Abstract
The current global novel coronavirus disease 2019 (COVID-19) pandemic threatens to derail the uptake of human papillomavirus (HPV) vaccination in low- and lower-middle income countries with major disruptions to routine immunization and the introduction of new vaccines delayed. This has a major impact on the World Health Organization cervical cancer elimination strategy, where it is dependent on HPV vaccination as well as cervical cancer screening and treatment. We discuss current opportunities and barriers to achieve high uptake of HPV vaccination in low- and lower-middle income countries as well as the impact of COVID-19. Implementation of 4 key recommendations for HPV vaccination in low- and lower-middle income countries is needed: increased global financial investment; improved vaccine supply and accelerated use of a single-dose schedule; education and social marketing; and adoption of universal school-based delivery. With the commitment of the global health community, the adoption of these strategies would underpin the effective elimination of cervical cancer.
Cervical cancer is the fourth most common cancer in women worldwide, with 570 000 cases each year causing 311 000 deaths, mostly in low- and lower-middle income countries (LLMICs) (1, 2). It is caused by chronic infection with oncogenic genotypes of human papillomavirus (HPV), but unlike most cancers, it is largely preventable by vaccination. In 2018, the director-general of the World Health Organization (WHO) made a pledge to eliminate cervical cancer as a public health problem within the next century. Elimination is defined as an incidence of less than 4 cases per 100 000 women-years. Clear targets that have been set by 2030 to achieve the “elimination goal” include the following: 90% of girls to be immunized by 15 years of age; 70% of women between 35 and 45 years old to be screened at least once in a lifetime with a proficiency test; and 90% of women with high-grade cervical lesions or cervical cancer being treated (3).
Whereas some high-income countries (HICs) such as the United Kingdom and Australia are on track to eliminate cervical cancer within the next decade as a result of high HPV vaccine coverage rates and robust screening programs as well as access to treatment for precancerous and early stage cancerous lesions (4,5), the situation in LLMICs is far less optimistic. Of the 131 countries that have introduced the HPV vaccine (Romania, Lesotho, and Kazakhstan have since stopped; Japan suspended) (6), less than 20% of countries are LLMICs. Furthermore, 70% of the target global population of 9- to 14-year-old girls currently live in a country without an HPV immunization program (7). More than 44 million women will be diagnosed with cervical cancer in the next 50 years if primary and secondary prevention programs are not implemented in LLMICs (8). Although strategies to prevent, screen, and treat are necessary for cervical cancer elimination, primary prevention of HPV infection through HPV immunization in LLMICs is likely to have the greatest long-term impact and represents the most feasible approach to permanently reduce the global cervical cancer burden.
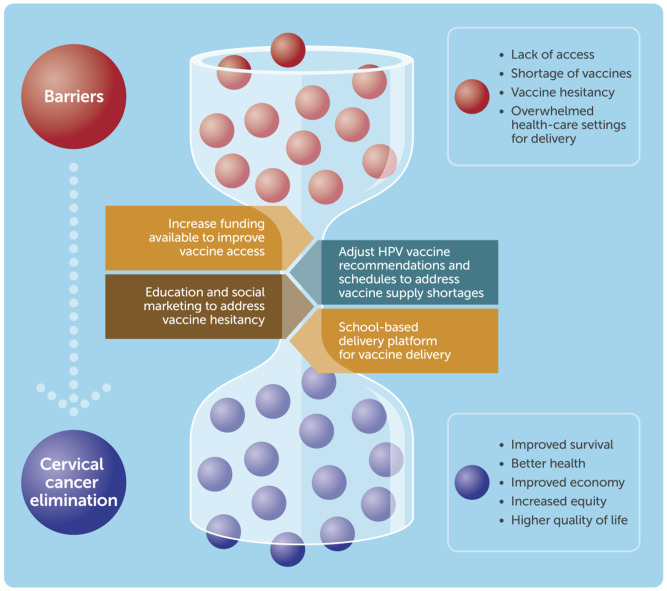
There has been a growing global momentum toward the introduction of the HPV vaccine in LLMICs. As of 2020, 52 LLMICs have conducted pilot or demonstration projects, and almost half have introduced HPV vaccine in their national program in the last 5 years (6). Bhutan and Rwanda were 2 of the first LLMICs to successfully introduce HPV vaccine, achieving high vaccine coverage (>90%) in the target population, demonstrating the feasibility of a successful HPV immunization program in LLMICs. Another 41 countries and territories are projected to introduce HPV vaccine in their national schedule by the end of 2023 (6). However, the uptake of HPV vaccination in LLMICs following successful pilot programs, as well as in those countries unable to introduce HPV vaccine in the near future, was already dependent on overcoming major barriers such as high vaccine and programmatic costs, HPV vaccine shortages, vaccine hesitancy, and suitable vaccine delivery strategies (Figure 1).
Figure 1.
Overcoming the barriers in the uptake of human papillomavirus (HPV) vaccination in low- and lower-middle-income countries
On March 11, 2020, the WHO declared novel coronavirus disease 2019 (COVID-19) a global pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The outbreak was first identified in Wuhan, China, in December 2019. As of October 7, 2020, more than 35 million cases and 1 million deaths due to COVID-19 have been reported in more than 188 countries and territories (9). Unprecedented public health and social measures (ie, closure of international borders, movement restrictions within countries, and partial or complete closure of schools and businesses, as well as strict quarantine practices) have been implemented across the globe to curb the spread of the virus and prevent overwhelming the health-care systems.
