SUMMARY:
Recent data suggest that DKA may contribute to cognitive impairment in children with type 1 DM. We measured the NAA/Cr ratio in a teenager during and following 2 separate episodes of DKA without clinically apparent cerebral edema. The NAA/Cr ratio decreased during DKA and improved following recovery. However, the NAA/Cr value was lower after the second episode of DKA (1.76) than after the first (1.97). These findings provide support for the hypothesis that neuronal injury may result from DKA.
Several studies suggest that type 1 DM can lead to long-term alterations in cognitive function, and some studies have documented structural abnormalities of the brain in individuals with type 1 DM.1,2 The cause of brain injury in type 1 DM is not well understood, but recent data suggest that DKA may be strongly associated with cognitive impairment in children.3 Children who experienced an episode of DKA showed significantly decreased memory capacity compared with children with type 1 DM without a history of DKA.3 These data suggest that DKA may be an important factor causing permanent cerebral injury.
Case Report
A 14-year-old boy with type 1 DM experienced 2 episodes of DKA, separated by 2 months, without clinically apparent cerebral edema. At the first presentation of DKA, his initial blood glucose level was 780 mg/dL; pH, 6.99; serum bicarbonate level, 8 mmol/L; and BUN level, 20 mg/dL. At the time of the second presentation, his initial blood glucose level was 986 mg/dL; pH, 6.98; serum bicarbonate level, 8 mmol/L; and BUN, 27 mg/dL. He had normal mental status at presentation and maintained normal mental status (hourly Glasgow Coma Scale scores of 15) during both episodes, suggesting that he did not develop clinically relevant cerebral edema.
This case review was approved by our institutional review board. MR spectroscopy was performed on a 3T imaging system (8-channel Excite HD, OS Version 12M5; GE Healthcare, Milwaukee, Wisconsin) at 2 time points: 9–12 hours after initial presentation of DKA and after recovery from the episode (>72 hours after treatment, after resolution of metabolic acidosis and ketosis). A single voxel (8 cm3) at the right basal ganglia was studied using a Probe-P sequence with a TR/TE of 1500/144 ms. The NAA peak was identified according to its chemical shift at 2.02 ppm, and Cr, at 3.02 ppm. The heights of the peaks, which reflect the relative corresponding metabolite concentrations, were used to calculate the ratio of NAA/Cr. The basal ganglia were chosen as the point of interrogation because this region is especially susceptible to injury caused by DKA-related cerebral edema.
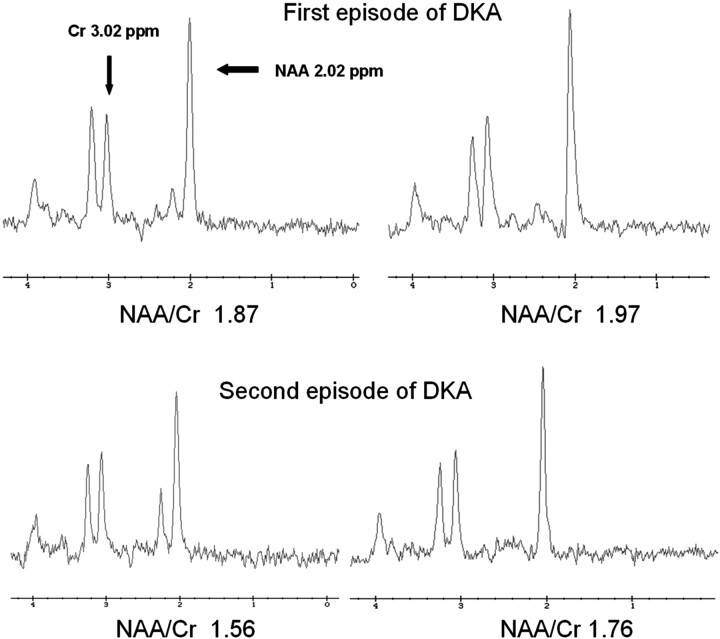
The NAA/Cr ratio was lower during acute DKA than after recovery in both episodes (1.87 versus 1.97 for the first episode, 1.56 versus 1.76 for the second episode, Fig 1). More important, the NAA/Cr ratio was lower after recovery from the second episode of DKA (1.76) than after recovery from the first episode (1.97).
Fig 1.
TR/TE 1500/144 ms single-voxel spectroscopy of the right basal ganglia in a 14-year-old boy during 2 episodes of DKA 2 months apart. The first episode of DKA is shown on the top 2 spectra, and second episode, on the bottom 2 spectra. The first of each pair of spectra was obtained during DKA treatment, 9–12 hours after beginning therapy. The second of the pair was obtained after recovery. During the acute DKA episode, the NAA/Cr ratio is decreased compared with the postrecovery spectra. In addition, the NAA/Cr ratio is lower (1.76) after recovery from the second episode of DKA compared with the postrecovery spectra from the first episode (1.97). This suggests a degree of permanent neuronal dysfunction or loss resulting from DKA.
