Abstract
Pancreatic cancer (PaCa) is one of the most aggressive types of cancer. Thus, the development of new and more effective therapies is urgently required. Escin, a pentacyclic triterpenoid from the horse chestnut, has been reported to exhibit antitumor potential by reducing cell proliferation and blocking the nuclear factor-κB (NF-κB) signaling pathway in several types of cancer. Our previous study reported that NF-κB enhanced the secretion of interleukin (IL)-8 and vascular endothelial growth factor (VEGF), thereby inducing angiogenesis in PaCa cell lines. In the present study, it was examined whether escin inhibited angiogenesis by blocking NF-κB activation in PaCa. It was initially confirmed that escin, at concentrations >10 µM, significantly inhibited the proliferation of several PaCa cell lines. Next, using immunocytochemical staining, it was found that escin inhibited the nuclear translocation of NF-κB. Furthermore, ELISA confirmed that NF-κB activity in the escin-treated PaCa cells was significantly inhibited and reverse transcription-quantitative PCR showed that the mRNA expression levels of tumor necrosis factor-α-induced IL-8 and VEGF were significantly suppressed following escin treatment in the PaCa cell lines. ELISA also showed that escin decreased the secretion of IL-8 and VEGF from the PaCa cells. Furthermore, tube formation in immortalized human endothelial cells was inhibited following incubation with the supernatants from escin-treated PaCa cells. These results indicated that escin inhibited angiogenesis by reducing the secretion of IL-8 and VEGF by blocking NF-κB activity in PaCa. In conclusion, escin could be used as a novel molecular therapy for PaCa.
Keywords: escin, pancreatic cancer, IL-8, VEGF, NF-κB, angiogenesis
Introduction
Pancreatic cancer (PaCa) is one of the most aggressive types of cancer. In 2018, PaCa was the 4th most common cause of all cancer-related deaths in Japan (1) and the third most common cause of death among all cancer-related deaths in the USA (2). In 2019, over 56,000 patients were newly diagnosed with PaCa and almost 46,000 patients died from PaCa in the USA (2). After diagnosis, 24% of patients survived for 1 year, and the 5-year overall survival rate was only 9% in 2019, globally (3). Furthermore, PaCa is highly resistant to chemotherapy (4); however, FOLFIRINOX and gemcitabine with nab-paclitaxel have been found to improve the median survival time to 11.1 months in France (2011) and 8.5 months in USA (2013), respectively (5–7). Thus, there is an urgent requirement to develop new therapeutic agents to reduce mortality rates in patients with PaCa.
Numerous natural compounds, such as Curcuma longa (turmeric) and Vitis vinifera (grape seed extract) have been reported to have anticancer effects (8,9). Escin, a natural triterpene saponin extracted from horse chestnuts (Aesculus hippocastanum), has been widely used to treat inflammation in Traditional Chinese Medicine in Korea, China, and Japan (10). Previous studies have found that escin had antitumor effects in various types of human cancer cell, including glioblastoma, lung adenocarcinoma, melanoma, hepatocellular carcinoma, PaCa, leukemia and osteosarcoma (10–14). In addition, escin has been reported to inhibit migration and invasion, and induced caspase-dependent apoptosis and autophagy (9,13). Escin may inhibit NF-κB activation (15,16); however, the specific mechanisms involved are unknown.
NF-κB is a transcription factor that was first discovered in 1986 and is reported that it plays essential roles in carcinogenesis-related angiogenesis (17). NF-κΒ is also involved in the creation of new blood vessels, which provides oxygen and nutrients to the tumor cells, and in the progression of the growth of malignant solid tumors (18). In solid tumors, angiogenesis occurs via several steps, including extracellular matrix remodeling, migration and proliferation of endothelial cells, and capillary tube formation (19). New blood vessels formed in the tumor allow tumor cells to circulate and metastasize to distant organs. Our previous study reported that the liver metastatic potential of PaCa cell lines was associated with angiogenesis and that NF-κB was homeostatically activated in PaCa cells, with high metastatic potential (19). These findings indicated that agents blocking NF-κB activation can decrease angiogenesis in PaCa, and additional studies have reported that tumor growth and angiogenesis in several types of cancer, including PaCa, were reduced by the inhibition of NF-κB activity (20,21). In previous reports, interleukin (IL)-8 and vascular endothelial growth factor (VEGF) have been identified as key mediators of angiogenesis in PaCa (22–24). In addition, our previous study showed that suppressing NF-κB activation decreased the secretion of both IL-8 and VEGF in PaCa (18). Furthermore, it was shown that several natural compounds inhibited angiogenesis by suppressing NF-κB activity, and reducing VEGF and IL-8 production (25,26). However, the mechanism of how escin affects the angiogenesis of PaCa is not fully understood.
The present study aimed to clarify whether escin inhibited angiogenesis in PaCa cells by suppressing nuclear translocation of NF-κB.
Materials and methods
Reagents
Escin (C55H86O24; CID 6476031) and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich (Merck KGaA). Escin solution (50 mM) was prepared in DMSO, stored as small amounts at −20°C, then thawed and diluted in cell culture medium as required. Recombinant human tumor necrosis factor (TNF)-α was purchased from R&D Systems Inc.
Cell lines and treatments
The human pancreatic adenocarcinoma cell lines, BxPC-3, AsPC-1 and SW1990, and the immortalized human endothelial cell line, EA.hy 926, were purchased from the American Type Culture Collection. The BxPC-3 and AsPC-1 cell lines were maintained in RPMI-1640 medium, while the SW1990 and EA.hy 926 cell lines were maintained in DMEM (both from Sigma-Aldrich; Merck KGaA). Each medium was replenished with 10% fetal bovine serum (FBS), 10 mg/ml streptomycin, 10,000 U/ml penicillin, and 25 µg amphotericin B (all from Gibco; Thermo Fisher Scientific, Inc.). All the cell lines were cultured at 37°C in a humidified incubator with 5% CO2.
