Summary.
Tumorigenesis proceeds through discrete steps where acquisition of genetic lesions and changes in the surrounding microenvironment combine to drive unrestricted neoplastic proliferation and metastasis. The ability of tumor-infiltrating immune cells to promote tumor growth via the provision of signals that enable tumor cell survival and proliferation as well as contribute to immune suppression is an active area of research. Recent efforts have provided us with mechanistic insights into how B cells can positively and negatively regulate immune responses. Negative regulation of immune responses in cancer can be mediated by regulatory B cells and is often a result of increased production of cytokines that can directly and indirectly affect anti-tumor immune function and cancer cell growth. Signals that lead to the expansion of regulatory B cells and the spectrum of their functional roles are not well understood and are the subject of active research by many groups. Here, we elaborate broadly on the history of regulatory B cells in cancer and summarize recent studies that have established genetic models for the study of regulatory B cell function and their potential for therapeutic intervention in the setting of solid cancers.
Keywords: Regulatory B cells, cancer, IL-35, tumor immunology
1. Introduction.
This review highlights the recent studies on the role of regulatory B cells (Breg) in cancer. We provide a brief discussion of the role of regulatory immune cells in development and disease, followed by a general summary of Breg cell phenotypes and function in solid malignancies. We discuss analysis of preclinical studies that demonstrate the detection methods, genetic models and functional studies on the role of IL-35 producing Breg cells in cancer growth and control of anti-tumor immune responses, and discuss the use of selective targeting of pathogenic B cell subsets. Finally, we highlight translational work describing phenotype, frequency, localization, and gene expression signature of Breg cells in patients with pancreatic cancer, that may enable studies of this B cell subset in many distinct cancer types.
2. Regulatory function in homeostasis.
The immune system serves to provide a potent barrier against invading pathogens. At the same time, there are mechanisms in place that ensure limited reactivity to potentially pathogenic self-antigens. Such protective measures are termed ‘tolerance’ and encompass a range of mechanisms such as self-antigen-induced apoptosis and anergy, which are largely executed on a cell autonomous level. Extrinsic control of self-reactivity is perpetuated by a collection of peripheral immune cells that suppress unwanted immune cell activity via soluble mediators such as cytokines, direct cell-to-cell contact via membrane bound checkpoint mediators, or via induction of additional regulatory cell types (1, 2). Several distinct regulatory cell types have been shown to contribute to tolerance against abundant self-antigens or those that may be temporarily overexpressed during key developmental processes. These include regulatory T cells (Treg), regulatory B cells (Breg), dendritic cells, macrophages, and myeloid derived suppressor cells (MDSC) among others (3, 4). Their tolerizing activity is shaped by maturation status, often accompanied by low expression of co-stimulatory machinery and microenvironmental cues (5, 6). For example, MDSCs are a heterogeneous population of immature myeloid cells which can suppress T cells, and in normal development are important in maintenance of immune tolerance at the maternal–fetal interface during pregnancy (7, 8). Myeloid cells also play an important role in tolerizing against the vast amounts of self-material that can be carried by apoptotic cells – this occurs via innate checkpoints such Tyro3, Axl and MerTK receptor tyrosine kinases (9).
Adaptive immune cell types also contribute to the maintenance of self-tolerance. Treg cells, characterized by expression of transcription factor Foxp3, are well known to be important regulators of peripheral T cell responses and mice that lack regulatory T cells develop severe autoimmune disease (10–12). New T cell subsets capable of regulating autoreactivity have been emerging such as follicular regulatory T cells, and CD8+ Treg; these may be important in restricting emergence of autoreactive B cells and limiting damage in models of type I diabetes respectively (13, 14). B cells can also contribute to tolerance via multiple mechanisms: by producing immunosuppressive cytokines and/or expressing checkpoint ligands that curb activation potential of T cell-mediated immune responses (15). It is well appreciated now that deficiencies in function of regulatory immune cells may lead to unchecked self-reactivity and is closely linked to severity of autoimmune and inflammatory conditions, such as rheumatoid arthritis, lupus erythematosus and multiple sclerosis (3, 4, 16–18).
3. Regulatory cells in cancer.
The immune contexture that results from continuous exposure of immune cells to tumor-derived factors contributes to cancerous outgrowth in many ways including directly promoting tumor growth, eliciting angiogenesis, supporting recruitment and expansion of additional inflammatory cell types, and fostering metastatic disease. In contrast to autoimmune diseases, the evolution of cancer is linked to changes in the tumor microenvironment that favor suppression of active immune responses (Figure 1) (19–24). This is often achieved by aberrant recruitment and expansion of immunosuppressive immune and stromal cells to the sites of the tumor growth (25–27). In this scenario, the ability of macrophages, MDSC, Tregs and Bregs to execute their normal homeostatic tolerogenic functions is hijacked by the cancer and results in local and systemic immunosuppression that directly counteracts immunotherapy efforts (28, 29).
Figure 1. Breg cells are suppressors of anti-tumor immunity.
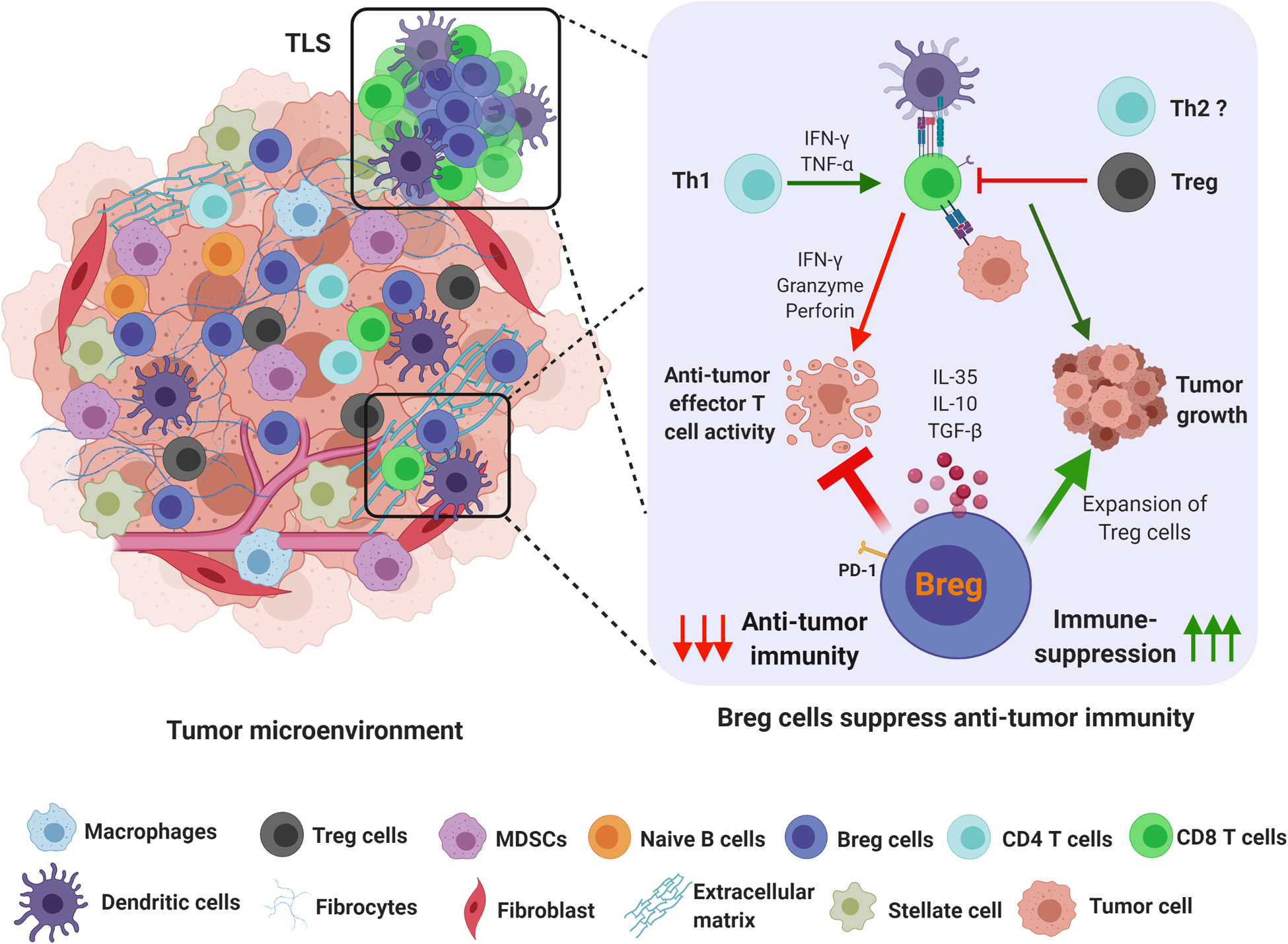
The tumor immune microenvironment is highly immunosuppressive. Recent reports indicate that apart from MDSC and regulatory T cells, subsets of immunosuppressive B cells (Breg) are important for controlling anti-tumor immune responses. Bregs expand intratumorally, in tertiary lymphoid structures, draining lymph nodes, and major secondary lymphoid organs, and can produce immune suppressive cytokines such as IL-35 and IL-10 in response to tumor-associated inflammation. B cell-derived IL-35 can dampen the effector T cell response by inhibiting effector CD4+ and CD8+ T cell responses and enhance the proliferation of Treg cells to simultaneously affect multiple arms of the anti-tumor immune response.
4. Multifaceted role for B cells in solid cancer pathogenesis.
Cancer immunotherapeutic approaches have been primarily focused on activating T cell-driven immunity, be it through adoptive T cell transfers, immune checkpoint blockade, or chimeric antigen receptor T cell therapy. The need to improve efficacy of such approaches has led to increased focus on other immune cell types within the tumor microenvironment. Mounting evidence supports an important role for B cells in the immunomodulation of cancer. It is clear that both anti- and pro-tumor effects can be attributed to B cells, although more work needs to be done to determine how such effects depend on the type of cancer, tumor microenvironment, and balance of B cell subsets that are present (30–36).
Early studies characterizing tumor-infiltrating lymphocytes (TILs) in non-small-cell lung tumors found that the percentage of B cells was associated with increased survival (37, 38). Additional research has highlighted a positive role for plasma cells in lung adenocarcinoma, as evidenced by associations with increased patient survival (39, 40). Analyses of RNA sequencing data from The Cancer Genome Atlas (TCGA) have also correlated high expression of B cell and plasma cell genes with increased patient survival in bladder, head and neck, melanoma, ovarian, and pancreatic adenocarcinoma (35, 41–48). In a model of pancreatic cancer, B cells were found to upregulate proinflammatory genes that may contribute to an anti-tumor response (49). While there is mounting evidence that B cells alone may provide valuable prognostic insight, biologically, the generation of effective anti-tumor immunity is likely tied to interactions between both B and T cells. In a murine 4T1 breast cancer model, the transfer of both activated B and T cells from tumor-draining lymph nodes yielded greater tumor regression than was observed when either population was transferred individually (50). Tertiary lymphoid structures (TLS), which contain aggregates of B and T lymphocytes in the tumor stroma, have garnered recent attention as a prognostic and predictive factor (44, 51). The presence of activated B cells within TLS have been associated with improved clinical outcome and response to immunotherapy in multiple cancer types. (52–58).
However, despite the promising implications of TLSs and plasma cells in a variety of cancers, a negative role for B cells is also observed across cancer types (35). Higher expression of IgG, B cell gene signatures, and plasma cell gene signatures from the TCGA were correlated with poor clinical prognosis in glioblastoma (42, 59, 60). Similarly, elevated expression of B cell-related genes in renal clear cell carcinoma correlated with decreased survival (42, 45). Both pro- and anti-tumorigenic properties have also been described in the same cancer type. In ductal breast cancer, immunohistochemical analysis revealed that high levels of CD138+ plasma cells were associated with decreased survival (61). However, B cell gene expression signatures have been correlated with improved metastasis-free survival using the TCGA datasets (41). Furthermore, analyses of tissue samples from HER2+ and triple negative breast cancer patients support the correlation between increased tumor-infiltrating B cells and improved clinical outcome (62). Collectively, these studies reflect the multifaceted functional properties of B cells. The capacity to promote antigen presentation, cytokine production, antibody-dependent cellular cytotoxicity, formation of TLS, and isotype switching to immune stimulatory antibodies such as IgG1 are thought to help drive an anti-tumoral response, whereas the presence of characteristically immunosuppressive B cell phenotypes and antibody isotypes have been linked to pro-tumor effects (36). The following section addresses several of these functional processes as they relate to anti- and pro-tumoral responses in cancer.
4.1. Antibody production.
Production of tumor-specific antibodies can promote the opsonization and phagocytosis of tumor cells by macrophages and dendritic cells, as well as induce the lysis of cancer cells via complement cascades and antibody-dependent cellular cytotoxicity (ADCC). Tumor-binding IgG antibodies in murine models of melanoma, pancreas, lung, and breast cancer facilitate phagocytosis by dendritic cells, and subsequently lead to enhanced presentation of tumor antigens to T cells (63). In vitro studies evaluating IgG antibody responses in melanoma demonstrated effective killing of cancer cells by ADCC as well (64). Furthermore, in a mouse model of triple negative breast cancer, activation of T cells and antibody production by B cells impacted the response to checkpoint blockade immunotherapy (55). It is also important to note that in addition to producing antibodies, plasma cells secrete a myriad of cytokines that can recruit, activate, or suppress other immune cell populations (65). The role of plasma cells in the tumor microenvironment has been reviewed in detail in the excellent review by Sharonov et al. (36).
4.2. Antigen presentation.
Although dendritic cells (DCs) are rightfully hailed as the masters of antigen presentation, DCs are often modified by tumors, hindering their capacity to present antigens. Therefore, the ability of B cells to present tumor-specific antigens to T cells may play a vital role in fostering anti-tumor immunity. For instance, dendritic cells harvested from cervical cancer patients were resistant to α-CD40 treatment and incapable of driving an anti-tumor response, whereas B lymphocytes taken from the same patients responded to α-CD40 treatment and consequently elicited secondary T cell responses (66). Moreover, in vitro antigen-presentation assays in a model of non-small-cell lung cancer supported the conclusion that activated B cells within the tumor were not only able to present antigen to CD4+ T cells, but also initiated an effector T cell phenotype (67). It should be noted, however, that exhausted tumor-infiltrating B cells in this same study led to the production of suppressive, regulatory FoxP3+ CD4+ T cells (67).
