Abstract
Objective.
To delineate the impact of treatment exposures and chronic health conditions on psychological, educational, and social outcomes in adolescent survivors of Wilms tumor.
Methods.
Parent reports from the Childhood Cancer Survivor Study were analyzed for 666 adolescent survivors of Wilms tumor and 698 adolescent siblings. Adjusting for race and household income, survivors were compared to siblings on the Behavior Problems Index and educational outcomes. Multivariable modified Poisson regression estimated relative risks (RR) for therapeutic exposures and chronic health conditions (CTCAE 4.03 graded) among survivors, adjusting for sex, race, income, and age at diagnosis.
Results.
Compared to siblings, adolescent survivors of Wilms tumor were more likely to take psychoactive medication (9.4% versus 5.1%, p<0.001) and utilize special education services (25.5% versus 12.6%, p<0.001) but did not differ significantly in emotional and behavioral problems. Survivors were less likely to be friendless (7.2% vs. 10.1%, p=0.04) but were more likely to have difficulty getting along with friends (14.5% vs. 7.8%, p<0.001). Among survivors, use of special education services was associated with abdomen plus chest radiation (RR=1.98, CI:1.18-3.34). Those with grade 2-4 cardiovascular conditions had higher risk for anxiety/depression (RR=1.95, CI:1.19-3.19), headstrong behaviors (RR=1.91, CI:1.26-2.89), and inattention (RR=1.56, CI:1.02-2.40).
Conclusions.
Adolescent survivors of Wilms tumor are similar to siblings with respect to mental health concerns overall but were more likely to require special education. Monitoring of psychosocial and academic problems through adolescence is warranted, especially among those treated with radiation to the abdomen plus chest or with cardiac conditions.
Keywords: Adolescent, childhood cancer, education, oncology, psychological, psycho-oncology, social, Wilms tumor
Introduction
Among survivors of Wilms tumor (WT) within the Childhood Cancer Survivor Study (CCSS), chronic health conditions and functional impairment are common, with cardio-pulmonary, endocrine, and renal conditions frequently identified.1–2 However, associations between chronic health conditions and psychological, educational, and social outcomes remain largely unknown. Studies reporting psychosocial late effects in long-term survivors of WT are difficult to interpret, as survivors of WT often serve as comparison or control groups because children with WT do not receive central nervous system-directed therapy, which is a known risk factor for poorer psychosocial outcomes among survivors of childhood cancer.1–8
Prior studies primarily described long-term survivors of WT as having fewer psychosocial problems compared to survivors of leukemia, lymphoma, other solid tumors, and central nervous system malignancies, with similar outcomes compared to siblings but worse outcomes compared to population norms.1–4 Survivors of WT are often reported to have no substantial cognitive deficits and lower rates of behavioral and academic concerns compared to other survivor groups.5–6, 9–12 However, more recent research suggests ongoing academic needs for survivors of WT,13 with survivors being less likely to graduate college or be employed as compared to siblings.1 Significant problems with peer and romantic relationships have been reported among adult survivors of WT as well.14–15
As patients diagnosed with WT are not exposed to cancer therapies traditionally associated with cognitive impairment, factors commonly associated with poor academic outcomes are not well-recognized. Moreover, there has been no assessment of how chronic health conditions resulting from diagnosis and treatment relate to these poor outcomes despite new research suggesting treatments such as radiation to the abdomen and chest may lead to cardiopulmonary concerns, which in turn can restrict blood flow to the brain and impact learning and socioemotional outcomes.16–22 We sought to identify the prevalence of psychological, educational, and social problems in adolescent survivors of WT as compared to an adolescent sibling sample within the CCSS cohort and to examine associated demographic, treatment, and chronic health predictors. We hypothesized higher frequency of psychological concerns, fewer close friends and less interaction with friends, and greater use of special education services as compared to siblings. Furthermore, we hypothesized worse psychological, social, and educational outcomes among survivors with radiation to the chest, higher dose of anthracyclines, and higher severity of chronic cardiac, pulmonary, and endocrine conditions (CTCAE grade).
Methods
Participants
Adolescent participants were identified from the CCSS, a retrospective cohort with longitudinal follow-up of survivors treated at one of 31 institutions across North America.23 Details regarding the cohort and study recruitment have been published previously.23–24 Eligibility for current analyses included diagnosis of WT, treatment between 1970-1999, survival at least five years post diagnosis, and age of 12-18 years at survey completion by a parent/guardian. A random sample of survivors’ siblings who were age 12-18 years at survey completion were also included.23–24Adolescent survivor and sibling surveys were all completed during the same time periods with the original cohort completing surveys between 1994 and 1996 and the expansion cohort completing surveys between 2009 and 2011. All aspects of this study were Institutional Review Board approved (Approval #006014), and all parents/guardians provided written consent prior to completing the study.
Outcomes and Exposures
Demographic, treatment, and chronic health conditions.
Parents of survivor and sibling participants provided information regarding age, sex, race, ethnicity, education status (including history of participation in a 504 plan or Individualized Education Plan for learning, concentration, and/or behavioral concerns), family income, use of psychoactive medications (e.g., antidepressants, attention-deficit/hyperactivity medications, antianxiety medications), and history of scoliosis. For survivors, information regarding treatment era, radiation therapy (RT), surgical procedures, and chemotherapy were abstracted from medical records at local institutions. Chronic health conditions (endocrine, cardiovascular, and respiratory) were graded according to a modification of the Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03,25 a previously published system in which each possible chronic condition is graded in terms of functional impact and intervention required on a scale from 1 (largely asymptomatic with no functional impairment) to 5 (death). Therefore, hypertension would be graded as a 1 if largely asymptomatic but assigned higher grading depending upon impairment and management needed.
Behavior Problems Index.