The COVID-19 pandemic has had profound impact on communities, causing disruptions to many essential health services, including the provision of routine immunization services (10). These are due to factors such as transport interruptions, health workers being unavailable because of safety and health concerns, travel restrictions, or redeployment to COVID-19 response duties, as well as a lack of personal protective equipment (PPE) (10). The restrictions on movement, or fear of being exposed to people with COVID-19, have also seen people reluctant to leave home to get immunized (10). It is estimated that 80 million children under the age of 1 year in at least 68 countries will miss out on essential vaccines, predisposing them to severe diseases (11). Gavi, the Vaccine Alliance, is a partnership of public and private sectors that help improve access to new and underused vaccines for the most vulnerable children in low-income countries (gross domestic product < US$3000). It is estimated that at least 24 million children and adolescents living in 21 Gavi-supported lower-income countries are also at risk of missing out on life-saving vaccines such as HPV vaccine because of postponed campaigns and delayed introduction of new vaccines (11). By June 2020, 45 out of 68 Gavi-supported vaccine introductions have already been affected by shipment delays and low vaccine stocks (10). Modeled data suggest that the deaths prevented by sustaining routine childhood immunization in Africa far outweigh the excess risk of COVID-19 deaths associated with vaccination clinic visits, especially for the vaccinated children (12). This is likely to be true for HPV vaccination, where the pandemic is exacerbating the barriers and threatening the progress already made toward HPV vaccine introduction in LLMICs over the last decade. Therefore, proactive deployment of intervention strategies is urgently needed by the global health community. In this viewpoint, we discuss the major barriers to HPV vaccination in LLMICs, the effects of COVID-19, and the strategies now needed to increase the uptake of HPV vaccination.
Barriers to HPV Vaccine Uptake and Impact of COVID-19 Pandemic
High Vaccine and Programmatic Costs
High vaccine and programmatic costs (including vaccine cold chain storage, staff, and transport of large quantities of vaccines) are major barriers to access HPV vaccine in many LLMICs. In the last 10 years, Gavi has enabled access to subsidized HPV vaccine (20% of the Gavi purchase price, currently around $4.50 per dose) and supported HPV vaccine programs for the poorest countries in the world. In 2016, Gavi initiated an accelerated HPV vaccine program where an estimated 40 million girls from Gavi-eligible countries were expected to receive the vaccine by 2020, thereby averting 900 000 deaths (13). However, this number was reduced to 14 million because of the increased global demand and subsequent shortage of HPV vaccine (see below; HPV Vaccine Supply). Although Gavi provides substantial resources to kick-start the HPV vaccine program, sustainable financing mechanisms and the commitment from governments are necessary for these countries as they graduate from Gavi status in the coming years to maintain their HPV immunization program. In most cases, the cost of HPV vaccine is critical. This is where financial support for vaccine procurement and/or price negotiation with vaccine manufacturers from international health agencies such as the Pan American Health Organization, Program for Appropriate Technology in Health, United Nations International Children's Emergency Fund (UNICEF), and the Gulf Cooperation Council become extremely important for countries planning to introduce HPV vaccine nationally but are not eligible for Gavi funding (ie, middle-income countries).
The COVID-19 pandemic has restricted vaccine access (not limited to HPV) in many LLMICs. The economic impact of COVID-19 has led to a global recession, which will undoubtedly lead to reduced national income and health budgets, increased public and private debt, and reduced access to vaccines and vaccine delivery services. Access to HPV vaccine, like other vaccines, will be even more challenging than before, and the recession threatens to roll back the hard-won progress that LLMICs have made in recent decades. Some LLMICs however, such as Lao People’s Democratic Republic and Uganda, have continued routine immunization services in the face of COVID-19 through support from Gavi and UNICEF, government leadership, and community engagement (14,15). Specifically, the support from global health leaders in vaccine procurement, PPE provision, diagnostics, training and communication campaigns, and most importantly, strong support and advocacy by government ministries is crucial in the continuation of immunization services in response to the COVID-19 pandemic. Lessons learned from these countries would help facilitate vaccine implementation in some of the poorest countries and most remote communities for the current pandemic and future outbreaks. Indeed, some of these countries (ie, Nepal, Niger, and Syria), with the help of UNICEF, have been able to continue immunization services for children (16). One of the highest global priorities now is to restore immunization services, including HPV vaccination, provided it is safe to do so with strict public health measures in place (ie, use of PPE and physical distancing).
Emerging evidence suggests that a single dose of HPV vaccine is immunogenic and sufficient for protection against HPV infection and potentially HPV-associated diseases over the long term (17‐21). Although considerable investment is still required for delivery of a single-dose HPV vaccine program, it remains highly cost-effective compared with other health interventions, and substantial cost-savings over the long-term will be achieved because of reductions in cervical cancer treatment costs (22,23). Formal randomized controlled trials are underway to examine a single-dose HPV schedule, and results from these studies are greatly anticipated within the next 5 years (24). The provision of a single dose of HPV vaccine during these difficult times may help bridge the issues of vaccine cost and logistical constraints. A second dose may be administered at a later time if needed, in the period following this pandemic.
