Abstract
BACKGROUND AND PURPOSE:
Cortical and white matter changes have been identified outside the MR imaging–visible cortical/subcortical tubers in the tuberous sclerosis complex. The aim of this study was to evaluate DTI changes in the corpus callosum and internal capsules and to correlate the DTI changes with cortical/subcortical tuber load.
MATERIALS AND METHODS:
Twelve TSC patients and 23 controls underwent MR imaging including DTI. FA, trace, D‖, and D⫠ of genu and splenium of corpus callosum and right and left internal capsules were assessed. The number and volume of cortical/subcortical tubers were correlated with DTI indices of corpus callosum and internal capsules.
RESULTS:
In the genu and splenium, FA was lower and trace (P < .01) and D⫠ were higher (P < .01), and in the internal capsules, trace was higher (P = .04) in TSC patients compared with controls. The total tuber volume correlated positively with trace of genu (r = 0.77, P < .01) and splenium (r = 0.69, P = .01) and with D⫠ of splenium (r = 0.68, P = .01), and negatively with FA of splenium (r = −0.60, P = .04) of corpus callosum. The left and right hemispheric tuber volume correlated positively with trace of left (r = 0.56, P = .05) and right (r = 0.67, P = .02) internal capsules.
CONCLUSIONS:
Our findings of reduced FA, elevated trace, and elevated D⫠ in the corpus callosum and internal capsules may be related to abnormalities in myelin. The correlations between tuber volume and DTI indices in corpus callosum and internal capsules suggested that more extensive malformation as demonstrated by larger tuber load was more likely to be associated with more severe DTI changes in the commissural and projection white matter.
Tuberous sclerosis complex is an autosomal dominant disorder, characterized by a variety of hamartomatous lesions in various organs, including the brain.1 Two distinct mutated or deleted genes have been identified in patients with TSC, that is, 9q34 (TSC1) and 16q13 (TSC2), and are responsible for the disease.2,3 The brain is the most frequently affected organ in TSC, and neurologic manifestations are a common cause of morbidity and mortality, including seizures, developmental delay, and neurobehavioral abnormalities, and are present in >80% of TSC patients.1 Four major neuroimaging and neuropathologic findings are observed in TSC patients: subependymal nodules, cortical tubers, subependymal giant cell astrocytomas, and white matter abnormalities. Besides the classic MR imaging findings, cortical and white matter changes have been identified outside the MR imaging–visible cortical/subcortical tubers on histology.4
DTI provides information on the microstructure of tissues and white matter tracts in vivo and therefore can be used to evaluate alterations in the microstructure of white matter in disease states, even when the white matter appears normal on structural imaging.5 Previous reports on quantitative MR imaging methods by using DWI and DTI in cerebral TSC have mostly concentrated on cortical tubers and white matter lesions.6–8 Recently, abnormalities in the NAWM have been reported by using DWI and DTI.9,10 However, it is uncertain if the changes in the NAWM are related to tuber load. Cortical tubers are a type of malformation of cortical development,11 and malformations affecting the cortex could affect the white matter as well.5,12 The corpus callosum is the largest commissural tract connecting the 2 cerebral hemispheres. The internal capsules contain major white matter tracts projecting from different lobes of the cerebrum to the thalami, brain stem, and spinal cord.13 Any lesions affecting the cortex and adjacent subcortical white matter could potentially affect the distal commissural and projection white matter. We hypothesize that the abnormalities in the normal-appearing white matter of commissural and projection pathways such as the corpus callosum and internal capsules are related to the cortical/subcortical tuber load. The aim of this study was to evaluate the DTI changes in the corpus callosum and internal capsules and to correlate the DTI changes with cortical/subcortical tuber load.
Materials and Methods
Patients
The study protocol had the approval of our institutional research ethics board. The inclusion criteria for the study were children between the ages of 4 and 18 years with established diagnosis of TSC according to clinical and imaging criteria.14 Twelve patients (9 boys and 3 girls; mean age, 9.2 years; age range, 4.8–16 years) underwent MR imaging including DTI between March 2008 to March 2009. All the patients had typical TSC findings in the brain, including cortical/subcortical tubers, white matter abnormalities, and subependymal nodules. Eleven patients had seizures, 8 had developmental delay, 4 had autistic spectrum disorder, and 1 had attention deficit/hyperactivity disorder. Twenty-three control subjects (13 boys and 10 girls; mean age, 11.1 years; age range, 5–17 years) with normal MR imaging and no histories of neurologic disorders were included in the study.
