Abstract
Social interactions play a key role in modulating the impact of stressful experiences. In some cases, social interactions can result in social buffering, the process in which the presence of one individual reduces the physiological and behavioral impact of stress in another individual. On the other hand, there is growing evidence that a key initiating factor of social buffering behaviors is the initiation of an anxiogenic state in the individual that was not directly exposed to the stress. This is referred to as stress contagion (a form of emotion contagion). Both processes involve the transmission of social information, suggesting that contagion and buffering could share similar neural mechanisms. In general, mechanistic studies of contagion and buffering are considered separately, even though behavioral studies show that a degree of contagion is usually necessary for social buffering behaviors to occur. Here we consider the extent to which the neuropeptides corticotropin releasing hormone and oxytocin are involved in contagion and stress buffering. We also assess the importance that frontal cortical areas such as the anterior cingulate cortex and infralimbic cortex play in these behavioral processes. We suggest that further work that directly compares neural mechanisms during stress contagion and stress buffering will be important for identifying what appear to be distinct but overlapping circuits mediating these processes.
Introduction
Communication is an essential function in social species. Individuals communicate to protect themselves and others from environmental threats like predators (Kikusui et al., 2006). In the context of danger, individuals of social species communicate with other group members to inform them of threats using vocal, visual, and pheromone cues (Owings et al., 2001). Indeed, this form of communication provides protection from these threats and favors group living in many species (Kikusui et al., 2006). An additional beneficial effect of social interactions is social buffering, a phenomenon by which the presence of affiliative social partners mitigates the physiological responses to stressors (Kiyokawa and Hennessy, 2018). This can be observed between parents and offspring, mates, conspecifics, and sometimes even between species (e.g. between a dog and its owner), and can facilitate faster recovery from stressors (Kikusui et al., 2006). Social buffering appears to be evolutionarily conserved, as it has been observed in non-human primates, rodents, birds, fish and even invertebrates (Oliveira and Faustino, 2017). In humans social support reduces risk for several mental illnesses including depression, anxiety, and substance use disorder (Cobb, 1976; Ozbay et al., 2007). The broad presence of social buffering across species suggests that the neural mechanisms underlying this phenomenon are also shared across taxa (Beery and Kaufer, 2015).
While stressed individuals can benefit from the presence of a partner, what does this mean for the partner? Multiple studies have shown that partners of stressed individuals exhibit behavioral and endocrine changes indicating that stress parameters can be transmitted from one individual to other members of the group (Carnevali et al., 2017; Dimitroff et al., 2017; Sterley et al., 2018). This phenomenon is called stress contagion or emotional contagion, which is the ability of a subject to match its emotional state to that of a conspecific in pain or distress (Briefer, 2018; Engert et al., 2019). From an evolutionary perspective, being able to transfer information from a stressed individual to naive partners could have important adaptive value. Cooperation and vigilance to potential threats among group members can reduce exposure to danger (De Waal and Preston, 2017), therefore increasing chances of survival. However, frequent and long-lasting attunement to the emotional state of a stressed partner could have a cost. For example, increased pain sensitivity (hyperalgesia) in one individual can be transferred to a naive conspecific (Ueda and Neyama, 2017) and post traumatic stress disorder (PTSD) has been reported in individuals that did not experienced trauma first hand, but know someone who has (Blanchard et al., 2004; Perlman et al., 2011; Wingen et al., 2011).
A common thread between social buffering and stress contagion is that both use social information in the context of threat perception to adapt to dynamic contexts (Oliveira and Faustino, 2017). This suggests that buffering and contagion may be complementary processes, sharing some of the same sensory and cognitive mechanisms related to social information processing and decision making. Indeed, stress contagion is thought to be a key first step in promoting behaviors that lead to social buffering (De Waal and Preston, 2017). New rodent models have allowed for investigations of the neuroendocrine mechanisms of stress contagion and related behaviors (Sterley and Bains, 2021). Here we review rodent models of stress contagion and social buffing with their corresponding neural mechanisms. The current evidence shows an intriguing degree of overlap in these processes and several exciting new avenues for inquiry.
2. Rodent models of stress contagion
Two general strategies have been used to assess the impact of observing a demonstrator that has been exposed to stress. The most widely used is the witness stress model, in which an observer animal witnesses a demonstrator that is directly exposed to a stressor. This model allows for the study of psychological stress without the component of physical harm, which can activate the immune system and have indirect effects (Carnevali et al., 2020; Warren et al., 2020). An important caveat of witness approaches is that it is unclear what elicits distress in observers – mirroring the affective state of the demonstrator (so called vicarious stress) or observing the stressful event itself. An alternate approach is the crossover stress approach, in which the observer is not exposed to the authentic stressor that the demonstrator experiences (Wethington, 2000; Carnevali et al., 2020). Here, because the observer has no direct exposure to the stressor, effects of demonstrator exposure on the observer should be solely based on the behavioral and physiological states of the demonstrator (Adriaense et al., 2020). Both witness and crossover stress models have been very useful for outlining the mechanisms through which affective states are transmitted between individuals.
2.1. Witness stress
The witness defeat paradigm has been successfully applied in several species for both males and females (Warren et al., 2020). In a typical study, a clear perforated plexiglas barrier is used to separate an observer from a demonstrator during stress exposure (e.g. footshocks or social defeat). The observer has visual, auditory, and olfactory experiences of the event, without direct exposure to the stressor (Sial et al., 2016). Effects of witness defeat are abolished if an opaque, non-perforated divider is used to separate the observer from the demonstrator during episodes of defeat. In both male (Warren et al., 2013) and female (Iñiguez et al., 2018) mice, witness stress exposure reduces social approach to an unfamiliar individual in a novel context. In females witness defeat also increases social vigilance (Duque-Wilckens et al., 2020), an anxiety-like behavior in which individuals avoid but attend an unfamiliar stimulus mouse. Increased anxiety-related behaviors were also observed in pregnant mice that witness defeat of a male cagemate (Miao et al., 2017). Witness defeat can also affect motivated behavior. In male mice, witness defeat enhances morphine preference in a two-bottle choice paradigm (Cooper et al., 2017). In female rats witness stress decreases sucrose preference (Finnell et al., 2018).
Witness defeat induces a broad range of physiological and neurobiological responses. Both male and female witnesses show acute stress responses such as increased corticosterone (Warren et al., 2013) and increased blood pressure (Finnell et al., 2017, 2018). Most investigations of the impact of witness defeat have focused on the mesolimbic dopamine system including the ventral tegmental area (VTA) (Warren et al., 2013) and nucleus accumbens (NAc) (Warren et al., 2014), in which males and females show distinct transcriptional responses to witness defeat. While this pathway can have important effects on social behavior and is stress sensitive, its role in stress contagion or buffering is unclear. Witness defeat can affect expression of corticotropin releasing hormone (CRH) (Finnell et al., 2018), a neuropeptide that has key effects on the hypothalamic-pituitary-adrenal (HPA) axis and anxiety related behavior (Flandreau et al., 2012). Importantly, CRH signaling has an important role in promoting passive coping responses (Bosch et al., 2008) and has been found to play a key role in modulating stress contagion (see section 3).
2.2. Crossover stress
Crossover stress effects have been observed across numerous species using different experimental designs. Some of the earliest descriptions of crossover stress was described in fish, in which chemicals released by skin can function as a signal of predator danger (Pfeiffer, 1977). When male and female zebrafish were exposed to skin extract in the home tank, they showed antipredator behavior, such as swimming faster, moving to deeper water, and remaining immobile (Fernandes Silva et al., 2019). Simply observing these defensive responses in demonstrators in another tank could also induce these responses in observers who were not directly exposed to the skin extract. The effects on observers were stronger if the demonstrators were familiar. Crossover stress effects have also been observed in rodents. In one study, male demonstrator rats were separated from their cagemates and repeatedly exposed to social defeat (Carnevali et al., 2017). After the socially defeated demonstrator returned to the homecage, within minutes the male cagemate’s heart rate increased, coinciding with increased sympathetic and decreased vagal nervous system output. These changes were absent in cagemates of control (stress naïve) demonstrators. In a social interaction test, both stressed demonstrators and their observers showed reduced social approach responses. Baseline corticosterone levels were also elevated in observers of stressed demonstrators compared to observers of control demonstrators. Interestingly, if an auditory cue was presented before each episode of social defeat, demonstrators but not observers exhibited conditioned freezing responses.
There is also evidence that there can be long-term effects of exposure to a stressed individual. An analysis of previously published data in male and female California mice suggests that social defeat stress exposure can impact the behavior of cagemates. The standard experimental design in adults is to randomly assign males or females to three episodes of social defeat on consecutive days. Two to four weeks later, a social interaction test is performed with a same-sex unfamiliar target mouse. In adults, social defeat reduces social approach (time within 8 cm of the caged target mouse) in female but not male California mice (Trainor et al., 2013; Duque-Wilckens et al., 2018). An analysis of 239 mice showed that social defeat reduced social approach to a greater extent in females than in males (Fig. 1A, F1,231=13.1, p<0.001). Interestingly, stressed mice that had at least one cagemate that was also exposed to social defeat showed stronger reductions in social approach compared to stressed mice housed with only unstressed cagemates (F1,116=4.0, p<0.05). There was no sex × cagemate interaction (F1,116=0.03, p =0.8), suggesting that this effect was present in both males and females. The impact of a stressed cagemate was limited to social contexts, as there were no differences in approach behavior in an acclimation phase, when the cage for the target mouse was empty (Fig. 1B). Consistent with the results reviewed above, these data suggest that negative affective states can be transferred across cagemates, but do not identify the underlying neural mechanisms. A series of studies indicate that hypothalamic neuropeptides and frontal cortex regions play a key role in stress contagion.