4.3. Spatial organization.
The recent evidence correlating the presence of B cells within cancer-associated TLS with improved patient survival and immunotherapy response highlights that B cells can be a key cell population for facilitating an effective anti-tumor immunity in response to immunotherapy (51, 56–58). Using genomic datasets of soft-tissue sarcoma biopsies, Petitprez et al. were able to create an immune signature that revealed a correlation between increased B cell presence within the tumors and an increase in patient survival. Most notably, the predictive effect on survival was independent of the level of CD8+ T cell presence. In a multi-center clinical trial, patients with a high B cell immune signature had a 50% overall response rate when treated with pembrolizumab (αPD-1), which was significantly higher than patients with low B cell tumor signatures. In melanoma, a similar co-occurrence of CD8+ T cells and CD20+ B cells aggregates correlated with longer patient survival (56). Again, the longer survival was attributed more to the presence of the CD20+ B cells than the presence of CD8+ T cells within the TLS. Melanoma patients that had CD8+CD20+ TLS structures within the tumor also responded better to αPD-1 and αCTLA-4 checkpoint blockade therapy. B cell populations within responding tumors were more clonally diverse and contained significantly more memory B cells and plasma cells indicating a robust immunological response in responding tumors (57). Collectively, these studies indicate that activated B cells in the context of organized immune intratumoral aggregates may confer a predictive outcome to survival checkpoint blockade responsiveness.
5. Immunosuppressive functions of B cells in preclinical cancer models.
Murine models of cancer are powerful preclinical tools for investigating mechanisms of tumor growth and testing the efficacy of therapeutic strategies (Figure 2). Syngeneic models allow for the investigation of the interplay of tumorigenesis and the immune system (68). Such models generally encompass two main approaches: syngeneic cell line implantation and genetically engineered mouse models (GEMM). Syngeneic cell line implantation allows for more flexibility as the cell lines can be injected either subcutaneously, which is more amenable to high throughput in vivo experiments, or orthotopically, which better reflects the native tumor microenvironment albeit more technically complicated. GEMMs provide the most translational mimicry of the human tumor microenvironment as cancer evolves from the native host tissue under the guidance of defined genetic alterations. GEMMs, however, can require a long development time for full disease onset. We have successfully utilized both GEMM and orthotopic cancer models to investigate the effects of pancreatic tumorigenesis on the immune response with a particular interest in B cell function (Table 1) (69–72). Both approaches result in the expansion of regulatory B cell and plasma cell subsets. However, the tumor microenvironment that these two systems develop may not be identical and as a result, expose B cells to tumor-associated factors for different durations. GEMMs allow for the development of potential neoantigens de novo over time, whereas syngeneic models may grow too rapidly for this process. As a result, B cells in syngeneic models may or may not clonally expand based on a cell line specific antigen which may not be translationally applicable to patients. Syngeneic cell lines, however, do have the advantage of being implantable in various genetic knockout models without need for extensive breeding. In the case of B cells, syngeneic tumors can be implanted in mice with deficiencies in cell surface receptors, co-stimulatory molecules, or cytokines. The utilization of both syngeneic and GEMM approaches will provide the most comprehensive understanding of the biology of B cells in cancer.
Figure 2. Understanding Bregs: pre-clinical models and translational potential.
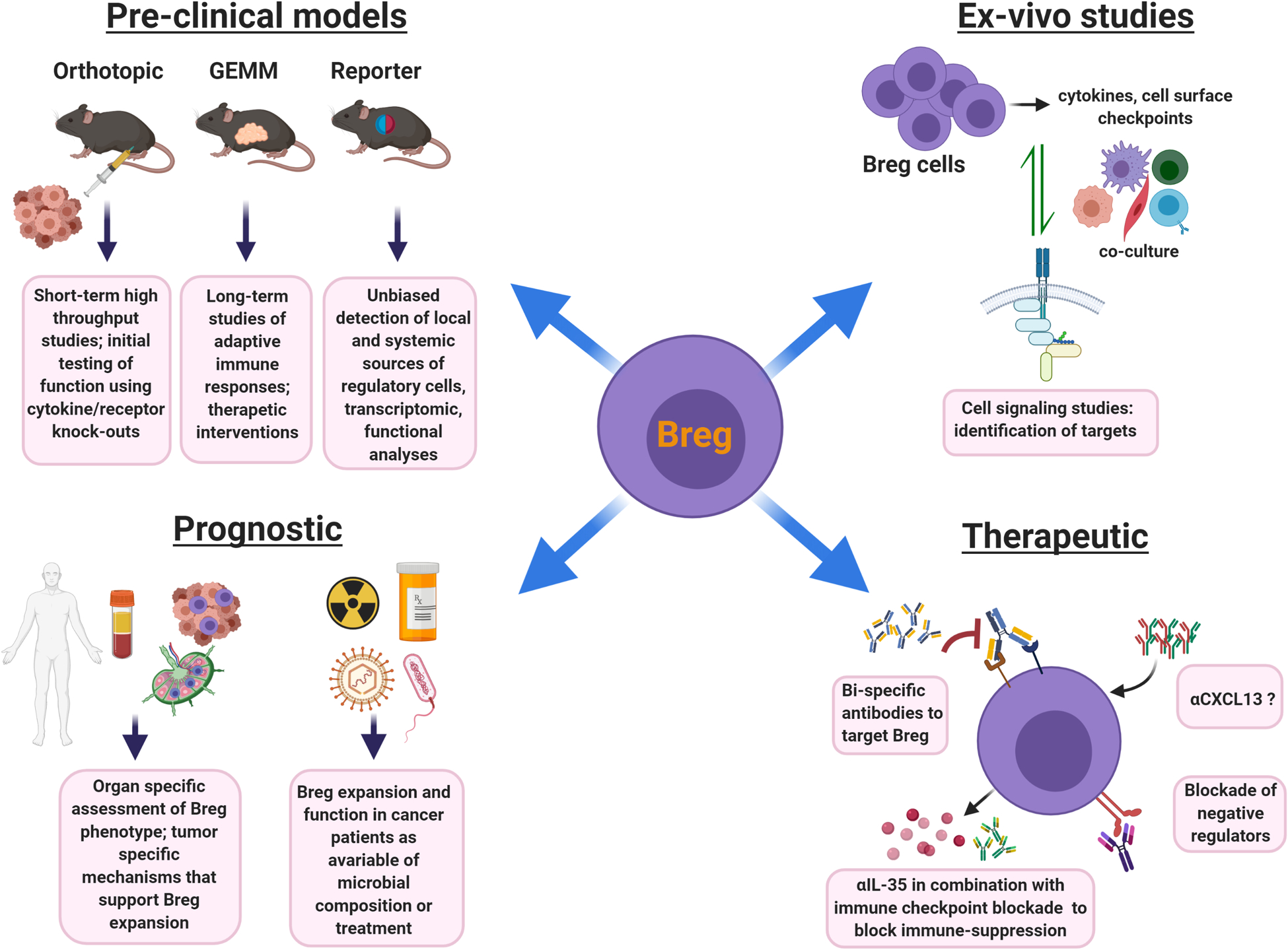
Pre-clinical models: Several types of in vivo modeling approaches can be utilized to study Breg biology in cancer. Orthotopic mouse models involving transplantation of syngeneic tumor cells are quick to generate and B cell-specific knock-out strains can be used to study gene functionality in tumor growth. Limitations include short-life span and the need to characterize the ability of existing cancer cell lines to initiate B cell responses. GEMM models take longer to develop and can be useful for long-term studies of adaptive immune responses, tertiary aggregate formation, and pre-clinical testing of Breg targeted therapeutics. Limitations are time-intensive breeding and the potential tolerization of the immune system to certain cancer-associated antigens. The reporter models are useful for unbiased identification of Breg subsets, as well as studies on timing, migration patterns, and localization of Bregs in cancer. Limitations include differential lifespan of reporter protein versus that of the cytokine. Signaling studies: Ex vivo analysis using co-culture of Breg cells, T cells, cancer cells, and/or other cellular components of the tumor microenvironment allows for the testing of crosstalk mechanisms between cell types. Prognostic studies: IL-35+ Breg frequency in peripheral blood of cancer patients can serve as a potential prognostic determinant of the immunosuppressive nature of the tumor microenvironment. More research needs to be done to determine how Breg phenotype and functional attributes may be adjusted based on their localization in blood, tumor, and/or lymphoid aggregates and secondary lymphoid tissues. Exposure to immuno-, chemo-, and radiation-therapy as well as the microbial contexture of the cancer patient may influence frequency and function of Bregs and should be further studied. Therapy: Strategies that specifically target Bregs and their functional outputs are more likely to boost the anti-tumor immune response. Neutralizing IL-35 antibody treatment could decrease Breg function and enhance the infiltration and cytotoxic activity of CD8+ T cells within tumors as well as synergize with checkpoint blockade. A better understanding of Breg-specific markers may lead to the development of antibodies to deplete these immunosuppressive subsets. Neutralizing α-CXCL13 can potentially block B cell infiltration into the tumor microenvironment, however, α-CXCL13 may also have prominent effect on conventional B cell function and formation of productive tertiary lymphoid structures, therefore such treatment may need to be tailored to tumors with prominent Breg activity.
Table 1.
Regulatory B cells identified in murine models of cancer
| Cancer Type | Surface Markers | Location | Effector Function | Reference |
|---|---|---|---|---|
| Pancreatic Ductal Adenocarcinoma | CD19+CD1dHiCD5+ | Tumor | IL-35 | 69 |
| Pancreatic Ductal Adenocarcinoma | CD19+CD1dHiCD5+CD21Hi | Tumor | IL-35 | 70, 71, 72 |
| Prostate Cancer | CD20+ | Tumor | Lymphotoxin | 77 |
| Prostate Cancer | CD19+CD20LoB220LoIgA+PD-L1+ | Tumor | IL-10 | 78 |
| Hepatocellular Carcinoma | CD19+B220LoCD138+IgA+ | Tumor | PD-L1 | 80 |
| Breast Cancer | CD19+B220+CD25+CD69Hi | Tumor | TGF-B | 82 |
| Breast Cancer | PD-L1Hi CD86Hi IAdHi CD62LHi LAP+CD44Lo | Tumor | TGF-B, PD-L1 | 83 |
| Squamous Carcinoma | CD19+CD21Hi | Tumor | IL-10 | 85 |
| Non-Hodgkin Lymphoma | CD19+CD1dHiCD5+ | Tumor | IL-10 | 86 |
Instances of regulatory B cells observed in murine models of cancer.
5.1. Suppressive effects of antibodies.
B cells have been found to play a prominent role in promoting tumor growth by multiple mechanisms including the B cell-specific function of antibody production. This mechanism was first outlined in a mouse model of HPV16-driven epithelial carcinogenesis (73). Interestingly, B cells are not recruited into the premalignant tissues in this model, but instead act from a distance via production of antibodies that circulate to the tumor. The mechanism surrounding the suppressive effect of antibodies in the HPV16 squamous carcinoma model was determined to be the engagement of Fc receptors (FcRy) on infiltrating macrophages and mast cells, overall activating a chronic pro-tumorigenic and pro-angiogenic program to help facilitate tumor growth (74). Depletion of B cells from this model using α-CD20 results in the infiltration of myeloid cells with an M1 anti-tumorigenic phenotype and importantly confers significant sensitivity to chemotherapy in a CD8-dependent fashion (75). A similar FcRy-dependent mechanism driving tumor growth has also been described in pancreatic ductal adenocarcinoma where B cell-dependent activation of macrophage FcRy signaling leads to a M2-type macrophage reprogramming (76). Both the B cell generation of antibodies in response to pancreatic inflammation and FcRy-mediated activation of macrophages were found to be dependent on PI3Ky activation of Bruton’s Tyrosine Kinase (BTK). Inhibition of BTK or PI3Ky led to a significant decrease in tumor burden in an orthotopic pancreatic cancer model while also causing an M1-type macrophage conversion and an increased CD8+ T cell response (76).
B cells have also been found to play a prominent role in the promotion of prostate cancer. In a TRAMP model of castration-resistant prostate cancer, CD20+ B cells were found to promote castration-resistant tumor growth in a direct manner through secretion of lymphotoxin which stimulated IKKα and STAT3 in tumor cells (77). B cells in prostate cancer have also been shown to inhibit chemotherapy-induced tumor regression (78). The source of resistance was identified to be infiltrating IgA+PD-L1+IL-10+ plasma cells, a separate population from the lymphotoxin-producing B cells previously described. The addition of oxaliplatin induced infiltration of the plasma cells into the tumor and their development was dependent on TGF-β receptor signaling and class switch recombination to IgA. Depletion of the plasma cells restored CD8+ T cell activation that is required for oxaliplatin-mediated tumor regression. It should be noted that expression of PD-L1 and IL-10 by B cells were also required to maintain tumor growth after oxaliplatin administration, concluding that IgA expression is not the sole immunosuppressive mechanism in this model. Collectively these and additional recent studies suggest that antibody-producing B cells play a crucial role in promoting tumor growth by suppression of cytotoxic T cell responses and identified B cells as mediators of tumor responsiveness to chemotherapy (79).
5.2. Immune checkpoints.
The PD-1/PD-L1 immune checkpoint is arguably the most currently studied mechanism in cancer-mediated immunosuppression and a role for this checkpoint has been recently extended to B cells. In a mouse model of hepatocellular carcinoma (HCC), Shalapour et al. identified an IgA+ population of plasma cells accumulating in the liver that suppressed CD8+ T cell responses through expression of PD-L1 (80). PD-L1 blockade significantly decreased HCC tumor size which correlated with increased T cell activation and decreased IgA+ plasma cell accumulation in the liver. The PD-L1+ B cells also expressed IL-10, but the role of IL-10 was not determined. Also, a population of pro-tumorigenic B cells was identified in hepatocellular carcinoma patients with high expression of PD-1 rather than the ligands PD-L1 or PD-L2 (81). These PD-1Hi B cells exhibit a CD5hiCD27hiCD38dimCD69+ surface marker phenotype and were incapable of producing IL-10 after BCR, CD40, and/or TLR9 stimulation. However, PD-1 activation induced IL-10 expression by HCC tumor B cells and PD-L1 blockade prevented IL-10 expression in vivo, decreased T cell suppression, and slowed disease progression. Additionally, PD-1Hi tumor B cells were incapable of differentiating into plasma cells, suggesting that this B cell subset is separate from previously identified murine regulatory plasma B cells (80). The PD-1/PD-L1 axis may be utilized by B cells in different ways within different tumor types. While PD-L1 mediated immunosuppression is mainly attributed to cancer cells and myeloid cells, these findings demonstrate the need to understand how the axis operates in all cell types within the microenvironment.
5.3. Anti-inflammatory cytokines.
B cell regulation of immune responses in cancer is not limited to the plasma cell compartment as B cell can use non-Ig-based mechanisms, predominantly cytokines. TGF-β is a cytokine that can suppress anti-tumorigenic immune responses. A population of B220+CD25+ B cells expressing high levels of TGF-β were found in a 4T1 mammary tumor model (82). Provision of TGF-β by B cells converted naïve CD4+ T cells into FoxP3+ Treg cells and suppressed CD8+ T cells (82). Similarly, EMT-6 mammary tumor-educated B cells were reported to suppress CD4+ and CD8+ T cell proliferation and IFNγ secretion both in vivo and in vitro in a TGF-β-dependent manner (83). The authors also found that TGF-β+ B cells were enriched for PD-L1. Combination blockade of the two inhibitory molecules led to a synergistic decrease in tumor growth (83).