MR Imaging
MR imaging was performed with a 3T magnet (Achieva; Phillips Medical System, Best, the Netherlands) and an 8-channel head coil. Imaging protocol consisted of a variety of sequences, including sagittal 3D T1-weighted sequence (TR/TE, 4.9/2.3 ms; section thickness, 1 mm; FOV, 22 cm; matrix, 220 × 220), axial and coronal T2-weighted images (TR/TE, 4200/80; section thickness, 3 mm; FOV, 22 cm; matrix, 400 × 272), and axial FLAIR (TR/TE, 10,000/140; section thickness, 3 mm; FOV, 22 cm; matrix, 316 × 290).
Diffusion Tensor Imaging
Axial DTI was performed on the same MR imaging by using single-shot diffusion-weighted echo-planar imaging in both patients and controls. The imaging parameters included 15 spatially isotropically arranged noncollinear directions, TR/TE of 11,000/55, section thickness of 2 mm, FOV of 22 cm, matrix of 112 × 112, and b-value of 1000 s/mm2 and 0 s/mm2. Echo-planar image distortion was corrected automatically on the scanner.
Data Analysis
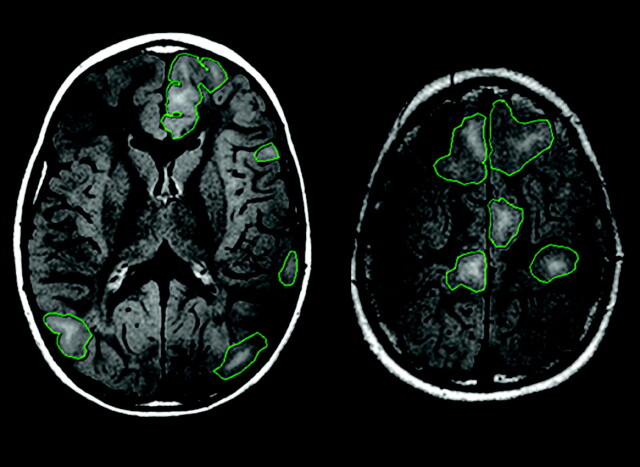
FLAIR was used to identify and assess the cortical/subcortical tubers, as FLAIR has previously been shown to be sensitive at identifying tubers in TSC patients.15 The number of cortical/subcortical tubers and their lobar distribution in the right and left cerebral hemispheres were assessed. The margins of the cortical/subcortical tubers were manually outlined on each section on the PACS system (GE Healthcare, Milwaukee, Wisconsin) (Fig 1) by a neuroradiologist (G.S.). The volume of each cortical/subcortical tuber was calculated by multiplying the area of each of the tubers by the section thickness. The hemispheric tuber volume was the sum of the volume of each tuber in 1 cerebral hemisphere. The total cerebral tuber volume was the sum of the volume of each tuber in both hemispheres.
Fig 1.
Axial FLAIR showing outlines of the MR imaging–visible cortical/subcortical tubers, which have been outlined manually to calculate the volume of the cortical/subcortical tubers.
DTI data processing was performed with DTIStudio (version 2.4; Johns Hopkins University, Baltimore, Maryland; http://www.mristudio.org). Diagonalization of the diffusion tensor yielded 6 independent tensor elements, 3 eigenvalues (λ1, λ2, and λ3) and 3 eigenvectors, which provide 3D information about the diffusivity of water molecules per voxel that can be represented graphically by an ellipsoid.16 From these data, the FA and trace maps were calculated. D‖ and D⊥, which were the average of λ2 and λ3, were also evaluated. ROIs were carefully drawn to outline the genu and splenium of corpus callosum, and right and left internal capsules on the directionally color-coded map (Fig 2). The same ROIs were then transposed onto FA, trace, and eigenvalue maps.
Fig 2.
