Abstract
BACKGROUND AND PURPOSE:
Therapeutic hypothermia is the current treatment for neonates with hypoxic-ischemic encephalopathy. It is believed to work by decreasing the brain temperature and reducing the baseline metabolism and energy demand of the brain. This study aimed to noninvasively assess brain temperature during the first month of life in neonates with hypoxic-ischemic encephalopathy treated with hypothermia.
MATERIALS AND METHODS:
Neonates with hypoxic-ischemic encephalopathy treated with hypothermia and healthy neonates were enrolled prospectively. MR imaging was used to identify the presence and extent of brain injury. MR imaging multivoxel spectroscopy was used to derive brain temperatures in the basal ganglia and white matter at different time points during the first month of life. Brain temperature measurements were compared between neonates with hypoxic-ischemic encephalopathy and healthy neonates.
RESULTS:
Forty-three term neonates with hypoxic-ischemic encephalopathy treated with hypothermia had a total of 74 spectroscopy scans, and 3 healthy term neonates had a total of 9 spectroscopy scans during the first month of life. Brain temperatures were lower in neonates with hypoxic-ischemic encephalopathy during hypothermia, compared with the healthy neonates (respectively, on day 1 of life: basal ganglia, 38.81°C ± 2.08°C, and white matter, 39.11°C ± 1.99°C; and on days 2–3 of life: basal ganglia, 38.25°C ± 0.91°C, and white matter, 38.54°C ± 2.79°C). However, neonates with hypoxic-ischemic encephalopathy who developed brain injury had higher brain temperatures during hypothermia (respectively, on day 1 of life: basal ganglia, 35.55°C ± 1.31°C, and white matter, 37.35°C ± 2.55°C; and on days 2–3 of life: basal ganglia, 35.20°C ± 1.15°C, and white matter, 35.44°C ± 1.90°C) compared with neonates who did not develop brain injury (respectively, on day 1 of life: basal ganglia, 34.46°C ± 1.09°C, and white matter, 33.97°C ± 1.42°C; and on days 2–3 of life: basal ganglia, 33.90°C ± 1.34°C, and white matter, 33.07°C ± 1.71°C). Also, brain temperatures tended to remain slightly higher in the neonates who developed brain injury around day 10 of life and around 1 month of age.
CONCLUSIONS:
Therapeutic hypothermia using current guidelines decreased the brain temperature of neonates with hypoxic-ischemic encephalopathy during the first days of life but did not prevent an early increase of brain temperature in neonates with hypoxic-ischemic encephalopathy who developed brain injury despite this treatment.
Therapeutic hypothermia is the current standard treatment for term neonates with hypoxic-ischemic encephalopathy to try to prevent the development of brain injury.1–4 Although this treatment has reduced the rate of death and neurologic impairment in neonates with hypoxic-ischemic encephalopathy at 18 months of age,5–7 some neonates still develop brain injury and long-term neurologic sequelae. Induced hypothermia is believed to work by decreasing the brain temperature and thus reducing the baseline metabolism and energy demand of the brain.8 Therefore, it would be of great importance to monitor brain temperature for these neonates during and after hypothermia treatment.
In recent years, techniques using MR imaging have been developed to noninvasively measure brain temperature (referenced as “brain thermometry”) even in neonates. One such technique is proton MR spectroscopy (1H-MR spectroscopy), which can be used to estimate brain temperature by monitoring the chemical shift of water with temperature compared with the resonance frequency of a reference metabolite,9 such as N-acetyl aspartic acid. MR spectroscopy has been used for brain thermometry in healthy adults,10–12 in adults with stroke,13,14 and in adults with traumatic brain injury.15 However, until now, brain thermometry has been used only a few times with neonates,16,17 and even more rarely with neonates with hypoxic-ischemic encephalopathy.18,19 Bainbridge et al18 have demonstrated the feasibility of this technique with neonates using single-voxel MR spectroscopy placed in the thalamus and/or white matter to show the correlation between brain and rectal temperatures in healthy neonates; in addition, in their study, only 9 neonates had brain temperature measurements during hypothermia treatment. Wu et al19 studied 18 neonates with hypoxic-ischemic encephalopathy treated with hypothermia with single-voxel MR spectroscopy placed in the thalamus, basal ganglia, and/or cortical gray matter. They found that the initial degree of encephalopathy seemed to influence brain temperature measurements during and after hypothermia. Neither of these 2 studies correlated their brain temperature measurements with the extent of brain injury.
The present study hypothesized that neonates with hypoxic-ischemic encephalopathy who developed brain injury despite hypothermia treatment will have a higher brain temperature compared with neonates with hypoxic-ischemic encephalopathy who did not develop brain injury during and after treatment. Thus, the present study was designed to noninvasively assess brain temperature during the first month of life in term neonates with hypoxic-ischemic encephalopathy treated with hypothermia.