The most cited mechanism for immunosuppression by B cells in cancer has been secretion of the anti-inflammatory cytokine IL-10. IL-10 expression by B cells was upregulated after co-culture of splenocytes with irradiated murine thymoma and melanoma cells (84). The upregulation of IL-10 coincided with a decrease in secreted IFNγ, TNFα, and MCP-1 and was reversed in splenocytes from B cell deficient mice, indicating a B cell-mediated suppression of pro-inflammatory responses. The increase in IL-10 expression was heavily influenced by CD40-CD40L interactions between B cells and cancer cells. This mechanism was not present when B cells were cultured with MCA304 sarcoma cells, suggesting that engagement of IL-10 mediated suppression by B cells in cancer may be tumor-type specific. In a murine tumor model of TPA-induced squamous carcinoma, CD19+CD21Hi B cells produced IL-10 in a TNFα-dependent manner, which correlated with a decrease in CD8+ T cell effector function (85). IL-10 producing B cells were also reported in a model of non-Hodgkin B cell lymphoma and displayed a CD1dHiCD5+ surface marker phenotype. In this case, IL-10 from CD1dHiCD5+ B cells increased tumor growth by suppressing the phagocytic/effector activity of macrophages (86). Considering this evidence, we aimed to understand how IL-10+ B cells play a role in the growth of pancreatic tumors. Adoptive transfer of Il10−/− B cells into B cell deficient mice bearing orthotopic pancreatic tumors did not affect tumor growth compared to tumor-bearing mice reconstituted with WT B cells (69). Furthermore, orthotopic pancreatic tumors implanted in Il10−/− mice did not demonstrate any change in tumor growth compared to WT mice and did not display increased CD8+ T cell infiltration or activation, suggesting that IL-10 from B cells does not play in a functional role in this model (72). To this end, the recent discovery of the cytokine IL-35 as a mediator of immunosuppression by B cells in autoimmunity and inflammation has translated into an emerging area of interest in solid cancers and will be discussed in detail below.
Section 6: Identification and generation of immunosuppressive IL-35+/IL-10+ Bregs
6.1. Immunosuppressive cytokine IL-35.
Interleukin-35 (IL-35) is a heterodimeric cytokine within the IL-12 family, which also includes IL-12, IL-23, IL-27, and IL-39 (87, 88). IL-35 is comprised of a p35 alpha subunit, shared with IL-12, and an EBi3 beta subunit, shared with IL-27 (69, 87). The overlap of subunits between these cytokines not only contributes to unique functional interactions, but also influences how experimental models for studying IL-12 family cytokines are designed and interpreted.
The suppressive function of IL-35 was first elucidated within the context of autoimmune and inflammatory conditions (89–91). Collison et al. determined that the EBi3-p35 heterodimer was constitutively secreted by resting wild type Tregs in vitro (89). Furthermore, Ebi3−/− and Il12a−/− Treg cells had reduced regulatory activity in vitro and in a mouse model of inflammatory bowel disease (89). Additionally, a protein construct with covalently linked EBi3 and p35 inhibited the proliferation of CD4+ CD25− T effector cells and differentiation of Th17 cells in vitro, yet expanded CD4+ CD25+ Tregs (90). Following these studies describing the suppressive role of IL-35 secreted by Tregs, IL-35 production was detected in other cell types, including dendritic cells, B cells, and cancer cells (69, 92–97). IL-35 expression has been found in several human cancers (92, 96, 98–102). Among these studies, IL-35 was detected in patient tumor tissue, plasma, peripheral blood, and in human cancer cell lines using techniques including RT-PCR, enzyme-linked immunoassays, IHC analysis, western blot analysis, and intracellular cytokine staining. The impact of cancer cell autonomous IL-35 on cancer progression, survival rates, and response to treatment is still under investigation: overexpression of IL-35 was implicated in promoting tumor growth by enhancing myeloid cell accumulation and angiogenesis, mediating cell extravasation and increased metastasis, inducing regulatory T cells, and inhibiting apoptosis of cancer cells, although it is still not clear what role cancer cell-production of IL-35 has on immune responses (92, 96, 98–102).
In addition to identifying the cell populations responsible for secreting IL-35, understanding the mechanisms governing IL-35 signaling has been a key area of interest. One question in the field was whether heterodimer formation was necessary for functional activity. While more work is needed, studies conducted by the Egwuagu lab using models of inflammatory disease, including uveitis and experimental autoimmune encephalitis, helped elucidate the intrinsic immune-suppressive function of each IL-35 subunit (103–105). Unlike other cytokines in the IL-12 family, the alpha and beta subunits of IL-35 and IL-27 are not thought to be linked by a disulfide bond. To combat the consequent challenge of isolating heterodimers of IL-35 in vivo, Egwuagu et al. created recombinant mouse IL-35 that enabled expression of both EBi3 and p35 (103). In vitro experiments demonstrated that the monomeric and homodimer form of p35 suppressed both T and B cell proliferation, potentially by inducing cell cycle arrest or inhibiting STAT3 and STAT4 activation in IL-6 and IL-12 pathways, respectively (104). In B cells it seems that STAT3 and STAT4 are engaged by IL-35, yet in CD4+ T cells, activation of STAT1 and STAT4 was identified (106). EBi3 also inhibited lymphocyte proliferation, yet the complete heterodimer of IL-35 demonstrated the highest level of suppression (104). Furthermore, although EBi3 had little effect on the expression of IL-10, p35, and Ebi3 mRNAs in B cells, p35 upregulated these genes and consequently, induced the expansion of IL-10 and IL-35 producing regulatory B cells (104). In vivo, recombinant p35 and p35-p35 reduced the severity of experimental autoimmune uveitis and experimental autoimmune encephalitis as well (104, 105). Overall, although the suppressive effects were not as strong as heterodimeric IL-35, p35 alone displayed immune-regulatory functions and may have potential as a therapeutic (104). However, the synergy between EBi3 and p35, physiological factors regulating the stability of the heterodimer, and potential activity of monomers or homodimers still remain to be determined, as it is challenging to eliminate more than one subunit at one time without perturbing expression of multiple IL-12 family members.
Although our knowledge and understanding of IL-35 has recently expanded, there is still much to learn about the mechanistic function, regulation, localization, downstream immunosuppressive effects, and therapeutic potential of IL-35. As researchers continue to unravel the unique features of IL-35, the ability to identify relevant cytokine producing populations will be paramount. Many initial studies have relied on PCR to identify IL-35 secreting cell populations. However, gene expression does not always correlate with protein level. It is also difficult to discern cells that express both the p35 and EBi3 subunits of IL-35 by PCR. Therefore, moving forward, more biologically relevant, and sensitive techniques should be incorporated. Specifically, the implementation of ELISA, intracellular flow cytometry, reporter models, and reagents optimized for use in both animal and human samples will enable researchers to obtain a more accurate and detailed representation of IL-35 and its effects.
6.2. Identification of IL-35+ regulatory B cells.
While the identification of IL-35+ B cells is a relatively recent discovery, a suppressive function of B cells has been proposed for decades. B cells have been implicated in suppressing delayed hypersensitivity reactions (107, 108) and recovery from experimental autoimmune encephalitis (EAE) (109). Using models of inflammation, including EAE, colitis, and arthritis, the suppressive capability of B cells was linked to the production of IL-10 (110–112). IL-10 then became the hallmark cytokine of a subpopulation of B cells known for their regulatory function, and thus named regulatory B cells (Bregs). However, it is now appreciated that Bregs are a heterogeneous population, and as such, multiple IL-10 and IL-35 producing subsets have been identified. Adoptive transfer of murine transitional 2 marginal-zone precursor cells (T2-MZP) (B220+CD21HiCD23+IgMHi) was shown to prevent collagen-induced arthritis and diminish established disease through the production of IL-10 (113). Other groups have identified the expansion of IL-10+ T2-MZPs, as defined by the markers CD19+CD21HiCD24HiCD23HiCD1dHi, under conditions of inflammation induced by gut flora and arthritis (114). Furthermore, a population of CD5+CD1dHi B cells characterized by their selective ability to produce IL-10 (B10 cells) (115) was found in murine models of inflammation, EAE, Listeria infection, and reported to inhibit effector CD4+ T cells, monocytes, and DCs (115–117). Plasma cells (CD138+MHC-IILoB220+), Tim-1+ B cells (Tim-1+CD19+), and plasmablasts (CD138+CD44Hi) were also observed to secrete IL-10 and mediate immune suppression in mice (93, 118–121).
Recently, the suppressive functions of IL-35+ B cells, have been identified. Shen et al. reported IL-35 producing B cells (89, 90, 93). While mice lacking IL-35 expression in B cells survived longer than control mice after both primary infection and secondary challenge with Salmonella typhimurium, they were unable to recover from experimental autoimmune encephalomyelitis (93). These results are consistent with the role of IL-35 facilitating an anti-inflammatory response, which would be beneficial in autoimmunity but not in response to infection (93). In these models, CD138+MHC-IILo B220+ plasma cells were described as the primary source of B-cell-derived IL-35 and IL-10 (93). In a separate study, recombinant IL-35 induced a subset of suppressive CD19+CD5+B220Lo Bregs capable of producing both IL-35 and IL-10, and mice treated with IL-35 were protected from experimental autoimmune uveitis (94). Collectively, these early studies exploring the function of IL-35 in models of autoimmune disease and inflammation detailed a unique immunosuppressive function and ability to induce Treg and Breg populations.
Expanding the role of cytokine-producing Bregs beyond the scope of autoimmune disease, we detected a subset of immunosuppressive IL-10 and IL-35-producing CD1dhiCD5+ B cells in murine pancreatic cancer (69). Specifically, pancreatic ductal adenocarcinoma (PDAC), a cancer type with a prominent B cell presence, was found to be heavily dependent on the expression of IL-35 but not IL-10 for proper tumorigenesis in both human PDAC lesions and in a well-characterized oncogenic Kras-driven mouse model (69). Orthotopic pancreatic tumor growth in B cell-deficient mice was significantly inhibited compared to WT controls and tumor growth was rescued upon adoptive transfer of wild-type or IL10-deficient but not p35 deficient CD1dHiCD5+ B cells (69). In our recent studies, we expanded the definition of B regulatory cells to CD19+CD1dHiCD21HiCD5+ cells (70–72). Bregs under this definition produced both IL-10 and IL-35 and accumulated in the spleen and pancreas of mice from both a spontaneous and orthotopic models of pancreatic tumorigenesis (72). Corroborating these findings, work by Takahashi et al. showed that CD19+CD1dHiCD5+ Bregs and PD-L1+ B cells were increased in the spontaneous model of pancreatic cancer with overexpression of IL-1β in the pancreas (122). Treatment with an IL-35 neutralizing antibody led to decreased PanIN formation and a reduced frequency of PD-L1+ B cells, providing further evidence that IL-35 promote pancreatic tumorigenesis (122).
Our lab also utilized Il12aGFP and Ebi3Tom reporter mice to detect the subunits of IL-35 in vivo (70, 123). Using these models, we demonstrated that expression of both reporters was found in CD19+CD5+CD1dhiCD21hi Bregs, but also, to some extent, in myeloid cells, T cells, and other B cell subsets (70). Additionally, Ebi3Tom expression was found to be more tightly regulated than p35 in vivo and in vitro (70). Our results support previous observations showing low constitutive expression of p35 as opposed to more regulated EBi3 expression (93). Ultimately, stimuli leading to the upregulation of EBi3 expression, such as those fostering coordinated activation of the BCR, CD40, and TLR pathway, as we discuss in detail below, likely confer IL-35 expression (70). Hypothetically, under circumstances of low EBi3 expression, available p35 could be more available to bind p40 subunits, generating IL-12 and facilitating an inflammatory response. In the future, the use of reporter mice that have both Il12aGFP and Ebi3Tom will enable the identification of cells that express both IL-35 subunits simultaneously and may help address open questions in the field about the regulation, and persistence of this cytokine.
Unlike Tregs, which express FoxP3, a single transcription factor facilitating the development of Bregs across the distinct spectrum of pathological conditions has been elusive. This, and the myriad of phenotypic markers of Breg subpopulations, support the idea that Bregs are not lineage specific, but are instead derived from B cells at multiple stages of development in response to stimuli (124). As such, different inflammatory environments may provide unique stimuli and foster the development of distinct Breg populations. For instance, upregulation of the transcription factor aryl hydrocarbon receptor was shown to promote the generation of IL10+CD19+CD21HiCD24Hi Bregs and suppress pro-inflammatory gene expression in a model of murine arthritis (125). Furthermore, the conditions surrounding immune activation, including those driven by microbial contexture, may lead to differentially up- or down-regulated surface markers, potentially giving rise to inconsistencies between experimental models (124). In addition to characterizing distinct surface markers for the wide range of Breg subsets, discerning the localization of these populations is also critical. An understanding of cellular localization, be it within the tumor, spleen, pancreas, lymph nodes, or peripheral blood, can not only help elucidate local or systemic effects, but also provides valuable insight about potential targets for the development of therapeutics.
In line with the hypothesis that any B cell can become a Breg, some groups have identified populations of suppressive plasmablasts (93, 121). As discussed above, Shen et al. reported IL-35 and IL-10 producing CD138+ B cells that facilitated immune suppression (93). Furthermore, Matsumoto et al. documented the presence of suppressive IL-10 secreting plasmablasts in the draining lymph nodes of mice during EAE (121). We assessed whether plasma cell formation and immunosuppressive cytokine function may exist in the same B cell subset in cancer. To do this, we profiled splenic B220loCD138+ plasma cells from healthy mice and mice with orthotopically derived pancreatic cancer for p35 and IL-10 using intracellular cytokine staining. Treatment with α-CD40 and LPS did not upregulate p35 or IL-10 in plasma cells, suggesting plasma cells do not account for a cytokine-producing B cell subset in murine PDA (71). Overall, in our mouse models and human samples of pancreatic adenocarcinoma, production of IL-35 and IL-10 by murine plasma cells or human plasmablasts was not observed (71). These differences not only highlight the diversity of Breg phenotypes but may also support the idea that specific stimuli within the inflammatory environment induce differentiation of distinct Breg subsets.