The Behavior Problems Index (BPI)26 was utilized to explore psychosocial functioning. The BPI is comprised of a question subset from the Child Behavior Checklist (CBCL)27 and was initially developed for the National Health Interview Survey. For each item, parents were asked to indicate their child’s functioning within the past six months on a Likert scale from 1 (“Not True”) to 3 (“Often True”). An evaluation of validity and reliability of the BPI within the CCSS cohort indicated five domains comprised of 27 CBCL items including 1) Depression/Anxiety (e.g., fearful, sad), 2) Headstrong (e.g., strong temper, argumentative), 3) Attention Deficit (e.g., hyperactive, inattentive), 4) Peer Conflicts/Social Withdrawal (e.g., cheating, difficulties getting along with peers), and 5) Antisocial Behaviors (e.g., not getting involved/avoiding others).28 Three additional BPI items were utilized to describe aspects of social competence including the number of close friends, frequency of interacting with close friends, and ability to get along with peers; psychometrics of the social competence items have also been described.28
Statistical Analysis
Descriptive statistics were calculated for demographic, treatment, and chronic health conditions. Initial comparisons between survivors and siblings were completed via independent sample t-tests and chi-square statistics (or Fisher’s exact test). Findings were deemed significant if p<0.05. Mental health impairment was defined as a score ≥ 90th percentile of symptoms reported by siblings on any of the five BPI domains. For each psychological, educational and social outcome, univariate analyses of independent variables (e.g., chronic health conditions, chemotherapies, treatment era) were also conducted via independent sample t-tests and chi-square statistics (or Fisher’s exact test), and any independent variables at p<0.10 or any clinical variables of interest were then included in the multivariable models. A p<0.10 was chosen to ensure all variables potentially contributing to multivariable model variance were included. Since the frequency of impairment for psychosocial outcomes (measured by the five BPI domains) and involvement in special education all exceeded 10%, modified Poisson regression29 analyses were used to compare the frequency of psychosocial impairment and involvement in special education between survivors and siblings (with adjustment for race and family income) to avoid overestimation of relative risk, which could exist when instead using odds ratio from logistic regression analysis. Therefore, Modified Poisson regression models determined demographic, treatment-related, and chronic condition predictors of: 1) psychosocial outcomes, as measured by the five BPI domains, and 2) involvement in special education services. Two separate models were performed for each of the BPI subscales. Model 1 included pertinent demographics (e.g., sex, race, family income, and age at diagnosis) and treatment exposures, and Model 2 included pertinent demographic and chronic conditions (e.g., endocrine and cardiovascular systems). Two models were created to account for the overlapping variance that would exist between some of the treatment and chronic health condition variables in each model. Results were presented as relative risk (RR) with 95% confidence intervals (CIs). Multinomial logistic regression models determined demographic, treatment-related, or chronic conditions predictors of friendship variables. Results were presented as odds ratio (OR) with 95% confidence intervals (CIs). These analyses were conducted using Statistical Analysis System software (SAS 9.4, Cary NC).
Results
Sample Characteristics
This study included 666 adolescent survivors of WT diagnosed 1970-1999 (Mean[SD] age at survey=15.3[1.7] years, range 12-18 years; age at diagnosis=2.8[1.8] years, range=0.0–11.2 years; time since diagnosis=12.5[2.1] years, range=5.6–17.4 years) and 698 siblings (age at survey=15.4[1.7] years). Survivors were more likely than siblings to be non-White (p=0.005), reside in families with lower household income (p<0.001), have chronic endocrine or cardiovascular disorders (p’s<0.001), and be on dialysis (p<0.001; Table 1). Most survivors were between three and six years of age at diagnosis (56.0%), underwent total nephrectomy (93.2%), completed radiation (52.8%; no participants received cranial radiation), had treatment that included vincristine (95.0%), dactinomycin (95.3%), and/or doxorubicin (51.2%), and did not experience relapse or second cancer (93.5%). Survivor outcomes did not differ as a function of treatment era. There were no differences between siblings and survivors based on sex, ethnicity, history of chronic respiratory conditions, history of scoliosis, or history of kidney infections.
Table 1.
Demographic, diagnostic, and treatment characteristics of adolescent survivors of Wilms tumor and siblings
| Survivor n (%) |
Sibling n (%) |
p-value | Effect size† | Number of Observation | |
|---|---|---|---|---|---|
| Sex | |||||
| Female | 331 (49.7) | 333 (47.7) | 0.46 | 0.0199 | 1364 |
| Male | 335 (50.3) | 365 (52.3) | |||
| Age at Survey | |||||
| Mean (SD) | 15.3 (1.7) | 15.4 (1.7) | 0.64 | 0.0254 | 1364 |
| Median (Range) | 15.4 (12.0, 18.0) | 15.5 (12.0, 18.0) | |||
| Race | |||||
| Caucasian | 523 (80.8) | 581 (86.6) | 0.005 | 0.0780 | 1318 |
| Non-Caucasian | 124 (19.2) | 90 (13.4) | |||
| Ethnicity | |||||
| Hispanic | 35 (5.4) | 27 (5.7) | 0.84 | 0.0055 | 1318 |
| Non-Hispanic | 612 (94.6) | 633 (94.3) | |||
| Family Income | |||||
| <$40,000 | 160 (26.3) | 117 (17.6) | 0.0002 | 0.1162 | 1272 |
| $40,000 - $100,000 | 291 (47.8) | 323 (48.7) | |||
| >$100,000 | 158 (25.9) | 223 (33.6) | |||
| Education | |||||