Figure 1:
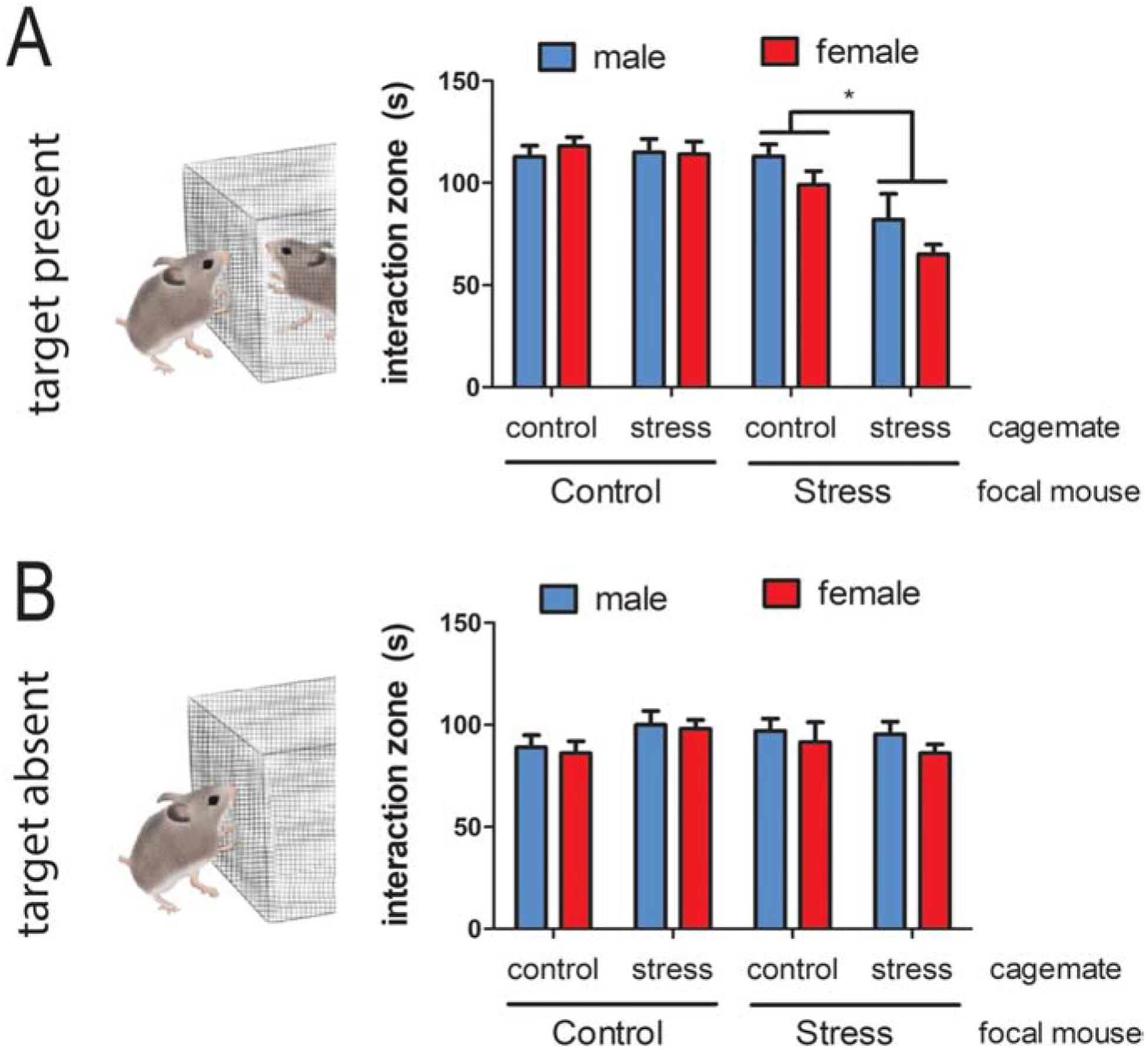
Effects of cagemates on the impact of social defeat on social interaction behavior in male and female California mice. A) Experimental design. B) Effects of defeat on social approach to an unfamiliar, same-sex target mouse in males and females were stronger when focal mice were housed with at least one stressed cagemate. C) There were no differences in time spent interacting with an empty cage when the target mouse was absent. * p < 0.05 main effect of cagemate.
3. Neuroendocrine circuits of stress contagion
To be influenced by the affective state of another, an individual first needs to notice the altered state. The detection and discrimination of affective states has an important impact on whether an individual shows avoidance or approach behavior. Two hypothalamic peptides, CRH and oxytocin, have been found to play a key role in modulating these responses. While CRH is best known for its role in controlling the release of glucocorticoids, this neuropeptide also has important effects on anxiety-related behaviors (Bangasser and Kawasumi, 2015; McCall et al., 2015). Oxytocin is usually considered to have anxiolytic properties, but emerging evidence suggests that its behavioral effects are more nuanced and often context-dependent (Shamay-Tsoory and Abu-Akel, 2016; Steinman et al., 2019). In addition to engaging the hypothalamus, observing a stressed demonstrator often leads to the recruitment of cortical regions that modulate decision making. The medial prefrontal cortex (mPFC) and insular cortex (IC) have emerged as key regions modulating behavioral responses in distressed individuals.
3.1. Corticotropin releasing hormone
Corticotropin releasing hormone neurons in the paraventricular nucleus of the hypothalamus (PVN) play a key role in facilitating stress contagion between a demonstrator and observer (Sterley et al., 2018). Male demonstrator C57BL6/J mice were randomly assigned to receive foot shocks or a novel environment as a control condition. Demonstrators were then returned to the homecage with an unstressed cagemate (observer). Male observers paired with stressed demonstrators showed enhanced investigation of the head and torso region compared to control demonstrators. However, there was no difference in allogrooming behavior between observers paired with stressed demonstrators and controls. This is a curious result, as allogrooming of demonstrators by observers is considered a stress-buffering response (see section 4.2). The lack of a difference in allogrooming between stress and control groups could be due to the less social C57BL6/J (compared to species like prairie voles), or because the control condition (novel environment, handling, etc) may have been more stressful than anticipated. Indeed, routine cage changes for husbandry can generate physiological stress responses (Rasmussen et al., 2011). Regardless, demonstrator exposure to foot-shock had strong effects on observer behavior.
Slice electrophysiology analyses of the PVN showed that in male and female observers paired with demonstrators previously exposed to footshock, CRH neurons were more sensitive to excitatory input than controls. One possible consequence of this enhanced sensitivity (potentiation) is enhanced release of CRH in mildly stressful contexts. Although this effect was observed in both males and females, the potentiation of CRH neurons to excitatory input was brief in females relative to males, suggesting that female cagemates may have had a stress buffering effect (see section 4.2). The potentiation of CRH neurons in observers was blocked by optical inhibition of CRH neurons in demonstrators. Potentiation of CRH neurons in observers could also be induced through optical activation of CRH neurons in an unstressed demonstrator. Together, these results suggest that enhanced CRH signaling across multiple brain regions may contribute to generating anxiogenic states in observers.
In male observers, inhibition of CRH type 1 receptors (CRHR1) blocked stress-induced potentiation of CRH neurons. This suggests that locally released CRH binds to CRHR1, creating a positive feedback loop that enhances sensitivity to excitatory input. An alternative possibility is that CRH synthesized outside of the hypothalamus could activate CRHR1 receptors. The extended amygdala encompasses both the amygdala and bed nucleus of the stria terminalis (BNST) (Alheid and Heimer, 1988), and plays a key role in modulating behavioral responses to threat (Shackman and Fox, 2016). Within the central nucleus of the amygdala (CeA), there is a population of CRH producing neurons that can facilitate anxiogenic states (Pomrenze et al., 2019), and witness defeat exposure increases CRH content in the CeA (Finnell et al., 2018). One of the many actions of CRHR1 activation is an enhancement of brain derived neurotrophic factor (BDNF) signaling (Hauger et al., 2009), which is also enhanced by witness defeat. Upregulations in CRH-BDNF signaling likely contribute to stress contagion, as BDNF has anxiogenic effects in the extended amygdala (Greenberg et al., 2014). Together, these results show that enhanced CRH signaling across multiple brain regions contributes to generating anxiogenic states in observers.
3.1. Oxytocin
Multiple lines of evidence suggest that oxytocin enhances the salience of both positive and negative social contexts (Shamay-Tsoory and Abu-Akel, 2016; Steinman et al., 2019), and that oxytocin plays a key role on stress contagion. For example, male and female mice that observe a demonstrator exposed to footshock showed exaggerated freezing behavior but this effect was blocked by systemic oxytocin receptor (OTR) antagonist administration (Pisansky et al., 2017). Oxytocin neurons in the PVN projecting to the CeA appear to be particularly important for discriminating affective states. In one study male or female observer mice were given a choice to interact with a stressed (exposure to a conditioned stimulus that was paired with foot shock) or relieved (23 hr water restriction +1 hr water access) demonstrator versus a neutral one (Ferretti et al., 2019). Here, both males and females showed preferences for demonstrators in altered emotional states (stress or relief) versus neutral demonstrators. However, these preferences were blocked by chemogenetic inhibition of PVN oxytocin neurons projecting to CeA. In contrast, chemogenetic inhibition of PVN oxytocin neurons projecting to NAc, mPFC, or the CA2 region of the hippocampus had no effect on performance in the affective discrimination task. However, the CeA is not the only region in which oxytocin can facilitate recognition of affective states.