HPV Vaccine Supply
Because of the increased global demand of HPV vaccine (more than double the demand in 2018 compared with 2017) as a result of more publicly funded programs and a move toward gender-neutral vaccination and multi-age cohort catch-up campaigns, there is now a global shortage of HPV vaccine (25). Many LLMICs now have to delay the introduction of the HPV vaccine. For example, in 2018, the Ethiopian government had planned for a multi-age cohort HPV vaccine national introduction (9-14 years old) but were only able to vaccinate 14-year-old girls because of the global vaccine shortage (Mulholland K, personal communication). Although the vaccine manufacturers are currently expanding their production including building new facilities to meet global demand, this still requires long lead times (up to 4 years), including the necessary regulatory approvals (26). Production is not expected to meet demand until 2024 at the earliest. Reallocating vaccines from one country to another is also complicated and unlikely given differing regulatory requirements across countries.
It is uncertain if the COVID-19 pandemic has affected the timeline of vaccine production to meet global demand. At the recent Global Vaccine Summit in May 2020, HPV vaccine manufacturers (existing and new) committed to provide sufficient supply of HPV vaccines for Gavi-supported countries by 2025, enabling 84 million girls much-needed access to this vaccine (27). Although this positive news may not necessarily provide immediate relief during the pandemic, countries where routine immunization of HPV vaccine has been disrupted can initiate plans for future catch-up campaigns to adolescents who may have missed HPV vaccination during the COVID-19 pandemic. In the longer term, access to HPV vaccines will also improve with the development of new, low-cost, and quicker-to-produce HPV vaccines that are expected to come onto the market over the next few years. One of these is a bivalent HPV vaccine (Cecolin®, Xiamen Innovax Biotech CO., LTD., China) recently licensed in China, and a further 2 candidates are currently in phase III clinical trials (a bivalent vaccine from Walvax Biotechnology Co. Ltd, China, and a quadrivalent vaccine from Serum Institute of India, India) (28).
Ensuring sufficient supply of HPV vaccines and rollout of targeted catch-up programs will be key to ensure the most vulnerable populations are not predisposed to cervical cancer and other HPV-associated diseases. The use of single-dose HPV vaccine schedules or delaying the second dose of vaccine by at least 2 to 3 years once the results of the single-dose trials are available would alleviate the vaccine supply issue (26). Female-only HPV programs should be the priority during this period, with WHO (Strategic Advisory Group of Experts on Immunization Committee) recommending temporary suspension of gender-neutral and multibirth cohort vaccination programs, although this may be difficult in countries with an established HPV vaccine program. These recommendations are endorsed by the International Papillomavirus Society (IPVS), the preeminent society for basic science, clinical, and public health research on papillomavirus (26). Temporary suspension of gender-neutral vaccination programs is unlikely to have a major impact on the cervical cancer elimination strategy, although the changing recommendation for established gender-neutral programs may cause public confusion. It is therefore important to highlight that girls in LMICs, where cervical cancer burden is highest, are most in need of the vaccine but currently do not have access to it. Vaccinating girls will have the greatest impact on the global burden of cervical cancer because cervical cancer is the most important disease caused by HPV. A catch-up vaccination program for boys may be offered to accelerate cervical cancer elimination and prevent other HPV-associated diseases when vaccine supply meets demand (29). Such drastic changes in the current thinking are needed if we are to meet the challenge of cervical cancer elimination within the time frame, against the backdrop of COVID-19.
Community Awareness and Attitudes Toward HPV Vaccine
Public knowledge and awareness of HPV-associated diseases and HPV vaccination are crucial for vaccine acceptance among adolescents and their parents. This is particularly true in many LLMICs as well as some HICs, where vaccine uptake is low even in regions where HPV vaccine is available. For example, in the United States, the vaccine coverage in girls for a 3, 2, and 1 dose of HPV vaccine is 63%, 52%, and 42%, respectively (30). This low coverage is partly because of the vaccine being given in private health clinics as part of their national immunization program (ie, opportunistic and the need for reimbursement costs), increasing HPV-vaccine specific hesitancy with inconsistent recommendation by health-care providers, and gaps in parents’ knowledge around HPV vaccination (31‐33). A systematic review of 16 studies conducted in sub-Saharan Africa found low levels of knowledge and awareness of HPV, HPV vaccine, and cervical cancer, yet a high level of willingness to be vaccinated (34).
Vaccine hesitancy has been flagged as one of the top 10 threats to global health by the WHO. Similar to vaccines such as for measles, it has had a dramatic effect on HPV vaccine uptake and coverage in some countries (35). HPV vaccine hesitancy has surfaced based on unfounded claims that HPV vaccines are unsafe and promote promiscuity (36,37). Misinformation through stories from social and traditional media and conversations about people who believed they were harmed by HPV vaccine has also led to parents declining timely vaccination (38). Vaccine-hesitant parents also cite other issues such as their child being too young, low risk of a child getting HPV infection, and mistrust of vaccines (39‐41). Many studies analyzing large HPV vaccination datasets, as well as WHO and US Centers for Disease Control and Prevention, have found the vaccine to be safe (42,43). Despite this, HPV vaccine coverage in Japan has plummeted from 70% to less than 1% since 2013 because of unconfirmed reports of severe adverse events following HPV vaccination (44). Recent modeling data suggest that this crisis is estimated to result in a further 5000 cervical cancer deaths based on the current screening coverage of 30%-40% (45). A pilot HPV vaccine introduction program in Mongolia, where the cervical cancer burden is one of the highest in Asia (46), was also disrupted because of unsubstantiated negative claims about the vaccine, resulting in one-third of target population missing out (47). It was only after years of strong advocacy from health professionals and researchers, as well as quality local data on the effectiveness of HPV vaccine, that the Mongolian government is now considering introducing the vaccine nationally (47,48).
