Abstract
Coronavirus disease (COVID-19) is a global pandemic. The COVID-19 outbreak caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has overloaded healthcare systems that need medication to be rapidly established, at least to minimize the incidence of COVID-19. The coinfection with other microorganisms has drastically affected human health. Due to the utmost necessity to treat the patient infected with COVID-19 earliest, poor diagnosis and misuse of antibiotics may lead the world where no more drugs are available even to treat mild infections. Besides, sanitizers and disinfectants used to help minimize widespread coronavirus infection risk also contribute to an increased risk of antimicrobial resistance. To ease the situation, zinc supplements’ potentiality has been explored and found to be an effective element to boost the immune system. Zinc also prevents the entry of the virus by increasing the ciliary beat frequency. Furthermore, the limitations of current antiviral agents such as a narrow range and low bioavailability can be resolved using nanomaterials, which are considered an important therapeutic alternative for the next generation. Thus, the development of new antiviral nanoagents will significantly help tackle many potential challenges and knowledge gaps. This review paper provides profound insight into how COVID-19 and antimicrobial resistance (AMR) are interrelated and the possible implications and current strategies to fight the ongoing pandemic.
Keywords: COVID-19, antimicrobial resistance, nanomaterials, immune system, antiviral agents
Graphical abstract
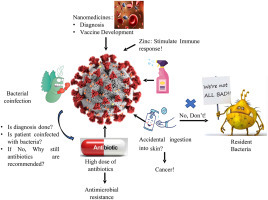
1. Introduction
The ongoing pandemic has clinically affected more than 113 million lives worldwide; by February 2021, India is on to the second most-affected country after the United States. Nearly 89 million of those infected have recovered, more than 2 million have died, and about 21 million are still battling the infection [1]. This pandemic is primarily caused by a coronavirus belonging to the family of Corona viridae. These family members are enveloped positive-sense and single-stranded RNA viruses that have caused mild respiratory diseases in the human population. However, in some cases, they may cause severe infections, including the central nervous system, and gastrointestinal and respiratory disorders [2,3].
There are four genera of Coronaviruses (CoVs): alpha, beta, gamma, and delta. The β-CoVs are further classified into four subgroups: A, B, C, and D. Two alpha and four β-CoVs strains are known that can infect humans. The four major structural proteins of β-CoVs are spike (S), envelope (E), membrane (M), and nucleocapsid (N) protein depicted in Fig. 1 . They range in size from 26 to 32 kb, the largest known viral RNA genome [4]. The latest 2019 Coronavirus Diseases (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is closely linked to SARS-CoV and is 79.5% identical to the genomic sequence [5,6]. SARS-CoV-2 is spherical and has spike-like glycoproteins (S proteins) protruding from the surface of the virion that plays a vital role in the virus binding to the host cell receptor and also mediates the fusion of the host cell membrane with the virus. The S protein subdomains S1 and S2 are responsible for initial attachment to the host cell receptor and fusion with the cell membrane. The S1 subdomain is divergent across the receptor-binding domain (RBD) coronaviruses, while S2 is more conserved and has the fusion machinery that allows the virus to reach the host cell [6].
Fig. 1.
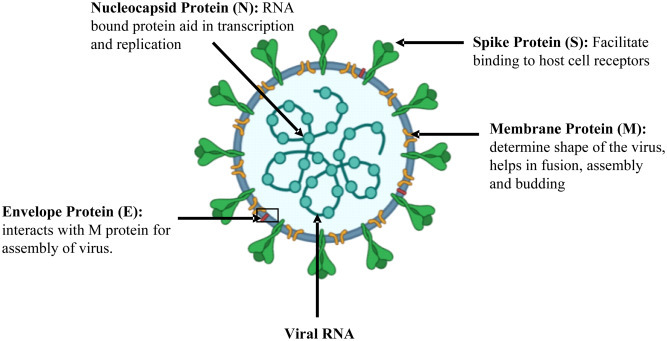
Schematic diagram of SARS-CoV-2 virus structure, component, and its role in virus propogation.
Human health, economic, and social status have been seriously affected by the SARS-CoV-2 virus. Coronaviruses are responsible for 10–15% of acute respiratory diseases (ARD) [7]. Epidemiological surveillance indicates that coronavirus infections in adults and coinfections may be more frequent [8]. With the start of the COVID-19 pandemic, the use of hand sanitizers has been dramatically increased, undoubtedly having a positive impact on people's hygiene. On the other hand, this practice can lead to selecting resistant bacteria and ultimately contribute even more to an increased risk of antimicrobial resistance (AMR) [9]. Pidot et al. have studied antimicrobial resistance in Enterococcus faecium against alcohol-based hand sanitizers. Researchers have conducted the test in 139 hospital isolates from 1997 to 2015 and found that the isolates of E. faecium were ten times more resistant to alcohol than the untreated ones after 2010 [10]. Similar findings from other parts of the world were found due to the overuse of alcohol-based hand sanitizer. Escherichia coli and Pseudomonas aeruginosa were reported to be 48% and 64% resistant to all available sanitizers on the market, respectively [11]. It is well known that the misuse and overuse of antibiotics in medicinal and agricultural have put the world in an alarming situation where there are no new antibiotics to treat the present and extremely drug-resistant bacterial infections. Despite COVID-19 being a viral infection, antibiotics are commonly used at a rising pace to support and prevent infections. This review paper focuses on providing deep insight into how COVID-19 and AMR are an interlinked and present focus areas working to combat the ongoing pandemic.
2. COVID-19 and coinfections! How severe is it?
Coinfections with other microorganisms such as bacteria, fungi, and other viruses are commonly associated with respiratory viral infections. Coinfections are directly associated with a rise in morbidity and mortality rates, requiring early diagnosis and specific treatment. For example, most deaths were due to coinfections during the influenza epidemic in 1918, with other bacteria mainly due to Streptococcus pneumonia [12]. Still, the occurrence of SARS-CoV-2 bacterial coinfections is not well defined, and there is a knowledge gap in the understanding and lack of evidence to support the bacteria–virus association [13]. Given the abundant clinical evidence backed by mechanistic research in animal models, this knowledge gap is puzzling, showing that respiratory viral infections predispose patients to bacterial coinfections. Moreover, most deaths were due to associated bacterial infection in the 1918 flu pandemic. Similar observations were made during the last three twentieth-century pandemic influenza: the 1957 H2N2, the 1968–1969 H3N2, and the 2009–2010 H1N1 disease outbreaks [14,15].