Materials and Methods
Patients
We conducted a prospective cohort study of term neonates with hypoxic-ischemic encephalopathy admitted to our neonatal intensive care unit at the Montreal Children's Hospital, McGill University, from 2010 to 2015 who met the criteria for induced hypothermia:1,3 1) gestational age of 36 weeks or older and birth weight of ≥1800 g; 2) evidence of fetal distress (eg, a history of an acute perinatal event, cord pH ≤ 7.0, or base deficit ≤ −16 mEq/L); 3) evidence of neonatal distress (eg, an Apgar score of ≤5 at 10 minutes, postnatal blood gas pH within the first hour of life of ≤7.0 or a base deficit of ≤ −16 mEq/L, or continuous need for ventilation with the initiation at birth and duration for at least 10 minutes); and 4) evidence of moderate or severe encephalopathy obtained by a standardized neurologic examination and/or by an amplitude-integrated electroencephalogram. The initial background pattern of the amplitude-integrated electroencephalogram2,20 was assessed on admission and collected to classify the neonates. Eligible neonates received whole-body cooling to an esophageal temperature of 33.5°C, initiated by 6 hours of life and continued for 72 hours. Three additional healthy term neonates were included as controls. The research protocol was approved by the institutional review board, and informed parental consent was obtained in all cases.
Brain MR Imaging
As per standard clinical protocol at our institution, a brain MR imaging was performed for all these neonates after hypothermia treatment was completed, usually around day 10 of life. In addition, since 2010, when possible (ie, when the parents consented for their neonates to have additional MRIs, when the neonates were hemodynamically stable, and when a team of a nurse and a respiratory therapist was available to go to the MR imaging), neonates were enrolled in an MR imaging research study, and MR imaging scans were performed on day 1 of life, on days 2–3 of life, around day 10 of life, and around 1 month of age. These time points were chosen to ensure the absence of antenatal brain injury (day 1 of life), to assess early patterns of injury (days 2–3 of life), and to define the extent of definitive brain injuries (around day 10 of life and around 1 month of life). Neonates receiving hypothermia had the hypothermia therapy maintained during the MR imaging scan without any adverse events.21 Any ventilation, pressor support, or sedation was maintained during the MR imaging process, and additional sedation was avoided. The healthy term neonates had normal imaging findings at the same time points; they were kept at regular temperature during the MR imaging.
The MR imaging was performed with a 3T clinical system (Achieva X; Philips Healthcare, Best, the Netherlands). Each MR imaging study included 3D T1-weighted gradient-echo (TR, 24 ms; TE, 4.6 ms; matrix size, 180 × 180; FOV, 180 mm; flip angle, 30°; sagittal sections, 104; section thickness, 1.0 mm; and multiplanar reformations in axial and coronal planes), TSE high-resolution T2-weighted (TR, 5000 ms; TE, 90 ms; TSE factor, 15; matrix size, 300 × 300; FOV, 150 mm; flip angle, 90°; axial sections, 27; section thickness, 3.0 mm), and a single-shot echo-planar diffusion-tensor imaging sequence (TR, 5937.8 ms; TE, 69 ms; matrix size, 100 × 100; FOV, 180 mm; sensitivity encoding factor, 2; directions, 32; b-value, 750 s/mm2; axial sections, 64; section thickness, 2.2 mm). In addition, if time allowed and the neonates were still sleeping, neonates enrolled in the MR imaging research study underwent a 1H-MR spectroscopy with a 2D point-resolved spectroscopy sequence (TR, 2000 ms; TE, 288 ms; FOV, 180 × 180 mm; bandwidth, 2000 Hz; 1024 points; voxel size, 15 × 15 × 15 mm). The multivoxels were placed over the whole cerebrum at the level of the basal ganglia. Data acquisition was performed at room temperature (22°C ± 1°C). This sequence was used to noninvasively measure absolute brain temperature in the neonates.
Pediatric neuroradiologists, who were blinded to the clinical conditions of the infants, interpreted the MR imaging studies of the asphyxiated neonates treated with hypothermia. They reported the presence and extent of brain injury in the cerebrum according to a previously described MR imaging scoring system.22 The MR imaging scores obtained around day 10 of life were used as the reference to determine the extent of the brain injury for each patient.23,24
Temperature Measurements in Neonates
MR spectra data were obtained with the previously mentioned 2D point-resolved spectroscopy sequence. On the basis of the principle that temperature affects the hydrogen bonding between water molecules,9 which leads to a shift in the spectral position of the water signal relative to a reference metabolite that has less sensitivity to temperature changes—such as N-acetyl aspartate—on MR spectroscopy,11,17,25,26 brain temperature was derived by analyzing the chemical shift differences (δ ppm) between the water peak and the NAA spectrum.19 This method has been demonstrated previously to have an accuracy of ±0.5°C and a precision of 0.3°C.14,17,26 The chemical shift differences were plotted against the temperature to obtain the calibration line for estimating the brain temperature of the neonates.19 The software package jMRUI (Version 5.2; www.mrui.uab.es/mrui/mrui_download/)27,28 was used to estimate the position of the water peak and NAA in each of these voxels and thus measure the chemical shift differences. A 2D fast Fourier transform was applied to the signals as the preprocessing steps in the software. To remove undesirable resonance frequencies, we used a Hankel Lanczos Singular Value Decomposition29 filter, implemented in jMRUI.
The multivoxels were placed over the whole cerebrum of the neonates at the level of basal ganglia (Fig 1). For each MR multivoxel spectra acquired for the neonate, brain temperature was subsequently measured in the voxels located in the basal ganglia (ie, a voxel in the thalamus and a voxel in the remaining basal ganglia) and the white matter (ie, a voxel in the anterior white matter and a voxel in the posterior white matter) on each side of the brain (Fig 1). Measurements were obtained on the right and left sides of the brain in these different ROIs and then averaged. The voxels containing noisy and poor signals were excluded manually from the data. Then, for each scan, brain temperature was estimated with a previously described calibration equation.30
Fig 1.