Education around the benefits of HPV vaccination and understanding the link between HPV infection and HPV diseases are essential in improving vaccine acceptance. The cultural context and current social norms in a given country or community also need to be considered to allay any anxiety; for example, in ethnic minority populations, cultural sensitivities regarding beliefs and values of sexual activity may result in reluctance to receive HPV vaccine (49). Language barriers can also make the informed consent process difficult (50). These barriers are not insurmountable, and educational interventions targeted toward adolescents, parents, and health providers can be effective approaches to improve knowledge and willingness to be vaccinated (51‐54). In addition, an opt-out approach for the vaccine program may also improve vaccine coverage. The success of these strategies has not only been seen in HICs but also led to increased rates of HPV vaccine uptake and broad acceptance in LLMICs such as India, Peru, Uganda, Vietnam, and Fiji (55‐57). Global partners such as WHO and the United Nations (UN) Population Fund, UN Women (United Nations Entity for Gender Equality and the Empowerment of Women), and the IPVS as well as local health practitioners who are generally well regarded and trusted in providing health recommendations continue to educate and advocate for HPV immunization. For school-based delivery programs, teachers and principals as well as the Ministry of Education will need to communicate the benefits of HPV vaccination.
Education is essential but not sufficient to combat vaccine hesitancy. Governments and health policy makers play an important role by implementing policies to improve vaccine uptake (ie, cash transfer incentives), as well as working with media platforms (including social media), to quell any anti-vaccine misinformation, by providing and showing only credible, science-based information (35). Increasing transparency in policy-making decisions related to vaccinations by focusing on safety and trust issues is one way to improve public awareness and confidence (58).
In the current COVID-19 pandemic, the acute uncertainty around the virus and the massive influx of misinterpreted, manipulated, and malicious information have caused an “infodemic” problem, with rising anxiety and conspiracy theories among the general public (59). Recent surveys of up to 2200 individuals in France and the United States suggest that 1 in 4 will not use a vaccine against SARS-CoV-2 when it becomes available (60,61), and only 30% of people would be willing to receive the vaccine soon after it becomes available (61). Surprisingly, more than one-third of those who would refuse vaccination were among low-income people (who are generally more exposed to infectious diseases) and were young women (aged 18-35 years, who play a crucial role regarding childhood vaccination) (60). These individuals are also most likely to be hesitant about routine child and adolescent vaccination, which is a major concern for public health. Additional intervention research is needed to improve vaccine acceptance for COVID-19 vaccine and also all other vaccines in the routine immunization program.
There are more than 180 COVID-19 vaccines in development. The success or failure of any COVID-19 vaccine is likely to be crucial for vaccination acceptance more broadly. A safe and effective COVID-19 vaccine will improve vaccine confidence in many people and may lead to higher overall vaccine uptake. However, a failed vaccine, one in which there are major postlicensure safety signals, will risk public backlash with devastating consequences for other COVID-19 vaccines as well as routine childhood vaccines, including HPV (62). Like with HPV vaccine, communications and trust in the government and its health systems are going to be crucial in countering vaccine hesitancy and any conspiracy theories. The public health community will also have an important role in assisting families and communities to distinguish between facts and conspiracy theories around COVID-19 and vaccination in general (59). This includes designing information materials that specifically address concerns around COVID-19 and educational campaigns regarding vaccination using both traditional and social media platforms to advise on the benefits and risks, as well as targeting misinformation (61).
Delivery Strategies for HPV Vaccine
The target population for HPV immunization is young adolescents (younger than 15 years of age) receiving 2 or potentially even 1 dose of HPV vaccine. High vaccine coverage is key to ensuring the success of any HPV vaccine program. School-based programs generally yield higher coverage than health center delivery in both HICs and LLMICs (63,64). The 3-dose coverage rate in Bhutan dropped from 99% to around 68% when the country switched from a school-based program to routine delivery through health centers; the government subsequently reverted back to a school-based program and achieved greater than 90% coverage (65). Although school attendance is generally lower in LLMICs, particularly as adolescents grow older, the final year of primary school may be considered an ideal time for vaccination to minimize the need to capture out-of-school children, thereby maximizing coverage. Modeled data have found an increased benefit of HPV vaccination among 9-year-old girls as compared with previous estimates of cervical cancer burden, amounting to a further 51% increase in cervical cancer deaths averted, with the greatest benefit in the WHO African region (66). Primary school completion rates among LLMICs are relatively high, at around 62% for sub-Saharan Africa and 88% for South Asia, giving confidence that a primary school-based program would reach most girls (67), although community outreach programs are still crucial in capturing out-of-school girls. School-based HPV vaccine programs need to be driven by government sectors including the departments of health and education to ensure high vaccine coverage and success of the program.