Discussion
In this adolescent with type 1 DM, we observed a decrease in NAA/Cr ratios in the basal ganglia during each of 2 episodes of DKA. NAA/Cr ratios improved after recovery from each episode of DKA but did not return to baseline values. This pattern implies a component of permanent neuronal dysfunction or injury resulting from DKA.
NAA is a putative neuronal marker. Decreases in NAA may result from decreased neuronal viability or function, or neuronal loss. A previous study has demonstrated acute decreases in NAA levels in ischemic brain tissue, making NAA a valuable marker of brain injury.4 NAA levels have also been useful in predicting the degree of injury and potential recoverability of brain damage following head injury.4 Reduction of cerebral NAA may be reversible; thus, it can be used as a dynamic marker of neuronal dysfunction and integrity.5
The basal ganglia are a region thought to be particularly susceptible to injury caused by DKA-related cerebral edema.6 The vulnerability of the basal ganglia has been hypothesized to be related to the high adenosine triphosphate demand of this region.6 Some studies suggest that cerebral hypoperfusion may occur during DKA, resulting from dehydration and cerebral vasoconstriction related to hypocapnia.7 Hyperglycemia may also potentiate ischemic neuronal injury. The observed decrease in NAA/Cr during DKA correlates with these hypotheses and suggests that neuronal injury may result from DKA, even in the absence of clinically apparent cerebral edema or substantial mental status changes during DKA.
Previous studies by our group have documented consistently lower NAA/Cr values during DKA treatment compared with values measured after resolution of DKA.8 The increase in NAA/Cr after recovery from DKA implies a degree of neuronal recovery. In our previous studies, however, no comparison MR spectroscopy data were available before the DKA episodes; therefore, it was not possible to determine whether the recovery from DKA was complete. In the patient reported here, it appears that the neuronal recovery from DKA may not have been complete. The patient's NAA/Cr ratio after the second episode of DKA did not return to the baseline measurement obtained after his first DKA episode. It is, therefore, possible that repeated DKA episodes may result in progressive neurologic injury, which may lead to neurologic and/or cognitive decline in children with type 1 DM.
Supporting this possibility, a recent study found that a history of DKA was the strongest predictor of cognitive dysfunction in children with type 1 DM.3 Children with a history of DKA performed poorly on tests of contextual memory compared with children with type 1 diabetes who had never experienced DKA. Most children in the study had only experienced 1 DKA episode, typically at the time of diagnosis of diabetes. Differences in memory function between the 2 groups persisted after adjusting for age, duration of diabetes, episodes of hypoglycemia, and measures of glycemic control. Unfortunately, the patient reported here did not have neurocognitive testing performed, and the memory deficits reported in the study cited above are subtle and unlikely to be detectable without specific testing. Therefore, we cannot correlate the neuroimaging findings with cognitive data for this specific patient.
In summary, these data suggest that DKA may cause neuronal dysfunction or injury from which the patient recovers partially, but not completely. It is, therefore, possible that repeated DKA episodes may lead to progressive neurologic and/or cognitive decline in children with type 1 DM, and further study is warranted.
Abbreviations
- BUN
blood urea nitrogen
- Cr
creatine
- DKA
diabetic ketoacidosis
- DM
diabetes mellitus
- NAA
N-acetylaspartate
Footnotes
This work was supported by the National Institutes of Health grant 1R01 NS048610–01A1.
References
- 1. Naguib JM, Kulinskaya E, Lomax CL, et al. Neuro-cognitive performance in children with type 1 diabetes: a meta-analysis. J Pediatr Psychol 2009; 34: 271–82 [DOI] [PubMed] [Google Scholar]
- 2. Biessels GJ, Deary IJ, Ryan CM. Cognition and diabetes: a lifespan perspective. Lancet Neurol 2008; 7: 184–90 [DOI] [PubMed] [Google Scholar]
- 3. Ghetti S, Lee J, Holtpatrick C, et al. Diabetic ketoacidosis and memory impairment in children with type 1 diabetes. J Pediatr 2010; 156: 109–14 [DOI] [PubMed] [Google Scholar]
- 4. Moffett JR, Ross B, Arun P, et al. N-acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neruobiol 2007; 81: 89–131. Epub 2007 Jan 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Demougeot C, Garnier P, Mossiat C, et al. N-acetylaspartate, a marker of both cellular dysfunction and neuronal loss: its relevance to studies of acute brain injury. J Neurochem 2001; 77: 408–15 [DOI] [PubMed] [Google Scholar]
- 6. Muir AB, Quisling RG, Yang MC, et al. Cerebral edema in childhood diabetic ketoacidosis: natural history, radiographic findings and early identification. Diabetes Care 2004; 27: 1541–46 [DOI] [PubMed] [Google Scholar]
- 7. Glaser NS, Wootton-Gorges SL, Marcin JP, et al. Mechanism of cerebral edema in children with diabetic ketoacidosis. J Pediatr 2004; 145: 164–71 [DOI] [PubMed] [Google Scholar]
- 8. Wootton-Gorges SL, Buonocore MH, Kupperman N, et al. Cerebral proton magnetic resonance spectroscopy in children with diabetic ketoacidosis. AJNR Am J Neuroradiol 2007; 28: 895–99 [PMC free article] [PubMed] [Google Scholar]