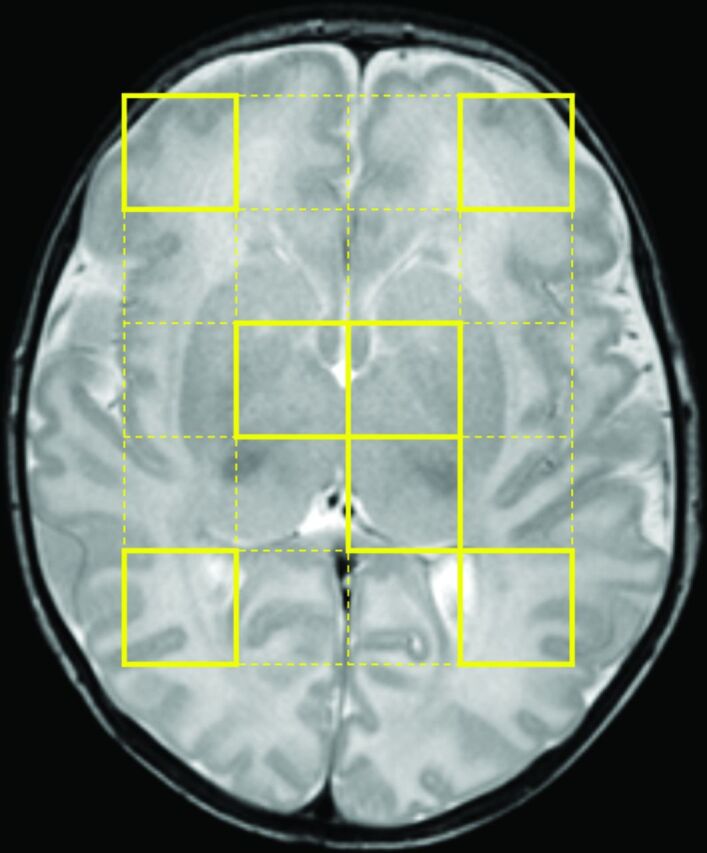
ROIs. T2-weighted image shows where the multivoxels were placed over the whole cerebrum at the level of basal ganglia (yellow dotted lines). In this example, temperature was measured in a total of 7 voxels—that is, in the voxels in the left thalamus, the remaining basal ganglia bilaterally, and the anterior and posterior white matter bilaterally (yellow lines).
Statistical Analysis
In healthy neonates, differences of brain temperature in the basal ganglia and white matter across time were assessed for statistical significance with Kruskal-Wallis tests.
For the first comparison of brain temperature measurements between neonates with hypoxic-ischemic encephalopathy treated with hypothermia and healthy neonates, the neonates with hypoxic-ischemic encephalopathy treated with hypothermia were categorized into 2 subgroups according to their initial degree of encephalopathy on an amplitude-integrated electroencephalogram (ie, moderate versus severe). For the second analysis, the same neonates were categorized into 2 subgroups based on the presence or absence of brain injury in the ROI on their conventional MR imaging performed around day 10 of life. We assessed the following differences at each time point for statistical significance with Kruskal-Wallis tests: 1) between neonates with hypoxic-ischemic encephalopathy treated with hypothermia with initial moderate encephalopathy versus those with initial severe encephalopathy versus healthy neonates, and 2) neonates with hypoxic-ischemic encephalopathy treated with hypothermia who developed brain injury versus those without brain injury versus healthy neonates. For multiple comparisons, the Dunn post hoc comparison tests comparing the 3 groups at each time point were applied to adjust the α level as necessary. An overall (2-sided) α level of .05 was used to indicate statistical significance.
Results
Forty-three term neonates with hypoxic-ischemic encephalopathy treated with hypothermia had a total of 74 spectroscopy scans over the first month of life. Fifty-three percent (23/43) of these neonates had an initial moderate encephalopathy by amplitude-integrated electroencephalography. Fifty-two percent (12/23) did not develop brain injury, 13% (3/23) developed basal ganglia injury, 26% (6/23) developed watershed injury, and 9% (2/23) developed near-total injury. Forty-nine percent (21/43) of these neonates had an initial severe encephalopathy by an amplitude-integrated electroencephalogram. Thirty-five percent (7/20) did not develop brain injury, 10% (2/20) developed basal ganglia injury, 5% (1/20) developed watershed injury, and 50% (10/20) developed near-total injury. In addition, 3 healthy term neonates had a total of 9 spectroscopy scans during the first month of life. Seventeen percent (8/46) of the neonates enrolled in the present study had a spectroscopy scan on day 1 of life, 35% (16/46) had a spectroscopy scan on days 2–3 of life, 76% (35/46) had a spectroscopy scan around day 10 of life, and 35% (16/46) had a spectroscopy scan around 1 month of age. Among the 8 voxels where temperature was measured, a mean of 1.30 ± 1.56 voxels per patient was excluded manually from the data because they contained noisy and poor signals.