Cytotoxicity assay
The cytotoxicity of escin was assessed with a Premix WST-1 Cell Proliferation Assay System (Takara Bio, Inc.) according to the manufacturer's protocols. Briefly, BxPC-3, AsPC-1 and SW1990 cell lines were seeded at 2×103 cells/100 µl/well in 96-well plates and cultured for 1 day. Then, various concentrations of escin (0–30 µM) and DMSO (equivalent to the concentration contained in 30 µM escin) were added to the cells. After incubation for 72 h, the absorbance at 450 nm was measured using a SpectraMax 340 spectrophotometer (Molecular Devices, LLC).
Immunocytochemical analysis for NF-κB p65 localization
The BxPC-3, AsPC-1 and SW1990 cell lines were initially seeded at 1×104 cells/chamber in a 4-chamber slide glass and cultured overnight. The cells were then treated with escin (10 µM) for 2 h and stimulated with TNF-α (1 ng/ml) for 15 min before the end of the incubation. The cells that had not been treated were used as controls. The cells were then washed with PBS and fixed with 4% paraformaldehyde for 20 min at room temperature. Next, the cells were washed and permeabilized with 0.1% Triton-X for 3 min and incubated with blocking buffer (3% BSA, FUJIFILM Wako Pure Chemical Corporation) for 1 h at room temperature. The cells were probed with anti-NF-κB p65 antibody (cat. no. 8242T; Cell Signaling Technology, Inc.) overnight at 4°C. Subsequently, the cells were washed and incubated with Alexa Fluor® 488 goat anti-rabbit IgG (cat. no. ab150077; Abcam) for 1 h at room temperature. Primary and secondary antibodies were used at 1:400 and 1:500 dilution with 3% BSA, respectively. The nuclei were visualized with DAPI staining at room temperature for 10 min. Images of the stained slides were captured using a BZ-X710 fluorescent microscope at ×100 (Keyence Corporation).
Nuclear protein extraction and NF-κB p65 activity assays
The BxPC-3, AsPC-1 and SW1990 cell lines were seeded at 2×106 in 100-mm dishes with 10% FBS and cultured to ~80% confluence. The cells were then treated with escin (10 µM) in 5% FBS for 2 h and stimulated with or without TNF-α (5 ng/ml) for 30 min before the end of the incubation. Nuclear extracts were obtained from the cells using a Nuclear Extraction kit (Active Motif, Inc.). The concentrations of intranuclear proteins were measured with a Pierce BCA protein assay kit (Thermo Fisher Scientific, Inc.). Nuclear extracts were stored at −80°C until further use. The NF-κB activity was determined using a Trans AM NF-κB p65/p50 Transcription Factor Assay kit (cat. no. 40096; Active Motif, Inc.) according to the manufacturer's protocols. A total of 4 µg nuclear extract was used for the NF-κB activity assays.
Western blot analysis of NF-κB p65 and NF-κB phosphorylated (p)p65
The BxPC-3 cells were seeded at 2×106 in 100-mm dishes with 10% FBS and cultured to ~80% confluence. The cells were then treated with escin (10 µM) in 5% FBS for 2 h and stimulated with or without TNF-α (5 ng/ml) for 30 min before the end of the incubation. Nuclear and cytoplasmic extracts were obtained from the cells using a Nuclear Extraction kit (Active Motif, Inc.). The concentrations of each protein were measured with a Pierce BCA protein assay kit (Thermo Fisher Scientific, Inc.). A total of 20 µg each protein extract was denatured at 90°C for 5 min and separated on 10% Mini-PROTEAN TGX Precast gels (Bio-Rad Laboratories, Inc.). The protein bands were transferred to nitrocellulose membranes and blocked in iBind Flex Solution (iBind Flex Buffer, iBind Flex Additive and distilled water; Thermo Fisher Scientific, Inc.) for 15 min at room temperature. The primary and secondary antibody reactions were performed using the iBind Flex Western System (Thermo Fisher Scientific, Inc.) for 2.5 h at room temperature according to the manufacturer's instructions. The membranes were incubated with anti-p65 (1:1,000; cat. no. 8242S; Cell Signaling Technology, Inc.), -p-p65 (1:1,000; cat. no. 3033S; Cell Signaling Technology, Inc.), -GAPDH (1:2,000; cat. no. SC-47724; Santa Cruz Biotechnology Inc.) and -TATA-box binding protein (TBP; 1:1,000; cat. no. 22006-1-AP; ProteinTech Group, Inc.) primary antibodies, then HRP-conjugated goat anti-rabbit polyclonal secondary antibody (1:2,000; cat. no. P0448; Agilent Technologies, Inc.). The protein-antibody complexes were visualized with a SuperSignal West Pico Chemiluminescent Substrate, SuperSignal West Femto Chemiluminescent Substrate, or Pierce ECL Western Blotting Substrate (all from Thermo Fisher Scientific, Inc.). The immunoreactive protein bands were detected using an Amersham Imager 600 (Cytiva).
Reverse transcription-quantitative PCR (RT-qPCR)
The BxPC-3, AsPC-1 and SW1990 cell lines were seeded at 1×105 in 6-well plates with 10% FBS and cultured to ~80% confluence. Then, the cells were treated with or without 10 µM escin and 5% FBS for 1 h and stimulated with TNF-α (1 ng/ml) for 15 min before the end of the incubation. Total RNA was extracted from the cell pellets using a RNeasy Plus Mini kit (Qiagen GmbH) according to the manufacturer's protocols and quantified using a NanoDrop® 1000 (Thermo Fisher Scientific, Inc.). Total RNA (1 µg) was reverse transcribed using a Super Script III First-Strand Synthesis Super Mix for RT-qPCR (Invitrogen; Thermo Fisher Scientific, Inc.) following the manufacturer's protocols. RT-qPCR was performed using a Taqman Fast Advanced Master Mix and Taqman Gene Expression Assays for VEGF (Hs00900055_m1), IL-8 (Hs01553824_g1) and GAPDH (Hs99999905_m1) on a 7900HT Fast Real-Time PCR System (all from Applied Biosystems; Thermo Fisher Scientific, Inc.). The following thermocycling conditions were used: Initial denaturation at 95°C for 20 sec, followed by 40 cycles at 95°C for 1 sec and 60°C for 20 sec. The expression levels of VEGF and IL-8 were standardized to those of GAPDH in each sample, using the relative standard curve method (27).