The insular cortex has dense expression of OTR and is extensively connected with sensory regions as well as frontal and limbic structures (Levy and Yizhar, 2018). In a study on adult male rats, unstressed individuals were given a choice to interact with stressed or non-stressed demonstrators (Rogers-Carter et al., 2018b). Interestingly, observer responses to demonstrators were age dependent. Observers approached stressed juveniles more than naive juveniles, but stressed adults were avoided in favor of naive adults. Optogenetic inhibition of excitatory neurons or pharmacological inhibition of OTR in the insular cortex of observer rats disrupted this selective behavior toward both juveniles and adults. This suggests that oxytocin signaling in the insular cortex plays an important role in either recognizing stress related cues or choosing whether to approach or avoid stressed animals (Rogers-Carter et al., 2018b). In a follow-up study, a projection from insular cortex to NAc was necessary for observers to show a preference for stressed male juveniles, but not for the avoidance of stressed male adults. Chemogenetic stimulation of insular cortex terminals in the NAc increased exploration toward juveniles that are naïve, but not toward adults (Rogers-Carter et al., 2019). Interestingly, activation of OTR within the NAc also plays an important role in promoting social approach in both male and female rodents (Dölen et al., 2013; Williams et al., 2020). Together the studies by Rogers-Carter and colleagues indicate that exposure to a stressed individual leads to oxytocin release in the insular cortex, which affects the excitability of insular cortex output neurons, resulting in an age-dependent approach or avoidance response toward stressed individuals (Rogers-Carter et al., 2018b, 2019). The insular cortex is not the only cortical region that has an important function for the evaluation of affective states.
3.2. Medial Prefrontal Cortex
The medial prefrontal cortex (mPFC) plays an important role in behavioral responses in an affective state discrimination task in which observer male or female mice chose to interact with demonstrators in different affective states (Scheggia et al., 2020). In one comparison, observers could choose between a non-stressed control mouse (neutral) or a mouse that experienced 23 hrs of water deprivation with 1 hr of ad libitum water access immediately before testing (relieved). In a second comparison, observers could choose between a non-stressed mouse or a stressed mouse that experienced 15 min of restraint stress immediately before the test. The authors found that observers initially spent more time exploring the demonstrators in the stress or relief conditions versus control mice, showing that observes could discriminate altered affective states. Olfactory cues, but not visual or auditory cues, were sufficient for this discrimination. Investigative responses were similar toward cotton balls scented with body odors from the stressed or relieved animals, though the stress odor was now avoided instead of approached. Importantly, these findings were observed in both male and female mice. Recording of neural activity in the mPFC of observers during the task showed increased firing during interactions with stressed or relieved demonstrators, compared to neutral ones. Somatostatin-expressing (SOM+) interneurons in mPFC had increased activity when mice were evaluating demonstrator mice, and optogenetic inhibition of these SOM+ cells specifically during the emotional-discrimination task abolished the preference for the emotionally altered demonstrator. General sociability was not changed by inhibition of SOM+ neurons. Activating mPFC SOM+ cells in the observer when interacting with one of two neutral demonstrators caused increased time spent with the activation-paired neutral demonstrator, thus inducing discrimination where there should be none. This suggests that top-down control from the mPFC over other limbic brain areas plays a key role for identifying affective states (Yasui et al., 1991). Recent work has demonstrated that CRHR1 receptors in the mPFC have important effects on cognitive function (Hupalo and Berridge, 2016), but the role of these receptors in modulating social behavior has not been considered. This would be worth investigating in light of findings that observers paired with stressed demonstrators had more reactive CRH neurons in the PVN (Sterley et al., 2018). The source of CRH in the mPFC is likely local (Swanson et al., 1983), so it would also be interesting to examine the extent to which different populations of CRH neurons coordinate activity.
The mPFC also has strong projections to the insular cortex (Yasui et al., 1991), but this specific circuit has not yet been tested in an affective state recognition task. It’s interesting that strong effects of the mPFC SOM+ were observed when optogenetic inhibition of PVN neurons projecting to mPFC were found to have no effect on affective state recognition in mice (Ferretti et al., 2019), especially since OTR is expressed in neurons within the mPFC (Nakajima et al., 2014). Although it is possible that OTR in the mPFC do not modulate affective state recognition, another possible explanation for these results is that oxytocin release in the mPFC may not be activity dependent (Johnson and Young, 2017). Thus, future study is needed to determine whether OTR in the mPFC are unnecessary for the discrimination of affective states.
4. Social buffering of stress
In the previous section, the impact of a stressed demonstrator on stress-naïve observers was considered. Here we consider the other side of this interaction, how a stress-naïve observer could impact the affective state of a stressed demonstrator, often referred to as social buffering. Interestingly, it has been suggested that stress contagion is a first step toward social buffering behaviors (De Waal and Preston, 2017). One form of social buffering is consolation behavior, defined as increased affiliative contact in response to, and directed toward a distressed individual that produces a calming effect (Clay and Waal, 2013; Burkett et al., 2016). These actions of the observer are elicited by the demonstrator and a major consequence of these interactions is reduced physiological and behavioral responses to stress in the demonstrator. The primary method for studying mechanisms of buffering behavior in observers and demonstrators is the cross over stress design reviewed above.
4.1. Mechanisms underlying stress buffering by observers
Several lines of evidence suggest that induction of anxiogenic states in observers by demonstrators is a key step in social buffering. In a common paradigm observer male or female rats were given an opportunity to either free a trapped cagemate from a restrainer or receive a food reward (Bartal et al., 2011). Typically observers choose to free the cagemate, but when observers were treated with the anxiolytic drug midazolam, helping behavior toward the trapped cagemate decreased without impairing the instrumental act to receive the food reward (Bartal et al., 2016). This suggests that the induction of an anxiogenic state in observers is important for initiating stress-buffering behaviors. Similar results were seen in prairie voles. When male or female demonstrator voles were exposed to a fear conditioning paradigm, both demonstrators and observers exhibited increased autogrooming behavior in response to exposure of the tone (Burkett et al., 2016). Conditioned autogrooming is an anxiety-related behavior that is kappa opioid receptor dependent (Williams et al., 2018). Importantly, demonstrator voles exposed to foot-shock received more allogrooming from the observer than control demonstrators, which were not exposed to foot-shock. Similar results were found in male and female mandarin voles, which are also monogamous (Tai et al., 2001). Males and females were exposed to social defeat or control conditions and then returned to their home cage with an unstressed mate (Li et al., 2019a). In both male and female observers, exposure to a stressed partner increased c-fos immunoreactivity in circuits important for anxiety including the BNST, PVN, basolateral amygdala (BLA), and CeA. Together, these results suggest it is necessary for observers to experience some degree of stress contagion in order to induce helping or allogrooming behavior.
Several lines of evidence suggest that oxytocin plays an important role in establishing the salience of a distressed demonstrator. For example, exposure to a stressed demonstrator increases the activity of PVN oxytocin neurons in male and female mandarin vole observers as measured by oxytocin/c-fos colocalizations (Li et al., 2019a). At least some of the effects of oxytocin on observer behavior is mediated by cortical regions. One of these regions is the anterior cingulate cortex (ACC), which human imaging data suggests is a key node in neural circuits related to empathy (Lamm et al., 2011). Observer prairie voles exposed to a stressed demonstrator had increased immediate early gene expression in the ACC compared to controls (Burkett et al., 2016). Similar results were observed in mandarin voles (Li et al., 2019a). Site-specific infusions of an OTR antagonist showed that inhibition of OTR in ACC but not NAc or prelimbic cortex reduced demonstrator-directed grooming by observers in prairie voles (Burkett et al., 2016). Work in mandarin voles also showed that oxytocin infusions into the ACC promote allogrooming by observers and also indicated a role for D2/D3 dopamine receptors (Li et al., 2019b). Interestingly, OTR/D2 receptor interactions in the NAc have also been shown to be important for the formation of pair bonds (Liu and Wang, 2003). Finally, in mandarin voles infusions of the GABA receptor antagonist bicuculline into ACC also reduced allogrooming by male observers of stressed female demonstrators (Li et al., 2019a). These studies show an important role for oxytocin in the ACC in promoting consolation related behavior, but it’s less clear whether these effects are driven by increasing salience of a distressed demonstrator or a more direct effect on consolation-related behaviors. Either way, exposure to a stressed demonstrator has important effects on an observer’s physiological stress responses and behavior.
4.2. Effects of stress buffering on demonstrators
After consolation-related behaviors are initiated by observers, these interactions have important effects on the stressed demonstrator. Some of the most in depth analyses of these effects have been performed in prairie voles (Lieberwirth and Wang, 2016). Females exposed to a 1 hour restraint stress had significantly lower corticosterone levels 30 minutes later if they recovered with a pair bonded partner compared to females that recovered alone (Smith and Wang, 2014; Donovan et al., 2018). Females that recovered with a partner also showed reduced anxiety-related behavior both in the home cage and in an elevated plus maze. Hypothalamic infusions of oxytocin could mimic effects of social buffering in females recovering alone while infusions of an OTR antagonist blunted effects of social buffering in females that recovered with a partner (Smith and Wang, 2014). Pair housing also blunted corticosterone responses in male rats exposed to social defeat compared to single housed rats (Patki et al., 2014). In mice however, effects of recovering in a familiar social context are stronger in females than males (Sterley et al., 2018). This stronger effect of stress buffering in females was found to be mediated by enhanced vasopressin signaling (Loewen et al., 2020). While vasopressin has been found to exaggerate HPA activity in males, these effects are generally weaker or absent in females (Viau et al., 2005). Overall, the effects of consolation-related behavior on HPA responses are consistent with prior findings showing that both group housing (Blume et al., 2008) and hypothalamic oxytocin infusions (Windle et al., 2004) decreased stress-induced PVN CRH neuronal activation in rats. Interestingly, the effects of oxytocin on the HPA axis may be indirect.
In male rats and mice, OTR are not expressed in the majority of parvocellular CRH-positive neurons (Dabrowska et al., 2013; Winter and Jurek, 2019). This suggests that oxytocin may act primarily on neurons that regulate CRH neurons. Supporting this hypothesis, intra-hypothalamic infusions of oxytocin increased c-fos/GABA colocalizations while decreasing c-fos/CRH colocalizations in the PVN of female prairie voles (Smith et al., 2016). However, in slice preparations of the hypothalamus from male and female mice, oxytocin bath had no effect on inhibitory post-synaptic currents (Jamieson et al., 2017). Although it is possible that there could be species differences in inhibitory inputs of CRH regulation, an alternate possibility is that oxytocin acts on inhibitory neurons within the BNST to inhibit CRH neurons. Since these neurons would not be present in a hypothalamus slice preparation, this could explain the lack of effects of oxytocin on inhibitory inputs (Dong et al., 2001). While there are still some uncertainties in the exact mechanisms of action, it seems clear that while recovering from a stressor, oxytocin released from the hypothalamus functions as an anxiolytic and promotes negative feedback in the HPA axis (Neumann et al., 2000).