6.3. Function of IL-35+ regulatory B cells.
To understand the mechanisms of IL-35-mediated suppression in cancer, we analyzed established mouse models of PDAC with a deficiency for IL-35 (72). We determined that genetic ablation of IL-35 in mice results in reduced orthotopic pancreatic tumor growth by way of increased T cell activation (Figure 1) (72). Murine pancreatic tumors in II12a−/− and Ebi3−/− mice had a significant increase in intratumoral CD4+ and CD8+ T cells that express TNFα and/or IFNγ, and concurrently contained significantly fewer FoxP3+ CD4+ T cells, overall resulting in significantly smaller tumors. While these models provide insight into the B cell specific role of IL-35, it is important to consider the subunit overlap within the IL-12 family cytokines. We utilized p40−/− and IL27Rα−/− mice as controls to account for the overlapping use of p35 and Ebi3 in IL-12 and IL-27 heterodimers (72). Furthermore, administration of aPD-1 in Il12a−/− mice resulted in a synergistic reduction in pancreatic tumor growth and increased T cell activation compared to Il12a−/− controls. Treatment of WT mice with aPD-1 did not result in decreased pancreatic tumor growth, similar to clinical findings, suggesting IL-35 inhibition confers sensitivity to checkpoint blockade (72).
Next, to understand the role of B cell-specific IL-35 expression in T cell suppression, orthotopic pancreatic tumors were implanted in mice with a genetic ablation of either p35 (Bp35−/−) or Ebi3 (BEbi3−/−) specifically in B cells, generated by bone marrow chimerism and CD19-Cre recombination, respectively (71, 93, 123, 126). Using Bp35−/− and BEbi3−/− mice, we demonstrated that B cell ablation of IL-35 resulted in smaller tumors, whereas Treg specific ablation of IL-35 (TregEbi3−/−) did not affect tumor growth (71). Furthermore, the combination of α–PD-1 with B cell–specific loss of IL-35 significantly reduced tumor growth compared to BEbi3+/– mice treated with either isotype or α–PD-1 or BEbi3–/– mice treated with isotype control. Additionally, tumors in B cell IL-35 knockout mice contained a significant increase in IFNγ+ and TNFα+ CD4+ and CD8+ T cells, and had decreased frequencies CD4+FoxP3+ T cells (71). This data supports prior studies demonstrating the functional role of IL-35 to suppress the proliferation and activation of CD4+ T effector cells and induce the expansion of Tregs (89, 90, 126–130). Previous work also demonstrated that IL-35 signals through STAT1/STAT4 heterodimers in CD4+ T cells, yet interestingly, we observed phosphorylation of STAT1, STAT3, and STAT4 in CD8+ T cells in the presence of recombinant IL-35 (71, 106). To elucidate the impact of IL-35 on CD8+ T cell function, we analyzed the expression of IFNγ and two chemokine receptors expressed on effector T cells, CXCR3 and CCR5, that have been linked to intratumoral CD8+ T cell infiltration (Figure 3) (131, 132). Recombinant IL-35 suppressed expression of IFNγ, CXCR3, and CCR5, in OVA-specific CD8+ T cells, supporting a role for IL-35 in reducing CD8+ T cell activation and tumor infiltration (71). To clarify the signaling mechanism driving STAT3 activation in CD8+ T cells, T cells were sorted by singular expression of the IL-35 receptor chain subunits, IL12Rβ2 or gp130 (71). Treatment with rIL-35 revealed STAT1 and STAT3 activation in gp130+CD8+ T cells, but only STAT4 activation in the IL12Rβ2+CD8+ subset (71). Moreover, the gp130+ T cells downregulated of IFNγ, CXCR3, and CCR5, supporting the conclusion that the mechanism of IL-35 mediated inhibition of CD8+ T cells is driven by STAT3 (Figure 3) (71). Inhibition of STAT3 activity in CD8+ T cells resulted in decreased pancreatic tumor growth with a significant increase in the infiltration of CD8+ T cells (71). Treatment of tumor-bearing mice with a neutralizing IL-35 antibody resulted in a significant decrease in pSTAT3+ CD8+ T cells within the tumors and an increased frequency of CXCR3+ and CCR5+ CD8+ T cells (71). Collectively, this evidence connects B cell-specific expression of IL-35 to activation of STAT3 followed by decreased infiltration and activation of CD8+ T cells and decreased tumor growth suggests that this axis may be a promising therapeutic target to augment anti-tumor T cell responses in combination with checkpoint blockade (71).
Figure 3. IL-35+ Bregs control CD8+ T cell activity in pancreatic cancer.
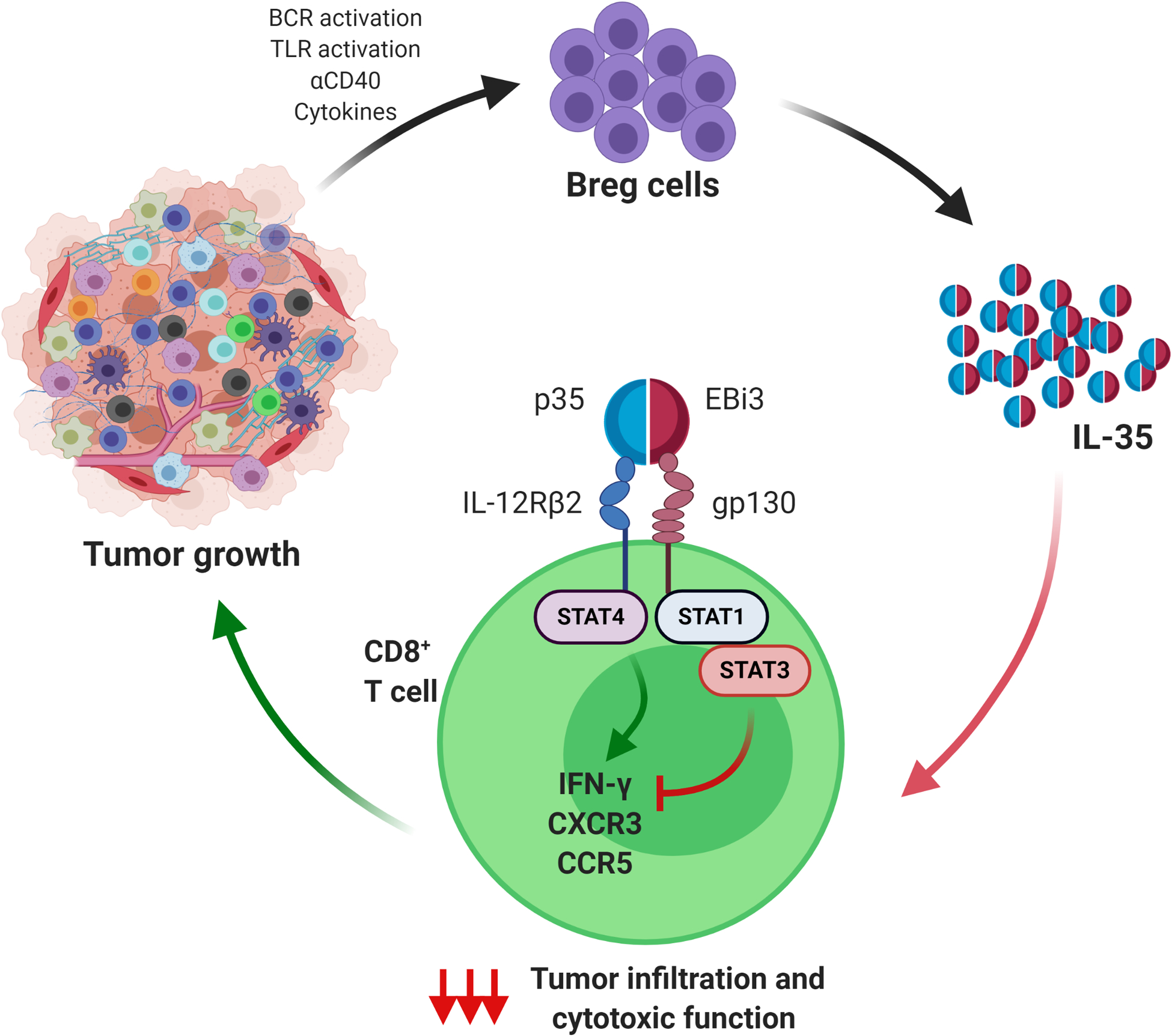
Breg-derived IL-35 (formed by the dimeric subunits p35 and EBi3) signals to CD8+ T cells via IL-12Rβ2 and gp130 heterodimeric receptors. IL-35-gp130-mediated signaling enhances STAT3 activity in CD8+ T cells. Activated STAT3 dampens expression of chemokine receptors CXCR3 and CCR5 and expression of the effector cytokine IFNγ in CD8+ T cells. This results in decreased ability of CD8+T cells to infiltrate tumors and produce functional responses. While stimulation of IL-12Rβ2 is known to activate STAT4 and potentiate cytotoxic function of CD8+ T cells, the presence of IL-35-activated STAT3 leaves the STAT4 signaling arm ineffective and the overall result is the reduction of cytotoxic capacity of CD8+ T cells.
6.4. IL-35 vs IL-10: functional redundancy and divergence.
IL-10 and IL-35 have each been shown to contribute to immune suppression, however, the functional interplay between these cytokines in Bregs remains unclear. Mounting evidence suggests subpopulations of cells secreting either IL-35 or IL-10 may utilize distinct mechanisms to facilitate immune suppression (133, 134). Tumor growth in B cell-deficient mice that received adoptively transferred Il10−/− B cells was similar to that of WT mice, yet we observed decreased tumor growth in B cell-deficient mice that received Il12a−/− B cells (69). Moreover, recent data demonstrated that IL-10 and IL-35 may be functionally divergent with only partially overlapping and non-redundant regulation (134). Separate populations of Treg cells expressing IL-10 or IL-35 were found using triple reporter mice (Il10GFP;Ebi3Tom;Foxp3Cre-YFP) (134). This supports previous data from IL-35 reporter mice (Ebi3-Dre-Thy1.1) that characterized distinct populations of IL-10 and IL-35 producing Treg subsets with different transcription factors, activation status, geographic location, and effector functions (133). Furthermore, human Treg cells from healthy donors and those with non-small cell lung cancer showed divergent expression of IL-10 and IL-35, as opposed to being double positive for each cytokine (134). However, TCR stimulation of purified Tregs in vitro was sufficient to induce both Il10 and Ebi3 expression, providing evidence for a model of developmental plasticity with transitory states instead of discrete subsets (134). Functionally, while IL-10 exhibited a more dominant role limiting effector function and IL-35 limited memory differentiation, both cytokines showed overlapping ability to induce inhibitory receptors such as PD-1, LAG3, TIM3, TIGIT, and 2B4 (134). Collectively, these results highlight an adaptive plasticity in which Tregs may be intrinsically capable of producing either IL-10 or IL-35 yet are influenced by environmental cues to favor the expression of one or the other (134). Similarly, Shen et al. found three distinct subpopulations of plasma cells differentiated by their level of CD138 and CD22 that expressed mRNA for either IL-10 or the two subunits of IL-35 during Salmonella infection, yet rarely expressed transcripts for both cytokines (93). Moving forward, the use of reporter mice to distinguish IL-10 and IL-35 expression in B cells will provide further insight into the coordinated mechanisms of IL-10 and IL-35 in the tumor microenvironment, and may help inform the development of combination therapies to enhance the efficacy of immunotherapy.
7. Cancer-associated inflammation as a prerequisite for Breg expansion and function
The release of cancer-associated inflammatory molecules within the tumor microenvironment are key initiators of the immune responses and recruitment of many immune cell types. We are beginning to understand how these microenvironmental cues may trigger differential responses from B cells within tumors and secondary lymphatic organs. Cancer-associated inflammation appears to increase the number of regulatory B cells from either the existing population or inducing differentiation of naïve B cells into a regulatory phenotype. Additionally, secretion of IL-10 and IL-35 is unlikely constitutive, and is instead induced by inflammation (135). It is still unclear what mechanisms cancer-associated inflammatory molecules cause these events to occur. B cells are equipped with multiple innate and adaptive antigen receptors as well as a plethora of inflammatory cytokine receptors which drive their differentiation and differentiation. In this section, we will discuss how these receptors dictate regulatory B cell function in cancer.
7.1. Toll-like receptors.
Toll-like receptors (TLRs) are a class of receptors that can recognize bacterial and viral pathogen-associated molecular patterns and direct expression of regulatory and/or inflammatory genes in response. In vitro stimulation of splenic B cells with TLR2, 4, or 9 agonists is sufficient to significantly upregulate MyD88-dependent IL-10 expression, suggesting that engagement of TLRs on B cells can drive their regulatory properties (93, 136–138). Prior research also implicates TLR stimulation in the expression of IL-35 as well. Il12a in naïve splenic B cells is constitutively high in vitro, but not Ebi3 (93). Using splenic Il12aGFP and Ebi3Tom B cells, we also reported a similar finding of constitutive Il12aGFP expression and absent Ebi3Tom expression in vitro (70). TLR stimulation of Il12aGFP B cells resulted in the upregulation of GFP, but only to a moderate amount due to the high basal expression level (70, 93). TLR stimulation of Ebi3Tom B cells, however, revealed a highly significant upregulation of Ebi3Tom expression indicating that the EBi3 subunit is more tightly regulated in B cells than p35 (70). The role of TLRs in IL-35 expression should also be considered in the context of simultaneous CD40 or BCR stimulation as both pathways synergistically increase Il12a and more significantly EBi3 beyond TLR stimulation alone (70, 93). (70). The requirement for this proposed synergy in disease has been demonstrated in an infection model, but the application to other diseases is currently not known (93). The role of TLRs in activation of Bregs in cancer has not been robustly addressed in vivo, but TLR activation by cancer-associated byproducts is entirely plausible. Endogenous TLR ligands are highly abundant within the tumor microenvironment in multiple forms. Traditionally, TLR ligands acting as pattern associated molecular patterns (PAMPs) are present on the cell surface of bacteria and fungi. Recent discoveries into the role the microenvironment plays in tumor growth and immune modulation implicate microbiota-driven stimulation of B cell TLRs within tumors or secondary lymphoid organs (139). Pancreatic cancer models have recently revealed that bacteria infiltrate the pancreas during tumorigenesis and accelerate disease progression (140). Cell-free gut extracts from mice bearing pancreatic tumors revealed a significant increase in the amount of TLR ligands present. The effect that such cancer microbiome-associated TLR ligands have specifically on B cells still needs to be explored but could serve as a source of TLR-dependent induction of regulatory B cell function in cancer. TLR ligands also take on the form of damage associated molecular patterns (DAMPs), which are molecules released by damaged or dying cells capable of binding various TLRs. Two prominent DAMPs, HMGB1 and S100A9, have been found in pancreatic ductal fluid from patients with pancreatic adenocarcinoma and could hypothetically serve as a B cell TLR agonist as well (141). It will be important to examine the extent of TLR function on B cell function in cancer going forward as many current therapies such as radiation and chemotherapy result in the generation of DAMPs within tumors. The use of preclinical mouse models with conditional B cell deletion of specific TLRs or key signaling proteins such as MyD88 or TRIF can provide insight into these functions in vivo.
