Abstract
BACKGROUND AND PURPOSE:
The arterial spin-labeling method for CBF assessment is widely available, but its accuracy is not fully established. We investigated the accuracy of a whole-brain arterial spin-labeling technique for assessing the mean parenchymal CBF and the effect of aging in healthy volunteers. Phase-contrast MR imaging was used as the reference method.
MATERIALS AND METHODS:
Ninety-two healthy volunteers were included: 49 young (age range, 20–30 years) and 43 elderly (age range, 65–80 years). Arterial spin-labeling parenchymal CBF values were averaged over the whole brain to quantify the mean pCBFASL value. Total CBF was assessed with phase-contrast MR imaging as the sum of flows in the internal carotid and vertebral arteries, and subsequent division by brain volume returned the pCBFPCMRI value. Accuracy was considered as good as that of the reference method if the systematic difference was less than 5 mL/min/100 g of brain tissue and if the 95% confidence intervals were equal to or better than ±10 mL/min/100 g.
RESULTS:
pCBFASL correlated to pCBFPCMRI (r = 0.73; P < .001). Significant differences were observed between the pCBFASL and pCBFPCMRI values in the young (P = .001) and the elderly (P < .001) volunteers. The systematic differences (mean ± 2 standard deviations) were −4 ± 14 mL/min/100 g in the young subjects and 6 ± 12 mL/min/100 g in the elderly subjects. Young subjects showed higher values than the elderly subjects for pCBFPCMRI (young, 57 ± 8 mL/min/100 g; elderly, 54 ± 7 mL/min/100 g; P = .05) and pCBFASL (young, 61 ± 10 mL/min/100 g; elderly, 48 ± 10 mL/min/100 g; P < .001).
CONCLUSIONS:
The limits of agreement were too wide for the arterial spin-labeling method to be considered satisfactorily accurate, whereas the systematic overestimation in the young subjects and underestimation in the elderly subjects were close to acceptable. The age-related decrease in parenchymal CBF was augmented in arterial spin-labeling compared with phase-contrast MR imaging.
Using well-established perfusion imaging techniques, such as PET, SPECT, or other techniques such as perfusion CT, cerebral blood flow can be quantified within parenchymal tissue and expressed in milliliters per minute per 100 g of brain tissue (mL/min/100 g). These methods require injection of a contrast agent or a radioactive tracer. However, radiotracers are associated with exposure to ionizing radiation, CT contrast agents are nephrotoxic, and perfusion studies of this kind cannot be repeated until the contrast medium or tracer disappears. Using arterial spin-labeling (ASL) MR imaging,1 it is possible to assess parenchymal CBF (pCBF) noninvasively. Recent developments have enabled quantitative assessment of whole-brain perfusion with ASL within a few minutes.2,3 The accuracy of pCBF estimates obtained by using ASL, however, is still a subject of discussion.4 Age- and sex-related differences in pCBF have been found by using ASL, PET, and SPECT.5–10 However, these effects are still not fully understood, and no consensus has been established from previously published data.11,12
Total CBF is defined by the 4 arteries that supply the brain (ie, the internal carotid arteries and the vertebral arteries [VAs]). The blood flow of these arteries, and thus the total CBF, can be measured with good accuracy at the level of the foramen magnum by using 2D phase-contrast MR imaging (PCMRI).13,14 Using high-resolution morphologic MR imaging data and postprocessing software, the total volume of the brain parenchymal tissue can be assessed. Total CBF can be obtained accurately with PCMRI (shown here as pCBFPCMRI values),14 and brain parenchymal volume can be measured accurately15 from the T1 sequence. By dividing flow by volume, pCBFPCMRI can be estimated with expected good accuracy and used as a reference to evaluate the accuracy of pCBF obtained via ASL (pCBFASL).
The aim of this study was to investigate the accuracy of a clinically implemented pseudocontinuous ASL method for assessing pCBF in 92 healthy individuals by using PCMRI as the reference method. The effects of aging and sex on pCBF were assessed by using both methods, and the results were compared.
Materials and Methods
Subjects
A total of 111 subjects, recruited by advertisement in a daily newspaper, were included in this prospective study. The subjects were defined as healthy if they had no neurologic or cardiac disease, hypertension, peripheral vascular disease, or renal disease. Eleven subjects were excluded after the physical examination because of a Mini-Mental State Examination score of <28 points (n = 3),16 electrocardiogram changes (n = 1), a blood pressure of >160/90 mm Hg (n = 1), or neurologic issues (n = 6). After the MR imaging examination, 8 subjects were excluded because of claustrophobia (n = 3) or technical problems or missing MR imaging data (n = 5). The remaining 92 healthy subjects were categorized in 1 of 2 different age groups (ie, 49 subjects in the healthy young [HY] group [age range, 20–30 years; mean age ± standard deviation, 25 ± 2 years; 27 women] and 43 subjects in the healthy elderly [HE] group [age range, 65–80 years; mean age ± standard deviation, 71 ± 4 years; 23 women]). In addition, the 92 subjects were classified according to sex (ie, the study group included 50 healthy women and 42 healthy men). The research protocol used in this study was approved by the ethical review board of Umeå University. Each patient provided oral and written informed consent.