| In Elementary/Middle School | 346 (53.2) | 284 (46.6) | <0.0001 | 0.1560 | 1260 |
| In High School | 261 (40.2) | 316 (51.8) | |||
| Other | 43 (6.6) | 10 (1.6) | |||
| Special education‡ | |||||
| Yes | 157 (25.5) | 80 (12.6) | <0.0001 | 0.1655 | 1252 |
| No | 458 (74.5) | 557 (87.4) | |||
| Use of Psychoactive Medications | |||||
| Yes | 62 (9.4) | 35 (5.1) | 0.0002 | 0.0844 | 1347 |
| No | 595 (90.6) | 655 (94.9) | |||
| Age at Diagnosis (years) | |||||
| < 3 | 257 (38.6) | -- | -- | ||
| 3 to ≤ 6 | 373 (56.0) | -- | -- | ||
| > 6 | 36 (5.4) | -- | -- | ||
| Treatment Era | |||||
| 1970-1979 | 37 (5. 6) | -- | -- | ||
| 1980-1989 | 431 (64.7) | -- | -- | ||
| 1990-1999 | 198 (29.7) | -- | -- | ||
| Treatment Modalities | |||||
| Surgery§ | |||||
| Partial nephrectomy | 16 (4.3) | -- | -- | ||
| Total nephrectomy | 345 (93.2) | -- | -- | ||
| Bilateral nephrectomy | 9 (2.5) | -- | -- | ||
| Chemotherapy | |||||
| Vincristine | |||||
| Yes | 572 (95.0) | -- | -- | ||
| No | 30 (5.0) | -- | -- | ||
| Dactinomycin | |||||
| Yes | 574 (95.3) | -- | -- | ||
| No | 28 (4.7) | -- | -- | ||
| Doxorubicin | |||||
| Yes | 308 (51.2) | -- | -- | ||
| No | 294 (48.8) | -- | -- | ||
| Cyclophosphomide | |||||
| Yes | 55 (9.1) | -- | -- | ||
| No | 547 (90.9) | -- | -- | ||
| Carboplatin | |||||
| Yes | 10 (1.7) | -- | -- | ||
| No | 592 (98.3) | -- | -- | ||
| Etoposide | -- | -- | |||
| Yes | 39 (6.5) | -- | -- | ||
| No | 563 (93.5) | -- | -- | ||
| Radiation | |||||
| No radiation treatment | 285 (47.2) | -- | -- | ||
| Radiation to the abdomen | 188 (31.1) | -- | -- | ||
| Radiation to the abdomen plus chest | 118 (19.5) | -- | -- | ||
| Radiation to another location | 13 (2.2) | -- | -- | ||
| Second Malignancy or Recurrence | |||||
| Yes | 43 (6.5) | -- | -- | ||
| Any Endocrine Condition | |||||
| CTCAE Grade 0/1 | 615 (92.3) | 687 (98.4) | < 0.0001 | 0.1459 | 1364 |
| CTCAE Grade 2/3/4 | 51 (7.7) | 11 (1.6) | |||
| Any Heart and Vascular Condition | |||||
| CTCAE Grade 0/1 | 587 (88.1) | 676 (96.8) | < 0.0001 | 0.1663 | 1364 |
| CTCAE Grade 2/3/4 | 79(11.9) | 22 (3.2) | |||
| Any Respiratory Condition | |||||
| CTCAE Grade 0/1 | 623 (93.5) | 650 (93.1) | 0.76 | 0.0084 | 1364 |
| CTCAE Grade 2/3/4 | 43 (6.5) | 48 (6.9) | |||
| History of Kidney Infections | |||||
| Yes | 19 (2.9) | 10 (1.4) | 0.06 | 0.0511 | 1348 |
| No | 632 (97.1) | 687 (98.6) | |||
| History of Dialysis | |||||
| Yes | 11 (1.7) | 0 (0.0) | 0.0003 | 0.0935 | 1345 |
| No | 642 (98.3) | 692 (100.0) | |||
| History of Scoliosis | |||||
| Yes | 3 (0.5) | 1 (0.1) | 0.3610 | 0.0287 | 1358 |
| No | 657 (99.5) | 697 (99.1) |
Note. Percentages calculated on total number of participants for whom data was available.
Cramer’s V for categorical variable; Cohen’s d for continuous variable
Adjusted for race and family income
These data only include survivors diagnosed 1970-86
Survivor and Sibling Comparisons
Survivors were more likely to use psychoactive medication (9.4% versus 5.1%, p<0.001) compared to siblings (Table 1). When controlling for race and family income, they were also more likely to participate in special education (25.5% versus 12.6%, p<0.001). Fewer survivors were reported as having no friends or one friend compared to siblings (7.2% versus 10.1%, p=0.04); however, survivors were more likely than siblings to get along worse with their peers (14.5% versus 7.8%, p<0.0001). Survivors and siblings did not vary significantly in rates of depression/anxiety symptoms, headstrong behavior, inattention, social withdrawal, or antisocial behavior (Table 2).
Table 2.
Behavior Problems Index comparisons for survivors and siblings
| Overall Comparisons | Impairment Comparisons | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Survivor | Sibling | Survivor | Sibling | ||||||||
| Variable | N | M (SD) | N | M (SD) | P-value | Effect size† | Category | N (%) | N (%) | P-value | Effect size† |
| Depression/Anxiety | 646 | 8.51 (2.66) | 696 | 8.07 (2.41) | 0.08 | 0.1751 | No | 558 (86.38) | 624 (89.66) | 0.50 | 0.0505 |
| Yes | 88 (13.62) | 72 (10.34) | |||||||||
| Headstrong | 645 | 7.36 (2.51) | 697 | 7.03 (2.32) | 0.25 | 0.1353 | No | 526 (81.55) | 601 (86.23) | 0.20 | 0.0637 |
| Yes | 119 (18.45) | 96 (13.77) | |||||||||
| Attention Deficit | 646 | 6.81 (2.28) | 694 | 6.42 (2.09) | 0.11 | 0.1790 | No | 518 (80.19) | 596 (85.88) | 0.33 | 0.0760 |
| Yes | 128 (19.81) | 98 (14.12) | |||||||||
| Peer Conflict/Social Withdrawal | 645 | 4.69 (1.35) | 695 | 4.5 (1.19) | 0.11 | 0.1517 | No | 535 (82.95) | 612 (88.06) | 0.08 | 0.0727 |
| Yes | 110 (17.05) | 83 (11.94) | |||||||||
| Antisocial Behaviors | 646 | 7.38 (2.14) | 696 | 7.1 (1.93) | 0.30 | 0.1371 | No | 559 (86.53) | 625 (89.8) | 0.65 | 0.0506 |
| Yes | 87 (13.47) | 71 (10.20) | |||||||||
Note. Adjusted for race and family income, N = number of subjects; M = mean; SD = standard deviation
Cramer’s V for categorical variable; Cohen’s d for continuous variable
Analyses of Psychological Outcomes
Survivors who received RT to a location other than the abdomen and/or chest (RR=2.56, CI:1.04-6.33) were more likely to engage in headstrong behaviors than those without RT (Table 3). History of RT, age at diagnosis, and history of receiving doxorubicin did not significantly impact antisocial behaviors, anxiety/depression, inattention, or social withdrawal when controlling for sex, race, and family income. Survivors with grade 2-4 cardiovascular conditions were more likely to have anxiety/depression symptoms (RR=1.95, CI:1.19-3.19), headstrong behavior (RR=1.91, CI:1.26-2.89), or inattention (RR=1.56, CI:1.02-2.40) compared to survivors with grade 0/1 conditions. History of cardiac conditions did not significantly impact antisocial behaviors or social withdrawal, controlling for sex, race, family income, and age at diagnosis.