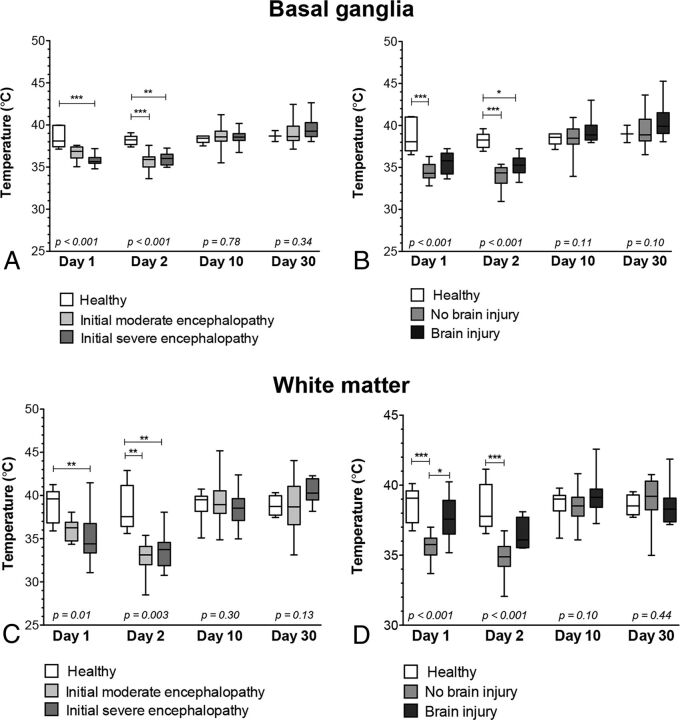
In healthy neonates, brain temperature remained similar in the basal ganglia (average, 38.50°C ± 1.21°C; comparison between time points, P = .89) and in the white matter (average, 38.88°C ± 1.86°C; comparison between time points, P = .76) during the first month of life (Fig 2). In the same neonates, brain temperature was not different between the basal ganglia and white matter (P = .23).
Fig 2.
Comparison of brain temperature values in the basal ganglia and white matter according to the initial degree of encephalopathy and the presence of brain injury. Brain temperature values in the basal ganglia according to the initial degree of encephalopathy (A) and according to the presence of brain injury (B). Brain temperature values in white matter according to the initial degree of encephalopathy (C) and the presence of brain injury (D). Box-and-whisker plots (median, minimum, and maximum in degrees Celsius). Significance was derived from Kruskal-Wallis tests, followed by the Dunn post hoc comparison tests for multiple comparisons: The asterisk indicates P < .05; 2 asterisks, P < .01; 3 asterisks, P < .001.
When we compared the brain temperature results of the neonates with hypoxic-ischemic encephalopathy—according to their initial degree of encephalopathy—and the healthy neonates, brain temperatures were significantly different in the 3 groups (moderate encephalopathy, severe encephalopathy, and healthy) on day 1 of life (respectively, basal ganglia, P < .001, and white matter, P = .01) and on days 2–3 of life (respectively, basal ganglia, P < .001, and white matter, P = .003) (Fig 2). Neonates with moderate encephalopathy tended to have lower brain temperatures (respectively, basal ganglia, 35.82°C ± 1.31°C, and white matter, 36.84°C ± 0.83°C) on day 1 of life compared with healthy neonates (respectively, basal ganglia, 38.81°C ± 2.08°C, and white matter, 38.76°C ± 1.25°C); in addition, the brain temperatures of neonates with moderate encephalopathy were significantly lower on days 2–3 of life (respectively, basal ganglia, 34.07°C ± 1.52°C, and white matter, 34.87°C ± 1.12°C) compared with healthy neonates (respectively, basal ganglia, 38.2°C 5 ± 0.91°C, and white matter, 38.40°C ± 1.76°C). Neonates with severe encephalopathy had significantly lower brain temperatures on day 1 of life (respectively, basal ganglia, 34.42°C ± 1.10°C, and white matter, 36.22°C ± 1.81°C) and on days 2–3 of life (respectively, basal ganglia, 34.77°C ± 1.12°C, and white matter, 35.23°C ± 1.16°C) compared with healthy neonates. Brain temperatures did not differ between the neonates with moderate encephalopathy and the neonates with severe encephalopathy. Brain temperatures did not differ in the 3 groups around day 10 of life (respectively, basal ganglia, P = .78, and white matter, P = .30) and around 1 month of life (respectively, basal ganglia, P = .34, and white matter, P = .13) (Fig 2).
When we compared the brain temperature results of the neonates with hypoxic-ischemic encephalopathy—according to the presence or absence of brain injury—and the healthy neonates, we found that the brain temperatures were significantly different in the 3 groups (neonates with hypoxic-ischemic encephalopathy who did not develop brain injury, neonates with hypoxic-ischemic encephalopathy who developed brain injury, and healthy neonates) on day 1 of life and on days 2–3 of life (P < .001) (Fig 2). Neonates with hypoxic-ischemic encephalopathy who did not develop brain injury had significantly lower brain temperatures on day 1 of life (respectively, basal ganglia, 34.46°C ± 1.09°C, and white matter, 33.97°C ± 1.42°C) and on days 2–3 of life (respectively, basal ganglia, 33.90°C ± 1.34°C, and white matter, 33.07°C ± 1.71°C) compared with healthy neonates. Neonates who developed brain injury had higher brain temperatures on day 1 of life (respectively, basal ganglia, 35.55°C ± 1.31°C, and white matter, 37.35°C ± 2.55°C) and on days 2–3 of life (respectively, basal ganglia, 35.20°C ± 1.15°C, and white matter, 35.44°C ± 1.90°C) compared with neonates with hypoxic-ischemic encephalopathy who did not develop brain injury; however, the brain temperatures of neonates who developed brain injury were not different compared with those in healthy neonates, except on days 2–3 of life in the basal ganglia.