ELISA
The BxPC-3, AsPC-1 and SW1990 cell lines were seeded at 1×105 cells/well in a 6-well plate containing cell specific medium as aforementioned, supplemented with 10% FBS and incubated overnight at 37°C. The culture media were then changed, and cells were incubated and stimulated with TNF-α (1 ng/ml) for 48 h in the presence of escin (10 µM). In addition, the BxPC-3 and SW1990 cells were also incubated for an additional 48 h in the presence of different doses of escin (0–10 µM) with 5% FBS after culturing overnight as aforementioned. The culture media were then collected and centrifuged at 400 × g for 5 min at 4°C to discard particulates and stored at −80°C until further use. The concentrations of IL-8 and VEGF were then measured using appropriate ELISA kits (R&D Systems, Inc.) following the manufacturer's protocols.
Tube formation assay for angiogenesis
Tube formation was determined using the EA.hy 926 cell line and angiogenesis assays using Matrigel (Corning, Inc.). The BxPC-3 and SW1990 cell lines were seeded at 1×105 cells/well in 6-well plates containing medium (RPMI-1640 and DMEM, respectively) supplemented with 10% FBS and incubated overnight at 37°C. The culture media were then changed, and the cells were incubated for an additional 48 h with or without escin (10 µM) in 2% FBS. The cell supernatants were then collected and centrifuged at 400 × g for 5 min at 4°C to discard particulates. Matrigel was added to a 96-well plate (50 µl/well) at 4°C and incubated for 30 min at 37°C for the Matrigel to solidify. The EA.hy 926 cells (1.2×104 cells/well) were added on top of the Matrigel. The cells were then incubated with mixed medium (50 µl of the RPMI-1640 medium with 2% FBS and 50 µl of the aforementioned supernatant per well) for 16 h to form capillary-like structures. The cells incubated with RPMI medium with 2% FBS only were used as the control. The EA.hy 926 cells (1.2×104 cells/well) were also added on top of the Matrigel and incubated with RPMI medium (2% FBS) containing 100 ng/ml recombinant IL-8 and VEGF (both from R&D Systems Inc.), respectively. The number of endotubes were counted under a confocal microscope (×40). A total of 4 fields of view were analyzed per sample.
Statistical analysis
All experiments were performed in triplicate. All the experimental data are represented as the mean ± SD. Comparisons between two groups were assessed using unpaired t-tests, while comparisons between multiple groups were determined using one-way analysis of variance with Bonferroni's post hoc test for subsequent comparison of individual groups. P<0.05 was considered to indicate a statistically significant difference.
Results
Escin suppresses the proliferation of the PaCa cells
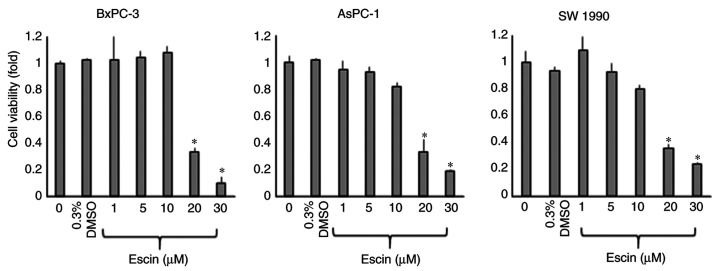
The short-term effects of escin on the proliferation of the PaCa cells (BxPC-3, AsPC-1 and SW1990) were examined using a WST-1 assay after incubating the cells with various concentrations of escin for 72 h. The proliferation of all the PaCa cell lines was significantly inhibited by >10 µM escin (Fig. 1). The half-maximal inhibitory concentration (IC50) values were calculated from the results of the WST-1 assays and were 17.2, 15.9 and 14.1 µM for the BxPC-3, AsPC-1 and SW1990 cells, respectively. To avoid the effect of cytotoxicity induced by escin, the concentration of escin was set at less than the IC50 value in the subsequent experiments.
Figure 1.
Cytotoxic effects of escin on PaCa cell lines. There were PaCa cell lines (BxPC-3, AsPC-1 and SW1990) treated with different concentrations of escin for 72 h, and the viability of each cell line was assessed with a WST-1 assay. Values are expressed as the mean ± SD. *P<0.05 vs. 0 µM escin.
Escin inhibits TNF-α-induced translocation of NF-κB
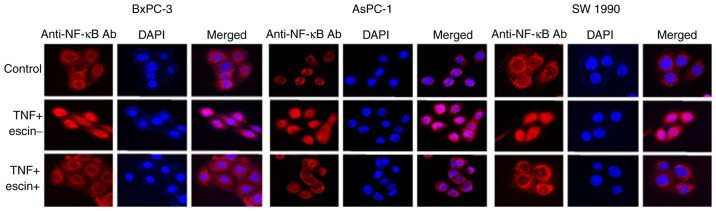
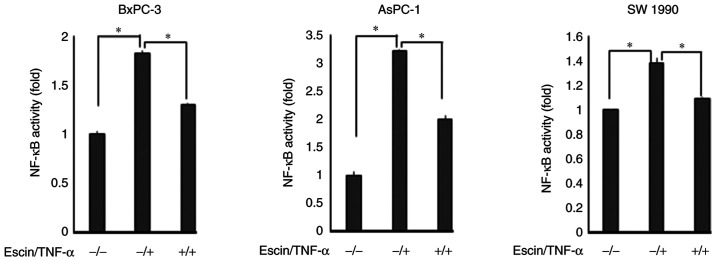
Immunocytochemical analysis was subsequently performed to examine whether escin affected TNF-α-induced translocation of p65 to the nucleus in the PaCa cells (Fig. 2). In cells treated with TNF-α alone, p65 translocated into the nucleus, while in cells treated with escin, p65 remained in the cytoplasm. The activity of p65 translocated into the nucleus was then determined using ELISA (Fig. 3). The results demonstrated that escin significantly reduced the TNF-α-induced activity of p65 in the nucleus. The activity of p65 was higher in cells treated with both escin and TNF-α compared with that in cells that were untreated; however, it is considered that the activation of p65 by TNF-α stimulation was beyond the range of suppression by escin. These results supported the hypothesis that escin inhibited the translocation of p65. To further support the results that escin inactivated NF-κB, western blot analysis was also performed to determine the protein expression levels of p65 and p-p65 in the cytoplasm and nucleus from the BxPC-3 cells, which were found to be the most sensitive to escin in immunocytotochemical staining. Escin notably reduced TNF-α-induced activation of both total p65 and p-p65 in the cytoplasm and the nucleus (Fig. S1).