Incorporating HPV immunization with other school and adolescent health programs, as well as the co-administration of other adolescent vaccines, can also alleviate logistical constraints (68). There is also the possibility of vaccinating younger children (younger than 9 years old) and infants if it works, because the coverage of childhood vaccinations (ie, Hepatitis B, polio) is generally high, and co-administration of vaccines can also reduce health-services burden in LLMICs (40). This might negate some of the stigmatism and vaccine hesitancy associated with HPV vaccination during adolescence (ie, childhood vaccination to prevent adult cancer). Furthermore, in many LLMICs, child marriage and/or child sexual abuse still occur at an alarming rate, with serious impact in life (ie, early pregnancy, mental health, disrupted education, and limiting opportunities for career and vocational advancement). Approximately 15% of girls were married by the age of 15 years in West and Central Africa (69), and a study conducted in Cambodia, Haiti, Kenya, Malawi, Swaziland, Tanzania, and Zimbabwe found that more than 25% of children and adolescents had experienced some form of sexual violence (70). Therefore, offering HPV vaccination to younger children (younger than 9 years old) may actually protect a large proportion of girls in LLMICs who are at an increased risk of cervical cancer (71). A phase III randomized controlled trial in the Gambia is currently ongoing to assess the immunogenicity of 9-valent HPV (9vHPV) in girls aged 4 to 8 years (41).
During the COVID-19 pandemic, school closures have been widespread with disruption of routine and/or catch-up immunizations, including HPV, tetanus, diphtheria, and pertussis, that are normally given at schools. School closures also have had an indirect impact on health care, with health-care workers unavailable to work because of the need to care for their children (72). Catch-up immunization campaigns and community outreach programs will therefore be needed to ensure children and adolescents are protected from vaccine-preventable diseases. A multi-age cohort HPV vaccination may be able to overcome the delayed vaccine introduction or uptake as long as the vaccine access and supply issues are addressed.
Uptake of HPV Vaccination After COVID-19
The COVID-19 pandemic is causing disruptions to livelihoods and threatening years of progress in health, education, and life opportunities for children and adolescents worldwide. This is particularly true for immunization programs, where this pandemic has exposed gross inequalities in health. For HPV vaccination in particular, this is likely to lead to heightened inaccessibility to the vaccine in many LLMICs, which will have major consequences. The indirect effect of economic downturns as a result of COVID-19 will expose many families to extreme poverty that may lead to increased risk of early marriage and early sexual activity in LLMICs. Further, food insecurity and starvation might trigger migration and vulnerability of girls (eg, pressures to sell sex in some places). These factors all heighten risks for sexually transmitted infections and HPV before any catch-up vaccine becomes available.
In this context of escalating sexual and reproductive health risks for girls, the uptake of HPV vaccination in LLMICs is a pressing agenda (Figure 1). The following recommendations address the major barriers to HPV vaccination that have worsened as a result of the pandemic.
Increase the funding available for HPV vaccines to address high vaccine and programmatic costs. This requires commitments from both governments and the donor community with the engagement of multilateral organizations such as WHO and alliances such as Gavi, Pan American Health Organization, Program for Appropriate Technology in Health, United Nations, UNICEF, and the World Bank.
Adjust HPV vaccine recommendations and schedules to address HPV vaccine supply shortages. This requires accelerated progress to a single-dose regime while awaiting results from randomized controlled trials (anticipated in the next few years), delay in the second vaccine dose, a focus on female-only vaccination in single-age cohorts in countries planning to introduce HPV vaccine, and fast-track of WHO prequalification process for new HPV vaccine candidates.
Provide education and social marketing of the HPV vaccine in local contexts to address low community awareness and unfavorable attitudes toward HPV vaccine. National governments, with the support of UN agencies, and working with young people and their families, should develop strategies to educate and address vaccine hesitancy and fears, as well as increase awareness about HPV and HPV-associated diseases.
Make schools the primary delivery platform for the delivery of the HPV vaccine. For most countries, retention rates to the end of primary school are very high so that school-based vaccination could provide close to universal coverage. For those countries with lower retention rates, complementary community-based delivery will be needed.
Conclusions
Although a comprehensive approach of prevent, screen, and treat is needed to eliminate cervical cancer within the lifetime of today’s girls, HPV vaccination will have the greatest long-term impact. The tools to achieve this are within the reach of the global health community. Taking HPV vaccination to scale presents an unparalleled opportunity for health gain while working toward one of the Sustainable Development Goals 2030 (to reduce premature mortality from noncommunicable diseases by one-third through prevention and treatment). Furthermore, HPV vaccination prevents not only a majority of cervical cancers but also a large proportion of other HPV-related anogenital cancers (ie, vulva, vagina, penile, and anal) and some oropharyngeal cancers, as well as anogenital warts. With the pandemic, gains in HPV vaccine from the global health community and governments are pressing. A failure to act will lead to countless avoidable cases of HPV-associated disease and deaths as well as consuming billions of treatment dollars that could otherwise be diverted to health problems where prevention is not yet possible.
Funding
This work was supported by Victorian Government’s Medical Research Operational Infrastructure Support Program. FMR, SMG, GCP, and PVL are supported by Australian National Health and Medical Research Council fellowships.
Notes
Role of the funders: The funders had no role in the writing or submission of the article for publication.
Author contributions: ZQT, GCP, and PVL conceptualized the idea and wrote the original draft. All authors reviewed and edited the article.
Disclosures: SMG has received grants through her institution from Merck and has delivered lectures and received speaking fees from MSD for work performed in her personal time. All other authors report no conflicts of interest.
Acknowledgements: We thank Bill Reid from the Royal Children’s Hospital Creative Studio for assistance with the figure.
Data Availability
No new data were generated or analyzed in support of this research.