MR Imaging
Each subject was scanned by using a 3T MR imaging unit (Discovery MR 750; GE Healthcare, Milwaukee, Wisconsin) supplied with a 32-channel head coil.
Three-dimensional time-of-flight angiography was performed to visualize the ICAs and the VAs. TOF angiography was used to position a perpendicular PCMRI plane at the cervical (C1–C2) level. The 2D PCMRI data were acquired with the following parameters: TR, 9 ms; TE, 5 ms; section thickness, 5 mm; flip angle, 15°; FOV, 180 × 180 mm2; acquisition matrix, 512 × 512; in-plane resolution, 0.35 × 0.35 mm2; views per segment, 6; velocity encoding, 70 cm/s; and NEX, 2. Thirty-two velocity-coded and magnitude images throughout the entire cardiac cycle were collected. A peripheral pulse signal was used for retrospective cardiac gating. The acquisition time of the PCMRI was approximately 2 minutes 30 seconds, depending on the subject's heart rate.
Whole-brain perfusion data were obtained by using a 3D pseudocontinuous ASL (pCASL) method implemented by the manufacturer (Appendix). In summary, the pCASL was applied, followed by an interleaved 3D stack of spiral fast spin-echo readout with background suppression.3 The pCASL parameters were as follows: sampling points on 8 spirals, 512; FOV, 240 × 240 mm2; true in-plane resolution, 3.75 mm17–19; reconstructed matrix, 128 × 128; TR, 4674 ms; TE, 10 ms; NEX, 3; section thickness, 4 mm; labeling plane positioned at the base of the cerebellum; labeling duration, 1500 ms; postlabeling delay, 1525 ms20; sections covering the whole brain, 40–44; control/label pairs, 30; and acquisition time, 4 minutes 31 seconds.
High-resolution T1-weighted data for assessing brain parenchymal volume were collected by using a sagittal 3D fast-spoiled gradient-echo sequence to image the whole brain with 176 sections, a section thickness of 1 mm, a TR of 7 ms, a TE of 2 ms, a flip angle of 10°, a FOV of 250 × 250 mm, an acquisition matrix of 256 × 256, and an acquisition time of 5 minutes 20 seconds.
MR Imaging Data Postprocessing and Analysis
Brain Parenchymal Tissue Segmentation.
The T1-weighted data were processed by using the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm.html) and default parameters of SPM8 software (http://www.fil.ion.ucl.ac.uk/spm/) by using Matlab R2013b (MathWorks, Natick, Massachusetts). The segmentation method of VBM8 is based on an adaptive maximum a posteriori approach,21 and tissue compartments were classified into gray matter, white matter, and CSF. GM and WM segmentations were inspected visually to ensure quality of the segmentation. No severe missegmentation of brain parenchymal tissue was observed, and therefore no data were omitted from the analysis. However, in a few of the elderly subjects, the periventricular WM was misclassified as GM, but the segmentation did not alter the whole-brain segmentation, and no manual correction had to be performed.
ASL Measurement of Parenchymal Cerebral Blood Flow.
The pCASL pCBF maps (in mL/min/100 g) were computed by the postprocessing FuncTool software (version 10.4.04; GE Healthcare) that was based on a general kinetic model for ASL.22 The details of the manufacturer's implementation method to quantify the pCBF maps are shown in the Appendix.
Using the SPM8 software, GM and WM masks were co-registered to the ASL data and down-sampled to the same pixel size as that of the reconstructed ASL data. The GM and WM masks were then smoothed in-plane with a Gaussian kernel (3.25 × 3.25 mm2 full width at half maximum) to create a resolution identical to the true spatial resolution of ASL (On-line Fig 1). Erosion was applied to exclude the 2 outer pixel layers from the brain mask (GM and WM) to avoid ASL artifacts and inclusion of the skull. Each pixel in the brain mask contains the volume fractions of GM (FGM) and WM (FWM). The brain parenchymal volume was calculated as the sum of the GM and WM volumes. The mean pCBF from the ASL data (pCBFASL) was estimated by using equation 1:
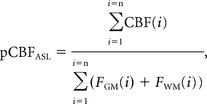 |
where FGM and FWM are the volume fractions of GM and WM, respectively, CBF(i) is the cerebral blood flow (mL/min/100 g of brain tissue) within the ith ASL pixel, and n is the number of pixels that contain brain tissue (FGM + FWM > 0%). Our aim was to estimate the perfusion in the entire parenchymal tissue. No partial volume correction was thus necessary to separate perfusion signal from individual WM and GM voxels.