Bengoechea and Bamford envision three nonmutually exclusive bacterial/SARS-CoV-2 coinfection scenarios: secondary SARS-CoV-2 following bacterial infection/colonization; combined viral/bacterial pneumonia; secondary bacterial “super-infection” after SARS-CoV-2 depicted in Fig. 2 [15]. Highly qualitative and time-dependent, underlying mechanisms of these scenarios involve dynamic interactions among three (virus, host, and bacteria) different entities. However, it is evident that in a combination of viral/bacterial pneumonia, the SARS-CoV-2 immune response is probably altered. They completely postulate that any coinfection situation would eventually exacerbate the clinical outcome severity of COVID-19. SARS-CoV-2 may strengthen bacterial colonization and attachment to the host tissue, and the combined infections may lead to increased degradation and pathophysiology of the tissue. Systemic transmission of the virus/bacterial copathogens can be facilitated by airway dysfunction, cytopathology, and tissue degradation caused by SARS-CoV-2 or bacterial coinfection, significantly raising blood infection risk. Virus-mediated bacterial infection improvement is not uncommon. The airway epithelium invasion by respiratory pathogens is increased by rhinovirus and influenza virus infections [16].
Fig. 2.
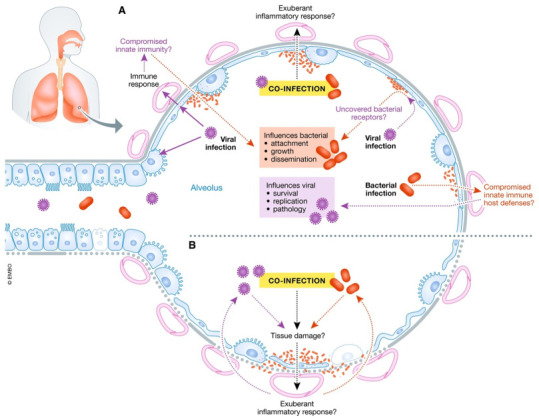
The relationship between SARS-CoV-2 in coinfections, bacteria, and the host. (A) The virulence factors of SARS-CoV-2 interact with the lungs and elicit an immune response. These interactions can compromise innate immunity at several levels, leading to increased bacterial attachment, growth, and dissemination. Bacterial receptors mediating bacterial attachment can be discovered by a viral infection. An exuberant inflammatory response can result from coinfection. It is also possible that the form of SARS-CoV-2-induced immune response can allow bacteria to thrive in the lungs. On the other hand, SARS-CoV-2 infection may be predisposed to bacterial colonization because the innate immune host defenses can be downregulated, allowing survival, development, and pathology of the virus. (B) Coinfection may aggravate tissue damage, and exuberant inflammatory response may further aggravate SARS-CoV-2-induced lung damage. Reproduced with permission from ref. [15]. Copyright 2020 Published under the terms of the CC BY 4.0 license.
The exponentially rising concern of SARS-CoV-2 has put everything else in the shade, including the ever-growing AMR, the global issue. Even after this pandemic, problems will persist, which may be exacerbated if proper measures are not taken to treat and recommend antibiotics. A current study on COVID-19 has reported 3.5% of bacterial coinfections and nearly 14% of secondary infections. The bacterial coinfection rate varies with the patient's populations and the criticality of the diseases. The majority of the affected people with COVID-19 have preliminary symptoms of high fever, cough, and shortness of breath. Despite COVID-19 being a viral infection, the doctor has to prescribe the antibiotics since high fever, radiological infiltrates, and cough are hallmarks of bacterial acquired pneumonia, which required immediate antibiotic treatment. Even patients with mild symptoms of COVID-19 had been prescribed azithromycin along with hydroxychloroquine despite the lack of evidence supporting the efficacy of these drugs [12]. The urgency of treatment, absence of antiviral drugs with proven efficacy, and uncertainty about COVID-19 infection have worsened the situation.
3. COVID-19 and AMR
The effect of COVID-19 on AMR is one dimension that required requisite attention. The SARS-CoV-2 outbreak is internationally superimposed on the ongoing multidrug-resistant bacteria pandemic. According to the National Institute for Health and Care Excellence (NICE) guidelines for treating severe pneumonia, patients will be given broad-spectrum antibiotics (amoxicillin or doxycycline) as an alternative in the United Kingdom regardless of cause. About 15 to 50% of bacterial isolates from a blood sample are resistant to as a minimum one antimicrobial group and collective resistance to various antimicrobial groups in most European countries, building it probable that broad pragmatic antibiotic treatment would have a limited impact on hospital-acquired infections [17,18]. Almost all COVID-19 severe patients, in this case, antibiotics treatment may have minimal effect. Ominously, clinical evidence also indicates that insufficient broad-spectrum scientific utilization of antibiotics may be correlated, at least in sepsis cases with higher mortality [19].
Unluckily, a considerable rise in AMR by the extensive utilization of antibiotics in patients with COVID-19 as the pandemic continues. Intensive care units (ICUs) are epicenters for AMR growth, even in a typical scenario. With an already elevated prevalence of multidrug-resistant strains in certain hospital environments, this could have catastrophic implications [20]. Multidrug-resistant bacteria are also transmitting SARS-CoV-2 in hospitals, contributing to a rise in death due to the insufficient armory of antibiotics for hospital-acquired infections treatment. After surgery, transplantation, or chemotherapy, the treatment of patients may be significantly affected. The transmission of AMR to the community should not be ignored and the direct effect on the healthcare environment. The more considerable amount of released antimicrobials from hospital wastewater leads to increased antimicrobial in the ecosystem, impacting resistance levels in animals (animal feed and wildlife) and agricultural and natural systems [15,21,22]. All in all, particularly in these difficult times, antibiotic stewardship standards should not be relaxed. It is important to immediately determine a need for antibiotic treatment and avoid it if not needed. Ideally, if antibiotics are essential, the microbiology lab should suggest which ones are most suitable based on the microorganism and the resistance pattern [23].