Table 3.
Demographics and treatment exposures (Model 1) and demographics and chronic conditions (Model 2) as risk factors for domains of the Behavior Problems Index
| Variable | Category | Antisocial (N=526) |
Anxiety/Depression (N=526) |
Head Strong (N=525) |
Attention Deficit (N=526) |
Social Withdrawal (N=525) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | ||
| Model 1 | |||||||||||
| Sex | Female | 1.00 | . | 1.00 | . | 1.00 | . | 1.00 | . | 1.00 | . |
| Male | 1.90 | 1.12-3.22 | 0.90 | 0.56-1.43 | 1.24 | 0.82-1.87 | 1.13 | 0.79-1.62 | 0.85 | 0.57-1.27 | |
| Race | Caucasian | 1.00 | . | 1.00 | . | 1.00 | . | 1.00 | . | 1.00 | . |
| Non-Caucasian | 2.02 | 1.18-3.45 | 2.00 | 1.19-3.38 | 0.94 | 0.55-1.62 | 1.91 | 1.30-2.82 | 1.23 | 0.74-2.05 | |
| Household Income | >$100,000 | 1.00 | . | 1.00 | . | 1.00 | . | 1.00 | . | 1.00 | . |
| $40,000-$100,000 | 2.34 | 1.06-5.16 | 1.49 | 0.80-2.78 | 1.31 | 0.77-2.21 | 1.33 | 0.79-2.25 | 1.41 | 0.85-2.35 | |
| <$40,000 | 3.05 | 1.33-6.98 | 1.57 | 0.79-3.11 | 1.59 | 0.88-2.87 | 2.08 | 1.22-3.56 | 1.25 | 0.68-2.28 | |
| Age at Diagnosis | Per year increased | 1.07 | 0.92-1.25 | 1.14 | 0.99-1.32 | 1.03 | 0.91-1.16 | 1.01 | 0.90-1.13 | 0.97 | 0.85-1.12 |
| Radiation | No radiation | 1.00 | . | 1.00 | . | 1.00 | . | 1.00 | . | 1.00 | . |
| Radiation to another location | 1.62 | 0.55-4.76 | 0.52 | 0.06-4.40 | 2.56 | 1.04-6.33 | 1.23 | 0.57-2.67 | 0.81 | 0.22-2.97 | |
| Radiation to the abdomen | 1.37 | 0.72-2.62 | 0.90 | 0.43-1.89 | 1.35 | 0.76-2.41 | 0.77 | 0.44-1.34 | 0.79 | 0.40-1.55 | |
| Radiation to the abdomen plus chest | 0.61 | 0.24-1.55 | 0.86 | 0.35-2.09 | 1.29 | 0.64-2.59 | 1.03 | 0.57-1.88 | 0.56 | 0.25-1.22 | |
| Doxorubicin | No | 1.00 | . | 1.00 | . | 1.00 | . | 1.00 | . | 1.00 | . |
| Yes | 0.82 | 0.44-1.52 | 0.74 | 0.36-1.55 | 0.81 | 0.46-1.42 | 1.14 | 0.69-1.88 | 1.15 | 0.62-2.15 | |
| Model 2 | |||||||||||
| Sex | Female | 1.00 | . | 1.00 | . | 1.00 | . | 1.00 | . | 1.00 | . |
| Male | 1.44 | 0.92-2.26 | 0.84 | 0.55-1.30 | 1.09 | 0.76-1.57 | 1.18 | 0.84-1.67 | 0.83 | 0.57-1.21 | |
| Race | Caucasian | 1.00 | . | 1.00 | . | 1.00 | . | 1.00 | . | 1.00 | . |
| Non-Caucasian | 1.68 | 1.05-2.70 | 2.00 | 1.26-3.18 | 1.03 | 0.65-1.65 | 1.68 | 1.16-2.43 | 1.24 | 0.80-1.94 | |
| Family Income | >$100K | 1.00 | . | 1.00 | . | 1.00 | - | 1.00 | . | 1.00 | . |
| $40K-$100K | 2.57 | 1.23-5.37 | 1.28 | 0.73-2.26 | 1.37 | 0.84-2.20 | 1.44 | 0.87-2.37 | 1.44 | 0.88-2.36 | |
| <$40K | 3.04 | 1.40-6.62 | 1.30 | 0.70-2.41 | 1.65 | 0.97-2.79 | 2.19 | 1.31-3.65 | 1.42 | 0.82-2.47 | |
| Age at Diagnosis | Per year increased | 1.02 | 0.90-1.15 | 1.15 | 1.02-1.31 | 1.02 | 0.92-1.13 | 1.01 | 0.91-1.12 | 0.97 | 0.86-1.10 |
| Hormonal Systems | CTCAE Grade 0/1 | 1.00 | . | 1.00 | . | 1.00 | . | 1.00 | . | 1.00 | . |
| CTCAE Grade 2/3/4 | 0.93 | 0.38-2.27 | 0.64 | 0.28-1.46 | 0.64 | 0.30-1.39 | 1.34 | 0.83-2.16 | 1.10 | 0.58-2.11 | |
| Heart and Vascular Systems | CTCAE Grade 0/1 | 1.00 | . | 1.00 | . | 1.00 | . | 1.00 | . | 1.00 | . |
| CTCAE Grade 2/3/4 | 1.11 | 0.58-2.12 | 1.95 | 1.19-3.19 | 1.91 | 1.26-2.89 | 1.56 | 1.02-2.40 | 1.21 | 0.71-2.08 | |
Note. RR = relative risk; CI = confidence interval
Use of psychoactive agents and history of kidney infections or dialysis were not included in multivariable modeling due to infrequent occurrence. However, in univariate analyses, survivors who were taking psychoactive medications were more likely to demonstrate depression/anxiety symptoms (RR=3.20, CI:2.10-4.86), social withdrawal (RR=2.26, CI:1.50-3.41), headstrong behaviors (RR=1.91, CI:1.25-2.90), and inattention (RR=3.14, CI:2.30-4.29).