Figure 2.
Escin suppresses the nuclear translocation of p65 induced by TNF-α. The BxPC-3, AsPC-1 and SW1990 cell lines were initially treated with escin (10 µM) for 2 h, then stimulated with TNF-α (1 ng/ml) for 15 min before the end of incubation. Magnification; ×100. Ab, antibody; NF-κB, nuclear factor-κB; TNF, tumor necrosis factor.
Figure 3.
Escin suppresses TNF-α-induced NF-κB activation. The BxPC-3, AsPC-1 and SW1990 cell lines were seeded and incubated to ~80% confluence, then treated with escin (10 µM) and stimulated with or without TNF-α before the end of the incubation. *P<0.05. TNF, tumor necrosis factor; NF-κB, nuclear factor-κB.
Escin downregulates TNF-α-induced mRNA expression levels of IL-8 and VEGF
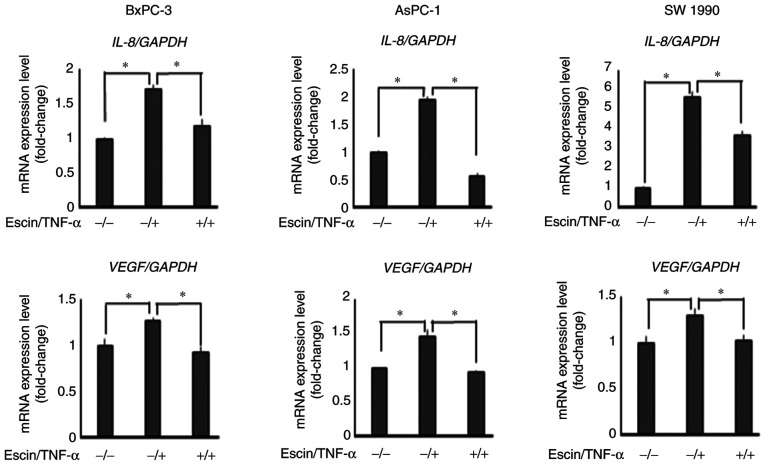
RT-qPCR revealed that escin (10 µM) significantly decreased TNF-α-induced mRNA expression levels of IL-8 and VEGF in PaCa cells (Fig. 4).
Figure 4.
Escin downregulates TNF-α-induced mRNA expression level of IL-8 and VEGF. The BxPC-3, AsPC-1 and SW1990 cell lines were first treated with escin, then stimulated with TNF-α before the end of the incubation. The relative mRNA expression levels of VEGF and IL-8 were standardized to the expression level of GAPDH in each sample. *P<0.05. VEGF, vascular endothelial growth factor; IL, interleukin.
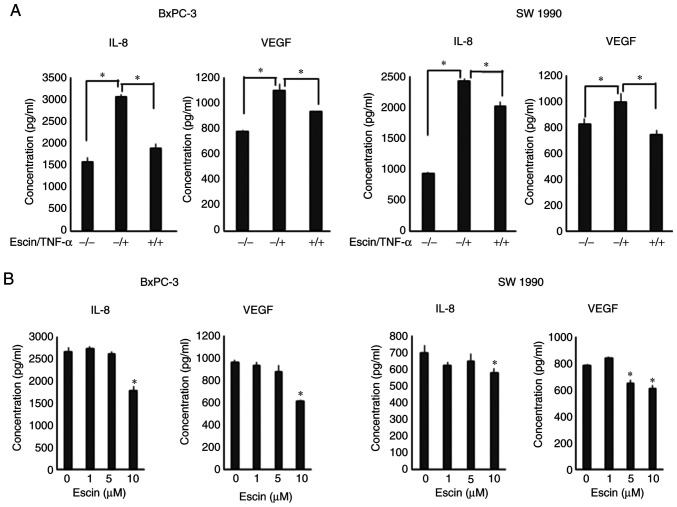
Escin suppresses the secretion of IL-8 and VEGF in the PaCa cells
Next, ELISA was performed to evaluate the protein secretion levels of IL-8 and VEGF in PaCa cells. Escin (10 µM) significantly suppressed TNF-α-induced protein secretion of IL-8 and VEGF in the PaCa cells (Fig. 5A). With respect to the AsPC-1 cell line, secretion levels of both VEGF and IL-8 were significantly enhanced by the treatment with TNF, and the enhancement was significantly inhibited by escin (Fig. S2); however, the basal secretion levels of both the angiogenic cytokines were lower compared with that in the other cell lines. Therefore, the AsPC-1 cell line was excluded from the next experiment. In addition, escin also suppressed the secretion of these proteins in a dose-dependent manner in the BxPC-3 and SW1990 cells (Fig. 5B). With respect to the inhibition of IL-8 production, there were statistically significant differences; however, the inhibition of TNF-α-induced IL-8 production in the SW1990 cell line by escin was slightly lower compared with the inhibition of VEGF production.
Figure 5.
Escin suppresses the secretion of IL-8 and VEGF in the PaCa cells. (A) BxPC-3 and SW1990 cell lines were cultured and treated with TNF-α for 48 h in the presence of escin. *P<0.05. (B) BxPC-3 and SW1990 cell lines were incubated for 48 h in the presence of various doses of escin. *P<0.05 vs. 0 µM. The concentrations of IL-8 and VEGF in the cell supernatants were measured with ELISA kits. VEGF, vascular endothelial growth factor; IL, interleukin.