7.2. B cell receptor.
The role of the BCR in the suppression of pro-inflammatory responses in cancer has yet to be clearly defined, but existing data from autoimmune disease models suggests that the BCR plays a critical role in this process. MD4 mice, which harbor a transgenic BCR for hen egg lysozyme, are unable to resolve MOG-induced EAE (110). The resolution of EAE was found to be dependent on the production of IL-10 by B cells. MD4 mice have significantly fewer splenic IL-10+ B cells in homeostatic conditions, suggesting that antigen recognition by the BCR is critical for the development of regulatory B cells as well (136). Stimulation of the BCR alone in vitro is not sufficient to induce IL-10 expression, however, combination of BCR , CD40 and TLR4 stimulation significantly increases both IL-10 and IL-35 expression in B cells, suggesting that BCR recognition of antigen alone is not sufficient to regulate an anti-inflammatory B cell response (70, 93, 142).
The role of the BCR in various cancers has been focused on the recognition of antigen and subsequent production of antibodies, however, it is still not well understood if the secretion of immunosuppressive cytokines by B cells in cancer is based on cognate interactions with the BCR. A B cell could release these cytokines after direct interaction with a tolerizing self-antigen released by a tumor and/or interaction with a T cell specific for the same self-antigen to quell inflammation. Better understanding of the antigenic strength, the nature of the antigen (mutated tumor antigen or tolerizing self-antigen), timing of antigenic exposure and local environment may provide insight into why some Breg cells present as plasma cells and some do not. Moving forward it will be important to examine the phenotype of B cells that produce immunosuppressive cytokines in response to cancer and what peptide sequences the BCR is able to recognize. This could be approached using syngeneic tumor implantation in cytokine reporter models for IL-10 (143, 144) and IL-35 (70, 123) to isolate activated regulatory B cells and decipher their characteristics via surface markers, transcriptomes, and BCR sequencing.
7.3. CD40.
The co-stimulatory molecule CD40 is essential for proper maturation of B cells to eliminate pathogens and accumulating evidence suggests a role for CD40 in Breg cell function as well. While CD40 is not essential for IL-10 production by splenic B cells, transgenic expression of CD40L on B cells can significantly increase the generation of IL-10+ B cells (136). Furthermore, splenic Transitional 2 (T2) B cells isolated from MRL/lpr lupus-prone mice significantly upregulate IL-10 production in response to CD40 stimulation ex vivo (145). Mounting evidence suggests that CD40 engagement is a key factor in whether a B cell expresses IL-10 or IL-35. Splenic B cells isolated from Il10-eGFP reporter mice and stimulated with LPS display a significantly larger frequency of GFP+ B cells when compared to LPS+α-CD40 stimulated B cells (93). Interestingly, Il10 mRNA expression in LPS+α-CD40 treated B cells was similar to that of naïve B cells, but Ebi3 expression was significantly upregulated (93). These findings collectively suggest that CD40 stimulation regulates IL-10 vs. IL-35 expression in B cells. CD40 agonism alone is sufficient to significantly induce EBi3 production in B cells, which agrees with prior data demonstrating that Ebi3 is a direct target gene of CD40 (70, 93, 146). Il12a expression, however, is not significantly upregulated after CD40 agonism (93). Collectively these data suggest that CD40 stimulation strongly promotes IL-35 expression by primarily upregulating Ebi3.
What remains uncertain is how CD40 stimulation affects different B cell subsets in the context of disease. LPS+α-CD40 stimulation in vitro consistently induces IL-35 expression specifically in CD1dHiCD5+ B cells, but not in CD1dLoCD5− B cells (71, 72, 93). Bone marrow chimeric mice containing Cd40−/− B cells display more severe EAE pathology than controls suggesting a loss of regulatory potential during inflammation (110). Furthermore, myelin oligodendrocyte glycoprotein (MOG) immunized mice supplemented with Cd40−/− CD1dHiCD5+ B cells experienced more severe EAE than mice supplemented with WT CD1dHi CD5+ B cells (147). These data suggest that CD40 signaling promotes regulatory function within CD1dHiCD5+ B cells and contributes to limiting disease severity, but the effect of B cell CD40 loss on regulatory function and cancer outcome are currently unknown. B cell deficient mice supplemented with Cd40−/− splenic B cells do not display overall altered subcutaneous MC38 tumor-growth compared to mice supplemented with WT splenic B cells suggesting that B cell-specific CD40 is not essential in tumor growth (148). However, this finding may be model-specific, as CD40L+ EL4 thymoma cells co-cultured with Cd40−/− B cells produce significantly less IL-10 than co-cultured WT B cells (84). Suppression of co-cultured CD8+ T cells and NK1.1+ T cells with B cells and EL4 cells was found to be IL-10 dependent, but the in vivo effect of CD40 deletion on tumor immunity was not examined. Use of agonistic CD40 to promote the anti-inflammatory properties of B cells has been successfully seen in a mouse model of Lupus. MRL/lpr lupus mice treated with α-CD40 experienced significantly increased survival attributed to increased IL-10 producing B cells, an increased frequency of T2 B cells, and a decrease in circulating α-dsDNA immunoglobulins (145). In preclinical models of pancreatic cancer, use of agonistic CD40 combined with immune checkpoint blockade resulted in the complete regression of tumors (149). The effect of CD40 agonism on B cells specifically was not determined, but the resolution of disease leads one to hypothesize that α-CD40 did not strongly activate the immunosuppressive functions of B cells. Activation of splenic B cells ex vivo with CD40L and subsequent transfer to mice bearing B16.F10 melanoma or E.G7 lymphoma tumors displayed slowed tumor growth compared to unstimulated controls further suggesting that B cell CD40 agonism is anti-tumorigenic (150). These studies suggest that engagement of CD40 on B cells is important for their regulatory capacity, but this is also disease specific. While CD40 is important in IL-10 and IL-35 function, B cell differentiation into antibody-secreting cells is also heavily dependent on CD40, so future investigation into what molecular mechanisms determine B cell fate in response to CD40 stimulation is incredibly important.
7.4. Cytokines.
Resolution of the immune response by anti-inflammatory immune cells limits the extent of the response and evidence suggests that pro-inflammatory cytokines can directly regulate regulatory B cell function. This was demonstrated in a murine model of arthritis, where IL-10 producing B cells capable of suppressing auto-reactive T cell function arise in response to increased environmental IL-6 and IL-1β (114). Many cancers are highly dependent on pro-inflammatory cytokines for the recruitment of pro-angiogenic and mesenchymal cell types necessary for proper tumor growth. These cytokines have both autocrine effects within the tumor microenvironment, but also paracrine effects as well. In orthotopic pancreatic cancer mouse models, knockdown of tumor cell-derived IL-1β results in smaller tumors containing fewer CD1dHi CD5+ regulatory B cells and concurrently more CD8+ T cells expressing IFNγ and Granzyme B (151). Additionally, pancreatic tumor-bearing mice administered with a neutralizing IL-1β antibody displayed similar results while also synergizing with α-PD-1 treatment to further reduce tumor burden (151). In vitro, IL-6 and IL-1β stimulation in combination with agonistic α-CD40 results in a significant increase in the expression of IL-35 subunits Il12a and Ebi3 in addition to IL-10 suggesting that cancer-associated inflammatory cytokines promote regulatory B cell function and expansion in pancreatic cancer (152).
IL-21 is another pro-inflammatory cytokine shown to play a role in the promotion of regulatory function in B cells. Recombinant IL-21 significantly upregulates the frequency of IL-10 producing B cells in vitro, specifically B cells with a CD1dHiCD5+ phenotype (147). Using a MOG-induced model of EAE, mice receiving adoptively transferred Il21r−/− B cells developed more severe EAE pathology and increased MOG-TCR CD4+ T cell proliferation suggesting that IL-21 drives autoimmune T cell suppression in vivo. IL-21, however, is an essential cytokine for the maturation of B cells from the germinal center to plasma cells. The differential effects of IL-21 on different B cell populations may be explained by the observation that CD1dLoCD5− B cells do not upregulate IL-10 in response to IL-21 (147) and could perhaps follow a conventional plasma cell developmental path. In a mouse model of triple negative breast cancer, IL-21 provided by follicular CD4+ T cells results in the development of tumor-specific plasma cells that in turn resulted in increased long-term survival (55). This, however, is in stark contrast to studies in pancreatic cancer, skin cancer, and prostate cancer where plasma cells play a pro-tumorigenic role and increase tumor burden. Although the role of IL-21 in these models was not examined, the potential of IL-21’s effect on B cell fate may not only be B cell population dependent, but also cancer type dependent as well. Additionally, the location of IL-21 stimulation may play an important role as Lindner et al. demonstrated that IL-21 activated B cells within tumors of various human carcinomas present a regulatory phenotype of IL-10+ CD25+ and IDO+ (153).
8. Mechanisms & markers of immune suppression by B cells in human cancer.
Corroborating data from murine models, studies in human cancer have described multiple regulatory B cell phenotypes. Without evidence for a distinct lineage marker, the origin of human Bregs remains unclear. It is not fully understood whether the suppressive function of Bregs is intrinsic to pre-defined subsets, or if instead, any B cell has the capacity to become regulatory in the presence of certain environmental stimuli. To date, human Breg subsets have been identified in a wide variety of B cell subsets (Table 2). Furthermore, production of immunosuppressive cytokines has been consistently observed in Bregs derived from immature B cells as opposed to mature cells like plasmablasts (71, 96). Differences in sampling location (peripheral blood versus intratumoral) may contribute to the observed variation in Breg phenotype, although more work is needed to clarify potential site-specific dependence. Additionally, the assortment of techniques used to identify and purify Breg subsets may contribute to the described phenotypic variation. Iwata et al. first identified a population of IL-10 secreting B cells in human blood samples from patients with various autoimmune diseases (154). This distinct subset of B cells was characterized by the ability to express IL-10 following ex vivo stimulation and by the presence of CD5+CD24hiCD27+ surface markers (154). IL-10 secreting B cells have since been described in numerous human cancers and are oftentimes linked to cancer progression. For instance, peripheral blood samples from patients with esophageal cancer contained elevated levels of IL-10 producing CD19+CD5+ B cells compared to healthy controls (155). Higher frequencies of IL-10 producing B cells were observed in late-stage disease samples than early-stage samples suggesting a role for regulatory B cells in the progression of esophageal cancer (155). Similarly, increased levels of CD19+CD24+CD38+ B cells were detected in the peripheral blood and bone marrow of patients with acute myeloid leukemia and linked to reduced overall survival (156). IL-10 producing B cells were also detected in the tumors of patients with gastric cancer, breast cancer, head and neck squamous carcinoma, and esophageal squamous carcinoma (157–160). However, functional analysis of this subpopulation still needs to be conducted. Enhanced cancer progression was also attributed to B regulatory cells in a cohort of chronic lymphocytic leukemia patients treated with rituximab (161). Since TGF-β producing tumor-evoked Bregs express were CD20Lo, Bodogai et al. noted that treatment with rituximab enriched for the immunosuppressive CD20Lo Bregs in leukemia patients and consequently led to enhanced cancer progression (161).
Table 2.
Regulatory B cells identified in human cancer
| Cancer Type | Surface Markers | Location | Effector Function | Reference |
|---|---|---|---|---|
| Pancreatic Ductal Adenocarcinoma | CD20+ | Tumor | IL-35 | 72 |
| Pancreatic Ductal Adenocarcinoma | CD19+CD24HiCD38Hi | Peripheral Blood | IL-35, IL-10 | 71 |
| Hepatocellular Carcinoma | CD19+CD5HiCD27HiCD38Dim | Tumor | PD-1, IL-10 | 81 |
| Oesophageal Carcinoma | CD19+CD5+ | Peripheral Blood | IL-10 | 157 |
| Ovarian Carcinoma | CD19+CD20+ | Ascites | IL-10 | 160 |
| Ovarian Carcinoma | CD19+ | Tumor | Granzyme B | 155 |
| Tongue Squamous Carcinoma | CD19+ | Tumor, Lymph Node | IL-10 | 20 |
| Gastric Cancer | CD19+CD24HiCD38Hi | Peripheral Blood, Tumor | IL-10, TGF-β1 | 161 |
| Gastric Cancer | CD19+CD24HiCD27+ | Peripheral Blood, Tumor | IL-10 | 162 |
| Gastric Cancer | CD19+ | Peripheral Blood | IL-35 | 101 |
| Breast Carcinoma | CD19+ | Tumor | Granzyme B | 155 |
| Breast Carcinoma | CD19+ | Tumor | IL-10, CD25 | 159 |
| Cervical Carcinoma | CD19+ | Tumor | Granzyme B | 155 |
| Head & Neck Squamous Carcinoma | CD19+CD24HiCD38Hi | Tumor | IL-10, CD25 | 160 |
| Esophageal Squamous Carcinoma | CD19+ | Tumor | IL-10, PD-1 | 161 |
Instances of regulatory B cells observed in human cancer.
The correlation between high frequencies of regulatory B cells and increased cancer progression, may also be due to IL-10’s role in facilitating regulatory T cell (Treg) development. In ascites from ovarian cancer patients, increased IL-10 producing B cells were not only associated with advanced disease, but were also positively correlated with the frequencies of FoxP3+CD4+ T cells and negatively correlated with the frequency of IFNy+CD8+ T cells (162). Zhou et al. detected increased infiltrating CD19+IL-10+ Bregs in tongue squamous cell carcinoma tumors compared to the surrounding tissue via IHC and the increased Breg frequency was associated with decreased patient survival (20). Additionally, Bregs induced by tongue squamous cell carcinoma cells in vitro were able to convert effector CD4+CD25− T cells into Tregs through an IL-10 driven mechanism (20). As seen with IL-10, TGF-β has been shown to facilitate the conversion of effector T cells into Tregs in the context of cancer (20, 162, 163). Wang et al. reported that although Bregs did not hinder the proliferation of T cells, Bregs were positively correlated with Treg frequency and suppressed CD4+ T cell IFNγ and TNFα secretion (163). Increased TGF-β mRNA expression and TGF-β1 was detected in CD19+CD24HiCD38Hi Breg cells and Treg conversion was inhibited by the addition of neutralizing α-TGF-β (163). Together, these studies highlight the importance of further elucidating the crosstalk between Breg and Treg cells, and their role in shaping the tumor microenvironment.
B cells in human tumors are heterogeneous. CD38+CD1d+IgM+CD147+ B cells uniquely characterized by granzyme B expression have been identified in breast, cervical, and ovarian tumors (153). Linder et al. demonstrated that these granzyme B+ cells were induced by IL-21, and functionally inhibited T cell proliferation by degrading the T cell receptor zeta chain in vitro and were found adjacent to IL-21+ T cells in tumors (153). Overall, these findings support a role for IL-21 in Breg induction and draw attention to granzyme B’s immunomodulatory capacity within the tumor microenvironment. Another distinct subset of PD-1Hi CD5HiCD24−/+CD27Hi/+CD38Dim Bregs was identified in human hepatocellular carcinoma (81). Like other conventional Breg subsets, increased infiltration of PD-1Hi Bregs was significantly correlated with disease progression in patients (81). Additionally, PD-1Hi Bregs were shown to secrete IL-10 and suppress tumor-specific immunity upon activation of the PD-1/PD-L1 axis (81). Understanding of the development, and functional significance of the myriad of Breg subpopulations is still an active area of research.