At least some of the behavioral effects of social buffering appear to be mediated by the infralimbic subregion of the frontal cortex (IL). An intriguing study used an activity-dependent molecular tagging system that allowed for selective optogenetic control of neurons that respond to social stimuli (Gutzeit et al., 2020). In ArcCreETT2 mouse line, the promoter for the immediate early gene Arc is used to drive the expression of a Cre construct that contains an estrogen receptor (ER) ligand binding domain (Denny et al., 2014). Thus, to induce Cre recombination, the cell must be active and in the presence of a strong ER ligand. This allows the experimenter precise control over when and where Cre is expressed. By infusing a Cre-dependent virus expressing channelrhodopsin, socially responsive neurons in the IL could be tagged with channelrhodopsin by combining an injection of tamoxifen (a selective estrogen receptor modulator) with a social interaction test. Those neurons could subsequently be reactivated using optical stimulation. Male and female demonstrator mice that were exposed to foot-shock had increased c-fos expression in the IL when recovering with a familiar cagemate versus a novel object. Demonstrators that recovered with a cagemate also showed reduced anxiety-related behaviors. Neurons in the IL were tagged with channelrhodopsin, and were then optically re-activated after stress exposure in the absence of a cagemate. Interestingly, reactivating these neurons reduced anxiety-related behaviors in both conditioned and unconditioned contexts. This suggest that IL neurons play a key role in translating social experiences into an anxiolytic effect. This effect showed some specificity to stress-related contexts, as activating these neurons in a real-time place preference revealed no evidence for rewarding or aversive properties of these neurons. Currently the cellular phenotypes of these IL neurons are unknown, so it is unclear whether oxytocin acts directly or indirectly to modulate the activity of IL neurons that mediate effects of social buffering in demonstrators.
5. Overlap in Mechanisms of Contagion in Observers and Buffering in Demonstrators
To assess the extent to which neuroendocrine mechanisms of stress contagion and social buffering overlap, a useful starting point is to compare findings from observers that engage in consolation related behaviors and demonstrators receiving social buffering. Table 1 presents an overview of the brain regions identified in the currently reviewed literature. Below we focus on mechanisms that have received the most attention to date, oxytocin signaling and neural circuits within the frontal cortex.
Table 1:
Overview of the effects found in the currently reviewed literature.
| Emotional recognition and approach | Change/Function | Animal | Reference |
|---|---|---|---|
| Proposed ↓ top-down prefrontal control | |||
| PVN à CeA | OT projection necessary for discriminating unfamiliar conspecifics based on emotional state | Observer mouse | Ferretti et al., 2019 |
| IC | ↑ activity and signaling via OTRs to recognize stress signals or choosing to interact with stressed individuals | Observer rat | Rogers-Carter et al., 2018a |
| IC à NAcc | ↑ OT activity to investigate stressed juveniles | Male observer rat | Rogers-Carter et al., 2019 |
| Stress contagion | |||
| PVN-CRH neurons | ↑ activity and STP of glutamate synapses | Demonstrator and observer mice | Sterley et al., 2018 |
| Striatum and dorsal hippocampus | ↓ c-Fos positive cells | Male CSDS and WDS mice | Cooper et al., 2017 |
| Dorsal hippocampus | ↑ ΔFosB positive cells | Male CSDS and WDS mice | Cooper et al., 2017 |
| Dorsal and ventral striatum and PFC | ↑ ΔFosB positive cells | Male CSDS mice | Cooper et al., 2017 |
| ↑ ERK2 transcription | |||
| NAcc | ↑ dendritic spine density | Male CDSD mice | Warren et al., 2014 |
| CeA | ↑ CRF and i nterleukin-13 | Female WDS mice | Finnell et al., 2018 |
| Hippocampus and mPFC | ↓ BDNF | Pregnant female WDS mice | Miao et al., 2017 |
| Amygdala | ↑ BDNF | Pregnant female WDS mice | Miao et al., 2017 |
| ACC | ↓ neuronal activity and OTR, D2R and 5HT1AR expression | Male demonstrator mice | Li et al., 2019b |
| Social buffering | |||
| PVN | Demonstrator mice | Takahashi et al., 2013; Kiyokawa et al., 2014; Smith and Wang, 2014; Lieberwirth and Wang, 2016; Smith et al., 2016 | |
| ↓ CRH neuron activity | |||
| NAcc | Inhibition of ↓ OT receptor binding | Demonstrator mice | Donovan et al., 2018 |
| LA | ↓ Fos expression and activity | Demonstrator rats | Fuzzo et al., 2015; Kiyokawa et al., 2014 |
| IL-PFC | ↑ c-Fos when interacting with conspecific leading to reduced freezing | Demonstrator mice | Gutzeit et al., 2020 |
| Social buffering provider (partner/observer) | |||
| ACC | ↑ c-Fos and OT + GABA colocalization OTR signaling in ACC to mediate consolation to distressed partners | Observer prairie and mandarin voles | Burkett et al., 2016; Li et al., 2019a |
| PVN | ↑ c-Fos and OT + GABA colocalization | Observer mandarin voles | Li et al., 2019a |
| BNST/PVN/BA/BLA/CeA/habenular nucleus | ↑ c-Fos expression | Observer mandarin voles after consoling partner | Li et al., 2019a |
| Medial preoptic area | ↑ c-Fos activity | Female observer mandarin vole after consoling partner | Li et al., 2019a |
| MeA | ↑ c-Fos activity | Male observer mandarin vole after consoling partner | Li et al., 2019a |
5.1. Oxytocin in observers versus demonstrators
An intriguing theme is that oxytocin signaling plays key role in both the initiation of consolation-related behaviors by observers and the stress-buffering effects induced by this behavior in demonstrators. In observers, the role of oxytocin is usually assumed to be a key factor in promoting social approach to the demonstrator. However, this occurs while observers are in an anxiogenic state, as anxiolytics reduce consolation-related behaviors. At present, it is unclear whether oxytocin facilitates the salience of a negative social context (a distressed demonstrator) or whether oxytocin specifically promotes consolation behaviors. An additional possibility is that oxytocin may promote behavioral coordination between the observer and demonstrator (Spengler et al., 2017; Jiang and Platt, 2018; Monari et al., 2020). It is also unknown whether the behavioral effects of oxytocin in demonstrators are mediated by effects on the HPA axis or by increasing the salience of interactions with the stress naïve observer. It’s likely that behavioral effects of oxytocin in observers and demonstrators occur through multiple mechanisms (Carter et al., 2020). An emerging finding is that oxytocin acting within different neural circuits can have virtually opposing effects on behavior. One reason for this is that oxytocin appears to increase the salience of positive and negative social experiences.
One goal of the social salience hypothesis is to reconcile apparently disparate findings that oxytocin administration can sometimes promote anxiety while at other times reduce anxiety (Bartz et al., 2011; Shamay-Tsoory and Abu-Akel, 2016). Increasing evidence suggests that this capability is mediated by different neural circuits (Grinevich et al., 2016; Steinman et al., 2019). Oxytocin acting within the medial PFC (Sabihi et al., 2014) and CeA (Viviani et al., 2011; Knobloch et al., 2012) generally reduces measures of anxiety whereas oxytocin acting in the BNST (Duque-Wilckens et al., 2018, 2020; Martinon et al., 2019) or LS (Guzman et al., 2014) (but see (Zoicas et al., 2014)) increase measures of anxiety. This raises the possibility that oxytocin release could occur in different brain circuits to enhance the salience of a distressed demonstrator or a helping observer. Optogenetics (Knobloch et al., 2012) or antisense knockdown (Duque-Wilckens et al., 2020) can be used to determine the source of behaviorally active oxytocin. However, stressors often increase the activity of multiple populations of oxytocin neurons (Steinman et al., 2016; Nasanbuyan et al., 2018) and microdialysis analyses often show that oxytocin release is elevated in multiple brain regions (Nishioka et al., 1998; Engelmann et al., 1999). This suggests that oxytocin release may be widespread across the brain in both demonstrators and observers. If this is true, how could such a similar neuroendocrine signal produce such different behavioral effects in demonstrators and observers?
A potentially important mechanism for diverse behavioral actions of oxytocin is through its role as a neuromodulator (Stoop, 2012). Unlike neurotransmitters that directly increase or decrease the excitability of a neuron, neuromodulators alter the effects of other events within the neuron (Kupfermann, 1979). While activation of OTR can enhance excitatory input in neurons in the MeA (Terenzi and Ingram, 2005) and lateral CeA (Huber et al., 2005), in deep layers of the spinal cord OTR can have inhibitory effects on neural activity (Eliava et al., 2016). This diversity in the effects of OTR is possible because of its diverse signaling capacity, as OTR can lead to coupling through excitatory Gq pathways or inhibitory Gi/Go pathways (Busnelli and Chini, 2018). The majority of our understanding of the molecular transduction of OTR comes from cell culture experiments that allow precise assessments of the downstream effects of OTR activation (Busnelli et al., 2012; Passoni et al., 2016), which are currently impossible in vivo. However the in vitro approaches allowed for the identification of biased agonists, which can selectively induce OTR-Gq coupling or OTR-Gi coupling (Busnelli et al., 2013). Future studies could use these biased agonists to assess the extent to which OTR uses different signaling pathways in different neural circuits to increase social salience, initiate consoling behaviors, or reduce anxiety related behaviors.