Brain temperatures did not differ for neonates with hypoxic-ischemic encephalopathy with moderate encephalopathy and neonates with hypoxic-ischemic encephalopathy with severe encephalopathy. For all 3 groups, brain temperatures did not differ around day 10 of life (respectively, basal ganglia, P = .11, and white matter, P = .10) and around 1 month of life (respectively, basal ganglia, P = .10, and white matter, P = .44) (Fig 2). However, brain temperatures tended to remain higher around day 10 of life (in the basal ganglia, 39.34°C ± 1.35°C, and in the white matter, 40.10°C ± 2.36°C) and around 1 month of age (in the basal ganglia, 40.58°C ± 2.15°C) in the neonates who developed brain injury compared with those who did not develop brain injury (in the basal ganglia: day 10 of life, 38.31°C ± 1.69°C; 1 month of age, 39.25 ± 1.78°C; and in the white matter: day 10 of life, 38.74°C ± 1.84°C).
Discussion
In this study, we investigated the evolution of brain temperature during the first month of life in neonates with hypoxic-ischemic encephalopathy treated with hypothermia. To the best of our knowledge, this is the largest cohort of term neonates with hypoxic-ischemic encephalopathy treated with hypothermia for whom brain thermometry has been used. This also is the first time that multivoxel spectroscopy was used to perform brain thermometry of neonates with hypoxic-ischemic encephalopathy treated with hypothermia. Previous studies used single-voxel spectroscopy18,19 and had to acquire different spectra in the different ROIs, which increased the duration of the MR imaging scans for these patients who often are critically sick. In addition, brain temperature could potentially vary between the times that the different voxels were scanned. Using multivoxel spectroscopy enabled a simultaneous measurement of brain temperature in different ROIs with the same sequence, without prolonging the scan duration. Most interesting, with multivoxel spectroscopy, we did not find consistent differences between the brain temperature measurements performed on the basal ganglia and white matter, contrary to some other studies.18,19 In our study, we averaged the measurements performed on the thalami and the remaining basal ganglia on both sides as “basal ganglia” measurements and the measurements performed on the anterior and posterior white matter on both sides as “white matter” measurements. It also is possible that measurements on the white matter were sometimes “contaminated” to varying degrees by the cortical gray matter because it often was technically challenging to pick a voxel located solely in the white matter.
As expected, hypothermia treatment with current guidelines lowered the brain temperatures of the neonates with hypoxic-ischemic encephalopathy compared with the healthy neonates, as has also been suggested by other studies.19 However, the decrease of brain temperature during hypothermia treatment was not uniform for all the neonates with hypoxic-ischemic encephalopathy. Therapeutic hypothermia did not prevent an early increase of brain temperature in neonates with hypoxic-ischemic encephalopathy who developed brain injury—despite these neonates being actively cooled to around 33.5°C—compared with those who did not develop brain injury. These results are similar to those found in adults with traumatic brain injury, which showed that brain temperature dissociates from core body temperature and increases when brain injury develops.10 The increased brain temperatures in the neonates who developed brain injury probably correspond to an increased brain perfusion despite hypothermia treatment, which has been previously reported in these neonates despite hypothermia treatment.31–33 The increased brain temperatures in the neonates who developed brain injury may also correspond to the often-described phenomenon of luxury perfusion during which brain perfusion exceeds the metabolic demands, which leads to an increase of cerebral oxygen delivery.34–37 Thus, the current hypothermia guidelines did not seem to prevent the development of brain injury when increased brain temperatures were found during the first days of life. These neonates with increased brain temperatures may be candidates for adjustments in their hypothermia therapy or for adjunctive therapies.19,33
Brain temperatures tended to remain slightly higher in the basal ganglia and in the white matter around day 10 of life, and in the basal ganglia around 1 month of age in the neonates who developed brain injury compared those not who did not. Similar results were found when measuring brain perfusion in these neonates38: Brain perfusion remained somewhat increased in the brain regions with brain injury around day 10 of life and around 1 month of life. It has been hypothesized that these results may be due to increased blood vessels rather than the tone adjustments of the first days of life.38
The analysis of brain temperatures according to the initial degree of encephalopathy did not permit a prediction of the outcome of these neonates because no difference occurred in the brain temperatures of the neonates with initial moderate encephalopathy and those with initial severe encephalopathy recorded on an amplitude-integrated electroencephalogram. These results are consistent with those found when measuring the regional cerebral oxygen saturation in these neonates,35 in which the regional cerebral oxygen saturation did not differ for neonates with initial moderate encephalopathy and those with initial severe encephalopathy. However, our results were different compared with those in a previous study by Wu et al,19 who studied 18 neonates with hypoxic-ischemic encephalopathy treated with hypothermia (including 14 with initial moderate encephalopathy and 4 with initial severe encephalopathy based on a modified Sarnat score) and found that the initial degree of encephalopathy seemed to influence the brain temperature measurements during and after hypothermia. Unfortunately, they did not report the correlation between the brain temperature measurements and the extent of brain injury in each of their neonates, so it is impossible to know whether all their neonates with initial severe encephalopathy developed brain injury. In our study, only 65% of the neonates with an initial severe encephalopathy developed brain injury. This finding might help explain why our results were different from theirs, in addition to our sample size being larger. Also, most of their scans were performed on days 2–3 of life,19 and our scans were usually performed on days 1 and 2 of life; similarly, previous studies have shown that the regional cerebral oxygen saturation did not differ for neonates with hypoxic-ischemic encephalopathy with moderate-versus-severe encephalopathy, except on day 3 of life.35
