Y, et al. Potent neutralization of MERS-CoV by human neutralizing monoclonal antibodies to the viral spike glycoprotein. Sci Transl Med. 2014;6:234r–259r. doi: 10.1126/scitranslmed.3008140. [DOI] [PubMed] [Google Scholar]
- 116.Niu P, Zhang S, Zhou P, Huang B, Deng Y, Qin K, et al. Ultrapotent human neutralizing antibody repertoires against Middle East respiratory syndrome coronavirus from a recovered patient. J Infect Dis. 2018;218:1249–1260. doi: 10.1093/infdis/jiy311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tian X, Li C, Huang A, Xia S, Lu S, Shi Z, et al. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg Microbes Infect. 2020;9:382–385. doi: 10.1080/22221751.2020.1729069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mair-Jenkins J, Saavedra-Campos M, Baillie JK, Cleary P, Khaw F, Lim WS, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211:80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu STH, Lin HM, Baine I, Wajnberg A, Gumprecht JP, Rahman F, et al. Convalescent plasma treatment of severe COVID-19: a propensity score-matched control study. Nat Med. 2020;26:1708–1713. doi: 10.1038/s41591-020-1088-9. [DOI] [PubMed] [Google Scholar]
- 120.Li L, Zhang W, Hu Y, Tong X, Zheng S, Yang J, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020;324:460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang Y, Jiang W, He Q, Wang C, Wang B, Zhou P, et al. A retrospective cohort study of methylprednisolone therapy in severe patients with COVID-19 pneumonia. Signal Transduct Target Ther. 2020;5:57. doi: 10.1038/s41392-020-0158-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Edalatifard M, Akhtari M, Salehi M, Naderi Z, Jamshidi A, Mostafaei S, et al. Intravenous methylprednisolone pulse as a treatment for hospitalised severe COVID-19 patients: results from a randomised controlled clinical trial. Eur Respir J. 2020;56(6):2002808. doi: 10.1183/13993003.02808-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sterne J, Murthy S, Diaz JV, Slutsky AS, Villar J, Angus DC, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Stone JH, Frigault MJ, Serling-Boyd NJ, Fernandes AD, Harvey L, Foulkes AS, et al. Efficacy of tocilizumab in patients hospitalized with COVID-19. N Engl J Med. 2020;383(24):2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hermine O, Mariette X, Tharaux PL, Resche-Rigon M, Porcher R, Ravaud P, et al. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med. 2020;20:e206820. doi: 10.1001/jamainternmed.2020.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Salvarani C, Dolci G, Massari M, Merlo DF, Cavuto S, Savoldi L, et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181(1):24–31. doi: 10.1001/jamainternmed.2020.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cinatl J, Morgenstern B, Bauer G, Chandra P, Rabenau H, Doerr HW. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet. 2003;361:2045–2046. doi: 10.1016/S0140-6736(03)13615-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fiore C, Eisenhut M, Krausse R, Ragazzi E, Pellati D, Armanini D, et al. Antiviral effects of glycyrrhiza species. Phytother Res. 2008;22(2):141–148. doi: 10.1002/ptr.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hoever G, Baltina L, Michaelis M, Kondratenko R, Baltina L, Tolstikov GA, et al. Antiviral activity of glycyrrhizic acid derivatives against SARS-coronavirus. J Med Chem. 2005;48(4):1256–1259. doi: 10.1021/jm0493008. [DOI] [PubMed] [Google Scholar]
- 131.Wang M, Sun G, Wang X. Experimental study on effects of Chinese herbal preparations on SARS virus. Chin J Basic Med Tradit Chin Med. 2004;10:38–39. [Google Scholar]
- 132.Li S, Chen C, Zhang H, Guo H, Wang H, Wang L, et al. Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antivir Res. 2005;67(1):18–23. doi: 10.1016/j.antiviral.2005.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lu Y, Jiang Y, Ling L, Zhang Y, Li H, Chen D. Beneficial effects of Houttuynia cordata polysaccharides on "two-hit" acute lung injury and endotoxic fever in rats associated with anti-complementary activities. Acta Pharm Sin B. 2018;8:218–227. doi: 10.1016/j.apsb.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lau K, Lee K, Koon C, Cheung CS, Lau C, Ho HM, et al. Immunomodulatory and anti-SARS activities of Houttuynia cordata. J Ethnopharmacol. 2008;118:79–85. doi: 10.1016/j.jep.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jiang D, Chen W, Xu J, Yu T, Lu P, Mu X. Effect of herbs for prevention and cure of SARS on neutrophil cAMP-phosphodiesterase activity. Chin J Vet Med. 2006;42:38–39. [Google Scholar]