Analyses of Educational Outcomes
Survivors who received RT to the abdomen plus chest (RR=1.98, CI:1.18-3.34) were more likely to be in special education than those with no history of RT (Supplemental Table 1). Treatment with doxorubicin was not associated with special education, controlling for sex, race, family income, or age at diagnosis. History of endocrine or cardiovascular chronic conditions did not impact involvement in special education services beyond the influence of sex, race, family income, and age at diagnosis. Survivors were more likely to be in special education if they had problems with antisocial behavior, anxiety/depression symptoms, headstrong behavior, inattention, or social withdrawal (p’s<0.05; Figure 1).
Figure 1.
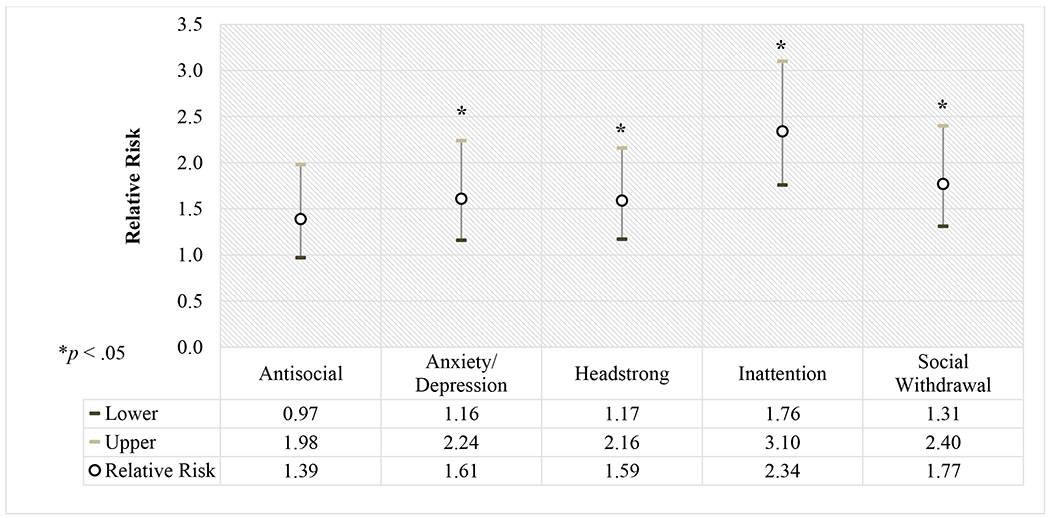
Relative risk and 95% confidence intervals for use of special education according to internalizing and externalizing behaviors, adjusting for sex, race, and family income
Analyses of Social Outcomes
History of doxorubicin or RT was not associated with number of friendships, time spent with friends, or ability to get along with peers when controlling for sex, race, family income, and age at diagnosis. History of endocrine or cardiovascular chronic conditions also were not associated with number of friendships, time spent with friends, or ability to get along with peers after adjusting for the same covariates. Survivors who had problems with anxiety/depression symptoms (OR=5.27, CI:2.34-11.90), headstrong behavior (OR=3.74, CI:1.80-7.74), inattention (OR=3.28, CI:1.56-6.91), or social withdrawal (OR=8.35, CI:3.91-17.85) were more likely to have one or no friends as compared to four or more friends (Supplemental Table 2). Those who demonstrated problems with anxiety/depression (OR=3.08, CI:1.56-6.08), headstrong behaviors (OR=2.12, CI:1.14-3.93), or social withdrawal (OR=4.34, CI:2.31-8.14) were also more likely to spend less than one hour per week with friends as compared to four or more hours weekly. Survivors with antisocial behavior (OR=0.31, CI:0.14-0.67), anxiety/depression (OR=0.41, CI:0.20-0.83), headstrong behavior (OR=0.36, CI:0.20-0.67), inattention (OR=0.44, CI:0.25-0.80), or social withdrawal (OR=0.12, CI:0.05-0.30) were less likely to be described as getting along better with peers.
Discussion
Study results indicated adolescent survivors of WT were similar to siblings with regard to most aspects of psychosocial functioning. However, nearly one quarter of survivors had histories of being in special education at some point due to concerns with learning, concentration, and/or emotional/behavioral concerns. Survivors were more likely to be in special education if they had radiation to the abdomen plus chest or problems with internalizing or externalizing behaviors. Although only a small percentage of patients received radiation to the abdomen plus chest, and these patients likely had higher disease burden overall, it is important to explore several possibilities that may explain the association with radiation. It is possible higher risk for academic concerns can be partially attributed to increased preschool/school absences, reduced exposure to preschool, and/or disruptions in learning pre-academic skills due to medical appointments and reduced interactions with peers to avoid infection risks. Academic accommodations may have been in place during treatment that then simply continued into later years of education. However, these possibilities likely do not fully account for high numbers of survivors in special education. Although causal relationships are outside the scope of this study, there is growing literature linking pediatric cardiovascular conditions, which can be caused by radiation to the abdomen and chest, and learning problems. Cardiovascular conditions, including hypertension, can impact blood flow to the brain, leading to negative impacts on learning, executive functioning, and mood.16–18 Radiation to one part of the body may impact other areas including the brain. Even a small-to-moderate impact of radiation can have lasting effects that impact developing brain structures; new research has found the impact to be especially detrimental to young children being treated for cancer, the same age group as most children being treated for WT.19 Animal models, which have examined why tumors distant from a radiation site may regress (the Abscopal effect),20 recently demonstrated such effects can impact brain tissue and cognitive function.21–22 Additional research with directly-assessed cardiovascular function is required to determine whether such treatment is associated with reduced blood flow to the brain, potentially via impact of baroreceptors in the aortic arch, or increased systemic inflammation associated mild cardiovascular pathology.