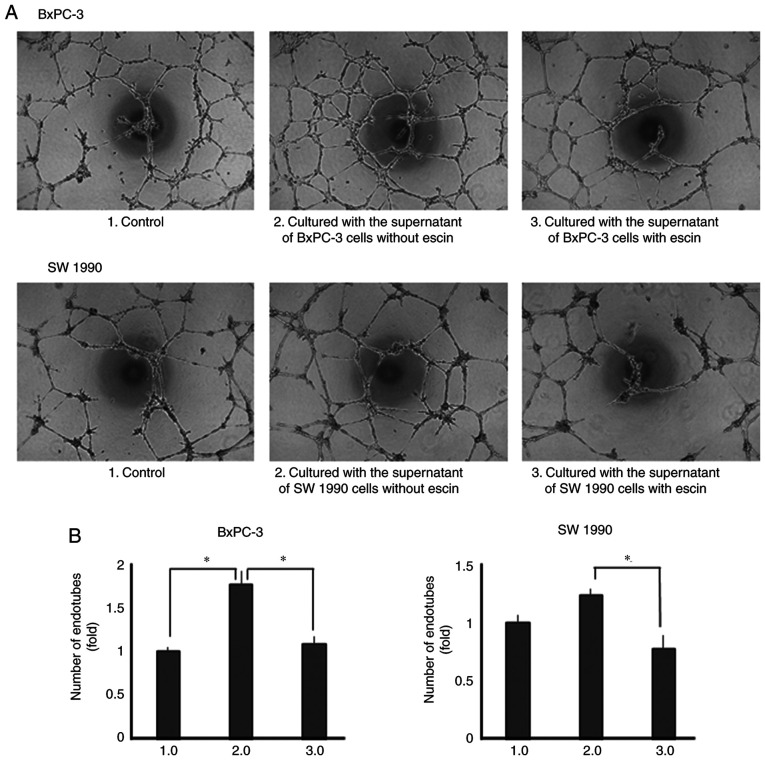
Escin inhibits tube formation in human endothelial cells
Lastly, the effects of escin on tube formation in human endothelial cells were determined. Tube formation was enhanced when the cells were incubated with recombinant IL-8 and VEGF (Fig. S3). Tube formation was also enhanced when incubated with the supernatants from the untreated PaCa cells. However, tube formation was significantly decreased when the cells were incubated with supernatants from escin-treated PaCa cells (Fig. 6A and B).
Figure 6.
Escin suppresses angiogenesis in the EA.hy 926 cell line using Matrigel. (A) Tube formation assays in the human endothelial cells using Matrigel under different conditions and representative images are shown (magnification, ×40). (B) Number of endotubes was counted and statistically analyzed between the different experimental groups. *P<0.05.
Discussion
The aim of the present study was to clarify whether escin, a natural compound extracted from horse chestnut, inhibited the angiogenesis of PaCa by blocking NF-κB activation. The results showed that escin suppressed cell proliferation and NF-κB activation, and reduced the secretion of VEGF and IL-8 in several human PaCa cell lines. In addition, escin suppressed human endothelial cell line tube formation induced by PaCa cell supernatants.
The progressive growth and metastasis of solid malignant tumors, including PaCa, depend on angiogenic factors released from the tumor and stromal cells (28). Various pro-angiogenic molecules, including VEGF and IL-8, secreted by PaCa cells have been identified and reported to mediate angiogenesis of PaCa (23,24,29). Our previous study demonstrated that the higher the potential of liver metastasis is, the more IL-8 is secreted in PaCa cell lines (30).
In a previous study, NF-κB was reported to induce the expression of various proteins (e.g. VEGF and IL-8) that are involved in cell survival, apoptosis, proliferation, metastasis and angiogenesis (31). Furthermore, ~70% of PaCa cells exhibit constitutive activation of NF-κB (32), and the constitutive activity of NF-κB in PaCa plays important roles in resistance to chemotherapy. Several reports have demonstrated that high activity of the NF-κB signaling pathway was involved in chemoresistance in PaCa (33–35).
In addition, our previous study demonstrated that suppressing the activation of NF-κB using the proteasome inhibitor MG132 blocked the production of pro-angiogenic molecules, such as VEGF and IL-8 (18). These findings were consistent with the results from the present study, that escin reduced the production of pro-angiogenic molecules, including VEGF and IL-8, by blocking NF-κB activation in PaCa cell lines. Therefore, it logically follows that inhibiting the production of these angiogenic factors by blocking NF-κB activity may suppress angiogenesis in PaCa. Recently, NF-κB inhibitors, such as bortezomib, have been administered to patients with multiple myeloma (36). Unfortunately, these inhibitors frequently have undesired side-effects, which prevent their widespread use (36). Thus, novel agents that suppress NF-κB activity with reduced toxicity are required.
The present study has focused on the effects of natural products, which are commonly considered safe and less toxic. It has been reported that several natural products, including curcumin, sesamin, zerumbone and escin, have anticancer effects (9,26,37,38). In PaCa, natural products exhibit anticancer effects by inhibiting NF-κB (39). In addition, a previous study showed that curcumin, which is a natural compound that also inhibits NF-kB, similar to escin, had a synergistic effect on tumor suppression when used in combination with gemcitabine (40). Therefore, it was hypothesized that escin also has a similar effect by blocking NF-κB. Furthermore, escin has already been widely used clinically to prevent inflammatory edema due to inflammation caused by trauma, such as surgery and fractures (41). Notably, orally administered escin has been shown to be safe, whereas injection of escin may cause phlebitis and allergies in animal models (42).