To understand which subsets of human B cells may contribute to the regulatory function in pancreatic cancer, we characterized B cells from the blood of from treatment-naïve patients with pancreatic cancer and healthy volunteers. (71). QPCR analysis of sorted B cells identified two distinct Breg subpopulations: an IL-10 producing CD19+CD24HiCD27+ B10 subset and an IL-10+IL-35+ producing CD19+CD24HiCD38Hi immature B cell subset akin to identified murine Bregs (71). The frequency of CD19+CD24HiCD38Hi cells was determined to not only be higher in patients with PDAC, but also to produce elevated levels of IL-10 and IL-35 compared to healthy controls (71). Moreover, immature B cells and CD4+ T cells isolated from PDAC patient blood were determined to be the main producers of IL-35 as determined by flow cytometry (71). To assess CD8+ T cell and IL-35+ immune cell infiltration, the expression of CD8+ T cell-specific pSTAT3 and CXCR3 was examined using multiplex immunofluorescence in EBi3 high and EBi3 low areas of 11 PDA patient samples (71). Most IL-35+ B cells were in tertiary lymphoid aggregates, implicating these regions as potential sites of active immune suppression (71). Corroborating the observed activation of pSTAT3 in CD8+ T cells, the percentage of pSTAT3+CD8+ T cells was higher in areas rich with EBi3+ immune cells (71). Inversely, lower percentages of CXCR3+CD8+ T cells were found in regions with increased IL-35 production, suggesting IL-35 may inhibit CD8+ T cell infiltration by downregulating CXCR3 through a STAT3-mediated pathway (71).
To elucidate the correlation between IL-35+ B cells and cytotoxicity in human pancreatic cancer, RNAseq was performed on regulatory and conventional B cells from both PDAC patient and healthy volunteer PBMCs. A tumor-associated Breg signature was calculated using expression values of genes differentially upregulated in Bregs compared to conventional B cells, and was found to inversely correlate with the cytotoxic CD8+ T cell index in The Cancer Genome Atlas pancreatic adenocarcinoma cohort (71). The Breg signature was also negatively correlated with anti-tumor T cell activity in many other cancer types (71). Taken together, this data highlights a subset of CD19+CD24HiCD38Hi IL-35 and IL-10 producing B cells in human pancreatic cancer and identifies a STAT3-mediated signaling pathway that may facilitate CD8+T cell exclusion from the tumor microenvironment (71).
9. Therapeutic potential.
Reflecting on the dual functionality of B cells, therapeutic strategies involving the activation and depletion of B cells have each been explored (Figure 2) (164). One branch of recent therapeutic strategies has centered around enhancing B cell stimulation of T cells. Vaccination with antigen-loaded CD40+ B cells resulted in the induction of specific T cell responses (150). Similarly, α-CD40 activated B cells from cervical cancer patients triggered anti-tumor T cell responses (66). Additionally, CpG-ODN (165), a fusion cytokine of GM-CSF and IL-4 (166), and tumor-derived autophagosomes known as “DRibbles” (167, 168), have all been implemented as therapies to activate B cells and foster the successful activation of T cells.
On the other hand, depletion of B cells as a means of mitigating their regulatory function in cancer has also been evaluated. While the broad depletion of CD20+ cells using α-CD20 was deemed advantageous in select studies on colon cancer, melanoma, and cutaneous T cell lymphoma, other studies identified small, or in some cases, detrimental effects (161, 169–173). This potentially reflects ability of B cells to both promote and/or inhibit tumor growth (174, 175). CD20 antibodies do not reliably discern between effector and regulatory B cells. Attempts at targeted reduction of Breg populations has been the recent focus of several therapies, but seem to target mechanisms common to all B cells (161, 176–181). With mounting evidence supporting an immunosuppressive role for B cells in cancer, tailored treatments targeting Breg function have promising clinical implications. Implementing an IL-35 blockade in combination with immunotherapy may be another therapeutic avenue to explore (71). Additionally, based on our findings supporting a role for STAT3 activation in facilitating reduced CD8+ T cell infiltration, incorporation of a STAT3 inhibitor may help enhance CD8+ T cell mediated responses (71). While more work is needed to develop these strategies, selectively targeting Bregs and their regulatory functions, has the potential to increase efficacy of cancer immunotherapies.
10. Conclusion and outstanding questions.
While recent findings suggest that rational targeting of B cell function may be beneficial for cancer immunotherapy, there is still a lot to be understood. There is a clear need to identify tumor-associated drivers that promote effector versus regulatory B cells. This could be enabled by engineering pre-clinical models that make use of well-defined antigens, allow enough time to generate both conventional and innate B cell responses, and test the functional relevance of specific B cell subsets in cancer. Use of regulatory cytokine reporter mice will help to discern the timing and localization of Bregs and could be further fleshed out in the patient population using multiplex staining on existing tumor samples. Studies should also be designed that address the role of the tumor microenvironment composition on the polarization of B cells.
Available patient data needs to be harnessed to understand how B cell function may be regulated in distinct cancer types by studying relevance of driver mutations, mutational load, and microenvironment contexture. Identification of tumor-associated antigens and an enhanced investigation into immunoglobulin repertoire data will enable better understanding of how tumors drive functional specification of B cell subsets. This could be achieved by single cell sequencing in combination with B cell receptor profiling to identify unique markers, transcription factors, and/or chemokine pathways that define Breg populations. Better modeling and mechanistic understanding of how tertiary lymphoid structures form in cancer will allow for vaccination strategies that favor formation or expansion of functional TLS without eliciting Breg responses. It would also be informative to understand to which extent activated Bregs cycle in and out of tumor bed to influence systemic anti-tumor immune responses. In addition, B cells express a multitude of receptors that respond to environmental cues, such as changes in microbial composition. Thus, the influence of bacterial and viral history with regards to shaping of B cell responses in cancer should be carefully considered. Commonly used cancer treatments, such as chemo and radiation therapy likely affect B cell numbers and functionality, and these effects are not well understood. Investigations into how current immunotherapeutic approaches affect the balance of pro- and anti-tumor B cell responses will also be of tremendous benefit. The field of regulatory B cell biology in cancer has a lot of work left to do, but the momentum is clearly here, and we should look forward to more mechanistic insights into contributions of B cells to cancer emergence and progression.
Acknowledgements:
This work was supported in part by R37 CA230786 (Y.P-G.), University Cancer Research Fund at the University of North Carolina at Chapel Hill, V Scholar Plus Award (Y.P-G.), Concern Foundation Conquer Cancer Now Award (Y.P-G), and 1F31CA239494-01A1 (D.M.). The authors have no personal or financial interests to disclose. Figures created using BioRender.com.
References
- 1.Zhang Q, Vignali DA. Co-stimulatory and Co-inhibitory Pathways in Autoimmunity. Immunity. 2016;44(5):1034–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sela U, Olds P, Park A, Schlesinger SJ, Steinman RM. Dendritic cells induce antigen-specific regulatory T cells that prevent graft versus host disease and persist in mice. J Exp Med. 2011;208(12):2489–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papp G, Boros P, Nakken B, Szodoray P, Zeher M. Regulatory immune cells and functions in autoimmunity and transplantation immunology. Autoimmun Rev. 2017;16(5):435–44. [DOI] [PubMed] [Google Scholar]
- 4.Cao T, Shao S, Fang H, Li B, Wang G. Role of Regulatory Immune Cells and Molecules in Autoimmune Bullous Dermatoses. Front Immunol. 2019;10:1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaehn PS, Zaenker KS, Schmitz J, Dzionek A. Functional dichotomy of plasmacytoid dendritic cells: antigen-specific activation of T cells versus production of type I interferon. Eur J Immunol. 2008;38(7):1822–32. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Gonzalez P, Ubilla-Olguin G, Catalan D, Schinnerling K, Aguillon JC. Tolerogenic dendritic cells for reprogramming of lymphocyte responses in autoimmune diseases. Autoimmun Rev. 2016;15(11):1071–80. [DOI] [PubMed] [Google Scholar]
- 7.Boros P, Ochando J, Zeher M. Myeloid derived suppressor cells and autoimmunity. Hum Immunol. 2016;77(8):631–6. [DOI] [PubMed] [Google Scholar]
- 8.Kang X, Zhang X, Liu Z, et al. Granulocytic myeloid-derived suppressor cells maintain feto-maternal tolerance by inducing Foxp3 expression in CD4+CD25-T cells by activation of the TGF-beta/beta-catenin pathway. Mol Hum Reprod. 2016;22(7):499–511. [DOI] [PubMed] [Google Scholar]
- 9.Graham DK, DeRyckere D, Davies KD, Earp HS. The TAM family: phosphatidylserine sensing receptor tyrosine kinases gone awry in cancer. Nature reviews Cancer. 2014;14(12):769–85. [DOI] [PubMed] [Google Scholar]
- 10.Toda A, Piccirillo CA. Development and function of naturally occurring CD4+CD25+ regulatory T cells. J Leukoc Biol. 2006;80(3):458–70. [DOI] [PubMed] [Google Scholar]
- 11.Dwivedi M, Kemp EH, Laddha NC, et al. Regulatory T cells in vitiligo: Implications for pathogenesis and therapeutics. Autoimmun Rev. 2015;14(1):49–56. [DOI] [PubMed] [Google Scholar]
- 12.Brunkow ME, Jeffery EW, Hjerrild KA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nature genetics. 2001;27(1):68–73. [DOI] [PubMed] [Google Scholar]
- 13.Dhaeze T, Peelen E, Hombrouck A, et al. Circulating Follicular Regulatory T Cells Are Defective in Multiple Sclerosis. J Immunol. 2015;195(3):832–40. [DOI] [PubMed] [Google Scholar]
- 14.Christoffersson G, von Herrath M. Regulatory Immune Mechanisms beyond Regulatory T Cells. Trends Immunol. 2019;40(6):482–91. [DOI] [PubMed] [Google Scholar]
- 15.Yang M, Rui K, Wang S, Lu L. Regulatory B cells in autoimmune diseases. Cell Mol Immunol. 2013;10(2):122–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kleinewietfeld M, Hafler DA. Regulatory T cells in autoimmune neuroinflammation. Immunol Rev. 2014;259(1):231–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Komatsu N, Takayanagi H. Regulatory T cells in Arthritis. Prog Mol Biol Transl Sci. 2015;136:207–15. [DOI] [PubMed] [Google Scholar]
- 18.Tao JH, Cheng M, Tang JP, et al. Foxp3, Regulatory T Cell, and Autoimmune Diseases. Inflammation. 2017;40(1):328–39. [DOI] [PubMed] [Google Scholar]
- 19.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nature reviews Cancer. 2016;16(5):275–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou X, Su YX, Lao XM, Liang YJ, Liao GQ. CD19(+)IL-10(+) regulatory B cells affect survival of tongue squamous cell carcinoma patients and induce resting CD4(+) T cells to CD4(+)Foxp3(+) regulatory T cells. Oral Oncol. 2016;53:27–35. [DOI] [PubMed] [Google Scholar]
- 21.Lorenzo-Sanz L, Munoz P. Tumor-Infiltrating Immunosuppressive Cells in Cancer-Cell Plasticity, Tumor Progression and Therapy Response. Cancer Microenviron. 2019;12(2–3):119–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeNardo DG, Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol. 2019;19(6):369–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Binnewies M, Roberts EW, Kersten K, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24(5):541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morrison AH, Byrne KT, Vonderheide RH. Immunotherapy and Prevention of Pancreatic Cancer. Trends Cancer. 2018;4(6):418–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G, Bar-Sagi D. Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell. 2012;21(6):836–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tcyganov E, Mastio J, Chen E, Gabrilovich DI. Plasticity of myeloid-derived suppressor cells in cancer. Current opinion in immunology. 2018;51:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakaguchi S, Mikami N, Wing JB, et al. Regulatory T Cells and Human Disease. Annu Rev Immunol. 2020;38:541–66. [DOI] [PubMed] [Google Scholar]
- 28.Sica A, Massarotti M. Myeloid suppressor cells in cancer and autoimmunity. J Autoimmun. 2017;85:117–25. [DOI] [PubMed] [Google Scholar]
- 29.Lindau D, Gielen P, Kroesen M, Wesseling P, Adema GJ. The immunosuppressive tumour network: myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology. 2013;138(2):105–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gunderson AJ, Coussens LM. B cells and their mediators as targets for therapy in solid tumors. Exp Cell Res. 2013;319(11):1644–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balkwill F, Montfort A, Capasso M. B regulatory cells in cancer. Trends Immunol. 2013;34(4):169–73. [DOI] [PubMed] [Google Scholar]
- 32.Sarvaria A, Basar R, Mehta RS, et al. IL-10+ regulatory B cells are enriched in cord blood and may protect against cGVHD after cord blood transplantation. Blood. 2016;128(10):1346–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuen GJ, Demissie E, Pillai S. B lymphocytes and cancer: a love-hate relationship. Trends Cancer. 2016;2(12):747–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee KE, Spata M, Bayne LJ, et al. Hif1a Deletion Reveals Pro-Neoplastic Function of B Cells in Pancreatic Neoplasia. Cancer Discov. 2016;6(3):256–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wouters MCA, Nelson BH. Prognostic Significance of Tumor-Infiltrating B Cells and Plasma Cells in Human Cancer. Clin Cancer Res. 2018;24(24):6125–35. [DOI] [PubMed] [Google Scholar]
- 36.Sharonov GV, Serebrovskaya EO, Yuzhakova DV, Britanova OV, Chudakov DM. B cells, plasma cells and antibody repertoires in the tumour microenvironment. Nat Rev Immunol. 2020;20(5):294–307. [DOI] [PubMed] [Google Scholar]
- 37.Riemann D, Wenzel K, Schulz T, et al. Phenotypic analysis of T lymphocytes isolated from non-small-cell lung cancer. Int Arch Allergy Immunol. 1997;114(1):38–45. [DOI] [PubMed] [Google Scholar]
- 38.Al-Shibli KI, Donnem T, Al-Saad S, et al. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res. 2008;14(16):5220–7. [DOI] [PubMed] [Google Scholar]
- 39.Lohr M, Edlund K, Botling J, et al. The prognostic relevance of tumour-infiltrating plasma cells and immunoglobulin kappa C indicates an important role of the humoral immune response in non-small cell lung cancer. Cancer Lett. 2013;333(2):222–8. [DOI] [PubMed] [Google Scholar]