5.2. Do neural circuits of stress buffering overlap in observers and demonstrators?
Whereas there is good evidence that similar brain regions are activated in demonstrators and observers during stress contagion, it is less clear whether similar overlap occurs during stress buffering. Studies of the neural circuits of social buffering have focused primarily on observers of stressed demonstrators. There is strong evidence for an important role of the ACC in promoting consolation related behavior in both male and female observers (Burkett et al., 2016; Li et al., 2019a) and the insular cortex is also important for driving context-dependent approach behaviors in observers (Rogers-Carter et al., 2018b). However, the extent to which activity in the ACC or insular cortex is affected in demonstrators on the receiving end of these interactions is unknown. On the other hand, the few studies focusing on neural circuits in demonstrators have focused on the action of hypothalamic oxytocin (Smith and Wang, 2014). While oxytocin neurons appear to be activated in observers of stressed demonstrators (Li et al., 2019a), the extent to which other mechanisms are activated in both observers and demonstrators is unclear. There is some evidence that unlike stress contagion, there may be important differences in the neural circuits activated in demonstrators and observers during social buffering.
For example, optogenetic activation of neurons in the IL mimicked the effects of stress buffering by an observer in stressed male and female demonstrators (Gutzeit et al., 2020). In contrast, in mandarin voles, male and female observers did not show increased c-fos in IL upon exposure to a stressed partner (Li et al., 2019a). Similarly, prairie vole observers of stressed demonstrators showed no increases in c-fos in the adjacent prelimbic cortex (Burkett et al., 2016). Although it’s possible that distinct neural mechanisms are activated in observers and demonstrators during social buffering, the temporal resolution of c-fos analyses may not be fine enough to detect subtle, time-dependent patterns. For example, it’s likely that after consoling behaviors reduce distress in demonstrators, a similar effect could occur in observers. Brain imaging studies in humans showed increased synchrony in brain activity in parents and children while playing a cooperative game (Reindl et al., 2018). The simultaneous use of fiber photometry to assess neural activity in regions such as ACC, IL, and insular cortex in both observers and demonstrators would be an ideal approach to determine whether similar synchrony occurs during social buffering. The improved temporal resolution of photometry over immediate-early gene analysis could also help for discerning whether changes in activity within the ACC of observers occur before matching with the emotional state of the partner or after consolation behaviors are initiated. An addition issue is that changes in neural activity may be limited to specific cell types or projections. Implementing fiber photometry would allow for activity monitoring of specific cell types or groups of neurons that project to a specific location. This could be especially useful for understanding oxytocin-CRH interactions. Studies reviewed above indicate that stressed demonstrators induce enhanced release of both CRH and oxytocin in observers, yet most research indicates that activation of OTR has inhibitory effects on the CRH system (Winter and Jurek, 2019). One possible explanation for these results is that exposure to a stressed demonstrator triggers an initial surge in CRH release which is followed by release of oxytocin in the observer. Finer temporal resolution in the activity of these neurons could help resolve this conundrum.
6. Future Directions
The development of new experimental paradigms in animal models and humans has opened new doors for the study of the neuroendocrine mechanisms of the complex behavioral processes associated with stress contagion and stress buffering. Groundbreaking studies reviewed above have detailed how the hypothalamic neuropeptide CRH mediates stress contagion in observers and how oxytocin contributes to stress buffering in demonstrators (Figure 2). In a few cases, preliminary neural circuits have been identified, primarily in frontal cortex regions and IC. In many respects these pioneering discoveries have raised as many questions as answers. Exactly which behavioral processes oxytocin modulates to promote consoling behavior (social salience, approach, motor patterns) is still unknown, suggesting that additional innovation in behavioral tasks is needed. The simultaneous quantification of neural activity in demonstrators and observers could provide key insights into potential overlap in neural circuits for consolation and stress buffering. These types of studies could have high translational value for interpreting human imaging data, and could set the stage for manipulation-based studies that could determine the extent to which these circuits modulate behavior. However, there are some significant barriers to applying results from rodent mechanistic studies. While CRH and oxytocin are clearly important mechanisms in rodents, it is extremely difficult to measure these neuropeptides in humans, particularly within the brain. Indeed, the methods to accurately quantify OTR protein in post-mortem human brain have only recently become available (Freeman et al., 2017). Innovative approaches for combining post-mortem gene expression with imaging data can provide clues to oxytocin (and eventually CRH) sensitive brain circuits in the human brain (Quintana et al., 2019), but new methods are needed to assess the extent to which these gene networks are activated during specific affective states. Finally, preclinical studies indicate that aspects of a demonstrator’s identity such as age or familiarity are important factors affecting an observer’s behavior (Rogers-Carter et al., 2018b, 2018a), yet the mechanisms that modulate these responses have not been identified. Although these processes likely involve cortical circuits discussed above such at ACC and IC, other systems are likely involved. For example, vasopressin and V1a receptors play an important role in social recognition (Albers, 2012), and so are well situated to modulate context-dependent responses. Continued cooperation between basic scientists and neuroscientists working with human subjects will be needed to gain a better understanding of the evolutionary conserved social neural systems in vertebrate brains. This could lead to new approaches for promoting social interactions that could have a profound improvement on our general health.
Figure 2:
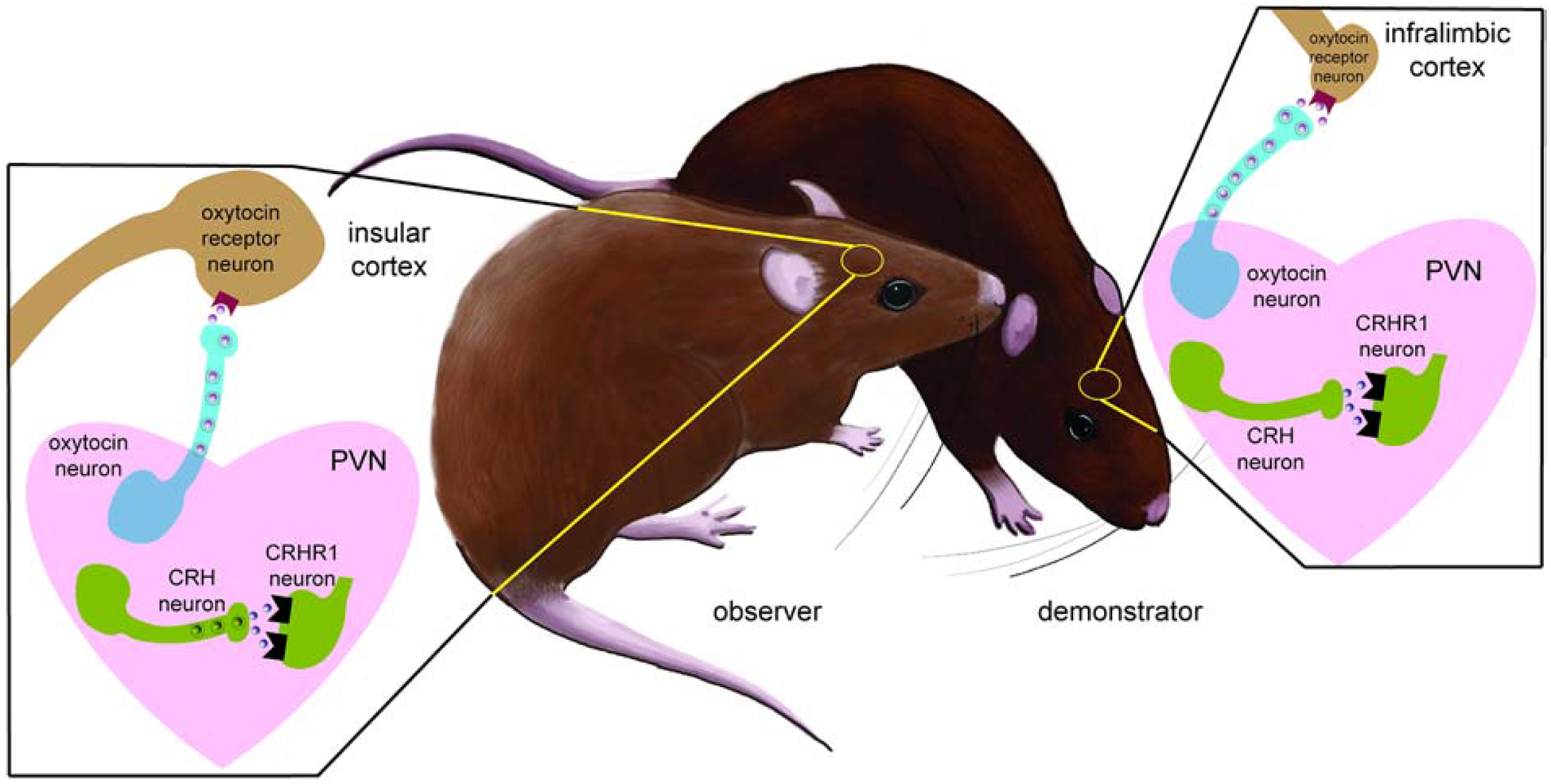
Neuroendocrine mechanisms of social contagion and stress buffering. In demonstrators stress-exposure increases the sensitivity of CRH neurons to excitatory input and increased activation of CRHR1 receptors. Observers exposed to stressed demonstrators show a similar increase in CRH activity. Increased activity of oxytocin neurons results in the activation of OTR within the insular cortex to drive consolation related behaviors. These behaviors can blunt physiological stress responses and induce anxiolytic effects in demonstrators. At least some of these effects are mediated through the actions of oxytocin or activation of neurons within the infralimbic cortex. Currently it is unknown whether oxytocin acts specifically within the infralimbic cortex.
Highlights.