To the best of our knowledge, this is the first study associating escin with suppression of the production of angiogenic factors, such as VEGF and IL-8, by inhibiting NF-κB activity. When the study was started, it was hypothesized that escin might have anti-angiogenic effects by reducing the production of VEGF and IL-8 in the PaCa cells (at lower concentrations); however, direct pro-apoptotic effects on PaCa cells (at higher concentrations) were also found. At a concentration of 20 µM, escin inhibited cell proliferation in several PaCa cell lines. To eliminate the cytotoxicity of escin, all experiments were performed at concentrations of escin below its IC50. No significant cytotoxicity was observed at a concentration of 10 µM by WST-1 assays. It was found that 10 µM escin reduced TNF-α-induced NF-κB activation. It is conceivable that the pathway via which TNF induces the production of IL-8 includes not only NF-κB signaling, but also other pathways. For example, the TNF/TRAF2 axis induces not only NF-κB activity, but also AP-1 activity in PaCa (43). AP-1 also regulates IL-8 production in PaCa (44). In the present study, it was found that escin significantly suppressed IL-8 and VEGF production; however, it may be a partial suppression. Accordingly, it can be concluded from the results that by inactivating NF-κB, escin caused downregulation of VEGF and IL-8 production, affecting PaCa-induced angiogenesis.
PaCa is generally referred to as an ischemic tumor based on diagnostic imaging findings at the microscopic level; however, there are numerous reports showing the associations between microvessel density and the prognosis of PaCa (45–47). In fact, some reports have demonstrated the effectiveness of anti-angiogenic treatment for PaCa (48,49). Furthermore, it was previously demonstrated by our research group that new treatment targeting angiogenesis inhibited the tumor growth of PaCa in vivo (25,50). Thus, it was examined whether escin inhibited angiogenesis in human endothelial cells. The EA.hy 926 cell line is an immortalized endothelial cell line that has previously been used to estimate angiogenic potential (51). The results from the present study indicated that culturing EA.hy 926 cells with supernatants from the PaCa cell lines, which may contain VEGF and IL-8, enhanced the angiogenic potential of the EA.hy 926 cells; however, culturing the cells with supernatants from escin-treated PaCa cells, which may contain less VEGF and IL-8, suppressed angiogenic potential. To the best of our knowledge, no study has demonstrated the marked effects of escin on PaCa-induced angiogenesis.
In conclusion, the results from the present study indicated that a low concentration of escin inhibited angiogenesis by reducing the secretion of VEGF and IL-8 by suppressing NF-κB activation in PaCa. As escin did not affect the normal proliferating function of the cells, escin may be a safer and less toxic compound compared with other available treatments, such as gemcitabine. Therefore, escin may have important applications as an effective therapeutic agent for PaCa; however, further investigation is required, such as in vivo experiments, before escin can be used in a clinical setting. These animal experiments with nude mice will be performed in future investigations.
Supplementary Material
Acknowledgements
Not applicable.
Glossary
Abbreviations
- PaCa
pancreatic cancer
- NF-κB
nuclear factor-κB
- IL-8
interleukin-8
- VEGF
vascular endothelial growth factor
- RT-qPCR
reverse transcription-quantitative PCR
- IC50
half-maximal inhibitory concentration
Funding Statement
The current study was supported by Grants-in-Aid for Scientific Research (Japan Society for the Promotion of Science; grant no. 19K18157).
Funding
The current study was supported by Grants-in-Aid for Scientific Research (Japan Society for the Promotion of Science; grant no. 19K18157).
Availability of data and materials
The data generated or analyzed during this study are included in the published article.
Authors' contributions
KO and YM contributed to the conception and design of the study, analyzed and interpreted the data, and wrote and reviewed the manuscript. KO, YM, GU, YH, HI, KS, KT, MM, HT and ST designed the study. KO, YA, TK, YH, HI and GU acquired the data. KO, YH and YM confirm the authenticity of all the raw data. YA, TK, YH, MM and RO wrote the methods section of the manuscript. YM, HT, RO and ST provided technical support (advising on determining concentration and time) in performing the RT-PCR, immunocytochemistry and angiogenesis assays. YM and ST supervised the study. All authors read the final manuscript and are equally responsible for all aspects of the study, ensuring that the integrity or accuracy of all part of the study.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.National Cancer Center Japan, Center for Cancer Control and Information Services. https://ganjoho.jp/reg_stat/statistics/stat/summary.html 2020
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 3.Rawla P, Sunkara T, Gaduputi V. Epidemiology of pancreatic cancer: Global trends, etiology and risk factors. World J Oncol. 2019;10:10–27. doi: 10.14740/wjon1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao H, Le D, Yang LX. Current status in chemotherapy for advanced pancreatic adenocarcinoma. Anticancer Res. 2013;33:1785–1791. [PubMed] [Google Scholar]