It is noted that a subset of children with WT have WAGR (Wilms tumor, aniridia, genitourinary anomalies, and range of abilities) syndrome, a cancer predisposition syndrome associated with intellectual disability, learning problems, mental health, and social concerns.30 We could not differentiate adolescents with WAGR within our data, but only seven in 1000 cases of WT (i.e., <1%) are attributed to WAGR, meaning this syndrome cannot fully account for the large number of survivors with academic concerns.30–31
Survivors were less likely than siblings to have few friends but were also found to get along more poorly with peers despite spending similar amounts of time together. Interestingly, number of friends, time spent with friends, and ability to get along with friends were unrelated to treatment variables or history of chronic health conditions in multivariable modeling. By comparison, current difficulties with mood or disruptive behaviors were related to having fewer friends, spending less time with friends, and getting along worse with friends. Given that treatment factors and chronic health factors were unrelated to social outcomes, it may be that the young age (i.e., <6 years of age) at which most survivors of WT undergo treatment may interfere with typical socialization.32 That is, the process of learning developmentally appropriate social skills may be disrupted by clinic visits and hospitalizations, with survivors spending more time with adults than peers during socially formative years.
Although no differences were found for internalizing or externalizing behaviors, survivors were more likely to take psychoactive medication than siblings. These findings are difficult to reconcile, given we would reasonably expect to find greater depression/anxiety symptoms and disruptive behaviors for survivors if they are also taking more psychoactive medications. Several factors may explain this finding. Notably, survivors taking psychoactive medications were reported as having fewer friends and getting along more poorly with friends, so it is possible that children with WT diagnoses were placed on psychoactive medication for adjustment concerns better captured by facets of social functioning rather than emotional/behavioral symptoms. Moreover, a history of taking psychoactive medication, which may have occurred earlier in the child’s life, does not necessarily equate to current concerns. Another possibility is use of psychoactive medications has resulted in successful treatment of psychological concerns for some survivors, thus resulting in lower levels of parent-reported internalizing and externalizing behaviors.
Study Limitations
This study’s design and findings must be considered within the context of its limitations. Generalizing outcomes from survivors diagnosed 1970-1999 to more recent survivors is a potential limitation. However, treatment of WT has not changed substantially over the past two decades with regard to the scope of chemotherapy agents used (Vincristine, Actinomycin, and Doxorubicin).33 Although there has been refinement in therapy duration, drug schedules, and radiation use and dosing over time, we believed it was still reasonable to utilize the CCSS sample as comparison to children currently in treatment given the relatively limited variation with regard to chemotherapy exposure. We are not able to assess variables beyond treatment exposures such as radiation therapy, anesthesia or surgical techniques, including supportive care measures that may influence toxicity and long-term psychologic adjustments. Another potential limitation is all psychosocial, educational, and demographic data, including current medications, were from parent reports. While parent perspectives on adolescents’ well-being is valuable, consideration of adolescents’ self-perceptions are equally important given that parent-adolescent perceptions may vary; therefore, it is recommended that self-report also be collected when identifying socioemotional concerns among adolscents.34 We further recommend inclusion of validated measures in multiple languages as a means of sampling more racially and ethnically diverse participants, as use of only English measures may have contributed to the primarily White sample of both survivors and siblings.
One study strength was the ability to compare survivors’ and siblings’ outcomes. Unfortunately, the siblings were not necessarily related to adolescents with WT. While it may have been possible to match survivors and siblings within the same families as a means of further limiting variance attributed to race and income, this would have limited the size of the comparison sample and biased them to adjusting only for survivors of WT from multiple child households. We also adjusted for the race and household income differences between the sibling and the survivor groups. While the current siblings group served as a reasonable comparison sample, better than a comparison from the general population, further research of siblings of survivors of WT is merited. This may be particularly important given that all siblings of survivors of childhood cancer, including WT, have been identified in previous research as unique populations with their own emotional and other psychosocial needs resulting from having a sibling with cancer.35 Finally, a large number of analyses were conducted with a range of effect sizes, leading to a need for additional study regarding the functional impact associated with medical and psychosocial risk factors identified in this study. As one of the first studies including a large sample of adolescent survivors of WT, we encourage replication of results.
Lastly, it is possible that ways in which chronic health conditions are assessed in the CCSS may not be sensitive enough to identify all nuances impacting psychosocial and educational outcomes for adolescent survivors of WT. Moreover, the relatively small sample size and young age of the survivors limited analysis of specific chronic health conditions. For example, we would expect a relationship between cardiac health conditions and a history of radiation to the chest such that both may negatively impact psychosocial or educational outcomes. This is because radiation to the chest is likely to cause deleterious cardiovascular effects, which in turn negatively impacts blood flow and creates a risk of learning and mental health concerns. However, our analyses revealed only higher grade cardiovascular conditions (but not radiation to the chest) were related to worse psychological outcomes. It is possible higher rates of hypertension among survivors of WT with a history of nephrectomy, a toxicity that can occur independent of radiation exposure in this population, contributes to associations between cardiovascular conditions and worse psychological, learning, and executive functioning outcomes.1, 17 Such findings suggest need for direct measurement of cardiac functioning to elucidate the finding indicating cardiac conditions are related to worse psychosocial or educational outcomes. Future researchers should consider evaluating vascular outcomes independent of cardiac outcomes to delineate the impact of particular exposures among WT survivors with potentially overlapping toxicity.
Clinical Implications
Annual mental health and neurocognitive screening is currently recommended for all survivors of childhood cancer.36–37 Our results suggest adolescent survivors of WT with a history of RT and/or higher grade cardiovascular problems may benefit from greater attention to mental health, social, and academic needs despite having what is often considered overall to be lower intensity treatment plans as compared to many survivors of other childhood cancers. Survivors have reported intervention for psychological needs is equally important to attending to physical health concerns; however, few receive dedicated long-term psychosocial follow-up care, with even fewer receiving serial comprehensive assessments.37 Screenings should begin when age appropriate, such as transitioning to kindergarten/school, and continue annually. While there is no consistent consensus on screening methodologies, several validated options exist such as PROMIS® (Patient-Reported Outcomes Measurement Information System)38–39 and the Psychosocial Assessment Tool (PAT) Version 3.40 Importantly, immediate feedback should be provided directly to survivors and their families with necessary referrals to pediatric psychologists for mental health interventions; pediatric neuropsychologists for assessment of cognitive, executive functioning, and learning problems; occupational therapy for life skills training; and school liaisons to advocate for school accommodations. As stipulated in the current psychosocial care standards for all survivors of childhood cancer, interventions designed to facilitate age-appropriate development of social skills should also be available in oncology clinics and inpatient hospital settings to reduce the likelihood of atypical social development caused by disrupted socialization experiences.32 Such social skills groups can be facilitated by child life specialists and pediatric psychologists.