The improved utilization of hand sanitizers and antibacterial soaps as protection against SARS-CoV-2 is one final factor to consider. Although do not argue against this practice, it emphasizes that they may contain extra chemicals that do not affix safety but may boost antimicrobial bacterial resistance instead. To develop resistance against disinfectants, bacteria manipulate efflux pumps, and these same efflux pumps lead to AMR [24]. In any event, to prevent the selection of bacteria with increased tolerance/resistance to antimicrobials, the public must adhere to the manufacturer's instructions for proper use.
4. Case studies
Zhu et al. showed that among 257 COVID-19 patients used in the study, 243 (94.2%) were coinfected [25]. Bacterial coinfection was dominant when tested for 39 test microorganisms, including bacteria, viruses, and fungi, using pathogen-specific real-time PCR techniques. S. pneumoniae, followed by Klebsiella pneumoniae and Haemophilus influenzae, were the most widespread. However, they are conditional pathogenic but are pathogenic if the immune response of COVID-19 infected patients is not successful or decreased. Patients were divided into four groups, of which 8.5% were asymptomatic cases, 30.4% were mild cases, 54.5% were moderate cases, and 6.6% were severe cases. Among the extreme groups, the highest rates of coinfection were found with bacteria and fungi. Therefore, simultaneous screening for other respiratory pathogens is required while testing SARS-CoV-2for proper treatment and diagnosis.
He et al. studied both COVID-19 positive and negative pneumonia patients to examine bacterial pathogen coinfection [26]. The 2216 patients were diagnosed with pneumonia based on chest X-ray, blood routine, and blood biochemistry tests. Out of 2216, 292 patients were COVID-19 positive based on the RT-PCR technique. For the analysis, 194 were selected out of 292 COVID-19 positive pneumonia patients, and 212 chosen out of 1924 COVID-19 negative pneumonia patients. Bordetella pertussis infection rate in COVID-19 positive patients was 10.31%, significantly higher than 4.25% in COVID-19 negative patients. Mycoplasma pneumonia and Moraxella catarrhalis were only found in COVID-19 negative pneumonia patients. B. pertussis enhances the pathogen's transmission while coughing and also cause various respiratory disorders that require extensive care in treating COVID-19 patients. Pseudomonas aeruginosa was present in both COVID-19 positive and negative patients in significantly higher number compared to S. pneumoniae, B. pertussis, Streptococcus pyogenes, Staphylococcus aureus, Neisseria meningitides, H. influenzae, M. pneumonia, and M. catarrhalis. P. aeruginosa is an opportunistic pathogen responsible for some pulmonary diseases. That is why it is necessary to prevent P. aeruginosa infection while treating COVID-19 patients. Nearly 50% of COVID-19 patients were coinfected with bacterial pathogens. When one or several bacteria infect and harm the upper respiratory tract, the other bacteria become prone to quickly infect the area, complicating the diagnosis and treatment efficiency. As a result, certain precautions should be taken to treat coinfection with common bacterial pathogens, especially B. pertussis [26].It is essential to explain and further validate why B. pertussis increased in COVID-19 positive patients in the next study.
A retrospective analysis of the clinical and microbiological characteristics of 140 COVID-19 patients admitted to a German university hospital between February and April 2020, focusing on bacterial coinfections and antimicrobial therapy, was conducted by Rotheand their group [27]. According to the implemented local ABS guidelines, the most widely used antibiotic regimen was ampicillin/sulbactam (41.5%) with a median period of 6 (range 1–13) days. In all cases, urinary antigen tests were negative for Legionella pneumophila and Streptococcus peumoniae. Coinfections with Enterobacterales (34.0%) and Aspergillus fumigates (18.0%) have been found in critically ill patients admitted to intensive care units (n = 50). Blood cultures obtained at admission showed a 4.2% diagnostic yield. In COVID-19 patients, bacterial and fungal coinfections are rare and are primarily prevalent in critically ill patients. To avoid antimicrobial overuse, more studies are required to examine the effect of antimicrobial therapy on clinical results in COVID-19 patients.
5. Risk factors associated
COVID-19 can affect anyone and cause symptoms from the asymptomatic carrier or mildly affected to severe, resulting in multiple organ failure. The preexistence of medical conditions commonly called risk factors is more likely to be at higher risks. According to CDC report, October 2020, the potential risk factors associated with the severity of the diseases are cancer, hypertension, thalassemia, cerebrovascular disease, asthma (moderate-to-severe), cystic fibrosis, neurologic conditions, pulmonary fibrosis, immune-compromised state, chronic kidney disease, liver disease, type 1 and 2 diabetes mellitus, chronic obstructive pulmonary disease (COPD), sickle cell disease, severe obesity (BMI ≥ 40 kg/m2). Table 1 depicts the list of risk factors for COVID-19.
Table 1.
Preexisting risk comorbidities associated with change in levels of biological markers.
| Preexisting risk comorbidities | Signs and symptoms | Clinical factors |
Lifestyle and demographic factors | Reference | |
|---|---|---|---|---|---|
| Increased level | Decreased level | ||||
| Hypertension Cardiovascular Diseases COPD Diabetes Malignancy Chronic gastritis and gastric ulcers Chronic Kidney Abnormal liver function HIV infection Disease Cerebrovascular diseases |
Fever and Fatigue Dry cough Anorexia Dyspnea Gastrointestinal Myalgia Diarrhoea Nausea Dizziness Headache Vomiting Abdominal pain |
Neutrophil counts d-dimer level Leukocytes C-Reactive protein White blood cell count Creatine kinase level Blood Urea nitrogen Aspartate aminotransferase Alanine aminotransferase IL-6 |
Platelets Procalcitonin Lymphocytes Eosinophils Haemoglobin Albumin |
Higher age Smoking Male gender |
[[28], [29], [30], [31]] |
Momtazmanesh et al. analyzed data of 54 studies to identify: (i) the prevalence of newly developed and preexisting cardiovascular disorders, hypertension, diabetes mellitus; (ii) the possible increase in cardiac and inflammatory biomarkers; and (iii) the correlation of diseases understudy with disease severity and mortality. The study found that the heart was the second most affected organ by COVID-19 after the lungs relative to the liver and kidneys. More than one-fourth of the patients developed Cardiovascular disease (CVD) and arrhythmia, which raised the mortality rate 20 times. Patients with preexisting heart disease had an 8-fold mortality rate and almost 3.5-fold need for admission to the ICU [32]. In other studies, the data of COVID-19 pregnant women with COVID-19 during late pregnancy and their neonates were examined [33]. COVID-19 enhanced placenta inflammatory responses, and possible organ dysregulation and coagulation disorders were observed in fetuses and neonates.