- 5.Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul J, Gourgou-Bourgade S, de la Fouchardière C, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 6.Von Hoff DD, Ervin T, Arena FP, hiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torphy RJ, Fujiwara Y, Schulick RD. Pancreatic cancer treatment: Better, but a long way to go. Surg Today. 2020;50:1117–1125. doi: 10.1007/s00595-020-02028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z, Dabrosin C, Yin X, Fuster MM, Arreola A, Rathmell WK, Generali D, Nagaraju GP, El-Rayes B, Ribatti D, et al. Broad targeting of angiogenesis for cancer prevention and therapy. Semin Cancer Biol. 2015;35(Suppl 1):S224–S243. doi: 10.1016/j.semcancer.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta SC, Kim JH, Prasad S, Aggarwal BB. Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev. 2010;29:405–434. doi: 10.1007/s10555-010-9235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwak H, An H, Alam MB, Choi WS, Lee SY, Lee SH. Inhibition of migration and invasion in melanoma cells by β-escin via the ERK/NF-κB signaling pathway. Biol Pharm Bull. 2018;41:1606–1610. doi: 10.1248/bpb.b18-00251. [DOI] [PubMed] [Google Scholar]
- 11.Çiftçi GA, Işcan A, Kutlu M. Escin reduces cell proliferation and induces apoptosis on glioma and lung adenocarcinoma cell lines. Cytotechnology. 2015;67:893–904. doi: 10.1007/s10616-015-9877-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harford-Wright E, Bidère N, Gavard J. β-escin selectively targets the glioblastoma-initiating cell population and reduces cell viability. Oncotarget. 2016;7:66865–66879. doi: 10.18632/oncotarget.11784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rimmon A, Vexler A, Berkovich L, Earon G, Ron I, Lev-Ari S. Escin chemosensitizes human pancreatic cancer cells and inhibits the nuclear factor-kappaB signaling pathway. Biochem Res Int. 2013;2013:251752. doi: 10.1155/2013/251752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu J, Yu W, Liu B, Wang Y, Shao J, Wang J, Xia K, Liang C, Fang W, Zhou C, Tao H. Escin induces caspase-dependent apoptosis and autophagy through the ROS/p38 MAPK signalling pathway in human osteosarcoma cells in vitro and in vivo. Cell Death Dis. 2017;8:e3113. doi: 10.1038/cddis.2017.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang YW, Wang SJ, Zhou YN, Pan SH, Sun B. Escin augments the efficacy of gemcitabine through down-regulation of nuclear factor-κB and nuclear factor-κB-regulated gene products in pancreatic cancer both in vitro and in vivo. J Cancer Res Clin Oncol. 2012;138:785–797. doi: 10.1007/s00432-012-1152-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harikumar KB, Sung B, Pandey MK, Guha S, Krishnan S, Aggarwal BB. Escin, a pentacyclic triterpene, chemosensitizes human tumor cells through inhibition of nuclear factor-kappaB signaling pathway. Mol Pharmacol. 2010;77:818–827. doi: 10.1124/mol.109.062760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pramanik K, Makena M, Bhowmick K, Pandey M. Advancement of NF-κB signaling pathway: A novel target in pancreatic cancer. Int J Mol Sci. 2018;19:3890. doi: 10.3390/ijms19123890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuo Y, Sawai H, Ochi N, Yasuda A, Sakamoto M, Takahashi H, Funahashi H, Takeyama H, Guha S. Proteasome inhibitor MG132 inhibits angiogenesis in pancreatic cancer by blocking NF-kappaB activity. Dig Dis Sci. 2010;55:1167–1176. doi: 10.1007/s10620-009-0814-4. [DOI] [PubMed] [Google Scholar]
- 19.Bussolino F, Mantovani A, Persico G. Molecular mechanisms of blood vessel formation. Trends Biochem Sci. 1997;22:251–256. doi: 10.1016/S0968-0004(97)01074-8. [DOI] [PubMed] [Google Scholar]
- 20.Sosmitha G, Bano S, Javadi M, Lu F, Clarissa EH, Frank A, Kwang Seok A, Gautam S, Ajaikumar BK. Zerumbone as an anti-cancer agent. Molecules. 2019;24:734. doi: 10.3390/molecules24040734. [DOI] [Google Scholar]
- 21.Meteoglu I, Erdogdu IH, Meydan N, Erkus M, Barutca S. NF-KappaB expression correlates with apoptosis and angiogenesis in clear cell renal cell carcinoma tissues. J Exp Clin Cancer Res. 2008;27:53. doi: 10.1186/1756-9966-27-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Itakura J, Ishiwata T, Friess H, Fujii H, Matsumoto Y, Büchler MW, Korc M. Enhanced expression of vascular endothelial growth factor in human pancreatic cancer correlates with local disease progression. Clin Cancer Res. 1997;3:1309–1316. [PubMed] [Google Scholar]
- 23.Shi Q, Abbruzzese JL, Huang S, Fidler IJ, Xiong Q, Xie K. Constitutive and inducible interleukin 8 expression by hypoxia and acidosis renders human pancreatic cancer cells more tumorigenic and metastatic. Clin Cancer Res. 1999;5:3711–3721. [PubMed] [Google Scholar]
- 24.Shi Q, Le X, Abbruzzese JL, Peng Z, Qian CN, Tang H, Xiong Q, Wang B, Li XC, Xie K. Constitutive Sp1 activity is essential for differential constitutive expression of vascular endothelial growth factor in human pancreatic adenocarcinoma. Cancer Res. 2001;61:4143–4154. [PubMed] [Google Scholar]
- 25.Saito K, Matsuo Y, Imafuji H, Okubo T, Maeda Y, Sato T, Shamoto T, Tsuboi K, Morimoto M, Takahashi H, et al. Xanthohumol inhibits angiogenesis by suppressing nuclear factor-κB activation in pancreatic cancer. Cancer Sci. 2018;109:132–140. doi: 10.1111/cas.13441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsuboi K, Matsuo Y, Shamoto T, Shibata T, Koide S, Morimoto M, Guha S, Sung B, Aggarwal BB, Takahashi H, Takeyama H. Zerumbone inhibits tumor angiogenesis via NF-κB in gastric cancer. Oncol Rep. 2014;31:57–64. doi: 10.3892/or.2013.2842. [DOI] [PubMed] [Google Scholar]
- 27.Bustin S. Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): Trends and problems. J Mol Endocrinol. 2002;29:23–39. doi: 10.1677/jme.0.0290023. [DOI] [PubMed] [Google Scholar]
- 28.Folkman J. Angiogenesis and angiogenesis inhibition: An overview. Exs. 1997;79:1–8. doi: 10.1007/978-3-0348-9006-9_1. [DOI] [PubMed] [Google Scholar]
- 29.Brown LF, Berse B, Jackman RW, Tognazzi K, Manseau EJ, Senger DR, Dvorak HF. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in adenocarcinomas of the gastrointestinal tract. Cancer Res. 1993;53:4727–4735. [PubMed] [Google Scholar]