- 136.Kostoff RN. Literature-related discovery: Potential treatments and preventatives for SARS. Technol Forecast Soc Change. 2011;78:1164–1173. doi: 10.1016/j.techfore.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ryu YB, Park SJ, Kim YM, Lee JY, Seo WD, Chang JS, et al. SARS-CoV 3CLpro inhibitory effects of quinone-methide triterpenes from Tripterygium regelii. Bioorg Med Chem Lett. 2010;20:1873–1876. doi: 10.1016/j.bmcl.2010.01.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Park J, Kim JH, Kwon JM, Kwon H, Jeong HJ, Kim YM, et al. Dieckol, a SARS-CoV 3CLpro inhibitor, isolated from the edible brown algae Ecklonia cava. Bioorg Med Chem. 2013;21:3730–3737. doi: 10.1016/j.bmc.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Park JY, Jeong HJ, Kim JH, Kim YM, Park SJ, Kim D, et al. Diarylheptanoids from Alnus japonica inhibit papain-like protease of severe acute respiratory syndrome coronavirus. Biol Pharm Bull. 2012;35:2036–2042. doi: 10.1248/bpb.b12-00623. [DOI] [PubMed] [Google Scholar]
- 140.Kim DW, Seo KH, Curtis-Long MJ, Oh KY, Oh JW, Cho JK, et al. Phenolic phytochemical displaying SARS-CoV papain-like protease inhibition from the seeds of Psoralea corylifolia. J Enzyme Inhib Med Chem. 2014;29(1):59–63. doi: 10.3109/14756366.2012.753591. [DOI] [PubMed] [Google Scholar]
- 141.Park JY, Ko JA, Kim DW, Kim YM, Kwon HJ, et al. Chalcones isolated from Angelica keiskei inhibit cysteine proteases of SARS-CoV. J Enzyme Inhib Med Chem. 2016;31:23–30. doi: 10.3109/14756366.2014.1003215. [DOI] [PubMed] [Google Scholar]
- 142.Park JY, Kim JH, Kim YM, Jeong HJ, Kim DW, Jeong HJ, et al. Tanshinones as selective and slow-binding inhibitors for SARS-CoV cysteine proteases. Bioorg Med Chem. 2012;20:5928–5935. doi: 10.1016/j.bmc.2012.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Niu M, Wang RL, Wang ZX, Zhang P, Bai ZF, Jing J, et al. Rapid establishment of traditional Chinese medicine prevention and treatment of 2019-nCoV based on clinical experience and molecular docking. Chin J Chin Materia Med. 2020;45:1213–1218. doi: 10.19540/j.cnki.cjcmm.20200206.501. [DOI] [PubMed] [Google Scholar]
- 144.Zhang Y, Tang D, Shu B, Li W, Zhang J, Li Y, et al. Evaluation and analysis on the SARS-CoV-2-induced injuries in multiple organs and the intervention of traditional Chinese medicine based on renin-angiotensin system. Modern Tradit Chin Med Materia Med-World Sci Tech. 2020;22:264–269. [Google Scholar]
- 145.Jin X, Guan R, Mao J, Wang Y, Wang F, Li C, et al. Exploration on material basis of Qingfei Paidu Decoction with multi-target system against COVID-19 based on CADD. Chin Tradit Herb Drugs. 2020;51:1984–1995. [Google Scholar]
- 146.Zong Y, Yao W, Ju W. The intervention effect investigation of Chinese medicine monomer on cytokine storm induced by COVID-19 based on interleukin-6 receptor. Chin J Hosp Pharma. 2020;40:1182–1188. [Google Scholar]
- 147.Huang J, Zhang B, Lin Z. Intervention effect of Chinese medicine on interleukin cytokines and thinking on its prevention and treatment for inflammatory storm of COVID-19. Pharmacol Clin Chin Materia Med. 2020;36:23–28. [Google Scholar]
- 148.Liu J, Fan M, Sun K, Sun R. Exploring active ingredients and function mechanism of Chaihu Guizhi Ganjiang Decoction against coronavirus disease 2019 based on molecular docking technology. Chin Tradit Herb Drugs. 2020;51:1704–1712. [Google Scholar]
- 149.Chen R, Wang T, Li K, Shang R, Song J, Zhang J. Characteristics and application of immune-regulating and antiviral Chinese materia medica. Chin Tradit Herb Drugs. 2020;51:1412–26 (in Chinese).
- 150.Li J, Wei B, Li K, Su X, Zhang Z. Natural product research and development. Nat Prod Res Dev. 2020;32:1981–91 (in Chinese).
- 151.Ma J, Huo X, Chen X, Zhu W, Yao M, Qian Y. Study on screening potential traditional Chinese medicines against 2019-nCoV based on Mpro and PLP. Chin J Chin Materia Med. 2020;1219–1224. (in Chinese). [DOI] [PubMed]
- 152.Liu C, Zhu X, Lu Y, Zhang X, Jia X, Yang T. Potential treatment of Chinese and western medicine targeting Nsp14 of SARS-CoV-2. J Pharm Anal. 2020 doi: 10.1016/j.jpha.2020.08.002.10.1016/j.jpha.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Müller C, Schulte FW, Lange-Grünweller K, Obermann W, Madhugiri R, Pleschka S, et al. Broad-spectrum antiviral activity of the eIF4A inhibitor silvestrol against corona- and picornaviruses. Antivir Res. 2018;150:123–129. doi: 10.1016/j.antiviral.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jo S, Kim H, Kim S, Shin DH, Kim MS. Characteristics of flavonoids as potent MERS-CoV 3C-like protease inhibitors. Chem Biol Drug Des. 2019;94:2023–2030. doi: 10.1111/cbdd.13604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chang F, Yen C, Ei-Shazly M, Lin W, Yen M, Lin K, et al. Anti-human coronavirus (anti-HCoV) triterpenoids from the leaves of Euphorbia neriifolia. Nat Prod Commun. 2012;7:1415–1417. [PubMed] [Google Scholar]
- 156.Wenga J, Linb C, Hsueh-Chou Laic D, Line Y, Wange C, Tsai YC, et al. Antiviral activity of Sambucus FormosanaNakai ethanol extract and related phenolic acid constituents against human coronavirus NL63. Virus Res. 2019;273:197767. doi: 10.1016/j.virusres.2019.197767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Liang A, Ling Y, Liu H. Application of traditional Chinese medicine in the prevention and treatment of SARS. Pharm J Chin People's Lib Army. 2003;19:367–369. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.