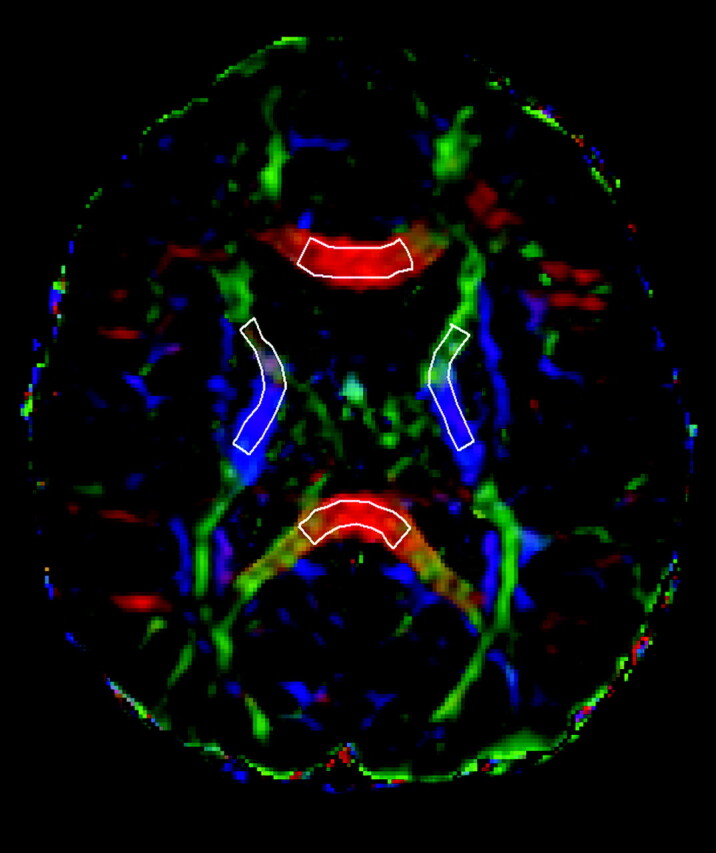
Selected ROIs are carefully drawn over the fractional anisotropy color-encoded images, in the genu and splenium of corpus callosum and in the internal capsules.
Statistical Analysis
Data were analyzed by using SAS 9.1 (SAS Institute, Cary, North Carolina). The age of patients and controls was compared by using a t test. Generalized estimating equations were used to compare FA, trace, D‖, and D⫠ of the genu and splenium of corpus callosum, as well as internal capsules of the patients versus controls, after adjusting for age and side of measurements. The right and left hemispheric tuber volume and the number of cortical/subcortical tubers were correlated with FA, trace, D‖, and D⫠ of the right and left internal capsules, respectively, in patients with TSC by using the Pearson correlation coefficient. The total tuber volume and the total number of the cortical/subcortical tubers were also correlated with FA, trace, D‖, and D⊥ of the genu and splenium of corpus callosum by using the Pearson correlation coefficient. Due to the exploratory nature of this study, no correction for type I error was made, and statistical significance was set at P < .05.
Results
Patients
There was no significant difference between the age of TSC patients and controls (P > .05).
MR Imaging
There were 314 cortical/subcortical tubers assessed in the 12 patients, varying from 3 to 39 tubers in each patient (mean of 26 cortical/subcortical tubers per subject). The distribution of cortical/subcortical tubers was as follows: 144 in frontal lobes, 64 in parietal lobes, 42 in temporal lobes, 57 in occipital lobes, and 7 in insular cortex. The mean right hemispheric cortical/subcortical tuber volume was 75.4 ± 84.7 cm3 and the mean left hemispheric cortical/subcortical tuber volume was 66.3 ± 63.4 cm3. The mean total cerebral cortical/subcortical tuber volume was 141.7 ± 141.0 cm3. The mean number of cortical/subcortical tubers in the right hemisphere was 13.0 ± 6.2 and in the left hemisphere was 13.1 ± 7.4.
Diffusion Tensor Imaging
Patients with TSC had significantly higher trace in the genu (P < .01) and splenium (P < .01) of corpus callosum, as well as in the internal capsules (P = .04), compared with controls (Table 1). FA was significantly lower in the genu (P < .01) and splenium (P < .01) of corpus callosum in TSC patients than in controls. D⊥ was higher in the genu (P < .01) and splenium (P < .01) of corpus callosum in TSC patients compared with controls. There were no significant differences in D‖ in the genu and splenium of corpus callosum and internal capsules in TSC patients compared with controls (P = .33, P = .41, and P = .06, respectively).