Moreover, the IL-6 level was elevated in COVID-19 patients and was about 2.9 times higher in complicated conditions than noncomplicated patients. The IL-6 release is associated with cytokine release syndrome, which is a systemic inflammation response. The recombinant humanized monoclonal antibody, tocilizumab against the IL-6 receptor, could be a potentially effective treatment in COVID-19 severe cases [25,34,35].
6. Current focused area to fight the COVID-19
The unexpected outburst of the COVID-19 (coronavirus disease) all over the world was a devastating pandemic. Limited medication or vaccine is available to inhibit the novel coronavirus. Therefore, the best method to control the increasing infection rate is to avoid exposure to the virus [11,36]. The overall picture is depicted in Fig. 3 . The government and health sectors from all over the world have led down specific preventive measures to prevent the spread of this virus, namely country lockdown, isolate itself, self-quarantine, maintain social distancing, wearing face mask to cover mouth and nose, periodic hand washing with soaps/detergents, use of alcohol-based hand sanitizers (ABHS), avoid to touching face among others [37,38]. With the start of the COVID-19 outbreak, hand sanitizers have been significantly elevated, which has positive effects on people's hygienic conditions.
Fig. 3.
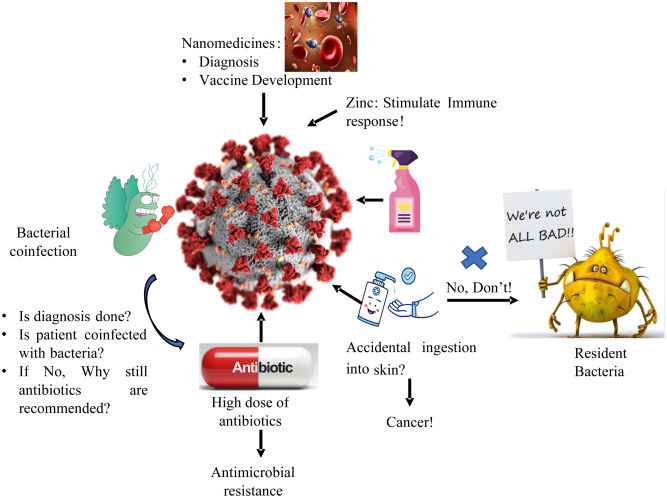
Schematic diagram of the current scenario of COVID-19.
On the other hand, this practice may select resistant bacteria, ultimately contributing to elevated AMR threat even more [9]. Moreover, the microbial flora of the human body also has a significant role in human health. Overuse of ABHS may result in an imbalance between the useful and pathogenic bacteria in the digestive system, causing inflammatory bowel diseases, obesity, autism, etc. [39]. Apart from the development of resistant bacteria, the use of ABHS is associated with many other severe issues like poisoning via ingestion, fire hazard, and organ toxicity via skin absorption [37]. Apart from this, repeated use of hand sanitizers and disinfectants may result in various health issues such as changes in skin texture, dryness (cutaneous xerosis), irritant contact dermatitis (ICD), and allergic contact dermatitis (ACD) [40]. These side effects are due to the chemical composition of sanitizers and disinfectants that need to be overcome by the other strategy like boosting the immune system by giving some supplement, developing nanomedicine and the vaccine.
Zinc is an essential trace element for the development and function of the immune cells. Zinc is involved in various biological processes as a cofactor, signaling molecule, structure, and regulatory component of the metabolism of carbohydrates and lipids. It also helps in the proper functioning of the cardiovascular, nervous, and reproductive systems [41]. Zinc deficiency results in altered and dysfunction of humoral and cell-mediated immune responses, mostly T-cell mediated, resulting in increased susceptibility to inflammatory and infectious diseases [42,43]. Under zinc inadequacy condition, organisms are more prone to toxin-producing bacteria and enteroviral pathogens, that trigger guanylate and adenylate cyclases, animating chloride discharge, causing diarrhea and reducing retention of supplements, consequently worsening a previously undermined mineral status [41,44]. The fact that zinc insufficiency is liable for 16% of all profound respiratory infections worldwide offers a first clear indication that zinc deficiency is associated with the risk of disease and the extreme progression of COVID-19 indicating possible zinc benefits supplementation [45]. Also, zinc has been shown to have a significant effect on viral infections by modulating the viral particle entry, fusion with the target cell membrane, replication, viral protein translation and processing, further release of viruses, and destabilizing the viral envelope, including those involved in respiratory system pathology [41]. Zn2+ cations have been shown to inhibit SARS-coronavirus RNA polymerase (RNA dependent RNA polymerase, RdRp) activity, especially in combination with Zn ionophore pyrithione, by reducing its replication [45]. Low-dose zinc supplementation with small amounts of zinc ionophores pyrithione or hinokitol-reduced RNA synthesis by inhibiting RdRp in equine arteritis virus (EAV), poliovirus, picornavirus, influenza, and SARS-CoV [3]. Recent studies have demonstrated the efficacy of chloroquine antiviral activity in treating COVID-19 enhanced by zinc supplementation. However, further investigation of the intimate mechanisms of its antiviral activity is needed. These significant findings indicate that in the treatment of COVID-19, Zn2+ may be considered as the specific antiviral agent [41].