PCMRI Measurement of Vessel Velocity and Parenchymal Cerebral Blood Flow.
PCMRI data were analyzed by using Segment software version 1.8 (Mediviso, Lund, Sweden). The magnitude images were used to delineate manually the cross-section areas of the ICAs and VAs. The positions and sizes of the cross-section areas were kept constant during the cardiac cycle. For each vessel, the flow rate was computed as the mean velocity multiplied by the cross-section area.
The blood flow rates of the bilateral ICAs and VAs were summed, and the derived blood flow rate was averaged over the cardiac cycle to estimate the total CBF (reported in milliliters per minute). Thereafter, the parenchymal cerebral blood flow from PCMRI (pCBFPCMRI) was calculated in milliliters per minute per 100 g of brain tissue by using equation 2:
where tCBF is total CBF, BPV is brain parenchymal volume, and ρ is the brain tissue density (1.05 g/mL).23
Furthermore, the velocity of blood in the labeling plane directly affects the labeling efficiency of the pCASL and thus also the pCBF quantification.3,13 To investigate the effect of the mean velocity of the bilateral ICAs and VAs on the ASL data, mean velocities were computed and correlated to the difference between pCBFPCMRI and pCBFASL.
Statistical Analysis
SPSS Statistics version 18 (IBM, Armonk, New York) was used to perform statistical analysis. Variables were expressed as means ± standard deviation. The Shapiro-Wilk test was used to test the normal distribution of the measured parameters. Differences between the groups were investigated by using the unpaired Student t test. For the comparison between pCBFASL and pCBFPCMRI values, linear regression analysis and Bland-Altman plots were used.24 Differences between the pCBFPCMRI and pCBFASL values were tested by using the paired Student t test. The accuracy was defined as the systematic bias and the random difference (mean difference ± 2 standard deviations) between the 2 methods. We considered the accuracy of ASL-based pCBF measurement to be good if the systematic difference against the reference method was <5 mL/min/100 g and if the randomized difference was less than or equal to ±10 mL/min/100 g, which corresponds to the limits of agreement previously shown for repeated measurements with PCMRI14 and for repeated measurements with ASL.19 A P value of <.05 was considered statistically significant.
Results
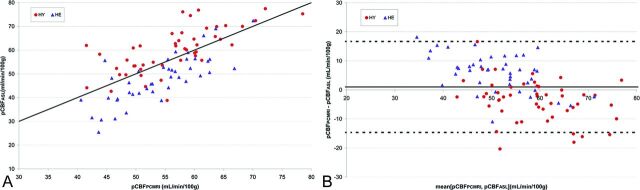
Comparison of PCMRI and ASL
The pCBF values obtained by PCMRI and ASL are shown in the Table, and a comparison between the pCBFPCMRI and pCBFASL values is displayed in the Figure. There was a significant correlation between pCBFPCMRI and pCBFASL values (r = 0.73; P < .001). Corresponding correlation coefficients (r) when the HY and HE groups were compared separately were 0.73 (P < .001) and 0.78 (P < .001), respectively. As shown in the Table, there was a significant difference between pCBFASL and pCBFPCMRI for HE (P < .001) and HY (P = .001) subjects but not for the whole group (P = .34). For all subjects, the mean bias and the limits of agreement between the 2 methods were 1 ± 16 mL/min/100 g (mean ± 2 standard deviations). The mean bias (pCBFPCMRI − pCBFASL) showed an underestimation by ASL in HE subjects (6 ± 12 mL/min/100 g) and an overestimation in HY subjects (−4 ± 14 mL/min/100 g), whereas it was similar for healthy men and women (2 ± 19 and 0 ± 16 mL/min/100 g, respectively; P = .256).
Comparison of pCBFPCMRI and pCBFASL with respect to age and sex
| Subjects | pCBFPCMRI (Mean ± SD) (mL/min/100 g) | pCBFASL (Mean ± SD) (mL/min/100 g) | P Value |
|---|---|---|---|
| HY (n = 49) | 57 ± 8 | 61 ± 10 | <.05a |
| HE (n = 43) | 54 ± 7 | 48 ± 10 | <.001a |
| HW (n = 50) | 58 ± 8 | 58 ± 12 | .94 |
| HM (n = 42) | 53 ± 6 | 51 ± 11 | .16 |
| All (n = 92) | 56 ± 8 | 55 ± 12 | .34 |
Note:—HW indicates healthy women; HM, healthy men.