Table 1:
Mean trace, FA, D‖, and D⊥ of the genu and splenium of corpus callosum and internal capsules of patients with TSC and controls
| Mean Trace × 10−3 (SD) |
Mean FA (SD) |
Mean D‖ × 10−3 (SD) |
Mean D⊥ × 10−3 (SD) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TSC | Controls | P Value | TSC | Controls | P Value | TSC | Controls | P Value | TSC | Controls | P Value | |
| Genu of corpus callosum | 3.36 (0.21) | 3.03 (0.18) | <.01 | 0.75 (0.06) | 0.81 (0.02) | <.01 | 2.34 (0.09) | 2.26 (0.17) | .33 | 0.49 (0.12) | 0.38 (0.03) | <.01 |
| Splenium of corpus callosum | 3.18 (0.21) | 2.85 (0.18) | <.01 | 0.79 (0.08) | 0.85 (0.35) | <.01 | 2.33 (0.17) | 2.25 (0.18) | .41 | 0.42 (0.12) | 0.30 (0.05) | <.01 |
| Internal capsules | 2.85 (0.13) | 2.73 (0.13) | .04 | 0.69 (0.04) | 0.70 (0.03) | .95 | 1.88 (0.11) | 1.80 (0.08) | .06 | 0.49 (0.04) | 0.46 (0.04) | .35 |
The total tuber volume correlated positively with trace of the genu (r = 0.77, P < .01) and splenium (r = 0.69, P = .01) of corpus callosum, and with D⊥ of the splenium of corpus callosum (r = 0.68, P = .01) (Table 2). The total tuber volume correlated negatively with FA of the splenium of corpus callosum (r = −0.60, P = .04). There was a tendency for left hemispheric tuber volume to correlate positively with trace (r = 0.56, P = .05) of the left internal capsule. There was significant positive correlation between left hemispheric tuber volume and D‖ (r = 0.63, P = .02) of the left internal capsule. The right hemispheric tuber volume correlated positively with the trace (r = 0.67, P = .02) and D‖ (r = 0.59, P = .04) of the right internal capsule. There were no significant correlations between total tuber number or hemispheric tuber number and trace, FA, D⫠, and D‖ of the genu and splenium of corpus callosum or internal capsules, respectively.
Table 2:
Pearson correlation coefficient between hemisphere tuber volume and hemispheric tuber number with DTI indices of internal capsules, as well as between total tuber volume and total tuber number with DTI indices of genu and splenium of corpus callosum
| Correlation | Mean Trace (P Value) | Mean FA (P Value) | Mean D‖ (P Value) | Mean D⊥ (P Value) | |
|---|---|---|---|---|---|
| Left internal capsule | Left hemispheric tuber volume | r = 0.56 (P = .05) | r = 0.36 (P = .25) | r = 0.63 (P = .02) | r = 0.06 (P = 0.84) |
| Left hemispheric tuber number | r = −0.16 (P = .61) | r = 0.43 (P = .16) | r = 0.13 (P = .68) | r = −0.38 (P = .22) | |
| Right internal capsule | Right hemispheric tuber volume | r = 0.67 (P = .02) | r = 0.02 (P = .94) | r = 0.59 (P = .04) | r = 0.23 (P = .46) |
| Right hemispheric tuber number | r = 0.04 (P = .89) | r = 0.06 (P = .86) | r = 0.01 (P = .99) | r = −0.06 (P = .84) | |
| Genu of corpus callosum | Total tuber volume | r = 0.77 (P < .01) | r = −0.35 (P = .25) | r = 0.53 (P = .07) | r = 0.43 (P = .16) |
| Total tuber number | r = 0.44 (P = .15) | r = −0.20 (P = .53) | r = −0.23 (P = .47) | r = 0.14 (P = .66) | |
| Splenium of corpus callosum | Total tuber volume | r = 0.69 (P = .01) | r = −0.60 (P = .04) | r = −0.11 (P = .74) | r = 0.68 (P = .01) |
| Total tuber number | r = 0.19 (P = .55) | r = −0.40 (P = .19) | r = −0.34 (P = .27) | r = 0.40 (P = .19) |
Discussion
White matter abnormalities are a well-recognized finding on conventional MR imaging of TSC patients.17,18 DWI and DTI give additional, quantitative information about the microstructure of the brain, in particular of the white matter, and they have been used to evaluate brain abnormalities in TSC patients. Several studies have found altered diffusion index in the cortical tubers and white matter lesions associated with TSC.4,6,7,19 Piao et al7 assessed 14 children and adults with TSC and found reduced FA and increased ADC in the white matter lesions in patients with TSC. Karadag et al6 evaluated 7 children with TSC and also found reduced FA and higher ADC values in the white matter lesions. Using diffusion-weighted MR imaging, Firat et al19 found elevated ADC in white matter lesions in 6 children with TSC. The reduced FA and elevated ADC could be related to hypomyelination, gliosis, and heterotopic cells,20 which may lead to loss of structural barriers to water motion.