Nanotechnology is a multidisciplinary area of engineering, chemistry, physics, and medicine. The application of nanotechnology in the field of medicine is referred to as nanomedicines [46]. Nanomedicines are defined as nanoscale tools or with the ability of early detection and prevention of diseases, direct the bioactive molecule to its target of action, and monitor its release to ensure optimum concentration over the desired time frame. Nanomedicine is often reported as nanodrugs, nanoconstructs, nanoparticles, nanocarriers, nanomaterials, or nanotherapeutics [[47], [48], [49], [50], [51], [52]]. Nanoparticles (NPs) have been widely used over the decades due to their nanoscale size, versatility, multifunctionality, adaptability, enhanced solubility, personalized medicines, early diagnosis, and disease prevention [[53], [54], [55], [56]]. Nanomedicine has been successfully used to improve care in a wide variety of diseases such as neurological, cancer, cardiovascular, and infectious diseases, including HBV, HIV-1, respiratory syncytial virus, and influenza virus [[57], [58], [59]]. Nanotechnology may hold tremendous promise in the detection, treatment, and prevention of COVID-19 [60,61]. Moreover, several patents related to CoV have been registered in the field of nanotechnology [57]. The ongoing pandemic impact on the world is terrifying, and vaccination is the most promising solution to prevent the spread and fight the novel CoV outbreaks. To design the vaccine, it is essential first to define the antigen, adjuvant, manufacturing, and delivery device. The antigen is a foreign material specific to the pathogen administered to initiate the host and adjuvant’s immune response as a stimulatory agent to boost the host immune response [6]. Nanoparticles enable multiple antigen presentation, stabilizing antigens from degradation by host cell system upon administration, and acting as carriers for target-based delivery. Two fundamental issues adopted technology for vaccine design must address the efficient delivery of antigen to the dendritic cells following the activation of dendritic cells to trigger adaptive immunity [62]. The ability of nanoparticles to deliver multiple antigens helps promote antigen-presenting cells to function more effectively, thereby increasing the immunogenicity and potency of administered vaccines via recognition by T cell receptors [63]. Identifying the target is the first step to design the vaccine to halt infectious microorganisms’ pathogenesis. The significant targets identified for CoV vaccine development are the interacting site of viral S protein with the host cell receptor (ACE2), viral protease, RNA polymerase (RdRp), and host cell-induced protease (TMPRSS2) for priming of S protein. Research suggests that S protein subunits, RBD of the S1 subunit, and S2 subunit derivatives are the prime target epitomes for the SARS/MERS vaccine development. However, knowledge of SARS-CoV-2 specific antigen(s) for under trial vaccine candidates is limited [6,64,65]. Gold NPs (AuNPs) are commonly employed in nano-vaccines due to their ability to serve as adjuvants in immunization in addition to being an antigen carrier. However, an extensive study on the use of nanoparticles for promising CoVs vaccine development is needed [57].
The development of a vaccine focuses on antigen identification and delivery to achieve a robust immune response. Previous experiences with SARS-CoV and MERS-CoV have allowed the fast growth of immunizing agent candidates [66]. As of 16 Dec 2020, the clinical evaluation includes 56 COVID-19 candidate vaccines, of which 13 are in phase III trials as listed in Table 2 . There are another 166 candidate vaccines in preclinical evaluation. Phase III trials usually require 30,000 or more participants. All high candidate vaccines are going to be administered through intramuscular injection [67]. Covishield, mRNA-1273, Comirnaty BNT162b2, and sputnik V have been submitted to regulatory bodies to get authorized for emergency use. Among these four, two vaccines, mRNA-1273 and Comirnaty BNT162b2, have reported more than 90% protection efficacy based on their ongoing phase III clinical trials study as listed in Table 3 . They have also been granted emergency authorization for their COVID-19 vaccines by the Food and Drug Administration (FDA) [66].
Table 2.
COVID-19 vaccine candidates in Phase III trials [67].
| Vaccine | Type | Location |
|---|---|---|
| Sinovac | Inactivated virus | Brazil |
| Beijing Institute of Biological Products/Sinopharm | Inactivated virus | China |
| University of Oxford/AstraZeneca | Viral vector | USA |
| Gamaleya Research Institute | Viral vector | Russia |
| Novavax | Protein subunit | The United Kingdom |
| Moderna/NIAID | RNA | USA |
| Medicago Inc | Virus Like Particles | Canada |
| Wuhan Institute of Biological Products/Sinopharm | Inactivated virus | United Arab Emirates |
| Bharat Biotech | Inactivated virus | India |
| CanSino Biological Inc./Beijing Institute of Biotechnology | Viral vector⁎ | Pakistan |
| Janssen Pharmaceutical Companies | Viral vector | USA, Brazil, Colombia, Peru, Mexico, Philippines, South Africa |
| Anhui Zhifei Longcom Biopharma/Institute of Microbiology, Chinese Academy of Sciences | Protein subunit | China |
| BioNTech/Fosun Pharma/Pfizer | RNA | USA, Argentina, Brazil |
Single dose vaccination (all listed vaccines require two doses).
Table 3.
Potential COVID-19 vaccines for immunization [67].
| Manufacturer | Type | Emergency use authorization status | How effective | Storage | Dose |
|---|---|---|---|---|---|
| Astra Zeneca, University of Oxford (Covishield) | Viral Vector | – | 62–90% | 4 °C | 2 shots, research on- going |
| Moderna TX, Inc. (mRNA-1273) | mRNA | USA | 95% | −20 °C | 2 shots, 28 days apart |
| Pfizer, Inc., and BioNTech (Comirnaty BNT162b2) | mRNA | Canada, UK,EMA, Switzerland, USA | 95% | −70 °C | 2 shots, 21 days apart |
| Gamaleya (Sputnik V) | Viral vector | Russia | 92% | 4 °C | 2 shots |
Current diagnostic tests for CoV are based on CT scans and nucleic acid testing using real-time reverse transcriptase-polymerase chain reaction (qRT-PCR). The first and most critical step for subsequent molecular diagnosis is the practical and robust extraction of nucleic acids from complex clinical samples. However, it is currently still highly labor-intensive and time-consuming. Due to the immediate priority of rapid, responsive, and affordable diagnosis of COVID-19, nanotechnology can contribute to quick, efficient, and accurate extraction of nucleic acid by exploiting the magnetic properties of NPs to avoid cross-infection false-negative results. Zhao and his colleagues recently developed a rapid method for extracting nucleic acid using carboxyl polymer-coated magnetic nanoparticles (pcMNPs). The interaction between carboxyl groups of the polymer and nucleic acids allows efficient binding and absorption of RNA molecules to the pcMNPs. The method combines the virus lysis and RNA binding steps into one, and the pcMNPs-RNA complexes can be directly introduced into subsequent RT-PCR reactions, thereby reducing the operating time and risk of contamination by the elimination elution step. Due to its simplicity, satisfactory performances, and robustness, this method provides a promising alternative to decreasing the labor-intensity and reducing the possibility of false-negative results in the current RT-PCR-based SARS-CoV-2 diagnosis [68].