Value is significant.
Figure.
A, Scatterplot of parenchymal cerebral blood flow determined by ASL (pCBFASL) versus PCMRI-determined parenchymal cerebral blood flow (pCBFPCMRI). The solid black line is the identity line. The correlation coefficient r is 0.73 (P < .001). B, Corresponding Bland-Altman plot. The horizontal dashed lines represent the 95% confidence intervals of the difference between pCBFPCMRI and pCBFASL.
The difference between pCBFPCMRI and pCBFASL showed significant correlation with the mean velocities of the VAs (r = −0.47; P < .001; On-line Fig 2) and ICAs (r = −0.35; P = .001).
Dependence of pCBFASL and pCBFPCMRI on Age and Sex
pCBFASL values were significantly higher in HY than in HE subjects (P < .001; Table). A similar pattern emerged for pCBFPCMRI values, but the difference was less evident (P = .05; Table). Therefore, the percent decrease of the mean pCBF with aging was lower in pCBFPCMRI (−5%) than in pCBFASL (−21%). The pCBFPCMRI and pCBFASL values were significantly higher in women (pCBFPCMRI, 9% [P = 4 × 10−4]; pCBFASL, 12% [P = .002]).
Discussion
Methods for assessing cerebral perfusion by using ASL are available on most modern MR imaging scanners, but their accuracy is still not fully established. Using high-spatial-resolution PCMRI as the reference method, the accuracy of ASL was investigated in this study, and the dependencies on age and sex were quantified and compared. A good correlation was found between the reference method and ASL, but a significant difference between the mean values was observed for both HE (approximately −11%) and HY (5%) subjects. Consequently, the observed effect of aging on pCBF was estimated as much lower in values obtained from PCMRI than in those from ASL (5% vs 21%). Because of ASL overestimation in HY subjects and underestimation in HE subjects, no significant difference between pCBFPCMRI and pCBFASL values was observed in the group as a whole (Table).
In this study, high-resolution PCMRI with an in-plane resolution of 0.35 mm was used, which represents >8 pixels per diameter for the internal carotid and vertebral arteries. With high spatial resolution (>4 pixels per diameter) and by using similar MR imaging parameters (velocity encoding, TE, TR, and section thickness) as in the present study, it was shown previously that PCMRI can accurately (<10% error) measure the blood flow in ICAs and VAs and thus can be considered a criterion-standard technique for measuring total CBF.14
The ASL sequence used in this study had a short MR imaging acquisition time (<5 minutes for whole-brain coverage), and we used a pseudocontinuous arterial-labeling scheme with 3D segmented readout and background suppression, which is considered one of the best ASL approaches for assessing pCBF.2 It is important to emphasize that in this study, the ASL data were obtained with a commercially available ASL sequence, and the CBF estimates were quantified by using the manufacturer's postprocessing software without any additional corrections, as was also done in previous studies.19,25 It should be mentioned that the CBF quantification model used in this study was slightly different than the model proposed in a recent consensus article concerning ASL for clinical applications (Appendix).2
Jain et al26 reported results from a group of children that were similar to ours (ie, a significant correlation of pCBF values determined by PCMRI and ASL). Other studies have found moderate to good correlations (r = 0.4–0.8) between pseudocontinuous ASL and PET imaging for pCBF measurements.4,27,28 On the contrary, Henriksen et al29 showed a large underestimation of ASL-based pCBF (75%) compared with the estimation by PCMRI and no correlation between the 2 methods. One explanation for this result might be that they used a model-free pulsed-ASL method. The results of our study further support the use of pseudocontinuous ASL. PET is most likely a good method for comparison with ASL, because it is possible to perform intermodal comparisons of global and regional brain perfusion measurements. A recent study found a relatively low correlation between ASL and PET for measuring pCBF in GM at resting state,4 but it is not feasible to repeat such a study with a large number of volunteers.
In accordance with the results of our study, previous ASL studies found a difference of mean pCBF (17%) or perfusion in GM (20%–30%) between HY and HE subjects.5,6 In these previous ASL studies, no age-specific postlabeling delay was used, and the postlabeling delays that were used ranged from 800 to 1700 ms. PET and SPECT studies have revealed similar results.9,10 Our findings confirm that pCBF decreases with age in healthy adults. However, the magnitude of the decline in pCBF as determined by ASL was approximately 4 times larger than that determined by PCMRI, and these results bring into question previous observations regarding the magnitude of decrease in ASL-derived pCBF that is associated with healthy aging. Aging causes general brain atrophy and cortical thinning, which may increase the partial volume effects,30,31 and aging also leads to increased arterial transit time.32 Potentially, such changes influence the accuracy of ASL.5 Increasing the postlabeling delay for elderly subjects could remedy some ASL inaccuracies.2 Furthermore, the CBF quantification model used in our study (see Appendix and equation 3) assumes that the longitudinal relaxation time of gray matter (T1GM) and the brain-to-blood partition coefficient (λ) are constant. However, previous studies have provided no real consensus with regard to a possible effect of aging on T1GM.33,34 Furthermore, it is known that λ values are higher in the neonatal brain than in the adult brain.35 Hence, we cannot rule out the possibility that the brain-to-blood partition coefficient varies over a life span between 25 and 71 years of age.