Social contagion and social buffering are behavioral processes that require social communication
Recent evidence suggests that at least some neuroendocrine mechanisms are shared
Oxytocin and corticotropin releasing hormone play important roles in social contagion and buffering
Acknowledgements
This work supported by Brain and Behavior Foundation New Investigator Award to NDW, NSF IOS 1937335 and NIH R01 MH121829 to BCT
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adriaense JEC, Koski SE, Huber L, and Lamm C (2020). Challenges in the comparative study of empathy and related phenomena in animals. Neuroscience and Biobehavioral Reviews 112, 62–82. doi: 10.1016/j.neubiorev.2020.01.021. [DOI] [PubMed] [Google Scholar]
- Albers HE (2012). The regulation of social recognition, social communication and aggression: Vasopressin in the social behavior neural network. Hormones and Behavior 61, 283–292. doi: 10.1016/j.yhbeh.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Alheid GF, and Heimer L (1988). New perspectives in basal forebrain organization of special relevance for neropsychiatric disorders: the striatopallidal amygdaloid and corticopetal components of the substantia innominata. Neuroscience 27, 1–39. [DOI] [PubMed] [Google Scholar]
- Bangasser DA, and Kawasumi Y (2015). Cognitive disruptions in stress-related psychiatric disorders: A role for corticotropin releasing factor (CRF). Hormones and Behavior 76, 125–135. doi: 10.1016/j.yhbeh.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartal IBA, Shan H, Molasky NMR, Murray TM, Williams JZ, Decety J, et al. (2016). Anxiolytic treatment impairs helping behavior in rats. Frontiers in Psychology 7, 1–14. doi: 10.3389/fpsyg.2016.00850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartal IB-A, Decety J, and Mason P (2011). Empathy and Pro-Social Behavior in Rats. Science 334, 1427–1430. doi: 10.1126/science.1210789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, and Ochsner KN (2011). Social effects of oxytocin in humans: context and person matter. Trends Cogn. Sci. (Regul. Ed.) 15, 301–309. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Beery AK, and Kaufer D (2015). Stress, social behavior, and resilience: Insights from rodents. Neurobiology of Stress 1, 116–127. doi: 10.1016/j.ynstr.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard EB, Kuhn E, Rowell DL, Hickling EJ, Wittrock D, Rogers RL, et al. (2004). Studies of the vicarious traumatization of college students by the September 11th attacks: effects of proximity, exposure and connectedness. 42, 191–205. doi: 10.1016/S0005-7967(03)00118-9. [DOI] [PubMed] [Google Scholar]
- Blume A, Bosch OJ, Miklos S, Torner L, Wales L, Waldherr M, et al. (2008). Oxytocin reduces anxiety via ERK 1/2 activation: local effect within the rat hypothalamic paraventricular nucleus. Eur. J. Neurosci 27, 1947–1956. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Nair HP, Ahern TH, Neumann ID, and Young LJ (2008). The CRF system mediates increased passive stress-coping behavior following the loss of a bonded partner in a monogamous rodent. Neuropsychopharmacology 34, 1406–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briefer EF (2018). Vocal contagion of emotions in non-human animals. Proceedings of the Royal Society B: Biological Sciences 285, 20172783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkett JP, Andari E, Johnson ZV, Curry DC, Waal F. B. M. de, and Young LJ (2016). Oxytocin-dependent consolation behavior in rodents. Science 351, 375–378. doi: 10.1126/science.aac4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busnelli M, Bulgheroni E, Manning M, Kleinau G, and Chini B (2013). Selective and potent agonists and antagonists for investigating the role of mouse oxytocin receptors. J Pharmacol Exp Ther 346, 318–327. doi: 10.1124/jpet.113.202994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busnelli M, and Chini B (2018). Molecular Basis of Oxytocin Receptor Signalling in the Brain: What We Know and What We Need to Know. Curr Top Behav Neurosci 35, 3–29. doi: 10.1007/7854_2017_6. [DOI] [PubMed] [Google Scholar]
- Busnelli M, Saulière A, Manning M, Bouvier M, Galés C, and Chini B (2012). Functional selective oxytocin-derived agonists discriminate between individual G protein family subtypes. J Biol Chem 287, 3617–3629. doi: 10.1074/jbc.M111.277178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnevali L, Montano N, Statello R, Coudé G, Vacondio F, Rivara S, et al. (2017). Social stress contagion in rats: Behavioural, autonomic and neuroendocrine correlates. Psychoneuroendocrinology 82, 155–163. doi: 10.1016/j.psyneuen.2017.05.017. [DOI] [PubMed] [Google Scholar]
- Carnevali L, Montano N, Tobaldini E, Thayer JF, and Sgoifo A (2020). The contagion of social defeat stress: Insights from rodent studies. Neuroscience and Biobehavioral Reviews 111, 12–18. doi: 10.1016/j.neubiorev.2020.01.011. [DOI] [PubMed] [Google Scholar]
- Carter CS, Kenkel WM, MacLean EL, Wilson SR, Perkeybile AM, Yee JR, et al. (2020). Is Oxytocin “Nature’s Medicine”? Pharmacol Rev 72, 829–861. doi: 10.1124/pr.120.019398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay Z, and Waal De FBM (2013). Bonobos Respond to Distress in Others: Consolation across the Age Spectrum. PLoS ONE 8, e55206. doi: 10.1371/journal.pone.0055206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb S (1976). Social support as a moderator of life stress. Psychosomatic Medicine 38, 300–314. doi: 10.1097/00006842-197609000-00003. [DOI] [PubMed] [Google Scholar]
- Cooper SE, Kechner M, Caraballo-Pérez D, Kaska S, Robison AJ, and Mazei-Robison MS (2017). Comparison of chronic physical and emotional social defeat stress effects on mesocorticolimbic circuit activation and voluntary consumption of morphine. Scientific Reports 7, 1–14. doi: 10.1038/s41598-017-09106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowska J, Hazra R, Guo JD, DeWitt S, and Rainnie DG (2013). Central CRF neurons are not created equal: Phenotypic differences in CRF-containing neurons of the rat paraventricular hypothalamus and the bed nucleus of the stria terminalis. Frontiers in Neuroscience 7. doi: 10.3389/fnins.2013.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Waal FBM, and Preston SD (2017). Mammalian empathy: Behavioural manifestations and neural basis. Nature Reviews Neuroscience 18, 498–509. doi: 10.1038/nrn.2017.72. [DOI] [PubMed] [Google Scholar]
- Denny CA, Kheirbek MA, Alba EL, Tanaka KF, Brachman RA, Laughman KB, et al. (2014). Hippocampal Memory Traces Are Differentially Modulated by Experience, Time, and Adult Neurogenesis. Neuron 83, 189–201. doi: 10.1016/j.neuron.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitroff SJ, Kardan O, Necka EA, Decety J, Berman MG, and Norman GJ (2017). Physiological dynamics of stress contagion. Scientific Reports 7, 1–8. doi: 10.1038/s41598-017-05811-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dölen G, Darvishzadeh A, Huang KW, and Malenka RC (2013). Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 501, 179–184. doi: 10.1038/nature12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Watts AG, and Swanson LW (2001). Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. Journal of Comparative Neurology 436, 430–455. [DOI] [PubMed] [Google Scholar]
- Donovan M, Liu Y, and Wang Z (2018). Anxiety-like behavior and neuropeptide receptor expression in male and female prairie voles: The effects of stress and social buffering. Behavioural Brain Research 342, 70–78. doi: 10.1016/j.bbr.2018.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque-Wilckens N, Steinman MQ, Busnelli M, Chini B, Yokoyama S, Pham M, et al. (2018). Oxytocin Receptors in the Anteromedial Bed Nucleus of the Stria Terminalis Promote Stress-Induced Social Avoidance in Female California Mice. Biol. Psychiatry 83, 203–213. doi: 10.1016/j.biopsych.2017.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque-Wilckens N, Torres LY, Yokoyama S, Minie VA, Tran AM, Petkova SP, et al. (2020). Extra-hypothalamic oxytocin neurons drive stress-induced social vigilance and avoidance. Proc Natl. Acad. Sci. U.S.A 117, 26406–26413. doi: 10.1101/2020.06.02.129981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wethington E (2000). “Contagion of Stress,” in Advances in Group Processes 17, 229–253. [Google Scholar]
- Eliava M, Melchior M, Knobloch-Bollmann HS, Wahis J, Gouveia MS, Tang Y, et al. (2016). A new population of parvocellular oxytocin neurons controlling magnocellular neuron activity and inflammatory pain processing. Neuron 89, 1291–12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann M, Ebner K, Landgraf R, Holsboer F, and Wotjak CT (1999). Emotional stress triggers intrahypothalamic but not periperal release of oxytocin in male rats. J. Neuroendocrinol 11, 867–872. [DOI] [PubMed] [Google Scholar]
- Engert V, Linz R, and Grant JA (2019). Psychoneuroendocrinology Embodied stress: The physiological resonance of psychosocial stress. Psychoneuroendocrinology 105, 138–146. doi: 10.1016/j.psyneuen.2018.12.221. [DOI] [PubMed] [Google Scholar]
- Fernandes Silva P, Garcia de Leaniz C, and Luchiari AC (2019). Fear contagion in zebrafish: a behaviour affected by familiarity. Animal Behaviour 153, 95–103. doi: 10.1016/j.anbehav.2019.05.004. [DOI] [Google Scholar]
- Ferretti V, Maltese F, Contarini G, Nigro M, Bonavia A, Huang H, et al. (2019). Oxytocin Signaling in the Central Amygdala Modulates Emotion Discrimination in Mice. Current Biology 29, 1938–1953.e6. doi: 10.1016/j.cub.2019.04.070. [DOI] [PubMed] [Google Scholar]