- 40.Gentles AJ, Newman AM, Liu CL, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015;21(8):938–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iglesia MD, Vincent BG, Parker JS, et al. Prognostic B-cell signatures using mRNA-seq in patients with subtype-specific breast and ovarian cancer. Clin Cancer Res. 2014;20(14):3818–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iglesia MD, Parker JS, Hoadley KA, et al. Genomic Analysis of Immune Cell Infiltrates Across 11 Tumor Types. J Natl Cancer Inst. 2016;108(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mose LE, Selitsky SR, Bixby LM, et al. Assembly-based inference of B-cell receptor repertoires from short read RNA sequencing data with V’DJer. Bioinformatics. 2016;32(24):3729–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kroeger DR, Milne K, Nelson BH. Tumor-Infiltrating Plasma Cells Are Associated with Tertiary Lymphoid Structures, Cytolytic T-Cell Responses, and Superior Prognosis in Ovarian Cancer. Clin Cancer Res. 2016;22(12):3005–15. [DOI] [PubMed] [Google Scholar]
- 45.Charoentong P, Finotello F, Angelova M, et al. Pan-cancer Immunogenomic Analyses Reveal Genotype-Immunophenotype Relationships and Predictors of Response to Checkpoint Blockade. Cell Rep. 2017;18(1):248–62. [DOI] [PubMed] [Google Scholar]
- 46.Bolotin DA, Poslavsky S, Davydov AN, et al. Antigen receptor repertoire profiling from RNA-seq data. Nature biotechnology. 2017;35(10):908–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Isaeva OI, Sharonov GV, Serebrovskaya EO, et al. Intratumoral immunoglobulin isotypes predict survival in lung adenocarcinoma subtypes. J Immunother Cancer. 2019;7(1):279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Griss J, Bauer W, Wagner C, et al. B cells sustain inflammation and predict response to immune checkpoint blockade in human melanoma. Nat Commun. 2019;10(1):4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spear S, Candido JB, McDermott JR, et al. Discrepancies in the Tumor Microenvironment of Spontaneous and Orthotopic Murine Models of Pancreatic Cancer Uncover a New Immunostimulatory Phenotype for B Cells. Front Immunol. 2019;10:542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Q, Lao X, Pan Q, et al. Adoptive transfer of tumor reactive B cells confers host T-cell immunity and tumor regression. Clin Cancer Res. 2011;17(15):4987–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sautes-Fridman C, Petitprez F, Calderaro J, Fridman WH. Tertiary lymphoid structures in the era of cancer immunotherapy. Nature reviews Cancer. 2019;19(6):307–25. [DOI] [PubMed] [Google Scholar]
- 52.Di Caro G, Bergomas F, Grizzi F, et al. Occurrence of tertiary lymphoid tissue is associated with T-cell infiltration and predicts better prognosis in early-stage colorectal cancers. Clin Cancer Res. 2014;20(8):2147–58. [DOI] [PubMed] [Google Scholar]
- 53.Hiraoka N, Ino Y, Yamazaki-Itoh R, et al. Intratumoral tertiary lymphoid organ is a favourable prognosticator in patients with pancreatic cancer. Br J Cancer. 2015;112(11):1782–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Silina K, Soltermann A, Attar FM, et al. Germinal Centers Determine the Prognostic Relevance of Tertiary Lymphoid Structures and Are Impaired by Corticosteroids in Lung Squamous Cell Carcinoma. Cancer Res. 2018;78(5):1308–20. [DOI] [PubMed] [Google Scholar]
- 55.Hollern DP, Xu N, Thennavan A, et al. B Cells and T Follicular Helper Cells Mediate Response to Checkpoint Inhibitors in High Mutation Burden Mouse Models of Breast Cancer. Cell. 2019;179(5):1191–206 e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cabrita R, Lauss M, Sanna A, et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. 2020;577(7791):561–5. [DOI] [PubMed] [Google Scholar]
- 57.Helmink BA, Reddy SM, Gao J, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020;577(7791):549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Petitprez F, de Reynies A, Keung EZ, et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature. 2020;577(7791):556–60. [DOI] [PubMed] [Google Scholar]
- 59.Zhang C, Li J, Wang H, Song SW. Identification of a five B cell-associated gene prognostic and predictive signature for advanced glioma patients harboring immunosuppressive subtype preference. Oncotarget. 2016;7(45):73971–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Klopfenstein Q, Truntzer C, Vincent J, Ghiringhelli F. Cell lines and immune classification of glioblastoma define patient’s prognosis. Br J Cancer. 2019;120(8):806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mohammed ZM, Going JJ, Edwards J,. The relationship between lymphocyte subsets and clinico-pathological determinants of survival in patients with primary operable invasive ductal breast cancer. Br J Cancer. 2013;109(6):1676–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Garaud S, Buisseret L, Solinas C, et al. Tumor infiltrating B-cells signal functional humoral immune responses in breast cancer. JCI Insight. 2019;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carmi Y, Spitzer MH, Linde IL, et al. Allogeneic IgG combined with dendritic cell stimuli induce antitumour T-cell immunity. Nature. 2015;521(7550):99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gilbert AE, Karagiannis P, Dodev T, et al. Monitoring the systemic human memory B cell compartment of melanoma patients for anti-tumor IgG antibodies. PloS one. 2011;6(4):e19330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dang VD, Hilgenberg E, Ries S, Shen P, Fillatreau S. From the regulatory functions of B cells to the identification of cytokine-producing plasma cell subsets. Current opinion in immunology. 2014;28:77–83. [DOI] [PubMed] [Google Scholar]
- 66.Rossetti RAM, Lorenzi NPC, Yokochi K, et al. B lymphocytes can be activated to act as antigen presenting cells to promote anti-tumor responses. PloS one. 2018;13(7):e0199034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bruno TC, Ebner PJ, Moore BL, et al. Antigen-Presenting Intratumoral B Cells Affect CD4(+) TIL Phenotypes in Non-Small Cell Lung Cancer Patients. Cancer Immunol Res. 2017;5(10):898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Olson B, Li Y, Lin Y, Liu ET, Patnaik A. Mouse Models for Cancer Immunotherapy Research. Cancer Discov. 2018;8(11):1358–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pylayeva-Gupta Y, Das S, Handler JS, et al. IL35-Producing B Cells Promote the Development of Pancreatic Neoplasia. Cancer Discov. 2016;6(3):247–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Michaud D, Mirlekar B, Bischoff S, et al. Pancreatic cancer-associated inflammation drives dynamic regulation of p35 and Ebi3. Cytokine. 2020;125:154817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mirlekar B, Michaud D, Lee SJ, et al. B cell-Derived IL35 Drives STAT3-Dependent CD8(+) T-cell Exclusion in Pancreatic Cancer. Cancer Immunol Res. 2020;8(3):292–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mirlekar B, Michaud D, Searcy R, Greene K, Pylayeva-Gupta Y. IL-35 hinders endogenous anti-tumor T cell immunity and responsiveness to immunotherapy in pancreatic cancer. Cancer Immunol Res. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7(5):411–23. [DOI] [PubMed] [Google Scholar]
- 74.Andreu P, Johansson M, Affara NI, et al. FcRgamma activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell. 2010;17(2):121–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Affara NI, Ruffell B, Medler TR, et al. B cells regulate macrophage phenotype and response to chemotherapy in squamous carcinomas. Cancer Cell. 2014;25(6):809–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gunderson AJ, Kaneda MM, Tsujikawa T, et al. Bruton Tyrosine Kinase-Dependent Immune Cell Cross-talk Drives Pancreas Cancer. Cancer Discov. 2016;6(3):270–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ammirante M, Luo JL, Grivennikov S, Nedospasov S, Karin M. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature. 2010;464(7286):302–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shalapour S, Font-Burgada J, Di Caro G, et al. Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy. Nature. 2015;521(7550):94–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lu Y, Zhao Q, Liao JY, et al. Complement Signals Determine Opposite Effects of B Cells in Chemotherapy-Induced Immunity. Cell. 2020;180(6):1081–97 e24. [DOI] [PubMed] [Google Scholar]
- 80.Shalapour S, Lin XJ, Bastian IN, et al. Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity. Nature. 2017;551(7680):340–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xiao X, Lao XM, Chen MM, et al. PD-1hi Identifies a Novel Regulatory B-cell Population in Human Hepatoma That Promotes Disease Progression. Cancer Discov. 2016;6(5):546–59. [DOI] [PubMed] [Google Scholar]
- 82.Olkhanud PB, Damdinsuren B, Bodogai M, et al. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4(+) T cells to T-regulatory cells. Cancer Res. 2011;71(10):3505–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang Y, Morgan R, Chen C, et al. Mammary-tumor-educated B cells acquire LAP/TGF-beta and PD-L1 expression and suppress anti-tumor immune responses. Int Immunol. 2016;28(9):423–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Inoue S, Leitner WW, Golding B, Scott D. Inhibitory effects of B cells on antitumor immunity. Cancer Res. 2006;66(15):7741–7. [DOI] [PubMed] [Google Scholar]
- 85.Schioppa T, Moore R, Thompson RG, et al. B regulatory cells and the tumor-promoting actions of TNF-alpha during squamous carcinogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(26):10662–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Horikawa M, Minard-Colin V, Matsushita T, Tedder TF. Regulatory B cell production of IL-10 inhibits lymphoma depletion during CD20 immunotherapy in mice. The Journal of clinical investigation. 2011;121(11):4268–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vignali DA, Kuchroo VK. IL-12 family cytokines: immunological playmakers. Nat Immunol. 2012;13(8):722–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Manning AA, Zhao L, Zhu Z, et al. IL-39 acts as a friend to pancreatic cancer. Med Oncol. 2018;36(1):12. [DOI] [PubMed] [Google Scholar]
- 89.Collison LW, Workman CJ, Kuo TT, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450(7169):566–9. [DOI] [PubMed] [Google Scholar]
- 90.Niedbala W, Wei XQ, Cai B, et al. IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. Eur J Immunol. 2007;37(11):3021–9. [DOI] [PubMed] [Google Scholar]
- 91.Devergne O, Birkenbach M, Kieff E. Epstein-Barr virus-induced gene 3 and the p35 subunit of interleukin 12 form a novel heterodimeric hematopoietin. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(22):12041–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nicholl MB, Ledgewood CL, Chen X, et al. IL-35 promotes pancreas cancer growth through enhancement of proliferation and inhibition of apoptosis: evidence for a role as an autocrine growth factor. Cytokine. 2014;70(2):126–33. [DOI] [PubMed] [Google Scholar]
- 93.Shen P, Roch T, Lampropoulou V, et al. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature. 2014;507(7492):366–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang RX, Yu CR, Dambuza IM, et al. Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nat Med. 2014;20(6):633–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dixon KO, van der Kooij SW, Vignali DA, van Kooten C. Human tolerogenic dendritic cells produce IL-35 in the absence of other IL-12 family members. Eur J Immunol. 2015;45(6):1736–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huang C, Li N, Li Z, et al. Tumour-derived Interleukin 35 promotes pancreatic ductal adenocarcinoma cell extravasation and metastasis by inducing ICAM1 expression. Nat Commun. 2017;8:14035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Koda Y, Nakamoto N, Chu P-S, et al. Plasmacytoid dendritic cells protect against immune-mediated acute liver injury via IL-35. Journal of Clinical Investigation. 2019;129(8):3201–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang Z, Liu J-Q, Liu Z, et al. Tumor-Derived IL-35 Promotes Tumor Growth by Enhancing Myeloid Cell Accumulation and Angiogenesis. The Journal of Immunology. 2013;190(5):2415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Long J, Zhang X, Wen M, et al. IL-35 over-expression increases apoptosis sensitivity and suppresses cell growth in human cancer cells. Biochem Biophys Res Commun. 2013;430(1):364–9. [DOI] [PubMed] [Google Scholar]
- 100.Wang K, Liu J, Li J. IL-35-producing B cells in gastric cancer patients. Medicine (Baltimore). 2018;97(19):e0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hao S, Chen X, Wang F, et al. Breast cancer cell-derived IL-35 promotes tumor progression via induction of IL-35-producing induced regulatory T cells. Carcinogenesis. 2018;39(12):1488–96. [DOI] [PubMed] [Google Scholar]
- 102.Zhu J, Yang X, Wang Y, Zhang H, Guo Z. Interleukin-35 is associated with the tumorigenesis and progression of prostate cancer. Oncol Lett. 2019;17(6):5094–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Egwuagu CE, Yu CR, Sun L, Wang R. Interleukin 35: Critical regulator of immunity and lymphocyte-mediated diseases. Cytokine Growth Factor Rev. 2015;26(5):587–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dambuza IM, He C, Choi JK, et al. IL-12p35 induces expansion of IL-10 and IL-35-expressing regulatory B cells and ameliorates autoimmune disease. Nat Commun. 2017;8(1):719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Choi JK, Dambuza IM, He C, et al. IL-12p35 Inhibits Neuroinflammation and Ameliorates Autoimmune Encephalomyelitis. Front Immunol. 2017;8:1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Collison LW, Delgoffe GM, Guy CS, et al. The composition and signaling of the IL-35 receptor are unconventional. Nat Immunol. 2012;13(3):290–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Katz SI, Parker D, Turk JL. B-cell suppression of delayed hypersensitivity reactions. Nature. 1974;251(5475):550–1. [DOI] [PubMed] [Google Scholar]
- 108.Neta R, Salvin SB. Specific suppression of delayed hypersensitivity: the possible presence of a suppressor B cell in the regulation of delayed hypersensitivity. J Immunol. 1974;113(6):1716–25. [PubMed] [Google Scholar]
- 109.Wolf SD, Dittel BN, Hardardottir F, Janeway CA Jr. Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. J Exp Med. 1996;184(6):2271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3(10):944–50. [DOI] [PubMed] [Google Scholar]
- 111.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic Intestinal Inflammatory Condition Generates IL-10-Producing Regulatory B Cell Subset Characterized by CD1d Upregulation. Immunity. 2002;16(2):219–30. [DOI] [PubMed] [Google Scholar]
- 112.Mauri C, Gray D, Mushtaq N, Londei M. Prevention of arthritis by interleukin 10-producing B cells. J Exp Med. 2003;197(4):489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Evans JG, Chavez-Rueda KA, Eddaoudi A, Meyer-Bahlburg A, Rawlings DJ, Ehrenstein MR, et al. Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol. 2007;178(12):7868–78. [DOI] [PubMed] [Google Scholar]