Conclusion
Adolescent survivors of WT have been relatively dismissed within the literature as a group with few, if any, psychological, social, or educational concerns following the completion of cancer-directed care. Results of the current study indicate that while survivors and siblings demonstrated similar rates of mood and behavioral concerns, survivors have higher rates of taking psychoactive medications, problems with friendships, and participating in special education than siblings. Overall, monitoring of psychosocial and academic problems through adolescence is warranted, especially among those treated with radiation to the abdomen plus chest or with chronic cardiac conditions.
Supplementary Material
Supplemental Table 1. Demographics and treatment exposures (Model 1) and demographics and chronic conditions (Model 2) as risk factors for special education
Supplemental Table 2. Association of Behavior Problems Index outcomes with social outcomes
Acknowledgements
This work was supported by the National Cancer Institute (CA55727, GTA). Support to St. Jude Children’s Research Hospital was also provided by a Cancer Center Support (CORE) grant (CA21765) and the American Lebanese-Syrian Associated Charities. All data were part of the Childhood Cancer Survivor Study. Information about accessing these data can be found at https://ccss.stjude.org/. Portions of this study were presented previously in 2017 at the American Society of Clinical Oncology (ASCO) Annual Meeting, at the Congress of the International Society of Paediatric Oncology (SIOP), and at the International Conference on Long-Term Complications of Treatment of Children and Adolescents for Cancer.
Abbreviations:
- WT
Wilms tumor
- CCSS
Childhood Cancer Survivor Study
- CTCAE
Common Terminology Criteria for Adverse Events Version 4.03
- BPI
Behavior Problems Index
- CBCL
Child Behavior Checklist
- RR
Relative Risk
- CI
Confidence Interval
- OR
Odds Ratio
- RT
Radiation therapy
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to disclose in relation to the completion of this study or writing of this manuscript.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- 1.Termuhlen AM, Tersak JM, Liu Q, Yasui Y, Stovall M, Weathers R, et al. Twenty-five year follow-up of childhood Wilms Tumor: A report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2011;57:1210–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeltzer LK, Recklitis C, Buchbinder D, Zebrack B, Tsao JC, Lu Q, et al. Psychological status in childhood cancer survivors: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2396–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peretz NM, Goldberg H, Kuten A, Meller I, Krivoi E, Lorber A, et al. Long-term sequelae of malignant tumors in childhood: Consequences of late side effects. Harefuah. 2001;140:95–100. [PubMed] [Google Scholar]
- 4.Nathan P, Ness K, Greenberg M, Hudson M, Wolden S, Davidoff A, et al. Health-related quality of life in adult survivors of childhood Wilms tumor or neuroblastoma: A report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2007;49:704–715. [DOI] [PubMed] [Google Scholar]
- 5.Buizer AI, De Sonneville LMJ, Van Den Heuvel-Eibrink MM, Van Den Heuvel-Eibrink MM, Nijokiktjien C, Veerman AJP. Visual control in survivors of childhood acute lymphoblastic leukemia treated with chemotherapy only. J Int Neuropsychol Soc. 2005;11:554–565. [DOI] [PubMed] [Google Scholar]
- 6.Buizer AI, De Sonneville LMJ, Van Den Heuvel-Eibrink MM, Veerman AJ. Chemotherapy and attentional dysfunction in survivors of childhood acute lymphoblastic leukemia: Effect of treatment intensity. Pediatr Blood Cancer. 2005;45:281–290. [DOI] [PubMed] [Google Scholar]
- 7.Krull KR, Brinkman TM, Li C, Armstrong GT, Ness KK, Srivastava DK, et al. Neurocognitive outcomes decades after treatment for childhood acute lymphoblastic leukemia: A report from the St. Jude Lifetime Cohort Study. J Clin Oncol. 2013;31:4407–4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krull KR, Cheung YT, Liu W, Fellah S, Reddick WE, Brinkman TM. Chemotherapy pharmacodynamics and neuroimaging and neurocognitive outcomes in long-term survivors of childhood acute lymphoblastic leukemia. J Clin Oncol. 2016;34:2644–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halberg FE Kramer JH, Moore IM, Wara WM, Matthay KK, Ablin AR. Prophylactic cranial irradiation dose effects on late cognitive function in children treated for acute lymphoblastic leukemia. Int J Radiat Oncol Biol Phys. 1992;22:13–16. [DOI] [PubMed] [Google Scholar]
- 10.Waber DP, Urion DK, Tarbell NJ, Niemeyer C, Gelber R, Sallan SE. Late effects of central nervous system treatment of acute lymphoblastic leukemia in childhood are sex-dependent. Dev Med Child Neurol. 1990;32:238–248. [DOI] [PubMed] [Google Scholar]
- 11.Buizer AI, De Sonneville LMJ, Van Den Heuvel-Eibrink MM, Veerman AJ. Behavioral and educational limitations after chemotherapy for childhood acute lymphoblastic leukemia or Wilms tumor. Cancer. 2006;106:2067–2075. [DOI] [PubMed] [Google Scholar]
- 12.Lahteenmaki PM Sankila R, Pukkala E, Kyyronen P, Harila-Saari A. Scholastic achievement of children with lymphoma or Wilms tumor at the end of comprehensive education – A nationwide, register-based study. Int J Cancer. 2008;123:2401–2405. [DOI] [PubMed] [Google Scholar]
- 13.Mohrmann C, Henry J, Hauff M, Hayashi RJ. Neurocognitive outcomes and school performance in solid tumor cancer survivors lacking therapy to the central nervous system. J Pers Med. 2015;5:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mackie E, Hill J, Kondryn H, McNally R. Adult psychosocial outcomes in long-term survivors of acute lymphoblastic leukaemia and Wilms’ tumour: A controlled study. Lancet. 2000;355:1310–1314. [DOI] [PubMed] [Google Scholar]