In this study, we have found reduced FA, increased trace and D⫠ in the NAWM in the genu and splenium of corpus callosum, as well as increased trace in the internal capsule in children with TSC. Our findings of abnormal DTI indices in these areas of NAWM were similar to findings by other investigators. Makki et al10 evaluated 6 children with TSC and found significant increase in mean diffusivity, decrease in anisotropy, and increase in D⫠ that was greater than the increase in D‖ in the NAWM of corpus callosum, internal capsules, and external capsules. Garaci et al21 evaluated 18 children and adults with TSC by using DWI, and they found increased ADC in the supratentorial NAWM, specifically in the occipital white matter, frontal white matter, centrum semiovale, parietal white matter, and corona radiata. Using DWI, Arulrajah et al9 also found increased ADC in the NAWM within the frontal, parietal, and occipital lobes and pons in 23 children and adults with TSC.
We have found increased D⫠ in the NAWM in the genu and splenium of corpus callosum. Animal models with absent myelin have demonstrated an increase in D⫠,22 suggesting that changes in D⫠ could potentially be used to differentiate myelin loss from axonal injury, which was associated with elevated D‖. Diffuse hypomyelination of the cerebral white matter was thought to occur in TSC patients.9,10,23 The white matter microstructural changes in NAWM in TSC patients may be related to an abnormal myelin packing due to giant cells, gliosis, and myelination defects.10 Using ex vivo postmortem MR imaging and neuropathologic examination, Boer et al24 reported that all areas of T2 high signal intensity on MR imaging were found to correspond with areas of demyelination. However, the areas of reduced myelin attenuation appeared to be more extensive on histologic examination compared with the MR imaging visible abnormality.
In this study, there was a positive correlation between trace in the genu and splenium of corpus callosum and total cortical/subcortical tuber volume, as well as between trace in the internal capsules and hemispheric cortical/subcortical tuber volume. Peng et al23 observed a negative correlation between D‖ in the superior longitudinal fasciculus and the number of tubers in the frontal and parietal lobes. Any malformation that traversed the cerebral mantle could disrupt the white matter tracts.12 Corpus callosum is the major commissural tract in the brain connecting the right and left cerebral hemispheres, and the internal capsules contain projection tracts connecting the cerebrum to the thalamus, brain stem, and spinal cord.13 Malformations in the cortical/subcortical tubers may exert secondary changes in the commissural and projection white matter tracts projecting to/from these lesions, and therefore account for the significant correlation between trace in the corpus callosum and internal capsules with cortical/subcortical tuber volume. Previously, we have investigated children with malformations of cortical development and found abnormal white matter tracts projecting to/from malformations of cortical development,5,12 even when the deep white matter demonstrated normal signal intensity on structural MR imaging. Similarly, Lee et al25 also found abnormal association white matter tracts projecting to/from focal cortical dysplasia.
There were greater correlations between FA and D⫠ of the splenium of corpus callosum with total cortical/subcortical tuber volume compared with FA and D⫠ of the genu of corpus callosum with total cortical/subcortical tuber volume. The genu of corpus callosum contains white matter tracts from the prefrontal and premotor area,26,27 and the splenium of corpus callosum contains white matter tracts from the parietal, temporal, and occipital lobes.27 The total number of cortical/subcortical tubers in the parietal, temporal, and occipital lobes combined was greater than the total number of tubers in the frontal lobes. The greater correlations between FA and D⫠ of the splenium of corpus callosum with cortical/subcortical tuber volume could be related to the greater tuber load within the parietal, temporal, and occipital lobes combined.
Ridler et al28 found a significant reduction in the white matter volume involving the longitudinal fasciculi and other major intrahemispheric tracts of 10 patients with TSC compared with controls. However, they did not find a correlation between white matter volume and number of cortical/subcortical tubers. We have also not found a significant correlation between tuber number and DTI indices of NAWM in the corpus callosum and internal capsules, suggesting that tuber number was a less sensitive indicator of tuber load than tuber volume. We have assessed the white matter by using DTI, as DTI indices were a more sensitive marker of abnormal microstructure of the white matter compared with white matter volume.