- 30.Sawai H, Funahashi H, Okada Y, Matsuo Y, Sakamoto M, Yamamoto M, Takeyama H, Manabe T. Interleukin-1alpha enhances IL-8 secretion through p38 mitogen-activated protein kinase and reactive oxygen species signaling in human pancreatic cancer cells. Med Sci Monit. 2005;11:BR343–BR350. [PubMed] [Google Scholar]
- 31.Aggarwal BB. Nuclear factor-kappaB: The enemy within. Cancer Cell. 2004;6:203–208. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Xiong HQ, Abbruzzese JL, Lin E, Wang L, Zheng L, Xie K. NF-kappaB activity blockade impairs the angiogenic potential of human pancreatic cancer cells. Int J Cancer. 2004;108:181–188. doi: 10.1002/ijc.11562. [DOI] [PubMed] [Google Scholar]
- 33.Arlt A, Schäfer H. NFkappaB-dependent chemoresistance in solid tumors. Int J Clin Pharmacol Ther. 2002;40:336–347. doi: 10.5414/CPP40336. [DOI] [PubMed] [Google Scholar]
- 34.Sebens S, Arlt A, Schäfer H. NF-kappaB as a molecular target in the therapy of pancreatic carcinoma. Recent Results Cancer Res. 2008;177:151–164. doi: 10.1007/978-3-540-71279-4_17. [DOI] [PubMed] [Google Scholar]
- 35.Holcomb B, Yip-Schneider M, Schmidt CM. The role of nuclear factor kappaB in pancreatic cancer and the clinical applications of targeted therapy. Pancreas. 2008;36:225–235. doi: 10.1097/MPA.0b013e31815b3207. [DOI] [PubMed] [Google Scholar]
- 36.Chen D, Frezza M, Schmitt S, Kanwar J, Dou QP. Bortezomib as the first proteasome inhibitor anticancer drug: Current status and future perspectives. Curr Cancer Drug Targets. 2011;11:239–253. doi: 10.2174/156800911794519752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aggarwal BB, Van Kuiken ME, Iyer LH, Harikumar KB, Sung B. Molecular targets of nutraceuticals derived from dietary spices: Potential role in suppression of inflammation and tumorigenesis. Exp Biol Med (Maywood) 2009;234:825–849. doi: 10.3181/0902-MR-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harikumar KB, Sung B, Tharakan ST, Pandey MK, Joy B, Guha S, Krishnan S, Aggarwal BB. Sesamin manifests chemopreventive effects through the suppression of NF-kappaB-regulated cell survival, proliferation, invasion, and angiogenic gene products. Mol Cancer Res. 2010;8:751–761. doi: 10.1158/1541-7786.MCR-09-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yue Q, Gao G, Zou G, Yu H, Zheng X. Natural products as adjunctive treatment for pancreatic cancer: Recent trends and advancements. Biomed Res Int. 2017;2017:8412508. doi: 10.1155/2017/8412508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kunnumakkara AB, Guha S, Krishnan S, Diagaradjane P, Gelovani J, Aggarwal BB. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res. 2007;67:3853–3861. doi: 10.1158/0008-5472.CAN-06-4257. [DOI] [PubMed] [Google Scholar]
- 41.Gallelli L. Escin: A review of its anti-edematous, anti-inflammatory, and venotonic properties. Drug Des Devel Ther. 2019;13:3425–3437. doi: 10.2147/DDDT.S207720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li M, Lu C, Zhang L, Zhang J, Du Y, Duan S, Wang T, Fu F. Oral administration of escin inhibits acute inflammation and reduces intestinal mucosal injury in animal models. Evid Based Complement Alternat Med. 2015;2015:503617. doi: 10.1155/2015/503617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trauzold A, Röder C, Sipos B, Karsten K, Arlt A, Jiang P, Martin-Subero JI, Siegmund D, Müerköster S, Pagerols-Raluy L, et al. CD95 and TRAF2 promote invasiveness of pancreatic cancer cells. FASEB J. 2005;19:620–622. doi: 10.1096/fj.04-2984fje. [DOI] [PubMed] [Google Scholar]
- 44.Sclabas GM, Fujioka S, Schmidt C, Li Z, Frederick WA, Yang W, Yokoi K, Evans DB, Abbruzzese JL, Hess KR, et al. Overexpression of tropomysin-related kinase B in metastatic human pancreatic cancer cells. Clin Cancer Res. 2005;11:440–449. [PubMed] [Google Scholar]
- 45.Stipa F, Lucandri G, Limiti MR, Bartolucci P, Cavallini M, Di Carlo V, D'Amato A, Ribotta G, Stipa S. Angiogenesis as a prognostic indicator in pancreatic ductal adenocarcinoma. Anticancer Res. 2002;22:445–449. [PubMed] [Google Scholar]
- 46.Benckert C, Thelen A, Cramer T, Weichert W, Gaebelein G, Gessner R, Jonas S. Impact of microvessel density on lymph node metastasis and survival after curative resection of pancreatic cancer. Surg Today. 2012;42:169–176. doi: 10.1007/s00595-011-0045-0. [DOI] [PubMed] [Google Scholar]
- 47.Amin Z, Theis B, Russell RC, House C, Novelli M, Lees WR. Diagnosing pancreatic cancer: The role of percutaneous biopsy and CT. Clin Radiol. 2006;61:996–1002. doi: 10.1016/j.crad.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 48.Annese T, Tamma R, Ruggieri S, Ribatti D. Angiogenesis in pancreatic cancer: Pre-clinical and clinical studies. Cancers (Basel) 2019;11:381. doi: 10.3390/cancers11030381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Z, Ji S, Zhang B, Liu J, Qin Y, Xu J, Yu X. Role of angiogenesis in pancreatic cancer biology and therapy. Biomed Pharmacother. 2018;108:1135–1140. doi: 10.1016/j.biopha.2018.07.042. [DOI] [PubMed] [Google Scholar]
- 50.Matsuo Y, Raimondo M, Woodward TA, Wallace MB, Gill RA, Tong Z, Burdick MD, Yang Z, Strieter RM, Hoffman RM, Guha S. CXC-chemokine/CXCR2 biological axis promotes angiogenesis in vitro and in vivo in pancreatic cancer. Int J Cancer. 2009;125:1027–1037. doi: 10.1002/ijc.24383. [DOI] [PubMed] [Google Scholar]
- 51.Yang HL, Chang HC, Lin SW, Kumar KJS, Liao CH, Wang HM, Lin KY, Hseu YC. Antrodia salmonea inhibits TNF-α-induced angiogenesis and atherogenesis in human endothelial cells through the down-regulation of NF-κB and up-regulation of Nrf2 signaling pathways. J Ethnopharmacol. 2014;151:394–406. doi: 10.1016/j.jep.2013.10.052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated or analyzed during this study are included in the published article.