- Finnell JE, Lombard CM, Padi AR, Moffitt CM, Wilson LB, Wood CS, et al. (2017). Physical versus psychological social stress in male rats reveals distinct cardiovascular, inflammatory and behavioral consequences. PLoS ONE 12, 1–24. doi: 10.1371/journal.pone.0172868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnell JE, Muniz BL, Padi AR, Lombard CM, Moffitt CM, Wood CS, et al. (2018). Essential Role of Ovarian Hormones in Susceptibility to the Consequences of Witnessing Social Defeat in Female Rats. Society of Biological Psychiatry doi: 10.1016/j.biopsych.2018.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flandreau EI, Ressler KJ, Owens MJ, and Nemeroff CB (2012). Chronic overexpression of corticotropin-releasing factor from the central amygdala produces HPA axis hyperactivity and behavioral anxiety associated with gene-expression changes in the hippocampus and paraventricular nucleus of the hypothalamus. Psychoneuroendocrinology 37, 27–38. doi: 10.1016/j.psyneuen.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM, Smith AL, Goodman MM, and Bales KL (2017). Selective localization of oxytocin receptors and vasopressin 1a receptors in the human brainstem. Soc Neurosci 12, 113–123. doi: 10.1080/17470919.2016.1156570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg GD, Laman-Maharg A, Campi KL, Voigt H, Orr VN, Schaal L, et al. (2014). Sex differences in stress-induced social withdrawal: role of brain derived neurotrophic factor in the bed nucleus of the stria terminalis. Front Behav Neurosci 7, 223. doi: 10.3389/fnbeh.2013.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinevich V, Knobloch-Bollmann HS, Eliava M, Busnelli M, and Chini B (2016). Assembling the Puzzle: Pathways of Oxytocin Signaling in the Brain. Biological Psychiatry 79, 155–164. doi: 10.1016/j.biopsych.2015.04.013. [DOI] [PubMed] [Google Scholar]
- Gutzeit VA, Ahuna K, Santos TL, Cunningham AM, Sadsad Rooney M, Muñoz Zamora A, et al. (2020). Optogenetic reactivation of prefrontal social neural ensembles mimics social buffering of fear. Neuropsychopharmacology 45, 1068–1077. doi: 10.1038/s41386-020-0631-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman YF, Tronson NC, Sato K, Mesic I, Guedea AL, Nishimori K, et al. (2014). Role of oxytocin receptors in modulation of fear by social memory. Psychopharmacology (Berl) 231, 2097–2105. doi: 10.1007/s00213-013-3356-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauger RL, Risbrough V, Oakley RH, Olivares-Reyes JA, and Dautzenberg FM (2009). Role of CRF Receptor Signaling in Stress Vulnerability, Anxiety, and Depression. Ann N Y Acad Sci 1179, 120–143. doi: 10.1111/j.1749-6632.2009.05011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber W, Veinante P, and Stoop R (2005). Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science 308, 245–248. [DOI] [PubMed] [Google Scholar]
- Hupalo S, and Berridge CW (2016). Working Memory Impairing Actions of Corticotropin-Releasing Factor (CRF) Neurotransmission in the Prefrontal Cortex. Neuropsychopharmacology 41, 2733–2740. doi: 10.1038/npp.2016.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez SD, Flores-ramirez FJ, Riggs LM, Alipio JB, Hernandez MA, Sanchez DO, et al. (2018). Vicarious Social Defeat Stress Induces Depression-related Outcomes in Female Mice. Biological Psychiatry 83, 9–17. doi: 10.1016/j.biopsych.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson BB, Nair BB, and Iremonger KJ (2017). Regulation of hypothalamic corticotropin-releasing hormone neurone excitability by oxytocin. Journal of Neuroendocrinology 29, e12532. doi: 10.1111/jne.12532. [DOI] [PubMed] [Google Scholar]
- Jiang Y, and Platt ML (2018). Oxytocin and vasopressin flatten dominance hierarchy and enhance behavioral synchrony in part via anterior cingulate cortex. Scientific Reports 8, 8201. doi: 10.1038/s41598-018-25607-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ZV, and Young LJ (2017). Oxytocin and vasopressin neural networks: implications for social behavioral diversity and translational neuroscience. Neurosci Biobehav Rev 76, 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikusui T, Winslow JT, and Mori Y (2006). Social buffering: Relief from stress and anxiety. Philosophical Transactions of the Royal Society B: Biological Sciences 361, 2215–2228. doi: 10.1098/rstb.2006.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyokawa Y, and Hennessy MB (2018). Comparative studies of social buffering: A consideration of approaches, terminology, and pitfalls. Neuroscience and Biobehavioral Reviews 86, 131–141. doi: 10.1016/j.neubiorev.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch HS, C. A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, et al. (2012). Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron 73, 553–566. [DOI] [PubMed] [Google Scholar]
- Kupfermann I (1979). Modulatory actions of neurotransmitters. Annu Rev Neurosci 2, 447–465. doi: 10.1146/annurev.ne.02.030179.002311. [DOI] [PubMed] [Google Scholar]
- Lamm C, Decety J, and Singer T (2011). Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage 54, 2492–2502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Levy DR, and Yizhar O (2018). Stress and sociability. 12. [DOI] [PubMed] [Google Scholar]
- Li LF, Yuan W, He ZX, Wang LM, Jing XY, Zhang J, et al. (2019a). Involvement of oxytocin and GABA in consolation behavior elicited by socially defeated individuals in mandarin voles. Psychoneuroendocrinology 103, 14–24. doi: 10.1016/j.psyneuen.2018.12.238. [DOI] [PubMed] [Google Scholar]
- Li L, Yuan W, He Z, Ma H, Xun Y, Meng L, et al. (2019b). Reduced Consolation Behaviors in Physically Stressed Mandarin Voles: Involvement of Oxytocin, Dopamine D2, and Serotonin 1A Receptors Within the Anterior Cingulate Cortex. International Journal of Neuropsychopharmacology. doi: 10.1093/ijnp/pyz060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberwirth C, and Wang Z (2016). The neurobiology of pair bond formation, bond disruption, and social buffering. Current Opinion in Neurobiology 40, 8–13. doi: 10.1016/j.conb.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, and Wang ZX (2003). Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience 121, 537–544. doi: 10.1016/s0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- Loewen SP, Baimoukhametova DV, and Bains JS (2020). Sex-Specific Vasopressin Signaling Buffers Stress-Dependent Synaptic Changes in Female Mice. J Neurosci 40, 8842–8852. doi: 10.1523/JNEUROSCI.1026-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon D, Lis P, Roman AN, Tornesi P, Applebey SV, Buechner G, et al. (2019). Oxytocin receptors in the dorsolateral bed nucleus of the stria terminalis (BNST) bias fear learning toward temporally predictable cued fear. Transl Psychiatry 9, 140. doi: 10.1038/s41398-019-0474-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall JG, Al-Hasani R, Siuda ER, Hong DY, Norris AJ, Ford CP, et al. (2015). CRH Engagement of the Locus Coeruleus Noradrenergic System Mediates Stress-Induced Anxiety. Neuron 87, 605–620. doi: 10.1016/j.neuron.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Z, Mao F, Liang J, Szyf M, and Wang Y (2017). Anxiety-Related Behaviours Associated with microRNA-206–3p and BDNF Expression in Pregnant Female Mice Following Psychological Social Stress. Molecular Neurobiology. doi: 10.1007/s12035-016-0378-1. [DOI] [PubMed] [Google Scholar]
- Monari PK, Rieger NS, Hartfield K, Schefelker J, and Marler CA (2020). Oxytocin promotes convergence in personality between members of a monogamous pair. bioRxiv, 2020.11.20.390245. doi: 10.1101/2020.11.20.390245. [DOI] [Google Scholar]
- Nakajima M, Görlich A, and Heintz N (2014). Oxytocin modulates female sociosexual behavior through a specific class of prefrontal cortical interneurons. Cell 159, 295–305. doi: 10.1016/j.cell.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasanbuyan N, Yoshida M, Takayanagi Y, Inutsuka A, Nishimori K, Yamanaka A, et al. (2018). Oxytocin-Oxytocin Receptor Systems Facilitate Social Defeat Posture in Male Mice. Endocrinology 159, 763–775. doi: 10.1210/en.2017-00606. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Wigger A, Torner L, Holsboer F, and Landgraf R (2000). Brain Oxytocin Inhibits Basal and Stress-Induced Activity of the Hypothalamo-Pituitary-Adrenal Axis in Male and Female Rats: Partial Action Within the Paraventricular Nucleus. 12, 235–243. [DOI] [PubMed] [Google Scholar]
- Nishioka T, Anselmo-franci JA, Li P, Callahan MF, and Morris M (1998). Stress increases oxytocin release within the hypothalamic paraventricular nucleus. 57–61. [DOI] [PubMed] [Google Scholar]
- Oliveira RF, and Faustino AI (2017). Social information use in threat perception: Social buffering, contagion and facilitation of alarm responses. Communicative & Integrative Biology 10, e1325049. doi: 10.1080/19420889.2017.1325049. [DOI] [Google Scholar]
- Owings DH, Coss RG, McKernon D, Rowe MP, and Arrowood PC (2001). Snake-Directed Antipredator Behavior of Rock Squirrels (Spermophilus variegatus): Population Differences and Snake-Species Discrimination. Behaviour 138, 575–595. [Google Scholar]
- Ozbay F, Johnson DC, Dimoulas E, Morgan CA, Charney D, and Southwick S (2007). Social support and resilience to stress: from neurobiology to clinical practice. Psychiatry (Edgmont) 4, 35–40. [PMC free article] [PubMed] [Google Scholar]
- Passoni I, Leonzino M, Gigliucci V, Chini B, and Busnelli M (2016). Carbetocin is a functional selective Gq agonist that does not promote oxytocin receptor recycling after inducing barrestin-independent internalisation. J. Neuroendocrinol 28, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patki G, Solanki N, and Salim S (2014). Witnessing traumatic events causes severe behavioral impairments in rats. International Journal of Neuropsychopharmacology 755, 2017–2029. doi: 10.1017/S1461145714000923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman SE, Friedman S, Galea S, Nair HP, Erős-sarnyai M, Stellman SD, et al. (2011). Short-term and medium-term health effects of 9 / 11. The Lancet 378, 925–934. doi: 10.1016/S0140-6736(11)60967-7. [DOI] [PubMed] [Google Scholar]