Women had higher pCBFPCMRI (9%) and pCBFASL (12%) values than the men in this study. Similar results have been reported, with pCBF values being 9%–15% higher in women.5,10,36 Because various imaging modalities have indicated the same relative difference, it can be regarded as reliable, and it indicates that the accuracy of the ASL method was not affected by differences related to sex. The T1 relaxation of blood (T1b) can influence the accuracy of ASL perfusion measurements.26,37 Previous studies have shown higher mean T1b values (6%–9%) in women than in men, and the lower blood hematocrit level in women than in men may explain this observed sex difference in mean T1b.38–40 In a previous study, Piechnik et al40 found significantly higher mean T1b values in women than in men (1577 vs 1491 ms, respectively). Using equation 3 in the Appendix and T1b for men and for women, we estimated that the relative sex difference in pCBFASL values in our data decreased from 12% to approximately 4%, which then is less than the PCMRI findings. In the same study, Piechnik et al40 found no differences in T1b values between HY (20–30 years) and HE (60–70 years) subjects, which indicates that the T1b effect is not the dominating factor in explaining the large effect of aging on pCBFASL.
Motion artifacts during the ASL scan were not corrected, which might be a source of error in the pCBFASL estimates. Another factor that might influence the ASL perfusion accuracy is the location of the labeling plane. In the present study, the labeling plane was located at the base of the cerebellum and should be oriented perpendicularly to the cerebral feeding arteries. This placement was difficult to achieve; manually placing the labeling plane for ASL was not possible, because the current commercial implementation of pCASL does not allow it. Furthermore, the tortuosity of cerebral arteries increases with age, which may partly explain the underestimation of pCBFASL in HE subjects.41 For 5 HE subjects, pCBFASL values were unreasonably low (ie, of the order of 20–30 mL/min/100 g; Fig 1A). When we excluded these HE subjects from the analysis, we observed a minor increase (from 46 to 48 mL/min/100 g) in the mean pCBFASL in HE subjects, which did not change our main conclusions. Furthermore, we visually inspected the labeling-plane position in these 5 HE subjects with respect to the geometry of the feeding cerebral arteries by using TOF angiography. In 3 subjects, the labeling plane was close to parallel with the VAs, and ASL data showed a very low CBFASL in the posterior region (see example in On-line Fig 3). However, on 2 other HE subjects with a low pCBFASL, the labeling plane was close to perpendicular to both ICAs and VAs, and thus its position should not cause the low CBFASL that we observed in the posterior regions (On-line Fig 3). In future studies, it will be important to investigate in detail how the tortuosity of ICAs and VAs can alter the estimation of pCBF values with ASL.
The tortuosity of ICAs and VAs is also challenging for the PCMRI method, in which misalignment of the PCMRI plane can cause an underestimation of the total cerebral blood flow.42 In our study, this potential problem was partially avoided by careful manual placement of the PCMRI planes in the TOF angiogram. A potential source of pCBF overestimation in PCMRI is the inclusion of extracerebral blood flow of the anterior spinal artery and ophthalmic arteries. The lumen of the spinal artery has been reported to be small (diameter, <1 mm), and the total blood flow rate of the slightly larger ophthalmic artery is approximately 22 mL/min in healthy adults.43,44 Hence, we estimated that the total blood flow of these extracerebral arteries could represent 3%–4% of potential pCBF overestimation by using our reference PCMRI method.
Finally, pCBF quantification in white matter by ASL is problematic because of the low signal-to-noise ratio45 and its long and nonuniform arterial transit time.46,47 If white matter pCBF shows a systematic bias, it would affect the mean whole-brain value.
Conclusions
For mean parenchymal cerebral blood flow, a high degree of correlation was found between the ASL and PCMRI (reference) methods. For HY adults, the accuracy of pCBF assessment determined by ASL was good with regard to the systematic difference, though the randomized difference against the PCMRI method was outside of the limits according to our criteria. There were both systematic underestimation and a similarly large randomized difference in results for the HE subjects. Consequently, age-related reductions in pCBF became augmented with ASL compared with the reference method.