7. Conclusion
There is also a limited understanding of the long-term consequences of COVID-19 disease. Researchers in the hunt for effective antiviral drugs and preventive treatment strategies have been increasingly interested in the recent COVID-19 outbreak. There is still an urgent need to classify and characterize bacterial coinfections during COVID-19 in this fast-evolving area. Human health has been drastically impacted by coinfection with other microorganisms. The ability of zinc supplements has been investigated and may play an important role for boosting the immune system and limiting entry of viruses to ease the situation. Nanomaterials are considered an important therapeutic option for the next generation, and can overcome the drawbacks of current antiviral agents, such as a limited range and low bioavailability. Also, some vaccines are in preclinical evaluation and four of them have been authorized for emergency used by FDA.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
CrediT author statement
Nikky Goel: Conceptualization; Writing - original draft. Razi Ahmad: Conceptualization; Writing - review & editing. Huma Fatima-Review; Sunil K. Khare-Supervision; Review.
Funding Source
The authors gratefully acknowledge the financial grant provided by the Indian Institute of Technology Delhi (IITD) for carrying out this work. NG gratefully appreciates the financial assistance provided by the Ministry of Human Resource Development (MHRD), Govt. of India and IIT Delhi. HF is grateful for the financial support provided by the Ministry of Science and Technology, Department of Science and Technology (DST) Government of India, INSPIRE Fellowship no. (IF190004).
Availability of data and materials
Not applicable.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.https://www.worldometers.info/coronavirus/
- 2.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fehr A.R., Channappanavar R., Perlman S. Middle East respiratory syndrome: emergence of a pathogenic human coronavirus. Annu Rev Med. 2017;68:387–399. doi: 10.1146/annurev-med-051215-031152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denison M.R., Graham R.L., Donaldson E.F., Eckerle L.D., Baric R.S. Coronaviruses: an RNA proofreading machine regulates replication fidelity and diversity. RNA Biol. 2011;8(2):270–279. doi: 10.4161/rna.8.2.15013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shin M.D., Shukla S., Chung Y.H., Beiss V., Chan S.K., Ortega-Rivera O.A., et al. COVID-19 vaccine development and a potential nanomaterial path forward. Nat Nanotechnol. 2020;15(8):646–655. doi: 10.1038/s41565-020-0737-y. [DOI] [PubMed] [Google Scholar]
- 7.Corman V.M., Lienau J., Witzenrath M. Coronaviruses as the cause of respiratory infections. Internist. 2019;60(11):1136–1145. doi: 10.1007/s00108-019-00671-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunter J., Arentz S., Goldenberg J., Yang G., Beardsley J., Mertz D., et al. Rapid review protocol: zinc for the prevention or treatment of COVID-19 and other coronavirus-related respiratory tract infections. Integrat Med Res. 2020;9(3) doi: 10.1016/j.imr.2020.100457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egyir B., Obeng-Nkrumah N., Kyei G.B. COVID-19 pandemic and antimicrobial resistance: another call to strengthen laboratory diagnostic capacity in Africa. Afr J Lab Med. 2020;9(1):1–4. doi: 10.4102/ajlm.v9i1.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pidot S.J., Gao W., Buultjens A.H., Monk I.R., Guerillot R., Carter G.P., et al. Increasing tolerance of hospital Enterococcus faecium to handwash alcohols. Sci Transl Med. 2018;10(452):1–10. doi: 10.1126/scitranslmed.aar6115. [DOI] [PubMed] [Google Scholar]
- 11.Mahmood A., Eqan M., Pervez S., Alghamdi H.A., Tabinda A.B., Yasar A., et al. COVID-19 and frequent use of hand sanitizers; human health and environmental hazards by exposure pathways. Sci Total Environ. 2020;742 doi: 10.1016/j.scitotenv.2020.140561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Langford B.J., So M., Raybardhan S., Leung V., Westwood D., MacFadden D.R., et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26(12):1622–1629. doi: 10.1016/j.cmi.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rawson T.M., Moore L.S.P., Zhu N., Ranganathan N., Skolimowska K., Gilchrist M., et al. Bacterial and fungal co-infection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. 2020;71(9):2459–2468. doi: 10.1093/cid/ciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morris D.E., Cleary D.W., Clarke S.C. Secondary bacterial infections associated with influenza pandemics. Front Microbiol. 2017;8:1041. doi: 10.3389/fmicb.2017.01041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bengoechea J.A., Bamford C.G.G. SARS-CoV-2, bacterial co-infections, and AMR: the deadly trio in COVID-19? EMBO Mol Med. 2020;12 doi: 10.15252/emmm.202012560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bosch A.A.T.M., Biesbroek G., Trzcinski K., Sanders E.A.M., Bogaert D. Viral and bacterial interactions in the upper respiratory tract. PLoS Pathog. 2013;9(1) doi: 10.1371/journal.ppat.1003057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sutherland T., Mpirimbanyi C., Nziyomaze E., Niyomugabo J.-P., Niyonsenga Z., Muvunyi C.M., et al. Widespread antimicrobial resistance among bacterial infections in a Rwandan referral hospital. PLoS One. 2019;14(8) doi: 10.1371/journal.pone.0221121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehrad B., Clark N.M., Zhanel G.G., Lynch Iii J.P. Antimicrobial resistance in hospital-acquired gram-negative bacterial infections. Chest. 2015;147(5):1413–1421. doi: 10.1378/chest.14-2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rhee C., Kadri S.S., Dekker J.P., Danner R.L., Chen H.-C., Fram D., et al. Prevalence of antibiotic-resistant pathogens in culture-proven sepsis and outcomes associated with inadequate and broad-spectrum empiric antibiotic use. JAMA Netw Open. 2020;3(4) doi: 10.1001/jamanetworkopen.2020.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ventola C.L. The antibiotic resistance crisis: part 1: causes and threats. Pharm Therap. 2015;40(4):277. [PMC free article] [PubMed] [Google Scholar]