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2. Arbyn M, Weiderpass E, Bruni L, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. 2020;8(2):e191–e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eliminating cervical cancer. Editorial. Lancet. 2020;395(10221):312. [DOI] [PubMed] [Google Scholar]
- 4. Torjesen I. HPV vaccine: high coverage could eradicate cervical cancer within decades, say researchers. BMJ. 2019;365:l4450. [DOI] [PubMed] [Google Scholar]
- 5. Hall MT, Simms KT, Lew JB, et al. The projected timeframe until cervical cancer elimination in Australia: a modelling study. Lancet Public Health. 2019;4(1):e19–e27. [DOI] [PubMed] [Google Scholar]
- 6. PATH. Global HPV Vaccine Introduction Overview. https://path.azureedge.net/media/documents/Global_HPV_Vaccine_Intro_Overview_Slides_webversion_2020May.pdf; May 4, 2020. Accessed July 23, 2020.
- 7. UNICEF WHO. Progress and Challenges with Achieving Universal Immunization Coverage. https://www.who.int/immunization/monitoring_surveillance/who-immuniz.pdf; July 15, 2020. Accessed July 23, 2020.
- 8. Simms KT, Steinberg J, Caruana M, et al. Impact of scaled up human papillomavirus vaccination and cervical screening and the potential for global elimination of cervical cancer in 181 countries, 2020-99: a modelling study. Lancet Oncol. 2019;20(3):394–407. [DOI] [PubMed] [Google Scholar]
- 9. John Hopkins University. Coronavirus Resource Center. https://coronavirus.jhu.edu/map.html. Accessed August 30 2020.
- 10. Gavi. Overview of COVID-19 Situation in GAVI-Supported Countries and GAVI’s Response 30th June 2020. https://www.gavi.org/vaccineswork/30-june-2020-overview-covid-19-situation-gavi-supported-countries-gavi-response; June 30, 2020. Accessed July 23, 2020.
- 11. World Health Organization. Atleast 80 Million Children Under One At Risk of Diseases such as Diphtheria, Measles and Polio as COVID-19 Disrupts Routine Vaccination Efforts, Warn Gavi, WHO and UNICEF. https://www.who.int/news-room/detail/22-05-2020-at-least-80-million-children-under-one-at-risk-of-diseases-such-as-diphtheria-measles-and-polio-as-covid-19-disrupts-routine-vaccination-efforts-warn-gavi-who-and-unicef; May 22, 2020. Accessed July 23, 2020.
- 12. Abbas K, Procter SR, van Zandvoort K, et al. Routine childhood immunisation during the COVID-19 pandemic in Africa: a benefit-risk analysis of health benefits versus excess risk of SARS-CoV-2 infection. Lancet Glob Health. 2020;8(10):e1264–e1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gavi. Human Papillomavirus Vaccine Support. https://www.gavi.org/types-support/vaccine-support/human-papillomavirus. Accessed September 12, 2019.
- 14. World Health Organization. Lao Health System Continues to Offer Immunization Services Despite COVID-19 Pandemic. https://www.who.int/laos/news/detail/24-04-2020-lao-health-system-continues-to-offer-immunization-services-despite-covid-19-pandemic; April 24, 2020. Accessed September 1, 2020.
- 15. UNICEF. Despite COVID-19 Challenges, Uganda Receives Polio and Pentavalent Vaccines. https://www.unicef.org/uganda/press-releases/despite-covid-19-challenges-uganda-receives-polio-and-pentavalent-vaccines; April 24, 2020. Accessed September 1, 2020.
- 16. UNICEF. The superheroes delivering vaccines when it’s more important than ever before. https://www.unicef.org.au/blog/unicef-in-action/november-2020/in-pictures-vaccines-during-covid19; November 16, 2020. Accessed December 7, 2020.
- 17. Markowitz LE, Naleway AL, Klein NP, et al. Human papillomavirus vaccine effectiveness against HPV infection: evaluation of one, two, and three doses. J Infect Dis. 2020;221(6):910–918. [DOI] [PubMed] [Google Scholar]
- 18. Brotherton JM, Budd A, Rompotis C, et al. Is one dose of human papillomavirus vaccine as effective as three? A national cohort analysis. Papillomavirus Res. 2019;8:100177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kreimer AR, Struyf F, Del Rosario-Raymundo MR, et al. Efficacy of fewer than three doses of an HPV-16/18 AS04-adjuvanted vaccine: combined analysis of data from the Costa Rica Vaccine and PATRICIA trials. Lancet Oncol. 2015;16(7):775–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Basu P. Efficacy of a single dose human papillomavirus vaccine–findings from the Indian Cohort Study. In: International Papillomavirus Conference (virtual); July 20-24, 2020.
- 21. Kreimer AR, Sampson JN, Porras C, et al. Evaluation of durability of a single-dose of the bivalent HPV vaccine: the CVT Trial. J Natl Cancer Inst. 2020;112(10):1038–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Burger EA, Campos NG, Sy S, Regan C, Kim JJ.. Health and economic benefits of single-dose HPV vaccination in a Gavi-eligible country. Vaccine. 2018;36(32):4823–4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Horton S, Gelband H, Jamison D, Levin C, Nugent R, Watkins D.. Ranking 93 health interventions for low- and middle-income countries by cost-effectiveness. PLoS One. 2017;12(8):e0182951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kreimer AR, Herrero R, Sampson JN, et al. Evidence for single-dose protection by the bivalent HPV vaccine-Review of the Costa Rica HPV vaccine trial and future research studies. Vaccine. 2018;36(32):4774–4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. World Health Organization. Global Market STUDY HPV. https://www.who.int/immunization/programmes_systems/procurement/mi4a/platform/module2/WHO_HPV_market_study_public_summary.pdf; September 2018. Accessed March 26, 2020.