Supplementary Material
ABBREVIATIONS:
- ASL
arterial spin-labeling
- HE
healthy elderly
- HY
healthy young
- pCASL
pseudocontinuous ASL
- pCBF
parenchymal CBF
- PCMRI
phase-contrast MRI
- VA
vertebral artery
APPENDIX
The true in-plane spatial resolution of the pCASL sequence was 3.75 mm.17–19 Background suppression pulses were achieved by saturating the imaged volume before labeling and by applying 4 nonselective inversion pulses at 1500 ms, 680 ms, 248 ms, and 57 ms before readout.48 A reference image was obtained 2000 ms after saturation in the same sequence as the rest of the ASL data.
The following description of the CBF quantification method was provided by the manufacturer of the MR imaging scanner:
where PLD is the postlabeling delay time (1525 ms); τ is the labeling duration (1500 ms); α is a combination of inversion efficiency (0.8) and background suppression efficiency (0.75)48 resulting in an overall labeling efficiency of 0.6; λ is the tissue-to-blood partition coefficient (0.9 mL/g)35; T1b and T1GM are the longitudinal relaxation times of blood (1600 ms) and GM (1200 ms), respectively; Tsat is the saturation time (2000 ms)18; S0 is the reference image signal (obtained voxelwise); and ΔS is the ASL difference image signal. The scaling factor 6000 was used to convert to CBF units (mL/min/100 g). In our study, it was assumed that WM perfusion can be calculated by using the model described above and setting parameters. Compared with the quantification proposed by Alsop et al,2 a term for compensation of the imperfect relaxation in the reference image is added.
Footnotes
Disclosures: Ronnie Wirestam—RELATED: Grant: Swedish Research Council,* Comments: Government funding for part of my salary as university professor. Anders Eklund—RELATED: Grant: Swedish Research Council.* *Money paid to institution.
This work was supported by Swedish Research Council (grants 621-2011-5216 and 13514), European Union Objective 2 Norra Norrland (project 148273 CMTF), County Council of Västerbotten and Swedish Heart and Lung Foundation (grant 20110383), and the Swedish Brain Foundation.
REFERENCES
- 1. Williams DS, Detre JA, Leigh JS, et al. Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc Natl Acad Sci U S A 1992;89:212–16 10.1073/pnas.89.1.212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alsop DC, Detre JA, Golay X, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 2014. April 8. [Epub ahead of print] 10.1002/mrm.25197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dai W, Garcia D, de Bazelaire C, et al. Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn Reson Med 2008;60:1488–97 10.1002/mrm.21790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heijtel DF, Mutsaerts HJ, Bakker E, et al. Accuracy and precision of pseudo-continuous arterial spin labeling perfusion during baseline and hypercapnia: a head-to-head comparison with 15O H2O positron emission tomography. Neuroimage 2014;92:182–92 10.1016/j.neuroimage.2014.02.011 [DOI] [PubMed] [Google Scholar]
- 5. Parkes LM, Rashid W, Chard DT, et al. Normal cerebral perfusion measurements using arterial spin labeling: reproducibility, stability, and age and gender effects. Magn Reson Med 2004;51:736–43 10.1002/mrm.20023 [DOI] [PubMed] [Google Scholar]
- 6. Asllani I, Habeck C, Borogovac A, et al. Separating function from structure in perfusion imaging of the aging brain. Hum Brain Mapp 2009;30:2927–35 10.1002/hbm.20719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Biagi L, Abbruzzese A, Bianchi MC, et al. Age dependence of cerebral perfusion assessed by magnetic resonance continuous arterial spin labeling. J Magn Reson Imaging 2007;25:696–702 10.1002/jmri.20839 [DOI] [PubMed] [Google Scholar]
- 8. Chen JJ, Rosas HD, Salat DH. Age-associated reductions in cerebral blood flow are independent from regional atrophy. Neuroimage 2011;55:468–78 10.1016/j.neuroimage.2010.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Leenders KL, Perani D, Lammertsma AA, et al. Cerebral blood flow, blood volume and oxygen utilization. Normal values and effect of age. Brain 1990;113:27–47 10.1093/brain/113.1.27 [DOI] [PubMed] [Google Scholar]
- 10. Slosman DO, Chicherio C, Ludwig C, et al. (133)Xe SPECT cerebral blood flow study in a healthy population: determination of T-scores. J Nucl Med 2001;42:864–70 [PubMed] [Google Scholar]