- 21.Nielsen K.M., Gjoen T., Asare N.Y.O., Lunestad B.T., Ytrehus B., Yazdankhah S.P., et al. Opinion of the panel on microbial ecology of the Norwegian Scientific Committee for Food and Environment. 2018. Antimicrobial resistance in wildlife potential for dissemination. VKM report. [Google Scholar]
- 22.Prestinaci F., Pezzotti P., Pantosti A. Antimicrobial resistance: a global multifaceted phenomenon. Pathog Glob Health. 2015;109(7):309–318. doi: 10.1179/2047773215Y.0000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leekha S., Terrell C.L., Edson R.S. Mayo clinic proceedings. Elsevier; 2011. General principles of antimicrobial therapy; pp. 156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amsalu A., Sapula S.A., De Barros Lopes M., Hart B.J., Nguyen A.H., Drigo B., et al. Efflux pump-driven antibiotic and biocide cross-resistance in Pseudomonas aeruginosa isolated from different ecological niches: a case study in the development of multidrug resistance in environmental hotspots. Microorganisms. 2020;8(11):1647. doi: 10.3390/microorganisms8111647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu X., Ge Y., Wu T., Zhao K., Chen Y., Wu B., et al. Co-infection with respiratory pathogens among COVID−2019 cases. Virus Res. 2020;285 doi: 10.1016/j.virusres.2020.198005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He F., Xia X., Nie D., Yang H., Jiang Y., Huo X., et al. Respiratory bacterial pathogen spectrum among COVID-19 infected and non-COVID-19 virus infected pneumonia patients. Diagn Microbiol Infect Dis. 2020;98(4) doi: 10.1016/j.diagmicrobio.2020.115199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rothe K., Feihl S., Schneider J., Wallnofer F., Wurst M., Lukas M., et al. Rates of bacterial co-infections and antimicrobial use in COVID-19 patients: a retrospective cohort study in light of antibiotic stewardship. Eur J Clin Microbiol Infect Dis. 2020:1–11. doi: 10.1007/s10096-020-04063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo W., Li M., Dong Y., Zhou H., Zhang Z., Tian C., et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020;36(7) doi: 10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qu R., Ling Y., Zhang Y.-H.-Z., Wei L.-Y., Chen X., Li X.-M., et al. Platelet-to-lymphocyte ratio is associated with prognosis in patients with coronavirus disease-19. J Med Virol. 2020;92(9):1533–1541. doi: 10.1002/jmv.25767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryu Y.B., Jeong H.J., Kim J.H., Kim Y.M., Park J.-Y., Kim D., et al. Biflavonoids from Torreya nucifera displaying SARS-CoV 3CLpro inhibition. Bioorg Med Chem. 2010;18(22):7940–7947. doi: 10.1016/j.bmc.2010.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Momtazmanesh S., Shobeiri P., Hanaei S., Mahmoud-Elsayed H., Dalvi B., Rad E.M. Cardiovascular disease in COVID-19: a systematic review and meta-analysis of 10,898 patients and proposal of a triage risk stratification tool. Egypt Heart J. 2020;72(1):1–17. doi: 10.1186/s43044-020-00075-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou J., Wang Y., Zhao J., Gu L., Yang C., Wang J., et al. The metabolic and immunological characteristics of pregnant women with COVID-19 and their neonates. Eur J Clin Microbiol Infect Dis. 2020:1–10. doi: 10.1007/s10096-020-04033-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang C., Wu Z., Li J.-W., Zhao H., Wang G.-Q. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020;55(5) doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the Cytokine Storm’in COVID-19. J Infect. 2020;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adhikari S.P., Meng S., Wu Y.-J., Mao Y.-P., Ye R.-X., Wang Q.-Z., et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty. 2020;9(1):1–12. doi: 10.1186/s40249-020-00646-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Atolani O., Baker M.T., Adeyemi O.S., Olanrewaju I.R., Hamid A.A., Ameen O.M., et al. COVID-19: critical discussion on the applications and implications of chemicals in sanitizers and disinfectants. EXCLI J. 2020;19:785. doi: 10.17179/excli2020-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng V.C.C., Wong S.-C., Chuang V.W.M., So S.Y.C., Chen J.H.K., Sridhar S., et al. The role of community-wide wearing of face mask for control of coronavirus disease 2019 (COVID-19) epidemic due to SARS-CoV-2. J Infect. 2020;81(1):107–114. doi: 10.1016/j.jinf.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Himabindu C.S.H.C.S., Tanish B.T.B., Kumari N.P.K.N.P., Nayab S.N.S. Hand sanitizers: is over usage harmful? World J Curr Med Pharm Res. 2020;2(4):296–300. [Google Scholar]
- 40.Beiu C., Mihai M., Popa L., Cima L., Popescu M.N. Frequent hand washing for COVID-19 prevention can cause hand dermatitis: management tips. Cureus. 2020;12(4) doi: 10.7759/cureus.7506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skalny A.V., Rink L., Ajsuvakova O.P., Aschner M., Gritsenko V.A., Alekseenko S.I., et al. Zinc and respiratory tract infections: perspectives for COVID-19. Int J Mol Med. 2020;46(1):17–26. doi: 10.3892/ijmm.2020.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alexander J., Tinkov A., Strand T.A., Alehagen U., Skalny A., Aaseth J. Early nutritional interventions with zinc, selenium and vitamin D for raising anti-viral resistance against progressive COVID-19. Nutrients. 2020;12(8):2358. doi: 10.3390/nu12082358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chilvers M.A., McKean M., Rutman A., Myint B.S., Silverman M., O’Callaghan C. The effects of coronavirus on human nasal ciliated respiratory epithelium. Eur Respir J. 2001;18(6):965–970. doi: 10.1183/09031936.01.00093001. [DOI] [PubMed] [Google Scholar]