- 15.Sadak KT, Kitchey ML, Dome JS. Paediatric genitourinary cancers and late effects of treatment. Nat Rev Urol. 2013;10:15–25. [DOI] [PubMed] [Google Scholar]
- 16.Klouda LK, Franklin WJ, Saraf A, Parekh DR, Schwartz DD. Neurocognitive and executive functioning in adult survivors of congenital heart disease. Congenit Heart Dis. 2017;12:91–98. [DOI] [PubMed] [Google Scholar]
- 17.Cha SD, Patel HP, Hains DS, Mahan JD. The effects of hypertension on cognitive function in children and adolescents. Int J Pediatric. 2012;2012:891094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vuotto SC, Krull KR, Li C, Oeffinger KC, Green DM, Patel SK, et al. Impact of chronic disease on emotional distress in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Cancer. 2017;123:521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones RM, Pattwell SS. Future considerations for pediatric cancer survivorship: Translational perspectives from developmental neuroscience. Dev Cogn Neurosci. 2019;38:100657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siva S, MacManus MP, Martin RF, Martin OA. Abscopal effects of radiation therapy: A clinical review for the radiobiologist. Cancer Lett. 2015;356:82–90. [DOI] [PubMed] [Google Scholar]
- 21.Mancuso M, Giardullo P, Leonardi S, Pasquali E, Casciati A, De Stefano I, et al. Dose and spatial effects in long-distance radiation signaling in vivo: Implications for abscopal tumorigenesis. Int J Radiat Oncol Biol Phys. 2013;85:813–819. [DOI] [PubMed] [Google Scholar]
- 22.Cui M, Xiao H, Li Y, Dong J, Luo D, Li H, et al. Total abdominal irradiation exposure impairs cognitive function involving miR-34a-5p/BDNF axis. Biochim Biophys Acta. 2018;1863:2333–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robison LL, Armstrong GT, Boice JD, Chow EJ, Davies SM, Donaldson SS, et al. The Childhood Cancer Survivor Study: A National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;10:2308–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robison LL, Mertens AC, Boice JD, Breslow NE, Donaldson SS, Green DM, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: A multi-institutional collaborative project. Med Pediatr Oncol. 2002;38:229–239. [DOI] [PubMed] [Google Scholar]
- 25.Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. [DOI] [PubMed] [Google Scholar]
- 26.Zill N, Peterson J. Behavior Problems Index. Washington, DC: Child Trends Inc; 1986. [Google Scholar]
- 27.Achenbach TM. Manual for the Child Behavior Checklist. Burlington: University of Vermont, Department of Psychiatry; 1991. [Google Scholar]
- 28.Schultz KA, Ness KK, Whitton J, Recklitis C, Zebrack B, Robison LL, et al. Behavioral and social outcomes in adolescent survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2007;25:3649–3656. [DOI] [PubMed] [Google Scholar]
- 29.Zou GA. Modifed Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. [DOI] [PubMed] [Google Scholar]
- 30.Han JC, Thurm A, Williams CG, Joseph LA, Zein WM, Brooks BP, et al. Association of brain-derived neurotrophic factor (BDNF) haploinsufficiency with lower adaptive behavior and reduced cognitive functioning in WAGR/11p13 deletion syndrome. Cortex. 2013;4:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Institutes of Health: Genetics Home Reference: WAGR syndrome. 2018. Available from: https://ghr.nlm.nih.gov/condition/wagr-syndrome#statistics [Google Scholar]
- 32.Christiansen HL, Bingen K, Hoag JA, Karst JS, Valzquez-Martin B, Barakat LP. Providing children and adolescents social interaction as a standard of care in pediatric oncology. Pediatr Blood Cancer. 2015; 62:S724–S749. [DOI] [PubMed] [Google Scholar]
- 33.Green DM: The evolution of treatment for Wilms tumor. J Ped Surg. 2013;48:14–19. [DOI] [PubMed] [Google Scholar]
- 34.Yeh CH, Chang CW, Chang PC. Evaluating quality of life in children with cancer using children’s self-reports and parent-proxy reports. Nurs Res. 2005;54:354–362. [DOI] [PubMed] [Google Scholar]
- 35.Gerhardt CA, Lehmann V, Long KA, Alderfer MA. Supporting siblings as a standard of care in pediatric oncology. Pediatr Blood Cancer. 2015;62:S750–S804. [DOI] [PubMed] [Google Scholar]
- 36.Annet RD, Patel SK, Phipps S. Monitoring and assessment of neuropsychological outcomes as a standard of care in pediatric oncology. Pediatr Blood Cancer. 2015;62:S460–S513. [DOI] [PubMed] [Google Scholar]
- 37.Lown EA, Phillips F, Schwartz LA, Rosenberg AR, Jones B. Psychosocial follow-up in survivorship as a standard of care in pediatric oncology. Pediatr Blood Cancer. 2015;62:S514–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cook KF, Jensen SE, Schalet BD, Beaumont JL, Amtmann D, Czajkowski S, et al. PROMIS measures of pain, fatigue, negative affect, physical function, and social function demonstrated clinical validity across a range of chronic conditions. J Clin Epidemiol. 2016;73:89–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schalet BD, Pilkonis PA, Yu L, Dodds N, Johnston KL, Yount S, et al. Clinical validity of PROMIS depression, anxiety, and anger across diverse clinical samples. J Clin Epidemiol. 2016;73:119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kazak AE, Hwang WT, Chen FF, Askins MA, Carlson O, Argueta-Ortiz F, et al. Screening for family psychosocial risk in pediatric cancer: Validation of the Psychosocial Assessment Tool (PAT) Version 3. J Pediatr Psychol. 2018;43:737–748. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Demographics and treatment exposures (Model 1) and demographics and chronic conditions (Model 2) as risk factors for special education
Supplemental Table 2. Association of Behavior Problems Index outcomes with social outcomes