- 26. Garland SM, Stanley M, Giuliano AR, et al. IPVS statement on “Temporary HPV vaccine shortage: implications globally to achieve equity.” Papillomavirus Res. 2020;9:100195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gavi. HPV vaccine manufacturers commit to provide enough supply to immunise at least 84 million girls in Gavi countries. https://www.gavi.org/news/media-room/hpv-vaccine-manufacturers-commit-provide-enough-supply-immunise-least-84-million; June 3, 2020. Accessed June 9, 2020.
- 28. UNICEF. Human Papillomavirus Vaccine: Supply and Demand Update. https://www.unicef.org/supply/reports/human-papillomavirus-hpv-vaccine-supply-and-demand-update; October 2020. Accessed December 7, 2020.
- 29. Elfstrom KM, Lazzarato F, Franceschi S, Dillner J, Baussano I.. Human papillomavirus vaccination of boys and extended catch-up vaccination: effects on the resilience of programs. J Infect Dis. 2016;213(2):199–205. [DOI] [PubMed] [Google Scholar]
- 30. Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(33):850–858. [DOI] [PubMed] [Google Scholar]
- 31. Chuang E, Cabrera C, Mak S, Glenn B, Hochman M, Bastani R.. Primary care team- and clinic level factors affecting HPV vaccine uptake. Vaccine. 2017;35(35):4540–4547. [DOI] [PubMed] [Google Scholar]
- 32. Bednarczyk RA, Ellingson MK, Omer SB.. Human papillomavirus vaccination before 13 and 15 years of age: analysis of national immunization survey teen data. J Infect Dis. 2019;220(5):730–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stokley S, Jeyarajah J, Yankey D, et al. ; Centers for Disease Control and Prevention (CDC). Human papillomavirus vaccination coverage among adolescents, 2007-2013, and postlicensure vaccine safety monitoring, 2006-2014–United States. MMWR Morb Mortal Wkly Rep. 2014;63(29):620–624. [PMC free article] [PubMed] [Google Scholar]
- 34. Perlman S, Wamai RG, Bain PA, Welty T, Welty E, Ogembo JG.. Knowledge and awareness of HPV vaccine and acceptability to vaccinate in sub-Saharan Africa: a systematic review. PLoS One. 2014;9(3):e90912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vaccine hesitancy: a generation at risk. Editorial. Lancet Child Adolesc Health .2019;3(5):281. [DOI] [PubMed] [Google Scholar]
- 36. Grimes RM, Benjamins LJ, Williams KL.. Counseling about the HPV vaccine: desexualize, educate, and advocate. J Pediatr Adolesc Gynecol. 2013;26(4):243–248. [DOI] [PubMed] [Google Scholar]
- 37. Nicol AF, Andrade CV, Russomano FB, Rodrigues LL, Oliveira NS, Provance DW Jr.. HPV vaccines: a controversial issue? Braz J Med Biol Res. 2016;49(5):e5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Margolis MA, Brewer NT, Shah PD, Calo WA, Gilkey MB.. Stories about HPV vaccine in social media, traditional media, and conversations. Prev Med. 2019;118:251–256. [DOI] [PubMed] [Google Scholar]
- 39. Cunningham-Erves J, Koyama T, Huang Y, et al. Providers’ perceptions of parental human papillomavirus vaccine hesitancy: cross-sectional study. JMIR Cancer. 2019;5(2):e13832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Guignard A, Praet N, Jusot V, Bakker M, Baril L.. Introducing new vaccines in low- and middle-income countries: challenges and approaches. Expert Rev Vaccines. 2019;18(2):119–131. [DOI] [PubMed] [Google Scholar]
- 41. Clarke E (Medical Research Council, London School of Hygiene and Tropical Medicine). HPV Vaccination in Africa–new delivery schedules alias the HANDS HPV vaccine trial (HPV). https://clinicaltrials.gov/ct2/show/NCT03832049; January 27, 2020. Accessed May 28, 2020.