- 44.Woodworth B.A., Zhang S., Tamashiro E., Bhargave G., Palmer J.N., Cohen N.A. Zinc increases ciliary beat frequency in a calcium-dependent manner. Am J Rhinol Allergy. 2010;24(1):6–10. doi: 10.2500/ajra.2010.24.3379. [DOI] [PubMed] [Google Scholar]
- 45.Wessels I., Rolles B., Rink L. The potential impact of zinc supplementation on COVID-19 pathogenesis. Front Immunol. 2020;11:1712. doi: 10.3389/fimmu.2020.01712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tabish T.A., Hamblin M.R. Multivalent nanomedicines to treat COVID-19: a slow train coming. Nano Today. 2020;35 doi: 10.1016/j.nantod.2020.100962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahmad R., Khatoon N., Sardar M. Antibacterial effect of green synthesized TiO2 nanoparticles. Adv Sci Lett. 2014;20(7–8):1616–1620. [Google Scholar]
- 48.Ahmad R., Mohsin M., Ahmad T., Sardar M. Alpha amylase assisted synthesis of TiO2 nanoparticles: structural characterization and application as antibacterial agents. J Hazard Mater. 2015;283:171–177. doi: 10.1016/j.jhazmat.2014.08.073. [DOI] [PubMed] [Google Scholar]
- 49.Ahmad R., Srivastava S., Ghosh S., Khare S.K. Phytochemical delivery through nanocarriers: a review. Colloids Surf B Biointerfaces. 2021;197 doi: 10.1016/j.colsurfb.2020.111389. [DOI] [PubMed] [Google Scholar]
- 50.Alam S., Ahmad R., Pranaw K., Mishra P., Khare S.K. Asparaginase conjugated magnetic nanoparticles used for reducing acrylamide formation in food model system. Bioresour Technol. 2018;269:121–126. doi: 10.1016/j.biortech.2018.08.095. [DOI] [PubMed] [Google Scholar]
- 51.Fornaguera C., Garcia-Celma M.J. Personalized nanomedicine: a revolution at the nanoscale. J Personal Med. 2017;7(4):12. doi: 10.3390/jpm7040012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sadaf A., Ahmad R., Ghorbal A., Elfalleh W., Khare S.K. Synthesis of cost-effective magnetic nano-biocomposites mimicking peroxidase activity for remediation of dyes. Environ Sci Pollut Res. 2019;27:27211–27220. doi: 10.1007/s11356-019-05270-3. [DOI] [PubMed] [Google Scholar]
- 53.Ahmad R., Sardar M. Enzyme immobilization: an overview on nanoparticles as immobilization matrix. Biochemi Analyt Biochem. 2015;4(2):1. [Google Scholar]
- 54.Ghosh S., Ahmad R., Gautam V.K., Khare S.K. Cholesterol-oxidase-magnetic nanobioconjugates for the production of 4-cholesten-3-one and 4-cholesten-3, 7-dione. Bioresour Technol. 2018;254:91–96. doi: 10.1016/j.biortech.2018.01.030. [DOI] [PubMed] [Google Scholar]
- 55.Ghosh S., Ahmad R., Khare S.K. Immobilization of cholesterol oxidase: an overview. Open Biotechnol J. 2018;12(1):176–188. [Google Scholar]
- 56.Soares S., Jo Sousa, Pais A., Vitorino C. Nanomedicine: principles, properties, and regulatory issues. Front Chem. 2018;6:360. doi: 10.3389/fchem.2018.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abd Ellah N.H., Gad S.F., Muhammad K., Batiha E.G., Hetta H.F. Nanomedicine as a promising approach for diagnosis, treatment and prophylaxis against COVID-19. Nanomedicine. 2020;15(21):2085–2102. doi: 10.2217/nnm-2020-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ahmad R., Mishra A., Sardar M. Simultaneous immobilization and refolding of heat treated enzymes on TiO2 nanoparticles. Adv Sci Eng Med. 2014;6(12):1264–1268. [Google Scholar]
- 59.Ghosh S., Ahmad R., Khare S.K. Refolding of thermally denatured cholesterol oxidases by magnetic nanoparticles. Int J Biol Macromol. 2019;138:958–965. doi: 10.1016/j.ijbiomac.2019.07.103. [DOI] [PubMed] [Google Scholar]
- 60.Campos E.V.R., Pereira A.E.S., de Oliveira J.L., Carvalho L.B., Guilger-Casagrande M., de Lima R., et al. How can nanotechnology help to combat COVID-19? Opportunities and urgent need. J Nanobiotechnol. 2020;18(1):1–23. doi: 10.1186/s12951-020-00685-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bhavana V., Thakor P., Singh S.B., Mehra N.K. COVID-19: pathophysiology, treatment options, nanotechnology approaches, and research agenda to combating the SARS-CoV2 pandemic. Life Sci. 2020;261 doi: 10.1016/j.lfs.2020.118336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reddy S.T., Van Der Vlies A.J., Simeoni E., Angeli V., Randolph G.J., O’Neil C.P., et al. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol. 2007;25(10):1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 63.Hashemzadeh A., Avan A., Ferns G.A., Khazaei M. Vaccines based on virus-like nano-particles for use against middle east respiratory syndrome (MERS) coronavirus. Vaccine. 2020;38(36):5742–5746. doi: 10.1016/j.vaccine.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chauhan G., Madou M.J., Kalra S., Chopra V., Ghosh D., Martinez-Chapa S.O. Nanotechnology for COVID-19: therapeutics and vaccine research. ACS Nano. 2020;14(7):7760–7782. doi: 10.1021/acsnano.0c04006. [DOI] [PubMed] [Google Scholar]
- 65.Jafarzadeh A., Chauhan P., Saha B., Jafarzadeh S., Nemati M. Contribution of monocytes and macrophages to the local tissue inflammation and cytokine storm in COVID-19: lessons from SARS and MERS, and potential therapeutic interventions. Life Sci. 2020;257 doi: 10.1016/j.lfs.2020.118102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Riel D., de Wit E. Next-generation vaccine platforms for COVID-19. Nat Mater. 2020;19(8):810–812. doi: 10.1038/s41563-020-0746-0. [DOI] [PubMed] [Google Scholar]
- 67.https://www.who.int/docs/default-source/coronaviruse/risk-comms-updates/update45-vaccines-developement.pdf?sfvrsn=13098bfc_5
- 68.Zhao Z., Cui H., Song W., Ru X., Zhou W., Yu X. A simple magnetic nanoparticles-based viral RNA extraction method for efficient detection of SARS-CoV-2. bioRxiv. 2020 doi: 10.1101/2020.02.22.961268. Preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