In addition to the brain temperature measurements at different time points during the first month of life, another strength of the present study is that brain temperature measurements at the same time points in healthy neonates were also included as comparisons with those of neonates with hypoxic-ischemic encephalopathy. Most interesting, the brain temperature in the healthy neonates was, on average, above 38°C, which is higher than the normal target core temperature; these results were consistent with previously described results16 and also with the consensus that brain temperature is typically 0.5°C–1°C higher than core temperature.39–41 Similarly, our brain temperature measurements performed during hypothermia were, on average, at least above 34°C in those neonates who did not develop brain injury, which is higher than the target core temperature (ie, 33.5°C) during the treatment, a finding that was also consistent with previously described results.19
Of note, neonates with hypoxic-ischemic encephalopathy received whole-body cooling in this study. Brain temperature measurements may yield different results in neonates with hypoxic-ischemic encephalopathy treated with head cooling because it has been previously demonstrated in neonatal swine that head cooling creates a temperature gradient across the brain (ie, cooler periphery and warmer central structures) compared with no temperature gradient in those treated with whole-body cooling.42,43
Instead of just comparing our results with previous studies of brain perfusion and/or regional cerebral oxygen saturation measurements, it would have been interesting to correlate directly the brain temperature measurements of these neonates with the brain perfusion measurements by MR arterial spin-labeling and/or with the measurement of regional cerebral oxygen saturation by near-infrared spectroscopy, to better understand the physiopathology underlying the development of brain injury in these neonates. However, these other measurements were not always consistently performed for the neonates enrolled in this study. Another limitation of our study was that the body temperatures of the neonates at each time point were not collected systematically, especially with respect to the MR imaging performed during the hypothermia treatment, so they could not be correlated with the neonates' body temperatures. Other studies have shown that body and brain temperatures correlate in healthy subjects but that a dissociation exists between the 2 in brain injury.10 In addition, the sample size, even if it is the largest described so far with respect to neonates with hypoxic-ischemic encephalopathy treated with hypothermia, was not sufficient to study, in more detail, the impact of sedation and antiepileptic medications that were sometimes used with these neonates. The sample size of healthy term neonates was also small; thus, the statistical power of our comparisons and the conclusions that can be drawn were limited. Of note, none of the neonates presented with seizures during the MR imaging studies, and minimal sedation was used for all of them as per the standard of care at our institution.
Conclusions
Therapeutic hypothermia using current guidelines decreased brain temperature in neonates with hypoxic-ischemic encephalopathy during the first days of life but did not prevent an early increase of brain temperature in neonates with hypoxic-ischemic encephalopathy who developed brain injury. Noninvasive monitoring of brain temperatures may permit a better understanding of the development of brain injury despite hypothermia in neonates with hypoxic-ischemic encephalopathy. Further studies are needed to determine whether noninvasive measuring of brain temperature may permit a selection of the neonates who may be candidates for adjustments in their hypothermia therapy or for adjunctive therapies.
Acknowledgments
The authors thank the families and their neonates for participating in the study. Special thanks are also expressed to the neonatal intensive care unit nurses, the neonatal intensive care unit respiratory therapists, and the MR imaging technicians who have made this study possible. The authors also thank Mr Wayne Ross Egers for his professional English correction of the manuscript.
Footnotes
Disclosures: Guillaume Gilbert—UNRELATED: Employment: Philips Healthcare, Comments: clinical scientist who participates in and supports clinical research performed with academic partners. Pia Wintermark—RELATED: Grant: New Investigator Research Grant from the SickKids Foundation and the Canadian Institutes of Health Research Institute of Human Development, Child and Youth Health, Comments: Pia Wintermark receives research grant funding from the Fonds de la recherche en santé du Québec Clinical Research Scholar Career Award Junior 2, and the New Investigator Research Grant from the SickKids Foundation and the Canadian Institutes of Health Research Institute of Human Development, Child and Youth Health.* *Money paid to the institution.
Pia Wintermark receives research grant funding from the Fonds de la recherche en santé du Québec Clinical Research Scholar Career Award Junior 2 (32814) and the New Investigator Research Grant from the SickKids Foundation and the Canadian Institutes of Health Research Institute of Human Development, Child and Youth Health (NI13-049R).