One of the potential weaknesses of our study was that we have used the MR imaging–visible lesion to define the cortical/subcortical tuber volume. The histologic abnormalities of cortical/subcortical tubers frequently extend beyond the MR imaging–visible lesion. By using the abnormal signal intensity on MR imaging to define the margin of the cortical/subcortical tuber, we may have underestimated the volume of the cortical/subcortical tuber. The relationship of abnormal DTI indices in NAWM with neurocognitive performance or autism is not clear at present; future studies are required to elucidate this relationship. At present it is not known if these white matter changes are reversible. Novel therapeutic agents such as rapamycin have been reported to improve cognitive performance and reduce seizure frequency in patients with TSC.29,30 Further studies are necessary to determine if such therapeutic agents could affect cortical/subcortical tuber load and reduce or reverse the changes in NAWM.
Conclusions
This study showed reduced FA, elevated trace, and elevated D⫠ in the genu and splenium of corpus callosum and internal capsules. These findings may be related to abnormalities in myelin. There was a positive correlation between total cortical/subcortical tuber volume and trace of the genu and splenium of corpus callosum, as well as hemispheric cortical/subcortical tuber volume and trace of the internal capsules. Malformations within the cortical/subcortical tubers could result in microstructural changes in the commissural and projection white matter tracts connecting to/from the abnormal cortex, with larger tuber load demonstrating more severe changes in the distal white matter.
Abbreviations
- ADC
apparent diffusion coefficient
- D‖
axial diffusivity
- D⊥
radial diffusivity
- DTI
diffusion tensor imaging
- DWI
diffusion-weighted imaging
- FA
fractional anisotropy
- FLAIR
fluid-attenuated inversion recovery
- NAWM
normal-appearing white matter
- ROI
region of interest
- TSC
tuberous sclerosis complex
References
- 1. Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med 2006;355:1345–56 [DOI] [PubMed] [Google Scholar]
- 2. European Chromosome 16 Tuberous Sclerosis Consortium. Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell 1993;75:1305–15 [DOI] [PubMed] [Google Scholar]
- 3. van Slegtenhorst M, de Hoogt R, Hermans C, et al. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science 1997;277:805–08 [DOI] [PubMed] [Google Scholar]
- 4. Chandra PS, Salamon N, Huang J, et al. FDG-PET/MRI coregistration and diffusion-tensor imaging distinguish epileptogenic tubers and cortex in patients with tuberous sclerosis complex: a preliminary report. Epilepsia 2006;47:1543–49 [DOI] [PubMed] [Google Scholar]
- 5. Widjaja E, Zarei Mahmoodabadi S, Otsubo H, et al. Subcortical alterations in tissue microstructure adjacent to focal cortical dysplasia: detection at diffusion-tensor MR imaging by using magnetoencephalographic dipole cluster localization. Radiology 2009;251:206–15 [DOI] [PubMed] [Google Scholar]
- 6. Karadag D, Mentzel HJ, Gullmar D, et al. Diffusion tensor imaging in children and adolescents with tuberous sclerosis. Pediatr Radiol 2005;35:980–83 [DOI] [PubMed] [Google Scholar]
- 7. Piao C, Yu A, Li K, et al. Cerebral diffusion tensor imaging in tuberous sclerosis. Eur J Radiol 2009;71:249–52 [DOI] [PubMed] [Google Scholar]
- 8. Sener RN. Tuberous sclerosis: diffusion MRI findings in the brain. Eur Radiol 2002;12:138–43 [DOI] [PubMed] [Google Scholar]
- 9. Arulrajah S, Ertan G, Jordan L, et al. Magnetic resonance imaging and diffusion-weighted imaging of normal-appearing white matter in children and young adults with tuberous sclerosis complex. Neuroradiology 2009;51:781–86 [DOI] [PubMed] [Google Scholar]