- 11. Meltzer CC, Cantwell MN, Greer PJ, et al. Does cerebral blood flow decline in healthy aging? A PET study with partial-volume correction. J Nucl Med 2000;41:1842–48 [PubMed] [Google Scholar]
- 12. Aanerud J, Borghammer P, Chakravarty MM, et al. Brain energy metabolism and blood flow differences in healthy aging. J Cereb Blood Flow Metab 2012;32:1177–87 10.1038/jcbfm.2012.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aslan S, Xu F, Wang PL, et al. Estimation of labeling efficiency in pseudocontinuous arterial spin labeling. Magn Reson Med 2010;63:765–71 10.1002/mrm.22245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wåhlin A, Ambarki K, Hauksson J, et al. Phase contrast MRI quantification of pulsatile volumes of brain arteries, veins, and cerebrospinal fluids compartments: repeatability and physiological interactions. J Magn Reson Imaging 2012;35:1055–62 10.1002/jmri.23527 [DOI] [PubMed] [Google Scholar]
- 15. Valverde S, Oliver A, Cabezas M, et al. Comparison of 10 brain tissue segmentation methods using revisited IBSR annotations. J Magn Reson Imaging 2015;41:93–101 10.1002/jmri.24517 [DOI] [PubMed] [Google Scholar]
- 16. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 17. Pienaar R, Paldino MJ, Madan N, et al. A quantitative method for correlating observations of decreased apparent diffusion coefficient with elevated cerebral blood perfusion in newborns presenting cerebral ischemic insults. Neuroimage 2012;63:1510–18 10.1016/j.neuroimage.2012.07.062 [DOI] [PubMed] [Google Scholar]
- 18. Järnum H, Steffensen EG, Knutsson L, et al. Perfusion MRI of brain tumours: a comparative study of pseudo-continuous arterial spin labelling and dynamic susceptibility contrast imaging. Neuroradiology 2010;52:307–17 10.1007/s00234-009-0616-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mutsaerts HJ, Steketee RM, Heijtel DF, et al. Inter-vendor reproducibility of pseudo-continuous arterial spin labeling at 3 Tesla. PLoS One 2014;9:e104108 10.1371/journal.pone.0104108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Melzer TR, Watts R, MacAskill MR, et al. Arterial spin labelling reveals an abnormal cerebral perfusion pattern in Parkinson's disease. Brain 2011;134:845–55 10.1093/brain/awq377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rajapakse JC, Giedd JN, Rapoport JL. Statistical approach to segmentation of single-channel cerebral MR images. IEEE Trans Med Imaging 1997;16:176–86 10.1109/42.563663 [DOI] [PubMed] [Google Scholar]
- 22. Buxton RB, Frank LR, Wong EC, et al. A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn Reson Med 1998;40:383–96 10.1002/mrm.1910400308 [DOI] [PubMed] [Google Scholar]
- 23. Torack RM, Alcala H, Gado M, et al. Correlative assay of computerized cranial tomography CCT, water content and specific gravity in normal and pathological postmortem brain. J Neuropathol Exp Neurol 1976;35:385–92 10.1097/00005072-197607000-00001 [DOI] [PubMed] [Google Scholar]
- 24. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307–10 [PubMed] [Google Scholar]
- 25. Bron EE, Steketee RM, Houston GC, et al. ; Alzheimer's Disease Neuroimaging Initiative. Diagnostic classification of arterial spin labeling and structural MRI in presenile early stage dementia. Hum Brain Mapp 2014;35:4916–31 10.1002/hbm.22522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jain V, Duda J, Avants B, et al. Longitudinal reproducibility and accuracy of pseudo-continuous arterial spin-labeled perfusion MR imaging in typically developing children. Radiology 2012;263:527–36 10.1148/radiol.12111509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Golen LW, Kuijer JP, Huisman MC, et al. Quantification of cerebral blood flow in healthy volunteers and type 1 diabetic patients: comparison of MRI arterial spin labeling and [(15)O]H2O positron emission tomography (PET). J Magn Reson Imaging 2014;40:1300–09 10.1002/jmri.24484 [DOI] [PubMed] [Google Scholar]
- 28. Zhang K, Herzog H, Mauler J, et al. Comparison of cerebral blood flow acquired by simultaneous [15O]water positron emission tomography and arterial spin labeling magnetic resonance imaging. J Cereb Blood Flow Metab 2014;34:1373–80 10.1038/jcbfm.2014.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Henriksen OM, Larsson HB, Hansen AE, et al. Estimation of intersubject variability of cerebral blood flow measurements using MRI and positron emission tomography. J Magn Reson Imaging 2012;35:1290–99 10.1002/jmri.23579 [DOI] [PubMed] [Google Scholar]