- 114.Rosser EC, Oleinika K, Tonon S, et al. Regulatory B cells are induced by gut microbiota-driven interleukin-1beta and interleukin-6 production. Nat Med. 2014;20(11):1334–9. [DOI] [PubMed] [Google Scholar]
- 115.Yanaba K, Bouaziz J-D, Haas KM, et al. A Regulatory B Cell Subset with a Unique CD1dhiCD5+ Phenotype Controls T Cell-Dependent Inflammatory Responses. Immunity. 2008;28(5):639–50. [DOI] [PubMed] [Google Scholar]
- 116.Matsushita T, Horikawa M, Iwata Y, Tedder TF. Regulatory B cells (B10 cells) and regulatory T cells have independent roles in controlling experimental autoimmune encephalomyelitis initiation and late-phase immunopathogenesis. J Immunol. 2010;185(4):2240–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Horikawa M, Weimer ET, DiLillo DJ, et al. Regulatory B cell (B10 Cell) expansion during Listeria infection governs innate and cellular immune responses in mice. J Immunol. 2013;190(3):1158–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Neves P, Lampropoulou V, Calderon-Gomez E, et al. Signaling via the MyD88 adaptor protein in B cells suppresses protective immunity during Salmonella typhimurium infection. Immunity. 2010;33(5):777–90. [DOI] [PubMed] [Google Scholar]
- 119.Ding Q, Yeung M, Camirand G, et al. Regulatory B cells are identified by expression of TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice. Journal of Clinical Investigation. 2011;121(9):3645–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xiao S, Brooks CR, Zhu C, et al. Defect in regulatory B-cell function and development of systemic autoimmunity in T-cell Ig mucin 1 (Tim-1) mucin domain-mutant mice. Proceedings of the National Academy of Sciences. 2012;109(30):12105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Matsumoto M, Baba A, Yokota T, et al. Interleukin-10-producing plasmablasts exert regulatory function in autoimmune inflammation. Immunity. 2014;41(6):1040–51. [DOI] [PubMed] [Google Scholar]
- 122.Takahashi R, Macchini M, Sunagawa M, et al. Interleukin-1beta-induced pancreatitis promotes pancreatic ductal adenocarcinoma via B lymphocyte-mediated immune suppression. Gut. 2020. [DOI] [PubMed] [Google Scholar]
- 123.Turnis ME, Sawant DV, Szymczak-Workman AL, et al. Interleukin-35 Limits Anti-Tumor Immunity. Immunity. 2016;44(2):316–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity. 2015;42(4):607–12. [DOI] [PubMed] [Google Scholar]
- 125.Piper CJM, Rosser EC, Oleinika K, et al. Aryl Hydrocarbon Receptor Contributes to the Transcriptional Program of IL-10-Producing Regulatory B Cells. Cell Rep. 2019;29(7):1878–92 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rickert RC, Roes J, Rajewsky K. B lymphocyte-specific, Cre-mediated mutagenesis in mice. Nucleic Acids Res. 1997;25(6):1317–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Collison LW, Pillai MR, Chaturvedi V, Vignali DA. Regulatory T cell suppression is potentiated by target T cells in a cell contact, IL-35- and IL-10-dependent manner. J Immunol. 2009;182(10):6121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pillai S, Mattoo H, Cariappa A. B cells and autoimmunity. Current opinion in immunology. 2011;23(6):721–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wirtz S, Billmeier U, McHedlidze T, Blumberg RS, Neurath MF. Interleukin-35 mediates mucosal immune responses that protect against T-cell-dependent colitis. Gastroenterology. 2011;141(5):1875–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kochetkova I, Golden S, Holderness K, Callis G, Pascual DW. IL-35 stimulation of CD39+ regulatory T cells confers protection against collagen II-induced arthritis via the production of IL-10. J Immunol. 2010;184(12):7144–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li J, Ybarra R, Mak J, et al. IFNgamma-induced Chemokines Are Required for CXCR3-mediated T-Cell Recruitment and Antitumor Efficacy of Anti-HER2/CD3 Bispecific Antibody. Clin Cancer Res. 2018;24(24):6447–58. [DOI] [PubMed] [Google Scholar]
- 132.Mikucki ME, Fisher DT, Matsuzaki J, et al. Non-redundant requirement for CXCR3 signalling during tumoricidal T-cell trafficking across tumour vascular checkpoints. Nat Commun. 2015;6:7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wei X, Zhang J, Gu Q, et al. Reciprocal Expression of IL-35 and IL-10 Defines Two Distinct Effector Treg Subsets that Are Required for Maintenance of Immune Tolerance. Cell Reports. 2017;21(7):1853–69. [DOI] [PubMed] [Google Scholar]
- 134.Sawant DV, Yano H, Chikina M, et al. Adaptive plasticity of IL-10(+) and IL-35(+) Treg cells cooperatively promotes tumor T cell exhaustion. Nat Immunol. 2019;20(6):724–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li X, Mai J, Virtue A, et al. IL-35 is a novel responsive anti-inflammatory cytokine--a new system of categorizing anti-inflammatory cytokines. PloS one. 2012;7(3):e33628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yanaba K, Bouaziz JD, Matsushita T, Tsubata T, Tedder TF. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J Immunol. 2009;182(12):7459–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lampropoulou V, Hoehlig K, Roch T, et al. TLR-activated B cells suppress T cell-mediated autoimmunity. J Immunol. 2008;180(7):4763–73. [DOI] [PubMed] [Google Scholar]
- 138.Mishima Y, Oka A, Liu B, et al. Microbiota maintain colonic homeostasis by activating TLR2/MyD88/PI3K signaling in IL-10-producing regulatory B cells. The Journal of clinical investigation. 2019;129(9):3702–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Riquelme E, Zhang Y, Zhang L, et al. Tumor Microbiome Diversity and Composition Influence Pancreatic Cancer Outcomes. Cell. 2019;178(4):795–806 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pushalkar S, Hundeyin M, Daley D, et al. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov. 2018;8(4):403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ochi A, Nguyen AH, Bedrosian AS, et al. MyD88 inhibition amplifies dendritic cell capacity to promote pancreatic carcinogenesis via Th2 cells. J Exp Med. 2012;209(9):1671–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Matsumoto M, Fujii Y, Baba A, et al. The calcium sensors STIM1 and STIM2 control B cell regulatory function through interleukin-10 production. Immunity. 2011;34(5):703–14. [DOI] [PubMed] [Google Scholar]
- 143.Kamanaka M, Kim ST, Wan YY, et al. Expression of interleukin-10 in intestinal lymphocytes detected by an interleukin-10 reporter knockin tiger mouse. Immunity. 2006;25(6):941–52. [DOI] [PubMed] [Google Scholar]
- 144.Madan R, Demircik F, Surianarayanan S, et al. Nonredundant roles for B cell-derived IL-10 in immune counter-regulation. J Immunol. 2009;183(4):2312–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Blair PA, Chavez-Rueda KA, Evans JG, et al. Selective targeting of B cells with agonistic anti-CD40 is an efficacious strategy for the generation of induced regulatory T2-like B cells and for the suppression of lupus in MRL/lpr mice. J Immunol. 2009;182(6):3492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dadgostar H, Zarnegar B, Hoffmann A, et al. Cooperation of multiple signaling pathways in CD40-regulated gene expression in B lymphocytes. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(3):1497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yoshizaki A, Miyagaki T, DiLillo DJ, et al. Regulatory B cells control T-cell autoimmunity through IL-21-dependent cognate interactions. Nature. 2012;491(7423):264–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shah S, Divekar AA, Hilchey SP, et al. Increased rejection of primary tumors in mice lacking B cells: inhibition of anti-tumor CTL and TH1 cytokine responses by B cells. Int J Cancer. 2005;117(4):574–86. [DOI] [PubMed] [Google Scholar]
- 149.Morrison AH, Diamond MS, Hay CA, Byrne KT, Vonderheide RH. Sufficiency of CD40 activation and immune checkpoint blockade for T cell priming and tumor immunity. Proceedings of the National Academy of Sciences of the United States of America. 2020;117(14):8022–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wennhold K, Weber TM, Klein-Gonzalez N, et al. CD40-activated B cells induce anti-tumor immunity in vivo. Oncotarget. 2017;8(17):27740–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Das S, Shapiro B, Vucic EA, Vogt S, Bar-Sagi D. Tumor Cell-Derived IL1beta Promotes Desmoplasia and Immune Suppression in Pancreatic Cancer. Cancer Res. 2020;80(5):1088–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Das S, Bar-Sagi D. BTK signaling drives CD1d(hi)CD5(+) regulatory B-cell differentiation to promote pancreatic carcinogenesis. Oncogene. 2019;38(17):3316–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lindner S, Dahlke K, Sontheimer K, et al. Interleukin 21-Induced Granzyme B-Expressing B Cells Infiltrate Tumors and Regulate T Cells. Cancer Research. 2013;73(8):2468–79. [DOI] [PubMed] [Google Scholar]
- 154.Iwata Y, Matsushita T, Horikawa M, et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117(2):530–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Qian L, Bian G-R, Zhou Y, et al. Short communication Clinical significance of regulatory B cells in the peripheral blood of patients with oesophageal cancer. Central European Journal of Immunology. 2015;2:263–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lv Y, Wang H, Liu Z. The Role of Regulatory B Cells in Patients with Acute Myeloid Leukemia. Med Sci Monit. 2019;25:3026–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ishigami E, Sakakibara M, Sakakibara J, et al. Coexistence of regulatory B cells and regulatory T cells in tumor-infiltrating lymphocyte aggregates is a prognostic factor in patients with breast cancer. Breast Cancer. 2019;26(2):180–9. [DOI] [PubMed] [Google Scholar]
- 158.Lechner A, Schlosser HA, Thelen M, et al. Tumor-associated B cells and humoral immune response in head and neck squamous cell carcinoma. Oncoimmunology. 2019;8(3):1535293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mao Y, Wang Y, Dong L, et al. Circulating exosomes from esophageal squamous cell carcinoma mediate the generation of B10 and PD-1(high) Breg cells. Cancer Sci. 2019;110(9):2700–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Murakami Y, Saito H, Shimizu S, et al. Increased regulatory B cells are involved in immune evasion in patients with gastric cancer. Sci Rep. 2019;9(1):13083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bodogai M, Lee Chang C, Wejksza K, et al. Anti-CD20 Antibody Promotes Cancer Escape via Enrichment of Tumor-Evoked Regulatory B Cells Expressing Low Levels of CD20 and CD137L. Cancer Research. 2013;73(7):2127–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wei X, Jin Y, Tian Y, et al. Regulatory B cells contribute to the impaired antitumor immunity in ovarian cancer patients. Tumor Biology. 2015;37(5):6581–8. [DOI] [PubMed] [Google Scholar]
- 163.Wang WW, Yuan XL, Chen H, et al. CD19+CD24hiCD38hiBregs involved in downregulate helper T cells and upregulate regulatory T cells in gastric cancer. Oncotarget. 2015;6(32):33486–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Largeot A, Pagano G, Gonder S, Moussay E, Paggetti J. The B-side of Cancer Immunity: The Underrated Tune. Cells. 2019;8(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sorrentino R, Morello S, Forte G, et al. B Cells Contribute to the Antitumor Activity of CpG-Oligodeoxynucleotide in a Mouse Model of Metastatic Lung Carcinoma. American Journal of Respiratory and Critical Care Medicine. 2011;183(10):1369–79. [DOI] [PubMed] [Google Scholar]
- 166.Deng J, Yuan S, Pennati A, et al. Engineered Fusokine GIFT4 Licenses the Ability of B Cells to Trigger a Tumoricidal T-cell Response. Cancer Research. 2014;74(15):4133–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Li W, Zhou M, Ren H, et al. Tumor-derived autophagosomes (DRibbles) induce B cell activation in a TLR2-MyD88 dependent manner. PloS one. 2013;8(1):e53564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ren H, Zhao S, Li W, et al. Therapeutic antitumor efficacy of B cells loaded with tumor-derived autophagasomes vaccine (DRibbles). J Immunother. 2014;37(8):383–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Barbera-Guillem E, Nelson MB, Barr B, et al. B lymphocyte pathology in human colorectal cancer. Experimental and clinical therapeutic effects of partial B cell depletion. Cancer Immunol Immunother. 2000;48(10):541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Winkler JK, Schiller M, Bender C, Enk AH, Hassel JC. Rituximab as a therapeutic option for patients with advanced melanoma. Cancer Immunol Immunother. 2018;67(6):917–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pinc A, Somasundaram R, Wagner C, et al. Targeting CD20 in Melanoma Patients at High Risk of Disease Recurrence. Molecular Therapy. 2012;20(5):1056–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Theurich S, Schlaak M, Steguweit H, et al. Targeting Tumor-Infiltrating B Cells in Cutaneous T-Cell Lymphoma. J Clin Oncol. 2016;34(12):e110–6. [DOI] [PubMed] [Google Scholar]
- 173.Aklilu M, Stadler WM, Markiewicz M, et al. Depletion of normal B cells with rituximab as an adjunct to IL-2 therapy for renal cell carcinoma and melanoma. Annals of Oncology. 2004;15(7):1109–14. [DOI] [PubMed] [Google Scholar]
- 174.JahrsdÖrfer B, Blackwell SE, Wooldridge JE, et al. B-chronic lymphocytic leukemia cells and other B cells can produce granzyme B and gain cytotoxic potential after interleukin-21-based activation. Blood. 2006;108(8):2712–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kemp TJ, Moore JM, Griffith TS. Human B Cells Express Functional TRAIL/Apo-2 Ligand after CpG-Containing Oligodeoxynucleotide Stimulation. The Journal of Immunology. 2004;173(2):892–9. [DOI] [PubMed] [Google Scholar]
- 176.Pal Singh S, Dammeijer F, Hendriks RW. Role of Bruton’s tyrosine kinase in B cells and malignancies. Molecular Cancer. 2018;17(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Tao H, Lu L, Xia Y, et al. Antitumor effector B cells directly kill tumor cells via the Fas/FasL pathway and are regulated by IL-10. European Journal of Immunology. 2015;45(4):999–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lee-Chang C, Bodogai M, Martin-Montalvo A, et al. Inhibition of Breast Cancer Metastasis by Resveratrol-Mediated Inactivation of Tumor-Evoked Regulatory B Cells. The Journal of Immunology. 2013;191(8):4141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Song SS, Yuan PF, Li PP, et al. Protective Effects of Total Glucosides of Paeony on N-nitrosodiethylamine-induced Hepatocellular Carcinoma in Rats via Down-regulation of Regulatory B Cells. Immunol Invest. 2015;44(6):521–35. [DOI] [PubMed] [Google Scholar]
- 180.Wang Z, Cheng Q, Tang K, et al. Lipid mediator lipoxin A4 inhibits tumor growth by targeting IL-10-producing regulatory B (Breg) cells. Cancer Letters. 2015;364(2):118–24. [DOI] [PubMed] [Google Scholar]
- 181.Wejksza K, Lee-Chang C, Bodogai M, et al. Cancer-Produced Metabolites of 5-Lipoxygenase Induce Tumor-Evoked Regulatory B Cells via Peroxisome Proliferator–Activated Receptor α. The Journal of Immunology. 2013;190(6):2575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]