- 10. Makki MI, Chugani DC, Janisse J, et al. Characteristics of abnormal diffusivity in normal-appearing white matter investigated with diffusion tensor MR imaging in tuberous sclerosis complex. AJNR Am J Neuroradiol 2007;28:1662–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barkovich AJ, Kuzniecky RI, Jackson GD, et al. A developmental and genetic classification for malformations of cortical development. Neurology 2005;65:1873–87 [DOI] [PubMed] [Google Scholar]
- 12. Widjaja E, Blaser S, Miller E, et al. Evaluation of subcortical white matter and deep white matter tracts in malformations of cortical development. Epilepsia 2007;48:1460–69 [DOI] [PubMed] [Google Scholar]
- 13. Wakana S, Jiang H, Nagae-Poetscher LM, et al. Fiber tract-based atlas of human white matter anatomy. Radiology 2004;230:77–87 [DOI] [PubMed] [Google Scholar]
- 14. Roach ES, Gomez MR, Northrup H. Tuberous Sclerosis Complex Consensus Conference: revised clinical diagnostic criteria. J Child Neurol 1998;13:624–28 [DOI] [PubMed] [Google Scholar]
- 15. Takanashi J, Sugita K, Fujii K, et al. MR evaluation of tuberous sclerosis: increased sensitivity with fluid-attenuated inversion recovery and relation to severity of seizures and mental retardation. AJNR Am J Neuroradiol 1995;16:1923–28 [PMC free article] [PubMed] [Google Scholar]
- 16. Pierpaoli C, Jezzard P, Basser PJ, et al. Diffusion tensor MR imaging of the human brain. Radiology 1996;201:637–48 [DOI] [PubMed] [Google Scholar]
- 17. Braffman BH, Bilaniuk LT, Naidich TP, et al. MR imaging of tuberous sclerosis: pathogenesis of this phakomatosis, use of gadopentetate dimeglumine, and literature review. Radiology 1992;183:227–38 [DOI] [PubMed] [Google Scholar]
- 18. Griffiths PD, Bolton P, Verity C. White matter abnormalities in tuberous sclerosis complex. Acta Radiol 1998;39:482–86 [DOI] [PubMed] [Google Scholar]
- 19. Firat AK, Karakas HM, Erdem G, et al. Diffusion weighted MR findings of brain involvement in tuberous sclerosis. Diagn Interv Radiol 2006;12:57–60 [PubMed] [Google Scholar]
- 20. Yagishita A, Arai N. Cortical tubers without other stigmata of tuberous sclerosis: imaging and pathological findings. Neuroradiology 1999;41:428–32 [DOI] [PubMed] [Google Scholar]
- 21. Garaci FG, Floris R, Bozzao A, et al. Increased brain apparent diffusion coefficient in tuberous sclerosis. Radiology 2004;232:461–65 [DOI] [PubMed] [Google Scholar]
- 22. Song SK, Sun SW, Ramsbottom MJ, et al. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 2002;17:1429–36 [DOI] [PubMed] [Google Scholar]
- 23. Peng SS, Lee WT, Wang YH, et al. Cerebral diffusion tensor images in children with tuberous sclerosis: a preliminary report. Pediatr Radiol 2004;34:387–92 [DOI] [PubMed] [Google Scholar]
- 24. Boer K, Troost D, Jansen F, et al. Clinicopathological and immunohistochemical findings in an autopsy case of tuberous sclerosis complex. Neuropathology 2008;28:577–90 [DOI] [PubMed] [Google Scholar]
- 25. Lee SK, Kim DI, Mori S, et al. Diffusion tensor MRI visualizes decreased subcortical fiber connectivity in focal cortical dysplasia. Neuroimage 2004;22:1826–29 [DOI] [PubMed] [Google Scholar]
- 26. Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum: a postmortem morphological study. Brain 1989;112:799–835 [DOI] [PubMed] [Google Scholar]
- 27. Hofer S, Frahm J. Topography of the human corpus callosum revisited: comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage 2006;32:989–94 [DOI] [PubMed] [Google Scholar]
- 28. Ridler K, Bullmore ET, De Vries PJ, et al. Widespread anatomical abnormalities of grey and white matter structure in tuberous sclerosis. Psychol Med 2001;31:1437–46 [DOI] [PubMed] [Google Scholar]
- 29. Wong M. Mammalian target of rapamycin (mTOR) inhibition as a potential antiepileptogenic therapy: from tuberous sclerosis to common acquired epilepsies. Epilepsia 2009;51:27–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ehninger D, de Vries PJ, Silva AJ. From mTOR to cognition: molecular and cellular mechanisms of cognitive impairments in tuberous sclerosis. J Intellect Disabil Res 2009;53:838–51 [DOI] [PMC free article] [PubMed] [Google Scholar]