- 30. Kety SS. Human cerebral blood flow and oxygen consumption as related to aging. J Chronic Dis 1956;3:478–86 10.1016/0021-9681(56)90146-1 [DOI] [PubMed] [Google Scholar]
- 31. Good CD, Johnsrude IS, Ashburner J, et al. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage 2001;14:21–36 10.1006/nimg.2001.0786 [DOI] [PubMed] [Google Scholar]
- 32. Scheel P, Ruge C, Schöning M. Flow velocity and flow volume measurements in the extracranial carotid and vertebral arteries in healthy adults: reference data and the effects of age. Ultrasound Med Biol 2000;26:1261–66 10.1016/S0301-5629(00)00293-3 [DOI] [PubMed] [Google Scholar]
- 33. Cho S, Jones D, Reddick WE, et al. Establishing norms for age-related changes in proton T1 of human brain tissue in vivo. Magn Reson Imaging 1997;15:1133–43 10.1016/S0730-725X(97)00202-6 [DOI] [PubMed] [Google Scholar]
- 34. Breger RK, Yetkin FZ, Fischer ME, et al. T1 and T2 in the cerebrum: correlation with age, gender, and demographic factors. Radiology 1991;181:545–47 10.1148/radiology.181.2.1924802 [DOI] [PubMed] [Google Scholar]
- 35. Herscovitch P, Raichle ME. What is the correct value for the brain–blood partition coefficient for water? J Cereb Blood Flow Metab 1985;5:65–69 10.1038/jcbfm.1985.9 [DOI] [PubMed] [Google Scholar]
- 36. Esposito G, Van Horn JD, Weinberger DR, et al. Gender differences in cerebral blood flow as a function of cognitive state with PET. J Nucl Med 1996;37:559–64 [PubMed] [Google Scholar]
- 37. Varela M, Hajnal JV, Petersen ET, et al. A method for rapid in vivo measurement of blood T1. NMR Biomed 2011;24:80–88 10.1002/nbm.1559 [DOI] [PubMed] [Google Scholar]
- 38. Wu WC, Jain V, Li C, et al. In vivo venous blood T1 measurement using inversion recovery true-FISP in children and adults. Magn Reson Med 2010;64:1140–47 10.1002/mrm.22484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Qin Q, Strouse JJ, van Zijl PC. Fast measurement of blood T1 in the human jugular vein at 3 Tesla. Magn Reson Med 2011;65:1297–304 10.1002/mrm.22723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Piechnik SK, Ferreira VM, Lewandowski AJ, et al. Normal variation of magnetic resonance T1 relaxation times in the human population at 1.5 T using ShMOLLI. J Cardiovasc Magn Reson 2013;15:13 10.1186/1532-429X-15-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bullitt E, Zeng D, Mortamet B, et al. The effects of healthy aging on intracerebral blood vessels visualized by magnetic resonance angiography. Neurobiol Aging 2010;31:290–300 10.1016/j.neurobiolaging.2008.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhao M, Charbel FT, Alperin N, et al. Improved phase-contrast flow quantification by three-dimensional vessel localization. Magn Reson Imaging 2000;18:697–706 10.1016/S0730-725X(00)00157-0 [DOI] [PubMed] [Google Scholar]
- 43. Sheehy NP, Boyle GE, Meaney JF. Normal anterior spinal arteries within the cervical region: high-spatial-resolution contrast-enhanced three-dimensional MR angiography. Radiology 2005;236:637–41 10.1148/radiol.2362040804 [DOI] [PubMed] [Google Scholar]
- 44. Ambarki K, Hallberg P, Jóhannesson G, et al. Blood flow of ophthalmic artery in healthy individuals determined by phase-contrast magnetic resonance imaging. Invest Ophthalmol Vis Sci 2013;54:2738–45 10.1167/iovs.13-11737 [DOI] [PubMed] [Google Scholar]
- 45. van Osch MJ, Teeuwisse WM, van Walderveen MA, et al. Can arterial spin labeling detect white matter perfusion signal? Magn Reson Med 2009;62:165–73 10.1002/mrm.22002 [DOI] [PubMed] [Google Scholar]
- 46. Pohmann R. Accurate, localized quantification of white matter perfusion with single-voxel ASL. Magn Reson Med 2010;64:1109–13 10.1002/mrm.22476 [DOI] [PubMed] [Google Scholar]
- 47. Lu K, Liu T, Wong EC, et al. Regional white matter perfusion measurement using an optimized pseudo-continuous ASL MRI. In: Proceedings of the 17th Annual Meeting of the International Society of Magnetic Resonance in Medicine [abstract 4401]. Honolulu, Hawaii. April 18–24, 2009 [Google Scholar]
- 48. Garcia DM, Duhamel G, Alsop DC. Efficiency of inversion pulses for background suppressed arterial spin labeling. Magn Reson Med 2005;54:366–72 10.1002/mrm.20556 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.