References
- 1. Azzopardi D, Strohm B, Marlow N, et al. ; TOBY Study Group. Effects of hypothermia for perinatal asphyxia on childhood outcomes. N Engl J Med 2014;371:140–49 10.1056/NEJMoa1315788 [DOI] [PubMed] [Google Scholar]
- 2. Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet 2005;365:663–70 [DOI] [PubMed] [Google Scholar]
- 3. Shankaran S, Pappas A, McDonald SA, et al. ; Eunice Kennedy Shriver NICHD Neonatal Research Network. Childhood outcomes after hypothermia for neonatal encephalopathy. N Engl J Med 2012;366:2085–92 10.1056/NEJMoa1112066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Simbruner G, Mittal RA, Rohlmann F, et al. ; neo.nEURO.network Trial Participants. Systemic hypothermia after neonatal encephalopathy: outcomes of neo.nEURO.network RCT. Pediatrics 2010;126:e771–78 10.1542/peds.2009-2441 [DOI] [PubMed] [Google Scholar]
- 5. Edwards AD, Brocklehurst P, Gunn AJ, et al. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ 2010;340:c363 10.1136/bmj.c363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jacobs SE, Berg M, Hunt R, et al. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev 2013;CD003311 10.1002/14651858.CD003311.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shah PS. Hypothermia: a systematic review and meta-analysis of clinical trials. Semin Fetal Neonatal Med 2010;15:238–46 10.1016/j.siny.2010.02.003 [DOI] [PubMed] [Google Scholar]
- 8. Polderman KH. Induced hypothermia and fever control for prevention and treatment of neurological injuries. Lancet 2008;371:1955–69 10.1016/S0140-6736(08)60837-5 [DOI] [PubMed] [Google Scholar]
- 9. Hindman JC. Proton resonance shift of water in the gas and liquid states. J Chem Phys 1966;44:4582 10.1063/1.1726676 [DOI] [Google Scholar]
- 10. Childs C, Hiltunen Y, Vidyasagar R, et al. Determination of regional brain temperature using proton magnetic resonance spectroscopy to assess brain-body temperature differences in healthy human subjects. Magn Reson Med 2007;57:59–66 10.1002/mrm.21100 [DOI] [PubMed] [Google Scholar]
- 11. Corbett R, Laptook A, Weatherall P. Noninvasive measurements of human brain temperature using volume-localized proton magnetic resonance spectroscopy. J Cereb Blood Flow Metab 1997;17:363–69 10.1097/00004647-199704000-00001 [DOI] [PubMed] [Google Scholar]
- 12. Covaciu L, Rubertsson S, Ortiz-Nieto F, et al. Human brain MR spectroscopy thermometry using metabolite aqueous-solution calibrations. J Magn Reson Imaging 2010;31:807–14 10.1002/jmri.22107 [DOI] [PubMed] [Google Scholar]
- 13. Karaszewski B, Wardlaw JM, Marshall I, et al. Measurement of brain temperature with magnetic resonance spectroscopy in acute ischemic stroke. Ann Neurol 2006;60:438–46 10.1002/ana.20957 [DOI] [PubMed] [Google Scholar]
- 14. Marshall I, Karaszewski B, Wardlaw JM, et al. Measurement of regional brain temperature using proton spectroscopic imaging: validation and application to acute ischemic stroke. Magn Reson Imaging 2006;24:699–706 10.1016/j.mri.2006.02.002 [DOI] [PubMed] [Google Scholar]
- 15. Marino S, Ciurleo R, Bramanti P, et al. 1H-MR spectroscopy in traumatic brain injury. Neurocrit Care 2011;14:127–33 10.1007/s12028-010-9406-6 [DOI] [PubMed] [Google Scholar]
- 16. Cady EB, D'Souza PC, Penrice J, et al. The estimation of local brain temperature by in vivo 1H magnetic resonance spectroscopy. Magn Reson Med 1995;33:862–67 10.1002/mrm.1910330620 [DOI] [PubMed] [Google Scholar]
- 17. Cady EB, Penrice J, Robertson NJ. Improved reproducibility of MRS regional brain thermometry by ‘amplitude-weighted combination.’ NMR Biomed 2011;24:865–72 10.1002/nbm.1634 [DOI] [PubMed] [Google Scholar]
- 18. Bainbridge A, Kendall GS, De Vita E, et al. Regional neonatal brain absolute thermometry by 1H MRS. NMR Biomed 2013;26:416–23 10.1002/nbm.2879 [DOI] [PubMed] [Google Scholar]
- 19. Wu TW, McLean C, Friedlich P, et al. Brain temperature in neonates with hypoxic-ischemic encephalopathy during therapeutic hypothermia. J Pediatr 2014;165:1129–34 10.1016/j.jpeds.2014.07.022 [DOI] [PubMed] [Google Scholar]
- 20. al Naqeeb N, Edwards AD, Cowan FM, et al. Assessment of neonatal encephalopathy by amplitude-integrated electroencephalography. Pediatrics 1999;103:1263–71 10.1542/peds.103.6.1263 [DOI] [PubMed] [Google Scholar]
- 21. Wintermark P, Labrecque M, Warfield SK, et al. Can induced hypothermia be assured during brain MRI in neonates with hypoxic-ischemic encephalopathy? Pediatr Radiol 2010;40:1950–54 10.1007/s00247-010-1816-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Barkovich AJ, Hajnal BL, Vigneron D, et al. Prediction of neuromotor outcome in perinatal asphyxia: evaluation of MR scoring systems. AJNR Am J Neuroradiol 1998;19:143–49 [PMC free article] [PubMed] [Google Scholar]