- Pfeiffer W (1977). The Distribution of Fright Reaction and Alarm Substance Cells in Fishes. Copeia 1977, 653–665. doi: 10.2307/1443164. [DOI] [Google Scholar]
- Pisansky MT, Hanson LR, Gottesman II, and Gewirtz JC (2017). Oxytocin enhances observational fear in mice. Nature Communications 8, 2102. doi: 10.1038/s41467-017-02279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomrenze MB, Giovanetti SM, Maiya R, Gordon AG, Kreeger LJ, and Messing RO (2019). Dissecting the Roles of GABA and Neuropeptides from Rat Central Amygdala CRF Neurons in Anxiety and Fear Learning. Cell Reports 29, 13–21.e4. doi: 10.1016/j.celrep.2019.08.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana DS, Rokicki J, van der Meer D, Alnæs D, Kaufmann T, Córdova-Palomera A, et al. (2019). Oxytocin pathway gene networks in the human brain. Nature Communications 10, 668. doi: 10.1038/s41467-019-08503-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen S, Miller MM, Filipski SB, and Tolwani RJ (2011). Cage Change Influences Serum Corticosterone and Anxiety-Like Behaviors in the Mouse. Journal of the American Association for Laboratory Animal Science 50, 479–483. [PMC free article] [PubMed] [Google Scholar]
- Reindl V, Gerloff C, Scharke W, and Konrad K (2018). Brain-to-brain synchrony in parent-child dyads and the relationship with emotion regulation revealed by fNIRS-based hyperscanning. NeuroImage 178, 493–502. doi: 10.1016/j.neuroimage.2018.05.060. [DOI] [PubMed] [Google Scholar]
- Rogers-Carter MM, Djerdjaj A, Culp AR, Elbaz JA, and Christianson JP (2018a). Familiarity modulates social approach toward stressed conspecifics in female rats. PLOS ONE 13, e0200971. doi: 10.1371/journal.pone.0200971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers-Carter MM, Djerdjaj A, Gribbons KB, Varela JA, and Christianson JP (2019). Insular cortex projections to nucleus accumbens core mediate social approach to stressed juvenile rats. Journal of Neuroscience 39, 8717–8729. doi: 10.1523/JNEUROSCI.0316-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers-Carter MM, Varela JA, Gribbons KB, Pierce AF, McGoey MT, Ritchey M, et al. (2018b). Insular cortex mediates approach and avoidance responses to social affective stimuli. Nature Neuroscience 21, 404–414. doi: 10.1038/s41593-018-0071-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabihi S, Durosko NE, Dong SM, and Leuner B (2014). Oxytocin in the prelimbic medial prefrontal cortex reduces anxiety-like behavior in female and male rats. Psychoneuroendocrinology 45, 31–42. doi: 10.1016/j.psyneuen.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheggia D, Managò F, Maltese F, Bruni S, Nigro M, Dautan D, et al. (2020). Somatostatin interneurons in the prefrontal cortex control affective state discrimination in mice. Nature Neuroscience 23, 47–60. doi: 10.1038/s41593-019-0551-8. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, and Fox AS (2016). Contributions of the central extended amygdala to fear and anxiety. J. Neurosci 36, 8050–8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, and Abu-Akel A (2016). The Social Salience Hypothesis of Oxytocin. Biological Psychiatry 79, 194–202. doi: 10.1016/j.biopsych.2015.07.020. [DOI] [PubMed] [Google Scholar]
- Sial OK, Warren BL, Alcantara LF, Parise EM, and Bolaños-Guzmán CA (2016). Vicarious social defeat stress: Bridging the gap between physical and emotional stress. Journal of Neuroscience Methods 258, 94–103. doi: 10.1016/j.jneumeth.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AS, Tabbaa M, Lei K, Eastham P, Butler MJ, Linton L, et al. (2016). Local oxytocin tempers anxiety by activating GABAA receptors in the hypothalamic paraventricular nucleus. Psychoneuroendocrinology 63, 50–58. doi: 10.1016/j.psyneuen.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AS, and Wang Z (2014). Hypothalamic oxytocin mediates social buffering of the stress response. Biological Psychiatry 76, 281–288. doi: 10.1016/j.biopsych.2013.09.017.Hypothalamic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spengler FB, Scheele D, Marsh N, Kofferath C, Flach A, Schwarz S, et al. (2017). Oxytocin facilitates reciprocity in social communication. Soc Cogn Affect Neurosci 12, 1325–1333. doi: 10.1093/scan/nsx061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman MQ, Duque-Wilckens N, Greenberg GD, Hao R, Campi KL, Laredo SA, et al. (2016). Sex-Specific Effects of Stress on Oxytocin Neurons Correspond With Responses to Intranasal Oxytocin. Biological Psychiatry 80, 406–414. doi: 10.1016/j.biopsych.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman MQ, Duque-Wilckens N, and Trainor BC (2019). Complementary Neural Circuits for Divergent Effects of Oxytocin: Social Approach Versus Social Anxiety. Biol. Psychiatry 85, 792–801. doi: 10.1016/j.biopsych.2018.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterley TL, Baimoukhametova D, Füzesi T, Zurek AA, Daviu N, Rasiah NP, et al. (2018). Social transmission and buffering of synaptic changes after stress. Nature Neuroscience 21, 393–403. doi: 10.1038/s41593-017-0044-6. [DOI] [PubMed] [Google Scholar]
- Sterley T-L, and Bains JS (2021). Social communication of affective states. Current Opinion in Neurobiology in press. [DOI] [PubMed] [Google Scholar]
- Stoop R (2012). Neuromodulation by Oxytocin and Vasopressin. Neuron 76, 142–159. doi: 10.1016/j.neuron.2012.09.025. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, and Vale WW (1983). Organization of Ovine Corticotropin-Releasing Factor Immunoreactive Cells and Fibers in the Rat Brain: An Immunohistochemical Study. NEN 36, 165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Tai FD, Wang TZ, and Zhao YJ (2001). Mating system of mandarin vole (Lasiopodomys mandarinus). Acta Zool. Sinica 47, 266–273. [Google Scholar]
- Terenzi MG, and Ingram CD (2005). Oxytocin-induced excitation of neurones in the rat central and medial amygdaloid nuclei. Neuroscience 134, 345–354. doi: 10.1016/j.neuroscience.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Trainor BC, Takahashi EY, Campi KL, Florez SA, Greenberg GD, Laman-Maharg A, et al. (2013). Sex differences in stress-induced social withdrawal: Independence from adult gonadal hormones and inhibition of female phenotype by corncob bedding. Hormones and Behavior 63. doi: 10.1016/j.yhbeh.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda H, and Neyama H (2017). LPA1 receptor involvement in fibromyalgia-like pain induced by intermittent psychological stress, empathy. Neurobiology of Pain 1, 16–25. doi: 10.1016/j.ynpai.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viau V, Bingham B, Davis J, Lee P, and Wong M (2005). Gender and Puberty Interact on the Stress-Induced Activation of Parvocellular Neurosecretory Neurons and Corticotropin-Releasing Hormone Messenger Ribonucleic Acid Expression in the Rat. Endocrinology 146, 137–146. doi: 10.1210/en.2004-0846. [DOI] [PubMed] [Google Scholar]
- Viviani D, Charlet A, van den Burg E, Robinet C, Hurni N, Abatis M, et al. (2011). Oxytocin selectively gates fear responses through distinct outputs from the central amygdala. Science 333, 104–107. [DOI] [PubMed] [Google Scholar]
- Warren BL, Mazei-Robison MS, Robison AJ, and Iñiguez SD (2020). Can I Get a Witness? Using Vicarious Defeat Stress to Study Mood-Related Illnesses in Traditionally Understudied Populations. Biological Psychiatry. doi: 10.1016/j.biopsych.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren BL, Sial OK, Alcantara LF, Greenwood MA, Brewer JS, Rozofsky JP, et al. (2014). Altered gene expression and spine density in nucleus accumbens of adolescent and adult male mice exposed to emotional and physical stress. Developmental Neuroscience 36, 250–260. doi: 10.1159/000362875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren BL, Vialou VF, Iñiguez SD, Alcantara LF, Wright KN, Feng J, et al. (2013). Neurobiological sequelae of witnessing stressful events in adult mice. Biological Psychiatry 73, 7–14. doi: 10.1016/j.biopsych.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AV, Duque-Wilckens N, Ramos-Maciel S, Campi KL, Bhela SK, Xu CK, et al. (2020). Social approach and social vigilance are differentially regulated by oxytocin receptors in the nucleus accumbens. Neuropsychopharmacology 45, 1423–1430. doi: 10.1038/s41386-020-0657-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AV, Laman-Maharg A, Armstrong CV, Ramos-Maciel S, Minie VA, and Trainor BC (2018). Acute inhibition of kappa opioid receptors before stress blocks depression-like behaviors in California mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 86, 166–174. doi: 10.1016/j.pnpbp.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle RJ, Kershaw YM, Shanks N, Wood SA, Lightman SL, and Ingram CD (2004). Oxytocin attenuates stress-induced c-fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo-pituitary-adrenal activity. J. Neurosci 24, 2974–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingen G van, Geuze E, Vermetten E, and Fernández G (2011). Perceived threat predicts the neural sequelae of combat stress. Molecular Psychiatry 16, 664–671. doi: 10.1038/mp.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter J, and Jurek B (2019). The interplay between oxytocin and the CRF system: regulation of the stress response. Cell and Tissue Research 375, 85–91. doi: 10.1007/s00441-018-2866-2. [DOI] [PubMed] [Google Scholar]
- Yasui Y, Breder CD, Saper CB, and Cechetto DF (1991). Autonomic responses and efferent pathways from the insular cortex in the rat. J Comp Neurol 303, 355–374. doi: 10.1002/cne.903030303. [DOI] [PubMed] [Google Scholar]
- Zoicas I, Slattery DA, and Neumann ID (2014). Brain Oxytocin in Social Fear Conditioning and Its Extinction: Involvement of the Lateral Septum. Neuropsychopharmacology 39, 3027–3035. doi: 10.1038/npp.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]