- 42. Global Advisory Committee on Vaccine Safety, 30 November - 1 December 2016. Wkly Epidemiol Rec. 2017;92(2):13–20. [PubMed] [Google Scholar]
- 43. Markowitz LE, Gee J, Chesson H, Stokley S.. Ten years of human papillomavirus vaccination in the United States. Acad Pediatr. 2018;18(2):S3–S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hanley SJ, Yoshioka E, Ito Y, Kishi R.. HPV vaccination crisis in Japan. Lancet. 2015;385(9987):2571. [DOI] [PubMed] [Google Scholar]
- 45. Simms KT, Hanley SJB, Smith MA, Keane A, Canfell K.. Impact of HPV vaccine hesitancy on cervical cancer in Japan: a modelling study. Lancet Public Health. 2020;5(4):e223-e34. [DOI] [PubMed] [Google Scholar]
- 46. Chimed T, Sandagdorj T, Znaor A, et al. Cancer incidence and cancer control in Mongolia: results from the National Cancer Registry 2008-12. Int J Cancer. 2017;140(2):302–309. [DOI] [PubMed] [Google Scholar]
- 47. Batmunkh T, von Mollendorf C, Tulgaa K, et al. HPV genoprevalence and HPV knowledge in young women in Mongolia, five years following a pilot 4vHPV vaccination campaign. Papillomavirus Res. 2019;8:100175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Batmunkh T, Dalmau MT, Munkhsaikhan ME, et al. A single dose of quadrivalent human papillomavirus (HPV) vaccine is immunogenic and reduces HPV detection rates in young women in Mongolia, six years after vaccination. Vaccine. 2020;38(27):4316–4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Batista Ferrer H, Trotter CL, Hickman M, Audrey S.. Barriers and facilitators to uptake of the school-based HPV vaccination programme in an ethnically diverse group of young women. J Public Health .2016;38(3):569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Howard N, Mounier-Jack S, Gallagher KE, et al. The value of demonstration projects for new interventions: the case of human papillomavirus vaccine introduction in low- and middle-income countries. Hum Vacc Immunother. 2016;12(9):2475–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liu CR, Liang H, Zhang X, et al. Effect of an educational intervention on HPV knowledge and attitudes towards HPV and its vaccines among junior middle school students in Chengdu, China. BMC Public Health. 2019;19(1):488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Perkins RB, Zisblatt L, Legler A, Trucks E, Hanchate A, Gorin SS.. Effectiveness of a provider-focused intervention to improve HPV vaccination rates in boys and girls. Vaccine. 2015;33(9):1223–1229. [DOI] [PubMed] [Google Scholar]
- 53. Dixon BE, Zimet GD, Xiao S, et al. An educational intervention to improve HPV vaccination: a cluster randomized trial. Pediatrics. 2019;143(1):e20181457. [DOI] [PubMed] [Google Scholar]
- 54. Reiter PL, Katz ML, Paskett ED. HPV vaccination among adolescent females from Appalachia: implications for cervical cancer disparities. Cancer Epidemiol Biomarkers Prev. 2012;21(12):2220–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. LaMontagne DS, Barge S, Le NT, et al. Human papillomavirus vaccine delivery strategies that achieved high coverage in low- and middle-income countries. Bull World Health Organ. 2011;89(11):821–830B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wadhera P, Evans JL, Stein E, et al. ; on behalf of the YWHS Collaborative. Human papillomavirus knowledge, vaccine acceptance, and vaccine series completion among female entertainment and sex workers in Phnom Penh, Cambodia: the Young Women’s Health Study. Int J STD Aids. 2015;26(12):893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. La Vincente SF, Mielnik D, Jenkins K, et al. Implementation of a national school-based human papillomavirus (HPV) vaccine campaign in Fiji: knowledge, vaccine acceptability and information needs of parents. BMC Public Health. 2015;15(1):1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Patel PR, Berenson AB.. Sources of HPV vaccine hesitancy in parents. Vaccines. 2013;9(12):2649–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Larson HJ. A call to arms: helping family, friends and communities navigate the COVID-19 infodemic. Nat Rev Immunol. 2020;20(8):449–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. COCONEL Group. A future vaccination campaign against COVID-19 at risk of vaccine hesitancy and politicisation. Lancet Infect Dis. 2020;20(7):769–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Schaffer DeRoo S, Pudalov NJ, Fu LY.. Planning for a COVID-19 vaccination program. JAMA. 2020;323(24):2458. [DOI] [PubMed] [Google Scholar]
- 62. Harrison EA, Wu JW.. Vaccine confidence in the time of COVID-19. Eur J Epidemiol. 2020;35(4):325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wigle J, Coast E, Watson-Jones D.. Human papillomavirus (HPV) vaccine implementation in low and middle-income countries (LMICs): health system experiences and prospects. Vaccine. 2013;31(37):3811–3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Toh ZQ, Licciardi PV, Russell FM, Garland SM, Batmunkh T, Mulholland EK.. Cervical cancer prevention through HPV vaccination in low- and middle-income countries in Asia. Asian Pac J Cancer Prev. 2017;18(9):2339–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Dorji T, Tshomo U, Phuntsho S, et al. Introduction of a national HPV vaccination program into Bhutan. Vaccine. 2015;33(31):3726–3730. [DOI] [PubMed] [Google Scholar]
- 66. Abbas KM, van Zandvoort K, Brisson M, Jit M.. Effects of updated demography, disability weights, and cervical cancer burden on estimates of human papillomavirus vaccination impact at the global, regional, and national levels: a PRIME modelling study. Lancet Glob Health .2020;8(4):e536–e544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. UNICEF. Education overview. https://data.unicef.org/topic/education/overview/; October 2019. Accessed March 4, 2020.
- 68. Noronha AS, Markowitz LE, Dunne EF.. Systematic review of human papillomavirus vaccine coadministration. Vaccine. 2014;32(23):2670–2674. [DOI] [PubMed] [Google Scholar]
- 69. UNICEF. Child marriage. https://data.unicef.org/topic/child-protection/child-marriage/; April 2020. Accessed July 31, 2020.
- 70. Sumner SA, Mercy AA, Saul J, et al. ; Centers for Disease Control and Prevention. Prevalence of sexual violence against children and use of social services–seven countries. MMWR Morb Mortal Wkly Rep. 2015;64(21):565–569. 2015 [PMC free article] [PubMed] [Google Scholar]
- 71. Garland SM, Subasinghe AK, Jayasinghe YL, et al. HPV vaccination for victims of childhood sexual abuse. Lancet. 2015;386(10007):1919–1920. [DOI] [PubMed] [Google Scholar]
- 72. Viner RM, Russell SJ, Croker H, et al. School closure and management practices during coronavirus outbreaks including COVID-19: a rapid systematic review. Lancet Child Adolesc Health. 2020;4(5):397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analyzed in support of this research.