- 23. Executive summary: neonatal encephalopathy and neurologic outcome, second edition—Report of the American College of Obstetricians and Gynecologists' Task Force on Neonatal Encephalopathy. Obstet Gynecol 2014;123:896–901 10.1097/01.AOG.0000445580.65983.d2 [DOI] [PubMed] [Google Scholar]
- 24. Boudes E, Tan X, Saint-Martin C, et al. MRI obtained during versus after hypothermia in asphyxiated newborns. Arch Dis Child Fetal Neonatal Ed 2015;100:F238–42 10.1136/archdischild-2014-306550 [DOI] [PubMed] [Google Scholar]
- 25. Corbett RJ, Laptook AR, Tollefsbol G, et al. Validation of a noninvasive method to measure brain temperature in vivo using 1H NMR spectroscopy. J Neurochem 1995;64:1224–30 [DOI] [PubMed] [Google Scholar]
- 26. Weis J, Covaciu L, Rubertsson S, et al. Noninvasive monitoring of brain temperature during mild hypothermia. Magn Reson Imaging 2009;27:923–32 10.1016/j.mri.2009.01.011 [DOI] [PubMed] [Google Scholar]
- 27. Naressi A, Couturier C, Castang I, et al. Java-based graphical user interface for MRUI, a software package for quantitation of in vivo/medical magnetic resonance spectroscopy signals. Comput Biol Med 2001;31:269–86 10.1016/S0010-4825(01)00006-3 [DOI] [PubMed] [Google Scholar]
- 28. Stefan D, Di Cesare F, Andrasescu A, et al. Quantitation of magnetic resonance spectroscopy signals: the jMRUI software package. Meas Sci Technol 2009;20:104035 10.1088/0957-0233/20/10/104035 [DOI] [Google Scholar]
- 29. Pijnappel WW, van den Boogaart A, de Beer R, et al. SVD-based quantification of magnetic resonance signals. J Magn Reson 1992;97:122–34 [Google Scholar]
- 30. Kuroda K, Takei N, Mulkern RV, et al. Feasibility of internally referenced brain temperature imaging with a metabolite signal. Magn Reson Med Sci 2003;2:17–22 10.2463/mrms.2.17 [DOI] [PubMed] [Google Scholar]
- 31. De Vis JB, Hendrikse J, Petersen ET, et al. Arterial spin-labelling perfusion MRI and outcome in neonates with hypoxic-ischemic encephalopathy. Eur Radiol 2015;25:113–21 10.1007/s00330-014-3352-1 [DOI] [PubMed] [Google Scholar]
- 32. Massaro AN, Bouyssi-Kobar M, Chang T, et al. Brain perfusion in encephalopathic newborns after therapeutic hypothermia. AJNR Am J Neuroradiol 2013;34:1649–55 10.3174/ajnr.A3422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wintermark P, Hansen A, Gregas MC, et al. Brain perfusion in asphyxiated newborns treated with therapeutic hypothermia. AJNR Am J Neuroradiol 2011;32:2023–29 10.3174/ajnr.A2708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kusaka T, Ueno M, Miki T, et al. Relationship between cerebral oxygenation and phosphorylation potential during secondary energy failure in hypoxic-ischemic newborn piglets. Pediatr Res 2009;65:317–22 10.1203/PDR.0b013e318194fa73 [DOI] [PubMed] [Google Scholar]
- 35. Peng S, Boudes E, Tan X, et al. Does near-infrared spectroscopy identify asphyxiated newborns at risk of developing brain injury during hypothermia treatment? Am J Perinatol 2015;32:555–64 10.1055/s-0034-1396692 [DOI] [PubMed] [Google Scholar]
- 36. Tichauer KM, Wong DY, Hadway JA, et al. Assessing the severity of perinatal hypoxia-ischemia in piglets using near-infrared spectroscopy to measure the cerebral metabolic rate of oxygen. Pediatr Res 2009;65:301–06 10.1203/PDR.0b013e318194faa6 [DOI] [PubMed] [Google Scholar]
- 37. Toet MC, Lemmers PM, van Schelven LJ, et al. Cerebral oxygenation and electrical activity after birth asphyxia: their relation to outcome. Pediatrics 2006;117:333–39 10.1542/peds.2005-0987 [DOI] [PubMed] [Google Scholar]
- 38. Shaikh H, Lechpammer M, Jensen FE, et al. Increased brain perfusion persists over the first month of life in term asphyxiated newborns treated with hypothermia: does it reflect activated angiogenesis? Transl Stroke Res 2015;6:224–33 10.1007/s12975-015-0387-9 [DOI] [PubMed] [Google Scholar]
- 39. Bertolizio G, Mason L, Bissonnette B. Brain temperature: heat production, elimination and clinical relevance. Paediatr Anaesth 2011;21:347–58 10.1111/j.1460-9592.2011.03542.x [DOI] [PubMed] [Google Scholar]
- 40. Kiyatkin EA. Brain temperature homeostasis: physiological fluctuations and pathological shifts. Front Biosci (Landmark Ed) 2010;15:73–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rango M, Arighi A, Bresolin N. Brain temperature: what do we know? Neuroreport 2012;23:483–87 10.1097/WNR.0b013e3283534a60 [DOI] [PubMed] [Google Scholar]
- 42. Iwata S, Iwata O, Thornton JS, et al. Superficial brain is cooler in small piglets: neonatal hypothermia implications. Ann Neurol 2006;60:578–85 10.1002/ana.20978 [DOI] [PubMed] [Google Scholar]
- 43. Laptook AR, Shalak L, Corbett RJ. Differences in brain temperature and cerebral blood flow during selective head versus whole-body cooling. Pediatrics 2001;108:1103–10 10.1542/peds.108.5.1103 [DOI] [PubMed] [Google Scholar]